Abstract
Cold shock induction of cspB has been shown to be primarily regulated at the mRNA level. Here, we demonstrate that the induction of cspB at low temperature also requires the translational cis-acting element called the downstream box (DB). Full induction of cspB at low temperature is achieved in the presence of both the Shine-Dalgarno sequence and DB. We propose that the DB sequence functions as a translational enhancer for the biosynthesis of CspB to bypass the inhibitory effect in translation caused by cold shock.
Previously, we have shown that the cold shock induction of cspB is regulated at the level of transcription (2). Herein, we demonstrate that the translational cis-acting element called the downstream box (DB) is essential for the induction of cspB after a temperature downshift. The presence of a DB sequence in the cspB mRNA was reported earlier by Mitta et al. (13). The role of the DB in translation initially proposed by Sprengart and coworkers (16) indicates that the DB sequence is complementary to a region in the penultimate stem of the 16S rRNA. Thereby, translation initiation is enhanced by an increase in the affinity of the ribosome for a DB-containing mRNA (16).
It has been demonstrated that shifting Escherichia coli cells to low temperature results in a global inhibition of cellular protein synthesis (8). However, under these conditions, maximal levels of cold shock proteins are observed. Therefore, it has been speculated that cold shock mRNAs, unlike the majority of cellular mRNAs, are capable of bypassing the protein synthesis inhibitory effect triggered by cold shock (9). In addition, it has been demonstrated that after a temperature downshift, translation is inhibited at the initiation step (9). In this regard, Jones and Inouye (9) have proposed that cold shock mRNAs may be efficiently translated due to a higher affinity of these mRNAs for the ribosome at low temperature. Recently, we have demonstrated that artificial DB sequences inserted in the lacZ gene cause a significant translational enhancement (3). Herein, we examined the role of the DB in the translational induction of cspB, a major cold shock gene of E. coli, at low temperature.
Induction of cspB at low temperature requires DB.
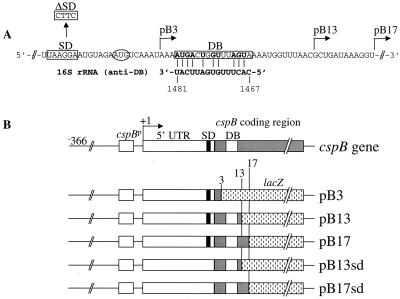
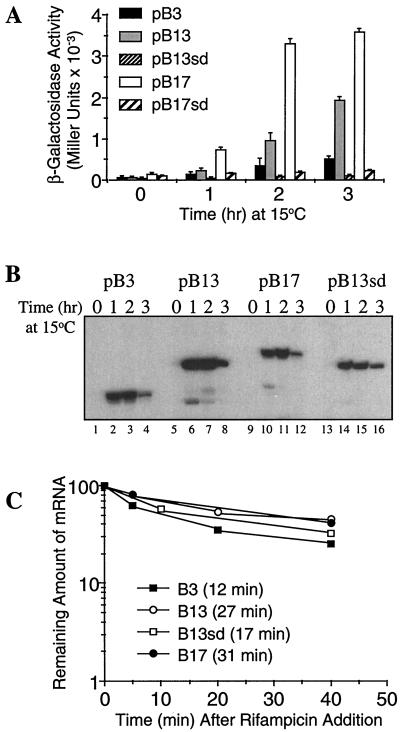
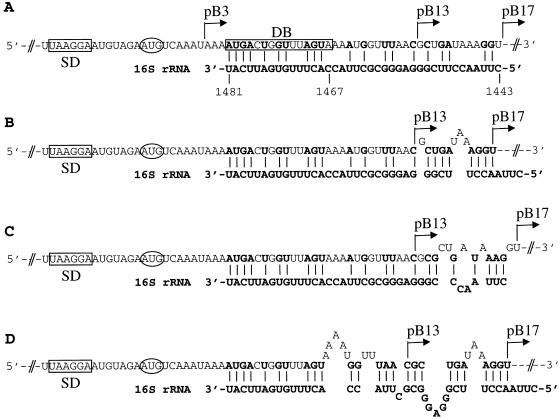
E. coli AR137, a pcnB mutant which maintains pBR322 derivative plasmids at a low copy number (11), was transformed with a series of cspB-lacZ translational fusions (Fig. 1). Figure 1A shows the cspB-DB sequence located 5 codons downstream of the initiation codon and its complementarity to the anti-DB sequence in the 16S rRNA (from base 1467 to 1481). Figure 1B shows a series of translational fusions in which cspB was fused to the lacZ gene after codons 3, 13, and 17 to create pB3, pB13 and pB17, respectively. Figure 2A shows β-galactosidase activity at various time points after a temperature shift from 37 to 15°C. After 2 and 3 h at 15°C, pB13 and pB17 show the highest levels of β-galactosidase activity, indicating that a region from codons 3 and 13 (containing DB) (see Fig. 1A) plays a major role in high-level expression of cspB at low temperature. Primer extension analysis (Fig. 2B) shows that the mRNA levels of pB3 and pB17 between 1 and 2 h at 15°C are almost identical, while the β-galactosidase activity of pB17 is seven times higher than that of pB3 (Fig. 2A). This indicates that the differences in the sequence downstream of the initiation codon cause a significant effect on the efficiency of mRNA translation but not on the amount of the mRNAs. Based on the amounts of mRNAs estimated using a phosphorimager and the increments of β-galactosidase activity between 1 and 2 h after cold shock induction, the translational capability of pB17 was calculated to be 6 times higher than that of pB13 and 18 times higher than that of pB3 (Table 1). This could be explained by the potential for additional base pairing between the cspB mRNA (pB17) and the 16S rRNA, as shown in Fig. 3. There are an additional G-C base pair and four extra base pairs immediately after codon 13. However, if mismatches in either cspB mRNA and/or 16S rRNA are incorporated, the number of G-C base pairs could increase up to four and five together with other base pairs (Fig. 3B through D). These extra nucleotides of pB17 complementary to the 16S rRNA possibly increases the affinity of the pB17 mRNA for the ribosome and in turn enhances its translation as compared with pB13. This suggests that a longer DB (pB17) could be more effective for translation than a shorter DB (pB13). In addition, it could be that specific base pairs between the mRNA and the 16S rRNA and/or specific mRNA conformations may be important for a higher translational activity.
FIG. 1.
Construction of cspB-lacZ fusions. (A) cspB-DB–anti-DB complementarity. The cspB-DB sequence is boxed and encompasses the region from codons 5 to 9 (13). The AUG codon is circled, the SD sequence is boxed, and the L-shaped arrows show the positions where the cspB gene was fused to lacZ. (B) Translational cspB-lacZ fusion constructs. On the top, the E. coli cspB gene is depicted from its 5′-end. In pB3, pB13, and pB17, cspB is fused to lacZ at residue +177 (3rd codon), +200 (13th codon), and +212 (17th codon), respectively. The cspB DNA fragments were amplified by PCR using synthetic oligonucleotide primers containing a BamHI site at the 5′ end. A plasmid, pSJ7 (10) carrying the wild-type cspB gene was used as a template DNA to create the PCR fragments B3, B13, and B17. The 5′-end oligonucleotide primer used in each of the above PCR reactions is 5′-CCGGATCCAGCTTTAATATAGCT-3′. The 3′-end oligonucleotide primers for the PCR products B3, B13, and B17 are 5′-CCGGATCCAGATTTGACATTCTACA-3′, 5′-CCGGATCCAGGTTAAACCATTTT-3′, and 5′-CCGGATCCAGACCTTTATCAGCGTT-3′, respectively. A deletion of the SD sequence in the cspB gene was created by site-directed mutagenesis using the QuickChange site-directed mutagenesis kit (Stratagene). The PCR reaction was carried out using the pSJ7 plasmid as a template and the oligonucleotides Bsd1 (5′-GAAAGGCTCAAGTTACTTCATGTAGAATG-3′) and Bsd2 (5′-CATTCTAC ATGAAGTAACTTGAGCCTTTC-3′) to create pSJsd. Then, pSJsd was used as a template to make the PCR fragments B13sd and B17sd. The 5′ and 3′-end oligonucleotide primers used in these PCR reactions are the same as the one used for the PCR fragments B13 and B17. All the above PCR products were cloned at the BamHI site of the pRS414 vector (10, 15) to create the pB3, pB13, pB13sd, pB17, and pB17sd constructs.
FIG. 2.
Enhancement of cspB translation by the DB. (A) β-Galactosidase activity of the cspB-lacZ constructs obtained before (time zero) and 1, 2, and 3 h after temperature shift from 37 to 15°C. E. coli AR137 cells transformed with pB3, pB13, pB13sd, pB17, and pB17sd were grown in Luria-Bertani medium, and at mid-log phase (optical density at 600 nm = 0.4) cultures were shifted from 37 to 15°C. β-Galactosidase activity was measured as described by Miller (12). (B) mRNA levels of pB3, pB13, pB13sd, and pB17 after temperature shift from 37 to 15°C. The cspB-lacZ mRNAs were detected by primer extension as described previously (13) at the same time points described above (panel A). (C) Stabilities of pB3, pB13, pB13sd, and pB17 mRNA. E. coli AR137 cells transformed with pB3, pB13, pB13sd, and pB17 were grown under the same conditions as those described above. At mid-log phase the culture was shifted to 15°C, and after 30 min rifampin was added to a final concentration of 0.2 mg/ml (time zero). Total RNA was extracted at 5, 10, 20, and 40 min after rifampin addition. The cspB-lacZ mRNA was detected by primer extension as described previously (13).
TABLE 1.
Effect of the DB on translation of cspB at low temperaturea
| cspB-lacZ construct | Avg amt of mRNA between 1 and 2 h at 15°C (m) | Difference in lacZ U between 1 and 2 h at 15°C (Z) | Z/m | Relative DB effect |
|---|---|---|---|---|
| pB3 | 11.2 | 201 | 18 | 1 |
| pB13 | 13.4 | 723 | 54 | 3 |
| pB13sd | 7.2 | 32 | 4.5 | 0.25 |
| pB17 | 8.1 | 2,554 | 315 | 18 |
The mRNA amount of the cspB-lacZ constructs after 1 and 2 h of cold shock at 15°C (see Fig. 2B) was estimated by phosphorimager analysis, and an average value was calculated and termed m. The difference in the β-galactosidase activities of the cspB-lacZ constructs between 1 and 2 h at 15°C was calculated from Fig. 2A (Z). The relative effect of the DB was then estimated by the ratio of Z to m by taking the Z/m ratio of pB3 as 1.
FIG. 3.
Additional base pairing between the cspB mRNA and the 16S rRNA. (A) Additional base pairs between the pB17 mRNA (within the sequence from codon 13 to 17) and the 16S rRNA with no mismatches. (B) pB17 mRNA (within the sequence from codon 13 to 17)-16S rRNA base pairs with two mismatches in the cspB mRNA. (C) Same as in panel B with three mismatches in the pB17 mRNA and one mismatch in the 16S rRNA. (D) Additional base pairs between pB17 mRNA and 16S rRNA with multiple mismatches in both of them allowed.
It has been shown that mRNA stability plays an important role in the cold shock inducibility of cspA (1, 5, 6). Figure 2C shows the stabilities of the mRNAs from pB3, pB13, pB13sd, and pB17. Their half-lives were calculated to be 12, 27, 17, and 31 min, respectively. It has been shown that efficiently translated lacZ mRNA is more stable than those translated less efficiently due to the protection by ribosomes (7). Thus, the greater stability of pB13 and pB17 mRNA in comparison with that of pB3 could result from ribosome protection due to their higher translational efficiency.
Requirement of the SD sequence for the DB activity.
In order to know if the Shine-Dalgarno (SD) sequence is required for the DB sequence to exert its function as a translational enhancer, the SD sequence from pB13 and pB17 was mutated from AGGA to CTTC to create pB13sd and pB17sd, respectively (Fig. 1A). The low levels of β-galactosidase units from the SD deletion constructs pB13sd and pB17sd demonstrate that the SD sequence is required for DB-enhanced cspB expression (Fig. 2A). When the SD sequence was deleted from pB13, the translational efficiency became even lower than that of pB3 at low temperature; the translational efficiency of pB13sd was reduced to 25% of that of pB3 (Table 1). In addition, although the mRNA half-lives of pB3 (12 min) and pB13sd (17 min) are similar, the β-galactosidase activity of pB3 is four times higher than that of pB13sd. This result clearly indicates that the role of the DB in translation initiation is dependent on the SD sequence.
Inhibition of translation by antibiotics (4) or by temperature downshift (8) triggers the induction of the major cold shock proteins, CspA, CspB, and CspG. The presence of a translational enhancer, such as DB, in an mRNA could explain the preferred synthesis of these cold shock proteins when global protein synthesis is inhibited. Here we demonstrate that the DB is an essential translational cis-acting element for the induction of cspB at low temperature and that the activity of DB as a translational enhancer is dependent on the SD sequence. Furthermore, it is speculated that additional base pairings over the conventional DB base pairings between mRNA and 16S rRNA further enhance translation. These results are consistent with our recent findings about the role of the DB as an SD-dependent translational enhancer obtained using an artificial perfectly matching DB (3).
Acknowledgments
The present work was supported by a grant from the National Institute of Health (GM19043).
REFERENCES
- 1.Brandi A, Pietroni P, Gualerzi C O, Pon C L. Post-transcriptional regulation of CspA expression in Escherichia coli. Mol Microbiol. 1996;19:231–240. doi: 10.1046/j.1365-2958.1996.362897.x. [DOI] [PubMed] [Google Scholar]
- 2.Etchegaray J-P, Jones P G, Inouye M. Differential thermoregulation of two highly homologous cold-shock genes, cspA and cspB, of Escherichia coli. Genes Cell. 1996;1:171–178. doi: 10.1046/j.1365-2443.1996.d01-231.x. [DOI] [PubMed] [Google Scholar]
- 3.Etchegaray J-P, Inouye M. Translational enhancement by an element downstream of the initiation codon in Escherichia coli. J Biol Chem. 1999;274:10079–10085. doi: 10.1074/jbc.274.15.10079. [DOI] [PubMed] [Google Scholar]
- 4.Etchegaray J-P, Inouye M. CspA, CspB, and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature under conditions that completely block protein synthesis. J Bacteriol. 1999;181:1827–1830. doi: 10.1128/jb.181.6.1827-1830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang L, Jiang W, Bae W, Inouye M. Promoter-independent cold-shock induction of cspA and its derepression at 37°C by mRNA stabilization. Mol Microbiol. 1997;23:355–364. doi: 10.1046/j.1365-2958.1997.2351592.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg D, Azar I, Opphenheim A B. Differential mRNA stability of the cspA gene in the cold-shock response in Escherichia coli. Mol Microbiol. 1996;19:241–248. doi: 10.1046/j.1365-2958.1996.363898.x. [DOI] [PubMed] [Google Scholar]
- 7.Iost I, Dreyfus M. The stability of Escherichia coli lacZ mRNA depends upon the simultaneity of its synthesis and translation. EMBO J. 1995;14:3252–3261. doi: 10.1002/j.1460-2075.1995.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones P G, Inouye M. The cold-shock response—a hot topic. Mol Microbiol. 1994;11:811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones P G, Inouye M. RbfA, a 30S ribosomal binding factor, is a cold-shock protein whose absence triggers the cold-shock response. Mol Microbiol. 1996;21:1207–1218. doi: 10.1111/j.1365-2958.1996.tb02582.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee S J, Xie A, Jiang W, Etchegaray J-P, Jones P G, Inouye M. Family of the major cold-shock protein, CspA (CS7.4) of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol Microbiol. 1994;11:833–839. doi: 10.1111/j.1365-2958.1994.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 11.Lopilato J, Bortuer S, Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduces plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986;205:285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- 12.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 352–355. [Google Scholar]
- 13.Mitta M, Fang L, Inouye M. Deletion analysis of cspA of E. coli: requirement of the AT-rich UP element for cspA transcription and downstream box in the coding region for its cold-shock induction. Mol Microbiol. 1997;26:321–335. doi: 10.1046/j.1365-2958.1997.5771943.x. [DOI] [PubMed] [Google Scholar]
- 14.Sarmientos P, Sylvester J E, Contente S, Cashel M. Differential stringent control of the tandem E. coli: ribosomal RNA promoters from RNA operon expressed in vivo in multicopy plasmids. Cell. 1983;32:1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- 15.Simmons R W, Houman F, Klechner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 16.Sprengart M L, Fuchs E, Porter A G. The downstream box: an efficient and independent translation initiation signal in Escherichia coli. EMBO J. 1996;15:665–674. [PMC free article] [PubMed] [Google Scholar]