Abstract
Bladder cancer is any tumor that originates in the urinary bladder. It is the most prevalent tumor of the urinary system, with urothelial carcinoma being the most prevalent histologic subtype. It impacts both men and women. The development of bladder cancer was influenced by several risk factors, including advanced age, male sex, cigarette smoking, and occupational and environmental toxin exposure. Bladder tumors may manifest as gross or microscopic hematuria, which is assessed using cystoscopy, urine analysis, and other specialized tests. Due to the large number of cases related to environmental causes, bladder cancer is an appropriate target for public health preventative interventions. Cessation of smoking, adequate occupational safety procedures, diet, weight loss, and schistosomiasis prevention may mitigate the rising global incidence.
Keywords: etiology, smoking tobacco, urothelial cell carcinoma, bladder ca, urinary bladder ca
Introduction and background
Bladder cancer, commonly referred to as urinary bladder cancer, is the tenth most prevalent cancer worldwide, and its prevalence is gradually increasing globally, particularly in industrialized nations. It continues to be the most prevalent malignancy of the urinary system [1]. The primary function of the bladder, a cavernous organ found in the lower abdomen, is to hold urine until micturition. Urothelial cells, which are specialized transitional epithelial cells that line the urinary bladder and urinary tract, accommodate the volume of urine generated by flattening under pressure. Additionally, the bladder is lined with smooth muscle that may relax to accept larger amounts and tighten to evacuate urine through the urethra [2]. The urothelial cells that line the bladder and urinary tract are always exposed to environmental chemicals that could cause mutations. The kidneys filter these chemicals out of the urine. It is not surprising that these urothelial cells, mostly found in the bladder, cause the majority of cancer cases, particularly in the developed world. Most bladder cancers may be dated directly to exposure to environmental and occupational toxins, with tobacco smoke being the most prevalent. Men's higher exposure to cigarette smoke and occupational hazards may help explain the fourfold gender disparity in bladder cancer incidence. Following tobacco use, the likelihood of bladder cancer is next only to the risk of lung cancer [1,3]. Unlike other malignancies, bladder cancer is seldom identified unintentionally during an autopsy. Eighty-five percent of patients with newly diagnosed bladder cancer have painless gross hematuria, and microscopic hematuria is present in virtually all patients [4]. Typically, hematuria is intermittent and may be associated with Valsalva movements. Therefore, a comprehensive evaluation of hematuria for bladder cancer consists of a focused history and physical examination in addition to diagnostic modalities such as bladder endoscopies, upper tract imaging, and urine culture. Although survival rates have improved as a result of earlier detection, robotic surgical methods, and the advent of immunotherapy, bladder cancer continues to be a major and growing contributor to the global cancer burden, particularly in wealthy nations [1]. On average, a patient's lifetime treatment costs for bladder cancer are higher than for any other malignancy. The overall treatment cost ranges from $129,000 to $251,000 per patient. It is anticipated that annual directed medical expenses will surpass $4 billion in the United States and €4.9 billion in the European Union (EU) [5]. Therefore, an improved understanding of the epidemiology and risk factors behind bladder cancer is essential for its prevention and reduction of its burden. This non-systematic literature review focuses on identifying the primary causes and risk factors of bladder cancer.
Review
Epidemiology
Incidence
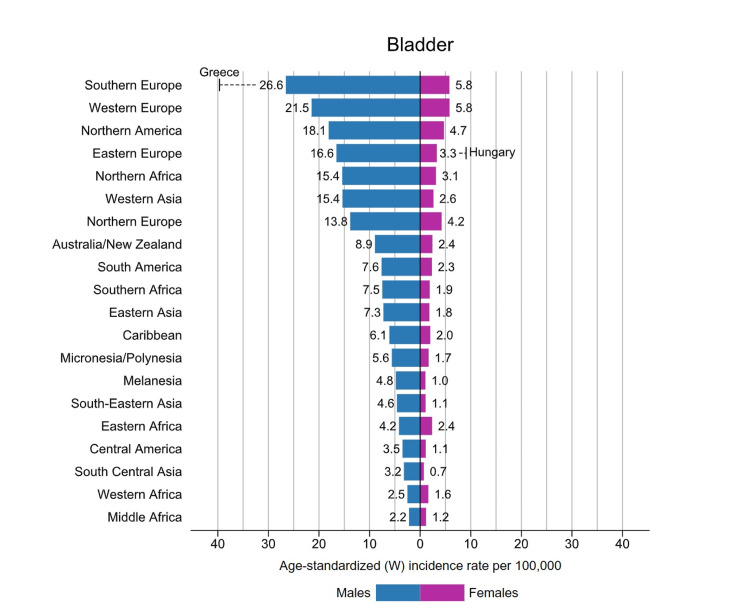
As per Global Cancer Incidence, Mortality, and Prevalence (GLOBOCAN) data, an additional 573,000 cases of bladder cancer were identified in 2020. This accounts for around 3% of new cancer diagnoses. Most countries with a high incidence of bladder cancer are located in Southern and Western Europe and North America. Worldwide, Greece has the most significant incidence of bladder cancer among males, whereas Hungary has the most significant number of females. Southern Europe, where approximately 26.6 per 100,000 males and 5.8 per 100,000 females are diagnosed with bladder cancer each year, has the most significant incidence of bladder cancer among the global population [1], as shown in Figure 1. The territories with the lowest prevalence rate of bladder cancer are Middle Africa, South Central Asia, and Western Africa, which are largely comprised of countries with a human development index below the average, a consequence of less manufacturing exposure to chemicals and restricted access to tobacco [1].
Figure 1. Bladder cancer incidence rates by region and gender in 2020.
Source: reference [1].
In the United Kingdom, an estimated 12,400 instances of bladder cancer were diagnosed in 2020, constituting 2.7% of all cancer diagnoses. Thus, bladder cancer is the ninth most prevalent cancer in the United Kingdom [1]. Due to a more significant frequency of smoking and an older population, the prevalence rate of bladder cancer has continued to climb in several European countries, including Germany, and is projected to rise even more. Nevertheless, several nations have achieved substantial success in prevention, with New Zealand's incidence decreasing by roughly 10% over the previous decade [6]. Bladder cancer is nearly four times more prevalent in males than females, with incidence rates of 9.5 per 100,000 males and 2.4 per 100,000 females globally. Urinary bladder cancer is the sixth most prevalent and ninth most lethal tumor among males [1]. This disparity is most likely linked to gender disparities in cigarette use, which may also possibly clarify why cancer rates are growing among women in industrialized nations [1]. While bladder cancer is the tenth most prevalent malignancy in the world, it is the thirteenth most lethal, claiming an estimated 212,536 lives in 2020. This accounts for 2.1% of all cancer fatalities. Mortality rates follow incidence rates regarding the gender imbalance, with a mortality rate of 3.3 per 100,000 males, which is nearly four times higher than the mortality rate of 90 per 100,000 females globally (Table 1). Between birth and age 74, the cumulative probability of dying from bladder cancer is 0.3% for males and 0.08% for females. Geographical and temporal trends of bladder cancer incidence globally tend to mirror the prevalence of tobacco use. However, infection with Schistosoma haematobium and other chemicals exposure may be substantial contributors in some populations such as Egypt [1].
Table 1. Total global bladder cancer mortality in 2020.
Source: reference [1].
| Rank | Country | Number |
| World | 212,536 | |
| 1 | Egypt | 6,170 |
| 2 | Tunisia | 822 |
| 3 | Libya | 242 |
| 4 | Poland | 5,026 |
| 5 | Mali | 426 |
| 6 | Slovakia | 629 |
| 7 | Latvia | 271 |
| 8 | São Tomé and Príncipe | 5 |
| 9 | Algeria | 1,861 |
| 10 | Serbia | 931 |
Due in part to breakthroughs in treatment (e.g., endoscopic resection, adjuvant instillation of chemotherapy, and IV immunotherapy), mortality rates have decreased primarily in the most developed settings, except for countries experiencing a rapid economic shift, such as those in Central and South America, some Central, Southern, and Eastern European countries, and the Baltic states [7,8]. According to data from the United Kingdom, 77.8% of males survived bladder cancer in their first year of diagnosis [9]. According to other statistics collected from the United States, the five-year survival rate was 77.1%. However, these percentages increase to nearly 96% for patients with an in-situ illness, reduce to nearly 69% for patients with local disease, and decrease even further to 36% and 4% for regional and metastatic diseases, respectively with a five-year survival [10].
Etiology
The wall of the urinary bladder consists of four components: mucosa, submucosa, muscularis, and serosa. The typical urothelium of the mucosa is composed of a seven-cell thick layer of stratified, non-squamous, homogeneous cells with big umbrella cells on top. Tumors arising from the urothelial cells compromise most bladder cancer, with a rough estimation of 90%. They are formally known as transitional cell carcinoma. Other non-urothelial bladder malignancies that can arise include squamous cell carcinoma (SCC), small cell carcinoma, adenocarcinoma, and other tumors with mixed histology [11,12]. Due to urothelial direct exposure, the urothelial subtype is strongly related to exposure to chemicals, such as workers exposed or tobacco smoking. On the other hand, squamous cell subclass instances are more prevalent in Africa, perhaps due to schistosomiasis, a protozoal illness that causes urinary bladder irritation and inflammation [13].
Risk factors
Tobacco
Tobacco is the primary recognized cause of bladder cancer, accounting for 30-40% of all cases of urothelial carcinoma and up to two-thirds of all bladder cancer. There are more than 1 billion active smokers worldwide, and smokers have two-to-three greater chances of developing bladder cancer [14]. Tobacco smoke has recognized carcinogens, including beta-naphthylamine and polycyclic aromatic hydrocarbons. The metabolism of these particles in the bladder and throughout the system results in the creation of DNA adducts and persistent genetic mutation. These mutations may activate oncogenes or inhibit tumor suppressor genes, encouraging carcinogenesis. It has been demonstrated that some hereditary genotypes associated with defective detoxification enzymes enhance the cancer predisposition in smokers [15]. Although cigarettes are the most prevalent tobacco product connected with bladder cancer incidence, pipe and cigar consumption have also been linked to the development of urothelial carcinoma [16]. One study demonstrated that quitting smoking reduces the risk of urothelial carcinoma. Those who have quit smoking for 1-3 years had a 2.6 relative risk compared to a 1.1 relative risk for those who have quit smoking for more than 15 years [17].
Gender
As noted previously, about three-quarters of bladder cancer cases occur in men with a greater incidence rate than in women [1]. Several theories have been suggested to explain the increase in male bladder cancer incidence. First, smoking is far more prevalent among males than females worldwide [18]. Although exposure to carcinogens may not account for variations between genders, the physiological breakdown of carcinogens could be distinct [19]. Enzymes involved in aromatic amine degradation and foreign material detoxification have been linked to carcinogen metabolism related to bladder cancer. It has been demonstrated that these enzymes are expressed differently in men and women [20]. In addition, variations in sex steroid synthesis and receptor expression underlie gender disparities. Age at menarche greater than 15 years, parity compared to nulliparous women, and use of estrogen or progestin medication have been linked to lower bladder cancer risk in women, suggesting that sex steroid exposure reduces bladder cancer risk [21]. From a tumor biology perspective, the androgen receptor (AR) has been linked to the genesis and progression of bladder cancer. AR expression appears to be downregulated in bladder cancer immunohistochemistry investigations, and this downregulation tends to increase with increasing tumor stage and grade [22]. In both males and females, current smokers develop bladder cancer six years earlier than current non-smokers.
Genetics and Hereditary
Although studies have failed to recognize important germline genetic factors underpinning sporadic bladder cancer, genome-wide correlation studies have identified several genetic loci having a small correlation with a genetic predisposition to bladder cancer [23]. Among these, N-acetyltransferase 2 (NAT2) and deletion of glutathione S-transferase (GSTM1) are both connected with the ability to metabolize aromatic amines and so play a significant role in the subgroup of individuals with environmental carcinogen exposure. Also, both appear to have a cancer-causing relationship with cigarette smoking [24]. Although first-degree relatives of bladder cancer patients have a twofold greater chance of getting urothelial bladder cancer, families at high risk for bladder cancer are extremely rare. The absence of a Mendelian inheritance pattern in hereditary bladder cancer renders traditional family-tree linkage analyses ineffective. Probability favors a complex explanation, with particular genes amplifying environmental stressors [25]. An increase in the incidence of urothelial and squamous bladder cancer has been related to mutations in the tumor-suppressor gene phosphatase and tensin homolog (PTEN) and the DNA mismatch repair gene MutS homolog 2 (MSH2), which are seen in Cowden and Lynch syndromes, respectively [26,27].
Age
One of the hallmarks of bladder cancer is its tendency to affect the elderly population. In the United States, more than 90% of people diagnosed with bladder cancer are over 55 years old, with a median age of diagnosis of 73 [28]. This suggests a disease progression that takes decades after contact with toxins to override cellular tumor-suppressor systems and results in carcinogenesis.
Occupational and Environmental Exposure
As with the skin and lungs, the bladder is a constantly exposed organ that is consequently susceptible to environmental toxins and inflammation. The second major avoidable risk factor for bladder cancer is occupational exposure to carcinogens such as aromatic amines (2-naphthylamine, 4-aminobiphenyl, and benzidine), which are responsible for 5-10% of all cases of bladder cancer [29]. These compounds are frequently used in manufacturing dyes, paints, metals, rubber, and petroleum goods. Working very closely with chemicals and dyes carries the highest lifetime risk. The vocations most at risk for exposure to aromatic amines include tobacco, dye, rubber professionals, hairdressers, painters, and leather workers. Those who deal with polycyclic aromatic hydrocarbons, such as chimney sweeps, nurses, alumni workers, petroleum workers, and sailors, are also in danger [29,30].
Infections and Pathogens
Multiple researchers have hypothesized that persistent or recurring bacterial UTIs may raise the chance of developing bladder cancer. Numerous epidemiologic studies suggest that chronic UTIs may be associated with a modestly increased risk of bladder cancer. However, these findings are flummoxed by chronic intermittent catheterization, chronic inflammatory bladder stone formation, and many other risk factors, such as smoking status and occupation [31]. Additionally, recurrent gonorrhea infections have been linked to the development of bladder cancer. One prospective research indicated that males with a history of gonorrhea have a roughly twofold increased chance of developing bladder cancer, and these tumors are more prone to being aggressive [32]. This is linked to the generation of carcinogens such as nitrosamines. Another important pathogen is schistosomiasis, a protozoan illness prevalent in as many as 76 poor nations, affecting as many as 236 million people. Schistosomes are blood flukes that parasitize both mammals and intermediate hosts (e.g., freshwater snails). However, Schistosoma haematobium (S. haematobium) is the only human schistosome associated with squamous cell bladder cancer. S. haematobium resides in the venules of the mammalian urinary bladder, where they produce inflammation and tissue fibrosis by laying their eggs. The process of SCC formation presumably includes a proinflammatory immune response of the T-helper cell-2 type [33]. In countries of the Middle East and Africa where schistosomiasis is predominant, SCC of the bladder is the second most prevalent form of cancer after hepatocellular carcinoma, which is also related to the disease [34].
Diet
Dietary variables have been extensively studied concerning bladder cancer incidence. Initial retrospective studies revealed a lower incidence of bladder cancer with higher water consumption [30]. In contrast, the European Prospective Investigation of Cancer and Nutrition (EPIC) found no association between total fluid consumption and bladder cancer risk. Nonetheless, there remains disagreement around fluid intake. In addition to fluid consumption, many carcinogens are absorbed through food and expelled in the urine, leading to significant contact with the urothelium and an elevated risk of bladder cancer. However, no solid evidence supports the intake of a particular diet or food group to reduce the risk of bladder cancer. For example, meat consumption has been linked to a higher risk of developing different types of cancer. However, among more than half a million participants in the EPIC trial, the red meat diet was not linked to bladder cancer risk [35].
Medical Illness
Medical disorders may raise the risk of bladder cancer either directly or indirectly through the toxicity of therapy. Typically, persistent inflammation and the formation of keratinizing squamous metaplasia drive direct carcinogenesis. In addition, it has been hypothesized that bladder stones, urinary outflow obstruction, repeated UTIs, and irritation from direct catheter damage contribute to the development of metaplasia and increase the risk of SCC of the bladder [36]. Bladder cancer is often caused by unforeseen side effects of medicinal therapy. One of those drugs includes pioglitazone. It is a thiazolidinedione-class antidiabetic medication that reduces glucose levels in individuals with non-insulin-dependent diabetes. In 2005, the PROactive randomized trial reported the unexpected discovery that the pioglitazone group had more bladder cancer cases than the placebo group [37]. Another important risk factor is chemotherapy. It eliminates cancerous cells by producing DNA damage and cell death; nevertheless, chemotherapy might produce dysregulation in normal cells in organs with a fast cell turnover. Cyclophosphamide is the only chemotherapy that has been proved to induce bladder cancer. Phosphoramide mustard is the major mutagenic metabolite that causes bladder cancer caused by cyclophosphamide. Patients who had cyclophosphamide therapy have a 4.5-fold increased risk of developing bladder cancer, which appears to be dose-dependent and is greatest among those who received more than 20 grams [38]. The genesis of urothelial carcinoma following radiation therapy does not seem to be age-dependent; nevertheless, the estimated latency time is 15-30 years [39]. External beam radiation treatment for cervical cancer is related to a two- to fourfold increased incidence of subsequent bladder cancer compared to the non-irradiated group [40].
BMI
A growing body of data indicates that obesity, defined as BMI above 30 kg/m2, may be carcinogenic, and multiple cancer types correlate with increased BMI. Multiple studies have demonstrated that increasing BMI is a significant risk factor for bladder tumor progression [41]. One reason for this relationship is the high correlation between smoking and obesity; nevertheless, even after correcting the smoking status, BMI remains related to bladder cancer. According to research on how obesity may promote carcinogenesis, obesity increases insulin production, which may stimulate tumor growth. Expansion of adipose tissue increases the synthesis of proinflammatory proteins and cytokines (such as tumor necrosis factor and interleukin-6) while decreasing the synthesis of the protein adiponectin. These metabolic irregularities result in hyperinsulinemia and insulin resistance [42]. To control normal glucose levels, these alterations might cause pancreatic beta cells to increase insulin synthesis, resulting in hyperinsulinemia. Hyperinsulinemia augments the expression of insulin-like growth factor 1 [43], which in turn causes an imbalance in cell proliferation, death, and angiogenesis [42], hence impacting the development of bladder cancer [44].
Lowering risk and prevention
According to recent studies and analyses, nearly 82% of bladder cancer diagnoses from the last 20 years might be related to known preventive factors. Only 7% of bladder cancer occurrences are anticipated to be caused by inherited genetic factors [45]. Bladder cancer is an ideal target for public health preventative measures due to the high proportion of cases attributed to established environmental factors. Cigarette smoking is by far the largest potential risk for bladder cancer in the industrialized world, accounting for up to two-thirds of all cases. It has been established that quitting smoking reduces the incidence of cancer. Similarly, second-hand smoking exposure raises danger and must be eliminated [46]. The second highest avoidable health risk for bladder cancer is occupational exposure. Those in the manufacturing, transportation, firefighting, and hair styling industries should take precautions to minimize chemical exposure through aerosols and touch [30]. The degree to which a fruit-and-vegetable-rich diet can mitigate bladder cancer is still up for debate. Physical exercise has been proven to have a minor protective impact against bladder cancer irrespective of smoking and BMI. Its benefit may be augmented if incorporated into a weight loss program [47]. In endemic places, sanitizing, drinking water, showering, and avoiding swimming and wading in freshwater might substantially reduce the incidence of schistosomiasis [48]. The mass use of the anthelmintic drug praziquantel might control the illness and substantially reduce the chance of contracting bladder cancer [49]. Regular screening for bladder cancer in the general population is not recommended by any major professional organizations at this time. In individuals who are at medium risk, no screening test has been demonstrated to reduce the chance of dying from bladder cancer. People at a very high risk of developing bladder cancer, such as those who have had the disease in the past, those who have specific congenital abnormalities in the bladder, and those who have been subjected to certain toxins at work, may be advised to undergo testing for the disease [40,50]. These tests may include a urine analysis, cytology, a urine test for tumor indicators such as bladder tumor-associated antigen (BTA), UroVysion, ImmunoCyt, and a cystoscopy of the bladder [51].
Conclusions
Bladder cancer is one of the most prevalent cancers in the world. Its incidence is on the rise, particularly in Europe and other industrialized nations, although deaths are down globally due to increased prevention, early detection, and treatment. The prognosis for those with metastatic disease is not good. Tobacco smoking is the largest contributing factor to the urothelial subtype, which accounts for 90% of all cases. Two-thirds of all bladder cancer occurrences are attributable to smoking, which increases the likelihood of the illness by two to three times. In contrast, SCC of the bladder, which represents a minority of occurrences, is prevalent in Africa and the Middle East and is strongly linked to schistosomiasis, a protozoal infection. Occupational and environmental interaction with carcinogenic substances is the second largest risk factor behind smoking. Approximately 82% of bladder cancer cases are attributable to modifiable risk factors. Therefore, prevention initiatives, such as smoking cessation, appropriate workplace safety practices, nutrition, weight reduction, and schistosomiasis prevention, might considerably alleviate the increasing global incidence of bladder cancer.
Acknowledgments
All listed authors contributed equally to the work and should be considered co-first authors.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Urinary bladder contraction and relaxation: physiology and pathophysiology. Andersson KE, Arner A. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 3.The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Wong MC, Fung FD, Leung C, Cheung WW, Goggins WB, Ng CF. Sci Rep. 2018;8:1129. doi: 10.1038/s41598-018-19199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol-driven haematuria clinic. Edwards TJ, Dickinson AJ, Natale S, Gosling J, McGrath JS. BJU Int. 2006;97:301–305. doi: 10.1111/j.1464-410X.2006.05976.x. [DOI] [PubMed] [Google Scholar]
- 5.Economic burden of bladder cancer across the European Union. Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Eur Urol. 2016;69:438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Epidemiology of bladder cancer. Silverman DT, Hartge P, Morrison AS, Devesa SS. https://pubmed.ncbi.nlm.nih.gov/1556044/ Hematol Oncol Clin North Am. 1992;6:1–30. [PubMed] [Google Scholar]
- 7.EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Babjuk M, Burger M, Zigeuner R, et al. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bladder cancer incidence and mortality: a global overview and recent trends. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Cancer survival in England - adults diagnosed. [ Jul; 2022 ];https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed 2019
- 10.Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2019. [ Jul; 2022 ];Areas S. http://SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. Available from https://seer.cancer.gov/statistics-network/explorer/. 2019
- 11.Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A. Ann Oncol. 2014;25:40–48. [Google Scholar]
- 12.The current classification of urothelial neoplasms. Grignon DJ. Mod Pathol. 2009;22:0. doi: 10.1038/modpathol.2008.235. [DOI] [PubMed] [Google Scholar]
- 13.Bladder cancer. Mushtaq J, Thurairaja R, Nair R. https://doi.org/10.1016/j.mpsur.2019.07.003 Surgery. 2019;37:529–537. [Google Scholar]
- 14.Global and regional patterns of tobacco smoking and tobacco control policies. Islami F, Stoklosa M, Drope J, Jemal A. Eur Urol Focus. 2015;1:3–16. doi: 10.1016/j.euf.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US) enters for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53017/ Atlanta (GA): Centers for Disease Control and Prevention (US); 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 16.The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Cumberbatch MG, Rota M, Catto JW, La Vecchia C. Eur Urol. 2016;70:458–466. doi: 10.1016/j.eururo.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Smoking cessation and survival in lung, upper aero-digestive tract and bladder cancer: cohort study. Koshiaris C, Aveyard P, Oke J, Ryan R, Szatkowski L, Stevens R, Farley A. Br J Cancer. 2017;117:1224–1232. doi: 10.1038/bjc.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evaluation of gender-based disparities in time from initial haematuria presentation to upper tract urothelial carcinoma diagnosis: analysis of a nationwide insurance claims database. Chappidi MR, Kates M, Tosoian JJ, Johnson MH, Hahn NM, Bivalacqua TJ, Pierorazio PM. https://doi.org/10.1111/bju.13878. BJU Int. 2017;120:377–386. doi: 10.1111/bju.13878. [DOI] [PubMed] [Google Scholar]
- 19.The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Hemelt M, Yamamoto H, Cheng KK, Zeegers MP. Int J Cancer. 2009;124:412–419. doi: 10.1002/ijc.23856. [DOI] [PubMed] [Google Scholar]
- 20.Gender, smoking, glutathione-S-transferase variants and bladder cancer incidence: a population-based study. Karagas MR, Park S, Warren A, Hamilton J, Nelson HH, Mott LA, Kelsey KT. Cancer Lett. 2005;219:63–69. doi: 10.1016/j.canlet.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Daugherty SE, Lacey JV Jr, Pfeiffer RM, Park Y, Hoover RN, Silverman DT. Int J Cancer. 2013;133:462–472. doi: 10.1002/ijc.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Androgen receptor signaling in bladder cancer. Li P, Chen J, Miyamoto H. Cancers (Basel) 2017;9 doi: 10.3390/cancers9020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genetic susceptibility to bladder cancer risk and outcome. Gu J, Wu X. Per Med. 2011;8:365–374. doi: 10.2217/PME.11.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. García-Closas M, Malats N, Silverman D, et al. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hereditary bladder cancer. Kiemeney LA. Scand J Urol Nephrol Suppl. 2008:110–115. doi: 10.1080/03008880802283755. [DOI] [PubMed] [Google Scholar]
- 26.Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. J Med Genet. 2010;47:464–470. doi: 10.1136/jmg.2010.076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Riegert-Johnson DL, Gleeson FC, Roberts M, Tholen K, Youngborg L, Bullock M, Boardman LA. Hered Cancer Clin Pract. 2010;8:6. doi: 10.1186/1897-4287-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer statistics, 2020. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 29.Contemporary occupational carcinogen exposure and bladder cancer: a systematic review and meta-analysis. Cumberbatch MG, Cox A, Teare D, Catto JW. JAMA Oncol. 2015;1:1282–1290. doi: 10.1001/jamaoncol.2015.3209. [DOI] [PubMed] [Google Scholar]
- 30.Epidemiology and risk factors of urothelial bladder cancer. Burger M, Catto JW, Dalbagni G, et al. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Vermeulen SH, Hanum N, Grotenhuis AJ, et al. Br J Cancer. 2015;112:594–600. doi: 10.1038/bjc.2014.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonorrhoea and male bladder cancer in a prospective study. Michaud DS, Platz EA, Giovannucci E. Br J Cancer. 2007;96:169–171. doi: 10.1038/sj.bjc.6603510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Insight into the molecular basis of Schistosoma haematobium-induced bladder cancer through urine proteomics. Bernardo C, Cunha MC, Santos JH, et al. Tumour Biol. 2016;37:11279–11287. doi: 10.1007/s13277-016-4997-y. [DOI] [PubMed] [Google Scholar]
- 34.Relationship between schistosomiasis and bladder cancer. Mostafa MH, Sheweita SA, O'Connor PJ. Clin Microbiol Rev. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fluid intake and the risk of urothelial cell carcinomas in the European Prospective Investigation into Cancer and Nutrition (EPIC) Ros MM, Bas Bueno-de-Mesquita HB, Büchner FL, et al. Int J Cancer. 2011;128:2695–2708. doi: 10.1002/ijc.25592. [DOI] [PubMed] [Google Scholar]
- 36.Chronic indwelling urinary catheter increase the risk of bladder cancer, even in patients without spinal cord injury. Ho CH, Sung KC, Lim SW, Liao CH, Liang FW, Wang JJ, Wu CC. Medicine (Baltimore) 2015;94:0. doi: 10.1097/MD.0000000000001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pioglitazone and bladder cancer risk: a systematic review and meta-analysis. Tang H, Shi W, Fu S, Wang T, Zhai S, Song Y, Han J. Cancer Med. 2018;7:1070–1080. doi: 10.1002/cam4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin's lymphoma. Travis LB, Curtis RE, Glimelius B, et al. J Natl Cancer Inst. 1995;87:524–530. doi: 10.1093/jnci/87.7.524. [DOI] [PubMed] [Google Scholar]
- 39.Second primary cancer after treatment for cervical cancer. An international cancer registries study. Kleinerman RA, Boice JD Jr, Storm HH, et al. Cancer. 1995;76:442–452. doi: 10.1002/1097-0142(19950801)76:3<442::aid-cncr2820760315>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Bladder cancer risk following primary and adjuvant external beam radiation for prostate cancer. Chrouser K, Leibovich B, Bergstralh E, Zincke H, Blute M. J Urol. 2005;174:107–110. doi: 10.1097/01.ju.0000163459.57305.a1. [DOI] [PubMed] [Google Scholar]
- 41.Estimating the impact of body mass index on bladder cancer risk: stratification by smoking status. Choi JB, Lee EJ, Han KD, Hong SH, Ha US. Sci Rep. 2018;8:947. doi: 10.1038/s41598-018-19531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Ferlay J, Soerjomataram I, Dikshit R, et al. Int J Cancer. 2015;136:0. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 43.Overweight, obesity, and cancer risk. Bianchini F, Kaaks R, Vainio H. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 44.Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. Zhao H, Grossman HB, Spitz MR, Lerner SP, Zhang K, Wu X. J Urol. 2003;169:714–717. doi: 10.1097/01.ju.0000036380.10325.2a. [DOI] [PubMed] [Google Scholar]
- 45.Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP. Eur J Epidemiol. 2016;31:811–851. doi: 10.1007/s10654-016-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passive smoking and lung cancer in Japanese non-smoking women: a prospective study. Kurahashi N, Inoue M, Liu Y, Iwasaki M, Sasazuki S, Sobue T, Tsugane S. Int J Cancer. 2008;122:653–657. doi: 10.1002/ijc.23116. [DOI] [PubMed] [Google Scholar]
- 47.The association between physical activity and bladder cancer: systematic review and meta-analysis. Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. Br J Cancer. 2014;110:1862–1870. doi: 10.1038/bjc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prevention and control of schistosomiasis: a current perspective. Inobaya MT, Olveda RM, Chau TN, Olveda DU, Ross AG. Res Rep Trop Med. 2014;2014:65–75. doi: 10.2147/RRTM.S44274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halting Schistosoma haematobium - associated bladder cancer. Botelho MC, Alves H, Richter J. Int J Cancer Manag. 2017;10:0. doi: 10.5812/ijcm.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Periodic urine cytology surveillance of bladder tumor incidence in dyestuff workers. Yamaguchi N, Tazaki H, Okubo T, Toyama T. Am J Ind Med. 1982;3:139–148. doi: 10.1002/ajim.4700030204. [DOI] [PubMed] [Google Scholar]
- 51.Three-year follow-up of bladder tumours found on screening. Whelan P, Britton JP, Dowell AC. Br J Urol. 1993;72:893–896. doi: 10.1111/j.1464-410x.1993.tb16292.x. [DOI] [PubMed] [Google Scholar]