Abstract
Background
Although a high prevalence of pulmonary embolism (PE) has been reported in association with coronavirus disease 2019 (COVID-19) in critically ill patients, nationwide data on the outcome of hospitalised patients with COVID-19 and PE are still limited. Thus, we investigated seasonal trends and predictors of in-hospital death in patients with COVID-19 and PE in Germany.
Methods
We used a German nationwide inpatient sample to analyse data on hospitalisations among COVID-19 patients with and without PE during 2020, and to detect changes in PE prevalence and case fatality in comparison with 2019.
Results
We analysed 176 137 COVID-19 hospitalisations in 2020; PE was recorded in 1.9% (n=3362) of discharge certificates. Almost one-third of patients with COVID-19 and PE died during the in-hospital course (28.7%) compared with COVID-19 patients without PE (17.7%). Between 2019 and 2020, numbers of PE-related hospitalisations were largely unchanged (98 485 versus 97 718), whereas the case fatality rate of PE increased slightly in 2020 (from 12.7% to 13.1%; p<0.001). Differences in case fatality were found between PE patients with and without COVID-19 in 2020 (28.7% versus 12.5%; p<0.001), corresponding to a 3.1-fold increased risk of PE-related death (OR 3.16, 95% CI 2.91–3.42; p<0.001) in the presence of COVID-19.
Conclusions
In Germany, the prevalence of PE events during hospitalisations was similar in 2019 and 2020. However, the fatality rate among patients with both COVID-19 and PE was substantially higher than that in those with only one of these diseases, suggesting a life-threatening additive prognostic impact of the COVID-19–PE combination.
Short abstract
This study demonstrated a considerable impact of COVID-19 on adverse outcomes of patients with pulmonary embolism in 2020, which should guide attention to this special population with regard to antithrombotic prevention and diagnostic strategies https://bit.ly/3zz3OjZ
Introduction
The first patient cases of pneumonia caused by a previously unknown virus were identified in China by the end of 2019 [1, 2]. The fast spread of infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) resulted in a global pandemic [1]. Since the beginning of the pandemic, deaths related to COVID-19 surpassed 6 million people worldwide and more than 120 000 in Germany as of March 2022 [3]. Most often, COVID-19 patients present with respiratory symptoms but may also suffer from chest pain and haemoptysis. These symptoms largely overlap with the typical symptoms observed in the clinical presentation of patients with acute pulmonary embolism (PE). In this context, thrombotic and thromboembolic complications such as PE have been described as frequent and relevant complications of COVID-19 infection across several countries in 2020 [4–7]. From a pathophysiological point of view, it is still a matter of debate whether venous thromboembolism (VTE), in situ immunothrombosis or both may cause contrast-filling defects in computed tomography pulmonary angiography (CTPA) when PE is diagnosed [8, 9]. COVID-19-associated local and systemic inflammation in combination with traditional predisposing factors for VTE such as immobilisation, hypovolaemia as well as endothelial damage are assumed to provoke VTE and/or immunothrombosis in patients with severe COVID-19 infection. However, if acute PE is confirmed, regardless of COVID-19 status, treatment should follow the general risk-adapted guidelines for the management of acute PE [10]. Studies have suggested that the incidence and fatality rates of patients with COVID-19 and PE vary among countries, and seem to be considerably higher compared with PE patients without COVID-19 [11–14]. Unselected data of nationwide studies of hospitalised patients with COVID-19 and PE are missing in Germany. The aim of this analysis was to provide comprehensive and precise information on patient characteristics, regional and seasonal differences, and outcomes of hospitalised patients with COVID-19 and PE in Germany in 2020, and to compare changes of PE prevalence and case fatality between 2019 and 2020.
Methods
Data source
Statistical analyses were performed on our behalf by the Research Data Center (RDC) of the Federal Bureau of Statistics (Wiesbaden, Germany). Aggregated statistics were provided from RDC on the basis of our SPSS codes (SPSS Statistics for Windows version 20.0; IBM, Armonk, NY, USA), which we had supplied to the RDC (source: RDC of the Federal Statistical Office and the Statistical Offices of the federal states, Diagnosis-Related Groups (DRG) Statistic 2019–2020, own calculations) [15, 16].
With this data analysis of the German nationwide inpatient sample, we aimed to analyse temporal trends of all hospitalised patients with a confirmed COVID-19 diagnosis (International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) code U07.1) and an additional diagnosis of PE (ICD-10 code I26) during the observational period between 1 January 2020 and 31 December 2020 and identify independent predictors of in-hospital death. To allow a comparison of PE prevalence between 2020 and previous years, all patients with the diagnosis of PE in 2019 and 2020 were analysed for this additional comparison.
Study oversight and support
Since our study did not comprise direct access by the investigators to individual patient data but only access to summarised results provided by the RDC, approval by an ethics committee as well as patients’ informed consent were not required, in accordance with German law [15, 16].
Coding of diagnoses, procedures and definitions
Diagnosis- and procedure-related remuneration was introduced in Germany in 2004. Coding according the German DRG system, with coding of patient data on diagnoses, coexisting conditions, surgeries as well as on procedures/interventions and transferring these codes to the Institute for the Hospital Remuneration System, is mandatory for German hospitals to get their remuneration. Patients’ diagnoses are coded according to ICD-10, German Modification (ICD-10-GM). In parallel, surgical, diagnostical and interventional procedures were coded according to OPS (Operationen- und Prozedurenschlüssel) codes.
To obtain data regarding coexisting conditions and complications, we used the available diagnostic and procedural codes for acute and chronic conditions (OPS and ICD-10-GM codes), which are presented with related ICD and OPS coding in supplementary table S1.
Statistical analysis
Differences in patient characteristics between the groups of hospitalised COVID-19-patients with PE versus without PE and patients who died during the in-hospital course and those who were discharged alive were calculated with the Wilcoxon–Whitney U-test for continuous variables and Fisher's exact or the Chi-squared test for categorical variables, as appropriate. Temporal trends regarding hospitalisations of COVID-19 and PE, and in-hospital mortality over time, were estimated by means of linear regression analyses. Logistic regression models were calculated to investigate associations between patients’ characteristics as well as adverse events, on one hand, and 1) need for mechanical ventilation or 2) in-hospital death, on the other. In order to test the (in)dependence of the findings on confounding parameters, multivariate regression models were adjusted for age, sex, cancer, heart failure, coronary artery disease, peripheral artery disease, COPD, essential arterial hypertension, hyperlipidaemia, renal insufficiency (glomerular filtration rate <60 mL·min−1·1.73 m−2), diabetes mellitus and atrial fibrillation/flutter. Tested variables were not adjusted on their own. The results were presented as odds ratios and 95% confidence intervals. All statistical analyses were performed with SPSS Statistics for Windows version 20.0; p-values <0.05 (two-sided) were considered to be statistically significant.
Results
COVID-19 patients with and without PE: baseline characteristics
In total, 176 137 cases with confirmed COVID-19 infection were hospitalised in Germany during 2020. The majority were male (n=92 188 (52.3%)) and aged ≥70 years (n=94 329 (53.6%)). In total, 31 607 (17.9%) patients with COVID-19 died.
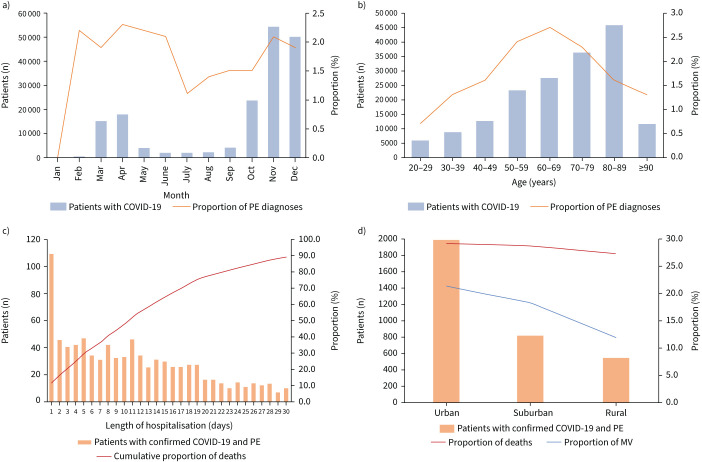
A minority of patients with COVID-19 had an additional diagnosis of PE (n=3362 (1.9%)). The proportion of PE in patients with COVID-19 infection did not change significantly on a monthly basis during 2020, with the highest numbers in spring and winter (figure 1a). Regarding age distribution, PE proportion increased with age, revealing a peak in the fifth to seventh decades of life (figure 1b). Similar to the baseline characteristics, the majority of patients with COVID-19 and PE were male (n=2062 (61.3%)) and more than half of them were aged ≥70 years (n=1717 (51.1%)). Cardiovascular risk factors and comorbidities such as obesity (299 (8.9%) versus 9084 (5.3%); p<0.001), malignancies (217 (6.5%) versus 8784 (5.1%); p<0.001) and chronic renal insufficiency (1119 (33.3%) versus 47 822 (27.7%); p<0.001) were more prevalent in patients with COVID-19 and PE compared with patients with COVID-19 without PE (table 1). A multivariate regression analysis revealed that male sex (OR 1.35, 95% CI 1.10–1.49; p>0.001), obesity (OR 1.88, 95% CI 1.66–2.12; p<0.001) and cancer (OR 1.25, 95% 1.09–1.44; p<0.001) were independently associated with an increased risk for diagnosis of PE in patients with COVID-19.
FIGURE 1.
Temporal and regional trends regarding hospitalised patients with COVID-19 infection and pulmonary embolism (PE) in 2020. a) Temporal trends regarding total numbers of hospitalised patients with COVID-19 and proportion of PE stratified by months. b) Age (decade)-dependent total numbers of hospitalised patients with COVID-19 and proportion of PE. c) Time trends regarding total numbers of hospitalised patients with COVID-19 and PE and cumulative proportion of deaths stratified by hospitalisation days. d) Regional trends regarding total numbers of hospitalised patients with COVID-19 and PE, proportion of deaths and proportion of mechanical ventilation (MV).
TABLE 1.
Characteristics, medical history, presentation and in-hospital adverse events (AEs) of the 176 137 hospitalised patients with confirmed COVID-19 infection in Germany in 2020 stratified for the presence of pulmonary embolism (PE)
| COVID-19 infection without PE | COVID-19 infection with PE | p-value | |
| Patients | 172 775 (98.1) | 3362 (1.9) | |
| Age (years) | 71.0 (55.0–82.0) | 70.0 (59.0–80.0) | 0.219 |
| Age ≥70 years | 92 612 (53.6) | 1717 (51.1) | 0.004* |
| Female | 82 649 (47.8) | 1300 (38.7) | <0.001* |
| In-hospital stay (days) | 8.0 (4.0–14.0) | 12.0 (6.0–21.0) | <0.001* |
| VTE risk factors | |||
| Obesity | 9084 (5.3) | 299 (8.9) | <0.001* |
| Diabetes mellitus | 44 371 (25.7) | 861 (25.6) | 0.935 |
| Thrombophilia | 398 (0.2) | 44 (1.3) | <0.001* |
| Surgery | 46 597 (27.0) | 1398 (41.6) | <0.001* |
| Cancer | 8784 (5.1) | 217 (6.5) | <0.001* |
| Comorbidities | |||
| Coronary artery disease | 25 199 (14.6) | 375 (11.2) | <0.001* |
| Peripheral artery disease | 5554 (3.2) | 86 (2.6) | <0.001* |
| Atrial fibrillation/flutter | 33 595 (19.4) | 565 (16.8) | <0.001* |
| COPD | 11 953 (6.9) | 201 (6.0) | 0.035* |
| Chronic renal insufficiency# | 47 822 (27.7) | 344 (10.2) | <0.001* |
| Essential arterial hypertension | 80 906 (46.8) | 1574 (46.8) | 1.000 |
| Charlson Comorbidity Index | 4.1 (2.0–6.0) | 4.5 (2.0–6.0) | <0.001* |
| Respiratory manifestations of COVID-19 | |||
| Pneumonia | 104 078 (60.2) | 2835 (84.3) | <0.001* |
| ARDS | 10 834 (6.3) | 760 (22.6) | <0.001* |
| Mild ARDS | 513 (0.3) | 16 (0.5) | <0.001* |
| Moderate ARDS | 2603 (1.5) | 161 (4.8) | <0.001* |
| Severe ARDS | 7337 (4.2) | 575 (17.1) | <0.001* |
| Treatment | |||
| Intensive care unit | 25 728 (14.9) | 1325 (39.4) | <0.001* |
| Mechanical ventilation | 11 504 (6.7) | 638 (19.0) | <0.001* |
| ECMO | 1283 (0.7) | 153 (4.6) | <0.001* |
| Dialysis | 5271 (3.1) | 304 (9.0) | <0.001* |
| Systemic thrombolysis | 285 (0.2) | 173 (5.1) | <0.001* |
| Surgical embolectomy | 0 | 7 (0.2) | <0.001* |
| AEs during hospitalisation | |||
| Transfusion of blood constituents | 13 249 (7.7) | 625 (18.6) | <0.001* |
| Deep vein thrombosis | 1275 (075) | 508 (15.1) | <0.001* |
| Acute kidney failure | 21 296 (12.3) | 779 (23.2) | <0.001* |
| Severe liver disease | 3961 (2.3) | 178 (5.3) | <0.001* |
| Myocarditis | 217 (0.1) | 9 (0.3) | 0.044* |
| Stroke (ischaemic or haemorrhagic) | 3068 (1.8) | 128 (3.8) | <0.001* |
| Intracerebral bleeding | 535 (0.3) | 41 (1.2) | <0.001* |
| Gastrointestinal bleeding | 2869 (1.7) | 79 (2.3) | 0.003* |
| Right ventricular dysfunction | 0 | 706 (21.0) | <0.001* |
| Shock | 5933 (3.4) | 453 (13.5) | <0.001* |
| Cardiopulmonary resuscitation | 2584 (1.5) | 275 (8.2) | <0.001* |
| Case fatality | 30 643 (17.7) | 964 (28.7) | <0.001* |
Data are presented as n (%) or median (range), unless otherwise stated. VTE: venous thromboembolism; ARDS: acute respiratory distress syndrome; ECMO: extracorporeal membrane oxygenation. #: glomerular filtration rate <60 mL·min−1·1.73 m−2. *: statistically significant (p<0.05).
Of the total population hospitalised in relation to the diagnosis of COVID-19, 60.7% (n=106 913) exhibited pneumonia and 6.6% (n=11 594) exhibited acute respiratory distress syndrome (ARDS). Regarding the development of pneumonia between COVID-19 patients with versus without PE, the unadjusted risk ratio was 1.4 and, in the same comparison regarding the development of ARDS, the unadjusted risk ratio was 3.6. More than one-third of patients with COVID-19 and PE had to be treated in an intensive care unit (ICU). Regarding parameters indicating the severity of PE, right ventricular dysfunction was present in 706 (21.0%) patients, shock in 453 (13.5%) patients and cardiopulmonary resuscitation was provided in 275 (8.2%) patients with COVID-19 and PE (table 1).
COVID-19 patients with PE: regional differences and predictors of case fatality
In total, 964 (28.7%) patients with COVID-19 and PE died as opposed to patients without PE, with a case fatality rate of 17.7% (n=30 643) (table 1). More than one-third of deaths (36.2%) of patients with COVID-19 and PE occurred during the first 7 days of hospitalisation and >60% during the first 14 days (figure 1c). The case fatality rate increased substantially with patient age, with a peak in the ninth decade of life (supplementary figure S1a). In this context, elderly patients in the eighth (n=613 (74.7%)) and ninth (n=528 (70.9%)) decades of life underwent diagnostic work-up with CTPA less frequently than patients in the fifth (n=166 (82.2%)) or sixth (n=438 (78.9%)) decades (supplementary figure S1b).
Some regional differences in admissions and treatment approaches were evident in Germany. Most patients with confirmed COVID-19 and with PE were treated in hospitals in urban areas (59.1% (n=1989)), with a comparable case fatality (29.1% (n=579)) compared with hospitals in suburban (28.6% (n=234)) or rural areas (27.2% (n=151)). There was a considerably higher rate of mechanical ventilation in urban and suburban areas versus rural areas (21.3% (n=424) versus 18.2% (n=149) versus 11.9% (n=66)) (figure 1d).
Nonsurvivors were older, more often had comorbidities such as obesity, diabetes mellitus and coronary artery disease, and had ∼30% higher prevalence of ARDS (table 2). Parameters of PE-related severity such as right ventricular dysfunction, shock as well as cardiopulmonary resuscitation were more often presented in deceased patients. Consequently, nonsurvivors were more often treated in an ICU, and they more frequently necessitated extracorporeal membrane oxygenation (ECMO), mechanical ventilation and haemodialysis. Several independent predictors of in-hospital case fatality were detected in a multivariate logistic regression model. Briefly, age ≥70 years, right ventricular dysfunction, dialysis, ECMO, ARDS, intracerebral bleeding, shock and cardiopulmonary resuscitation had a strong association with increased case fatality (table 3).
TABLE 2.
Characteristics, medical history, presentation and in-hospital adverse events (AEs) of the 3362 hospitalised patients with confirmed COVID-19 infection and concomitant pulmonary embolism (PE) in Germany in 2020 stratified for the presence of in-hospital mortality
| Survivors | Nonsurvivors | p-value | |
| Patients | 2398 (71.3) | 964 (28.7) | |
| Age (years) | 67.0 (57.0–79.0) | 75.0 (65.0–82.0) | 0.001* |
| Age ≥70 years | 1097 (45.7) | 620 (64.3) | <0.001* |
| Female | 971 (40.5) | 329 (34.1) | 0.001* |
| In-hospital stay (days) | 12.0 (7.0–22.0) | 11.0 (5.0–19.0) | 0.001* |
| VTE risk factors | |||
| Obesity | 197 (8.2) | 102 (10.6) | 0.032* |
| Diabetes mellitus | 544 (22.7) | 317 (32.9) | <0.001* |
| Thrombophilia | 27 (1.1) | 17 (1.8) | 0.178 |
| Surgery | 849 (35.4) | 549 (57.0) | <0.001* |
| Cancer | 135 (5.6) | 82 (8.5) | 0.003* |
| Comorbidities | |||
| Coronary artery disease | 233 (9.7) | 142 (14.7) | <0.001* |
| Peripheral artery disease | 43 (1.8) | 43 (4.5) | <0.001* |
| Atrial fibrillation/flutter | 293 (12.2) | 272 (28.2) | <0.001* |
| COPD | 121 (5.0) | 80 (8.3) | 0.001* |
| Chronic renal insufficiency# | 202 (8.4) | 142 (14.7) | <0.001* |
| Essential arterial hypertension | 1125 (46.9) | 449 (46.6) | 0.879 |
| Charlson Comorbidity Index | 4 (2.0–5.0) | 6.0 (4.0–8.0) | <0.001* |
| Respiratory manifestations of COVID-19 | |||
| Pneumonia | 1988 (82.9) | 847 (87.9) | <0.001* |
| ARDS | 354 (14.8) | 406 (42.1) | <0.001* |
| Mild ARDS | Censored | Censored | 0.052 |
| Moderate ARDS | 96 (4.0) | 65 (6.7) | 0.001* |
| Severe ARDS | 239 (10.0) | 336 (34.9) | <0.001* |
| Treatment | |||
| Intensive care unit | 760 (31.7) | 565 (58.6) | <0.001* |
| Mechanical ventilation | 352 (14.7) | 286 (29.7) | <0.001* |
| ECMO | 50 (2.1) | 121 (12.6) | <0.001* |
| Dialysis | 90 (3.8) | 214 (22.4) | <0.001* |
| Systemic thrombolysis | 53 (2.2) | 120 (12.4) | <0.001* |
| Surgical embolectomy | Censored | Censored | 1.000 |
| AEs during hospitalisation | |||
| Transfusion of blood constituents | 277 (11.6) | 348 (36.1) | <0.001* |
| Deep vein thrombosis | 411 (17.1) | 97 (10.1) | <0.001* |
| Acute kidney failure | 318 (13.3) | 461 (47.8) | <0.001* |
| Severe liver disease | 57 (2.4) | 121 (12.6) | <0.001* |
| Stroke (ischaemic or haemorrhagic) | 52 (2.2) | 76 (7.9) | <0.001* |
| Intracerebral bleeding | 13 (0.5) | 28 (2.9) | <0.001* |
| Gastrointestinal bleeding | 42 (1.8) | 37 (3.8) | 0.001* |
| Right ventricular dysfunction | 379 (15.8) | 327 (33.9) | <0.001* |
| Shock | 131 (5.5) | 322 (33.4) | <0.001* |
| Cardiopulmonary resuscitation | 40 (1.7) | 235 (24.4) | <0.001* |
Data are presented as n (%) or median (range), unless otherwise stated. VTE: venous thromboembolism; ARDS: acute respiratory distress syndrome; ECMO: extracorporeal membrane oxygenation; #: glomerular filtration rate <60 mL·min−1·1.73 m−2. *: statistically significant (p<0.05).
TABLE 3.
Associations of baseline characteristics, comorbidities and adverse events (AEs) with in-hospital mortality in patients with pulmonary embolism and confirmed COVID-19 infection (n=3362; 964 died during in-hospital stay (28.7%)): univariable and multivariable logistic regression models
| Univariate | Multivariate# | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age ≥70 years | 2.137 (1.832–2.494) | <0.001* | 2.039 (1.760–2.490) | <0.001* |
| Female | 0.761 (0.651–0.890) | 0.001* | 0.594 (0.499–0.707) | <0.001* |
| Obesity | 1.322 (1.028–1.700) | 0.030* | 1.633 (1.238–2.156) | 0.001* |
| Comorbidities | ||||
| Coronary artery disease | 1.605 (1.284–2.007) | <0.001* | 0.929 (0.720–1.198) | 0.570 |
| Cancer | 1.558 (1.172–2.073) | 0.002* | 1.541 (1.140–2.083) | 0.005* |
| Peripheral artery disease | 2.557 (1.664–3.929) | <0.001* | 1.465 (0.918–2.340) | 0.110 |
| Heart failure | 2.501 (2.100–2.978) | <0.001* | 1.840 (1.522–2.224) | <0.001* |
| COPD | 1.703 (1.271–2.283) | <0.001* | 1.278 (0.932–1.751) | 0.128 |
| Arterial hypertension | 0.859 (0.849–1.146) | 0.859 | 0.811 (0.688–0.957) | 0.013* |
| Renal insufficiency | 1.878 (1.493–2.362) | <0.001* | 1.147 (0.886–1.484) | 0.297 |
| Diabetes mellitus | 1.670 (1.416–1.969) | <0.001* | 1.420 (1.185–1.704) | <0.001* |
| Atrial fibrillation | 2.824 (2.345–3.401) | <0.001* | 1.994 (1.634–2.432) | <0.001* |
| Charlson Comorbidity Index | 1.370 (1.327–1.414) | <0.001* | 1.210 (1.207–1.225) | <0.001* |
| Clinical presentation | ||||
| Right ventricular dysfunction | 2.735 (2.301–3.250) | <0.001* | 2.575 (2.129–3.115) | <0.001* |
| NYHA FC III | 1.400 (1.281–1.529) | <0.001* | 0.946 (0.832–1.077) | 0.403 |
| NYHA FC IV | 3.342 (2.473–4.516) | <0.001* | 1.593 (1.112–2.282) | 0.011* |
| Serious AEs through hospitalisation | ||||
| Stroke | 3.861 (2.691–5.540) | <0.001* | 3.444 (2.340–5.069) | <0.001* |
| Dialysis | 7.317 (5.644–9.487) | <0.001* | 7.994 (5.995–10.659) | <0.001* |
| Acute renal failure | 5.995 (5.043–7.126) | <0.001* | 5.785 (4.779–7.002) | <0.001* |
| ARDS | 4.201 (3.543–4.982) | <0.001* | 5.721 (4.666–7.016) | <0.001* |
| Mild ARDS | 0.165 (0.022–1.251) | 0.081 | 0.149 (0.019–1.195) | 0.073 |
| Moderate ARDS | 1.734 (1.254–2.397) | 0.001* | 1.701 (1.195–2.420) | 0.003* |
| Severe ARDS | 4.833 (4.004–5.834) | <0.001* | 6.448 (5.179–8.027) | <0.001* |
| MSCOVID | 20.383 (6.123–67.850) | <0.001* | 24.273 (7.039–83.696) | <0.001* |
| Post-COVID | 0.620 (0.207–1.860) | 0.394 | 0.999 (0.325–3.066) | 0.998 |
| Severe liver disease | 5.895 (4.261–8.155) | <0.001* | 5.658 (3.982–8.039) | <0.001* |
| ECMO | 6.740 (4.803–9.458) | <0.001* | 10.334 (7.107–15.027) | <0.001* |
| Pneumonia | 1.493 (1.197–1.862) | <0.001* | 1.698 (1.339–2.115) | <0.001* |
| Deep venous thrombosis | 0.541 (0.428–0.684) | <0.001* | 0.550 (0.429–0.706) | <0.001* |
| Intracerebral bleeding | 5.488 (2.831–10.641) | <0.001* | 5.531 (2.708–11.297) | <0.001* |
| Gastrointestinal bleeding | 2.239 (1.430–3.506) | <0.001* | 1.885 (1.170–3.039) | 0.009* |
| Admission to ICU | 3.052 (2.615–3.561) | <0.001* | 3.887 (3.238–4.666) | <0.001* |
| Mechanical ventilation | 2.452 (2.051–2.931) | <0.001* | 2.618 (2.142–3.199) | <0.001* |
| Transfusion of erythrocytes | 4.326 (3.608–5.187) | <0.001* | 4.365 (3.570–5.338) | <0.001* |
| Shock | 8.680 (6.957–10.829) | <0.001* | 10.658 (8.310–13.671) | <0.001* |
| Cardiopulmonary resuscitation | 19.003 (13.453–26.842) | <0.001* | 23.561 (16.286–34.085) | <0.001* |
NYHA FC: New York Heart Association Functional Class; ARDS: acute respiratory distress syndrome; MSCOVID: multisystem inflammatory syndrome COVID-19; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit. #: adjusted for age, sex, cancer, coronary artery disease, heart failure, COPD, arterial hypertension, renal insufficiency, diabetes mellitus, atrial fibrillation, peripheral arterial disease and hyperlipidaemia. *: statistically significant at least in the multivariate regression model (p<0.05).
Diagnosis of PE: comparison of the pre-COVID-19 and COVID-19 era
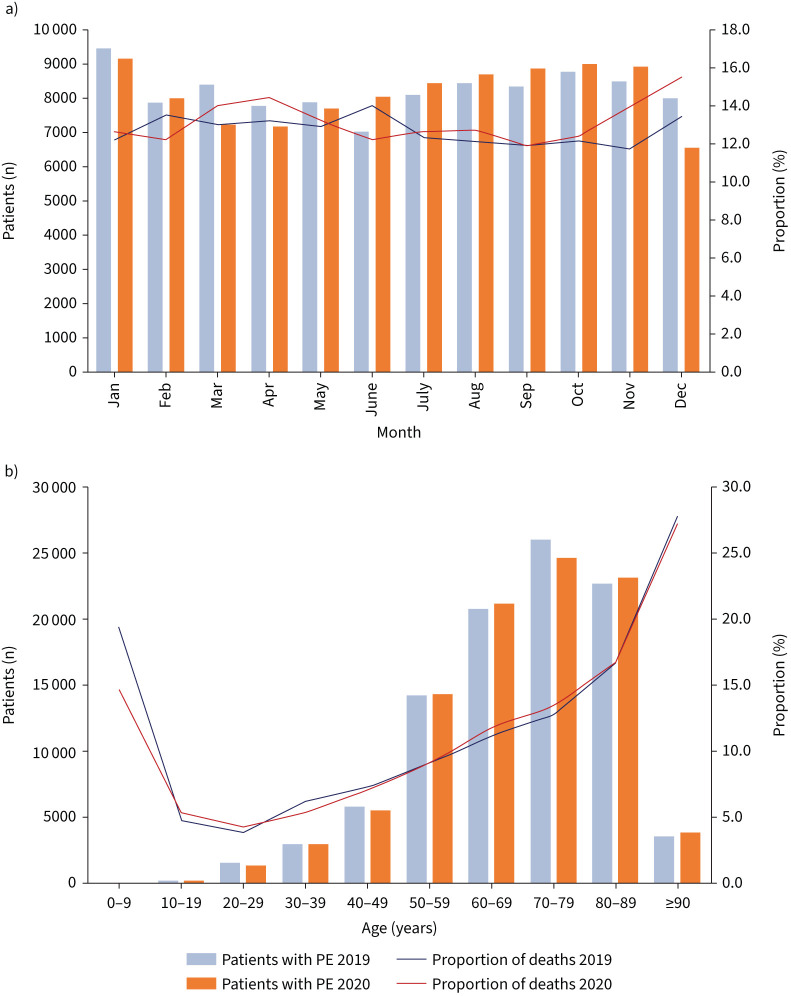
The number of hospitalisations for PE was slightly higher in 2019 (n=98 485) than in 2020 (n=97 718) (figure 2a and b), whereas the case fatality of patients with PE was lower in 2019 compared with 2020 (12.7% versus 13.1%). The difference in case fatality rates between the two years was most pronounced in the months of November and December (11.7% and 13.4%, respectively, in 2019, increasing to 13.9% and 15.5%, respectively, in 2020), being in association with the second wave of COVID-19 at the end of 2020. These results are supported by the multivariate regression analysis, which showed a slightly higher risk of case fatality for patients hospitalised with the diagnosis of PE in 2020 compared with 2019 (OR 1.03, 95% CI 1.01–1.06; p=0.018). Parameters indicating severe disease such as right ventricular dysfunction, shock, cardiopulmonary resuscitation, systemic thrombolysis, intracranial bleeding and mechanical ventilation were distributed equally in both years.
FIGURE 2.
Changes in temporal trends in pulmonary embolism (PE) diagnosis regarding the pre-COVID era in 2019 and the COVID era in 2020. a) Temporal trends regarding total numbers of hospitalised patients with PE in 2019 compared with 2020 with respective proportions of deaths stratified for months. b) Age (decade)-dependent total numbers of hospitalised patients with PE in 2019 compared with 2020 with respective proportion rates of deaths.
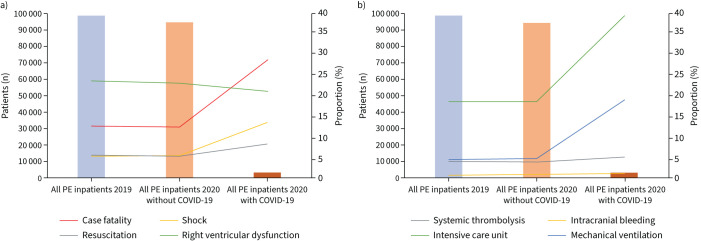
Considering 2020 singularly, PE patients with and without COVID-19 infection demonstrated substantial differences (supplementary table S1). With regard to adverse outcomes, patients with co-prevalence of PE and COVID-19 infection had a higher case fatality rate compared with patients with PE without COVID-19 infection (28.7% versus 12.5%; p<0.001) (figure 3a and supplementary table S1). In a univariate regression model, COVID-19 infection was associated with a 2.8-fold increased risk of case fatality in patients with PE (OR 2.81, 95% CI 1.66–2.12; p<0.001). When adjusting for several parameters, COVID-19 infection was still associated with a 3.1-fold increased risk of case fatality in patients with PE in the multivariate regression model (OR 3.16, 95% CI 2.91–3.42; p<0.001) (supplementary table S3). In this context, patients with PE and COVID-19 more often had right ventricular dysfunction, shock and cardiopulmonary resuscitation (figure 3a). Additionally, patients with PE and concomitant COVID-19 infection exhibited increased frequency of treatment in the ICU, mechanical ventilation and systemic thrombolysis (figure 3b and supplementary table S1).
FIGURE 3.
Total numbers of hospitalised patients with pulmonary embolism (PE) with respective parameters indicating severe in-hospital course stratified for 2019 and 2020 as well as for COVID-19 infection. Further parameters are presented in supplementary table S1.
Discussion
The aim of the present study was to examine patient characteristics, regional and seasonal differences, and outcomes of hospitalised patients with COVID-19 and PE in German hospitals during 2020. Additionally, PE adverse outcomes were compared with 2019 in order to assess the impact of COVID-19 on the in-hospital course of patients with PE. The main results of the study can be summarised as follows: 1) in COVID-19 patients, the prevalence of PE was 1.9% and did not change over the months of 2020; 2) male sex, obesity and cancer were independently associated with an increased risk for PE in patients with COVID-19 infection; 3) case fatality was considerably higher (28.7%) in patients with COVID-19 and PE as opposed to COVID-19 patients without PE (17.7%); 4) COVID-19 patients with PE suffered especially from ARDS compared with COVID-19 patients without PE, and were more often treated on the ICU with a higher rate of mechanical ventilation, dialysis and ECMO; 5) the numbers of hospitalisations for PE were largely unchanged between 2019 and 2020, while the case fatality rate was slightly higher in 2020 in accordance with peak numbers of the COVID-19 pandemic; 6) PE patients with COVID-19 infection more often demonstrated right ventricular dysfunction, shock, cardiopulmonary resuscitation and case fatality (28.7% versus 12.5%) in contrast to PE patients without COVID-19; and 7) COVID-19 infection was associated with a 3.1-fold increased risk of case fatality in patients with PE.
Several studies estimated the proportion of patients diagnosed with PE among those hospitalised with COVID-19 infection, but the rates varied widely between 0.5% and 61.5% across all risk categories [12, 17–19]. A recent meta-analysis included 23 177 patients of 66 studies and estimated a 7.8% (95% CI 6.2–9.4%) overall prevalence of COVID-19-related PE [20]. Our analysis demonstrated a proportion of 1.9%, which corresponds to an in-hospital incidence rate of 2.9 per 100 000 infections per year. The prevalence of PE diagnosis increased with age and the peak occurred between the fifth and seventh decades of life; this peak occurred at considerably older ages compared with previously published PE studies from the USA, Canada and Europe [21, 22]. In the literature, autopsy reports in particular have proposed the hypothesis of pulmonary microvascular immunothrombosis, according to findings of thrombosis in the small vessels and capillaries of the lung [19, 23]. It has to be kept in mind that detection of PE in COVID-19 patients strongly depends on the use of CTPA [24–28]. Indeed, specific recommendations on the appropriate use of CTPA in the diagnostic pathway of PE in COVID-19 are still lacking [29]. Our results showed age-dependent differences regarding the use of CTPA in COVID-19 patients, with less frequent use in the elderly, which may have contributed to underdiagnosis of PE in this age group (supplementary figure S1).
Several studies investigated whether the risk factors for PE in patients with COVID-19 differ from those for PE without COVID-19 infection [7, 13]. A recent meta-analysis found that among others, male sex and obesity represent risk factors for PE in COVID-19 as opposed to age and common comorbidities with no association regarding PE occurrence [30]. Our data support these previous results by finding that PE occurrence in COVID-19 infection is associated with risk factors such as male sex, obesity and cancer, but interestingly not age.
The prevalence of PE was higher in COVID-19 patients in the ICU than in those hospitalised in general wards [31, 32]. A recent meta-analysis has shown that PE is significantly associated in COVID-19 patients with mechanical ventilation and ICU admission [33]. In our analysis, results demonstrated that more than one-third (39.4%) of patients with COVID-19 and PE were treated in the ICU as opposed to 14.9% of COVID-19 patients without PE. The frequent coexistence of COVID-19 and PE in critically ill patients may reflect the high burden of thromboembolic complications in those patients. It can also be assumed that in COVID-19 patients who die early after admission or who cannot be transported to the radiology department for contrast CT angiography due to instability or limited resources during a wave of the pandemic, PE might be underdiagnosed as the cause of death. As expected, the manifestation of the respiratory infection was a strong predictor of in-hospital case fatality in our cohort, since pneumonia and ARDS were both independently associated with an unfavourable course of illness. In particular, COVID-19 patients with multisystem inflammatory syndrome (MSCOVID) had a 24-fold increased risk for in-hospital mortality, indicating that a cytokine storm could further trigger the coagulation cascade and predispose to immunothrombosis [34]. In this context, transfer of the sickest patients to larger tertiary hospitals in urban areas for escalation of intensive treatment (including mechanical ventilation) may explain the higher case fatality rate compared with the suburban and rural medical supply.
Several studies reported a substantial increase in the number of all-cause and cardiovascular mortalities during the COVID-19 pandemic [35, 36]. COVID-19 itself was the main cause of death or a concomitant cause in 90% of excess deaths [6]. In this context, numbers of PE-related deaths also increased during the pandemic in several countries [6, 37, 38]. Our findings from Germany revealed no increase in the prevalence of PE between 2019 and 2020, but we did observe a slightly higher case fatality for PE in 2020 with seasonal characteristics following the pandemic waves; this is opposed to reports from Italy and France, but in line with nationwide data from Denmark [6, 37, 39]. However, considering PE patients with COVID-19 infection, case fatality was dramatically increased compared with PE patients without COVID-19 infection. Comparable results were found in Denmark, but were in contrast to findings from Spain, which found no difference between the groups [18]. Those differences might be explained by the larger, unselected dataset of nationwide data in Denmark and in our study [37]. In general, the higher rate of case fatality in PE patients with COVID-19 has to be considered in the context of the haemodynamic effect of systemic inflammation. Those systemic effects are also reflected in our results, by showing a higher rate of shock, need for cardiopulmonary resuscitation and mechanical ventilation in PE patients with COVID-19 compared with patients without COVID-19. Overall, COVID-19 was associated with a 3.1-fold increased risk for an adverse outcome in patients with PE, which underlines the importance to pay particular attention for this special patient population and to establish optimal antithrombotic strategies that may minimise the risk of thromboembolic events in COVID-19 patients.
Although this study includes data collected on a national level, with almost 200 000 adult patients of all ages hospitalised with PE and more than 180 000 hospitalised with COVID-19, we recognise that it has several limitations. First, as our results are based on administrative and retrospective data, we cannot exclude misclassification or inconsistencies. Additionally, this analysis of the German nationwide inpatient sample was not pre-specified; therefore, our findings can only be considered to be hypothesis generating. Second, patients with confirmed COVID-19 infection who died out of hospital or were diagnosed post-mortem were not included in the nationwide inpatient sample. Third, the nationwide inpatient sample does not report long-term outcomes after discharge from hospital. Fourth, changes in treatment recommendations such as prophylactic or even therapeutic doses as well as anti-inflammatory regimens were not considered in this analysis due to missing codes in the nationwide sample.
Our findings demonstrated a considerable impact of COVID-19 infection on adverse outcomes of patients with PE in 2020, which should guide our attention to this special population with regard to antithrombotic prevention and diagnostic strategies. However, a roughly constant but high case fatality in patients hospitalised with PE was found in both 2019 and 2020.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00619-2022.Supplement (379.7KB, pdf)
Shareable PDF
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.02447-2022
Conflict of interest: L. Hobohm reports lecture/consultant fees from MSD and Actelion, outside the submitted work. I. Sagoschen reports lecture/consultant fees from Hamilton Medical and Novalung, outside the submitted work. S. Barco reports lecture/consultant fees from Bayer HealthCare, Concept Medical, BTG Pharmaceuticals, INARI, Boston Scientific and LeoPharma; institutional grants from Boston Scientific, Bentley, Bayer HealthCare, INARI, Medtronic, Concept Medical, Bard and Sanofi; and financial support for travel/congress costs from Daiichi Sankyo, BTG Pharmaceuticals and Bayer HealthCare, outside the submitted work. I.T. Farmakis reports no conflict of interests. U. Fedeli reports no conflict of interests. S. Koelmel reports no conflict of interests. T. Gori reports consultancy and lecture honoraria from Abbott Vascular and Boston Scientific. C. Espinola-Klein reports lecture fees and/or travel costs from Amarin Germany, Amgen GmbH, Bayer Vital, Boehringer Ingelheim, BMS, Daiichi Sankyo, Leo Pharma, MSD, Novartis Pharma, Pfizer Pharma GmbH and Sanofi-Aventis GmbH. T. Münzel is PI of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine Main, Mainz, Germany. S. Konstantinides reports institutional grants and personal lecture/advisory fees from Bayer AG, Daiichi Sankyo, Boehringer Ingelheim and Boston Scientific; institutional grants from Inari Medical and Actelion; and personal lecture/advisory fees from MSD and BMS/Pfizer. K. Keller reports no conflict of interests.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins University . Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu Date last accessed: 31 March 2022.
- 4.Hobohm L, Sagoschen I, Barco S, et al. . Trends and risk factors of in-hospital mortality of patients with COVID-19 in Germany: results of a large nationwide inpatient sample. Viruses 2022; 14: 275. doi: 10.3390/v14020275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Tacquard C, Severac F, et al. . High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–1098. doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voci D, Fedeli U, Farmakis IT, et al. . Deaths related to pulmonary embolism and cardiovascular events before and during the 2020 COVID-19 pandemic: an epidemiological analysis of data from an Italian high-risk area. Thromb Res 2022; 212: 44–50. doi: 10.1016/j.thromres.2022.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauvel C, Weizman O, Trimaille A, et al. . Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020; 41: 3058–3068. doi: 10.1093/eurheartj/ehaa500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dam LF, Kroft LJM, van der Wal LI, et al. . Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res 2020; 193: 86–89. doi: 10.1016/j.thromres.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middleton EA, He XY, Denorme F, et al. . Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020; 136: 1169–1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinides SV, Meyer G. The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2019; 40: 3453–3455. doi: 10.1093/eurheartj/ehz726 [DOI] [PubMed] [Google Scholar]
- 11.Benito N, Filella D, Mateo J, et al. . Pulmonary thrombosis or embolism in a large cohort of hospitalized patients with Covid-19. Front Med 2020; 7: 557. doi: 10.3389/fmed.2020.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. . Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020; 324: 799–801. doi: 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bompard F, Monnier H, Saab I, et al. . Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 2020; 56: 2001365. doi: 10.1183/13993003.01365-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceriani E, Combescure C, Le Gal G, et al. . Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost 2010; 8: 957–970. [DOI] [PubMed] [Google Scholar]
- 15.Reinohl J, Kaier K, Reinecke H, et al. . Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med 2015; 373: 2438–2447. doi: 10.1056/NEJMoa1500893 [DOI] [PubMed] [Google Scholar]
- 16.Keller K, Hobohm L, Ebner M, et al. . Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J 2020; 41: 522–529. doi: 10.1093/eurheartj/ehz236 [DOI] [PubMed] [Google Scholar]
- 17.Pellicori P, Doolub G, Wong CM, et al. . COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev 2021; 3: CD013879. doi: 10.1002/14651858.CD013879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miro O, Jimenez S, Mebazaa A, et al. . Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur Heart J 2021; 42: 3127–3142. doi: 10.1093/eurheartj/ehab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contou D, Pajot O, Cally R, et al. . Pulmonary embolism or thrombosis in ARDS COVID-19 patients: a French monocenter retrospective study. PLoS One 2020; 15: e0238413. doi: 10.1371/journal.pone.0238413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan BK, Mainbourg S, Friggeri A, et al. . Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax 2021; 76: 970–979. doi: 10.1136/thoraxjnl-2020-215383 [DOI] [PubMed] [Google Scholar]
- 21.Barco S, Mahmoudpour SH, Valerio L, et al. . Trends in mortality related to pulmonary embolism in the European Region, 2000–15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med 2020: 8; 277–287. doi: 10.1016/S2213-2600(19)30354-6 [DOI] [PubMed] [Google Scholar]
- 22.Barco S, Valerio L, Ageno W, et al. . Age-sex specific pulmonary embolism-related mortality in the USA and Canada, 2000–18: an analysis of the WHO Mortality Database and of the CDC Multiple Cause of Death database. Lancet Respir Med 2021; 9: 33–42. doi: 10.1016/S2213-2600(20)30417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermann M, Verleden SE, Kuehnel M, et al. . Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suh YJ, Hong H, Ohana M, et al. . Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology 2021; 298: E70–E80. doi: 10.1148/radiol.2020203557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R, Ni L, Di X, et al. . Systematic review and meta-analysis of the prevalence of venous thromboembolic events in novel coronavirus disease-2019 patients. J Vasc Surg Venous Lymphat Disord 2021; 9: 289–298. doi: 10.1016/j.jvsv.2020.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boonyawat K, Chantrathammachart P, Numthavaj P, et al. . Incidence of thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Thromb J 2020; 18: 34. doi: 10.1186/s12959-020-00248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malas MB, Naazie IN, Elsayed N, et al. . Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 2020; 29: 100639. doi: 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porfidia A, Valeriani E, Pola R, et al. . Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res 2020; 196: 67–74. doi: 10.1016/j.thromres.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porfidia A, Talerico R, Mosoni C, et al. . CT pulmonary angiography for the diagnosis of pulmonary embolism in patients with COVID-19: when, why, and for who? Radiology 2021; 299: E287. doi: 10.1148/radiol.2021210400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui LY, Cheng WW, Mou ZW, et al. . Risk factors for pulmonary embolism in patients with COVID-19: a systemic review and meta-analysis. Int J Infect Dis 2021; 111: 154–163. doi: 10.1016/j.ijid.2021.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roncon L, Zuin M, Barco S, et al. . Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med 2020; 82: 29–37. doi: 10.1016/j.ejim.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiezia L, Boscolo A, Poletto F, et al. . COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 2020; 120: 998–1000. doi: 10.1055/s-0040-1710018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez CA, Sun CK, Tsai IT, et al. . Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: a systematic review and meta-analysis. Sci Rep 2021; 11: 16025. doi: 10.1038/s41598-021-95512-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGonagle D, O'Donnell JS, Sharif K, et al. . Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020; 2: e437–e445. doi: 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam N, Shkolnikov VM, Acosta RJ, et al. . Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ 2021; 373: n1137. doi: 10.1136/bmj.n1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilinski A, Emanuel EJ. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA 2020; 324: 2100–2102. doi: 10.1001/jama.2020.20717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tankere P, Cottenet J, Tubert-Bitter P, et al. . Impact of COVID-19 and lockdowns on pulmonary embolism in hospitalized patients in France: a nationwide study. Respir Res 2021; 22: 298. doi: 10.1186/s12931-021-01887-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aktaa S, Wu J, Nadarajah R, et al. . Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: multi-sourced population-based health records cohort study. Thromb Res 2021; 202: 17–23. doi: 10.1016/j.thromres.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sindet-Pedersen C, Olesen JB, Blanche P, et al. . Effect of government interventions to contain the COVID-19 pandemic on incidence of pulmonary embolism – a Danish nationwide register-based cohort study. Thromb Res 2021; 199: 97–100. doi: 10.1016/j.thromres.2020.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00619-2022.Supplement (379.7KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00619-2022.Shareable (485.9KB, pdf)