Abstract
Objective to implement Universal Neonatal Hearing Screening (UNHS) in a tertiary academic hospital and identify associated risk factors. Prospective study. Screening tests with Otoacoustic Emissions (OAE) were done among newborns, prior to hospital discharge. In babies who fail OAE twice, Brain Response Audiometry (BERA) was done, failing which they were referred to higher ENT center for repeat testing and hearing rehabilitation. A total 2323 babies were admitted in the neonatal unit during the study period. Only 773 babies (a third) could be screened for the first OAE, two thirds being lost to study right at inception!! Among the 773 neonates, in the “at risk” group of 301 neonates, 31(10%) and in the “not at risk” group of 472 neonates, 30 (6%) were lost to follow up respectively. The occurrence of hearing loss in this study population was 1.3 per 1000. Risk factors were noted in 38.9% of this subgroup with occurrence of hearing loss in “at risk” group being 3.32 per 1000. The implementation of UNHS in a developing country like India, has multiple challenges including infrastructural and non-compliance to follow up. In the meantime, the possibility of compromising ‘at-risk” neonates, who are significantly more prone to hearing loss, both neonatal and delayed onset, is an additional grave reality which needs deep considerationin this Herculean task of attaining “universality”.
Keywords: At risk neonates, Brain stem evoked response audiometry, Otoacoustic emissions, Lost to follow up, Universal neonatal hearing screening
Introduction
Hearing impairment is one of the most prevalent sensory deficits in India affecting approximately 5–6 infants in every 1000 neonates [1].According to the Rehabilitation Council of India 4 out of every 1000 live births have severe to profound hearing loss [2]. Though hearing screening was initially done only for the high-risk neonates, it was not representative as 50% of infants born with hearing loss have no known risk factors [3, 4].Hearing loss, if not detected at an early stage and treated can adversely affect the speech and language development of children [3, 5]. Hence Joint Committee on Infant Hearing (JCIH)in 2007 recommended Universal Neonatal Hearing Screening (UNHS), irrespective of risk factors; for early hearing loss detection and intervention with the 1-3-6 guidelines (diagnose hearing loss by 3 months of age, fitted with hearing aids within 1 month of diagnosis, and enroll in early intervention by 6 months of age), thus achieving best optimal age for hearing rehabilitation [6, 7]. Aprospective study from India, noted that the average delay between age of suspicion, confirmation and rehabilitation of congenital hearing loss was 19.59 months, 24.82 months and 29.28 months respectively. In majority of them (70.48%) hearing loss was suspected after 12 months of age [8].
Otoacoustic emissions (OAE) are fast, efficient, and frequency-specific measurements of peripheral auditory sensitivity [9]. Its effectiveness is reduced with ambient noise, vernix in ear canal, or middle ear pathology. Besides, they are insufficient for screening infants who are at risk for auditory neuropathy/dysynchrony, especially those in neonatal intensive care unit NICU [10].To overcome this, the other hearing screening test suggested is Automated Auditory Brainstem Response (AABR) which compares an infant's waveform with that of a template developed from normative Auditory Brainstem Response (ABR) or Brainstem Evoked Response Audiometry (BERA) on infant data. A ‘pass’ or ‘fail’ response is determined from this comparison for both these tests [11]. However, when compared to AABR, OAE have been shown to be highly cost effective [9]. The confirmatory test for hearing loss is BERA/ABR.
Though neonatal hearing screening was started in India by early 1970′s, Government of India launched the National Program for Prevention and Control of Deafness in 2006, aim being to identify babies with bilateral severe-profound hearing losses by 6 months of age and initiate rehabilitation by 9 months of age. In India, more than 60 districts established hearing screening program for newborns since 2006 [7, 12]. Under this program a two-part protocol for infant hearing screening was implemented, as 75% of the population live in rural areas and over 50% of births occur at home. The first protocol was for every baby born or admitted to hospital soon after birth an institution based OAE screeningto be done, preferably while admitted and if discharged prior first OAE appointment to be given at first immunization 6 weeks later, failing which re-tested after one month. If second time OAE fails, baby is referred for BERA (ABR) testing. The second protocol was for those not born in hospitals a community-based OAE screening to be done by trained health care worker when baby attends clinic for immunization at 6 weeks of age, failing this screening to be referred for formal OAE screening to the district hospital. If second OAE fails baby is referred for BERA (ABR) testing [12]. Though these protocols were in place, in 2011, Kumar and Mahapatra reported that only 38.09% of the medical institutions in India carried out newborn hearing screening [13].
In recent years, there has been reports from developed countries, especially USA, having reached the acceptable strategy around 98% of screening coverage in 2016 [14].However, with delays, loss to follow-up and documentation, failed Newborn Hearing Screening (NHS) has been reported globally, raising concern about failing the acceptable screening coverage of 95% for achieving UNHS [15–20].
Our study was thus undertaken at our institutional level in an attempt to implement Universal Neonatal Hearing Screening (UNHS) besides identifying the prevalence of hearing loss among neonates among both ‘at risk’ and ‘not at risk’.
Methods
This prospective study was conducted in newborn babies of the neonatal unit of the pediatric department in Pondicherry Institute of Medical Sciences from October 2016 to July 2018, following clearance by the Institutional Research Committee and Ethics Committee (REC/IEC:RC/16/103).The labor room and neonatology departments were contacted daily by the ENT department, newborns identified and noted by maintaining a Newborn Registry. All newborn babies delivered in this hospital and those that were admitted after delivery elsewhere were included in the study were planned to be screened prior to discharge or within three months after birth. Babies’ parents were informed regarding benefits of hearing screening, procedure of the screening test and need for follow-up. Consent was taken if willing for hearing screening.
Detailed report form including contact, maternal, perinatal and birth history were noted by the primary investigator. Prior to the screening, external auditory canal was checked to rule out debris. The first screening test of OAE was done in the ENT outpatient (in a quiet area), preferably prior to discharge. A two-step protocol OAE followed by BERA was used to screen the babies as per government protocol (Fig. 1). All babies were screened using LABAT Echolab OAE. Transient Evoked Otoacoustic Emissions (TEOAEs) was recorded using 80 ms click stimuli presented at 80 dBSPL. The responses were analyzed for 1000 Hz, 2000 Hz, 3000 Hz, 4000 Hz and 5000 Hz. A signal to noise ratio (SNR) of 6 dB of higher in any of the three above frequencies with overall reproducibility of 90% or more were considered ‘pass’. BERA testing was done in a sound proof room with baby was either in deep sleep or was given light sedation with pedichloryl after consulting with the pediatrician by Echolab Ep-X, using click stimuli presented at rated of 11.1/second and responses was recorded using one channel recording. If BERA revealed normal hearing threshold infant was discharged from the study while those who failed was referred to higher ENT center for hearing rehabilitation program.
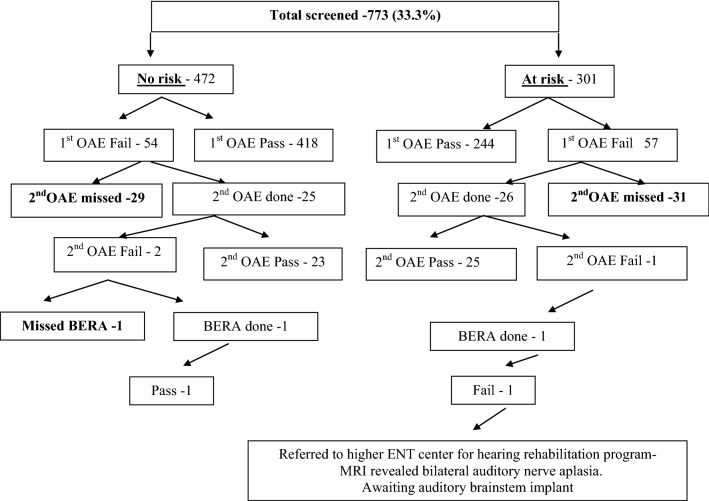
Fig. 1.
Flow chart representing neonates evaluated in the study
Results
There were 2323 newborn babies in neonatal unit during our study period. However, 1611 babies missed the testing which constitute 69% of the hospital neonates with the screening coverage being only 31%. The study was therefore conducted on 773 newborns. Among them 364 (47.1%) were females and 409 (52.9%) were males. No risk factors were noted in 472 neonates (61.06%) while 301(38.9%) had risk factors and they formed the two groups. Among the latter group 135 neonates (44.8%) had more than one risk factor. Table 1 show associated risk factors identified.
Table 1.
Risk factors encountered in the study
| Risk factors* | No. of newborns | Percentage |
|---|---|---|
| Premature delivery | 92 | 11.90 |
| Consanguineous marriage of parents | 88 | 11.38 |
| Exposure to ototoxic medications | 81 | 10.48 |
| Birth weight less than 2.5 | 73 | 9.44 |
| Hyperbilirubinemia | 63 | 8.1 |
| Delayed birth cry | 61 | 7.89 |
| Assisted ventilation | 53 | 6.86 |
| NICU stay for more than 5 days | 48 | 6.21 |
| Prenatal hypertension | 36 | 4.66 |
| Craniofacial anomaly | 19 | 2.5 |
On analyzing the neonates as the two groups, 54 (11.4%) and 57 (18.9%)who were of ‘notat risk’ and ‘at risk’failed first OAE respectively. Among these neonates30 (6%) and 31 (10%) of the former and latter group respectively missed their second OAE appointment. Follow up of the rest of the neonates revealed normal OAE in 432 neonates in the ‘not at risk’ group with one neonate in the ‘at risk group’ requiring BERA. This patient had profound sensorineural hearing loss and referral to higher center for rehabilitation (Fig. 1).
Discussion
Various barriers that prevent families from achieving the JCIH 1-3-6 benchmarks for Universal Neonatal hearing screening (UNHS)have been identified, more so in developing countries, though reported in developed countries as well. These include family or provider- related issues. A retrospective study from South Africa, reported that only 57.4% neonates had been screened. The most frequent reasons for screening refusal were related to costs (especially it being not included in birthing protocol or associated medical insurance), in addition to caregiver knowledge of UNHS. There were also provider related issues like health care professional knowledge and team collaboration [15]. Another retrospective study form Gujarat in India reported that from among the 2534 neonates who underwent DPOE, 26.9% from first to second screening and 26.6% from second screening to BERA (for confirmation) among high risk infants, were lost to follow up. The main reason for follow-up failure was both parental unwillingness for testing, long distance between house and hospital, change of address and financial constraints along with foregoing parental daily wages [16]. A recent longitudinal descriptive study fromThailand reported that among the total of 6234 live births, almost one quarter of infants was lost to follow-up at different stages of the study, exceeding the benchmark of 5% maximum set by the American Academy of Pediatrics.The main reasons given in this study by parents for failing to attend either a second screening or a diagnostic appointment included work constraints, a belief that their infants did not have a hearing problem or lack of transport [17]. A systematic review of follow up in new born hearing screening from high and upper middle-income countries published in 2016 reported overall ‘lost to follow up’ around 20.5%. Inadequate knowledge, distance and difficulties due to work constraints were the major reasons for failure for follow up [18].
The issue of loss to follow-up is a problem in developed countries as well. Cunningham et al. in the irretrospective study from US noted that 24% did not pass the newborn hearing screening completion by one month. They reported that low-income, rural, and minority infants are at risk for loss to follow up. Birth in a facility that charges a re-screening fee was associated with completion of follow-up screening [19]. In the rich Arabian Peninsula too, the performance indicators fell below the international benchmark of UNHS, the causative factors being related to communication, delays in the appointment system, and inefficient follow up tracking the data [20].
In our study too, among 2323 babies who were admitted, 1611 babies missed the screening test. This constituted 69% of study population, screening coverage being only31%. OAE instrument failure was a major concern as there was prolonged delay in repair and replacement of malfunctioning parts. Equipment failure which needed either as stand-by similar equipment or an alternative place for doing pending tests, were both not considered during procedure planning and was indeed a major constraint to the program! However, during the 10 months when OAE screener was available, it was noted that, among the 955 neonates admitted, only 773 babies were screened (81.95%) and 182 neonates (19.05%) failed to get OAE done. The main problems encountered for the first OAE screening being missed prior to discharge, in spite of equipment functioning were non-willingness for test, venue being away from the neonatal ward, lack of attendant and/or ward attender accompanying babies to test venue and unplanned discharges requested by parents. Considering the screening appointments being missed after discharge, the reasons were cost factor of travel to venue which is far away, loss of daily wages during visit or babies’ hearing appeared normal.
Among the 773 babies screened in our study, 472 (61.06%) had no risk factors and 301 (38.93%) had risk factors with 134 (44.8%) in the latter group having multiple risk factors. The one patient who was diagnosed to have profound hearing loss by BERA in our study was from the ‘at risk’ group. Common risk factors noted in our study (Table 1) was similar to other reports. They were premature delivery, hyperbilirubinemia, assisted ventilation and NICU more than five days admission [21–23], consanguineous marriage of parents [24], exposure to ototoxic medications and birth weight less than 2.5 kg [24–26]. Reports of prenatal hypertension [24] and craniofacial anomaly [22, 26] have also been noted similar to the present study. In their multivariable discrete-time survival analysis Butcher et al. in 2020 reported that risk was noted to be increased in those with neonatal illness, with or without admission to neonatal care, of Bangladeshi or Pakistani ethnicity or born to younger mothers [27].
In our study, one neonate among 773 screened who failed BERA was noted which indicates a prevalence of 1.3 per 1000 population. Considering the number who underwent the tests and missing data, occurrence of hearing loss in our study was 3.32 per 1000 in the “risk” group, while there was none in the “not at risk” group. In the recent past, there has been various publications globally confirming that the hearing loss is more prevalent in the ‘at risk’ neonates. To name a few, hearing loss in ‘not at risk’ versus ‘at risk” neonate was 0.69 versus 1.689 per 1000 [26] and 3.81 versus 26.67 per 1000 [28]. Moreover, the prevalence of hearing loss among high risk babies,confirmation by BERA revealed 8.8% per 1000 babies with hearing loss in a cross-sectional study conducted in tertiary care centerin India [29].Recently a systematic review with meta-analysis estimating prevalence of UNHS-detected permanent childhood hearing loss in very highly-developed countries showed prevalence was 1.1 per 1000 screened children with 6.9 times higher among those admitted to NICU [30].
It now appears that Neonatal Screening Program has two vital issues to be addressed:
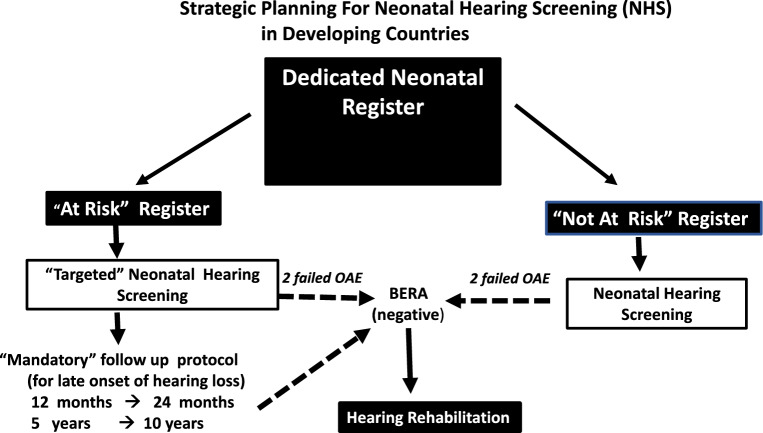
The cohort of ‘at risk’ neonates in addition to ‘not at risk’. Researchers in India have suggested “targeted newborn hearing screening” as a valuable option when resources are constrained [31]. Adedicated ‘Risk Register’ with a ‘Targeted Hearing Screening” protocol for ongoing scheduled hearing assessment appears to be essential (Fig. 2).
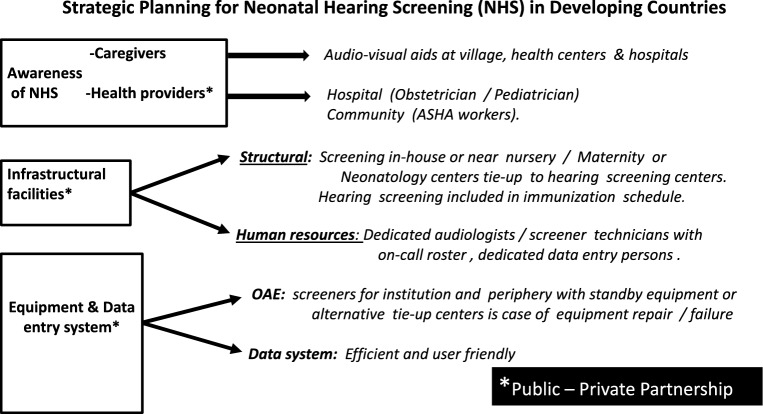
Delays and lost to follow up in Neonatal Hearing Screening. To overcome the above issue, the most commonly used strategy suggested by Ravi et al. following their systemic analysis of published studies was to improve parental education by distributing education material, increase communication between parents and medical professionals and the use of an adequate data management system with tracking system for storage of patient data [18]. In our country, considering available data confounded by ongoing financial constraints, a constructive approach towards Neonatal Hearing Screening is to be considered. The following strategic planning, within the Indian scenario of birthing at institutional or at community levels, within both “at risk and ‘not at risk’ neonatal cohorts, are being suggested in Fig. 2 and 3. Awareness for early diagnosis and implementation of hearing rehabilitation, among caregivers, though does not negate the cost of travel to test venue or loss of daily wages pay, does improve chance getting test being done!! Likewise would the increase in awareness among health providers, infrastructural facilities in terms of structural, human resources and equipment with a public–private partnership (Fig. 3).
Fig. 2.
Strategic planning for neonatal hearing screening in developing countries
Fig. 3.
Strategic planning for neonatal hearing screening (NHS) in developing countries
WHO recommends UNHS to be “cost-sensitive”, its benefits needing to outweigh the costs, considering the reality of low health spending in many developing countries. The cost-effectiveness of UNHS is indeed a “double-edged sword” [7].
Conclusion
Universal Neonatal/Newborn Hearing Screening (UNHS) is being adopted world-wide since its introduction in the US by early 90′s.Though hearing screening is mandatory in neonatal screening package, the high loss to follow-up rate and non-availability of funding is a threat to the overall success to neonatal hearing screening (NHS) programs in India. With recent overwhelming evidence that “at risk” neonates have significantly more chance of hearing loss at birth or later in childhood, necessitating ongoing audiological evaluation,‘targeted newborn hearing screening’ to this vulnerable cohort is to be initiated urgently in addition to implementing strategic planning of UNHS in our [31] financial constrain-ridden background. An advisory and leadership role of Otolaryngologists of India with public–private partnership of the stakeholders is the need of the hour in achieving the long-awaited goal of UNHS in India.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References:
- 1.Garg S, Singh R, Khurana D. Infant hearing screening in India: Current status and way forwards. Int J Prev Med. 2015;6:113. doi: 10.4103/2008-7802.170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kundu CL (2000). Status of Disability in India 2000 New Delhi: Rehabilitation Council of India, Ministry of Welfare, Government of India] In National Sample Survey Organization, Disabled Persons in India, NSS 58th Round. Report no. 485 (58/26/1), National Sample Survey Organization, Ministry of Statistics and Programme Implementation, Government of India, New Delhi 2003
- 3.DelaneyAM and Roland P.Newborn Hearing Screening: Overview, Prevalence of Hearing Loss, The High-Risk Register [Internet]. Emedicine.medscape.com. 2020 [cited 26 July 2016]. Available from: https://emedicine.medscape.com/article /836646-overview.
- 4.Sukumaran TU. Newborn hearing screening program Indian paediatrics. 2011;48(5):351. doi: 10.1007/s13312-011-0079-9. [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102(5):1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 6.Joint Committee on Infant Hearing Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 7.Newborn and infant hearing screening: current issues and guiding principles for action. Geneva: World Health Organization; 2010. Available from: https://www.who.int/blindness/publications/Newborn_and_Infant_Hearing_Screenig. Report.pdf [cited 2018 Jul 3].
- 8.Suresh K, Arif Ali K, Mary K. Challenges in the detection and intervention of childhood deafness: experience from a developing country. Int J Biomed Res. 2015;6(01):40–45. doi: 10.7439/ijbr.v6i1.1485. [DOI] [Google Scholar]
- 9.Gelfand S. Audiological screening chapter 13. In: Sydor A, editor. Essentials of Audiology. 4. New York: Thieme; 2016. pp. 353–354. [Google Scholar]
- 10.Jedrzejczak WW, Konopka W, Kochanek K, Skarzynski H. Otoacoustic emissions in newborns evoked by 0.5 kHz tone bursts. Int J PediatrOtorhinolaryngol. 2015;79(9):1522–1526. doi: 10.1016/j.ijporl.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Johnson LC, Toro M, Vishnja E, et al. Age and other factors affecting the outcome of aabr screening in neonates. HospPediatr. 2018;8(3):141–147. doi: 10.1542/hpeds.2017-0060. [DOI] [PubMed] [Google Scholar]
- 12.Directorate General of Health Services . National Programme for Prevention and Control of Deafness. Ministry of Health and Family Welfare, New Delhi: Project Proposal; 2006. [Google Scholar]
- 13.Kumar S, Mohapatra B. Status of a newborn hearing screening program in India. Int J PediatrOtorhinolaryngol. 2011;75(1):20–26. doi: 10.1016/j.ijporl.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Annual Data Early Hearing Detection and Intervention (EHDI) Program [internet]. Atlanta: Centers for Disease Control and Prevention; 2016. Available from: https://www.cdc.gov/ncbddd/hearingloss/ehdi-data2016.html [cited 2019 Jul 3].
- 15.Scheepers L, Swanepoel D, Roux T. why parents refuse newborn hearing screening and default on follow-up rescreening—a south African perspective. Int J PediatrOtorhinolaryngol. 2014;78(4):652–658. doi: 10.1016/j.ijporl.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Hansashree YS, Bhatt SH, Nimbalkar S, Mishra G. Non-compliance with neonatal hearing screening follow-up in rural western India. Indian Pediatr. 2018;55(6):482–484. doi: 10.1007/s13312-018-1338-9. [DOI] [PubMed] [Google Scholar]
- 17.Pitathawatchai P, Khaimook W, Kirtsreesakul V. Pilot implementation of newborn hearing screening programme at four hospitals in southern Thailand. Bull World Health Organ. 2019;97(10):663–671. doi: 10.2471/BLT.18.220939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravi R, Gunjawate D, Yerraguntla K, Lewis L, Driscoll C, Rajashekhar B. Follow-up in newborn hearing screening – a systematic review. Int J PediatrOtorhinolaryngol. 2016;90:29–36. doi: 10.1016/j.ijporl.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham M, Thomson V, McKiever E, Dickinson LM, Furniss A, Allison MA. Infant, maternal and hospital factors’ role in loss to follow-up after failed newborn hearing screening. AcadPediatr. 2018;18(2):188–195. doi: 10.1016/j.acap.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Kolethekkat AA, Al Abri R, HIaiwah O, AI Harasi, Sulaiman AA, AI Bahlani H, AI Jaradi M, Mathew J. Limitations and drawbacks of the hospital-based universal neonatal hearing screening program: First report from the Arabian Peninsula and insights. Int J PediatrOtorhinolaryngol. 2020, 132:109926. 10.1016/j.ijporl.2020.109926 [DOI] [PubMed]
- 21.Kumar P, Adhisivam B, Bhat V, Bharathi B, Francis F, Mondal N. Screening for hearing loss among high risk neonates– experience from a tertiary care center. CurrPediatr Res. 2016;20(1&2):43–46. [Google Scholar]
- 22.Wroblewska-Seniuk K, Greczka G, Dabrowski P, Szyfter-Harris J, Mazela J. Hearing impairment in premature newborns—analysis based on the national hearing screening database in Poland. PLoS ONE. 2017 doi: 10.1371/journal.pone.0184359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair VS, Das P, Soundararajan P. Prevalence and risk factors of hearing impairment among neonates admitted in NICU in a tertiary care center in South India. Int J ContempPediatr. 2018;5(4):1342–1347. [Google Scholar]
- 24.Yilmazer R, ZahideYazici M, Erdim I, Kaya H, OzcanDalbudak S, Kayhan T. Follow-up results of newborns after hearing screening at a training and research hospital in Turkey. J Intl AdvOtol. 2016;12(1):55–60. doi: 10.5152/iao.2015.1736. [DOI] [PubMed] [Google Scholar]
- 25.Nair M, Girish S, Nair SS, Sameer P. Risk factors and prevalence of hearing impairment among neonates in a South Indian Tertiary Neonatal Centre. Journal of Medical Science and Clinical Research. 2018;6(02):665–675. doi: 10.18535/jmscr/v6i2.103. [DOI] [Google Scholar]
- 26.Parab SR, Khan MM, Kulkarni S, Ghaisas V, Kulkarni P. Neonatal screening for prevalance of hearing impairment in rural areas. Indian J Otolaryngol Head Neck Surg. 2018;70(3):380–386. doi: 10.1007/s12070-018-1386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butcher E, Dezateux C, Knowles R. Risk factors for permanent childhood hearing impairment. Arch Dis Child. 2020;105(2):187–189. doi: 10.1136/archdischild-2018-315866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Gupta SC, Sinha VR. Universal hearing screening in newborns using otoacoustic emissions and brainstem evoked response in Eastern Uttar Pradesh. Indian J Otolaryngol Head Neck Surg. 2017;69(3):296–299. doi: 10.1007/s12070-017-1081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachdeva K, Sao T. Outcomes of newborn hearing screening program: a hospital based study. Indian J Otolaryngol Head Neck Surg. 2017;69(2):194–198. doi: 10.1007/s12070-017-1062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butcher E, Dezateux C, Cortina-Borja M, Knowles R. Prevalence of permanent childhood hearing impairment identified by universal newborn hearing screening: a systematic review and meta-analysis. Revue d'Épidémiologieet de Santé Publique. 2018;66:S313. doi: 10.1016/j.respe.2018.05.200. [DOI] [Google Scholar]
- 31.Mehar V, Somani P, Singh K, Munjal VR. Targeted hearing screening in newborns. Int J ContempPediatrics. 2015;3(1):159–163. [Google Scholar]