Abstract
Opioid receptors belong to the class A G-protein-coupled receptors and are activated by alkaloid opiates such as morphine, and endogenous ligands such as endorphins and enkephalins. Opioid receptors are widely distributed in the human body and are involved in numerous physiological processes through three major classical opioid receptor subtypes; the mu, delta and kappa along with a lesser characterized subtype, opioid receptor-like (ORL1). Opioids are the most potent analgesics and have been extensively used as a therapeutic drug for the treatment of pain and related disorders. Chronic administration of clinically used opioids is associated with adverse effects such as drug tolerance, addiction and constipation. Several investigations attempted to identify the molecular signaling networks associated with endogenous as well as synthetic opiates, however, there is a paucity of a cumulative depiction of these signaling events. Here, we report a systemic collection of downstream molecules pertaining to four subtypes of opioid receptors (MOR, KOR, DOR and ORL1) in the form of a signaling pathway map. We manually curated reactions induced by the activation of opioid receptors from the literature into five categories- molecular association, activation/inhibition, catalysis, transport, and gene regulation. This led to a dataset of 180 molecules, which is collectively represented in the opioid receptor signaling network following NetPath criteria. We believe that the public availability of an opioid receptor signaling pathway map can accelerate biomedical research in this area because of its high therapeutic significance. The opioid receptors signaling pathway map is uploaded to a freely available web resource, WikiPathways enabling ease of access (https://www.wikipathways.org/index.php/Pathway:WP5093).
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-021-00653-z.
Keywords: Opioid signaling, NetPath, Post-translational modifications, Protein–protein interactions, Nociception, Opioid tolerance
Introduction
The 1800s, heralded the discovery of opiates when Friedrich Serturner first discovered the active ingredient of opium as a sleep-inducing molecule, which was named by him as morphine (Krishnamurti and Rao 2016). That was the beginning of opioid pharmacology and since then, opioid derivatives have been used for medicinal as well as recreational purposes. Opiates function by interacting with opioid receptors, which are intriguing members of the G protein-coupled receptor (GPCR) family. Known to be associated with pain behavior and nociception, opioid peptides and their receptors are expressed throughout the nociceptive neural circuitry (Al-Hasani and Bruchas 2011). The biochemical and pharmacological characterization has led to the classification of opioid receptors into four subtypes: Mu (μ), Delta (δ), Kappa (κ) and opioid receptor like-1 (ORL1) (Dhawan et al. 1996). All the four receptor subtypes have unique pharmacological significance consisting of highly selective agonists and antagonists (Goldstein and Naidu 1989). Several preclinical and clinical studies have also been conducted to analyze the endogenous opioid framework as well as the agonist activity of synthetic opiates (Bohn et al. 2002; Gavériaux-Ruff 2013; Stein 2016; Altarifi et al. 2017).
The opioid receptor genes OPRM1, OPRD1, OPRK1 and OPRL1 encoding for the μOR (MOR-1), δOR (DOR-1), κOR (KOR-1), and OPRL1 (ORL1), respectively. These proteins are abundantly present as integral membrane proteins expressed on the neuronal cell surface in the mammalian brain. Like most of the G-protein-coupled receptor proteins, opioid receptors possess seven hydrophobic transmembrane domains (TM I–VII). These include three intracellular (i1–i3) [identical in 20–23 amino acids in all opioid receptors], three extracellular (e1–e3) loops and a glycosylated amino and carboxyl terminus (Jordan et al. 2000). Transmembrane (TM) domains II, III, V, VI, and VII, the three intracellular loops, and a short region of the C-terminal tail are all quite similar among opioid receptors. However, the extracellular loops (ECL) and N-terminal tails, display almost no homology (Jordan et al. 2000; Zöllner and Stein 2007). Opioid receptors are activated by endogenous as well as exogenously administered opiate compounds/ligands. Pro-enkephalin, pro-opiomelanocortin, pro-dynorphin, and pre-pro-N/OFQ are the four pro-hormone precursors that are cleaved to produce endogenous opioid peptides (pp-noc) (Janecka et al. 2004). The list of other agonists of the four opioid subtypes including the synthetic ligands is provided in Table 1.
Table 1.
Classification of opioid receptors and their respective ligands
| Official name and Gene Symbol | Aliases | Endogenous ligands/ Atypical opioid peptides | Synthetic ligands |
|---|---|---|---|
| Delta opioid receptor OPRD1 | DOR, DOR1, Dor-1, δOR, DOP, OPRD,D-OR-1 | β-Endorphin, [Met]-Enkephalin, [Leu]- Enkephalin, Dermenkephalin, Deltorphin-I, Deltorphin-II | DADLE, Etorphine, DSLET, DTLET, BUBU, DPDPE, DPLPE, SNC80 YKFA, BW373U86, TAN-67, UFP-512, Oxycodone |
| Mu opioid receptor OPRM1 | MOR, MOR-1, LMOR, MOP, HMOP, OPRM, MOR-1, M-OR-1, µOR | β-Endorphin, Endomorphin-I, Endomorphin-II, Metorphamide, β-Casomorphin, Morphiceptin, Dermorphin, Hemorphin-4 | DAMGO, Morphine, Fentanyl, Loperamide, DALDA, ADA-DER, DAS-DER, TAPA, ADAMB, YKFA, Buprenorphine, Tramadol, Remifentanil, Methadone |
| Kappa opioid receptor OPRK1 | KOR, KOR-1, KOR1, KOP, K-OR-1, OPRK, κOR | Dynorphin A, Dynorphin B, Metorphamide | U50,488, Salvinorin-A, LPK-26, Oxycodone, U69,593, TRK820, Nalfurafine, BRL52537, 6` GNTI |
| Nociceptin receptor OPRL1 | ORL1, OOR, KOR3, KOR-3, NOCIR, NOP, NOPr, OPRL | Nociceptin/OrphaninFQ |
The opioid receptors are primarily implicated in antinociceptive signaling in the central nervous system (CNS), where they are expressed in descending pathways of pain-modulation, comprising the locus coeruleus, medulla, and periaqueductal grey area. They also show a wide distribution in non-neuronal tissues including neuroendocrine, immune, and ectodermal cells (Zöllner and Stein 2007). These receptors are functionally linked to analgesia, feelings of euphoria, respiratory depression, reproduction, growth, myosis, constipation, and changes in the endocrine as well as immune systems (Anand and Montgomery 2018). Opiates, such as morphine or heroin (a chemically synthesized derivative), are extremely effective pain relievers on one hand and highly addictive drugs on the other (Rosenblum et al. 2008). Thus, the opioid receptors are also well known for the adverse reactions due to chronic use of opiates, such as tolerance and dependence, in addition to respiratory depression and addiction (Morgan and Christie 2011).
The activation cascade of prototypical Gi/G0-coupled opioid receptors follows the conventional GPCR signaling system, which activates intracellular G proteins consisting of three subunits: α, β and γ (Reisine et al. 1996). Interestingly, these receptors activate two main transducing pathways upon stimulation, with varying degrees of preference: the β-arrestin or/and the G-protein pathway (Al-Hasani and Bruchas 2011). The β-arrestin signaling pathway regulates opioid receptor signaling via desensitization and internalization. However, the G-protein signaling pathway is the “classical” signaling route and promotes different effects depending on the opioid receptor subtype. This cascade regulates adenylyl cyclase, phosphatidylinositol-3 kinase, the MAP kinase pathway, Ca2+channels, and GIRK channels exerting its activation onto further signaling molecules.
Though the signaling modalities of opioid receptor stimulation by different agonists have been studied extensively, a compendium of the molecules encompassing the opioid signaling system remains elusive. The availability of a pathway map portraying the signal transduction associated with the stimulation of opioid receptors will provide immense information that can be utilized for further research on opioid signaling.
Materials and methods
An extensive literature search was performed using PubMed to screen the processes that are involved in the stimulation of opioid receptors. Biochemical reactions upon opioid ligand stimulation were annotated into five categories which include, (1) catalysis (post-translational modification, cleavage, and binding), (2) molecular association (protein–protein interactions), (3) protein translocation/transport across subcellular compartments, (4) activation/inhibition and (5) gene regulation at mRNA/protein level (Kandasamy et al. 2010). For annotation of these reactions, we adhered to the criteria of NetPath pathway annotation as described previously in galanin-galanin receptor signaling network (Gopalakrishnan et al. 2021), serotonin (Sahu et al. 2018), oxytocin–oxytocin receptor signaling (Chatterjee et al. 2016), oncostatin M (Dey et al. 2013), AGE/RAGE signaling (Soman et al. 2013), IL33 signaling (Pinto et al. 2018), and MIF signaling (Subbannayya et al. 2015) pathways. After curation, all the reactions were reviewed manually.
The annotated information included the information about the model organism/cell line used for the experiment, type of experiment (in vivo, in vitro), site and residue of the post-translational modifications, subcellular localization of the proteins, upregulation or downregulation of gene expression as provided in the literature (Kandasamy et al. 2009; Raju et al. 2011). We also provide a brief comment on the annotated reaction for providing a clear context of the reaction. The reactions were then connected appropriately to develop a signaling map of the modules pertaining to the activation of the opioid receptor upon stimulation by the agonists/ligands. The map thus generated was exported to WikiPathways, a freely available pathway resource. (https://www.wikipathways.org/index.php/WikiPathways).
Results and discussion
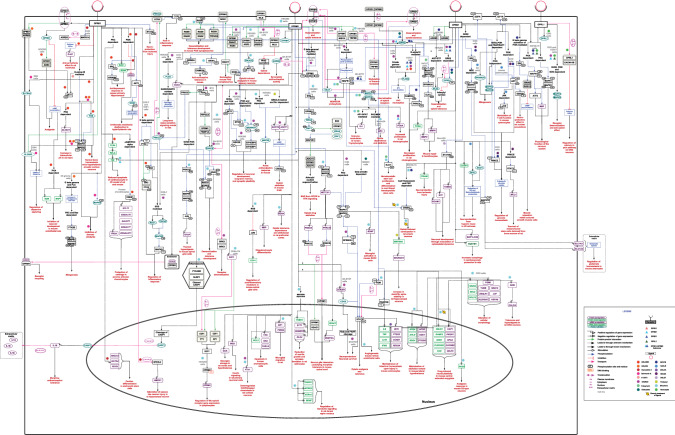
The literature search on PubMed using the search term ‘("Delta opioid receptor" OR "OPRD1" OR "OPRD" OR "Kappa opioid receptor" OR "OPRK1" OR "K-OR-1" OR "KOR" OR "KOR-1" OR "OPRK" OR "Mu opioid receptor" OR "OPRM1" OR "LMOR" OR "M-OR-1" OR "MOP" OR "MOR" OR "MOR1" OR "OPRM" OR "opiate receptor like 1" OR "OPRL1" OR "KOR-3" OR "NOCIR" OR "OOR" OR "ORL1") AND ("signaling" OR "pathway" OR "signalling")’ resulted in the 6,181 papers. Of which 209 papers were selected for annotation due to the availability of information pertaining to the signaling of the opioid receptor system. The annotation of reactions as per NetPath criteria resulted in the identification of a total of 464 reactions (Kandasamy et al. 2009; Raju et al. 2011). This includes reactions, 178 gene regulation reactions at the transcriptional level and 97 at the translational level, 32 activation/inhibition reactions, 61 catalysis reactions, 30 protein translocation reactions, and 66 molecular association reactions (See also Supplementary Table 1). These reactions were utilized for the generation of a detailed opioid-opioid receptor signal transduction map. A pictorial representation of this consolidated map is provided in Fig. 1. This map is available to the scientific community on WikiPathways, an open-source biological pathway database (https://www.wikipathways.org/index.php/Pathway:WP5093). The data is presented in BioPAX level 3 format (OWL), a standard community exchange format (Demir et al. 2010). The pathway data can be downloaded from the WikiPathways in PNG, PDF and SVG image formats as well as the gene lists in.txt format (Kelder et al. 2009).
Fig. 1.
Pictorial representation of opioid signaling from four receptor subtypes
Opioid-opioid receptor-mediated signal transduction
The binding of opioid agonists to the receptor leads to the conformational rearrangements or dissociation of the trimeric G protein complex into Gα- and Gβγ-subunits, which is one of the major stages of the signal transduction pathway that activates a number of downstream signaling cascades (Nagi et al. 2015; Stein 2016). An established mechanism of GPCR stimulation is the generation of the secondary messenger Cyclic Adenosine 3', 5'-Monophosphate (cAMP), from Adenosine 5' Triphosphate (ATP) activating protein kinase A (PKA), further activating CREB (cAMP response element-binding protein) by phosphorylation. For instance, as depicted in the map, KOR regulates the characteristics of endothelial cells (EC) and vascular progenitors through the inhibition of cAMP in a PKA-dependent manner by modifying the EC differentiation and vascular pathfinding (Yamamizu et al. 2011). Similarly, stimulation of DOR terminated the Gαi signaling via Gβγ- dependent PI3K/Src pathway resulting in cAMP inhibition, which facilitates muscle relaxation (Huang et al. 2014). On the contrary, MOR plays a crucial role in the development of opioid addiction through the activation of adenylyl cyclase ensuing in an increased production of cAMP, modulating the activity of CREB in mouse striatum and prefrontal cortex (Yang et al. 2014). CREB belongs to a family of transcription factors that alter the transcription of certain genes, thereby implementing the function of opioid receptors (Shao et al. 2016). This is one of the key regulating pathways critical for memory formation and pain modulation as well as in long-term potentiation, a phenomenon that underpins synaptic plasticity (Impey et al. 1998).
The activated opioid receptors aid in the recruitment of other signal transduction cascades including mitogen-activated protein kinases (MAPKs), of which ERK1/2 is the most commonly studied opioid-induced MAPK cascade (Al-Hasani and Bruchas 2011). Opioid receptor agonists have selective preferences for the G-protein pathway or the β-arrestin pathway when it comes to ERK activation. In the case of MOR, morphine and methadone, for example, activate ERK through the β-arrestin independent, PKC-dependent pathway, whereas etorphine and fentanyl do so through the β-arrestin-dependent pathway (Zheng et al. 2008). DOR activates ERK1/2 through Gβγ and Ras signaling and does not always require receptor internalization or phosphorylation (Kramer and Simon 2000). On the other hand, the PI3-kinase, PKC, and dependence of intracellular calcium are involved in the KOR-mediated activation of ERK1/2 (Bohn et al. 2000a). On the contrary, only one study on ORL1 suggested the mechanism of ERK1/2 activation to be Gi/G0, PI3K and SOS-dependent in CHO and COS-7 cells (Hawes et al. 1998). Several studies point towards the opiate-regulated ERK1/2 to be associated with mitogenesis, regulation of dopamine as well as glucose homeostasis, desensitization and tolerance development (Wilson et al. 1997; Olianas et al. 2011; Qiu et al. 2014; Kivell et al. 2014).
We also curated other MAPKs which play an important role in the signal transduction of opiates including, c-Jun N-terminal kinase (JNK) and p38 MAPK. The opioid receptors demonstrate JNK signaling in a subtype or ligand-specific manner. A PI3-kinase mechanism is observed downstream of DOR inducing protein kinase B/AKT-dependent JNK phosphorylation (Shahabi et al. 2006), while JNK activity is PI3kinase-independent under other receptor subtypes (Kam et al. 2004a). Additionally, JNK activation through MOR was also shown to require PKC activity (Melief et al. 2010). Interestingly, an opioid-dependent p38 MAPK phosphorylation was mostly demonstrated by all four subtypes (Zhang et al. 1999; Rozenfeld-Granot et al. 2002; Robinson and McDonald 2015). As provided in the literature and depicted in the map, SNC80 and DPDPE stimulated DOR led to the activation of p38 in a Gi/0/Src/MEK-dependent manner. This resulted in the upregulation of SLC1A2/A3 proteins and was found to be associated with the regulation of glutamate homeostasis in mouse astrocyte cultures (Liang et al. 2014). PKA-dependent phosphorylation of p38MAPK through OPRL1 led to an increase in MBP protein expression along with the phosphorylation of ATF2, aiding in the physiological functioning of the nervous system (Zhang et al. 1999). As shown in previous studies and represented in the map, the PLD2-dependent phosphorylation of MAPK14 facilitates the endocytosis of DOR and MOR (Yang et al. 2010), whereas, KOR stimulation induced phosphorylation of MAPK14 influences the crosstalk between orexin receptor and KOR, thus enabling dopaminergic neuronal response (Robinson and McDonald 2015).
Ligand-dependent opioid receptor regulation
One of the challenges of opioid signaling studies is that receptor trafficking and regulation vary depending upon the agonist. For instance, in contrast to DAMGO, which promotes robust internalization of MOR receptor, morphine is capable of promoting a weak receptor internalization (Finn and Whistler 2001; Charfi et al. 2014)). KOR which also shares trafficking similar to MOR, gets phosphorylated, desensitized and internalized by agonists U50,488 and dynorphin 1–17 and not by etorphine and levorphanol (Blake et al. 1997). Interaction studies of these GPCRs have suggested the role of the C-terminal tail of DOR, MOR, KOR to be crucial for arrestin 2/3 binding. Arrestins assist in determining the fate of the receptor by internalization (Bohn et al. 2000b; Moulédous et al. 2012; Schmid et al. 2013). Additionally, receptor phosphorylation is known to be related to the ability of the agonist to induce internalization. Phosphorylation of DOR at Ser363 regulates the abilities of the DOR agonists DPDPE and TIPP to activate ERK by G-protein- or β-arrestin-dependent pathways. While TIPP operated through the β-arrestin1/2- pathway, DPDPE used G-protein as the primary mediator to activate the ERK cascade in an Src-dependent manner (Audet et al. 2005; Xu et al. 2010). In the signaling map, we also provide a signaling cascade of varied proliferation mechanisms by DOR and MOR. A DPDPE-induced DOR activation resulted in decreased cell proliferation through the phosphorylation of CAMK2A and PKCα, along with downregulation of PCNA in rat BDL cholangiocytes. On the contrary, DAMGO-stimulated MOR to cause an upregulation of PCNA resulting in increased proliferation in rat BDL cholangiocytes (Marzioni et al. 2006).
The activation of Src kinase under the KOR, DOR and MOR exerts varied physiological outcomes. Upon stimulation, KOR regulates the inflammatory response by inducing a Gβγ-dependent activation of Src resulting in JNK activation routed via RAC1 and CDC42 in THP1 cells (Kam et al. 2004b). Interestingly, under DOR, the Src cascade activates MAPK1/3 which in turn phosphorylates MAPK14, leading to the maintenance of glutamate homeostasis in mouse astrocyte cultures (Liang et al. 2014). Remarkably, the phosphorylation of MOR at Tyr336 by Src serves as a docking site for the Grb/SOS/Ras/Raf1, thus triggering the conversion of MOR from a classic Gi/0-coupled receptor to a receptor tyrosine kinase-like entity (Zhang et al. 2013).
The agonist-induced MOR stimulation leads to activation of three different effectors of phosphoinositide 3-kinase (PI3K)-dependent signaling cascade which includes the phosphorylation of p70S6 kinase and the repressors of mRNA translation, 4EBP1 and 4EBP2, associated with neuronal survival and translational control (Polakiewicz et al. 1998). The signaling cascade involving Gi/G0, Src, PI3K, and Akt through an activated DOR receptor-stimulated glucose transport (Liang et al. 2014). Furthermore, the calcium-Cx43 pathway modulated calcium influx through KOR activation with U50,488H, resulting in antiarrhythmic effects (Shi et al. 2013). Internalization of the channels mediated by nociceptin is accompanied by a significant decrease in calcium associated with long-term regulation of calcium influx in the pain pathway (Altier et al. 2006). Activation of the transcription factor, signal transducer and activator of transcription 3 (STAT3) by phosphorylation has varying fates depending on the receptor subtype. In the case of KOR-stimulated rat hippocampus and MOR-activated mouse retinal endothelial cells, phosphorylation of STAT3 leads to neuroprotection and angiogenesis, respectively (Chen et al. 2006; Fang et al. 2013). However, stimulation of DOR by SNC80 resulted in upregulation of MCL1, downstream to activated STAT3, aiding in the survival of rat mesenchymal stem cells (Higuchi et al. 2012). Mitogenesis was observed to be emanated through different cascades under KOR and DOR. A Gβγ/PLC/PKC/Ras-mediated ERK1/2 phosphorylation led to mitogenesis upon KOR activation in C6 glioma cells, whereas, mitogenesis was achieved by DOR via Gi/0-PI3K and FRAP/RAFT-dependent p70S6 kinase phosphorylation as well as PI3K-independent ERK1/2 phosphorylation (Wilson et al. 1997; Bohn et al. 2000a). As represented in the map, opiate dependence and addiction upon MOR stimulation followed the route of a PI3K/MEK-dependent upregulation of ARC in mouse striatum and Neuro2A cells (Ziółkowska et al. 2005).
The evidence on ligand-directed signaling or biased agonism reported for opioid receptor has been a significant advancement in opioid pharmacology. Biased agonism suggests the binding of specific ligands to the receptor, which stimulates a specific activation of the downstream cascades in a preferential manner. This distinguishes the desirable functions of the receptor from the adverse drug-induced responses (Schmid et al. 2017; Faouzi et al. 2020). For instance, in a demonstration of functional selectivity on KOR, the partial agonist 6’GNTI (6′-Guanidinonaltrindole) displayed biased coupling to G protein over β-arrestin2 recruitment-mediated receptor internalization in CHO cells and mouse primary neuronal cultures (Schmid et al. 2013). Similarly, a strong agonist of MOR, TRV130 (oliceridine; N-[(3-methoxythiophen-2-yl)methyl]-2-[(9 R)-9-pyridin-2-yl-6-oxaspiro[4.5]decan-9-yl]ethanamine), elicits potent and efficacious G-protein coupling when compared to morphine, however, shows weaker β-arrestin-2 recruitment and reduced receptor internalization in rodents (Altarifi et al. 2017). This led to the development of G-protein‐biased opioid receptor agonists as safer analgesics that activate G-proteins better than they recruit arrestins (Kliewer et al. 2020). However, there is a growing interest that challenges this hypothesis and suggests the involvement of G-protein-dependent components in developing side effects such as opioid-induced respiratory depression (Montandon et al. 2016).
Chronic opioid administration and receptor tolerance
Prolonged treatment with opiates results in opioid tolerance that causes a diminution of its effect; hence, a higher dose is needed to maintain the function. The tolerance development to opiates due to prolonged treatment is thought to be associated with the desensitization of the opioid receptors. GPCR desensitization is caused by three different mechanisms; receptor phosphorylation, receptor internalization and/or sequestration, and receptor downregulation indicated by a reduced number of receptors (Rajagopal and Shenoy 2018). One of the important cascades curated from literature involves NOTCH1, downstream of chronic morphine treatment induced MOR activation which explains the interactions between neurons and glia eventually leading to morphine tolerance (Sanna et al. 2020). Another module downstream of MOR activation is that of the adenylyl cyclase 5 and 6 associated with the receptor leading to phosphorylation of KRAS, SRC, RAF1 and the activation of MAP2K1-mediated opioid tolerance (Zhang et al. 2013). Interestingly, we also observed PRKCE and GRK2/3-mediated receptor desensitization and tolerance under MOR stimulation (Mandyam et al. 2003). In the case of DOR, however, we observed a PLD2 activation mediated receptor desensitization and opioid tolerance along with GRK2 dependent receptor phosphorylation and desensitization. The signaling involved in the achievement of tolerance for MOR, DOR and KOR is also depicted in the pathway map (Fig. 1). Additionally, we observed the receptor recycling of MOR mediated by the ECE2 enzyme, which ideally binds to the internalized MOR and cleaves off the ligand thus freeing the receptor to be recycled to the plasma membrane (Gupta et al. 2015).
Overall, the opioid receptor pathway map highlights nociception, antinociceptive activity, receptor desensitization and tolerance development, cellular homeostasis, cell survival and protection from injury. A few interesting signaling cascades include regulation of genes associated with neuronal and synaptic plasticity upon MOR activation as well as keratinocyte wound repair, cardiomyocyte protection and muscle relaxation under DOR stimulation. Interestingly, protection of cartilage, inhibition of vascular development, antidepressant response and antiarrhythmic effects were found distinctly under KOR.
Conclusions
A detailed map of opioid receptor signaling will enable the scientific community with readily available data on opioid signal transduction. It will serve as a knowledge base for information pertaining to the activation of opioid receptors through different agonists/ligands. The map provides an overview of the known downstream signaling events derived from the literature, associated with the execution of opiate functions. This map attempts to categorize and characterize the versatility of the opiate-opioid receptor signaling system in mammals which will accentuate opioid research.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Department of Biotechnology, Government of India for research support to the Institute of Bioinformatics, Bangalore. Lathika Gopalakrishnan and Oishi Chatterjee are recipients of Inspire Fellowship from the Department of Science and Technology (DST), Government of India. We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka for the support to the Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017). Rajesh Raju is a recipient of the Young Scientist Award (YSS/2014/000607) from the Science and Engineering Research Board, Department of Science and Technology (DST), Government of India.
Abbreviations
- DOR
Delta opioid receptor
- MOR
Mu opioid receptor
- KOR
Kappa opioid receptor
- OPRL
Opioid like receptor
- GPCR
G protein-coupled receptor
- DADLE
[D-Ala2, D-Leu5]-Enkephalin
- DALDA
Tyr-D-Arg-Phe-Lys-NH2
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-Enkephalin
- OPRD1
δ-Opioid receptor gene
- OPRK1
κ-Opioid receptor gene
- OPRM1
μ-Opioid receptor gene
- PKA
Protein kinase A
- PKC
Protein kinase C
- PTX
Pertussis toxin
- GRK
G Protein-Coupled Receptor Kinase
Declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lathika Gopalakrishnan, Email: lathika@ibioinformatics.org.
Oishi Chatterjee, Email: oishi@ibioinformatics.org.
Namitha Ravishankar, Email: namitharavi12@gmail.com.
Sneha Suresh, Email: sneha108ss@gmail.com.
Rajesh Raju, Email: rajrrnbt@gmail.com.
Anita Mahadevan, Email: mahadevan.anita@gmail.com.
T. S. Keshava Prasad, Email: keshav@yenepoya.edu.in.
References
- Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiol. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, David B, Muchhala KH, et al. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol. 2017;31:730–739. doi: 10.1177/0269881116689257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Khosravani H, Evans RM, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- Anand JP, Montgomery D. Multifunctional opioid ligands. Handb Exp Pharmacol. 2018;247:21–51. doi: 10.1007/164_2018_104. [DOI] [PubMed] [Google Scholar]
- Audet N, Paquin-Gobeil M, Landry-Paquet O, et al. Internalization and Src activity regulate the time course of ERK activation by delta opioid receptor ligands. J Biol Chem. 2005;280:7808–7816. doi: 10.1074/jbc.M411695200. [DOI] [PubMed] [Google Scholar]
- Blake AD, Bot G, Li S, et al. Differential agonist regulation of the human kappa-opioid receptor. J Neurochem. 1997;68:1846–1852. doi: 10.1046/j.1471-4159.1997.68051846.x. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Belcheva MM, Coscia CJ. Mitogenic signaling via endogenous kappa-opioid receptors in C6 glioma cells: evidence for the involvement of protein kinase C and the mitogen-activated protein kinase signaling cascade. J Neurochem. 2000;74:564–573. doi: 10.1046/j.1471-4159.2000.740564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, et al. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nat. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charfi I, Nagi K, Mnie-Filali O, et al. Ligand- and cell-dependent determinants of internalization and cAMP modulation by delta opioid receptor (DOR) agonists. Cell Mol Life Sci. 2014;71:1529–1546. doi: 10.1007/s00018-013-1461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee O, Patil K, Sahu A, et al. An overview of the oxytocin-oxytocin receptor signaling network. J Cell Commun Signal. 2016;10:355–360. doi: 10.1007/s12079-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Farooqui M, Gupta K. Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc Res. 2006;3:171–180. doi: 10.2174/156720206778018767. [DOI] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, et al. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G, Radhakrishnan A, Syed N, et al. Signaling network of Oncostatin M pathway. J Cell Commun Signal. 2013;7:103–108. doi: 10.1007/s12079-012-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan BN, Cesselin F, Raghubir R, et al. International union of pharmacology. XII classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- Fang S, Xu H, Lu J, et al. Neuroprotection by the kappa-opioid receptor agonist, BRL52537, is mediated via up-regulating phosphorylated signal transducer and activator of transcription-3 in cerebral ischemia/reperfusion injury in rats. Neurochem Res. 2013;38:2305–2312. doi: 10.1007/s11064-013-1139-4. [DOI] [PubMed] [Google Scholar]
- Faouzi A, Varga BR, Majumdar S. Biased opioid ligands. Mol. 2020 doi: 10.3390/molecules25184257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Gavériaux-Ruff C. Opiate-induced analgesia: contributions from mu, delta and kappa opioid receptors mouse mutants. Curr Pharm Des. 2013;19:7373–7381. doi: 10.2174/138161281942140105163727. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- Gopalakrishnan L, Chatterjee O, Raj C, et al. An assembly of galanin-galanin receptor signaling network. J Cell Commun Signal. 2021;15:269–275. doi: 10.1007/s12079-020-00590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Fujita W, Gomes I, et al. Endothelin-converting enzyme 2 differentially regulates opioid receptor activity. Br J Pharmacol. 2015;172:704–719. doi: 10.1111/bph.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes BE, Fried S, Yao X, et al. Nociceptin (ORL-1) and mu-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem. 1998;71:1024–1033. doi: 10.1046/j.1471-4159.1998.71031024.x. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Ii M, Zhu P, Ashraf M. Delta-opioid receptor activation promotes mesenchymal stem cell survival via PKC/STAT3 signaling pathway. Circ J. 2012;76:204–212. doi: 10.1253/circj.cj-11-0309. [DOI] [PubMed] [Google Scholar]
- Huang J, Nalli AD, Mahavadi S, et al. Inhibition of Gαi activity by Gβγ is mediated by PI 3-kinase-γ- and cSrc-dependent tyrosine phosphorylation of Gαi and recruitment of RGS12. Am J Physiol Gastrointest Liver Physiol. 2014;306:G802–G810. doi: 10.1152/ajpgi.00440.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Janecka A, Fichna J, Janecki T. Opioid receptors and their ligands. Curr Top Med Chem. 2004;4:1–17. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Cvejic S, Devi LA. Opioids and their complicated receptor complexes. Neuropsychopharmacol. 2000;23:S5–S18. doi: 10.1016/S0893-133X(00)00143-3. [DOI] [PubMed] [Google Scholar]
- Kam AYF, Chan ASL, Wong YH. Phosphatidylinositol-3 kinase is distinctively required for mu-, but not kappa-opioid receptor-induced activation of c-Jun N-terminal kinase. J Neurochem. 2004;89:391–402. doi: 10.1111/j.1471-4159.2004.02338.x. [DOI] [PubMed] [Google Scholar]
- Kam AYF, Chan ASL, Wong YH. Kappa-opioid receptor signals through Src and focal adhesion kinase to stimulate c-Jun N-terminal kinases in transfected COS-7 cells and human monocytic THP-1 cells. J Pharmacol Exp Ther. 2004;310:301–310. doi: 10.1124/jpet.104.065078. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Keerthikumar S, Raju R, et al. PathBuilder–open source software for annotating and developing pathway resources. Bioinf. 2009;25:2860–2862. doi: 10.1093/bioinformatics/btp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, et al. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11:R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelder T, Pico AR, Hanspers K, et al. Mining biological pathways using WikiPathways web services. PLoS ONE. 2009;4:e6447. doi: 10.1371/journal.pone.0006447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell B, Uzelac Z, Sundaramurthy S, et al. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacol. 2014;86:228–240. doi: 10.1016/j.neuropharm.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Gillis A, Hill R, et al. Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol. 2020;177:2923–2931. doi: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer HK, Simon EJ. mu and delta-opioid receptor agonists induce mitogen-activated protein kinase (MAPK) activation in the absence of receptor internalization. Neuropharmacol. 2000;39:1707–1719. doi: 10.1016/s0028-3908(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Krishnamurti C, Rao SC. The isolation of morphine by Serturner. Indian J Anaesth. 2016;60:861–862. doi: 10.4103/0019-5049.193696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Chao D, Sandhu HK, et al. δ-Opioid receptors up-regulate excitatory amino acid transporters in mouse astrocytes. Br J Pharmacol. 2014;171:5417–5430. doi: 10.1111/bph.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Standifer KM. Mu-opioid-induced desensitization of opioid receptor-like 1 and mu-opioid receptors: differential intracellular signaling determines receptor sensitivity. J Pharmacol Exp Ther. 2003;306:965–972. doi: 10.1124/jpet.103.051599. [DOI] [PubMed] [Google Scholar]
- Marzioni M, Alpini G, Saccomanno S, et al. Endogenous opioids modulate the growth of the biliary tree in the course of cholestasis. Gastroenterol. 2006;130:1831–1847. doi: 10.1053/j.gastro.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Ren J, Victoria NC, et al. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiol. 2016;124:641–650. doi: 10.1097/ALN.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulédous L, Froment C, Dauvillier S, et al. GRK2 protein-mediated transphosphorylation contributes to loss of function of μ-opioid receptors induced by neuropeptide FF (NPFF2) receptors. J Biol Chem. 2012;287:12736–12749. doi: 10.1074/jbc.M111.314617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi K, Charfi I, Pineyro G. Kir3 channels undergo arrestin-dependant internalization following delta opioid receptor activation. Cell Mol Life Sci. 2015;72:3543–3557. doi: 10.1007/s00018-015-1899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Onali P. δ-Opioid receptors stimulate GLUT1-mediated glucose uptake through Src- and IGF-1 receptor-dependent activation of PI3-kinase signalling in CHO cells. Br J Pharmacol. 2011;163:624–637. doi: 10.1111/j.1476-5381.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SM, Subbannayya Y, Rex DAB, et al. A network map of IL-33 signaling pathway. J Cell Commun Signal. 2018;12:615–624. doi: 10.1007/s12079-018-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, et al. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhao W, Wang Y, et al. FK506-binding protein 12 modulates μ-opioid receptor phosphorylation and protein kinase C(ε)-dependent signaling by its direct interaction with the receptor. Mol Pharmacol. 2014;85:37–49. doi: 10.1124/mol.113.087825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Shenoy SK. GPCR desensitization: Acute and prolonged phases. Cell Signal. 2018;41:9–16. doi: 10.1016/j.cellsig.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R, Nanjappa V, Balakrishnan L, et al. NetSlim: high-confidence curated signaling maps. Database (oxford) 2011;2011:bar032. doi: 10.1093/database/bar032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T, Law SF, Blake A, Tallent M. Molecular mechanisms of opiate receptor coupling to G proteins and effector systems. Ann N Y Acad Sci. 1996;780:168–175. doi: 10.1111/j.1749-6632.1996.tb15121.x. [DOI] [PubMed] [Google Scholar]
- Robinson JD, McDonald PH. The orexin 1 receptor modulates kappa opioid receptor function via a JNK-dependent mechanism. Cell Signal. 2015;27:1449–1456. doi: 10.1016/j.cellsig.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld-Granot G, Toren A, Amariglio N, et al. MAP kinase activation by mu opioid receptor in cord blood CD34(+)CD38(-) cells. Exp Hematol. 2002;30:473–480. doi: 10.1016/s0301-472x(02)00786-5. [DOI] [PubMed] [Google Scholar]
- Sahu A, Gopalakrishnan L, Gaur N, et al. The 5-Hydroxytryptamine signaling map: an overview of serotonin-serotonin receptor mediated signaling network. J Cell Commun Signal. 2018;12:731–735. doi: 10.1007/s12079-018-0482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MD, Borgonetti V, Galeotti N. μ opioid receptor-triggered Notch-1 activation contributes to morphine tolerance: role of neuron-glia communication. Mol Neurobiol. 2020;57:331–345. doi: 10.1007/s12035-019-01706-6. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell. 2017;171:1165–1175.e13. doi: 10.1016/j.cell.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Groer CE, et al. Functional selectivity of 6’-guanidinonaltrindole (6’-GNTI) at κ-opioid receptors in striatal neurons. J Biol Chem. 2013;288:22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabi NA, McAllen K, Sharp BM. delta opioid receptors stimulate Akt-dependent phosphorylation of c-jun in T cells. J Pharmacol Exp Ther. 2006;316:933–939. doi: 10.1124/jpet.105.091447. [DOI] [PubMed] [Google Scholar]
- Shao X-M, Sun J, Jiang Y-L, et al. Inhibition of the cAMP/PKA/CREB pathway contributes to the analgesic effects of electroacupuncture in the anterior cingulate cortex in a rat pain memory model. Neural Plast. 2016;2016:5320641. doi: 10.1155/2016/5320641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q-X, Zhang L-J, Yao Y, et al. κ-opioid receptor activation prevents against arrhythmias by preserving Cx43 protein via alleviation of intracellular calcium. Am J Ther. 2013;20:493–501. doi: 10.1097/MJT.0b013e3182456676. [DOI] [PubMed] [Google Scholar]
- Soman S, Raju R, Sandhya VK, et al. A multicellular signal transduction network of AGE/RAGE signaling. J Cell Commun Signal. 2013;7:19–23. doi: 10.1007/s12079-012-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. Opioid receptors. Annu Rev Med. 2016;67:433–451. doi: 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- Subbannayya T, Leal-Rojas P, Barbhuiya MA, et al. Macrophage migration inhibitory factor-a therapeutic target in gallbladder cancer. BMC Cancer. 2015;15:843. doi: 10.1186/s12885-015-1855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Burt AR, Milligan G, Anderson NG. Mitogenic signalling by delta opioid receptors expressed in rat-1 fibroblasts involves activation of the p70s6k/p85s6k S6 kinase. Biochem J. 1997;325(1):217–222. doi: 10.1042/bj3250217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Hong M-H, Zhang L-S, et al. Serine 363 of the {delta}-opioid receptor is crucial for adopting distinct pathways to activate ERK1/2 in response to stimulation with different ligands. J Cell Sci. 2010;123:4259–4270. doi: 10.1242/jcs.073742. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Furuta S, Katayama S, et al. The κ opioid system regulates endothelial cell differentiation and pathfinding in vascular development. Blood. 2011;118:775–785. doi: 10.1182/blood-2010-09-306001. [DOI] [PubMed] [Google Scholar]
- Yang H-Y, Wu Z-Y, Wood M, et al. Hydrogen sulfide attenuates opioid dependence by suppression of adenylate cyclase/cAMP pathway. Antioxid Redox Signal. 2014;20:31–41. doi: 10.1089/ars.2012.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Seifert A, Wu D, et al. Role of phospholipase D2/phosphatidic acid signal transduction in micro- and delta-opioid receptor endocytosis. Mol Pharmacol. 2010;78:105–113. doi: 10.1124/mol.109.063107. [DOI] [PubMed] [Google Scholar]
- Zhang L, Loh HH, Law P-Y. A novel noncanonical signaling pathway for the μ-opioid receptor. Mol Pharmacol. 2013;84:844–853. doi: 10.1124/mol.113.088278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xin SM, Wu GX, et al. Endogenous delta-opioid and ORL1 receptors couple to phosphorylation and activation of p38 MAPK in NG108-15 cells and this is regulated by protein kinase A and protein kinase C. J Neurochem. 1999;73:1502–1509. doi: 10.1046/j.1471-4159.1999.0731502.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law P-Y. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziółkowska B, Urbański MJ, Wawrzczak-Bargieła A, et al. Morphine activates Arc expression in the mouse striatum and in mouse neuroblastoma Neuro2A MOR1A cells expressing mu-opioid receptors. J Neurosci Res. 2005;82:563–570. doi: 10.1002/jnr.20661. [DOI] [PubMed] [Google Scholar]
- Zöllner C, Stein C. Opioids. Handb Exp Pharmacol. 2007 doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.