Abstract
Early pregnancy loss (EPL) is a common complication of assisted reproductive technology treatment; however, the exact factors involved in EPL are not fully understood. This study aimed to evaluate the risk factors for EPL in fresh in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycles. This retrospective cohort study was conducted on all couples who underwent clinical pregnancy in fresh IVF/ICSI cycles from January to December 2019 at a single large reproductive medical center. In total, 954 cycles were included in this study. Univariate and multivariate logistic regression analyses were performed to evaluate relevant risk factors for EPL. Curve fitting and threshold analyses were used to explore the association between risk factors and EPL. Compared with women with a normal total antral follicle count (AFC) (≥10, <15), those with a low AFC (<10) had a higher risk of EPL (odds ratio 2.97, 95% confidence interval: 1.38–6.38, P < .05). Patients with an estradiol/progesterone ratio (E2/P) ≥ 1.1 had significantly lower odds of EPL than women with E2/P < 1.1 (odds ratio 0.51, 95% confidence interval 0.28–0.91, P < .05). E2/P and serum human chorionic gonadotropin (hCG) levels negatively correlated with EPL. By using a 2-piecewise regression model, the inflection point of serum hCG level was 599.9 IU/L. Our results showed that lower AFC, E2/P, and serum hCG levels were associated with a higher EPL risk in fresh IVF/ICSI cycles.
Keywords: early pregnancy loss, IVF/ICSI, risk factor
1. Introduction
Infertility is a disease that affects up to 15.5% of reproductive-aged couples.[1] With the development of assisted reproductive technology (ART),[2] an increasing number of patients with infertility are receiving ART for help, such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). Although the clinical pregnancy rate has gradually improved over the past decade, early pregnancy loss (EPL) is a common complication,[3] with an EPL rate higher than that of natural conception, up to 29%.[4] Molecular karyotypic abnormalities are the most important cause of miscarriage, and a woman’s age is a significant factor influencing karyotypic abnormalities.[5] Therefore, ART treatment may not present an increased risk for chromosomal abnormalities occurring in EPL, but the incidence of fetal aneuploidy could increase significantly with advancing maternal age.[6] Maternal age, paternal age, controlled ovarian hyperstimulation (COH) protocol, and serum human chorionic gonadotropin (hCG) levels 14 days after transfer[7] were all related to pregnancy loss. However, some unknown factors may affect pregnancy outcomes during IVF/ICSI cycles. Thus, it is essential to explore the exact factors influencing EPL, thereby improving the ART strategy.
Although many studies have assessed risk factors for EPL in ART pregnancies,[8,9] few large-scale studies have compared the differences between EPL and patients with ongoing pregnancy, which may be applied to counsel pregnant women on ART about risk factors and help doctors improve the protocols.
The study aimed to explore the EPL risk in women who conceived with IVF/ICSI in fresh cycles by analyzing a retrospective cohort of pregnancies. We explored the significant risk factors for EPL risk. This study could be useful in identifying patients at a high risk of EPL and may shed light on the modification of ART protocols to minimize the risk of EPL.
2. Materials and Methods
2.1. Study population
This was a retrospective, cohort study. A total of 954 embryo transfer (ET) cycles were carried out, resulting in clinical pregnancies at the Reproductive Medicine Center of the First Hospital of Lanzhou University. Detailed information on maternal and paternal characteristics, ART treatment procedures, and follow-up outcomes was entered into the electronic database by the clinical support staff. The records of all patients who conceived from January to December 2019 by IVF/ICSI treatment were screened. Only patients with singleton pregnancies were included in the study. The exclusion criteria were multiple pregnancies that showed more than 1 gestational sac on ultrasonography, ectopic pregnancy, preimplantation genetic testing, and those involving donor oocytes or semen. The indications for IVF/ICSI included tubal, male, endocrine, and immune factors.
2.2. Ethics statement
This study was approved by the Ethics Committee of the First Hospital of Lanzhou University (approval number LDYYSZLLKH2022-01). The written informed consent was excused by the institutional review board.
2.3. Outcome variables
A total of 954 patients with clinical pregnancy were divided into 2 groups according to their early pregnancy outcomes: ongoing pregnancy group (>12 weeks of gestation) and EPL group. Clinical pregnancy was defined as the presence of a gestational sac on ultrasonography. The primary outcome was EPL, defined as pregnancy loss before the 12th gestational week after confirmation of a gestational sac on ultrasound. Ongoing pregnancy was defined as the presence of a gestational sac and a fetal heartbeat after 12 weeks of gestation.
Clinical and laboratory data were extracted directly from our electronic medical records, including maternal age, maternal body mass index (BMI), paternal age, infertility type, duration of infertility, basal follicle-stimulating hormone (FSH), basal luteinizing hormone (LH), basal estradiol (E2), basal prolactin (PRL), basal thyroid-stimulating hormone (TSH), carbohydrate antigen 125 (CA-125), total antral follicle count (AFC), COH protocol, gonadotropin (Gn) stimulation days, total Gn dose, estradiol/progesterone ratio (E2/P) on hCG administration day, number of retrieved oocytes, number of metaphase-2 oocytes, endometrial thickness, fertilization methods(IVF or ICSI), and number of transferred embryos. hCG was measured 14 days after transfer in a single laboratory. All variables had <5% missing data.
2.4. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables as N (%). Univariate and multivariate logistic regression analyses were performed to evaluate risk factors for EPL. Smooth curve fitting was performed to analyze the relationships among AFC, E2/P, serum hCG level, and EPL risk. A 2-piecewise linear regression model was used to examine the threshold effect of serum hCG levels on EPL risk. The threshold level (i.e., inflection point) was determined by trial and error, including the selection of inflection points along with a predefined interval and then choosing the inflection point that gave the maximum model likelihood. All statistical analyses were performed using EmpowerStats, version 2018-05-05 (X&Y Solutions, Inc., Boston, MA, USA) and R software.
3. Results
Between January and December 2019, a total of 954 IVF/ICSI fresh transfer cycles from patients resulted in clinical pregnancy. Among the conception cycles, 132 (13.8%) patients had EPL and 822 (86.2%) had ongoing pregnancies. The baseline characteristics of the 2 groups are shown in Table 1. Significant differences were found in maternal age, paternal age, type of infertility, and total AFC.
Table 1.
Basal characteristics of clinical pregnancies conceived in fresh IVF/ICSI cycles.
| Ongoing pregnancy | Early pregnancy loss | P | |
|---|---|---|---|
| N | 822 | 132 | |
| Maternal age (y) | <.001 | ||
| <35 | 641 (77.98%) | 87 (65.91%) | |
| ≥35, <40 | 147 (17.88%) | 25 (18.94%) | |
| ≥40 | 34 (4.14%) | 20 (15.15%) | |
| Maternal BMI (kg/m2) | .988 | ||
| <18.5 | 73 (9.06%) | 12 (9.45%) | |
| ≥18.5, <24 | 544 (67.49%) | 85 (66.93%) | |
| ≥24 | 189 (23.45%) | 30 (23.62%) | |
| Paternal age (y) | .002 | ||
| <35 | 585 (71.25%) | 77 (58.33%) | |
| ≥35, <40 | 162 (19.73%) | 31 (23.48%) | |
| ≥40 | 74 (9.01%) | 24 (18.18%) | |
| Type of infertility | .004 | ||
| Primary | 478 (58.15%) | 59 (44.70%) | |
| Secondary | 344 (41.85%) | 73 (55.30%) | |
| Duration of infertility (y) | 4.15 ± 4.73 | 4.17 ± 3.11 | .964 |
| Basal FSH (IU/L) | 6.70 ± 2.20 | 6.21 ± 2.04 | .067 |
| Basal LH (IU/L) | 5.66 ± 3.46 | 5.00 ± 2.54 | .110 |
| Basal E2 (pmol/mL) | 43.52 ± 28.98 | 48.58 ± 37.48 | .174 |
| Basal PRL (μg/L) | 19.26 ± 10.02 | 17.76 ± 8.22 | .220 |
| Basal TSH (IU/L) | 4.35 ± 8.95 | 4.33 ± 6.56 | .982 |
| CA-125 (U/mL) | 20.68 ± 16.26 | 20.28 ± 12.85 | .841 |
| Total AFC | .002 | ||
| <5 | 188 (23.30%) | 49 (37.69%) | |
| ≥10, <15 | 274 (33.95%) | 32 (24.62%) | |
| ≥15 | 345 (42.75%) | 49 (37.69%) |
Values are mean ± standard deviation or number (percentage).
AFC = total antral follicle count, BMI = body mass index, CA-125 = carbohydrate antigen 125, E2 = estradiol, FSH = follicle-stimulating hormone, IVF/ICSI = in vitro fertilization/intracytoplasmic sperm injection, LH = luteinizing hormone, PRL = prolactin, TSH = thyroid-stimulating hormone.
Treatment information for the ongoing pregnancy and EPL groups is shown in Table 2. Women with EPL received a higher dose of Gn (3382.94 ± 1464.77 vs 3114.43 ± 1325.50, P < .05) and retrieved less E2/P (2.21 ± 1.08 vs 2.48 ± 1.09, P < .05) and number of metaphase-2 oocytes. On day 14 after transfer, the ongoing pregnancy group had significantly higher serum hCG levels than the EPL group (1011.51 ± 789.25 vs 495.19 ± 569.19, P < .001). However, no differences in the COH protocol, Gn stimulation days, number of retrieved oocytes, endometrial thickness, fertilization method, and number of the transferred embryos were identified.
Table 2.
Treatment information of clinical pregnancies conceived in fresh IVF/ICSI cycles.
| Ongoing pregnancy | Early pregnancy loss | P | |
|---|---|---|---|
| N | 822 | 132 | |
| COH protocol | .278 | ||
| GnRH-a prolonged protocol | 303 (36.86%) | 44 (33.33%) | |
| GnRH-a short protocol | 1 (0.12%) | 1 (0.76%) | |
| GnRH-a long protocol | 459 (55.84%) | 72 (54.55%) | |
| GnRH antagonist protocol | 42 (5.11%) | 10 (7.58%) | |
| Minimal stimulation protocol | 15 (1.82%) | 5 (3.79%) | |
| Other protocol | 2 (0.24%) | 0 (0.00%) | |
| Gn stimulation days | 13.07 ± 1.91 | 12.61 ± 2.04 | .114 |
| Total Gn dose(IU) | 3114.43 ± 1325.50 | 3382.94 ± 1464.77 | .037 |
| Total Gn dose(IU) | .014 | ||
| <3150.73 | 508 (63.03%) | 65 (51.59%) | |
| ≥3150.73 | 298 (36.97%) | 61 (48.41%) | |
| E2/P | 2.48 ± 1.09 | 2.21 ± 1.08 | .009 |
| Number of retrieved oocytes | .176 | ||
| <6 | 70 (8.52%) | 13 (9.85%) | |
| ≥6, <15 | 401 (48.78%) | 74 (56.06%) | |
| ≥15 | 351 (42.70%) | 45 (34.09%) | |
| Number of metaphase-2 oocytes | .024 | ||
| <7 | 144 (17.52%) | 34 (25.76%) | |
| ≥7 | 678 (82.48%) | 98 (74.24%) | |
| Endometrial thickness(cm) | .201 | ||
| <0.8 | 13 (1.59%) | 5 (3.79%) | |
| ≥0.8, <1.2 | 451 (55.00%) | 68 (51.52%) | |
| ≥1.2 | 356 (43.41%) | 59 (44.70%) | |
| Fertilization methods | 0.211 | ||
| IVF | 550 (66.91%) | 81 (61.36%) | |
| ICSI | 272 (33.09%) | 51 (38.64%) | |
| Number of transferred embryos | .195 | ||
| 1 | 55 (6.69%) | 11 (8.33%) | |
| 2 | 707 (86.01%) | 106 (80.30%) | |
| 3 | 60 (7.30%) | 15 (11.36%) | |
| Serum hCG level(IU/L) | 1011.51 ± 789.25 | 495.19 ± 569.02 | <.001 |
Values are mean ± standard deviation or number (percentage).
COH = controlled ovarian hyperstimulation, Gn = gonadotropin, hCG = human chorionic gonadotropin, IVF/ICSI = in vitro fertilization/intracytoplasmic sperm injection.
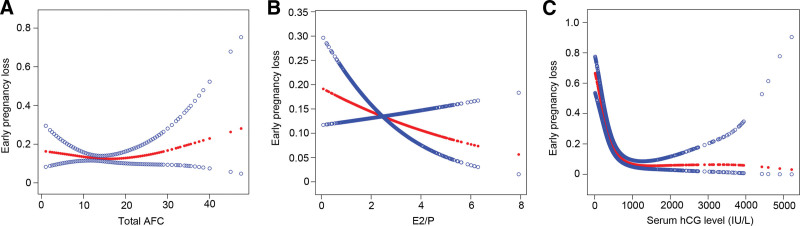
Univariate logistic regression analysis revealed that maternal age, paternal age, infertility type, total AFC, total Gn dose, E2/P, number of metaphase-2 oocytes, and serum hCG levels were associated with the EPL risk (Table 3). After including these variables in the same model, total AFC, E2/P, and serum hCG levels were still independently associated with the EPL risk, whereas maternal age, paternal age, infertility type, total Gn dose, and number of metaphase-2 oocytes were no longer related (Table 3). Compared with women with normal AFC (≥ 10, <15), those with low AFC (<10) had a higher risk of EPL (OR 2.97, 95% confidence interval (CI) 1.38–6.38, P < .01); however, patients with high (AFC ≥ 15) showed no difference in EPL risk. We further applied a 2-piecewise linear regression model to examine the threshold effect of AFC on EPL using a smoothing function (Fig. 1A). We found the inflection point was 13, but the difference was not significant.
Table 3.
Risk of early pregnancy loss by univariate and multivariate logistic regression analyses.
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Maternal age (y) | ||||
| <35 | Reference | Reference | ||
| ≥35, <40 | 1.25 (0.78, 2.02) | .3565 | 0.80 (0.40, 1.58) | .5215 |
| ≥40 | 4.33 (2.39, 7.87) | <.001 | 2.33 (0.90, 6.04) | .0816 |
| Paternal age (y) | ||||
| <35 | Reference | Reference | ||
| ≥35, <40 | 1.45 (0.93, 2.28) | .1045 | 1.32 (0.74, 2.34) | .3503 |
| ≥40 | 2.46 (1.47, 4.14) | <.001 | 1.33 (0.57, 3.09) | .5057 |
| Type of infertility | ||||
| Primary | Reference | Reference | ||
| Secondary | 1.72 (1.19, 2.49) | .0041 | 1.44 (0.91, 2.28) | .1158 |
| Total AFC | ||||
| <10 | 2.23 (1.38, 3.62) | .0011 | 2.97 (1.38, 6.38) | .0054 |
| ≥10, <15 | Reference | |||
| ≥15 | 1.22 (0.76, 1.95) | .4173 | 1.10 (0.64, 1.92) | .7242 |
| Total Gn dose (IU) | ||||
| <3150.73 | Reference | Reference | ||
| ≥3150.73 | 1.60 (1.10, 2.33) | .0147 | 1.34 (0.67, 2.69) | .4062 |
| E2/P | ||||
| <1.1 | Reference | Reference | ||
| ≥1.1 | 0.45 (0.26, 0.78) | .0043 | 0.51 (0.28, 0.91) | .0225 |
| Number of metaphase-2 oocytes | ||||
| <7 | Reference | Reference | ||
| ≥7 | 0.61 (0.40, 0.94) | .0252 | 0.82 (0.45, 1.48) | .5016 |
| Serum hCG level (IU/L) | ||||
| <591.7 | Reference | Reference | ||
| ≥591.7 | 0.19 (0.12, 0.28) | <.001 | 0.44 (0.21, 0.92) | .0304 |
AFC = total antral follicle count, CI = confidence interval, COH = controlled ovarian hyperstimulation, Gn = gonadotropin, hCG = human chorionic gonadotropin, IVF/ICSI = in vitro fertilization/intracytoplasmic sperm injection, OR = odds ratio.
Figure 1.
(A) Multivariate-adjusted smoothing spline plots of early pregnancy loss rate by the total AFC. (B) Multivariate-adjusted smoothing spline plots of early pregnancy loss rate by E2/P. (C) Multivariate-adjusted smoothing spline plots of pregnancy loss rate by the serum hCG level. The red lines represent the smooth curve fits between variables. Analyses were adjusted for maternal age, paternal age, infertility type, total Gn dose, and number of metaphase-2 oocytes. AFC = antral follicle count, E2/P = estradiol/progesterone ratio, hCG = human chorionic gonadotropin.
Patients with E2/P ≥ 1.1 had significantly lower odds of EPL than women with E2/P < 1.1 (OR 0.51, 95% CI 0.28–0.91, P < .05). Additionally, smooth curve fitting clearly showed a linear independent association between E2/P and EPL risk (Fig. 1B). No threshold or saturation effect was observed in this association. With increasing E2/P, the EPL risk decreases linearly.
Serum hCG levels were negatively correlated with EPL. As shown in Figure 1C, the smoothing curve showed a nonlinear relationship between the EPL risk and serum hCG level. Using a 2-piecewise regression model (Table 4), the inflection point was 599.9 IU/L. On the left of the inflection point, the serum hCG level negatively correlated with EPL risk (OR 0.9945, 95% CI 0.9933–0.9958, P < .0001). By contrast, there was no correlation between the serum hCG level and EPL risk on the right of the inflection point (OR 0.9998, 95% CI 0.9993–1.0003, P = .4293).
Table 4.
Threshold effect analysis of serum hCG level on EPL using piecewise linear regression.
| Models | Risk of EPL | |
|---|---|---|
| Adjusted OR(95% CI) | P | |
| Model I | ||
| One line slope | 0.9981 (0.9976, 0.9986) | <.001 |
| Model II | ||
| Inflection point (K) | 599.9 | |
| Slope 1: hCG < 599.9 IU/L | 0.9945 (0.9933, 0.9958) | <.001 |
| Slope 2: hCG ≥ 599.9 IU/L | 0.9998 (0.9993, 1.0003) | .429 |
| Slope 2-Slope 1 | 1.0053 (1.0037, 1.0069) | <.001 |
| A log likelihood ratio test | <.001 | |
Adjusted for maternal age, paternal age, type of infertility, total Gn dose, number of metaphase-2 oocytes.
CI = confidence interval, early pregnancy loss, EPL, hCG = human chorionic gonadotropin, OR = odds ratio.
4. Discussion
This retrospective cohort study focused on the risk factors of EPL throughout fresh IVF/ICSI cycles, including 954 clinical pregnancies, 132 of which had EPL. The main findings of this study were that total AFC, E2/P, and serum hCG levels were associated with a high EPL risk in the ART/ICSI population.
Studies addressing factors related to EPL in fresh IVF/ICSI have reported varying results. Sunkara demonstrated that the pregnancy loss rate was higher with advanced age, secondary infertility, and female-related infertility than with younger age, male-related infertility, and unexplained cause.[10] However, an earlier study that analyzed 1196 pregnancies found that the effects of age, obesity, and other risk factors were not significant in the logistic regression analysis.[8] The adverse effects of age on EPL risk have been reported in many studies.[10–12] This may be attributed to oocyte aneuploidy, oocyte deterioration, and endocrine variations that occur with advanced age. This study also indicated that paternal and maternal age was significantly different between the ongoing pregnancy and EPL groups. However, in the logistic regression analysis after adjusting for confounders, the differences were no longer significant.
AFC is an ultrasound measure of pretreatment small antral follicles in both the ovaries. This is a good predictor of ovarian reserve during ART. Therefore, the AFC measurement is recommended at the beginning of a cycle.[13] Studies on AFC in the EPL are limited. Keane et al[14] reported that pregnancy loss rates appeared to be dependent on AFC, but this was not significant (P > .05). Our study demonstrated a nonlinear relationship between AFC and EPL; therefore, further work is necessary.
COH produces P and supra-physiological levels of E2, both of which alter oocyte quality, endometrial function, or both and ultimately influence IVF/ICSI outcomes. Endometrial receptivity is regulated by complex interactions between E2 and P. Supra-physiological steroid hormone levels alter endometrial E2/P ratios and subsequently lead to impaired endometrial receptivity. Few studies have evaluated the effect of E2/P on IVF/ICSI outcomes, including clinical pregnancy,[15] ongoing pregnancy,[16] and live birth rates.[16,17] However, to the best of our knowledge, no study has evaluated the association between E2/P and EPL. We observed a negative and strong relationship between E2/P and EPL risk. Besides, the result of the smooth curve fitting suggested that the association was linear in the whole range of E2/P. In other words, the risk of EPL decreases proportionally with the increase in the E2/P ratio, without any saturation or threshold effect. Hence, our study may be an important addition to this field, but further studies are needed to strengthen this finding.
HCG is a key factor in embryo implantation in the first trimester of pregnancy. In early pregnancy, hCG is secreted primarily by trophoblasts and performs multiple roles in the establishment of pregnancy, including the promotion of angiogenesis,[18] decidualization,[19] maternal immune tolerance at the maternal–fetal interface,[20] and trophoblast invasion.[21] Previous studies have established an association between serum hCG levels and EPL. Hu et al observed that the hCG level 14 days after transfer was significantly higher in the live birth group than in the miscarriage group, especially EPL, but the cutoff value of prediction was not determined.[7] Zhang et al[22] reported that the serum β-hCG level of live births was significantly higher than that of spontaneous miscarriage (596.8 IU/L vs 357.15 IU/L; P < .001). Those were consistent with our study, but a definite cutoff value for EPL prediction remains unestablished. In our study, we found that hCG was negatively related to EPL risk when hCG was < 599.9 IU/L. However, the potential mechanism linking hCG and EPL should be considered in the future.
This study had some limitations. First, this study was limited by the retrospective nature of the analysis. Second, although multiple pregnancies were excluded in this study, multiple ETs were still included, which may have interfered with the outcome. However, we believe that these data will be of interest to the clinicians.
5. Conclusion
In conclusion, risk factors associated with EPL in fresh IVF/ICSI cycles were identified through a retrospective study. Lower AFC, E2/P, and serum hCG levels were associated with a higher EPL risk. Hopefully, these findings may offer some suggestions for the population at risk of EPL and contribute to future basic studies on its etiology.
Acknowledgments
We thank all participants of this study and the staff of the Reproductive Medicine Center from the First Hospital of Lanzhou University for their help with technical assistance and information service.
Author contributions
Conceptualization: Liyan Wang and Xuehong Zhang
Data curation: Lin Wang, Panpan Jin, and Rui Zhang
Formal analysis: Liyan Wang, Rui Zhang, and Yanbiao Jiang
Software: Liyan Wang
Writing—original draft: Liyan Wang
Writing—review and editing: Xia Yang and Xuehong Zhang
Abbreviations:
- AFC =
- antral follicle count
- ART =
- assisted reproductive technology
- BMI =
- body mass index
- CA-125 =
- carbohydrate antigen 125
- COH =
- controlled ovarian hyperstimulation
- E2 =
- estradiol
- E2/P =
- estradiol/progesterone ratio
- EPL =
- early pregnancy loss
- ET =
- embryo transfer
- FSH =
- follicle-stimulating hormone
- Gn =
- gonadotropin
- hCG =
- human chorionic gonadotropin
- ICSI =
- intracytoplasmic sperm injection
- IVF =
- in vitro fertilization
- LH =
- luteinizing hormone
- PRL =
- prolactin
- SD =
- standard deviation
- TSH =
- thyroid-stimulating hormone
How to cite this article: Wang L, Wang L, Yang X, Jin P, Zhang R, Jiang Y, Zhang X. Risk factors related to early pregnancy loss in fresh IVF/ICSI: An analysis of 954 embryo transfer cycles. Medicine 2022;101:34(e30166).
This work was supported by the Gansu Youth Science and Technology Project (2019-0406-JCC-0138), Gansu TCM Project (GZK-2018-45), and the First Hospital of Lanzhou University Fund (ldyyn2020-4).
The authors of this work have nothing to disclose.
All the data will be available upon motivated request to the corresponding author of the present paper. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Kawwass JF, Badell ML. Maternal and fetal risk associated with assisted reproductive technology. Obstet Gynecol. 2018;132:1. [DOI] [PubMed] [Google Scholar]
- [2].Kushnir VA, Barad DH, Albertini DF, et al. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. 2017;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gravino G, Caruana-Finkel L. Abortion and methods of reproductive planning: the views of Malta’s medical doctor cohort. Sex Reprod Health Matters. 2019;27:1683127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165:1380–8. [DOI] [PubMed] [Google Scholar]
- [5].Li G, Jin H, Niu W, et al. Effect of assisted reproductive technology on the molecular karyotype of missed abortion tissues. Biosci Rep. 2018;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qin JZ, Pang LH, Li MQ, et al. Risk of chromosomal abnormalities in early spontaneous abortion after assisted reproductive technology: a meta-analysis. PLoS One. 2013;8:e75953e75953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu L, Du J, Lv H, et al. Influencing factors of pregnancy loss and survival probability of clinical pregnancies conceived through assisted reproductive technology. Reprod Biol Endocrinol. 2018;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Winter E, Wang J, Davies MJ, et al. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17:3220–3. [DOI] [PubMed] [Google Scholar]
- [9].Hipp H, Crawford S, Kawwass JF, et al. First trimester pregnancy loss after fresh and frozen in vitro fertilization cycles. Fertil Steril. 2016;105:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sunkara SK, Khalaf Y, Maheshwari A, et al. Association between response to ovarian stimulation and miscarriage following IVF: an analysis of 124 351 IVF pregnancies. Hum Reprod. 2014;29:1218–24. [DOI] [PubMed] [Google Scholar]
- [11].Yi Y, Lu G, Ouyang Y, et al. A logistic model to predict early pregnancy loss following in vitro fertilization based on 2601 infertility patients. Reprod Biol Endocrinol. 2016;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang AM, Xu X, Han Y, et al. Risk factors for different types of pregnancy losses: analysis of 15,210 pregnancies after embryo transfer. Front Endocrinol (Lausanne). 2021;12:683236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ersahin AA, Arpaci H, Ersahin SS, et al. AFC vs. AMH: prediction of ovarian response in women with endometrioma undergoing controlled ovarian stimulation. Eur Rev Med Pharmacol Sci. 2017;21:2499–503. [PubMed] [Google Scholar]
- [14].Keane K, Cruzat VF, Wagle S, et al. Specific ranges of anti-Mullerian hormone and antral follicle count correlate to provide a prognostic indicator for IVF outcome. Reprod Biol. 2017;17:51–9. [DOI] [PubMed] [Google Scholar]
- [15].Arora R, Chan C, Ye XY, et al. Progesterone, progesterone/estradiol and ART outcomes in day-5 transfer cycles. Gynecol Endocrinol. 2018;34:59–63. [DOI] [PubMed] [Google Scholar]
- [16].Cetinkaya ES, Berker B, Aytac R, et al. The value of the progesterone-to-estradiol ratio on the day of hCG administration in predicting ongoing pregnancy and live birth rates in normoresponders undergoing GnRH antagonist cycles. Eur J Obstet Gynecol Reprod Biol. 2013;170:452–7. [DOI] [PubMed] [Google Scholar]
- [17].Golbasi H, Ince O, Golbasi C, et al. Effect of progesterone/estradiol ratio on pregnancy outcome of patients with high trigger-day progesterone levels undergoing gonadotropin-releasing hormone antagonist intracytoplasmic sperm injection cycles: a retrospective cohort study. J Obstet Gynaecol. 2019;39:157–63. [DOI] [PubMed] [Google Scholar]
- [18].Zygmunt M, Herr F, Keller-Schoenwetter S, et al. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–6. [DOI] [PubMed] [Google Scholar]
- [19].Makrigiannakis A, Vrekoussis T, Zoumakis E, et al. The role of HCG in implantation: a mini-review of molecular and clinical evidence. Int J Mol Sci . 2017;18:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schumacher A. Human chorionic gonadotropin as a pivotal endocrine immune regulator initiating and preserving fetal tolerance. Int J Mol Sci . 2017;18:2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zygmunt M, McKinnon T, Herr F, et al. HCG increases trophoblast migration in vitro via the insulin-like growth factor-II/mannose-6 phosphate receptor. Mol Hum Reprod. 2005;11:261–7. [DOI] [PubMed] [Google Scholar]
- [22].Zhang Q, Yan J, Tang R, et al. Serum human chorionic gonadotropin level measured 17 days after oocyte retrieval can predict final clinical pregnancy outcomes in IVF/ICSI treatment cycles. J Reprod Med. 2014;59:285–92. [PubMed] [Google Scholar]