Abstract
Background
Brolucizumab has recently been approved in Europe as a novel treatment for patients with neovascular age-related macular degeneration (nAMD). We report on early experiences with real-world outcomes of switch to brolucizumab therapy in previously anti-vascular endothelial growth factor (anti-VEGF)-treated patients.
Methods
Patients with recalcitrant nAMD were switched to brolucizumab therapy. Functional and structural parameters 4 weeks after first brolucizumab injection were evaluated including best-corrected visual acuity (BCVA (logMAR)), foveal centre point (FCP (µm)), central subfield retinal thickness (CSRT (µm)) and macular volume (mm³).
Results
Sixty-three eyes of 57 patients with nAMD (52.6% females) with a mean (±SD) age of 79.5±6.7 years were included. Mean change of BCVA was 0.03±0.14 logMAR (p=0.115). Significant reductions were recorded for FCP with a mean (±SD) change of −66.81±72.63 µm, −66.76±60.71 µm for CSRT and −0.27±0.24 mm³ for macular volume (all p<0.001). Intraocular inflammation was observed in seven eyes of seven patients, including one case of retinal vasculitis.
Conclusions
The results of the SHIFT study indicate that switch to brolucizumab may represent a treatment option in patients with nAMD poorly responsive to other anti-VEGF agents. Further long-term analyses appear prudent to assess efficacy and safety of brolucizumab in a routine clinical setting.
Keywords: Brolucizumab, drugs, imaging, macula, neovascularisation
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the elderly in industrialised countries.1 With the advent of anti-vascular endothelial growth factor (VEGF) therapy, the visual outcome of patients with neovascular AMD (nAMD) has been improved and measurable reductions of legal blindness incidence have emerged.2 3
Besides the anticipated worldwide increase of AMD prevalence due to demographical changes with longer life expectancy, the burden for both patients and caregivers is high when managing patients with repetitive intravitreal injections and monitoring visits over a long period of time in a chronic disease. Various real-world studies have shown visual outcomes to be inferior compared with the results from prospective randomised clinical trials (RCTs).4 Undertreatment is one of the major factors in part driven by non-adherence. In addition, some patients and certain subphenotypes of nAMD do not respond favourably.5–12 Therefore, more efficacious agents with longer durability represent an important unmet need.
Brolucizumab (Novartis), a single-chain antibody fragment, was recently approved for the treatment of nAMD in October 2019 and in February 2020 by the regulatory agencies in the USA and the European Union, respectively, as well as in other countries.13 Potential benefits of brolucizumab are assumed to be related to its low molecular weight with subsequent better tissue penetration as well as higher molar concentration.13–15 Two pivotal trials have recently shown non-inferiority of brolucizumab to the comparator aflibercept with regard to visual outcome.13 Post hoc analyses demonstrated overall favourable anatomical effects.16–18 However, safety signals have been reported in both RCTs and postmarketing reports, which included the occurrence of intraocular inflammation (IOI) and retinal vasculitis with or without occlusion.13 18–25
The aim of the SHIFT study was to report early real-world experiences in a single European clinical centre of brolucizumab treatment for nAMD with regard to both functional and anatomical disease control as well as adverse effects following approval in February 2020 in Europe.
Methods
The SHIFT study is a retrospective, observational, monocentre study of patients with exudative AMD who received 6 mg brolucizumab intravitreal therapy between 16 March 2020 and 15 October 2020, at the Department of Ophthalmology, University of Bonn, Germany, in routine clinical care. All patients were previously treated repetitively because of recalcitrant fluid accumulations on optical coherence tomography (OCT) despite frequent dosing with other anti-VEGF agents, including ranibizumab, aflibercept and bevacizumab. Recalcitrant fluid was defined as persistent fluid accumulations despite a high frequency of intravitreal injections of other anti-VEGF agents over a longer period of time prior to the switch to brolucizumab. The day of the first intravitreal brolucizumab injection was regarded as the baseline visit.
At each visit, best-corrected visual acuity (BCVA) determination and complete ophthalmic examination, including slit-lamp examination and funduscopy following pupil dilation, was performed. Signs of IOI and/or retinal vasculitis were recorded if present. Retinal imaging was performed at each visit with combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography (SD-OCT) (Spectralis HRA2+OCT, Heidelberg Engineering, Heidelberg, Germany) with acquisition of central 30°×30° near-infrared reflectance (λ=820 nm, ART (automatic real time) at least 15 frames). SD-OCT volume raster scans consisted of at least 19 B-scans (distance between neighbouring B-scans ≤240 µm) and a field size of at least 20°×15° (centred to the fovea) and included at least 5 frames (ART mode).
Assessment of structural outcomes
Morphological effects were quantified to assess structural efficacy of brolucizumab. Three parameters were determined: foveal centre point (FCP), defined as the distance (µm) between the internal limiting membrane (ILM) and Bruch’s membrane (BM); central subfield retinal thickness (CSRT), defined as the mean retinal thickness (µm) between ILM and BM of the circular area within 1 mm diameter around the centre of the fovea; as well as macular volume, defined as the mean volume of the retina in a circular area within 3 mm diameter around the fovea. For thickness and volume quantification, the automated segmentation of the Heidelberg Eye Explorer software V.2 (Heidelberg Engineering, V.2.4.1, Heidelberg, Germany) was carefully reviewed and, if indicated, manually corrected in each B-scan. Furthermore, a thorough evaluation of fluid distribution within the retina was performed with qualitative assessment of presence of subretinal, intraretinal and/or subretinal pigment epithelial (sub-RPE) fluid.
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics V.25). The baseline characteristics are summarised by mean±SD and range (minimum–maximum) for normally distributed continuous variables.
BCVA was measured in decimals and for the purpose of this analysis converted to the logarithm of the minimum angle of resolution (logMAR).26–28
Functional and structural outcomes were determined at baseline and at first visit after switch to brolucizumab (visit 1). The outcomes were differences for each functional (logMAR) and structural (FCP, CSRT and macular volume) parameter between visit 1 and baseline. Descriptive values of these outcomes are shown with mean±SD and (95% CI). Linear mixed-effects models with the change of functional or structural parameter as the response variable were used to analyse the effects of treatment with brolucizumab. To account for dependencies between measurements from the same patient, patient-specific random intercepts and slopes (analysis of longitudinal data) were included in the mixed-effects models. P values smaller than 0.05 were considered significant.
Results
Study cohort
Between 16 March 2020 and 15 October 2020, a total of 207 brolucizumab injections were performed with a mean (±SD) of 3.09±1.55 (range 1–7) brolucizumab injections per eye. Sixty-three eyes of 57 patients (30 females, 52.6%) with a mean (±SD) age of 79.5±6.7 (58–94) years were treated with at least one brolucizumab intravitreal injection (baseline) and were further assessed at the first visit after brolucizumab injection (visit 1) after a mean of 4.3±0.8 weeks (2.9–7.7). Table 1 summarises the results of descriptive analysis of the study cohort.
Table 1.
Study cohort characteristics at baseline (BSL) and visit 1 (V1)
| BSL | V1 | |||
| Per patient | n=57 | |||
| Mean±SD | Range | |||
| Age (years) (mean±SD) | 79.46±6.65 | 58–94 | ||
| N | % | |||
| Gender, female (n) (%) | 30 | 52.6 | ||
| Per eye (mean±SD) | n=63 | |||
| Mean±SD | Range | Mean±SD | Range | |
| BCVA (logMAR) | 0.39±0.28 | 0–1.2 | 0.41±0.31 | 0–1.3 |
| FCP (µm) | 363.32±133.03 | 89–826 | 296.51±113.96 | 88–738 |
| CSRT (µm) | 409.43±112.32 | 224–784 | 342.67±99.05 | 163–707 |
| Macular volume (mm³) | 2.72±0.51 | 2.07–4.63 | 2.46±0.44 | 1.9–4.31 |
BCVA, best-corrected visual acuity; CSRT, central subfield retinal thickness; FCP, foveal centre point.
During the entire observation period, patients treated with brolucizumab were seen across a mean follow-up of 16.04±6.86 (2.86–27.43) weeks. All patients had received other anti-VEGF agents before switching to brolucizumab. Treatment history comprised a mean of 32.5±25.6 (3–126) anti-VEGF injections per eye over a mean period of 4.2±3.3 (0.3–13.4) years.
Functional outcome
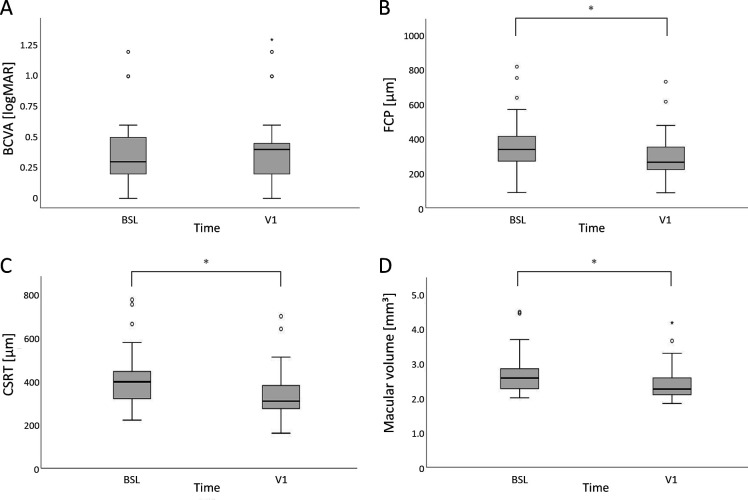
Mean BCVA was 0.39±0.28 (0–1.2) logMAR at baseline. After switch to brolucizumab, a mean change in visual acuity of 0.03±0.14 (95% CI −0.01;0.06) logMAR was detected at visit 1 (p=0.115). See table 1 for absolute values at baseline and visit 1 and table 2 for the change of functional outcome under brolucizumab treatment. A graphical outcome representation is provided in figure 1.
Table 2.
Functional and structural outcomes after switch to brolucizumab
| Outcome | Mean±SD | 95% CI | P value |
| Change BCVA (logMAR) | 0.03±0.14 | (−0.01 to 0.06) | 0.115 |
| Change FCP (µm) | −66.81±72.63 | (−85.10 to −48.52) | <0.001 |
| Change CSRT (µm) | −66.76±60.71 | (−82.05 to −51.47) | <0.001 |
| Change macular volume (mm³) | −0.27±0.24 | (−0.33 to −0.20) | <0.001 |
BCVA, best-corrected visual acuity; CSRT, central subfield retinal thickness; FCP, foveal centre point.
Figure 1.
Boxplots (each box: median; 75% and 25% quartiles; whiskers: 10% and 90% quantiles; outliers are defined as sample outliers 1.5 times (circles) and 3 times (star) the IQR above the upper quartile and below the lower quartile) at baseline (BSL) and visit 1 (V1) for (A) best-corrected visual acuity (BCVA (logMAR)), (B) focal centre point (FCP (µm)), (C) central subfield retinal thickness (CSRT (µm)) and (D) macular volume (mm³). Structural outcomes (B–D) showed a significant change after brolucizumab treatment.
Anatomical outcomes
Regarding structural quantitative parameters, mean (±SD) FCP was 363.32±133.03 (89–826) µm, mean CSRT was 409.43±112.32 (224–784) µm and mean macular volume was 2.72±0.51 (2.07–4.63) mm³ at baseline. At visit 1, mean change in FCP was −66.81±72.63 (−85.1; −48.5) µm, in CSRT −66.76±60.71 (−82.05; −51.47) µm and in macular volume −0.27±0.24 (−0.33; −0.2) mm³ (all: p<0.001). See table 1 for absolute values at baseline and visit one and table 2 for the changes of structural parameters under brolucizumab treatment. A graphical representation of the outcomes is provided in figure 1.
Assessment of qualitative SD-OCT parameters
For the following analysis, two eyes of two patients with frequent previous injections but without intraretinal, subretinal fluid or sub-RPE fluid at baseline were excluded. Sixty-one eyes (96.8%) of 55 patients presented at baseline with intraretinal and/or subretinal and/or sub-RPE fluid (eyes with subretinal, intraretinal and sub-RPE fluid: n=7; eyes with only subretinal and intraretinal fluid: n=4; eyes with subretinal and sub-RPE fluid: n=18; eyes with intraretinal and sub-RPE fluid: n=6; eyes with only subretinal fluid: n=13; eyes with only intraretinal fluid: n=12, eyes with only sub-RPE fluid: n=1) detected by SD-OCT imaging. At visit 1, absence of fluid in any retinal compartment was detected in 18 eyes (29.5%) of 18 patients.
At baseline, 27 eyes of 27 patients demonstrated (with or without fluid in any other compartment) intraretinal and 42 eyes of 38 patients demonstrated subretinal fluid, respectively. After initiation of brolucizumab treatment (visit 1), 11 eyes (40.7%) of 11 patients with intraretinal and 24 eyes (57.1%) of 21 patients with subretinal fluid at baseline showed complete resolution. Out of the 30 eyes of 27 patients demonstrating sub-RPE fluid at baseline, complete sub-RPE fluid resolution at visit 1 was detectable in only 6 eyes (20%) of 6 patients. For exemplary cases of structural response to brolucizumab treatment, see figures 2–5.
Figure 2.
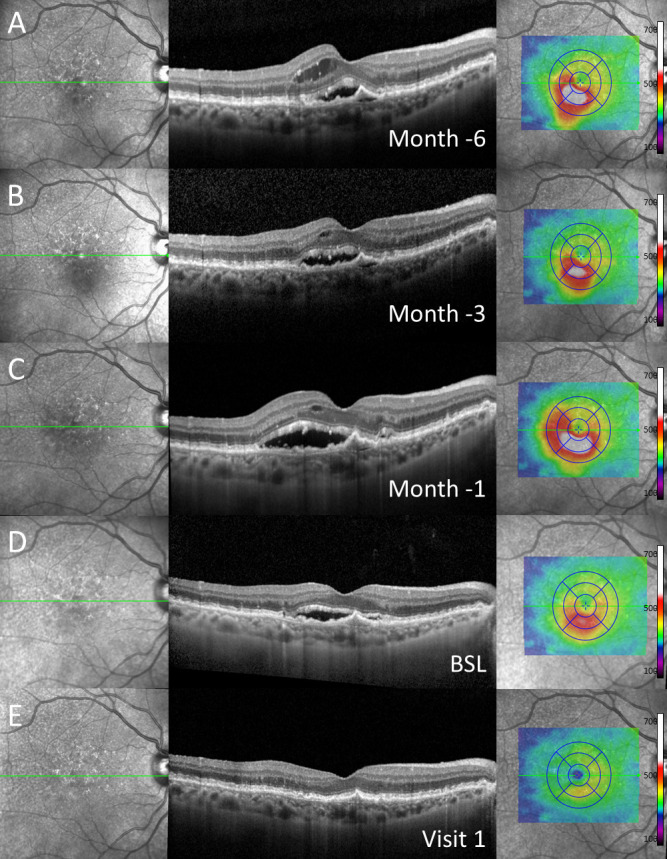
Exemplary case of a patient with subretinal fluid at baseline (BSL) (D) and both intraretinal and subretinal fluid 1 (C), 3 (B) and 6 (A) months in historical imaging before baseline despite regular anti-vascular endothelial growth factor (anti-VEGF) treatment as demonstrated in (from left to right) near-infrared imaging, spectral-domain optical coherence tomography through the fovea and colour-coded two-dimensional thickness map for total retinal thickness. One month after switch to brolucizumab (E, visit 1), complete resolution of subretinal fluid was detected. Note: Before switch to brolucizumab, the patient received repetitive, high-frequency intravitreal injections of other anti-VEGF agents over a longer period of time.
Figure 3.
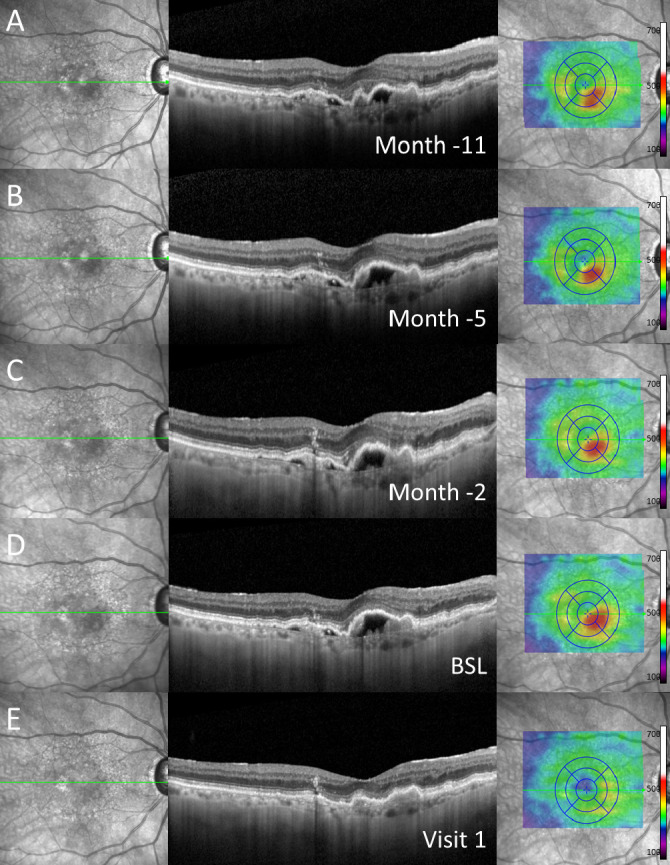
Exemplary case of a patient with subretinal pigment epithelial (sub-RPE) fluid at baseline (BSL) (D) and in historical imaging up to 11 months (A–C) before switch to brolucizumab as demonstrated in (from left to right) near-infrared imaging, spectral-domain optical coherence tomography through the fovea and colour-coded two-dimensional thickness map for total retinal thickness. At visit 1 (E), complete resolution of sub-RPE fluid was detected. Note: Before switch to brolucizumab, the patient received repetitive, high-frequency intravitreal injections of other anti-vascular endothelial growth factor agents over a longer period of time.
Figure 4.
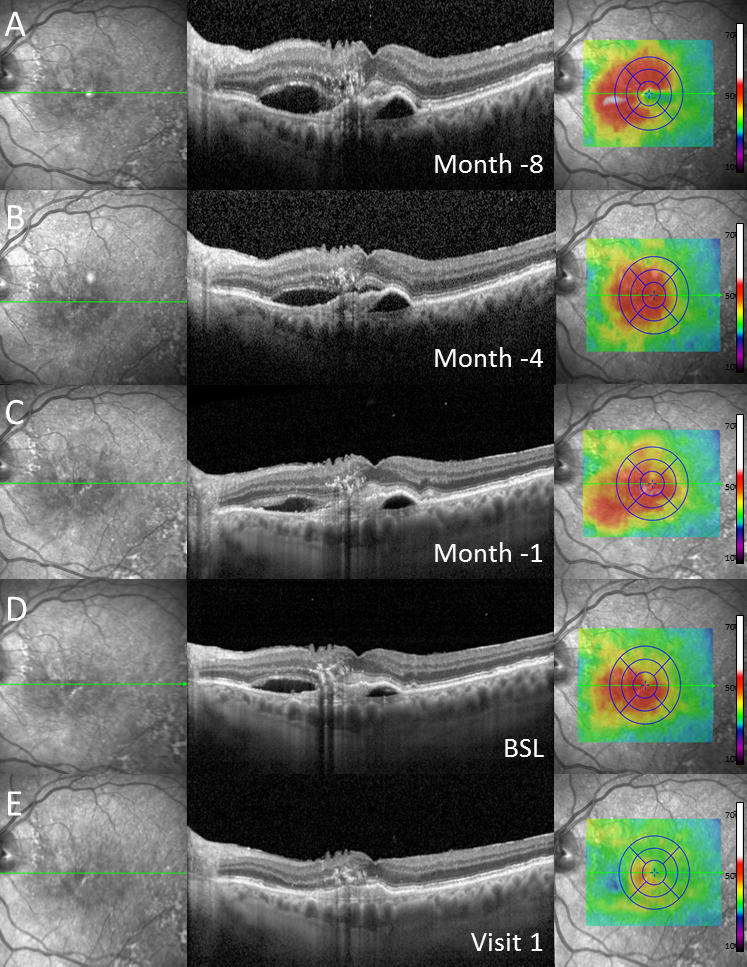
Exemplary case of a patient with subretinal pigment epithelial (sub-RPE) and subretinal fluid at baseline (BSL) (D) as well as in historical imaging up to 8 months (A–C) before switch to brolucizumab as demonstrated in (from left to right) near-infrared imaging, spectral-domain optical coherence tomography through the fovea and colour-coded two-dimensional thickness map for total retinal thickness. Retinal imaging at visit 1 (E) revealed complete resolution of subretinal and sub-RPE fluid. Note: Before switch to brolucizumab, the patient received repetitive, high-frequency intravitreal injections of other anti-vascular endothelial growth factor agents over a longer period of time.
Figure 5.
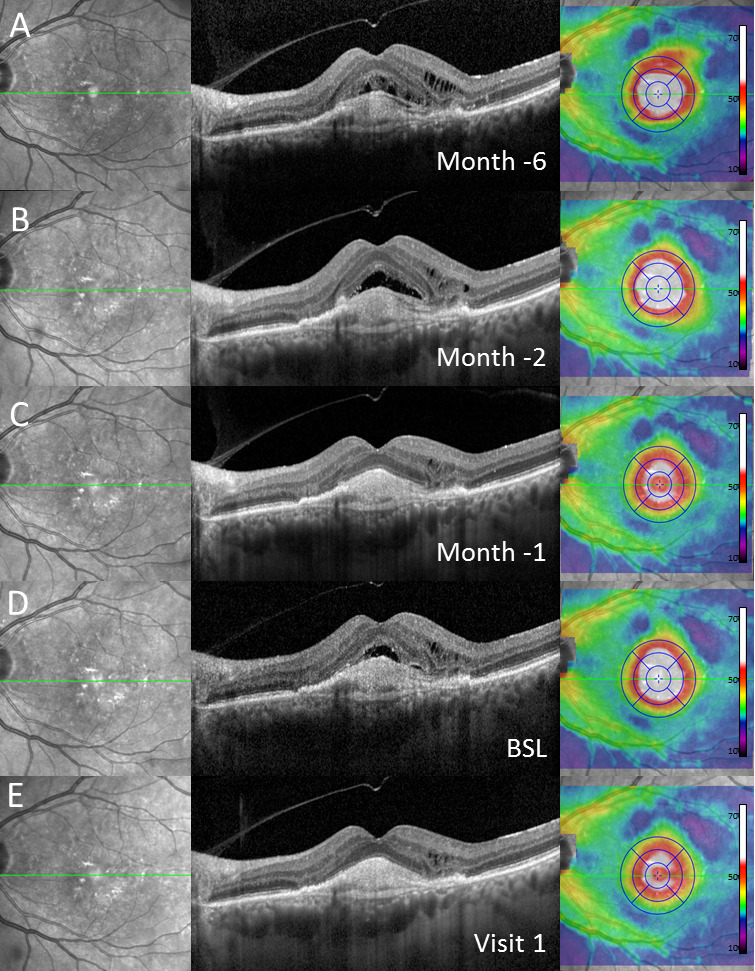
Exemplary case of a patient with intraretinal and subretinal fluid at baseline (BSL) (D) and in historical images 1 (C), 2 (B) and 6 (A) months before switch to brolucizumab as demonstrated in (from left to right) near-infrared imaging, spectral-domain optical coherence tomography through the fovea and colour-coded two-dimensional thickness map for total retinal thickness. One month after switch (E, visit 1), complete resolution of subretinal and incomplete resolution of intraretinal fluid was demonstrated. Note: Before switch to brolucizumab, the patient received repetitive, high-frequency intravitreal injections of other anti-vascular endothelial growth factor agents over a longer period of time.
Adverse events
During the observation period, adverse events were reported in eight eyes (12.7%) of eight patients. In one patient, a vitreous haemorrhage occurred immediately following the intravitreal injection, which was considered as a procedure-related and not drug-related event. Vitreous haemorrhage resolved spontaneously and required no further therapeutic intervention.
Out of the 63 eyes of 57 patients treated with brolucizumab, 7 eyes (11.1%) of 7 patients (3.38% per 207 given injections) with a mean age of 80.7±9.1 years (4 females, 57.1%) developed various degrees of IOI, which was suspected to be drug related. Out of the seven eyes with IOI, anterior uveitis with anterior chamber cells only was noted in two patients. Four eyes had additional signs of intermediate uveitis with occurrence of anterior and vitreous cells. In one patient, segmental periarteriolar sheathing was noted without concurrent retinal vascular occlusion (figure 6). Due to IOI development, four patients sought medical attention at an unscheduled visit, while three patients presented at a regularly scheduled visit. Once signs of IOI development were detected, treatment with brolucizumab for nAMD was discontinued and patients were switched back to an alternate anti-VEGF drug.
Figure 6.
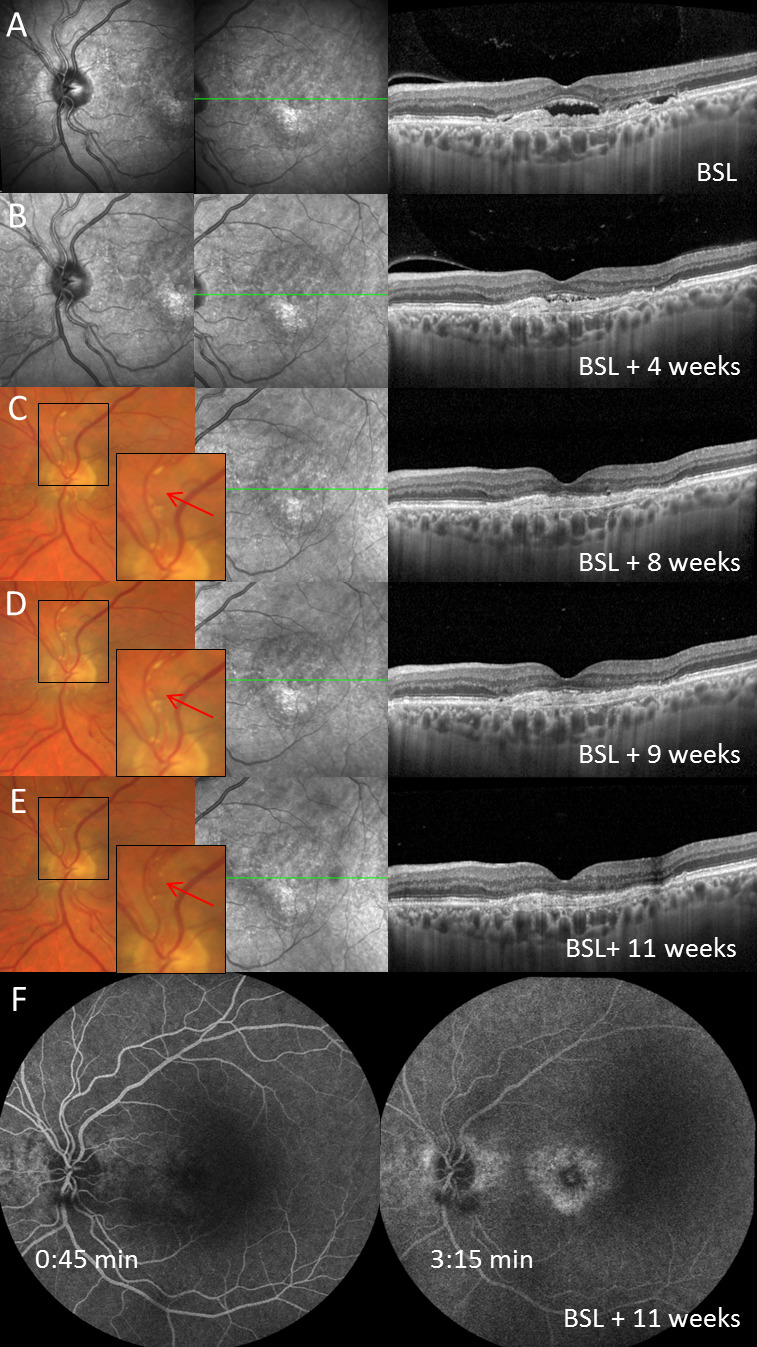
Patient with first brolucizumab injection at baseline (BSL, row A), first follow-up visit (visit 1) and second brolucizumab injection 4 weeks later (row B) and development of non-occlusive retinal vasculitis with segmental perivascular sheathing 4 weeks following the second brolucizumab injection (row C). Further follow-up visits (row D–F) as presented by multimodal imaging (from left to right: near-infrared imaging (row A–C) or digital colour fundus photography (row D and E) of the optic nerve head, near-infrared imaging of the macula and spectral-domain optical coherence tomography (SD-OCT) through the fovea). The intra-arterial plaque secondary to retinal vasculitis and its disappearance after treatment initiation is marked by a red arrow in row C, D and E. Seven weeks after first diagnosis of retinal vasculitis, fluorescein angiography did not show signs of vascular occlusion or retinal ischaemia (row F). Of note, subretinal fluid completely resolved after two intravitreal injections of brolucizumab (BSL and V1) in SD-OCT imaging.
Regarding the time of IOI development, four patients developed signs of IOI after the first brolucizumab injection with occurrence of first symptoms after a mean of 19.0±6.5 (12–25) days. Medical attention was sought after a mean of 23.8±6.4 (13–29) days after injection. In the three patients with IOI development after the second brolucizumab injection, first symptoms occurred after a mean of 7.3±5.6 (2–15) days. Medical advice was sought after 8.7±5.2 (5–16) days. During the observation period, no patient receiving at least three brolucizumab injections developed any signs of IOI.
Initial symptoms differed between the patients with IOI development. Eye redness as the initial symptom was reported by four patients, while three reported floaters and/or cloudy vision. One patient reported a decrease in visual acuity. None of the patients with documented adverse events reported earlier episodes of uveitis or ocular autoimmune disease, except for one patient who had a history of bilateral macular oedema interpreted as Irvine-Gass syndrome following routine cataract surgery with intraocular lens implantation.
Treatment was initiated promptly according to the severity of the documented IOI. In cases with only mild IOI and without signs of posterior uveitis or vasculitis, topical therapy with corticosteroid eye drops (dexamethasone or prednisolone) was initiated with application ranging from four times a day up to every hour and ointment (prednisolone) before hours of sleep. In four patients, topical therapy was supplemented by a subconjunctival injection of dexamethasone (8 mg). In the single patients who presented with vasculitis and in three patients with more severe IOI, additional systemic therapy with corticosteroids was initiated in a body weight adapted dose (ie, 60 or 80 mg) and subsequently tapered off according to IOI activity. None of the seven patients demonstrated persistent clinical relevant change in visual acuity once IOI had ceased.
Discussion
Efficacious therapy with maintaining visual acuity gains during the upload phase in the long-term management of patients with chronic nAMD still represents an unmet need. Recently, brolucizumab was approved as a new anti-VEGF agent for intravitreal administration in patients with nAMD. However, only limited data of its use in a real-world setting are available to date.16 17 20–22
Herein, we report early experiences with brolucizumab use in clinical routine in a single centre in Europe following approval in February 2020 (SHIFT study). Our findings indicate that initiation of intravitreal brolucizumab therapy in previously treated patients with nAMD (switch) may be an option in particular with regard to the morphological effects in recalcitrant cases who previously received multiple anti-VEGF injections without satisfactory resolution of fluid in various anatomical compartments. A significant reduction on average of retinal thickness parameters, including FCP, CSRT and macular volume, was observed demonstrating a favourable response on morphological signs for disease activity. Although differences in study design and analysis methods limit a direct comparison, our findings mirror a recent real-world report by Sharma et al, who also showed improvement in structural outcomes in 42 eyes of 42 patients with nAMD over a mean (±SD) observational period of 7.2±3.6 weeks.16 In accordance with our study, Avaylon and colleagues have also found beneficial structural outcomes at first visit following switch to brolucizumab.17 Although the analysis of this group is based on a rather small cohort of only six patients, our results confirm the observations by Avaylon et al of improvement of various OCT characteristics.
When interpreting the functional outcome in our study, it needs to be considered that the SHIFT cohort comprises previously treated patients only, with up to 126 previous intravitreal anti-VEGF injections (with a mean (±SD) of 32.5±25.6). This might not only impact the BCVA before initial injection of brolucizumab of 0.39±0.28 logMAR but may also explain the lack of BCVA improvement despite significant change in structurally defined efficacy outcome parameters. This phenomenon has been observed before in other switch studies. Given the longstanding ocular history of exudative AMD in our cohort, underlying structural, in part irreversible damage may contribute to a limited potential of visual recovery.29–32 The phenomenon of disconnect between morphology and function has been observed in other switch studies of patients with nAMD.29–32
Since the approval of brolucizumab, safety signals of development of IOI following brolucizumab treatment, ranging from anterior chamber cells to retinal vasculitis with or without occlusion and with or without moderate and severe visual loss, have been reported.13 19 21 23 33 The pathogenesis of the inflammatory events is not yet understood and needs further investigations which are ongoing. In the pivotal phase-III HAWK and HARRIER studies of treatment-naïve nAMD patients, an overall rate of 4.6% of any IOI was reported following a review by a safety review committee (SRC).24 The rate of the development of retinal vasculitis was reported to be 3.3% (36/1088) and that of concomitant retinal vasculitis and retinal vascular occlusion was 2.1% (23/1088), respectively.13 The overall incidence of at least moderate vision loss due to IOI was <1%. Recently, the American Society of Retina Specialists referred to post approval, reported IOI cases that the majority (88%) was of female gender in accordance with other published reports of brolucizumab cases.18 20–22 In line with these previous reports, IOI occurred in our small cohort more frequently in female patients, while the case of retinal arteriolar vasculitis was detected in a male patient who showed a favourable outcome in the absence of retinal vascular occlusion and resolution of the intraocular inflammation following steroid therapy.
In the patients reported here, anti-inflammatory treatment was initiated promptly following detection of IOI and included topical, subconjunctival and systemic corticosteroid therapy. In none of the patients functional deficits occurred following IOI resolution. In the meantime, recommendations have been published on the management of patients with nAMD who develop IOI following brolucizumab treatment.25
Various limitations need to be considered for this study. This is an observational study in a relatively small cohort of 57 patients. An important limitation of our study is the short review period post switch to brolucizumab of 1 month. In this real-world study, we applied a treat & extend scheme immediately after the first injection and did not perform three fixed 4-weekly injections post switch to brolucizumab as proposed in other switch studies. Therefore, in this relatively small cohort, data analyses beyond 1 month would be based on a variable number of injections after switch and would not allow a meaningful analysis and comparison between patients. Accordingly, the same applies to an analysis of the treatment history before switch to brolucizumab. Furthermore, no treatment-naïve patients were investigated compromising any overall conclusion on structural and visual outcome following treatment. Finally, the study cannot address the questions of potentially longer durability of brolucizumab compared with other anti-VEGF agents in particular during a treat & extend regimen. To determine this, both longitudinal assessments in real-world settings and randomised clinical nAMD trials are warrant.
In summary, the SHIFT study reports real-world early experiences outside RCTs with brolucizumab in previously anti-VEGF-treated patients with nAMD. A beneficial morphological effect was recorded pointing towards a strong antihyperpermeability effect of this agent. Safety issues with regard to IOI remain a concern and require further investigations with regard to the underlying mechanisms as well as risk mitigation measures.
Footnotes
ST and FGH contributed equally.
Correction notice: This paper has been corrected since it was published online first.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: Non-financial support from Heidelberg Engineering to ST, LB, RL, MS and FGH; from CenterVue to ST and FGH; from Optos to ST, LB, RL, MS and FGH; from Carl Zeiss Meditec to ST, LB, RF, FGH and MS. Grant and personal fees from Allergan, Novartis, Bayer and Heidelberg Engineering to ST and FGH; from Apellis, Carl Zeiss Meditec, Acucela, Genentech/Roche, Boehringer-Ingelheim, LIN Bioscience, Pixium, Kanghong, Oxurion, Grayburg Vision, Stealth BioTherapeutics, Geuder and Iveric Bio to FGH. JN: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to this study are included in the article.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study does not contain personal information from any identifiable individual. Because this study is a retrospective analysis of data obtained in clinical routine care at an academic university setting, no ethics approval is required.
References
- 1. Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet 2012;379:1728–38. 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 3. Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol 2012;153:209–13. 10.1016/j.ajo.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 4. Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:220–6. 10.1136/bjophthalmol-2014-305327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eghøj MS, Sørensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol 2012;96:21–3. 10.1136/bjo.2011.203893 [DOI] [PubMed] [Google Scholar]
- 6. Forooghian F, Chew EY, Meyerle CB, et al. Investigation of the role of neutralizing antibodies against bevacizumab as mediators of tachyphylaxis. Acta Ophthalmol 2011;89:e206–7. 10.1111/j.1755-3768.2009.01773.x [DOI] [PubMed] [Google Scholar]
- 7. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal 2014;2:e106–16. 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 8. Schmidt-Erfurth U, Chong V, Loewenstein A, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of retina specialists (EURETINA). Br J Ophthalmol 2014;98:1144–67. 10.1136/bjophthalmol-2014-305702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol 2015;38:620–7. 10.1016/j.jfo.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 10. Ehlken C, Helms M, Böhringer D, et al. Association of treatment adherence with real-life Va outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol 2018;12:13–20. 10.2147/OPTH.S151611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang S, Zhao J, Sun X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Devel Ther 2016;10:1857–67. 10.2147/DDDT.S97653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol 2012;96:14–20. 10.1136/bjo.2011.204685 [DOI] [PubMed] [Google Scholar]
- 13. Dugel PU, Singh RP, Koh A, et al. Hawk and harrier: Ninety-Six-Week outcomes from the phase 3 trials of Brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2021;128:30570–4. 10.1016/j.ophtha.2020.06.028 [DOI] [PubMed] [Google Scholar]
- 14. Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology 2017;124:1296–304. 10.1016/j.ophtha.2017.03.057 [DOI] [PubMed] [Google Scholar]
- 15. Holz FG, Dugel PU, Weissgerber G, et al. Single-Chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration: a randomized controlled study. Ophthalmology 2016;123:1080–9. 10.1016/j.ophtha.2015.12.030 [DOI] [PubMed] [Google Scholar]
- 16. Sharma A, Kumar N, Parachuri N, et al. Brolucizumab-early real-world experience: brew study. Eye 2021;35:1045–7. 10.1038/s41433-020-1111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avaylon J, Lee S, Gallemore RP. Case series on initial responses to intravitreal brolucizumab in patients with recalcitrant chronic wet age-related macular degeneration. Int Med Case Rep J 2020;13:145–52. 10.2147/IMCRJ.S252260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal Brolucizumab. J Vitreoretin Dis 2020;4:269–79. 10.1177/2474126420930863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brolucizumab Safety information - Post-marketing data. Available: https://www.brolucizumab.info/post-marketing-data [Accessed 16 Nov 2020].
- 20. Jain A, Chea S, Matsumiya W, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep 2020;18:100687. 10.1016/j.ajoc.2020.100687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of Brolucizumab. Ophthalmology 2020;127:1345–59. 10.1016/j.ophtha.2020.04.017 [DOI] [PubMed] [Google Scholar]
- 22. Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep 2020;18:100680. 10.1016/j.ajoc.2020.100680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma A, Kumar N, Parachuri N. Brolucizumab-Related retinal vasculitis: emerging disconnect between clinical trials and real world. Springer us 2020. [DOI] [PMC free article] [PubMed]
- 24. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal Occlusion-Related events with Brolucizumab: post hoc review of hawk and harrier. Ophthalmology 2020. 10.1016/j.ophtha.2020.11.011. [Epub ahead of print: 15 Nov 2020]. [DOI] [PubMed] [Google Scholar]
- 25. Holz FG, Heinz C, Wolf A. Intraocular inflammation with brolucizumab use: patient management, diagnosis, therapy. Ophthalmologe 2021;118:1–3. 10.1007/s00347-021-01321-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khoshnood B, Mesbah M, Jeanbat V, et al. Transforming scales of measurement of visual acuity at the group level. Ophthalmic Physiol Opt 2010;30:816–23. 10.1111/j.1475-1313.2010.00766.x [DOI] [PubMed] [Google Scholar]
- 27. Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol 2003;135:194–205. 10.1016/s0002-9394(02)01825-1 [DOI] [PubMed] [Google Scholar]
- 28. Kniestedt C, Stamper RL. Visual acuity and its measurement. Ophthalmol Clin North Am 2003;16:155–70. 10.1016/S0896-1549(03)00013-0 [DOI] [PubMed] [Google Scholar]
- 29. Gale RP, Pearce I, Eter N, et al. Anatomical and functional outcomes following switching from aflibercept to ranibizumab in neovascular age-related macular degeneration in Europe: SAFARI study. Br J Ophthalmol 2020;104:493–9. 10.1136/bjophthalmol-2019-314251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mantel I, Gillies MC, Souied EH. Switching between ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration. Surv Ophthalmol 2018;63:638–45. 10.1016/j.survophthal.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 31. García-Layana A, Figueroa MS, Araiz J, et al. Treatment of exudative age-related macular degeneration: focus on aflibercept. Drugs Aging 2015;32:797–807. 10.1007/s40266-015-0300-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ho VY, Yeh S, Olsen TW, et al. Short-Term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol 2013;156:23–8. 10.1016/j.ajo.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Study of safety and efficacy of Brolucizumab 6 Mg dosed every 4 weeks compared to aflibercept 2 Mg dosed every 4 weeks in patients with retinal fluid despite frequent anti-VEGF injections (merlin). Available: https://clinicaltrials.gov/ct2/show/NCT03710564 [Accessed 16 Nov 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to this study are included in the article.