We read the Comment by Amit Saxena and colleagues1 regarding the clinical efficacy and seroreactivity of an additional COVID-19 vaccine dose in patients with systemic lupus erythematosus with great interest.1 Samples were analysed at a mean of 44·7 days (SD 31·7) after the additional dose, and rates of breakthrough infections and anti-SARS-CoV-2 spike protein antibody titres were compared between those who did and did not receive an additional vaccine dose after the initial vaccination series. Although there were fewer breakthrough infections after the additional vaccine dose, there was no association between the antibody titre reported after that vaccine dose and infection.
Although there is no defined threshold of antibody titres that confers protection against SARS-CoV-2 infection, antibody results are used to manage individuals receiving immunosuppressive medications that might affect the serological response to vaccination.
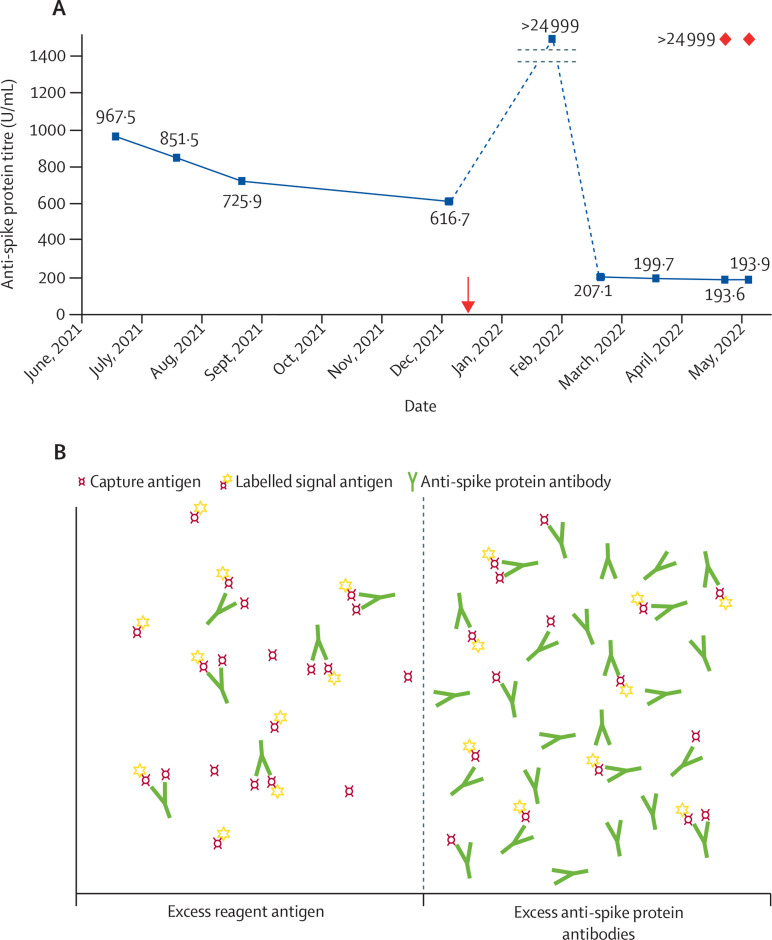
We serially assessed anti-SARS-CoV-2 spike protein antibody titres using Roche Cobas2 (appendix) in patients with nephrotic syndrome receiving immunosuppression at the time of COVID-19 vaccination. Several patients had a rapid decline of antibody titres to less than 200 U/mL after a third COVID-19 vaccine dose (figure ) which appeared incongruent with typical antibody decay. This raised concerns of short-lived humoral immunity associated with immunosuppression or urinary losses of immunoglobulin. Consultation with the clinical laboratory highlighted assay interference as a possible cause for the discrepant results. The prozone effect was confirmed by diluting the samples and retesting, which produced the expected titres of more than 24 999 U/mL (figure). Briefly, very high antibody concentrations might bind to both the capture and signal reagent antigens separately, disrupting immune complex formation, and leading to falsely low results. Dilution of the sample overcomes this effect.
Figure.
Spurious decline of anti-SARS-CoV-2 spike protein antibody titres due to the prozone effect
(A) Serial results for a selected patient. Orange arrow indicates administration of the third dose of the BNT162b2 mRNA vaccine (Pfizer–BioNTech). The February, 2022, sample was probably more than 250 U/mL and underwent autodilution as per analyser programming. Samples from March and April, 2022, and the two samples from May, 2022, were less than 250 U/mL; therefore, they did not undergo autodilution. Manual dilution of the two samples in May, 2022, (red markers) gave expected results. Results of 25 000 or more were reported as more than 24 999. The dotted line indicates y axis not in scale. (B) Under normal conditions, the reagent antigens are in excess. Antibodies in the patient's sample bind one capture antibody and one signal antibody, forming a double antigen sandwich. This produces a signal that is proportional to the antibody concentration in the sample. If the antibodies are in extreme excess, few double antigen sandwiches are formed, yielding a signal that is falsely low.
These cases emphasise the importance of clinical correlation with laboratory results and highlight the limitations of antibody testing in the management of SARS-CoV-2. Clinicians should be aware of the reasons for spurious results, particularly if it influences clinical management (ie, modification of immunosuppressive treatments, additional booster doses, or pre-exposure prophylaxis). Of note, these limitations should be considered when characterising antibody dynamics after COVID-19 vaccination in larger cohorts, if such data are used to inform vaccine booster policies or to understand factors associated with breakthrough infections. The manufacturer now recommends initial sample dilution when measuring anti-spike protein antibodies after the additional vaccine dose.
We declare no competing interests.
Supplementary Material
References
- 1.Saxena A, Engel A, Banbury B, et al. Breakthrough SARS-CoV-2 infections, morbidity, and seroreactivity following initial COVID-19 vaccination series and additional dose in patients with SLE in New York City. Lancet Rheumatol. 2022 doi: 10.1016/S2665-9913(22)00190-4. published online July 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loving HS, Stallcup P, Burbelo P, et al. Early antibody temporal responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vaccinated subjects determined by the cobas 6000 spike Assay. Arch Pathol Lab Med. 2022;146:9–10. doi: 10.5858/arpa.2021-0407-LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.