Abstract
Background: An exaggerated blood pressure response (EBPR) during exercise testing is not well defined, and several blood pressure thresholds are used in different studies and recommended in different guidelines. Methods: Competitive athletes of any age without known arterial hypertension who presented for preparticipation screening were included in the present study and categorized for EBPR according to American Heart Association (AHA), European Society of Cardiology (ESC), and American College of Sports Medicine (ACSM) guidelines as well as the systolic blood pressure/MET slope method. Results: Overall, 1137 athletes (mean age 21 years; 34.7% females) without known arterial hypertension were included April 2020–October 2021. Among them, 19.6%, 15.0%, and 6.8% were diagnosed EBPR according to ESC, AHA, and ACSM guidelines, respectively. Left ventricular hypertrophy (LVH) was detected in 20.5% of the athletes and was approximately two-fold more frequent in athletes with EBPR than in those without. While EBPR according to AHA (OR 2.35 [95%CI 1.66–3.33], p < 0.001) and ACSM guidelines (OR 1.81 [95%CI 1.05–3.09], p = 0.031) was independently (of age and sex) associated with LVH, EBPR defined according to ESC guidelines (OR 1.49 [95%CI 1.00–2.23], p = 0.051) was not. In adult athletes, only AHA guidelines (OR 1.96 [95%CI 1.32–2.90], p = 0.001) and systolic blood pressure/MET slope method (OR 1.73 [95%CI 1.08–2.78], p = 0.023) were independently predictive for LVH. Conclusions: Diverging guidelines exist for the screening regarding EBPR. In competitive athletes, the prevalence of EBPR was highest when applying the ESC (19.6%) and lowest using the ACSM guidelines (6.8%). An association of EBPR with LVH in adult athletes, independently of age and sex, was only found when the AHA guideline or the systolic blood pressure/MET slope method was applied.
Keywords: arterial hypertension, exercise hypertension, blood pressure, exercise testing
1. Introduction
Arterial hypertension is the most important and most common cardiovascular risk factor (CVRF) for morbidity and mortality worldwide [1,2,3,4]. The prevalence of arterial hypertension is high [5], affecting approximately 78 million adults in the United States of America [6]. While the prevalence of arterial hypertension increases substantially with age [7,8,9,10], its prevalence in athletes is low, at approximately 3% [11].
Diagnosis of arterial hypertension by resting blood pressure is well defined. In Europe, a systolic blood pressure (BP) value of ≥140 mmHg and a diastolic BP value of ≥90 mmHg are the defined thresholds of arterial hypertension [12,13,14,15]. In contrast, an exaggerated blood pressure response (EBPR) during treadmill and bicycle exercise testing is not well defined and poorly recognized, and several blood pressure thresholds were used in the different studies and are recommended in different guidelines [9,14,16,17,18,19,20,21,22]. While the American Heart Association (AHA) guideline [23] (EBPR threshold: systolic peak BP >210 mmHg in men, >190 mmHg in women, and/or >90 mmHg diastolic peak BP in both sexes) and the European Society of Cardiology (ESC) guideline [22,24] (EBPR threshold: systolic peak BP >220 mmHg in men, >200 mmHg in women, and/or >85 mmHg in men and 80 mmHg in women for diastolic peak BP) used sex-specific EBPR thresholds, the American College of Sports Medicine (ACSM) guideline [20,21] (EBPR threshold: systolic peak BP >225 mmHg and/or >90 mmHg for diastolic peak BP in both sexes) recommends the same systolic and diastolic thresholds values for both sexes.
However, for arterial-hypertension-naïve individuals with EBPR during the exercise testing, it was shown that these individuals are at increased risk of developing both arterial hypertension as well as cardiovascular events in the future, underlining the importance of this phenomenon [1,4,17,25,26,27,28,29,30,31,32,33,34,35,36,37].
In the context of arterial hypertension, it is well known that an increase in left ventricular mass and left ventricular hypertrophy (LVH) are associated with cardiovascular disease (CVD) as well as an elevated number of cardiovascular events and mortality [37,38]. Despite the development of the heart in highly trained athletes, a septal thickness of ≥13 mm was observed in only a very small number of athletes and should be considered as LVH in athletes [22,39,40,41].
Thus, the objectives of the present study were to evaluate (I) how prevalent EBPR is in athletes and (II) which definition of an EBPR during exercise testing was best associated with LVH in athletes without known arterial hypertension.
2. Materials and Methods
We performed a retrospective analysis of athletes of any age without known arterial hypertension who presented at the Department of Sports Medicine (Medical Clinic VII) of the University Hospital Heidelberg (Germany) for their preparticipation screening examination between April 2020 and October 2021.
2.1. Enrolled Subjects
Athletes were eligible for this study if they performed regular training for competition, were able to perform an exercise test at our department, had no contraindications for exercise testing, and had no known diagnosis of arterial hypertension. Exclusion criteria were a known diagnosis of arterial hypertension and contraindications regarding performing exercise testing [22,23].
2.2. Ethical Aspects
The requirement for informed consent was waived as we used only anonymized retrospective data routinely collected during the health screening process. Studies in Germany involving a retrospective analysis of diagnostic standard data of anonymized patients do not require an ethics statement.
2.3. Definitions
Arterial hypertension at rest was defined according to the ESC guidelines [42]. In all athletes, a transthoracic echocardiography was performed. Investigated echocardiographic parameters were defined according to current guidelines [22,43].
LVH was defined as (I) septal or posterior left ventricular (LV) wall diameter ≥13 mm [22,40] or (II) LV mass >162 g in female or >224 g in male individuals [43]. LV mass was computed according the established 2D echocardiography area-length method: LV mass = 0.80 × (1.04 × [(septal LV wall thickness + LV end-diastolic diameter + posterior LV wall thickness)3 − (LV end-diastolic diameter)3]) + 0.6 g [43]. LVH was considered to be present if one or both of the definitions applied.
EBPR was defined on the basis of the peak BP values during exercise testing according to three different guidelines and the systolic BP/MET slope method:
American Heart Association (AHA) guidelines [23]: systolic peak BP >210 mmHg in men, >190 mmHg in women, and/or >90 mmHg diastolic peak BP in both sexes.
European Society of Cardiology (ESC) guidelines [22,24]: systolic peak BP >220 mmHg in men, >200 mmHg in women, and/or >85 mmHg in men and 80 mmHg in women for diastolic peak BP.
The American College of Sports Medicine (ACSM) guidelines [20,21]: systolic peak BP >225 mmHg and/or >90 mmHg, for diastolic peak BP in both sexes.
The systolic BP/MET slope method [44,45,46,47]: The Δ regarding systolic BP was calculated as maximum systolic BP during exercise—systolic BP at rest and was indexed by the increase in MET from rest (Δ regarding MET was calculated as peak MET-1) to obtain the systolic BP/MET slope [46]. In accordance with previous studies, a cutoff value > 6.2 mmHg/MET was used to define an EBPR [44,46]. The MET value was calculated based on the athletes’ VO2 maximum values during exercise testing as recommended by the ACSM guideline (MET = VO2max/3.5 mL·kg−1·min−1) [48].
Exercise testing was performed according to current guidelines with electrocardiogram (ECG) and BP measurements at the end of every load level. The exercise test was stopped if the athlete was at their maximum capacity or stopping criteria according to current guidelines [22,23].
Obesity was defined as body mass index (BMI) ≥30 kg/m2 according to the World Health Organization.
2.4. Statistics
Athletes categorized as athletes with EBPR according to the three aforementioned guidelines and the systolic BP/MET slope method were compared to those athletes not categorized as EBPR (normal BP response during the exercise test) with the help of the Wilcoxon–Mann–Whitney U test for continuous variables and Fisher’s exact or chi2 test for categorical variables, as appropriate. Data of continuous variables were presented as median and interquartile range and categorical variables as absolute numbers with related percentages.
We performed univariate and multivariate logistic regression models to investigate the association between EBPR (defined according to the three guidelines) as well as BP values at rest and maximum values during exercise on the one hand and LVH on the other hand. Multivariate regression models were adjusted for age and sex in order to prove the independence of the statistical results of athletes’ age and sex. Results of the logistic regressions are presented as odds ratio (OR) and 95% Confidence interval (CI).
All statistical analyses were carried out with the use of SPSS software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY, USA). Only the p values < 0.05 (two-sided) were considered to be statistically significant. No adjustment for multiple testing was applied to the present analysis.
3. Results
3.1. Athletes’ Characteristics
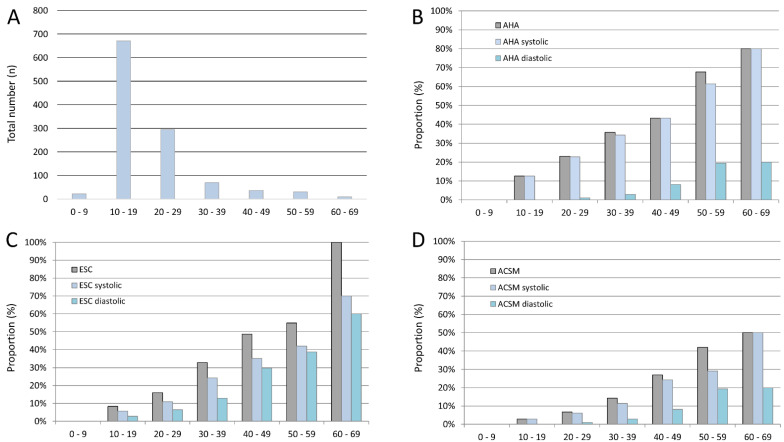
Overall, 1137 athletes (mean age 21 years; median 18 years (IQR 15/25); 395 (34.7%) females) without known arterial hypertension were included in the present study between April 2020 and October 2021. Most included athletes were in the second or third decade of life (Figure 1A). Among them, CVRF were rare, with nicotine abuse reported in 34 (3.0%) and obesity detected in 14 (1.2%) athletes. LVH was diagnosed in 233 athletes (regardless of athletes’ sex: 20.5%; 87 female athletes (22.0%); 146 male athletes (19.7%)). Median past training period was 8 (IQR 5/12) years.
Figure 1.
Included numbers of athletes and proportion of blood pressure deviations stratified for age by decade. Panel (A) Total numbers of included athletes stratified for age by decade. Panel (B) Proportion of athletes with exaggerated blood pressure response according to American Heart Association (AHA) guideline stratified for age by decade. Panel (C) Proportion of athletes with exaggerated blood pressure response according to European Society of Cardiology (ESC) guideline stratified for age by decade. Panel (D) Proportion of athletes with exaggerated blood pressure response according to American College of Sports Medicine (ACSM) guideline stratified for age by decade.
3.2. Prevalence of Exaggerated Blood Pressure Response (EBPR) during Exercise Testing
Overall, 223 athletes (regardless of athletes’ sex: 19.6%; 74 female athletes (18.7%); 149 male athletes (20.1%)) had a diagnosis of EBPR according to AHA guidelines (Table 1), 171 (regardless of athletes’ sex: 15.0%; 66 female athletes (16.7%); 105 male athletes (14.2%)) according to ESC guidelines (Table 2), and 77 (regardless of athletes’ sex: 6.8%; 11 female athletes (2.8%); 66 male athletes (8.9%)) according to ACSM guidelines (Table 3).
Table 1.
Patient characteristics of the 1137 examined athletes without known arterial hypertension stratified for exaggerated blood pressure response according to AHA guideline.
| Parameters | Normal Blood Pressure Response According to AHA Classification (n = 914; 80.4%) | Exaggerated Blood Pressure Response According to AHA Classification (n = 223; 19.6%) | p-Value |
|---|---|---|---|
| Age (in years) | 17.0 (15.0/22.0) | 22.0 (18.0/33.0) | <0.001 |
| Female sex | 321 (35.1%) | 74 (33.2%) | 0.586 |
| Body height (cm) | 174.0 (166.9/181.0) | 179.0 (173.0/184.0) | <0.001 |
| Body weight (kg) | 67.0 (57.6/77.7) | 75.8 (68.0/85.8) | <0.001 |
| Body mass index (kg/m2) | 22.0 (20.2/24.1) | 23.4 (22.0/25.4) | <0.001 |
| Body fat (%) | 11.3 (8.5/16.4) | 11.9 (9.0/16.3) | 0.140 |
| Leading athletes at a regional or national level | 707 (77.4%) | 146 (65.5%) | <0.001 |
| Training years | 8.0 (5.0/11.0) | 11.0 (6.0/15.0) | <0.001 |
| Cardiovascular risk factors | |||
| Nicotine abuse | 20 (2.2%) | 14 (6.3%) | 0.003 |
| Obesity | 8 (0.9%) | 6 (2.7%) | 0.039 |
| Blood pressure values | |||
| Systolic blood pressure (mmHg) | 115.0 (110.0/120.0) | 120.0 (115.0/130.0) | <0.001 |
| Diastolic blood pressure (mmHg) | 70.0 (60.0/75.0) | 70.0 (70.0/80.0) | <0.001 |
| Maximum systolic blood pressure during exercise (mmHg) | 180.0 (160.0/190.0) | 220.0 (210.0/230.0) | <0.001 |
| Maximum diastolic blood pressure during exercise (mmHg) | 70.0 (70.0/80.0) | 80.0 (70.0/85.0) | <0.001 |
| Exercise parameters | |||
| VO2 maximum during exercise | 45.5 (39.9/50.5) | 44.0 (37.2/49.5) | 0.031 |
| Respiratory exchange ratio (RER) |
1.15 (1.10/1.20) | 1.15 (1.11/1.21) | 0.864 |
| Maximum lactate value | 9.46 (7.79/11.2) | 9.21 (7.61/11.24) | 0.861 |
| Echocardiographic parameters | |||
| Left ventricular hypertrophy | 151 (16.5%) | 82 (36.8%) | <0.001 |
| Left ventricular mass | 158.8 (128.0/200.4) | 194.2 (164.1/220.8) | <0.001 |
| Aortic valve regurgitation | 48 (5.3%) | 26 (11.7%) | 0.001 |
| Mitral valve regurgitation | 474 (51.9%) | 153 (68.6%) | <0.001 |
| Tricuspid valve regurgitation | 115 (12.6%) | 43 (19.3%) | 0.027 |
| Pulmonary valve regurgitation | 91 (10.0%) | 17 (7.6%) | 0.311 |
| Heart volume in total (mL) | 760.5 (625.8/906.3) | 910.3 (770.2/1004.5) | <0.001 |
| Heart volume related to body weight (mL/kg) | 11.4 (10.2/12.4) | 11.7 (10.6/12.8) | 0.003 |
| Left ventricular ejection fraction (%) | 65.0 (62.0/69.0) | 66.0 (62.0/69.0) | 0.140 |
| Left ventricular end-diastolic diameter (cm) | 49.0 (45.0/53.0) | 51.0 (48.0/54.0) | <0.001 |
| Left atrial area (cm2) | 13.5 (11.1/15.4) | 15.2 (12.9/17.6) | <0.001 |
| Right atrial area (cm2) | 13.2 (11.0/15.5) | 15.1 (13.3/17.7) | <0.001 |
| Tricuspid annular plane systolic excursion (TAPSE, cm) | 2.46 (2.20/2.70) | 2.6 (2.3/2.9) | <0.001 |
| Systolic pulmonary artery pulmonary pressure (mmHg) | 20.0 (17.0/23.0) | 20.3 (17.0/23.6) | 0.274 |
| E/A quotient | 2.7 (1.9/3.7) | 2.6 (1.8/3.6) | 0.215 |
| E/E’ quotient | 4.7 (4.0/5.7) | 4.8 (4.0/5.7) | 0.606 |
Table 2.
Patient characteristics of the 1137 examined athletes without known arterial hypertension stratified for exaggerated blood pressure response according to ESC guideline.
| Parameters | Normal Blood Pressure Response According to ESC Classification (n = 966; 85.0%) | Exaggerated Blood Pressure Response According to ESC Classification (n = 171; 15.0%) | p-Value |
|---|---|---|---|
| Age (in years) | 17.0 (15.0/22.0) | 26.0 (18.0/42.0) | <0.001 |
| Female sex | 329 (34.1%) | 66 (38.6%) | 0.251 |
| Body height (cm) | 175.0 (167.0/182.0) | 179.0 (171.0/184.0) | <0.001 |
| Body weight (kg) | 68.2 (58.3/78.5) | 75.8 (66.4/84.0) | <0.001 |
| Body mass index (kg/m2) | 22.1 (20.2/24.2) | 23.7 (22.3/25.5) | <0.001 |
| Body fat (%) | 11.0 (8.5/16.0) | 13.0 (9.5/17.2) | <0.001 |
| Leading athletes at a regional or national level | 754 (78.1%) | 99 (57.9%) | <0.001 |
| Training years | 8.0 (5.0/11.0) | 11.0 (7.0/16.0) | <0.001 |
| Cardiovascular risk factors | |||
| Nicotine abuse | 19 (2.0%) | 15 (8.8%) | <0.001 |
| Obesity | 8 (0.8%) | 6 (3.5%) | 0.011 |
| Blood pressure values | |||
| Systolic blood pressure (mmHg) | 115.0 (110.0/120.0) | 120.0 (110.0/130.0) | <0.001 |
| Diastolic blood pressure (mmHg) | 70.0 (60.0/75.0) | 75.0 (70.0/80.0) | <0.001 |
| Maximum systolic blood pressure during exercise (mmHg) | 180.0 (160.0/195.0) | 220.0 (210.0/230.0) | <0.001 |
| Maximum diastolic blood pressure during exercise (mmHg) | 70.0 (70.0/80.0) | 85.0 (80.0/90.0) | <0.001 |
| Exercise parameters | |||
| VO2 maximum during exercise | 45.6 (40.1/50.6) | 42.0 (35.1/49.1) | <0.001 |
| Respiratory exchange ratio (RER) |
1.15 (1.10/1.20) | 1.15 (1.11/1.21) | 0.497 |
| Maximum lactate value | 9.42 (7.71/11.2) | 9.28 (7.96/11.07) | 0.933 |
| Echocardiographic parameters | |||
| Left ventricular hypertrophy | 177 (18.3%) | 56 (32.7%) | <0.001 |
| Left ventricular mass | 164.3 (132.6/200.8) | 188.0 (153.2/219.7) | <0.001 |
| Aortic valve regurgitation | 50 (5.2%) | 24 (14.0%) | <0.001 |
| Mitral valve regurgitation | 506 (52.4%) | 121 (70.8%) | <0.001 |
| Tricuspid valve regurgitation | 123 (12.7%) | 35 (20.5%) | 0.022 |
| Pulmonary valve regurgitation | 94 (9.7%) | 14 (8.2%) | 0.526 |
| Heart volume in total (mL) | 774.4 (634.6/919.0) | 883.0 (728.4/982.6) | <0.001 |
| Heart volume related to body weight (mL/kg) | 11.5 (10.3/12.5) | 11.5 (10.3/12.5) | 0.790 |
| Left ventricular ejection fraction (%) | 65.0 (62.0/68.0) | 66.0 (63.0/69.0) | 0.012 |
| Left ventricular end-diastolic diameter (cm) | 50.0 (46.0/53.0) | 51.0 (47.0/54.0) | 0.004 |
| Left atrial area (cm2) | 13.6 (11.3/15.6) | 15.0 (12.6/15.6) | <0.001 |
| Right atrial area (cm2) | 13.4 (11.1/15.7) | 15.0 (12.9/17.7) | <0.001 |
| Tricuspid annular plane systolic excursion (TAPSE, cm) | 2.50 (2.20/2.80) | 2.6 (2.4/2.9) | <0.001 |
| Systolic pulmonary artery pulmonary pressure (mmHg) | 20.0 (17.0/23.0) | 21.0 (18.0/24.1) | 0.018 |
| E/A quotient | 2.7 (2.0/3.7) | 2.2 (1.6/3.3) | <0.001 |
| E/E’ quotient | 4.7 (4.0/5.7) | 4.9 (4.1/6.0) | 0.167 |
Table 3.
Patient characteristics of the 1137 examined athletes without known arterial hypertension stratified for exaggerated blood pressure response according to ACSM guideline.
| Parameters | Normal Blood Pressure Response According to ACSM Classification (n = 1060; 93.2%) | Exaggerated Blood Pressure Response According to ACSM Classification (n = 77; 6.8%) | p-Value |
|---|---|---|---|
| Age (in years) | 18.0 (15.0/23.0) | 29.0 (19.5/48.5) | <0.001 |
| Female sex | 384 (36.2%) | 11 (14.3%) | <0.001 |
| Body height (cm) | 175.0 (167.0/182.0) | 181.0 (175.3/186.5) | <0.001 |
| Body weight (kg) | 68.4 (58.8/78.5) | 80.3 (75.0/87.9) | <0.001 |
| Body mass index (kg/m2) | 22.2 (20.4/24.2) | 24.4 (23.0/26.3) | <0.001 |
| Body fat (%) | 11.3 (8.6/16.7) | 11.5 (9.2/14.0) | 0.884 |
| Leading athletes at a regional or national level | 817 (77.1%) | 36 (46.8%) | <0.001 |
| Training years | 8.0 (5.0/11.0) | 13.0 (8.5/18.8) | <0.001 |
| Cardiovascular risk factors | |||
| Nicotine abuse | 27 (2.5%) | 7 (9.1%) | 0.006 |
| Obesity | 10 (0.9%) | 4 (5.2%) | 0.012 |
| Blood pressure values | |||
| Systolic blood pressure (mmHg) | 115.0 (110.0/120.0) | 125.0 (120.0/135.0) | <0.001 |
| Diastolic blood pressure (mmHg) | 70.0 (60.0/75.0) | 80.0 (70.0/80.0) | <0.001 |
| Maximum systolic blood pressure during exercise (mmHg) | 180.0 (160.0/200.0) | 230.0 (230.0/240.0) | <0.001 |
| Maximum diastolic blood pressure during exercise (mmHg) | 75.0 (70.0/80.0) | 80.0 (80.0/90.0) | <0.001 |
| Exercise parameters | |||
| VO2 maximum during exercise | 45.4 (39.8/50.4) | 43.2 (35.8/49.5) | 0.040 |
| Respiratory exchange ratio (RER) |
1.15 (1.11/1.20) | 1.15 (1.11/1.21) | 0.515 |
| Maximum lactate value | 9.40 (7.75/11.21) | 9.41 (7.85/11.16) | 0.974 |
| Echocardiographic parameters | |||
| Left ventricular hypertrophy | 203 (19.2%) | 30 (39.0%) | <0.001 |
| Left ventricular mass | 164.3 (132.8/200.8) | 207.1 (181.4/227.7) | <0.001 |
| Aortic valve regurgitation | 60 (5.7%) | 14 (18.2%) | <0.001 |
| Mitral valve regurgitation | 571 (53.9%) | 56 (72.7%) | 0.001 |
| Tricuspid valve regurgitation | 141 (13.3%) | 17 (22.1%) | 0.090 |
| Pulmonary valve regurgitation | 101 (9.5%) | 7 (9.1%) | 1.000 |
| Heart volume in total (mL) | 774.6 (642.5/919.0) | 965.4 (829.4/1047.0) | <0.001 |
| Heart volume related to body weight (mL/kg) | 11.5 (10.3/12.5) | 11.7 (10.4/12.6) | 0.350 |
| Left ventricular ejection fraction (%) | 65.0 (62.0/69.0) | 66.0 (62.0/72.0) | 0.037 |
| Left ventricular end-diastolic diameter (cm) | 49.0 (46.0/53.0) | 52.0 (49.5/54.5) | <0.001 |
| Left atrial area (cm2) | 13.6 (11.4/15.7) | 15.7 (14.4/18.2) | <0.001 |
| Right atrial area (cm2) | 13.5 (11.2/15.8) | 16.5 (14.0/18.5) | <0.001 |
| Tricuspid annular plane systolic excursion (TAPSE, cm) | 2.50 (2.20/2.80) | 2.6 (2.3/2.9) | 0.001 |
| Systolic pulmonary artery pulmonary pressure (mmHg) | 20.0 (17.0/23.0) | 22.0 (20.0/25.0) | <0.001 |
| E/A quotient | 2.7 (1.9/3.7) | 2.1 (1.5/3.2) | <0.001 |
| E/E’ quotient | 4.7 (4.0/5.7) | 5.1 (4.1/6.4) | 0.080 |
3.3. Comparison of Athletes with and without Exaggerated Blood Pressure Response (EBPR) during Exercise Testing
While the proportions of female athletes with and without EBPR according to ESC and AHA guidelines were widely balanced, comprising approximately 1/3 of the athletes with EBPR, the proportion of male athletes with EBPR according to ACSM was distinctly higher, with 85.7% of all individuals with EBPR (Table 3). CVRF nicotine abuse and obesity were both more prevalent in athletes with EBPR regardless of which definition of EBPR was chosen (Table 1, Table 2 and Table 3). The criteria regarding full effort during the exercise test did not differ between athletes with and without EBPR (Table 1, Table 2 and Table 3).
The proportion of athletes with EBPR increased with inclining age regardless of the chosen definition. Notably, EBPR was more often diagnosed due to maximum systolic in comparison to maximum diastolic blood pressure values during exercise (Figure 1B–D).
3.4. Prevalence of Left Ventricular Hypertrophy (LVH) in Athletes
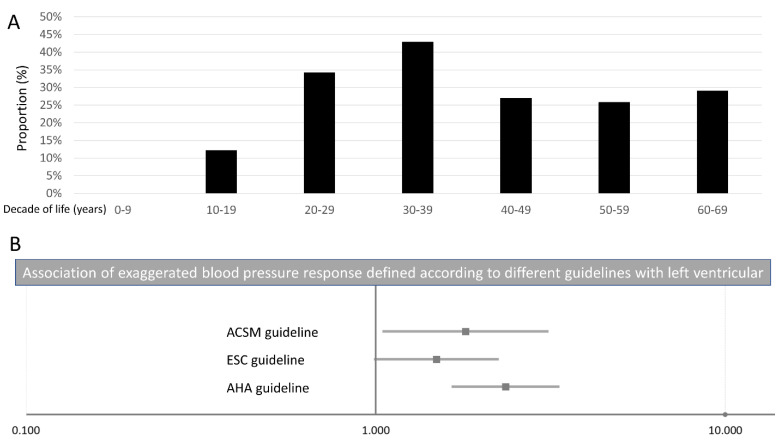
LVH was approximately two-fold more frequent in athletes with EBPR than in those without (risk ratios (RR) 2.2, 1.8, and 2.0 when using the definitions of AHA guidelines, ESC guidelines, and ACSM guidelines, respectively).
Interestingly, aortic valve regurgitation and mitral valve regurgitation were both more prevalent in athletes with EBPR (Table 1, Table 2 and Table 3).
3.5. Association of Exaggerated Blood Pressure Response (EBPR) during Exercise Testing and Left Ventricular Hypertrophy (LVH) in Athletes
In addition, we computed logistic regression models in order to analyse associations between EBPR defined according to the different guidelines on the one hand and LVH on the other hand. While EBPR according to the definition of the AHA guidelines (OR 2.35 (95%CI 1.66–3.33), p < 0.001) and the ACSM guidelines (OR 1.81 (95%CI 1.05–3.09), p = 0.031) were independently (of age and sex) associated with LVH, EBPR defined according to the ESC guidelines (OR 1.49 (95%CI 1.00–2.23), p = 0.051) was not independently associated with LVH (Figure 2B, Table 4).
Figure 2.
Exaggerated blood pressure response and left ventricular hypertrophy. Panel (A) Proportion of left ventricular hypertrophy stratified for age by decades. Panel (B) Association of exaggerated blood pressure response according to AHA, ESC, and ACSM guidelines with left ventricular hypertrophy.
Table 4.
Association between of exaggerated blood pressure response, blood pressure values at rest, and maximum value during exercise on the one hand and left ventricular hypertrophy on the other hand (univariate and multivariate logistic regression model).
| Left Ventricular Hypertrophy | ||||
|---|---|---|---|---|
| Univariate Regression Model | Multivariate Regression Model (Adjusted for Age and Sex) |
|||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| AHA guideline classification of exaggerated blood pressure response | 2.939 (2.127–4.060) | <0.001 | 2.351 (1.660–3.328) | <0.001 |
| ESC guideline classification of exaggerated blood pressure response | 2.171 (1.517–3.107) | <0.001 | 1.493 (0.998–2.232) | 0.051 |
| ACSM guideline classification of exaggerated blood pressure response | 2.695 (1.663–4.367) | <0.001 | 1.805 (1.054–3.093) | 0.031 |
| Systolic blood pressure/MET slope (>6.2 mmHg/MET) | 2.120 (1.449–3.101) | <0.001 | 2.257 (0.403–12.655) | 0.355 |
| Systolic blood pressure at rest (mmHg) | 1.023 (1.010–1.036) | <0.001 | 1.016 (1.001–1.030) | 0.033 |
| Diastolic blood pressure at rest (mmHg) | 1.025 (1.007–1.043) | 0.005 | 1.011 (0.992–1.030) | 0.253 |
| Maximum systolic blood pressure during exercise (mmHg) | 1.024 (1.018–1.030) | <0.001 | 1.026 (1.019–1.033) | <0.001 |
| Maximum diastolic blood pressure during exercise (mmHg) | 1.023 (1.007–1.040) | 0.005 | 1.006 (0.989–1.024) | 0.470 |
In addition, LVH was associated with systolic BP at rest and maximum systolic BP during exercise, but not with diastolic BP values (Table 4).
3.6. Prevalence of Exaggerated Blood Pressure Response (EBPR) during Exercise Testing and Left Ventricular Hypertrophy (LVH) in Adult Athletes
When focusing on the adult athletes only, 598 athletes (33.1% females; median age 23.0 (19.0–29.0) years) aged 18 years or older remained in the analysis. Among these, 180 (30.1%) had an LVH.
According to the guideline definitions, 170 (regardless of athletes’ sex: 28.4%; 54 female athletes (27.3%); 116 male athletes (29.0%)) athletes were classified as EBPR according to AHA guidelines, 137 (regardless of athletes’ sex: 22.9%; 54 female athletes (27.3%); 83 male athletes (20.8%)) according to ESC guidelines, and 65 (regardless of athletes’ sex: 10.9%; 11 female athletes (5.6%); 54 male athletes (13.5%)) according to ACSM guidelines.
3.7. Association of Exaggerated Blood Pressure Response (EBPR) during Exercise Testing and Left Ventricular Hypertrophy (LVH) in Adult Athletes
In adult athletes, only the definition of EBPR according to AHA guidelines was independently predictive for LVH (univariate: OR 1.88 (95%CI 1.29–2.74), p = 0.001; multivariate: OR 1.96 (95% CI 1.32–2.90), p = 0.001). EBPR according to the ESC (univariate: OR 1.40 (95% CI 0.94–2.10), p = 0.100; multivariate: OR 1.44 (95%CI 0.93–2.22), p = 0.104) as well as ACSM guidelines (univariate: OR 1.64 (95% CI 0.97–2.79), p = 0.067; multivariate: OR 1.73 (95% CI 0.98–3.07), p = 0.060) were not associated with LVH independently of age and sex.
3.8. Prevalence of Exaggerated Blood Pressure Response (EBPR) during Exercise Testing Identified by Systolic BP/MET Slope Method with a Cutoff Value > 6.2 mmHg/MET
When using the systolic BP/MET slope method with a cutoff value > 6.2 mmHg/MET to define an EBPR in those 639 athletes, who underwent spiroergometric testing, we detected 386 athletes (60.4%) with normal BP response and 253 athletes with EBPR (regardless of athletes’ sex: 39.6%; 80 female athletes (36.5%); 173 male athletes (41.2%)) (Table 5). LVH was more prevalent in athletes with than without EBPR (29.6% vs. 16.6%, p < 0.001).
Table 5.
Patient characteristics of the 639 examined athletes with spiroergometry and without known arterial hypertension stratified for exaggerated blood pressure response according to systolic blood pressure/MET slope.
| Parameters | Normal Blood Pressure Response According to Systolic Blood Pressure/MET Slope (≤6.2 mmHg/MET) (n = 386; 60.4%) | Exaggerated Blood Pressure Response According to Systolic Blood Pressure/MET Slope (>6.2 mmHg/MET) (n = 253; 39.6%) | p-Value |
|---|---|---|---|
| Age (in years) | 18.0 (15.0/22.0) | 24.0 (18.0/36.5) | <0.001 |
| Female sex | 139 (36.0%) | 80 (31.6%) | 0.253 |
| Body height (cm) | 175.0 (168.0/182.0) | 178.0 (170.0/184.0) | 0.014 |
| Body weight (kg) | 66.8 (58.0/77.7) | 76.0 (66.0/85.9) | <0.001 |
| Body mass index (kg/m2) | 21.7 (20.2/24.0) | 23.8 (22.3/26.0) | <0.001 |
| Body fat (%) | 12.4 (8.2/16.6) | 12.2 (9.2/17.1) | 0.003 |
| Leading athletes at a regional or national level | 295 (76.4%) | 135 (53.4%) | <0.001 |
| Training years | 7.0 (5.0/10.0) | 10.0 (5.0/14.0) | <0.001 |
| Cardiovascular risk factors | |||
| Nicotine abuse | 8 (2.1%) | 18 (7.1%) | 0.003 |
| Obesity | 1 (0.3%) | 9 (3.6%) | 0.001 |
| Blood pressure values | |||
| Systolic blood pressure (mmHg) | 120.0 (110.0/125.0) | 120.0 (110.0/125.0) | 0.908 |
| Diastolic blood pressure (mmHg) | 70.0 (60.0/75.0) | 70.0 (65.0/80.0) | 0.003 |
| Maximum systolic blood pressure during exercise (mmHg) | 170.0 (155.0/180.0) | 210.0 (190.0/220.0) | <0.001 |
| Maximum diastolic blood pressure during exercise (mmHg) | 70.0 (65.0/80.0) | 80.0 (70.0/80.0) | <0.001 |
| Exercise parameters | |||
| VO2 maximum during exercise | 47.5 (42.1/51.5) | 41.9 (36.2/47.0) | <0.001 |
| Respiratory exchange ratio (RER) |
1.15 (1.10/1.19) | 1.15 (1.11/1.21) | 0.037 |
| Maximum lactate value | 9.36 (7.67/11.24) | 9.51 (7.89/11.24) | 0.533 |
| Echocardiographic parameters | |||
| Left ventricular hypertrophy | 64 (16.6%) | 75 (29.6%) | <0.001 |
| Left ventricular mass | 163.6 (132.3/199.3) | 188.1 (153.4/220.6) | <0.001 |
| Aortic valve regurgitation | 20 (5.2%) | 22 (8.7%) | 0.080 |
| Mitral valve regurgitation | 203 (52.6%) | 169 (66.8%) | <0.001 |
| Tricuspid valve regurgitation | 46 (12.0%) | 51 (20.2%) | 0.010 |
| Pulmonary valve regurgitation | 34 (8.8%) | 25 (9.9%) | 0.647 |
| Heart volume in total (mL) | 772.0 (639.0/908.5) | 896.4 (732.9/1000.0) | <0.001 |
| Heart volume related to body weight (mL/kg) | 11.4 (10.2/12.4) | 11.4 (10.2/12.3) | 0.803 |
| Left ventricular ejection fraction (%) | 65.0 (62.0/69.0) | 66.0 (63.0/69.0) | 0.041 |
| Left ventricular end-diastolic diameter (cm) | 50.0 (46.0/53.0) | 51.0 (47.0/54.0) | <0.001 |
| Left atrial area (cm2) | 13.5 (11.0/15.3) | 14.9 (12.6/17.4) | <0.001 |
| Right atrial area (cm2) | 13.3 (11.1/15.5) | 14.9 (12.8/17.9) | <0.001 |
| Tricuspid annular plane systolic excursion (TAPSE, cm) | 2.40 (2.20/2.70) | 2.60 (2.40/2.90) | <0.001 |
| Systolic pulmonary artery pulmonary pressure (mmHg) | 20.0 (16.5/23.0) | 21.5 (18.0/24.0) | 0.002 |
| E/A quotient | 2.5 (1.9/3.4) | 2.4 (1.6/3.6) | 0.111 |
| E/E’ quotient | 4.7 (4.0/5.7) | 4.9 (4.1/5.9) | 0.193 |
3.9. Association of Exaggerated Blood Pressure Response (EBPR) during Exercise Testing Identified by Systolic BP/MET Slope Method with a Cutoff Value > 6.2 mmHg/MET and Left Ventricular Hypertrophy (LVH) in Athletes
Systolic BP/MET slope > 6.2 mmHg/MET was associated with LVH in the univariate regression analysis (OR 2.12 (95% CI 1.45–3.10), p < 0.001), but this association remained not significant after adjustment for age and sex (OR 2.26 (95% CI 0.40–12.66), p = 0.355). Sex-specific analyses revealed a significant association of systolic BP/MET slope > 6.2 mmHg/MET with LVH in male (OR 2.348 (95%CI 1.472–3.746), p < 0.001) in contrast to female athletes (OR 1.706 (95%CI 0.878–3.315), p = 0.115).
In contrast, in the 398 adult athletes with spiroergometric evaluation, systolic BP/MET slope > 6.2 mmHg/MET was associated with LVH in both, the univariate (OR 1.67 (95% CI 1.07–2.60), p = 0.023) as well as multivariate logistic regression analysis adjusted for age and sex (OR 1.73 (95% CI 1.08–2.78), p = 0.023). However, sex-specific analyses also revealed sex-specific differences in adult athletes. While systolic BP/MET slope > 6.2 mmHg/MET was associated with LVH in male adult athletes (OR 1.848 (95% CI 1.079–3.166), p = 0.025), in females, no association was seen (OR 1.325 (95% CI 0.603–2.913), p = 0.484).
4. Discussion
Arterial hypertension is accompanied by substantially increased cardiovascular morbidity and mortality [2,4,7,9,17,49,50,51].
Among individuals who were not categorized as patients with arterial hypertension [12,13,14,15] a number of individuals revealed EBPR during exercise testing. The consequences of this phenomenon are not well elucidated, and study results are inconsistent. In previous investigations, a large number of different definitions of EBPR were used, hampering a clear interpretation of study results [1,4,17,25,26,27,28,29,30,31,32,33,34,35,36,37]. However, several studies have shown that individuals without known arterial hypertension who present with EBPR during the exercise testing are at increased risk to develop arterial hypertension in the future and might also be prone to develop cardiovascular events [1,4,17,25,26,27,28,29,30,31,32,33,34,35,36,37]. Three guideline definitions are currently available and valid: the AHA [23], the ESC [22,24], and the ACSM guidelines [20,21]. In this context, it is widely unclear from which study sample these definitions were derived and whether these definitions were able to predict cardiovascular morbidity, e.g., LVH, in athletes.
Thus, the objectives of our present study were to evaluate the prevalence of EBPR in athletes and which definition regarding EBPR during exercise testing was best/strongest associated with LVH in athletes without known arterial hypertension.
The main results of the study can be summarized as follows:
-
(I)
EBPR was diagnosed between 6.8% and 19.6% of all athletes in our study according to the different guideline recommendations. Prevalence was highest when categorized according to the ESC guidelines (19.6%) and lowest according to the ACSM guidelines (6.8%).
-
(II)
CVRF, such as nicotine abuse and obesity, were more prevalent in athletes with EBPR.
-
(III)
The proportion of athletes with EBPR increased with inclining age regardless of the chosen definition.
-
(IV)
EBPR was more often diagnosed due to maximum systolic in comparison to maximum diastolic BP values during exercise.
-
(V)
Only the EBPR definition of the AHA guideline was able to predict LVH independently of age and sex in both the overall sample as well as in adult athletes as the only guideline recommended threshold.
-
(VI)
In addition, the recently implemented systolic BP/MET slope method with a cutoff value > 6.2 mmHg/MET to define an EBPR, was able to predict LVH in adult athletes independently of age and sex.
Our study results reveal a large variation regarding the prevalence of EBPR according to the different guideline definitions in athletes without known arterial hypertension (variation of 12.8% according to different guideline recommendations). The prevalence was highest when categorized according to the ESC guidelines [22,24] (19.6%) and lowest when classified according to the ACSM guidelines [20,42] (6.8%). In contrast to the study of Caselli at al. [24], who reported that only a rate of 7.5% of the 1876 investigated athletes had an EBPR defined according to the ESC guidelines, we identified a frequency of 19.6% in the athletes presenting with EBPR according the ESC guidelines’ definition. However, the differences between our results and the aforementioned study might be based on differences regarding the performance level of the examined athletes and athletes’ ages in both studies.
As expected, CVRF, such as nicotine abuse and obesity, were in our study more prevalent in those athletes with EBPR. This finding is in line with the literature, reporting a close relation between obesity and elevated blood pressure [52,53]. Arterial hypertension is frequently observed in individuals who are obese [53]. In addition, smoking was strongly associated with arterial hypertension in several studies [54,55].
The proportion of athletes with EBPR increased significantly with inclining age regardless of the chosen definition. In this context, studies underlined a physiological increase in BP with age [4,56,57,58]. While at birth, the systolic and diastolic BP values are on average at levels of 70 mmHg and 50 mmHg, respectively [4,56,58], BP values rise progressively throughout childhood and adolescence [4,56,57,58]. As aforementioned, BP is substantially determined by body weight, and it is of key interest that BP in childhood has a strong impact on adult BP levels [4,57,58]. Individuals aged ≥70 years reach an average systolic BP of approximately 140 mmHg. Diastolic BP tends also to rise with the aging process but the intense of this increase is less steep and after the 50th life year, diastolic mean BP either inclines only slightly or even declines [4,56]. These changes in BP reflect normal age-dependent development, while BP deviations due to arterial hypertension could be detected in every period of life [4,56]. The association between a growing burden of arterial hypertension with increasing age is well known and described [4,6,56,59]. While in Germany, 10–35% of the citizens aged between 30 and 60 were diagnosed with arterial hypertension, the frequency increases to higher than 65% in people aged 60 years and older [8]. In light of the quoted literature, an age-dependent increase regarding the proportion of athletes with EBPR might be expected but could also be interpreted as an increasing number of athletes who might have undiagnosed or masked arterial hypertension.
In stress situations, the BP rises from resting to stress level depending on the exercise intensity and the affecting stressor [4,17,19,60]. The BP responses to exercise are a result of cardiac output and peripheral vascular resistance [61]. Cardiac output is elevated to provide oxygenated blood and nutrition for the active regions of the body according to increased demand [62]. During physical activity, BP values increase, whereby the rise in systolic BP values becomes more pronounced compared to diastolic BP. BP values generally increase to an exercise dependent and predetermined individual limit [1,4,17,61]. Normal systolic BP response in progressive exercise testing on a bicycle stress test comprise a systolic BP increase of approximately 7 to 10 mmHg per 25 watt workload incline [19]. Expected maximal BP values in bicycle testing are approximately 200/100 mmHg in healthy untrained adults in the general population and approximately 215/105 mmHg in those individuals who are older than 50 years [16]. Notably, only systolic BP values, not diastolic values, could be reliably measured with the standardly used non-invasive methods [1].
Thus, in our present study, it is of outstanding importance that EBPR was more often diagnosed due to maximum systolic in comparison to maximum diastolic BP values during exercise, although all of the guideline recommendations defined a diastolic threshold regarding EBPR [20,21,22,23,24].
Although three different guideline recommendations for the definition of EBPR are available, only the EBPR definition of the AHA guidelines [23] was able to predict LVH independently of age and sex in both the overall sample as well as in adult athletes only in our study. Nevertheless, despite this result, we do not think that the definition of EBPR as systolic BP > 210 mmHg in men, > 190 mmHg in women, and/or > 90 mmHg diastolic peak BP in both sexes [23] is well suited to identify individuals at risk and deduce further consequences as a singular diagnostic tool in athletes. From the experiences of daily routine in sports medicine, the defined systolic BP values regarding EBPR are too low for exercise testing in male and female athletes. In accordance with these experiences of daily practice, it has been reported in the literature that very fit and powerful athletes reach physiologically higher BP values during competition as well as exercise testing [4,16,19,63]. Although, systolic BP values ≥ 250 mmHg and diastolic BP values ≥ 120 mmHg were defined as stopping criteria for bicycle ergometry exercise testing [16,63,64]—especially in young athletes, who exceed these thresholds within their normal sports practice—a stop of the exercise testing even at this higher and rigid recommended thresholds (250/120 mmHg) seems limited in its usefulness and the decision to stop should be made individually [16,19,63].
In order to encounter these only-in-part useful definitions of EBPR for athletes, a workload-indexed EBPR definition was introduced by different authors with promising results [44,45,46,47]. Our study confirmed these results—that an EBPR defined according to the systolic BP/MET slope method with a cutoff value > 6.2 mmHg/MET was able to predict LVH in adult athletes independently of age and sex. A threshold of 6.2 mmHg/MET was chosen since a systolic BP/MET slope >6.2 mmHg/MET was in the study of Hedman et al. associated with a 27% higher risk for mortality during a 20-year observational period in males compared to those with <4.3 mmHg/MET [44,46]. However, we detected sex-specific differences regarding this associations between EBPR defined according to the systolic BP/MET slope method with a cutoff value >6.2 mmHg/MET and LVH with significant associations in males and missing associations in females. In accordance, several studies revealed sex-specific differences regarding blood pressure response in males and females [65,66,67]. In studies, men had significantly higher systolic BP values at 50%, 75%, and 100% of maximum exercise efforts [67].
Nevertheless, although these recommended EBPR thresholds—defined by the three guidelines—seem only in part to be suitable for athletes (but more for the general untrained population), an identified EBPR and especially a prolonged and delayed decline in blood pressure after exercise testing could provide clues regarding a masked arterial hypertension or development of a manifest arterial hypertension in the future [4,63].
In athletes with EBPR and/or a prolonged and delayed decline in blood pressure after exercise testing, a 24 h blood pressure measurement could give important and valuable additional diagnostic information [15]. Where the threshold regarding EBPR in athletes from which further diagnostic procedures should be implemented is still controversial [16,19,63].
5. Conclusions
EBPR was diagnosed in between 6.8% and 19.6% of all athletes without known arterial hypertension. Prevalence was highest when athletes were categorized according to ESC guidelines (19.6%) and lowest when categorized according to ACSM guidelines (6.8%). The proportion of athletes with EBPR increased with inclining age regardless of the chosen definition. Only the EBPR definition of the AHA guidelines and the systolic blood pressure/MET slope method were associated with LVH independently of age and sex in adult athletes. However, the prognostic value of this association remains to be elucidated by sufficiently powered in-depth long-term studies. Such studies are also necessary to further evaluate the importance of the identification of EBPR in athletes and the significance of actual EBPR guidelines as diagnostic tools in young athletes.
Author Contributions
Conceptualization, K.K. and B.F.-B.; Data curation, K.K. and J.T.; Formal analysis, K.K.; Investigation, K.K.; Methodology, K.K.; Project administration, K.K.; Supervision, K.K.; Visualization, K.K.; Writing—original draft, K.K.; Writing—review & editing, K.K., K.H., L.d.C.C., J.T., F.S., C.S., F.H. and B.F.-B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The requirement for informed consent was waived as we used only anonymized retrospective data routinely collected during the health screening process. Studies in Germany involving a retrospective analysis of diagnostic standard data of anonymized patients do not require an ethics statement.
Informed Consent Statement
The requirement for informed consent was waived as we used only anonymized retrospective data routinely collected during the health screening process. Studies in Germany involving a retrospective analysis of diagnostic standard data of anonymized patients do not require an ethics statement.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mancia G., Fagard R., Narkiewicz K., Redon J., Zanchetti A., Bohm M., Christiaens T., Cifkova R., De Backer G., Dominiczak A., et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur. Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 2.Schmieder R.E. End organ damage in hypertension. Dtsch. Arztebl. Int. 2010;107:866–873. doi: 10.3238/arztebl.2010.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Keller K., Stelzer K., Ostad M.A., Post F. Impact of exaggerated blood pressure response in normotensive individuals on future hypertension and prognosis: Systematic review according to PRISMA guideline. Adv. Med. Sci. 2017;62:317–329. doi: 10.1016/j.advms.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Wolf-Maier K., Cooper R.S., Banegas J.R., Giampaoli S., Hense H.W., Joffres M., Kastarinen M., Poulter N., Primatesta P., Rodriguez-Artalejo F., et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 6.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., et al. Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks F.M., Campos H. Dietary therapy in hypertension. N. Engl. J. Med. 2010;362:2102–2112. doi: 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 8.Mahfoud F., Himmel F., Ukena C., Schunkert H., Bohm M., Weil J. Treatment strategies for resistant arterial hypertension. Dtsch. Arztebl. Int. 2011;108:725–731. doi: 10.3238/arztebl.2011.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A.M., Kjeldsen S.E., Laurent S., et al. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J. Hypertens. 2007;25:1751–1762. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 10.Moebus S., Hanisch J., Bramlage P., Losch C., Hauner H., Wasem J., Jockel K.H. Regional differences in the prevalence of the metabolic syndrome in primary care practices in Germany. Dtsch. Arztebl. Int. 2008;105:207–213. doi: 10.3238/artzebl.2008.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niebauer J., Borjesson M., Carre F., Caselli S., Palatini P., Quattrini F., Serratosa L., Adami P.E., Biffi A., Pressler A., et al. Recommendations for participation in competitive sports of athletes with arterial hypertension: A position statement from the sports cardiology section of the European Association of Preventive Cardiology (EAPC) Eur. Heart J. 2018;39:3664–3671. doi: 10.1093/eurheartj/ehy511. [DOI] [PubMed] [Google Scholar]
- 12.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018;71:2199–2269. doi: 10.1016/j.jacc.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Cuspidi C. Is exaggerated exercise blood pressure increase related to masked hypertension? Am. J. Hypertens. 2011;24:861. doi: 10.1038/ajh.2011.82. [DOI] [PubMed] [Google Scholar]
- 14.Matthews C.E., Pate R.R., Jackson K.L., Ward D.S., Macera C.A., Kohl H.W., Blair S.N. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J. Clin. Epidemiol. 1998;51:29–35. doi: 10.1016/S0895-4356(97)00223-0. [DOI] [PubMed] [Google Scholar]
- 15.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D.L., Coca A., de Simone G., Dominiczak A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 16.Kindermann W. Arterielle Hypertonie. In: Kindermann W., Dickhuth H.-H., Niess A., Röcker K., Urhausen A., editors. Sportkardiologie. Volume 2. Steinkopf-Verlag; Darmstadt, Germany: 2007. pp. 227–240. [Google Scholar]
- 17.Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A.M., Kjeldsen S.E., Laurent S., et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur. Heart J. 2007;28:1462–1536. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 18.Le V.V., Mitiku T., Sungar G., Myers J., Froelicher V. The blood pressure response to dynamic exercise testing: A systematic review. Prog. Cardiovasc. Dis. 2008;51:135–160. doi: 10.1016/j.pcad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Sieira M.C., Ricart A.O., Estrany R.S. Blood pressure response to exercise testing. Apunts Med. Esport. 2010;45:191–200. [Google Scholar]
- 20.Lea & Febiger . American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Volume 4 Wolters Kluwer; Philadelphia, PA, USA: 1991. [Google Scholar]
- 21.Currie K.D., Floras J.S., La Gerche A., Goodman J.M. Exercise Blood Pressure Guidelines: Time to Re-evaluate What is Normal and Exaggerated? Sports Med. 2018;48:1763–1771. doi: 10.1007/s40279-018-0900-x. [DOI] [PubMed] [Google Scholar]
- 22.Pelliccia A., Sharma S., Gati S., Back M., Borjesson M., Caselli S., Collet J.P., Corrado D., Drezner J.A., Halle M., et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher G.F., Ades P.A., Kligfield P., Arena R., Balady G.J., Bittner V.A., Coke L.A., Fleg J.L., Forman D.E., Gerber T.C., et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation. 2013;128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 24.Caselli S., Serdoz A., Mango F., Lemme E., Vaquer Segui A., Milan A., Attenhofer Jost C., Schmied C., Spataro A., Pelliccia A. High blood pressure response to exercise predicts future development of hypertension in young athletes. Eur. Heart J. 2019;40:62–68. doi: 10.1093/eurheartj/ehy810. [DOI] [PubMed] [Google Scholar]
- 25.Laukkanen J.A., Kurl S., Rauramaa R., Lakka T.A., Venalainen J.M., Salonen J.T. Systolic blood pressure response to exercise testing is related to the risk of acute myocardial infarction in middle-aged men. Eur. J. Prev. Cardiol. 2006;13:421–428. doi: 10.1097/01.hjr.0000198915.83234.59. [DOI] [PubMed] [Google Scholar]
- 26.Laukkanen J.A., Rauramaa R. Systolic blood pressure during exercise testing and the risk of sudden cardiac death. Int. J. Cardiol. 2013;168:3046–3047. doi: 10.1016/j.ijcard.2013.04.129. [DOI] [PubMed] [Google Scholar]
- 27.Gupta M.P., Polena S., Coplan N., Panagopoulos G., Dhingra C., Myers J., Froelicher V. Prognostic significance of systolic blood pressure increases in men during exercise stress testing. Am. J. Cardiol. 2007;100:1609–1613. doi: 10.1016/j.amjcard.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 28.Kjeldsen S.E., Mundal R., Sandvik L., Erikssen G., Thaulow E., Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J. Hypertens. 2001;19:1343–1348. doi: 10.1097/00004872-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Schultz M.G., Otahal P., Cleland V.J., Blizzard L., Marwick T.H., Sharman J.E. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: A systematic review and meta-analysis. Am. J. Hypertens. 2013;26:357–366. doi: 10.1093/ajh/hps053. [DOI] [PubMed] [Google Scholar]
- 30.Allison T.G., Cordeiro M.A., Miller T.D., Daida H., Squires R.W., Gau G.T. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am. J. Cardiol. 1999;83:371–375. doi: 10.1016/S0002-9149(98)00871-6. [DOI] [PubMed] [Google Scholar]
- 31.Kurl S., Laukkanen J.A., Rauramaa R., Lakka T.A., Sivenius J., Salonen J.T. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke A J. Cereb. Circ. 2001;32:2036–2041. doi: 10.1161/hs0901.095395. [DOI] [PubMed] [Google Scholar]
- 32.Palatini P. Blood pressure behaviour during physical activity. Sports Med. 1988;5:353–374. doi: 10.2165/00007256-198805060-00002. [DOI] [PubMed] [Google Scholar]
- 33.Syme A.N., Blanchard B.E., Guidry M.A., Taylor A.W., Vanheest J.L., Hasson S., Thompson P.D., Pescatello L.S. Peak systolic blood pressure on a graded maximal exercise test and the blood pressure response to an acute bout of submaximal exercise. Am. J. Cardiol. 2006;98:938–943. doi: 10.1016/j.amjcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Wilson N.V., Meyer B.M. Early prediction of hypertension using exercise blood pressure. Prev. Med. 1981;10:62–68. doi: 10.1016/0091-7435(81)90006-2. [DOI] [PubMed] [Google Scholar]
- 35.Singh J.P., Larson M.G., Manolio T.A., O’Donnell C.J., Lauer M., Evans J.C., Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.CIR.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 36.Smith R.G., Rubin S.A., Ellestad M.H. Exercise hypertension: An adverse prognosis? J. Am. Soc. Hypertens. JASH. 2009;3:366–373. doi: 10.1016/j.jash.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Levy D., Garrison R.J., Savage D.D., Kannel W.B., Castelli W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 38.Okwuosa T.M., Soliman E.Z., Lopez F., Williams K.A., Alonso A., Ferdinand K.C. Left ventricular hypertrophy and cardiovascular disease risk prediction and reclassification in blacks and whites: The Atherosclerosis Risk in Communities Study. Am. Heart J. 2015;169:155–161. doi: 10.1016/j.ahj.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scharhag J., Lollgen H., Kindermann W. Competitive sports and the heart: Benefit or risk? Dtsch. Arztebl. Int. 2013;110:14–23. doi: 10.3238/arztebl.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galderisi M., Cardim N., D’Andrea A., Bruder O., Cosyns B., Davin L., Donal E., Edvardsen T., Freitas A., Habib G., et al. The multi-modality cardiac imaging approach to the Athlete’s heart: An expert consensus of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:353. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 41.Marwick T.H., Gillebert T.C., Aurigemma G., Chirinos J., Derumeaux G., Galderisi M., Gottdiener J., Haluska B., Ofili E., Segers P., et al. Recommendations on the use of echocardiography in adult hypertension: A report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) Eur. Heart J. Cardiovasc. Imaging. 2015;16:577–605. doi: 10.1016/j.echo.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D.L., Coca A., de Simone G., Dominiczak A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 43.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Bauer P., Kraushaar L., Hoelscher S., Weber R., Akdogan E., Keranov S., Dorr O., Nef H., Hamm C.W., Most A. Blood Pressure Response and Vascular Function of Professional Athletes and Controls. Sports Med. Int. Open. 2021;5:E45–E52. doi: 10.1055/a-1400-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer P., Kraushaar L., Dorr O., Nef H., Hamm C.W., Most A. Workload-indexed blood pressure response to a maximum exercise test among professional indoor athletes. Eur. J. Prev. Cardiol. 2021;28:1487–1494. doi: 10.1177/2047487320922043. [DOI] [PubMed] [Google Scholar]
- 46.Hedman K., Cauwenberghs N., Christle J.W., Kuznetsova T., Haddad F., Myers J. Workload-indexed blood pressure response is superior to peak systolic blood pressure in predicting all-cause mortality. Eur. J. Prev. Cardiol. 2020;27:978–987. doi: 10.1177/2047487319877268. [DOI] [PubMed] [Google Scholar]
- 47.Hedman K., Lindow T., Elmberg V., Brudin L., Ekstrom M. Age- and gender-specific upper limits and reference equations for workload-indexed systolic blood pressure response during bicycle ergometry. Eur. J. Prev. Cardiol. 2021;28:1360–1369. doi: 10.1177/2047487320909667. [DOI] [PubMed] [Google Scholar]
- 48.ACSM’s Guidelines for Exercise Testing: American College of Sports Medicine. Wolters Kluwer; Philadelphia, PA, USA: 2010. [Google Scholar]
- 49.MacMahon S., Peto R., Cutler J., Collins R., Sorlie P., Neaton J., Abbott R., Godwin J., Dyer A., Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 50.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 51.Middeke M. Antihypertensive drug therapy: Where do we stand? Der. Internist. 2015;56:230–239. doi: 10.1007/s00108-014-3570-2. [DOI] [PubMed] [Google Scholar]
- 52.Rocchini A.P., Key J., Bondie D., Chico R., Moorehead C., Katch V., Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N. Engl. J. Med. 1989;321:580–585. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 53.Heymsfield S.B., Wadden T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 54.Carter B.D., Abnet C.C., Feskanich D., Freedman N.D., Hartge P., Lewis C.E., Ockene J.K., Prentice R.L., Speizer F.E., Thun M.J., et al. Smoking and mortality--beyond established causes. N. Engl. J. Med. 2015;372:631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 55.Bowman T.S., Gaziano J.M., Buring J.E., Sesso H.D. A prospective study of cigarette smoking and risk of incident hypertension in women. J. Am. Coll. Cardiol. 2007;50:2085–2092. doi: 10.1016/j.jacc.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Whelton P.K. Epidemiology of hypertension. Lancet. 1994;344:101–106. doi: 10.1016/S0140-6736(94)91285-8. [DOI] [PubMed] [Google Scholar]
- 57.Jackson L.V., Thalange N.K., Cole T.J. Blood pressure centiles for Great Britain. Arch. Dis. Child. 2007;92:298–303. doi: 10.1136/adc.2005.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J., Rajadurai V.S., Tan K.W. Blood pressure standards for very low birthweight infants during the first day of life. Arch. Dis. Child. Fetal Neonatal Ed. 1999;81:F168–F170. doi: 10.1136/fn.81.3.F168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajjar I., Kotchen T.A. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA J. Am. Med. Assoc. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 60.Jordan J. Pathophysiology of hypertension: What are our current concepts? Der. Internist. 2015;56:219–223. doi: 10.1007/s00108-014-3572-0. [DOI] [PubMed] [Google Scholar]
- 61.Balady G.J., Arena R., Sietsema K., Myers J., Coke L., Fletcher G.F., Forman D., Franklin B., Guazzi M., Gulati M., et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 62.Schultz M.G., Sharman J.E. Exercise Hypertension. Pulse. 2014;1:161–176. doi: 10.1159/000360975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibbons R.J., Balady G.J., Bricker J.T., Chaitman B.R., Fletcher G.F., Froelicher V.F., Mark D.B., McCallister B.D., Mooss A.N., O’Reilly M.G., et al. ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J. Am. Coll. Cardiol. 2002;40:1531–1540. doi: 10.1016/S0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 64.Löllgen H., Gerke R. Belastungs-EKG (Ergometrie) Herzschr. Elektrophys. 2008;19:98–106. doi: 10.1007/s00399-008-0009-2. [DOI] [PubMed] [Google Scholar]
- 65.Bauer P., Kraushaar L., Dorr O., Nef H., Hamm C.W., Most A. Sex differences in workload-indexed blood pressure response and vascular function among professional athletes and their utility for clinical exercise testing. Eur. J. Appl. Physiol. 2021;121:1859–1869. doi: 10.1007/s00421-021-04656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reckelhoff J.F. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.HYP.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 67.Gleim G.W., Stachenfeld N.S., Coplan N.L., Nicholas J.A. Gender differences in the systolic blood pressure response to exercise. Am. Heart J. 1991;121:524–530. doi: 10.1016/0002-8703(91)90721-S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.