Background:
This systematic review evaluates the effect of exercise training in the treatment of patients with mild cognitive impairment (MCI).
Methods:
PubMed, Medline, EMBASE, Web of Science, and Cochrane Library databases were systematically searched up to Oct 2021 in order to identify randomized controlled trials (RCTs) which evaluated the effects of physical exercise in persons with MCI. Changes of cognitive and physical function were tested using pre- and postMMSE and TUG scores, and were compared with control intervention.
Results:
A total of 10 RCTs involving 635 MCI patients were included in the meta-analysis. Physical exercise improved MMSE scores (MD 0.71, 95 % CI 0.57 to 0.85, P < .00001, I2 = 95 %) and TUG performance (MD −0.82, 95 % CI −1.20 to −0.45, P < .00001, I2 = 12 %) in patients with MCI.
Conclusions:
This meta-analysis demonstrated a positive effect of exercise training in people with MCI in relation to cognitive and physical function. These findings suggest exercise interventions be persistent, and reveal that more high-quality researches are needed.
Keywords: cognition, exercise, meta-analysis, mild cognitive impairment
1. Introduction
Mild cognitive impairment (MCI) is an early condition in which someone has cognitive dysfunction associated with increasing age, which may lead to dementia.[1] The prevalence of MCI is reported to be between 15% and 20% in adults aged 60 years and older, and the annual incidence of progression to dementia increased to between 8% and 15% over the years.[2] For old adults diagnosed as MCI, cognitive impairment are associated with the individual age, while no interference in their activities of daily living is expected.[3] Ongoing researches have documented the accelerated cognitive decline may be affected by lifestyle factors, such as exercise, diet, sleep, work, recreation, and study.[4–6]
Exercise interventions has been proved to reduce risk of falls and fractures[7] and prevent dementia and delay cognitive decline[8] in people aged > 60 years. In general, investigation of physical training program such as aerobic, resistant, flexibility, strengthening, and balance exercise, provide clinical significance in improvement the outcome for patients with cognitive decline. Nevertheless, association of different exercise programs with cognitive and physical function in MCI still remains unclear.
Cognitive functions are fundamental to human beings, including functions of learning, perception, deduction, problem solving, memory, and others. The Mini-Mental State Examination (MMSE) is one of the best-known and most widely used tools to evaluate cognitive status in people with cognitive impairments.[9] Physical function can be defined as the ability to perform both basic and instrumental activities of daily living (ADL), is crucial for the community dwelling adults older than 60 years old. The physical function involves neuromotor, musculoskeletal, and the cardiorespiratory systems. The Timed Up and Go test is a commonly used assessment of patients’ basic functional mobility,[10] which is suggested to detecting differences in physical performance.[11]
The present meta-analysis pooled data from available clinical evidence to investigate the effect of exercise training on cognitive and physical alteration of MCI patients.
2. Method
2.1. Data sources and search strategy
This study was performed in accordance with the preferred reporting items for systematic reviews and Meta analyses (PRISMA) standards.[12] A systematic literature search was on PubMed, Medline, EMBASE, Web of Science and Cochrane Library databases for randomized controlled trials (RCTs) from the inception dates to Oct 1st, 2021. The search included the keywords “mild cognitive impairment,” “MCI,” “exercise,” “physical,” and “training.”
2.2. Eligibility criteria
The inclusion criteria were the following: (1) participants were diagnosed with mild cognitive impairment of any type and stage; (2) exercise interventions were performed without limitation to exercise types, intensity, or duration; (3) studies reporting the outcome of MMSE scores and TUG; (4) comparison with other control intervention.
2.3. Outcomes definition
The outcome of cognitive status was defined using the MMSE score, which comprises 5 domains (orientation, immediate memory, attention/concentration, delayed recall, language) and totaling 30 points.[9] The outcome of physical status was assessed by the TUG test, a performance-based measure to identify mobility and balance in older adults. Patients were required to rise from a standard armchair, walk ahead 3 meters, turn back, and sit down again. The time was recorded when the subjects completed the test.[10]
2.4. Data extraction and quality assessment
Two independent reviewers performed the study selection, data extraction and risk of bias evaluation. Information about the authors, publication year, number of participants, age, gender, exercise program, period of intervention, follow-up duration, MMSE scores, and TUG test was extracted. Mean value and standard deviations (SD) pre- and postintervention were calculated from each study for both experimental and control groups.
Review Manager 5.3 software (Cochrane Collaboration, UK) was used to assess the quality of the included studies according to the Cochrane criteria.[13] We assessed the following sources of publication bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases, each study were then marked as low risk, unclear risk and high risk. Any disagreements were resolved via the third reviewer.
2.5. Calculation of effect size (ES) and statistical analysis
The statistical analysis was carried out using Review Manager 5.3 software. Mean differences (MD) and standard deviations (SD) were used to calculate the ES. A standardized effect for each study, a pooled effect for the overall comparisons, and their 95% confidence intervals (CIs) were calculated using fixed-effect models. Heterogeneity among studies was determined using I2 statistic, with significance set at P < .05.[14] Changes of MMSE scores and TUG test were conducted to investigate the effect of exercise training for cognitive and physical function.
2.6. Ethics and dissemination
The ethical approval was not necessary for this meta-analysis.
3. Result
3.1. Literature search and study characteristics
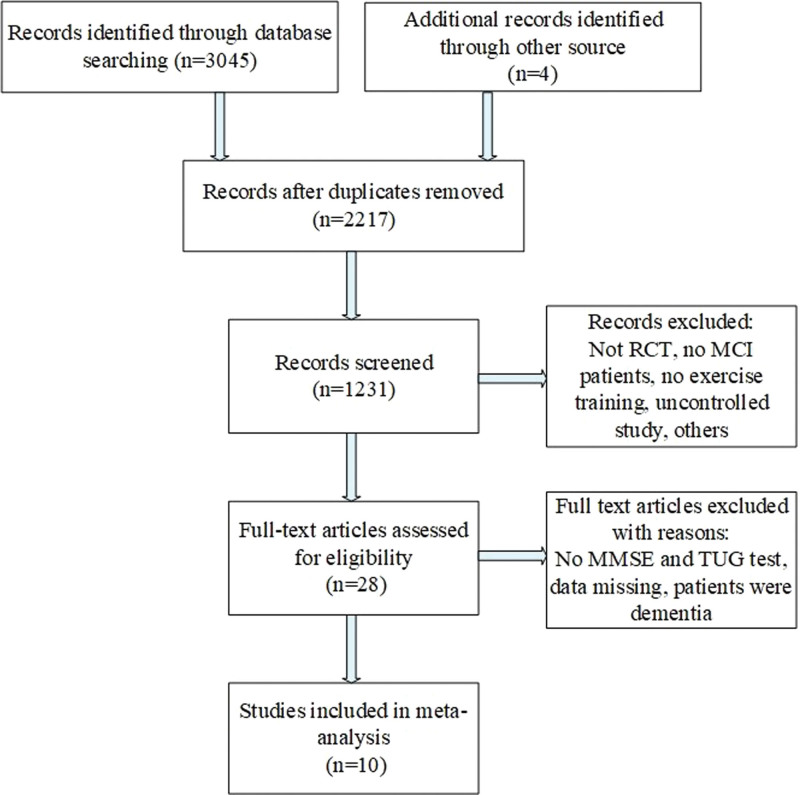
A total of 3049 titles and abstracts were screened, and the detailed steps of literature search were shown in Figure 1. The remaining 10 RCTs were included in this meta-analysis after removing the irrelevant records,[15–24] and the detailed characteristics were shown in Table 1.
Figure 1.
The study search, selection, and inclusion process.
Table 1.
Characteristics of the included studies.
| Author | Year | N | Age | Gender | Intervention | Duration | Follow-up |
|---|---|---|---|---|---|---|---|
| Varela, S. | 2012 | 48 | 78.3 ± 9.5 | 43.75% | 5 minutes warm-up, 20 minutes cycling, and 5 minutes cool-down | 3 months | 6 months |
| Suzuki, T. | 2013 | 100 | 75.4 ± 7.1 | 51.00% | 10 minutes warm-up, 20 minutes muscle strength exercise, aerobic exercise, postural balance retraining, and 60 minutes dual-task training | 6 months | 24 months |
| Lu, J. | 2016 | 45 | 69.7 ± 4.78 | 28.89% | 5 minutes warm-up, 50 minutes dumbbell-spinning exercises and 5 minutes cool-down | 3 months | 3 months |
| Lazarou, I. | 2017 | 129 | 66.9 ± 10.1 | 21.71% | 5 minutes warm-up, 45 minutes new material (figures/dances), and 10 minutes cool-down | 10 months | 10 months |
| Yoon, D. H. | 2017 | 30 | 76.0 ± 3.56 | NA | 10 minutes warm-up, 40 minutes elastic band training and 10 minutes cool-down | 3 months | 3 months |
| Bademli, K. | 2018 | 60 | 71.5 ± 6.0 | 41.67% | 10 min warm-up, 20 minutes rhythmic exercises, 10 minutes cool-down, and 40 minutes free walking | 20 weeks | 20 weeks |
| de Oliveira Silva, F. | 2019 | 19 | 75.9 ± 6.1 | 42.11% | 5 minute warm up, 20 minutes aerobic exercise, and 5-min warm-down | 3 months | 3 months |
| Goldberg, S. E. | 2019 | 60 | 76 (65–91) | 56.67% | Balance challenging, strength building, dual-task training and gait reeducation | 12 months | 12 months |
| Langoni, C. D. S. | 2019 | 52 | 72.3 ± 7.78 | 23.08% | Strength exercises and aerobic training | 6 months | 6 months |
| Lipardo, D. S. | 2020 | 92 | 69 ± 8.3 | 20.65% | Multicomponent exercise program on balance, strength, endurance, and flexibility | 3 months | 12 months |
N = number of participants, age = years, gender = male%, min = minutes.
A total of 635 patients diagnosed as MCI were included in this meta-analysis, the mean age was 73.1 and 36.61% were male. The exercise programs included aerobic, balance, strength, endurance, flexibility, and combination training, with different intensity. The length of the interventions ranged from 3 to 10 months, and the follow-up period ranged from 3 to 24 months.
3.2. Effects of exercise treatment on cognitive function
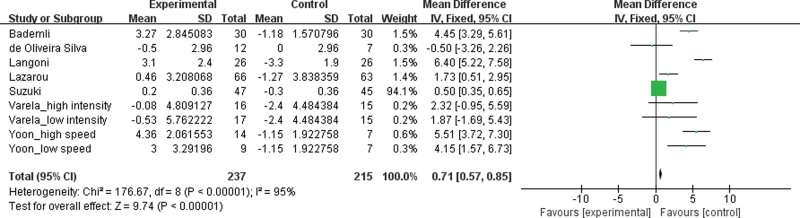
MMSE test was selected to evaluate the effects of exercise treatment on cognitive function in patients with MCI (see Fig. 2). The value for the change in overall effect size showed statistically significant (MD 0.71, 95% CI 0.57 to 0.85, P < .00001, I2 = 95 %), indicating an improvement on cognitive function.
Figure 2.
Effects of exercise treatment on cognitive function for patients with MCI.
3.3. Effects of exercise treatment on physical function
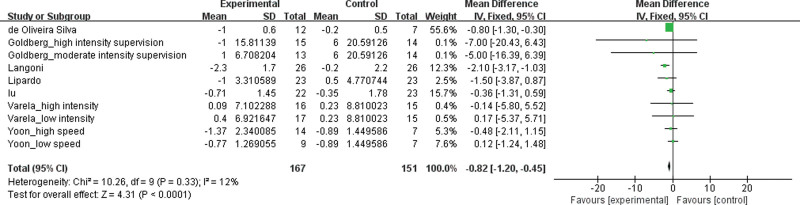
TUG test was selected to evaluate the effects of exercise treatment on physical function in patients with MCI (see Fig. 3). The value for the change in overall effect size showed statistically significant (MD −0.82, 95 % CI −1.20 to −0.45, P < .00001, I2 = 12 %), indicating an improvement on physical function. No significant heterogeneity was present among the papers (I2 = 12 %, P = .33).
Figure 3.
Effects of exercise treatment on physical function for patients with MCI.
3.4. Study quality
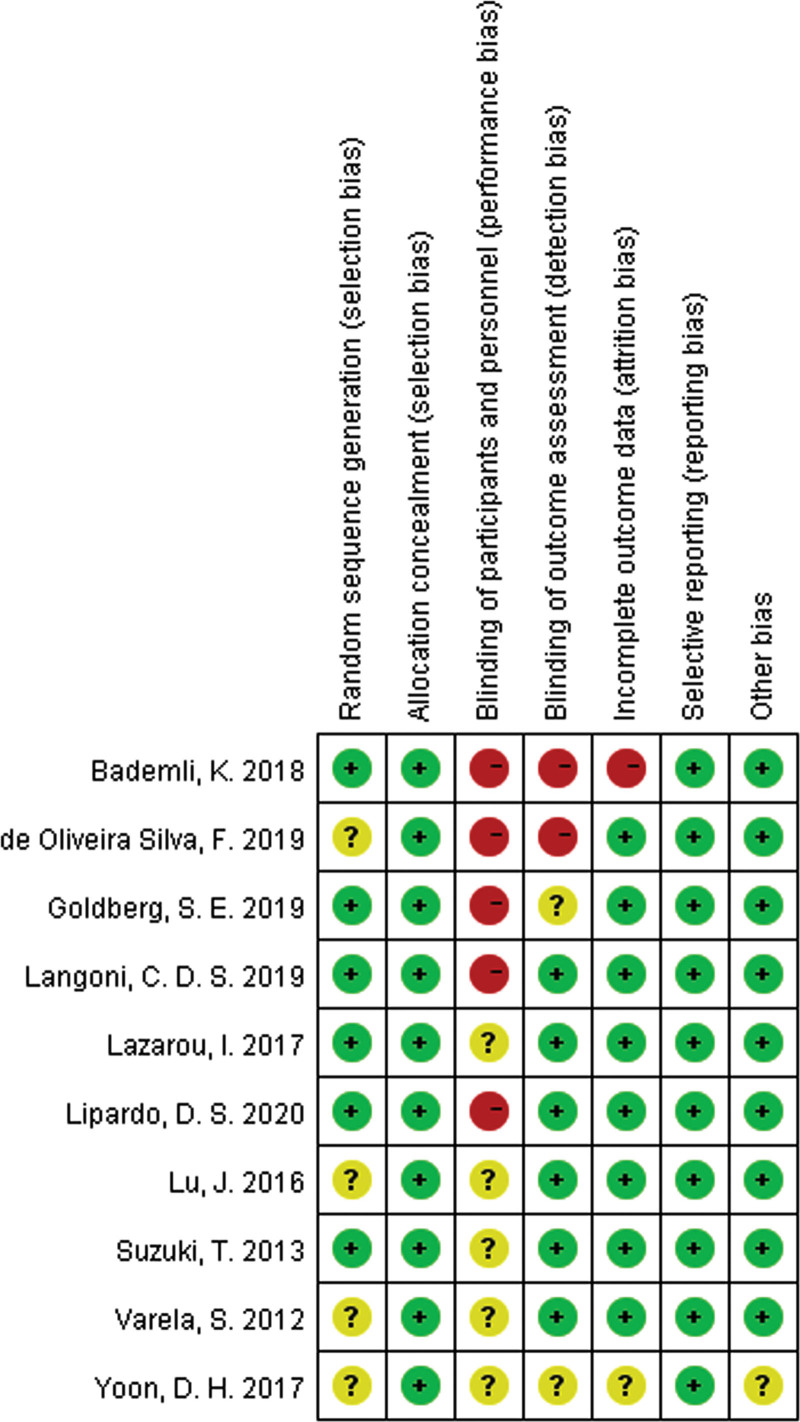
Details about the risks of bias of the included studies are shown in Figure 4. Because of the intervention, most of the studies used single-blinded, randomized and matched-pair controlled design, which may lead to high risks of performance bias. The study by Bademli[20] was not blind and the data is incomplete.
Figure 4.
Risk of bias summary: judgments about each risk of bias item for each included study.
4. Discussion
In this systematic review and meta-analysis, existing evidence from 10 RCTs demonstrated that any type of long-term exercises (aerobic, balance, strength, endurance, flexibility, and combination training), with any intensities, significantly benefit cognitive and physical performance in older adults with MCI.
The previous results of exercise interventions in elderly are controversial,[25] possible explanation for this situation could be the population, training protocol, therapy duration, and sample size. Considerable heterogeneity (I2 > 50%) in the results of MMSE scores was detected in most of the included studies, however, there were no insufficient data to conduct the subgroup analyses. Therefore, more researches with larger sample size are needed for the further study.
Although several studies have shown a beneficial effect of physical exercise in old adults,[26,27] this is the first study investigating the overall effect for both cognitive and physical performance in MCI patients. Cognitive and physical processes have always appeared in a highly interlinked fashion, which could be an effective target for rehabilitation interventions both directly and indirectly.[28] Physical exercise triggers several molecular and cellular cascades in hippocampus and cortex to exert beneficial effects, lead to the improved cognitive capacity and modulate disease progression.[29,30] Increased cerebral blood flow, activation of brain area, and production of neurotrophic factors have suggested to be possible mechanism for the exercise-induced change in cognitive function.[31]
Limitations of this study are as follows. Firstly, only 10 studies and 635 patients were included in the meta-analysis. The small sample size makes it hardly to determine the source of heterogeneity. Secondly, the included trials showed heterogeneity in cognitive performance. Finally, the quality of the included studies was not ideal, and further qualitative studies are necessary.
This systematic review demonstrated the exercise training in people with MCI is feasible and successful in improving cognitive and physical function. Furthermore, the standardization, professionalization, and popularization of exercise program to maximize the clinical use is critical, and finally promote the change in clinical practice.
Abbreviations:
- ADL =
- activities of daily living
- CI =
- confidence interval
- ES =
- effect sizes
- MCI =
- mild cognitive impairment
- MD =
- mean difference
- MMSE =
- The Mini-Mental State Examination
- PRISMA =
- Preferred Reporting Items for Systematic Reviews and Meta-analysis
- RCTs =
- Randomized controlled trials
- SD =
- standard deviations
- TUG =
- The Timed Up and Go test
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Zhou Y, Li L-D. Exercise training for cognitive and physical function in patients with mild cognitive impairment: A PRISMA-compliant systematic review and meta-analysis. Medicine 2022;101:34(e30168).
The authors have no conflict of interest to declare.
References
- [1].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- [2].Petersen RC. Mild cognitive impairment. Continuum (Minneap Minn). 2016;22:404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–70. [DOI] [PubMed] [Google Scholar]
- [4].Farhud DD. Impact of lifestyle on health. Iran J Public Health. 2015;44:1442–4. [PMC free article] [PubMed] [Google Scholar]
- [5].Deng JH, Huang KY, Hu XX, et al. Midlife long-hour working and later-life social engagement are associated with reduced risks of mild cognitive impairment among community-living Singapore elderly. J Alzheimers Dis. 2019;67:1067–77. [DOI] [PubMed] [Google Scholar]
- [6].Jang JW, Kim Y, Choi YH, et al. Association of nutritional status with cognitive stage in the elderly Korean population: the Korean brain aging study for the early diagnosis and prediction of Alzheimer’s disease. J Clin Neurol. 2019;15:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Souto Barreto P, Rolland Y, Vellas B, et al. Association of long-term exercise training with risk of falls, fractures, hospitalizations, and mortality in older adults: a systematic review and meta-analysis. JAMA Intern Med. 2019;179:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–61. [DOI] [PubMed] [Google Scholar]
- [9].Arevalo-Rodriguez I, Smailagic N, Roque-Figuls M, et al. Mini-mental state examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2021;7:CD010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- [11].Blankevoort CG, van Heuvelen MJ, Scherder EJ. Reliability of six physical performance tests in older people with dementia. Phys Ther. 2013;93:69–78. [DOI] [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Varela S, Ayan C, Cancela JM, et al. Effects of two different intensities of aerobic exercise on elderly people with mild cognitive impairment: a randomized pilot study. Clin Rehabil. 2012;26:442–50. [DOI] [PubMed] [Google Scholar]
- [16].Suzuki T, Shimada H, Makizako H, et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One. 2013;8:e61483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lu J, Sun M, Liang L, et al. Effects of momentum-based dumbbell training on cognitive function in older adults with mild cognitive impairment: a pilot randomized controlled trial. Clin Interv Aging. 2016;11:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lazarou I, Parastatidis T, Tsolaki A, et al. International ballroom dancing against neurodegeneration: a randomized controlled trial in Greek community-dwelling elders with mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2017;32:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yoon DH, Kang D, Kim HJ, et al. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr Gerontol Int. 2017;17:765–72. [DOI] [PubMed] [Google Scholar]
- [20].Bademli K, Lok N, Canbaz M, et al. Effects of physical activity program on cognitive function and sleep quality in elderly with mild cognitive impairment: a randomized controlled trial. Perspect Psychiatr Care. 2019;55:401–8. [DOI] [PubMed] [Google Scholar]
- [21].de Oliveira Silva F, Ferreira JV, Placido J, et al. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: a randomized controlled trial. Maturitas. 2019;126:28–33. [DOI] [PubMed] [Google Scholar]
- [22].Goldberg SE, van der Wardt V, Brand A, et al. Promoting activity, Independence and stability in early dementia (PrAISED): a, multisite, randomised controlled, feasibility trial. BMC Geriatr. 2019;19:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Langoni CDS, Resende TL, Barcellos AB, et al. Effect of exercise on cognition, conditioning, muscle endurance, and balance in older adults with mild cognitive impairment: a randomized controlled trial. J Geriatr Phys Ther. 2019;42:E15–22. [DOI] [PubMed] [Google Scholar]
- [24].Lipardo DS, Tsang WW. Effects of combined physical and cognitive training on fall prevention and risk reduction in older persons with mild cognitive impairment: a randomized controlled study. Clin Rehabil. 2020;34:773–82. [DOI] [PubMed] [Google Scholar]
- [25].de Labra C, Guimaraes-Pinheiro C, Maseda A, et al. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chantanachai T, Sturnieks DL, Lord SR, et al. Risk factors for falls in older people with cognitive impairment living in the community: systematic review and meta-analysis. Ageing Res Rev. 2021;71:101452. [DOI] [PubMed] [Google Scholar]
- [27].Bao W, Sun Y, Zhang T, et al. Exercise programs for muscle mass, muscle strength and physical performance in older adults with Sarcopenia: a systematic review and meta-analysis. Aging Dis. 2020;11:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wajda DA, Mirelman A, Hausdorff JM, et al. Intervention modalities for targeting cognitive-motor interference in individuals with neurodegenerative disease: a systematic review. Expert Rev Neurother. 2017;17:251–61. [DOI] [PubMed] [Google Scholar]
- [29].Rizzo FR, Guadalupi L, Sanna K, et al. Exercise protects from hippocampal inflammation and neurodegeneration in experimental autoimmune encephalomyelitis. Brain Behav Immun. 2021;98:13–27. [DOI] [PubMed] [Google Scholar]
- [30].Liang J, Wang H, Zeng Y, et al. Physical exercise promotes brain remodeling by regulating epigenetics, neuroplasticity and neurotrophins. Rev Neurosci. 2021;32:615–29. [DOI] [PubMed] [Google Scholar]
- [31].Maass A, Duzel S, Brigadski T, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142–54. [DOI] [PubMed] [Google Scholar]