Abstract
Background
Although naloxone prevents opioid overdose deaths, few patients prescribed opioids receive naloxone, limiting its effectiveness in real-world settings. Barriers to naloxone prescribing include concerns that naloxone could increase risk behavior and limited time to provide necessary patient education.
Objective
To determine whether pharmacy-based naloxone co-dispensing affected opioid risk behavior. Secondary objectives were to assess if co-dispensing increased naloxone acquisition, increased patient knowledge about naloxone administration, and affected opioid dose and other substance use.
Design
Cluster randomized pragmatic trial of naloxone co-dispensing.
Setting
Safety-net health system in Denver, Colorado, between 2017 and 2020.
Participants
Seven pharmacies were randomized. Pharmacy patients (N=768) receiving opioids were followed using automated data for 10 months. Pharmacy patients were also invited to complete surveys at baseline, 4 months, and 8 months; 325 survey participants were enrolled from November 15, 2017, to January 8, 2019.
Intervention
Intervention pharmacies implemented workflows to co-dispense naloxone while usual care pharmacies provided usual services.
Main Measures
Survey instruments assessed opioid risk behavior; hazardous drinking; tobacco, cannabis, and other drug use; and knowledge. Naloxone dispensings and opioid dose were evaluated using pharmacy data among pharmacy patients and survey participants. Intention-to-treat analyses were conducted using generalized linear mixed models accounting for clustering at the pharmacy level.
Key Results
Opioid risk behavior did not differ by trial group (P=0.52; 8-month vs. baseline adjusted risk ratio [ARR] 1.07; 95% CI 0.78, 1.47). Compared with usual care pharmacies, naloxone dispensings were higher in intervention pharmacies (ARR 3.38; 95% CI 2.21, 5.15) and participant knowledge increased (P=0.02; 8-month vs. baseline adjusted mean difference 1.05; 95% CI 0.06, 2.04). There was no difference in other substance use by the trial group.
Conclusion
Co-dispensing naloxone with opioids effectively increased naloxone receipt and knowledge but did not increase self-reported risk behavior.
Trial Registration
Registered at ClinicalTrials.gov; Identifier: NCT03337100
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-07356-6.
KEY WORDS: naloxone, prescription opioids, risk compensation, overdose, substance use
INTRODUCTION
Naloxone prevents overdose fatalities by reversing opioid-induced respiratory depression. Expanding access to naloxone has been an important strategy to address the opioid overdose crisis. However, despite recommendations by national organizations and legislative efforts to expand access,1–3 naloxone uptake among patients prescribed opioids has remained low.4–8 In addition, as overdose deaths related to fentanyl and other synthetic opioids increased exponentially from 2013 to 2019, fentanyl co-involvement in prescription opioid-related deaths also became increasingly common,9 thus emphasizing the need for interventions to increase naloxone uptake among patients prescribed opioids.
Barriers to naloxone prescribing include identifying appropriate candidates and the time required to educate patients on overdose risk and naloxone administration.10–13 Pharmacists can address these barriers by using standing orders to identify patients prescribed opioids, co-dispense naloxone without individual prescriptions, and educate patients.14 However, naloxone access may still be limited if physicians and pharmacists have concerns about risk compensation,10,15,16 the concept that possessing naloxone encourages behaviors that increase overdose risk, such as taking more opioids than prescribed or using them with alcohol. In addition, patients may hesitate to accept naloxone if they think this action represents an admission of risk behavior.17,18 While prior studies have shown that risk behavior is reduced following overdose education and naloxone receipt,19,20 these studies were observational.
To address barriers to naloxone acquisition by patients prescribed opioids, we conducted a pharmacy-randomized trial to determine whether naloxone co-dispensing with opioids would affect patient risk behavior, naloxone dispensings, opioid dose, and patient knowledge about opioid overdose prevention and naloxone. The hypotheses were that co-dispensing would increase naloxone dispensings and knowledge and could either increase or decrease risk behavior and opioid dose.
METHODS
Study Design
The naloxone co-dispensing trial was a cluster randomized pragmatic trial21 in which the co-dispensing intervention was implemented at the pharmacy level with patients nested within pharmacies. Study outcomes were assessed among pharmacy patients and the subset of patients who responded to surveys (participants). Ethical approval was granted by the Kaiser Permanente Colorado Institutional Review Board, to which the Colorado Multiple Institutional Review Board ceded (Protocol). Trial reporting followed the Consolidated Standards of Reporting Trials guideline. An independent data and safety monitoring board periodically reviewed the trial’s conduct. The trial was registered with ClinicalTrials.gov as “The Impact of Co-Dispensing Naloxone to Patients Prescribed Chronic Opioid Therapy” (NCT03337100).
Study pharmacies were based at Denver Health (DH), a safety-net healthcare system in Colorado, USA, that provides primary care, addiction treatment, paramedic, urgent care, emergency, and hospital services.
Randomization and Masking
DH pharmacies were eligible if they stocked naloxone for outpatient dispensing, had a naloxone standing order but had not yet implemented co-dispensing, and their leaders agreed to randomization. For feasibility, the study biostatistician (S.X.) randomized similarly sized pharmacies in 3 waves between October 2017 and April 2018 using computerized block randomization (SAS STAT PROC PLAN procedure, version 9.4, SAS Institute Inc). Pharmacies within each wave were randomized to early co-dispensing (hereafter called the intervention) or co-dispensing after 10 months of usual pharmacy services (hereafter called usual care). This design effectively allowed the intervention to be compared to usual care over 10 months. Eligible pharmacy patients receiving opioids were recruited to complete surveys before co-dispensing began in intervention pharmacies in each wave. After implementing, intervention pharmacies continued co-dispensing for at least 10 months while usual care pharmacies provided usual pharmacy services. In all pharmacies, patients could request naloxone under a standing order or be prescribed naloxone. Naloxone was routinely prescribed in Opioid Safety Clinic visits,22 opioid use disorder treatment, or the emergency department to address the risk of repeat overdoses. In both trial groups, insured patients paid for naloxone according to their pharmacy benefit plan and deductibles, whereas uninsured patients could obtain naloxone at no or low cost through indigent care or financial assistance programs. Patient costs generally ranged from $0 to $80 and up to $3 with Medicaid. Study investigators were blinded to patients’ and participants’ trial groups until the end of follow-up.
Study Population
The study population included patients receiving opioids from randomized pharmacies and a subset of patients who agreed to surveys. Pharmacy patients were eligible if they received a extended-release, long-acting (ER/LA) opioid or ≥84 opioid pills, excluding buprenorphine and tramadol, from a randomized pharmacy in the prior 60 days; were English-speaking; aged 18 years or older; and had at least one DH primary care visit in the prior 18 months. Patients with a do-not-resuscitate order or enrolled in hospice care were excluded. Eligible patients were followed for outcomes included in DH’s electronic health records, linked paramedic records, and linked state vital records. Due to minimal risk procedures, eligible patients were followed using automated and linked records with a waiver of consent. All eligible patients were recruited to participate in surveys by email, phone, mail, and in person. Participants received $20, $25, and $30 gift cards for baseline, 4-month, and 8-month surveys, respectively. Baseline surveys were administered by mail, emailed web link, telephone, or in person, and follow-up surveys were administered by mail, emailed web link, or telephone. Research Electronic Data Capture (REDCap)23,24 was used for survey data capture and management. To maximize disclosure of sensitive behaviors, we used self-administered survey instruments25 and reassured participants that their responses were confidential, would not be shared with their doctors, and would not affect their health care, insurance, or access to opioid pain medicines. When reporting results, we distinguish patients followed using automated or linked records from the subset who provided consent for surveys (i.e., participants). In each wave, survey follow-up was complete before usual care pharmacies began co-dispensing.
Co-Dispensing Intervention Procedures
The pharmacy-level naloxone co-dispensing intervention was designed to offer and dispense naloxone to patients receiving chronic opioid therapy,26 but, first, pharmacy staff needed efficient screening criteria. Therefore, the study team and operational leaders developed pragmatic co-dispensing criteria of an opioid dispensing of ≥84 pills (≥3 pills per day per 28-day prescription) or any (ER/LA) opioid pill or patch, whether a first-time prescription or a refill. Tramadol was excluded because of its low risk of respiratory depression.27Buprenorphine-containing products were excluded because, when the study was conducted, opioid use disorder treatment providers had distinct naloxone protocols, and buprenorphine was rarely used for pain.
Pharmacists in intervention pharmacies received training on workflow, presenting naloxone to patients, and answering common questions (see Appendix). Under a standing order, pharmacy staff offered naloxone to any patient requesting an eligible opioid fill. If the patient accepted naloxone, pharmacy staff collected payment, dispensed naloxone, counseled patients on naloxone administration,14 and provided written educational material on overdose prevention and naloxone in English and Spanish.
Outcomes
The primary trial outcome was self-reported opioid risk behavior, assessed using the Opioid-Related Behaviours In Treatment (ORBIT) instrument and measured among participants. The ORBIT is a self-administered 10-item scale that was psychometrically validated among 426 people prescribed long-term opioid therapy or who had opioid use disorder.28 The instrument measures clinically meaningful behaviors (e.g., using opioids for purposes other than pain and obtaining them from others), which providers identified as behaviors that could be exacerbated if naloxone were provided to patients.10 While the ORBIT has clinician face validity, its low respondent burden, focus on recent risk behavior, and ability to quantify change over time make it useful for research.28,29 Endorsing one or more opioid risk behaviors, presented on a 5-point Likert scale, was considered positive.30 The ORBIT was modified to refer to the past 4 months and administered at baseline, 4 months, and 8 months.
Secondary risk behavior outcomes included cannabis, non-medical sedative, and other drugs (heroin, cocaine, methamphetamine, hallucinogens, inhalants, and other drugs) use based on survey items derived from the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST), which has been evaluated for concurrent, construct, and discriminative validity.31,32 Tobacco use was assessed using an item from the WHO ASSIST V3.0.33 Endorsing any use of a substance was positive for that substance. Hazardous drinking was assessed using the Alcohol Use Disorders Identification Test – Concise (AUDIT-C), with higher scores shown to be associated with higher mortality.34 In men, a score of ≥4 out of 12, and in women, a score of ≥3 were positive.35 Knowledge was assessed using the Prescription Opioid Overdose Knowledge Scale (Rx-OOKS), a psychometrically validated scale with a higher score representing greater knowledge.36 Missing Rx-OOKS item responses received a score of zero.
Other secondary outcomes measured in patients and participants included naloxone dispensings and opioid dose, ascertained using automated pharmacy data. Naloxone dispensings were assessed over the 10-month follow-up period, and the opioid dose was evaluated at baseline and 10 months. Participants also reported if they or their household members acquired naloxone and could indicate why they did not pick up naloxone in surveys. Before launching the study, surveys were cognitively tested with 11 patients and administered to 97 patients in 2 pilot studies.
Other outcomes measured in patients and participants included opioid overdoses, deaths, and, when available, urine drug screen positive for cocaine, heroin, or methamphetamines. Opioid overdoses were identified using the International Classification of Diseases (ICD)-10 CM codes from DH emergency department and hospital records and Denver county paramedic records. Survey participants could also report experiencing an opioid overdose. Finally, identifiers for all patients were linked to the Colorado Department of Public Health and the Environment vital records to identify deaths and causes of death. When available, medical records were reviewed to confirm opioid overdoses and the cause of death.
Race, ethnicity, education, income, and housing status were reported in surveys using questions derived from the Behavioral Risk Factor Surveillance System 2014.37 Other patient baseline characteristics were identified in electronic health records; clinical characteristics, such as an opioid use disorder diagnosis, were identified using ICD-10 codes.
Statistical Analysis
At a significance level of 0.05 and an intra-class correlation (ICC) of patients within pharmacies of 0.01, pre-specified power calculations indicated we could detect differences in ORBIT scores of 1.10 with a sample size of 300 and a SD of 2.8. However, ORBIT scores were skewed, with more than half of participants reporting no risk behavior at baseline and a mean score of 1.3 (SD=1.9) on a scale of 0 to 40. Since all ORBIT questions are clinically meaningful,28 we elected to dichotomize the ORBIT score as endorsing ≥1 opioid risk behavior vs. endorsing 0 behaviors.30 Revised power calculations indicated that with a sample size of 300, an ICC of 0.01, and the proportion endorsing ≥1 opioid risk behavior of 0.5, the study had 80% power to detect a minimum risk ratio of 1.4. Based on these parameters, the Cohen’s h was 0.32, suggesting that the study could detect a small to moderate effect size.38,39 The study was not powered to detect differences in opioid overdoses or deaths.
Baseline patient characteristics were presented by survey participation, and participant characteristics were reported separately by the trial group.
We used generalized linear mixed (GLM) models to assess differences in study outcomes between trial groups over time. Among participants, we analyzed the change in the proportion endorsing ≥1 opioid risk behavior and secondary substance use outcomes between trial groups with a binary distribution and log link function. Each model included the trial group, the survey time point, an interaction between the trial group and the survey time point, and the study wave. Covariates associated with baseline behavior at a significance level of 0.1 were considered potential confounders and included in the final models. Random effects accounted for pharmacy-level clustering and repeated measures. Adjusted risk ratios (ARR) and 95% CIs comparing outcomes between trial groups at each time point are reported. Unadjusted risk ratios and 95% CIs are reported at each time point for the primary outcome. If the interaction term was significant at alpha=0.1, we report differences and 95% CI between trial groups over time. The Rx-OOKS score was modeled as a continuous outcome, using a normal distribution and identity link function.
To account for missing survey data (e.g., 3–19% item or unit non-response for baseline and follow-up ORBIT surveys), multiple imputations were performed by replacing missing survey responses with a set of plausible values using the fully conditional specification method. The multiple imputation model included all variables considered in the analyses and the auxiliary variables potentially associated with missing data. Data were imputed 20 times, and imputation results were then combined.40
Receipt of at least one naloxone prescription was analyzed as a dichotomous outcome using a binary distribution and log link in separate models for patients and survey participants. Models included trial group, study wave, baseline covariates that had a bivariate association with the outcome (significance level of 0.1), and random effects for pharmacy. The analyses of opioid overdoses and deaths were similar to naloxone dispensings. Due to small numbers of opioid overdoses, propensity scores were used to address potential confounding in the patient population. Opioid dose over time was modeled using a log-normal distribution and identity link function.
All outcomes were analyzed by the intention-to-treat principle. A 5% significance level using 2-sided tests was applied in all analyses, and data were analyzed using SAS® Studio Software version 3.8 (SAS Institute Inc., Cary, NC).
Sensitivity Analyses
We conducted multiple sensitivity analyses for opioid risk behavior and naloxone dispensings. In addition, we conducted a sensitivity analysis combining urine drug screen results with self-reported drug use and a post hoc analysis examining the association between insurance and naloxone receipt. Details are included in the Appendix.
RESULTS
Randomized Pharmacies and Study Participants
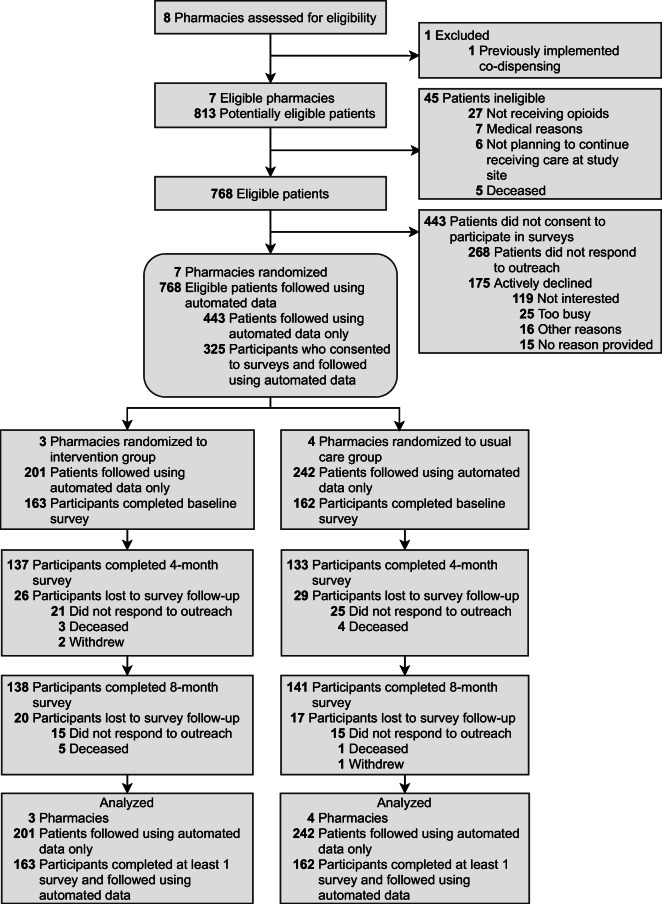
Seven pharmacies were eligible for randomization; 1 was ineligible because it was already co-dispensing(Fig. 1). Three pharmacies were randomized to the intervention and 4 to usual care (eTable 1). Within randomized pharmacies, 325 of 768 eligible patients (42.3%) were enrolled between November 15, 2017, and January 8, 2019, to participate in surveys. Nearly half of participants reported Hispanic, Latino/a, or Spanish origin (n=149; 45.8%), a quarter (n=80, 24.6%) had less than high school education, and over a third (n=123; 37.8%) had a household income less than $10,000. Demographic characteristics, insurance, clinical characteristics, opioid use disorder, prior naloxone receipt, and opioid dose were similar between survey participants (n=325) and non-participants(n=443; eTable 2). Compared to participants in usual care pharmacies, participants in intervention pharmacies were younger, less likely to have previously received naloxone, and more likely to be male, white, and have a prior opioid use disorder diagnosis (Table 1). Four- and 8-month surveys were completed by 84.9% (270/318) and 89.4% (279/312) of participants alive at each follow-up time point, respectively (Fig. 1); participants who completed both follow-up surveys (n=252) were similar to those who did not (n=73), except for income, insurance, and prior naloxone receipt (eTable 3).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of screening, enrollment, and follow-up of patients and survey participants
Table 1.
Baseline Demographic and Clinical Characteristics of Survey Participants by Trial Group
|
Participants N(%) |
||
|---|---|---|
| Characteristic |
Intervention pharmacies (n=163) |
Usual care pharmacies (n=162) |
| Age, mean (SD), y | 56.3 (10.9) | 59.8 (9.9) |
| Female | 77 (47.2) | 97 (59.9) |
| Race/ethnicity* | ||
| Hispanic | 58 (35.6) | 91 (56.2) |
| White, non-Hispanic | 61 (37.4) | 40 (24.7) |
| Black, non-Hispanic | 34 (20.9) | 20 (12.4) |
| All other racial and ethnic groups | 8 (4.9) | 6 (3.7) |
| Missing | 2 (1.2) | 5 (3.1) |
| Education* | ||
| Less than high school | 32 (19.6) | 48 (29.6) |
| Completed high school | 50 (30.7) | 45 (27.8) |
| Attended some college | 46 (28.2) | 48 (29.6) |
| Completed college or a higher degree | 27 (16.6) | 18 (11.1) |
| Missing | 8 (4.9) | 3 (1.9) |
| Annual household income, US dollars* | ||
| Less than 10,000 | 57 (35.0) | 66 (40.7) |
| 10,000 – 19,999 | 51 (31.3) | 57 (35.2) |
| 20,000 or more | 37 (22.7) | 21 (13.0) |
| Missing | 18 (11.0) | 18 (11.1) |
| Live in their own house/apartment* | 127 (77.9) | 120 (74.1) |
| Insurance | ||
| Commercial | 25 (15.3) | 23 (14.2) |
| Medicaid | 81 (49.7) | 73 (45.1) |
| Medicare | 39 (23.9) | 47 (29.0) |
| Uninsured | 18 (11.0) | 19 (11.7) |
| Modified Charlson comorbidity index, median (IQR)† | 2.0 (1.0–4.0) | 1.0 (0–3.0) |
| Opioid use disorder† | 40 (24.5) | 25 (15.4) |
| Alcohol use disorder† | 7 (4.3) | 12 (7.4) |
| Tobacco use or nicotine use disorder† | 71 (43.6) | 83 (51.2) |
| Prior naloxone receipt‡ | 27 (16.6) | 51 (31.5) |
| Average daily opioid dose, median morphine milligram equivalents (IQR)§ | 29.7 (17.0–55.8) | 35.7 (18.9–59.2) |
| Any opioid risk behavior (ORBIT)* | 83/162 (51.2) | 72/154 (46.8) |
| Hazardous drinking (AUDIT-C)* | 13/160 (8.1) | 14/157 (8.9) |
| Reported tobacco use* | 83/161 (51.6) | 83/162 (51.2) |
| Substance use (ASSIST)* | ||
| Cannabis | 69/162 (42.6) | 54/162 (33.3) |
| Other drugs|| | 3/163 (1.8) | 5/162 (3.1) |
| Non-medical sedatives | 30/160 (18.8) | 29/162 (17.9) |
| Knowledge (Rx-OOKS), mean (SD)* | 11.5 (4.7) | 11.3 (4.1) |
Abbreviations: ORBITopioid-related behaviors in treatment modified to refer to past 4 months; AUDIT-C Alcohol Use Disorders Identification Test - Concise; NIDA-modified ASSIST National Institute on Drug Abuse–modified Alcohol, Smoking and Substance Involvement Screening Test modified to refer to past 4 months; Rx-OOKS Prescription Opioid Overdose Knowledge Scale
* Assessed using survey measures; when denominators are indicated, the denominator is those who completed that baseline measure; Ethnicity was reported as “Hispanic, Latino/a, or Spanish origin”
† Assessed in the year prior to study enrollment
‡ Assessed using survey measure (past 4 months) and dispensing (past year)
§ Assessed in the 6 months prior to study enrollment
|| Other drugs include heroin, other illicit opioids, cocaine, methamphetamine, inhalants, and hallucinogens
Primary Outcomes
Compared with baseline, significantly fewer participants endorsed opioid risk behavior at 4 and 8 months in intervention pharmacies and at 8 months in usual care pharmacies (eFigure 1). Changes in opioid risk behavior over time were not significantly different by the trial group in unadjusted (eTable 4), adjusted (Table 2, eTable 5), and sensitivity (eTable 6) analyses.
Table 2.
Risk Behavior Among Survey Participants over Time by Trial Group
| Risk behavior | Crude proportion endorsing risk behavior (95% CI) | Adjusted risk ratio (95% CI) | Time x intervention P value | |
|---|---|---|---|---|
| Intervention pharmacies (n=163) | Usual care pharmacies (n=162) | |||
| Opioid risk behavior* | ||||
| Baseline | 51.1 (43.4, 58.8) | 46.9 (39.0, 54.8) | 1.07 (0.86, 1.34) | 0.52 |
| 4 months | 37.2 (29.4, 45.1) | 39.1 (31.0, 47.3) | 0.92 (0.68, 1.24) | |
| 8 months | 38.3 (29.9, 46.6) | 34.7 (26.6, 42.7) | 1.07 (0.78, 1.47) | |
| Hazardous drinking† | ||||
| Baseline | 8.0 (3.8, 12.1) | 9.2 (4.6, 13.7) | 0.80 (0.39, 1.66) | 0.58 |
| 4 months | 10.5 (5.6, 15.3) | 8.5 (4.1, 12.9) | 1.10 (0.54, 2.25) | |
| 8 months | 11.6 (6.5, 16.6) | 9.2 (4.6, 13.9) | 1.13 (0.56, 2.24) | |
| Tobacco use‡ | ||||
| Baseline | 51.8 (44.1, 59.5) | 51.2 (43.5, 58.9) | 1.05 (0.86, 1.29) | 0.46 |
| 4 months | 52.6 (44.9, 60.4) | 49.8 (42.1, 57.5) | 1.10 (0.89, 1.35) | |
| 8 months | 53.4 (45.6, 61.2) | 48.8 (41.1, 56.6) | 1.12 (0.91, 1.37) | |
| Cannabis use§ | ||||
| Baseline | 42.3 (34.7, 49.9) | 33.3 (26.1, 40.6) | 1.13 (0.86, 1.48) | 0.93 |
| 4 months | 40.1 (32.4, 47.8) | 31.1 (23.8, 38.4) | 1.15 (0.86, 1.54) | |
| 8 months | 41.3 (33.5, 49.1) | 33.8 (26.4, 41.1) | 1.11 (0.84, 1.47) | |
| Other drug use|| | ||||
| Baseline | 1.8 (0, 3.9) | 3.1 (0.4, 5.7) | 0.68 (0.12, 3.74) | 0.90 |
| 4 months | 1.4 (0, 3.4) | 1.9 (0, 3.9) | 0.90 (0.12, 6.54) | |
| 8 months | 2.5 (0.1, 5.0) | 5.0 (1.6, 8.4) | 0.56 (0.12, 2.56) | |
| Non-medical sedative use¶ | ||||
| Baseline | 19.0 (12.9, 25.1) | 17.9 (12.0, 23.8) | 1.04 (0.61, 1.77) | 0.62 |
| 4 months | 14.6 (8.6, 20.5) | 16.1 (10.1, 22.1) | 0.94 (0.50, 1.75) | |
| 8 months | 12.0 (6.8, 17.2) | 16.0 (10.0, 22.0) | 0.77 (0.40, 1.50) | |
*Opioid risk behavior model adjusted for education
† Hazardous drinking model adjusted for age, Charlson comorbidity index score, and income
‡ Tobacco use model adjusted for alcohol use and education
§ Cannabis use model adjusted for age, gender, race/ethnicity, education, and tobacco use
|| Other drug use includes heroin, other illicit opioids, cocaine, methamphetamine, inhalants, and hallucinogens. Model adjusted for alcohol use, tobacco use, and prior receipt of naloxone; modeled with logit link function
¶ Non-medical sedative use model adjusted for housing and tobacco use
Secondary Outcomes
Hazardous drinking, tobacco use, cannabis use, non-medical sedative use, and other drug use (measured by survey alone or survey plus urine drug screen results) were similar over time by trial group (Table 2, eTable 7). In intervention pharmacies, knowledge about overdose prevention and naloxone increased compared to usual care pharmacies (P=0.02; adjusted mean difference at 8 months vs. baseline 1.05; 95% CI 0.06, 2.04; Table 3) and between baseline and both follow-up time points (Table 3, eTable 8).
Table 3.
Prescription Opioid Overdose Knowledge Among Survey Participants over Time by Trial Group
| Rx-OOKS | Crude score Mean (95% CI) |
Adjusted difference* (95% CI) | Time x intervention P value* | |
|---|---|---|---|---|
| Intervention pharmacies (n=163) | Usual care pharmacies (n=162) | |||
| Baseline | 11.5 (10.7, 12.2) | 11.3 (10.7, 12.0) | −0.16 (−1.08, 0.76) | 0.02 |
| 4 months | 13.0 (12.3, 13.7) | 12.1 (11.4, 12.8) | 0.64 (−0.32, 1.61) | |
| 8 months | 13.7 (13.0, 14.4) | 12.4 (11.7, 13.0) | 1.05 (0.06, 2.04) | |
Abbreviations: Rx-OOKS Prescription Opioid Overdose Knowledge Scale
* Model adjusted for age, race/ethnicity, annual household income, baseline daily opioid dose, prior receipt of naloxone, previous opioid use disorder diagnosis, and previous alcohol use disorder diagnosis
By 10 months, the proportion of participants dispensed naloxone was more than threefold greater in intervention pharmacies than in usual care pharmacies (ARR 3.38; 95% CI 2.21, 5.15; Table 4). Sensitivity analyses yielded similar findings (eTable 9). Including baseline and follow-up dispensings and self-reported naloxone receipt and possession, 63.2% (n=103) of participants in the intervention arm had naloxone by the end of the study. By pharmacy, receipt of naloxone varied from 46.9 to 76.1% among participants in intervention pharmacies and 10.9 to 17.1% among participants in control pharmacies. Differences were not observed in the proportion of patients prescribed opioids at 8 months (intervention pharmacies 86.5%, usual care pharmacies 87.3%, P=0.83) or opioid dose over time (Table 4, eTable 10). Insurance was associated with naloxone uptake (P=0.02) among patients in the intervention pharmacies, with higher uptake among participants with Medicaid and Medicare than those with commercial insurance or no insurance (eTable 11).
Table 4.
Naloxone Dispensed and Opioid Dose over Time in Patients and Survey Participants by Trial Group
| Intervention pharmacies | Usual care pharmacies | Adjusted risk ratio* or difference† (95% CI) | Time x intervention P value | |
|---|---|---|---|---|
| Naloxone dispensings, no. dispensed / no. patients or participants (%)‡ | N/A | |||
| Patients§ | 188/364 (51.7) | 58/404 (14.4) | 3.75 (2.88, 4.88) | |
| Survey participants|| | 83/163 (50.9) | 25/162 (15.4) | 3.38 (2.21, 5.15) | |
| Opioid dose, median MME (IQR) | ||||
| Patients¶ | ||||
| Baseline** | 45.1 (23.0, 89.0) | 40.7 (24.2, 76.3) | 0.06 (−0.07, 0.20) | 0.48 |
| End of follow-up†† | 45.0 (13.5, 96.0) | 45.0 (18.7, 90.0) | −0.01 (−0.27, 0.25) | |
| Survey participants‡‡ | ||||
| Baseline§§ | 38.6 (19.5, 85.3) | 45.2 (24.3, 83.0) | −0.04 (−0.42, 0.35) | 0.12 |
| End of follow-up†† | 41.1 (8.0, 89.4) | 55.0 (20.0, 110.1) | −0.27 (−0.75, 0.22) | |
Abbreviations: MME Morphine milligram equivalents: IQR Interquartile range
*Reported for naloxone dispensings
†Reported for opioid dose; estimates are on a log scale
‡Some patients were dispensed naloxone more than once over the follow-up (intervention pharmacies: 212 dispensings among patients and 95 dispensings among survey participants; usual care pharmacies: 63 dispensings among patients and 26 dispensings among survey participants)
§Model adjusted for Charlson comorbidity index score, opioid use disorder, and baseline opioid dose
||Model adjusted for Charlson comorbidity index score and race/ethnicity
¶Model adjusted for gender, insurance type, prior receipt of naloxone, previous opioid use disorder diagnosis, and Charlson comorbidity index score
**Baseline dose was calculated within 45 days of study recruitment
††Follow-up ended 10 months from the time co-dispensing began in intervention pharmacies; opioid dose was calculated within 45 days of follow-up end
‡‡Model adjusted for gender, insurance type and prior receipt of naloxone
§§Baseline dose was calculated within 45 days of study enrollment
Other Outcomes
No fatal opioid overdoses occurred among participants. Two participants from intervention pharmacies experienced non-fatal opioid overdoses and none from usual care pharmacies. Among patients, opioid overdoses were not significantly different across trial groups (ARR 4.60; 95% CI 0.57, 36.94; eTable 12). All-cause mortality was similar across the trial groups for participants (ARR 1.33; 95% CI 0.34, 5.28) and patients (ARR 1.42; 95% CI 0.58, 3.44; eTable 12 and eTable 13). Cancer was the leading cause of death (n=12; 38.7%).
DISCUSSION
This pharmacy-randomized trial demonstrated that co-dispensing naloxone with opioids significantly increases naloxone acquisition and overdose knowledge among patients prescribed opioids, without any detectable increase in risk behavior. There was no effect on opioid dose. This pragmatic intervention could be applicable to a range of pharmacies and can help ensure naloxone is accessible to patients who need it in the event of an overdose.
While the USA has experienced declines in opioid prescribing since 2012,41 the absolute number of prescription opioid-involved overdoses has fallen only slightly relative to 2013.9 It is therefore imperative to continue to implement strategies to prevent opioid overdose deaths in clinical settings, particularly since general internists and other physicians have a duty to try to prevent adverse outcomes from the medications they prescribe.
Prior observational studies suggest that overdose education and naloxone provision reduce opioid risk behavior,6 emergency department visits,42 and heroin use.10,20 In this trial, naloxone co-dispensing did not impact opioid risk behaviors. Given these findings, concerns that naloxone could cause risk compensation among patients prescribed opioids are not supported. The trial results also suggest that education provided with co-dispensing increases knowledge about overdose and naloxone. If such knowledge is shared, it could positively impact the patient’s family and social networks.
Although the co-dispensing intervention was highly effective at increasing naloxone uptake, about 37% of participants in the intervention arm did not report having naloxone before or during the trial and were not dispensed naloxone during the trial. Since this was a pragmatic trial in real-world clinical settings, the lack of uptake may be partially attributed to inconsistent adherence to the study protocol by non-research pharmacists, cost barriers (8% of participants indicated cost was a reason they did not pick up naloxone),43 and no longer taking opioids. While co-dispensing achieved greater dispensing than a primary-care-based prescribing initiative (38%),42 waiving naloxone co-payments may further increase dispensing.
This trial occurred after the leading causes of opioid overdoses had shifted from prescription opioids to heroin and fentanyl.44 However, national data suggest that co-involvement of fentanyl and prescription opioids in overdose deaths is common.9 Approximately 20% of the patients in this trial had a prior diagnosis of an opioid use disorder, and co-dispensing significantly increased naloxone uptake among these patients. Therefore, in addition to harm reduction and addiction treatment settings, co-dispensing naloxone in a clinical setting could significantly impact fentanyl-related deaths among patients prescribed opioids.
We conducted this trial in a single US health system that serves a predominantly low-income population. Support and cooperation from clinical and pharmacy leadership were integral to the trial’s success. However, pharmacies in other health systems and countries may have distinct barriers to implementation that limit the feasibility of co-dispensing.45 Despite randomization, more patients with opioid use disorder were in intervention pharmacies. This imbalance may have been due to the pragmatic nature of this trial, the low number of pharmacies in this health system, or known limitations of ICD-10 codes for opioid use disorder.46–48 If this imbalance impacted our primary outcome of self-reported risk behavior, it would have biased the results towards finding an association rather than the null finding observed. The imbalance may also have resulted in a higher than the anticipated absolute number of opioid overdoses in co-dispensing pharmacies, but it was not significantly different from usual care pharmacies. While not significantly different by trial group, we also observed more deaths than expected, perhaps because we had no ethical or scientific reason to exclude patients with cancer from the trial. Naloxone effectively prevents death, so increasing naloxone uptake should reduce opioid overdose mortality, but, due to insufficient power, larger studies would be needed to formally evaluate the effects of co-dispensing on this outcome. To impact overdose mortality, naloxone co-dispensing could also be coupled with other interventions that could be delivered in pharmacy settings, such as buprenorphine for pain or opioid use disorder.
Opioid risk behavior was self-reported, and baseline opioid risk scores were low, suggesting that such behaviors could have been underreported. However, at baseline, 8% of participants disclosed hazardous drinking and more than a third disclosed marijuana use (see Table 2). Furthermore, we successfully surveyed 42% of the eligible population, a proportion that exceeds other health system trials (8–21%)49–52 and the survey participants appeared to be representative of the patient population. We observed no significant differences in opioid dose or deaths. While we did not have Spanish language surveys, intervention pharmacies co-dispensed naloxone to patients who primarily spoke Spanish and provided them with Spanish-language educational materials.
Naloxone is an efficacious medication that prevents opioid overdose fatalities. This randomized pragmatic trial demonstrated that barriers to naloxone acquisition, including knowledge gaps, can be overcome by implementing pharmacy-based naloxone co-dispensing with opioids.
Supplementary information
(DOCX 507 kb)
Acknowledgements
We appreciate the assistance and expertise of Kris Wain, MS, Edward Gardner, MD, Josh Durfee, MSPH, Kerry Broderick, MD, Brittany Irwin, PharmD, Kirk Bol, MSPH, David Edwards, John Strang, MBBS, FRCPsych, FRCP, MD, F.Med.Sci., Anna Williams, BSc, MSc, PhD, Courtney Kraus, MSPH, Catherine G. Derington, PharmD, and members of the Data Safety and Monitoring Board (William Henderson, PhD, MPH, Marta Brooks, PharmD, and Adam Abraham, MD).
Contributors
I.B. and J.G. formulated the research aims, acquired the financial support for the project, and oversaw the study implementation, data collection, and analysis. S.M, N.W., and M.S. managed data collection and the research activity execution. D.R., M.S., and S.M. coordinated intervention implementation. K.M. facilitated data collection. R.H. and J.B. advised intervention development and implementation. K.N. and S.X. applied statistical techniques to analyze the study data and verify the underlying data. I.B. prepared the initial draft of the manuscript. All authors discussed results and provided critical review and revisions to the final manuscript. All authors had full access to all the data in the study, and the corresponding author takes final responsibility for the decision to submit for publication.
Funders
Funding for this study was provided by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA042059 and supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors had full access to the complete data in the study and accept responsibility for publication.
Declarations
Conflict of Interest
Outside of the listed affiliations, I.B. reports royalties from UpToDate, and all remaining authors declare that they do not have a conflict of interest.
Footnotes
Prior Presentations
Baseline and preliminary results were presented at the College on Problems of Drug Dependence Virtual Meeting, June 22, 2020.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davis C, Carr D. State legal innovations to encourage naloxone dispensing. J Am Pharm Assoc. 2003;57(2):S180–S4. doi: 10.1016/j.japh.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. HHS recommends prescribing or co-prescribing naloxone to patients at high risk for an opioid overdose. HHS.Gov. December 19, 2018. https://www.hhs.gov/about/news/2018/12/19/hhs-recommends-prescribing-or-co-prescribing-naloxone-to-patients-at-high-risk-for-an-opioid-overdose.html. Accessed 29 Sep 2021.
- 4.Sohn M, Talbert JC, Huang Z, Lofwall MR, Freeman PR. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215-e. doi: 10.1001/jamanetworkopen.2019.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy GP, Jr, Haegerich TM, Evans ME, Losby JL, Young R, Jones CM. Vital signs: Pharmacy-based naloxone dispensing - United States, 2012-2018. MMWR Morb Mortal Wkly Rep. 2019;68(31):679–86. doi: 10.15585/mmwr.mm6831e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behar E, Rowe C, Santos G-M, Murphy S, Coffin PO. Primary care patient experience with naloxone prescription. Ann Fam Med. 2016;14(5):431–6. doi: 10.1370/afm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones CM, Compton W, Vythilingam M, Giroir B. Naloxone co-prescribing to patients receiving prescription opioids in the Medicare part d program, United States, 2016-2017. JAMA. 2019;322(5):462–4. doi: 10.1001/jama.2019.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choremis B, Campbell T, Tadrous M, Martins D, Antoniou T, Gomes T. The uptake of the pharmacy-dispensed naloxone kit program in Ontario: A population-based study. PLoS One. 2019;14(10):e0223589. doi: 10.1371/journal.pone.0223589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202–7. doi: 10.15585/mmwr.mm7006a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binswanger IA, Koester S, Mueller SR, Gardner EM, Goddard K, Glanz JM. Overdose education and naloxone for patients prescribed opioids in primary care: A qualitative study of primary care staff. J Gen Intern Med. 2015;30(12):1837–44. doi: 10.1007/s11606-015-3394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beletsky L, Ruthazer R, Macalino GE, Rich JD, Tan L, Burris S. Physicians' knowledge of and willingness to prescribe naloxone to reverse accidental opiate overdose: Challenges and opportunities. J Urban Health. 2007;84(1):126–36. doi: 10.1007/s11524-006-9120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martino JG, Smith SR, Rafie S, Rafie S, Marienfeld C. Physician and pharmacist: Attitudes, facilitators, and barriers to prescribing naloxone for home rescue. Am J Addict. 2020;29(1):65–72. doi: 10.1111/ajad.12982. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JD, Spicyn N, Matson P, Alvanzo A, Feldman L. Internal medicine resident knowledge, attitudes, and barriers to naloxone prescription in hospital and clinic settings. Subst Abus. 2016;37(3):480–7. doi: 10.1080/08897077.2016.1142921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dispense Supply of Emer Drugs for Overdose Victims, SB15-053 (COLO 2015) April 6, 2016. Accessed January 12, 2021. https://leg.Colorado.Gov/sites/default/files/images/olls/2015a_sl_78.Pdf.
- 15.Winograd RP, Werner KB, Green L, Phillips S, Armbruster J, Paul R. Concerns that an opioid antidote could “make things worse”: Profiles of risk compensation beliefs using the naloxone-related risk compensation beliefs (NaRRC-B) scale. Subst Abus. 2020;41(2):245–51. doi: 10.1080/08897077.2019.1616348. [DOI] [PubMed] [Google Scholar]
- 16.Green TC, Bowman SE, Zaller ND, Ray M, Case P, Heimer R. Barriers to medical provider support for prescription naloxone as overdose antidote for lay responders. Subst Use Misuse. 2013;48(7):558–67. doi: 10.3109/10826084.2013.787099. [DOI] [PubMed] [Google Scholar]
- 17.Mueller SR, Koester S, Glanz JM, Gardner EM, Binswanger IA. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017;32(3):277–83. doi: 10.1007/s11606-016-3895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green TC, Case P, Fiske H, Baird J, Cabral S, Burstein D, et al. Perpetuating stigma or reducing risk? Perspectives from naloxone consumers and pharmacists on pharmacy-based naloxone in 2 states. J Am Pharm Assoc. 2003;57(2, Supplement):S19–S27.e4. doi: 10.1016/j.japh.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Seal KH, Thawley R, Gee L, Bamberger J, Kral AH, Ciccarone D, et al. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: A pilot intervention study. J Urban Health. 2005;82(2):303–11. doi: 10.1093/jurban/jti053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JD, Campbell A, Metz VE, Comer SD. No evidence of compensatory drug use risk behavior among heroin users after receiving take-home naloxone. Addict Behav. 2017;71:104–6. doi: 10.1016/j.addbeh.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meurer WJ, Lewis RJ. Cluster randomized trials: Evaluating treatments applied to groups. JAMA. 2015;313(20):2068–9. doi: 10.1001/jama.2015.5199. [DOI] [PubMed] [Google Scholar]
- 22.Binswanger IA, Joseph N, Hanratty R, Gardner EM, Durfee J, Narwaney KJ, et al. Novel opioid safety clinic initiative to deliver guideline-concordant chronic opioid therapy in primary care. Mayo Clin Proc Innov Qual Outcomes. 2018;2(4):309–16. doi: 10.1016/j.mayocpiqo.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health. 2005;27(3):281–91. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]
- 26.Glanz JM, Narwaney KJ, Mueller SR, Gardner EM, Calcaterra SL, Xu S, et al. Prediction model for two-year risk of opioid overdose among patients prescribed chronic opioid therapy. J Gen Intern Med. 2018;33(10):1646–53. doi: 10.1007/s11606-017-4288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houmes R-J, Voets MA, Verkaaik A, Erdmann W, Lachmann B. Efficacy and safety of tramadol versus morphine for moderate and severe postoperative pain with special regard to respiratory depression. Anesth Analg. 1992;74(4):510–4. doi: 10.1213/00000539-199204000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Larance B, Bruno R, Lintzeris N, Degenhardt L, Black E, Brown A, et al. Development of a brief tool for monitoring aberrant behaviours among patients receiving long-term opioid therapy: The opioid-related behaviours in treatment (ORBIT) scale. Drug Alcohol Depend. 2016;159:42–52. doi: 10.1016/j.drugalcdep.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Paillard F, McKeown A, Burke LB, Edwards RR, Katz NP, et al. Instruments to identify prescription medication misuse, abuse, and related events in clinical trials: An ACTTION systematic review. J Pain. 2015;16(5):389–411. doi: 10.1016/j.jpain.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell G, Noghrehchi F, Nielsen S, Clare P, Bruno R, Lintzeris N, et al. Risk factors for indicators of opioid-related harms amongst people living with chronic non-cancer pain: Findings from a 5-year prospective cohort study. EClinicalMedicine. 2020;28:100592. doi: 10.1016/j.eclinm.2020.100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeely J, Strauss SM, Wright S, Rotrosen J, Khan R, Lee JD, et al. Test-retest reliability of a self-administered alcohol, smoking and substance involvement screening test (ASSIST) in primary care patients. J Subst Abus Treat. 2014;47(1):93–101. doi: 10.1016/j.jsat.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) Addiction. 2008;103(6):1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 33.WHO ASSIST Working Group The alcohol, smoking and substance involvement screening test (ASSIST): Development, reliability and feasibility. Addiction. 2002;97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 34.Harris AH, Bradley KA, Bowe T, Henderson P, Moos R. Associations between AUDIT-C and mortality vary by age and sex. Popul Health Manag. 2010;13(5):263–8. doi: 10.1089/pop.2009.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 36.Shoup JA, Mueller SR, Binswanger IA, Williams AV, Strang J, Glanz JM. Modifying and evaluating the opioid overdose knowledge scale for prescription opioids: A pilot study of the Rx-OOKS. Pain Med. 2020;21(10):2244–52. doi: 10.1093/pm/pnaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC). Behavioral risk factor surveillance system survey questionnaire. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2014. https://www.cdc.gov/brfss/about/brfss_faq.htm#:~:text=Suggested%20Citation%20for%20Online%20BRFSS,Prevention%2C%20%5Bappropriate%20year%5D
- 38.Yu X, Tam WW, Wong PT, Lam TH, Stewart SM. The patient health questionnaire-9 for measuring depressive symptoms among the general population in hong kong. Compr Psychiatry. 2012;53(1):95–102. doi: 10.1016/j.comppsych.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Titus JC, Schiller JA, Guthmann D. Characteristics of youths with hearing loss admitted to substance abuse treatment. J Deaf Stud Deaf Educ. 2008;13(3):336–50. doi: 10.1093/deafed/enm068. [DOI] [PubMed] [Google Scholar]
- 40.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, New Jersey: John Wiley & Sons; 2004. [Google Scholar]
- 41.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. U.S. Opioid dispensing rate maps. https://www.cdc.gov/drugoverdose/rxrate-maps/index.html. Accessed September 15, 2021.
- 42.Coffin PO, Behar E, Rowe C, Santos GM, Coffa D, Bald M, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165(4):245–52. doi: 10.7326/M15-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barenie RE, Gagne JJ, Kesselheim AS, Pawar A, Tong A, Luo J, et al. Rates and costs of dispensing naloxone to patients at high risk for opioid overdose in the United States, 2014-2018. Drug Saf. 2020;43(7):669–75. doi: 10.1007/s40264-020-00923-6. [DOI] [PubMed] [Google Scholar]
- 44.Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths — United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290–7. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strang J, McDonald R, Campbell G, Degenhardt L, Nielsen S, Ritter A, et al. Take-home naloxone for the emergency interim management of opioid overdose: The public health application of an emergency medicine. Drugs. 2019;79(13):1395–418. doi: 10.1007/s40265-019-01154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAdam-Marx C, Roland CL, Cleveland J, Oderda GM. Costs of opioid abuse and misuse determined from a Medicaid database. J Pain Palliat Care Pharmacother. 2010;24(1):5–18. doi: 10.3109/15360280903544877. [DOI] [PubMed] [Google Scholar]
- 47.Watson A, Simon DM, Peratikos MB, Stringer EA. Medical utilization surrounding initial opioid-related diagnoses by coding method. Am J Manag Care. 2020;26(2):e64-e68. [DOI] [PubMed]
- 48.Paulozzi LJ, Zhou C, Jones CM, Xu L, Florence CS. Changes in the medical management of patients on opioid analgesics following a diagnosis of substance abuse. Pharmacoepidemiol Drug Saf. 2016;25(5):545–52. doi: 10.1002/pds.3980. [DOI] [PubMed] [Google Scholar]
- 49.Friedly JL, Comstock BA, Turner JA, Heagerty PJ, Deyo RA, Sullivan SD, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014;371(1):11–21. doi: 10.1056/NEJMoa1313265. [DOI] [PubMed] [Google Scholar]
- 50.Bohnert AS, Bonar EE, Cunningham R, Greenwald MK, Thomas L, Chermack S, et al. A pilot randomized clinical trial of an intervention to reduce overdose risk behaviors among emergency department patients at risk for prescription opioid overdose. Drug Alcohol Depend. 2016;163:40–7. doi: 10.1016/j.drugalcdep.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D. Patient navigation improves cancer diagnostic resolution: An individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1629. [DOI] [PMC free article] [PubMed]
- 52.Bulger EM, Maier RV, Sperry J, Joshi M, Henry S, Moore FA, et al. A novel drug for treatment of necrotizing soft-tissue infections: A randomized clinical trial. JAMA Surgery. 2014;149(6):528–36. doi: 10.1001/jamasurg.2013.4841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 507 kb)