Abstract
Tocopheryl succinate (Tsuc) is a succinic acid ester of the well-known antioxidant α-tocopherol (T). Tsuc exhibits various biological activities, including tumor growth suppression via activation of cell signaling and prevention of lipid accumulation in mouse adipocyte 3T3-L1 cells. The latter findings suggest that Tsuc may be a drug candidate for the treatment of obesity. However, Tsuc was found to induce apoptosis of normal cells (in addition to cancer cells), demonstrating the need to reduce the cytotoxicity of Tsuc without losing the suppression effect on lipid accumulation. Based on our previous findings, we focused on the ester structure of Tsuc for controlling cytotoxicity. Herein, we examined the cytotoxicity and lipid accumulation suppression effect of various T ester derivatives. We found that the terminal carboxylic group is necessary for suppression of lipid accumulation. We synthesized tocopheryl glutarate (Tglu) and tocopheryl adipate (Tadi) by elongation of carbon atoms 1 and 2 of the dicarboxylic moiety, respectively. Tglu and Tadi did not show any cytotoxicity, and both esters suppressed lipid accumulation, although their suppression activities were weaker than that of Tsuc. Tadi showed a more potent lipid accumulation inhibitory effect than Tglu. Although Tadi inhibited lipogenesis and promoted lipolysis, lipolysis was induced at lower concentrations than inhibition of lipogenesis, suggesting that Tadi mainly affects lipolysis. Taken together, we succeeded in the reduction of cytotoxicity, without loss of the suppression effect on lipid accumulation, by elongation of the dicarboxylic moiety of Tsuc. Tadi may be a promising candidate as an anti-obesity drug.
Keywords: Tocopheryl ester, Cytotoxicity, Lipid accumulation suppression, Anti-obesity
Highlights
-
•
Successful development of a novel tocopheryl ester as an anti-obesity drug candidate.
-
•
Reduction of cytotoxicity by elongation of dicarboxylic moiety of tocopheryl esters.
-
•
A novel tocopheryl ester mainly induces lipolysis of accumulated lipids.
1. Introduction
Tocopheryl succinate (Tsuc: Fig. 1) is a succinic acid ester of α-tocopherol (T: Fig. 1), which is a well-known natural antioxidant. Tsuc exhibits various biological activities, such as inhibition of nuclear factor-kappa B (NF-κB) activation, activation of cell signaling enzyme protein kinase C (PKC) and tumor growth suppression [1], although the antioxidative activity was lost due to esterification of the phenolic hydroxyl group of T. Various reports of the anti-cancer effect of Tsuc have been published [[2], [3], [4], [5]]. We previously reported the suppression of tumor growth by intravenous administration of liposomes encapsulating Tsuc [6]. The mechanism of Tsuc's anti-cancer effect is thought to occur by induction of apoptosis via mitochondrial dysfunction or production of reactive oxygen species [7,8]. As Tsuc can be hydrolyzed to T and succinic acid in the body, accumulation toxicity of Tsuc might be lower than other anti-cancer chemotherapeutic agents, suggesting that Tsuc may be an ideal drug candidate for cancer treatment.
Fig. 1.
Chemical structures of α-tocopherol (T) and various tocopheryl esters used in this study.
We recently discovered a novel function of Tsuc, namely its ability to suppress lipid accumulation in mouse adipocyte 3T3-L1 cells without cytotoxicity to adipocytes [9]. The mechanism of suppression of lipid accumulation was suggested to occur via inhibition of lipogenesis and promotion of lipolysis [9]. Thus, Tsuc may also offer promise as a new drug candidate for the treatment of obesity. However, Tsuc induces apoptosis of normal mouse Swiss 3T3 fibroblasts (in addition to cancer cells, as described above), while adipocytes are resistant to this T ester. The cytotoxicity to normal cells limits the application of Tsuc as an anti-obesity drug. We previously reported that the chemical structure of the ester moiety conjugated to the phenolic hydroxyl group of tocopherol determines the cytotoxicity of tocopherol esters [10,11]. Tocopheryl oxalate and tocopheryl malonate, which contain dicarboxylic ester moieties with carbon chain lengths of 2 and 3 carbons, respectively, were found to exhibit more potent cytotoxicity than Tsuc. On the other hand, tocopheryl pimelate, which contains a dicarboxylic ester moiety with a carbon chain length of 7, and tocopheryl succinate ethylester, in which a terminal carboxylic group is esterified with ethanol, did not show any cytotoxicity. Moreover, cytotoxicity was not induced by either tocopheryl acetate (Tace, Fig. 1) or tocopheryl nicotinate (Tnic, Fig. 1). These findings suggest that modification of the ester moiety offers the opportunity to control the cytotoxicity of Tsuc. However, it is also possible that modification of the ester moiety could attenuate not only the cytotoxicity, but also the suppression effect on lipid accumulation, so that there is a need to balance between reducing the cytotoxicity while maintaining the suppression activity of lipid accumulation.
In the present study, we synthesized tocopheryl esters containing different ester moieties from Tsuc. We examined the cytotoxicity of the newly synthesized tocopheryl esters, namely tocopheryl butyrate (Tbut), tocopheryl glutarate (Tglu) and tocopheryl adipate (Tadi), as well as commercially available Tace and Tnic (Fig. 1) against the normal fibroblast 3T3-Swiss albino cells. We also evaluated the suppression effect of the tocopheryl esters on lipid accumulation in adipocyte 3T3-L1 cells. Further, the mechanism of the suppression effect of lipid accumulation was investigated.
2. Materials and methods
2.1. Materials
Tsuc, Oil Red O and egg phosphatidylcholine (egg PC) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). d-α-tocopherol (T) was obtained from Nacalai tesque (Kyoto, Japan). Other reagents were of the highest grade commercially available. Mouse fibroblast Swiss 3T3 cells were obtained from American Type Culture Collection. Mouse pre-adipocyte 3T3-L1 cells were purchased from Japanese Collection of Research Bioresources (JCBR) cell bank (Tokyo, Japan).
2.2. Synthesis of tocopheryl esters
Tbut was synthesized by the condensation of T and n-butyric acid. 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (106 mg, 0.553 mmol), triethylamine (185 μL, 1.33 mmol), and 4-dimethylaminopyridine (29.7 mg, 0.243 mmol) were added to solutions of T (95.0 mg, 0.221 mmol) and n-butyric acid (48.6 μL, 0.530 mmol) in dichloromethane (2.0 mL). The reaction completion was confirmed by thin layer chromatography (TLC). The reaction was completed when we checked after stirring for 3 days at room temperature. The reaction mixture was washed sequentially with sat. NaHCO3, water, 5% (w/v) KHSO4, and sat. NaCl (brine). The resultant solution was then dried over MgSO4, filtered and concentrated in vacuo to yield Tbut (99.5 mg, 90%) as a white turbid oil.
Tglu was synthesized by the condensation of T and glutaric anhydride in the presence of a catalytic amount of 4-dimethylaminopyridine. Glutaric anhydride (160 mg, 1.40 mmol) and 4-dimethylaminopyridine (5.7 mg, 0.047 mmol) were added to a solution of T (201 mg, 0.467 mmol) in tetrahydrofuran (3 mL) at room temperature. The reaction completion was confirmed by TLC. The reaction was completed after stirring for 40 h. Then, sat. NH4Cl (10 mL) was added, and the mixture was then extracted twice with 20 mL of ethyl acetate. The organic layer was washed twice with brine (15 mL), dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography using an Isolara™ automated purification system (Biotage, Sweden). The first step employed a linear gradient of n-hexane:AcOEt from 95:5 to 70:30, and the second step employed a linear gradient of n-hexane:AcOEt from 10:1 to 70:30 to yield Tglu (66.4 mg, 26%) as a white solid.
Tadi was synthesized by the condensation of T and adipoyl chloride. Adipoyl chloride (1.83 mL, 12.6 mmol) was added to a solution of T (544 mg, 1.26 mmol) in tetrahydrofuran (20 mL) and pyridine (3 mL) at 0 °C. The reaction completion was confirmed by TLC. The reaction was completed after stirring for 4 days at room temperature. The reaction mixture was filtered and concentrated in vacuo. The residue was dissolved in 20 mL of CHCl3 and washed with 1 M HCl (5 mL). The organic layer was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography using an Isolara™ automated purification system (Biotage, Sweden) employing a linear gradient of n-hexane:AcOEt from 95:5 to 70:30 to yield Tadi (103 mg, 15%) as a white solid.
The difference in the reaction time is due to the different reactive species. The reactive species are acyl 4-dimethylaminopyridinium derived from carboxylic acid activated with a condensing reagent and 4-dimethylaminopyridine in the synthesis of Tbut, acyl 4-dimethylaminopyridnium derived from acid anhydride and 4-dimethylaminopyridine in the synthesis of Tglu, and acyl pyridinium derived from acid chloride and pyridine in the synthesis of Tadi.
2.3. Culture of adipocyte 3T3-L1 cells
3T3-L1 cells (2 × 105 cells) were seeded on a 35 mm dish and cultured at 37 °C for 96 h in a CO2 incubator to confluency. Then, the culture medium was changed to a medium containing 467.5 μM isobutyl methyl xanthine, 0.93 μM dexamethasone and 1.637 μM insulin for induction of differentiation (day 0). After 2 days of culturing to induce differentiation, the medium was changed to a medium containing 1.637 μM insulin and 3.72 mg/L D-biotin for maturation. The maturation medium was changed every 2 days until day 8. The liposome suspension was added at day 0, and the cells were treated with the liposomes until day 8.
2.4. Observation and quantification of lipid accumulation
Accumulated lipids in adipocytes were stained with Oil Red O [9]. 3T3-L1 cells were fixed with 10% neutral buffered formalin solution. The cells were then washed once with 60% isopropanol, and incubated with 60% Oil Red O solution for 20 min at room temperature for staining of the accumulated lipids. The cells stained with Oil Red O were washed once with 60% isopropanol, and twice with distilled water. After washing, distilled water was added to the dish for observation of the cells stained with Oil Red O by a BiorevoI microscope (Keyence Co., Osaka, Japan). For extraction of Oil Red O, the cells were dried, and 100% isopropanol was added. The absorbance of the extracted solution was measured at 540 nm.
2.5. Preparation of liposomes containing tocopheryl esters
A chloroform solution containing 0.1 mL of 0.1 M tocopheryl ester ethanol solution and 49 μL 0.1 g/mL EPC ethanol solution in a glass test tube was dried under nitrogen gas. The 0.2 mL phosphate buffered saline (PBS) solution containing 8 μL 1 M NaOH was added to the resultant lipid film and incubated for 10 min at room temperature to hydrate the lipid membrane. To make the pH of PBS alkaline, NaOH was added according to the previous protocol for liposomes containing Tsuc [9]. The weak electrolyte carboxylic group of tocopheryl esters does not dissociate completely at neutral pH. However, in order to reproducibly prepare liposomes containing tocopheryl esters, it is necessary to completely dissociate the carboxyl groups of the tocopheryl esters in the liposome preparation process. Therefore, NaOH was added to PBS to completely dissociate the carboxylic group of tocopheryl esters. Then, the glass test tube was sonicated with an ultrasonic bath for 30 min to form liposomes. The diameters and zeta-potentials of the liposomes were measured using a Zetasizer Nano (Malvern Panalytical Ltd., U.K.); the data are summarized in Table 1.
Table 1.
Physicochemical properties of the liposomes containing tocopheryl esters.
| Liposomes | Diameter (nm) | Polydispersity index | Zeta-potential (mV) |
|---|---|---|---|
| egg PC-lipo | 140 ± 20 | 0.36 ± 0.04 | −4 ± 1 |
| T-lipo | 150 ± 40 | 0.43 ± 0.1 | −3 ± 2 |
| Tsuc-lipo | 100 ± 20 | 0.25 ± 0.02 | −38 ± 3 |
| Tglu-lipo | 110 ± 20 | 0.25 ± 0.02 | −40 ± 2 |
| Tadi-lipo | 100 ± 25 | 0.28 ± 0.1 | −36 ± 8 |
| Tace-lipo | 270 ± 230 | 0.43 ± 0.1 | −6 ± 4 |
| Tnic-lipo | 190 ± 60 | 0.40 ± 0.03 | −4 ± 1 |
Values are the average ± S.D. of the measurement of at least three preparations.
Liposomes containing Tbut could not prepared due to its structure. Ethanol solution of Tbut was used in this study.
2.6. Measurement of cell viability
Swiss 3T3 fibroblasts (1.5 × 105 cells/well) were seeded in 6-well plates and cultured at 37 °C in a CO2 incubator for 24 h. The liposome formulation was added to each well, and the plates were incubated at 37 °C for 24 h. The cells were then collected by treatment with trypsin for measurement of cell viability by the trypan blue staining method. Percent cell viability was obtained by dividing the number of cells stained with trypan blue by the total number of cells.
2.7. Evaluation of the effect of tocopheryl adipate on lipogenesis and lipolysis
To evaluate lipogenesis in 3T3-L1 cells, glycerol 3-phosphate dehydrogenase (GPDH) activity (Supplementary schema) was measured using the GPDH Assay Kit (TaKaRa Bio Inc., Shiga, Japan). Following treatment with liposomes from day 0 to day 8, the cells were then washed with PBS, and the cytoplasmic fraction was extracted using the GPDH Assay Kit, according to the manufacturer's protocol. To evaluate lipolysis activity, 3T3-L1 cells were cultured until day 4 for lipid accumulation, before the addition of liposomes. After 48 h, the glycerol amount in the culture medium (Supplementary schema) was measured using a Glycerol Colorimetric Assay Kit (Cayman Chemical, MI, U.S.A.).
2.8. Evaluation of the effect of tocopheryl adipate on PKC phosphorylation and the amount of peroxisome proliferator-activated receptor γ (PPARɤ)
Phosphorylation of PKC and amount of PPARɤ were evaluated by Western blotting. Cells were collected at day 8 using a cell scraper and solubilized with a lysis buffer. The protein concentration of the cell lysate was measured, and equal amounts of cellular proteins were subjected to Western blotting with anti-mouse phospho-PKC (rabbit anti-PKC alpha (phospho T497) ab278608 (abcam, Cambridge, UK)), or anti-mouse PPARɤ(69) antibody(Cell Signaling Technology, MA) used as the primary antibody. Goat anti-rabbit IgG H&L(HRP)ab6721 (abcam, Cambridge, UK) was used as the secondary antibody. Band intensity was quantified using the ImageJ software.
2.9. Statistical analysis
Statistical significance was determined by one-way ANOVA, followed by Tukey's honest significant difference test. p Values < 0.05 were considered significant.
3. Results and discussion
3.1. Effect of Tsuc on fibroblast viability and lipid accumulation in adipocytes
As Tsuc is a hydrophobic compound, a liposomal formulation of Tsuc was used in this study. The addition of Tsuc-lipo resulted in a dose-dependent decrease in viability of mouse fibroblast Swiss 3T3 cells (Fig. 2a), indicating that Tsuc results in substantial cytotoxicity to normal cells. On the other hand, lipid accumulation in mouse adipocyte 3T3-L1 cells was significantly reduced by addition of Tsuc-lipo (Fig. 2b). Treatment with Tsuc-lipo did not show substantial damage to adipocytes, although intracellular lipid accumulation was diminished, as shown by a decrease in the red color associated with Oil Red O-staining (Fig. 2c). These results are consistent with the results of our previous report [9], and suggest that Tsuc inhibits lipid accumulation in adipocytes, but has severe cytotoxicity against normal cells.
Fig. 2.
Effect of Tsuc-lipo, Tadi-lipo and Tglu-lipo on viability of mouse fibroblast Swiss 3T3 cells and lipid accumulation in mouse adipocyte 3T3-L1 cells
(a) Liposomes containing toropheryl esters were added to Swiss 3T3 cells, and cell viability was measured after 24 h. Values are the average of the results of at least three experiments. egg PC-lipo: egg PC liposomes. **vs control (non-treated cells) p < 0.01. (b) 3T3-L1 cells were treated with Tsuc-lipo from day 0 to day 8. The cells were stained with Oil Red O at day 8. The intracellular Oil Red O was extracted, and absorbance of the extracted solution was measured at 540 nm. Values are the average of the results of at least three experiments. *vs control p < 0.05, **vs control p < 0.01. (c) 3T3-L1 cells stained with Oil Red O were observed with a microscope. The black bar indicates 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Effects of various tocopheryl esters on fibroblast viability and adipocyte lipid accumulation
To identify other tocopheryl esters that inhibit lipid accumulation without cytotoxicity, we examined the effect of the commercially available tocopheryl esters Tace and Tnic (Fig. 1) on fibroblast viability and adipocyte lipid accumulation. The addition of Tace-lipo or Tnic-lipo to fibroblasts had no affect on cell viability, consistent with a previous report [10]. However, lipid accumulation in adipocytes remained unchanged by Tace-lipo and Tnic-lipo in this study.
To confirm the role of the carboxyl group on the lipid accumulation suppression effect, we synthesized a novel tocopheryl ester, Tbut, by deleting the terminal carboxyl group of Tsuc (Fig. 1). Liposomes containing Tbut were difficult to prepare, so that an ethanol solution of Tbut was added to the fibroblasts. Tbut did not exhibit any cytotoxicity against fibroblasts, similar to Tace and Tnic. However, Tbut did not suppress lipid accumulation in adipocytes in this study. These results suggest that the terminal carboxyl group plays an important role in the suppression effect of lipid accumulation and that a dicarboxylic moiety is necessary to suppress lipid accumulation. Although we don't have the evidence, a dicarboxylic moiety of tocopheryl ester might interact with target protein, such as PKC reported previously [12], by electrostatic interaction or hydrogen bonding.
In our previous study, tocopheryl pimelate, which contains a dicarboxylic moiety with a carbon chain length of 7 (which is longer than that of succinate), did not exhibit any cytotoxicity [10]. Therefore, elongation of the carbon chain in the dicarboxylic moiety was suggested as a means of attenuating the cytotoxicity. However, it's also possible that elongation of the carbon chain could also decrease the suppression effect on lipid accumulation. We therefore expected that a tocopheryl ester containing a dicarboxylic moiety with a carbon chain length between 4 (succinic acid) and 7 (pimelic acid) would show lipid accumulation suppression activity without cytotoxicity.
We synthesized tocopheryl adipate (Tadi), which contains a dicarboxylic moiety with a carbon chain length of 6, and subsequently prepared liposomes containing Tadi (Tadi-lipo). We measured the physicochemical properties of Tadi-lipo, and found the diameter and zeta-potential to be approximately 100 nm and −36 mV, respectively (Table 1). A negative charge indicates the presentation of the carboxylic acid terminal of Tadi on the surface of liposomes, similar to Tsuc-lipo. Even at incomplete dissociation of the carboxylic moiety of Tadi, the zeta-potential of Tadi-lipo was −36 mV. Therefore, if the carboxylic moiety of the tocopheryl ester dissociate completely, we expect that the zeta-potential might be over −50 mV, although we don't have evidence. We examined the effect of Tadi-lipo on the viability of fibroblasts. Cell viability did not change, even at a Tadi-lipo concentration of 100 μM (Fig. 2a). Thus, the cytotoxicity of Tscu-lipo was reduced by elongation of the dicarboxylic moiety. Next, we examined the effect of Tadi-lipo on lipid accumulation in adipocytes. Addition of 50 μM Tadi-lipo suppressed 40% of lipid accumulation, although the effect was not statistically significant (Fig. 2b). Furthermore, 100 μM Tadi-lipo significantly reduced >70% of the amount of accumulated lipid in adipocytes. Thus, Tadi-lipo suppressed lipid accumulation in a dose-dependent manner. In addition, the sizes of the lipid droplets in adipocytes treated with 100 μM Tadi-lipo (Fig. 2c) were smaller than those in non-treated adipocytes (Fig. 2c). Taken together, these results confirm that elongation of the dicarboxylic moiety by 2 carbons eliminates the cytotoxicity. Contrary to our initial concerns, lipid accumulation was significantly suppressed by Tadi-lipo, although the suppression activity of Tadi-lipo was lower than that of Tsuc.
Based on these results, we expected that tocopheryl glutarate (Tglu), containing a dicarboxylic moiety with a carbon chain length of 5, which is between that of succinic acid (4) and adipic acid (6), would show more potent suppression effects on lipid accumulation without cytotoxicity. The size and surface charge of Tglu-lipo were approximately 106 nm and −40 mV, respectively (Table 1). The physicochemical properties of Tglu-lipo were nearly the same as those of Tsuc-lipo and Tadi-lipo.
We examined the effect of Tglu-lipo on fibroblast viability. As shown in Fig. 2a, cell viability was approximately 90% at 100 μM Tglu. The cytotoxic effects of Tglu-lipo were more potent than those of Tadi-lipo. This result is not unexpected, because the length of the dicarboxylic moiety of Tglu is shorter than that of Tadi, as mentioned above. Next, we examined the effect of Tglu-lipo on lipid accumulation in adipocytes. Contrary to expectation, 50 μM Tglu-lipo did not suppress lipid accumulation, while 100 μM Tglu-lipo significantly reduced approximately 50% of lipid accumulation (Fig. 2b). The sizes of the lipid droplets accumulated in adipocytes treated with 100 μM Tglu-lipo (Fig. 2c) were smaller than those in non-treated adipocytes (Fig. 2c), e.g., Tadi-lipo-treated adipocytes (Fig. 2c). However, the lipid accumulation suppression effect of Tglu-lipo was weaker than that of Tadi-lipo, although the carbon chain length of the dicarboxylic moiety of Tglu is shorter than that of Tadi. A possible explanation for these findings could be due to the even number of carbon atoms in dicarboxylic moieties of Tsuc and Tadi, compared with the odd number of carbon atoms in Tglu. The orientation of the carboxylic group may affect the interaction between a dicarboxylic moiety of tocopheryl esters and target proteins such as PKCα reported previously [12], so that an even number of carbon atoms might be more suitable than an odd number for association with a trigger protein for suppression of lipid accumulation. Therefore, we focused on Tadi as a novel tocopheryl ester, which can suppress lipid accumulation without cytotoxicity.
3.3. Effects of Tadi on lipogenesis and lipolysis
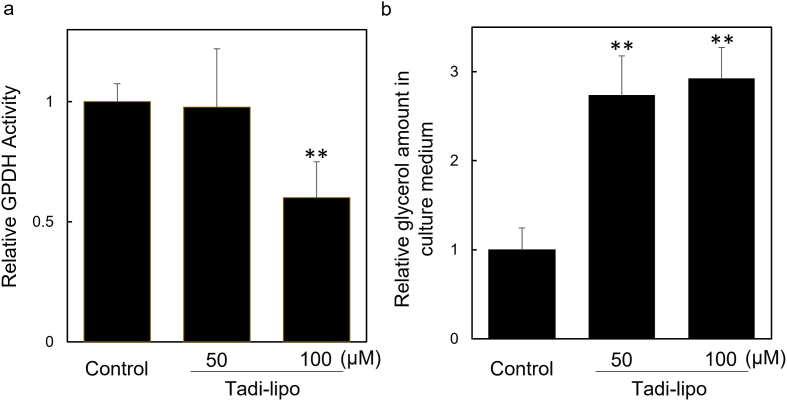
To better elucidate the mechanism of Tadi's suppression effect of lipid accumulation, we examined the effect of Tadi-lipo on GPDH activity, which indicates lipogenesis, and the amount of glycerol produced by lipolysis (Supplementary schema). In our previous report, 50 μM Tsuc-lipo significantly inhibited GPDH activity and increased the amount of glycerol in the culture medium. Moreover, 100 μM Tsuc-lipo completely prevented GPDH activity and further enhanced glycerol production [9]. These results suggest that Tsuc has a similar effect on both phenomena (lipogenesis and lipolysis). On the other hand, GPDH activity was not affected by 50 μM Tadi-lipo. GPDH activity was inhibited by only 50%, even at a concentration of 100 μM Tadi-lipo. However, the glycerol amount increased by 2.5 times at 50 μM Tadi-lipo, and was nearly the same at 100 μM Tadi-lipo (Fig. 3). These findings suggest that Tadi mainly affects lipolysis rather than lipogenesis. Since triglycerides in adipocytes would be hydrolyzed by lipolytic enzymes, such as hormone-sensitive lipase and adipose triglyceride lipase, it is possible that those enzymes are activated by Tadi. The mechanism by which Tadi activates lipolysis requires additional investigation in the future. It's likely that the inhibitory effect on lipogenesis was attenuated by elongation of the carbon chain of the dicarboxylic moiety of Tsuc, although both Tadi and Tsuc were found to enhance lipolysis. Taken together, these results suggest that structural changes of the ester moiety only affected lipogenesis, while the structural characteristics, which may be the terminal carboxylic moiety, responsible for lipolysis are different from those responsible for lipogenesis.
Fig. 3.
Effect of Tadi on GPDH activity and amount of glycerol in culture medium
(a) 3T3-L1 cells were treated with Tadi-lipo from day 0 to day 8, and then GPDH activity was measured. Values are the average of the results of at least three experiments. **vs control p < 0.01. (b) Tadi-lipo were added to 3T3-L1 cells at day 4. After 48 h, the amount of glycerol in the culture medium was measured. Values are the average of the results of at least three experiments. **vs control p < 0.01.
As mentioned in the introduction, tocopherol esters are not able to react with reactive oxygen species (ROS) because the tocopherol hydroxyl group, which is known as the active site with ROS, is esterified. Therefore, the tocopheryl esters’ effect on lipid metabolism does not appear to be due to reactions with ROS in this study.
3.4. Effects of Tsuc and Tadi on PKCα and PPARγ
We previously reported that Tsuc activates cell signaling related to protein PKC in vascular smooth muscle cells (VSMC) via direct interaction of the ester moiety of Tsuc with the kinase [12]. It was also reported that tocotrienol, which is one type of vitamin E, prevents lipid accumulation via regulation of lipid metabolism-related genes, including PPARγ [13]. PPARγ plays an important role in adipocyte differentiation and maintenance, and regulates various genes in lipogenesis (Supplementary schema) [14]. We therefore evaluated the effect of Tadi-lipo on PKCα and PPARγ by Western blotting, and compared the results with Tsuc-lipo (Fig. 4). Although the difference was not statistically significant due to large variability, we observed a trend towards a dose-dependent increase in phosphorylated PKCα and decrease in the amount of PPARγ by Tsuc-lipo. Tadi-lipo also showed a similar tendency in phosphorylation of PKCα, but was less effective than Tsuc-lipo. These results suggest that Tsuc also activates PKC in both VSMC and adipocytes. Furthermore, Tadi-lipo exhibits a slight effect on PKC, similar to Tsuc-lipo. Therefore, the effects of Tsuc-lipo and Tadi-lipo on PKC may partially contribute to the suppression of lipid accumulation found for these tocopheryl esters. Further investigation is needed to better elucidate the relationship between lipid accumulation suppression and the effect on PKC.
Fig. 4.
Effects of Tsuc and Tadi on PKCα and PPARγ
(a) Physphorylation of PKCα (p-PKCα) and the amount of PPARγ were evaluated by Western blotting of lysates from 3T3-L1 cells treated with Tsuc-lipo or Tadi-lipo from day 0 to day 8. Band intensity of (b) p-PKCα and (c) PPARγ were quantified using Image J. Values are the average of the results of five experiments.
4. Conclusion
In the present study, we developed novel tocopheryl esters, tocopheryl glutarate (Tglu) and tocopheryl adipate (Tadi), to eliminate the cytotoxicity of Tsuc without losing its ability to suppress lipid accumulation. Neither Tglu-lipo nor Tadi-lipo showed any cytotoxicity, and both were found to suppress lipid accumulation. Since the inhibitory effect of Tadi-lipo on lipid accumulation was higher than that of Tglu-lipo, we focused on Tadi-lipo as a potential new drug candidate for the treatment of obesity. Tadi-lipo inhibited both lipogenesis and stimulated lipolysis, although lipolysis was induced at a lower concentration of Tadi-lipo than inhibition of lipogenesis. Taken together, these results suggest that the suppression effect of Tadi-lipo on lipid accumulation is mainly due to the induction of lipolysis. Further, we succeeded in eliminating the cytotoxicity effects, without losing the suppression effect on lipid accumulation, via elongation of the dicarboxylic moiety of Tsuc. The newly developed Tadi is a likely candidate for an anti-obesity drug.
Author contributions
MY: Investigation, Formal analysis, Data curation; YS: Investigation, Data curation; MO: Writing - review & editing; MN: Investigation, Writing - review & editing; AS: Investigation, Writing - review & editing; AO: Supervision; SS: Supervision; KK: Conceptualization, Writing - original draft.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by the Research Program for the Development of Intelligent Tokushima Artificial Exosome (iTEX) from Tokushima University. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2022.101329.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
Data availability
No data was used for the research described in the article.
References
- 1.Majima D., Mitsuhashi R., Fukuta T., Tanaka T., Kogure K. Biological functions of alpha-tocopheryl succinate. J. Nutr. Sci. Vitaminol. 2019;65:S104–S108. doi: 10.3177/jnsv.65.S104. [DOI] [PubMed] [Google Scholar]
- 2.Fukuzawa K., Kogure K., Morita M., Hama S., Manabe S., Tokumura A. Enhancement of nitric oxide and superoxide generations by alpha-tocopheryl succinate and its apoptotic and anticancer effects. Biochemistry (Mosc.) 2004;69:50–57. doi: 10.1023/b:biry.0000016351.77553.74. [DOI] [PubMed] [Google Scholar]
- 3.Wang X.F., Dong L., Zhao Y., Tomasetti M., Wu K., Neuzil J. Vitamin E analogues as anticancer agents: lessons from studies with alpha-tocopheryl succinate. Mol. Nutr. Food Res. 2006;50:675–685. doi: 10.1002/mnfr.200500267. [DOI] [PubMed] [Google Scholar]
- 4.Koudelka S., Turanek Knotigova P., Masek J., Prochazka L., Lukac R., Miller A.D., Neuzil J., Turanek J. Liposomal delivery systems for anti-cancer analogues of vitamin E. J. Contr. Release. 2015;207:59–69. doi: 10.1016/j.jconrel.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Liang L., Qiu L. Vitamin E succinate with multiple functions: a versatile agent in nanomedicine-based cancer therapy and its delivery strategies. Int. J. Pharm. 2021;600 doi: 10.1016/j.ijpharm.2021.120457. [DOI] [PubMed] [Google Scholar]
- 6.Hama S., Utsumi S., Fukuda Y., Nakayama K., Okamura Y., Tsuchiya H., Fukuzawa K., Harashima H., Kogure K. Development of a novel drug delivery system consisting of an antitumor agent tocopheryl succinate. J. Contr. Release. 2012;161:843–851. doi: 10.1016/j.jconrel.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Sobotka O., Drahota Z., Kučera O., Endlicher R., Rauchová H., Červinková Z. The effect of alpha-tocopheryl succinate on succinate respiration in rat liver mitochondria. Physiol. Res. 2015;64:S609–S615. doi: 10.33549/physiolres.933219. [DOI] [PubMed] [Google Scholar]
- 8.Kogure K., Morita M., Nakashima S., Hama S., Tokumura A., Fukuzawa K. Superoxide is responsible for apoptosis in rat vascular smooth muscle cells induced by alpha-tocopheryl hemisuccinate. Biochim. Biophys. Acta. 2001;1528:25–30. doi: 10.1016/s0304-4165(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 9.Majima D., Mitsuhashi R., Yamasaki M., Kajimoto K., Fukuta T., Kogure K. Suppression of lipid accumulation in 3T3-L1 adipocytes by alpha-tocopheryl succinate. Biol. Pharm. Bull. 2021;44:46–50. doi: 10.1248/bpb.b20-00573. [DOI] [PubMed] [Google Scholar]
- 10.Kogure K., Hama S., Kisaki M., Takemasa H., Tokumura A., Suzuki I., Fukuzawa K. Structural characteristic of terminal dicarboxylic moiety required for apoptogenic activity of alpha-tocopheryl esters. Biochim. Biophys. Acta. 2004;1672:93–99. doi: 10.1016/j.bbagen.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Kogure K., Manabe S., Suzuki I., Tokumura A., Fukuzawa K. Cytotoxicity of alpha-tocopheryl succinate, malonate and oxalate in normal and cancer cells in vitro and their anti-cancer effects on mouse melanoma in vivo. J. Nutr. Sci. Vitaminol. 2005;51:392–397. doi: 10.3177/jnsv.51.392. [DOI] [PubMed] [Google Scholar]
- 12.Kogure K., Hama S., Goto S., Munakata T., Tokumura A., Fukuzawa K. alpha-Tocopheryl succinate activates protein kinase C in cellular and cell-free systems. J. Nutr. Sci. Vitaminol. 2003;49:310–314. doi: 10.3177/jnsv.49.310. [DOI] [PubMed] [Google Scholar]
- 13.Burdeos G.C., Nakagawa K., Abe T., Kimura F., Miyazawa T. Tocotrienol modulates crucial lipid metabolism-related genes in differentiated 3T3-L1 preadipocytes. Food Funct. 2014;5:2221–2227. doi: 10.1039/c4fo00463a. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Quiles M., Broekema M.F., Kalkhoven E. PPARgamma in metabolism, immunity, and cancer: unified and diverse mechanisms of action. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.