Abstract
Aims
The aim of this observational study was to report the distribution of glycoprotein B (gB) genotypes in the eyes of cytomegalovirus (CMV) positive patients with Posner-Schlossman syndrome (PSS), and to investigate their clinical characteristics and outcomes.
Methods
We collected aqueous humour samples from 165 patients clinically diagnosed with PSS between 2017 and 2019. PCR was performed to analyse the CMV DNA and identify the gB genotypes in the samples. Clinical characteristics and responses to antiviral treatment were compared among patients with different gB genotypes.
Results
CMV DNA was detected in 94 (56.97%) of the 165 aqueous humour specimens analysed. Owing to the quantity requirement for CMV gB genotype analysis, results could be obtained from only 14 specimens. CMV gB type 1 was detected in 11 samples (78.6%), whereas CMV gB type 3 was detected in three samples (21.4%). No other gB genotypes or mixed genotypes were detected. Overall, 9.1% (1/11) of the patients in the gB type 1 group and 66.7% (2/3) of the patients in the gB type 3 group had bilateral attacks (p=0.093). The concentration of anti-CMV immunoglobulin G (IgG) in the type 1 group was 0.94±0.79 s/co (ratio of aqueous humour CMV IgG/serum CMV IgG to aqueous humour albumin concentration/serum albumin concentration), whereas that in the type 3 group was 0.67±0.71 s/co.
Conclusion
Genotype 1 was the most prevalent genotype in the aqueous humour of CMV-infected patients with PSS. Bilateral attack was predominant among patients with gB genotype 3. CMV gB gene may be related to the pathogenicity of CMV virus strain in patients with PSS.
Keywords: glaucoma, anterior chamber, aqueous humour, treatment medical, inflammation
Introduction
The Posner-Schlossman syndrome (PSS) is clinically characterised by recurring unilateral uveitis and elevated intraocular pressure (IOP).1 A retrospective study reported that the annual incidence of PSS is about 3.91 per 100 000 persons and is relatively high in China.2 Unlike glaucoma, the IOP of patients with PSS is usually within the normal range during the non-onset period; however, PSS is prone to relapse accompanied by inflammatory responses in the anterior segment.3 Although administration of corticosteroids is a traditional method of relieving inflammation, its side effects include more frequent relapses and the risk of developing corticosteroid dependence, which may cause steroid-induced cataract and steroid-induced glaucoma over time.4 Eventually, repeated elevated IOP can damage the optic nerve and induce severe visual dysfunction. To facilitate effective treatment of PSS, its aetiology and pathophysiological mechanism need to be elucidated. The aetiology of PSS has been examined in some studies, which indicated that PSS may be associated with cytomegalovirus (CMV) infection,5–7 gene susceptibility,8 inflammatory cytokines9 10 and vascular endothelial dysfunction.11 Among these, CMV infection is considered a vital risk factor for PSS.5–7 12–17 Anti-CMV therapy has been used to treat patients with CMV-positive PSS.7 18 However, we found that in clinical practice, not all patients receive satisfactory curative effect after ganciclovir treatment, and their clinical manifestations are not always the same. The underlying causes of these irregularities were worth exploring.
CMV shows wide genetic diversity. Envelope glycoproteins play an important role in host immune response and virus replication.19 Presumably, the variability of the genes encoding these proteins contributes to the virulence of the strain. CMV glycoprotein B (gB), encoded by the UL55 gene, is the major envelope glycoprotein of CMV. It is considered to play a vital role in viral entry, viral spreading between cells, and fusion of infected cells.20 CMV genotyping based on the gB nucleotide sequence has been performed to analyse infections caused by CMV. There were four main specific sequence variations of gB, named gB genotype 1, gB genotype 2, gB genotype 3 and gB genotype 4. Genotyping of these CMV genes determines the characteristics of CMV in each disease. However, due to the rarity of PSS and the relatively low viral load of CMV in aqueous humour, little is known about the genotypic composition of CMV in patients with PSS. Differences in the genotypes of patients with PSS may account for differences in characteristics. In this study, we determined the frequency distribution of the CMV gB genotypes in the aqueous humour of patients with PSS and investigated the differences in the clinical characteristics of the patients according to their different CMV genotypes.
Materials and methods
Study design and sample collection
This observational study was approved by the Medical Ethics Committee of the Eye, Ear, Nose and Throat (EENT) Hospital of Fudan University (2017006–2), and performed in accordance with the tenets of the Declaration of Helsinki, revised in 2000. All participants provided written informed consent. The study was conducted at the EENT Hospital of Fudan University from July 2017 to May 2019.
Individuals diagnosed as PSS were enrolled. The diagnostic criteria for PSS were based on the following clinical manifestations during an attack: (1) recurrent mild inflammation in the anterior chamber; (2) characteristic keratic precipitates (KPs) and corneal oedema; (3) a history of transient elevated IOP; (4) no peripheral anterior or posterior synechiae; (5) no posterior inflammation and (6) open anterior angle. Exclusion criteria included previous antiviral treatment, intraocular surgery or penetrating ocular injury. General information on each patient, including sex, age and medical history, was collected. Glucocorticoid dependence was defined as the need for continuous use of glucocorticoids, and the aggravation or relapse of PSS-related inflammation when the dosage of the medication was reduced or the treatment was discontinued. Clinical data, including peak IOP, visual acuity, corneal endothelial cell density, KPs, Tyndall effect and vertical cup–disc ratio, were recorded. Relative corneal endothelial loss compared with the other eye was defined as the ratio of the number of corneal endothelial cells in the affected eye to that in the contralateral eye. Glaucomatous optic neuropathy is determined if any of these conditions are satisfied21: (1) vertical cup–disc ratio ≥0.7, (2) retinal nerve fibre layer defects correspond with thinning width of rim or localised notches and (3) splinter haemorrhages. Using the Lens Opacities Classification System III,22 cataract was defined as meeting any of the following conditions: (1) nuclear opalescence ≥3.0, (2) cortical cataract ≥3.0 and (3) posterior subcapsular cataract ≥2.0. When the patient is examined with a slit lamp microscope, the abnormal enhancement of the light beam through the aqueous humour to greyish white is considered a positive Tyndall sign. After topical anaesthesia was administered, a 27-gauge needle was used to extract 100–150 µL of aqueous humour from the eyes of the patients under the magnified view of a slit-lamp biomicroscope, and the samples were immediately stored at −80°C. All patients with PSS were in the acute onset stage and received antiviral treatment (2% ganciclovir eye drops four times a day) after anterior chamber paracentesis. Reducing IOP treatment and anti-inflammatory therapy were performed according to the condition. Patients were required to return to the clinic every 2 weeks for follow-up.
CMV DNA sequence analysis
Viral DNA was obtained from the sample with the QIAMP viral DNA extraction kit (Qiagen, Hilden, Germany), according to the instructions. DNA was eluted in the 100 µL buffer provided in the kit and stored at −40°C. Real-time PCR (RT-PCR) was used to detect CMV. The genes were amplified using the RT-PCR Kit (TaKaRa, Japan) and sequences were determined by sequencing the products. DNA Dynamo Sequence Analysis Software (Blue Tractor Software, Llanfairfechan, UK) and the Clustal W algorithm were used for sequence alignment and phylogenetic analysis. The clustering method used was the ‘unweighted pair group method with arithmetic mean’.23 Based on the results of other studies, the genotype pattern strains were categorised under UL55 (gB).24
Analysis of the concentration of antiviral immunoglobulin G in aqueous humour
As reported in our previous study,16 antiviral immunoglobulin (Ig) G in the aqueous humour and serum was detected using an ELISA kit (Virion/Serion, Germany). Scattering immunonephelometry was performed to examine albumin (Guosai Biotechnology Co, China). The level of CMV IgG in the aqueous humour was presented as a corrected ratio of aqueous humour CMV IgG/serum CMV IgG to aqueous humour albumin concentration/serum albumin concentration, abbreviated as s/co.
Statistical and data analysis
All analyses were performed using SPSS software, V.22. Fisher’s exact test was performed to compare the incidence of clinical features between patients infected with gB genotype 1 and genotype 3. Considering the insufficient sample size of the CMV gB3 group, descriptive statistics were mainly applied for preliminary analysis of the data.
Results
Viral analysis of the aqueous humour samples of patients with PSS
As shown in figure 1, a total of 165 aqueous humour specimens collected from patients with PSS were tested using RT-PCR analyses; 94 of the specimens were positive for CMV DNA (56.97%) and gB was detected in 14 specimens, which were tested further to ascertain the distribution of the virus subtypes.
Figure 1.
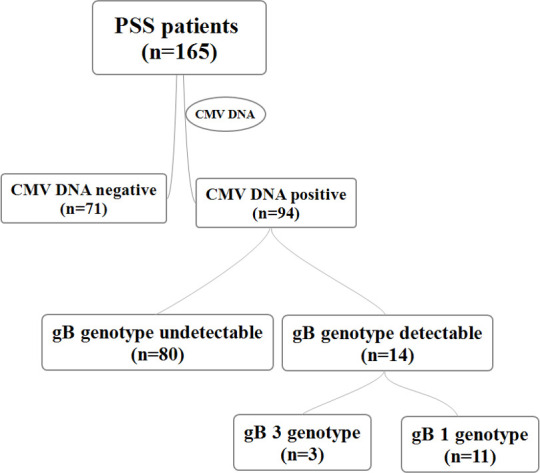
Distribution of gB genotypes among patients with the PSS. CMV, cytomegalovirus; gB, glycoprotein B; PSS, Posner-Schlossman syndrome.
Distribution of the CMV gB genotype
Phylogenetic analysis of the gB gene sequence was performed on 14 gB-positive samples according to the genotype sequence of the gB proteolytic site. CMV gB1 was detected in 11 specimens (78.6%) and CMV gB3 in three specimens (21.4%). No mixed infection with the gB1 and gB3 genotypes was detected. The concentration of anti-CMV IgG in the type 1 group and type 3 group was 0.94±0.79 s/co and 0.67±0.71 s/co, respectively.
Demographics and clinical characteristics of patients in the gB-positive group
There were more men among the 14 patients in the gB-positive group (85.7%, 12/14), as well as the gB genotype 1 (90.9%, 10/11) and gB genotype 3 (66.7%, 2/3) groups, than women. As presented in table 1, patients infected with CMV gB genotype 1 seemed to be younger at the time of the clinic visit (41.55±11.25 vs 52.33±14.19) and at the time of the first onset of disease (37±12 vs 47±17) than patients infected with CMV gB3; the former also seemed to have a shorter course of disease than the latter (4.7±2.8 vs 5.7±4.5). In the genotype 1 group, only 9.1% (1/11) of the patients had bilateral attacks, whereas 66.7% (2/3) of the patients in the genotype 3 group had bilateral attacks (p=0.093). The frequencies of onset in the genotype 1 group and genotype 3 group were 1.5±0.8 per year and 1.7±0.6 per year, respectively. Patients in the genotype 1 group seemed to have a higher dependence on glucocorticoids (63.6%, 7/11) than patients in the genotype 3 group (33.3%, 1/3).
Table 1.
Details and characteristics of CMV-infected patients with PSS
| Case | Sex | Unilateral/Bilateral | Age at the clinic visit | Age at the first onset | Frequency of attack (n/year) | Peak IOP | GC dependence | Anti-HCMV IgG (s/co) | CMV (copies/ mL) |
Response to antiviral therapy | CMV genotype |
| 1 | Male | Uni/OS | 54 | 51 | 1 | 46 | – | 1.5 | 2213 | Complete control | gB 1 |
| 2 | Male | Uni/OD | 32 | 29 | 3 | 45 | + | 0.59 | 15 311 | Poor control | gB 1 |
| 3 | Male | Uni/OD | 50 | 49 | 3 | 50 | + | 1.06 | 10 965 | Poor control | gB 1 |
| 4 | Male | Uni/OD | 42 | 38 | 1 | 50 | + | 2.39 | 28 510 | Poor control | gB 1 |
| 5 | Male | Uni/OS | 31 | 25 | 2 | 50 | – | 0.34 | 473 151 | Complete control | gB 1 |
| 6 | Male | Bi/OS | 62 | 56 | 2 | 50 | + | 0.76 | 2 585 235 | Complete control | gB 1 |
| 7 | Male | Uni/OD | 51 | 46 | 1 | 60 | – | 0.27 | 181 970 | Complete control | gB 1 |
| 8 | Female | Uni/OD | 28 | 25 | 1 | 46 | + | 0.28 | 142 889 | Complete control | gB 1 |
| 9 | Male | Uni/OS | 40 | 30 | 1 | 41 | – | 0.67 | 346 737 | Poor control | gB 1 |
| 10 | Male | Uni/OD | 31 | 22 | 1 | 48 | + | 2.29 | 307 256 | Poor control | gB 1 |
| 11 | Male | Uni/OS | 36 | 34 | 1 | 43 | + | 0.24 | 7 852 356 | Poor control | gB 1 |
| 12 | Female | Bi/OD | 37 | 31 | 1 | 45 | + | 0.27 | 407 380 | Complete control | gB 3 |
| 13 | Male | Uni/OS | 55 | 45 | 2 | 52 | – | 0.24 | 301 995 | Poor control | gB 3 |
| 14 | Male | Bi/OD | 65 | 64 | 2 | 48 | – | 1.49 | 38 238 | Poor control | gB 3 |
Bi, bilateral; CMV, cytomegalovirus; gB, glycoprotein B; GC-dependence, glucocorticoids-dependence; HCMV, human cytomegalovirus; IOP, intraocular pressure; OD, right eye; OS, left eye; PSS, Posner-Schlossman syndrome; Uni, unilateral.
The clinical characteristics of the patients according to their gB genotype distributions are shown in table 2. There was no visible difference in visual acuity (logarithm of mininal angle resolution (LogMAR)), cup–disk ratio, peak IOP or corneal endothelial cell loss (%) between groups. The rates of occurrence of the Tyndall effect in the anterior chamber (3/11, 27.3% vs 2/3, 66.7%) and iris depigmentation (8/11, 72.3% vs 3/3, 100%) were higher in the CMV type 3 group than in the CMV type 1 group. Coin-shaped KPs (shown in figure 2) were observed in 100% of patients infected with genotype 3 (3/3) and in 45.5% of patients infected with type 1 (5/11). Pigmented KPs were observed in 66.7% (2/3) of patients with type 3 infection, and in only 9.1% (1/11) of patients with type 1 infection (p=0.093).
Table 2.
Comparison of the clinical manifestations of patients in various gB genotype groups
| CMV gB genotype 1 | CMV gB genotype 3 | P value | |||
| Mean±SD | Range, median | Mean±SD | Range, median | ||
| N (%) | 11, 78.6% | 3, 21.4% | |||
| Male (n, %) | 10, 90.9% | 2, 66.7% | 0.396 | ||
| Age at the clinic visit (years) | 41.55±11.25 | 28–62, 40 | 52.33±14.19 | 37–65, 55 | |
| Age at the first onset (years) | 37±12 | 22–56, 34 | 47±17 | 31–64, 45 | |
| Course of disease (years) | 4.7±2.8 | 1–10, 4 | 5.7±4.5 | 1–10, 6 | |
| Bilateral (n, %) | 1, 9.1% | 2, 66.7% | 0.093 | ||
| Frequency of onset (n/year) | 1.5±0.8 | 1–3, 1 | 1.7±0.6 | 1–2, 2 | 0.209 |
| GC dependence (n, %) | 7, 63.6% | 1, 33.3% | 0.538 | ||
| Anti-HCMV IgG (s/co) | 0.94±0.79 | 0.24–2.39, 0.67 | 0.67±0.71 | 0.24–1.49, 0.27 | |
| Response to antiviral therapy (n of Cc, %) | 5, 45.5% | 1, 33.3% | 1.000 | ||
| VA (LogMAR) | 0.36±0.48 | 0.00–1.70, 0.22 | 0.23±0.32 | 0.00–0.6, 0.10 | |
| Ratio of C/D | 0.59±0.22 | 0.3–0.9, 0.5 | 0.47±0.12 | 0.4–0.6, 0.4 | |
| Peak IOP | 48±5 | 41–60, 48 | 48±3.5 | 45–52, 48 | |
| Corneal endothelial cell loss (%) | 0.14±0.09 | −0.03–0.27, 0.12 | 0.08±0.09 | −0.02–0.14, 0.12 | |
| KPs (n, %) | |||||
| Sheep-fat | 10, 90.9% | 2, 66.7% | 0.396 | ||
| Coin-shaped | 5, 45.5% | 3, 100% | 0.209 | ||
| Pigmented | 1, 9.1% | 2, 66.7% | 0.093 | ||
| Tyndall effect (n, %) | 3, 27.3% | 2, 66.7% | 0.505 | ||
| Iris depigmentation (n, %) | 8. 72.3% | 3, 100% | 1.000 | ||
| Cataract (n, %) | 4, 36.4% | 2, 66.7% | 0.583 | ||
| Glaucomatous optic neuropathy (n, %) | 8, 72.3% | 1, 33.3% | 0.505 | ||
Statistical analysis was performed using Fisher’s exact test.
C/D, cup to disk ratio; gB, glycoprotein B; GC, glucocorticoid; HCMV, human cytomegalovirus; IgG, immunoglobulin G; IOP, intraocular pressure; KPs, keratic precipitates; LogMAR, logarithm of mininal angle resolution; n of Cc, number of patients with the 'complete control' outcome; VA, visual acuity.
Figure 2.
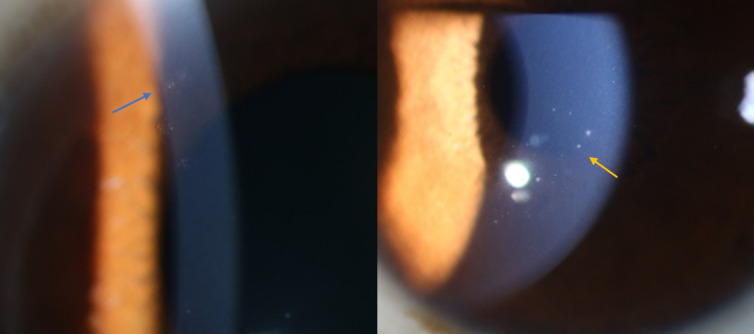
Typical morphology of keratic precipitates (KPs). The blue arrow indicates coin-shaped KPs. The yellow arrow indicates sheep-fat KPs.
Clinical outcomes after topical ganciclovir treatment
To evaluate the effect of antiviral therapy on PSS, we divided the outcomes into two categories, namely complete control and poor control. The outcomes were categorised based on records of IOP and inflammation within 1 year of treatment. When only antiviral therapy was performed, if IOP did not exceed 21 mm Hg and inflammation did not recur, the disease was considered to be under complete control; otherwise, it was considered to be poorly controlled. As shown in table 2, in the gB genotype 1 group, 45.5% of the patients (5/11) showed a good response to the antiviral treatment and 4 patients underwent anti-glaucoma surgery within a year to reduce their IOP. 33.3% of the patients with the gB genotype 3 (1/3) who only received antiviral therapy had stable IOP and good control of inflammation for up to a year, whereas the remaining two patients needed to be treated with additional hormones and drugs to reduce IOP.
Discussion
In this study, we determined the distribution of CMV gB genotypes in the aqueous humour of patients with PSS and investigated the differences in their clinical characteristics according to their different CMV genotypes. To the best of our knowledge, considering that PSS is generally considered as a rare condition and that the detection rate of CMV genome is relatively low in aqueous humour, the distribution of CMV gB genotypes in the aqueous humour of patients with PSS has not been published before. In addition, responses to antiviral treatment based on genotype distribution were also evaluated in this study.
In the present study, genotype 1 was the most prevalent genotype detected in the aqueous humour of patients with PSS. Only gB1 and gB3 were detected in this study; no other subtypes or mixed subtypes of genotype B were detected. A previous report of immunocompetent patients with CMV-related anterior uveitis showed that genotype 1 and genotype 3 were the common gB genotypes detected in the anterior chamber,25 a finding which is consistent with that of our study. However, the subset that causes inflammation in the anterior segment is distinct from the variants that cause posterior segment infection.26 27 In related studies of CMV infection in the posterior segment, gB genotype 2 was considered to be associated with human cytomegalovirus retinitis in patients with AIDS.28 It has been found that genotype 2 is the most common gB genotype in the blood,26 urine28 and ocular fluid27 of patients with retinitis. The distribution of the gB genotype varies according to immune status and may thus influence prognosis.29 In previous studies, the genotype 1 and genotype 3 were found to be more prevalent in immunocompetent individuals and were more associated with non-fatal outcomes than other gB genotypes, especially genotype 2.29 30 The skewed genotype distribution caused differences in virulence, which might explain the differences in clinical manifestations of the fundus and anterior ganglia infection by CMV. In contrast, differences in gB genotypes may also result in different invasion pathways. Retinal infection by CMV is regarded as the result of haematogenous spread of the virus,26 while few patients with PSS show viremia by CMV. Thus, we speculate that the pathogenesis of CMV in the eyes of patients with PSS may be different from the direct blood invasion observed in the eyes of patients with retinitis.
In the present study, bilateral onset was predominant among patients infected with the genotype 3 genotype; this seemed to be different from the characteristics of most patients with PSS who are considered to be suffered unilateral onset. gB3 has been found to be more common among immunocompetent patients with congenital infections than among those with postnatal infections.31 32 In addition, viruses with genotype 1 are considered to be weaker in lymphocyte tropism and virulence than those with genotype 2 and genotype 3.33 Therefore, we speculate that the clinical manifestations and prognoses of patients infected with genotype 1 CMV may be better than those of patients infected with other virus subtypes. We investigated whether there were other differences in the clinical characteristics of the two gB genotype groups, but no significant variations were found. Further studies with larger samples may be required to verify this finding.
The main limitation of this study is the small number of patients with genotype 3, which limited effective statistical analysis in this study. This is partly because of the generally low incidence of PSS, and partly because the viral load of CMV-infected patients with anterior uveitis is lower than that of patients with posterior uveitis or retinitis, which results in a lower acquired viral load after assay. In addition, the small quantity of aqueous humour samples (only 50–100 µL) yields a small number of available viruses.
In conclusion, this is the first report of the distribution of the gB genotypes of CMV in patients with PSS. CMV of genotype 1 was predominant in the aqueous fluid of patients with PSS. Patients infected with genotype 3 CMV mostly had bilateral attacks, but there was no significant difference in clinical characteristics between patients infected with different genotypes of CMV. The finding makes a significant contribution to the existing research and suggests CMV gB genotype may be an important target related to the virulence and pathogenicity of CMV strains in PSS.
Footnotes
Contributors: RZ acquired and analysed the data. RZ wrote the first draft of the manuscript. ZW participated to extract DNA from the samples and helped with analysis. QS and XF participated in the collection and input of data. XS revised the manuscript. XK contributed to the experimental design and revised the manuscript.
Funding: This work was supported by the Western Medicine Guidance Project of Shanghai Science and Technology Commission (grant number 19411961600), the Experimental Animal Research Project of Shanghai Science and Technology (grant number 201409006600) and the Double Excellent Project of Eye, Ear, Nose, and Throat Hospital (grant number SYB202003). The authors were funded by the Project of National Natural Science Foundation of China (grant numbers 81770922, 82070957, 81790641 and 81 430 007), the project of Shanghai Municipal Health Commission (grant number 201 740 204) and the Clinical Science and Technology Innovation Project of the Shanghai Shenkang Hospital Development Centre (grant number SHDC12017×18).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Posner A, Schlossman A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch Ophthal 1948;39:517–35. 10.1001/archopht.1948.00900020525007 [DOI] [PubMed] [Google Scholar]
- 2. Jiang JH, Zhang SD, Dai ML, et al. Posner-Schlossman syndrome in Wenzhou, China: a retrospective review study. Br J Ophthalmol 2017;101:1638–42. 10.1136/bjophthalmol-2016-309863 [DOI] [PubMed] [Google Scholar]
- 3. Accorinti M, Gilardi M, Pirraglia MP, et al. Cytomegalovirus anterior uveitis: long-term follow-up of immunocompetent patients. Graefes Arch Clin Exp Ophthalmol 2014;252:1817–24. 10.1007/s00417-014-2782-4 [DOI] [PubMed] [Google Scholar]
- 4. Jap A, Sivakumar M, Chee SP. Is Posner Schlossman syndrome benign? Ophthalmology 2001;108:913–8. 10.1016/S0161-6420(01)00551-6 [DOI] [PubMed] [Google Scholar]
- 5. Chee S-P, Jap A. Presumed Fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol 2008;146:883–9. 10.1016/j.ajo.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 6. Teoh S-B, Thean L, Koay E. Cytomegalovirus in aetiology of Posner-Schlossman syndrome: evidence from quantitative polymerase chain reaction. Eye 2005;19:1338–40. 10.1038/sj.eye.6701757 [DOI] [PubMed] [Google Scholar]
- 7. Su C-C, Hu F-R, Wang T-H, et al. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am J Ophthalmol 2014;158:1024–31. 10.1016/j.ajo.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 8. Zhao J, Zhu T, Chen W, et al. Human leukocyte Antigens-B and -C loci associated with Posner-Schlossman syndrome in a southern Chinese population. PLoS One 2015;10:e132179. 10.1371/journal.pone.0132179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pohlmann D, Schlickeiser S, Metzner S, et al. Different composition of intraocular immune mediators in Posner-Schlossman-Syndrome and Fuchs’ Uveitis. PLoS One 2018;13:e199301. 10.1371/journal.pone.0199301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohira S, Inoue T, Iwao K, et al. Factors influencing aqueous proinflammatory cytokines and growth factors in uveitic glaucoma. PLoS One 2016;11:e147080. 10.1371/journal.pone.0147080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen S-C, Ho W-J, Wu S-C, et al. Peripheral vascular endothelial dysfunction in Glaucomatocyclitic crisis: a preliminary study. Invest. Ophthalmol. Vis. Sci. 2010;51:272. 10.1167/iovs.09-3849 [DOI] [PubMed] [Google Scholar]
- 12. Megaw R, Agarwal PK. Posner-Schlossman syndrome. Surv Ophthalmol 2017;62:277–85. 10.1016/j.survophthal.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 13. Chan NS-W, Chee S-P, Caspers L, et al. Clinical features of CMV-Associated anterior uveitis. Ocul Immunol Inflamm 2018;26:107–15. 10.1080/09273948.2017.1394471 [DOI] [PubMed] [Google Scholar]
- 14. Miyanaga M, Sugita S, Shimizu N, et al. A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. Br J Ophthalmol 2010;94:336–40. 10.1136/bjo.2008.156422 [DOI] [PubMed] [Google Scholar]
- 15. Chee S-P, Bacsal K, Jap A, et al. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol 2008;145:834–40. 10.1016/j.ajo.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Zhai R, Sun Q, et al. Metabolomic profile of Posner–Schlossman syndrome: a gas chromatography time-of-flight mass spectrometry-based approach using aqueous humor. Front Pharmacol 2019;10. 10.3389/fphar.2019.01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan NS-W, Chee S-P, Caspers L, et al. Clinical features of CMV-Associated anterior uveitis. Ocul Immunol Inflamm 2018;26:107–15. 10.1080/09273948.2017.1394471 [DOI] [PubMed] [Google Scholar]
- 18. Cao G, Tan C, Zhang Y, et al. Digital droplet polymerase chain reaction analysis of common viruses in the aqueous humour of patients with Posner-Schlossman syndrome in Chinese population. Clin Exp Ophthalmol 2019;47:513-520. 10.1111/ceo.13440 [DOI] [PubMed] [Google Scholar]
- 19. Navarro D, Paz P, Tugizov S, et al. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 1993;197:143–58. 10.1006/viro.1993.1575 [DOI] [PubMed] [Google Scholar]
- 20. Tang J, Frascaroli G, Lebbink RJ, et al. Human cytomegalovirus glycoprotein B variants affect viral entry, cell fusion, and genome stability. Proc Natl Acad Sci U S A 2019;116:18021–30. 10.1073/pnas.1907447116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z, Guo C, Lin D, et al. Deep learning for automated glaucomatous optic neuropathy detection from ultra-widefield fundus images. Br J Ophthalmol 2021;105:1548–54. 10.1136/bjophthalmol-2020-317327 [DOI] [PubMed] [Google Scholar]
- 22. Chylack LT, Wolfe JK, Singer DM, et al. The lens opacities classification system III. The longitudinal study of cataract Study Group. Arch Ophthalmol 1993;111:831–6. 10.1001/archopht.1993.01090060119035 [DOI] [PubMed] [Google Scholar]
- 23. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994;22:4673–80. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chou SW, Dennison KM. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis 1991;163:1229–34. 10.1093/infdis/163.6.1229 [DOI] [PubMed] [Google Scholar]
- 25. Oka N, Suzuki T, Inoue T, et al. Polymorphisms in cytomegalovirus genotype in immunocompetent patients with corneal endotheliitis or iridocyclitis. J Med Virol 2015;87:1441–5. 10.1002/jmv.24239 [DOI] [PubMed] [Google Scholar]
- 26. Shepp DH, Match ME, Ashraf AB, et al. Cytomegalovirus glycoprotein B groups associated with retinitis in AIDS. J Infect Dis 1996;174:184–7. 10.1093/infdis/174.1.184 [DOI] [PubMed] [Google Scholar]
- 27. Peek R, Verbraak F, Bruinenberg M, et al. Cytomegalovirus glycoprotein B genotyping in ocular fluids and blood of AIDS patients with cytomegalovirus retinitis. Invest Ophthalmol Vis Sci 1998;39:1183–7. [PubMed] [Google Scholar]
- 28. Vogel J-U, Otte J, Koch F, et al. Role of human cytomegalovirus genotype polymorphisms in AIDS patients with cytomegalovirus retinitis. Med Microbiol Immunol 2013;202:37–47. 10.1007/s00430-012-0244-3 [DOI] [PubMed] [Google Scholar]
- 29. Fries BC, Chou S, Boeckh M, et al. Frequency distribution of cytomegalovirus envelope glycoprotein genotypes in bone marrow transplant recipients. J Infect Dis 1994;169:769–74. 10.1093/infdis/169.4.769 [DOI] [PubMed] [Google Scholar]
- 30. Trincado DE, Scott GM, White PA, et al. Human cytomegalovirus strains associated with congenital and perinatal infections. J Med Virol 2000;61:481–7. [DOI] [PubMed] [Google Scholar]
- 31. Yan H, Koyano S, Inami Y, et al. Genetic variations in the gB, UL144 and UL149 genes of human cytomegalovirus strains collected from congenitally and postnatally infected Japanese children. Arch Virol 2008;153:667–74. 10.1007/s00705-008-0044-7 [DOI] [PubMed] [Google Scholar]
- 32. Puhakka L, Pati S, Lappalainen M, et al. Viral shedding, and distribution of cytomegalovirus glycoprotein H (UL75), glycoprotein B (UL55), and glycoprotein N (UL73) genotypes in congenital cytomegalovirus infection. J Clin Virol 2020;125:104287. 10.1016/j.jcv.2020.104287 [DOI] [PubMed] [Google Scholar]
- 33. Meyer-König U, Vogelberg C, Bongarts A, et al. Glycoprotein B genotype correlates with cell tropism in vivo of human cytomegalovirus infection. J Med Virol 1998;55:75–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request.


