Abstract
The aim of this study was to test the effect of mixing doses of glutamate (Glu) and glutamine (Gln) on the growth, health and gut health of post-weaning piglets. One hundred twenty weaned piglets (24 ± 2 days of age) were assigned to 6 dietary groups: (1) standard diet (CO); (2) CO plus Glu (6 kg/Ton): 100Glu; (3) CO plus 75Glu + 25Gln; (4) CO plus 50Glu + 50Gln; (5) CO plus 25Glu + 75Gln and (6) CO plus 100Gln. At days 8 and 21, blood was collected for haematological and reactive oxygen metabolite analysis, intestinal mucosa for morphological and gene expression analysis, and caecal content for microbial analysis. Data were fitted using a Generalised Linear Model (GLM). Piglet growth increased linearly with an increase in Gln from d7 to d14. The Glu:Gln ratio had a quadratic effect on faecal consistency and days of diarrhoea, neutrophil% and lymphocyte%, and a positive linear effect on monocyte% in the blood at d8. The amino acids (AAs) reduced the intraepithelial lymphocytes in the jejunum, and 100Gln improved intestinal barrier integrity at d8. The caecal microbiota did not differ. Overall, this study suggested a favourable effect of mixing Glu and Gln (25 + 75–50 + 50) as a dietary supplementation in post-weaning piglets to benefit the immune and barrier function of the gut, resulting in an increase in faecal consistency and improvement of growth during the first 2 weeks post-weaning.
Subject terms: Physiology, Metabolism, Immunology
Introduction
Amino acids (AAs) are not only needed for protein production but can be considered precursors of energy, signalling molecules and microbiota modulators. Therefore, AAs can contribute to restoring gut health and, in turn, improving general health1. L-Glutamate (Glu) and L-Glutamine (Gln) are abundant AAs in the body and have traditionally been considered dispensable AAs. However, they are currently regarded as a conditionally indispensable nutrient under stress conditions, including the weaning of piglets2. These two AAs can benefit the gut health of piglets by acting as sources of energy for the intestinal cells, as precursors for promoting cell proliferation in the gut and as precursors for the immune cells3–6. Glutamate is mainly utilised as a source of energy by enterocytes3,4. However, it can be used as a precursor for the synthesis of Gln and glutathione4; thus, it plays a key role in preserving the gut from oxidative damage.
The beneficial effects of Gln on the gut are mainly related to its use as a source of energy for enterocytes5, as metabolic fuel for the immune cells (including lymphocytes and macrophages) and by supplying substrates for the synthesis of glucosamines, nucleic acids, nucleotides and adenosine triphosphate (ATP)6–8. Furthermore, Gln can modulate the phosphorylation of the mammalian target of rapamycin (mTOR) which is involved in the regulation of protein synthesis in the intestine9.
Previous studies have shown that the supplementation of Glu alone and Gln alone could improve the growth performance, and gut health of weaning piglets in terms of gut integrity, acting as modulators of the mucosal gene expression and gut microbial community1,10–14.
The metabolism of Glu and Gln is closely connected; in fact, Glu is the immediate product of the Gln metabolism, produced by the action of glutaminase; Glu can be combined with ammonia (NH3) to produce Gln by the action of glutamine synthetase in some tissues and cells, such as the liver and skeletal muscles7. Therefore, it is plausible that the combined supplementation of Glu and Gln could have synergistic effects15. However, to date, only a few studies have investigated the effect of mixing Glu and Gln supplementation on piglets, suggesting a positive effect on growth performance and gut morphology parameters15,16. Up to now, no study has investigated the effect of mixing Glu and Gln at different doses regarding the growth, health and gut eubiosis of post-weaning piglets. Therefore, in the present study, the hypothesis that mixing different doses of Glu and Gln affected the growth, immune response and gut health of post-weaning piglets was tested. The aims of the present study were to (1) investigate the various beneficial effects of mixing different doses of Glu and Gln on the intestinal health and growth of post-weaning piglets and to evaluate whether mixing doses of Glu and Gln could be more promising than providing a single AA, (2) elucidate the mode of action of mixing different doses of Glu and Gln on the gut health of post-weaning piglets and (3) identify the best supplementation ratio of Glu and Gln for sustaining piglets during the post-weaning phase.
Results
To test the hypothesis that mixing different doses of Glu and Gln affected the growth, immune response and gut health of post-weaning piglets an in vivo trial was carried out; the piglets were assigned to 6 groups fed (1) a standard diet (CO), (2) CO plus 6 kg/Ton of Glu alone (100Glu), (3) CO plus 75% of Glu (4.5 kg/Ton) + 25% of Gln (1.5 kg/Ton) (75Glu + 25Gln), (4) CO plus 50% of Glu and Gln (3 kg/Ton) (50Glu + 50Gln), (5) CO plus 25% of Glu (1.5 kg/Ton Glu) and 75% of Gln (4.5 kg/Ton) (25Glu + 75Gln) and (6) CO plus 6 kg/Ton og Gln (100Gln) .
Performance and faecal score
During the trial, one pig, two pigs and one pig from the 75Glu + 25Gln, 25Glu + 75Gln and 100Gln groups respectively, were excluded due to health impairment and a substantial reduction in feed intake. The results of the growth performance are reported in Table 1. The live body weight (BW) at day (d) 0 (d0) (weaning), d7 and d14 did not differ among the dietary groups. At d21 post-weaning, the ever-increasing level of Glu tended to linearly reduce the BW (P = 0.065). The average daily gain (ADG) from d0 to d7 was not affected by the diet. From d7 to d14, the AAs increased the ADG of the piglets as compared with the CO (Control vs. AAs addition, P = 0.050). In the period d14–d21, the diet generally affected the ADG (P = 0.026), and the CO group had a higher ADG as compared with the groups supplemented with the AAs (Control vs. AAs addition, P = 0.015). Furthermore, from d14 to d21, the inclusion of Gln tended to linearly increase the ADG (P = 0.073). From d0 to d21 a linear effect of the Glu-Gln ratio was observed for the ADG with a favourable effect of the highest dose of Gln (P = 0.051). The feed intake (FI) was not affected by diet for the periods d0–d7 or d7–d14. In the periods d14–d21 and d7–d21, the FI tended to have a linear effect on the Glu:Gln ratio, which increased the value of the highest dose of Gln (P = 0.069 and P = 0.089, respectively). The gain to feed (G:F) ratio was not affected by diet for the periods d0–d7, d7–d14 or d14–d21. From d7 to d21, the G:F ratio tended to have a linear effect on the Glu:Gln ratio (P = 0.051), and the 100 Glu group tended to have a lower G:F ratio than the other groups supplemented with mixed doses of AAs (P = 0.078).
Table 1.
Effect of the dietary supplementation (6 kg/T) with glutamate and glutamine in different ratio on growth performance of post-weaning piglets.
| Item | Diet1 | SEM | P-value | Contrasts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | 100Glu | 75Glu + 25Gln |
50Gln + 50Glu |
25Glu + 75Gln |
100Gln | Diet | Linear | Quadratic | CO vs AAs addition |
100Glu vs mixed addition |
100Gln vs mixed addition |
||
| Body weight, kg | |||||||||||||
| d0 | 6.94 | 6.98 | 6.77 | 7.00 | 7.07 | 7.03 | 0.21 | 0.932 | 0.393 | 0.935 | 0.807 | 0.756 | 0.664 |
| d7 | 7.79 | 7.63 | 7.53 | 7.86 | 7.77 | 7.84 | 0.24 | 0.914 | 0.486 | 0.533 | 0.434 | 0.350 | 0.769 |
| d8 | 7.57 | 7.57 | 7.61 | 7.90 | 7.48 | 8.04 | 0.36 | 0.821 | 0.468 | 0.804 | 0.689 | 0.820 | 0.352 |
| d14 | 8.48 | 8.45 | 8.27 | 8.96 | 9.52 | 8.91 | 0.47 | 0.441 | 0.152 | 0.566 | 0.473 | 0.383 | 0.995 |
| d21 | 11.01 | 10.33 | 10.47 | 10.82 | 11.76 | 11.26 | 0.52 | 0 .413 | 0.065 | 0.720 | 0.875 | 0.255 | 0.701 |
| Average daily gain, kg/day | |||||||||||||
| d0–d7 | 0.12 | 0.09 | 0.11 | 0.12 | 0.10 | 0.12 | 0.02 | 0.704 | 0.486 | 0.534 | 0.434 | 0.350 | 0.769 |
| d7–d14 | 0.10 | 0.14 | 0.15 | 0.20 | 0.21 | 0.18 | 0.04 | 0.234 | 0.201 | 0.444 | 0.050 | 0.300 | 0.980 |
| d0–d14 | 0.11 | 0.12 | 0.13 | 0.16 | 0.18 | 0.15 | 0.02 | 0.265 | 0.126 | 0.323 | 0.115 | 0.167 | 0.917 |
| d14–d21 | 0.36 | 0.27 | 0.31 | 0.27 | 0.32 | 0.34 | 0.02 | 0.026 | 0.073 | 0.620 | 0.015 | 0.257 | 0.221 |
| d0–d21 | 0.19 | 0.17 | 0.19 | 0.20 | 0.22 | 0.21 | 0.02 | 0.427 | 0.051 | 0.563 | 0.839 | 0.118 | 0.663 |
| Daily feed intake, kg/day | |||||||||||||
| d0–d7 | 0.15 | 0.15 | 0.16 | 0.16 | 0.15 | 0.15 | 0.01 | 0.962 | 0.600 | 0.495 | 0.689 | 0.369 | 0.890 |
| d7–14 | 0.30 | 0.32 | 0.30 | 0.35 | 0.36 | 0.33 | 0.02 | 0.271 | 0.239 | 0.447 | 0.142 | 0.436 | 0.832 |
| d14–d216 | 0.51 | 0.44 | 0.48 | 0.48 | 0.51 | 0.52 | 0.03 | 0.459 | 0.069 | 0.881 | 0.399 | 0.188 | 0.397 |
| d7–d217 | 0.41 | 0.38 | 0.39 | 0.41 | 0.44 | 0.43 | 0.03 | 0.610 | 0.089 | 0.656 | 0.889 | 0.230 | 0.654 |
| Gain to feed ratio | |||||||||||||
| d0–d7 | 0.08 | 0.10 | 0.10 | 0.11 | 0.09 | 0.10 | 0.01 | 0.572 | 0.527 | 0.286 | 0.434 | 0.471 | 0.371 |
| d7–14 | 0.07 | 0.12 | -0.04 | 0.31 | -0.19 | 0.61 | 0.42 | 0.821 | 0.526 | 0.486 | 0.813 | 0.853 | 0.246 |
| d14–d21 | 0.95 | 0.84 | 0.85 | 0.70 | 1.05 | 0.63 | 0.23 | 0.818 | 0.772 | 0.653 | 0.556 | 0.902 | 0.384 |
| d7–d21 | 0.56 | 0.52 | 0.59 | 0.56 | 0.60 | 0.61 | 0.03 | 0.300 | 0.051 | 0.711 | 0.570 | 0.078 | 0.447 |
Diet1 CO = standard diet; 100Glu = CO plus 6 kg/Ton Glu; 75Glu + 25Gln = CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln; 50Glu + 50Gln = CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln; 25Glu + 75Gln = CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln; 100Gln = CO plus 6 kg/Ton Gln. Significant values are in bold.
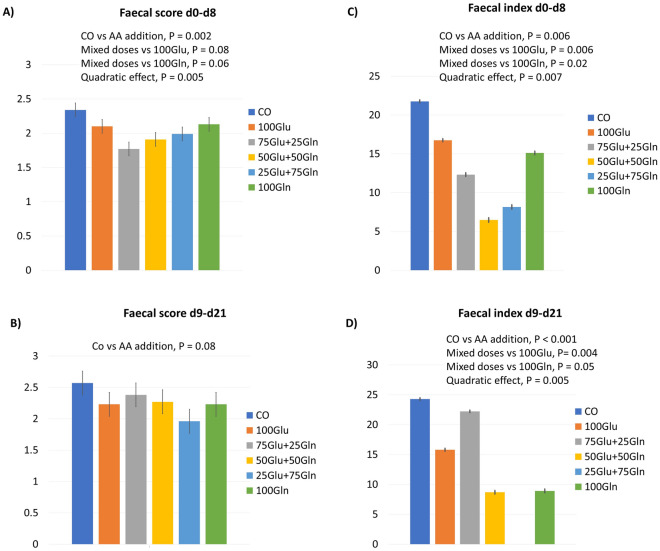
Figure 1 shows the effect of diet on the faecal score and the faecal index of the piglets. The faecal score from d0 to d8 was reduced in the groups supplemented with the AAs as compared with the CO group (P = 0.002). The groups having the mixed doses of Glu and Gln had lower faecal scores as compared with the groups having Glu alone (P = 0.08) and Gln alone (P = 0.06). There was a quadratic effect of the Glu:Gln ratio (P = 0.005). From d9 to d21, the faecal score tended to be reduced in the groups supplemented with the AAs as compared with the CO group (P = 0.08). The diarrhoea index was reduced in the groups supplemented with the AAs as compared with the CO group in both periods (d0–d8, P = 0.006; d9–d21, P < 0.001). For both periods, there was a quadratic effect of the Glu:Gln ratio (d0–d8, P = 0.0007; d9–d21, P = 0.005). For the period d9–d21, the Glu:Gln ratio also exerted a linear effect (P < 0.0001). For both periods, the groups having the mixed doses of Glu and Gln had a lower diarrhoea index (d0–d8, Glu alone vs. mixed addition P = 0.0006, Gln alone vs. mixed addition P = 0.02; d9–d21, Glu alone vs. mixed addition P = 0.0004, Gln alone vs. mixed addition P = 0.05).
Figure 1.
The effect of dietary supplementation (6 g/T) with Glu and Gln in different ratios on the faecal score and the faecal index of weaned piglets from d0 to d8 (A: faecal score; C: faecal index) and from d9 to d21 (B: faecal score; D: faecal index). The data of the faecal index were transformed from log values. Diet: CO = standard diet; 100Glu = CO plus 6 kg/Ton Glu; 75Glu + 25Gln = CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln; 50Glu + 50Gln = CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln; 25Glu + 75Gln = CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln; 100Gln = CO plus 6 kg/Ton Gln.
Blood parameters
Table 2 shows the effect of the dietary supplementation on blood values at d8. No difference was observed for the values of haemoglobin (HGB), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), platelets and the percentage of red cell distribution width (RDW) in the number of leukocytes, neutrophils, lymphocytes, basophils and the percentage of basophils. The level of the red blood cell (RBC) count tended to be higher in the Glu alone group (100Glu) as compared with the other groups supplemented with mixed doses of AAs (P = 0.059). For hematocrit % (HCT %), a quadratic effect of the Glu:Gln ratio (P = 0.023) was observed, and the 100Glu group had a higher value as compared with the other groups supplemented with mixed doses of AAs (P = 0.025). The level of mean corpuscular volume (MCV) was higher in the Gln alone group (100Gln) as compared with the other groups supplemented with mixed doses of AAs (P = 0.049) (Table 2). Considering the white cell fraction, the diet influenced the number and the percentage of eosinophils (P = 0.011; P = 0.010, respectively). For the eosinophil count, a linear effect of the Glu:Gln ratio was observed (P = 0.026). For the percentage of eosinophils, a higher value was observed in the Gln alone group (100Gln) as compared with the other groups supplemented with mixed doses of AAs (P = 0.012), and a negative linear effect of Glu addition was observed (P = 0.012). A trend and a linear effect for the Glu:Gln ratio was observed regarding the number and the percentage of monocytes (P = 0.068; P = 0.028, respectively), and the Gln alone group tended to have a lower value of this white cell fraction as compared with the groups receiving the mixed doses of AAs (P = 0.063; P = 0.075, respectively). The diet tended to affect the percentage of neutrophils (P = 0.075); a quadratic effect of the Glu:Gln ratio was observed (P = 0.012) in which the 50Glu + 50Gln group had the highest value. The diet tended to affect the percentage of lymphocytes (P = 0.1); a quadratic effect of the Glu:Gln ratio was observed (P = 0.012) in which the 50Glu + 50Gln group had the lowest value. In addition, the Gln alone group (100Gln) tended to have a higher lymphocyte as percentage compared with the other groups supplemented with AAs (P = 0.092) (Table 2).
Table 2.
Effect of the dietary supplementation (6 kg/T) with glutamate and glutamine in different ratio on haematological parameters of piglets at 8 days post-weaning.
| Item1 | Diet2 | SEM | P-value | Contrasts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | 100Glu | 75Glu + 25Gln |
50Glu + 50Gln |
25Glu + 75Gln |
100Gln | Diet | Linear | Quadratic | CO vs AAs addition |
100Glu vs mixed addition |
100Gln vs mixed addition |
||
| RBC, M/µL | 6.92 | 7.07 | 6.81 | 6.75 | 6.76 | 6.8 | 0.1 | 0.530 | 0.180 | 0.181 | 0.552 | 0.059 | 0.869 |
| HGB g/dL | 11.9 | 12 | 11.6 | 11.7 | 11.7 | 11.8 | 0.2 | 0.708 | 0.567 | 0.142 | 0.687 | 0.108 | 0.458 |
| HCT, % | 37.8 | 38.1 | 36.3 | 36.6 | 36.5 | 37.6 | 0.6 | 0.201 | 0.680 | 0.023 | 0.291 | 0.025 | 0.130 |
| MCV, fl | 54.8 | 54.3 | 53.3 | 54.4 | 54.1 | 55.4 | 0.6 | 0.326 | 0.156 | 0.172 | 0.499 | 0.604 | 0.049 |
| MCH, pg | 17.2 | 17.2 | 17.1 | 17.3 | 17.3 | 17.5 | 0.3 | 0.946 | 0.332 | 0.819 | 0.884 | 0.771 | 0.438 |
| MCHC, g/dL | 31.5 | 31.6 | 32.1 | 31.9 | 32 | 31.5 | 0.3 | 0.492 | 0.782 | 0.133 | 0.252 | 0.237 | 0.157 |
| RDW, % | 25.4 | 25.4 | 24.5 | 24.9 | 25.1 | 24.6 | 0.8 | 0.947 | 0.696 | 0.866 | 0.583 | 0.766 | 0.553 |
| Platelets, K/µL | 600 | 614 | 665 | 668 | 640 | 580 | 44 | 0.638 | 0.515 | 0.121 | 0.473 | 0.386 | 0.130 |
| Leukocytes, K/µL | 15.66 | 15.07 | 13.76 | 13.92 | 15.67 | 14.07 | 0.91 | 0.457 | 0.976 | 0.797 | 0.234 | 0.717 | 0.555 |
| Neutrophils, K/µL | 7.39 | 6.94 | 6.63 | 7.77 | 7.66 | 6.58 | 0.55 | 0.468 | 0.864 | 0.167 | 0.633 | 0.515 | 0.220 |
| Lymphocytes, K/µL | 7.46 | 7.3 | 6.4 | 5.47 | 7.1 | 6.85 | 0.57 | 0.136 | 0.912 | 0.069 | 0.752 | 0.139 | 0.427 |
| Monocytes, K/µL | 0.46 | 0.59 | 0.44 | 0.45 | 0.54 | 0.32 | 0.08 | 0.178 | 0.068 | 0.783 | 0.943 | 0.196 | 0.063 |
| Eosinophils, K/µL | 0.31 | 0.19 | 0.27 | 0.18 | 0.33 | 0.29 | 0.04 | 0.011 | 0.026 | 0.961 | 0.113 | 0.107 | 0.460 |
| Basophils, K/µL | 0.04 | 0.04 | 0.03 | 0.05 | 0.04 | 0.04 | 0.01 | 0.965 | 0.796 | 0.807 | 0.956 | 0.943 | 0.943 |
| Neutrophils, % | 47.35 | 46.16 | 47.71 | 54.8 | 47.5 | 45.87 | 2.36 | 0.075 | 0.918 | 0.012 | 0.675 | 0.159 | 0.131 |
| Lymphocytes, % | 47.14 | 48.12 | 46.44 | 39.66 | 46.85 | 49.29 | 2.53 | 0.104 | 0.738 | 0.012 | 0.695 | 0.195 | 0.092 |
| Monocytes, % | 3.16 | 4.22 | 3.56 | 3.71 | 3.3 | 2.39 | 0.55 | 0.291 | 0.028 | 0.599 | 0.643 | 0.268 | 0.075 |
| Eosinophils, % | 2.03 | 1.21 | 2.04 | 1.39 | 2.11 | 2.12 | 0.23 | 0.010 | 0.012 | 0.764 | 0.297 | 0.279 | 0.012 |
| Basophils, % | 0.32 | 0.29 | 0.27 | 0.4 | 0.24 | 0.33 | 0.07 | 0.718 | 0.815 | 0.839 | 0.822 | 0.910 | 0.724 |
| ROM, mmol H2O2/L | 24.07 | 21.75 | 21.07 | 22.63 | 24.12 | 22.57 | 1.29 | 0.487 | 0.260 | 0.703 | 0.236 | 0.564 | 0.980 |
| GPX-1, ng/mL | 557 | 640 | 609 | 545 | 558 | 522 | 61.42 | 0.727 | 0.321 | 0.434 | 0.775 | 0.301 | 0.478 |
| GSH, uM | 0.31 | 0.14 | 0.39 | 0.18 | 0.21 | 0.17 | 0.11 | 0.640 | 0.439 | 0.810 | 0.449 | 0.377 | 0.495 |
1RBC, red blood cells, HGB, haemoglobin, HCT, haematocrit, MCV, mean corpuscular volume, MCH, mean corpuscular haemoglobin, MCHC, mean corpuscular haemoglobin concentration, RDW, red cell distribution width.
Diet2 CO = standard diet; 100Glu = CO plus 6 kg/Ton Glu; 75Glu + 25Gln = CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln; 50Glu + 50Gln = CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln; 25Glu + 75Gln = CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln; 100Gln = CO plus 6 kg/Ton Gln. Significant values are in bold.
No difference due to diet was observed for any of the parameters, except for HGB for which the CO group tended to have a lower value as compared with the groups supplemented with AAs (P = 0.079) and for the percentage of eosinophils for which the diet showed a trend (P = 0.057); no significant contrasts were determined (Supplementary Table 1).
Caecal microbiota
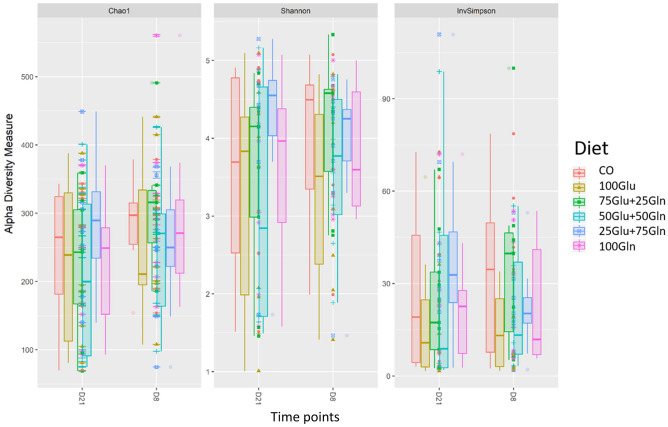
A total of 4,098,584 quality checked reads on 103 samples were obtained resulting in 3523 different amplicon sequence variants (ASVs). The rarefaction curves relative to all the samples are shown in Supplementary Fig. 1. Considering the overall samples, eighteen different phyla were identified in the caecum; the most abundant phylum was Firmicutes (70%) followed by Bacteroidetes (17%). At the family level, eighty different genera were assigned; the most abundant were Peptostreptococcaceae (18%), and Eryspelotrichaceae (17%). At the genus level, the most abundant assigned genera were Turicibacter (15%) and Terrisporobacter (13%). Figure 2 shows the Alpha diversity indices of the dietary groups at the two time points. The dietary groups showed a fairly constant ASVs distribution in the caecum; the Alpha index values (Chao1, Shannon and InvSimpson) were not affected by diet. Time did not influence the Shannon and InvSimpson indices, but reduced the Chao1 index from d8 (265) to d21 (231) (P = 0.008). For the Beta diversity index (Bray–Curtis distance), a clear effect of time was observed (P = 0.01; R2 = 0.068). Two well-defined clusters regarding time were shown in the Non-Metric Multidimensional Scaling (NMDS) plot of the Bray–Curtis distance matrix (Supplementary Fig. 2). Diet did not affect the Beta diversity index. Results for the different taxa of the dietary groups at the family and genera levels at d8 and d21 are reported in Table 3. At d8, the 100Gln group had a lower abundance of family Fusobacteriaceae as compared with the CO group (adj.p < 0.0001); considering the comparison between the Glu alone group vs. the mixed addition groups, the 100Glu group had a lower relative abundance of Enterococcaceae (adj.p < 0.0001) and Lactobacillaceae (adj.p < 0.0001), and a higher abundance of Erysipelotrichaceae (adj.p = 0.02). Considering the comparison between the Gln alone group vs. the mixed addition groups, the 100Gln group had a lower abundance of Fusobacteriaceae (adj.p < 0.0001) and a higher level of Clostridiales_vadinBB60_group (adj.p = 0.001). Considering the comparisons at the genus level at d8, the CO group had a higher relative abundance of Selenomonas (adj.p = 0.007) and Mogibacterium (adj.p < 0.0001) than the 100Glu group, a higher relative abundance of Pelistega (adj.p < 0.0001) and Selenomonas (adj.p = 0.006) than the 50Glu + 50Gln group, a higher relative abundance of Selenomonas (adj.p = 0.004) and Blautia (adj.p = 0.023) than the 25Glu + 75Gln group and a higher relative abundance of Fusobacterium (adj.p < 0.0001) and UB1819 (Family Ruminococcaceae) (adj.p < 0.0001) as compared with the 100Gln group. The comparison between the Glu alone group or the Gln alone group vs. the AAs mixed diets showed a decrease in the relative abundance of Pediococcus (adj.p < 0.0001), Enterococcus (adj.p < 0.0001) and Lactobacillus (adj.p = 0.008), in the 100Glu group, and a decrease in Pediococcus (adj.p < 0.05) and Fusobacterium (adj.p < 0.0001) in the 100Gln group. At d21, the CO group had a lower abundance of Fibrobacteraceae vs. each of the groups supplemented with AAs (adj.p < 0.0001) and a lower relative abundance of Bacteroidales_RF16_group (adj.p = 0.028) and Peptococcaceae as compared with the 100Gln group (adj.p = 0.037). Considering the comparisons at the genus level at d21, the CO group had a lower relative abundance of Selenomonas_3 as compared with the 100Glu group (adj.p = 0.006) and the 25Glu + 75Gln groups (adj.p = 0.007), and a lower relative abundance of Succiniclasticum as compared with the groups supplemented with AAs (adj.p < 0.0001).
Figure 2.
Alpha diversity indices per Diet and time points (day 21 and day 8 post-weaning). Diet: CO = standard diet; 100Glu = CO plus 6 kg/Ton Glu; 75Glu + 25Gln = CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln; 50Glu + 50Gln = CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln; 25Glu + 75Gln = CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln; 100Gln = CO plus 6 kg/Ton Gln.
Table 3.
Effect of the dietary supplementation (6 kg/T) with glutamate and glutamine in different ratio on microbial taxa composition of piglets at 8- and 21-days post-weaning.
| Comparison | Base Mean1 |
log2 Fold Change2 |
lfcSE3 | P Value4 |
Padj5 | Taxa |
|---|---|---|---|---|---|---|
| Day 8 | ||||||
| Family level | ||||||
| 100Gln vs CO | 22.87 | − 21.51 | 2.96 | < 0.0001 | < 0.0001 | Fusobacteriaceae |
|
Mixed additions vs Glu |
10.78 | 21.06 | 3.41 | < 0.0001 | < 0.0001 | Enterococcaceae |
| 1989.71 | 2.75 | 0.63 | < 0.0001 | < 0.0001 | Lactobacillaceae | |
| 7931.63 | − 2.77 | 0.84 | 0.001 | 0.02 | Erysipelotrichaceae | |
|
Mixed additions vs Gln |
25.76 | 23.38 | 2.14 | < 0.0001 | < 0.0001 | Fusobacteriaceae |
| 495.19 | − 3.31 | 0.94 | < 0.0001 | 0.001 | Clostridiales_vadinBB60_group | |
| Genus level | ||||||
| 100Glu vs CO | 19.62 | − 21.65 | 2.78 | < 0.0001 | < 0.0001 | Mogibacterium |
| 9.07 | − 21.07 | 5.28 | < 0.0002 | 0.007 | Selenomonas | |
| 50Glu + 50Gln vs CO | 6.7 | − 18.56 | 3.91 | < 0.0003 | < 0.0001 | Pelistega |
| 9.07 | − 21.16 | 5.28 | < 0.0004 | 0.006 | Selenomonas | |
| 25Glu + 75 Gln vs CO | 9.07 | − 21.96 | 5.14 | < 0.0005 | 0.004 | Selenomonas |
| 740.85 | − 3.7 | 1 | < 0.0006 | 0.023 | Blautia | |
| 100Gln vs CO | 34.79 | − 20.86 | 3.03 | < 0.0007 | < 0.0001 | Fusobacterium |
| 8.33 | − 21 | 3.12 | < 0.0008 | < 0.0001 | UBA1819, Family Ruminococcaceae | |
|
Mixed additions vs Glu |
57.99 | 23.33 | 2.92 | < 0.0011 | < 0.0001 | Pediococcus |
| 11.38 | 21.04 | 3.41 | < 0.0012 | < 0.0001 | Enterococcus | |
| 1809.38 | 2.7 | 0.7 | < 0.0013 | 0.008 | Lactobacillus | |
|
Mixed additions vs Gln |
37.65 | 24.02 | 2.21 | < 0.0009 | < 0.0001 | Fusobacterium |
| 59.71 | 9.67 | 2.53 | < 0.0010 | 0.013 | Pediococcus | |
| Day 21 | ||||||
| Family level | ||||||
| 100Glu vs CO | 10.809 | 18.989 | 2.53 | < 0.0001 | < 0.0001 | Fibrobacteraceae |
| 75Glu + 25Gln vs CO | 10.809 | 18.689 | 2.59 | < 0.0001 | < 0.0001 | Fibrobacteraceae |
| 50Glu + 50Gln vs CO | 10.809 | 20.141 | 2.52 | < 0.0001 | < 0.0001 | Fibrobacteraceae |
| 25Glu + 75Gln vs CO | 10.809 | 20.892 | 2.65 | < 0.0001 | < 0.0001 | Fibrobacteraceae |
| 100Gln vs CO | 10.809 | 19.677 | 2.58 | < 0.0001 | < 0.0001 | Fibrobacteraceae |
| 101.951 | 5.117 | 1.53 | 0.001 | 0.028 | Bacteroidales_RF16_group | |
| 109.322 | 2.14 | 0.68 | 0.002 | 0.037 | Peptococcaceae | |
| Genus level | ||||||
| 100Glu vs CO | 18.045 | 21.269 | 5.08 | < 0.0001 | 0.006 | Selenomonas_3 |
| 25Glu + 75Gln vs CO | 18.045 | 22.13 | 5.35 | < 0.0001 | 0.007 | Selenomonas_3 |
| CO vs AAs addition | 7.378 | − 21.523 | 4.13 | < 0.0001 | < 0.0001 | Succiniclasticum |
baseMean1 = mean of normalized taxa counts averaged over all samples from both conditions. log2FoldChange2 = log2 Fold Change. The sign is relative to the first group identified in the comparison. lfcSE3 = log2 Fold change standard error. P value4 = Wald statistic value. Padj5 = Benjamini–Hochberg adjusted p value.
Intestinal morphology
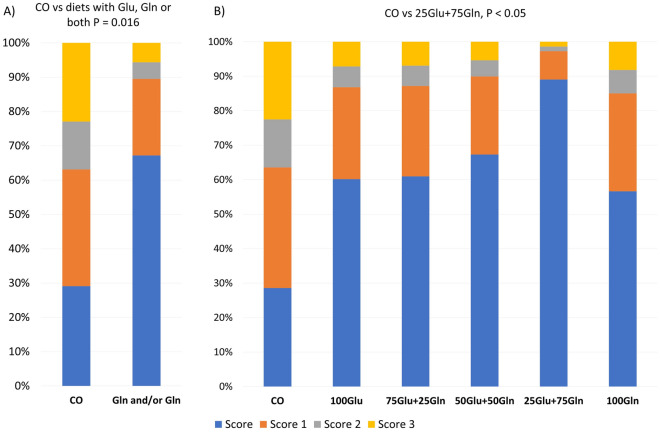
Table 4 shows the effect of dietary supplementation on the intestinal morphology measurements at d8 and d21. At d8, no difference due to diet was observed for villus height, villus width, crypt depth, and the number of goblet cells and duodenal glands. However, at d8, in the duodenum, the number of duodenal glands was higher in the 100Gln group as compared with the other groups supplemented with mixed doses of AAs (P = 0.048); a quadratic effect of the Glu:Gln ratio was observed (P = 0.048). At d8, in the jejunum, the number of goblet cells was lower in the CO group as compared with the 50Glu + 50Gln group (P = 0.05); in the ileum, the villus height tended to be higher in the 100Glu group as compared with the other groups supplemented with mixed doses of AAs (P = 0.097). The villus width tended to be higher in the CO group as compared with the 100Glu group (P = 0.079) and the number of goblet cells tended to increase linearly with the Gln dose (P = 0.090). No difference due to diet was observed for the interstitial score in any of the intestinal segments at d8. Diet influenced the intraepithelial score in the jejunum at d8; the CO group had a higher probability of having a higher intraepithelial lymphocyte score as compared with the groups supplemented with Glu, Gln or both (P < 0.016), and the 25Glu + 75Gln group showed a lower probability of a high intraepithelial lymphocyte score as compared with the CO group (P = 0.05) (Fig. 3). At d21, no differences due to diet were observed for villus height, villus width, crypt depth, and the number of goblet cells and duodenal glands (Table 4). At d 21, in the duodenum, the crypt depth tended to be higher in the 100Gln group as compared with the other groups supplemented with mixed doses of AAs (P = 0.083). In the jejunum, the villus width tended to be higher in the CO group as compared with the 25Glu + 75Gln group (P = 0.081) at d21. No difference due to diet was observed for the interstitial and intraepithelial scores in any of the intestinal tracts at d21.
Table 4.
Effect of the dietary supplementation (6 kg/T) with glutamate and glutamine in different ratio on the intestinal morphology of piglets at 8- and 21-days post-weaning.
| Item | Diet | SEM | P-value | Contrasts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | 100Glu | 75Glu + 25Gln |
50Glu + 50Gln |
25Glu + 75Gln |
100Gln | Diet | Linear | Quadratic | CO vs AAs additions |
100Glu vs mixed addition |
100Gln vs mixed addition |
||
| Day 8 | |||||||||||||
| Duodenum | |||||||||||||
| Villus height, (µm) | 402 | 398 | 415 | 421 | 457 | 387 | 42.95 | 0.990 | 0.773 | 0.518 | 0.783 | 0.512 | 0.381 |
| Villus width, (µm) | 122 | 115 | 106 | 112 | 118 | 120 | 9.69 | 0.816 | 0.946 | 0.189 | 0.441 | 0.751 | 0.411 |
| Crypt depth, (µm) | 334 | 342 | 307 | 322 | 336 | 338 | 37.32 | 0.988 | 0.592 | 0.917 | 0.772 | 0.655 | 0.637 |
| Duodenal glands, N | 160 | 127 | 109 | 140 | 127 | 196 | 30.48 | 0.423 | 0.888 | 0.048 | 0.771 | 0.655 | 0.048 |
| Jejunum | |||||||||||||
| Villus height, (µm) | 325 | 313 | 323 | 357 | 278 | 316 | 32.2 | 0.582 | 0.665 | 0.710 | 0.820 | 0.862 | 0.920 |
| Villus width, (µm) | 113.5 | 95.6 | 94.5 | 121.2 | 107.6 | 93.1 | 9.17 | 0.286 | 0.660 | 0.720 | 0.341 | 0.334 | 0.220 |
| Crypt depth, (µm) | 224 | 253 | 287 | 227 | 210 | 217 | 37.28 | 0.711 | 0.464 | 0.337 | 0.723 | 0.786 | 0.551 |
| Goblet cells, N | 16.5a | 17.1ab | 25.2ab | 34.1b | 28.8ab | 27.3ab | 6.73 | 0.356 | 0.110 | 0.280 | 0.169 | 0.123 | 0.787 |
| Ileum | |||||||||||||
| Villus height, (µm) | 243 | 286 | 235 | 255 | 236 | 249 | 23.28 | 0.609 | 0.609 | 0.913 | 0.733 | 0.097 | 0.797 |
| Villus width, (µm) | 104 | 82.8 | 91.9 | 107 | 88.2 | 86.4 | 8.39 | 0.173 | 0.425 | 0.829 | 0.079 | 0.189 | 0.313 |
| Crypt depth, (µm) | 209 | 249 | 243 | 221 | 213 | 193 | 24.98 | 0.544 | 0.325 | 0.167 | 0.597 | 0.406 | 0.244 |
| Goblet cells, N | 19.5 | 19.6 | 25.7 | 33.7 | 32.1 | 34.3 | 8.35 | 0.615 | 0.090 | 0.784 | 0.314 | 0.247 | 0.682 |
| Day 21 | |||||||||||||
| Duodenum | |||||||||||||
| Villus height, (µm) | 539 | 523 | 564 | 499 | 553 | 512 | 34.53 | 0.707 | 0 .712 | 0.813 | 0.794 | 0.679 | 0.560 |
| Villus width, (µm) | 157 | 135 | 133 | 138 | 141 | 141 | 14.43 | 0.882 | 0.697 | 0.384 | 0.236 | 0.900 | 0.854 |
| Crypt depth, (µm) | 344 | 292 | 338 | 340 | 343 | 427 | 36.78 | 0.374 | 0.110 | 0.135 | 0.915 | 0.238 | 0.083 |
| Duodenal glands, N | 173 | 148 | 186 | 173 | 191 | 155 | 22.58 | 0.73 | 0.180 | 0.135 | 0.915 | 0.238 | 0.289 |
| Jejunum | |||||||||||||
| Villus height, (µm) | 417 | 361 | 387 | 399 | 414 | 368 | 29.98 | 0.642 | 0.776 | 0.973 | 0.293 | 0.251 | 0.394 |
| Villus width, (µm) | 107.8a | 98.1ab | 111.4b | 100.7ab | 92.5ab | 110.8ab | 6.34 | 0.272 | 0.815 | 0.345 | 0.414 | 0.630 | 0.243 |
| Crypt depth, (µm) | 261 | 250 | 277 | 252 | 218 | 279 | 25.42 | 0.647 | 0.872 | 0.615 | 0.814 | 0.962 | 0.351 |
| Goblet cells, N | 25.2 | 23.5 | 29.2 | 28.1 | 35 | 22 | 5.98 | 0.688 | 0.825 | 0.603 | 0.687 | 0.279 | 0.240 |
| Ileum | |||||||||||||
| Villus height, (µm) | 306 | 324 | 319 | 260 | 331 | 329 | 22.98 | 0.196 | 0.681 | 0.318 | 0.771 | 0.429 | 0.369 |
| Villus width, (µm) | 103 | 113 | 105 | 108 | 104 | 102 | 7.47 | 0.912 | 0.647 | 0.507 | 0.653 | 0.399 | 0.689 |
| Crypt depth, (µm) | 232 | 216 | 258 | 210 | 216 | 224 | 19.1 | 0.471 | 0.600 | 0.879 | 0.718 | 0.565 | 0.861 |
| Goblet cells, N | 21.6 | 28.4 | 19 | 27.5 | 28.8 | 19.6 | 4.95 | 0.548 | 0.999 | 0.404 | 0.528 | 0.549 | 0.382 |
Diet1 CO = standard diet; 100Glu = CO plus 6 kg/Ton Glu; 75Glu + 25Gln = CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln; 50Glu + 50Gln = CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln; 25Glu + 75Gln = CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln; 100Gln = CO plus 6 kg/Ton Gln. Significant values are in bold.
Figure 3.
The effect of the diet on the cumulative probabilities of having each score for the intraepithelial lymphocytes in the jejunum of piglets at 8 days post-weaning. (A) Comparison between the CO group and the groups supplemented with AAs; (B) Cumulative probabilities of having each score in each dietary group. Diet: CO = standard diet; 100Glu = CO plus 6 kg/Ton Glu; 75Glu + 25Gln = CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln; 50Glu + 50Gln = CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln; 25Glu + 75Gln = CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln; 100Gln = CO plus 6 kg/Ton Gln.
Intestinal gene expression
No difference due to diet was observed for the expression of the Innate Immune Signal Transduction Adaptor (MyD88), Nuclear Factor Kappa B Subunit (NFKB2), Tumor Necrosis Factor (TNF), C-X-C Motif Chemokine Ligand 8 (IL8;gene CXCL8), Occludin (OCLN), Tight Junction Protein 1(ZO-1), Mucin 13, Cell Surface Associated (MUC13), Glutathione Peroxidase 2 (GPX-2) and Glutamate-Ammonia Ligase (GLUL) and Regenerating Family Member 3 Gamma (REG3G) in the jejunal mucosa of piglets. Some effects or trends for GLUL, ZO-1 and GPX2 were found with the orthogonal contrasts as shown in Table 5. The CO group tended to have a lower expression of GLUL as compared with the groups supplemented with AAs (P = 0.088). The expression of ZO-1 was higher in the 100Gln group as compared with the groups supplemented with mixed doses of AAs (P = 0.025); a linear tendency of the diet was observed (P = 0.079). The expression of GPX-2 tended to be higher in the 100Glu group as compared with the other groups supplemented with AAs (P = 0.086).
Table 5.
Effect of the dietary supplementation (6 g/T) with glutamate and glutamine in the different ratios on the jejunal gene expression of piglets at 8 days post-weaning.
| Gene1 | Diet2 | SEM | P-value | Contrasts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | 100Glu | 75Glu + 25Gln |
50Glu + 50Gln |
25Glu + 75Gln |
100Gln | Diet | Linear | Quadratic | CO vs AAs addition |
100Glu vs mixed addition |
100Gln vs mixed addition |
||
| GLUL | 1.17 | 1.78 | 1.38 | 1.64 | 1.55 | 1.48 | 0.21 | 0.409 | 0.501 | 0.251 | 0.088 | 0.338 | 0.834 |
| OCLN | 1.09 | 0.95 | 0.91 | 0.95 | 1.11 | 1.04 | 0.11 | 0.727 | 0.736 | 0.263 | 0.449 | 0.778 | 0.678 |
| ZO-1 | 1.07 | 1.26 | 1.19 | 1.16 | 1.19 | 1.47 | 0.11 | 0.188 | 0.079 | 0.394 | 0.132 | 0.518 | 0.025 |
| GPX-2 | 1.64 | 3.37 | 1.60 | 2.70 | 1.79 | 1.68 | 0.69 | 0.308 | 0.538 | 0.351 | 0.415 | 0.086 | 0.648 |
| Myd88 | 1.02 | 1.01 | 0.99 | 1.16 | 0.96 | 0.97 | 0.07 | 0.331 | 0.704 | 0.338 | 0.977 | 0.727 | 0.418 |
| TNF | 1.11 | 1.06 | 1.37 | 0.98 | 0.94 | 0.90 | 0.15 | 0.359 | 0.175 | 0.364 | 0.728 | 0.849 | 0.296 |
| MUC13 | 1.16 | 1.22 | 1.10 | 1.15 | 1.35 | 1.47 | 0.16 | 0.632 | 0.161 | 0.322 | 0.578 | 0.905 | 0.169 |
| IL8 | 1.10 | 1.12 | 1.00 | 1.27 | 0.97 | 0.89 | 0.18 | 0.731 | 0.432 | 0.466 | 0.810 | 0.858 | 0.367 |
| NFKB2 | 1.09 | 1.08 | 0.96 | 1.04 | 1.23 | 1.18 | 0.13 | 0.759 | 0.375 | 0.388 | 0.974 | 0.993 | 0.496 |
| REG3G | 1.00 | 1.01 | 0.36 | 0.90 | 0.85 | 2.25 | 0.48 | 0.925 | 0.407 | 0.887 | 0.382 | 0.811 | 0.586 |
Gene1: Glutamate-Ammonia Ligase (GLUL); Occludin (OCLN); Tight Junction Protein 1 (ZO-1); Glutathione Peroxidase 2 (GPX-2); Innate Immune Signal Transduction Adaptor (MyD88); Tumor Necrosis Factor (TNF); Mucin 13 Cell Surface Associated (MUC13); C-X-C Motif Chemokine Ligand 8 (IL8/ CXCL8); Nuclear Factor Kappa B Subunit 2 (NFKB2); Regenerating Family Member 3 Gamma (REG3G). Diet2 CO = standard diet; 100Glu = CO plus 6 kg/Ton Glu; 75Glu + 25Gln = CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln; 50Glu + 50Gln = CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln; 25Glu + 75Gln = CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln; 100Gln = CO plus 6 kg/Ton Gln. Significant values are in bold.
Discussion
Supporting the integrity, functionality and morphology of the gut is a key strategy for sustaining piglets during the weaning transition period1. The results obtained in the present study demonstrated that mixing doses of Glu and Gln can benefit gut health and the growth of post-weaning piglets during the first 2 weeks post-weaning, sustaining the thesis that these AAs play a crucial role in supporting and restoring the gut health of piglets.
The results suggested that both AAs were able to improve piglet growth during the more acute inflammatory period (until the second week post-weaning) as compared with the control diet, the piglets of which recovered in the third week post-weaning17,18. The recovery of the control group could have been due to compensatory or “catch up” growth which occur when animals are supplied with sufficient nutrition after restricting feed or following adverse conditions, such as weaning19. In fact, it is known that transient anorexia and gut inflammation can increase piglet morbidity in the first weeks post-weaning while, after 2 weeks, the gut can recover, and improve the digestion and absorption of nutrients, promoting piglet growth20. The faster recovery of piglets supplemented with Glu and Gln could have been related to their functional roles regarding gut health which will be discussed later in detail. The supplementation of Gln was associated with more promising effects than that of Glu; in fact, a linear effect with higher values regarding the ADG and the G:F ratio for the groups receiving more Gln than Glu was observed considering the overall period. to date, no direct comparison between supplementation using different quantities of Glu and Gln has been reported in the literature. However, previous studies have suggested a positive effect of mixed doses of Glu and Gln during the pre- and post-weaning phases (0.88% of 50% of Glu:Gln ratio21) and later in the piglets’ life16. In addition, Liu et al.22 observed that both Glu and Gln dietary supplementation (1%) increased the level of RNA in the skeletal muscle of piglets at first week post-weaning, suggesting that these AAs can sustain the protein synthesis in muscles and improve growth performance under stressful conditions.
In the present study, the faecal score and the complete blood cell formula were analysed to investigate the effect of Glu and Gln on the health of piglets. Overall, both the faecal score and the blood cell formula data suggested that the piglets did not suffer from severe illness. Moreover, the increase in the faecal score could have been due to greater water secretion in the small intestine, derived from the alteration of the osmotic gradients and not from a pathogen infection which could be linked to secretory or osmotic diarrhoea23. Both the number of days with diarrhoea and the faecal score values showed a quadratic effect for the ratio of Glu to Gln supplementation in the diet, with the 50 to 50 mixed dose having the lowest value. According to the literature, Gln can have a positive effect on sodium absorption; its supplementation can improve water intestinal absorption, leading to a reduction in loose faeces in other species9,24 and in post-weaning piglets (1% of Gln)25. This mechanism of glutamine-dependent electroneutral sodium absorption could explain the reduced number of days with loose faeces and the reduced faecal score of piglets supplemented with AAs26. Furthermore, the quadratic effect observed in the present study suggested that a synergy between Glu and Gln could be present.
The diet did not influence the blood parameters and, only for a few parameters including eosinophils, neutrophils at d8, lymphocytes at d8 and eosinophils at d21, was a trend found. An effect of the Glu:Gln ratios was found on some white blood cells with a more evident effect at d8 than at d21. Glutamine is known to be an important fuel for cell proliferation, not only for enterocytes, but also for immune cells, such as lymphocytes, macrophages, and neutrophils27,28. The results obtained in the present study confirmed this fact. Moreover, the 100Gln group tended to have a higher percentage of lymphocytes. On the contrary, the level of monocytes tended to be lower in the 100Gln group; it could be hypothesised that Gln contributed to a greater differential ratio of monocytes to macrophages29 (not analysed). In addition, monocytes are among the cells with a higher expression of glutamate dehydrogenase (GLUD1), controlling their Gln catabolism30. However, there is no direct evidence of increased production of monocytes with Glu. The interaction of the AAs supplementation on some blood parameters related to the immune response was also observed. The mixed doses of Glu and Gln influenced the neutrophil %/lymphocyte %; this confirmed the interaction between the two AAs in their catabolism (via glutaminase and glutamate dehydrogenase31 and, therefore, in enhancing the immune functions.
To explain the results observed regarding the growth and health of piglets in depth, the effect of the synergy of the two AAs was investigated in the small intestine mucosal morphological structure during inflammation. The results regarding the morphological structure showed that the contribution of AAs supplementation was more consistent at d8 than at d21, which is reasonable as, from weaning to d8, a higher acute inflammatory phase due to weaning transition occurs in piglets32. Furthermore, the results showed a different response according to the doses of Glu and Gln, and the sites of the intestinal tract. In particular, at d8, Gln increased the number of the duodenal gland and goblet cells in the ileum; this was in accordance with the results observed by Xing et al. (2017)33. An explanation could be the stimulation of the differentiation of stem cells into goblet cells by Gln, as indicated by an in vitro study in which increased differentiation into Mucin 2 secreting cells was observed when Gln availability was increased34. In the present study, the effect of Glu was mainly exerted in the ileum in which it tended to improve the villus height. Finally, a beneficial interaction between Glu and Gln (50Glu + 50Gln) was highlighted by an increasing number of goblet cells in the jejunum. As previously suggested by Windmueller and Spaeth3, and Cabrera et al.21, the present results indicated that Glu and Gln could have a synergistic effect on the post-weaning recovery of the intestinal mucosa.
The intestinal mucosal structure is closely connected to positive intestinal integrity and barrier function. The results obtained in the present study suggested that Gln could linearly increase the expression of ZO-1 in the jejunum, confirming the beneficial effect of Gln on Zonula Occludens (ZO) family members which, together with occludin, claudins, and actin, provide a scaffold for the assembly and regulation of the expression and distribution of the tight junction (TJ) complex in the intestinal mucosa. This result confirmed the previous hypotheses that Gln could contribute to regulating the expression of the TJ proteins, thereby conferring a beneficial effect on the mucosal barrier function (in vivo, post-weaning piglets12 and in vitro35). Glutamine can benefit the tight junction proteins by means of several mechanisms: (1) a direct effect on the enterocytes, providing energy which helps to maintain the integrity of the epithelial barrier; (2) increasing the functional integrity of the mitochondria, via the activation of heat shock proteins (HSPs) expressed by enterocytes, particularly HSP72, and; (3) preventing apoptosis under heat stress by means of its regulation of the mTOR and p38 MAP kinase pathways.36 In fact, when there was Gln deficiency, dysfunctional mitochondria and the accumulation of mtROS induced epithelial barrier defects and disrupted the tight junction proteins37. In the present study, Gln also tended to reduce the expression of GPX2 in the jejunum. The GPX2 gene (also called GI-GPx) encodes the most expressed selenoprotein of the glutathione peroxidase family in the intestine which stimulates the reduction of hydrogen peroxide derived from inflammation in the gut, thereby protecting the cells against oxidative damage38. In agreement with the present study, previous evidence from Zhang et al.39 showed that Gln reduced the expression of GPX2 protein in the jejunum of post-weaning piglets. In the present study, this effect in reducing the GPX2 expression in the gut did not result in a variation of Glutathione Peroxidase 1 (GPX-1) and Reactive Oxygen Metabolite (ROM) levels in the blood of piglets. However, it should be noted that the results of the gene expression are strictly related to the sampling time and the specific tissue; thus, they do not always agree with the pathway metabolites investigated in the peripheral blood. Furthermore, it appears that GPX2 is not only related to oxidative stress but is involved in counteracting inflammatory responses in the gut40 by means of the regulation of the NF-kB pathway38.
It should be emphasised that, in the present study, supplementation with Glu and Gln and, in particular, the 25%Glu + 75%Gln diet, reduced the probability of having intraepithelial lymphocytes (IELs) in the jejunal mucosa at d8. Intraepithelial lymphocytes are a T-cell subpopulation located above the basement membrane and in between the intestinal epithelial cells, below the intercellular tight junctions connecting the intestinal epithelial cells. Their main role is to kill infected epithelial cells and protect the intestine against pathogens. Previous studies have suggested modulation of the IELs by Glu and Gln as reported by Lobley et al.41. Although the studies in the literature have reported an increase in IELs by Glu and Gln, it should be noted that they were assessing the effect of AAs using acute challenge models, such as sepsis or colitis, characterised by severe impairment of the barrier function and immunity of the gut42–44. In the present study, the results of the faecal score and growth performance suggested that the piglets in the 25%Glu + 75%Gln group were not under severe health impairment; thus, the result obtained for the IELs suggested a lower inflammatory status of these animals as compared with the animals in the other groups.
Results based on in vitro studies have suggested that AAs, including Glu and Gln, can be utilised by intestinal bacteria. Data have suggested that from 22 to 40% of Gln is utilised by bacteria in the jejunal and ileal tracts45. The results obtained in the present study suggested that the AAs supplementation did not profoundly influence the microbial composition in the large intestine of weaned piglets; in fact, no differences in the alpha and beta diversity indices were found. Nevertheless, some minor differences in the abundance of some taxa were observed. After 1 week, the supplementation of Gln reduced the bacteria belonging to the Fusobacteriaceae family, which is known to include Glu and Gln-fermenting bacteria with some differences at the species level46. At the same timepoint, the supplementation of Glu reduced the bacteria belonging to the Enterococcaceae and Lactobacillaceae families (mainly Pediococcus, Enterococcus and Lactobacillus genera) compared with the mixed doses of the AAs. Previous studies suggested that Lactobacillus species display glutaminase activity (responsible for the conversion of Gln to Glu)47,48 which can explain the increase in Lactobacillus genus in the groups in which Gln was available; less clear pieces of evidence have been reported for Enterococcus and Pediococcus species which, however, are known to be able to utilize both Gln and Glu49,50. In addition, it should be considered that both Gln and Glu play a key role in pH homeostasis and stationary phase survival of lactic acid bacteria which may have contributed to the different selection of these bacteria, however additional studies are needed to fully explain the present results.
After 3 weeks of dietary supplementation, the relative abundance of Fibrobacteraceae was promoted in all the groups supplemented with AAs. Fibrobacteraceae is a small common bacterial family capable of adhering to lignocellulosic fragments, degrading cellulose in the rumen51,52, being present in the intestinal microbiota of piglets53,54; this benefits the host by fermenting dietary fibre into short-chain fatty acids (SCFAs). At the genus level, the relative abundance of Selenomonas was reduced at d8 and increased (in a dose-dependent way) at d21 in the groups supplemented with AAs. Selenomonas is implicated in the fermentation of soluble carbohydrates55; however, it has also been recognised as a proteolytic bacterium56, capable of fermenting Glu and Gln46.
The comparison between CO and the groups supplemented with the AAs showed a decrease in the control group of the genus Succiniclasticum which is known for its ability to ferment succinate and convert it into propionate.57.
Overall, the results obtained suggested that, after 3 weeks, the supplementation of AAs could favour the growth of fermenting AA bacteria in the large intestine without compromising the gut microbial ecosystem.
The results obtained in the present study suggested that both Glu and Gln were able to improve piglet growth during the more acute inflammatory periods (until the second week post-weaning). Supplementation with Gln was more promising, especially related to better maintenance of the intestinal barrier integrity as the groups receiving more Gln had better results. A favourable effect of mixing the Glu and Gln was observed on the immune parameters, gut morphological structure and barrier function of the small intestine. These effects contributed to reducing the number of days with loose faeces and increasing the faecal consistency in piglets, mainly in the first period post-weaning. In conclusion, the present study suggested that supplementation with Glu and Gln at a ratio from 50% Glu–50% Gln to 25% Glu–75% Gln would maintain gut health and the growth of post-weaning piglets.
Material and methods
Animals, study design and sampling
The in vivo trial was approved by the Ethics Committee for Experiments on Animals of the University of Bologna, Italy and by the Italian Ministry of Health (Authorisation n. 503/2018-PR, released 2 July 2018 in compliance with art. 31 of the D.lgs. 26/2014), and complied with the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines58. At weaning (d0), 120 piglets (24 ± 2 days of age; initial BW 6.96 kg ± 0.11 kg) were moved to the experimental facility of the University of Bologna. The in vivo trial was carried out in two consecutive batches of 60 piglets each.
The piglets were assigned to one of the six experimental diets (10 replicates per diet, 2 piglets/each replicate) based on the litter of origin and BW: (1) standard diet (CO); (2) CO plus 6 kg/Ton Glu (100Glu); (3) CO plus 4.5 kg/Ton Glu and 1.5 kg/Ton Gln (75Glu + 25Gln); (4) CO plus 3 kg/Ton Glu plus 3 kg/Ton Gln (50Glu + 50Gln); (5) CO plus 1.5 kg/Ton Glu and 4.5 kg/Ton Gln (25Glu + 75Gln); (6) CO plus 6 kg/Ton Gln (100Gln). The diets did not include antimicrobials, zinc oxide or growth promoters. To design the diets, the nutrient values were estimated using EvaPig® software (v. 1.4.0.1; INRA, Saint-Gilles, France). Amino acid supplementation was provided on-top. The diet composition and chemical composition in terms of AAs are reported in Supplementary Table 2.
The piglets were housed in cages with a mesh floor. Room temperature was kept controlled from 30 °C at the beginning of the trial to 25 °C at the end of the trial, with a 1 °C decrease every 3 days. The piglets had free access to feed and water throughout the experimental period; feed was ad libitum supplied in a dry feeder.
At d8 (acute phase) and d21 post-weaning (recovery phase), half of the pigs were slaughtered. The piglets were deeply anaesthetised with Pentothal Sodium® (10 mg/kg BW, MSD Animal Health S.r.l., MI, Italy) and sacrificed using an intracardiac injection of Tanax® (0.5 mL/ kg BW, MSD Animal Health S.r.l., MI, Italy) in compliance with the ARRIVE guidelines.
The piglets were weighed at d0 and weekly until d21. Faecal consistency was scored daily using a 5-score scale (1: hard and dry; 2: well-formed firm faeces; 3: formed faeces; 4: pasty faeces; 5: watery faeces)59. A consistency score > 3 was defined as a clinical sign of diarrhoea. The faecal index was calculated as follows: number of days with diarrhoea/number of days of the period × 100.
At d8 and d21, after a 2 h-fasting, two samples of blood were collected from the vena cava of the piglets using a vacutainer (DB VACUTAINER 20G PRECISION GLIDE III; DB, Milan, Italy) into a K3 EDTA tube (Vacutest Kima S.r.l., PD, Italy) in order to process the sample for the blood composition, and into a tube containing a clot activator to obtain serum (Vacutest Kima S.r.l, PD, Italy). The blood was drawn by placing the piglets in dorsal recumbency, and securing their head, hinds and forelimbs.
At slaughter, 5 g of caecum content was collected into a sterile tube, snap-frozen in liquid nitrogen and then preserved at − 80 °C. Intestinal segments of the duodenum, jejunum and ileum were sampled and fixed in 10% buffered formalin, and paraffin embedded using an automatic processor60. For paraffin embedding, the tissue fragments were dehydrated using a complete alcohol series and were finally clarified in xylene. The sections were stained with hematoxylin–eosin for morphometric evaluation. Furthermore, at d8, the mucosa of the distal jejunum was gently scraped, snap-frozen into liquid nitrogen and then preserved at − 80 °C61.
Blood analysis
A total of 19 haematological parameters (erythrocyte traits: RBCs, HGB, haematocrit (HCT), MCV, MCH, MCHC, red cell distribution width, RDW; leukocyte traits: white blood cell count, leukocytes, neutrophils, lymphocytes, monocytes, eosinophils, basophils, (K/µL and %), and platelet traits: platelet count) were detected using laser impedimetric cytometry.
The concentration of the ROMs was detected colourimetrically from the serum samples. The concentration of GPX-1 was assessed in the serum samples of the piglets at d8 using a sandwich enzyme immunoassay kit (SEA295Po, Cloud-Clone Corporation, Katy, TX, USA) according to the manufacturer’s instructions.
Microbial analysis
Total bacterial DNA for microbiota analysis was extracted from samples collected from the caeca using a FastDNA™ Spin Kit for Soil (MP Biomedicals, LLC, Santa Ana, CA, USA). The quantity and quality of the DNA were evaluated using a Nanodrop ND 1000 spectrophotometer (Nanodrop Technologies Inc., Wilmington, DE, USA). Next Generation Sequencing (NGS) was carried out as reported by Luise et al. (2019)62.
Morphological analysis
The paraffin block was cut into 4 µm thick sections and then fixed to slides. The sections were hydrated using a xylene and alcohol series, then stained with hematoxylin and eosin, and examined at 10 × using a Leica DM2500 microscope with a Leica DFC7000 T camera (Leica Microsystems, Wetzlar, Germany). Measurements of each anatomical district were taken in a casually selected area using LAS X software (Leica Microsystems, Wetzlar, Germany). For the evaluation of each measurement (length of the villi, width of the villi, depth of the crypts and number of the goblet cells), three villi and three crypts were chosen randomly for each section, making sure that the villi used were in perfect morphological condition, without distortion, absence of epithelium or folds in the tissue60. For each tract, an inflammatory evaluation was carried out, using a scale based on a four-point score. For the epithelium (Intraepithelial lymphocytes), the four-point score was assigned as follows: 0 = normal conditions, under which up to 10 leukocytes were observed; 1 = presence of some leukocytes (20–30) in transcytosis without epithelial swelling; 2 = presence of leukocyte infiltration (30–70) with signs of epithelial alteration or degeneration and micro-erosions, and 3 = severe infiltration of leukocytes (> 70) with epithelial erosion loss or ulcerations with villi surface denudation. For the mucosal chorion (Interstitial lymphocytes) or sub-mucosal area, the four-point score was assigned as follows: 0 = normal conditions, under which up to 20 leukocytes were observed; 1 = presence of some leukocytes (30–50) in transcytosis or in the perivascular area; 2 = leukocyte infiltration (50–90) with signs of perivasculitis and collagen shrinkage, and 3 = severe infiltration of leukocytes (> 90) with areas of necrosis63.
Gene expression analysis
Total RNA was extracted using the GeneJET RNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA) and treated to remove contaminating DNA using a TURBO DNA-free™ DNA Removal Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the recommended protocol. A total of 1000 ng of RNA was then converted into complementary DNA using a High-Capacity RNA-to-cDNA™ Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Duplex Real-Time PCR reactions contained 2 µl cDNA and 8 µl mix containing primers, probes (Supplementary Table 3) and 2X TaqMan Mastermix, and was run in triplicate on the Applied Biosystems QuantStudio™ 7 Flex Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) with the following thermocycler settings: 50 °C for 2 min, 95 °C for 2 min and 40 cycles of 95 °C for 1 s and 60 °C for 20 s. Hydroxymethylbilane synthase (HMBS) was used as a housekeeping gene. QuantStudio Design and Analysis Software v2.5 (Thermo Fisher Scientific, Waltham, MA, USA) was used for determining the gene expression cycle threshold (Ct) values. For each sample, the Ct value of the HMBS gene was subtracted from the Ct value of the target gene (ΔCt). The average ΔCt value of the reference animals was then subtracted from the ΔCt value of all the samples (ΔΔCt). The expression of the target gene was given as fold change calculated by 2-ΔΔ Ct.
Bioinformatic and statistical analysis
Statistical analysis was carried out using SAS version 9.3 (SAS Inst. Inc., Cary, NC, USA). The GLM procedure was used to fit the measurements carried on piglets with a linear model including batch, litter of origin, sex of piglets and diet. Orthogonal contrasts were used to assess the effect of Glu/Gln supplementation as follows: CO vs. AAs addition: (CO vs. 100Glu, 75Glu + 25Gln, 50Glu + 50Gln, 25Glu + 75Gln, 100Gln), Glu alone vs. mixed addition (100Glu vs. 75Glu + 25Gln, 50Glu + 50Gln, 25Glu + 75Gln), Gln alone vs. mixed addition (100Gln vs. 75Glu + 25Gln, 50Glu + 50Gln, 25Glu + 75Gln). In addition, the linear and quadratic effects of AAs supplementation were tested. Data regarding the faecal index were log-transformed before the statistical analysis.
Data regarding the inflammatory evaluation of the intestinal mucosa, such as categorical response variables in numerical order, were assessed using the Generalised Linear Mixed Model (GLIMMIX) procedure of SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), considering a multinomial distribution and calculating the cumulative probabilities of having each score for each experimental diet.
Microbiota analysis was carried out using the DADA2 pipeline64. Taxonomic categories were assigned using the Silva Database (release 138) as a reference65. Alpha (Shannon, Chao1 and InvSimpson indices) and Beta diversity (calculated as the Bray Curtis distance matrix), as well as the abundance of taxonomic categories, were analysed utilising R software 3.666 using the PhyloSeq67, Vegan68 and DESeq269 packages. The Alpha diversity indices were analysed using an ANOVA model (lm function) including batch, diet, time point (d8 and d21) and the interaction between diet and time-point as factors. Beta diversity was analysed using a PERMANOVA Adonis test model (adonis.test function) which included batch, diet, time point, and the interaction between diet and time point as factors. The effects on Beta diversity were visualised using a Non-Metric Multidimensional Scaling (NMDS) approach (plot_ordination function). The analysis was then carried out in the dataset composed of each single time point. Furthermore, orthogonal contrasts were carried out to assess the effect of Glu/Gln supplementation on Alpha and Beta diversity. The differences in taxonomic abundances among the diets were analysed using the DESeq2 package, based on negative binomial generalised linear models, including and applying the Benjamini–Hochberg method for multiple testing correction (estimateSizeFactors function)69. Statistical significance was set at P ≤ 0.05 and tendencies at P ≤ 0.10.
Ethics declarations
The procedures complied with Italian law pertaining to experimental animals and were approved by the Ethic-Scientific Committee for Experiments on Animals of the University of Bologna, Italy and by the Italian Ministry of Health (Approval No. 503/2018 of 2 July, 2018). The study was carried out in compliance with the ARRIVE guidelines.
Supplementary Information
Author contributions
D.L.: Conceptualisation, Methodology, Formal analysis, Visualisation, Writing—Original draft preparation. F.C.: Methodology, Formal analysis. T.C.D.: Conceptualisation, Writing—Review & Editing. L.G.: Formal analysis. G.R.: Formal analysis. W.L.: Writing—Review & Editing. P.B.: Conceptualisation, Formal analysis, Supervision, Writing—Review & Editing. P.T.: Conceptualisation, Methodology, Funding acquisition, Supervision, Writing—Review & Editing.
Data availability
The raw reads obtained are publicly available at the NCBI Sequence Read Archive (SRA) under the project number PRJNA798542. The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors have read the Journal’s policy and have the following competing interests. Co-authors T.C.D and W.L. are associated with Metex Noovistago which partially financed the project and they provided support for experimental design. The authors D.L., F.C., L.G., G.R., P.B. and P.T. have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-18330-5.
References
- 1.Chalvon-Demersay T, et al. Functional amino acids in pigs and chickens: Implication for gut health. Front. Vet. Sci. 2021;8:663727. doi: 10.3389/fvets.2021.663727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu G, et al. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014;2:387–417. doi: 10.1146/annurev-animal-022513-114113. [DOI] [PubMed] [Google Scholar]
- 3.Windmueller HG, Spaeth AE. Intestinal metabolism of glutamine and glutamate from the lumen as compared to glutamine from blood. Arch. Biochem. Biophys. 1975;171:662–672. doi: 10.1016/0003-9861(75)90078-8. [DOI] [PubMed] [Google Scholar]
- 4.Reeds PJ, et al. Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am. J. Physiol. Endocrinol. Metab. 1997;273:E408–E415. doi: 10.1152/ajpendo.1997.273.2.E408. [DOI] [PubMed] [Google Scholar]
- 5.Watford M. Glutamine and glutamate: Nonessential or essential amino acids? Anim. Nutr. 2015;1:119–122. doi: 10.1016/j.aninu.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newsholme P, Newsholme EA. Rates of utilization of glucose, glutamine and oleate and formation of end-products by mouse perioneal macrophages in culture. Biochem. J. 1989;261:211–218. doi: 10.1042/bj2610211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newsholme, P., Procopio, J., Ramos Lima, M. M., Pithon-Curi, T. C. & Curi, R. Glutamine and glutamate: Their central role in cell metabolism and function. Cell Biochem. Funct.21, 1–9 (2003). [DOI] [PubMed]
- 8.Blachier F, Boutry C, Bos C, Tomé D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009;90:814–821. doi: 10.3945/ajcn.2009.27462S. [DOI] [PubMed] [Google Scholar]
- 9.Coëffier M, Hecketsweiler B, Hecketsweiler P, Déchelotte P. Effect of glutamine on water and sodium absorption in human jejunum at baseline and during PGE1-induced secretion. J. Appl. Physiol. 2005;98:2163–2168. doi: 10.1152/japplphysiol.00761.2004. [DOI] [PubMed] [Google Scholar]
- 10.Kyoung H, et al. Dietary glutamic acid modulates immune responses and gut health of weaned pigs. Animals. 2021;11:504. doi: 10.3390/ani11020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin M, et al. L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS ONE. 2014;9:e111950. doi: 10.1371/journal.pone.0111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, et al. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 2015;145:25–31. doi: 10.3945/jn.114.202515. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, et al. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J. Nutr. 2008;138:1025–1032. doi: 10.1093/jn/138.6.1025. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. L-Glutamine supplementation slleviates constipation during late gestation of mini sows by modifying the microbiota composition in feces. Biomed Res. Int. 2017;2017:4862861. doi: 10.1155/2017/4862861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira, A. de O., Nogueira, E. T., Kutschenko, M., Rostagno, H. S. & Lopes, D. C. Inclusion of glutamine associated with glutamic acid in the diet of piglets weaned at 21 days of age. Rev. Bras. Saúde Prod. Anim. Salvador15, 881–896 (2014).
- 16.Molino JP, et al. L-glutamine and L-glutamate in diets with different lactose levels for piglets weaned at 21 days of age. R. Bras. Zootec. 2012;41:98–105. doi: 10.1590/S1516-35982012000100015. [DOI] [Google Scholar]
- 17.Collins CL, et al. Post-weaning and whole-of-life performance of pigs is determined by live weight at weaning and the complexity of the diet fed after weaning. Anim. Nutr. 2017;3:372–379. doi: 10.1016/j.aninu.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluske JR. Invited review: Aspects of gastrointestinal tract growth and maturation in the pre- and postweaning period of pigs. J. Anim. Sci. 2016;94:399–411. doi: 10.2527/jas.2015-9767. [DOI] [Google Scholar]
- 19.Ju D, et al. The role of protein restriction and interaction with antibiotics in the regulation of compensatory growth in pigs: Growth performance, serum hormone concentrations, and messenger RNA levels in component tissues of the endocrine growth axis. Domest. Anim. Endocrinol. 2021;74:106524. doi: 10.1016/j.domaniend.2020.106524. [DOI] [PubMed] [Google Scholar]
- 20.Pluske, J. R., Le Dividich, J. & Verstegen, M. W. A. Weaning the Pig: Concepts and Consequences. (Wageningen Academic Publishers, 2003).
- 21.Cabrera RA, et al. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J. Anim. Sci. Biotechnol. 2013;4:29. doi: 10.1186/2049-1891-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Peng J, Xiong Y, Zhou S, Cheng X. Effects of dietary glutamine and glutamate supplementation on small intestinal structure, active absorption and DNA, RNA concentrations in skeletal muscle tissue of weaned piglets during d 28 to 42 of age. Asian Australas. J. Anim. Sci. 2002;15:238–242. doi: 10.5713/ajas.2002.238. [DOI] [Google Scholar]
- 23.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Investig. 2003;111:931–943. doi: 10.1172/JCI200318326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima AAM, et al. Effects of an alanyl-glutamine-based oral rehydration and nutrition therapy solution on electrolyte and water absorption in a rat model of secretory diarrhea induced by cholera toxin. Nutrition. 2002;18:458–462. doi: 10.1016/S0899-9007(02)00775-X. [DOI] [PubMed] [Google Scholar]
- 25.Zou XT, Zheng GH, Fang XJ, Jiang JF. Effects of glutamine on growth performance of weanling piglets. Czech J. Anim. Sci. 2006;51:444–448. [Google Scholar]
- 26.Rhoads, J. M., Keku, E. O., Bennett, L. E., Quinn, J. & Lecce, J. G. Development of L-glutamine-stimulated electroneutral sodium absorption in piglet jejunum. Am. J. Physiol. Gastrointest. Liver Physiol.259, 99–107 (1990). [DOI] [PubMed]
- 27.Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001;131:2515–2522. doi: 10.1093/jn/131.9.2515S. [DOI] [PubMed] [Google Scholar]
- 28.Manhart N, et al. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. Ann. Surg. 2001;234:92–97. doi: 10.1097/00000658-200107000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogero MM, et al. Dietary glutamine supplementation affects macrophage function, hematopoiesis and nutritional status in early weaned mice. Clin. Nutr. 2008;27:386–397. doi: 10.1016/j.clnu.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Schilling JD. Macrophages fuel skeletal muscle regeneration. Immunometabolism. 2021;3:e210013. doi: 10.20900/immunometab20210013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pithon Curi TC, De Melo MP, De Azevedo RB, Zorn TMT, Curi R. Glutamine utilization by rat neutrophils: Presence of phosphate-dependent glutaminase. Am. J. Physiol. Cell Physiol. 1997;273:1124–1129. doi: 10.1152/ajpcell.1997.273.4.C1124. [DOI] [PubMed] [Google Scholar]
- 32.Pié S, et al. Weaning is associated with an upregulation of expression of inflamatory cytokines in the intestine of piglets. J. Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- 33.Xing S, et al. Effects of alanyl-glutamine supplementation on the small intestinal mucosa barrier in weaned piglets challenged with lipopolysaccharide. Can. J. Anim. Sci. 2017;98:144–155. doi: 10.5713/ajas.16.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Tseng SH, Yao CL, Li C, Tsai YH. Distinct effects of growth hormone and glutamine on activation of intestinal stem cells. J. Parenter. Enter. Nutr. 2018;42:642–651. doi: 10.1177/0148607117709435. [DOI] [PubMed] [Google Scholar]
- 35.Beutheu S, Ghouzali I, Galas L, Déchelotte P, Coëffier M. Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin. Nutr. 2013;32:863–869. doi: 10.1016/j.clnu.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Hu ZY, Li SL, Cao ZJ. Short communication: Glutamine increases autophagy of liver cells in weaned calves. J. Dairy Sci. 2012;95:7336–7339. doi: 10.3168/jds.2012-5881. [DOI] [PubMed] [Google Scholar]
- 37.Jackson DN, Theiss AL. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes. 2020;11:285–304. doi: 10.1080/19490976.2019.1592421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brigelius-Flohé R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006;387:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, et al. Dietary glutamine supplementation enhances expression of ZO-1 and occludin and promotes intestinal development in Min piglets. Acta Agric. Scand. A Anim. Sci. 2017;67:15–21. [Google Scholar]
- 40.Trevisi P, et al. Molecular networks affected by neonatal microbial colonization in porcine jejunum, luminally perfused with enterotoxigenic Escherichia coli, F4ac fimbria or Lactobacillus amylovorus. PLoS ONE. 2018;13:e0202160. doi: 10.1371/journal.pone.0202160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lobley GE, Hoskin SO, McNeil CJ. Glutamine in animal science and production. J. Nutr. 2001;131:2525S–2531S. doi: 10.1093/jn/131.9.2525S. [DOI] [PubMed] [Google Scholar]
- 42.Tung JN, et al. Glutamine modulates CD8αα+ TCRαβ+ intestinal intraepithelial lymphocyte expression in mice with polymicrobial sepsis. Nutrition. 2013;29:911–917. doi: 10.1016/j.nut.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Nose K, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: A potential mechanism for the preservation of epithelial barrier function. J. Interface Cytokine Res. 2010;30:67–79. doi: 10.1089/jir.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vicario M, Amat C, Rivero M, Moretó M, Pelegrí C. Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J. Nutr. 2007;137:1931–1937. doi: 10.1093/jn/137.8.1931. [DOI] [PubMed] [Google Scholar]
- 45.Dai Z-L, Zhang J, Wu G, Zhu W-Y. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 2010;39:1201–1215. doi: 10.1007/s00726-010-0556-9. [DOI] [PubMed] [Google Scholar]
- 46.Dai Z-L, Wu G, Zhu W-Y. Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front. Biosci. 2011;16:1768–1786. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- 47.Vermeulen N, Gänzle MG, Vogel RF. Glutamine deamidation by cereal-associated lactic acid bacteria. J. Appl. Microbiol. 2007;103:1197–1205. doi: 10.1111/j.1365-2672.2007.03333.x. [DOI] [PubMed] [Google Scholar]
- 48.Botta C, et al. Genomic assessment in Lactobacillus plantarum links the butyrogenic pathway with glutamine metabolism. Sci. Rep. 2017;7:15975. doi: 10.1038/s41598-017-16186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veith N, et al. Using a genome-scale metabolic model of Enterococcus faecalis V583 to assess amino acid uptake and its impact on central metabolism. Appl. Environ. Microbiol. 2015;81:1622–1633. doi: 10.1128/AEM.03279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gänzle MG. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015;2:106–117. doi: 10.1016/j.cofs.2015.03.001. [DOI] [Google Scholar]
- 51.Raut MP, Couto N, Karunakaran E, Biggs CA, Wright PC. Deciphering the unique cellulose degradation mechanism of the ruminal bacterium Fibrobacter succinogenes S85. Sci. Rep. 2019;9:16542. doi: 10.1038/s41598-019-52675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gharechahi, J., Vahidi, M. F., DIng, X. Z., Han, J. L. & Salekdeh, G. H. Temporal changes in microbial communities attached to forages with different lignocellulosic compositions in cattle rumen. FEMS Microbiol. Ecol.96, fiaa069 (2020). [DOI] [PubMed]
- 53.Castillo M, Martín-Orúe SM, Nofrarías M, Manzanilla EG, Gasa J. Changes in caecal microbiota and mucosal morphology of weaned pigs. Vet. Microbiol. 2007;124:239–247. doi: 10.1016/j.vetmic.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 54.Correa F, et al. Effect of dietary supplementation with a blend of protected aromatic compounds, including benzoic acid, on growth performance and faecal microbial profile of weaned piglets as an alternative to Zinc Oxide. Livest. Sci. 2021;246:104455. doi: 10.1016/j.livsci.2021.104455. [DOI] [Google Scholar]
- 55.Hespell, R. B., Paster, B. J. & Dewhirst, F. E. The Genus Selenomonas. in The Prokaryotes: Volume 4: Bacteria: Firmicutes, Cyanobacteria (eds. Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H. & Stackebrandt, E.) 982–990 (Springer US, 2006). 10.1007/0-387-30744-3_33.
- 56.Pieper R., Villodre Tudela C., Taciak M., Bindelle J., Pérez J. F., Zentek J. Health relevance of intestinal protein fermentation in young pigs. Animal Health Research Reviews. 2016;17(2):137–147. doi: 10.1017/S1466252316000141. [DOI] [PubMed] [Google Scholar]
- 57.Scheifinger CC, Wolin MJ. Propionate formation from cellulose and soluble sugars by combined cultures of Bacteroides succinogenes and Selenomonas ruminantium. Appl. Microbiol. 1973;26:789–795. doi: 10.1128/am.26.5.789-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.du Sert, N. P. et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol.18, e3000411 (2020). [DOI] [PMC free article] [PubMed]
- 59.Luise D, Lauridsen C, Bosi P, Trevisi P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 2019;10:53. doi: 10.1186/s40104-019-0352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desantis S, Mastrodonato M, Accogli G, Rossi G, Crovace AM. Effects of a probiotic on the morphology and mucin composition of pig intestine. Histol. Histopathol. 2019;34:1037–1050. doi: 10.14670/HH-18-106. [DOI] [PubMed] [Google Scholar]
- 61.Luise D, et al. Long-term administration of formic acid to weaners: Influence on intestinal microbiota, immunity parameters and growth performance. Anim. Feed Sci. Technol. 2017;232:160–168. doi: 10.1016/j.anifeedsci.2017.06.015. [DOI] [Google Scholar]
- 62.Luise D, et al. Effect of Mucine 4 and Fucosyltransferase 1 genetic variants on gut homoeostasis of growing healthy pigs. J. Anim. Physiol. Anim. Nutr. (Berl) 2019;103:801–812. doi: 10.1111/jpn.13063. [DOI] [PubMed] [Google Scholar]
- 63.Puglisi F, et al. Activation of PI3-kinase/Akt induced small bowel cell apoptosis during laparoscopic ischaemia-reperfusion of swine jejunum. Acta Chir. Belg. 2009;109:216–223. doi: 10.1080/00015458.2009.11680408. [DOI] [PubMed] [Google Scholar]
- 64.Callahan BJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quast C, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.R Core Team. R: A Language and Environment for Statistical Computing. (2021).
- 67.McMurdie PJ, Holmes S, Kindt R, Legendre P, O’Hara R. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dixon P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003;14:927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x. [DOI] [Google Scholar]
- 69.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw reads obtained are publicly available at the NCBI Sequence Read Archive (SRA) under the project number PRJNA798542. The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.