Abstract
Diabetic kidney disease (DKD) is a serious microvascular complication of diabetes and is the leading cause of end-stage renal disease (ESRD). Persistent proteinuria is an important feature of DKD, which is caused by the destruction of the glomerular filtration barrier (GFB). Glomerular endothelial cells (GECs) and podocytes are important components of the GFB, and their damage can be observed in the early stages of DKD. Recently, studies have found that crosstalk between cells directly affects DKD progression, which has prospective research significance. However, the pathways involved are complex and largely unexplored. Here, we review the literature on cellular crosstalk of GECs and podocytes in the context of DKD, and highlight specific gaps in the field to propose future research directions. Elucidating the intricates of such complex processes will help to further understand the pathogenesis of DKD and develop better prevention and treatment options.
Keywords: Diabetic kidney disease, Glomerular endothelial cell, Podocyte, Cellular crosstalk, High glucose
Introduction
Diabetic kidney disease (DKD) is a serious microvascular complication of diabetes and the main cause of end-stage renal disease (ESRD) (Shaw et al. 2010). Therapies for early stage DKD are limited. Traditional methods include strict control of blood glucose and use of renin–angiotensin system (RAS) antagonists, which can reduce proteinuria to a certain extent. Some emerging therapies are increasingly being applied in clinical setting, including sodium-glucose cotransporter 2 (SGLT2) inhibitors (Hanai and Babazono 2020), dipeptidyl peptidase-4 (DPP-4) inhibitor (Guo et al. 2016), and glucagon-like protein 1 receptor (GLP-1R) agonists (Greco et al. 2019). However, they can only delay the progression of DKD and cannot prevent the progression to ESRD. Potential therapeutic targets and current clinical trial results are shown in Table 1. The metabolism in patients with DKD is highly disordered, which complicates the treatment of ESRD. Therefore, DKD is considered a major health and financial burden (Wada and Makino 2012).
Table 1.
Potential therapeutic targets of DKD
| Target | Medication | Current clinical trial result | Reference |
|---|---|---|---|
| Mineralocorticoid receptor | Finerenone | Effective | Bakris et al. (2019) |
| SGLT2 | Dapagliflozin | Effective | Mosenzon et al. (2019) |
| GLP-1 | Liraglutide | Effective | Thornton et al. (2017) |
| DPP-4 | Linagliptin | Effective | Perkovic et al. (2020) |
| AGEs | Pyridorin | Ineffective | Lewis et al. (2012) |
| Vascular adhesion protein-1 | ASP8232 | Effective | Dick de Zeeuw et al. 2018) |
| Nuclear 1 factor (erythroid-derived 2)–related factor 2 | Bardoxolone methyl | Ineffective | de Zeeuw et al. (2013) |
| Janus kinase | Baricitinib | Effective | Tuttle et al. (2018) |
| Vitamin D receptor | Paricalcitol | Effective | Dick de Zeeuw et al. (2010) |
| Methylxanthine phosphodiesterase | Pentoxifylline | Effective | Shan et al. (2012) |
| Endothelin receptor | Atrasentan | Effective | Heerspink et al. (2019) |
| Apoptosis signal-regulating kinase 1 | Selonsertib | Effective | Chertow et al. (2019) |
| C–C chemokine receptor type 2 | CCX140-B | Effective | Dick de Zeeuw et al. (2015) |
| TGF-β1, TNF-α, NF-κB | Tocotrienol-rich vitamin E | Effective | Koay et al. (2021) |
| miRNA | Unproved |
Jiang et al. (2020) Cheng et al. (2020) Kölling et al. (2017) |
|
| Endoplasmic reticulum (ER) stress | Unproved | Xiong et al. (2020) | |
| Caveolin-1 | Unproved | Van Krieken and Krepinsky (2017) | |
| Complement | Unproved |
Tang et al. (2020a) Ling Li et al. (2015) Li et al. (2014) |
Owing to the destruction of the glomerular filtration barrier (GFB), persistent proteinuria is an important feature of DKD (Phillips and Steadman 2002). The GFB includes fenestrated endothelium and its associated glycocalyx, glomerular basement membrane (GBM), and podocyte foot processes with slit diaphragms (SDs). Damage to any part of the GFB can lead to persistent proteinuria. Glycosaminoglycans, proteoglycans, and adsorbed plasma components form a surface-bound (‘endothelial glycocalyx’) and loosely adherent matrix that covers the luminal surface of glomerular endothelial cells (GECs), which is called the endothelial surface layer (ESL) (Salmon et al. 2012). GECs are covered with glycocalyx to form an osmotic barrier that prevents the development of proteinuria under healthy conditions (Satchell and Braet 2009). GECs serve as the primary barrier against exposure to hyperglycaemia, to which they are particularly vulnerable (Lassén and Daehn 2020). Podocytes are highly specialised pericytes that surround glomerular capillaries, and their dysfunction is critical to the pathophysiology of DKD (Torban et al. 2019). Nephrin, a podocyte protein, is essential for the structural integrity of the SD between podocytes. Podocin, another SD protein, is encoded by NPSH2 in podocytes. The lack of expression of nephrin and podocin has negative effects on the phenotype and function of podocytes (Saleem et al. 2002). The role of GECs and podocytes in the pathophysiology of DKD has been reported (Sol et al. 2020; Zhang et al. 2020b).
The mechanism of kidney cell damage caused by diabetes is highly complex, and it mainly includes hemodynamic factors and metabolic abnormalities (Arora and Singh 2013). Hemodynamic pathways include the renin–angiotensin–aldosterone system (RAAS), urotensin systems, and endothelin (ET). Metabolic factors are associated with advanced glycation end-products (AGEs), reactive oxygen species (ROS), and nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), etc. (Volpe et al. 2018). Hemodynamic and metabolic factors activate various pathways, including the mitogen-activated protein kinase (MAPK), Notch, NF-κB, Wnt, protein kinase C (PKC), and various cytokine signalling pathways (Tesch 2017; Zeng et al. 2019). A meta-analysis of genetic association studies of diabetic nephropathy (DN) further revealed six signalling pathways, namely, the RAAS, pyruvate metabolism, adipocytokine signalling pathway, cytokine–cytokine receptor interaction, and renal cell carcinoma- and type II diabetes mellitus (T2DM)-related pathways (Tziastoudi et al. 2020). These mechanisms lead to the characteristic pathological changes, including thickening of GBM, accumulation of extracellular matrix (ECM), and diffuse or nodular mesangial expansion. Kimmelstiel–Wilson lesions are characteristic pathological changes and are associated with a poor prognosis (Hong et al. 2007).
In recent years, studies have found that cellular crosstalk directly affects the progression of DKD, and it has prospective research significance (Chen et al. 2020; Gil et al. 2020; Mahtal etal. 2021). Thus, research on crosstalk of GECs and podocytes may further reveal the mechanism of DKD and guide its treatment. Here, we review available literature on the crosstalk of GECs and podocytes, and highlight the aspects of the field that require further investigation. Herein, we first deal with the crosstalk of GECs, describing the different molecules and signalling pathways involved. Similarly, we describe the crosstalk of podocytes. This review may help guide future research on elucidating the complex pathogenesis of DKD.
Cellular crosstalk of GECs.
Transforming growth factor-β (TGF-β)
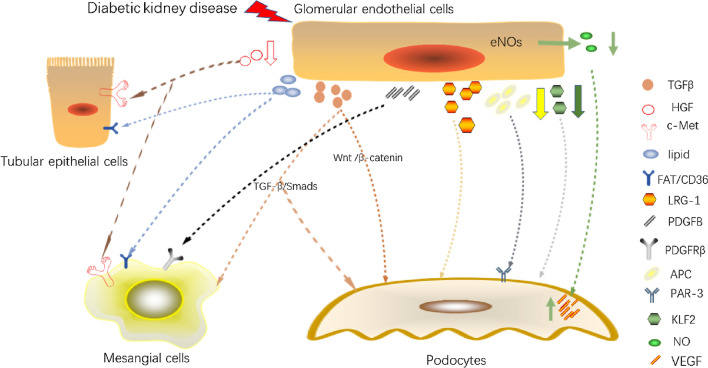
TGF-β is an important growth factor with a variety of regulatory effects. TGF-β1 is the most abundantly expressed isoform and is associated with susceptibility to various diseases (Martelossi Cebinelli et al. 2016). Monica et al. reported that TGF-β1 plays a leading role in fibrosis and immunity (Lodyga and Hinz 2020). Wu et al. treated GECs with high glucose (HG) and used their supernatant to culture podocytes. In the cultured podocytes, the expression of epithelial markers nephrin, zonula occludens-1 (ZO-1), and Wilms’ tumour 1 (WT-1) decreased, and the expression of mesenchymal markers including α-smooth muscle actin (α-SMA), desmin, and fibroblast-specific protein-1 (FSP-1) increased compared with those in podocytes co-cultured with exosomes from normal glucose (NG)-treated GECs. They demonstrated that HG-treated GECs can secrete exosomes in a paracrine manner, mediating podocyte epithelial–mesenchymal transition (EMT). The increased levels of TGF-β1, Wnt1, β-catenin, and Snail demonstrated the activation of the Wnt/β-catenin signalling pathway (which can lead to EMT) in exosome-treated podocytes. On this basis, further experiments proved that exosomes secreted by HG-treated GECs are rich in TGF-β1 mRNA. In an HG environment, GECs cause podocyte dysfunction through increases in TGF-β1 expression and activation of Wnt/β-catenin signalling (Wu et al. 2017).
Han et al. showed that the genetic deletion of bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI), a negative regulator of TGF-β signal transduction, in diabetic mice can accelerate DKD progression (Lai et al. 2020). They observed that BAMBI is expressed in podocytes and GECs, and TGF-β1 is expressed in GECs by single-cell RNA-Seq. By specifically knocking out BAMBI in GECs (EC-Bambi-/-) and in podocytes (Pod-Bambi-/-), they found that activin receptor-like kinase 1 (ALK1)-Smad1/5 is activated in EC-Bambi-/- diabetic mice, resulting in GEC proliferation, whereas ALK5-Smad3 is activated in Pod-Bambi-/- diabetic mice, resulting in podocyte damage. Interestingly, the damage and loss of podocytes marked by WT-1 in EC-Bambi-/- diabetic mice were comparable with those in Pod-Bambi-/- diabetic mice, whereas GEC proliferation was only observed in EC-Bambi-/- diabetic mice, demonstrating that GEC-to-podocyte crosstalk is complex and occurs via the TGF-β/Smad1/5 signalling pathway. Furthermore, by measuring 8-oxo-2'-deoxyguanosine, they demonstrated that the oxidative stress in EC-Bambi-/- diabetic mice and Pod-Bambi-/- diabetic mice is aggravated, leading to the disappearance of podocyte foot processes, loss of podocytes and proliferation of GECs.
Jin et al. demonstrated that HG activates TGF-β-dependent Smad signalling, which promotes the synthesis of collagen and results in ECM production (Li et al. 2003), which is related to Kimmelstiel–Wilson lesions (Glick et al. 1992). Wu et al. showed that exosomes released by HG-treated GECs can promote the expression and proliferation of α-SMA, type IV collagen, and fibronectin in mesangial cells (MCs). Furthermore, they found that exosomes released by HG-treated GECs were rich in TGF-β1 mRNA. Finally, they used TGF-β1 small interfering RNA to decrease TGF-β1 expression in GEC-derived exosomes and found that MCs were not activated. Overall, TGF-β1 from HG-treated GECs was demonstrated to mediate MC activation and ECM protein overproduction through the TGF-β1/Smads signalling pathway (Wu et al. 2016). Crosstalk of GECs with podocytes and MCs through TGF-β is one of the reasons that TGF-β is considered a key cytokine in the development of glomerular fibrosis in patients with DKD. Urine TGF-β and serum TGF-β1 can predict renal function in DN (Shaker and Sadik, 2013; Verhave et al. 2013). In conclusion, the role of TGF-β in DKD is important and complex, and requires further exploration.
Leucine-rich α-2-glycoprotein 1 (LRG-1)
LRG-1 is a secreted glycoprotein that mediates protein interaction and is widely involved in signal transduction (Zhang et al. 2020a). Plasma LRG-1 is considered a marker of DKD progression (Liu et al. 2017). Fu et al. conducted a transcriptome analysis of GECs from diabetic mice and nondiabetic controls and found that the expression of genes related to angiogenesis and endothelial proliferation pathways were up-regulated in diabetic mice, including LRG-1 (Fu et al. 2018). LRG-1, which is mainly expressed in GECs, with no obvious expression in podocytes, promotes the progression of DKD by enhancing angiogenesis through TGF-β/ALK1 (Hong et al. 2019). LRG-1 ablation in DKD not only reduces glomerular angiogenesis but also attenuates foot process disappearance, podocyte loss, and mesangial expansion. Thus, LRG1 seems to participate in the crosstalk between GECs and podocytes; however, the specific mechanism remains unclear.
Hepatocyte growth factor (HGF)
HGF was originally discovered as a mitogen in adult rat liver cells. c-mesenchymal-epithelial transition factor (c-Met) is an HGF receptor, and HGF/c-Met plays a role in promoting liver repair (Nakamura 1994). In the kidney, HGF/c-Met has also been shown to induce kidney regeneration and repair (Igawa et al. 1993). Through transcriptome analysis, Tang et al. found that HGF is one of the differentially expressed genes (DEGs) in the glomeruli and tubules of patients with DKD and healthy controls, and its expression in patients with DKD is down-regulated compared with that in the healthy controls (Tang et al. 2020b). Moreover, correlation analysis revealed that HGF may serve as a biomarker for diagnosing DKD. In normal glomeruli, GECs express HGF, and MCs hardly express c-Met. In DKD, HGF increases briefly and then drops below the basic level (Mizuno and Nakamura 2004). This may be due to the damage to GECs caused by HG. In contrast, the expression of c-Met increases significantly in HG-treated MCs. HGF exerts a paracrine effect, and the combination of HGF and c-Met can protect MCs from HG-mediated oxidative stress. The levels and activities of malondialdehyde, glutathione, antioxidant enzymes, and glucose-6-phosphate dehydrogenase revealed that HGF/c-Met inhibits the production of ROS and increases their clearance (Li et al. 2006). This anti-oxidative stress effect is achieved by inhibiting PKA and activating PKG (Hui et al. 2010). HGF can also inhibit the production of TGF-β1, thus playing a protective role (Mizuno and Nakamura 2004). Furthermore, Li et al. added exogenous HGF to HG-treated tubular epithelial cells (TECs) and found that c-Met is also up-regulated in TECs, playing a role in alleviating DKD (Xing and Muxun, 2007). However, Lucia et al. considered that c-Met activation in TECs is independent of HGF (Mesarosova et al. 2017). Thus, the role of HGF/c-Met requires further exploration.
Endothelial nitric oxide synthase (eNOS)
eNOS-derived nitric oxide (NO) can relax blood vessels (Sol et al. 2020) and block the activation of cytokines such as tumour necrosis factor to protect GECs (Takahiko Nakagawa et al. 2011). Fu et al. conducted a transcriptome analysis on GECs from streptozotocin (STZ)-induced diabetic wildtype and diabetic eNOS-null mice and compared DEGs (Fu et al. 2018). Pathway analysis has shown that eNOS deficiency is related to abnormal angiogenesis, cytoskeletal organization, and epigenetic regulation. eNOS is expressed in GECs but not in podocytes. Darren et al. demonstrated that the lack of eNOS in diabetic mice can cause podocyte damage including the disappearance of foot processes and the accumulation of vacuoles, pseudocysts, and electron-dense droplets in the cytoplasm (Yuen et al. 2012). eNOS deficiency has been shown to play a role in DN via the activation of RAAS or increased RAAS sensitivity (Takahashi and Harris, 2014). Takahiko et al. found that eNOS knockout in diabetic mice results in an increased expression of vascular endothelial growth factor (VEGF) in podocytes (T. Nakagawa 2008). Diabetic eNOS-knockout mice have abnormal angiogenesis, GBM thickening, mesangial expansion, macrophage infiltration, and Kimmelstiel–Wilson-like lesions in the kidney. These findings indicate that the absence of eNOs may be related to Kimmelstiel–Wilson lesions.
Kruppel-like factors (KLFs)
KLFs are involved in a wide range of cellular processes, such as cell differentiation, angiogenesis, erythropoiesis, and immunomodulation (Bai et al. 2007; Pearson et al. 2008). Moreover, their deficiency leads to serious damage of the cardiovascular system (Chiplunkar et al. 2013; Lee et al. 2006). In DKD, KLFs are regarded as protective factors (Zhong et al. 2018), although the specific mechanism of KLFs in DKD is still unclear. KLF2 is expressed in GECs but not in podocytes (Mallipattu et al. 2017). A previous study showed that KLF2 expression is inhibited in HG-treated GECs (Lee et al. 2012). Zhong et al. performed microarray gene expression analysis on the glomeruli of diabetic rats and found that many DEGs are GEC-specific genes, KLF2 being one of them. Furthermore, they induced diabetes by STZ in GEC-specific KLF2 knockout mice (KO-STZ) and found that the expression of podocyte-specific genes encoding nephrin, podocin, and synaptopodin in the kidney decreased compared with that in wild-type diabetic mice. Moreover, podocyte foot processes and the number of podocytes (marked by WT-1) decreased in KO-STZ mice (Zhong et al. 2015). This indicates that KLF2 is involved in the interactions between GECs and podocytes. The specific mechanism remains unclear, and may be related to key molecules such as eNOS, thrombomodulin, endothelin-1(Slater et al. 2012).
Platelet-derived growth factor B (PDGFB)/platelet-derived growth factor receptor β
The PDGFB/PDGFRβ axis plays an angiogenic role in kidney tumours, causing tumour endothelial cell proliferation and migration (Cumpănas et al. 2016). Changes in the expression of PDGFB and/or its receptors are associated with many kidney diseases. The expression of PDGFB/PDGFRβ is significantly increased in DN (Langham et al. 2003) and mesangial proliferative glomerulonephritis (Matsuda et al. 1997). The over-expression of PDGFB is one of the reasons for ECM protein production (Floege et al. 1993). By inactivating PDGFB/PDGFRβ in developing mice, Betsholtz et al. demonstrated that PDGFB/PDGFRβ can promote the survival and recruitment of MCs and the production of mesangial matrix (Betsholtz, 1995). PDGFB can stimulate the rapid mobilisation of intracellular calcium, increase cyclin A and cyclin dependent kinase 2, and reduce p27, leading to considerable cell proliferation (Shankland et al. 1997; Wallmon et al. 1993). In addition, through synergy with TGF, it promotes the synthesis of mesangial matrix (Throckmorton et al. 1995). Lindahl et al. found that PDGFB is mainly expressed in GECs, whereas PDGFRβ is mainly expressed in MCs (Lindahl et al. 1998). Defective MC ingrowth and, consequently, failure of capillary loop development have been found in PDGFB mutant mice with inhibited PDGFB expression in GECs (Levéen et al. 1994). Overall, the findings suggest that the paracrine function of PDGFB controls the development of MCs. Moreover, by measuring α-SMA and proliferating cell nuclear antigen, Eunjin et al. demonstrated that inhibition of PDGFB and its receptors can inhibit mesangial proliferation in diabetic rats (Sohn et al. 2014). Further evidence indicating that the PDGFB/PDGFRβ axis is the main mediator of GEC-to-MC crosstalk in DKD was provided by Jia et al. (Fu et al. 2015).
Lipids and fatty acid translocase (FAT/CD36)
In DKD, lipid deposits in the glomeruli and renal tubules can be observed, and abnormal lipid metabolism is nephrotoxic (Thongnak et al. 2020). The deposition of lipids in the kidney is related to glomerular hypertrophy and interstitial fibrosis, causing various types of cell damage (Jiang et al. 2005). Lipid deposition is part of the original description of Kimmelstiel-Wilson lesions (Gröne, 1999). Although GECs are not prone to lipid accumulation, they play an important role in the transport of lipids to other tissues in the kidney. GECs are the main source of lipid supply to glomerular cells (Hagberg et al. 2010). Importantly, lipid delivery between cells may amplify the pathogenic effect. To our knowledge, the mechanism behind cellular crosstalk through lipids remains unreported. However, we speculate that FAT/CD36 may be involved.
FAT/CD36 is a cell-surface protein and a member of the class B scavenger receptor family. It can bind to oxidised low-density lipoproteins, long-chain fatty acids, phospholipids, and collagen (Bonen et al. 2004; Martin et al. 2011), and directly promote the transport of fatty acids across the plasma membrane (Schneider et al. 2014). Furthermore, its overexpression in muscle and liver cells can cause muscle and liver steatosis (Bechmann et al. 2010; Ibrahimi et al. 1999). The increase in FAT/CD36 permanently fixed on the cell membrane is considered the main factor leading to the early onset of T2DM (Steinbusch et al. 2011). Aiko et al. have shown that in the state of hyperglycaemia, the endothelial cell glycocalyx is damaged, causing MCs to express LDL and FAT/CD36 receptors (de Vries et al. 2014). Down-regulating the expression of CD36 can reduce the deposition of free fatty acids in MCs, and reduce oxidative stress and fibrosis (Y. Su et al. 2019). Moreover, Lei et al. measured the levels of CD36 and peroxisome proliferator-activated receptor γ (PPARγ) separately in TECs cultured in HG and an AKT pathway inhibitor and evaluated the lipid content in TECs by Oil Red O staining. They demonstrated that HG can promote FAT/CD36 expression in TEC by upregulating the levels of PPARγ (Feng et al. 2017). Increased expression of FAT/CD36 leads to lipid deposition in kidney cells and exerts lipotoxicity through the Wnt/β-catenin pathway in DKD (X. Li et al. 2019). Nevertheless, the mechanism of GEC crosstalk with other cells through lipids needs to be further clarified.
Activated protein C (APC)
APC is an effector enzyme involved in the protein C pathway. It not only acts as an inhibitor of the coagulation system but also as a regulator of inflammation, tissue remodelling, and apoptosis (Suzuki et al. 2004). Berend et al. reported that the levels of pro-apoptotic proteins p53 and Bax substantially increased in diabetic wild-type mouse glomeruli, whereas the levels in diabetic APC-high mice were normal, indicating that APC regulates the mitochondrial apoptosis pathway in DKD (Isermann et al. 2007). The protective effects of APC on GEC are mainly mediated through endothelial protein C receptor (EPCR) and protease activated receptor-1 (PAR-1). The protective effect of APC on podocytes in DKD is through PAR-3 by inhibiting the RhoA signalling pathway and requires the participation of integrin β3 (Madhusudhan et al. 2020, 2012). However, in patients with diabetes, the level of APC, regulated by endothelial thrombomodulin, is decreased compared with physiologic conditions (Tan et al. 2016), leading to activation of the mitochondrial apoptotic pathway in podocytes (Isermann et al. 2007). Maestroni et al. also indicated that APC is involved in the crosstalk between GECs and podocytes in DKD (Maestroni and Zerbini 2018). Meanwhile, Berend et al. revealed that APC can also play a protective role in DKD by regulating caspase activation and nitrosative stress.
A summary of the cellular crosstalk of GECs is shown in Fig. 1.
Fig. 1.
Cellular crosstalk of glomerular endothelial cells. (TGF-β, transforming growth factor-β; HGF, hepatocyte growth factor; c-Met, c-mesenchymal-epithelial transition factor; FAT, fatty acid translocase; LRG-1, leucine-rich α-2-glycoprotein 1; PDGFB, platelet-derived growth factor B; PDGFRβ, platelet-derived growth factor receptor β; APC, activated protein C; PAR-3, protease activated receptor-3; KLF2, Kruppel -like factor 2; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; VEGF, vascular endothelial growth factor; Smad, small mother against decapentaplegic; Wnt, Wingless and Int-1;)
Cellular crosstalk of podocytes
VEGF
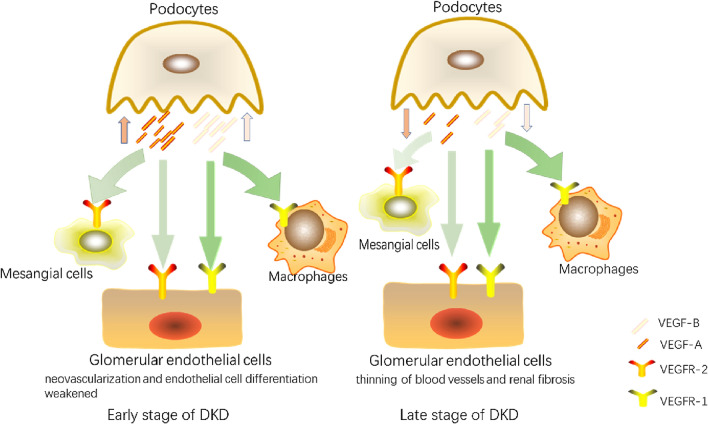
Podocyte damage is considered the main feature of DKD and includes podocyte hypertrophy, foot process shedding, and podocyte apoptosis (Bose et al. 2017; Su et al. 2010). In the glomerulus, podocytes produce large amounts of VEGF. Podocyte-produced VEGF regulates the structure and function of adjacent GECs by binding to VEGF receptors 1 and 2 (VEGFR1 and VEGFR2) and neuropilin-1/2 (NP1/NP2) (Melincovici et al. 2018). VEGF, which has both autocrine and paracrine effects (Stieger et al. 2011), is considered an important factor in diabetes and its complications. Plasma VEGF can be used as a marker of DKD progression(Aly et al. 2019). The VEGF family consists of several members. Among them, VEGF-A plays a major role in vasculogenesis (Tufro and Veron, 2012), and VEGF-B is related to the promotion of lipid accumulation and lipotoxicity (Karpanen et al. 2008). Early studies have shown that VEGF has a complicated pathogenic mechanism in DKD. In the early stage, human kidney biopsies show high VEGF expression. In contrast, the loss of podocytes in the late stage leads to low VEGF expression (Shulman et al. 1996).
Although the highest expression of the VEGF-A receptor VEGFR-2 is found in GECs, it is also expressed in podocytes (Villegas et al. 2005). Systemic loss of VEGFR-2 leads to abnormal GECs. Karen et al. inhibited VEGFR-2 expression in podocytes and found that autocrine signal transduction of podocytes through VEGFR-2 had no major effect on glomerular function (Sison et al. 2010). This showed that the paracrine function of VEGF-A plays a major role in regulating glomerular function. Du et al. identified 142 DEGs between DN and normal glomeruli through the GSE30122 and GSE1009 databases, including VEGF-A (Du et al. 2021a). Meanwhile, Masahiro et al. observed that fenestrations of GECs were reduced, the subendothelial space was enlarged, and staining of plasmalemmal vesicle-associated protein-1 was generally positive in VEGF-A-overexpressing podocytes, indicating the overexpression of VEGF-A in podocytes can weaken the differentiation of GECs (Suyama et al. 2018). In addition, the lack of VEGF-A in diabetic mouse podocytes can cause GEC damage, including shedding of GECs and loss of capillary loops (Sivaskandarajah et al. 2012). The combination of VEGF-A expression in podocytes and VEGFR-2 expression in GECs plays a role in the cellular crosstalk of DKD.
In recent years, the inhibition of VEGF-B signalling has been suggested as a promising option for the treatment of T2DM and its complications (Hagberg et al. 2012). Woroniecka et al. conducted analysis using Affymetrix expression arrays on micro-dissected glomeruli and tubules from healthy subjects and patients with DKD, and found that VEGF-B was up-regulated in the glomeruli of patients with DKD (Woroniecka et al. 2011). Inhibition of VEGF-B signalling in DKD mouse models has been found to prevent pathological lipid deposition; improve podocyte abundance; and reduce mesangial expansion, hyaline arteriole degeneration, and glomerular sclerosis (GS). Furthermore, VEGFR1 and NP-1 (receptors of VEGF-B) expressed on GECs (Falkevall et al. 2017). The expression analysis of whole kidney lysate showed that with the progression of DKD, the transcription level of fatty acid transport protein 4 (Fatp4) increases in the glomeruli. VEGF-B may play a pathogenic role through Fatp4. Thus, the crosstalk between podocytes and GECs through VEGF-B is one of the mechanisms of lipotoxicity in DKD.
Moreover, macrophage infiltration in DKD is partially due to the increased expression of VEGF in podocytes. Waichi et al. demonstrated that VEGF produced by podocytes in diabetic eNOS-deficient mice regulates actin recombination and mediates macrophage migration through VEGFR1. Furthermore, they found that the p38 MAPK-specific blocker 31,169 can supress the migration of macrophages caused by VEGF, indicating that this process is dependent on p38 MAPK (Sato et al. 2008). Meanwhile, other studies have suggested that VEGF can inhibit pericyte function and vascular maturation by inducing the VEGF-R2/PDGF-Rβ complex (Greenberg et al. 2008). Similarly, the expression of VEGFR2 has been detected in MCs. Masahiro et al. found upregulation of VEGF in podocytes leading to significant down-regulation of MC markers, α-SMA, desmin, and PDGFRβ, indicating that MCs are reduced (Suyama et al. 2018). A decrease of MCs leads to GBM damage, aneurysm and cluster collapse. Although the levels of PDGFB did not change substantially, the immunoreactivity of phosphorylated PDGFRβ is substantially reduced. These results indicate that PDGFRβ may be involved in the crosstalk of podocytes with MCs through VEGF.
The role of VEGF in cellular crosstalk is illustrated in Fig. 2.
Fig. 2.
Cellular crosstalk of VEGF produced by podocyte in DKD. (DKD, diabetic kidney disease; VEGF-A, vascular endothelial growth factor A; VEGF-B, vascular endothelial growth factor B; VEGFR-1, vascular endothelial growth factor receptor 1; VEGFR-2, vascular endothelial growth factor receptor 2;)
Angiopoietin (Ang)/Tyrosine kinase receptor-2 (Tie-2)
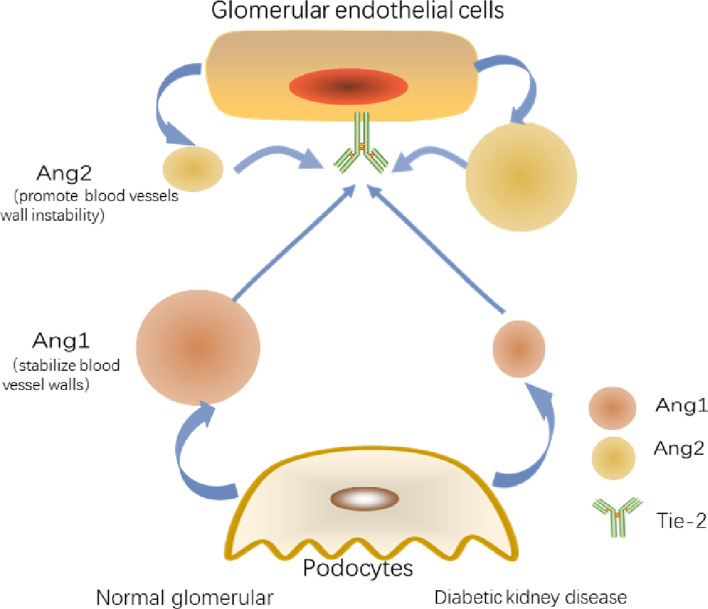
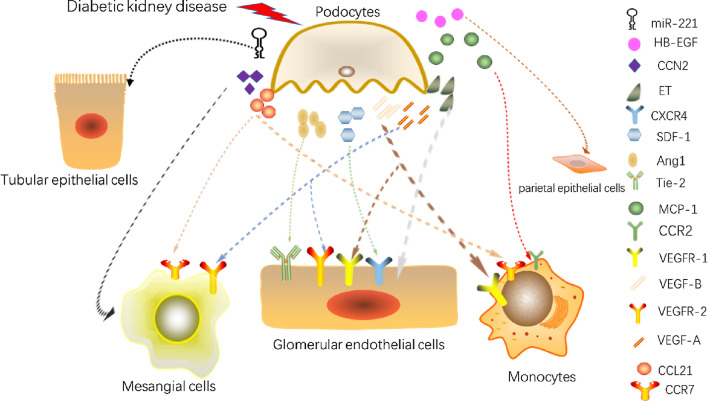
In DKD, Ang-1 can prevent damage to the capillary wall from hyperglycaemia, and Ang-2 expression is directly proportional to the degree of endothelial damage. Ang-1 is mainly expressed by podocytes, while Ang2 is mainly produced by GECs and antagonises Ang1 through competitive inhibition of Tie-2 receptors (Satchell et al. 2002). However, Tie-2 is localised on GECs (Yuan et al. 2000). Ang-2 is an antagonistic ligand for Tie-2 on GECs, inhibiting the binding of Ang-1 to Tie-2 in an autocrine fashion (Maisonpierre et al. 1997). Belinda et al. emphasised the role of Ang2 in glomerular damage and aggravation of proteinuria (Davis et al. 2007). Cecile et al. reported that a reduced level of Ang1 in diabetic mice leads to vascular instability, albuminuria, and GEC proliferation. Moreover, supplementation of Ang1 did not reverse the down-regulation of nephrin in DKD, but increased Tie-2 phosphorylation and significantly reduced albuminuria, VEGFR2 phosphorylation, and nephrin phosphorylation. No significant difference in the Ang2 level was observed between non-diabetic and diabetic mice (Dessapt-Baradez et al. 2014). Yoshihiko et al. found that Ang2 was significantly increased in diabetic mice kidney. Tumstatin peptide can improve glomerular hypertrophy, glomerular matrix expansion, and monocyte accumulation in diabetic mice. In diabetic mice treated with tumstatin, Ang2 expression was substantially reduced and nephrin level was restored. However, the Ang1 level did not change substantially in the kidneys of diabetic mice in response to tumstatin (Yamamoto et al. 2004). Importantly, the difference in the expression levels of Ang2 and Ang1 plays a role in the pathogenesis of DKD (Gnudi 2016). Serum Ang2 is an effective predictor of DKD(Sokolovska et al. 2020).
The role of Ang in cellular crosstalk is shown in Fig. 3.
Fig. 3.
Ang-1 and Ang-2 in DKD. (Ang1, angiopoietin 1; Ang2, angiopoietin 2; Tie-2, tyrosine kinase receptor-2;)
Endothelin (ET)
ET has a significant regulatory effect on the kidneys under physiological and pathological conditions. The main members of the ET family include ET-1–3. Among them, ET-1 is involved in kidney disease (DKD (Zeravica et al. 2016), focal segmental glomerulosclerosis (Daehn et al. 2014), etc.). It has two receptor (ETR) subtypes, endothelin receptor-A (ET-A) and endothelin receptor-B (ET-B). Margien et al. found that ET-A antagonists can improve GECs mitochondrial oxidative damage, proteinuria, and podocyte damage in DN (Boels et al. 2016). Several studies have reported various mechanisms of ET-1 in cellular crosstalk. Qi et al. demonstrated that ET-1/ET-A in GECs promotes the progression of DKD by mediating mitochondrial dysfunction(Qi et al. 2017). Marjolein et al. showed that glomerulosclerosis and podocyte loss are attenuated in podocyte-specific ETR-deficient (Pod-ETRKO) diabetic mice. They treated podocytes and GECs with ET-1 and found that heparanase expression increased in podocytes, whereas that in GECs was not affected. This is due to the difference in ETR distribution in podocytes and GECs. Podocytes express both ET-A and ET-B, whereas GECs only express ET-B. ET-1 plays an autocrine role by binding to ET-A to mediate the increased expression of heparanase in podocytes. Heparanase acts on the ESL of GECs to reduce its hardness and induce proteinuria (Garsen et al. 2016). Olivia et al. found the same phenomenon in Pod-ETRKO diabetic mice. ET-1 binds to its receptor and mediates rapid calcium transients. Thrombin or ET-1 was used to stimulate podocytes and variations in Ca2+ were measured. Calcium transients decreased with the addition of ET-B and ET-A selective inhibitors separately, illustrating that ET-A and ET-B play the same role, and that their downstream pathways involve NF-κB and β-catenin (Lenoir et al. 2014). Despite possible differences in the mechanisms, the observations confirm the crosstalk between podocytes and GECs through ET.
Stromal cell-derived factor-1 (SDF-1)/ CXC chemokine receptor 4(CXCR4)
Chemokines are 8–12 kDa peptides that play roles in cell activation, differentiation, and transportation. SDF-1, also known as C-X-C motif chemokine ligand 12 (CXCL12), plays roles in osteoarthritis (Wang et al. 2017), and chronic skin inflammation (Zgraggen et al. 2014) through its interactions with CXC chemokine receptors 4 (CXCR4) and 7 (CXCR7) (Hunger et al. 2012; Puchert and Engele, 2014). The SDF-1/CXCR4 axis has a crosstalk effect between the subchondral bone and articular cartilage in osteoarthritis (Qin et al. 2019). It is related to cancer metastasis (Gelmini et al. 2008) such as breast cancer (Duan et al. 2020), non-small cell lung cancer (Otsuka and Bebb 2008), and gastric cancer (Zhao et al. 2011), and promotes the development of glomerular blood vessels (Floege et al. 2009). SDF-1α is an isoform of SDF-1 (Gahan et al. 2012). The SDF-1α/ CXCR4 pathway has been considered a protective mediator in cell and tissue repair in DKD. SDF-1α expression is considerably reduced in DKD compared with that in normal condition (Chang et al. 2017; Lovshin et al. 2017). Some researchers speculate that SDF-1/CXCR4 may be involved in the cellular crosstalk of DKD. Yoshitsugu et al. observed developing kidney and CXCR4-knockout mice by immunohistochemistry (IHC) and demonstrated the important role of SDF-1/CXCR4 in the development of renal blood vessels. Podocytes were found to express CXCL12 and GECs expressed CXCR4 in mature glomeruli (Takabatake et al. 2009). CXCL12- null and CXCR4-null kidneys showed a similar pathology, including glomerular capillary malformations, abnormal angiogenesis, and decreased MC number. Moreover, linagliptin improved the renal outcome of diabetic mice by increasing the expression of SDF-1, and this protective effect could be eliminated by CXCR4 blockers (Takashima et al. 2016). These results suggest that the SDF-1/CXCR4 axis plays a protective crosstalk role between podocytes and GECs in DKD.
Secondary lymphoid tissue chemokine (SLC)/C–C chemokine receptor type 7 (CCR7)
SLC, also called C–C chemokine ligand 21 (CCL21), can promote healing in vitro injury models. Banas et al. clarified the localisation of SLC and CCR7 by IHC staining. They revealed that podocytes specifically express SLC, whereas its receptor, CCR7, is located on MCs (Banas et al. 2002). Migration assays confirmed that SLC/CCR7 mediates the directional migration of MCs to podocytes. Culturing MCs at different SLC concentrations confirmed that SLC/CCR7 mediates the proliferation of MCs. Analysis using a Fas/CD95-mediated cell death test showed that SLC/CCR7 can promote the survival of MCs. Furthermore, Banas et al. confirmed that SLC/CCR7 enhances cell adhesion and firmness (Bernhard Banas et al. 2004). SLC/CCR7 increased the phosphorylation of glycogen synthase kinase-3 and PKB. This shows that the downstream pathway of SLC/CCR7 involves the activation of integrin-linked kinase. In a previous study, CCL21 was found to be up-regulated in podocytes that were damaged as a result of systemic polyomavirus administration. HG also increased CCL21 mRNA level in podocytes (Valiño-Rivas et al. 2016). In addition, SLC/CCR7 can recruit macrophages. Blocking SLC/CCR7 can reduce macrophage aggregation and renal fibrosis (T. Wada et al. 2007). It was observed that CCR7 was also present in M1 macrophages. Torres et al. found that A2B adenosine receptor (A2BAR) antagonist can reduce the characteristic symptoms of GS, macrophage infiltration, and mesangial expansion in diabetic rats. Moreover, transcriptome analysis of chemokines and other related genes in DN rats treated with A2BAR antagonist showed considerable decrease in CCL21 compared with that in the control group (Torres et al. 2020).
Monocyte chemotactic protein 1 (MCP-1)/ C–C chemokine receptor type 2 (CCR2)
The presence of MCP-1 (also known as C–C chemokine ligand 2 [CCL2]) in urine is considered a potential marker of DN (Siddiqui et al. 2020; Titan et al. 2012). CCR2, MCP-1 receptor, is mainly expressed in monocytes (Awad et al. 2011). The pathogenic role of MCP-1/CCR2 in DKD is widely recognised. Tarabra et al. demonstrated that MCP-1 not only recruits monocytes, but also reduces the expression of nephrin and synaptopodin, causing podocyte damage in DKD (Tarabra et al. 2009). Lack of MCP-1 in mice (F. Y. Chow et al. 2006) and the use of CCR2 antagonists(Sullivan et al. 2013) can reduce macrophage aggregation in the kidney and proteinuria. Priscila et al. showed that renal tubular reabsorption proteins, AGEs, angiotensin II, and pro-inflammatory cytokines induce podocytes to produce MCP-1 (Calle and Hotter, 2020). Fiona et al. showed that MCP-1 is mainly expressed in podocytes in DKD (F. Chow et al. 2004). Gu et al. incubated podocytes with AGE, and measured dichlorofluorescein-sensitive intracellular ROS production by confocal microscopy, and proved that AGE stimulates podocytes to express MCP-1 through the ROS signalling pathway. MCP-1 expressed by podocytes promotes DN progression by recruiting macrophages (Gu et al. 2006). This may be related to MAPK phosphatase 1 (Kim et al. 2012), NADPH oxidase 4(Ullevig et al. 2012), and RAS (Kato et al. 1999). Infiltration of inflammatory cells (such as monocytes and macrophages) are signs of DKD progression (Furuta et al. 1993). Blocking MCP-1/CCR2 can reduce infiltration of macrophages and mesangial matrix expansion of DKD (Du et al. 2021b). Through prospective follow-up in 83 patients with DN and analysis of relevant urine biomarkers, Verhave et al. reported that urine MCP-1 could independently predict the progression of renal function (Verhave et al. 2013).
Cellular communication network factor 2 (CCN2)
CCN2, also known as connective tissue growth factor (CTGF), is a cytokine that plays an important role in chronic renal fibrosis, promoting EMT and inhibiting matrix degradation (Grotendorst et al. 2004; Yang et al. 2004). Nguyen et al. conducted a prospective study and found that plasma CCN2 has a significant effect on the poor prognosis of patients with T1DM nephropathy (McLennan et al. 2013). In DKD, the expression of CCN2 in podocytes and MCs is significantly up-regulated compared with that in physiological condition. Yokoi et al. established podocyte-specific CCN2 transgenic diabetic mice and found more severe podocyte damage, mesangial expansion, and lower podocin expression than those in diabetic wild-type mice (Yokoi et al. 2008). Moreover, CCN2 in the mesangial region also increased significantly in transgenic mice compared with that in wild-type diabetic mice. These findings showed that podocytes affect MCs through CCN2, leading to the accumulation of glomerular mesangial matrix and progressive glomerulosclerosis in diabetic mice. Furthermore, they found that fibronectin and collagen did not increase significantly in transgenic mice, whereas the expression and activity of matrix metalloproteinase-2 (an ECM-degrading enzyme) decreased. Therefore, CCN2 produced by podocytes does not directly increase the production of ECM, but inhibits the degradation of ECM.
Heparin-binding epidermal growth factor-like growth factor (HB-EGF)
HB-EGF is a ligand of the epidermal growth factor receptor (EGFR), and it is increased in the kidney of diabetic rats, which can be counteracted by strict control of blood glucose (Lee et al. 1995). Uttarwar et al. showed that HB-EGF mediated glucose-induced EGFR activation in MCs (Uttarwar et al. 2011). In a study that used a three-dimensional (3D) multiscale model to simulate the glomerular microenvironment, HB-EGF, ET-1, and TGF-β were found to be accumulated under diabetic conditions (HG), whereas VEGF-A and APC expression was attenuated. Moreover, in diabetic conditions, damaged podocytes secreted HB-EGF, which stimulated the activation and proliferation of parietal epithelial cells (PECs) and interfered with their compensatory differentiation into podocytes (Tan et al. 2016). This may be related to EGFR transactivation. Under physiological conditions, PECs can differentiate to supplement the loss of podocytes (Poulsom and Little, 2009). Under DKD conditions, this supplementary repair effect is impaired by the crosstalk between podocytes and PECs through HB-EGF.
miR-221
miRNAs are small endogenous RNAs of approximately 21 nucleotides that are involved in protein–protein and protein–RNA interactions (Krol et al. 2010), and participate in important biological processes such as cell growth, tissue differentiation, cell proliferation, and apoptosis (Sayed and Abdellatif, 2011). miRNAs can regulate the expression of target genes after transcription (Gommans and Berezikov, 2012), and one single miRNA can simultaneously target multiple genes within the same cell signalling pathway (Correia de Sousa et al. 2019). Anthony et al. and Christian et al. have emphasised the paracrine role of miRNAs (Bär et al. 2019; Leung, 2015). miRNAs are emerging biomarkers of DKD, potentially important regulatory factors, and feasible targets for clinical diagnosis and therapeutic interventions (Alvarez and Distefano 2013). For example, urinary miR-3137 and miR-4270 have been described as potential biomarkers for DKD (Li et al. 2020). In addition, urinary miR-196a level is associated with the progression of kidney damage in patients with DN (An et al. 2020). In T2DM, the level of miR-152-3p is significantly correlated with indicators of the severity of kidney disease (Nasser et al. 2020). Urine miR-27b-3p and miR-1228-3p are associated with the progression of renal fibrosis in DN (Conserva et al. 2019). Cui et al. reported that miR-17-5p, miR-20a and miR-106a can be used as biomarkers for DKD (Cui et al. 2018).
Su et al. co-cultured labelled podocytes by PKH26, a fluorescent dye, with HG-treated proximal TECs (PTECs) and red fluorescence was observed in the PTECs, indicating crosstalk between them. This crosstalk can be inhibited in co-culture with podocytes supplemented with GW4869 (a EV secretion inhibitor). In PTECs treated with extracellular vesicles (EVs) produced by HG-induced podocytes, the expression of epithelial markers, E-cadherin and ZO-1 was absent, whereas the expression of mesenchymal markers such as vimentin and α-SMA was up-regulated. At the same time, PTECs are morphologically manifested by a decrease in the number of microvilli and mitochondria and an increase in the volume density of the rough ER. Furthermore, they transfect the labelled miR-221 mimics into the podocytes, co-cultured with PTECs under HG conditions, and found the presence of the labelled miR-221 mimics in PTECs. These results support the hypothesis that miR-221 plays a role in crosstalk in DKD. Moreover, podocyte miR-221 directly targets Dickkopf-2, an inhibitor of Wnt signalling, in the PTECs, resulting in the damage of these cells (Su et al. 2020).
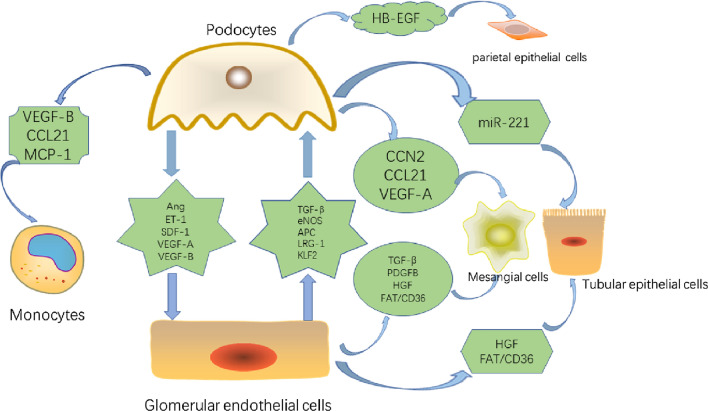
Cellular crosstalk of podocytes is summarised in Fig. 4.
Fig. 4.
Cellular crosstalk of podocytes. (HB-EGF, heparin-binding epidermal growth factor-like growth factor; CCN2, cellular communication network factor 2; ET, endothelin; CXCR4, CXC chemokine receptor 4; SDF-1, stromal cell-derived factor-1; Ang1, angiopoietin 1; Tie-2, angiopoietin receptor-2; MCP-1, monocyte chemotactic protein 1; CCR2, C–C chemokine receptor type 2; VEGFR-1, vascular endothelial growth factor receptor 1; VEGF-B, vascular endothelial growth factor B; VEGFR-2, vascular endothelial growth factor receptor 2; VEGF-A, vascular endothelial growth factor A; CCL21, C–C chemokine ligand 21; CCR7, C–C chemokine receptor type 7;)
Conclusions
Many studies have reported the characteristics and important pathogenic effects of GEC and podocyte damage in DKD. Here, we have reviewed the studies demonstrating that cellular crosstalk from these cell types exacerbates damage and promotes DKD progression (Table 2 and Fig. 5). MCs and TECs also play a cellular crosstalk role in DKD and promote DKD progression. We summarised the factors involved in the cellular crosstalk of these two types of cells in Table 3. The main conclusions are:
Under HG conditions, GEC-to-podocyte crosstalk causes podocyte dysfunction through a variety of disturbed signalling pathways, leading to DKD development. These include, TGF-β1/Wnt/β-catenin and TGF-β/Smad signalling, and LRG-1, KLF, eNOS, and APC signalling.
Podocyte-to-GEC crosstalk results in lipotoxicity and alterations in the structure and function of GECs. This crosstalk involves diverse molecules, including VEGF, Ang/Tie-2, ET, and SDF-1/CXCR4.
Abnormal GEC and podocyte signalling affects other cells, such as MCs, proximal tubular epithelial cells, and macrophages, promoting DKD progression.
Table 2.
Crosstalk of endothelial cells and podocytes to other cells
| Factor | Crosstalk cell | Reference | |
|---|---|---|---|
| Glomerular endothelial cells | TGF-β | Podocytes |
Wu et al. (2017) Lai et al. (2020) |
| eNOS |
Yuen et al. (2012) Takahashi and Harris (2014) Nakagawa, 2008) |
||
| LRG1 | Hong et al. (2019) | ||
| KLF2 | Zhong et al. (2015) | ||
| APC |
Isermann et al. (2007) Madhusudhan et al. (2012) |
||
| TGF-β | Mesangial cells | Wu et al. (2016) | |
| PDGFB |
Levéen et al. (1994) Sohn et al. (2014) Fu et al. (2015) |
||
| HGF |
Mizuno and Nakamura, 2004) Li et al. (2006) |
||
| FAT/CD36 |
Hagberg et al. (2010) de Vries et al. (2014) |
||
| HGF | Tubular epithelial cells | Xing and Muxun, 2007) | |
| FAT/CD36 |
Hagberg et al. (2010) Feng et al. (2017) |
||
| Podocytes | VEGF-A | Endothelial cells | Sivaskandarajah et al. (2012) |
| Ang |
Satchell et al. (2002) Yuan et al. (2000) Dessapt-Baradez et al. (2014) Yamamoto et al. (2004) |
||
| VEGF-B | Falkevall et al. (2017) | ||
| ET-1 |
Garsen et al. (2016) Lenoir et al. (2014) |
||
| SDF-1 |
Takabatake et al. (2009) Takashima et al. (2016) |
||
| VEGF-A | Mesangial cells |
Shulman et al. (1996) Suyama et al. (2018) |
|
| CCL21 | Banas et al. (2002; Bernhard Banas et al. (2004; Valiño-Rivas et al. (2016) | ||
| CCN2 | Yokoi et al. (2008) | ||
| VEGF-B | Monocytes | Sato et al. (2008) | |
| CCL21 | Torres et al. (2020) | ||
| MCP-1 |
Calle and Hotter, 2020) Gu et al. (2006) |
||
| miR-221 | Proximal tubular epithelial cells | Su et al. (2020) | |
| HB-EGF | Parietal epithelial cells | Tan et al. (2016) |
Fig. 5.
Cellular crosstalk of endothelial cells and podocytes in diabetic kidney disease. (HB-EGF, heparin-binding epidermal growth factor-like growth factor; TGF-β, transforming growth factor-β; HGF, hepatocyte growth factor; FAT, fatty acid translocase; LRG-1, leucine-rich α-2-glycoprotein 1; PDGFB, platelet-derived growth factor B; APC, activated protein C; KLF2, Kruppel -like factor 2; eNOS, endothelial nitric oxide synthase; VEGF-A, vascular endothelial growth factor A; VEGF-B, vascular endothelial growth factor B; MCP-1, monocyte chemotactic protein 1; ET-1, endothelin-1; SDF-1, stromal cell-derived factor-1; Ang, angiopoietin; CCL21, C–C chemokine ligand 21; CCN2, cellular communication network factor 2;)
Table 3.
Crosstalk of mesangial cells and tubular epithelial cells
| Factor/ Signal pathway | Crosstalk cell | Reference | |
|---|---|---|---|
| Mesangial cells | α8-integrin | Podocytes | Hartner et al. (2010) |
| ER-associated degradation | Fujimoto et al. (2020) | ||
| TGF-β | Wang et al. (2018) | ||
| Tubular epithelial cells | B cell lymphoma 2-interacting mediator (Bim) | Podocytes | Xu et al. (2020) |
| Sirt1 | Hasegawa et al. (2013) | ||
| Gremlin | Marchant et al. (2015) | ||
| Ang | Endothelial cells | Rizkalla et al. 2005) | |
| miR-196b-5p | Fibroblast | Hu et al. (2020) | |
| MCP-1 | Monocytes | Chow et al. (2006) |
Furthermore, we identified the following unclear/unexplored aspects in the field:
TGF-β and VEGF are key cytokines in the process of DKD. However, their role is complex and requires further investigation.
Lipotoxicity is considered to promote DKD progression. GECs are considered to be a source of lipid deposits in other cells. We speculate that FAT/CD36 is involved in lipid transport in kidney cells. However, the specific mechanism is currently unknown and needs to be explored, as this will help to further guide the prevention and treatment of DKD.
The pathogenic crosstalk factors of DKD include TGF-β, LRG-1, FAT/CD36, PDGFB, VEGF-B, ET-1, SLC, MCP-1, CCN2, miR-221, and HB-EGF. The protective factors include eNOS, KLF2, APC, HGF, Ang1, VEGF-A, and SDF-1. Decreased expression of protective factors and upregulation of pathogenic factors lead to DKD progression. Although these factors have been shown to be involved in the cellular crosstalk of DKD, their specific mechanisms need to be further elucidated.
The role of miRNAs in DKD has been described. While the expression of some miRNAs is upregulated in DKD, the expression of others is downregulated. As emerging biomarkers of DKD, miRNAs have research significance in the cellular crosstalk of DKD. However, the role of miRNAs in cellular crosstalk needs to be further elucidated.
In conclusion, a better understanding of the cellular crosstalk of GECs and podocytes will help to further reveal the pathogenesis of DKD and develop better prevention and treatment options. Cellular crosstalk in DKD is highly complex and remains largely unknown. Efforts should be made toward elucidating the intricates of such complex processes.
Acknowledgements
SJ wishes to thank Mr. Feng Gao for his constant encouragement and support throughout.
Authors’ contributions
Shan Jiang participated in the literature search and writing of the manuscript. Manyu Luo and Xue Bai participated in the literature review and made important revisions. Ping Nie, Yuexin Zhu and Hangxi Cai are responsible for making the tables and pictures. Bing Li and Ping Luo is responsible for writing and editing the manuscript. All authors have read and approved the final manuscript.
Funding
This study was funded in part by grants from the National Natural Science Foundation of China (81970628), Science and Technology Development Plan Project of Jilin province (20200201488JC, 20190304042YY), the Jilin Province Sanitation and Health Technology Innovation Project(2018J048), and National Clinical Research Center for Kidney Diseases, Chinese PLA General Hospital (No. kfkt202020).
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bing Li, Email: 115674160@qq.com.
Ping Luo, Email: luopingjen@163.com.
References
- Alvarez ML, Distefano JK. The role of non-coding RNAs in diabetic nephropathy: potential applications as biomarkers for disease development and progression. Diabetes Res Clin Pract. 2013;99(1):1–11. doi: 10.1016/j.diabres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Aly MH, Arafat MA, Hussein OA, Elsaid HH, Abdel-Hammed AR. Study of Angiopoietin-2 and vascular endothelial growth factor as markers of diabetic nephropathy onset in Egyptians diabetic patients with non-albuminuric state. Diabetes Metab Syndr. 2019;13(2):1623–1627. doi: 10.1016/j.dsx.2019.03.016. [DOI] [PubMed] [Google Scholar]
- An Y, Zhang C, Xu F, Li W, Zeng C, Xie L, Liu Z. Increased urinary miR-196a level predicts the progression of renal injury in patients with diabetic nephropathy. Nephrol Dial Transplant. 2020;35(6):1009–1016. doi: 10.1093/ndt/gfy326. [DOI] [PubMed] [Google Scholar]
- Arora MK, Singh UK. Molecular mechanisms in the pathogenesis of diabetic nephropathy: An update. Vascul Pharmacol. 2013;58(4):259–271. doi: 10.1016/j.vph.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Awad AS, Kinsey GR, Khutsishvili K, Gao T, Bolton WK, Okusa MD. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am J Physiol Renal Physiol. 2011;301(6):F1358–1366. doi: 10.1152/ajprenal.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178(12):7632–7639. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Nowack C, et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol. 2019;50(5):333–344. doi: 10.1159/000503713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas B, Wörnle M, Berger T, Nelson PJ, Cohen CD, Kretzler M, et al. Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol. 2002;168(9):4301–4307. doi: 10.4049/jimmunol.168.9.4301. [DOI] [PubMed] [Google Scholar]
- Banas B, Wörnle M, Merkle M, Gonzalez-Rubio M, Schmid H, Kretzler M, et al. Binding of the chemokine SLC/CCL21 to its receptor CCR7 increases adhesive properties of human mesangial cells. Kidney Int. 2004;66(6):2256–2263. doi: 10.1111/j.1523-1755.2004.66037.x. [DOI] [PubMed] [Google Scholar]
- Bär C, Thum T, de Gonzalo-Calvo D. Circulating miRNAs as mediators in cell-to-cell communication. Epigenomics. 2019;11(2):111–113. doi: 10.2217/epi-2018-0183. [DOI] [PubMed] [Google Scholar]
- Bechmann LP, Gieseler RK, Sowa JP, Kahraman A, Erhard J, Wedemeyer I, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010;30(6):850–859. doi: 10.1111/j.1478-3231.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Role of platelet-derived growth factors in mouse development. Int J Dev Biol. 1995;39(5):817–825. [PubMed] [Google Scholar]
- Boels MG, Avramut MC, Koudijs A, Dane MJ, Lee DH, van der Vlag J, et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65(8):2429–2439. doi: 10.2337/db15-1413. [DOI] [PubMed] [Google Scholar]
- Bonen A, Campbell SE, Benton CR, Chabowski A, Coort SL, Han XX, et al. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc. 2004;63(2):245–249. doi: 10.1079/PNS2004331. [DOI] [PubMed] [Google Scholar]
- Bose M, Almas S, Prabhakar S. Wnt signaling and podocyte dysfunction in diabetic nephropathy. J Investig Med. 2017;65(8):1093–1101. doi: 10.1136/jim-2017-000456. [DOI] [PubMed] [Google Scholar]
- Calle P, Hotter G. Macrophage phenotype and fibrosis in diabetic nephropathy. Int J Mol Sci. 2020;21(8):2806. doi: 10.3390/ijms21082806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YP, Sun B, Han Z, Han F, Hu SL, Li XY, et al. Saxagliptin attenuates albuminuria by inhibiting podocyte epithelial- to-mesenchymal transition via SDF-1α in diabetic nephropathy. Front Pharmacol. 2017;8:780. doi: 10.3389/fphar.2017.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Lv LL, Liu BC, Tang RN. Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif. 2020;53(3):e12763. doi: 10.1111/cpr.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Wang D, Wang F, Liu J, Huang B, Baker MA, et al. Endogenous miR-204 protects the kidney against chronic injury in hypertension and diabetes. J Am Soc Nephrol. 2020;31(7):1539–1554. doi: 10.1681/ASN.2019101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow GM, Pergola PE, Chen F, Kirby BJ, Sundy JS, Patel UD. Effects of selonsertib in patients with diabetic kidney disease. J Am Soc Nephrol. 2019;30(10):1980–1990. doi: 10.1681/ASN.2018121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiplunkar AR, Lung TK, Alhashem Y, Koppenhaver BA, Salloum FN, Kukreja RC, et al. Krüppel-like factor 2 is required for normal mouse cardiac development. PloS one. 2013;8(2):e54891. doi: 10.1371/journal.pone.0054891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65(1):116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69(1):73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- Conserva F, Barozzino M, Pesce F, Divella C, Oranger A, Papale M, et al. Urinary miRNA-27b-3p and miRNA-1228-3p correlate with the progression of Kidney Fibrosis in Diabetic Nephropathy. Sci Rep. 2019;9(1):11357. doi: 10.1038/s41598-019-47778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs' Action through miRNA Editing. Int J Mol Sci. 2019;20(24):6249. doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Cui Y, Fu Y, Ma S, Zhang S. Microarray analysis reveals gene and microRNA signatures in diabetic kidney disease. Mol Med Rep. 2018;17(2):2161–2168. doi: 10.3892/mmr.2017.8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumpănas AA, Cimpean AM, Ferician O, Ceausu RA, Sarb S, Barbos V, et al. The Involvement of PDGF-B/PDGFRβ Axis in the Resistance to Antiangiogenic and Antivascular Therapy in Renal Cancer. Anticancer Res. 2016;36(5):2291–2295. [PubMed] [Google Scholar]
- Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124(4):1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B, Dei Cas A, Long DA, White KE, Hayward A, Ku CH, et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol. 2007;18(8):2320–2329. doi: 10.1681/ASN.2006101093. [DOI] [PubMed] [Google Scholar]
- de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2(5):417–426. doi: 10.1016/S2213-8587(14)70065-8. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. The Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369(26):2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3(9):687–696. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Renfurm RW, Bakris G, Rossing P, Perkovic V, Hou FF, et al. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2018;6(12):925–933. doi: 10.1016/S2213-8587(18)30289-4. [DOI] [PubMed] [Google Scholar]
- Dessapt-Baradez C, Woolf AS, White KE, Pan J, Huang JL, Hayward AA, et al. Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. J Am Soc Nephrol. 2014;25(1):33–42. doi: 10.1681/ASN.2012121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Yang J, Meng L. Screening and identification of differentially expressed genes between diabetic nephropathy glomerular and normal glomerular via bioinformatics technology. Comb Chem High Throughput Screen. 2021;24(5):645–655. doi: 10.2174/1386207323999200821163314. [DOI] [PubMed] [Google Scholar]
- Du Q, Fu Y-X, Shu A-M, Lv X, Chen Y-P, Gao Y-Y, et al. Loganin alleviates macrophage infiltration and activation by inhibiting the MCP-1/CCR2 axis in diabetic nephropathy. Life Sciences. 2021;272:118808. doi: 10.1016/j.lfs.2020.118808. [DOI] [PubMed] [Google Scholar]
- Duan L, Lu Y, Xie W, Nong L, Jia Y, Tan A, Liu Y. Leptin promotes bone metastasis of breast cancer by activating the SDF-1/CXCR4 axis. Aging (albany NY) 2020;12(16):16172–16182. doi: 10.18632/aging.103599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkevall A, Mehlem A, Palombo I, Heller Sahlgren B, Ebarasi L, He L, et al. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab. 2017;25(3):713–726. doi: 10.1016/j.cmet.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Feng L, Gu C, Li Y, Huang J. High glucose promotes CD36 expression by upregulating peroxisome proliferator-activated receptor γ levels to exacerbate lipid deposition in renal tubular cells. Biomed Res Int. 2017;2017:1414070–1414070. doi: 10.1155/2017/1414070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J, Eng E, Young BA, Alpers CE, Barrett TB, Bowen-Pope DF, Johnson RJ. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest. 1993;92(6):2952–2962. doi: 10.1172/JCI116918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J, Smeets B, Moeller MJ. The SDF-1/CXCR4 axis is a novel driver of vascular development of the glomerulus. J Am Soc Nephrol. 2009;20(8):1659–1661. doi: 10.1681/ASN.2009060621. [DOI] [PubMed] [Google Scholar]
- Fu J, Lee K, Chuang PY, Liu Z, He JC. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015;308(4):F287–297. doi: 10.1152/ajprenal.00533.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wei C, Zhang W, Schlondorff D, Wu J, Cai M, et al. Gene expression profiles of glomerular endothelial cells support their role in the glomerulopathy of diabetic mice. Kidney Int. 2018;94(2):326–345. doi: 10.1016/j.kint.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto D, Kuwabara T, Hata Y, Umemoto S, Kanki T, Nishiguchi Y, et al. Suppressed ER-associated degradation by intraglomerular cross talk between mesangial cells and podocytes causes podocyte injury in diabetic kidney disease. FASEB J. 2020;34(11):15577–15590. doi: 10.1096/fj.202000078RR. [DOI] [PubMed] [Google Scholar]
- Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, Yoshinaga K. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21(5):480–485. doi: 10.1016/S0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- Gahan JC, Gosalbez M, Yates T, Young EE, Escudero DO, Chi A, et al. Chemokine and chemokine receptor expression in kidney tumors: molecular profiling of histological subtypes and association with metastasis. J Urol. 2012;187(3):827–833. doi: 10.1016/j.juro.2011.10.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsen M, Lenoir O, Rops ALWMM, Dijkman HB, Willemsen B, van Kuppevelt TH, et al. Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol. 2016;27(12):3545–3551. doi: 10.1681/ASN.2015091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmini S, Mangoni M, Serio M, Romagnani P, Lazzeri E. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J Endocrinol Invest. 2008;31(9):809–819. doi: 10.1007/BF03349262. [DOI] [PubMed] [Google Scholar]
- Gil CL, Hooker E, Larrivée B. Diabetic Kidney Disease, Endothelial Damage, and Podocyte-Endothelial Crosstalk. Kidney Medicine. 2020;3(1):105–115. doi: 10.1016/j.xkme.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick AD, Jacobson HR, Haralson MA. Mesangial deposition of type I collagen in human glomerulosclerosis. Hum Pathol. 1992;23(12):1373–1379. doi: 10.1016/0046-8177(92)90057-A. [DOI] [PubMed] [Google Scholar]
- Gnudi L. Angiopoietins and diabetic nephropathy. Diabetologia. 2016;59(8):1616–1620. doi: 10.1007/s00125-016-3995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommans WM, Berezikov E. Controlling miRNA regulation in disease. Methods Mol Biol. 2012;822:1–18. doi: 10.1007/978-1-61779-427-8_1. [DOI] [PubMed] [Google Scholar]
- Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De Cosmo S. GLP-1 Receptor Agonists and Kidney Protection. Medicina (kaunas) 2019;55(6):233. doi: 10.3390/medicina55060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröne HJ. Glomerular lipids in non-hereditary forms of glomerulopathy/glomerulonephritis. Nephrol Dial Transplant. 1999;14(6):1595–1598. doi: 10.1093/ndt/14.6.1595. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. Faseb j. 2004;18(3):469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- Gu L, Hagiwara S, Fan Q, Tanimoto M, Kobata M, Yamashita M, et al. Role of receptor for advanced glycation end-products and signalling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytes. Nephrol Dial Transplant. 2006;21(2):299–313. doi: 10.1093/ndt/gfi210. [DOI] [PubMed] [Google Scholar]
- Guo H, Fang C, Huang Y, Pei Y, Chen L, Hu J. The efficacy and safety of DPP4 inhibitors in patients with type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;121:184–191. doi: 10.1016/j.diabres.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464(7290):917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490(7420):426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- Hanai K, Babazono T. CREDENCE: a silver lining in the dark cloud of diabetic nephropathy. J Diabetes Investig. 2020;11(3):527–529. doi: 10.1111/jdi.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartner A, Cordasic N, Menendez-Castro C, Volkert G, Yabu JM, Kupraszewicz-Hutzler M, et al. Lack of {alpha}8-integrin aggravates podocyte injury in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299(5):F1151–1157. doi: 10.1152/ajprenal.00058.2010. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19(11):1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerspink HJL, Parving H-H, Andress DL, Bakris G, Correa-Rotter R, Hou F-F, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. The Lancet. 2019;393(10184):1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- Hong D, Zheng T, Jia-qing S, Jian W, Zhi-hong L, Lei-shi L. Nodular glomerular lesion: a later stage of diabetic nephropathy? Diabetes Res Clin Pract. 2007;78(2):189–195. doi: 10.1016/j.diabres.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Hong Q, Zhang L, Fu J, Verghese DA, Chauhan K, Nadkarni GN, et al. LRG1 Promotes Diabetic Kidney Disease Progression by Enhancing TGF-β-Induced Angiogenesis. J Am Soc Nephrol. 2019;30(4):546–562. doi: 10.1681/ASN.2018060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Li X, Peng C, Gao R, Ma L, Hu J, et al. miR-196b-5p-enriched extracellular vesicles from tubular epithelial cells mediated aldosterone-induced renal fibrosis in mice with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001101. doi: 10.1136/bmjdrc-2019-001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger C, Ödemis V, Engele J. Expression and function of the SDF-1 chemokine receptors CXCR4 and CXCR7 during mouse limb muscle development and regeneration. Exp Cell Res. 2012;318(17):2178–2190. doi: 10.1016/j.yexcr.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274(38):26761–26766. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- Igawa T, Matsumoto K, Kanda S, Saito Y, Nakamura T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol. 1993;265(1 Pt 2):F61–69. doi: 10.1152/ajprenal.1993.265.1.F61. [DOI] [PubMed] [Google Scholar]
- Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13(11):1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- Jiang T, Liebman SE, Lucia MS, Li J, Levi M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005;68(6):2608–2620. doi: 10.1111/j.1523-1755.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- Jiang ZH, Tang YZ, Song HN, Yang M, Li B, Ni CL. miRNA-342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. Int J Mol Med. 2020;45(1):45–52. doi: 10.3892/ijmm.2019.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpanen T, Bry M, Ollila HM, Seppänen-Laakso T, Liimatta E, Leskinen H, et al. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res. 2008;103(9):1018–1026. doi: 10.1161/CIRCRESAHA.108.178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Luyckx VA, Ots M, Lee K-W, Ziai F, Troy JL, et al. Renin-angiotensin blockade lowers MCP-1 expression in diabetic rats. Kidney Int. 1999;56(3):1037–1048. doi: 10.1046/j.1523-1755.1999.00643.x. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ullevig SL, Zamora D, Lee CF, Asmis R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc Natl Acad Sci U S A. 2012;109(41):E2803–2812. doi: 10.1073/pnas.1212596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay YY, Tan GCJ, Phang SCW, Ho JI, Chuar PF, Ho LS, et al. A Phase IIb randomized controlled trial investigating the effects of tocotrienol-rich vitamin E on diabetic kidney disease. Nutrients. 2021;13(1):258. doi: 10.3390/nu13010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling M, Kaucsar T, Schauerte C, Hübner A, Dettling A, Park JK, et al. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol Ther. 2017;25(1):165–180. doi: 10.1016/j.ymthe.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Lai H, Chen A, Cai H, Fu J, Salem F, Li Y, et al. Podocyte and endothelial-specific elimination of BAMBI identifies differential transforming growth factor-β pathways contributing to diabetic glomerulopathy. Kidney Int. 2020;98(3):601–614. doi: 10.1016/j.kint.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langham RG, Kelly DJ, Maguire J, Dowling JP, Gilbert RE, Thomson NM. Over-expression of platelet-derived growth factor in human diabetic nephropathy. Nephrol Dial Transplant. 2003;18(7):1392–1396. doi: 10.1093/ndt/gfg177. [DOI] [PubMed] [Google Scholar]
- Lassén E, Daehn IS. Molecular Mechanisms in Early Diabetic Kidney Disease: Glomerular Endothelial Cell Dysfunction. Int J Mol Sci. 2020;21(24):9456. doi: 10.3390/ijms21249456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Shin SJ, Lin SR, Tan MS, Tsai JH. Increased expression of heparin binding epidermal growth-factor-like growth factor mRNA in the kidney of streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 1995;207(1):216–222. doi: 10.1006/bbrc.1995.1175. [DOI] [PubMed] [Google Scholar]
- Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, et al. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11(6):845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Lee HY, Youn SW, Oh BH, Kim HS. Krüppel-like factor 2 suppression by high glucose as a possible mechanism of diabetic vasculopathy. Korean Circ J. 2012;42(4):239–245. doi: 10.4070/kcj.2012.42.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir O, Milon M, Virsolvy A, Hénique C, Schmitt A, Massé J-M, et al. Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol. 2014;25(5):1050–1062. doi: 10.1681/ASN.2013020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AKL. The Whereabouts of microRNA actions: cytoplasm and beyond. Trends Cell Biol. 2015;25(10):601–610. doi: 10.1016/j.tcb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8(16):1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, et al. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):131–136. doi: 10.1681/ASN.2011030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63(6):2010–2019. doi: 10.1046/j.1523-1755.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- Li H, Jiang T, Lin Y, Zhao Z, Zhang N. HGF protects rat mesangial cells from high-glucose-mediated oxidative stress. Am J Nephrol. 2006;26(5):519–530. doi: 10.1159/000097368. [DOI] [PubMed] [Google Scholar]
- Li L, Yin Q, Tang X, Bai L, Zhang J, Gou S, et al. C3a receptor antagonist ameliorates inflammatory and fibrotic signals in type 2 diabetic nephropathy by suppressing the activation of TGF-β/smad3 and IKBα pathway. PloS one. 2014;9(11):e113639. doi: 10.1371/journal.pone.0113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen L, Zang J, Tang X, Liu Y, Zhang J, et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64(5):597–610. doi: 10.1016/j.metabol.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang T, Geng J, Wu Z, Xu L, Liu J, et al. Advanced oxidation protein products promote lipotoxicity and tubulointerstitial fibrosis via CD36/β-catenin pathway in diabetic nephropathy. Antioxid Redox Signal. 2019;31(7):521–538. doi: 10.1089/ars.2018.7634. [DOI] [PubMed] [Google Scholar]
- Li X, Xu R, Liu X, Xu L, Xue M, Cheng Y, et al. Urinary miR-3137 and miR-4270 as potential biomarkers for diabetic kidney disease. J Clin Lab Anal. 2020;34(12):e23549. doi: 10.1002/jcla.23549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Hui Yu, Hong ZJ, yuan H, Qi C, Nong Z. HGF suppresses high glucose-mediated oxidative stress in mesangial cells by activation of PKG and inhibition of PKA. Free Radic Biol Med. 2010;49(3):467–473. doi: 10.1016/j.freeradbiomed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, et al. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125(17):3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Pek SLT, Ang K, Tavintharan S, Lim SC. Plasma leucine-rich α-2-glycoprotein 1 predicts rapid eGFR decline and albuminuria progression in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2017;102(10):3683–3691. doi: 10.1210/jc.2017-00930. [DOI] [PubMed] [Google Scholar]
- Lodyga M, Hinz B. TGF-β1 – A truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol. 2020;101:123–139. doi: 10.1016/j.semcdb.2019.12.010. [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Rajasekeran H, Lytvyn Y, Lovblom LE, Khan S, Alemu R, et al. Dipeptidyl peptidase 4 inhibition stimulates distal tubular natriuresis and increases in circulating SDF-1α(1–67) in patients with type 2 diabetes. Diabetes Care. 2017;40(8):1073–1081. doi: 10.2337/dc17-0061. [DOI] [PubMed] [Google Scholar]
- Madhusudhan T, Wang H, Straub BK, Gröne E, Zhou Q, Shahzad K, et al. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood. 2012;119(3):874–883. doi: 10.1182/blood-2011-07-365973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudhan T, Ghosh S, Wang H, Dong W, Gupta D, Elwakiel A, et al. Podocyte integrin-β (3) and activated protein C coordinately restrict rhoa signaling and ameliorate diabetic nephropathy. J Am Soc Nephrol. 2020;31(8):1762–1780. doi: 10.1681/ASN.2019111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni S, Zerbini G. Glomerular endothelial cells versus podocytes as the cellular target in diabetic nephropathy. Acta Diabetol. 2018;55(11):1105–1111. doi: 10.1007/s00592-018-1211-2. [DOI] [PubMed] [Google Scholar]
- Mahtal N, Lenoir O, Tharaux PL. Glomerular endothelial cell crosstalk with podocytes in diabetic kidney disease. Frontiers Med. 2021;8:659013. doi: 10.3389/fmed.2021.659013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Mallipattu SK, Estrada CC, He JC. The critical role of Krüppel-like factors in kidney disease. Am J Physiol Renal Physiol. 2017;312(2):F259–F265. doi: 10.1152/ajprenal.00550.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant V, Droguett A, Valderrama G, Burgos ME, Carpio D, Kerr B, et al. Tubular overexpression of Gremlin in transgenic mice aggravates renal damage in diabetic nephropathy. Am J Physiol Renal Physiol. 2015;309(6):F559–568. doi: 10.1152/ajprenal.00023.2015. [DOI] [PubMed] [Google Scholar]
- Martelossi Cebinelli GC, Paiva Trugilo K, Badaró Garcia S, Brajão de Oliveira K. TGF-β1 functional polymorphisms: a review. Eur Cytokine Netw. 2016;27(4):81–89. doi: 10.1684/ecn.2016.0382. [DOI] [PubMed] [Google Scholar]
- Martin C, Chevrot M, Poirier H, Passilly-Degrace P, Niot I, Besnard P. CD36 as a lipid sensor. Physiol Behav. 2011;105(1):36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shikata K, Makino H, Sugimoto H, Ota K, Akiyama K, et al. Gene expression of PDGF and PDGF receptor in various forms of glomerulonephritis. Am J Nephrol. 1997;17(1):25–31. doi: 10.1159/000169067. [DOI] [PubMed] [Google Scholar]
- McLennan SV, Abdollahi M, Twigg SM. Connective tissue growth factor, matrix regulation, and diabetic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(1):85–92. doi: 10.1097/MNH.0b013e32835b4889. [DOI] [PubMed] [Google Scholar]
- Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–467. [PubMed] [Google Scholar]
- Mesarosova L, Ochodnicky P, Leemans JC, Florquin S, Krenek P, Klimas J. High glucose induces HGF-independent activation of Met receptor in human renal tubular epithelium. J Recept Signal Transduct Res. 2017;37(6):535–542. doi: 10.1080/10799893.2017.1365902. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Nakamura T. Suppressions of chronic glomerular injuries and TGF-beta 1 production by HGF in attenuation of murine diabetic nephropathy. Am J Physiol Renal Physiol. 2004;286(1):F134–143. doi: 10.1152/ajprenal.00199.2003. [DOI] [PubMed] [Google Scholar]
- Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T. Uncoupling of VEGF with NO as a mechanism for diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S67–69. doi: 10.1016/j.diabres.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, Li Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol. 2011;7(1):36–44. doi: 10.1038/nrneph.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T. Hepatocyte growth factor as mitogen, motogen and morphogen, and its roles in organ regeneration. Princess Takamatsu Symp. 1994;24:195–213. [PubMed] [Google Scholar]
- Nasser M, Ibrahim KMF, Elnabawi WM, Ahmed TM. Overexpression of serum micro-RNA 152–3p in type 2 diabetes mellitus with a significant elevation in progressive nephropathy. Egypt J Immunol. 2020;27(2):81–92. [PubMed] [Google Scholar]
- Otsuka S, Bebb G. The CXCR4/SDF-1 chemokine receptor axis: a new target therapeutic for non-small cell lung cancer. J Thorac Oncol. 2008;3(12):1379–1383. doi: 10.1097/JTO.0b013e31818dda9d. [DOI] [PubMed] [Google Scholar]
- Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Krüppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40(10):1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Perkovic V, Toto R, Cooper ME, Mann JFE, Rosenstock J, McGuire DK, et al. Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: secondary analysis of the carmelina randomized trial. Diabetes Care. 2020;43(8):1803–1812. doi: 10.2337/dc20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol. 2002;17(1):247–252. doi: 10.14670/HH-17.247. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Little MH. Parietal epithelial cells regenerate podocytes. J Am Soc Nephrol. 2009;20(2):231–233. doi: 10.1681/ASN.2008121279. [DOI] [PubMed] [Google Scholar]
- Puchert M, Engele J. The peculiarities of the SDF-1/CXCL12 system: in some cells, CXCR4 and CXCR7 sing solos, in others, they sing duets. Cell Tissue Res. 2014;355(2):239–253. doi: 10.1007/s00441-013-1747-y. [DOI] [PubMed] [Google Scholar]
- Qi H, Casalena G, Shi S, Yu L, Ebefors K, Sun Y, et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes. 2017;66(3):763–778. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H-J, Xu T, Wu H-T, Yao Z-L, Hou Y-L, Xie Y-H, et al. SDF-1/CXCR4 axis coordinates crosstalk between subchondral bone and articular cartilage in osteoarthritis pathogenesis. Bone. 2019;125:140–150. doi: 10.1016/j.bone.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Rizkalla B, Forbes JM, Cao Z, Boner G, Cooper ME. Temporal renal expression of angiogenic growth factors and their receptors in experimental diabetes: role of the renin–angiotensin system. J Hypertens. 2005;23(1):153. doi: 10.1097/00004872-200501000-00026. [DOI] [PubMed] [Google Scholar]
- Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW. Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol. 2002;161(4):1459–1466. doi: 10.1016/S0002-9440(10)64421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23(8):1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296(5):F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell SC, Harper SJ, Tooke JE, Kerjaschki D, Saleem MA, Mathieson PW. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J Am Soc Nephrol. 2002;13(2):544. doi: 10.1681/ASN.V132544. [DOI] [PubMed] [Google Scholar]
- Sato W, Kosugi T, Zhang L, Roncal CA, Heinig M, Campbell-Thompson M, et al. The pivotal role of VEGF on glomerular macrophage infiltration in advanced diabetic nephropathy. Lab Invest. 2008;88(9):949–961. doi: 10.1038/labinvest.2008.60. [DOI] [PubMed] [Google Scholar]
- Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91(3):827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- Schneider H, Staudacher S, Poppelreuther M, Stremmel W, Ehehalt R, Füllekrug J. Protein mediated fatty acid uptake: synergy between CD36/FAT-facilitated transport and acyl-CoA synthetase-driven metabolism. Arch Biochem Biophys. 2014;546:8–18. doi: 10.1016/j.abb.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Shaker OG, Sadik NAH. Transforming growth factor beta 1 and monocyte chemoattractant protein-1 as prognostic markers of diabetic nephropathy. Hum Exp Toxicol. 2013;32(10):1089–1096. doi: 10.1177/0960327112470274. [DOI] [PubMed] [Google Scholar]
- Shan D, Wu HM, Yuan QY, Li J, Zhou RL, Liu GJ (2012) Pentoxifylline for diabetic kidney disease. Cochrane Database Syst Rev [DOI] [PMC free article] [PubMed]
- Shankland SJ, Floege J, Thomas SE, Nangaku M, Hugo C, Pippin J, et al. Cyclin kinase inhibitors are increased during experimental membranous nephropathy: Potential role in limiting glomerular epithelial cell proliferation in vivo. Kidney Int. 1997;52(2):404–413. doi: 10.1038/ki.1997.347. [DOI] [PubMed] [Google Scholar]
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Shulman K, Rosen S, Tognazzi K, Manseau EJ, Brown LF. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996;7(5):661–666. doi: 10.1681/ASN.V75661. [DOI] [PubMed] [Google Scholar]
- Siddiqui K, Joy SS, George TP, Mujammami M, Alfadda AA. Potential role and excretion level of urinary transferrin, KIM-1, RBP, MCP-1 and NGAL markers in diabetic nephropathy. Diabetes Metab Syndr Obes. 2020;13:5103–5111. doi: 10.2147/DMSO.S282166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol. 2010;21(10):1691–1701. doi: 10.1681/ASN.2010030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61(11):2958–2966. doi: 10.2337/DB11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater SC, Ramnath RD, Uttridge K, Saleem MA, Cahill PA, Mathieson PW, et al. Chronic exposure to laminar shear stress induces Kruppel-like factor 2 in glomerular endothelial cells and modulates interactions with co-cultured podocytes. Int J Biochem Cell Biol. 2012;44(9):1482–1490. doi: 10.1016/j.biocel.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Sohn E, Kim J, Kim CS, Jo K, Lee YM, Kim JS. Root of Polygonum cuspidatum extract reduces progression of diabetes-induced mesangial cell dysfunction via inhibition of platelet-derived growth factor-BB (PDGF-BB) and interaction with its receptor in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2014;14:477. doi: 10.1186/1472-6882-14-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovska J, Stefanovics J, Gersone G, Pahirko L, Valeinis J, Kalva-Vaivode S, et al. Angiopoietin 2 and neuropeptide Y are associated with diabetic kidney disease in type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2020;128(10):654–662. doi: 10.1055/a-1079-4711. [DOI] [PubMed] [Google Scholar]
- Sol M, Kamps J, van den Born J, van den Heuvel MC, van der Vlag J, Krenning G, Hillebrands JL. Glomerular endothelial cells as instigators of glomerular sclerotic diseases. Frontiers Pharmacol. 2020;11:573557. doi: 10.3389/fphar.2020.573557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch LK, Schwenk RW, Ouwens DM, Diamant M, Glatz JF, Luiken JJ. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell Mol Life Sci. 2011;68(15):2525–2538. doi: 10.1007/s00018-011-0690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger N, Worthmann K, Schiffer M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes Metab Res Rev. 2011;27(3):207–215. doi: 10.1002/dmrr.1164. [DOI] [PubMed] [Google Scholar]
- Su J, Li SJ, Chen ZH, Zeng CH, Zhou H, Li LS, Liu ZH. Evaluation of podocyte lesion in patients with diabetic nephropathy: wilms' tumor-1 protein used as a podocyte marker. Diabetes Res Clin Pract. 2010;87(2):167–175. doi: 10.1016/j.diabres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Su Y, Chen Q, Ma K, Ju Y, Ji T, Wang Z, et al. Astragaloside IV inhibits palmitate-mediated oxidative stress and fibrosis in human glomerular mesangial cells via downregulation of CD36 expression. Pharmacol Rep. 2019;71(2):319–329. doi: 10.1016/j.pharep.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Su H, Qiao J, Hu J, Li Y, Lin J, Yu Q, et al. Podocyte-derived extracellular vesicles mediate renal proximal tubule cells dedifferentiation via microRNA-221 in diabetic nephropathy. Mol Cell Endocrinol. 2020;518:111034. doi: 10.1016/j.mce.2020.111034. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Miao Z, Dairaghi DJ, Krasinski A, Wang Y, Zhao BN, et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am J Physiol Renal Physiol. 2013;305(9):F1288–1297. doi: 10.1152/ajprenal.00316.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama M, Miyazaki Y, Matsusaka T, Sugano N, Ueda H, Kawamura T, et al. Forced expression of vascular endothelial growth factor-A in podocytes decreases mesangial cell numbers and attenuates endothelial cell differentiation in the mouse glomerulus. Clin Exp Nephrol. 2018;22(2):266–274. doi: 10.1007/s10157-017-1450-5. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Gabazza EC, Hayashi T, Kamada H, Adachi Y, Taguchi O. Protective role of activated protein C in lung and airway remodeling. Crit Care Med. 2004;32(5 Suppl):S262–265. doi: 10.1097/01.CCM.0000129668.96935.A8. [DOI] [PubMed] [Google Scholar]
- Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20(8):1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, and Harris RC (2014). Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. J Diabetes Res, 590541. [DOI] [PMC free article] [PubMed]
- Takashima S, Fujita H, Fujishima H, Shimizu T, Sato T, Morii T, et al. Stromal cell–derived factor-1 is upregulated by dipeptidyl peptidase-4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int. 2016;90(4):783–796. doi: 10.1016/j.kint.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Tan H, Yi H, Zhao W, Ma JX, Zhang Y, Zhou X. Intraglomerular crosstalk elaborately regulates podocyte injury and repair in diabetic patients: insights from a 3D multiscale modeling study. Oncotarget. 2016;7(45):73130–73146. doi: 10.18632/oncotarget.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Wang X, Deng T, Ge H, Xiao X. Identification of C3 as a therapeutic target for diabetic nephropathy by bioinformatics analysis. Sci Rep. 2020;10(1):13468. doi: 10.1038/s41598-020-70540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Dong XY, Zeng ZG, Feng Z. Gene expression-based analysis identified NTNG1 and HGF as biomarkers for diabetic kidney disease. Medicine (Baltimore) 2020;99(1):e18596. doi: 10.1097/MD.0000000000018596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabra E, Giunti S, Barutta F, Salvidio G, Burt D, Deferrari G. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes. 2009;58(9):2109–2118. doi: 10.2337/db08-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch GH. Diabetic nephropathy – is this an immune disorder? Clin Sci. 2017;131(16):2183–2199. doi: 10.1042/CS20160636. [DOI] [PubMed] [Google Scholar]
- Thongnak L, Pongchaidecha A, Lungkaphin A. Renal lipid metabolism and lipotoxicity in diabetes. Am J Med Sci. 2020;359(2):84–99. doi: 10.1016/j.amjms.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Thornton SN, Regnault V, Lacolley P. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(22):2196–2197. doi: 10.1056/NEJMc1713042. [DOI] [PubMed] [Google Scholar]
- Throckmorton DC, Brogden AP, Min B, Rasmussen H, Kashgarian M. PDGF and TGF-β mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int. 1995;48(1):111–117. doi: 10.1038/ki.1995.274. [DOI] [PubMed] [Google Scholar]
- Titan SM, Vieira JM, Jr, Dominguez WV, Moreira SR, Pereira AB, Barros RT, Zatz R. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications. 2012;26(6):546–553. doi: 10.1016/j.jdiacomp.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Torban E, Braun F, Wanner N, Takano T, Goodyer PR, Lennon R, et al. From podocyte biology to novel cures for glomerular disease. Kidney Int. 2019;96(4):850–861. doi: 10.1016/j.kint.2019.05.015. [DOI] [PubMed] [Google Scholar]
- Torres Á, Muñoz K, Nahuelpán Y, Saez A-PR, Mendoza P, Jara C et al (2020) Intraglomerular monocyte/macrophage infiltration and macrophage–myofibroblast transition during diabetic nephropathy is regulated by the a2b adenosine receptor. Cells 9(4):1051 [DOI] [PMC free article] [PubMed]
- Tufro A, Veron D. VEGF and podocytes in diabetic nephropathy. Semin Nephrol. 2012;32(4):385–393. doi: 10.1016/j.semnephrol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle KR, Brosius FC, 3rd, Adler SG, Kretzler M, Mehta RL, Tumlin JA, et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant. 2018;33(11):1950–1959. doi: 10.1093/ndt/gfx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziastoudi M, Stefanidis I, Zintzaras E. The genetic map of diabetic nephropathy: evidence from a systematic review and meta-analysis of genetic association studies. Clin Kidney J. 2020;13(5):768–781. doi: 10.1093/ckj/sfaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol. 2012;32(2):415–426. doi: 10.1161/ATVBAHA.111.238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttarwar L, Peng F, Wu D, Kumar S, Gao B, Ingram AJ, Krepinsky JC. HB-EGF release mediates glucose-induced activation of the epidermal growth factor receptor in mesangial cells. Am J Physiol Renal Physiol. 2011;300(4):F921–931. doi: 10.1152/ajprenal.00436.2010. [DOI] [PubMed] [Google Scholar]
- Valiño-Rivas L, Gonzalez-Lafuente L, Sanz AB, Ruiz-Ortega M, Ortiz A, Sanchez-Niño MD. Non-canonical NFκB activation promotes chemokine expression in podocytes. Sci Rep. 2016;6:28857–28857. doi: 10.1038/srep28857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Krieken R, Krepinsky JC. Caveolin-1 in the pathogenesis of diabetic nephropathy: potential therapeutic target? Curr DiabRep. 2017;17(3):19. doi: 10.1007/s11892-017-0844-9. [DOI] [PubMed] [Google Scholar]
- Verhave JC, Bouchard J, Goupil R, Pichette V, Brachemi S, Madore F, Troyanov S. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract. 2013;101(3):333–340. doi: 10.1016/j.diabres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Villegas G, Lange-Sperandio B, Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int. 2005;67(2):449–457. doi: 10.1111/j.1523-1755.2005.67101.x. [DOI] [PubMed] [Google Scholar]
- Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119–119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2012;124(3):139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- Wada T, Sakai N, Matsushima K, Kaneko S. Fibrocytes: a new insight into kidney fibrosis. Kidney Int. 2007;72(3):269–273. doi: 10.1038/sj.ki.5002325. [DOI] [PubMed] [Google Scholar]
- Wallmon A, Fellström B, Larsson R, Floege J, Topley N, Ljunghall S. PDGF-BB, but not PDGF-AA, stimulates calcium mobilization, activation of calcium channels and cell proliferation in cultured rat mesangial cells. Exp Nephrol. 1993;1(4):238–244. [PubMed] [Google Scholar]
- Wang K, Li Y, Han R, Cai G, He C, Wang G, Jia D. T140 blocks the SDF-1/CXCR4 signaling pathway and prevents cartilage degeneration in an osteoarthritis disease model. PloS one. 2017;12(4):e0176048. doi: 10.1371/journal.pone.0176048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Y, Tang L-Q, Wei W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFβ1-PI3K/AKT pathway. Eur J Pharmacol. 2018;824:185–192. doi: 10.1016/j.ejphar.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60(9):2354–2369. doi: 10.2337/db10-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-M, Gao Y-B, Cui F-Q, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open. 2016;5(4):484–491. doi: 10.1242/bio.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gao Y, Xu L, Dang W, Yan H, Zou D, et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep. 2017;7(1):9371. doi: 10.1038/s41598-017-09907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Muxun Z. Expression of c-met stimulated by high glucose in human renal tubular epithelial cells and its implication. J Huazhong Univ Sci Technolog Med Sci. 2007;27(2):161–163. doi: 10.1007/s11596-007-0213-z. [DOI] [PubMed] [Google Scholar]
- Xiong S, Han Y, Gao P, Zhao H, Jiang N, Sun L. AdipoRon protects against tubular injury in diabetic nephropathy by inhibiting endoplasmic reticulum stress. Oxid Med Cell Longev. 2020;2020:6104375. doi: 10.1155/2020/6104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zhou X, Xie T, Zhou Y, Zhang Q, Jiang S, et al. Renal tubular Bim mediates the tubule-podocyte crosstalk via NFAT2 to induce podocyte cytoskeletal dysfunction. Theranostics. 2020;10(15):6806–6824. doi: 10.7150/thno.43145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Maeshima Y, Kitayama H, Kitamura S, Takazawa Y, Sugiyama H, et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes. 2004;53(7):1831–1840. doi: 10.2337/diabetes.53.7.1831. [DOI] [PubMed] [Google Scholar]
- Yang M, Huang HC, Li JZ, Wang HY. Connective tissue growth factor synergistically with transforming growth factor beta 1 to promote renal fibrosis. Zhonghua Yi Xue Za Zhi. 2004;84(7):569–573. [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73(4):446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Suri C, Landon DN, Yancopoulos GD, Woolf AS. Angiopoietin-2 is a site-specific factor in differentiation of mouse renal vasculature. J Am Soc Nephrol. 2000;11(6):1055–1066. doi: 10.1681/ASN.V1161055. [DOI] [PubMed] [Google Scholar]
- Yuen DA, Stead BE, Zhang Y, White KE, Kabir MG, Thai K, et al. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol. 2012;23(11):1810–1823. doi: 10.1681/ASN.2011121170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LF, Xiao Y, Sun L. A glimpse of the mechanisms related to renal fibrosis in diabetic nephropathy. Adv Exp Med Biol. 2019;1165:49–79. doi: 10.1007/978-981-13-8871-2_4. [DOI] [PubMed] [Google Scholar]
- Zeravica R, Ilincic B, Cabarkapa V, Sakac V, Crnobrnja V, Stosic Z. PLASMA ENDOTHELIN-1 LEVELS AND ALBUMINURIA IN PATIENTS WITH TYPE 2 DIABETES MELLITUS. Med Pregl. 2016;69(5–6):140–145. doi: 10.2298/MPNS1606140Z. [DOI] [PubMed] [Google Scholar]
- Zgraggen S, Huggenberger R, Kerl K, Detmar M. An important role of the SDF-1/CXCR4 axis in chronic skin inflammation. PloS one. 2014;9(4):e936654. doi: 10.1371/journal.pone.0093665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Fang H, Chen J, He L, Chen Y. Role of VEGF-A and LRG1 in abnormal angiogenesis associated with diabetic nephropathy. Front Physiol. 2020;11:1064–1064. doi: 10.3389/fphys.2020.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wen Z, Han L, Zheng Y, Wei Y, Wang X, et al. Research progress on the pathological mechanisms of podocytes in diabetic nephropathy. J Diabetes Res. 2020;2020:7504798–7504798. doi: 10.1155/2020/7504798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG, Zhao B, Xu HM. CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World J Gastroenterol. 2011;17(19):2389–2396. doi: 10.3748/wjg.v17.i19.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F, Chen H, Wei C, Zhang W, Li Z, Jain MK, et al. Reduced Krüppel-like factor 2 expression may aggravate the endothelial injury of diabetic nephropathy. Kidney Int. 2015;87(2):382–395. doi: 10.1038/ki.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F, Lee K, He JC. Role of Krüppel-like factor-2 in kidney disease. Nephrology (carlton) 2018;23(Suppl 4):53–56. doi: 10.1111/nep.13456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.