Abstract
Purpose
Safely postponing the use of chemotherapy is important for quality of life maintenance in patients with hormone receptor-positive advanced breast cancer. In previous studies, a combination of cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) and fulvestrant prolonged the time to chemotherapy (TTC). In this study, we used real-world data to evaluate TTC in the context of CDK4/6i therapy.
Methods
We performed a retrospective chart review of women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated at the Aichi Cancer Center Hospital. The patients were categorized into having received CDK4/6i therapy first (n = 41), second (n = 33), and none at all (n = 67). The change in TTC among the groups was examined.
Results
The median follow-up time was 13.8, 27.5, and 30.3 months in the CDK4/6i (first), CDK4/6i (second), and non-CDK4/6i groups, respectively. The median progression-free survival (PFS) with first-line therapy for metastasis was 30.0, 11.9, and 13.0 months, respectively (CDK4/6i [first] vs. non-CDK4/6i; p = 0.018, CDK4/6i [second] vs. non-CDK4/6i; p = 0.383). The median TTC was not reached in the CDK4/6i (first) group, was 39.1 months in the CDK4/6i (second) group, and was 44.2 months in the non-CDK4/6i group (CDK4/6i [first] vs. non-CDK4/6i; p = 0.880; CDK4/6i [second] vs. non-CDK4/6i; p = 0.407). The non-CDK4/6i group with TTC ≥ 60 months included more cases of secondary endocrine therapy resistance (p = 0.017), no perioperative chemotherapy (p = 0.021), and a longer disease-free interval (p = 0.093).
Conclusion
Although PFS was significantly longer in the CDK4/6i (first) group than in the non-CDK4/6i group, TTC did not significantly differ among the three groups in real-world data. The non-CDK4/6i group showed a long TTC in patients with late recurrence and low risk at the primary lesion site, who benefited greatly from hormone monotherapy.
Keywords: Breast Neoplasms; Cyclin-Dependent Kinases; Neoplasm Metastasis; Receptors, Estrogen
INTRODUCTION
Approximately 70% of breast cancers are hormone receptor (HR)-positive. Endocrine therapy is the first-line treatment for metastatic or advanced HR-positive breast cancer. The development of cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6is) has greatly benefited patients with HR-positive and human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer. Three CDK4/6is, palbociclib, abemaciclib, and ribociclib, considerably prolonged patient progression-free survival (PFS) when combined with a nonsteroidal aromatase inhibitor, as shown in the PALOMA-2, MONARCH-3, and MONALEESA-2 studies respectively [1,2,3]. Therapy with these nonsteroidal aromatase inhibitors also improved PFS when administered in combination with fulvestrant in patients whose tumors had progressed during previous endocrine therapy administration in the PALOMA-3, MONARCH-2, and MONALEESA-3 studies [4,5,6]. In addition, MONARCH-2 and MONALEESA-3 reported positive overall survival (OS) results, particularly upon abemaciclib or ribociclib use. CDK4/6is have become the standard first-line treatment for HR-positive and HER2-negative advanced breast cancer.
Postponing the use of chemotherapy for as long as possible is important for maintaining the quality of life of patients with advanced HR-positive breast cancer [7]. Although CDK4/6i have contributed to prolonged PFS and OS, approximately half of the treatments administered after discontinuation of CDK4/6i are chemotherapeutic [8,9]. In the PALOMA-3, MONARCH-2, and MONALEESA-3 studies, the addition of CDK4/6i prolonged both the time to chemotherapy (TTC) and chemotherapy-free survival (CFS); however, whether these results are also applicable in real-world settings is unclear [8,9,10].
In Japan, palbociclib and abemaciclib treatments have been covered under insurance since November 2017 and 2018, respectively. However, ribociclib has not been approved yet for use in Japan. In this study, we analyzed the changes in patient TTC and CFS in the context of CDK4/6i use.
METHODS
Patients
We conducted a retrospective chart review of women with estrogen receptor (ER)-positive and HER2-negative advanced breast cancer, either de novo or recurrent, who were treated at the Aichi Cancer Center Hospital from 2011 to 2020. Patient characteristics were retrieved from clinical records and reviewed. The patients were divided into three groups: CDK4/6i (first), CDK4/6i (second), and non-CDK4/6i.
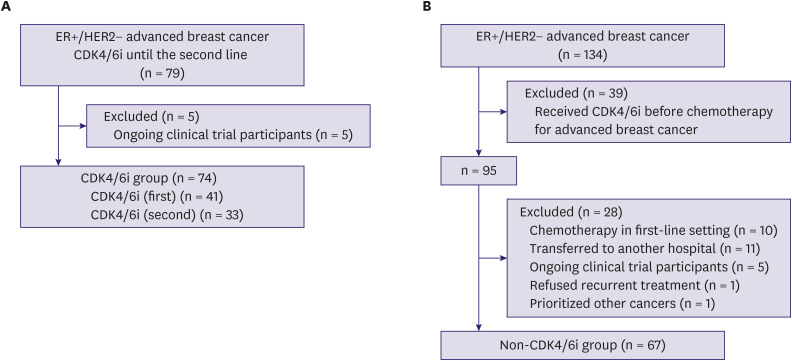
The CDK4/6i group comprised patients who received CDK4/6i until second-line treatment without prior chemotherapy for advanced breast cancer. We collected data from 79 patients diagnosed with advanced breast cancer between January 2016 and December 2020. Patients were excluded from the study if they were participating in an ongoing clinical trial (n = 5). Finally, 74 patients were included in the CDK4/6i group. Of these patients, 41 (55%) received first-line CDK4/6i (CDK4/6i [first] group), and 33 (45%) received second-line CDK4/6i (CDK4/6i [second] group) (Figure 1A).
Figure 1. CONSORT diagram. (A) CDK4/6i group and (B) non-CDK4/6i group.
CDK4/6i = cyclin-dependent kinase 4 and 6 inhibitor; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2.
The non-CDK4/6i group comprised patients diagnosed with advanced breast cancer between November 2011 and December 2015. Records of 134 patients were retrieved. Patients were excluded from this study if they 1) received CDK4/6i before chemotherapy for advanced breast cancer (n = 39), 2) received chemotherapy as first-line treatment (n = 10), 3) were transferred to another hospital immediately after recurrence (n = 11), 4) participated in a clinical trial (n = 5), 5) refused recurrent treatment (n = 1), or 6) received prioritized treatment for other cancers (n = 1). After exclusions, 67 patients were included in the non-CDK4/6i group (Figure 1B).
Treatment
Palbociclib was administered at a dose of 125 mg/day for 3 weeks, followed by a 1 week drug holiday, abemaciclib 150 mg twice daily, and everolimus 10 mg once daily. Letrozole, anastrozole, exemestane, and tamoxifen were administered at doses of 2.5, 1, 25, and 20 mg, respectively. One 500 mg dose of fulvestrant was administered by intramuscular injection on days 1 and 15 of the first cycle and then on day one of each subsequent cycle (28 days). The dose was reduced based on the occurrence of adverse events.
Assessment
We analyzed PFS following first-line treatment of metastases, TTC, and CFS in the CDK4/6i (first), CDK4/6i (second), and non-CDK4/6i groups.
Statistical analysis
All statistical analyses were performed using Stata version 15.1 (Stata Corporation, College Station, USA). The duration of therapy was used to calculate TTC and CFS. TTC was defined as the period from the start of first-line treatment with endocrine therapy to the date of chemotherapy initiation. CFS was defined as the period from the start of the first-line treatment with endocrine therapy to the date of chemotherapy initiation or death. Patients with primary endocrine therapy resistance, as defined in the MONARCH-2 study, were those whose disease relapsed during the first 2 years of neoadjuvant or adjuvant endocrine therapy or progressed while receiving endocrine therapy for advanced breast cancer during the first 6 months. Patients not considered to have primary endocrine therapy resistance were defined as having secondary resistance [5]. Statistical analyses were performed using updated data, with a cut-off date of August 24, 2021. The median TTC and CFS were estimated using the Kaplan-Meier method, and significance was determined using a generalized Wilcoxon test. We used the χ2 test to compare categorical variables and the Mann-Whitney U test to compare continuous variables. All reported p-values were two-sided, and statistical significance was set at p < 0.05.
Ethical approval
This study was reviewed and approved by the Institutional Review Board (2021-1-082) and was conducted in accordance with the 1964 Declaration of Helsinki. Informed consent was obtained in the form of an opt-out form accessible on the institutional website.
RESULTS
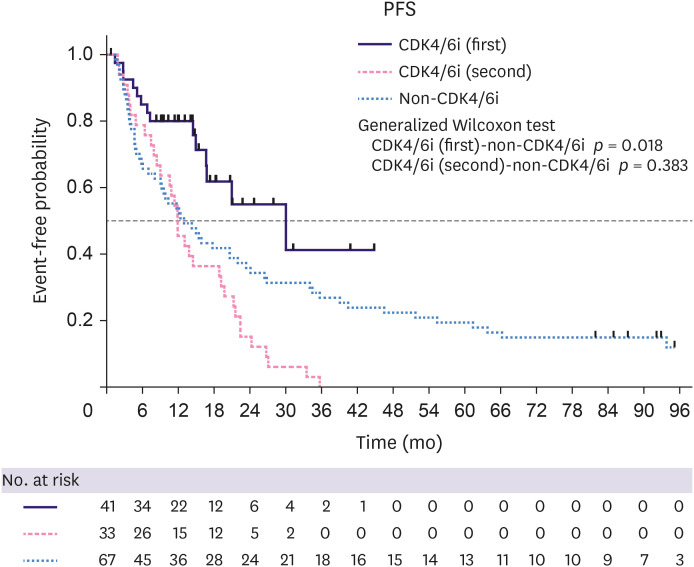
Patient characteristics are shown in Table 1, among which no significant differences were observed, except in median age, menopausal status, disease stage at initial diagnosis, and median PFS with first-line therapy for metastatic disease. The median age and the number of women who had reached menopause in the CDK4/6i (first) group were lower than those of the non-CDK4/6i group (p = 0.003 and p = 0.004, respectively). Eleven patients (33%) in the CDK4/6i (second) group and seven patients (10%) in the non-CDK4/6i group presented with de novo stage IV disease. De novo stage IV tumors were more common in the CDK4/6i (second) group than in the non-CDK4/6i group. Endocrine therapy, in combination with a CDK4/6 inhibitor in the CDK4/6i (first) group, included aromatase inhibitors (51%) and fulvestrant (49%). The median PFS in first-line therapy for metastatic disease was 30.0 months in the CDK4/6i (first) group, 11.9 months in the CDK4/6i (second) group, and 13.0 months in the non-CDK4/6i group (CDK4/6i [first] vs. non-CDK4/6i; p = 0.018, CDK4/6i [second] vs. non-CDK4/6i; p = 0.383) (Table 1, Figure 2).
Table 1. Patient characteristics.
| Characteristics | CDK4/6i (first) | CDK4/6i (second) | Non-CDK4/6i | p1* | p2* | ||
|---|---|---|---|---|---|---|---|
| All patients | 41 | 33 | 67 | ||||
| Median age (range) | 51 (34–78) | 57 (35–81) | 63 (27–88) | 0.003 | 0.076 | ||
| Menopausal status† | 0.004 | 0.518 | |||||
| Pre- or perimenopause | 21 (51%) | 10 (30%) | 16 (24%) | ||||
| Post | 20 (49%) | 23 (70%) | 50 (75%) | ||||
| Disease stage at initial diagnosis‡ | 0.409 | 0.023 | |||||
| I | 9 (22%) | 3 (9%) | 14 (21%) | ||||
| II | 19 (46%) | 14 (42%) | 26 (39%) | ||||
| III | 6 (15%) | 5 (15%) | 18 (27%) | ||||
| IV | 7 (17%) | 11 (33%) | 7 (10%) | 0.351§ | 0.006§ | ||
| Histological type | 0.676 | 0.107 | |||||
| Invasive ductal carcinoma | 37 (90%) | 27 (82%) | 62 (93%) | ||||
| Invasive lobular carcinoma | 4 (10%) | 6 (18%) | 5 (7%) | ||||
| Histological grade‖ | 0.570 | 0.179 | |||||
| Low/Intermediate | 31 (76%) | 27 (82%) | 42 (63%) | ||||
| High | 9 (22%) | 6 (18%) | 16 (24%) | ||||
| Progesterone receptor status¶ | 0.074 | 0.871 | |||||
| Negative | 2 (5%) | 5 (15%) | 11 (16%) | ||||
| Positive | 39 (95%) | 28 (85%) | 56 (84%) | ||||
| Postoperative metastatic lymph nodes** | 0.870 | 0.624 | |||||
| Negative | 15 (37%) | 8 (24%) | 25 (37%) | ||||
| Positive | 19 (46%) | 14 (42%) | 34 (51%) | ||||
| Metastatic site at the time of relapse†† | 0.143 | 0.552 | |||||
| Visceral disease‡‡ | 25 (61%) | 13 (39%) | 29 (43%) | ||||
| Liver involvement | 14 | 6 | 9 | ||||
| Bone only | 12 (29%) | 9 (27%) | 21 (31%) | ||||
| Other | 4 (10%) | 11 (33%) | 15 (22%) | ||||
| Median DFI (range) | 44.3 (10–245) | 53.9 (3–146) | 54.0 (10–294) | 0.496 | 0.992 | ||
| ≤ 12 months | 1 (2%) | 2 (6%) | 2 (3%) | 0.637 | 0.559 | ||
| > 12 and ≤ 60 months | 21 (51%) | 11 (33%) | 31 (46%) | ||||
| > 60 months | 12 (29%) | 9 (27%) | 27 (40%) | ||||
| Prior chemotherapy for neoadjuvant or adjuvant treatment§§ | 0.835 | 0.875 | |||||
| Yes | 20 (49%) | 13 (39%) | 36 (54%) | ||||
| No | 14 (34%) | 9 (27%) | 23 (34%) | ||||
| Endocrine therapy with neoadjuvant or adjuvant treatment‖‖ | 0.059 | 0.402 | |||||
| Aromatase inhibitors | 10 (24%) | 10 (30%) | 26 (39%) | ||||
| Tamoxifen | 21 (51%) | 10 (30%) | 20 (30%) | ||||
| Aromatase inhibitors and tamoxifen | 1 (2%) | 2 (6%) | 7 (10%) | ||||
| None | 2 (5%) | 0 (0%) | 6 (9%) | ||||
| Primary endocrine therapy for metastatic disease | - | 0.394 | |||||
| Aromatase inhibitors | - | 16 (48%) | 35 (52%) | ||||
| Tamoxifen | - | 10 (30%) | 23 (34%) | ||||
| Fulvestrant | - | 7 (21%) | 7 (11%) | ||||
| Other | - | 0 (0%) | 2 (3%) | ||||
| Median PFS of first-line for metastatic disease (range) | 30.0 (0.5–44) | 11.9 (2–36) | 13.0 (1–115) | 0.027¶¶ | 0.377¶¶ | ||
| Endocrine therapy resistance | - | - | |||||
| Primary | - | 10 (30%) | - | ||||
| Secondary | - | 23 (70%) | - | ||||
| Type of CDK4/6i | - | - | |||||
| Palbociclib | 23 (56%) | 20 (61%) | - | ||||
| Abemaciclib | 18 (44%) | 13 (39%) | - | ||||
| Endocrine therapy in combination with CDK4/6i | - | - | |||||
| Aromatase inhibitors | 21 (51%) | 13 (39%) | - | ||||
| Fulvestrant | 20 (49%) | 20 (61%) | - | ||||
CDK4/6i = cyclin-dependent kinase 4 and 6 inhibitor; DFI = disease-free interval; PFS = progression-free survival.
*p1-value is the p-value of the CDK4/6i (first) group vs. that of the non-CDK4/6i group, and p2-value is the p-value of the CDK4/6i (second) group vs. that of the non-CDK4/6i group.
†Menopausal status was not available for one patient in the non-CDK4/6i group.
‡Disease stage at initial diagnosis was not available for two patients in the non-CDK4/6i group.
§p1- and p2-values indicate the p-value of non-stage IV versus stage IV in each group.
∥Histological grade was not available for one and nine patients in the CDK4/6 (first) and non-CDK4/6i groups, respectively.
¶Allred scores of 3–8 are considered positive.
**Number of postoperative metastatic lymph node was not available for one patient in the non-CDK4/6i group.
††Metastatic sites were not available for two patients in the non-CDK4/6i group.
‡‡Viscera refers to the lungs, liver, pleura, and adrenal glands.
§§Prior chemotherapy for neoadjuvant or adjuvant treatment was not available for one patient in the non-CDK4/6i group.
∥∥Endocrine therapy with neoadjuvant or adjuvant treatment was not available for one patient in the non-CDK4/6i group.
¶¶Median PFS after first-line treatment for metastatic disease was assessed using the generalized Wilcoxon test.
Figure 2. Kaplan–Meier plots of PFS in the first-line setting.
PFS = progression-free survival; CDK4/6i = cyclin-dependent kinase 4 and 6 inhibitor.
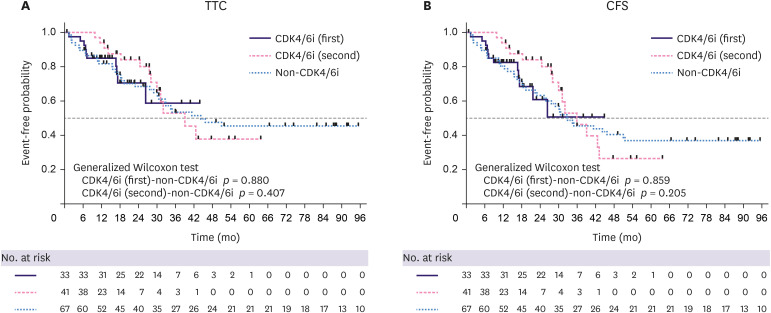
At the time of data cut-off for TTC analysis, the treatment for 57 patients with advanced breast cancer was changed to chemotherapy (10 of 41 patients [24%] in the CDK4/6i [first] group, 13 of 33 [39% in the CDK4/6i (second) group, and 34 of 67 [51%] in the non-CDK4/6i group). The median length of follow-up was 13.8 months (9.8–43.6 months) for all patients in the CDK4/6i (first) group, 27.5 months (0.5–63.4 months) in the CDK4/6i (second) group, and 30.3 months (1.4–117.0 months) in the non-CDK4/6i group. The median TTC was not reached in the CDK4/6i (first) group, 39.1 months in the CDK4/6i (second) group, and 44.2 months in the non-CDK4/6i group (Figure 3A). The median TTC did not significantly differ between the groups (CDK4/6i [first] vs. non-CDK4/6i; p = 0.880; CDK4/6i [second] vs. non-CDK4/6i; p = 0.407).
Figure 3. Kaplan–Meier plots of (A) TTC and (B) CFS.
TTC = time to chemotherapy; CFS = chemotherapy-free survival; CDK4/6i = cyclin-dependent kinase 4 and 6 inhibitor.
At the cut-off point, 50 patients died (4 of 41 patients [10%] in the CDK4/6i [first] group, 33 patients [21%] in the CDK4/6i [second] group, and 39 of 67 patients [58%] in the non-CDK4/6i group). The median CFS was not reached in the CDK4/6i (first) group, was 35.9 months in the CDK4/6i (second) group, and was 32.9 months in the non-CDK4/6i group (Figure 3B). Group median CFS was also not significantly different (CDK4/6i [first] vs. non-CDK4/6i; p = 0.859; CDK4/6i [second] vs. non-CDK4/6i; p = 0.205).
The treatment line at the time of data cut-off or chemotherapy initiation in each group is shown in the supplementary table (Supplementary Table 1). There were 31 patients in the CDK4/6i (first) group, 20 patients in the CDK4/6i (second) group, and 33 patients in the non-CDK4/6i group who were not receiving chemotherapy at the time of the data cut-off for TTC data analysis. Twenty-five and 12 patients in the CDK4/6i (first) and CDK4/6i (second) groups, respectively, received CDK4/6i on the cut-off day. The median endocrine treatment lines at the data cut-off for patients who did not receive chemotherapy during the observation period were 2, 3, and 2 in the CDK4/6i (first), CDK4/6i (second), and non-CDK4/6i groups, respectively. The median number of treatment lines at the start of chemotherapy during the observation period was 3, 4, and 3 in the CDK4/6i (first), CDK4/6i (second), and non-CDK4/6i groups, respectively. There was no apparent difference between the median endocrine treatment line and the median treatment line at chemotherapy initiation in patients treated with or without CDK4/6i. In the non-CDK4/6i group, eight of 39 (21%) patients had never received chemotherapy for advanced breast cancer before they died of the disease. This was due to patient preferences or advanced age.
In the non-CDK4/6i group, no patient received chemotherapy 60 months after the start of first-line treatment with endocrine therapy (Figure 3A). Table 2 shows the patient characteristics of the groups with TTC < 60 months and TTC ≥ 60 months among the non-CDK4/6i group. The TTC ≥ 60 months group tended to have a lower histological grade, no postoperative metastatic lymph nodes, and a longer disease-free interval (DFI) than the TTC < 60 months group. Furthermore, significantly more cases without perioperative chemotherapy (p = 0.021) and secondary endocrine therapy resistance (p = 0.017) were found in the TTC ≥ 60 months group. We also observed a long TTC in cases with late recurrence and low risk at the primary lesion, such as low histological grade, no postoperative metastatic lymph nodes, long DFI, no perioperative chemotherapy, and secondary endocrine therapy resistance.
Table 2. Patient characteristics in the TTC < 60 and TTC ≥ 60 months groups.
| Characteristics | TTC < 60 months | TTC ≥ 60 months | p | ||
|---|---|---|---|---|---|
| All patients | 46 | 21 | |||
| Median age (range) | 63 (27–88) | 62 (32–76) | 0.398 | ||
| < 60 | 28 (61%) | 9 (43%) | 0.169 | ||
| ≥ 60 | 18 (39%) | 12 (57%) | |||
| Menopausal status* | 0.472 | ||||
| Pre- or perimenopause | 10 (22%) | 6 (29%) | |||
| Post | 36 (78%) | 14 (30%) | |||
| Disease stage at initial diagnosis† | 0.103 | ||||
| I | 7 (15%) | 7 (33%) | |||
| II | 18 (39%) | 8 (38%) | |||
| III | 16 (35%) | 2 (10%) | |||
| IV | 4 (9%) | 3 (14%) | 0.463‡ | ||
| Histological type | 0.570 | ||||
| Invasive ductal carcinoma | 42 (91%) | 20 (95%) | |||
| Invasive lobular carcinoma | 4 (9%) | 1 (5%) | |||
| Histological grade§ | 0.083 | ||||
| Low/intermediate | 27 (59%) | 15 (71%) | |||
| High | 14 (30%) | 2 (10%) | |||
| Progesterone receptor status‖ | 0.629 | ||||
| Negative | 6 (13%) | 5 (24%) | |||
| Positive | 40 (87%) | 16 (76%) | |||
| Postoperative metastatic lymph node¶ | 0.054 | ||||
| Negative | 14 (30%) | 11 (52%) | |||
| Positive | 27 (59%) | 7 (33%) | |||
| Metastatic site at the time of relapse** | 0.375 | ||||
| Visceral†† | 22 (48%) | 7 (33%) | |||
| Liver involvement | 9 | 0 | |||
| Bone only | 12 (26%) | 9 (43%) | |||
| Other | 10 (22%) | 5 (24%) | |||
| Median DFI (range) | 49.4 (10–294) | 79.6 (10–215) | 0.093 | ||
| < 60 months | 25 (54%) | 8 (38%) | 0.282 | ||
| ≥ 60 months | 17 (37%) | 10 (48%) | |||
| Chemotherapy with neoadjuvant or adjuvant treatment‡‡ | 0.241 | ||||
| Anthracycline and taxane | 18 (39%) | 4 (19%) | |||
| Anthracycline only | 5 (11%) | 1 (5%) | |||
| Taxane only | 3 (7%) | 1 (5%) | |||
| Other | 3 (7%) | 1 (5%) | |||
| None | 12 (26%) | 11 (52%) | 0.021§§ | ||
| Endocrine therapy with neoadjuvant or adjuvant treatment‖‖ | 0.031 | ||||
| Aromatase inhibitors | 20 (43%) | 6 (29%) | |||
| Tamoxifen | 15 (33%) | 5 (24%) | |||
| Aromatase inhibitors and tamoxifen | 5 (11%) | 2 (10%) | |||
| None | 1 (2%) | 5 (24%) | |||
| Endocrine therapy resistance | 0.017 | ||||
| Primary | 23 (50%) | 4 (19%) | |||
| Secondary | 23 (50%) | 17 (81%) | |||
TTC = time to chemotherapy; DFI = disease-free interval.
*Menopausal status was not available for one patient in the TTC ≥ 60 months group.
†Disease stage at initial diagnosis was not available for one patient each in the TTC < 60 months and TTC ≥ 60 months groups.
‡p-value is that of non-stage IV versus stage IV patients in the two groups.
§Histological grade was not available for five patients in the TTC < 60 months group and four patients in the TTC ≥ 60 months group.
∥Allred scores of 3–8 were considered positive.
¶Postoperative metastatic lymph nodes were not available for one patient in the TTC < 60 months group.
**Metastatic site at the time of relapse was not available for two patients in the TTC < 60 months group.
††Viscera refers to the lungs, liver, pleura, and adrenal glands.
‡‡Chemotherapy with neoadjuvant or adjuvant treatment was not available for one patient in the TTC < 60 months group.
§§p-value is that of the chemotherapy group versus the non-chemotherapy group.
∥∥Endocrine therapy with neoadjuvant or adjuvant treatment was not available for one patient in the TTC < 60 months group.
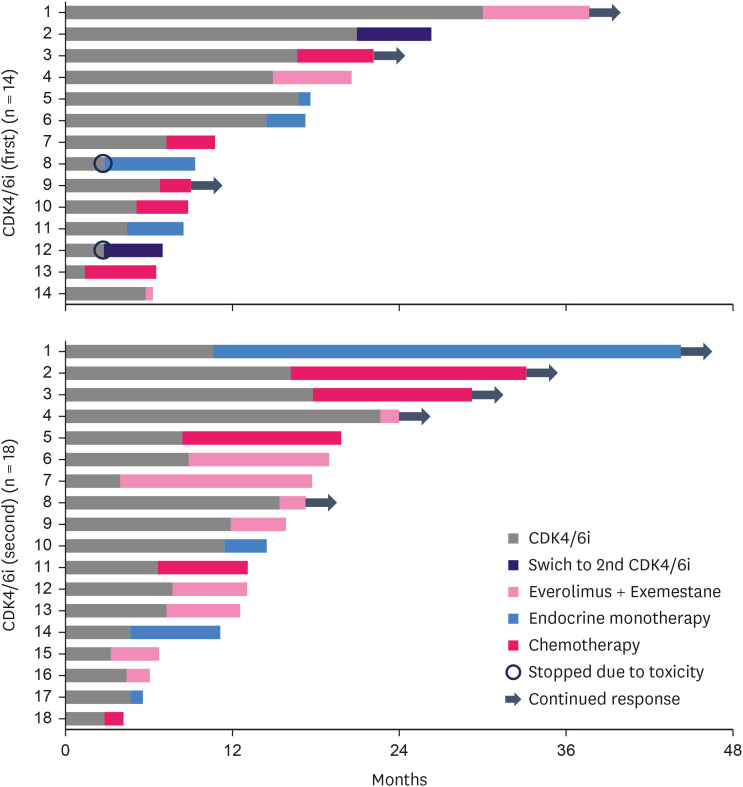
Post-discontinuation therapy for CDK4/6i is shown in Figure 4. A total of 37 patients in the CDK4/6i group discontinued CDK4/6i treatment. Of these, 32 (86.5%) received post-discontinuation therapy at our institution, with 14 and 18 patients in the first- and second-line settings, respectively. Of the 32 patients who received post-discontinuation therapy, 35.7% in the CDK4/6i (first) group and 27.8% in the CDK4/6i (second) group received chemotherapy. Endocrine monotherapy was administered to 35.7% of the patients in the CDK4/6i group (first) and 22.2% in the CDK4/6i (second) group. The percentage of patients who received mTOR inhibitors in conjunction with endocrine therapy was 21.4% in the CDK4/6i (first) group and 50.0% in the CDK4/6i (second) group. Endocrine therapy was administered to 60%–70% of the patients, regardless of the CDK4/6i line. mTOR inhibitors and endocrine therapy have been used as recurrent third-line treatments in many cases. Figure 4 shows detailed treatment outcomes after CDK4/6i treatment failure. Discontinuation of CDK4/6i occurred mainly because of disease progression and adverse effects in only two cases. Endocrine monotherapy involved administration of fulvestrant, anastrozole, tamoxifen, and medroxyprogesterone acetate. Capecitabine is the most frequently administered chemotherapeutic drug. The duration of response to post-discontinuation therapy for CDK4/6i varied from 1 to 34 months, regardless of the treatment type.
Figure 4. Swimmer plot of patients who progressed on CDK4/6i by treatment category.
CDK4/6i = cyclin-dependent kinase 4 and 6 inhibitor.
DISCUSSION
In this study, TTC and CFS did not differ significantly between patients with ER-positive, HER2-negative advanced breast cancer, regardless of whether CDK4/6i was administered before chemotherapy for advanced breast cancer. In the PALOMA-2 study, in which CDK4/6i was used as the first-line treatment for advanced breast cancer, PFS and TTC were significantly different between the palbociclib and letrozole and placebo–letrozole groups (p < 0.001 and p < 0.005, respectively) [1,11]. Similar to the results of the PALOMA-2 study, PFS differed significantly between the CDK4/6i (first) and non-CDK4/6i groups in this study (p = 0.018). Fulvestrant was administered to approximately half of the patients in the CDK4/6i (first) group, which differed from that in the PALOMA-2 study. According to the results of the PARSIFAL trial, this may not have affected PFS [12]. One reason for the lack of difference in TTC is that TTC in the non-CDK4/6i group may have been excessively prolonged. However, the median number of endocrine treatment lines in the non-CDK4/6i group was the same as in the CDK4/6i (first) group.
In the non-CDK4/6i group, some patients continued endocrine therapy for > 60 months without chemotherapy. We observed a long TTC in cases with late recurrence and low-risk primary lesion, such as those with secondary endocrine therapy resistance, no perioperative chemotherapy, no postoperative metastatic lymph nodes, low histological grade, and long DFI. Such cases may have had a prolonged TTC in the non-CDK4/6i group; as such, early CDK4/6i treatment may not always be necessary for ER-positive, HER2-negative breast cancer. Selecting cases in which CDK4/6i can be omitted would avoid unnecessary adverse effects and economic burden [7].
There is no clear evidence for benefit from post-CDK4/6i treatment discontinuation therapy. Patients in the PALOMA-3 study who had undergone previous endocrine therapy had immediate subsequent chemotherapy (55.6%), mTOR inhibitors (16.1%), and CDK4/6 inhibitors (2.4%) [9]. Thus, more than half of the patients were administered third-line chemotherapy after discontinuation of CDK4/6i. In this study, 27.8% of the patients were treated with CDK4/6i in the second-line setting; thus, chemotherapy was used more often in the PALOMA-3 study.
In Japan, tumor markers such as CA15-3 and CEA are occasionally evaluated regularly during postoperative follow-up, as recurrence can occur in patients without subjective symptoms. The timing of recurrence detection may differ between Europe, the United States, and Japan, and differences in tumor burden at the start of recurrence treatment may be related to the duration of endocrine therapy in patients with ER-positive and HER2-negative advanced breast cancer. Endocrine therapy is initiated when the tumor burden is small; therefore, chemotherapy may be administered less frequently in an early line setting.
We observed no obvious relationship between the duration of response to CDK4/6i and subsequent treatment. Everolimus and exemestane are often used at our institution as next-line therapies after receiving CDK4/6is. In some cases, a long-term response could be obtained with endocrine therapy alone; in other cases, endocrine monotherapy had to be discontinued after approximately 1 month, as shown in Figure 4.
Clinical data on TTC and CFS based on the use of CDK4/6is for ER-positive and HER2-negative advanced breast cancers are limited. As CDK4/6i was approved approximately 3 years ago in Japan, the median follow-up in this study was short, the sample size was small, and the number of events was low. There was also an imbalance in patient characteristics due to the retrospective nature of this study. In addition, TTC and CFS were defined in this study as the start of first-line treatment with endocrine therapy, which differs from the definitions used in the PALOMA2,3 and MONARCH2,3 studies, making direct comparison difficult.
In conclusion, although PFS was significantly longer in the CDK4/6i (first) group than in the non-CDK4/6i group, TTC did not differ significantly among the three groups based on real-world data. In the non-CDK4/6i group, we observed good responses and clinical outcomes in low-risk cases, such as in cases with secondary endocrine therapy resistance, no perioperative chemotherapy, no postoperative metastatic lymph nodes, low histological grade, and long DFI, which may reduce unnecessary adverse effects as well as economic burden.
Footnotes
Presentation: This paper was presented at the 29th Annual Meeting of the Japanese Breast Cancer Society (JBCS).
Conflict of Interest: Iwata received lecture fees from Pfizer Inc. and Eli Lilly and Company. Hattori received lecture fees from Eli Lilly and Company. The other authors have no conflicts of interest to declare.
- Conceptualization: Iwata H.
- Investigation: Yoshimura A, Sawaki M, Hattori M, Kotani H, Kataoka A, Horisawa N, Ozaki Y, Nozawa K, Takatsuka D, Isogai A.
- Supervision: Iwata H.
- Visualization: Endo Y. Writing - original draft.
- Writing - review & editing: Endo Y.
SUPPLEMENTARY MATERIAL
Treatment line
References
- 1.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 2.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 3.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 5.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman PA, Toi M, Neven P, Sohn J, Grischke EM, Andre V, et al. Health-related quality of life in MONARCH 2: abemaciclib plus fulvestrant in hormone receptor-positive, HER2-negative advanced breast cancer after endocrine therapy. Oncologist. 2020;25:e243–e251. doi: 10.1634/theoncologist.2019-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical Trial. JAMA Oncol. 2020;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 11.Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llombart-Cussac A, Pérez-García JM, Bellet M, Dalenc F, Gil-Gil M, Ruíz-Borrego M, et al. Fulvestrant-palbociclib vs letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor-positive, ERBB2-negative advanced breast cancer: a randomized clinical trial. JAMA Oncol. 2021;7:1791–1799. doi: 10.1001/jamaoncol.2021.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment line