Abstract
Chaperonins participate in the facilitated folding of a variety of proteins in vivo. To see whether the same spectrum of target proteins can be productively folded by the double-ring prokaryotic chaperonin GroEL-GroES and its single-ring human mitochondrial homolog, Hsp60-Hsp10, we expressed the latter in an Escherichia coli strain engineered so that the groE operon is under strict regulatory control. We found that expression of Hsp60-Hsp10 restores viability to cells that no longer express GroEL-GroES, formally demonstrating that Hsp60-Hsp10 can carry out all essential in vivo functions of GroEL-GroES.
Chaperonins are ubiquitous, essential, multisubunit ATPases in which the subunits form a cylindrical structure (a ring) enclosing a central cavity. They are thought to assist the folding of their target proteins by providing a sequestered environment conducive to correct folding in which extended proteins can fold while shielded from nonproductive interactions with other proteins (reviewed in references 2, 3, and 7). Two classes of chaperonin have been defined on the basis of sequence relationships and the requirement for cochaperonin (9): class I chaperonins (found in prokaryotes and the organelles descended from them), which function in conjunction with a cochaperonin, and class II chaperonins (found in archaebacteria and in the cytosol of eukaryotes), which have no requirement for cochaperonin. Only one kind of chaperonin molecule generally exists in any given cellular compartment; therefore, any chaperonin must be capable of facilitating the correct folding of a range of target proteins. For example, in Escherichia coli, the chaperonin GroEL (together with its cochaperonin GroES) is thought to participate in the facilitated folding of 2 to 7% of newly synthesized proteins (4, 11). Because these substrates are likely to be diverse in sequence, it follows that the binding of target protein by chaperonin must depend on properties common to misfolded, unfolded, or partially folded target molecules (such as, for example, exposed hydrophobic surfaces) that are recognized by corresponding surfaces lining the chaperonin’s central cavity (1).
The wide range of target proteins bound by chaperonins suggests that chaperonin-mediated folding could be so nonspecific that any chaperonin might facilitate the folding of any target protein. However, this is not the case. Class I chaperonins are incapable of facilitating the productive folding of actins and tubulins, target proteins which can proceed to the native state only via an interaction with the class II chaperonin found in the cytosol of eukaryotes (18). Conversely, the eukaryotic cytosolic chaperonin does not bind unfolded malate dehydrogenase, which is recognized and folded by class I chaperonins (2). These observations are examples of chaperonin specificity, a phenomenon which probably reflects the coevolution of chaperonins and their principal target proteins (10).
Among class I chaperonins, a major difference exists in the mechanism of the facilitated folding reaction. In E. coli, the chaperonin GroEL functions by a two-stroke engine action whereby the binding and hydrolysis of ATP by one chaperonin ring controls the release of the cochaperonin GroES from the opposite ring (8, 12, 17, 19, 24). In contrast, the mitochondrial homolog of GroEL-GroES, Hsp60-Hsp10, functions as a single ring, without any transition through a double-ring intermediate (15). Given this difference and the sequence divergence between GroEL and human Hsp60 (21) (the two proteins are 51% identical in sequence), we decided to explore the possibility that these chaperonins might not recognize an identical set of target proteins. Here we show that Hsp60-Hsp10 can replace GroEL-GroES in E. coli cells. Thus, despite their differences in sequence and mechanism, Hsp60-Hsp10 can facilitate the productive folding of all essential proteins that are dependent on GroEL-GroES. However, the inability of Hsp60-Hsp10 to support the maturation of GroE-dependent bacteriophage lambda and T4 proteins suggests that some specificity differences may indeed exist between these two chaperonin systems.
Transformation into MGM100 of constructs engineered for the expression of various chaperonins and cochaperonins.
MGM100, a derivative of the K-12 strain MG1655 described in reference 14, has been engineered so that the expression of the groE operon, although remaining in a single copy on the chromosome, is under the control of the inducible arabinose promotor PBAD. Since expression from PBAD is between 100- and 1,000-fold higher on arabinose broth than on glucose broth (6) and the amount of GroE produced by MGM100 growing on arabinose is less than that produced by MG1655 from PgroE (see below), the amount of GroE produced by MGM100 growing on glucose will be negligible. Consistent with this, MGM100 is inviable in the absence of exogenously supplied arabinose, confirming that GroEL and GroES are essential for growth. To determine whether Hsp60-Hsp10 can substitute for GroEL-GroES or whether the apical, substrate binding domain of Hsp60 can substitute for the cognate domain of GroEL, we introduced into MGM100 a series of plasmid constructs in which the groE promoter was used to express GroEL, Hsp60, or chaperonins and cochaperonins together. Constructs included those expressing Hsp60-Hsp10, GroEL-GroES, or one of two chimeric assemblies of GroEL and Hsp60 together with Hsp10 (15). The chimeras consist of an Hsp60-derived apical domain fused to either a wild-type GroEL equatorial domain (Hsp60-GroEL) or to a mutant GroEL, SR1 (23), engineered so that ring-ring interactions are abolished (Hsp60-GroELSR1).
Plasmid transformants of MGM100 were selected on either glucose (no production of chromosome-encoded chaperonin) or arabinose (to induce chromosome-encoded expression of GroEL-GroES). In the presence of glucose, normal progeny of uniform colony size were obtained with all constructs expressing both a chaperonin and a suitable cochaperonin (Table 1). It follows that Hsp60-Hsp10 and the chimeric proteins plus Hsp10 can substitute for GroEL-GroES in all essential folding reactions and that the double-ringed, two-stroke engine mechanism utilized by GroE is not required for folding essential chaperonin-dependent proteins in E. coli. The failure of plasmids expressing GroEL alone or Hsp60 alone to yield transformants on glucose proves that the MGM100 chromosome is not supplying the cochaperonin needed to support colony formation. In vitro, GroEL can function with either GroES or Hsp10, but Hsp60 will fold target proteins only in conjunction with its cognate cochaperonin, Hsp10 (22). Since GroEL coexpressed with Hsp10 can replace GroEL-GroES in MGM100, we conclude that GroEL can also function with Hsp10 in vivo.
TABLE 1.
Effects of temperature and chaperonin expression on growth of MGM100a
| Plasmid | Original transformants
|
Restreaked single colonies
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 37°C
|
40°C
|
42°C
|
43°C
|
|||||||
| Ara | Glc | Ara | Glc | Ara | Glc | Ara | Glc | Ara | Glc | |
| Controlb | +++ | − | +++ | − | ++ | − | + | − | − | − |
| pHsp60 | Mixed | − | +++ | − | NT | NT | NT | NT | − | − |
| pHsp60-Hsp10 | Mixed | +++ | +++ | +++ | ++ | +++ | + | ++ | − | − |
| pHsp60-GroEL-Hsp10 | +++ | +++ | +++ | +++ | NT | NT | NT | NT | − | − |
| pHsp60-GroELSR1-Hsp10 | +++ | +++ | +++ | +++ | NT | NT | NT | NT | − | − |
| pGroEL | +++ | − | +++ | − | NT | NT | NT | NT | − | − |
| pGroEL-Hsp10 | +++ | +++ | +++ | +++ | NT | NT | NT | NT | +++ | +++ |
| pGroES-GroEL | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
Fresh transformants of MG1655 were streaked on LB medium containing ampicillin (50 μg/ml) and either 0.2% glucose or 0.2% arabinose and examined after overnight incubation. +++, large colonies; ++, small colonies; +, patch growth without distinguishable single colonies; −, no growth; NT, not tested. “Mixed” indicates a mixture of different-size colonies.
Control plasmid was either the vector plasmid pET3d or pBR325.
MGM100(pHSP60-Hsp10) transformants selected on glucose were transferred to broth, and their growth was compared with that of MGM100(pBR325) (Fig. 1). The latter strain, as previously found (14), lyses when grown without arabinose. In contrast, MGM100(pHsp60-Hsp10) continues to grow exponentially at the same rate on glucose as MGM100(pBR325) on arabinose, indicating that Hsp60-Hsp10 can support normal exponential growth of E. coli in the absence of endogenous GroEL-GroES.
FIG. 1.
Growth in LB plus 0.2% arabinose (open symbols) or glucose (closed symbols) of MGM100 plasmid transformants at 37°C. Cultures grown overnight on LB plus arabinose were diluted 1:1,000 into LB plus arabinose or glucose. Shown are data for pBR325 (triangles) and pHsp60-Hsp10 (circles). All cultures were diluted 10-fold into a prewarmed medium 140, 225, and 325 min after inoculation to maintain exponential growth, except for the MGM100(pBR325) glucose culture, which was diluted only at 140 min.
When selected on a medium containing arabinose (to allow the expression of endogenous GroEL-GroES), transformants were obtained for all the constructs tested (Table 1). However, in contrast to the uniform progeny obtained with plasmids that did not encode Hsp60, those encoding either Hsp60-Hsp10 or Hsp60 alone gave rise to progeny displaying a range of colony sizes, many very small, suggesting that the coexpression of Hsp60 and GroEL can be deleterious. This could be either a consequence of the coassembly of subunits derived from the two chaperonins resulting in the generation of partially functional or nonfunctional mixed oligomers or a mutual inhibition of assembly such that reduced numbers of active chaperonin molecules are formed. Note that the plasmids encoding chimeric chaperonins did not cause growth inhibition on arabinose. If these chimeric chaperonins (15) form mixed oligomers, these mixed oligomers must be functional.
All plasmids were also transformed into MG1655, the groE+ parent of MGM100. In contrast to MGM100 transformants, all MG1655 transformant colonies were of normal size. Since the two strains differ principally in that MG1655 can produce more GroE (by responding to stress—see below) than MGM100, the intolerance of MGM100 for the coproduction of GroE and Hsp60 is likely to be due to the limited amount of GroE that it produces.
Hsp60-Hsp10 can support growth at temperatures up to 42°C, but coproduction with GroEL-GroES inhibits growth.
To further explore the ability of Hsp60-Hsp10 to support E. coli growth, we streaked single colonies of the transformants onto a medium containing either arabinose or glucose and incubated them at various temperatures. All strains grew in the presence of arabinose at 37°C, and strains expressing compatible chaperonin and cochaperonin pairs also grew in the presence of glucose (Table 1). As expected, strains expressing Hsp60 alone, GroEL alone, or no chaperonin failed to grow at any temperature on glucose.
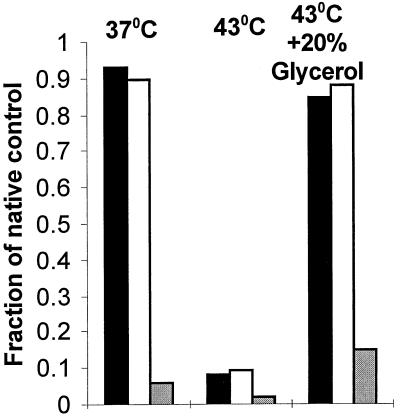
At 43°C, strains expressing GroEL together with either GroES or Hsp10 from plasmids grew well on both media. However, strains expressing Hsp60 (or chimeric chaperonins containing a GroEL-derived equatorial domain) together with Hsp10 failed to grow on glucose at 43°C, although growth of MGM100 synthesizing Hsp60-Hsp10 was evident at temperatures up to 42°C. Failure to grow at 43°C seems unlikely to be attributable to the mechanistic incompetence of Hsp60-Hsp10, because in in vitro folding assays (done as described in reference 15), this chaperonin folds malate dehydrogenase with the same efficiency as GroEL-GroES at either 37 or 43°C (Fig. 2). It seems more likely that insufficient Hsp60 is produced in E. coli from our constructs to supply the higher levels of chaperonin needed for high temperature growth. That higher amounts of chaperonin are indeed required at high temperatures is evident from the failure of MGM100 to grow at 43°C, even in the presence of arabinose. Since MGM100 is dependent on GroE expressed from PBAD, a promoter that is not upregulated by temperature (13), the failure of MGM100 to grow at high temperatures indicates that more GroE is needed to grow at these temperatures than is produced from PBAD.
FIG. 2.
Recovery of malate dehydrogenase enzymatic activity (expressed as a fraction of a native control) obtained in in vitro folding reactions (15) done at 37 or 42°C in the presence of either GroEL-GroES (black bars), Hsp60-Hsp10 (white bars), or no chaperonin (gray bars).
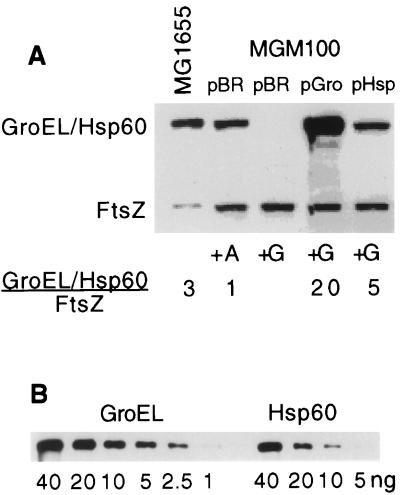
To determine whether MGM100 does indeed produce relatively low levels of Hsp60 from these plasmid constructs, we performed a Western blot analysis on extracts of cells grown at 37°C (Fig. 3). Cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes, and the blots were probed with a polyclonal antiserum (Stressgen, Inc.) that detects GroEL and Hsp60. FtsZ, which is not affected by GroE depletion, was measured by using an anti-FtsZ antibody so as to provide a measure of overall protein in the extracts. Bound antibody was detected with the chemiluminescence ECL detection kit (Amersham, Inc.). We found, firstly, that MGM100 induced with 0.2% arabinose produces only one-third as much GroEL as that produced from the groE promoter in MG1655, the parent of MGM100. This would not be increased at high temperatures (13) and probably accounts for the MGM100 temperature sensitivity. The presence of pGroES-GroEL increases GroEL levels 20-fold. In contrast, MGM100(pHsp60-Hsp10) produces less than twice as much Hsp60 as MG1655 produces GroEL, despite transcription from identical promoters in the two cases and the high copy number of pHsp60-Hsp10. It is not clear whether this is a consequence of a relatively reduced translation of the human protein (the gene contains codons that are rare in E. coli) or of the instability of Hsp60.
FIG. 3.
Amounts of chaperonin 60 measured by Western blotting. (A) MGM100 harboring pBR325 (labelled pBR), pGroES-GroEL (pGro), or pHsp60- Hsp10 (pHsp), grown overnight in LB plus arabinose, were diluted 1:2,000 into LB plus glucose (+G) or arabinose (+A) and sampled after 3 h. MG1655 was grown in LB. All cultures were grown at 37°C. Densitometry of the image shown in panel A and also of others (not shown) on which the extracts were diluted were used to determine protein concentrations. The values for Hsp60 were corrected for the fact (see panel B) that it reacts less well with anti-GroEL. (B) Indicated amounts of purified proteins were used to construct a standard curve for sample measurement. Anti-GroEL detects GroEL with about three times the sensitivity with which it detects Hsp60.
The growth of MGM100(pHsp60-Hsp10) observable on glucose appears to be inhibited by arabinose at temperatures between 40 and 42°C. This behavior confirms the observation made above that subunits derived from GroEL and Hsp60 can interact, either to form inactive mixed multimers or to interfere with the assembly of multimers of the other type. Although stable mixed GroEL-Hsp60 multimers form when the parent chaperonins are mixed, dissociated, and allowed to reassociate in vitro (16) we do not know whether they are biologically active. Since there is only just enough Hsp60 produced on glucose to support growth at higher temperatures, the loss of Hsp60 to inactive mixed multimers could reduce the supply to below that critical level. MG1655(pHsp60-Hsp10), on the other hand, grows satisfactorily at all temperatures tested. We infer that this is because MG1655, which produces GroE from its native promoter and is therefore induced to produce high levels of GroE at 43°C, can form enough homo-oligomers to support growth at high temperatures, even in the presence of high levels of Hsp60.
In order to further examine the effect of Hsp60-Hsp10 production on the colony growth rate, aliquots of serial dilutions of MGM100(pBR325), MGM100(pHsp60-Hsp10), and MGM100(pGroES-GroEL) were plated on broth plus arabinose or glucose and incubated at 37 or 42°C. Colonies were counted after 1 or 2 days (Table 2). When GroE is supplied from a plasmid, MGM100 grows rapidly and forms an equal number of colonies on both media at each temperature. In contrast, when MGM100 is relying on GroE expressed from the PBAD promoter, growth is satisfactory at 37°C but poor at higher temperatures; this is indicated both by slowed colony growth and reduced viability. When Hsp60-Hsp10 (but not GroE) are available, the colony growth rate is reduced at both temperatures, but the final viability is not. However, when both the Hsp and GroE proteins are produced, growth is slower at both temperatures than growth on glucose, and the final viability at high temperatures, already low because of insufficient GroE, is not enhanced. We conclude that MGM100 does better with Hsp60-Hsp10 alone, compared to the situation in which GroE is also expressed. Since this incompatibility between chaperonins is not observed during exponential growth in liquid, these growth limitations most likely take effect during the postexponential stages of growth.
TABLE 2.
Effects of Hsp60-Hsp10 expression on MGM100 colony formationa
| Plasmid | Relative colony number
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 37°C
|
42°C
|
|||||||
| Glc
|
Ara
|
Glc
|
Ara
|
|||||
| 1 day | 2 days | 1 day | 2 days | 1 day | 2 days | 1 day | 2 days | |
| pGroES-GroEL | 1.0 | 1* | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| pHsp60-pHsp10b | 0.7 | 1* | 0.3 | 1.0 | 0.6 | 0.9 | 0.1 | 0.5 |
| pBR325 | <10−4 | <10−4 | 1.0 | 1* | <10−4 | <10−4 | 0.2 | 0.4 |
Colonies grown on arabinose were suspended in a buffer, and aliquots of dilutions were spotted onto LB plus 0.2% arabinose or glucose. Numbers of colonies of dilutions yielding between 10 and 100 colonies were counted and normalized, for each strain, to the number in the sample labelled (*).
Results are averages of three separate measurements. Note that colonies at 1 day on Ara are very small compared to others.
Hsp60-Hsp10 is limited (relative to GroEL-GroES) in its ability to support bacteriophage growth.
Mutations in the groE genes were originally identified because they prevented bacteriophage growth. GroEL-GroES are required for bacteriophage λ head assembly (5) and for bacteriophage T5 tail assembly (25). GroEL, but not GroES, is needed for phage T4 head assembly; this is because T4 encodes a protein, gp31, which substitutes for GroES in the chaperonin cycle (20). In order to explore potential differences in the abilities of the E. coli and mitochondrial chaperonins to facilitate the folding of bacteriophage proteins, we examined the growth of these three bacteriophages in MGM100(pHsp60-Hsp10) as follows. To make plating cells, MGM100 with plasmid was grown in selective conditions overnight in Luria broth (LB) with arabinose or glucose and reinoculated into the same medium on the following day. For T4, chilled late-log-phase cells were used. For λ, log-phase cells were centrifuged and resuspended in 0.01% MgCl2; these cells were also used for T5. Phage dilutions were preincubated with 0.1 ml of plating cells, suspended in 2.5 ml of BBL (1% trypticase, 0.5% NaCl)–0.7% top agar containing 0.2% arabinose or glucose and plated on sugar-supplemented LB or BBL plates. The results are presented in Table 3. Note that plaques of reduced size are observed in all cases when both Hsp60-Hsp10 and GroEL are present, providing further evidence of incompatibility between the two chaperonins.
TABLE 3.
Support of bacteriophage growth by MGM100(pHsp60-Hsp10) at 37°Ca
| Plasmid | Relative numbers of plaques with phage (medium)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λv (LB)
|
λv (BBL)
|
T4 (LB)
|
T4 (BBL)
|
T5 (LB)
|
||||||
| Ara | Glc | Ara | Glc | Ara | Glc | Ara | Glc | Ara | Glc | |
| pBR325 | 1* | 1.1 | 1.0 | 1* | 1 | |||||
| pHsp60-Hsp10 | 0.9 | <2 × 10−3 | 1.1 | 0.7 | 0.8 | <2 × 10−3 | NT | <2 × 10−3 | 0.6 | 0.5 |
| pGroES-GroEL | 1.0 | 0.8 | 1.1 | 1.0 | 0.4 | 0.9 | NT | 1.1 | 0.9 | 0.6 |
MGM100 containing the indicated plasmid and grown for plating on LB plus ampicillin (50 μg/ml) plus 0.2% arabinose or, where it would support growth, 0.2% glucose was used as described. Numbers of plaques varied between 200 and 800 per plate; italicized numbers indicate that plaques were small. T4 and λ experiments were repeated several times and normalized, respectively, to values labelled (*). NT, not tested.
Hsp60-Hsp10 is clearly able to support the growth of T5, although the efficiency of plating may be reduced slightly. The mitochondrial chaperonin does not, however, support the growth of either λ or T4 on LB. Reduced numbers of very small lambda plaques were obtained on a less rich medium (BBL) where cell growth is slow, indicating that Hsp60-Hsp10 can assist the folding of λ proteins, albeit not very efficiently. T4 plaques were not obtained on either medium, even when 108 phages were added to a single plate. Hsp60 is unable to use GroES as a cochaperonin, and it is possible that it also cannot interact with gp31. This may be the reason why T4 growth is not supported by the mitochondrial chaperonin, rather than because gp23, the T4 GroEL substrate, fails to interact with Hsp60.
The data presented here formally demonstrate that the mitochondrial homolog of GroEL-GroES, Hsp60-Hsp10, can replace GroEL-GroES in growing E. coli cells. It follows that there are no target proteins essential for growth that cannot be productively folded by both these chaperonins. We cannot exclude the possibility that there are differences in folding efficiency or in target specificity for inessential proteins. Indeed, the reduced efficiency with which Hsp60-Hsp10 supports the growth of phages and the reduced efficiency of colony formation of cells dependent on Hsp60-Hsp10 suggest that there may be some such differences. We conclude that the single-ring mechanism of action used by Hsp60-Hsp10 can adequately replace the double-ring two-stroke mechanism characteristic of GroEL-GroES. We also find that the coexpression of GroES-GroEL and Hsp60 affects growth in a manner most easily explained by the hypothesis that subunits derived from the two chaperonins can interact, either to inhibit the completion of ring formation or by coassembly to form functionally compromised mixed multimers.
Acknowledgments
This work was supported by a grant (to N.J.C.) from the National Institutes of Health. M.M. and N.M. thank the U.K. Medical Research Council for financial support.
We also thank S. McAteer for technical assistance.
REFERENCES
- 1.Braig K, Otwinowski Z, Hegde R, Boisvert D, Joachimiak A, Horwich A L, Sigler P B. The crystal structure of the bacterial chaperonin GroEL at 2.8A. Nature. 1994;367:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 2.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 3.Ellis R J. The chaperonins. San Diego, Calif: Academic Press; 1996. [Google Scholar]
- 4.Ellis R J, Hartl F-U. Protein folding in the cell: competing models of chaperonin function. FASEB J. 1996;10:20–26. doi: 10.1096/fasebj.10.1.8566542. [DOI] [PubMed] [Google Scholar]
- 5.Georgopoulos C, Hendrix R W, Casjens S W, Kaiser A D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973;76:45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- 6.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartl F-U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 8.Kad N M, Ranson N A, Cliff M J, Clarke A R. Asymmetry, commitment and inhibition in the GroE ATPase cycle impose alternating functions on the two GroEL rings. J Mol Biol. 1998;278:267–278. doi: 10.1006/jmbi.1998.1704. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Willison K R, Horwich A R. Cystosolic chaperonin subunits have a conserved ATPase domain but diverged polypeptide-binding domains. Trends Biochem Sci. 1994;19:543–548. doi: 10.1016/0968-0004(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 10.Lewis S A, Tian G, Cowan N J. Chaperonin-mediated folding of actin and tubulin. Trends Cell Biol. 1997;7:479–484. doi: 10.1016/S0962-8924(97)01168-9. [DOI] [PubMed] [Google Scholar]
- 11.Lorimer G H. A quantitative assessment of the role of the chaperonin proteins in protein folding in vivo. FASEB J. 1996;10:5–9. doi: 10.1096/fasebj.10.1.8566548. [DOI] [PubMed] [Google Scholar]
- 12.Lorimer G H. Folding with a two-stroke motor. Nature. 1997;388:720–723. doi: 10.1038/41892. [DOI] [PubMed] [Google Scholar]
- 13.McAteer, S., and M. Masters. Unpublished data.
- 14.McLennan N, Masters M. GroE is vital for cell-wall synthesis. Nature. 1998;392:139. doi: 10.1038/32317. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen K L, Cowan N J. A single ring is sufficient for productive chaperonin-mediated folding in vivo. Mol Cell. 1998;2:93–100. doi: 10.1016/s1097-2765(00)80117-3. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen, K. L., and N. J. Cowan. 1998. Unpublished observations.
- 17.Rye H S, Burston S G, Fenton W A, Beechem J M, Xu Z, Sigler P B, Horwich A L. Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL. Nature. 1997;388:792–798. doi: 10.1038/42047. [DOI] [PubMed] [Google Scholar]
- 18.Tian G, Vainberg I E, Tap W D, Lewis S A, Cowan N J. Specificity in chaperonin-mediated protein folding. Nature. 1995;375:250–253. doi: 10.1038/375250a0. [DOI] [PubMed] [Google Scholar]
- 19.Todd J M, Viitanen P V, Lorimer G H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994;265:659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- 20.van der Vies S M, Gatenby A A, Georgopoulos C. Bacteriophage T4 encodes a co-chaperonin that can substitute for Escherichia coli GroES in protein folding. Nature. 1994;368:654–656. doi: 10.1038/368654a0. [DOI] [PubMed] [Google Scholar]
- 21.Venner T J, Singh B, Gupta R H. Nucleotide sequences and novel structural features of human and Chinese hamster Hsp60 (chaperonin) gene families. DNA Cell Biol. 1990;9:545–552. doi: 10.1089/dna.1990.9.545. [DOI] [PubMed] [Google Scholar]
- 22.Viitanen P V, Lorimer G H, Seetheram R, Gupta R S, Oppenheim J, Thomas J O, Cowan N J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J Biol Chem. 1992;267:695–698. [PubMed] [Google Scholar]
- 23.Weissman J S, Hohl C M, Kovalenko O, Kashi Y, Chen X, Braig K, Saibil H R, Fenton W A, Horwich A L. Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83:577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 24.Xu Z, Horwich A L, Sigler P. The crystal structure of the assymetric GroEL-GroES-(ADP)7 complex. Nature. 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- 25.Zweig M, Cummings D J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973;80:505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]