Abstract
COVID-19, caused by SARS-CoV-2, is characterised by a broad spectrum of symptom severity that requires varying amounts of care according to the different stages of the disease. Intervening at the onset of mild to moderate COVID-19 symptoms in the outpatient setting would provide the opportunity to prevent progression to a more severe illness and long-term complications. As early disease symptoms variably reflect an underlying excessive inflammatory response to the viral infection, the use of anti-inflammatory drugs, especially non-steroidal anti-inflammatory drugs (NSAIDs), in the initial outpatient stage of COVID-19 seems to be a valuable therapeutic strategy. A few observational studies have tested NSAIDs (especially relatively selective COX-2 inhibitors), often as part of multipharmacological protocols, for early outpatient treatment of COVID-19. The findings from these studies are promising and point to a crucial role of NSAIDs for the at-home management of people with initial COVID-19 symptoms.
Introduction
The COVID-19 pandemic continues to pose major threats to global public health.1 The more transmissible variants of SARS-CoV-2 were responsible for the four major waves of infections that spread across the globe starting in early 2020, with omicron (B.1.1.529) becoming the dominant variant after the summer of 2021, followed by the emergence of other omicron sublineages in 2022 (BA.2, BA.3, BA.4, and BA.5).1, 2
As a result, communities and hospitals around the world have been pushed to their limits.3 In a global effort that is unparalleled in modern history, there has been a race to find drugs and biological treatments to save the lives of patients who are hospitalised or severely ill and to develop vaccines.4, 5 Indeed, COVID-19 vaccines have contributed to the reduced risk of SARS-CoV-2 infection, and provide protection against severe disease caused by the SARS-CoV-2 variants, including omicron.6 Nonetheless, to reduce severe COVID-19-related illness, overcrowding of hospitals, and treatment costs,7 there has also been a focus on how primary care physicians can treat initial mild to moderate symptoms in outpatients with COVID-19. This approach would provide an opportunity to intervene before infected individuals develop severe illness. Since mild to moderate symptoms of COVID-19 might reflect an underlying excessive inflammatory response to the viral infection, the use of anti-inflammatory drugs in the community during the early stage of COVID-19 appears to be a valuable therapeutic option. However, anti-inflammatory therapy for managing people with COVID-19 at home continues to be a matter of debate, in terms of effectiveness and possible important side-effects.
In this Review, we briefly describe the pathogenic mechanisms underlying the inflammatory processes of early stage COVID-19 and discuss the rationale for using non-steroidal anti-inflammatory drugs (NSAIDs), as well as evidence regarding their risk-to-benefit balance in the home and community setting. We also examine whether outpatient pharmacological interventions with corticosteroids could mitigate the ongoing inflammatory process, potentially protecting against the risk of progression towards more severe illness.
Maladaptive hyperinflammatory response to SARS-CoV-2 infection
As with other coronaviruses, the human-to-human transmission of SARS-CoV-2 occurs primarily through direct or indirect respiratory tract exposure to infected droplets or aerosols generated during sneezing and coughing.8 To enter target cells, the spike subunit of SARS-CoV-2 engages the host protease ACE2 as an entry receptor, after being primed by the cellular serine protease TMPRSS2.9 Lysis of infected cells and SARS-CoV-2 replication in the host cells are associated with the release of inflammatory cytokines (such as TNF-α, IL-6, and IL-8) and free radicals, the induction of IFN-γ, and the recruitment and activation of leukocyte subsets, which in turn further release cytokines, chemokines, and other inflammatory mediators that determine the early inflammatory response.10 It has been hypothesised that the inflammatory environment of SARS-CoV-2 infection could also be sustained by the enhanced availability of angiotensin-II as the result of the net downregulation of ACE2, due to continuous recycling of this receptor upon viral cell entry.11 Since ACE2 degrades angiotensin-II, ACE2 deficiency causes angiotensin-II accumulation, which in turn (by binding to the angiotensin-I receptor) triggers inflammatory processes by stimulating proliferation of mononuclear cells and promoting the recruitment of proinflammatory cells.11, 12 Moreover, the uptake of an antibody-opsonised virus by the Fcγ receptors of monocytes and macrophages results in inflammatory cell death, which halts the production of infectious virus but causes systemic inflammation that contributes to COVID-19 pathogenesis.13
Later in the disease course, viral replication can occur in the lower respiratory tract.14 The apoptotic death response of the infected epithelial cells is associated with vascular leakage within alveoli, which induces initial local inflammation and the recruitment of immune cells to eliminate extracellular virus and destroy virus-infected cells.15 Proinflammatory cytokines further recruit leukocytes within the lung, contributing to the propagation of the inflammatory response, as shown in patients with COVID-19 progression to severe disease.16 Furthermore, evidence indicates that proinflammatory CD68 macrophages bearing ACE2 on their surface can be directly infected by SARS-CoV-2,17 and their virus-induced activation appears to be relevant to the initiation and spread of the hyperinflammatory response.18 Notably, CD163 monocyte-derived macrophages also accumulate in the lungs.19 As shown in patients with COVID-19-related acute respiratory distress syndrome, although these cells further contribute to inflammation in the lung, they are also reprogrammed by the virus towards a profibrotic transcriptional and proteomic phenotype, resulting in pulmonary fibrosis.19
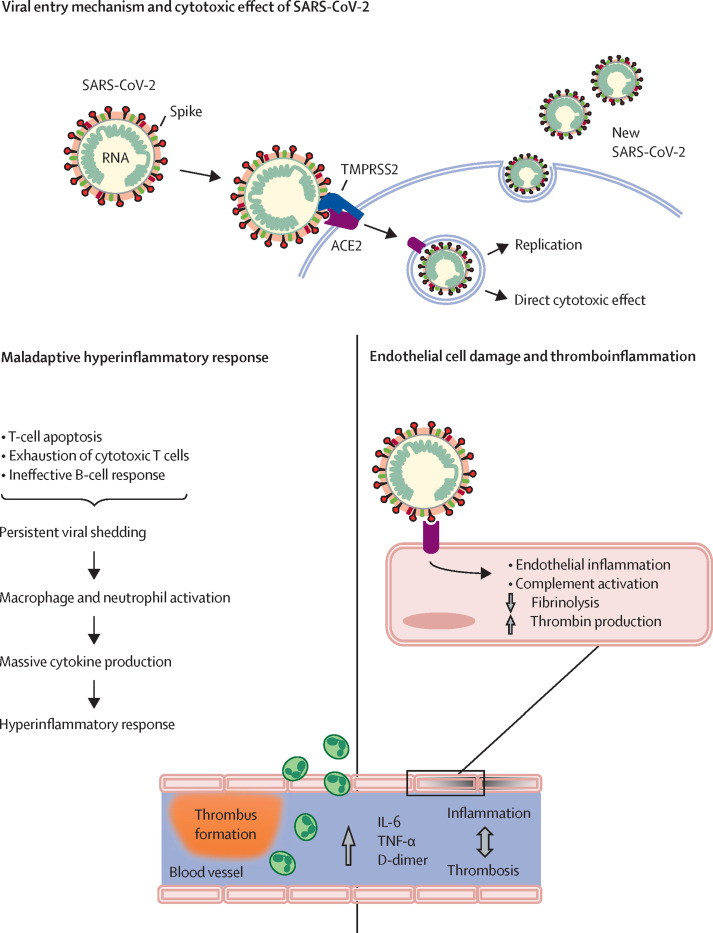
Together, the available evidence highlights the crucial role of the dysregulation of the innate and adaptive immune response, and the associated maladaptive hyperinflammatory response, in the initiation and exacerbation of COVID-19 disease (figure 1 ). Thus, the timing of intervention is likely to be an important determinant in the therapeutic response. Although monoclonal antibodies20, 21, 22 or polyclonal convalescent plasma23 have been shown to reduce the incidence of disease progression and hospitalisation when given to outpatients with COVID-19 within 5–9 days of disease onset, these therapies are sometimes unavailable or scarce (such as in low-income and middle-income countries) or ineffective because of antibody-resistant variants.24 Therefore, a different option would be to target the inflammatory processes underlying the mild to moderate symptoms at COVID-19 onset with currently available anti-inflammatory drugs.
Figure 1.
Proposed maladaptive hyperinflammatory response to SARS-CoV-2 infection
SARS-CoV-2 enters target host cells by interacting through its spike subunit with ACE2, after being primed by TMPRSS2. The virus induces cell damage through direct cytotoxic effects and after newly formed virions are released by exocytosis into the extracellular compartment. In addition, a dysregulated immune response eventually leads to the recruitment and activation of macrophages and neutrophils, with the release of cytokines, chemokines, and other inflammatory mediators determining hyperinflammation. At the same time, activation of the complement system and excessive cytokine production activate endothelial cells and disrupt vascular integrity leading to microthrombi deposition and microvascular dysfunction.
Rationale for using NSAIDs in the treatment of early stage COVID-19
Prostanoids and COVID-19
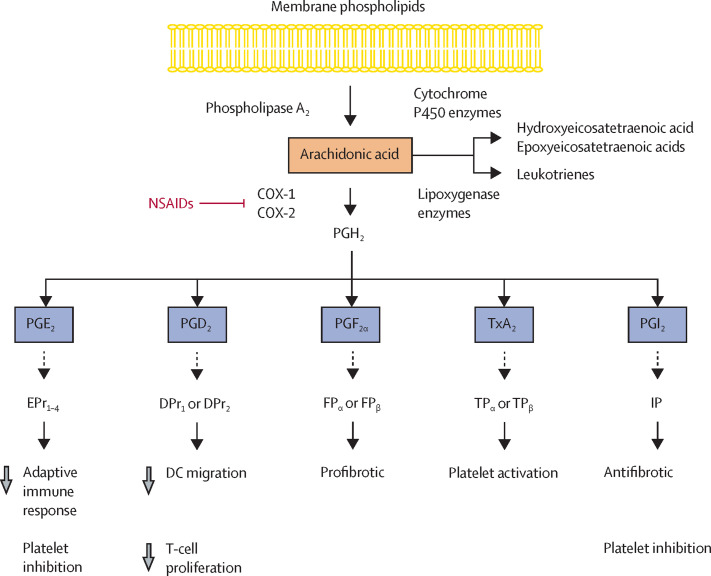
NSAIDs are one of the most commonly used classes of medicines worldwide—taken to reduce pain, control fever, and treat a broad range of inflammatory diseases, including osteoarthritis and rheumatoid arthritis.25 The principal therapeutic effect of NSAIDs relates to their capability to inhibit the cyclooxygenase activity of two enzymes, PTGS1 and PTGS2 (also known as COX-1 and COX-2).26 This inhibition of COX-1 and COX-2 eventually suppresses the formation of prostanoids—metabolites of arachidonic acid, a fatty acid present in cell membrane phospholipids (figure 2 ).27 Prostanoids, which include prostaglandin E2 (PGE2), D2 (PGD2), and F2α (PGF2α), thromboxane A2 (TxA2), and prostacyclin (PGI2), elicit a wide variety of biological effects involved in homoeostatic and normal tissue function after binding to their receptors. These biological effects include vascular tone, platelet function, kidney function, and gastrointestinal protection, but also effects implicated in pathophysiological processes including infection, thrombosis, and inflammation.28 Several initial small studies have shown that concentrations of PGE2, PGF2α, and TxA2 in biological samples from inpatients with COVID-19 are higher than in healthy controls, although no data are available regarding prostanoid concentrations in outpatients with SARS-CoV-2 infection.29, 30, 31, 32 Nonetheless, caution should be taken in interpreting these findings, which could be skewed by the use of questionable assays and an insufficient consideration of factors that influence prostanoid concentrations, including the use of NSAIDs, corticosteroids, or other drugs, besides possible artifacts created during sample collections.33
Figure 2.
Prostanoid production and NSAIDs
The 20-carbon fatty acid arachidonic acid is released from membrane phospholipids by phospholipase A2, which is activated by physical, chemical, and inflammatory stimuli. Arachidonic acid is converted by COX-1 and COX-2 to the unstable intermediate PGH2. By tissue-specific isomerases, PGH2 is metabolised to bioactive prostanoids, which include PGE2, PGD2, PGF2α, TxA2, and PGI2. After binding to their receptors (EPr1–4, DPr1, DPr2, FPα, FPβ, TPα, TPβ, and IP), prostanoids elicit a wide variety of biological effects involved in homoeostatic and normal tissue function but also implicated in pathophysiological processes including infection, thrombosis, and inflammation. The principal therapeutic effect of NSAIDs is related to their capability to inhibit the cyclooxygenase activity of COX-1 and COX-2 enzymes, eventually suppressing the formation of prostanoids. DC=dendritic cell. NSAIDs=non-steroidal anti-inflammatory drugs.
Coronaviruses, including SARS-CoV-2, enhance the expression of genes encoding for COX-1, COX-2, and cytosolic prostaglandin E synthase (PTGES).34 For example, SARS-CoV-2 infection upregulates COX-2 expression in vivo in the K18-hACE2 strain of mice, as well as in vitro in human cells.35 Similarly, COX-1, COX-2, and PTGES have been shown to be upregulated in peripheral blood mononuclear cells isolated from patients with COVID-19 compared with healthy controls.36
However, cyclooxygenase enzymes trigger the cell release of prostanoids with varying effects on host immune function, at least as reported in experimental models of coronavirus infections.37 Pharmacological inhibition of the PGE2 receptors EPr2 and EPr4 improved survival after influenza A virus infection, an effect attributed to prevention of PGE2-induced inhibition of type I interferon production and apoptosis in macrophages, thereby directly allowing mouse and human macrophages to restrict viral replication.37 However, the differences between the various PGE2 receptors allow for adaptable patterns of response by various cell types at different stages of immunity.38 Indeed, EPr2 and EPr4 mediate the anti-inflammatory and immune-suppressive activity of PGE2, whereas antigen-induced mast cell degranulation and IL-6 production are enhanced by PGE2 in a mechanism involving EPr1 and EPr3.39 Nonetheless, the biology of PGE2 and its receptors in the broader context of infectious diseases (such as acute vs chronic virus infections) and its pathogen-specific effects remain to be determined.40 PGD2 acts through its two receptors, DPr1 and DPr2.41 DPr1 inhibition enhanced the migration of dendritic cells to the lungs and lymph nodes, and subsequent T-cell proliferation, which increased survival in older but not younger mice after SARS-CoV infection.42
Although these findings are very preliminary, they suggest that prostanoids play a role in SARS-CoV-2 infection and support the hypothesis that therapies that modulate prostanoid biosynthesis could be beneficial, particularly during the early phase of COVID-19.
Do NSAIDs enhance susceptibility to SARS-CoV-2 infection?
Early on in the COVID-19 pandemic, there was debate on whether the use of NSAIDs, particularly ibuprofen, would enhance susceptibility to SARS-CoV-2 infection and exacerbate COVID-19 symptoms.43 The potential mechanism whereby NSAIDs could theoretically harm people with COVID-19 is through the upregulation of ACE2. This speculation arose from minimal findings in rats with streptozotocin-induced diabetes treated with ibuprofen.44 More recent in-vitro studies from the past 2 years, however, have highlighted the antiviral activity of some NSAIDs, albeit only at high concentrations, which could contribute to their efficacy in treating COVID-19, besides their anti-inflammatory and analgesic properties. For example, naproxen has been shown to prevent SARS-CoV-2 nucleoprotein oligomerisation and to inhibit viral replication in Vero E6 cells and human pulmonary epithelium.45 Other reports from in silico analyses from transcriptomic databases of kidney tissue from rats treated with various NSAIDs found that naproxen, nimesulide, diclofenac, meloxicam, and piroxicam markedly reduced ACE2 expression.46 Moreover, high concentrations of ibuprofen and flurbiprofen reduced SARS-CoV-2 replication in vitro.47 Data based on small population cohorts and observational and retrospective studies have consistently shown no association between ongoing NSAID therapy and increased mortality48, 49, 50, 51 or worsening of clinical outcomes48, 49, 51, 52, 53 in people with COVID-19 in the general population (ie, inpatient and outpatient population; appendix p 1). Similarly, in people with pre-existing rheumatic disease who were chronically exposed to NSAIDs, the ongoing use of these medicines did not increase the risk of hospitalisation for COVID-19 or COVID-19-related death compared with non-users.50, 54 Consistently, in people with osteoarthritis, chronic NSAID treatment was not associated with an increased risk of SARS-CoV-2 infection.55 There was also no evidence of increased risk of mortality associated with NSAIDs in small retrospective studies of hospitalised patients with COVID-19 who chronically used these drugs before admission,56, 57 or in ibuprofen users before SARS-CoV-2 infection.49 In contrast with a retrospective cohort study of 1824 adults hospitalised with COVID-19 in South Korea,58 findings from a matched, prospective, multicentre cohort study based on the International Severe Acute Respiratory and Emerging Infection Consortium Clinical Characterisation Protocol UK dataset documented that in 8410 patients hospitalised with a PCR-confirmed or highly suspected SARS-CoV-2 infection, NSAID use (including ibuprofen) in the 2 weeks preceding admission was not associated with higher in-hospital mortality or increased COVID-19 severity compared with no NSAID use.59 Furthermore, a systematic review and meta-analysis of 40 comparative studies that evaluated over 4·8 million adult cases substantiated that the use of NSAIDs (such as COX-2 inhibitors, ibuprofen, or aspirin) was not significantly associated with a higher risk of SARS-CoV-2 infection, or a greater probability of intensive care unit admission, mechanical ventilation, or administration of supplemental oxygen.60
In summary, growing evidence is becoming available and most of it concludes that for patients with COVID-19 NSAID use does not enhance susceptibility to SARS-CoV-2 infection, nor does it appear to confer an increased risk of worse outcomes.
Relatively selective COX-2 inhibitors for early COVID-19 symptoms
COX-2 is a crucial enzyme involved in several physiological and pathological processes.61 It plays a central role in viral infections and regulates the expression rates of several serum proteins.62 In a model of H3N2 influenza A viral infection, mice knocked-out for the gene encoding for COX-2 had less severe infection (as evidenced by the blunted inflammatory and cytokine response), attenuated weight loss, and improved survival compared with wild-type mice.63 Similarly, hyperinduction of COX-2 has been documented in the epithelial cells of autoptic lung tissue samples of patients who died of avian influenza H5N1 infection, along with increased concentrations of TNF-α and other pro-inflammatory cytokines.64
NSAIDs inhibit both COX-1 and COX-2 enzymes.65 The COX-2 selectivity of a particular drug is a continuous variable in relation to the relative drug concentration required to inhibit COX-1 and COX-2 enzymes in whole blood assays by 50%.65 In a small prospective study of 44 patients with next-generation sequencing-confirmed or PCR-confirmed COVID-19 admitted to hospital (82% of whom had moderate symptoms), the administration of the relatively selective COX-2 inhibitor celecoxib for 7–14 days as adjuvant therapy prevented clinical deterioration and improved chest CT grading compared with standard therapy.29 Similarly, in a retrospective study of hospitalised patients with COVID-19 and pneumonia, a median of 3 days of treatment with etoricoxib reduced the risk of disease progression compared with the control group.66 Although these findings were observed in hospitalised patients, some of them had mild or moderate COVID-19 symptoms at admission, a condition similar to that of non-hospitalised people with early mild or moderate symptoms of SARS-CoV-2 infection, thus supporting the use of relatively selective COX-2 inhibitors also for outpatients with COVID-19.
Substantial overlap in COX-2 selectivity is found among some COX-2 inhibitors (eg, celecoxib) and some traditional NSAIDs (eg, nimesulide).65, 67 The experimental evidence that celecoxib decreased cytokine concentrations (TNF-α, G-CSF, and IL-6) in bronchoalveolar lavage fluid in mice with influenza A infection,68 and the overlap in COX-2 selectivity between this COX-2 inhibitor and nimesulide, provided the rationale for recommending these two drugs for early outpatient treatment of COVID-19 symptoms if not contraindicated.69 Moreover, compared with other COX-2 inhibitors (including etoricoxib, rofecoxib, valdecoxib, and robenacoxib), celecoxib has higher lipophilicity, which would predict higher biological activity70 and, therefore, should be the preferential COX-2 inhibitor for at-home treatment of early COVID-19 symptoms.
In an observational matched-cohort study, we looked at the outcomes of 90 consecutive consenting patients with mild to moderate COVID-19 treated at home by their family physicians according to a treatment algorithm based on NSAIDs (with a priority for relatively selective COX-2 inhibitors), and compared them with outcomes for 90 age-matched, sex-matched, and comorbidities-matched patients who received other therapeutic regimens.71 Early treatment according to the proposed recommendation regimen almost completely prevented the need for hospitalisation due to a progression to more severe illness (two of 90 patients), compared with patients in the control cohort who were treated according to their family physician's assessments (13 of 90 patients). This result translated into a reduction of over 90% in the overall number of days of hospital stay and related treatment costs. Moreover, symptoms (such as anosmia, ageusia, or dysgeusia) persisted less frequently and for a shorter period in the recommended algorithm cohort than in the control cohort.71 These results were substantiated by a further matched-cohort study in 216 outpatients with mild to moderate COVID-19, managed by their family doctors, showing that the adoption of the outpatient treatment algorithm based on relatively selective COX-2 inhibitors during the early phase of the illness reduced the incidence of subsequent hospitalisation (primary outcome) and related costs.72 Nonetheless, future randomised studies will be required to consolidate these positive observational findings.
Although more selective inhibition of COX-2 is desirable to reduce the gastrointestinal toxicity seen with less selective COX-2 inhibitors, physicians might be aware that NSAID use has been associated with higher rates of cardiovascular events compared with placebo.73 Moreover, nimesulide has been associated with a risk of hepatotoxicity; however, this risk is low when the drug is administered at the recommended time and daily dose.74 Furthermore, long-term NSAID treatment could cause nephrotoxicity, which is exacerbated by fever and dehydration.75, 76 This risk is particularly true for people older than 65 years, who might already have reduced kidney function related to ageing or concomitant chronic kidney disease, and for whom the maintenance of renal blood flow is highly dependent on vasodilator prostaglandins. Thus, family physicians should counsel older patients to hydrate adequately while taking NSAIDs and use these drugs for the shortest time possible.
Other NSAIDs for early stage COVID-19
Anecdotal observations from researchers in Egypt reported that 17 people with PCR-confirmed or rapid IgM test-confirmed or suspected COVID-19 who were managed at home and took ibuprofen or diclofenac—NSAIDs with similar inhibitory activity of COX-1 and COX-2 enzymes65, 77—recovered from most early symptoms (sore throat, dry cough, mild dyspnoea, fever <38°C) within 5 days.78, 79 However, a prospective cohort study reported that ibuprofen, given to 40 people with COVID-19, did not lower their risk of hospitalisation compared with that of 357 non-NSAID users.48 Yet, the possibility that people with more severe symptoms were more likely to initiate ibuprofen, and thus be at higher risk of worse disease outcomes, cannot be excluded.
The data regarding indometacin are more convincing. A powerful NSAID that non-selectively inhibits COX-1 and COX-2 enzymes,80 indometacin has mostly been used to treat musculoskeletal inflammatory conditions.81 In vitro studies have shown both the antiviral effect of indometacin against several viruses, including SARS-CoV,82, 83, 84 and its ability to lower thrombin-induced production of IL-6,85 a cytokine that is upregulated during COVID-19.86 Notably, it has been speculated that indometacin could also modulate bradykinin effects in people with COVID-19, possibly improving the COVID-19-induced dry cough or other symptoms related to the biological activity of bradykinin.87 Using real-world data, Gordon and colleagues first reported the beneficial outcome in a cohort of 103 people with PCR-confirmed SARS-CoV-2 infection who happened to start a course of indometacin compared with a matched group who started a prescription of celecoxib (n=103).88 New users of indometacin were less likely than the matched new users of celecoxib to require hospitalisation or inpatient services. Similarly, in another real-world retrospective study involving 158 participants with a PCR-confirmed diagnosis of early stage COVID-19, early treatment at home (within 3 days of symptom onset vs later) with a multipharmacological regimen (including indometacin) shortened symptom duration and reduced the risk of hospitalisation.89 Although these findings could not be attributed to indometacin specifically, given the multipharmacological treatment, they further highlight the value of early COVID-19 patient management with therapy including NSAIDs. An open-label randomised clinical trial in India evaluated the efficacy and safety of indometacin for patients hospitalised with COVID-19 with mild to moderate symptoms including fever, cough, and myalgia at admission.90 None of the 103 patients randomly assigned to indometacin developed desaturation (SpO2 ≤93%); however, desaturation did occur in 20 of the 107 patients allocated to paracetamol. Patients who received indometacin also had more rapid symptom relief than those given paracetamol, with most symptoms disappearing in half the time. Although the trial involved only hospitalised patients, the mild to moderate symptoms at admission are those commonly seen in the early phase of COVID-19, so the beneficial effect of indometacin could also be expected for outpatients with COVID-19. Nonetheless, neither indometacin nor paracetamol was administered as a stand-alone treatment, but along with a standard of care including ivermectin and other adjuvant therapies.
Aspirin is an affordable, globally available drug that at low doses irreversibly inhibits the COX-1 enzyme. In addition to being a key enzyme in the synthesis of pro-inflammatory prostaglandins, COX-1 is responsible for the production of TxA2, which promotes platelet aggregation.91 Thus, in patients with COVID-19, low-dose aspirin has mainly been proposed as a preventive approach against the thromboembolic risk associated with this disease, and the related increased mortality.92, 93 Available studies on the use of aspirin as an antithrombotic drug for COVID-19 mainly involve hospitalised patients with varying disease severity. Consistent with small retrospective studies in China94 and Iran,95 a large propensity-score matched study reported a lower cumulative incidence of in-hospital death for patients with COVID-19 given low-dose aspirin during hospitalisation than for those who received no antiplatelet therapy.96 Similarly, the beneficial effect of short-term use of low-dose aspirin is supported by the findings of a retrospective cohort study involving 412 adult patients hospitalised with COVID-19-related acute respiratory distress syndrome, showing that aspirin administration, started within 24 h of admission or 7 days before hospital admission, was independently associated with a reduced risk of mechanical ventilation, intensive care unit admission, and in-hospital mortality.97 A meta-analysis of ten retrospective studies, including 56 696 hospitalised patients with COVID-19,94, 97, 98, 99, 100, 101, 102 indicated a lower likelihood of dying for those who had taken aspirin than for non-aspirin users.103 Moreover, in contrast to findings of the large RECOVERY trial (n=7351),104 an observational cohort study of 111 269 US adults hospitalised with moderate COVID-19 showed that early aspirin use was associated with a lower risk of in-hospital mortality after 28 days than was no aspirin use.105 Together, these studies on aspirin use as an antithrombotic agent in patients hospitalised for COVID-19 are encouraging, although not conclusive. However, the observation that the beneficial effects of aspirin were also documented in patients with moderate COVID-19105 suggests a usefulness of this anti-inflammatory drug even for managing outpatients with early stage COVID-19. In this setting, aspirin could be considered an alternative treatment to relatively selective COX-2 inhibitors, indometacin, or other NSAIDs when signs of toxicity or contraindications to these drugs are brought to the attention of family doctors. Indeed, aspirin has been shown to reduce the plasma concentrations of cytokines in patients with chronic stable angina,106 and even to have antiviral activity against RNA viruses in the respiratory tract.107 Thus, these aspirin properties could help to reduce the mild or moderate symptoms induced by the underlying inflammatory processes.
Corticosteroids for early stage COVID-19
Synthetic corticosteroids are widely available medications used to treat chronic inflammatory and autoimmune diseases. At the cellular level, they exert anti-inflammatory actions mainly through inhibition of the NF-κB pathway.108 The effects of corticosteroids on type I interferons, which play a key role in host immune response to viral infections, remain incompletely characterised. Routine doses of oral corticosteroids do not appear to suppress type I interferon production in patients with systemic lupus erythematosus.109 However, there is in vitro evidence that these medications impair the antiviral immune response in human airway cells by interfering with the type I interferon signalling pathway, thereby enhancing viral replication.110, 111 Corticosteroids also dampen cellular immunity by inhibiting the development of type 1 helper T cells, CD8 T cells, and natural killer cells.112
Evidence from preclinical studies suggests that corticosteroids can directly modulate endothelial function, with effects depending on the inflammatory environment. In particular, under physiological conditions, corticosteroids could lead to endothelial function impairment by reducing vascular nitric oxide availability.113, 114 By contrast, under inflammatory conditions, they were found to exert protective effects on the inflammation-associated endothelial cell dysfunction, possibly by reducing IL-6 and TNF-α expression.115 Thus, timing and inflammatory milieu seem to influence responses to corticosteroid treatment.
Chronic use of corticosteroids in autoimmune or obstructive pulmonary diseases and in COVID-19
Chronic use of systemic or inhaled corticosteroids has been associated with increased odds of hospitalisation54 or mortality 116, 117, 118, 119 in people with COVID-19 and rheumatic diseases, inflammatory bowel disease, asthma, or chronic obstructive pulmonary disease. However, the more serious outcomes observed in patients on corticosteroids could be confounded by treatment indication, whereby underlying disease activity or severity could itself negatively affect COVID-19 sequelae.119, 120, 121 Thus, uncertainty remains around the effects of chronic use of corticosteroid on COVID-19 outcomes.
Systemic corticosteroids for COVID-19 management
Based on evidence from previous coronavirus outbreaks (eg, SARS-CoV and MERS-CoV), the use of systemic corticosteroids in COVID-19 was initially discouraged due to the potential risk of secondary infections, long-term complications, and delayed viral clearance.122 However, the RECOVERY trial, published in 2021, showed reduced mortality with dexamethasone in patients hospitalised with COVID-19 who required mechanical ventilation or supplemental oxygen, but not among those receiving no respiratory support.123 Largely based on these findings, WHO guidance strongly recommended systemic corticosteroids in patients with severe and critical COVID-19.124 Data regarding the use of these drugs during the initial phase of COVID-19, when patients are not hospitalised, are scant. Anecdotal evidence from case reports and observational studies suggests that early outpatient treatment with systemic corticosteroids at the time of viral replication (ie, within the first few days of symptoms onset) is associated with an increased risk of delayed recovery and worse clinical outcomes.125, 126, 127, 128, 129 Systemic corticosteroids cause widespread immunosuppression, which could be useful during the late inflammatory phase of severe COVID-19 but cause harm in the early disease phase by suppressing host antiviral responses.130 Nonetheless, further studies are needed to pinpoint the optimal timing of corticosteroid initiation during the disease course.
Inhaled corticosteroids for COVID-19 management
Inhaled corticosteroids have been proposed as an early COVID-19 treatment on the basis of their targeted anti-inflammatory effects in the lung and their antiviral properties.131, 132, 133 To date, five randomised controlled trials have explored treatment with inhaled corticosteroids in outpatients with COVID-19 within 7–14 days of the onset of mild to moderate symptoms (table 1 ). The STOIC study showed that among 146 outpatients with mild COVID-19, inhaled budesonide reduced the composite endpoint of emergency department assessment or hospitalisation, and improved time to recovery, compared with usual care.134 The larger PRINCIPLE study found that in 1856 outpatients with COVID-19 at high risk of disease progression, inhaled budesonide improved time to recovery and reduced the combined endpoint of hospital admission or death compared with usual care, although statistical significance was not achieved for the combined endpoint.135 The COVERAGE study, which aimed to test inhaled ciclesonide in outpatients with COVID-19 at risk of disease aggravation, was prematurely stopped after the first interim analysis revealed no efficacy with regard to reducing the primary composite endpoint of a need for oxygen therapy at home, hospitalisation, or death.138 Notably, these randomised controlled trials had an open-label design, and inhaled medications are known to have placebo effects in respiratory diseases,139 which could have introduced bias in subjective endpoints. Two double-blind, placebo-controlled, randomised controlled trials have examined the effects of inhaled ciclesonide in patients with early stage COVID-19 in the community. An industry-sponsored study involving 400 people with mild to moderate COVID-19 disease found that inhaled ciclesonide did not decrease time to symptom alleviation, but it did reduce the combined endpoint of emergency department visit or hospitalisation.137 Instead, the CONTAIN study showed that among 203 young outpatients (median age of 35 years) with COVID-19, the combination of inhaled and intranasal ciclesonide did not improve the resolution of symptoms, nor did it reduce the incidence of hospitalisation.136 Overall, evidence from randomised controlled trials suggests that the use of inhaled corticosteroids in outpatients with COVID-19 does not adversely affect clinical outcomes nor does it increase the risk of side-effects compared with usual care or placebo. Nonetheless, the effectiveness of these medications in early COVID-19 management remains ill-defined.
Table 1.
Randomised controlled trials that examined inhaled corticosteroids for outpatient treatment of COVID-19
| Exposure | Timing of exposure | Total patients | Age, years* | Sex, % | Primary outcome | Inhaled corticosteroid group | Control group | Between-group difference, p value | |
|---|---|---|---|---|---|---|---|---|---|
| Ramakrishnan et al (2021);134 STOIC (open-label) | Inhaled budesonide (1600 μg/day) until self-reported symptom recovery or primary outcome achievement | ≤7 days from symptom onset | 146 | 45 (13) | 57% female, 43% male | Combined endpoint of COVID-19-related emergency department assessment or hospital admission | Two (3%) of 73 patients | 11 (15%) of 73 patients | Difference in proportions 12·3% (95% CI 3·3 to 21·3); p=0·009 |
| Yu et al (2021);135 PRINCIPLE (open-label) | Inhaled budesonide (1600 μg/day) for 14 days | ≤14 days from symptom onset | 1856 | 64·2 (7·6)† | 52% female, 48% male† | Time to first self-reported recovery by 28 days; combined endpoint of hospital admission or death by 28 days | 11·8 days (95% BCI 10·0 to 14·1); 6·8% (95% BCI 4·1 to 10·2) | 14·7 days (95% BCI 12·3 to 18·0); 8·8% (95% BCI 5·5 to 12·7) | HR 1·21 (95% BCI 1·08 to 1·36); HR 0·75 (95% BCI 0·55 to 1·03) |
| Ezer et al (2021);136 CONTAIN (placebo-controlled) | Inhaled (1200 μg/day) and intranasal (200 μg/day) ciclesonide for 14 days | ≤6 days from symptom onset | 203 | 35 (27–47) | 54% female, 46% male | Resolution of symptoms by day 7 | 42 (40%) of 105 patients | 34 (35%) of 98 patients | Adjusted risk difference 5·5% (95% CI −7·8 to 18·8), p=NS‡ |
| Clemency et al (2022);137 Covis Pharma (placebo-controlled) | Inhaled ciclesonide (640 μg/day) for 30 days | Positive SARS-CoV-2 test ≤72 h | 400 | 43·3 (16·9) | 55% female, 45% male | Time to alleviation of COVID-19-related symptoms by day 30 | 19·0 days (95% CI 14·0 to 21·0) | 19·0 days (95% CI 16·0 to 23·0) | HR 1·08 (95% CI 0·84 to 1·38), p=NS‡ |
| Duvignaud et al (2022);138 COVERAGE (open-label) | Inhaled ciclesonide (640 μg/day) for 10 days | ≤7 days from symptom onset | 217 | 63 (59–68) | 51% female, 49% male | Combined endpoint of hospital admission, oxygen therapy at home, or death by day 14 | 18 (16%) of 110 patients | 13 (12%) of 107 patients | p=NS§ |
BCI=Bayesian credible interval. HR=hazard ratio. NS=not significant.
Data are mean (SD) or median (IQR).
Data refer to SARS-CoV-2-positive participants.
Significance threshold was 0·05.
Significance threshold was not specified.
Discussion and conclusions
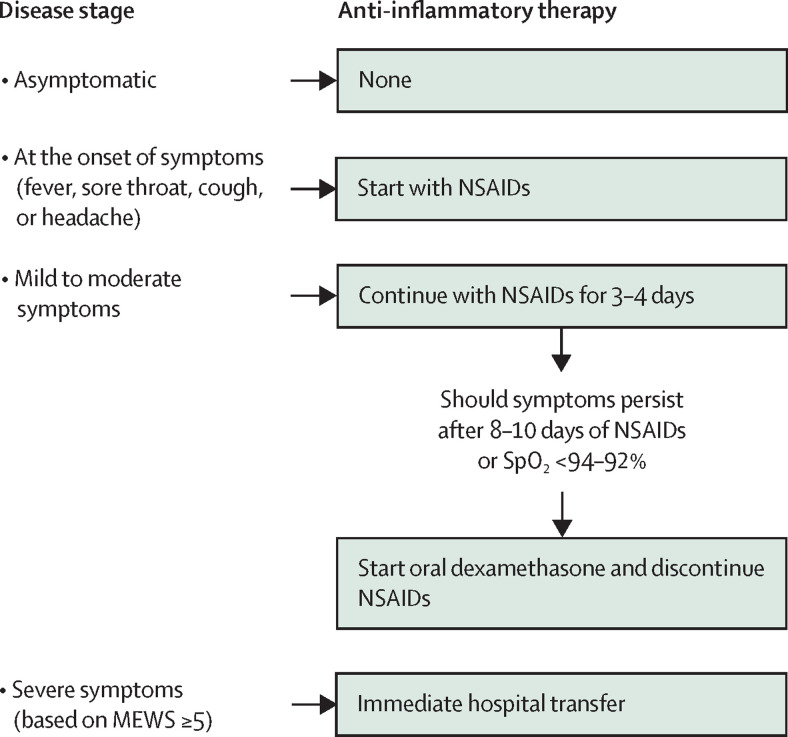
Several recommendations on how to treat people at home with COVID-19 with mild to moderate symptoms have been proposed, starting with the use of anti-inflammatory drugs.69, 140, 141, 142 The main recommended NSAIDs are relatively selective COX-2 inhibitors, indometacin, ibuprofen, and aspirin—often as part of a multipharmacological protocol. Some of the recommendations suggest paracetamol as a safe therapy for the early management of pain and fever in people with COVID-19. However, one should consider that (besides being a negligible anti-inflammatory drug)143 at relatively low doses paracetamol reduces plasma and tissue glutathione concentrations, which might exacerbate COVID-19.144, 145 Very few researchers have formally tested their proposed recommendations for outpatients with symptomatic COVID-19 through observational studies, although those that have show encouraging results.71, 72, 89, 146 In particular, findings from our studies71, 72 have corroborated the treatment protocol recommendations for early outpatient treatment of COVID-19 we have previously proposed on the basis of growing knowledge on the pathophysiology underlying the mild to moderate symptoms encountered at disease onset (figure 3 ).69 These treatment recommendations are based on three pillars: intervene at the very onset of symptoms at home; start therapy as early as possible after the family doctor has been contacted by the patient (without waiting for the results of a nasopharyngeal swab); and rely on NSAIDs, especially relatively selective COX-2 inhibitors (table 2 ). The overlap in COX-2 selectivity between celecoxib and nimesulide was the rationale for recommending these two drugs for the early outpatient treatment of COVID-19 symptoms (table 2). Aspirin or ibuprofen are the alternative treatments to these relatively selective COX-2 inhibitors, if these COX-2 inhibitors are not available or when signs of toxicity or contraindications to these drugs are evident according to clinical characteristics and the medical history of the patient. Treatment with NSAIDs should continue for 3–4 days, but if symptoms persist it could be extended for a maximum of 8–12 days, if not contraindicated (table 2). Moreover, given the metabolic pathway of these NSAIDs involving, among others, the cytochrome 3A4, family physicians should consider the risk of potential drug interactions, especially for patients with COVID-19 who started antiviral therapy with remdesivir or ritonavir-boosted nirmatrelvir. In this case, potential strategies include adjusting the NSAID dose, increasing monitoring for potential adverse reactions, or temporary NSAID withholding. These NSAIDs should be given to treatment-naive patients who are older than 65 years for the shortest time possible, and under adequate hydration. They can be prescribed to pregnant women but only in the first few months of gestation, according to the summary of product characteristics.147 Celecoxib, ibuprofen, and nimesulide should be avoided in children younger than 12 years, whereas aspirin should be taken only under prescription and at the dose recommended by the family doctor.
Figure 3.
Options for at-home anti-inflammatory therapy in adults with COVID-19 depending on disease stage
MEWS=Modified Early Warning Score (an international, universal scoring scale from multiple parameters including respiratory rate, SpO2, heart rate, systolic blood pressure, consciousness). NSAIDs=non-steroidal anti-inflammatory drugs. SpO2=oxygen saturation.
Table 2.
Recommendations for anti-inflammatory agents for early COVID-19 symptoms in adults
| When to use | Dosage | Time of exposure | ||
|---|---|---|---|---|
| NSAIDs | ||||
| Relatively selective COX-2 inhibitors | ||||
| Nimesulide | At the onset of symptoms (fever, cough, sore throat, headache) | 100 mg orally twice a day | For 3–4 days, if symptoms persist, continue for a maximum of 12 days | |
| Celecoxib | At the onset of symptoms (fever, cough, sore throat, headache) | Initial oral dose of 400 mg followed by a second dose of 200 mg on the first day; in the following days, 200 mg/day up to a maximum of 400 mg/day | For 3–4 days, if symptoms persist, continue for a maximum of 12 days | |
| Other NSAIDs | ||||
| Ibuprofen | At the onset of symptoms (fever, cough, sore throat, headache) | 400 mg orally twice a day | For 3–4 days | |
| Aspirin | At the onset of symptoms (fever) or with laboratory signs of hepatotoxicity associated with nimesulide or contraindications to celecoxib | 500 mg orally twice a day | For 3–4 days, if symptoms persist, continue for a maximum of 8 days | |
| Corticosteroids* | ||||
| Dexamethasone | Should fever persist after 8–10 days of NSAID treatment, or when oxygen saturation <94–92% occurs | 8 mg orally for 3 days, then tapered to 4 mg for a further 3 days, and then to 2 mg for 3 days | Duration of treatment depends on the clinical evolution of the disease | |
The recommended drugs can be used unless contraindicated according to summary of product characteristics.
At the start of corticosteroid treatment, NSAIDs should be discontinued. NSAID=non-steroidal anti-inflammatory drug.
Overall, our studies and other observational studies indicate that anti-inflammatory therapy, especially NSAIDs, is crucial for the management of outpatients with early symptoms of COVID-19, since the mitigation of these symptoms protects against progression towards a more severe illness that would eventually require hospitalisation, placing a huge burden on the hospital system (appendix p 1).
Search strategy and selection criteria
References for this Review were identified through searches of PubMed for articles published from Jan 1, 2020, to May 25, 2022, with the terms “COVID-19”, “SARS-CoV-2”, “anti-inflammatory agents”, “non-steroidal anti-inflammatory drugs”, “NSAIDs”, “COX-2 inhibitors”, “corticosteroids”, “glucocorticoids” “pathogenesis”, and “hyperinflammation”. All articles published in English and Italian resulting from these searches, and relevant references cited in those articles, were reviewed.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Kerstin Mierke (Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy) for her help with English language editing of the manuscript. We would also like to thank Martinelli Ginetto for their support realising the study. Finally, we would like to express our sincere gratitude to Fondazione MEI Onlus for their generous contribution to this study.
Contributors
NP and MC did the data search for this Review. All authors wrote the text, reviewed and edited the manuscript, and made substantial contributions to discussions of the content.
Supplementary Material
References
- 1.Johns Hopkins Center for Systems Science and Engineering COVID-19 map—Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html
- 2.US Centers for Disease Control and Prevention Omicron variant: what you need to know. https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html
- 3.Perico N, Fagiuoli S, Di Marco F, et al. Bergamo and COVID-19: how the dark can turn to light. Front Med. 2021;8 doi: 10.3389/fmed.2021.609440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367:1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 5.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (omicron) variant predominance—Los Angeles County, California, November 7, 2021–January 8, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:177–181. doi: 10.15585/mmwr.mm7105e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagiuoli S, Lorini FL, Remuzzi G. Adaptations and lessons in the province of Bergamo. N Engl J Med. 2020;382:e71. doi: 10.1056/NEJMc2011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui KPY, Cheung M-C, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020;8:687–695. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Ortega M, Lorenzo O, Suzuki Y, Rupérez M, Egido J. Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens. 2001;10:321–329. doi: 10.1097/00041552-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Junqueira C, Crespo Â, Ranjbar S, et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606:576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Shen C, Li J, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146:119–127. doi: 10.1016/j.jaci.2020.04.027. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Xie J, Zhao L, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen SF, Ho Y-C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wendisch D, Dietrich O, Mari T, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184:6243–6261. doi: 10.1016/j.cell.2021.11.033. e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan DJ, Gebo KA, Shoham S, et al. Early outpatient treatment for COVID-19 with convalescent plasma. N Engl J Med. 2022;386:1700–1711. doi: 10.1056/NEJMoa2119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callaway E. COVID antibody drugs work best when given as early as possible. Nature. 2022 doi: 10.1038/d41586-022-00893-y. published online March 30. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Zhao S, Lane N. Preference for nonsteroidal antiinflammatory drugs over acetaminophen by rheumatic disease patients: a survey of 1,799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum. 2000;43:378–385. doi: 10.1002/1529-0131(200002)43:2<378::AID-ANR18>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs—differences and similarities. N Engl J Med. 1991;324:1716–1725. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- 27.Samuelsson B. An elucidation of the arachidonic acid cascade. Discovery of prostaglandins, thromboxane and leukotrienes. Drugs. 1987;33(suppl 1):2–9. doi: 10.2165/00003495-198700331-00003. [DOI] [PubMed] [Google Scholar]
- 28.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong W, Chen Y, You K, et al. Celebrex adjuvant therapy on coronavirus disease 2019: an experimental study. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.561674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazancioglu S, Yilmaz FM, Bastug A, et al. Assessment of galectin-1, galectin-3, and prostaglandin E2 levels in patients with COVID-19. Jpn J Infect Dis. 2021;74:530–536. doi: 10.7883/yoken.JJID.2021.020. [DOI] [PubMed] [Google Scholar]
- 31.Yalçın Kehribar D, Cihangiroğlu M, Sehmen E, et al. The assessment of the serum levels of TWEAK and prostaglandin F2α in COVID-19. Turk J Med Sci. 2020;50:1786–1791. doi: 10.3906/sag-2006-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archambault A-S, Zaid Y, Rakotoarivelo V, et al. High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients. FASEB J. 2021;35 doi: 10.1096/fj.202100540R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grosser T, Naji A, FitzGerald GA. Urinary prostaglandin metabolites: an incomplete reckoning and a flush to judgment. Circ Res. 2018;122:537–539. doi: 10.1161/CIRCRESAHA.118.312616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theken KN, FitzGerald GA. Bioactive lipids in antiviral immunity. Science. 2021;371:237–238. doi: 10.1126/science.abf3192. [DOI] [PubMed] [Google Scholar]
- 35.Chen JS, Alfajaro MM, Chow RD, et al. Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. J Virol. 2021;95:e00014–e00021. doi: 10.1128/JVI.00014-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan Q, Li P, Ye X, et al. Longitudinal peripheral blood transcriptional analysis reveals molecular signatures of disease progression in COVID-19 patients. J Immunol. 2021;206:2146–2159. doi: 10.4049/jimmunol.2001325. [DOI] [PubMed] [Google Scholar]
- 37.Coulombe F, Jaworska J, Verway M, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomi K, Zhu FG, Marshall JS. Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J Immunol. 2000;165:6545–6552. doi: 10.4049/jimmunol.165.11.6545. [DOI] [PubMed] [Google Scholar]
- 40.Sander WJ, O'Neill HG, Pohl CH. Prostaglandin E2 as a modulator of viral infections. Front Physiol. 2017;8:89. doi: 10.3389/fphys.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FitzGerald GA. Misguided drug advice for COVID-19. Science. 2020;367 doi: 10.1126/science.abb8034. [DOI] [PubMed] [Google Scholar]
- 44.Qiao W, Wang C, Chen B, et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131:97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 45.Terrier O, Dilly S, Pizzorno A, et al. Antiviral properties of the NSAID drug naproxen targeting the nucleoprotein of SARS-CoV-2 coronavirus. Molecules. 2021;26 doi: 10.3390/molecules26092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Mdkhana B, et al. Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020;94:4037–4041. doi: 10.1007/s00204-020-02869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Bruin N, Schneider A-K, Reus P, et al. Ibuprofen, flurbiprofen, etoricoxib or paracetamol do not influence ACE2 expression and activity in vitro or in mice and do not exacerbate in-vitro SARS-CoV-2 infection. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abu Esba LC, Alqahtani RA, Thomas A, Shamas N, Alswaidan L, Mardawi G. Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study. Infect Dis Ther. 2021;10:253–268. doi: 10.1007/s40121-020-00363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26:1259.e5–1259.e7. doi: 10.1016/j.cmi.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong AY, MacKenna B, Morton CE, et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021;80:943–951. doi: 10.1136/annrheumdis-2020-219517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund LC, Kristensen KB, Reilev M, et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kragholm K, Gerds TA, Fosbøl E, et al. Association between prescribed ibuprofen and severe COVID-19 infection: a nationwide register-based cohort study. Clin Transl Sci. 2020;13:1103–1107. doi: 10.1111/cts.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi MH, Ahn H, Ryu HS, et al. Clinical characteristics and disease progression in early-stage COVID-19 patients in South Korea. J Clin Med. 2020;9 doi: 10.3390/jcm9061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandan JS, Zemedikun DT, Thayakaran R, et al. Nonsteroidal antiinflammatory drugs and susceptibility to COVID-19. Arthritis Rheumatol. 2021;73:731–739. doi: 10.1002/art.41593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288:469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruce E, Barlow-Pay F, Short R, et al. Prior routine use of non-steroidal anti-inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID-19. J Clin Med. 2020;9 doi: 10.3390/jcm9082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeong HE, Lee H, Shin HJ, Choe YJ, Filion KB, Shin J-Y. Association between nonsteroidal antiinflammatory drug use and adverse clinical outcomes among adults hospitalized with coronavirus 2019 in South Korea: a nationwide study. Clin Infect Dis. 2021;73:e4179–e4188. doi: 10.1093/cid/ciaa1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drake TM, Fairfield CJ, Pius R, et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021;3:e498–e506. doi: 10.1016/S2665-9913(21)00104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Q, Zhao S, Gan L, et al. Use of non-steroidal anti-inflammatory drugs and adverse outcomes during the COVID-19 pandemic: a systematic review and meta-analysis. EClinicalMedicine. 2022;46 doi: 10.1016/j.eclinm.2022.101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baghaki S, Yalcin CE, Baghaki HS, Aydin SY, Daghan B, Yavuz E. COX2 inhibition in the treatment of COVID-19: review of literature to propose repositioning of celecoxib for randomized controlled studies. Int J Infect Dis. 2020;101:29–32. doi: 10.1016/j.ijid.2020.09.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu L, Li R, Pan Y, et al. High-throughput screen of protein expression levels induced by cyclooxygenase-2 during influenza A virus infection. Clin Chim Acta. 2011;412:1081–1085. doi: 10.1016/j.cca.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 63.Carey MA, Bradbury JA, Seubert JM, Langenbach R, Zeldin DC, Germolec DR. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J Immunol. 2005;175:6878–6884. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- 64.Lee SMY, Cheung C-Y, Nicholls JM, et al. Hyperinduction of cyclooxygenase-2-mediated proinflammatory cascade: a mechanism for the pathogenesis of avian influenza H5N1 infection. J Infect Dis. 2008;198:525–535. doi: 10.1086/590499. [DOI] [PubMed] [Google Scholar]
- 65.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 66.Ong SWX, Tan WYT, Chan Y-H, et al. Safety and potential efficacy of cyclooxygenase-2 inhibitors in coronavirus disease 2019. Clin Transl Immunology. 2020;9 doi: 10.1002/cti2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singla AK, Chawla M, Singh A. Nimesulide: some pharmaceutical and pharmacological aspects—an update. J Pharm Pharmacol. 2000;52:467–486. doi: 10.1211/0022357001774255. [DOI] [PubMed] [Google Scholar]
- 68.Carey MA, Bradbury JA, Rebolloso YD, Graves JP, Zeldin DC, Germolec DR. Pharmacologic inhibition of COX-1 and COX-2 in influenza A viral infection in mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suter F, Perico N, Cortinovis M, Remuzzi G. A recurrent question from a primary care physician: how should I treat my COVID-19 patients at home? An update. Clin Med Invest. 2020;5:1–9. [Google Scholar]
- 70.Starek M, Plenis A, Zagrobelna M, Dąbrowska M. Assessment of lipophilicity descriptors of selected NSAIDs obtained at different TLC stationary phases. Pharmaceutics. 2021;13:440. doi: 10.3390/pharmaceutics13040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suter F, Consolaro E, Pedroni S, et al. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: a retrospective observational matched-cohort study. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Consolaro E, Suter F, Rubis N, et al. A home-treatment algorithm based on anti-inflammatory drugs to prevent hospitalization of patients with early COVID-19: a matched-cohort study (COVER 2) Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.785785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donati M, Conforti A, Lenti MC, et al. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol. 2016;82:238–248. doi: 10.1111/bcp.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Donnan PT, Bell S, Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18:256. doi: 10.1186/s12882-017-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clavé S, Rousset-Rouvière C, Daniel L, Tsimaratos M. The invisible threat of non-steroidal anti-inflammatory drugs for kidneys. Front Pediatr. 2019;7:520. doi: 10.3389/fped.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelleni MT. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelleni M. Breakthrough: ibuprofen/nitazoxanide/azithromycin: a battle changer personalized COVID-19 telemedicine five days protocol. 2020. https://www.authorea.com/users/318758/articles/460853-breakthrough-ibuprofen-nitazoxanide-azithromycin-a-battle-changer-personalized-covid-19-telemedicine-five-days-protocol?commit=52337e49b98b96fae21dab166828780e5f544a80
- 80.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 81.Nalamachu S, Wortmann R. Role of indomethacin in acute pain and inflammation management: a review of the literature. Postgrad Med. 2014;126:92–97. doi: 10.3810/pgm.2014.07.2787. [DOI] [PubMed] [Google Scholar]
- 82.Amici C, Di Caro A, Ciucci A, et al. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir Ther. 2006;11:1021–1030. [PubMed] [Google Scholar]
- 83.Schröer J, Shenk T. Inhibition of cyclooxygenase activity blocks cell-to-cell spread of human cytomegalovirus. Proc Natl Acad Sci USA. 2008;105:19468–19473. doi: 10.1073/pnas.0810740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bahrami H, Daryani NE, Haghpanah B, et al. Effects of indomethacin on viral replication markers in asymptomatic carriers of hepatitis B: a randomized, placebo-controlled trial. Am J Gastroenterol. 2005;100:856–861. doi: 10.1111/j.1572-0241.2005.41144.x. [DOI] [PubMed] [Google Scholar]
- 85.Bour AM, Westendorp RG, Laterveer JC, Bollen EL, Remarque EJ. Interaction of indomethacin with cytokine production in whole blood. Potential mechanism for a brain-protective effect. Exp Gerontol. 2000;35:1017–1024. doi: 10.1016/s0531-5565(00)00128-5. [DOI] [PubMed] [Google Scholar]
- 86.Russell B, Moss C, George G, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alkotaji M, Al-Zidan RN. Indomethacin: can it counteract bradykinin effects in COVID-19 patients? Curr Pharmacol Rep. 2021;7:102–106. doi: 10.1007/s40495-021-00257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon DE, Hiatt J, Bouhaddou M, et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370 doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fazio S, Bellavite P, Zanolin E, McCullough PA, Pandolfi S, Affuso F. Retrospective study of outcomes and hospitalization rates of patients in Italy with a confirmed diagnosis of early COVID-19 and treated at home within 3 days or after 3 days of symptom onset with prescribed and non-prescribed treatments between November 2020 and August 2021. Med Sci Monit. 2021;27 doi: 10.12659/MSM.935379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ravichandran R, Mohan SK, Sukumaran SK, et al. An open label randomized clinical trial of indomethacin for mild and moderate hospitalised COVID-19 patients. Sci Rep. 2022;12 doi: 10.1038/s41598-022-10370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72:619–633. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Q, Huang N, Li A, et al. Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haji Aghajani M, Moradi O, Amini H, et al. Decreased in-hospital mortality associated with aspirin administration in hospitalized patients due to severe COVID-19. J Med Virol. 2021;93:5390–5395. doi: 10.1002/jmv.27053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meizlish ML, Goshua G, Liu Y, et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am J Hematol. 2021;96:471–479. doi: 10.1002/ajh.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132:930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 98.Yuan S, Chen P, Li H, Chen C, Wang F, Wang DW. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J Cell Mol Med. 2021;25:1263–1273. doi: 10.1111/jcmm.16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Merzon E, Green I, Vinker S, et al. The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID-19 infection. FEBS J. 2021;288:5179–5189. doi: 10.1111/febs.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alamdari NM, Afaghi S, Rahimi FS, et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J Exp Med. 2020;252:73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 102.Sahai A, Bhandari R, Koupenova M, et al. SARS-CoV-2 receptors are expressed on human platelets and the effect of aspirin on clinical outcomes in COVID-19 patients. Res Sq. 2020 doi: 10.21203/Frs.3.rs-119031%2Fv1. published online Dec 23. (preprint) [DOI] [Google Scholar]
- 103.Srivastava R, Kumar A. Use of aspirin in reduction of mortality of COVID-19 patients: a meta-analysis. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abani O, Abbas A, Abbas F, et al. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:143–151. doi: 10.1016/S0140-6736(21)01825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chow JH, Rahnavard A, Gomberg-Maitland M, et al. Association of early aspirin use with in-hospital mortality in patients with moderate COVID-19. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. 1999;100:793–798. doi: 10.1161/01.cir.100.8.793. [DOI] [PubMed] [Google Scholar]
- 107.Glatthaar-Saalmüller B, Mair KH, Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract—an in vitro study. Influenza Other Respir Viruses. 2017;11:85–92. doi: 10.1111/irv.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 109.Guiducci C, Gong M, Xu Z, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas BJ, Porritt RA, Hertzog PJ, Bardin PG, Tate MD. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep. 2014;4 doi: 10.1038/srep07176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marcellini A, Swieboda D, Guedán A, et al. Glucocorticoids impair type I IFN signalling and enhance rhinovirus replication. Eur J Pharmacol. 2021;893 doi: 10.1016/j.ejphar.2020.173839. [DOI] [PubMed] [Google Scholar]
- 112.Shimba A, Ikuta K. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol. 2020;42:669–680. doi: 10.1007/s00281-020-00827-8. [DOI] [PubMed] [Google Scholar]
- 113.Schäfer SC, Wallerath T, Closs EI, et al. Dexamethasone suppresses eNOS and CAT-1 and induces oxidative stress in mouse resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H436–H444. doi: 10.1152/ajpheart.00587.2004. [DOI] [PubMed] [Google Scholar]
- 114.Rogers KM, Bonar CA, Estrella JL, Yang S. Inhibitory effect of glucocorticoid on coronary artery endothelial function. Am J Physiol Heart Circ Physiol. 2002;283:H1922–H1928. doi: 10.1152/ajpheart.00364.2002. [DOI] [PubMed] [Google Scholar]
- 115.Goodwin JE, Feng Y, Velazquez H, Sessa WC. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc Natl Acad Sci USA. 2013;110:306–311. doi: 10.1073/pnas.1210200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491. doi: 10.1053/j.gastro.2020.05.032. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shin YH, Shin JI, Moon SY, et al. Autoimmune inflammatory rheumatic diseases and COVID-19 outcomes in South Korea: a nationwide cohort study. Lancet Rheumatol. 2021;3:e698–e706. doi: 10.1016/S2665-9913(21)00151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schäfer M, Strangfeld A, Hyrich KL, et al. Response to: “Correspondence on factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician reported registry” by Mulhearn et al. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220134. published online March 1. [DOI] [PubMed] [Google Scholar]
- 121.Brain O, Satsangi J. Therapeutic decisions in inflammatory bowel disease in the SARS-Cov-2 pandemic. Gastroenterology. 2021;160:1883–1884. doi: 10.1053/j.gastro.2020.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lamontagne F, Agoritsas T, Siemieniuk R, et al. A living WHO guideline on drugs to prevent COVID-19. BMJ. 2021;372:n526. doi: 10.1136/bmj.n526. [DOI] [PubMed] [Google Scholar]
- 125.Arora K, Panda PK. Steroid harms if given early in COVID-19 viraemia. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ordinola Navarro A, Lopez Luis BA. Corticosteroids prescription for mild-moderate COVID-19 in primary care. J Infect Dev Ctries. 2021;15:1813–1815. doi: 10.3855/jidc.15069. [DOI] [PubMed] [Google Scholar]
- 127.Okoye C, Rogani S, Franchi R, et al. Pitfalls of early systemic corticosteroids home therapy in older patients with COVID-19 pneumonia. Geriatrics (Basel) 2022;7:21. doi: 10.3390/geriatrics7010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aggarwal A, Mittal A, Soneja M, et al. Role of systemic corticosteroids in preventing hypoxia among patients with mild COVID-19: an observational study. Drug Discov Ther. 2021;15:273–277. doi: 10.5582/ddt.2021.01081. [DOI] [PubMed] [Google Scholar]
- 129.Callejas Rubio JL, Aomar Millan I, Moreno-Higueras M, Martín Ripoll L, Yuste Osorio E, Ríos-Fernández R. Caution with the use of dexamethasone in patients with COVID-19 in its initial phases. Rev Clin Esp (Barc) 2021;221:592–595. doi: 10.1016/j.rceng.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matthay MA, Wick KD. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J Clin Invest. 2020;130:6218–6221. doi: 10.1172/JCI143331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nicolau DV, Bafadhel M. Inhaled corticosteroids in virus pandemics: a treatment for COVID-19? Lancet Respir Med. 2020;8:846–847. doi: 10.1016/S2213-2600(20)30314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8:436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pinna SM, Scabini S, Corcione S, Lupia T, De Rosa FG. COVID-19 pneumonia: do not leave the corticosteroids behind! Future Microbiol. 2021;16:317–322. doi: 10.2217/fmb-2020-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramakrishnan S, Nicolau DV, Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu L-M, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ezer N, Belga S, Daneman N, et al. Inhaled and intranasal ciclesonide for the treatment of COVID-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ. 2021;375 doi: 10.1136/bmj-2021-068060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern Med. 2022;182:42–49. doi: 10.1001/jamainternmed.2021.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duvignaud A, Lhomme E, Onaisi R, et al. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE) Clin Microbiol Infect. 2022;28:1010–1016. doi: 10.1016/j.cmi.2022.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wechsler ME, Kelley JM, Boyd IOE, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365:119–126. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Donno DR, Grattagliano I, Rossi A, et al. How to treat COVID-19 patients at home in the Italian context: an expert opinion. Infect Dis Rep. 2021;13:251–258. doi: 10.3390/idr13010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.WHO Home care for patients with suspected or confirmed COVID-19 and management of their contacts. Interim guidance. Aug 12, 2020. https://www.who.int/publications/i/item/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts
- 142.Pandolfi S, Chirumbolo S, Ricevuti G, et al. Home pharmacological therapy in early COVID-19 to prevent hospitalization and reduce mortality: time for a suitable proposal. Basic Clin Pharmacol Toxicol. 2022;130:225–239. doi: 10.1111/bcpt.13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ghanem CI, Pérez MJ, Manautou JE, Mottino AD. Acetaminophen from liver to brain: new insights into drug pharmacological action and toxicity. Pharmacol Res. 2016;109:119–131. doi: 10.1016/j.phrs.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khanfar A, Al Qaroot B. Could glutathione depletion be the Trojan horse of COVID-19 mortality? Eur Rev Med Pharmacol Sci. 2020;24:12500–12509. doi: 10.26355/eurrev_202012_24046. [DOI] [PubMed] [Google Scholar]
- 145.Sestili P, Fimognari C. Paracetamol-induced glutathione consumption: is there a link with severe COVID-19 illness? Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.579944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D'Amato G, Acanfora L, Delli Paoli L, D'Amato M. Preventive home therapy for symptomatic patients affected by COVID-19 and followed by teleconsultations. Multidiscip Respir Med. 2021;16:748. doi: 10.4081/mrm.2021.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.US National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.