Abstract
Serious bacterial infections (SBI) can lead to devastating complications with CD19 CAR T cells and cytokine release syndrome (CRS). Little is known about consequences of and risk factors for SBI with novel CAR T-cell constructs or with CRS complicated by HLH-like toxicities. We report on three patients with B-cell acute lymphoblastic leukemia treated with CD22 CAR T cells who developed SBI and CRS-associated HLH. Serum cytokine profiling revealed sustained elevations well beyond CRS resolution, suggesting ongoing systemic inflammation. Heightened inflammatory states converging with SBI contribute to poor outcomes, and recognition and prevention of extended inflammation may be needed to improve outcomes.
Keywords: ALL molecular diagnosis and therapy, ALL relapse, clinical trials, cytokines, immunotherapy, infections
1 |. INTRODUCTION
Chimeric antigen receptor T cells (CART) are effective for treating B-cell acute lymphoblastic leukemia (B-ALL)1 but are associated with cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis-like toxicities (carHLH).1,2 CD19 CART studies have correlated CRS severity and infection,3,4 but infectious risks of novel CART are unknown.
We report on three patients with B-ALL enrolled on the CD22 CART trial (NCT02315612) who developed serious bacterial infection (SBI) and carHLH following standard lymphodepletion (Supporting Methods) and CART infusion (Table 1, Figure 1A). In these patients, the convergence of SBI and carHLH contributed to heightened inflammatory states, predisposing patients to poor outcomes. These cases highlight the complex interplay between timing of bacteremia, CRS, immunosuppression, and the underlying inflammatory milieu in patients receiving novel CART.
TABLE 1.
Summary of presented case characteristics
| Case 1 | Case 2 | Case 3 | ||
|---|---|---|---|---|
| Demographics | Infection onset in relation to CRS (day 1st positive culture) | Concurrent with CRS onset (day +7) | After CRS resolution (day +19) | After CRS resolution (day +24) |
| Age/Sex | 4-Year-old male | 18-Year-old female | 26-Year-old male | |
| Relevant past medical history | Prior infectious disease complications | N/A | Clostridium difficile typhlitis bacteremia with septic shock | N/A |
| Most recent line of therapy | TACL 2017–002a (day −44) | Vincristine (day −40) | Vincristine (day −12) | |
| Relapsed vs. refractory (previous lines of therapy) | Refractory (2) | Relapsed (4) | Relapsed (2) | |
| Prior CAR T-cell therapy | Yes (CD19) | No | No | |
| Prior HSCT | No | Yes (MUD) | No | |
| Steroids in month prior to CAR | No | Yes | Yes | |
| ANC at lymphodepletion start | 370/μl | 7420/μl | 13,120/μl | |
| Hg at lymphodepletion start (days since pRBC transfusion) | 9.4 g/dl (8) | 10.7 g/dl (1) | 9.9 g/dl (16) | |
| Plt at lymphodepletion start (days since platelet transfusion) | 264,000/μl (NA) | 189,000/μl (17) | 228,000/μl (NA) | |
| Infectious disease | Antibiotic at the time of infectionb | Piperacillin/tazobactam (initiated with febrile neutropenia following CAR T-cell infusion) | Metronidazole (C. difficile prophylaxis) | Ciprofloxacin |
| ANC at the time of infection | N/Ac | 1020/μl | N/Ac | |
| Hg at time of infection (days since prior pRBC transfusion) | 10.4 g/dl (2) | 8.4 g/dl (6) | 8.6 g/dl (1) | |
| Plt at time of infection (days since prior platelet transfusion) | 167,000/μl (NA) | 53,000/μl (1) | 13,000/μl (2) | |
| Organism(s) by blood Cx | Bacillus cereus | Enterobacter clocae | Staphylococcus epidermidis Weeksella virosa | |
| Empiric antibiotics (organism sensitive?) | Meropenem + vancomycin (Y) | Ceftazidime + amphotericin (N) → transition to meropenem within 12 hours (Y) | Meropenem + linezolid + amphotericin + posaconazole (Y) | |
| CAR toxicities | CRS, max grade (onset) | 2 (day +7) | 3 (day +8) | 3 (day +4) |
| Peak ferritin (ng/ml) (day) | 349,750 (day+11) | 590,100 (day+17) | 271,600 (day+10) | |
| Clinical outcomes | Brief description | CR, but unable to proceed to HSCT; died from PD | Died from complications of sepsis | Achieved CR and proceeded to HSCTd |
Abbreviations: ANC, absolute neutrophil count; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; Hg, hemoglobin; HSCT, hematopoietic stem cell transplant; plt, platelet count; pRBC, packed red blood cells; MUD: matched unrelated donor
TACL 2017–002 (ixazomib, vincristine, doxorubicin, pegasparaginase, dexamethasone, and intrathecal methotrexate).
All patients on prophylactic acyclovir, micafungin, and pentamidine.
Differential not completed (total white blood cell count too low to complete differential).
Patient ultimately died from complications of treatment of post-HSCT relapse).
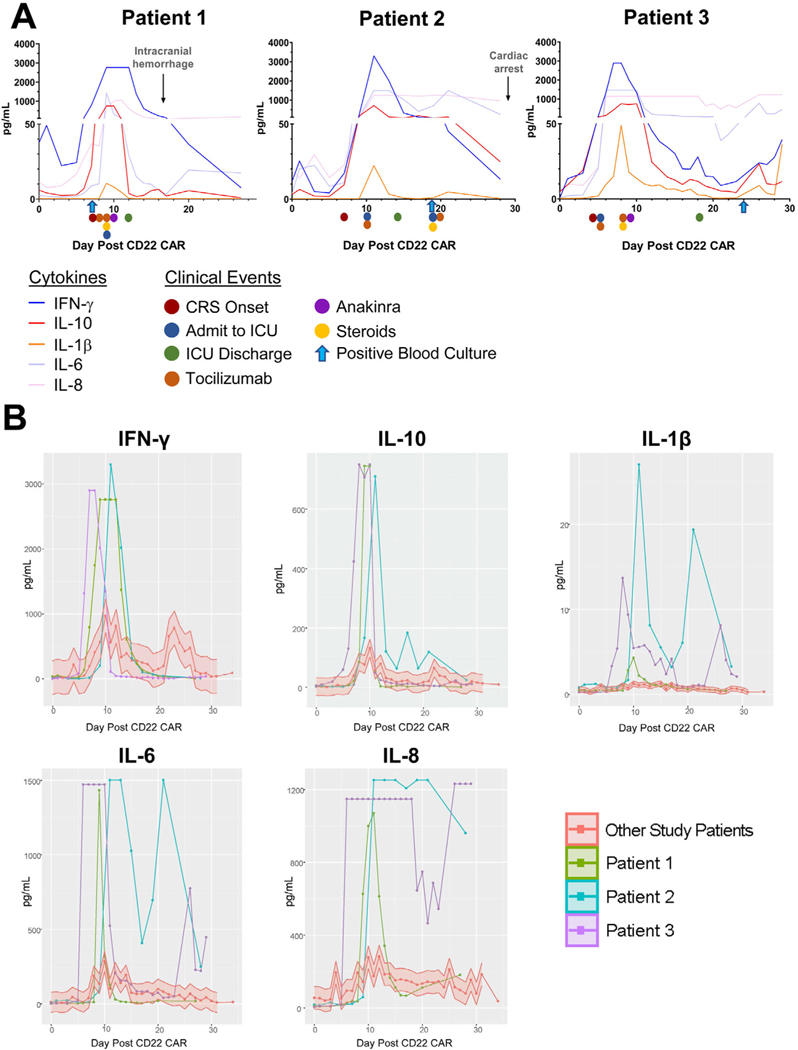
FIGURE 1.
Serum cytokine levels following CD22 chimeric antigen receptor (CAR) T-cell administration (day 0). (A) Clinical time course of three cases with important clinical changes indicated on appropriate days with arrows. (B) Serum cytokine levels for three patients displayed in relation to other patients with CRS in the CD22 CAR clinical trial cohort (red, shaded area representing 70% confidence interval); steroids = steroids initiated; anakinra = anakinra initiated. Serial cytokines were resulted using ELISA on 58 subjects
2 |. RESULTS
2.1 |. Patient 1
A 4-year-old boy with CD19-negative relapse following tisagenlecleucel developed culture-negative febrile neutropenia (day −2 to day +1), following lymphodepletion and CD22 CART infusion. On day +7, he had fever recrudescence, coinciding with CRS onset (grade 2: fever, tachycardia, hypoxia),5 and concurrent positive blood culture grew Bacillus cereus, which cleared within 24 hours on antimicrobials. He received tocilizumab (12 mg/kg, two doses) and methylprednisolone (1 mg/kg BID) for CRS, and he transferred to the intensive care unit (ICU). He defervesced and weaned off oxygen with CRS improvement, but on day +10 he developed carHLH (coagulopathy, transaminitis, hyperferritinemia) and initiated anakinra (3.3 mg/kg BID). He transferred out of the ICU on day +12 while continuing immunosuppression. He received daily cryoprecipitate for hypofibrinogenemia and had no signs of bleeding, normal PT/PTT, and platelet count remained above 50,000/μl.
Despite steady improvement in clinical and laboratory parameters and without signs of neurotoxicity, on day +17, he developed suddenonset hemiparesis. Head CT revealed a large right temporofrontal hematoma and hemorrhagic foci throughout the bilateral cerebral hemispheres. He underwent emergency decompressive craniectomy and had a protracted course but remained minimally responsive. As CD22 CART are used to bridge to hematopoietic stem cell transplant (HSCT); being unable to proceed to HSCT due to his neurologic sequelae, he passed away from CD22-negative relapse.
2.2 |. Patient 2
An 18-year-old girl with post-HSCT relapse and numerous prior SBI received lymphodepletion and CART without incident. She developed grade 3 CRS (fever, tachycardia, hypotension) on day +8. She received tocilizumab (8 mg/kg) and transferred to the ICU (day +10). Following stabilization, she transferred out of the ICU (day +14), but she subsequently developed carHLH (coagulopathy, transaminitis, hyperbilirubinemia, hyperferritinemia). On day +19, she developed non-neutropenic fever that rapidly progressed to shock. Following initiation with ceftazidime, antibiotics were broadened, and she also received tocilizumab and methylprednisolone (1 mg/kg q8h). Blood cultures revealed ceftazidime-resistant Enterobacter cloacae. Despite initial improvement, she developed multiorgan failure and died on day +39.
2.3 |. Patient 3
A 26-year-old man with relapsed B-ALL received lymphodepletion and CART without incident. On day +4, he developed grade 3 CRS (fever, hypoxia, hypotension). He received tocilizumab (8 mg/kg) and transferred to the ICU (day +5). On day +8, he developed carHLH (transaminitis, hyperferritinemia, coagulopathy, hyperbilirubinemia, hepatosplenomegaly), which was treated with a second tocilizumab dose, methylprednisolone (1 mg/kg q8h), and anakinra (1–2 mg/kg q6h). He also received broad-spectrum antibiotics for acalculous cholecystitis. He further developed atypical hemolytic uremic syndrome requiring transient renal replacement therapy (day +9) and began eculizumab (900 mg q72h) (day +16). He improved with these supportive measures and transferred out of the ICU (day +18).
On day +24 he developed febrile neutropenia, hemoptysis, and hypoxia. Chest CT identified pulmonary consolidations and bilateral ground-glass opacities. Blood cultures grew Staphylococcus epidermidis and Weeksella virosa, and bronchoscopy culture also grew W. virosa. Transient worsening hypoxia from infection led to increased steroid utilization. Following resolution, his restaging bone marrow demonstrated remission. He was discharged on day +46 and proceeded to allogeneic HSCT.
2.4 |. Patient cytokine levels
We previously established that patients with carHLH have higher peak cytokine values compared to those without carHLH.6 Serial evaluation of these three patients’ serum cytokine levels (Figure 1 and Supplementary Figure 1) revealed that cytokines associated with CRS (IFN-γ, IL-10, IL-6, IL-8) or sepsis (IL-1β)7 were amongst the highest values in comparison to the full CD22 CART cohort (Figure 1B). These patients had a sustained peak of several cytokines such as IL-8; or a bimodal peak of other cytokines (i.e., IL-6, IL-1β) at onset of CRS and then again with sepsis (Figure 1B).
3 |. DISCUSSION
CD22 CART is an emerging treatment for relapsed/refractory B-ALL, particularly for relapse following CD19 targeting.2,8 We previously demonstrated that carHLH was part of the CD22 CART toxicity profile,2 but have not evaluated this toxicity in the context of concurrent bacteremia. Thus, we highlight three exceptional cases where SBI in the context of carHLH may have converged to amplify toxicity, illustrating the adverse impact of inflammation and SBI in CART recipients.
Previous series assessing infectious complications of CD19 CART concluded that infection correlates with CRS severity but not cytokine levels.3,4 Serial cytokine profiling revealed that these three patients had a heightened and prolonged inflammatory state beyond CRS resolution, similar to what has been seen in our experience with carHLH in CD22 CART,2,6 and their infectious outcomes suggest that this inflammatory milieu could have contributed to risk of developing SBI.
HLH-associated complications, outside of CAR T-cell therapy, have particularly poor outcomes related to heighted inflammatory toxicities on end organs, particularly with concurrent infection. These manifestations may be due to an IL-1β-driven autocrine loop.9 Furthermore, this autocrine loop may increase IFN-γ and IL-6, which are key inflammatory regulators of CRS, and these cytokines were all elevated in our three patients.7,9 How the dual inflammatory processes of CRS and carHLH feed into and exacerbate each other requires elucidation, but our three patients raise provocative questions regarding how SBI may further compound this storm: did SBI with CRS onset trigger carHLH (patient 1), or did carHLH predispose patients to worse outcomes with bacteremia (patients 2 and 3)?
For patient 1, it is plausible that the confluence of bacteremia and CRS onset lowered the threshold for carHLH development. While B. cereus is notorious for central nervous system manifestations,10 delayed hemorrhage is unusual; however, the inflammatory state induced by this CART may have contributed to this atypical presentation. In contrast, for patients 2 and 3 persistent cytokine elevation preceded bacteremia, indicating that an inflammatory milieu was present despite improvement in CRS manifestations at the time of infection. This may have contributed to patient 2’s rapid decompensation with bacteremia. For patient 3, treatment with eculizumab, anakinra, and steroids for carHLH may have predisposed him to this particularly rare Weeksella infection.11
Treating patients with sepsis and HLH-like features with the IL-1 receptor antagonist, anakinra, may improve outcomes.9,12 We have increasingly used anakinra to treat this toxicity2 and will incorporate it into pre-emptive strategies for carHLH mitigation. Akin to the positive experiences of pre-emptive tocilizumab for prevention of severe CRS,13 we anticipate that carHLH prevention will reduce the overall inflammatory response and improve outcomes.
In conclusion, for these three patients treated with CD22 CART, SBI coinciding with CRS-associated carHLH may have resulted in poor outcomes. For patients receiving these experimental therapies, clinicians must be vigilant for evaluation of catastrophic bacteremia even after apparent CRS resolution. As novel CART emerge for patients with relapsed disease, an extensive, consistent approach to the evaluation, treatment, and prevention of infections and mitigation of inflammatory responses will be needed to optimize outcomes.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the study participants and their families, referring medical care teams, the faculty and staff of the NIH Clinical Center who provided their expertise in the management of the study participants, and the data managers and research nurses and patient care coordinators involved with this work. The authors particularly thank Dr. John Bennett, who critically reviewed the manuscript and gave insightful feedback and the NIH ID consult team for their ongoing support. This work was supported in part by the Intramural Research Program, National Cancer Institute and NIH Clinical Center, National Institutes of Health (ZIA BC 011823). Additional support was provided through the NIH Oxford-Cambridge Scholars Program.
Funding information
Intramural Research Program, National Cancer Institute and NIH Clinical Center, National Institutes of Health, Grant Number: ZIA BC 011823; NIH Oxford-Cambridge Scholars Program
Abbreviations:
- B-ALL
B-cell acute lymphoblastic leukemia
- CAR
chimeric antigen receptor
- carHLH
CAR T-cell-associated hemophagocytic lymphohistiocytosis
- CRS
cytokine release syndrome
- HSCT
hematopoietic stem cell transplant
- ICU
intensive care unit
- SBI
serious bacterial infection
Footnotes
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted in accordance with the ethical principles stated in the Belmont Report and the U.S. Common Rule. The clinical trial these patients were enrolled on was approved by the Central Institutional Review Board of the NCI, study number 15-C-0029. All three patients described are deceased. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.O’Leary MC, Lu X, Huang Y, et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019;25:1142–1146. [DOI] [PubMed] [Google Scholar]
- 2.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;38:1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JH, Romero FA, Taur Y, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25: 625–638. [DOI] [PubMed] [Google Scholar]
- 6.Ishii K, Pouzolles M, Chien CD, et al. Perforin-deficient CAR T cells recapitulate late-onset inflammatory toxicities observed in patients. J Clin Invest. 2020;130:5425–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diorio C, Shaw PA, Pequignot E, et al. Diagnostic biomarkers to differentiate sepsis from cytokine release syndrome in critically ill children. Blood Adv. 2020;4:5174–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bair H, Frank MJ, Craig J, et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR-refractory large B-cell lymphoma. Blood. 2021;137(17):2321–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansford JR, Phillips M, Cole C, Francis J, Blyth CC, Gottardo NG. Bacillus cereus bacteremia and multiple brain abscesses during acute lymphoblastic leukemia induction therapy. J Pediatr Hematol Oncol. 2014;36:e197–e201. [DOI] [PubMed] [Google Scholar]
- 11.Vaquera-Aparicio DN, Mascareñas-De Los Santos AH, Casillas-Vega N, et al. Bacteremia due to Weeksella virosa in a pediatric patient with embryonal rhabdomyosarcoma. Bol Med Hosp Infant Mex. 2020;77:149–152. [DOI] [PubMed] [Google Scholar]
- 12.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadauke S, Myers RM, Li Y, et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: a prospective clinical trial. J Clin Oncol. 2021;39(8):920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.