Abstract
The recent identification of the iron response regulator (Irr) in Bradyrhizobium japonicum raised the question of whether the global regulator Fur is present in that organism. A fur gene homolog was isolated by the functional complementation of an Escherichia coli fur mutant. The B. japonicum Fur bound to a Fur box DNA element in vitro, and a fur mutant grown in iron-replete medium was derepressed for iron uptake activity. Thus, B. japonicum expresses at least two regulators of iron metabolism.
Studies on the regulation of iron homeostasis in bacteria have focused on Fur, a global transcriptional regulator that represses target genes when bound to iron. The Irr (iron response regulator) protein from the bacterium Bradyrhizobium japonicum mediates iron control of heme biosynthesis and affects iron transport (9). Irr has low homology to Fur, and although it is not a functional homolog (9), its identification led us to ask whether B. japonicum also expresses a functional fur gene.
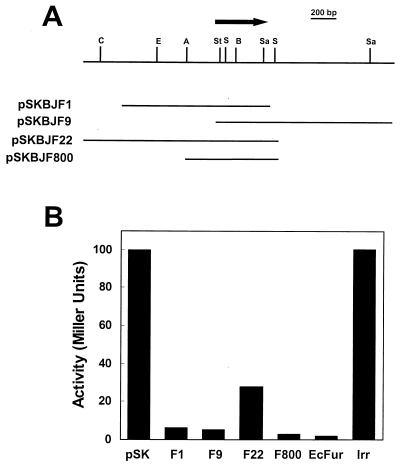
A B. japonicum genomic expression library was screened for clones that repressed the fiu::lacZ reporter gene fusion in Escherichia coli fur strain H1780 as discerned by the formation of white or light blue colonies in the presence of 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml and 100 μM FeCl3. Three overlapping library clones, pSKBJF1, pSKBJF9, and pSKBJF22, complemented the fur strain both on plates and in liquid cultures (Fig. 1). pSKBJF22 repressed less well, perhaps because the complementing gene (see below) was more distal from the plasmid-borne lacZ promoter. A 830-bp ApaI-Sau3AI subclone on pSKBJF800 including the overlapping region was also sufficient to complement the fur strain (Fig. 1). This fragment contained an open reading frame encoding a protein with homology to characterized Fur proteins from numerous organisms (Fig. 2). Among the proteins shown experimentally to be Fur, the B. japonicum protein was most homologous to that of E. coli, showing 39% identity and 49% similarity to E. coli Fur. Southern blot analysis indicated a single fur gene in the B. japonicum genome (data not shown). The complementation and homology indicate that the cloned B. japonicum DNA encodes a structural and functional homolog of Fur. A fur-like gene of Rhizobium leguminosarum was isolated (6) encoding a product with 77% similarity to B. japonicum Fur, indicating that the R. leguminosarum gene is a functional fur gene as well. Previous work indicates that the B. japonicum Irr is functionally distinct from bacterial Fur despite their modest homology to each other (9). Consistent with this, the plasmid-borne irr gene did not functionally complement the E. coli fur strain as did the B. japonicum fur gene (Fig. 1B).
FIG. 1.
Complementation of E. coli fur strain H1780 for in vivo repression of fiu::lacZ gene fusion by B. japonicum library clones. (A) B. japonicum genomic region represented by library plasmids pSKBJF1, pSKBJF9, and pSKBJF22, which complemented the fur strain on plates as described in the text. pSKBJF800 is an ApaI-Sau3AI-digested subclone of pSKBJF22. The arrow represents the open reading frame encoding the fur homolog. Restriction sites: A, ApaI; B, BglII; C, ClaI; E, EcoRI; Sa, SacI; S, SphI; St, StyI. (B) Repression of β-galactosidase activity in fur strain H1780 by B. japonicum library clones pSKBJF1 (F1), pSKBJF9 (F9), pSKBJF22 (F22), and pSKBJF800 (F800). Cells containing the library vector pBluescript SK(+) (pSK) were the control for unrepressed activity. The positive control was pMH15fur (EcFur), which contained the E. coli fur gene. Also shown is the result for B. japonicum irr gene on pSKSBIrr (Irr).
FIG. 2.
Deduced amino acid sequence of the B. japonicum Fur protein (bottom sequence) and alignment with other Fur proteins. Asterisks and colons denote amino acids that are identical or similar, respectively, at that position for all sequences. The sources of the proteins used, along with GenBank accession numbers, are as follows: E. coli (X02589), Klebsiella pneumoniae (L23871), Yersinia pestis (Z12101), Vibrio cholerae (M85154), Vibrio anguillarum (L19717), Vibrio parahaemolyticus (AB003752), Vibrio vulnificus (L06428), P. aeruginosa (L00604), Pseudomonas putida (X82037), Neisseria gonorrhoeae (L11361), Neisseria meningitidis (L19777), Bordetella pertussis (U11699), Haemophilus ducreyi (U37224), Campylobacter jejuni (AF052056), Campylobacter upsaliensis (L77075), Synechococcus sp. PCC 7942 (L41065), Legionella pneumophila (U06072), and R. leguminosarum (Y13657).
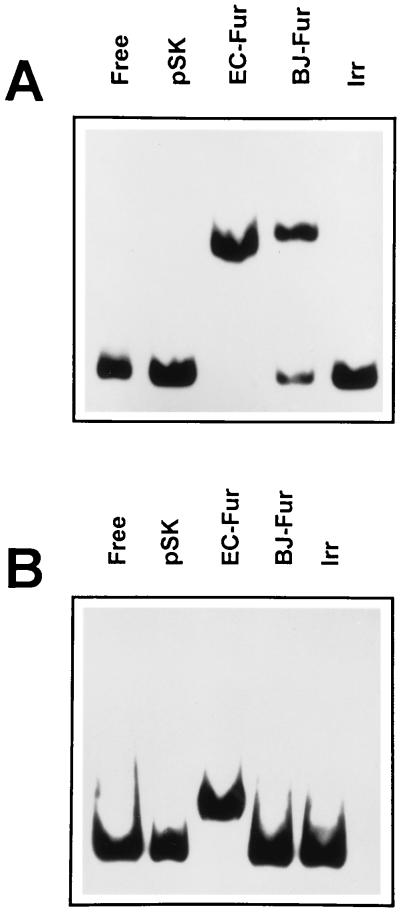
Complementation analysis showed that the B. japonicum Fur (BjFur) had repressor activity in vivo (Fig. 1); thus, we examined its DNA-binding activity in extracts from cells that overexpressed Fur by gel mobility shift assays with a double-stranded DNA probe that includes a “Fur box” consensus sequence recognized by E. coli Fur (EcFur) and other characterized Fur proteins (4, 5, 11). In addition, the assays were carried out in the presence or absence of Mn2+, which has been shown to substitute for Fe2+ as a cofactor of Fur in vitro (5). Mn2+ is used because Fe2+ is readily oxidized in air to Fe3+, which is not functional as a Fur cofactor. The results show that BjFur bound to the Fur box consensus element in the presence of metal (Fig. 3A) but not in its absence (Fig. 3B). EcFur showed metal-dependent DNA-binding activity as well (Fig. 3). However, in the absence of metal, the DNA-binding activity of EcFur produced a complex that was different from that observed in the presence of metal. Binding of E. coli apo-Fur to operator DNA with lower affinity in a gel mobility shift assay has been documented previously (1). Unlike BjFur, the B. japonicum Irr protein did not bind to the iron box element under any condition (Fig. 3), consistent with the conclusion that Irr is not functionally equivalent to Fur.
FIG. 3.
Metal-dependent binding of BjFur to the Fur box consensus sequence. Gel mobility shift assays were carried out in the presence (A) or absence (B) of 100 μM MnCl2. The DNA used in the assays was radiolabeled with 32P and contained the Fur box consensus sequence 5′-GATAATGATAATCATTATC-3′. The protein samples used were extracts of E. coli fur strain H1780 containing plasmids pBluescript SK(+) (pSK), pMH15fur (EC-Fur), pSKBJF800 (BJ-Fur), or pSKSBIrr (Irr). The DNA was also run in the absence of extract (Free).
E. coli fur has a Fur box element in its promoter and is modestly autoregulated, whereas the Pseudomonas aeruginosa fur gene does not (11). No recognizable Fur box element was found upstream of the fur gene, and an ApaI-StyI fragment that included 268 bp of DNA upstream of the fur open reading frame did not bind to BjFur in a gel mobility shift assay (data not shown). Thus, the B. japonicum fur gene does not appear to be autoregulated.
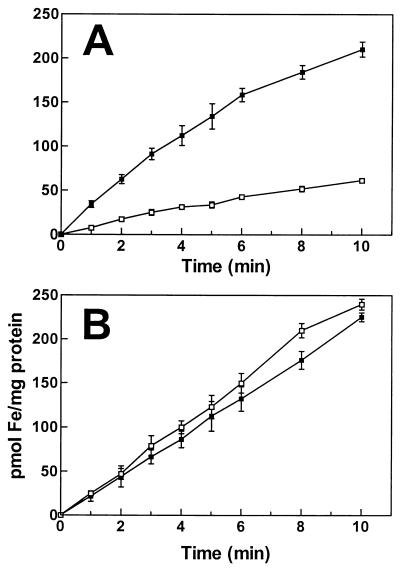
Many bacterial species, including B. japonicum (8, 9), express an inducible high-affinity iron uptake activity in response to iron limitation as a means of scavenging the metal. Fur proteins repress bacterial iron transport genes under iron-replete conditions; thus, a fur mutant of E. coli is derepressed for iron transport (for a review, see references 2 and 10). A B. japonicum fur null mutant of wild-type strain USDA I110 was constructed by the insertional inactivation of the fur gene of parent strain I110 with an Ω cassette to construct strain GEM4. Ferric citrate uptake activity was carried out as described previously (9). The fur strain showed similar iron uptake activity as the wild type when grown in iron-limited medium (Fig. 4). However, the mutant expressed a three- to fourfold higher initial rate of iron uptake activity than the parent strain when grown in high-iron medium. This modest derepression was similar to that observed for an E. coli fur mutant (10). A phenotype for the mutant strain showed that the cloned fur gene is functional in B. japonicum. The uptake activity of the fur strain grown in high-iron medium reached saturation by 10 to 15 min (Fig. 4 and data not shown), whereas activity induced in the wild type by iron deprivation is unchanged for at least 90 min (9). Irr is not expressed in high-iron medium (9); thus, it is possible that the iron transport system normally induced under iron limitation in the wild type was not fully expressed in the fur strain grown in high-iron medium.
FIG. 4.
Iron uptake activity in B. japonicum parent strain I110 (open squares) and fur strain GEM4 (closed squares). Cells were grown to mid-log phase in high-iron (A) or low-iron (B) medium, washed, and prepared as described in the text. At time zero, 59Fe (0.1 μM final concentration) was added to the cells. Aliquots were removed at various times, collected, and counted as described in the text. Each point represents the average of three separate samples; standard deviation bars are shown. As controls, 1- and 8-min samples of cells that remained on ice throughout the time course were taken, and no uptake was observed (data not shown). The data shown are typical of two independent experiments.
The identification of a functional fur gene in B. japonicum shows that Irr does not replace Fur, but rather both regulators function in that organism to regulate iron metabolism. Genes encoding Fur-like proteins in addition to bona fide Fur have been recently identified in E. coli and Bacillus subtilis; these proteins are involved in the maintenance of zinc homeostasis (Zur) (7, 12), or in a manganese-dependent response to oxidative stress (PerR) (3). Thus, there appears to be a family of functionally diverse Fur-like proteins, including Zur, PerR, and Irr, that are all involved in metal-dependent regulation but are distinct from Fur. These findings underscore the need for experimental evidence to correctly identify fur homologs obtained by nucleotide sequence information.
Nucleotide sequence accession number.
The nucleotide sequence of the ApaI-SauIIIA insert of pSKBJF800 was deposited in GenBank under accession no. AF052295.
Acknowledgments
We thank K. Hantke and M. Vasil for E. coli strains and plasmids.
This work was supported by National Science Foundation grant MCB-9722974 to M.R.O.
REFERENCES
- 1.Althaus E W, Outten C E, Olson K E, Cao H, O’Halloran T V. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry. 1999;38:6555–6569. doi: 10.1021/bi982788s. [DOI] [PubMed] [Google Scholar]
- 2.Braun V, Hantke V. Genetics of bacterial iron transport. In: Winkelmann G, editor. Handbook of microbial iron chelates. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 107–138. [Google Scholar]
- 3.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologs: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 4.Calderwood S B, Mekalanos J J. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operon fusion plasmid. J Bacteriol. 1988;170:1015–1017. doi: 10.1128/jb.170.2.1015-1017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lorenzo V, Giovannini F, Herrero M, Neilands J B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988;203:875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 6.De Luca N G, Wexler M, Pereira M J, Yeoman K H, Johnston A W B. Is the fur gene in Rhizobium leguminosarum essential? FEMS Microbiol Lett. 1998;168:289–295. doi: 10.1016/s0378-1097(98)00454-6. [DOI] [PubMed] [Google Scholar]
- 7.Gaballa A, Helmann J D. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol. 1998;180:5815–5821. doi: 10.1128/jb.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerinot M L, Meidl E J, Plessner O. Citrate as a siderophore in Bradyrhizobium japonicum. J Bacteriol. 1990;172:3298–3303. doi: 10.1128/jb.172.6.3298-3303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamza I, Chauhan S, Hassett R, O’Brian M R. The bacterial Irr protein is required for coordination of heme biosynthesis with iron availability. J Biol Chem. 1998;273:21669–21674. doi: 10.1074/jbc.273.34.21669. [DOI] [PubMed] [Google Scholar]
- 10.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 11.Ochsner U A, Vasil A I, Vasil M L. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol. 1995;177:7194–7201. doi: 10.1128/jb.177.24.7194-7201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patzer S I, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]