Abstract
In the present study, immunogenicity data in 61 vaccinated healthcare workers (HCWs) either infection naïve (naïve HCWs) or with infection of Delta and/or Omicron COVID-19 (experienced HCWs) were evaluated up to 270 days after the second dose of BNT162b2 vaccine and up to 90 days after a booster dose. A decrease in antibody levels at 270 days following administration of the second dose (p = 0.0335) was observed, although values did not fall below the positivity threshold (33.8 BAU/ml). After booster vaccination, antibody levels increased after 30 days (p = 0.0486), with much higher values than after first and second vaccination. Antibody levels then decreased at 60 and 90 days after the booster dose. A comparison between mean antibody levels of naïve and experienced HCWs revealed higher values in experienced HCWs, resulting from both natural and vaccination-induced immunity. A total of 14.7% of HCWs contracted the Omicron virus variant after the vaccine booster, although none showed severe symptoms. These results support that a booster dose results in a marked increase in antibody response that subsequently decreases over time.
Keywords: SARS-CoV-2, Booster vaccination, Humoral immune response, Neutralizing antibodies, Omicron variant, Immunisation safety
1. Introduction
The global coronavirus disease 2019 (COVID-19) epidemic is still ongoing [1]. Up to March 2022, more than 6 billion vaccinations had been administered worldwide, impacting positively on the control of the epidemic [2]. However, the observed reduction in antibody levels over time after vaccination and the rise in positive cases have led to the need for a further booster vaccination [3], [4].
The emergence of new variants has also been witnessed across the world; in recent months the Omicron variant (B.1.1.529), first detected in South Africa, has spread to many countries in a short space of time. In Italy, according to data from the Integrated Surveillance System of SARS-CoV-2 variants, the Delta variant was dominant (86.6%) between 23 October and 6 December 2021. Since December 2021, Italy has seen an increase in cases involving the Omicron variant, which shows higher transmissibility and lower infection severity. Currently, the prevalence of Omicron in Italy is at 99.2%, while that the Omicron BA.2 (B.1.1.529.2) is increasing and now stands at 44.1%. We previously evaluated the presence of antibodies to SARS-CoV-2 in samples from healthcare workers (HCWs) at different time points over 90 days after the second dose of BNT162b2 vaccine; we observed an initial increase in antibody levels, which subsequently decreased and reached a plateau at 60 days, with similar values detected after 90 days [5].
The aim of the present study was to evaluate antibody response in HCWs over more extended periods, up to 12 months from the first dose of vaccine, up to 270 days after the second dose, and up to 90 days after administration of the booster dose in order to trace the antibody trend in naïve HCWs and in HCWs with a Delta and/or Omicron COVID-19 infection.
2. Materials and methods
Immunogenicity was evaluated in 61 vaccinated Caucasian HCWs, 26 male and 35 female, aged 27 to 70 years at the Clinical Pathology Laboratories of the University of Campania “Luigi Vanvitelli” Hospital in Naples (Italy). None of the subjects were taking immunomodulatory drugs, and lifestyle, diet, and level of physical activity remained unchanged throughout the study period. All participants gave their informed consent to participate in the study. Of the 61 HCWs, 17 had a COVID-19 infection (experienced HCWs), five before the first vaccination in October and December 2020, three following the second dose, and two, three, and four about 50, 70, and 80 days after the booster vaccination, respectively; 44 were naïve HCWs, without previous infection.
A venous blood sample was taken 180 and 270 days after second-dose vaccination. In November 2021, 300 days after the second dose, all the HCWs received a booster dose (30 μg) of the BNT162b2 mRNA COVID-19 vaccine (Comirnaty; Pfizer). Venous blood was collected 30, 60, and 90 days after booster vaccination.
The DiaSorin Liaison SARS-CoV-2 TrimericS IgG (DiaSorin TriS IgG; DiaSorin) chemiluminescence immunoassay (CLIA) was used to quantify IgG antibodies in human serum against a trimeric S-protein antigen on a DiaSorin Liaison, as previously described [5].
Anti-SARS-CoV-2 IgG antibody levels were expressed in World Health Organization (WHO) International Standard (NIBSC code. 20/268) binding arbitrary units (BAU/ml). Samples were considered positive when values were ≥ 33.8 BAU/ml.
2.1. Statistical analysis
Data are reported as mean ± SD, unless otherwise stated. A comparison between the two groups was performed using Student's t-test. Significance was assumed for p-values less than 0.05.
3. Results
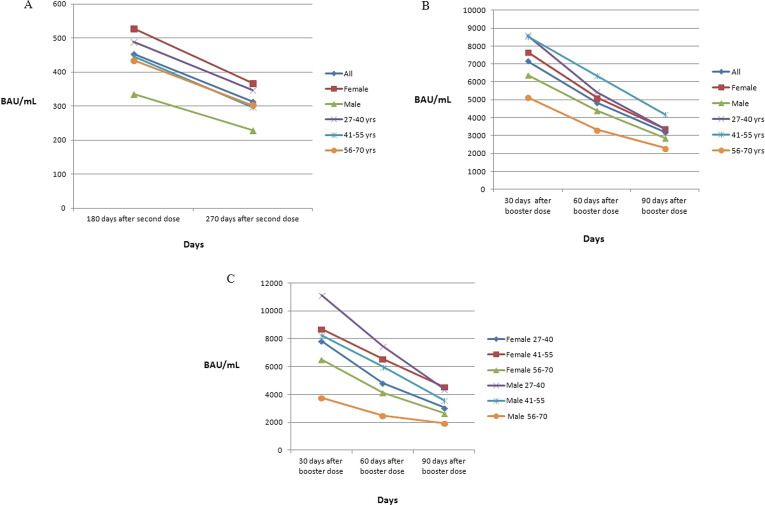
The mean values of serum levels of IgG antibodies to SARS-CoV-2 in the 44 naïve HCWs at 180 and 270 days after the second vaccine dose and according to sex and age are shown in Fig. 1 A. The overall mean value of IgG serum antibody levels to SARS-CoV-2 at 180 days after the second dose was 453.7 BAU/ml and decreased to 314 BAU/ml (p = 0.0335) at 270 days. No associations were found between antibody levels and sex, although higher levels were observed in female participants at both 180 and 270 days (decreasing from 528.2 BAU/ml to 367.9 BAU/ml) than in male participants (decreasing from 335.3 BAU/ml to 228.5 BAU/ml). A significant difference was observed in the 27–40 age group (p = 0.0202).
Fig. 1.
Mean values of anti-trimeric spike protein specific IgG antibodies (BAU/mL) in 44 naïve healthcare workers (HCWs): at 180 and 270 days after second dose of vaccine, according to sex and to age (A); at 30, 60, and 90 days after booster dose of vaccine, according to sex and to age (B); at 30, 60, and 90 days after booster dose of vaccine, according to sex and age combined (C). (*p less than 0.05).
After booster vaccination, high antibody levels were observed after 30 days; the mean values of serum levels of IgG antibodies at different time points and according to sex and age are illustrated in Fig. 1B. The overall mean value of IgG antibody serum levels at 30 days after the booster vaccination was 7162 BAU/ml and decreased to 4846 BAU/ml after 60 days, further decreasing to 3183 BAU/ml at 90 days. The difference in mean values of antibody levels of all HCWs was statistically significant between 30 and 60 days (p = 0.0486) and between 60 and 90 days (p = 0.0292). In addition, there was a significant difference in antibody levels in males between 60 and 90 days (p = 0.0307).
The mean values of IgG serum antibody levels at different time points in male and female naïve HCWs after the booster dose classified according to age are shown in Fig. 1C. No associations between antibody levels and age groups were observed, although a difference in the mean value of antibody levels was found to be statistically significant between 30 and 60 days (p = 0.0492) and between 60 and 90 days (p = 0.0075) in male participants aged 41–55 years.
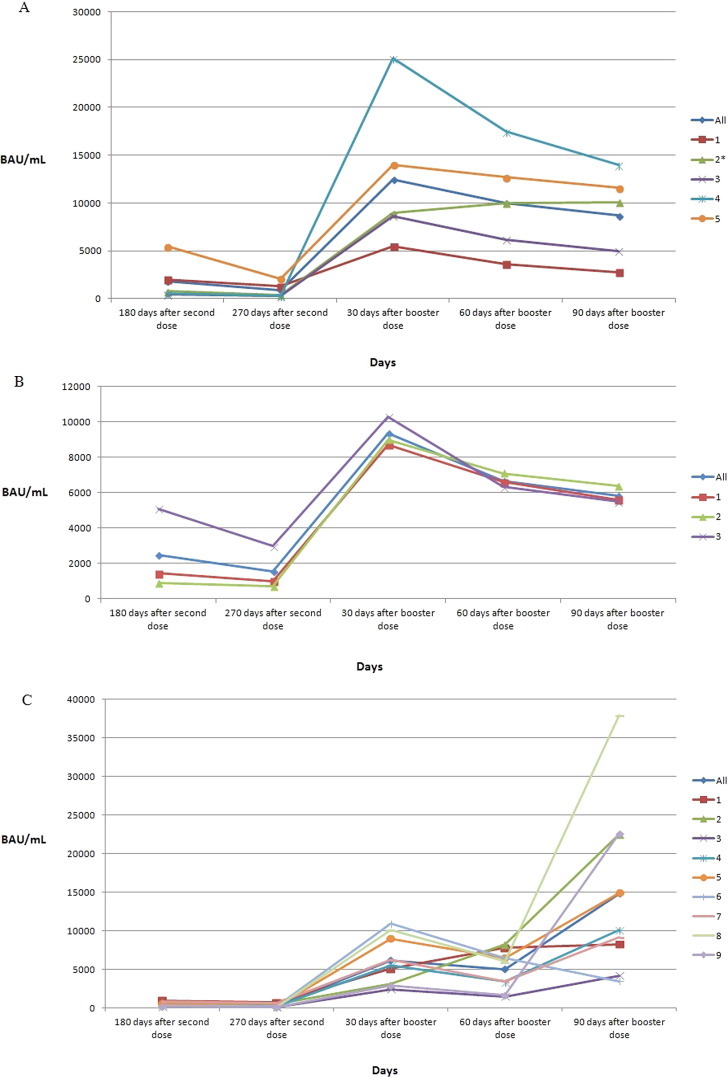
Seventeen experienced HCWs were infected by the COVID-19 Delta and/or Omicron variant. Antibody levels of 5/61 (8.2%) HCWs infected with the Delta variant of SARS-CoV-2 before vaccination are shown in Fig. 2 A. Of the 17 experienced HCWs, one was also infected with the Omicron variant after the booster dose. Antibody levels of 3/61 (4.9%) HCWs infected with the Delta variant after the second dose are shown in Fig. 2B. Antibody values of 9/61 HCWs (14.7%) infected with the Omicron variant after the booster dose are shown in Fig. 2C. None of the experienced HCWs showed severe symptoms.
Fig. 2.
Anti-trimeric spike protein specific IgG antibody (BAU/mL) values at 180 and 270 days after second dose of vaccine and at 30, 60, and 90 days after booster dose of vaccine in: 5 experienced HCWs infected by SARS-CoV-2 before vaccination in October–December 2020 (A); 3 experienced HCWs infected by SARS-CoV-2 ∼ 70–140 days after second dose (B); 9 experienced HCWs infected by SARS-CoV-2 ∼ 50–80 days after booster dose (C). * HCW also infected with Omicron variant ∼ 80 days after booster dose.
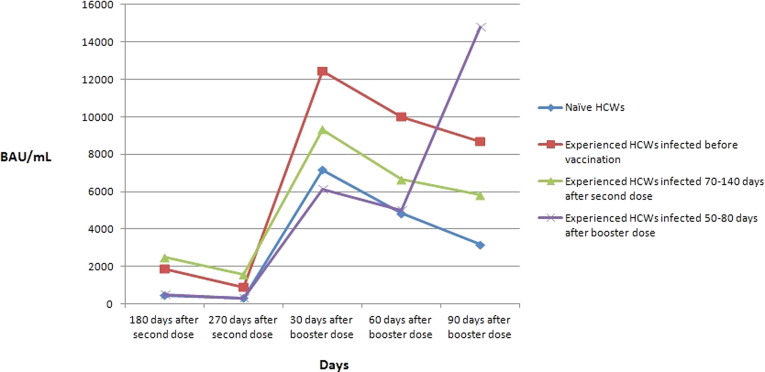
A comparison between mean antibody levels of naïve HCWs and experienced HCWs is illustrated in Fig. 3 . Higher values were observed in experienced HCWs, infected before vaccination and after the second dose, at all dosing times compared to naïve HCWs. In experienced HCWs infected after the booster dose, antibody levels increased significantly from 30 days to 90 days after booster administration, while values in naïve HCWs and experienced HCWs infected before vaccination and after the second dose decreased.
Fig. 3.
Comparison between mean values of anti-trimeric spike protein specific IgG antibodies (BAU/mL) in naïve HCWs and experienced HCWs infected by SARS-CoV-2 before vaccination, after second dose, and after booster dose.
4. Discussion
Since December 2020, several SARS-CoV-2 variants have emerged and have been classified by the WHO as variants of concern (VOC): Alpha (B.1.1.7), first detected in the United Kingdom; Beta (B.1.351), first identified in South Africa; Gamma (P.1), first detected in Brazil; Delta (B.1.617.2), first identified fin India; and Omicron (B.1.1.529). The Omicron variant has many mutations compared to the original virus, which could lead to significant changes in the antigenic properties of the virus [6], [7], [8], [9], [10], [11].
In a previous study we reported a decrease in antibody levels from 60 to 90 days after the second dose of BNT162b2 vaccine [5]. Here, we show that antibody levels continue to decrease at 180 and 270 days after the second dose (p = 0.0335), indicating that by nine months following second-dose administration of BNT162b2, humoral response was substantially reduced. A study conducted on 4868 HCWs up to six months after the second dose showed a considerable decrease in antibody levels [12]. Notably, in our study the values do not fall below the positivity threshold (33.8 BAU/ml), demonstrating the persistence of vaccine-induced antibody response.
After the booster vaccination, we found, as expected, that antibody levels increase after 30 days (p = 0.0486), with much higher values than after the first and second vaccination [5]. Our observations are in line with a previous study that tested the antibody levels of Italian HCWs receiving primary vaccination with BNT162b2 vaccine, followed by administration of a homologous second dose and one month later by a booster vaccination; the authors observed an antibody peak with values almost three times higher than those found one month after the second dose [13]. Therefore, SARS-CoV-2-specific memory lymphocytes induced by the first vaccination trigger a faster and more effective antibody response after a booster vaccine. However, at 60 and 90 days after the booster dose, antibody levels decline quickly, as reported in another study [14], showing that a booster dose induces high antibody levels which then undergo a considerable decrease over time. Evidence that vaccine effectiveness declines suggested the need for a fourth dose in individuals at risk, subsequently authorized by the Food and Drug Association (FDA) in March 2022 [15].
Since it was previously assumed that the immune response to two doses of BNT162b2 is lower in people aged 65 to 85 years than in those aged 18 to 55 years [16], a recent study assessed the antibody response in individuals aged 60 years and older after a third BNT162b2 dose and observed a significantly increased antibody titer without any correlation with age [17]. In our study, however, we found significant differences in the 27–40 age group after the second dose and in the male 41–55 subgroup after the booster dose.
In experienced HCWs, infected before vaccination, higher antibody levels that those in naïve HCWs were detected (Fig. 3). Previous studies showed that mRNA vaccines elicit rapid immune responses in seropositive individuals with post-vaccine antibody titers that are either comparable to or even exceed titers in naïve vaccinated individuals with two doses [18], as the values are the result of both natural immunity and immunity induced by vaccination.
Here, a total of 4.9% experienced HCWs contracted the virus between 70 and 140 days after the second dose, probably when antibody levels provide less protection, as reported in previous studies, where 0.3–3% of HCWs were infected after two doses of vaccine [19], [20]. The level of antibodies in our experienced HCWs was higher than in naïve HCWs, but lower than in those infected before vaccination (Fig. 3).
A study conducted using data from Maccabi Healthcare Services in Israel, in a population aged 40 years or older, found a positive rate for COVID-19 of 1.8% after the booster dose [21]. Our data show that 14.7% of HCWs contracted the Omicron variant, despite having received a booster dose and the presence of high antibody levels. However, none of these HCWs showed severe symptoms, and experienced only mild manifestations such as fatigue, colds, sore throat, low-grade fever, and joint pain.
Virological and efficacy studies are currently underway to assess the impact of the Omicron variant on protection conferred by vaccines. The Omicron variant is 2.8 times more infectious than the Delta variant [22] due to mutations in the spike protein associated with increased infectivity and antibody evasion [23].
In a study by Nemet et al, serum samples from subjects who received two or three doses of BNT162b2 vaccine were tested for the ability to neutralize the Omicron variant in vitro. Although neutralization activity was poor after two doses of the vaccine, substantial neutralization of the Omicron variant was found in samples from participants who received three doses [24].
A study evaluating the sensitivity of the Omicron variant to neutralization in serum pools from vaccinated and previously infected HCWs found an extensive, but incomplete, evasion of neutralizing antibody responses by the variant [25].
Another study showed that even after heterologous vaccination, the immunogenicity of BNT162b2 vaccine remains for up to 15 weeks after the booster dose [26]. A study in Japan also indicated that vaccination followed several months later by a SARS-CoV-2 infection offers better protection against the Omicron variant than when infection occurs soon after vaccination, thanks to long-lived cells that carry a memory of the pathogen and confer enhanced protection against later infections [27].
The role of T cells and their potential to provide long-term protection from re-infection with SARS-CoV-2 remains debated. A study investigating circulating leukocyte T cell response in healthy subjects, COVID-19-infected, and in healthy vaccinated subjects found a significant decrease in CD8+ T cells and an increase in CD4+/CD8+ ratio in the COVID-19-infected group compared with vaccinated individuals; healthy vaccinated subjects showed significant increased expression of CD8+ T cells and a reduction in CD4+/CD8+ ratio compared to those previously infected with COVID-19. In addition, an increase in central memory and terminal effector memory cells was observed [28], [29], [30], [31].
Monitoring humoral immune responses after booster COVID-19 vaccination allows us to analyze the kinetics of anti-SARS-CoV-2 antibodies and supports current policies promoting a booster vaccine to reinforce humoral immunity, especially in people at higher risk of developing severe illness. Our results support that a booster dose results in a marked increase in antibody response, although a small percentage of individuals can contract the Omicron variant. A fourth dose of vaccine has also been indicated by FDA for some specific categories.
The need still remains to test vaccines that are specific for the Omicron variant, and several institutions have in fact already started researching and developing Omicron variant-specific COVID-19 vaccines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.WHO. Coronavirus disease (COVID 19) Dashboard. Geneva: World Health Organization. Available from: https://covid19.who.int (accessed on March 21, 2022).
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of themRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike antibodywaning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levelsare highly predictive of immune protection from symptomatic SARSCoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 5.Vietri M.T., Albanese L., Passariello L., D'Elia G., Caliendo G., Molinari A.M., et al. Evaluation of neutralizing antibodies after vaccine BNT162b2. Preliminary data. J ClinVirol. 2022;146 doi: 10.1016/j.jcv.2021.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Tracking SARS-CoV-2 variants. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on March 30, 2022). [PubMed]
- 7.Vietri M.T., Riegler G., Ursillo A., Caserta L., Cioffi M., Molinari A.M. p53 codon 72 polymorphism in patients affected with ulcerative colitis. J Gastroenterol. 2007;42(6):456–460. doi: 10.1007/s00535-007-2026-z. [DOI] [PubMed] [Google Scholar]
- 8.Maiorino M.I., Schisano B., Di Palo C., Vietri M.T., Cioffi M., Giugliano G., et al. Interleukin-20 circulating levels in obese women: effect of weight loss. Nutr Metab Cardiovasc Dis. 2010;20(3):180–185. doi: 10.1016/j.numecd.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Bellastella G., Maiorino M.I., De Bellis A., Vietri M.T., Mosca C., Scappaticcio L., et al. Serum but not salivary cortisol levels are influenced by daily glycemic oscillations in type 2 diabetes. Endocrine. 2016;53(1):220–226. doi: 10.1007/s12020-015-0777-5. [DOI] [PubMed] [Google Scholar]
- 10.Napoli C., Benincasa G., Criscuolo C., Faenza M., Liberato C., Rusciano M. Immune reactivity during COVID-19: Implications for treatment. Immunol Lett. 2021;231:28–34. doi: 10.1016/j.imlet.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakasis A.D., Bitzogli K., Mouziouras D., Pouliakis A., Roumpoutsou M., Goules A.V., et al. Antibody responses after SARS-CoV-2 vaccination in patients with liver diseases. Viruses. 2022;14(2):207. doi: 10.3390/v14020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed]
- 13.Salvagno GL, Henry BM, Pighi L, De Nitto S, Gianfilippi G, Lippi G. Effect of BNT162b2 booster dose on anti-SARS-CoV-2 spike trimericIgG antibodies in seronegative individuals. Clin Chem Lab Med 2022. doi: 10.1515/cclm-2022-0212. [DOI] [PubMed]
- 14.Çağlayan D., Süner A.F., Şiyve N., Güzel I., Irmak Ç., Işik E., et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J Med Virol. 2022;94(5):2212–2221. doi: 10.1002/jmv.27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanne JH. Covid-19: Pfizer asks US regulator to authorise fourth vaccine dose for over 65s. BMJ 2022;376:o711. doi: 10.1136/bmj.o711. [DOI] [PubMed]
- 16.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of twoRNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eliakim-Raz N., Leibovici-Weisman Y., Stemmer A., Ness A., Awwad M., Ghantous N., et al. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326(21):2203–2204. doi: 10.1001/jama.2021.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ontañón J, Blas J, de Cabo C, Santos C, Ruiz-Escribano E, García A, et al. Influence of past infection with SARS-CoV-2 on the response to the BNT162b2 mRNA vaccine in health care workers: kinetics and durability of the humoral immune response. EBioMedicine 2021;73:103656.doi: 10.1016/j.ebiom.2021.103656. [DOI] [PMC free article] [PubMed]
- 19.Pilishvili T., Fleming-Dutra K.E., Farrar J.L., Gierke R., Mohr N.M., Talan D.A., et al. Interim estimates of vaccine effectiveness of pfizer-BioNTech and modernaCOVID-19 vaccines among health care personnel - 33U.S. Sites, January-March2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–758. doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouton T.C., Lodi S., Turcinovic J., Weber S.E., Quinn E., Korn C., et al. COVID-19 vaccine impact on rates of SARS-CoV-2 cases and post vaccination strain sequences among healthcare workers at an urban academic medical center: a prospective cohort study. Open Forum Infect Dis. 2021;8(10):ofab465. doi: 10.1093/ofid/ofab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patalon T., Gazit S., Pitzer V.E., Prunas O., Warren J.L., Weinberger D.M. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med. 2022;182(2):179–184. doi: 10.1001/jamainternmed.2021.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., Wang R., Gilby N.B., Wei G.W. Omicron (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62(2):412–422. doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemet I., Kliker L., Lustig Y., Zuckerman N., Erster O., Cohen C., et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 omicron infection. N Engl J Med. 2022;386(5):492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheward D.J., Kim C., Ehling R.A., Pankow A., Castro Dopico X., Dyrdak R., et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: a cross-sectional study. Lancet Infect Dis. 2022;22(6):813–820. doi: 10.1016/S1473-3099(22)00129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firinu D., Perra A., Campagna M., Littera R., Meloni F., Sedda F., et al. Evaluation of antibody response to heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2: an observational study. Vaccines (Basel) 2021;9(12):1478. doi: 10.3390/vaccines9121478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidik S.M. Immunity against Omicron from breakthrough infection could be a matter of timing. Nature. 2022 doi: 10.1038/d41586-022-00004-x. [DOI] [PubMed] [Google Scholar]
- 28.Grimaldi V., Benincasa G., Moccia G., Sansone A., Signoriello G., Napoli C. Evaluation of circulating leucocyte populations both in subjects with previous SARS-COV-2 infection and in healthy subjects after vaccination. J Immunol Methods. 2022;502 doi: 10.1016/j.jim.2022.113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marfella R., Sardu C., D'Onofrio N., Prattichizzo F., Scisciola L., Messina V., et al. Glycaemic control is associated with SARS-CoV-2 breakthrough infections in vaccinated patients with type 2 diabetes. Nat Commun. 2022;13(1):2318. doi: 10.1038/s41467-022-30068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cioffi M., Gazzerro P., Vietri M.T., Magnetta R., Durante A., D’Auria A., et al. Serum concentration of free T3, free T4 and TSH in healthy children. J Pediatr Endocrinol Metab. 2001;14(9):1635–1639. doi: 10.1515/jpem.2001.14.9.1635. [DOI] [PubMed] [Google Scholar]
- 31.Cioffi M., Riegler G., Vietri M.T., Pilla P., Caserta L., Carratù R., et al. Serum p53 antibodies in patients affected with ulcerative colitis. Inflamm Bowel Dis. 2004;10(5):606–611. doi: 10.1097/00054725-200409000-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.