Abstract
Background
Little is known about the joint associations of multiple lifestyle risk factors including smoking, low body mass index, physical inactivity, alcohol consumption, and low diet quality with risk of active tuberculosis.
Methods
We analyzed data from the Singapore Chinese Health Study, a prospective cohort study of 63 257 Chinese adults aged 45–74 years enrolled between 1993 and 1998. Incident cases of active tuberculosis were identified via linkage with the National TB Registry through 31 December 2016. Cox proportional hazards regression models were used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) of tuberculosis risk in relation to the combined scores of lifestyle risk factors.
Results
Compared with participants with none of the risk factors, the adjusted HRs (95% CI) of active tuberculosis for participants with 1, 2, 3, 4, and 5 risk factors were 1.24 (1.02–1.51), 1.84 (1.51–2.23), 2.52 (2.03–3.14), 4.07 (3.07–5.41), and 9.04 (5.44–15.02), respectively (Ptrend < .0001). The HR for those with 5 factors was ~1.5 times the product of individual risk estimates from the 5 factors on a multiplicative scale. The stepwise increase in risk of active tuberculosis with increasing number of lifestyle risk factors was significantly stronger in participants with diabetes than their counterparts without diabetes at recruitment (Pinteraction = .01).
Conclusions
Multiple lifestyle risk factors were associated with risk of active tuberculosis in a synergistic manner. Our findings highlight the importance of public health programs and interventions targeting these factors simultaneously to reduce the tuberculosis burden among the general population.
Keywords: tuberculosis, lifestyle factors, cohort
In the Singapore Chinese Health Study with a mean follow-up of 18.2 years, we found that increasing numbers of lifestyle risk factors were linearly and significantly associated with risk of active tuberculosis, especially among those with diabetes.
Tuberculosis, caused by Mycobacterium tuberculosis, is one of the top global causes of death from an infectious disease [1]. It is estimated that up to one-quarter of the global population are infected with M. tuberculosis and approximately 5–15% of the infected individuals will develop active tuberculosis over their lifetime [2, 3]. Even though the rate of active tuberculosis is much higher in specific groups, such as those on immunosuppressants or with human immunodeficiency virus (HIV) infection [4], as well as patients with comorbidities like diabetes or immunocompromised disorders [5], the risk of progression from latent infection to active disease in the general population is affected by factors such as cigarette smoking [6], alcohol consumption [7], low body mass index (BMI) [8], physical inactivity [9], and malnutrition [10].
These aforementioned factors are widely referred to in the literature as “lifestyle factors that can influence health” [11], and individual associations between these factors and risk of active tuberculosis have been extensively studied in different populations [6–9]. Previously, dyads of lifestyle risk factors have been shown to be related with active tuberculosis, and the risks ranged from 1.25 times for the smoking/alcohol combination to 6.43 times for the combination of diabetes and low BMI [12]. In the Singapore Chinese Health Study, we have previously reported that concurrent habits of heavy drinking and smoking were associated with a considerably higher risk of tuberculosis [13]. To the best of our knowledge, no epidemiological study has explored the joint associations of multiple lifestyle risk factors with risk of active tuberculosis. Moreover, lifestyle factors are typically clustered and may exert synergic or antagonistic effects in the development of active tuberculosis [14]. Therefore, understanding the associations of combined lifestyle risk factors with tuberculosis risk may be more informative for translating epidemiological findings to meaningful prevention strategies.
To address the research gaps, we aimed to examine the joint associations of multiple lifestyle factors, including smoking, BMI, physical activity, alcohol consumption, and diet quality, with risk of active tuberculosis using data from the Singapore Chinese Health Study cohort. A unique feature of this cohort is that our participants were born in the first half of the 20th century, a period when tuberculosis was highly prevalent in Singapore, and those who had acquired latent tuberculosis infection in those early years could be at risk of disease reactivation at an advanced age, which makes our cohort suitable for exploring factors associated with active tuberculosis in those with latent infection [15].
METHODS
Study Population
The design of the Singapore Chinese Health Study has been previously described [16]. Briefly, the cohort recruited 63 257 Chinese adults aged 45–74 years from 2 major dialect groups (Hokkien and Cantonese) between April 1993 and December 1998. Enrolled individuals were citizens or permanent residents of Singapore residing in government housing flats, where 86% of Singaporeans lived during the period of recruitment. At baseline, trained interviewers collected the information on demographics, anthropometrics, physical activity, smoking history, alcohol intake, habitual diet, and history of self-reported physician-diagnosed medical conditions including diabetes, hypertension, coronary heart disease, stroke, and cancer. In the second follow-up interview conducted between 2006 and 2010, an average of 12.7 years after recruitment, 39 528 participants updated information on their BMI, smoking, alcohol drinking, physical activity, and comorbid conditions. The Institutional Review Boards at the National University of Singapore approved this study, and all study participants gave informed consent.
Assessment of Individual Lifestyle Factors
All lifestyle factors of research interest were collected in the baseline survey and updated at the second follow-up interview, except for dietary data, which was only collected at baseline. Detailed assessment of 5 factors are provided in the Supplementary Methods. Briefly, cigarette smoking was determined by asking whether the participants had ever smoked at least 1 cigarette per day for 1 year or longer; for alcohol consumption, participants were asked in separate questions about their consumption of beer, wine, Western hard liquor, and Chinese hard liquor, and to choose from 8 categories of frequency (ranging from “never or hardly ever” to “2 or more times a day”) and 4 defined portion sizes. BMI was calculated using weight (kg) divided by height squared (m2). Physical activity was assessed by asking participants the number of hours per week engaged in moderate activities and strenuous sports. Habitual diet was assessed using a validated 165-item, semi-quantitative food-frequency questionnaire [16].
We have previously published that marine n–3 and n–6 fatty acids [17], as well as antioxidant vitamins and carotenoids from vegetables and fruits [18], were associated with a lower risk of active tuberculosis in this Singapore Chinese cohort. Consistent with our previous findings, we found that, after adjusting for energy intake using the residual method [19], lower intakes of fish/shellfish and vegetables/fruits (below median intake levels of the cohort) were independently associated with higher risk of active tuberculosis (Supplementary Table 1). As dietary guidelines are generally based on foods rather than nutrients [20], diet quality was assessed by incorporating intake of fish/shellfish and vegetables/fruits in the current analysis. Participants with intake levels lower than the median intake levels of both fish/shellfish and vegetables/fruits in this cohort were considered to have a low diet quality.
Computation of Combined Lifestyle Factor Score
We selected the 5 factors of cigarette smoking, BMI, physical activity, alcohol consumption, and dietary quality to create a combined lifestyle factor score on the basis of previously published work that had examined the impact of these risk factors on active tuberculosis [6–9, 13, 21]. The at-risk factors were defined as current smoking, underweight, physical inactivity, daily alcohol drinking, and low diet quality. Underweight was defined as BMI less than 20.0 kg/m2 [22]. Physical inactivity was categorized as less than 2 hours per week of any moderate activity or less than 0.5 hour per week of strenuous activity [23]. Low diet quality was identified as low intake for both fish/shellfish and vegetables/fruits. Each risk factor was coded as 1 (risk) or 0 (referent). The combined lifestyle factor score was the sum of the 5 individual risk factors, and ranged from 0 to 5, with a higher score indicating a higher number of risk factors. Among 37 596 participants who were free of tuberculosis when they attended the interviews at follow-up 2, we used the baseline diet plus updated information on the other 4 risk factors to construct an updated risk score.
Case Ascertainment and Follow-up
Active tuberculosis cases were identified through linkage of the cohort database with the National TB Notification Registry [24]. The notification of tuberculosis cases in Singapore is mandatory by law, and all suspected and confirmed tuberculosis cases must be reported to the Ministry of Health within 72 hours of the initial treatment for tuberculosis and/or the observation of laboratory-confirmed results. By law, all deaths in Singapore must be registered within 24 hours with the Singapore Registry of Births and Deaths. Hence, we had complete and valid information about death in this cohort via annual linkage with this registry. We updated information on migration in this cohort through regular recontact of our participants in follow-up interviews. However, migration was a rare event in this cohort, and only 52 participants were known to be lost to follow-up so far.
Statistical Analysis
A total of 60 245 participants (25 914 men; 43.0%) were included in the current analysis after excluding the prevalent tuberculosis cases (n = 3012) at baseline. Baseline characteristics of the participants were examined across the risk score. Participants were followed up until the diagnosis of active tuberculosis or until the occurrence of death, loss to follow-up, or 31 December 2016, whichever was earlier. Event-free survival probability by the number of risk factors at baseline was evaluated using the Kaplan-Meier method. We used Cox proportional hazards regression models to generate corresponding hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of individual risk factors and combined score with risk of active tuberculosis. The Schoenfeld residuals method was used to test the proportional hazards assumption of the Cox models and no violation was detected.
In the multivariable models, we adjusted for the following potential confounders: age at recruitment (years), year of recruitment (1993–1995, 1996–1998), sex, dialect group (Hokkien, Cantonese), level of education (no formal education, primary school, secondary school or higher), and daily energy intake (kcal/day). We also included tea intake (none, monthly, weekly, daily) in model 1 since we have previously published the inverse association between tea drinking and risk of active tuberculosis [25]. We further adjusted for history of physician-diagnosed diabetes, hypertension, coronary heart disease, stroke, and cancer in model 2. When analyzing the association between individual risk factor and risk of active tuberculosis, the other risk factors were also mutually adjusted in the models. The Ptrend was based on a test for linear trend using the score as an ordinal variable in the model. We then repeated the analysis by including combined risk score and comorbid conditions as time-varying covariates in the Cox regression model [26]. We also conducted stratified analyses by age at baseline (≥65 vs <65 years), sex (men vs women), and baseline diabetes status (yes vs no) in order to investigate the potential effect modification by these factors. In these analyses, we merged combined scores of 4 and 5 as 1 category due to the relatively small sample size of these 2 groups.
In addition, we examined the risk estimates of active tuberculosis according to possible combinations of risk factors. We excluded alcohol consumption in this analysis due to its relatively low prevalence in this cohort. The other 4 dichotomized risk factors yielded 16 combinations, and we used participants who had no risk factors as the reference group. We investigated for interaction between pairs of risk factors by including a product term comprising each pair of risk factors in Cox regression models. In addition, we assigned polytomous weights to each factor in the combined risk factor score and repeated the analyses (see Supplementary Methods).
Analyses were conducted using SAS software version 9.4 (SAS Institute). All of the P values quoted were 2-sided, and P < .05 was considered statistically significant.
RESULTS
During a mean (standard deviation [SD]) follow-up of 18.2 (5.9) years, 1358 incident cases of active tuberculosis were identified in our cohort. The sex-standardized and age-specific incidence rates of tuberculosis within this cohort were 90.0, 164.5, 247.4, and 260.0 per 100 000 person-years among participants aged less than 60 years, 60–65 years, 65–70 years, and 70 years and older, respectively. The mean age at tuberculosis diagnosis was 69.4 years (SD, 9.2 years). The proportions of participants with 0, 1, 2, 3, 4, and 5 risk factors were 14.7%, 47.3%, 27.8%, 8.4%, 1.9%, and 0.2%, respectively. At baseline, participants with a higher combined score were older, more likely to be men, less educated, have a lower BMI, and a lower frequency of tea intake (Table 1).
Table 1.
Baseline Characteristics of Participants by Combined Lifestyle Factor Score: The Singapore Chinese Health Study
| Combined Lifestyle Factor Score | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total | 0 | 1 | 2 | 3 | 4 | 5 |
| Number of participants | 60 245 (100.0%) | 8872 (14.7%) | 28 465 (47.3%) | 16 736 (27.8%) | 5039 (8.4%) | 1026 (1.7%) | 107(0.2%) |
| Number with active tuberculosis | 1358 (2.3%) | 132 (1.5%) | 441 (1.6%) | 464 (2.7%) | 225 (4.5%) | 79 (7.7%) | 17 (15.9%) |
| Men | 25 914 (43.0%) | 4411 (49.7%) | 9401 (33.0%) | 7841 (46.9%) | 3335 (66.2%) | 829 (80.8%) | 97 (90.7%) |
| Age at interview, y | 56.4 ± 8.0 | 55.5 ± 7.8 | 55.9 ± 7.9 | 57.1 ± 8.1 | 57.9 ± 8.1 | 58.5 ± 7.9 | 56.9 ± 7.8 |
| Dialect | |||||||
| Cantonese | 27 977 (46.4%) | 4412 (49.7%) | 13 449 (47.3%) | 7515 (44.9%) | 2145 (42.6%) | 410 (40.0%) | 46 (43.0%) |
| Hokkien | 32 268 (53.6%) | 4460 (50.3%) | 15 016 (52.8%) | 9221 (55.1%) | 2894 (57.4%) | 616 (60.0%) | 61 (57.0%) |
| Education | |||||||
| No formal education | 16 610 (27.6%) | 1620 (18.3%) | 8264 (29.0%) | 5005 (29.9%) | 1410 (28.0%) | 288 (28.1%) | 23 (21.5%) |
| Primary school (1–6 y) | 26 516 (44.0%) | 3517 (39.6%) | 12 318 (43.3%) | 7625 (45.6%) | 2473 (49.1%) | 525 (51.2%) | 58 (54.2%) |
| Secondary school and above | 17 119 (28.4%) | 3735 (42.1%) | 7883 (27.7%) | 4106 (24.5%) | 1156 (22.9%) | 213 (20.7%) | 26 (24.3%) |
| BMI, kg/m2 | 23.2 ± 3.3 | 24.0 ± 2.8 | 23.8 ± 3.1 | 22.5 ± 3.4 | 21.0 ± 3.3 | 19.3 ± 2.4 | 18.2 ± 1.3 |
| Total energy intake, kcal/d | 1551.9 ± 563.9 | 1626.8 ± 574.7 | 1512.5 ± 538.2 | 1552.7 ± 572.1 | 1613.4 ± 616.6 | 1658.4 ± 635.5 | 1795.3 ± 656.0 |
| Percentage reporting risk factora | |||||||
| Current smoker | 11 376 (18.9%) | NA | 1610 (5.7%) | 5210 (31.1%) | 3463 (68.7%) | 1093 (96.5%) | 107 (100%) |
| Underweight | 8916 (14.8%) | NA | 1319 (4.6%) | 4208 (25.1%) | 2486 (49.3%) | 903 (79.7%) | 107 (100%) |
| Physical inactivity | 43 469 (72.2%) | NA | 22 868 (80.3%) | 14 890 (89.0%) | 4630 (91.9%) | 974 (94.9%) | 107 (100%) |
| Daily alcohol consumption | 2030 (3.4%) | NA | 181 (0.6%) | 617 (3.7%) | 702 (13.9%) | 423 (41.2%) | 107 (100%) |
| Low diet quality | 15 902 (26.4%) | NA | 2487 (8.7%) | 8547 (51.1%) | 3836 (76.1%) | 925 (90.2%) | 107 (100%) |
| Tea intake | |||||||
| None | 24 859 (41.3%) | 2807 (31.6%) | 11 688 (41.1%) | 7542 (45.1%) | 2268 (45.0%) | 498 (48.5%) | 56 (52.3%) |
| Monthly | 7275 (12.1%) | 1024 (11.5%) | 3538 (12.4%) | 2040 (12.2%) | 558 (11.1%) | 108 (10.5%) | 7 (6.5%) |
| Weekly | 14 705 (24.4%) | 2576 (29.0%) | 7039 (24.7%) | 3758 (22.5%) | 1117 (22.2%) | 194 (18.9%) | 21 (19.6%) |
| Daily | 13 406 (22.3%) | 2465 (27.8%) | 6200 (21.8%) | 3396 (20.3%) | 1096 (21.8%) | 226 (22.0%) | 23 (21.5%) |
| History of chronic disease | |||||||
| Diabetes | 5401 (8.9%) | 805 (9.1%) | 2774 (9.8%) | 1453 (8.7%) | 329 (6.5%) | 35 (3.4%) | 5 (4.7%) |
| Hypertension | 14 493 (24.1%) | 2448 (27.6%) | 7617 (26.8%) | 3534 (21.1%) | 775 (15.4%) | 106 (10.3%) | 13 (12.2%) |
| Coronary heart disease | 2463 (4.1%) | 380 (4.3%) | 1200 (4.2%) | 675 (4.0%) | 175 (3.5%) | 30 (2.9%) | 3 (2.8%) |
| Stroke | 903 (1.5%) | 114 (1.3%) | 424 (1.5%) | 265 (1.6%) | 84 (1.7%) | 14 (1.4%) | 2 (1.9%) |
| Cancer | 823 (1.4%) | 108 (1.2%) | 407 (1.4%) | 216 (1.3%) | 70 (1.4%) | 18 (1.8%) | 4 (3.7%) |
Data are n (%) or mean ± standard deviation unless specified otherwise.
Abbreviations: BMI, body mass index; NA, not applicable.
Underweight was defined as BMI <20.0 kg/m2; physical inactivity was defined as moderate activity < 2 hours/week or strenuous activity <0.5 hour/week; low diet quality was defined as below the median intake levels of both fish/shellfish and vegetables/fruits.
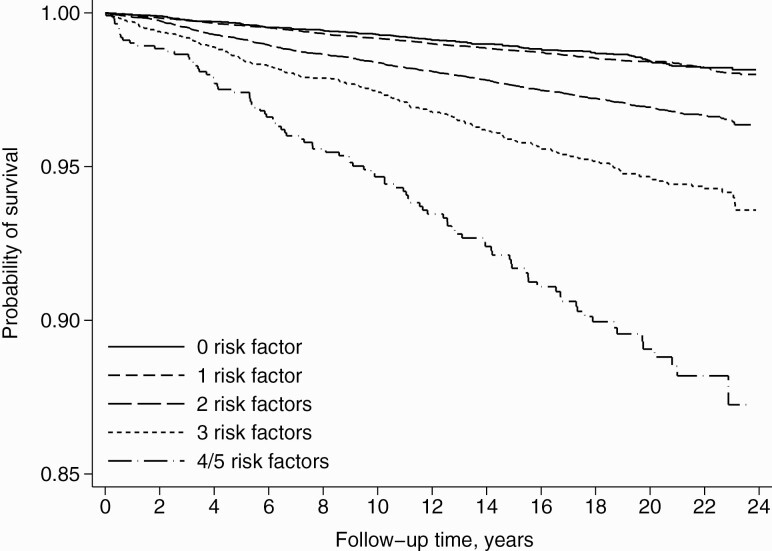
Each risk factor showed an independent association with a higher risk of active tuberculosis (Table 2 and Supplementary Table 2). In the Kaplan-Meier survival curves, increasing risk factor score was associated with decreasing survival (P for log-rank test < .0001) (Figure 1). After fully adjusting for potential confounders, compared with participants with 0 risk factors, the multivariate-adjusted HRs (95% CI) were 1.24 (1.02–1.51), 1.84 (1.51–2.23), 2.52 (2.03–3.14), 4.07 (3.07–5.41), and 9.04 (5.44–15.02) for those with 1, 2, 3, 4, and 5 risk factors, respectively (Ptrend < .0001). Assuming a multiplicative scale, the expected HR from the product of the individual association of the 5 risk factors was 5.94 (Table 2). Hence, the observed HR (95% CI) of 9.04 (5.44–15.02) from the combined effect of all 5 factors was approximately 1.5 times the expected HR.
Table 2.
Associations of Individual and Combined Risk Factors With Active Tuberculosis Risk: The Singapore Chinese Health Study
| HR (95% CI) | |||
|---|---|---|---|
| Cases/n | Model 1 | Model 2 | |
| Smoking status | |||
| Nonsmoking | 787/48 869 | 1.00 | 1.00 |
| Current smoking | 571/11 376 | 2.01 (1.78–2.26) | 1.96 (1.73–2.20) |
| BMI | |||
| ≥20.0 kg/m2 | 1036/51 329 | 1.00 | 1.00 |
| <20.0 kg/m2 | 322/8916 | 1.73 (1.53–1.97) | 1.67 (1.46–1.89) |
| Physical activitya | |||
| Active | 385/16 776 | 1.00 | 1.00 |
| Inactive | 973/43 469 | 1.15 (1.02–1.30) | 1.15 (1.02–1.30) |
| Alcohol consumption | |||
| Less than daily | 1258/58 215 | 1.00 | 1.00 |
| Daily drinking | 100/2030 | 1.36 (1.10–1.68) | 1.35 (1.09–1.66) |
| Diet qualityb | |||
| Hight diet quality | 879/44 343 | 1.00 | 1.00 |
| Low diet quality | 479/15 902 | 1.17 (1.05–1.31) | 1.17 (1.04–1.31) |
| Combined lifestyle factor score | |||
| 0 | 132/8872 | 1.00 | 1.00 |
| 1 | 441/28 465 | 1.24 (1.02–1.51) | 1.24 (1.02–1.51) |
| 2 | 464/16 736 | 1.86 (1.53–2.26) | 1.84 (1.51–2.23) |
| 3 | 225/5039 | 2.54 (2.05–3.16) | 2.52 (2.03–3.14) |
| 4 | 79/1026 | 4.08 (3.07–5.41) | 4.07 (3.07–5.41) |
| 5 | 17/107 | 9.39 (5.65–15.58) | 9.04 (5.44–15.02) |
| Ptrendc | <.0001 | <.0001 | |
The covariates in the model 1 included sex, age at recruitment, year of recruitment (1993–1995, 1996–1998), dialect group (Hokkien, Cantonese), level of education (no formal education, primary school, secondary school or higher), tea intake (none, monthly, weekly, daily), and total energy intake. In the analysis of individual risk factors, they were mutually adjusted in the model. Model 2 further adjusted for self-reported diabetes, hypertension, coronary heart disease, stroke, and cancer.
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Physical activity was defined as moderate activity ≥2 hours/week or strenuous activity ≥0.5 hour/week; physical inactivity was defined as moderate activity <2 hours/week or strenuous activity <0.5 hour/week.
High diet quality was defined as at or above the median intake levels of either fish/shellfish or vegetables/fruits, or at or above the median intake levels of both; low diet quality was defined as below the median intake levels of both fish/shellfish and vegetables/fruits.
P trend was based on a test for linear trend using the combined risk factor score as an ordinal variable in the model.
Figure 1.
Kaplan-Meier survival curves for active tuberculosis stratified by the number of risk factors at baseline.
We updated risk factor scores using data from interviews at follow-up 2 for 37 596 participants and found that 96% of these individuals had either no change in score or only had a 1-point difference between their baseline score and score at follow-up 2. In addition, the analysis with time-varying covariates yielded similar results as the main analysis that used baseline data only. Compared with those with 0 risk factors, the HRs (95% CI) were 1.18 (0.97–1.44), 1.82 (1.50–2.21), 2.51 (2.01–3.12), 4.03 (3.03–5.36), and 8.12 (4.88–13.50) for those with 1, 2, 3, 4, and 5 risk factors, respectively (Ptrend < .0001).
The association was significantly stronger in participants with baseline diabetes compared with their counterparts without diabetes (Pinteraction = .01), and the HR (95% CI) of active tuberculosis with 4–5 risk factors versus 0 risk factors among participants with diabetes (9.14 [4.27–19.57]) was much higher than their counterparts (4.34 [3.25–5.79]) (Table 3). No significant interaction was found with age or sex.
Table 3.
Stratified Analyses of Combined Lifestyle Factor Score With Active Tuberculosis Risk by Sex, Age and Diabetes Status: The Singapore Chinese Health Study
| Combined Lifestyle Factor Score | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4/5 | P trend | |
| Stratified by sexa | P interaction = .719 | |||||
| Men | ||||||
| Cases/n | 100/4411 | 263/9401 | 341/7841 | 198/3335 | 88/926 | |
| HR (95% CI) | 1.00 | 1.19 (.95–1.50) | 1.82 (1.46–2.29) | 2.58 (2.02–3.29) | 4.47 (3.33–5.98) | <.0001 |
| Women | ||||||
| Cases/n | 32/4461 | 178/19 064 | 123/8895 | 27/1704 | 8/207 | |
| HR (95% CI) | 1.00 | 1.31 (.90–1.92) | 1.87 (1.26–2.77) | 2.12 (1.26–3.56) | 4.83 (2.20–10.59) | <.0001 |
| Stratified by age at baselineb | P interaction = .096 | |||||
| Age <65 y | ||||||
| Cases/n | 89/7445 | 319/23 515 | 317/13 115 | 167/3821 | 71/844 | |
| HR (95% CI) | 1.00 | 1.30 (1.02–1.64) | 1.94 (1.53–2.46) | 3.00 (2.31–3.90) | 5.32 (3.87–7.32) | <.0001 |
| Age ≥65 y | ||||||
| Cases/n | 43/1427 | 122/4950 | 147/3621 | 58/1218 | 25/289 | |
| HR (95% CI) | 1.00 | 1.05 (.74–1.49) | 1.61 (1.14–2.28) | 1.66 (1.11–2.48) | 3.20 (1.93–5.29) | <.0001 |
| Stratified by baseline diabetesc | P interaction = .011 | |||||
| Without baseline diabetes | ||||||
| Cases/n | 108/8067 | 362/25 691 | 409/15 283 | 210/4710 | 86/1093 | |
| HR (95% CI) | 1.00 | 1.25 (1.00–1.55) | 1.93 (1.56–2.39) | 2.67 (2.11–3.38) | 4.34 (3.25–5.79) | <.0001 |
| With baseline diabetes | ||||||
| Cases/n | 24/805 | 79/2774 | 55/1453 | 15/329 | 10/40 | |
| HR (95% CI) | 1.00 | 1.17 (.74–1.85) | 1.37 (.84–2.22) | 1.52 (.79–2.91) | 9.14 (4.27–19.57) | <.0001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
The covariates included age at recruitment, year of recruitment (1993–1995, 1996–1998), dialect group (Hokkien, Cantonese), level of education (no formal education, primary school, secondary school or higher), tea intake (none, monthly, weekly, daily), total energy intake, self-reported diabetes, hypertension, coronary heart disease, stroke, and cancer.
The covariates included sex, year of recruitment (1993–1995, 1996–1998), dialect group (Hokkien, Cantonese), level of education (no formal education, primary school, secondary school or higher), tea intake (none, monthly, weekly, daily), total energy intake, self-reported diabetes, hypertension, coronary heart disease, stroke, and cancer.
The covariates included sex, age at recruitment, year of recruitment (1993–1995, 1996–1998), dialect group (Hokkien, Cantonese), level of education (no formal education, primary school, secondary school or higher), tea intake (none, monthly, weekly, daily), total energy intake, self-reported hypertension, coronary heart disease, stroke, and cancer.
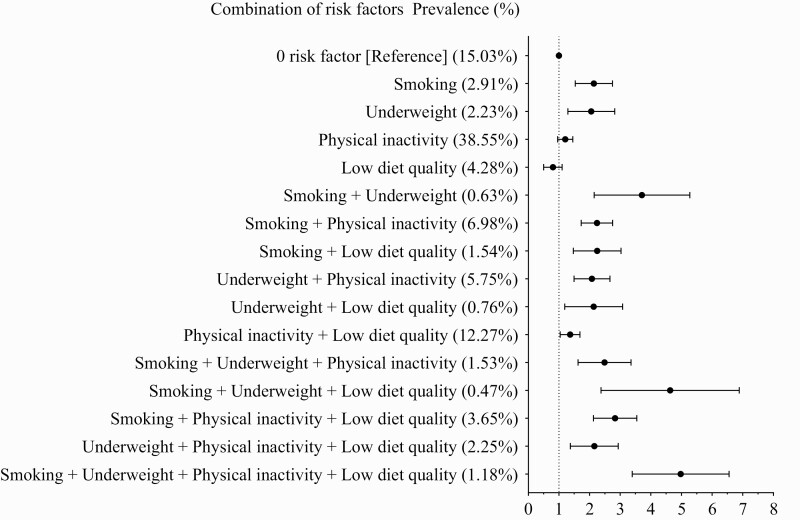
The distributions of 16 possible combinations from 4 risk factors (smoking, underweight, physical inactivity, and low diet quality) and their associations with risk of active tuberculosis are shown in Figure 2. We observed that the combination of all 4 factors (HR: 4.99; 95% CI: 3.69–6.74) was associated with the highest risk of active tuberculosis. Furthermore, we found significant interactions between smoking and alcohol drinking (Pinteraction = .02) and between smoking and poor diet quality (Pinteraction = .03), suggesting that the risk of active tuberculosis was synergistically increased in smokers who also drank alcohol or had a poor diet. Finally, the results were generally robust in the sensitivity analysis that assigned weighted points to each risk factor (Supplementary Table 3).
Figure 2.
Hazard ratios of active tuberculosis according to mutually exclusive combinations of risk factors (excluding alcohol consumption), the Singapore Chinese Health Study. The covariates included sex, age at recruitment, year of recruitment (1993–1995, 1996–1998), dialect group (Hokkien, Cantonese), level of education (no formal education, primary school, secondary school or higher), tea intake (none, monthly, weekly, daily), total energy intake, self-reported diabetes, hypertension, coronary heart disease, stroke, and cancer.
DISCUSSION
In this prospective cohort study of middle-aged and elderly Chinese adults, higher scores on risk factors, including cigarette smoking, underweight, physical inactivity, daily alcohol consumption, and low diet quality, were associated with a substantially increased risk of active tuberculosis. Compared with participants with 0 risk factors, those with 5 risk factors had a 9-fold increased risk of active tuberculosis, which was remarkedly higher than the product of the individual associations of these 5 factors on a multiplicative scale. Among those with 4–5 risk factors, the association was much stronger among participants with diabetes than those without diabetes at baseline.
Smoking and underweight have been identified as main risk factors for tuberculosis, especially in low- to middle-income countries [27]. A recent meta-analysis illustrated that current smokers experienced a 2.66-time higher risk of tuberculosis compared with never-smokers and there was a dose–response relationship for both intensity and duration of smoking with risk of tuberculosis [6]. Our study also showed that smoking was associated with approximately 2 times the risk of tuberculosis. In other studies, a log-linear inverse association was reported between BMI and tuberculosis risk, and a 1-unit increase in BMI was associated with a 13.8% lower risk of tuberculosis [8]. Similarly, our study showed that participants with BMI less than 20.0 kg/m2 had a considerably higher risk of active tuberculosis. In this study, we also found that daily alcohol drinking and physical inactivity were independently associated with increased tuberculosis risk, which concurred with previous studies that had also reported high alcohol consumption [7] and low level of physical exercise [9] to be associated with increased risk of tuberculosis. Although the associations of specific dietary factors with tuberculosis susceptibility have not been well established, our previous studies reported reduced risk of tuberculosis with increased dietary intake of vitamin A [18], vitamin C (among current smokers only) [18], and marine n–3 fatty acids [17], which were main components of vegetables, fruits, fish and shellfish, respectively. Furthermore, aligned with our findings (Supplementary Table 1), 2 previous studies also reported a positive association between inadequate intake of fruits and vegetables and tuberculosis risk [28, 29].
Our study suggested that smoking, as a strong risk factor for active tuberculosis, may interact with alcohol and poor diet quality to enhance the risk of active tuberculosis. It is well established that smokers tend to have a poor diet and drink more alcohol than nonsmokers [30]. Hence, improving dietary quality and reducing alcohol intake may have greater benefits in smokers, and public education programs on smoking cessation should also emphasize the importance of improving dietary quality and reducing alcohol intake as smokers quit the habit.
Our results provided unique insights into comprehensively understanding the association between multiple lifestyle factors and active tuberculosis risk in the general population. In particular, they have strong practical implication for public health in Asia, where many countries have the double burden of latent tuberculosis infection and increasing prevalence of diabetes, the latter being another important risk factor for tuberculosis [21, 31]. In our study, the co-existence of 4 or more risk factors augmented the risk of active tuberculosis substantially among individuals with diabetes, indicating that these risk factors might have a synergistic interaction with diabetes to further elevate the risk in this vulnerable group. Thus, lifestyle modifications are important and should be actively included in the management of patients with diabetes.
The strengths of this study include its prospective study design, large sample size, long duration of follow-up with minimal loss-to-follow-up rate, and stringent tuberculosis case ascertainment. In addition, the comprehensive collection of data on lifestyle, diet, and medical conditions enabled us to investigate multiple factors simultaneously in the same study population and to account for potential confounders. Furthermore, time-varying analysis with updated lifestyle risk factor scores further confirmed the robustness of the results. Nevertheless, several limitations in our study should be acknowledged. First, self-reported data in our study might introduce measurement errors. Second, since we could only adjust for education as a surrogate for socioeconomic status, the latter could still be a source of residual confounding. Third, we lacked information on HIV infection or other conditions related to immunodeficiency, although the prevalence of these conditions among Singaporeans was estimated to be very low [32]. Fourth, we used a simple dichotomization algorithm for each factor to compute the combined score. The advantage of this simple construction is that relevant findings may be easily applied to other populations. Nevertheless, our sensitivity analysis using a weighted score found similar results. Finally, caution should be taken when generalizing our estimates to other populations because of differences in the definition of these risk factors and other population characteristics.
Our results indicated that an increasing number of lifestyle risk factors was associated with a stepwise increase in risk of active tuberculosis in a prospective population-based cohort of Chinese men and women. These findings are particularly relevant for preventive strategies in the general population, considering the slow decline in tuberculosis incidence [33] and current limitations of effective strategies to control tuberculosis infection, such as availability of treatment or improvement in socioeconomic status and environmental factors, in countries with high tuberculosis burden [1]. Understanding the impact of combined risk factors on active tuberculosis risk further underscores the importance of multisectoral coordinated actions to promote smoking cessation, reduce underweight, avoid excessive alcohol use, increase physical activity, and improve diet quality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. A. P. and W.-P. K. contributed to the conception and design of the study. W.-P. K. and C. B. E. C. participated in the acquisition of data and analysis of data. H. Q. L. verified the data and drafted the manuscript. All authors were involved in the interpretation of the data, revising successive drafts of the manuscript, and approval of the final version. W.-P. K. has primary responsibility for final content.
Acknowledgments. The authors thank Ms. Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study. They also thank Dr. Jeffrey Cutter of the Ministry of Health and Dr. Kyi-Win Khin Mar of the National Tuberculosis Notification Registry in Singapore for assistance with the identification of tuberculosis cases in the cohort.
Disclaimer. The sponsors have no role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or in the decision to submit the article for publication.
Financial support. This work was supported by the US National Cancer Institute, National Institutes of Health (grant numbers UM1 CA182876, R01 CA144034). W.-P. K. is supported by the National Medical Research Council, Singapore (MOH-CSASI19nov-0001).
Contributor Information
Huiqi Li, Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Environment and Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China.
Cynthia B E Chee, Singapore Tuberculosis Control Unit, Tan Tock Seng Hospital, Singapore.
Tingting Geng, Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Environment and Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China.
An Pan, Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Environment and Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China; Key Laboratory of Systems Health Science of Zhejiang Province, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Hangzhou, Zhejiang Province, China.
Woon Puay Koh, Healthy Longevity Translational Research Program, Yong Loo Lin School of Medicine, National University of Singapore, Singaporeand; Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A∗STAR), Singapore.
References
- 1. World Health Organization. Global tuberculosis report 2021. Available at: https://www.who.int/publications/i/item/9789240037021. Accessed 26 October 2021.
- 2. Houben RM, Dodd PJ.. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med 2016; 13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Comstock GW, Livesay VT, Woolpert SF.. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 1974; 99:131–8. [DOI] [PubMed] [Google Scholar]
- 4. Corbett EL, Watt CJ, Walker N, et al. . The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003; 163:1009–21. [DOI] [PubMed] [Google Scholar]
- 5. Getahun H, Matteelli A, Abubakar I, et al. . Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015; 46:1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR.. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med 2007; 167:335–42. [DOI] [PubMed] [Google Scholar]
- 7. Simou E, Britton J, Leonardi-Bee J.. Alcohol consumption and risk of tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2018; 22:1277–85. [DOI] [PubMed] [Google Scholar]
- 8. Lonnroth K, Williams BG, Cegielski P, Dye C.. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–55. [DOI] [PubMed] [Google Scholar]
- 9. AlSharani F, Zafar M, Omer E, Al-Modeer MA, Abumelhah WSA-W.. Association of lifestyle with pulmonary tuberculosis among hospital patients in Asir region of Saudi Arabia. Ann Med Health Sci Res 2019; 9:741–7. [Google Scholar]
- 10. Cegielski JP, McMurray DN.. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8:286–98. [PubMed] [Google Scholar]
- 11. Kushner RF, Sorensen KW.. Lifestyle medicine: the future of chronic disease management. Curr Opin Endocrinol Diabetes Obes 2013; 20:389–95. [DOI] [PubMed] [Google Scholar]
- 12. Patra J, Jha P, Rehm J, Suraweera W.. Tobacco smoking, alcohol drinking, diabetes, low body mass index and the risk of self-reported symptoms of active tuberculosis: individual participant data (IPD) meta-analyses of 72,684 individuals in 14 high tuberculosis burden countries. PloS One 2014; 9:e96433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soh AZ, Chee CBE, Wang YT, Yuan JM, Koh WP.. Alcohol drinking and cigarette smoking in relation to risk of active tuberculosis: prospective cohort study. BMJ Open Respir Res 2017; 4:e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pednekar MS, Hakama M, Gupta PC.. Tobacco use or body mass—do they predict tuberculosis mortality in Mumbai, India? Results from a population-based cohort study. PLoS One 2012; 7:e39443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singapore Ministry of Health. Enhancing public health measures against tuberculosis. Singapore, Republic of Singapore: Ministry of Health, 2008. Available at:https://www.moh.gov.sg/diseases-updates/tuberculosis. Accessed 26 October 2021. [Google Scholar]
- 16. Hankin JH, Stram DO, Arakawa K, et al. . Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 2001; 39:187–95. [DOI] [PubMed] [Google Scholar]
- 17. Soh AZ, Chee CB, Wang YT, Yuan JM, Koh WP.. Dietary cholesterol increases the risk whereas PUFAs reduce the risk of active tuberculosis in Singapore Chinese. J Nutr 2016; 146:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soh AZ, Chee CBE, Wang YT, Yuan JM, Koh WP.. Dietary intake of antioxidant vitamins and carotenoids and risk of developing active tuberculosis in a prospective population-based cohort study. Am J Epidemiol 2017; 186:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willett WC. Nutritional epidemiology. 3rd ed. New York: Oxford University Press, 2013. [Google Scholar]
- 20. Food and Agriculture Organization of the United Nations; World Health Organization. The International Conference on Nutrition Rome. 1992. Available at: http://www.fao.org/3/v7700t/v7700t02.htm. Accessed 26 October 2021.
- 21. Soh AZ, Chee CBE, Wang YT, Yuan JM, Koh WP.. Diabetes and body mass index in relation to risk of active tuberculosis: a prospective population-based cohort. Int J Tuberc Lung Dis 2019; 23:1277–82. [DOI] [PubMed] [Google Scholar]
- 22. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–63. [DOI] [PubMed] [Google Scholar]
- 23. Jafar TH, Jin A, Koh WP, Yuan JM, Chow KY.. Physical activity and risk of end-stage kidney disease in the Singapore Chinese Health Study. Nephrology 2015; 20:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chee CB, James L.. The Singapore Tuberculosis Elimination Programme: the first five years. Bull World Health Organ 2003; 81:217–21. [PMC free article] [PubMed] [Google Scholar]
- 25. Soh AZ, Pan A, Chee CBE, Wang YT, Yuan JM, Koh WP.. Tea drinking and its association with active tuberculosis incidence among middle-aged and elderly adults: the Singapore Chinese Health Study. Nutrients 2017; 9:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ.. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int 2008; 74:994–7. [DOI] [PubMed] [Google Scholar]
- 27. Odone A, Houben RMGJ, White RG, Lönnroth K.. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014; 2:754–64. [DOI] [PubMed] [Google Scholar]
- 28. Fox GJ, Lee RS, Lucas M, et al. . Inadequate diet is associated with acquiring Mycobacterium tuberculosis infection in an Inuit community. a case-control study. Ann Am Thorac Soc 2015; 12:1153–62. [DOI] [PubMed] [Google Scholar]
- 29. Hemilä H, Kaprio J, Pietinen P, Albanes D, Heinonen OP.. Vitamin C and other compounds in vitamin C rich food in relation to risk of tuberculosis in male smokers. Am J Epidemiol 1999; 150:632–41. [DOI] [PubMed] [Google Scholar]
- 30. Koh WP, Yuan JM, Sun CL, Lee HP, Yu MC.. Middle-aged and older Chinese men and women in Singapore who smoke have less healthy diets and lifestyles than nonsmokers. J Nutr 2005; 135:2473–7. [DOI] [PubMed] [Google Scholar]
- 31. Martinez N, Kornfeld H.. Diabetes and immunity to tuberculosis. Eur J Immunol 2014; 44:617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nandar K, Ang LW, Tey J, et al. . Epidemiology of tuberculosis and HIV coinfections in Singapore, 2000-2014. HIV Med 2018; 19:59–644. [DOI] [PubMed] [Google Scholar]
- 33. Furin J, Cox H, Pai M.. Tuberculosis. Lancet 2019; 393:1642–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.