Abstract
Human gut microbial species found to associate with clinical responses to immune checkpoint inhibitors (ICIs) are often tested in mice using fecal microbiota transfer (FMT), wherein tumor responses in recipient mice may recapitulate human responses to ICI treatment. However, many FMT studies have reported only limited methodological description, details of murine cohorts, and statistical methods. To investigate the reproducibility and robustness of gut microbial species that impact ICI responses, we performed human to germ-free mouse FMT using fecal samples from patients with non-small cell lung cancer who had a pathological response or nonresponse after neoadjuvant ICI treatment. R-FMT mice yielded greater anti-tumor responses in combination with anti-PD-L1 treatment compared to NR-FMT, although the magnitude varied depending on mouse cell line, sex, and individual experiment. Detailed investigation of post-FMT mouse microbiota using 16S rRNA amplicon sequencing, with models to classify and correct for biological variables, revealed a shared presence of the most highly abundant taxa between the human inocula and mice, though low abundance human taxa colonized mice more variably after FMT. Multiple Clostridium species also correlated with tumor outcome in individual anti-PD-L1-treated R-FMT mice. RNAseq analysis revealed differential expression of T and NK cell-related pathways in responding tumors, irrespective of FMT source, with enrichment of these cell types confirmed by immunohistochemistry. This study identifies several human gut microbial species that may play a role in clinical responses to ICIs and suggests attention to biological variables is needed to improve reproducibility and limit variability across experimental murine cohorts.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03169-6.
Keywords: Gut microbiome, Immune checkpoint inhibitors, Neoadjuvant, Lung cancer, Fecal microbiota transfer, Biological variables
Introduction
The intestinal microbiome is a potentially modifiable factor that may impact checkpoint inhibitor success. Immune checkpoint inhibitors (ICIs), specifically monoclonal antibodies targeting CTLA-4 and PD-1/PD-L1, have revolutionized oncologic treatment across multiple tumor types by blocking the interaction of T cell inhibitory receptors with their respective ligands on tumor or immune cells [1] and thereby activating T lymphocyte-mediated immune responses. However, apart from melanoma, microsatellite instable colorectal cancer, Hodgkin’s lymphoma, and Merkel cell carcinoma, only ~ 15–30% of patients with advanced cancer respond to treatment, even among immunologically “responsive” cancer types [2]. In advanced stage non-small cell lung cancer (NSCLC), 17–21% of patients respond to ICIs alone, while ~ 48% respond to anti-PD-1 therapy when combined with chemotherapy as a first-line regimen in metastatic disease [3–9]. Despite increasingly favorable ICI response rates across tumors, the specific microbes and/or microbial communities in the gut that facilitate or hinder anti-tumor responses at a distant site remain unclear. Mechanistic studies in mice are critical to understanding the components of microbe to immunologic signaling and to moving the field beyond association; further murine models may provide insight into which gut microbiome modifications improve ICI response rates. In fact, recent data from two pilot clinical trials suggest that fecal microbiota transfer (FMT) from patients with responsive tumors to anti-PD-1 nonresponsive patients with melanoma may enhance the anti-tumor response to ICIs in some cases [10, 11]. Studies to improve ICI response rates are especially crucial in NSCLC, which is much less responsive to ICI therapy than melanoma.
To date, multiple studies have utilized human to murine FMT models with syngeneic tumor cell lines to evaluate association of gut microbiota with ICI tumor response [12]. Inoculation of human donor stool into germ-free (GF) or antibiotic-treated mice recapitulates the human tumor response in some mice. This murine FMT approach holds great potential in defining microbial influences on ICI beyond mere correlation and further may allow preclinical testing of approaches to enrich or de-enrich the microbiome for bacterial components helpful or harmful to ultimate anti-tumor immunity. While the limited murine FMT results published thus far show potentially concordant tumor responses with human donors, most models display variability in the tumor responses and confirmation of concordance between human and mouse microbiota correlates of response is often lacking. Moreover, the specific human microbes or communities that can establish in mice are not well defined, and the relationship of mouse colonization to anti-tumor responses remains unclear [13]. Additionally, while murine immune populations have been characterized as correlatives to human studies, little effort has focused on tumor transcriptional and/or tumor immune microenvironment (TiME) changes in response to FMT.
Herein, we detail studies of GF transplantable tumor models using FMT from two NSCLC patients who had dramatically different responses to checkpoint inhibitors. We hypothesized that in-depth study of transplantable tumor models in GF mice with FMT from patients with tumors that show a clear response (R) or nonresponse (NR) would provide insight to optimize this model for further study and provide initial mechanistic insight into transcriptional changes in the tumor. We identify several factors that affect tumor response in these models, such as sex, that have not previously been well defined in FMT murine models or directly compared using FMT with the same human samples. We identify human microbes that can establish in the mouse colon and how the composition of complex human microbial communities is altered when introduced into murine models. Finally, we utilize our models to: (1) identify human species enriched in our murine FMT models that display tumors that did not progress (stable or diminished growth rate) or progressed (increased growth rate) during ICI therapy; and (2) characterize transcriptional and immunologic changes associated with ICI response in the murine tumor. These data provide important results to consider in reproducibility and robustness of these murine models and identify several gut microbial species and transcriptional pathways that correlate with response and suggest that these species exert immune effects in the TiME to enhance the efficacy of anti-PD-L1 therapy.
Materials and methods
Human donor sample selection
Human samples used in murine studies were from patients enrolled in a multicenter, open-label single-arm phase IB/II study (NCT02259621) conducted at Johns Hopkins University. In this trial, patients received ipilimumab 1 mg/kg together with nivolumab 3 mg/kg intravenously once, 6 weeks prior to tumor resection. Two additional doses of nivolumab 3 mg/kg were given at approximately 4 and 2 weeks preoperatively [14]. Fecal samples used as human donor for murine FMT studies were collected prior to initiation of any treatment for both patients. Patient selection was made based on the availability of a pre-treatment stool sample and presence of an unambiguous response to treatment. For this in-depth analysis, we selected one complete pathological responder (R) and one nonresponder (NR) who has tumor progression on therapy.
Murine tumor models and growth curves
Six- to 15-week-old C57BL/6 GF mice, both male and female, were used in experiments. Mice received FMT of a 4% w/v human stool slurry in PBS, 100 µl using an 18 gauge needle (Cadence Science # 7904). Tumor cells were injected subcutaneously onto the right flank of mice 2 weeks after FMT (day 0). Culture methods and tumor cell preparation are described in supplementary methods. Once palpable, tumor growth was measured using sterile calipers every 3 days, typically starting at day 7 (with all tumors palpable by day 10) until end of experiment on days 21–22 for MC38 tumors and days 16–17 for B16F10 tumors. For each tumor, the shortest (a) and the longest (b) perpendicular diameters were measured and tumor volume was calculated using the formula: a2b/2. Mice were treated with the indicated isotype (rat IgG2b isotype control, anti-keyhole limpet hemocyanin, BioXcell, #BP0090) or anti-PD-L1 (B7-H1, clone 10F.9G2, BioXcell #BP0101) antibody by intra-peritoneal injection at 5 mg/kg every 3 days on days 10, 13, 16, and 19 for MC38 tumors or days 7, 10, 13 for B16F10 tumors. Fecal pellets were collected in a sterile manner 2 weeks after FMT (day 0) and at the end of the experiment (days 16–17, B16F10; days 21–22, MC38).
Analysis of 16S rRNA amplicon taxonomic data
Analysis of species transfer from human donor stool slurry into our murine mouse model was assessed with an operational taxonomic unit (OTU) abundance threshold of 0.01% and a ubiquity cutoff of 34%, where ubiquity was measured as the percentage of samples with a detectable amount of the OTU. Beta diversity was calculated using QIIME (v.1.8.0) to generate Bray–Curtis dissimilarity scores between samples. To identify species association with anti-PD-L1 mouse tumor response, murine tumors in each experiment were first categorized based on percent change in tumor volume from day 10 to the final day of tumor measurement (day 21 or 22, MC38) into murine tumor progressors (MT-P) with tumor growth > 25% or murine tumor nonprogressors (MT-NP) with tumor growth < 25%, measured from day 10 after cell injection to mouse harvest. Mann–Whitney U test was used to determine association of bacterial OTUs to the tumor response categories. For further analysis, a generalized linear model with adjustment for variation caused by experiment, cage, and age was applied to our analysis of OTU abundance and tumor response. Statistical analyses were performed using R (v3.6.3).
RNA sequencing
RNA was extracted from flash-frozen MC38 tumors harvested at the end of an experiment using a Purelink mRNA mini kit (Thermo Fisher Scientific). Quality control, library preparation, and RNA sequencing were performed by Novogene Corporation (Beijing, CN). Differential gene expression testing was done using DESeq2 (v1.30.1) with an FDR corrected p value cutoff of 0.05. Gene ontology of differentially abundant genes was analyzed using the Gene Ontology (GO) Project webtool (http://geneontology.org/, release 2021–02-01). Gene pathway analysis of the significantly differentially expressed genes between responding and progressing tumors was performed using the STRING database (V11.0) to identify GO annotated pathways enriched in responding tumors [15].
Immunohistochemistry
Formalin-fixed paraffin-embedded MC38 tumors were sectioned onto glass slides and immunohistochemistry performed using antibodies from Cell Signaling (Danvers, MA) (CD8α #98,941, KLRB1c #39,197, Cd11c #97,585, Alpha Smooth muscle actin #56,856). Antigen retrieval was performed for 30 min in a rice cooker. Slides were incubated with primary antibodies overnight at 4 °C. Primary antibody dilution, incubation with HRP-conjugated secondary antibodies, and staining with DAB were performed following protocols from Cell Signaling. Staining was quantified using HALO image analysis software (Indica Labs), and tumor margins were marked manually. For CD8 and KLRB1, data are shown as percent positive cells to total tumor cells. For CD11c, data are shown as percent positive of total area.
Statistical Analyses
All pairwise comparisons were performed using a Mann–Whitney test, with the exception of the flow cytometry data, which was analyzed using a Student's t test. P values are indicated in each figure legend. Tumor growth curves were analyzed using a two-way ANOVA and p value for both time and group are shown in each figure. Analysis comparing relative abundance in human vs murine samples was performed using Spearman correlation. Correlation coefficient and p value are shown with figure.
Results
Patient samples
To investigate human gut microbes that influence response to ICIs in lung cancer, we performed human to mouse FMT using pre-treatment stool samples from two patients with stage IIIA NSCLC enrolled in a neoadjuvant clinical trial of ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1). Both patients were male (ages 53, 72), previously untreated, and subsequently received ipilimumab and nivolumab once followed by nivolumab every 2 weeks over a period of 6 weeks prior to surgical resection [14]. Fig. S1 shows the pre-treatment and post-treatment computed tomography (CT) scans for both patients and relevant clinical characteristics. At the time of surgery, the tumor from the R patient showed a complete pathological response, whereas the tumor from the NR patient had metastatic progression during the 6 weeks of neoadjuvant therapy and was unable to undergo surgical resection. Both patients had comparable PD-L1 tumor expression scores based on clinical classifications (1–49%).
Significant effect of sex on enhanced ICI responsiveness in GF mice colonized with R vs NR microbiomes
Our initial goal was to determine if the pre-treatment human gut microbes of each patient would confer similar anti-tumor responses in syngeneic, heterotopic tumor GF murine models using human to mouse FMT, as these are common murine tumor models used to study human ICI responses [16, 17]. We also chose to test two syngeneic cell lines with different therapeutic sensitivities to immunotherapy, specifically B16F10 and MC38 cells. B16F10 cells are a non-immunogenic mouse melanoma cell line that is relatively resistant to anti-PD-L1 therapy, and MC38 cells are a moderately immunogenic mouse colon cancer cell line that is sensitive to anti-PD-L1 therapy. Figure 1a shows the overall schema for these experiments and the treatment days for the B16F10 and MC38 cell lines. B16F10 tumors responded to anti-PD-L1 with R-FMT with no response in NR-FMT mice, whereas MC38 tumors responded in both R-FMT and NR-FMT, with a significantly improved response in R-FMT mice (Figs. 1b and S2). When mice were separated by sex, tumors in female mice accounted for the response in B16F10 tumors and, similarly, females displayed enhanced responses in MC38 tumors compared to male mice (Fig. 1c). While a significant anti-tumor response to anti-PD-L1 treatment was observed with human R-FMT compared to NR-FMT in both cell lines, the MC38 model offered a greater dynamic range of tumor responses and was used for subsequent studies.
Fig. 1.
Fecal microbiota transfer (FMT) of human R donor stool enhances response to anti-PD-L1 treatment compared to human NR stool in syngeneic tumor-bearing germ-free (GF) mice. (a) Experimental schema for B16F10 and MC38 syngeneic GF mouse models: GF mice were given oral gavage of 4% w/v human stool on D-14, subcutaneous syngeneic tumor injections on D0, and isotype or 5 mg/kg anti-PD-L1 injections beginning on D10 and (b) tumors volumes at the end of experiment, either D19 (B16F10) or D21-22 (MC38). Graphs represent an aggregate of 3–4 individual experiments per cell line with each point representing a single mouse. (c) Data in B separated by sex of the mice. Mean and SD are shown. Statistical comparisons were performed using Mann–Whitney U Test: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
Fidelity of human to mouse FMT in the MC38 murine model
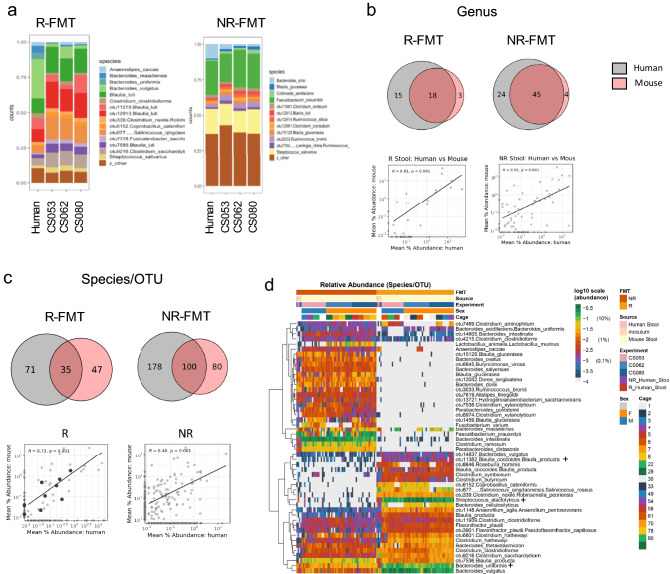
To examine the relationship between the human fecal inocula and subsequent murine colonization, we next examined the microbial composition in mice 2 weeks post-FMT using 16S rRNA amplicon sequencing with analysis using a high-resolution taxonomic assignment methodology (Table S1) (18). First, we compared the composition of the original human donor stools and the 4% w/v R and NR fecal slurries used to inoculate mice that were prepared independently for each experiment. Figure 2a shows the comparison of the relative abundance of the top species (> 1% relative abundance) between the donor stool and each inoculum. As expected, a similar microbial profile of species was present within each inocula when compared to the respective donor stool.
Fig. 2.
Relative abundance of shared genus and species-level OTUs between the human stools and colonized mice are correlated post-fecal microbiota transfer (FMT). (a) Original human stool and 4% w/v inoculation slurry for each experiment prepared independently from human responder (R-FMT) and nonresponder (NR-FMT) stool. CS053, CS062, CS080 represent independent experiments. Species above 1% relative abundance are shown. (b) Taxonomic comparison at the genus level for human and mouse by Venn diagram and using Spearman correlation (graphs in lower panels). Cutoffs for inclusion: relative abundance > 0.01% and ubiquity > 34%. (c) Taxonomic comparison at the species/operational taxonomic unit (OTU) level for human and mouse by Venn diagram and using Spearman correlation (graphs in lower panels). The inclusion criteria were the same as genus level. (d) Heatmap with relative species abundances shown for donor human stool, inocula for individual experiments, and individual mice. Statistical comparisons were performed using Mann–Whitney U Test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data shown in B, C, and D include all mice used in the MC38 model across 3 independent experiments. + indicates OTUs referenced in manuscript
Next, we compared average taxa abundance at the genus (Fig. 2b) and species/OTU level (Fig. 2c) to determine the proportion of human bacteria that established in individual GF mice 2 weeks post-FMT. In order to limit the impact of sequencing depth on low abundance OTUs, we imposed cutoffs for abundance and ubiquity. OTUs below an average abundance cutoff of 0.01% or found in fewer than 34% of samples were removed for this analysis (Fig. S3). At the genus level, we observed that bacteria in 55% (18/33) and 65% (45/69) of genera were concordant in the human donor stools and murine stools after R-FMT and NR-FMT, respectively (Fig. 2c). At the species/OTU level, concordance was lower (33% for R-FMT and 36% for NR-FMT). When looking at the bacterial composition in individual mice (Fig. 2d), relative abundance data demonstrate the overall similarity in murine colonization within FMT groups but also emphasizes the stark difference between the fecal microbiota present in the R versus NR patient, limiting the ability to make direct comparisons between the fecal microbiota compositions of the R-FMT and NR-FMT mice. Secondly, comparison of the taxonomic abundances within the human inocula vs colonization in the mouse recipients revealed possible important variations (Fig. 2d, second line, labeled “Source”). For example, Bacteroides uniformis was present at a low abundance in the human stool inocula for both the R and NR patients, but increased in relative abundance in the individual mice recipients (Fig. 2d, indicated with + sign). Others, such as OTU11382 (Blautia coccoides.Blautia producta), were undetected in the human donor samples, but detected at 0.00 to 0.47% abundance in the mice post-FMT (Fig. 2d, indicated with + sign, Table S1, column CN). Additional examples of bacteria that were discordant in abundance between the human inocula and subsequent murine colonization (i.e., low abundance in donor with high abundance in mouse and vice versa) are displayed in Table S2. Of interest, however, the relative abundance of shared genus and species-level OTUs between the human stools and colonized mice are correlated (Fig. 2b, C, lower graphs), suggesting that overall, human microbes that colonize mice largely maintain similar relative abundances within each host, particularly at the genus level.
Identification of experimental variables in the MC38 murine model
Given the variation in tumor response within each MC38 group of R-FMT or NR-FMT mice (Fig. 1) and having established the murine microbiome for individual mice (Fig. 2), we next examined each MC38 experiment in detail for additional factors that might affect tumor response, specifically experiment, cage effects, and age of the mice. Each individual experiment showed a significant anti-tumor response in anti-PD-L1 treated R-FMT mice but the degree of response varied across experiments (Fig. 3a). Further, the microbial composition of mice varied across experiments for both R-FMT and NR-FMT groups (Fig. 3b). While the majority of species/OTUs were present in all three experiments (consistent OTUs: R-FMT = 77; NR-FMT = 71), each contained or was lacking a unique subset of bacteria (Fig. 3b) and the variation across experiments is highlighted using principal coordinates analysis (PCoA, Fig. 3c). Further, the microbiome of mice within a single cage was more similar to mice in the same cage, with both overlap within and between experiments (Fig. 3d). Finally, with the complexity of breeding GF mice in sufficient number for these experiments, the age of the mice at the start of each experiment ranged from 6 to 15 weeks old. As the murine immune system typically matures over time [19, 20], we also examined whether the age of the mice impacted tumor response. Mice under 10 weeks of age responded to anti-PD-L1 treatment irrespective of FMT while mice over 10 weeks showed a greater effect with R-FMT (Fig. 3e). However, as these experiments were not designed to specifically test age, these results may be confounded by larger tumor growth seen in male mice less than 10 weeks of age (Fig. 3f). Therefore, we consider these exploratory data and further study of murine age and associated immune responses in this germ-free model is necessary before clear conclusions can be derived.
Fig. 3.
Multiple variables, including experiment, cage, and age, can affect anti-tumor responses in murine fecal microbiota transfer (FMT) in the MC38 syngeneic GF mouse model. (a) Individual MC38 tumor growth curves are shown for each experiment and condition (n = 3). ICI group is indicated by human responder (R) FMT or nonresponder (NR) FMT. Mean and SEM are shown. Statistical comparison performed across both time and group using two-way ANOVA: *** p < 0.001, **** p < 0.0001. (b) Species/OTU overlap from individual experiments. Number of reads: R = 8,143, NR = 15,000. (c) Principal coordinate analysis (PCoA) of species/OTU from each experiment. Dots represent cages within each experiment. (d) PCoA for each cage across experiments for R- and NR-FMT mice. (e) Final tumor volume for mice < 10 weeks old vs > 10 weeks old. N = 7–22 mice/condition. F. Final tumor volume data further subdivided by sex. N = 6–13 female mice/condition; 0–10 male mice/condition. Statistical comparisons were performed using Mann–Whitney U Test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Evaluation of bacteria associated with ICI response in the MC38 murine model
Empirically, we observed across all experiments that individual mice displayed variable rates of tumor growth or regression, regardless of whether inoculated with R- or NR-FMT. Therefore, we categorized each mouse based on individual MC38 tumor responses to anti-PDL1 treatment from day 10 to day 21/22 into two groups: murine tumor progressors (MT-P) with tumor growth > 25% and murine tumor nonprogressors (MT-NP) with tumor growth < 25% (see Methods, Figs. 1a, 3a). For R-FMT anti-PD-L1 treated mice, based on this criterion, 46% (13/28) were classified as MT-NP (Fig. 4a), and 5 OTUs were enriched in MT-NP mice (Fig. 4b). Enriched species included specific OTUs, such as Roseburia hominus, as well as two Citrobacter species (C. farmeri and C. amalonaticus) and two indeterminate OTUs in the genera Clostridium and Enterobacter. In contrast, 8 OTUs (from R-FMT) were enriched in the fecal microbiota of MT-P mice: C. braakii, two Blautia producta OTUs, two Clostridium OTUs (C. clostridioforme and C. symbiosum), Bacteroides uniformis, and two indeterminate OTUs for genera Anaerofilum and Enterococcus (Fig. 4b).
Fig. 4.
Species associated with MC38 tumor response in anti-PD-L1 treated R-FMT mice. C57BL/6 germ-free mice received human fecal microbiota transfers (FMT), followed by injection of MC38 tumor cells and treatment with the indicated isotype or anti-PD-L1 antibody. (a) R-FMT mice were categorized based on percent change in tumor volume: growth > 25% from day 10 were murine tumor progressors (MT-P, yellow, n = 15) and growth < 25% were murine tumor nonprogressors (MT-NP, blue, n = 13). (b) Bacterial species/OTUs enriched in MT-NP or MT-P. Statistical comparisons were performed using Mann–Whitney U Test: *p < 0.05, **p < 0.01. C. Correction for experimental variables (experiment, cage, and age) using a generalized linear model (GLM). OTUs above dotted line maintained significance after correction and are color coded based on direction of enrichment as shown in 4B. + indicates OTU label is truncated and full name is presented in Tables S3 and S4. Dotted line represents adjusted p < 0.05
Since we determined multiple variables affected the microbial composition across experiments in the MC38 model (Fig. 3), we applied a generalized linear model (GLM) to the significant OTUs determined using the Mann–Whitney U test (Fig. 4b) in MT-NP mice. In the GLM model, we included the factors we identified in Fig. 3 that impacted the results (i.e., experiment, cage, and age). In this adjusted GLM model, four species remained significant or gained significance (Fig. 4c). Unexpectedly, no bacteria associated with MT-NP mice in univariate analysis were confirmed in the GLM model. However, another Clostridium OTU (C. hathewayi) and Bacteroides massiliensis became enriched in MT-NP mice in the corrected GLM models. Two Clostridium OTUs remained significantly enriched in MT-P mice: C. clostridioforme and C. symbiosum. Full data for all OTUs are presented in Table S3 for R-FMT mice.
Subsequently, we applied the same strategy and analysis to anti-PD-L1-treated NR-FMT mice. While fewer mice responded with clearly diminishing tumor volumes, our categorization method was able to capture mice that also did not have tumor growth, similar to human patients with stable disease. For NR-FMT mice, 36% (11/30) were classified as MT-NP (Fig. 5a). In NR-FMT mice, 7 OTUs were enriched in MT-NP and 2 in MT-P mice by Mann–Whitney U test (Fig. 5b). Of these, 4 out of the 9 OTUs found to be significant in our univariate analysis maintained significance after correction in the GLM model, and 8 new OTUs gain significance after correction (Fig. 5c). Full data for all OTUs are presented in Table S4 for NR-FMT mice. Of note, there was no overlap in OTUs enriched in MT-NP or MT-P in R- and NR-FMT mice. We propose that this likely reflects the marked differences in the composition of the human R- and NR stools used for FMT (Fig. 2).
Fig. 5.
Species associated with MC38 tumor response in anti-PD-L1 treated NR-FMT mice. C57BL/6 germ-free mice received human fecal microbiota transfers (FMT), followed by injection of MC38 tumor cells and treatment with the indicated isotype or anti-PD-L1 antibody. (a) NR-FMT mice were categorized based on percent change in tumor volume: growth > 25% from day 10 were murine tumor progressors (MT-P, yellow, n = 15) and growth < 25% were murine tumor nonprogressors (MT-NP, blue, n = 13). (b) Bacterial species/OTUs enriched in MT-NP or MT-P. Statistical comparisons were performed using Mann–Whitney U Test: *p < 0.05, **p < 0.01. (c) Correction for experimental variables (experiment, cage, and age) using a generalized linear model (GLM). OTUs above dotted line maintained significance after correction and are color coded based on direction of enrichment as shown in 5B. + indicates OTU label is truncated and full name is presented in Tables S3 and S4. Dotted line represents adjusted p < 0.05
Gene expression analysis and validation of the TiME of MC38 tumors from R-FMT vs NR-FMT mice
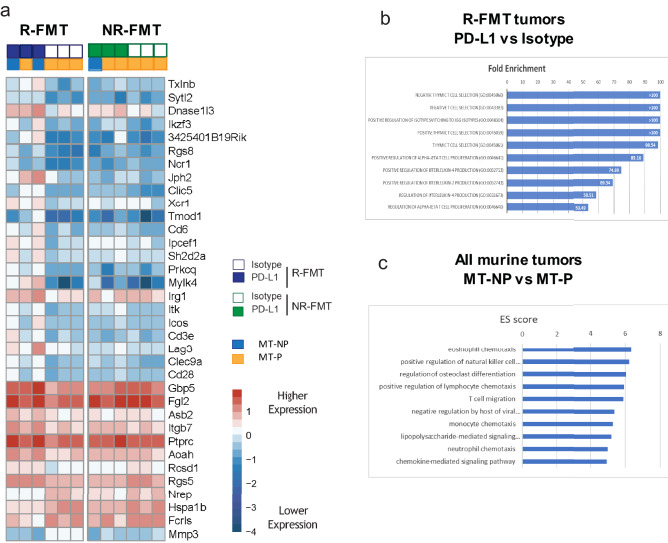
To begin to examine how the human R-FMT and NR-FMT microbes may impact immunotherapeutic responses, we performed RNAseq on tumors selected from CS062, in which we observed the most dramatic changes between the anti-PD-L1-treated mice inoculated with R-FMT vs NR-FMT (Fig. 3a). Raw sequencing data were normalized across all tumors (n = 12) and subsequently analyzed for differentially expressed genes (DEG), and our analysis was corrected for multiple hypothesis testing using false discovery rate (FDR; p < 0.05). Tumors in anti-PD-L1-treated R-FMT mice showed upregulation of multiple immune genes compared to isotype controls, but the same changes were not seen in tumors from NR-FMT mice (Fig. 6a, left panel vs right panel). Notable genes include Ikzf3 and Icos, both part of the inducible T cell co-stimulator (ICOS) pathway, as well as other checkpoint inhibitor genes such as Lymphocyte-activation gene 3 (Lag3). All DEGs corrected for multiple comparisons are presented in Table S5, with each comparison indicated in column A and the significant genes for that comparison shown in the same color block. Interestingly, several additional immune markers were upregulated in anti-PD-L1-treated R-FMT vs NR-FMT mice, including Lag3 and Il27 [interleukin (IL)-27] (Table S5, line 54 and line 63, respectively), which is also linked to the ICOS pathway and is part of a broad immune regulatory network [21]. Gene Ontology analysis on the DEGs from R-FMT mice (anti-PD-L1 vs isotype) revealed prominent upregulation of immune regulation genes and pathways, including T cell differentiation, stimulation, and proliferation and IL-2/IL-4 regulation (Fig. 6b shows top 10 pathways, Table S6 shows the full list of enriched pathways). The enrichment of T cell pathways was in agreement with immune profiling of CD45 + cells isolated from MC38 tumors collected from CS062 (n = 3 per group, n = 12 tumors total) by flow cytometry, which showed increased total and CD8 + T cell infiltration as well as more IFN-ɣ production in anti-PD-L1-treated R-FMT vs NR-FMT groups (Fig. S4). No significant pathways were found for the few DEGs in NR-FMT mice treated with anti-PD-L1 antibody (Table S5, lines 41–46). We also performed a similar analysis to compare DEGs in MT-NP vs. MT-P mice, irrespective of FMT donor, derived from Figs. 4a and 5a. Since far more DEGs were identified, we were able to use Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) analysis, which revealed significant enrichment in immune pathways including natural killer (NK) cell, lymphocyte, dendritic cell, and monocyte trafficking in responding tumors (Fig. 6c shows the top 10 pathways, Table S7 shows the full list of enriched pathways).
Fig. 6.
Murine MC38 tumors show upregulation of multiple immune pathways and other checkpoint inhibitors in mice receiving human responder fecal microbiota transfer (R-FMT). RNA was extracted from murine tumors and sequenced using an Illumina platform with normalization across all tumors (n = 12). (a) Significant differentially expressed genes (DEG) were identified using DESeq2 from R-FMT tumors treated with anti-PD-L1 vs isotype antibody. List shown in part A is derived from R-FMT mice but is also shown for all nonresponder (NR)-FMT mice. (b) Significant DEGs from R-FMT mice treated with anti-PD-L1 vs isotype antibody were analyzed by Gene Ontology (GO). Bars indicate fold enrichment for top 10 categories. (c) Significant DEGs from murine MT-NP vs MT = -P, independent of human FMT donor (n = 6), were analyzed by Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) and top 10 pathways are shown with their enrichment score (ES). Tables S5–7 display complementary data. Abbreviations: MT-P, murine tumor progressor; MT-NP, murine tumor nonprogressor
To validate the findings from our RNAseq analysis, we performed immunohistochemistry (IHC) on 36 tumors using antibodies for cytotoxic T cells (CD8), dendritic cells (DCs, CD11c), NK cells (KLRB1), and a stromal marker (smooth muscle actin). Quantification using the HALO image analysis platform confirmed that CD8 and NK cell infiltration, but not DC infiltration, aligned with MT-NP, but not MT-P, regardless of whether the inoculum was from R-FMT or NR-FMT (Fig. 7, row 1–3). Smooth muscle actin was also markedly increased in PD-L1-responding tumors, particularly in R-FMT tumors (Fig. 7, row 4), suggesting that the tumor stromal environment may also be modified by microbiome:immune interactions.
Fig. 7.
Murine tumors from anti-PD-L1 treated fecal microbiota transfer (human responder, R-FMT) mice showed increased CD8 + T cell and NK cell infiltration as well as increased smooth muscle actin. Representative images from each group are shown. Quantification for each stain is shown on the right of images. Mean and SEM are shown; n = 36 tumors across all groups. Statistical comparisons were performed using Mann–Whitney U Test: *p < 0.05, **p < 0.01. Antibodies: CD8α (CD8 + T cells), KLRB1 (NK cells), CD11c (dendritic cells), αSMA (smooth muscle actin). Abbreviations: MT-P, murine tumor progressor; MT-NP, murine tumor nonprogressor
Discussion
In this study, we performed human to mouse FMT in a GF mouse syngeneic tumor model to better define experimental variables in murine FMT models and to begin to identify, in these initial exploratory studies, microbes capable of influencing neoadjuvant ICI responses in humans. In this initial work, we chose to use pre-treatment stools from two patients with starkly differing outcomes to neoadjuvant ICI therapy. Compared to other studies, that mostly investigated the pre-treatment microbiome of individuals receiving ICI therapy for advanced cancer, the individuals herein were receiving neoadjuvant therapy as first-line treatment for resectable lung cancer. Hence, we hypothesized the patients we studied might provide novel insights compared to heavily pre-treated patients with potentially heterogeneous microbiomes after exposure to chemotherapy, other anti-cancer therapies, or the effects of metastatic disease. Further, potential predictors of response to combination anti-PD1/L1 with anti-CTLA-4, as opposed to anti-PD-L1 alone, are lacking in lung cancer. Here, as in other studies, FMT using human stools collected prior to ICI treatment largely reproduced therapeutic responses in heterotopic murine tumor models, as was observed in the original donors. These prior studies, however, failed to confirm that the species detected in the human donor stools and associated with ICI response actually colonized the mice and accounted for the reported ICI response of the mouse syngeneic tumor. Herein, we begin to address these gaps and further describe additional murine characteristics that affected murine tumor responses.
Our initial results suggested that use of MC38 cells, known to be more immunogenic and moderately responsive to ICI therapy, in our GF syngeneic mice present a more consistent and dynamic model (Fig. 1). Paradoxically, the mouse melanoma cell line, B16F10, is much less immunogenic than the mouse colon cancer cell line, MC38, whereas most human colon cancer is much less responsive to checkpoint inhibitors compared to melanoma. Interestingly, the MC38 tumor cell line is thought to have a high number of mutations, which may explain its immunogenicity in a similar manner to colorectal cancer with microsatellite instability, which is very responsive to checkpoint inhibitor therapy [22]. To first assess the species that colonized mice in this model post-FMT, we analyzed the stool composition of mice with MC38 tumors (Fig. 2). This cohort (n = 115 mice) is larger than those used to evaluate a pair of human fecal samples previously and addresses a knowledge gap regarding reproducibility in these murine models. Despite using similar inocula to perform R- and NR-FMT across experiments (Fig. 2a), the microbial composition of individual recipient mice varied between experiments with evident cage effects (Figs. 2d, 3b–d). Using both a defined minimum abundance and required percent presence in FMT mice as cutoffs, we found that the majority of genera were found in both human inocula and in the mice post-FMT (R-FMT: 55%; NR-FMT: 65%) (Fig. 2b). However, at the species level, this overlap was far less with only 33% and 36% of species shared between the human R stool and R-FMT mice and human NR stool and NR-FMT mice, respectively (Fig. 2c).
The percentages of human species that colonized the mice that we report are lower than those previously reported [13] with previous data suggesting that humans and conventional mice share 90% and 89% similarities in phyla and genera, respectively, but the abundance of specific microbes and ratio of Bacteroidetes:Firmicutes phyla differ [23]. Several factors could account for this discrepancy. First, we utilized a high-dimensional taxonomic assignment approach which is able to perform species-level identification at higher accuracy than previously available tools [18, 24]. Second, as in all microbiome studies, there are not standardized approaches to DNA extraction or sequencing methodology, and so technical differences and ongoing changes in technology development could contribute to the discrepancies. Finally, it is possible that in using frozen fecal samples, which is typical for human microbiome studies, more of our microbes simply lacked viability at the time of transfer. Thereby, the DNA of these microbes may have been detectable in the donor sample and inoculation slurry, but the microbe may not have been viable once introduced into the murine gut or not able to survive the acidic gastric environment in order to establish in the murine colon.
Notably, in our murine model, we observed that a few species grew better and even “bloomed” in the mice, in particular Streptococcus alactolyticus, and a Blautia coccoides.Blautia producta OTU (latter present in the mice but undetected in the donor samples) among others (Fig. 2d, indicated by + sign). As GF mice are checked by gram stain, culture, and qPCR to confirm sterility prior to inoculation, it seems likely these microbes were present at a very low concentrations in the human donor samples and were better able to propagate in the mouse colons, rather than being contaminants. These microbiome shifts have not been well described previously in murine heterotopic models assessing ICI efficacy, and thereby, could have been overlooked in interpreting results. While our study is limited in the number of human samples, which is certainly a caveat to broad interpretation and translation of these specific results to humans, we present these data in an effort to more closely scrutinize the microbiome shifts that occur when introducing human microbes into mice and to define experimental variables deserving attention in future experiments. Prior studies have focused on using FMT heterotopic models as a tool to define immunologic outcomes in tumors, but as our study indicates mice may not fully recapitulate the human microbial community. Based on these data, we suggest that these variables should be considered in the interpretation of future murine FMT studies.
Additionally, we were able to identify the murine variables that may impact ICI responses in heterotopic FMT models, contributing to inter-murine variability. While R-FMT led to a greater response to anti-PD-L1treatment in our GF syngeneic mouse models using both cell lines, we noted overall that efficacy was greater in female mice and the response with B16F10 cells occurred exclusively in females. While this difference has been described previously in conventional mice bearing murine microbiomes [25–27], therapeutic studies performed in mice typically are only reported in one sex, and if mixed cohorts are used details as to the mouse number of each sex used are often lacking. Males and females have, for example, differences in innate immunity, and retrospective data in human cohorts treated with checkpoint inhibitors suggest sex can impact clinical response in NSCLC and melanoma, although the underlying mechanisms are not known [28]. Sex can also influence baseline microbiomes in healthy and cancer patients [29], and age is another characteristic that may influence both ICI responses and microbiomes [30, 31]. Our data show that murine sex has a clear effect on the ICI tumor response, and the degree of response is also affected by tumor cell line (Fig. 1). We also present data to support experiment and cage effects on the microbiome profiles (Fig. 3), but our data on the impact of age are only exploratory as co-occurrence of other variables were confounding factors in that analysis. We suggest these data indicate that awareness of these variables and careful control of these specific factors, as well as mouse husbandry protocols, are likely necessary to obtain reproducible results. The impact of the sex of the human FMT donor on outcome is also unknown. In this paper, we used male donors given sample selection criteria and noted improved responses in female mice. The intersection of sex on human responses to checkpoint inhibitors is a topic for ongoing investigation.
To identify specific microbes, we took a novel approach by individually categorizing mice receiving R-FMT vs NR-FMT as to response (stable transplanted tumor size or regression) vs nonresponse (transplanted tumor growth) to anti-PDL1 treatment. In R-FMT mice using univariate analysis, we identified 5 OTUs enriched in mice with responding tumors and 8 OTUs in mice whose tumors progressed. However, upon adjustment in a GLM model for the variables that we identified as likely impacting our experiments (i.e., age, experiment, and cage), only 4 OTUS, including 3 Clostridia species (C. symbiosum, C. clostridioforme, and C. hathewayi), maintained or gained significance. Consistent with our results, both C. symbiosum and C. clostridioforme have been found enriched in nonresponder patients in patients with renal cell carcinoma [32, 33]. In contrast, our analysis of species associated with MC38 tumor response in anti-PDL1-treated NR-FMT mice identified greater concordance between the univariate and multivariate analyses. Overall, our finding that more species correlated with nonresponse than response is consistent with the findings of our recent meta-analysis in humans [24]. Nonetheless, neither our data nor prior data have provided a consistent and convincing group of bacteria as driving ICI response, either in humans or mice [24]. This strongly suggests that additional studies, both in humans and mice, are needed to understand how to predict when differing taxonomy yields similar biology, such as metabolism or host cell signaling, which may ultimately reflect functional redundancy in the microbiome.
Finally, in heterotopic murine tumor models studying ICI responses and the microbiome, there are limited data on the transcriptional changes associated with ICI responses in the tumors. Herein, the combination of mouse-colonizing microbes from the responder patient (R-FMT) with anti-PD-L1 treatment led to transcriptional changes involved in T cell differentiation, stimulation, and proliferation (Fig. 6). This is consistent with the known mechanism of anti-PD-L1 and was corroborated by our flow cytometry results (Fig. S4). These key changes did not occur with NR-FMT. Further, when we compared anti-PD-L1 treated tumors from mice receiving R-FMT vs NR-FMT, two key immune genes, Lag3 and Il27, were differentially expressed. LAG3 is an immune checkpoint regulator and expressed in a variety of immune cells, including effector T cells and Tregs, and several drugs targeting LAG3 are currently in clinical trials [34, 35]. IL27 is produced by antigen-presenting cells in response to TLR activation and modulates both myeloid cells and helper T cell activation [36]. Further, IL27 is thought to enhance immune-mediated anti-tumor responses through upregulation of Th1 and cytotoxic T cell responses [37]. We also found that a marker of NK cells (KLRB1) and myofibroblasts (smooth muscle actin) were enriched in responding tumors (Fig. 7), both findings are consistent with other studies [38, 39]. These data provide further evidence of how the gut microbiota, in the setting of anti-PD-L1 treatment, can result in transcriptional changes associated with T cell responses in tumors and provide evidence for a potential role for NK cells.
In summary, our murine studies illustrate the need to account for biological variables, including murine sex, when performing FMT using human fecal samples into GF mice in the quest to identify specific microbes enriched in tumor response vs nonresponse. We anticipate that control of the variables that impacted our experiments would also be important in specific pathogen-free mice in conventional mouse facilities. Our studies enabled us to begin to correlate FMT communities with transcriptional changes in the murine tumor that were further confirmed by flow cytometry and IHC, demonstrating the use of orthogonal experimental approaches can yield productive results. Key limitations of this work include the use of only two patients, although their fecal microbiota and physiologic impact were studied in some depth; relatively small sample sizes for some of the more detailed murine studies (e.g., RNAseq); and the use of a heterotopic tumor models, which is inherently limited in reproducing the TiME of an endogenous tumor model. Additional studies with more patient samples and additional endogenous tumor models are certainly needed to expand upon these results. Future studies focusing on defining whether FMT mouse models accurately recapitulate results in humans; identifying microbial community function in tumor response and nonresponse; and testing defined consortia, while considering both taxonomic composition and functional redundancy, are necessary to elucidate direct microbe:immune:tumor interactions and importance to response to ICI therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- FMT
Fecal microbiota transfer
- GF
Germ free
- ICI
Immune checkpoint inhibitor
- MT-P
Murine tumor progressors
- MT-NP
Murine tumor nonprogressors
- NR
Nonresponder
- NR-FMT
Nonresponder fecal microbiota transfer
- NSCLC
Non-small cell lung cancer
- OTU
Operational taxonomic unit
- PCoA
Principal coordinates analysis
- R
Responder
- R-FMT
Responder fecal microbiota transfer
- TiME
Tumor immune microenvironment
Author Contributions
FYS, JJG, FM, HD, JF, JRW, CMS, AT, RLB, JCD, and TCL were directly involved in data design, collection, and analysis. FYS, JJG, SG, FH, DMP, and CLS designed experiments and conceptual framework. JRW and FM performed analysis. JEC, JDS, JER, JN, and PMF contributed to the clinical trial design, implementation, data collection, and analysis. Manuscript was drafted by FYS, JJG, FM, and CLS. All authors reviewed and approved the final version of this manuscript.
Funding
This work was funded by the Bloomberg ~ Kimmel Institute for Cancer Immunotherapy (BKI) and a research grant from Bristol Myers Squibb. FYS was supported by NIH T32CA009071. JN was supported by International Association for the Study of Lung Cancer (IASLC), Lung Cancer Foundation of America, NCATS KL2TR001077, Johns Hopkins Institute for Clinical and Translational Research (ICTR). PMF and the clinical trial were supported by LUNGevity lung cancer foundation, ECOG-ACRIN, and IASLC. This work was supported by the Germ-free Murine Core, Flow Cytometry Technology Development Center, and Oncology Tissue services of Johns Hopkins University School of Medicine (P30 CA006973).
Availability of data and materials
The authors consent to sharing of data and materials. Sequencing data will be deposited with NCBI and made available for download.
Declarations
Conflict of interest
JRW reports equity ownership of Resphera Biosciences. JEC reports consultant fees for AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Flame Biosciences, Novartis, Regeneron, Guardant Health, and Jansen. JN reports research grants from Merck and AstraZeneca; consulting for AstraZeneca, Bristol-Myers Squibb, Takeda, Pfizer, Daiichi Sankyo, and Roche/Genentech; and honoraria from AstraZeneca and Bristol-Myers Squibb. PMF reports research grants from AstraZeneca, Bristol-Myers Squibb, Corvus, and Novartis. PMF reports consulting for Amgen, AstraZeneca, Bristol-Myers Squibb, Daiichi, Janssen, and Iteos. PMF reports serving as data safety monitoring board member for Flame Biosciences and Polaris. JEC reports consultant fees from AstraZeneca, Flame Biosciences, Genentech, Merck, Novartis, Jannsen. JDS has received consulting fees and honoraria from BMS, Merck, Roche, Amgen, AstraZeneca and Protalix Biotherapeutics; and he has received research grants from AstraZeneca, Merck, Roche and CLS therapeutics. JER has received advising/consulting fees from Oncocyte. DMP reports research support from AstraZeneca, Bristol Myers Squibb, and Compugen. DMP reports consulting for Aduro Biotech, Amgen, Astra Zeneca, Astellas, Bayer, Camden Partners, Compugen, DNAtrix, Dracen, Dynavax, Ervaxx, Five Prime Therapeutics, RAPT Therapeutics, Immunomic Therapeutics, Immunocore, Janssen, Merck, Potenza, Rock Springs Capital, Tizona, Trieza Therapeutics, Vaccitech, WindMil. DMP reports stock/ownership in Aduro Biotech, Dracen, Ervaxx, Five Prime Therapeutics, Tizona, Trieza Therapeutics, and WindMil. CLS reports research grants from Bristol-Myers Squibb and Janssen and personal fees from Ferring, outside the submitted work.
Consent to participate
All studies were approved by Johns Hopkins University Animal Care and Use Committee and Johns Hopkins Institutional Review Board.
Consent for publication
All authors have approved the following manuscript prior to submission.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fyza Y. Shaikh, Joell J. Gills and Fuad Mohammad contributed equally to this article.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 4.Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from keynote-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J, Horn L, Jackman D, et al (2017) Abstract CT077: Five-year follow-up from the CA209–003 study of nivolumab in previously treated advanced non-small cell lung cancer (NSCLC): Clinical characteristics of long-term survivors. In: Cancer Research. American Association for Cancer Research (AACR), pp CT077–CT077
- 6.MdABC HLPR, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMOA1910231. [DOI] [PubMed] [Google Scholar]
- 7.Ma S, Rm J, C F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMOA1716948. [DOI] [PubMed] [Google Scholar]
- 8.L P-A, TE C, M C,, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 9.LP A, A L, D V, et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell Lung Cancer. N Engl J Med 379:2040–2051. 10.1056/NEJMOA1810865 [DOI] [PubMed]
- 10.Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;80(371):602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 11.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;80(371):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaikh FY, Gills JJ, Sears CL. Impact of the microbiome on checkpoint inhibitor treatment in patients with non-small cell lung cancer and melanoma. EBioMedicine. 2019;48:642–647. doi: 10.1016/j.ebiom.2019.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg R, Toft MF, Metzdorff SB, et al. Human microbiota-transplanted C57BL/6 mice and offspring display reduced establishment of key bacteria and reduced immune stimulation compared to mouse microbiota-transplantation. Sci Rep. 2020;10:1–16. doi: 10.1038/s41598-020-64703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuss JE, Anagnostou V, Cottrell TR, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D S, AL G, D L, et al (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607–D613. 10.1093/NAR/GKY1131 [DOI] [PMC free article] [PubMed]
- 16.Routy B, Le Chatelier E, Derosa L, et al (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors [DOI] [PubMed]
- 17.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;80(359):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drewes JL, White JR, Dejea CM, et al. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. Npj Biofilms Microbiomes. 2017 doi: 10.1038/s41522-017-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson SJ, Andrews N, Ball D, et al. Does age matter? the impact of rodent age on study outcomes. Lab Anim. 2017;51:160–169. doi: 10.1177/0023677216653984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethna Z, Elhanati Y, Dudgeon CS, et al. Insights into immune system development and function from mouse T-cell repertoires. Proc Natl Acad Sci U S A. 2017;114:2253–2258. doi: 10.1073/pnas.1700241114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vignali DAA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722. doi: 10.1038/NI.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efremova M, Rieder D, Klepsch V, et al. (2018) Targeting immune checkpoints potentiates immunoediting and changes the dynamics of tumor evolution. Nat Commun. 2017;91(9):1–13. doi: 10.1038/s41467-017-02424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JC, Im SH. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med. 2020;52:1383–1396. doi: 10.1038/s12276-020-0473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaikh FY, White JR, Gills JJ, et al. A uniform computational approach improved on existing pipelines to reveal microbiome biomarkers of nonresponse to immune checkpoint inhibitors. Clin Cancer Res. 2021 doi: 10.1158/1078-0432.CCR-20-4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin P-Y, Sun L, Thibodeaux SR, et al. B7–H1–dependent sex-related differences in tumor immunity and immunotherapy responses. J Immunol. 2010;185:2747–2753. doi: 10.4049/jimmunol.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Jiang G, Jing N, et al. Downregulating testosterone levels enhance immunotherapy efficiency. Oncoimmunology. 2021 doi: 10.1080/2162402X.2021.1981570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty B, Byemerwa J, Shepherd J, et al. Inhibition of estrogen signaling in myeloid cells increases tumor immunity in melanoma. J Clin Invest. 2021 doi: 10.1172/JCI151347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19:737–746. doi: 10.1016/S1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 29.Byrd AL, Liu M, Fujimura KE, et al. Gut microbiome stability and dynamics in healthy donors and patients with non-gastrointestinal cancers. J Exp Med. 2020 doi: 10.1084/JEM.20200606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108:4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kugel CH, Douglass SM, Webster MR, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 2018;24:5347–5356. doi: 10.1158/1078-0432.CCR-18-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heshiki Y, Vazquez-Uribe R, Li J, et al. Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome. 2020;8:1–14. doi: 10.1186/s40168-020-00811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derosa L, Routy B, Fidelle M, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020;78:195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipson EJ, Tawbi HA-H, Schadendorf D, et al (2021) Relatlimab (RELA) plus nivolumab (NIVO) versus NIVO in first-line advanced melanoma: Primary phase III results from RELATIVITY-047 (CA224–047). 101200/JCO20213915_suppl9503 39:9503–9503. 10.1200/JCO.2021.39.15_SUPPL.9503
- 36.Kourko O, Seaver K, Odoardi N, et al. IL-27, IL-30, and IL-35: A Cytokine Triumvirate in Cancer. Front Oncol. 2019;9:969. doi: 10.3389/fonc.2019.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbi M, Carbotti G, Ferrini S. Dual roles of il-27 in cancer biology and immunotherapy. Mediators Inflamm. 2017;2017:1. doi: 10.1155/2017/3958069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galvani E, Mundra PA, Valpione S, et al. Stroma remodeling and reduced cell division define durable response to PD-1 blockade in melanoma. Nat Commun. 2020 doi: 10.1038/s41467-020-14632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu Q, Lin Y, Ma Y, et al (2021) Exploring the Emerging Role of the Gut Microbiota and Tumor Microenvironment in Cancer Immunotherapy. Front. Immunol. 11 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors consent to sharing of data and materials. Sequencing data will be deposited with NCBI and made available for download.