Abstract
Purpose:
To introduce a contouring guideline for the taste bud bearing tongue mucosa for head and neck cancer patients receiving radiotherapy.
Methods and Materials:
CT simulation images of oropharyngeal cancer patients were used to delineate both the whole tongue (extrinsic/intrinsic tongue muscles, floor of mouth) and the taste bud bearing ton-gue mucosa (method A: adaptation of the whole tongue structure; method B: axial adaptation of a mid-sagittal contour). Volumetric and dosimetric parameters of the whole tongue and the two methods of mucosal delineation, spatial overlap between methods A and B, and inter-observer variability for method B were calculated.
Results:
The study cohort was comprised of 70 patients with T1–4 N0–1 tonsillar (83%) and base of ton-gue (17%) cancers. Most of the comparative parameters between the whole tongue and mucosa (method A) significantly differed (mean, minimum, and maximum dose, V5–V70, D40–D90). The mean dose cal-culated for the whole tongue deviated on average 3.77 Gy compared to method A. No significant differ-ences were found between methods A and B of the taste bud bearing tongue mucosa structure, and none of the dosimetric parameters differed more than 1.03 Gy on average. The mean Dice similarity coefficient for both mucosal structures was 0.79 ± 0.05, and 0.63 ± 0.12 for the inter-observer analysis of method B.
Conclusions:
We defined two methods for delineating the taste bud bearing mucosa and both are equally satisfactory procedures. Either method is preferable over delineation of the whole tongue as organ at risk for taste impairment.
Taste impairment is a nearly ubiquitous patient-reported symptom during radiotherapy (RT) for head and neck cancer (HNC) and can affect appetite and subsequent nutritional status and quality of life [1,2]. Whereas over ninety percent of patients reporting moderate or severe problems tasting food in the acute phase [3], this symptom improves with time after RT for the majority of patients. However, many patients fail to recover the same pre-therapy quality of taste [4]. There is a large variation between individuals not only in the impairment of taste during treatment and posttherapy, but also regarding the degree of taste recovery [4]. Both must be further investigated regarding the influence of dose received by the taste buds, which are proven to be damaged by radiation in the preclinical setting [5].
In the clinical setting, a dose/toxicity relationship for taste impairment can be assumed based on increasing patient-reported taste impairment during the course of head and neck RT [6,7]. However, published studies on the dosimetric influence on taste have methodological limitations by not using exclusively the taste bud bearing tongue mucosa (superior mucosa of the mobile oral tongue) as the organ at risk (OAR) whose damage results in taste impairment. Instead, the prescribed dose to the tumor [8] or the dose received by either the entire oral cavity or whole tongue (including the deep musculature) [9–13] is used for analysis. In addition, some studies are from the 3D-RT era [8,12,13] with less complex dose distributions within the tongue than would be the case with more advanced techniques, such as highly conformal intensity-modulated radiation therapy (IMRT).
To better understand the impact of RT dose on taste impairment in HNC patients treated using IMRT, the aim of this study was to introduce a contouring guideline for the taste bud bearing tongue mucosa that can be used when evaluating the probability of normal tissue complications relating to taste. A systematic guideline such as outlined in this study could ultimately lead to a better understanding of the impact of RT dose on taste impairment. We also sought to demonstrate that delineation of the mucosa can provide more precision in dosimetric analysis than a surrogate such as the whole tongue.
Materials and methods
The study is part of an IRB-approved protocol at the Department of Radiation Oncology at The University of Texas MD Anderson Cancer Center to serially assess late radiation associated symptoms in head and neck cancer patients. All patients gave written informed consent to study participation, data collection and analysis.
Study procedure
Seventy oropharyngeal cancer (OPC) patients from the above mentioned study, who received definitive treatment with photon RT (2004–2014) were included. Only patients treated without major oral surgery, and without an oral stent during RT were included in this study. CT simulation images of the patients who were immobilized in a head, neck and shoulder thermoplastic mask were used for contouring of the whole tongue and the taste bud bearing tongue mucosa. CT images, structures and IMRT plans were then extracted as DICOM files from the treatment planning system Velocity (Varian Medical Systems, Palo Alto, CA). An inhouse developed MATLAB (The MathWorks, Natick, MA) code was used to calculate the volume of the structures and the following dosimetric parameters: mean dose, minimum and maximum doses, V5–V70 in 5 Gy intervals, and D5–D95 in 5–10% increments. Patient characteristics were extracted from the clinical patient database EPIC (Epic Systems Corporation, Verona, WI).
Contouring
Three structures have been contoured for every patient: the whole tongue, and the taste bud bearing tongue mucosa according to two different methods.
The whole tongue contour consisted of the extrinsic/intrinsic tongue muscles, including the floor of mouth for ease of delineation on CT. This corresponds to the extended oral cavity contour described by Brouwer et al. [14], but excluding the soft palate/uvula and possible air behind the mandible/maxilla depending on tongue position.
Two methods for contouring the taste bud bearing tongue mucosa have been developed; both considered covering the upper surface of the oral tongue, where the taste buds are mainly located. The first method, method A, is a relatively structured subtraction method from delineating the whole tongue. This method has been chosen to provide the best possible standardized approach for creating the taste bud bearing tongue mucosa structure, but the contouring is very time-consuming which might hamper the implementation of this new organ at risk (OAR) structure into clinical routine. Thus, another method, method B, which is quasifreehand, was created as an alternative.
Method A – Adaptation of whole tongue contour
Step 1: Contour the whole tongue including extrinsic and intrinsic muscles and floor of mouth (Fig. 1A).
Fig. 1.

Contouring of the taste bud bearing tongue mucosa according to method A. Detailed description in the Methods and Material section.
Step 2: Crop the inner part of the tongue leaving a hollow sphere of 5 mm (“cropped tongue”) (Fig. 1B).
Step 3: To define the posterior extension of the structure (border to the base of tongue), draw a line on the mid-sagittal image between two fixed points as visual guide: The lower border of the mandible will be the inferior fixed point, the tip of the dens axis or the inferior edge of the anterior tubercle of the atlas the superior fixed point (select the one where the line divides the tongue surface into anterior 2/3 and posterior 1/3 best). Point 1 will be the intersection of this line with the superior tongue surface (Fig. 1C).
Step 4: The anterior border of the structure will be defined as Point 2, which lies ventrally on the cropped tongue contour on the same level where the inner cropped tongue contour changes from more horizontal to more vertical direction (Fig. 1D). Now, draw a line on the mid-sagittal image between Point 1 and Point 2.
Step 5: Start with the most superior point on this line (Point V1, which might be either Point 1 or Point 2) and set the intersection of the coronal and axial plane (cross) exactly to this place (Fig. 1E).
Step 6: Move one axial slice down. Then navigate the coronal plane (longitudinal line) to the middle between Point V1 and Point V2, which is located at the intersection of the axial plane (sagittal axis) with the line between Point 1 and 2 (Fig. 1F).
Step 7: Delete all parts of the cropped tongue contour, which are located between the coronal plane (longitudinal line) and Point V1 (Fig. 1G–H) on the axial slice.
Step 8: Move forward with the cross to Point V2 and repeat the procedure from Step 5 on until reaching the anterior or posterior tongue surface (Fig. 1I–L).
Step 7: All slices below have to be deleted (Fig. 1M).
Special situations
If Point 2 is difficult to find, move one slice down from the most superior axial image where you can still see lower ventral teeth, and use the outer contour of the cropped tongue on this slice as Point 2.
In the case of no lower ventral teeth, move 1 cm up from the uppermost part of the ventral mandible, and use the outer contour of the cropped tongue on this slice as Point 2.
If Point 1 and 2 are at the same level, then delete all slices below (Suppl. Fig. A).
Method B – Axial adaptation of mid-sagittal tongue mucosa contour
Step 1 (equates to Step 3 of Method A): To define the posterior extension of the structure (border to the base of tongue), draw a line on the mid-sagittal image between two fixed points as visual guide: The lower border of the mandible will be the inferior fixed point, the tip of the dens axis or the inferior edge of the anterior tubercle of the atlas the superior fixed point (select the one where the line divides the tongue surface into anterior 2/3 and posterior 1/3 best). Point 1 will be the intersection of this line with the superior tongue surface (Fig. 2A).
Fig. 2.
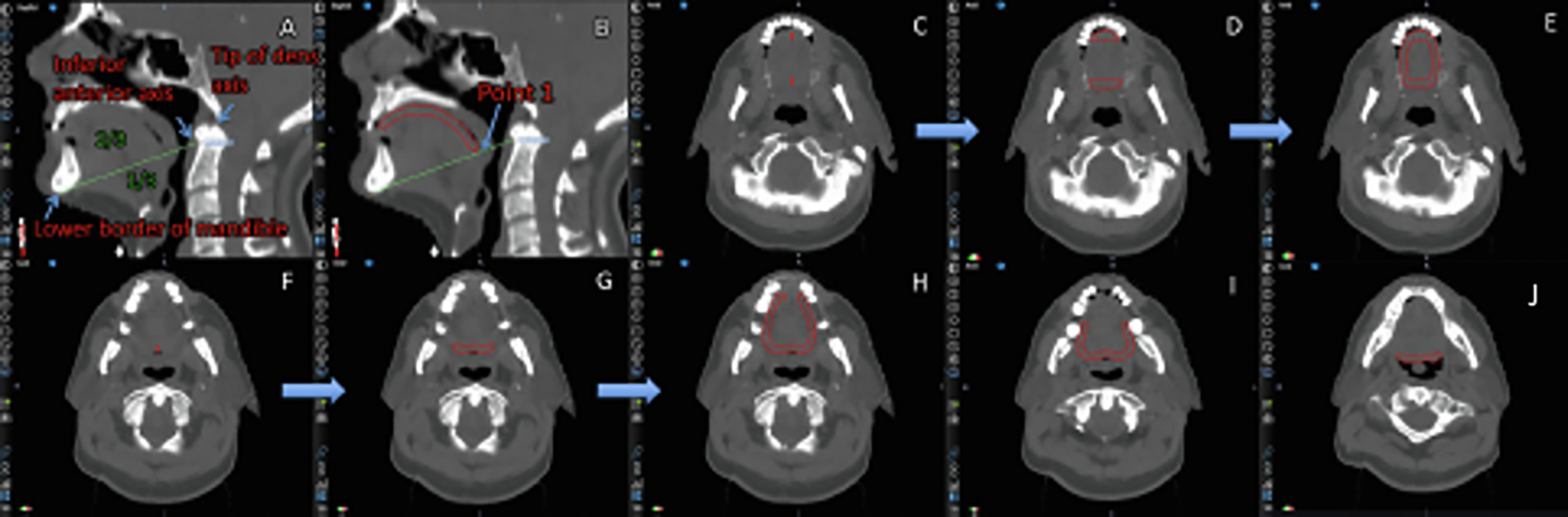
Contouring of the taste bud bearing tongue mucosa according to method B. Detailed description in the Methods and Material section.
Step 2 (Mid-sagittal contour): Draw a 5 mm thick contour from Point 1 along the upper surface of the tongue in anterior direction until reaching the level of the lower ventral teeth (or approximately 1 cm above the mandible in the case of no teeth) (Fig. 2B).
Step 3 (Lateral border): Extend the mid-sagittal contour laterally on the axial slices, thereby respecting the anatomy of the posterior border of the tongue (cave: contour might be wave-shaped) (Fig. 2C–D, Fig. 2F–G).
Step 4: Contours with a separate ventral and posterior part on axial slices have to be merged together. Therefore draw a 5 mm thick contour along the lateral border of the tongue (Fig. 2D–E).
Step 5: On lower slices with only a ventral OR posterior contour, extend the contour along the lateral border of the tongue with a diameter of 5 mm (Fig. 2G–H), thereby gradually reducing the length of the extension from upper to lower slices (Fig. 2H–J).
Inter-observer analysis
Nine observers contoured five different cases according to method B: two board-certified radiation oncologists, one board-certified radiologist, one medical doctor, one medical physicist, one technical physician, two medical students, and one undergraduate student. The cases had to be contoured in the same order for all observers: (1) a patient without dental artifacts, (2) with severe artifacts, (3) with mild artifacts, (4) with severe artifacts, and (5) without artifacts again. Assuming a better inter-observer agreement with method A due to the more standardized approach, a separate inter-observer analysis has not been performed.
Statistical analysis
All statistical procedures were done with IBM SPSS Statistics 24 (IBM, Armonk, NY) and MATLAB. Descriptive statistics were used to describe the patient cohort, the volume of the structures, and the dosimetric results. Dice similarity coefficients (DSC) and Jaccard indices were calculated to evaluate the spatial overlap of the two taste bud bearing tongue mucosa structures delineated according to methods A and B, and the inter-observer variability in contouring for method B, with values ranging from 0 (no overlap) to 1 (complete overlap) [15]. Paired-sample t-tests were used to compare the volumetric and dosimetric parameters between the different contouring methods (whole tongue vs. method A, method A vs. method B). A p-value <0.05 after Bonferroni correction was considered significant.
Results
Seventy patients treated with IMRT were included in the analysis. Fifty-eight (83%) had tonsil cancer and 12 (17%) had cancer of the BOT. The T-category of the primary tumor was: T1, 25 patients (36%), T2, 32 (46%), T3, 11 (16%), and T4, 2 patients (2%). Sixty patients (86%) were node positive.
IMRT dose prescribed to the primary tumor ranged from 64 Gy to 72 Gy. The majority was treated in 30 fractions (59%), and the rest treated in 33–40 fractions. Forty-seven patients (67%) were treated to both sides of the neck, while the others (33%) received treatment to the primary tumor and ipsilateral neck.
CT slice thickness was 2.5–3.0 mm in all but one case. Five patients (7%) had no dental artifacts, seven (10%) mild (no influence on contouring), 41 (59%) moderate (contouring affected, but whole tongue could be visualized with adaptation of the CT window level), and 17 (24%) severe imaging artifacts (precise contouring limited).
The border of the mobile tongue to the BOT was determined by a line from the inferior mandible to the anterior inferior atlas in 45 cases (64%), and to the tip of the dens axis in 25 (36%). Within those two groups eight patients had a slight adaptation of the posterior border of the taste bud bearing tongue mucosa structure: more anterosuperior: n = 7 (fixed point below atlas n = 6, fixed point at tip of dens axis n = 1); more posteroinferior: n = 1 (fixed point below atlas).
The mean volume of the whole tongue (123.6 cc, SD 24.6) differed significantly from the taste bud bearing tongue mucosa contoured according to method A (19.3 cc, SD 3.4) (Table 1). There was no significant difference in the volume of the taste bud bearing tongue mucosa if contoured according to method A or B (19.0 cc, SD 3.6) (Table 1); an example of the different volumes for the taste bud bearing tongue mucosa is depicted in Fig. 3.
Table 1.
Volume receiving a certain dose to the taste bud bearing tongue mucosa delineated according to method A and B, and the whole tongue.
| Taste bud bearing tongue mucosa |
Whole tongue |
||||
|---|---|---|---|---|---|
| Method A Mean dose ± SD | Method B Mean dose ± SD | Non-corrected p-value method A vs. method B | Mean dose ± SD | Non-corrected p-value method A vs. whole tongue | |
|
| |||||
| V5/Total volume [cc] | 19.33 ± 3.37 | 18.96 ± 3.60 | 0.082 | 123.55 ± 24.60 | <0.001* |
| V10 [cc] | 19.31 ± 3.37 | 18.93 ± 3.58 | 0.080 | 123.14 ± 24.65 | <0.001* |
| V15 [cc] | 19.01 ± 3.26 | 18.64 ± 3.44 | 0.084 | 121.62 ± 24.52 | <0.001* |
| V20 [cc] | 18.10 ± 3.55 | 17.77 ± 3.61 | 0.124 | 118.34 ± 25.04 | <0.001* |
| V25 [cc] | 16.82 ± 4.01 | 16.51 ± 3.86 | 0.119 | 112.44 ± 26.64 | <0.001* |
| V30 [cc] | 15.27 ± 4.42 | 14.97 ± 4.17 | 0.109 | 104.18 ± 28.66 | <0.001* |
| V35 [cc] | 13.35 ± 4.59 | 13.10 ± 4.32 | 0.135 | 94.15 ± 29.81 | <0.001* |
| V40 [cc] | 11.65 ± 4.45 | 11.44 ± 4.17 | 0.172 | 83.82 ± 29.52 | <0.001* |
| V45 [cc] | 10.08 ± 4.01 | 9.95 ± 3.79 | 0.304 | 73.49 ± 28.32 | <0.001* |
| V50 [cc] | 8.66 ± 3.49 | 8.58 ± 3.34 | 0.432 | 64.02 ± 26.70 | <0.001* |
| V55 [cc] | 7.36 ± 3.13 | 7.30 ± 3.00 | 0.511 | 54.90 ± 25.07 | <0.001* |
| V60 [cc] | 5.85 ± 2.93 | 5.83 ± 2.91 | 0.771 | 43.80 ± 24.04 | <0.001* |
| V65 [cc] | 4.32 ± 2.73 | 4.29 ± 2.76 | 0.517 | 30.83 ± 21.25 | <0.001* |
| V70 [cc] | 1.84 ± 2.52 | 1.84 ± 2.54 | 0.806 | 13.47 ± 18.90 | <0.001* |
Remains statistically significant after Bonferroni correction.
Fig. 3.

Comparison of delineation methods for the taste bud bearing tongue mucosa on a representative patient (orange: method A, red: method B).
Dose differences were highly significant between the whole tongue and the taste bud bearing tongue mucosa (method A) regarding mean dose, minimum dose, maximum dose, D40–D90 and V5–V70 (Fig. 4, Tables 1 and 2). On average, the dose to the whole tongue differed more than 2 Gy for mean dose, minimum dose, and D20–D95 compared to method A (range 2.57–6.09 Gy). The largest dose difference in a single patient was 30.90 Gy for D40.
Fig. 4.

Boxplot diagrams showing the difference in mean, minimum and maximum dose to the taste bud bearing tongue mucosa (method A and B, blue) and the whole tongue (yellow). Values on the y-axis are in Gy.
Table 2.
Dosimetric comparison between the taste bud bearing tongue mucosa method A, method B and the whole tongue.
| Mean dose ± SD taste bud bearing tongue mucosa |
p-value method A vs. B | Mean/median/max. dose deviation§ method B vs. A | Mean dose ± SD whole tongue | p-value method A vs. whole tongue | Mean/median/max. dose deviation§ whole tongue vs. method A | ||
|---|---|---|---|---|---|---|---|
| Method A | Method B | ||||||
|
| |||||||
| Mean dose [Gy] | 46.56 ± 7.17 | 46.74 ± 7.14 | 0.034 | 0.56/0.46/2.18 | 49.31 ± 7.92 | <0.001* | 3.77/2.61/19.61 |
| Minimum dose [Gy] | 18.12 ± 7.73 | 18.22 ± 7.59 | 0.432 | 0.58/0.38/4.33 | 15.44 ± 7.58 | <0.001* | 2.68/0.51/16.97 |
| Maximum dose [Gy] | 71.48 ± 3.30 | 71.45 ± 3.28 | 0.616 | 0.19/0.01/4.70 | 72.14 ± 2.93 | <0.001* | 0.66/0.29/6.85 |
| D5 [Gy] | 69.27 ± 3.34 | 69.24 ± 3.41 | 0.607 | 0.19/0.08/4.42 | 69.39 ± 3.26 | 0.511 | 0.83/0.38/6.62 |
| D10 [Gy] | 68.05 ± 4.12 | 68.07 ± 4.09 | 0.738 | 0.26/0.11/3.66 | 68.16 ± 4.04 | 0.699 | 1.28/0.60/11.16 |
| D20 [Gy] | 64.50 ± 6.39 | 64.58 ± 6.16 | 0.397 | 0.46/0.26/3.50 | 64.68 ± 6.32 | 0.710 | 2.57/1.36/21.57 |
| D30 [Gy] | 58.35 ± 8.60 | 58.62 ± 8.38 | 0.078 | 0.86/0.54/5.20 | 59.98 ± 8.76 | 0.027 | 4.27/3.30/27.80 |
| D40 [Gy] | 51.01 ± 9.90 | 51.47 ± 9.78 | 0.007 | 1.03/0.66/4.73 | 54.70 ± 10.17 | <0.001* | 5.36/3.63/30.90 |
| D50 [Gy] | 44.89 ± 10.31 | 45.30 ± 10.34 | 0.008 | 0.97/0.72/3.94 | 49.62 ± 10.76 | <0.001* | 5.91/3.67/30.48 |
| D60 [Gy] | 40.18 ± 10.09 | 40.93 ± 10.13 | 0.451 | 0.83/0.54/4.01 | 45.14 ± 10.87 | <0.001* | 6.09/3.87/28.87 |
| D70 [Gy] | 35.53 ± 9.27 | 35.60 ± 9.41 | 0.578 | 0.72/0.44/4.66 | 40.47 ± 10.38 | <0.001* | 5.88/3.75/21.92 |
| D80 [Gy] | 30.99 ± 8.83 | 31.18 ± 8.94 | 0.073 | 0.67/0.55/3.26 | 35.27 ± 9.58 | <0.001* | 5.15/3.81/19.66 |
| D90 [Gy] | 26.38 ± 8.40 | 26.52 ± 8.56 | 0.110 | 0.51/0.30/2.27 | 29.36 ± 9.13 | <0.001* | 4.05/3.23/16.20 |
| D95 [Gy] | 23.66 ± 8.17 | 23.87 ± 8.31 | 0.025 | 0.53/0.30/2.32 | 25.16 ± 9.11 | 0.008 | 3.78/3.18/12.68 |
Remains statistically significant after Bonferroni correction.
Dose deviation = √(dosemethodB − dosemethodA)2.
None of the dosimetric parameters (mean dose, minimum dose, maximum dose, D5–D95) differed significantly between method A and method B after Bonferroni correction (Tables 1 and 2), and none differed more than 1.03 Gy on average. The largest difference for an individual case was 5.2 Gy for D30 (Table 2).
Mean DSC was 0.79 (SD 0.05) for comparison of methods A and B of the taste bud bearing tongue mucosa structures (range: 0.68–0.92), while the mean Jaccard index was 0.65 (SD 0.07, range: 0.52–0.85).
Mean DSC and Jaccard index for the five cases contoured by the nine observers was 0.63 (SD 0.12) and 0.47 (SD 0.13), respectively (Suppl. Table B). Mean DSC and Jaccard index was higher when contoured by board-certified radiation oncologists and higher with no/mild artifacts than with severe artifacts (Suppl. Table B).
Mean dose, minimum and maximum dose differed on average less than 1 Gy, and median dose less than 2 Gy, for all cases contoured by the 9 observers according to method B compared to the contours according to method A provided by a board-certified radiation oncologist (Suppl. Table C). The difference between method A and the whole tongue structure ranged up to 10 Gy for the mean dose and up to 15 Gy for the median dose (Suppl. Table C).
Discussion
In this manuscript we propose two methods of contouring the taste bud bearing tongue mucosa and demonstrate that they lead to significant dosimetric differences compared with contouring the whole tongue. This underlines the importance of having a taste specific OAR structure instead of using the whole tongue or the entire oral cavity.
The guidelines for delineation of the two taste bud bearing tongue mucosa structures differ mainly in the time for contouring (approximately 9 vs. 4 min. for method A and B, respectively). Method A is more time consuming, as the whole tongue has to be contoured first. However, as method B was less structured, the concerns were whether the two methods would differ dosimetrically and whether the inter-observer variability for method B would be satisfactory. The dosimetric results were not significantly different between the two methods for all the parameters assessed after Bonferroni correction, although the spatial overlap measured with the DSC was only 0.79. Interestingly, the mean dose, minimum and maximum dose to the taste bud bearing tongue mucosa differed by less than 1 Gy on average between the nine observers contouring according to method B and the contours provided by a board-certified radiation oncologist according to method A, even though the observers had a wide range of expertise including not only board-certified radiation oncologists, but also others in the medical field as well as students. Assuming even less inter-observer variability with the highly standardized method A, we did not do comparative tests.
For both taste bud bearing tongue mucosa structures, we decided to use a 5 mm thickness for the contour. Although the taste buds are located in the upper 1 mm [16], the 5 mm approach was chosen to provide a structure large enough to be used not only for dosimetric analysis but also for evaluation of potential imaging biomarkers, i.e. radiomics. Dental artifacts in the CT images can hamper proper contouring, but are manageable in most cases with adjustment of the CT window level.
The taste bud bearing tongue mucosa contouring guideline has some limitations. Although we decided to define the border of the mobile tongue to the BOT using a line between two fixed points to allow for standardization of the contour, it was not possible to rely on one single approach. The decision of the radiation oncologist/dosimetrist on whether to take the tip of the dens axis or the inferior anterior atlas as one of the bony fixed points was based on the different head positions in the mask (ante-/retroflexion) and differing anatomy across patients. If not applied properly, this can lead to dosimetric discrepancies, especially in the volume receiving high doses in the posterior part of the mobile tongue in case of OPC or nasopharyngeal cancer. Even with these two options, in some patients this definition will still not fit perfectly and the border to the superior BOT will need adaptation (mainly in the anterosuperior direction).
Another limitation is that this guideline is only suitable for patients without major oral surgery and without positional oral stents. Patients with partial glossectomy however will already have taste difficulties due to the surgical resection and might benefit even more from applying strict dose constraints. A comparable guideline for those patients and for patients with oral stents would be desirable but requires consideration of the different anatomy or altered tongue position.
This guideline used CT simulation images because CT is mainly used in the clinic for delineation of the OARs. The guideline can be used for MRI as well, but it will be more challenging to define the bony fixed points in step 3 (method A)/step 1 (method B) of the contouring process due to the decreased visualization of bony structures.
As shown, the different contouring methods of the taste bud bearing tongue mucosa do not have significant dosimetric differences in most of the analyzed parameters, however, the inter-observer variability has been assessed only for method B. Furthermore, our study results do not necessarily mean that there would not be a difference between the taste bud bearing tongue mucosa contours when analyzing imaging biomarkers like the apparent diffusion coefficient (ADC) for example.
Finally, the tongue motion has to be considered. Ding et al. [17] could show that the image quality on MRI was significantly better in patients with tongue-depressing oral stents due to motion minimization during the scan compared to patients without stent. But as the application of one radiotherapy fraction does not last as long as an MRI sequence and patients are forced not to swallow during the treatment, the impact of variability of tongue position on dose received by the taste bud bearing tongue mucosa structure should be of minor impact.
In conclusion, two contouring methods of the taste bud bearing tongue mucosa are described. They were equally satisfactory for creating an OAR structure for taste without relevant difference in dosimetric parameters between each other and either is clearly preferred over the whole tongue contour.
Supplementary Material
Acknowledgement of grant or other financial support
S. Stieb is funded by the Swiss Cancer League (BIL KLS-4300-08-2017). B.A. McDonald receives research support from the National Institutes of Health/National Institute of Dental and Craniofacial Research (1F31DE029093-01A1) and a Dr. John J. Kopchick Fellowship through the MD Anderson UTHealth Graduate School of Biomedical Sciences. K. Wahid is supported by a training fellowship from The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences TL1 Program (Grant No. TL1 TR003169). J. Ventura is funded through a Research Supplement to Promote Diversity in Health-Related Research by the National Cancer Institute of the National Institutes of Health (R01CA218148). S.J. Frank is a paid consultant/advisory board member for Varian and advisory board member for Hitachi and Breakthrough Chronic Care. He is founder and director of and has received grants and personal fees from C4 Imaging. He reports grants from Eli Lilly, Elekta, grants and honoraria from Hitachi and personal fees from Boston Scientific and the National Comprehensive Cancer Center (NCCN). G.B. Gunn is supported by a philanthropic donation made by the Family of Paul W. Beach. C.D. Fuller received funding from the National Institute for Dental and Craniofacial Research Award (1R01DE025248-01/R56DE025248) and Academic-Industrial Partnership Award (R01 DE028290), the National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679), the NIH Big Data to Knowledge (BD2K) Program of the National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825), the NCI Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148), the NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672), the NIH/NCI Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50 CA097007) and the National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Program (R25EB025787). He has received direct industry grant support, speaking honoraria and travel funding from Elekta AB. None of the funding sources had an influence on study design, the collection, analysis and interpretation of data, the writing of the report, and the decision to submit the article for publication.
Footnotes
Conflicts of Interest
None.
Appendix A. Supplementary data
References
- [1].Kiss N, et al. , Taste function in adults undergoing cancer radiotherapy or chemotherapy, and implications for nutrition management: a systematic review. J Acad Nutr Diet, 2020. S2212–2672(20)31065–0. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [2].Baharvand M et al. Taste alteration and impact on quality of life after head and neck radiotherapy. J Oral Pathol Med 2013;42:106–12. [DOI] [PubMed] [Google Scholar]
- [3].Sio TT et al. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys 2016;95:1107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stieb S et al. Prospective observational evaluation of radiation-induced late taste impairment kinetics in oropharyngeal cancer patients: Potential for improvement over time?. Clin Transl Radiat Oncol 2020;22:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nguyen HM, Reyland ME, Barlow LA. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci 2012;32:3474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosenthal DI et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer 2014;120:1975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shi HB et al. Irradiation impairment of umami taste in patients with head and neck cancer. Auris Nasus Larynx 2004;31:401–6. [DOI] [PubMed] [Google Scholar]
- [8].Mossman KL. Gustatory tissue injury in man: radiation dose response relationships and mechanisms of taste loss. Br J Cancer Suppl 1986;7:9–11. [PMC free article] [PubMed] [Google Scholar]
- [9].Chen WC et al. Long-term taste impairment after intensity-modulated radiotherapy to treat head-and-neck cancer: correlations with glossectomy and the mean radiation dose to the oral cavity. Chem Senses 2019;44:319–26. [DOI] [PubMed] [Google Scholar]
- [10].Sapir E et al. Predictors of dysgeusia in patients with oropharyngeal cancer treated with chemotherapy and intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2016;96:354–61. [DOI] [PubMed] [Google Scholar]
- [11].Leitzen C et al. Change of taste during and after IM-/IG-radiotherapy for head and neck cancer patients. Laryngorhinootologie 2015;94:383–7. [DOI] [PubMed] [Google Scholar]
- [12].Kamprad F et al. Functional changes of the gustatory organ caused by local radiation exposure during radiotherapy of the head-and-neck Region. Strahlenther Onkol 2008;184:157–62. [DOI] [PubMed] [Google Scholar]
- [13].Yamashita H et al. Relation between acute and late irradiation impairment of four basic tastes and irradiated tongue volume in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006;66:1422–9. [DOI] [PubMed] [Google Scholar]
- [14].Brouwer CL et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol 2015;117:83–90. [DOI] [PubMed] [Google Scholar]
- [15].Allozi R et al. Tools for consensus analysis of experts’ contours for radiotherapy structure definitions. Radiother Oncol 2010;97:572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Srur E et al. Change of the human taste bud volume over time. Auris Nasus Larynx 2010;37:449–55. [DOI] [PubMed] [Google Scholar]
- [17].Ding Y et al. Prospective observer and software-based assessment of magnetic resonance imaging quality in head and neck cancer: Should standard positioning and immobilization be required for radiation therapy applications?. Pract Radiat Oncol 2015;5:e299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


