Abstract
Beach safety regulation is based on faecal indicators in water, leaving out sand and fungi, whose presence in both matrices has often been reported. To study the abundance, diversity and possible fluctuations of mycobiota, fungi from sand and seawater were isolated from the Portorož beach (Slovenia) during a 1-year period. Sand analyses yielded 64 species of 43 genera, whereas seawater samples yielded 29 species of 18 genera. Environmental and taxonomical data of fungal communities were analysed using machine learning approaches. Changes in the air and water temperature, sunshine hours, humidity and precipitation, air pressure and wind speed appeared to affect mycobiota. The core genera Aphanoascus, Aspergillus, Fusarium, Bisifusarium, Penicillium, Talaromyces, and Rhizopus were found to compose a stable community within sand, although their presence and abundance fluctuated along with weather changes. Aspergillus spp. were the most abundant and thus tested against nine antimycotics using Sensititre Yeast One kit. Aspergillus niger and A. welwitschiae isolates were found to be resistant to amphotericin B. Additionally, four possible human pollution indicators were isolated during the bathing season, including Meyerozyma, which can be used in beach microbial regulation. Our findings provide the foundations for additional research on sand and seawater mycobiota and show the potential effect of global warming and extreme weather events on fungi in sand and sea.
Keywords: beach sand, fungal diversity, health, influence of environmental factors, leisure activities, monitoring of sand, resistance to antimycotics, urban beach
1. Introduction
The fungal kingdom includes highly diverse group of ubiquitous organisms with heterotrophic metabolisms [1], obtaining energy from simple sugars to long-chained and cross-linked compounds, such as cellulose, lignin, polycyclic hydrocarbons, and even human-made plastic materials [2]. Fungi need oxygen to decompose organic matter; thus, they are usually present in water, and aerated layers of soil in all geographical regions [1,2]. On the other hand, some genera and species, described as extremophiles, have been isolated also from samples of rocky and sandy deserts [3,4], salterns [5,6], and brine [7]. Extremophiles do not only survive but are also able to propagate in habitats with combined harsh living conditions, such as high salinity, low water activity, prolonged UV-radiation, drought, and high or low temperatures [8,9]. Living conditions in many niches in nature often alternate, leading to selection of extremotolerant organisms [10]. One such habitat is beach sand. Like other soils, beach sand contains organic matter, originating from microorganisms, plants, animals, human activities, and the sea [11]. Water activity in the beach sand can be frequently low, sand can be exposed to prolonged UV-radiation and presence of salts [12,13]. The combinations of these factors contribute to the overall microbial load in beach sand. In the past, attention was mainly specific in the contest of health-related bacteria and viruses, particularly those causing gastrointestinal illnesses [14,15]. Recently, fungi in beach sand became a frequent subject of interest, especially as some detected taxa cause dermatomycoses, allergic reactions, otitis, and deep mycetoma [16]. Opportunistic environmental fungi came under scrutiny also due to the evolution of their genes encoding resistance to antimycotics, as recognised for the ubiquitous genera Aspergillus, Candida, and Fusarium, also regularly isolated from sand [17]. Thus, beach sand may pose a certain health risk for people spending a lot of time in close contact with it [13]. So far it has been recognised that beach sand harbours a great diversity of fungi including dermatophytes, dematiaceous fungi, plant- and insect-related fungi, halotolerant and xerotolerant fungi, and fungi related to anthropogenic pollution [13]. The filamentous genera Aspergillus, Cladosporium, Chrysosporium, Curvularia, Fusarium, Mucor, Penicillium, Rhizopus, Scedosporium, Scopulariopsis, Scytalidium, Stachybotrys, and Trichophyton usually prevailed [13,18,19,20] over the yeasts and yeast-like fungi Aureobasidium, Candida, Geotrichum, Exophiala, Metschnikowia, Rhodotorula, and Yarrowia [12,21]. Nevertheless, despite the diverse scientific approach and identification methods used, still little is known about the general structure of fungal communities in beach sand, in particular the identification of main core-genera and factors influencing their fluctuations, as well as the presence of possible indicator species, promoted after certain events. In addition, little is also known about the presence of fungicide-resistant strains colonising beach sand.
As a follow-up to the white paper “Beach sand and the potential for infectious disease transmission: observations and recommendations” [12], 13 countries initiated the European “Mycosands” project, with the general aim to shed some light onto fungal diversity in beach sands, as well as to understand the possible health risk they could pose to humans [13]. The initiative was limited to culture-specific approaches for the isolation and characterisation of fungi. The present study in Slovenia was conducted as a part of that initiative, during which we sampled the beach sand on an artificial beach that has no direct contact with seawater.
Samples were taken at the Central Portorož beach, located in the North Adriatic Sea, in the Gulf of Trieste, and subjected to the weather conditions of the Mediterranean Sea. This sandy beach, which was created artificially, was chosen for the study because it is one of the most popular beaches in Slovenia: This is due to its location in the center of an urban environment, surrounded by buildings, a main road and vegetation. Sand on the surface is regularly cleaned and mechanically aerated, starting a month before and lasting during the complete official bathing season (between June and September). The beach regularly receives the Blue Flag Award, a quality certification for being clean, safe and user-friendly.
Sampling was performed monthly during a 1-year period. For the monitoring, we focused particularly on environmental factors possibly affecting the presence of fungal taxa. Additionally, our aim was to determine whether sand harbours culturable fungi that could be associated with the presence of humans or certain environmental events (indicator fungi). Finally, as the most abundant fungi, the Aspergillus strains obtained during the official bathing season were tested against nine antifungals to determine possible resistance to antimycotics.
2. Materials and Methods
2.1. Sampling of Sand and Seawater
Sampling was conducted at the Central beach in Portorož, Slovenia, as a part of the wider European initiative “Mycosands” [13]. Sampling of the quartz sand and seawater was performed monthly between October 2018 and September 2019. Supratidal sand was sampled between 9 am and 10 am at a depth of 5–10 cm as pre-arranged in the Standard Operational Procedure (SOP). The SOP was adopted from Sabino et al. [19] and agreed among the leaders of the Mycosands project [13]. The sand was aseptically collected into sterile 50 mL Falcon flasks, and later homogenised in sterile plastic bags. The total weight of the final samples was ~200 g. Seawater samples were collected aseptically into 2 sterile 500 mL vessels (Golias, Ljubljana, Slovenia) at a depth of 20 cm in a 1 m-deep water column. All samples were labelled and transported to the laboratory in a cooler within 2 h of sampling, as described by Sabino et al. [19].
2.2. Cultivation of Fungi and Storage of Viable Strains
Forty grams of a crude sand sample were placed into sterile Erlenmeier flasks. Fungi were extracted from the sand with the addition of 40 mL of sterile distilled water and shaking for 30 min at 100 rpm. A total of 200 µL of the obtained sand extract and 200 µL of the collected seawater were plated in triplicate onto Sabouraud’s Dextrose agar (SDA) (Biolife, Milano, Italy) and Mycosel agar (Becton Dickinson, Heidelberg, Germany) with the addition of Cycloheximide and Chloramphenicol in order to prevent the overgrowth of bacteria. SDA plates were incubated for 5 to 7 days, and Mycosel plates were incubated for up to 21 days to also obtain slow-growing dermatophytes. Both media were incubated at 25, 30 and 37 °C. Additionally, 5 mL of sand extract and 20 mL of seawater samples were filtered using Millipore (Merck, Darmstadt, Germany) filters with a pore diameter of 0.45 µm. Filters were aseptically placed onto Malt Extract Agar (Biolife, Milano, Italy) supplemented with 10% (v/w) of NaCl and Chloramphenicol (0.05 g per liter). Plates were incubated for 5 to 7 days at 25, 30 and 37 °C for cultivation of halotolerant species.
After the incubation, morphotypical colonies were counted, and colony-forming units (CFU) per gram of crude sand and per liter of seawater were counted and finally reported as the average number of triplicates. Each of the morphotypes were then transferred to fresh SDA, Mycosel, or MEA + 10% NaCl and incubated for 5–20 days until visible growth.
All strains obtained in the study were permanently stored in the Ex-Culture Collection of the Infrastructural Centre Mycosmo, MRIC UL, Slovenia (http://www.ex-genebank.com/ (accessed on 16 August 2021)), at the Department of Biology, Biotechnical Faculty, University of Ljubljana.
2.3. DNA Extraction and Molecular Identification
Cultures were grown either on MEA or on Mycosel plates, until visible growth of mycelium (usually 5–7 days). DNA of filamentous fungi was extracted according to the protocol of Van den Ende and de Hoog [22] using mechanical lysis and chloroform extraction. Yeasts were grown on MEA for 3–5 days, before DNA was extracted with PrepMan Ultra reagent (Applied Biosystems, Foster City, California, USA) following the manufacturer’s instructions. The DNA samples obtained were stored at −20 °C prior their use in the identification process.
Fungi were identified by observing their macro- and micromorphological features. According to these, the final identification of filamentous fungi based on the rDNA nucleotide sequences of either the partial actin gene (act), the partial beta-tubulin gene exons and introns (benA), or the partial translation elongation factor 1-alpha (tef 1-α) and the whole internal transcribed spacer region (ITS = ITS1, 5.8S rDNA, ITS2). Identification of the yeasts was conducted based on the sequences of the large subunit ribosomal DNA (LSU = partial 28S rDNA, D1/D2 domains). The primers used for the amplification and sequencing were ACT-512F and ACT-783R (act) [23], Bt2a and Bt2b (benA) [24], EF1 and EF2 (tef 1-α) [25], ITS5 and ITS4 (ITS) [26], and NL1 and NL4 (LSU) [27]. Sequencing was performed at Microsynth AG, Austria. Sequences were obtained at and assembled by FinchTV 1.4 (Geospiza, PerkinElmer Inc., Seattle, Washington DC, USA) and adjusted with the Molecular Evolutionary Genetics Analysis (MEGA) software version 7.0 [28]. Identification of sequences was carried out using the BLAST algorithm on the NCBI web page [29] and finally compared with type strains from taxonomical database on Westerdijk Fungal Biodiversity Institute (Utrecht, The Netherlands). Fungal names were checked in Index Fungorum (www.indexfungorum.org (accessed on 16 August 2021)), and the sequences of representative strains obtained in the study were deposited in the GenBank database (NCBI).
2.4. Antifungal Susceptibility Testing of Selected Aspergillus Strains
Antifungal susceptibility testing of 10 Aspergillus strains was performed with the YeastOne YO10 Kit (ThermoFisher Scientific, Waltham, Massachusetts, USA) containing 9 antifungal agents: Amphotericin B (AB, dilution range 0.12–8 µg/mL); Caspofungin (CAS, dilution range 0.008–8 µg/mL); Fluconazole (FZ, dilution range 0.12–256 µg/mL); Itraconazole (IZ, dilution range 0.015–16 µg/mL); 5-Flucytosine (FC, dilution range 0.06–64 µg/mL); Voriconazole (VOR, dilution range 0.008–8 µg/mL); Anidulafungin (AND, dilution range 0.015–8 µg/mL); Micafungin (MF, dilution range 0.008–8 µg/mL); and Posaconazole (PZ, dilution range 0.008–8 µg/mL). The suspensions of conidia were diluted with YeastOne broth according to the manufacturer’s instructions to reach final inoculum between 0.5–5 × 104 CFU/mL. Each well of the dried YeastOne plates was rehydrated with 100 μL of the fungal suspension. The inoculated panels were incubated at 35 °C, and read after 24 and 48 h. Growth in the wells was indicated by color change from blue (negative) to pink (positive), and the minimal inhibitory concentration (MIC) was determined as the first concentration at which the growth indicator remained negative.
2.5. Using Machine Learning to Relate Environmental Conditions and Fungal Profiles
The data collected during 12 months of sampling were split into two distinct kinds. Variables of the first kind describe the environment and variables of the second kind describe the presence of fungi, i.e., the sampled fungal profiles. The variables of the first kind are the following: season, month, average air temperature (°C), maximal air temperature during the past 7 days before sampling (°C), average soil temperature at the depth of 5 cm (°C), average air humidity (%), air humidity in the sampling day (%), monthly rainfall rate (mm3), monthly sunshine hours (h), average daily sunshine hours (hh:mm), average air pressure (hPa), average wind speed (m/s), sea water temperature (°C) (data was obtained from ARSO [30] during 2018 and 2019), weather (sunny, dry, hot, cloudy, humid, rainy, foggy, cold, windy), unexpected events (flood, strong wind, heatwave, open celebration, construction work), bathing season, beach cleaning (which includes picking up waste and mechanical aeration), characteristics of beach sand (wet—coastal bottom sediment, humid—vadose zone sand, dry—supratidal sand), and pH (seawater, sand extract). The variables of the second kind record the presence and abundance of fungal species and taxonomical groups (genera, phylum, etc.), as well as functional/non-taxonomical fungal groups (human colonisers, dermatophytes, etc.). Detailed descriptive and numerical data of all variables are included in the Supplementary Material (Table S1).
2.6. Predictive Modelling
Predictive Clustering Trees (PCTs) [31] are a generalised version of standard decision trees belonging to the class of predictive models constructed by machine learning (ML). A decision tree of this kind can be viewed as a hierarchy of non-overlapping clusters. The PCT induction algorithm recursively splits the initial data (a large non-homogeneous cluster) into smaller, purer (more homogeneous) clusters. The splits (testing conditions) within the decision tree are selected by calculating the values of a heuristic function (the intra-cluster variance of the target variables) and maximising the reduction of its value: this reduction of variance can be also used to estimate variable importance in PCT ensemble approaches [32,33]. Many data splits are considered and those with the best heuristic values are the ones that are chosen to appear in the decision tree. Such data partitioning continues until a stopping criterion is met. Nodes at the bottom of the decision tree (technically, very small clusters) are called leaf nodes and provide predictions for the values of the target variables.
PCTs can be parametrised to address several ML tasks. Two of those tasks were addressed in our analysis, namely multi-target regression (MTR) and multi-label classification (MLC). We first declared specific fungi (and higher taxa from the corresponding taxonomy) and functional groups as labels (target variables with binary values) and trained MLC models to predict their presence/absence in the sand and water samples. We then consider the abundances (CFU/g) of the functional groups as real-valued targets and address the corresponding MTR task. Each of the three figures depicts one predictive model (a PCT) with leaf nodes giving predictions for multiple targets of interest, i.e., presence and abundance of different groups of fungi.
PCTs were trained using all available input features and were allowed to grow until all leaf nodes contained 2 and 4 examples (pre-pruning). The quality of the trained predictive models was evaluated by calculating appropriate metrics of predictive performance. Specifically, for the MTR setting, we calculated the relative root mean squared error (RRMSE) and for MLC setting, the weighted area under the precision recall curve (AUPRC). Since we do not have a separate test set, the predictive performance was estimated with a leave-one-out cross validation procedure.
3. Results
3.1. Artificially Designed Sandy Beach Harbours a Great Diversity of Fungi
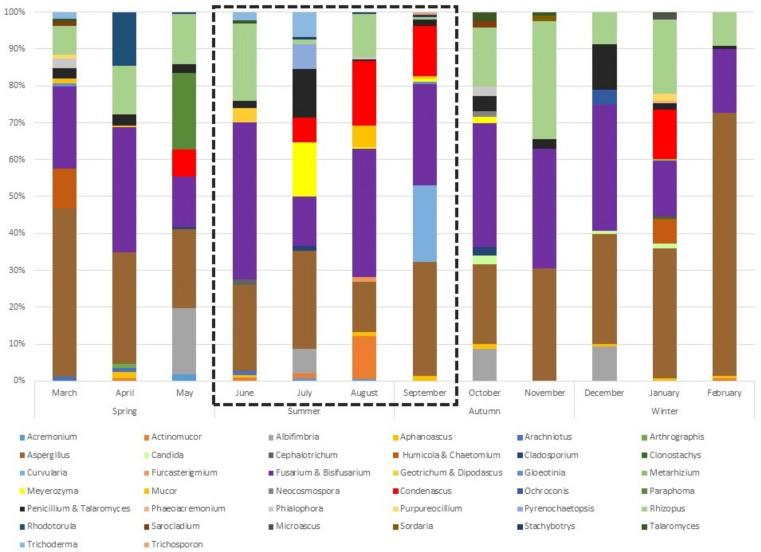
A 1-year-long survey with monthly sampling of beach sand provided insights into the diversity of culturable fungal species constituting the fungal community. Altogether, fungi isolated from the beach sand were classified into 43 genera and 64 species. The number of different genera isolated from sand in the spring varied between 10 and 13 (average = 11), in the summer 11–13 (average = 12), in the autumn 6–13 (average = 10), and in the winter 4–13 (average = 8). Two months stood out: in November, the number of genera dropped to 6 in comparison to September and October, and in January it rose in comparison to December and February to 13. The distribution and diversity of all identified genera over the period of 12 months is shown in Figure 1. Among them, 10 species, namely Actinomucor elegans, Aspergillus flavus, A. lentulus, A. terreus, Candida tropicalis, Fusarium solani, Microascus brevicaulis, Phialophora americana, Sarocladium kiliense, and Trichosporon asahii belonged to the microorganisms of Biosafety Level 2 (BSL-2) (Table 1).
Figure 1.
Relative abundance of cultured fungal genera (in %) in the samples taken each month during the period of 12 months. The official bathing season is indicated with black dashed square.
Table 1.
Fungal species isolated from beach sand and seawater in Portorož, Slovenia during the monitoring.
| Identification | Genetic Barcode | EXF-No. 1 | GenBank No. | Month | Habitat | Biosafety Level(BSL) 5 | Other Characteristics or Role in Habitats 6 |
|---|---|---|---|---|---|---|---|
| Acremonium masseei | ITS | EXF-14878 | MT280604 | August 2,3 | BS | No data | colonising plants, soil, freshwater |
| Acremonium sp. | ITS | EXF-14657 | MT280605 | July 2,3 | BS | BSL-1 | colonising plants, soil, freshwater |
| Actinomucor elegans | ITS | EXF-14384 EXF-14449 EXF-14621 EXF-14845 EXF-14849 EXF-14639 |
MT280606 MT280607 MT280608 MT280609 MT280610 MT280611 |
February, April, June 2,3, July 2,3, August 2,3 | BS | BSL-2 | colonising plants, soil, freshwater, insect-related, potential human pollution indicator |
| Albifimbria verrucaria | ITS | EXF-14006 EXF-14159 EXF-14548 EXF-14648 |
MT280612 MT280613 MT280614 MT280615 |
May 3, July 2,3, October 4, December | BS | BSL-1 | colonising plants, soil, freshwater |
| Alternaria sp. | ITS | EXF-13999 | MT280685 | October4 | SW | BSL-1 | colonising plants, soil, freshwater |
| Aphanoascus fulvescens | ITS | EXF-14459 EXF-14870 EXF-14871 |
MT280616 MT280617 MT280618 |
April, September 2,3 | SW | BSL-2 | dermatophyte, “core-genus” in the sand |
| Aphanoascus reticulisporus | ITS | EXF-14632 | MT280619 | June 2,3 | BS | BSL-1 | dermatophyte,“core-genus” in the sand |
| Aphanoascus terreus | ITS | EXF-13894 EXF-14308 EXF-14461 |
MT280627 MT280628MT280629 |
January, April, October 4 | BS | BSL-1 | dermatophyte,“core-genus” in the sand |
| Arachniotus flavoluteus | ITS | EXF-14420 EXF-14458 EXF-14628 |
MT280620 MT280621 MT280622 |
March, April, June 2,3 | BS | BSL-1 | dermatophyte |
| Arthrographis curvata | ITS | EXF-14460 | MT280623 | April | BS | BSL-1 | dermatophyte |
| Aspergillus calidoustus | benA | EXF-14640 | MT328464 | July 2,3 | BS | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus destruens | benA | EXF-14429 | MT328468 | February | SW | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus flavus | benA | EXF-13896 EXF-14002 EXF-13898 EXF-13905 EXF-13907 EXF-14163 EXF-14011 EXF-14285 EXF-14296 EXF-14383 EXF-14389 EXF-14404 EXF-14862 |
MT328469 MT328470 MT328471 MT328472 MT328473 MT328474 MT328475 MT328476 MT328477 MT328478 MT328479 MT328480 MT328481 |
January, February, March, May 3, June 2,3, July 2,3, August 2,3, September 2,3, October 4, November, December |
BS | BSL-2 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus floccosus | benA | EXF-14455 EXF-14615 |
MT328455 MT328456 |
April, June 2,3 | BS | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus jensenii | benA | EXF-14541 EXF-14542 |
MT328465 MT328466 |
May3 | SW | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus lentulus | ITS | EXF-13910 | MT280624 | October4 | BS | BSL-2 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus nidulans | benA | EXF-14003 EXF-13911 EXF-14014 EXF-14167 EXF-14298 EXF-14388 EXF-14392 EXF-14393 EXF-14405 EXF-14410 EXF-14452 EXF-14643 EXF-14850 EXF-14854 EXF-14465 |
MT328430 MT328431 MT328432 MT328433 MT328434 MT328435 MT328436 MT328437 MT328438 MT328439 MT328440 MT328441 MT328442 MT328443 MT328444 |
January, February, March, July 2,3, August 2,3, September 2,3, October 4, November, December |
BS | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus niger | benA | EXF-14015 EXF-14297 EXF-14558 EXF-14618 EXF-14873 |
MT328457 MT328458 MT328459 MT328460 MT328461 |
January, May 3, June 2,3, August 2,3, November | BS | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus pseudoglaucus | benA | EXF-14397 | MT328467 | March | SW | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus terreus | benA | EXF-14294 EXF-14387 EXF-14863 |
MT328445 MT328446 MT328447 |
January, February, September2,3 | BS | BSL-2 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus tubingensis | benA | EXF-14165 EXF-14289 EXF-14381 EXF-14453 EXF-14464 EXF-14555 EXF-14543 |
MT328448 MT328449 MT328450 MT328451MT328452 MT328453 MT328454 |
January, February, April, May3, December | BS, SW | BSL-1 | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aspergillus welwitchiae | benA | EXF-14400 EXF-14864 |
MT328462 MT328463 |
March, September 2,3 | BS | No data | dematiaceous, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Aureobasidium melanogenum | ITS | EXF-13886 | MT280686 | October4 | SW | BSL-1 | dematiaceous, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Aureobasidium pullulans | ITS | EXF-14611 EXF-14638 |
MT280687 MT280688 |
June 2,3, July 2,3 | SW | BSL-1 | dematiaceous, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Bisifusarium delphinoides | tef1 | EXF-14645 EXF-14166 |
MT292641 MT292642 |
July 2,3, December | BS | BSL-1 | “core-genus” in the sand, colonising plants, soil, freshwater |
| Candida parapsilosis | LSU | EXF-13884 EXF-13882 EXF-13881 EXF-14282 EXF-14300 |
MT273264 MT273265 MT273266 MT273267 MT273268 |
October 4, December, January | BS | BSL-1 | colonising humans, colonising plants, soil, freshwater |
| Candida tropicalis | LSU | EXF-14299 | MT273269 | January | BS | BSL-2 | colonising humans, colonising plants, soil, freshwater |
| Cephalotrichum gorgonifer | ITS | EXF-14630 | MT280625 | June 2,3 | BS | BSL-1 | rock-inhabiting |
| Chaetomidium fimeti | ITS | EXF-14301 | MT280626 | January | BS | BSL-1 | rock-inhabiting, dematiaceous, colonising plants, soil, freshwater |
| Cladosporium cladosporioides | act | EXF-13913 EXF-13912 EXF-14378 |
MT292643 MT292644 MT292645 |
February, October 4 | SW | BSL-1 | dematiaceous, psychrotolerant, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Cladosporium halotolerans | act | EXF-14442 EXF-14443 EXF-14004 EXF-14562 |
MT292646 MT292647 MT292648 MT292649 |
April, May 3 | BS, SW | BSL-1 | dematiaceous, psychrotolerant, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Cladosporium pseudocladosporioides | act | EXF-13897 EXF-14379 |
MT292650 MT292651 |
February, October4 | SW | BSL-1 | dematiaceous, psychrotolerant, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Cladosporium ramotenellum | act | EXF-13916 EXF-14380 |
MT292652 MT292653 |
February, October4 | SW | BSL-1 | dematiaceous, psychrotolerant, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Cladosporium sphaerospermum | act | EXF-14540 | MT292656 | May3 | SW | BSL-1 | dematiaceous, psychrotolerant, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Cladosporium tenellum | act | EXF-14001 | MT292654 | October4 | SW | BSL-1 | dematiaceous, psychrotolerant, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Cladosporium velox | act | EXF-13914 | MT292655 | October 4 | SW | BSL-1 | dematiaceous, psychrotolerant, halotolerant, BTEX degrader, colonising plants, soil, freshwater |
| Clonostachys rosea | ITS | EXF-14306 | MT280630 | January | BS | BSL-1 | insect-related, colonising plants, soil, freshwater |
| Condenascus tortuosus | ITS | EXF-14287 EXF-14553 EXF-14554 EXF-14653 EXF-14844 EXF-14858 |
MT280631 MT280632 MT280633 MT280634 MT280635 MT280636 |
January, May 3, July 2,3, August 2,3, September 2,3 | BS | No data | indicator in bathing season, colonising plants, soil, freshwater |
| Curvularia sp. | ITS | EXF-14855 EXF-14856 |
MT280637 MT280638 |
September 2,3 | BS | BSL-1 | dematiaceous, colonising plants, soil, freshwater |
| Cystofilobasidium macerans | LSU | EXF-14427 | MT273278 | February | SW | BSL-1 | psychrotolerant, halotolerant |
| Didymella glomerata | ITS | EXF-14613 | MT280689 | June 2,3 | SW | BSL-1 | dematiaceous, colonising plants, soil, freshwater |
| Dipodascus geotrichum | ITS | EXF-14631 EXF-14624 |
MT280640 MT280641 |
June 2,3 | BS | BSL-1 | colonising humans, plants |
| Exophiala xenobiotica | ITS | EXF-13891 | MT280690 | October 4 | SW | BSL-1 | rock-inhabiting, dematiaceous, BTEX degrader, colonising humans, freshwater |
| Furcasterigmium furcatum | ITS | EXF-14876 | MT280639 | August 2,3 | BS | BSL-1 | rock-inhabiting, colonising plants, soil, freshwater |
| Fusarium brachygibbosum | tef1 | EXF-14451 | MT292640 | April | BS | BSL-1 | “core-genus” in the sand, colonising plants, soil, freshwater |
| Fusarium ipomoeae | tef1 | EXF-14157 | MT292639 | December | BS | BSL-1 | “core-genus” in the sand, colonising plants, soil, freshwater |
| Fusarium nirenbergiae | tef1 | EXF-13888 EXF-14164 EXF-14385 EXF-14399 EXF-14411 EXF-14447 EXF-14448 EXF-14547 EXF-14549 EXF-14626 EXF-14641 EXF-14860 EXF-14843 |
MT292623 MT292624 MT292625 MT292626 MT292627 MT292628 MT292629 MT292630 MT292631 MT292632 MT292633 MT292634 MT292635 |
February, March, April, May 3, June 2,3, July 2,3, August 2,3, September 2,3, October 4, December |
BS | BSL-1 | “core-genus” in the sand, colonising plants, soil, freshwater |
| Fusarium solani | tef1 | EXF-14848 EXF-14005 EXF-14288 |
MT292636 MT292637 MT292638 |
January, August 2,3, October 4 | BS | BSL-2 | “core-genus” in the sand, colonising plants, soil, freshwater |
| Geotrichum silvicola | ITS | EXF-14629 | MT280642 | June 2,3 | BS | BSL-1 | colonising humans, colonising plants, soil, freshwater |
| Gliomastix roseogrisea | ITS | EXF-14564 | MT280643 | May 3 | BS | BSL-1 | BTEX degrader, dematiaceous, insect-related, colonising plants, soil, freshwater |
| Gloeotinia sp. | ITS | EXF-14407 EXF-14868 |
MT280644 MT280645 |
March, September 2,3 | BS | No data | psychrotolerant, colonising plants, soil, freshwater |
| Hortaea werneckii | ITS | EXF-14000 | MT280691 | October 4 | SW | BSL-1 | dematiaceous, halophilic |
| Humicola homopilata | ITS | EXF-14398 | MT280646 | March | BS | No data | rock-inhabiting, dematiaceous, colonising plants, soil, freshwater |
| Lenzites betulinus | ITS | EXF-14377 | MT280692 | February | SW | No data | colonising plants, soil, freshwater |
| Metarhizium marquandii | ITS | EXF-14305 | MT280647 | January | BS | BSL-1 | insect-related, colonising plants, soil, freshwater |
| Meyerozyma caribbica | LSU | EXF-14647 EXF-14650 EXF-14655 EXF-14881 EXF-14867 EXF-13879 |
MT273270 MT273271 MT273272 MT273273 MT273274 MT273275 |
July 2,3, August 2,3, September 2,3, October 4 | BS | BSL-1 | colonising humans, colonising plants, soil, freshwater, potential human pollution indicator |
| Meyerozyma guilliermondii | LSU | EXF-13885 | MT273279 | October 4 | SW | BSL-1 | colonising humans, colonising plants, soil, freshwater, potential human pollution indicator |
| Microascus brevicaulis | ITS | EXF-14307 | MT280648 | January | BS | BSL-2 | colonising plants, soil, freshwater |
| Mucor circinelloides | ITS | EXF-14403 EXF-14853 EXF-14450 EXF-14847 |
MT280649 MT280650 MT280651 MT280652 |
March, April, August 2,3, September 2,3 | BS | BSL-1 | colonising plants, soil, freshwater |
| Neocosmospora rubicola | ITS | EXF-13893 | MT280653 | October 4 | BS | No data | colonising plants, soil, freshwater |
| Neomicrosphaeropsis italica | ITS | EXF-14074 | MT280693 | November | SW | No data | colonising plants, soil, freshwater |
| Ochroconis sp. | ITS | EXF-14160 | MT280654 | December | BS | BSL-1 | dematiaceous, BTEX degrader, colonising plants, soil, freshwater |
| Papiliotrema fonsecae | LSU | EXF-14426 | MT273280 | February | SW | BSL-1 | halotolerant |
| Paraconiothyrium cyclothyrioides | ITS | EXF-13917 EXF-14007 EXF-14614 |
MT280694 MT280695 MT280696 |
June 2,3, October 4, November | SW | BSL-1 | halotolerant |
| Paraphoma radicina | ITS | EXF-14550 | MT280655 | May 3 | BS | BSL-1 | dematiceaous, colonising plants, soil, freshwater |
| Penicillium aurantiogriseum | benA | EXF-13906 EXF-14386 EXF-14402 EXF-14644 |
MT328501 MT328502 MT328503 MT328504 |
February, March, July 2,3, October 4 | BS | BSL-1 | psychrotolerant, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Penicillium crustosum | benA | EXF-14646 EXF-14444 |
MT328495 MT328496 |
April, July 2,3 | BS, SW | BSL-1 | psychrotolerant, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Penicillium glabrum | benA | EXF-14169 | MT328513 | December | BS | BSL-1 | psychrotolerant, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Penicillium griseofulvum | benA | EXF-13899 EXF-14170 EXF-14172 EXF-14156 EXF-14158 EXF-14423 EXF-14552 EXF-14563 |
MT328505 MT328506 MT328507 MT328508 MT328509 MT328510 MT328511 MT328512 |
March, May 3, October 4, December | BS | BSL-1 | psychrotolerant, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Penicillium oxalicum | benA | EXF-14292 EXF-14445 |
MT328493 MT328494 |
January, April | BS | BSL-1 | psychrotolerant, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Penicillium sizovae | benA | EXF-14880 | MT328498 | August 2,3 | BS | BSL-1 | psychrotolerant, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Penicillium sp. | benA | EXF-14610 | MT328497 | June 2,3 | SW | BSL-1 | psychrotolerant, halotolerant, “core-genus” in the sand, colonising plants, soil, freshwater |
| Phaeoacremonium iranianum | ITS | EXF-14302 | MT280656 | January | BS | No data | colonising plants, soil, freshwater |
| Phialophora americana | ITS | EXF-14409 EXF-14879 |
MT280659 MT280660 |
March, August 2,3 | BS | BSL-2 | dematiaceous, BTEX degrader, colonising plants, soil, freshwater |
| Phialophora sp. | ITS | EXF-13890 EXF-14073 |
MT280657 MT280658 |
October 4 | BS | BSL-1 | dematiaceous, colonising plants, soil, freshwater |
| Purpureocillium sp. | benA | EXF-14303 EXF-14421 |
MT328499 MT328500 |
January, March | BS | BSL-1/-2 | rock-inhabiting, halotolerant, colonising plants, soil, freshwater |
| Pyrenochaetopsis tabarestanensis | ITS | EXF-14642 | MT280661 | July 2,3 | BS | No data | colonising plants, soil, freshwater |
| Rhizopus arrhizus | ITS | EXF-13903 EXF-13909 EXF-14009 EXF-14012 EXF-14168 EXF-14290 EXF-14291 EXF-14293 EXF-14390 EXF-14454 EXF-14556 EXF-14627 EXF-14649 EXF-14846 EXF-14852 |
MT280662 MT280663 MT280664 MT280665 MT280666 MT280667 MT280668 MT280669 MT280670 MT280671 MT280672 MT280673 MT280674 MT280675 MT280676 |
January, February, April, May 3, June 2,3, July 2,3, August 2,3, September 2,3, October 4, November, December |
BS | BSL-2 | “core-genus” in the sand, colonising plants, soil, freshwater |
| Rhodotorula graminis | LSU | EXF-14539 | MT273282 | January | SW | BSL-1 | psychrotolerant, halotolerant, BTEX-degrader, colonising plants, soil, freshwater |
| Rhodotorula mucilaginosa | LSU | EXF-14446 | MT273276 | April | BS | BSL-1 | halotolerant, BTEX-degrader, colonising human, plants, soil, freshwater |
| Sarocladium kiliense | ITS | EXF-13880 EXF-14408 |
MT280677 MT280678 |
March, October4 | BS | BSL-2 | insect-related, colonising plants, soil, freshwater |
| Sordaria nodulifera | ITS | EXF-14010 | MT280679 | November | BS | No data | colonising plants, soil, freshwater |
| Stachybotrys sp. | ITS | EXF-14565 EXF-14654 EXF-14875 |
MT280680 MT280681 MT280682 |
May 3, July 2,3, August 2,3, September 2,3 | BS | BSL-1 | colonising plants, soil, freshwater, potential human pollution indicator |
| Stereum hirsutum | ITS | EXF-13915 | MT280697 | October4 | SW | BSL-1 | colonising plants, soil, freshwater |
| Talaromyces atroroseus | benA | EXF-14286 | MT328482 | January | BS | BSL-1 | insect-related, colonising plants, soil, freshwater, “core-genus” in the sand |
| Talaromyces pinophilus | benA | EXF-14406 EXF-13900 EXF-14017 EXF-13904 EXF-13902 EXF-14415 EXF-13901 EXF-14620 EXF-14633 |
MT328483 MT328484 MT328485 MT328486 MT328487 MT328488 MT328489 MT328490 MT328491 |
March, May 3, October 4, November | BS | BSL-1 | insect-related, colonising plants, soil, freshwater, “core-genus” in the sand |
| Talaromyces purpureogenus | benA | EXF-13895 | MT328492 | October 4 | BS | BSL-1 | insect-related, colonising plants, soil, freshwater, “core-genus” in the sand |
| Trichoderma citrinoviride | ITS | EXF-14412 EXF-14619 |
MT280683 MT280684 |
March, June 2,3 | BS | BSL-1 | colonising plants, soil, freshwater |
| Trichosporon asahii | LSU | EXF-14865 | MT273277 | September 2,3 | BS | BSL-2 | dermatophyte |
| Wickerhamomyces anomalus | LSU | EXF-14842 | MT273283 | August 2,3 | SW | BSL-2 | colonising humans, plants, soil, freshwater |
Legend: 1 EXF No., number designated to fungi in the EX Culture Collection of the Infrastructural Centre Mycosmo, Biotechnical Faculty, University of Ljubljana, Slovenia. 2 Official bathing season. 3 Beach cleaning, mechanical aeration. 4 A one-time event, flood. 5 Biosafety Level (BSL) data cited from de Hoog et al. [34] and ATCC [35]. 6 Fungal characteristics/role in habitats cited from Brandão et al. [13], de Hoog et al. [34] and Mycoses Study Group Education and Research Consortium, 2022 (https://drfungus.org/ (accessed on 16 August 2021)). BS—beach sand. SW—seawater.
3.2. Differences in Diversity of Culturable Fungi in Seawater and Beach Sand
We detected 18 genera and 29 different species of fungi in seawater samples. Amongst them, only Aphanoascus fulvescens and Wickerhamomyces anomalus were recognised as BSL-2 fungi. Interestingly, these neighboring environments had only three species in common (e.g., Aspergillus tubingensis, Cladosporium halotolerans, and Penicillium crustosum). The greatest diversity in the fungal community of seawater samples was observed in February, June, and October, with 5, 5, and 8 isolated genera respectively. The highest diversity was observed in October, with elevated numbers of the genus Cladosporium. During the same period, most Aspergillus species were isolated, while genera Aureobasidium, Paraconiothyrium, and Penicillium occurred during the summer and autumn. In addition, black yeasts (e.g., Exophiala and Hortaea) and human-related yeasts Meyerozyma and Wickerhamomyces were also isolated during the late summer and autumn months. However, their presence was only sporadic, which possibly indicates transient human pollution episodes.
3.3. Machine Learning Models Reveal Connections between Environmental Changes and Fungal Presence
Each fungal species was assigned into at least one group namely dermatophytes, dematiaceous fungi, rock-inhabiting fungi, halotolerant and halophilic fungi, psychrotolerant fungi, fungi related to plants, soil, and freshwater, fungi related to insects, BTEX (as benzene, toluene, ethylbenzene, and xylene) degraders, possible indicators for human presence, and the “core-fungi” of the sand (Table 1).
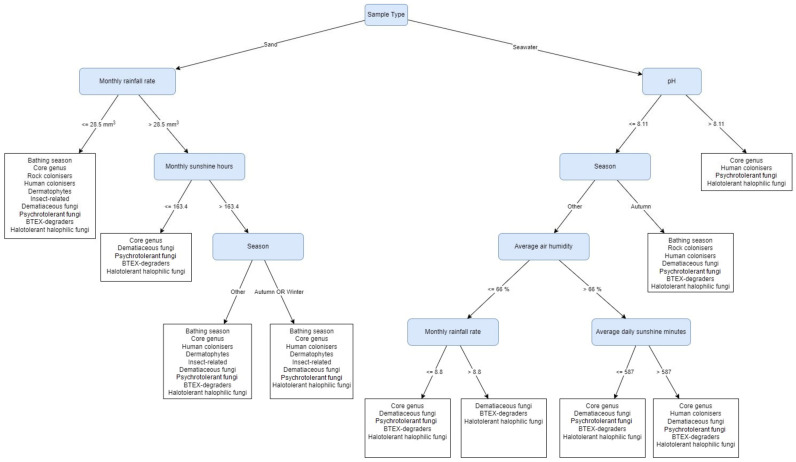
Figure 2 depicts a Predictive Clustering Tree (PCT) model that highlights the habitat, e.g., sand or seawater, as the first (most important) decisive factor that discriminates between both fungal communities. When looking into the fungal community of the sand (left section of the PCT depicted in Figure 2), the core genera, psychrotolerant fungi, dematiaceous fungi, and halotolerant/halophilic fungi are present in every leaf, regardless of the screened environmental factors. All non-taxonomical groups together are likely to be present in sand when the monthly rainfall rate is ≤28.5 mm3 (March, June, August, and January). Among them, rock-colonising genera appeared only under these conditions. Fungi related to the bathing season, human colonisers, dermatophytes, insects’ colonisers and rock colonisers are absent during the months when the monthly rainfall rate is >28.5 mm3 and the monthly sunshine hours are ≤163.4 h (May, November, and December). The last decision step following the monthly rainfall rate >28.5 mm3 and monthly sunshine hours ≤163.4 h is the season, which predicts the absence of BTEX-degrading fungi during the autumn and winter months, from September to February.
Figure 2.
The Predictive Clustering Tree (PCT) for multi-label classification (MLC), predicting the presence of fungal functional groups, isolated from beach sand and seawater. The decision process starts by separating samples based on their type (sand or seawater). From there on, Monthly rainfall rate, Monthly sunshine hours, Season, pH, Average air humidity and Average daily sunshine minutes are used to determine the prediction of the tree. For example, if samples come from seawater and their pH is higher than 8.11, the predicted labels (functional groups) are: Core genus, Human colonisers, Psychrotolerant fungi, and Halotolerant/halophilic fungi.
For fungal communities from seawater, pH is the first decision factor. The leaf with pH > 8.11 rules out BTEX degraders and dematiaceous fungi, while fungi related to the bathing season and rock-colonising fungi appear only during the autumn months when pH ≤ 8.11. Human-colonising fungi appeared in three leaves, independent of the pH of the water, yet their presence was influenced by the season, air humidity, and daily sunshine minutes. They can be present in seawater when pH > 8.11 (August, September), but can be isolated also when pH ≤8.11 during the autumn months (October, November), or when pH ≤8.11 in the other seasons, with average air humidity >66% and when average daily sunshine minutes exceeds 587 min (April, May). The group of halotolerant and halophilic fungi is present in every tree leaf, independent of all environmental factors included in the tree. On the other hand, the groups of fungi related to insects and dermatophytes did not appear in any of the tree leaves.
3.4. Factors Influencing the Stability of Fungal Community in Beach Sand
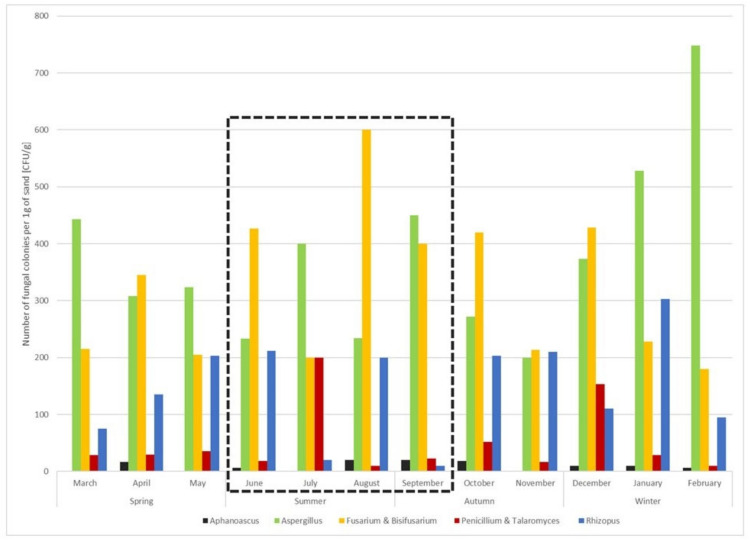
Fungal genera isolated from more than seven sand samples (>7/12) were recognised as the “core-genera”, representing the backbone of fungal community in the sand. They include the species of Aphanoascus fulvescens, A. reticulisporus, A. terreus, Aspergillus calidoustus, A. destruens, A. flavus, A. floccosus, A. jensenii, A. lentulus, A. nidulans, A. niger, A. pseudoglaucus, A. terreus, A. tubingensis, A. welwitchiae, Fusarium brachygibbosum, F. ipomoeae, F. nirenbergiae, F. solani, Bisifusarium delphinoides, Penicillium aurantiogriseum, P. crustosum, P. glabrum, P. griseofulvum, P. oxalicum, P. sizovae, Penicillium sp., Talaromyces atroroseus, T. pinophilus, T. purpureogenus, and Rhizopus arrhizus (Table 1, Figure 3). Although their numbers fluctuated over time, their presence was stable in comparison to the other genera in the community.
Figure 3.
Presence and numbers of “core-genera” in sand samples during the period of 12 months. The official bathing season is indicated with a black, dashed square.
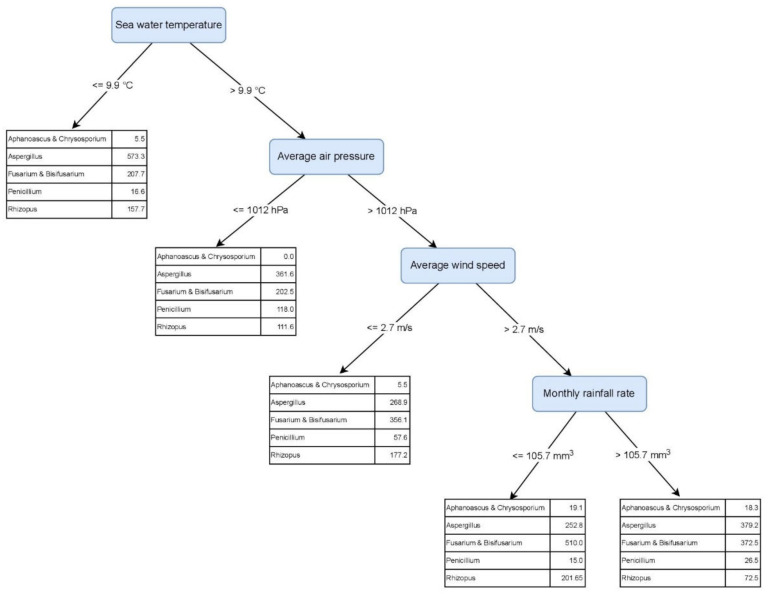
The machine learning-generated MTR tree describes the highest numbers of the genera Aphanoascus (19.2 CFU/g), Fusarium & Bisifusarium (510.0 CFU/g), and Rhizopus (201.7 CFU/g) in months when the seawater temperature was >9.9 °C, average air pressure >1012.0 mbar, average wind speed >2.7 m/s, and monthly rainfall rate ≤105.7 mm3 (August and October) (Figure 4). On the other hand, following the same environmental factors, the presence of Aspergillus and Penicillium was the lowest, with 252.8 CFU/g and 15.0 CFU/g respectively. The tree describes the highest Penicillium numbers (118.0 CFU/g) when seawater temperature was >9.9 °C and average air pressure ≤1012.0 mbar (May, July). The highest number of Aspergillus in beach sand is expected when seawater temperature is ≤9.9 °C (January–March) (Figure 4).
Figure 4.
Predictive Clustering Tree (PCT) for multi-target regression (MTR), predicting the abundance of the core-genera, isolated from beach sand and seawater. The model predicts the highest abundances for Aspergillus and Fusarium & Bisifusarium. Particularly high abundance of Aspergillus is predicted when sea water temperature is below or equal to 9.9 °C (leftmost leaf), while high abundance of Fusarium & Bisifusarium is predicted when sea water is warmer (>9.9 °C), air pressure is high (>1012 hPa), average wind speeds are higher than 2.7 m/s and monthly rainfall rate is below or equal to 105.7 mm3 (rightmost leaf). The genus Aphanoascus is always predicted in lowest quantities.
3.5. Potential Human Pollution Indicators for the Bathing Season
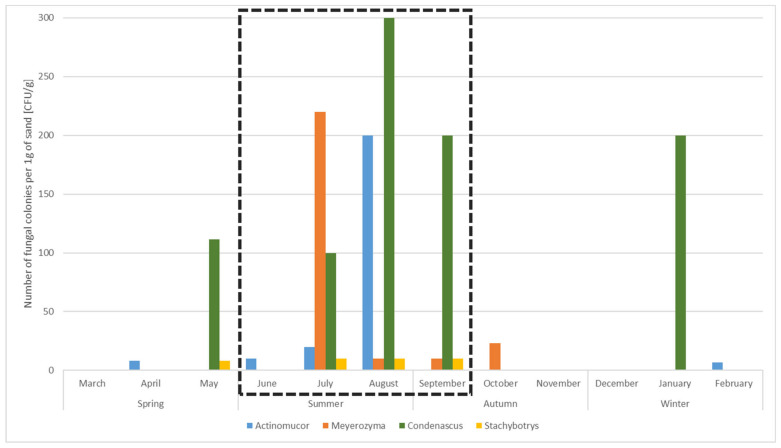
During the yearly monitoring, we observed a short-term elevated presence of four genera, including five species: Actinomucor elegans, Condenascus tortuosus, Meyerozyma caribbica, M. guilliermondii, and Stachybotrys sp. Their numbers increased mainly from May to October and were particularly elevated during the official bathing season from June to September. As their occurrence and increased number coincided with the official time of the bathing season, they could be used as potential human pollution indicators (Table 1, Figure 5).
Figure 5.
Presence and numbers of “potential human pollution indicator-genera” in sand samples during the period of 12 months. The official bathing season is indicated with a black, dashed square.
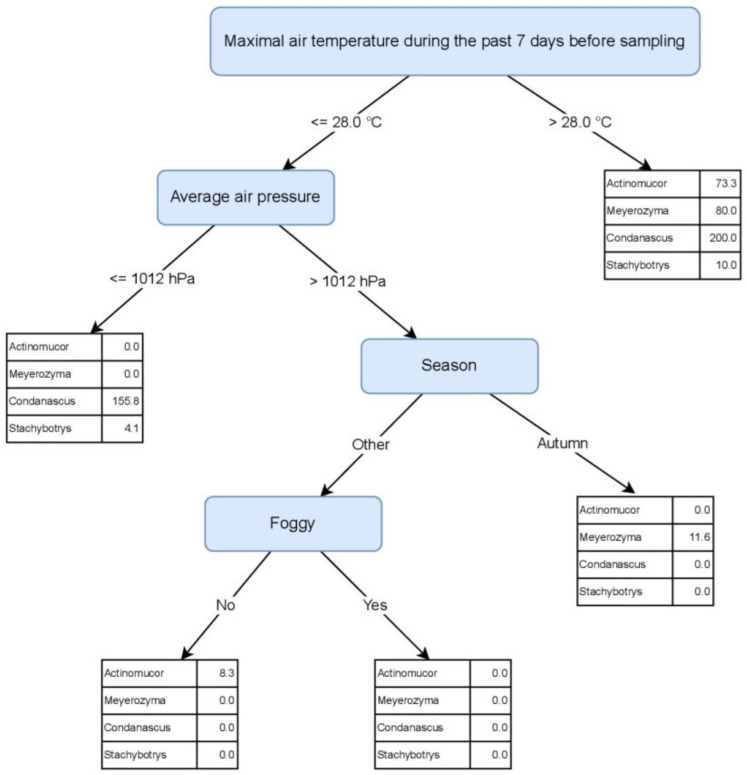
Figure 6 depicts a PCT model that predicts the abundance of the four genera based on environmental factors. Maximal air temperature during the past 7 days before sampling was identified as the most discriminating environmental factor. When its values are higher than 28 °C, the presence of all four indicator genera in the beach sand is predicted. This value corresponds with the summer season and the official bathing season. However, these indicator genera may be present in sand in lower numbers also outside the bathing season. The genera Condenascus and Stachybotrys were linked to the air pressure ≤1012.0 mbar, while Meyerozyma appears in autumn when the air pressure is >1012.0 mbar. Actinomucor is most likely isolated from sand when air pressure is >1012.0 mbar, during the whole year, except for the autumn months (Figure 6).
Figure 6.
Predictive Clustering Tree (PCT) for multi-target regression (MTR), predicting the abundances of potential human pollution indicators, isolated from beach sand and seawater. For the case when the Maximal air temperature during the past 7 days before sampling is higher than 28 °C, the predicted abundances (CFU/g) are given in the top-most leaf node of the tree (Actinomucor = 73.3 CFU/g, Meyerozyma = 80.0 CFU/g, Condenascus = 200.0 CFU/g, Stachybotrys = 10.0 CFU/g). If temperature is less than or equal to 28 °C, another test is applied on the Average air pressure attribute. This way, the decision tree is traversed and every sample eventually ends up in one of the leaf nodes of the tree, where predictions for fungal abundances are provided.
3.6. Aspergillus Niger Strains Isolated from Beach Sand Are Resistant to Amphotericin B
Fungi of the genus Aspergillus were with the monthly average number of 376 CFU/g also the most represented fungi in the beach sand samples. In total, 10 strains of Aspergillus spp, isolated during bathing season, were subjected to the antifungal susceptibility testing in order to evaluate possible risk for humans during their leisure activities on the beach. The detailed results carried out with Sensititre Yeas One Kit are summarised in Table 2.
Table 2.
The results of susceptibility testing for Aspergillus species isolated during bathing season against nine antifungals from Sensititre Yeast One Kit.
| Identification | EXF-No. | Range of Minimal Inhibitory Concentration (MIC) [µg/mL] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anidulafungin (AND), 24 h | Micafungin (MF), 24 h | Caspofungin (CAS), 24 h | Amphotericin B (AB), 48 h | 5-Flucytosine (FC), 48 h | Posaconazole (PZ), 48 h | Voriconazole (VOR), 48 h | Itraconazole (IZ), 48 h | Fluconazole (FZ), 48 h | ||
| Aspergillus flavus | EXF-14617 | 0.03 4 | 0.06 4 | 1 4 | 2–4 | >64 4 | 0.06 4 | 0.5–1 | 0.06 4 | ≥256 4 |
| EXF-14651 | ||||||||||
| EXF-14874 | ||||||||||
| EXF-14862 | ||||||||||
| ECOFF 1 | IE 2 | IE 2 | IE 2 | 4 | no data | 0.5 | 2 | 1 | / 3 | |
| Aspergillus nidulans | EXF-14643 | 0.03 4 | 0.12 4 | 0.25 4 | 2–4 | >64 4 | 0.06–0.12 | 0.12–0.25 | 0.03–0.06 | ≥256 4 |
| EXF-14850 | ||||||||||
| EXF-14854 | ||||||||||
| ECOFF 1 | IE 2 | IE 2 | IE 2 | 4 | no data | 0.5 | 1 | 1 | / 3 | |
| Aspergillus niger | EXF-14618 | 0.03 4 | 0.12 4 | 1 4 | 2 4 | 32 4 | 0.12 4 | 1 4 | 0.25 4 | ≥256 4 |
| EXF-14873 | ||||||||||
| ECOFF 1 | IE 2 | IE 2 | IE 2 | 0.5–1 | no data | 0.25–0.5 | 2 | 4 | / 3 | |
| Aspergillus welwitschiae | EXF-14864 | 0.015 | 0.06 | 0.5 | 2 | 16 | 0.12 | 0.5 | 0.12 | ≥256 |
| ECOFF 1 | no data | no data | no data | no data | no data | no data | no data | no data | no data | |
Legend: 1 ECOFF—Epidemiologic cut-off values for wild-type strains, as abbreviated by EUCAST [37]. 2 Insufficient evidence that the organism or group is a good target for therapy with the agent [37]. 3 No breakpoints. Susceptibility testing is not recommended [37]. 4 All tested strains had the same result; the numbers represent the average value for all strains of the same species. Red colour—resistant; growth of the strain was above ECOFF reported for the species [37]. Pink colour—possibly resistant; growth of the strain was above ECOFF reported for the taxonomically closest relative Aspergillus niger [37]. Green colour—susceptible; growth of the strain was below ECOFF reported for the species [37].
The minimum inhibitory concentration (MIC) for echinocandines, after 24 h was as follows: anidulafungin = 0.03 µg/mL, except for A. welwitschiae (0.015 µg/mL), micafungin = 0.06 µg/mL for A. flavus and A. welwitschiae, and 0.12 µg/mL for A. nidulans and A. niger. Aspergillus nidulans was the most susceptible to caspofungin (MIC = 0.25 µg/mL). After 48 h, MIC of echinocandines for all Aspergillus species was >8 µg/mL as observed by Siopi et al [36].
MIC for amphotericin B and 5-flucytosine for A. flavus and A. nidulans were 2–4 µg/mL and >64 µg/mL, respectively. These species are known to be intrinsically resistant to amphotericin B as reported by European Committee on Antimicrobial Susceptibility Testing (ECOFF = 4 µg/mL). Among the tested strains, only Aspergillus niger was resistant to amphotericin B (MIC = 2 µg/mL vs. ECOFF = 1 µg/mL). Additionally, its taxonomically close relative A. welwitschiae (section Nigri) yielded the same result (MIC = 2 µg/mL), but there is so far no known ECOFF data for this species.
Regarding tested triazoles, all Aspergillus species were the most susceptible to posaconazole and itraconazole, while all were resistant to fluconazole with MIC ≥ 256 µg/mL.
4. Discussion
Fungi as heterotrophs have crucial role in the circulation of organic matter in the environment. Many species are known to grow under elevated UV-radiation, desiccation and salinity, and break down long-chained hydrocarbons such as chitin, cellulose, lignin and crude oil, as well as on human-made materials, such as rubbers, silicone and different types of plastic [9,10,38,39]. Human-made materials are increasingly represented in the environment with a growing population, and frequently end up in the oceans [40,41]. Beach sand and sea bordering urban places usually meet many of the above-described conditions as explained in detail in the white paper “Beach sand and the potential for infectious disease transmission: observations and recommendations” [12]. During the last few decades, fungi have become frequently connected also to a variety of human diseases [34,42]. Contrary to viral or bacterial infections, the symptoms of fungal infections usually appear later and are therefore more difficult to relate to the primary environmental source [34,43]. Studies on fungal taxonomy and diversity in different habitats are thus important in order to fill the environmental gap in medical data. One of such studies is the European initiative “Mycosands”, investigating fungal diversity and abundance in beach sand and seawater, under different environmental conditions in order to assess the possible health risk fungi could pose to humans [13].
Compared to the other participating countries, Slovenia’s location possesses some geographical and natural specifics, for instance higher salinity and elevated concentrations of mercury [44]. Due to the location, sea flows, and the close proximity of major ports, pollutants such as petroleum may have been present [45]. The sampling site, the Central Beach Portorož, is a man-made beach with fine quartz sand, located in the northern Adriatic Sea (Gulf of Trieste) as a part of a touristic center, surrounded by buildings and a main traffic road with vegetation along the road; thus, we classified it as an “urban beach” [13]. Another characteristic is the concrete footpath, separating the sand from the sea, preventing the tidal wash-off of the sand. A month before and during the official bathing season (May–September), employees regularly pick up waste and mechanically aerate the sand. In addition, the beach regularly receives the Blue Flag Award as a synonym for its quality as a clean, safe, and user-friendly beach [46]. However, microbiological monitoring of the beach sand is not a part of the Bathing Water Directive [47], though the most recent World Health Organization guidelines for recreational water quality mention fungi and the risk assessment of non-generic parameters, when relevant [48]. To the best knowledge of the authors, this is the first report of fungal presence and diversity in beach sand and related seawater in Slovenia. During the monitoring, we identified 64 species from the sand, and 29 species from seawater samples. The results are comparable with previous global studies on sand, reporting a high diversity of fungi, even when sand is dry, exposed to the sun, and well-aerated [49,50,51].
4.1. Fungi Isolated from Seawater Differ from Sand Mycobiota
Interestingly, the results showed significant difference between the mycobiota of seawater and beach sand. Both habitats had only three species in common (i.e., Aspergillus tubingensis, Cladosporium halotolerans, and Penicillium crustosum). This difference was identified also with machine learning analysis, where the habitat appeared as the first, most decisive, factor (Figure 2). Besides salinity, UV radiation, temperature fluctuations and water flow, the concrete footpath between both environments could also affect the fungal biota, preventing the washing off of the sand and its possible mixing with seawater. Seawater samples expectedly yielded fewer fungal taxa in comparison to the sand [13], with only two species classified as BSL-2. The elevated number of taxa in seawater corresponded to high rainfall rated in February and June and flooding in October. This is particularly visible for the genera Aspergillus and Cladosporium isolated during these months, likely due to the washout from land [52]. In late summer and autumn, we isolated the melanised genera Aureobasidium, Exophiala, and Hortaea known to be well adapted to UV-irradiation and salinity, both of which are elevated during the late summer months [44,53]. Human-related yeasts Meyerozyma and Wickerhamomyces were expectedly isolated during this period, due to the official bathing season and presence of people [54], although their number in seawater was low.
According to the machine learning analysis, the pH is the first, most decisive, factor affecting the fungal community in seawater. The Northern Adriatic Sea has the lowest pH during the winter months and the highest during late spring and summer [55]. Our local measurements showed the peak of pH during the summer and early autumn, and its lowest values from October until June. The months with the highest pH (>8.11) yielded less fungi and particularly lacked dematiaceous, melanised fungi and BTEX degraders. The reason for this could be the negative effect of alkaline pH on pigment formation in certain genera [56]. More fungi were isolated from seawater with pH ≤ 8.11 during the autumn months, in particular fungi associated with the bathing season and rock-colonising fungi. In comparison to the sand samples, these groups appear in the seawater a month or two after their presence is noted in sand.
However, the pH of seawater does not affect the presence of human-colonising fungi, which is most related to the season, air humidity, and daily exposure to sunshine. They appeared in the seawater sporadically from August to November, but were present also in moist and sunny months in spring. This observation is in accordance with Kaewkrajay et al. [57], reporting the presence of the genera Candida, Meyerozyma, Rhodotorula and Wickerhamomyces in seawater [57]. Again, the time lapse of a month or two is visible when compared to the sand samples where these groups appear from June to October. The reason for the time lapse could be the precipitation and washout from the sand [58].
It is noteworthy that the group of halotolerant and halophilic fungi was present during the whole year, independently of environmental factors included in the study. These results were expected, since the seawater contains a stable community of halotolerant genera [53]. In addition, the models predict that the insect-related fungi and dermatophytes are not present (Figure 2), confirming their close connection to the terrestrial hosts.
4.2. Fungal Abundance in Beach Sand Varies According to Seasonal Weather Conditions
Monthly monitoring of the beach sand revealed the influence of seasonal weather on the fungal community. The highest fungal diversity in beach sand was described when the monthly rainfall rate was ≤28.5 mm3. Since the rock-colonising genera were also present, this suggests a possible washout of fungi during the rainy periods and a reestablishment of the community during the dry periods [58,59,60]. In months when the monthly rainfall rate was higher than 28.5 mm3 and consequently fewer sunshine hours were counted (≤163.4 h), fungi found during the bathing season, such as human colonisers, dermatophytes, insect-related fungi, and rock colonisers were absent in the beach sand. This may suggest both the washout of fungi and also the absence of hosts, e.g., humans and insects [13,61,62]. In addition, fungi reportedly connected to degradation of BTEX compounds are absent in the months from September to February, when the monthly rainfall rate is higher than 28.5 mm3, and the sunshine hours are lower than 163.4 h. This could be attributed to the winter season: lower temperatures, reduced beach activity, and less traffic on the roads and the sea nearby, with consequently less oil and gas pollution [63].
While there were differences observed for the above-mentioned fungal groups, the machine-learning model (PCT depicted in Figure 2) pointed out the presence of the core genera, psychrotolerant, dematiaceous, and halotolerant fungi in the beach sand regardless of the screened environmental factors. The results are expected, since sand is a known reservoir of Aspergillus, Penicillium, Cladosporium, Fusarium, Rhizopus, and dermatophytes [13,18]. The results relate fungal growth in the environment to longer sun exposure and moderate temperatures [64]. However, changes were noted in November, when the number of isolated genera dropped significantly and in January, when it elevated. The likely reason for lower diversity in November is the drop of monthly sunshine hours. This factor was previously reported as one of the most influencing for microbial communities in sand due to its double effect on daily temperatures and UV-irradiation [12,13].
On the other hand, the reason for higher fungal diversity in January sampling was a one-time event, the New Year celebration on the beach, which resulted also in the detection of human-related genera Candida and Rhodotorula [13,51,65]. In addition, 5 out of 10 species (Aspergillus flavus, A. terreus, Candida tropicalis, Fusarium solani, and Microascus brevicaulis) recognised as human opportunistic pathogens (BSL-2) [34] were isolated in January. This leads to the conclusion that one-time events could also significantly affect fungal abundance and diversity in beach sand.
4.3. Core Fungal Genera in the Sand Are Affected by the Seawater Temperature
During the study, we particularly focused on the constantly present core-genera and possible indicator fungi in sand samples, appearing mainly during the bathing season. The core-genera include the dermatophyte Aphanoascus, Aspergillus, Fusarium, Bisifusarium, Penicillium, Talaromyces, and Rhizopus. They usually colonise plants and plant debris, as well as other organic materials, and are often isolated from soil, dry beach sand, deserts, and dust [13,66]. Particular attention should be paid to the elevated numbers of thermotolerant species such as Aspergillus fumigatus, A. terreus, A. glaucus, A. nidulans, A. niger, A. sydowii, Fusarium solani, Rhizopus arrhizus, and dermatophyte Aphanoascus fulvescens, since these are frequently involved in a variety of fungal infections and can represent health hazards for beach-goers [18,34]. Despite forming stable fungal communities in the sand, the numbers of these genera fluctuated over time. The machine learning method found seawater temperature to be the first and most decisive factor in the changes of the core mycobiota (Figure 4). The changes in seawater temperature are slower than in the air or upper sand layers, and they affect the formation of the local Mediterranean coast microclimate [67]. During the period of January to March, when the seawater temperatures were ≤9.9 °C, Aspergillus species prevailed in sand. However, Aspergillus and Penicillium numbers were the lowest in months when seawater is warmer, the air pressure is higher, the wind is stronger, and the precipitation is ≤105.7 mm3. Air pressure is the variable that has a broad effect on weather and corresponds to the changes in wind speed and direction, as well as the rainy and sunny periods. Higher air pressure indicates sunny weather and lower air pressure is associated with precipitation. On the contrary, the above-described factors favor the growth of Aphanoascus, Fusarium, Bisifusarium, and Rhizopus, which is in accordance with previous studies, reporting elevated presence of these genera in warm and/or humid climate conditions [66,68,69,70]. Besides weather, also, the antagonism between Aspergillus and Penicillium versus Fusarium and Bisifusarium could affect the ratio of these genera in the sand [71].
4.4. Fungi in Sand as Possible Indicators of Anthropogenic Pollution during the Bathing Season
Another focus was the recognition of possible fungal indicators of human pollution, which appeared mainly during the bathing season. Indicator microorganisms appear after a certain cascade of events, e.g., Escherichia coli and enterococci indicate faecal pollution in the environment [15], while the data on fungal indicators are scarce. With the culturable approach we identified four fungal genera, namely Actinomucor, Condenascus, Meyerozyma, and Stachybotrys, that may serve also as potential human pollution indicators during the bathing season. Their numbers at the beach sand started to increase from May to October and reached the peak from June to September. Machine learning analysis yielded the air temperature >28 °C during the past 7 days before sampling as the most decisive factor for the presence of all four genera in the sand. The presence of the species Condenascus tortuosus is predicted to be the most numerous among all indicator fungi. It was isolated from May to September when the air temperatures are either higher than 28 °C or when T ≤ 28 °C and the air pressure is ≤1012.0 mbar (indicating warm and moist weather). This genus was described recently and the available data currently links it only to plants and soil, but not to human health [72]. Although isolated under the same cascade of environmental factors as Condenascus, the allergenic and mycotoxigenic fungus Stachybotrys was predicted in lowest numbers among indicator fungi. Its presence in beach sand was reported in many other studies, showing beach sand as a potential reservoir for this genus [13]. Actinomucor elegans is a ubiquitous fungus, related mainly to plants, soil, and insects. Machine learning predicts its presence in the beach sand mainly during the spring and summer months with higher air pressure (indicating sunny and warm weather), which can be related to plant growth and insects’ activity [62]. This species was recently added to the list of emerging clinically relevant fungi [73]; thus, its elevated presence in sand during the bathing season should not be neglected. Although all indicator genera were present during the bathing season, only the yeast Meyerozyma can be linked to the presence of humans, since it was isolated during the summer and autumn months with sunny, warm or hot weather, associated with the highest numbers of beach visits [13]. This result confirms previous observations on sand and consolidates the Meyerozyma species as a possible fungal indicator of human faecal pollution [13].
Besides possible indicators of anthropogenic pollution, the sporadic presence of thermotolerant species such as Arachniotus flavoluteus, Geotrichum spp., Mucor circinelloides, Phialophora americana, Trichoderma citrinoviride, and Trichosporon asahii was detected during the bathing season. These are known to cause mild to severe fungal infections, and should be taken into consideration if their numbers in beach sand elevate [34].
4.5. Beach Sand Harbours Amphotericin B-Resistant Strains of Aspergillus Section Nigri
Although beach sand harboured highly diverse mycobiota, the most numerous fungi belong to the genus Aspergillus. Nine species were isolated from the beach sand during the monitoring, while only four were present during the official bathing season. Interestingly, Aspergillus fumigatus was not isolated, although its presence is well documented in sand [12,13,51]. Only one strain of its sibling species, A. lentulus, was isolated in October after the flood event. Representatives of the genus Aspergillus can cause allergies, sinusitis, otitis, keratitis, but also life-threatening infections, such as invasive aspergillosis [34]. In addition, CDC takes it under scrutiny due to the fast development of resistance to antifungals, not only in hospitals, but also in the natural environment [74,75]. The isolates obtained during the bathing season were tested against nine antifungals, and the obtained results were compared with the recent EUCAST data [37,76] and the data from de Hoog et al. [34]. Echinocandines are not recommended clinical treatment against Aspergillus, and, as previously reported, Aspergillus strains showed aberrant growth endpoint after 48 h (in our case >8 µg/mL) [36,37,77]. In our study, MIC could not be determined for fluconazole, in accordance with EUCAST data [37]. EUCAST often reports intrinsic resistance for A. flavus, A. nidulans, and A. niger wild types against posaconazole, voriconazole, and itraconazol, but all strains from beach sand were susceptible to these antimycotics [37]. Aspergillus flavus and A. nidulans showed similar result against amphotericin B as previously reported for wild types (ECOFF = 4 µg/mL), but Aspergillus niger and its close relative A. welwitschiae (section Nigri) were resistant to this antimycotic with MIC = 2 µg/mL. ECOFF for A. niger is currently set between 0.5 and 1 µg/mL, while MIC values greater than 1 µg/mL are considered as strain’s resistance. Although the tests were carried out on a small number of isolates, the observed resistance for wild types of Aspergillus niger and possible resistance of A. welwitschiae against amphotericin B should be taken into consideration in further studies of beach sand.
5. Conclusions
To contribute to the knowledge on the mycobiota of sand and water, the present study investigated not only the abundance and diversity of the culturable fraction of the fungal community, but also the fluctuations of the most abundant genera through the year, and the possible fungal indicators for the bathing season. The data were analyzed with machine learning (ML) methods that can combine all factors screened, identifying combinations of the most important factors favoring certain fungal groups. The obtained ML models identify the weather conditions, i.e., air and water temperature, sunshine hours, precipitation, humidity, air pressure, and wind speed, as the key factors influencing sand and sea mycobiota. In addition, and for the first time, culturable methods yielded four genera that could be followed as potential human pollution indicators during the bathing season. Amongst them, and based on findings from other worldwide studies, we suggest the genus Meyerozyma as a candidate to be monitored as a human pollution indicator. Attention should be paid also to the whole-year present Aspergillus spp. strains. Resistance against amphotericin B was found in A. niger and possibly also in A. welwitschiae. Due to their role in fungal otitis, especially in children, this should encourage future studies on resistance in Aspergillus strains, isolated from beach sand and sea. Altogether, our findings lay the foundation for further research on sand and seawater mycobiota and suggest the potential effect of weather changes on the presence of opportunistic and resistant fungi in sand and sea. Diversity analyses based on sequencing methods could be used in the future to provide further insights into environmental fungal diversity and comparisons between beach sand and seawater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8080860/s1, Table S1: Descriptive and numerical variables used in the study of beach sand and seawater.
Author Contributions
Conceptualisation, J.B. and M.N.B.; methodology, J.B., M.N.B., M.B. and S.D.; software, M.B. and S.D.; validation, J.B., M.N.B., N.G.-C., M.B. and S.D.; Formal Analysis, M.N.B. and M.B.; investigation, M.N.B. and M.B.; resources, J.B., M.N.B., N.G.-C. and S.D.; data curation, M.N.B.; writing—original draft preparation, M.N.B. and M.B.; writing—review and editing, J.B., N.G.-C. and S.D.; visualisation, M.N.B. and M.B.; supervision, J.B., N.G.-C. and S.D.; project administration, J.B. and N.G.-C.; funding acquisition, J.B., M.N.B., N.G.-C. and S.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The work of Monika Novak Babič was supported by Slovenian Research Agency (ARRS) through the postdoctoral research project (grant number Z7-2668) and the research program, grant number P1-0198. The work of Sašo Džeroski was supported by Slovenian Research Agency (ARRS) through the program Knowledge Technologies (grant number P2-0103). The work of João Brandão received financial support from CESAM (UID/AMB/50017-POCI-01-0145-FEDER-007638) and CITAB (UID/AGR/04033/2019), via FCT/MCTES, from national funds (PIDDAC), co-founded by FEDER, (PT2020 Partnership Agreement and Compete 2020).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naranjo-Ortiz M.A., Gabaldón T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. Camb. Philos. Soc. 2019;94:1443–1476. doi: 10.1111/brv.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyde K.D., Xu J., Rapior S., Jeewon R., Lumyong S., Niego A.G.T., Abeywickrama P.D., Aluthmuhandiram J.V.S., Brahamanage R.S., Brooks S., et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. [DOI] [Google Scholar]
- 3.Murgia M., Fiamma M., Barac A., Deligios M., Mazzarello V., Paglietti B., Cappuccinelli P., Al-Qahtani A., Squartini A., Rubino S., et al. Biodiversity of fungi in hot desert sands. MicrobiologyOpen. 2019;8:e00595. doi: 10.1002/mbo3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleine C., Stajich E.J., de los Ríos A., Selbmann L. Beyond the extremes: Rocks as ultimate refuge for fungi in drylands. Mycologia. 2021;113:108–133. doi: 10.1080/00275514.2020.1816761. [DOI] [PubMed] [Google Scholar]
- 5.Zajc J., Zalar P., Plemenitaš A., Gunde-Cimerman N. The mycobiota of the salterns. Prog. Mol. Subcell. Biol. 2012;53:133–158. doi: 10.1007/978-3-642-23342-5_7. [DOI] [PubMed] [Google Scholar]
- 6.Azpiazu-Muniozguren M., Perez A., Rementeria A., Martinez-Malaxetxebarria I., Alonso R., Laorden L., Gamboa J., Bikandi J., Garaizar J., Martinez-Ballesteros I. Fungal diversity and composition of the continental solar saltern in Añana Salt Valley (Spain) J. Fungi. 2021;7:1074. doi: 10.3390/jof7121074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunde-Cimerman N., Zalar P. Fungi in solar salterns. Food Technol. Biotechnol. 2014;52:170–179. [Google Scholar]
- 8.Magan N. Fungi in extreme environments. In: Kubicek C., Druzhinina I., editors. Environmental and Microbial Relationships, The Mycota. 1st ed. Volume 4. Springer; Berlin/Heidelberg, Germany: 2007. pp. 85–103. [DOI] [Google Scholar]
- 9.Rampelotto P. Resistance of microorganisms to extreme environmental conditions and its contribution to astrobiology. Sustainability. 2010;2:1602–1623. doi: 10.3390/su2061602. [DOI] [Google Scholar]
- 10.Gostinčar C., Grube M., De Hoog S., Zalar P., Gunde-Cimerman N. Extremotolerance in fungi: Evolution on the edge. FEMS Microbiol. Ecol. 2009;71:2–11. doi: 10.1111/j.1574-6941.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 11.Pessoa M.F., Lidon F.C. Impact of human activities on coastal vegetation—A review. Emir. J. Food Agric. 2013;25:926–944. doi: 10.9755/ejfa.v25i12.16730. [DOI] [Google Scholar]
- 12.Solo-Gabriele H.M., Harwood V.J., Kay D., Fujioka R.S., Sadowsky M.J., Whitman R.L., Wither A., Caniça M., da Fonseca R.C., Duarte A., et al. Beach sand and the potential for infectious disease transmission: Observations and recommendations. J. Mar. Biol. Assoc. UK. 2016;96:101–120. doi: 10.1017/S0025315415000843. [DOI] [Google Scholar]
- 13.Brandão J., Gangneux J.P., Arikan-Akdagli S., Barac A., Bostanaru A.C., Brito S., Bull M., Çerikçioğlu N., Chapman B., Efstratiou M.A., et al. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021;781:146598. doi: 10.1016/j.scitotenv.2021.146598. [DOI] [PubMed] [Google Scholar]
- 14.Byappanahalli M.N., Nevers M.B., Korajkic A., Staley Z.R., Harwood V.J. Enterococci in the environment. Microbiol. Mol. Biol. Rev. 2012;76:685–706. doi: 10.1128/MMBR.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang J., Hur H.G., Sadowsky M.J., Byappanahalli M.N., Yan T., Ishii S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017;123:570–581. doi: 10.1111/jam.13468. [DOI] [PubMed] [Google Scholar]
- 16.Hurst C.J. Dirt and disease: The ecology of soil fungi and plant fungi that are infectious for vertebrates. In: Hurst C., editor. Understanding Terrestrial Microbial Communities. Volume 6. Springer; Cham, Switzerland: 2019. pp. 289–405. Advances in Environmental Microbiology. [DOI] [Google Scholar]
- 17.Friedman D.Z., Schwartz I.S. Emerging fungal infections: New patients, new patterns, and new pathogens. J. Fungi. 2019;5:67. doi: 10.3390/jof5030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romão D., Sabino R., Veríssimo C., Viegas C., Barroso H., Duarte A., Solo-Gabriele H., Gunde-Cimerman N., Babič M.N., Marom T., et al. Children and sand play: Screening of potential harmful microorganisms in sandboxes, parks, and beaches. Curr. Fungal Infect. Rep. 2015;9:155–163. doi: 10.1007/s12281-015-0230-5. [DOI] [Google Scholar]
- 19.Sabino R., Verissimo C., Cunha M.A., Wergikoski B., Ferreira F.C., Rodrigues R., Parada H., Falcão L., Rosado L., Pinheiro C., et al. Pathogenic fungi: An unacknowledged risk at coastal resorts? New insights on microbiological sand quality in Portugal. Mar. Pollut. Bull. 2011;62:1506–1511. doi: 10.1016/j.marpolbul.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Azevedo E., Figueira D., Caeiro M.F., Barata M. Biodiversity of filamentous fungi on soils and sands. In: Rai M., Kövics G., editors. Progress in Mycology. Springer; Dordrecht, The Netherlands: 2010. pp. 233–257. [Google Scholar]
- 21.Libkind D., Buzzini P., Turchetti B., Rosa C.A. Yeasts in continental and seawater. In: Buzzini P., Lachance M.A., Yurkov A., editors. Yeasts in Natural Ecosystems: Diversity. Springer; Cham, Switzerland: 2017. pp. 1–61. [Google Scholar]
- 22.Van den Ende A.H.G., de Hoog G.S. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud. Mycol. 1999;43:151–162. [Google Scholar]
- 23.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 24.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell K., Kistler H.C., Cigelnik E., Ploetz R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 27.Boekhout T., Kurtzman C.P. Principles and methods used in yeast classification, and an overview of currently accepted yeast genera. In: Wolf K., editor. Nonconventional Yeasts in Biotechnology. Springer; Berlin, Germany: 1996. pp. 1–81. [Google Scholar]
- 28.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 30.ARSO Slovenian Environmental Agency, Ministry of the Environment and Spatial Planning. [(accessed on 8 July 2022)]; Available online: https://meteo.arso.gov.si/met/sl/app/webmet/#webmet=vUHcs9WYkN3LtVGdl92LhBHcvcXZi1WZ09Cc1p2cvAncvd2LyVWYs12L3VWY0hWZy9SaulGdugXbsx3cs9mdl5WahxHf.
- 31.Blockeel H., De Raedt L. Top-down induction of first-order logical decision trees. Artif. Intell. 1998;101:285–297. doi: 10.1016/S0004-3702(98)00034-4. [DOI] [Google Scholar]
- 32.Petković M., Kocev D., Džeroski S. Feature ranking for multi-target regression. Mach. Learn. 2020;109:1179–1204. doi: 10.1007/s10994-019-05829-8. [DOI] [Google Scholar]
- 33.Kocev D., Vens C., Struyf J., Džeroski S. Tree ensembles for predicting structured outputs. Pattern Recognit. 2013;46:817–833. doi: 10.1016/j.patcog.2012.09.023. [DOI] [Google Scholar]
- 34.de Hoog G.S., Guarro J., Gené J., Ahmed S., Al-Hatmi A.M.S., Figueras M.J., Vitale R.G. Atlas of Clinical Fungi. 4th ed. Foundation Atlas of Clinical Fungi; Utrecht, The Netherlands: 2020. [Google Scholar]
- 35.ATCC American Type Culture Collection. [(accessed on 18 January 2022)]. Available online: https://www.atcc.org/
- 36.Siopi M., Pournaras S., Meletiadis J. Comparative evaluation of Sensititre YeastOne and CLSI M38-A2 reference method for antifungal susceptibility testing of Aspergillus spp. against echinocandins. J. Clin. Microbiol. 2017;55:1714–1719. doi: 10.1128/JCM.00044-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EUCAST European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0. [(accessed on 8 July 2022)]. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updatd_links_200924.pdf.
- 38.Lumibao C.Y., Formel S., Elango V., Pardue J.H., Blum M., Van Bael S.A. Persisting responses of salt marsh fungal communities to the Deepwater Horizon oil spill. Sci. Total Environ. 2018;642:904–913. doi: 10.1016/j.scitotenv.2018.06.077. [DOI] [PubMed] [Google Scholar]
- 39.Neto J.A.B., Gaylarde C., Beech I., Bastos A.C., da Silva Quaresma V., de Carvalho D.G. Microplastics and attached microorganisms in sediments of the Vitória bay estuarine system in SE Brazil. Ocean Coast. Manag. 2019;169:247–253. doi: 10.1016/j.ocecoaman.2018.12.030. [DOI] [Google Scholar]
- 40.Pérez-Alvelo K.M., Llegus E.M., Forestier-Babilonia J.M., Elías-Arroyo C.V., Pagán-Malavé K.N., Bird-Rivera G.J., Rodríguez-Sierra C.J. Microplastic pollution on sandy beaches of Puerto Rico. Mar. Pollut. Bull. 2021;164:112010. doi: 10.1016/j.marpolbul.2021.112010. [DOI] [PubMed] [Google Scholar]
- 41.Bridson J.H., Patel M., Lewis A., Gaw S., Parker K. Microplastic contamination in Auckland (New Zealand) beach sediments. Mar. Pollut. Bull. 2020;151:110867. doi: 10.1016/j.marpolbul.2019.110867. [DOI] [PubMed] [Google Scholar]
- 42.Pathakumari B., Liang G., Liu W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020;130:110550. doi: 10.1016/j.biopha.2020.110550. [DOI] [PubMed] [Google Scholar]
- 43.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klun K., Falnoga I., Mazej D., Šket P., Faganeli J. Colloidal organic matter and metal (loid) s in coastal waters (Gulf of Trieste, Northern Adriatic Sea) Aquat. Geochem. 2019;25:179–194. doi: 10.1007/s10498-019-09359-6. [DOI] [Google Scholar]
- 45.Perkovic M., Hribar U., Harsch R. Oil Pollution in Slovenian Waters: The Threat to the Slovene Coast, Possible Negative Influences of Shipping on an Environment and Its Cultural Heritage. In: Carpenter A., Kostianoy A., editors. Oil Pollution in the Mediterranean Sea: Part II. The Handbook of Environmental Chemistry. Volume 84. Springer; Cham, Switzerland: 2016. pp. 133–157. [DOI] [Google Scholar]
- 46.FEE Foundation for Environmental Education. Pure Water, Clean Coasts, Safety and Access for All. [(accessed on 11 July 2022)]. Available online: https://www.blueflag.global/
- 47.EEU Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing directive76/160/EEC. Off. J. Eur. Union. 2006;64:37–51. [Google Scholar]
- 48.WHO . Guidelines on Recreational Water Quality: Volume 1 Coastal and Fresh Waters. World Health Organization; Geneva, Switzerland: 2021. [PubMed] [Google Scholar]
- 49.Weiskerger C.J., Brandão J. Fungal contaminants in water and sand: A new frontier for quantitative microbial risk assessment. Curr. Opin. Environ. Sci. Health. 2020;16:73–81. doi: 10.1016/j.coesh.2020.03.001. [DOI] [Google Scholar]
- 50.Londoño C.O., Fernández R.R., Gulloso E.R.M. Identification of fungi dermatophytes in the coastal area of district of Riohacha, La Guajira. Contemp. Eng. Sci. 2018;11:4691–4699. doi: 10.12988/ces.2018.88433. [DOI] [Google Scholar]
- 51.Frenkel M., Yunik Y., Fleker M., Blum S.E., Sionov E., Elad D., Serhan H., Segal E. Fungi in sands of Mediterranean Sea beaches of Israel—Potential relevance to human health and well-being. Mycoses. 2020;63:1255–1261. doi: 10.1111/myc.13144. [DOI] [PubMed] [Google Scholar]
- 52.Michalska M., Kurpas M., Zorena K., Wąż P., Marks R. Mold and Yeast-Like Fungi in the Seaside Air of the Gulf of Gdańsk (Southern Baltic) after an Emergency Disposal of Raw Sewage. J. Fungi. 2020;7:219. doi: 10.3390/jof7030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gunde-Cimerman N., Ramos J., Plemenitaš A. Halotolerant and halophilic fungi. Mycol. Res. 2009;113:1231–1241. doi: 10.1016/j.mycres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Maciel N.O., Johann S., Brandão L.R., Kucharíková S., Morais C.G., Oliveira A.P., Freitas G.J., Borelli B.M., Pellizzari F.M., Santos D.A., et al. Occurrence, antifungal susceptibility, and virulence factors of opportunistic yeasts isolated from Brazilian beaches. Memórias Inst. Oswaldo Cruz. 2019;114 doi: 10.1590/0074-02760180566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luchetta A., Cantoni C., Cozzi S., Catalano G., Civitarese G. Dissolved carbon dioxide, nutrients and oxygen in the Adriatic Sea. A Regional Observing Effort. [(accessed on 10 July 2022)]. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1054.2313&rep=rep1&type=pdf.
- 56.Pombeiro-Sponchiado S.R., Sousa G.S., Andrade J.C., Lisboa H.F., Gonçalves R.C. Production of melanin pigment by fungi and its biotechnological applications. In: Blumenberg M., editor. Melanin. 1st ed. Volume 1. IntechOpen; London, UK: 2017. pp. 47–75. [Google Scholar]
- 57.Kaewkrajay C., Chanmethakul T., Limtong S. Assessment of diversity of culturable marine yeasts associated with corals and zoanthids in the Gulf of Thailand, South China Sea. Microorganisms. 2020;8:474. doi: 10.3390/microorganisms8040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sousa L., Camacho I.C., Grinn-Gofroń A., Camacho R. Monitoring of anamorphic fungal spores in Madeira region (Portugal), 2003–2008. Aerobiologia. 2016;32:303–315. doi: 10.1007/s10453-015-9400-8. [DOI] [Google Scholar]
- 59.Liu B., Fu R., Wu B., Liu X., Xiang M. Rock-inhabiting fungi: Terminology, diversity, evolution and adaptation mechanisms. Mycology. 2022;13:1–31. doi: 10.1080/21501203.2021.2002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abreu R., Figueira C., Romão D., Brandão J., Freitas M.C., Andrade C., Calado G., Ferreira C., Campos A., Prada S. Sediment characteristics and microbiological contamination of beach sand—A case-study in the archipelago of Madeira. Sci. Total Environ. 2016;573:627–638. doi: 10.1016/j.scitotenv.2016.08.160. [DOI] [PubMed] [Google Scholar]
- 61.Velegraki A., Efstratiou M.A., Arabatzis M., Prodromou P. A survey on the occurrence of dermatophytes in recreational beach sand; Proceedings of the International Society for Microbial Ecology (ISME); Copenhagen, Denmark. 19–24. August 2012. [Google Scholar]
- 62.Vega F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2008;98:277–279. doi: 10.1016/j.jip.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Alpar B., Unlu S. Volatile Aromatic Compounds (BTEX) in Sediments Offshore Zonguldak Industrial Region, Black Sea, Turkey. Asian J. Chem. 2010;22:3531. [Google Scholar]
- 64.van Rhijn N., Bromley M. The consequences of our changing environment on life threatening and debilitating fungal diseases in humans. J. Fungi. 2021;7:367. doi: 10.3390/jof7050367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loureiro S.T.A., Cavalcanti M.A.D.Q., Neves R.P., Passavante J.Z.D.O. Yeasts isolated from sand and sea water in beaches of Olinda, Pernambuco state, Brazil. Braz. J. Microbiol. 2005;36:333–337. doi: 10.1590/S1517-83822005000400005. [DOI] [Google Scholar]
- 66.Grishkan I., Lázaro R., Kidron G.J. Cultured microfungal communities in biological soil crusts and bare soils at the Tabernas Desert, Spain. Soil Syst. 2019;3:36. doi: 10.3390/soilsystems3020036. [DOI] [Google Scholar]
- 67.Zupančič N., Skobe S. Anthropogenic environmental impact in the Mediterranean coastal area of Koper/Capodistria, Slovenia. J. Soils Sediments. 2014;14:67–77. doi: 10.1007/s11368-013-0770-7. [DOI] [Google Scholar]
- 68.Nosratabadi M., Kordbacheh P., Kachuei R., Safara M., Rezaie S., Afshari M.A. Isolation of keratinophilic fungi from the soil of Greater Tunb, Abu-Musa, and Sirri islands in the Persian Gulf, Iran. Curr. Med. Mycol. 2017;3:13. doi: 10.29252/cmm.3.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richardson M.D., Rautemaa-Richardson R. Biotic environments supporting the persistence of clinically relevant Mucormycetes. J. Fungi. 2019;6:4. doi: 10.3390/jof6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timmusk S., Nevo E., Ayele F., Noe S., Niinemets Ü. Fighting Fusarium pathogens in the era of climate change: A conceptual approach. Pathogens. 2020;9:419. doi: 10.3390/pathogens9060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alwathnani H.A., Perveen K. Biological control of Fusarium wilt of tomato by antagonist fungi and cyanobacteria. Afr. J. Biotechnol. 2012;11:1100–1105. doi: 10.5897/AJB11.3361. [DOI] [Google Scholar]
- 72.Wang X.W., Bai F.Y., Bensch K., Meijer M., Sun B.D., Han Y.F., Crous P.W., Samson R.A., Yang F.Y., Houbraken J. Phylogenetic re-evaluation of Thielavia with the introduction of a new family Podosporaceae. Stud. Mycol. 2019;93:155–252. doi: 10.1016/j.simyco.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walther G., Wagner L., Kurzai O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi. 2019;5:106. doi: 10.3390/jof5040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.CDC Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED). Antifungal-Resistant Aspergillus. [(accessed on 11 July 2022)]; Available online: https://www.cdc.gov/fungal/diseases/aspergillosis/antifungal-resistant.html.
- 75.Espinel-Ingroff A., Fothergill A., Fuller J., Johnson E., Pelaez T., Turnidge J. Wild-type MIC distributions and epidemiological cutoff values for caspofungin and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document) Antimicrob. Agents Chemother. 2011;55:2855–2859. doi: 10.1128/AAC.01730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arendrup M.C., Friberg N., Mares M., Kahlmeter G., Meletiadis J., Guinea J., Andersen C.T., Arikan-Akdagli S., Barchiesi F., Chryssanthou E., et al. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST) Clin. Microbiol. Infect. 2020;26:1464–1472. doi: 10.1016/j.cmi.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Wang H.C., Hsieh M.I., Choi P.C., Wu C.J. Comparison of the Sensititre YeastOne and CLSI M38-A2 microdilution methods in determining the activity of amphotericin B, itraconazole, voriconazole, and posaconazole against Aspergillus species. J. Clin. Microbiol. 2018;56:e00780-18. doi: 10.1128/JCM.00780-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.