Abstract
The interaction of T4 phage-encoded anti-sigma factor, asiA, and Escherichia coli ς70 was studied by using the yeast two-hybrid system. Truncation of ς70 to identify the minimum region involved in the interaction showed that the fragment containing amino acid residues proximal to the C terminus (residues 547 to 603) was sufficient for complexing to asiA. Studies also indicated that some of the truncated C-terminal fragments (residues 493 to 613) had higher affinity for asiA as judged by the increased β-galactosidase activity. It is proposed that the observed higher affinity may be due to the unmasking of the binding region of asiA on the sigma protein. Advantage was taken of the increased affinity of truncated ς70 fragments to asiA in designing a coexpression system wherein the toxicity of asiA expression in E. coli could be neutralized and the complex of truncated ς70 and asiA could be expressed in large quantities and purified.
Anti-sigma proteins are known to play an important role in the regulation of gene expression in procaryotes (4). The T4 asiA protein, encoded by an early gene of the T4 phage, is responsible for switching off transcription at Escherichia coli promoters which are transcribed by the ς70 protein. This 10-kDa protein, originally described by Audrey Stevens as an inhibitor of E. coli transcription (22), was characterized by Brody and coworkers (1, 3, 16–18). The protein was shown to complex with both free ς70 and ς70 bound to the core enzyme. Additionally, they were also able to show that while asiA inhibited transcription at the ς70 promoters, it acted as a positive regulator in transcribing the middle genes of T4 phage in association with another T4-encoded protein, motA (17, 18).
Different approaches have been used to define the regions of ς70 interacting with asiA. While Colland et al. (6) and Severinova et al. (19) showed that region 4.2 of ς70 was involved in the interactions with asiA, studies using partial proteolysis led to the identification of 58 amino acids proximal to the C terminus as the site for interacting with asiA (20). The main approaches in these studies involved in vitro binding and protein cross-linking. In the present study, the yeast two-hybrid system was used to characterize the regions of ς70 involved in binding to asiA. This genetic system has been successfully used for studying the interactions of various proteins of procaryotic and eucaryotic origin (5, 7, 11–13, 23, 24). The two-hybrid system also provided a qualitative comparison of the binding affinities of asiA with the full-length E. coli ς70 protein and its truncated derivatives, which indicates that the asiA binding region which corresponds to region 4.2 of the native ς70 may be partially sequestered.
One of the major limitations in obtaining structural information regarding asiA is the toxicity of this protein when expressed in E. coli. The study of asiA-ς70 interaction by the two-hybrid system showed that the truncated forms of ς70 had higher affinity for asiA. Based on this observation, coexpression of a truncated ς70 fragment, ς70C121 (residues 493 to 613), with asiA in E. coli resulted in the neutralization of toxicity of asiA. The asiA-ς70C121 was shown to form a stable complex in E. coli, and this protein complex was purified to homogeneity by a single-step purification through an affinity column. The purified asiA-ς70 complex should be a good candidate for structural studies by nuclear magnetic resonance (NMR) and crystallography.
Yeast two-hybrid system detects asiA-ς70 interaction.
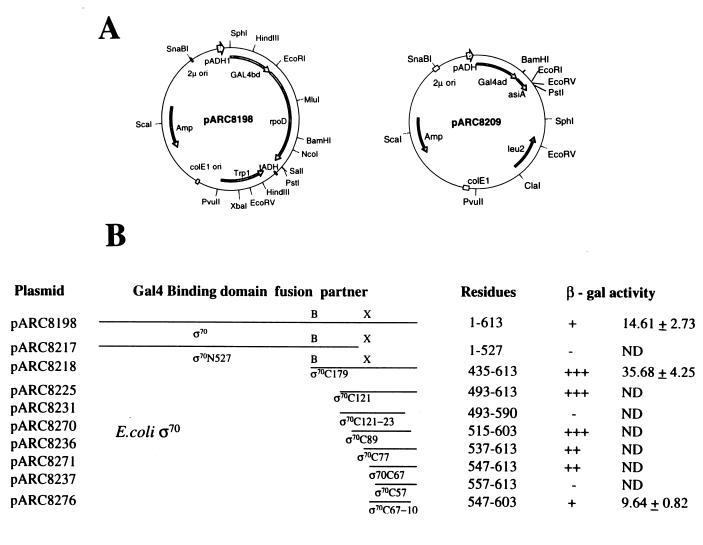
The yeast two-hybrid system was employed to delineate the regions of ς70 interacting with asiA. The yeast two-hybrid cloning vectors and the yeast strain Saccharomyces cerevisiae SFY526 were obtained from Clontech Laboratories Inc. Yeast transformations and β-galactosidase (β-gal) assays were done according to the recommendations of Clontech. A translation fusion of the entire coding sequence of ς70 of E. coli (21) with the binding domain of Gal4 protein encoded on pGBT9 was constructed (pARC8198; Fig. 1A). The gene encoding asiA was amplified by PCR and sequenced, and the encoded peptide was fused to the protein on the activation domain vector pGAD424 to obtain pARC8209 (Fig. 1A). Both of the recombinant plasmids were transformed into S. cerevisiae SFY526, the yeast strain containing the lacZ gene under a promoter regulated by Gal4 protein. In the β-gal assay with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as substrate on Whatman filter paper, the culture bearing Gal4BD:ς70- and Gal4AD:asiA-encoding constructs turned blue only after 90 min of exposure to X-Gal, as compared to the 5 min taken by the full-length Gal4 protein expressed from pCL1 (Fig. 1B). In a semiquantitative β-gal assay using liquid yeast culture, ς70 and asiA interaction produced 14.6 U of β-gal activity, which was in the same range as described previously (23) for interaction between Bacillus subtilis ςB and its regulators. The yeast culture bearing either Gal4BD:ς70- or Gal4AD:asiA-encoding constructs alone did not show any detectable activity in either of the assays, thereby confirming that the β-gal was produced due to the binding of ς70 with asiA. This observation further indicated that there were no other homologues of these proteins available in the yeast system which would have interfered in the binding of these proteins to each other.
FIG. 1.
Interaction of full-length and truncated E. coli ς70s with asiA in a yeast two-hybrid system. (A) Recombinant plasmids showing Gal4BD:ς70 and Gal4AD:asiA fusions in binding domain and activation domain vectors pGBT9 and pGAD424, respectively. The truncated rpoD fragments were generated by PCR and fused to Gal4BD in pGBT9 as EcoRI-SalI fragments to obtain recombinants pARC8217 through pARC8276 as shown in panel B. The position of the oligonucleotides corresponds to the amino acid numbers indicated. The nucleotide sequences will be made available on request. Only relevant restriction sites are shown. (B) β-gal activities obtained upon interaction of full-length and truncated ς70 fusions with Gal4AD:asiA fusions in S. cerevisiae SFY526. β-gal activity on Whatman membrane was approximated using a scale of + to +++, depending upon the time taken for appearance of blue in the presence of X-Gal (e.g., yeast culture showing blue in 30 min was marked +++ and the one showing blue in 90 min was marked +). At least five independent transformants were tested for β-gal activity. Numbers at right are units of β-gal activity in yeast liquid cultures calculated according to the method of Miller (15). Each value is the average of at least three independent experiments ± standard deviation. ND, not done; B, BamHI; X, XhoI.
C-terminal 57 residues of ς70 are enough for binding to asiA.
To delineate the regions of ς70 interacting with asiA, the gene fragments encoding N-terminal (residues 1 to 527) and C-terminal (residues 435 to 613) regions of ς70 were individually cloned into the binding domain vector pGBT9 (Fig. 1) to express Gal4BD:ς70N527 and Gal4BD:ς70C179 fusion proteins, respectively. Only the C-terminal region of ς70 (residues 435 to 613) bound to asiA, as indicated by the expression of β-gal. To further determine the minimum region of ς70 capable of binding to asiA, systematic deletions in the rpoD gene encoding the C-terminal region of ς70 were made. These gene fragments were amplified by PCR using Taq DNA polymerase (Bangalore Genei, Bangalore, India) and cloned into the binding domain vector pGBT9, and the sequence was confirmed (Fig. 1). It was found that the C-terminal 67 residues of ς70 (residues 547 to 613) were sufficient for binding to asiA. Further truncation of the rpoD gene by 30 bp, resulting in deletion of residues 547 to 557, led to the loss of binding. In the extreme C-terminal region, the deletion of 10 amino acids (residues 604 to 613) was tolerated but the extension of deletion to 23 amino acids (residues 491 to 613) was found to abolish the β-gal activity. The data obtained using the two-hybrid system delineated the asiA binding region on ς70 to amino acids 547 to 603, which is in close agreement with that reported earlier using in vitro protein binding assays (19, 20). Based on the studies with hydroxy radical footprinting of the asiA-ς70 complex, Colland et al. (6) had suggested that the amino acid residues present in the HTH motif (residues 572 to 588) of region 4.2 were involved in binding to asiA, but in the two-hybrid system we did not see any detectable level of β-gal activity in constructs encoding the C-terminal 57 amino acids (residues 557 to 613), suggesting that in addition to amino acids present in the HTH motif of region 4.2, some amino acids present in the surrounding region might also be important in binding to asiA.
The truncated ς70 fragments bind to asiA with higher affinity.
Since the β-gal activity in the yeast two-hybrid assay is a relative measure of the interaction between proteins (10), it provided a means to determine the relative avidity of binding between the different truncated ς70 forms and asiA. The constructs in binding domain plasmids carrying genes encoding Gal4BD:ς70C179, Gal4BD:ς70C121, and Gal4BD:ς70C89 (Fig. 1), when cotransformed with a Gal4AD:asiA-encoding plasmid, gave a positive reaction in 30 min on exposure to X-Gal, compared to the 90 min required to detect the interaction between full-length ς70 and asiA under similar conditions. This could be confirmed in a quantitative liquid β-gal assay, wherein a truncated ς70 fragment (Gal4BD:ς70C179) showed 35.6 U of β-gal activity compared to 14.6 U of activity shown by the full-length ς70 fusion. The β-gal activities obtained in the Whatman filter paper assay, with truncated ς70s ranging in size from 179 residues down to 89 residues, were similar but were reduced with smaller ς70 fragments.
The higher levels of β-galactosidase activity detected upon interaction between truncated ς70 fragments (e.g., ς70C179 or ς70C121) and asiA, compared to that of full-length ς70 and asiA, indicated an apparently higher affinity of the asiA protein for the truncated ς70 fragments. The asiA binding region overlaps with the −35 recognition domain (region 4.2) of ς70 (6). The same region of ς70 has also been shown to be masked by its N-terminal domain in its free state (8, 9). In addition, it has been postulated that this −35 domain becomes accessible for interaction with DNA only when ς70 binds to the core enzyme (8, 9). Taken together, these data suggest that the same N-terminal domain may be masking the asiA binding region on ς70 and would become unmasked in the holoenzyme conformation and in the C-terminal truncated forms of ς70. This could be responsible for the differences in affinity observed in the interaction of asiA with the full-length ς70 (lower affinity) and the truncated C-terminal fragments of ς70.
C-terminal ς70 fragments can neutralize asiA toxicity in E. coli.
Since the C-terminal fragments of ς70 showed higher affinity to asiA in the yeast two-hybrid system, it was postulated that coexpression of one of the C-terminal ς70 fragments with asiA will neutralize the asiA-mediated toxicity to E. coli cells due to preferential binding of the fragments to the overproduced asiA, thus leaving the native full-length E. coli ς70 free to perform the housekeeping functions. For this purpose, the gene fragments encoding the C-terminal 166 and 121 residues of ς70 were cloned individually behind the T7 promoter in a colE1-compatible vector, pACYC184. To express the C-terminal 121 amino acids of ς70, a 360-bp gene fragment of rpoD from pARC8225 (Fig. 1B) was cloned into EcoRI-SalI sites of pARC8173 (pET11D derivative) to obtain pARC8233. In a second step, a 700-bp BglII-SalI fragment from pARC8233 (which included the T7 promoter and a ribosome binding site) was cloned into BamHI-SalI sites of colE1-compatible vector pACYC184 to obtain pARC8234. A similar kind of strategy was used for cloning the full-length ς70 and C-terminal 166-amino-acid-encoding gene fragment of ς70 into pACYC184, and the resulting recombinant plasmids were designated pARC8116 and pARC8299, respectively. Expression of full-length and truncated ς70 fragments was confirmed in isopropyl-β-d-thiogalactopyranoside (IPTG)-induced cultures by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (Fig. 2). Expression of ς70 was found to be quite high even in uninduced cultures. The plasmid DNA bearing the glutathione S-transferase (GST):asiA-encoding gene (pARC8105) (GST was chosen because it provided an affinity tag for purification) was transformed into E. coli BL26(DE3) expressing ς70C166 or ς70C121. pARC8105 was constructed by cloning the 284-bp NcoI-BamHI fragment encoding asiA into the NcoI-BglII sites of pARC499 (a derivative of pGEX 3X with NcoI and BglII sites).
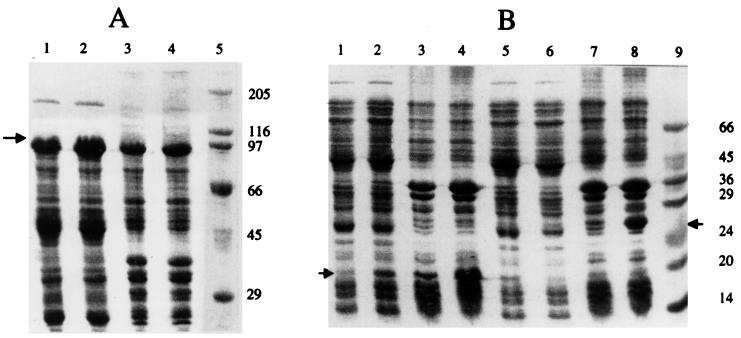
FIG. 2.
Analysis by SDS-PAGE of distribution of full-length ς70, ς70C121, and ς70C166 in soluble and inclusion body fractions. Cytosolic and inclusion body fractions were isolated as described in the text, and 100 μg of protein was applied in each lane. (A) Distribution of ς70. Lanes: 1 and 2, uninduced and induced cytosolic fractions, respectively; 3 and 4, uninduced and induced membrane-inclusion body fractions, respectively. (B) Lanes: 1 to 4, ς70C121; 5 to 8, ς70C166; 1 and 5, uninduced cytosolic fractions; 2 and 6, induced cytosolic fractions; 3 and 7, uninduced membrane-inclusion body fractions; 4 and 8, induced membrane-inclusion body fractions. The full-length ς70 fractions were run on SDS–10% PAGE gels, and the truncated ς70 fractions were run on a 12.5% gel. The protein size marker sizes are shown.
The analysis of E. coli transformants coexpressing both the proteins or the individual protein at different concentrations of IPTG is shown in Table 1. Cells coexpressing both GST-asiA and the C-terminal ς70 fragments (ς70C166 or ς70C121) were able to grow even at 100 μM concentrations of the inducer while, in contrast, induction with 20 μM IPTG of cells expressing GST-asiA alone was toxic to the cells. However, cells coexpressing full-length ς70 and GST-asiA failed to grow at 20 μM IPTG, indicating that coexpression of full-length ς70 failed to neutralize the asiA-mediated toxicity. Since overexpression of ς70 is known to form inclusion bodies (2, 21), all sigma-expressing cultures were fractionated into cystosolic (supernatant obtained by centrifugation at 100,000 × g) and membrane-inclusion body (pellet obtained by centrifugation at 100,000 × g) fractions and analyzed by SDS-PAGE. This analysis indicated that the induced ς70 protein or the ς70 fragments were distributed in nearly equal quantities in the two fractions (Fig. 2). Thus, the inability of full-length ς70 to neutralize asiA-mediated toxicity to E. coli cells was not due to the absence of soluble protein. In fact, full-length ς70 had larger amounts of soluble protein in the cytosolic fraction than did the truncated ς70C121 and ς70C166 proteins. Hence, the neutralizing effect of the truncated ς70 fragments was probably due to their higher binding affinity for the asiA protein.
TABLE 1.
Neutralization of GST:asiA toxicity in E. coli by truncated ς70 fragments
| Plasmid(s) | Protein(s) | Growth in LB plates containing IPTG at a concn (μM) ofa:
|
||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 50 | 100 | ||
| pARC8105 | GST:asiA | + | +/− | − | − | − |
| pARC8116 | ς70 | + | + | + | + | + |
| pARC8234 | ς70C121 | + | + | + | + | + |
| pARC8299 | ς70C166 | + | + | + | + | + |
| pARC8105 + pARC8116 | ς70 + GST:asiA | + | +/− | − | − | − |
| pARC8105 + pARC8234 | ς70C121 + GST:asiA | + | + | + | + | + |
| pARC8105 + pARC8299 | ς70C166 + GST:asiA | + | + | + | + | +/− |
Luria-Bertani plates containing appropriate antibiotics. +, −, and +/− indicate growth, no growth, and very slow growing colonies, respectively.
Overexpression and purification of asiA-ς70C121 complex in E. coli.
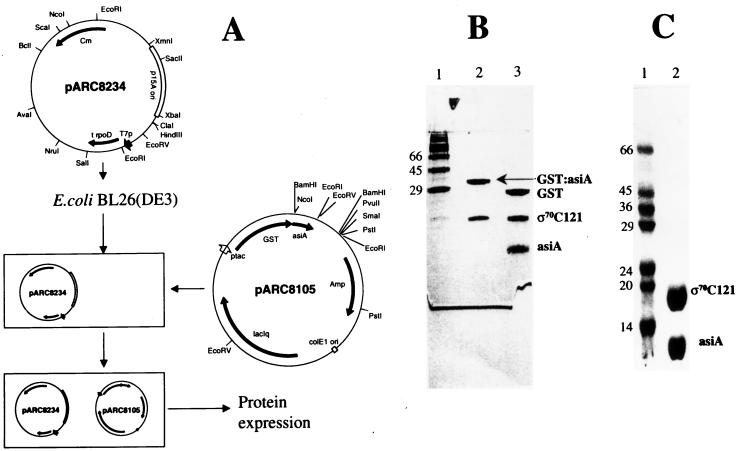
Since asiA overexpression is not tolerated by E. coli cells, earlier workers had partly overcome the problem by expressing asiA behind a T7 promoter either in combination with T7 lysozyme-encoding plasmid pLysE (14) or by inducing the expression of the protein with the help of phage CE6 (16). However, the quantities of asiA expressed and purified were limited, restricting the availability of the protein for crystallographic or NMR studies. We have taken advantage of the ability of C-terminal ς70 fragments to neutralize the toxicity of asiA by coexpression of the genes encoding ς70C121 and asiA followed by isolation of the fusion proteins. E. coli BL26(DE3) carrying the plasmids encoding GST:asiA (pARC8105) and ς70C121 (pARC8234) was grown at 30°C to an A600 of 0.5 and induced with 100 μM IPTG for 3 h. The pellet was washed with phosphate-buffered saline and lysed through a French pressure cell. The lysate was passed over a glutathione-Sepharose (Pharmacia) column. The bound proteins were eluted in accordance with the protocol of Pharmacia. As seen in Fig. 3B (lane 2), both GST:asiA and ς70C121 proteins were copurified through this procedure. The identities of the two proteins were confirmed by their reaction with specific antibodies (data not shown). Both the proteins were found to be present in roughly equimolar amounts, indicating that ς70C121 also binds to asiA at a 1:1 molar ratio, as has been observed for full-length ς70 (1). Upon Factor Xa cleavage and subsequent removal of GST protein through a glutathione affinity matrix, the ς70C121-asiA complex was purified to >95% purity (Fig. 3C, lane 2). The yield of purified protein complex was found to be 5 mg of E. coli culture/liter. The purified complex of the truncated ς70-asiA proteins isolated from the soluble fraction is suitable for studies on the structural elucidation of asiA and region 4.2 of ς70. These studies can also be extended to the modelling of the asiA surface interacting with region 4.2 of ς70 towards the design of novel inhibitors of transcription.
FIG. 3.
Coexpression and purification of GST:asiA-ς70C121 complex. (A) Design of coexpression system using two compatible plasmids. (B and C) Purification of asiA-ς70C121 complex from E. coli BL26(DE3) cells coexpressing GST:asiA and ς70C121. (B) Results of 10% SDS-PAGE. Lane 2, GST:asiA-ς70C121 complex obtained after purification through glutathione sepharose column; lane 3, factor Xa cleavage of GST:asiA. (C) Results of 15% SDS-PAGE. Lane 2, pure asiA-ς70C121 complex after removal of GST. The protein size markers are shown.
Acknowledgments
We thank all members of the transcription group for helpful discussions. Thanks are also due to Anand Kumar and Santanu Dutta for critical reading of the manuscript and R. Philomena for help in DNA sequencing.
REFERENCES
- 1.Adelman K, Orsini G, Colb A, Graziani L, Brody E N. The interaction between the asiA protein of bacteriophage T4 and the ς70 subunit of E. coli RNA polymerase. J Biol Chem. 1997;272:27435–27443. doi: 10.1074/jbc.272.43.27435. [DOI] [PubMed] [Google Scholar]
- 2.Borukhow S, Goldfarb A. Recombinant E. coli RNA polymerase: purification of individually overexpressed subunits and in-vitro assembly. Protein Expr Purif. 1993;4:503–511. doi: 10.1006/prep.1993.1066. [DOI] [PubMed] [Google Scholar]
- 3.Brody E N, Cassavetis G A, Ouhammouch M, Sanders G M, Geiduschek E P. Old phage, new insights: two recently recognised mechanisms of transcriptional regulation in bacteriophage T4 development. FEMS Microbiol Lett. 1995;128:1–8. doi: 10.1111/j.1574-6968.1995.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown K L, Hughes K T. The role of anti-sigma factors in gene regulation. Mol Microbiol. 1995;16:397–404. doi: 10.1111/j.1365-2958.1995.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 5.Chien C, Bartel P L, Sternglanz R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colland F, Orsini G, Brody E N, Buc H, Kolb A. The bacteriophage T4 asiA: a molecular switch for sigma 70 dependent promoters. Mol Microbiol. 1998;27:819–829. doi: 10.1046/j.1365-2958.1998.00729.x. [DOI] [PubMed] [Google Scholar]
- 7.Das A, Anderson L B, Xie Y-H. Delineation of interaction domains of Agrobacterium tumefaciens VirB7 and VirB9 by use of the yeast two-hybrid assay. J Bacteriol. 1997;179:3404–3409. doi: 10.1128/jb.179.11.3404-3409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombroski A J, Walter W A, Gross C A. Amino terminal amino acids modulate sigma factor DNA binding activity. Genes Dev. 1993;7:2446–2455. doi: 10.1101/gad.7.12a.2446. [DOI] [PubMed] [Google Scholar]
- 9.Dombroski A J, Walter W A, Record M J, Siegele D A, Gross C A. Polypeptides containing highly conserved regions of transcription initiation factor ς70 exhibit specificity of binding to DNA. Cell. 1992;70:501–512. doi: 10.1016/0092-8674(92)90174-b. [DOI] [PubMed] [Google Scholar]
- 10.Estojak J, Brent R, Golemis E A. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields S, Sternglanz R. The two hybrid system: an assay for protein-protein interactions. Trends Genet. 1994;10:286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 12.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 13.Golemis E, Gyuris J, Brent R. Interaction trap/two-hybrid system to identify interacting proteins. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1994. pp. 13.14.1–13.14.17. [Google Scholar]
- 14.Hinton M D, March-Amegadzie R, Gerber J S, Sharma M. Bacteriophage T4 middle transcription system: T4-modified RNA polymerase; asiA, a ς70 binding protein; and transcriptional activator MotA. Methods Enzymol. 1996;274:43–57. doi: 10.1016/s0076-6879(96)74007-7. [DOI] [PubMed] [Google Scholar]
- 15.Miller J. A short course in bacterial genetics: a laboratory manual and handbook for E. coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. [Google Scholar]
- 16.Orsini G, Ouhammouch M, Le Caer J P, Brody E N. The asiA bacteriophage T4 codes for the anti-sigma 70 protein. J Bacteriol. 1993;175:85–93. doi: 10.1128/jb.175.1.85-93.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouhammouch M, Orsini G, Brody E N. The asiA gene product of bacteriophage T4 is required for middle mode RNA synthesis. J Bacteriol. 1994;176:3956–3965. doi: 10.1128/jb.176.13.3956-3965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouhammouch M, Adelman K, Harvey S R, Orsini G, Brody E N. Bacteriophage T4 motA and asiA proteins suffice to direct E. coli RNA polymerase to initiate transcription at T4 middle promoters. Proc Natl Acad Sci USA. 1995;92:1451–1455. doi: 10.1073/pnas.92.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severinova E, Severinov K, Darst S A. Inhibition of Escherichia coli RNA polymerase by T4 AsiA. J Mol Biol. 1998;279:9–18. doi: 10.1006/jmbi.1998.1742. [DOI] [PubMed] [Google Scholar]
- 20.Severinova E, Severinov K, Fenyo D, Marr M, Brody E N, Roberts J W, Chait T, Darst S A. Domain organisation of Escherichia coli RNA polymerase ς70 subunit. J Mol Biol. 1996;262:637–647. doi: 10.1006/jmbi.1996.0604. [DOI] [PubMed] [Google Scholar]
- 21.Sharma, U. K., M. Balganesh, V. V. Vaidyanathan, R. K. Shandil, P. Kaur, and T. S. Balganesh. Unpublished data.
- 22.Stevens A. A salt promoted inhibitor of RNA polymerase isolated from T4 phage-infected E. coli. In: Losick R, Chamberlin M, editors. RNA polymerase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1976. pp. 617–627. [Google Scholar]
- 23.Voelker U, Voelker A, Haldenwang W G. The yeast two-hybrid system detects interactions between Bacillus subtilis ςB regulators. J Bacteriol. 1996;178:7020–7023. doi: 10.1128/jb.178.23.7020-7023.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White M A. The yeast two-hybrid system: forward and reverse. Proc Natl Acad Sci USA. 1996;93:10001–10003. doi: 10.1073/pnas.93.19.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]