Abstract
Background
The molecular genetic basis of pulmonary arterial hypertension (PAH) is heterogeneous, with at least 26 genes displaying putative evidence for disease causality. Heterozygous variants in the ATP13A3 gene were recently identified as a new cause of adult-onset PAH. However, the contribution of ATP13A3 risk alleles to child-onset PAH remains largely unexplored.
Methods and results
We report three families with a novel, autosomal recessive form of childhood-onset PAH due to biallelic ATP13A3 variants. Disease onset ranged from birth to 2.5 years and was characterised by high mortality. Using genome sequencing of parent–offspring trios, we identified a homozygous missense variant in one case, which was subsequently confirmed to cosegregate with disease in an affected sibling. Independently, compound heterozygous variants in ATP13A3 were identified in two affected siblings and in an unrelated third family. The variants included three loss of function variants (two frameshift, one nonsense) and two highly conserved missense substitutions located in the catalytic phosphorylation domain. The children were largely refractory to treatment and four died in early childhood. All parents were heterozygous for the variants and asymptomatic.
Conclusion
Our findings support biallelic predicted deleterious ATP13A3 variants in autosomal recessive, childhood-onset PAH, indicating likely semidominant dose-dependent inheritance for this gene.
Keywords: pediatrics, genetics, pulmonary heart disease
Background
Pulmonary arterial hypertension (PAH (MIM: 178 600)) is a disease of complex aetiology, clinically characterised by incomplete penetrance, sex bias and variable age of onset, both within and between families. While most families exhibit autosomal dominant transmission, rare examples of autosomal recessive inheritance have been observed.1 2 The major genetic risk factor for both familial (ie, heritable PAH (HPAH)) and idiopathic PAH (IPAH) is haploinsufficiency of the bone morphogenetic protein receptor 2 gene (BMPR2).1 2 However, approximately 80% of IPAH cases remain genetically undiagnosed. Recent sequencing studies have accelerated the identification of additional risk genes,3–5 including monoallelic ATP13A3 variants associated with adult-onset IPAH across genetic ancestries.3 4 6 7 ATP13A3 encodes a P-type ATPase, a cation transporter with a documented role in the polyamine transport system.8
Compared with adult-onset disease, childhood-onset PAH (cPAH) is marked by high morbidity, resistance to available therapies and poor survival.9 Emerging data also indicate a strikingly different genomic architecture and distribution of genetic variants.4 9 10 De novo variants cause ~15% of cPAH. Monoallelic heritable variants in BMPR2, TBX4, ABCC8 and GDF2 likely cause 2%–6% of HPAH/IPAH in children, while variants in KCNK3, ACVRL1, ENG and SMAD9 are rare causes of cPAH.9 Biallelic variants in GDF2 and KCNK3 have been identified in very early-onset, severe cPAH in at least two families.11 12 Herein, we identified biallelic variants in ATP13A3 as the genetic cause of cPAH in three families including five affected children presenting under 3 years of age.
Patient cohort
Family 1
Proband II.1 presented with right heart failure at age 2.5 years. Echocardiography established right ventricular (RV) dilatation and hypertrophy with right atrial enlargement. Right heart catheterisation (RHC) was deemed too risky and was not performed. The child was diagnosed with IPAH, treated with sildenafil and diuretics, but died at 4 years of age due to right heart failure. The brother of the proband, II.2, was monitored from birth. At 2.5 years of age, echocardiography revealed signs of suprasystemic pressure in the right ventricle with evidence of hypertrophy and dilation. There was also right-to-left shunting through a foramen ovale with percutaneous saturation of ~90%. RHC showed an elevated mean pulmonary artery pressure (mPAP) of 40 mm Hg. Despite triple therapy (dual oral bosentan and sildenafil supplemented with epoprostenol after 15 months), the patient required lung transplantation at age 4.5 years. He died at age 8.5 years after contracting pneumonia. Both parents (I.1 and I.2) and a sister (II.3) remain healthy with no signs of PAH. The family is of Slovenian descent (table 1).
Table 1.
Clinical phenotypes and ATP13A3 variants identified in childhood-onset, autosomal recessive PAH
| Case | Sex | Age at diagnosis | Age at death | mPAP (mm Hg) | PVRi (Woods units) | Treatment | Variant(s) | Mutation type | MAF gnomAD v2.1.1 (controls) | CADD score h38v1.6 | SIFT | PolyPhen2 (HumVar) | Previously-reported for PAH? | ACMG class |
| F1 II.1 | M | 2.5 years | 4 years | nk | Sildenafil and diuretics | c.2563G>A (p.Val855Met) rs1489314131 |
Missense (homozygous) | absent | 26.5 | D | D | Barozzi 201914 | LP | |
| F1 II.2 | M | 2.5 years | 8 years | 40 | Sildenafil, bosentan, intravenous epoprostenol and lung transplantation | c.2563G>A (p.Val855Met) rs1489314131 |
Missense (homozygous) | absent | 26.5 | D | D | Barozzi 201914 | LP | |
| F2 II.2 | M | 5 months | 11 months | 34* | 11.8 | Oxygen, diuretics, sildenafil, bosentan and intravenous treprostinil | c.2549dupT (p.Met850Ilefs13) rs1560082927 | Frameshift | 9.21e-6 (no homo) | 33 | D | D | Zhu 20194 | P |
| c.2227C>T (p.Arg743Cys) | Missense | absent | 32 | D | D | Zhu 20194 | LP | |||||||
| F2 II.4 | F | 7 days | 17 months | 51* | 11.8 | Oxygen, diuretics, sildenafil, bosentan and subcutaneous treprostinil | c.2549dupT (p.Met850Ilefs13) rs1560082927 | Frameshift | 9.21e-6 (no homo) | 33 | D | D | Zhu 20194 | P |
| c.2227C>T (p.Arg743Cys) | Missense | absent | 32 | D | D | Zhu 20194 | LP | |||||||
| F3 I.1 | F | 22 months | alive | 59* | 34.3 | Sildenafil, bosentan, treprostinil and digoxin | c.3079dupT (p.Trp1027Leufs9) rs746602775 | Frameshift | absent | absent | --- | -- | no | P |
| c.3685G>T (p.Glu1229) rs200914446 | Nonsense | absent | 26.4 | --- | --- | no | P |
Variant nomenclature according to transcript NM_001367549.1.
Deleteriousness predictions: CADD score>20: deleterious; SIFT or PolyPhen score=D: protein damaging.
ACMG class: LP: likely pathogenic; P: pathogenic.
*Non-responder to inhaled nitric oxide or oxygen. F1 II.1 and II.2 were not tested.
CADD, Combined Annotation Dependent Depletion; mPAP, mean pulmonary artery pressure; nk, not known; PAH, pulmonary arterial hypertension; PVRi, pulmonary vascular resistance index; SIFT, Sorting Intolerant from Tolerant.
Family 2
Proband II.2 was born at 30 weeks’ gestation, weighing 790 g. After 2 months in the neonatal intensive care unit, he was discharged home. At age 6 months, he was taken to his primary care provider due to delayed developmental milestones and episodes of cyanosis. Echocardiography demonstrated severe PAH with RV pressure greater than 71 mm Hg. There was no congenital heart disease. RHC showed a mPAP of 34 mm Hg with a pulmonary vascular resistance (PVR) index of 11.8 Woods units that did not respond to oxygen or inhaled nitric oxide (iNO). Following a cardiac arrest during recovery from the procedure, he was supported by extracorporeal membrane oxygenation for 10 days. Despite extubation and maintenance therapy, he remained in critical condition and died of sepsis and PAH at age 11 months.
The proband’s sibling, II.4, was born at 37 weeks’ gestation. Echocardiography on the first day of life showed an estimated near systemic RV systolic pressure. At age 1 week, she developed persistent cyanotic episodes, with oxygen saturations in the 70 s and respiratory distress. RV pressure was observed to be suprasystemic by echocardiography. She was initially treated with inhaled epoprostenol and NO, vasopressors and mechanical ventilation. She then received extracorporeal membrane oxygenation for 5 days, due to acute decompensation. She subsequently responded to oxygen, diuretics, sildenafil, bosentan and treprostinil and was discharged at age 2 months. Follow-up echocardiography showed near systemic RV pressure and moderate RV hypertrophy. RHC at age 9 months showed a mPAP of 51 mm Hg with a PVR index of 11.8 Woods units, and these did not improve substantively with iNO and oxygen. Vasoreactivity testing showed no response until inhaled epoprostenol was added to 100% oxygen and iNO, perhaps indicating more refractory disease requiring engagement of both cyclic AMP and GMP pathways to maximise vasodilatation. She died at age 17 months, after contracting a respiratory infection. Both parents (I.1 and I.2) and two siblings (II.1 and II.3) remain healthy with no signs of PAH. The family is of Ashkenazi Jewish ancestry (table 1).
Family 3
Proband II.3 presented with severe PAH and right heart failure at age 22 months. Echocardiography and chest CT angiogram established RV dilatation and hypertrophy with right atrial enlargement. RHC demonstrated suprasystemic pulmonary artery pressure, a PVR of 34.3 and a low cardiac index (CI 1.7) that was mildly responsive to oxygen and iNO (PVR decreased to 25.5). Given her severe presentation and pulmonary haemodynamics, balloon dilatation of her patent foramen ovale was performed. Triple therapy (subcutaneous remodulin, oral bosentan and oral sildenafil) was initiated for 1 year, but haemodynamic values were only modestly improved (PVR 12.5, CI 3.6), with a mild response to oxygen and iNO (PVR decreased to 9.8). Despite continued triple therapy, she re-presented with signs of right heart failure 6 months later. RHC showed suprasystemic pulmonary artery pressure and a PVR of 22 that was unresponsive to oxygen and iNO. An MRI demonstrated a RV ejection fraction of 49%. Given her malignant course that was refractory to therapy, a Potts shunt was performed via left thoracotomy. Three months after her Potts procedure, she has much improved exercise tolerance. Both parents (I.1 and I.2) and two siblings (II.1 and II.2) are asymptomatic, and her sibling II.2 had a normal echocardiogram. The family is of Anglo-European ancestry (table 1).
Methods
Patient recruitment
Family 1 was recruited for genetic analysis through the NIHR BioResource for Rare Diseases programme.13 Family 2 was enrolled into a genetic study of PAH reviewed by the Columbia University Irving Medical Center institutional review board. Family 3 was enrolled in a research protocol approved by the University of California San Francisco institutional review board. All family members who were genetically assessed provided informed consent for themselves or their children.
Molecular genetic analysis
For family 1, DNA was extracted from whole blood samples for I.1, I.2 and II.2 and from a dried blood spot from the proband II.1 using the truXTRAC DBS Kit (Covaris). Parent–offspring trio (I.1, I.2 and II.2) whole genome sequencing was performed as described.3 For family 2, chromosomal microarray analysis and clinical exome sequencing were performed using blood-derived DNA from II.2 and II.4. Reanalysis of exome sequencing data screened 19 PAH risk genes1 14 (ACVRL1, AQP1, ATP13A3, BMPR1B, BMPR2, CAV1, CD248, EFCABB4B, EIF2AK4, ENG, GDF2, KCNA5, KCNK3, KLF2, SMAD1, SMAD4, SMAD9, SOX17 and TBX4). Analysis of Family 3 proband II.1 employed targeted sequencing of 21 genes associated with PAH (ABCC8, ACVRL1, AQP1, ATP13A3, BMPR1B, BMPR2, CAV1, EIF2AK4, ENG, FOXF1, GDF2, KCNA5, KCNK3, KLF2, NFU1, NOTCH3, RASA1, SMAD4, SMAD9, SOX17 and TBX4).
Family 1 samples were sequenced using Illumina HiSeq (2500 or X) technology, generating paired-end reads. All samples had a minimum read-depth coverage of 15× in ≥95% of targeted regions. Quality control, read mapping, variant calling and annotation were all conducted as described.3 Likely pathogenic variants were independently confirmed by Sanger sequencing. Families 2 and 3 underwent clinical sequencing by GeneDx15 or Blueprint Genetics, respectively. Population allele frequencies were obtained from the Genome Aggregation Database (gnomAD) V.2.1.1 (controls) database. In silico pathogenicity predictions were assessed using Combined Annotation Dependent Depletion score, Sorting Intolerant from Tolerant and PolyPhen-2.16 Additional family members were genotyped by Sanger sequencing.
Results
Family 1
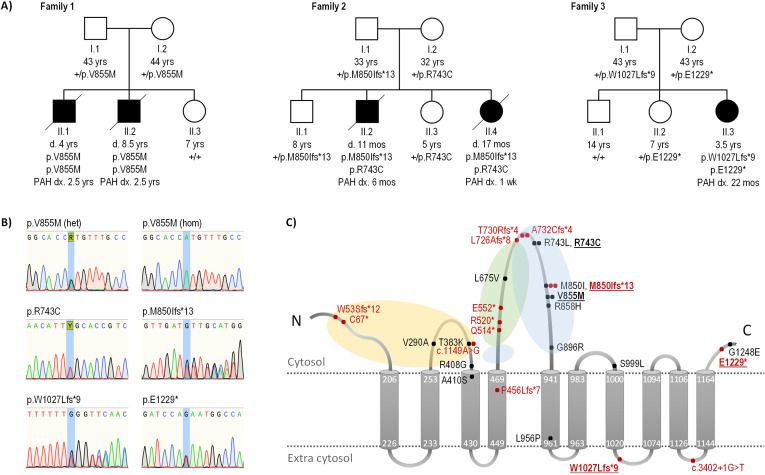
Trio analysis identified a homozygous ATP13A3 missense variant (NM_001367549.1: c.2563G>A; p.Val855Met) in affected child II.2, which was heterozygous in both parents (I.1 and I.2) (table 1). The variant is absent from gnomAD and a non-PAH European cohort (n=6385)3 and is predicted to be deleterious using in silico analysis (table 1). The deceased sibling (II.1) was homozygous for p.Val855Met and the unaffected sister (II.3) homozygous for the reference allele (figure 1A). The grandparents of the affected brothers are from nearby villages potentially indicating a common ancestor. The p.Val855Met variant resides in the likely kinase active cytoplasmic loop between the fourth and fifth transmembrane domains (figure 1B).17 Of note, a homozygous c.2563G>A substitution was recently reported in a Southern European cohort14 but variant cosegregation with affected relatives was not evaluated in this study.
Figure 1.
Rare deleterious ATP13A3 variants in biallelic and monoallelic PAH. (A) Family pedigrees for cases with early-onset, severe biallelic PAH. Filled boxes and circles indicate affected individuals. ATP13A3 mutation status is detailed for each individual. (B) Sequence chromatograms illustrating identified ATP13A3 variants in families 1–3. (C) ATP13A3 topology model with locations of variants identified in all PAH cases, both novel (bold typeface) and previously reported (Graf 2018, Zhu 2019, Wang, 2019, Lerche 2019, Gelinas 2020). Missense variants are in black, predicted truncating/splice variants in red. The number of filled circles at a variant location indicates the number of unrelated PAH cases identified with the variant. Yellow, green and blue shaded areas represent the actuator, nucleotide-binding and phosphorylation domains, respectively. Amino acids are numbered according to the longest transcript (NM_001367549.1; NP_001354478.1). +, wild type allele; d., age at death; dx., age at diagnosis; het, heterozygous; hom, homozygous; mos, months; PAH, pulmonary arterial hypertension.
Family 2
Targeted reanalysis detected compound heterozygous ATP13A3 variants in both affected children (II.2 and II.4): c.2227C>T; p.Arg743Cys and c.2549dupT; p.Met850Ilefs*13. Both parents (I.1 and I.2) and two unaffected siblings (II.1 and II.3) are heterozygous for one of the two variants (figure 1A) but remain asymptomatic. The p.Arg743Cys missense variant is absent from gnomAD and is predicted to be deleterious in silico (table 1). The variant resides in the same cytoplasmic loop as the family 1 variant (figure 1B), and a distinct monoallelic missense variant (c.2228G>T; p.Arg743Leu) impacting the same amino acid residue has been observed in an adult-onset IPAH case.4 ATP13A3 is highly constrained for loss-of-function variants (pLoF=1).18 The p.Met850Ilefs*13 frameshift is extremely rare (allele frequency 9.21e-6, with no homozygotes observed in gnomAD) and has been reported in a monoallelic adult-onset IPAH case.4 Of note, the two allele counts reported in gnomAD are from the Ashkenazi Jewish population similar to our family.
Family 3
Targeted screening identified compound heterozygous variants of ATP13A3 in the affected child (II.1): c.3079dupT; p.Trp1027Leufs*9 and c.3685G>T; p.Glu1229* (table 1). Both parents (I.1 and I.2) and one unaffected sibling (II.2) are heterozygous for one of the two variants; another unaffected sibling (II.1) is homozygous for the reference allele (figure 1A). The c.3079dupT mutant transcript is likely degraded by nonsense-mediated decay (NMD). However, the nonsense variant, c.3685G>T, is located in the final exon of the gene and is therefore predicted to escape NMD19 leading to the production of an abnormal, potentially damaging protein product.
Discussion
We report biallelic variants in ATP13A3 associated with very early age of PAH onset and high mortality in three independent families. The five variants are extremely rare or absent from gnomAD and predicted to be deleterious missense or loss-of-function variants. Some of the variants have been reported for monoallelic adult-onset PAH. Therefore, the relatively young parents and siblings of the affected children, who are currently asymptomatic heterozygous carriers, are at increased risk of developing PAH later in life. These data support a novel paradigm of semi-dominant inheritance for ATP13A3 in cPAH.
ATP13A3 encodes a transmembrane cation transporter, which was recently shown to transport polyamines.8 Polyamines are small metabolites required for normal cell growth and proliferation, and elevated concentrations have been reported in multiple cancers and, more recently, PAH.20 21 ATP13A3 is widely expressed in developing embryo and adult tissues,22 including pulmonary arterial smooth muscle cells (PASMC).3 Hypoxia, an environmental inducer of PAH, stimulates accumulation of spermine leading to increased PASMC proliferation in model systems. We hypothesise that ATP13A3 variants predicted to alter transporter function disturb polyamine homeostasis. However, additional experiments are required to assess the disease mechanism.
Identification of PAH causal genes and variants provides critical information to begin to tailor approaches for treatment and prognosis. In vitro analysis of patient-derived loss-of-function KCNK3 variants showed successful recovery of channel function in response to a phospholipase inhibitor, suggesting a novel therapeutic avenue for PAH.1 2 Similarly, experimental therapies targeting the polyamine transport system in cancer23 can now be tested in ATP13A3-targeted model systems relevant to PAH. An independent therapeutic approach includes adenoviral delivery of unmutated genes or molecular editing of mutated genes, in PAH relevant cell types, to restore normal gene expression or protein function, respectively. Finally, deep phenotyping and longitudinal follow-up of cases grouped by genetic diagnosis provides an opportunity to identify associated phenotypes to inform clinical care.
Emerging data from sequencing studies increases the utility of case-level genetic reanalysis. For family 2, the two affected children underwent extensive genetic testing without a diagnosis, but sequencing reanalysis facilitated the identification of biallelic variants in the newly identified ATP13A3 gene. Our study indicates that genetic reanalysis may be an appropriate strategy to prioritise genetic diagnoses when preterm infants do not follow the usual trajectory expected from the degree of prematurity or when clinical diagnoses are ambiguous.
Identification of biallelic ATP13A3 variants in three families with severe cPAH, in conjunction with recently identified monoallelic variants in adult-onset PAH, indicates semidominant inheritance for the ATP13A3 gene. Biallelic inheritance indicates a dose-dependent threshold effect with implications for prognosis and treatment strategies. Taken together, these findings demonstrate the growing importance of comprehensive genetic analyses in well-powered cPAH populations.
Acknowledgments
We would like to thank NIHR BioResource volunteers for their participation and gratefully acknowledge NIHR BioResource centers, NHS Trusts and staff for their contribution. We would like to thank the National Institute for Health Research and NHS Blood and Transplant.
Footnotes
RDM, CLW and MH contributed equally.
LS and WKC contributed equally.
Correction notice: The article has been corrected since it was published Online First. Names and affiliations have been amended, as well as table 1.
Contributors: Study concept and design, RDM, NWM, SG, LS, WKC; writing – original draft preparation, RDM, CLW, LS, WKC; writing – review and editing, RDM, CLW, MH, EC, JDC, MD, JE, JRF, WCG, ELG, MAH, YH, JEH-F, LN, LHR, SV, SG, NWM, LS, WKC; clinical diagnosis or clinical data gathering, EC, JDC, MD, JRF, WCG, ELG, CH, JEH-F, LN, LHR, SV, WKC; genetic data acquisition or analysis, RDM, CLW, MH, MB, JE, CH, YH, MAH, SG, LS, WKC. All authors have approved the final version of the manuscript.
Funding: LS is supported by an Academy of Medical Sciences Springboard award (SBF005/1115) and the Welcome Trust Institutional Strategic Support Fund (204809/Z/16/Z) awarded to St. George’s, University of London. The research was funded in part by a British Heart Foundation Special Project Grant and Personal Chair Award to NM, an MRC Experimental Challenge Award to NM and the Dinosaur Trust. This research was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014).
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health and Social Care.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol was approved by the UK NHS Research Ethics Committee (NIHR Bioresource – Rare Diseases, ref. 13/EE/0325), and the institutional review boards of Columbia University Irving Medical Center and the University of California San Francisco.
References
- 1. Southgate L, Machado RD, Gräf S, Morrell NW. Molecular genetic framework underlying pulmonary arterial hypertension. Nat Rev Cardiol 2020;17:85–95. 10.1038/s41569-019-0242-x [DOI] [PubMed] [Google Scholar]
- 2. Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, Trembath RC, Loyd JE. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 2019;53. 10.1183/13993003.01899-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gräf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, Hodgson J, Liu B, Salmon RM, Southwood M, Machado RD, Martin JM, Treacy CM, Yates K, Daugherty LC, Shamardina O, Whitehorn D, Holden S, Aldred M, Bogaard HJ, Church C, Coghlan G, Condliffe R, Corris PA, Danesino C, Eyries M, Gall H, Ghio S, Ghofrani H-A, Gibbs JSR, Girerd B, Houweling AC, Howard L, Humbert M, Kiely DG, Kovacs G, MacKenzie Ross RV, Moledina S, Montani D, Newnham M, Olschewski A, Olschewski H, Peacock AJ, Pepke-Zaba J, Prokopenko I, Rhodes CJ, Scelsi L, Seeger W, Soubrier F, Stein DF, Suntharalingam J, Swietlik EM, Toshner MR, van Heel DA, Vonk Noordegraaf A, Waisfisz Q, Wharton J, Wort SJ, Ouwehand WH, Soranzo N, Lawrie A, Upton PD, Wilkins MR, Trembath RC, Morrell NW. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 2018;9:1416. 10.1038/s41467-018-03672-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu N, Pauciulo MW, Welch CL, Lutz KA, Coleman AW, Gonzaga-Jauregui C, Wang J, Grimes JM, Martin LJ, He H, Shen Y, Chung WK, Nichols WC, PAH Biobank Enrolling Centers’ Investigators . Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med 2019;11:69. 10.1186/s13073-019-0685-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu N, Swietlik EM, Welch CL, Pauciulo MW, Hagen JJ, Zhou X, Guo Y, Karten J, Pandya D, Tilly T, Lutz KA, Rosenzweig E, Krishnan U, Coleman AW, Gonzaga-Juaregui C, Lawrie A, Trembath RC, Wilkins MR, Morrell NW, Shen Y, Gräf S, Nichols WC, Chung WK. Rare variant analysis of 4,241 pulmonary arterial hypertension cases from an international consortium implicate FBLN2, PDGFD and rare de novo variants in PAH. Genome Medicine 2021;10:80. 10.1186/s13073-021-00891-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X-J, Lian T-Y, Jiang X, Liu S-F, Li S-Q, Jiang R, Wu W-H, Ye J, Cheng C-Y, Du Y, Xu X-Q, Wu Y, Peng F-H, Sun K, Mao Y-M, Yu H, Liang C, Shyy JY-J, Zhang S-Y, Zhang X, Jing Z-C. Germline BMP9 mutation causes idiopathic pulmonary arterial hypertension. Eur Respir J 2019;53. 10.1183/13993003.01609-2018. [Epub ahead of print: 14 03 2019]. [DOI] [PubMed] [Google Scholar]
- 7. Lerche M, Eichstaedt CA, Hinderhofer K, Grünig E, Tausche K, Ziemssen T, Halank M, Wirtz H, Seyfarth H-J. Mutually reinforcing effects of genetic variants and interferon-β 1a therapy for pulmonary arterial hypertension development in multiple sclerosis patients. Pulm Circ 2019;9:204589401987219. 10.1177/2045894019872192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamouda NN, Van den Haute C, Vanhoutte R, Sannerud R, Azfar M, Mayer R, Cortés Calabuig Álvaro, Swinnen JV, Agostinis P, Baekelandt V, Annaert W, Impens F, Verhelst SHL, Eggermont J, Martin S, Vangheluwe P. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J Biol Chem 2021;296. 10.1074/jbc.RA120.013908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welch CL, Chung WK. Genetics and genomics of pediatric pulmonary arterial hypertension. Genes 2020;11:10. 10.3390/genes11101213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gelinas SM, Benson CE, Khan MA, Berger RMF, Trembath RC, Machado RD, Southgate L. Whole exome sequence analysis provides novel insights into the genetic framework of childhood-onset pulmonary arterial hypertension. Genes 2020;11. 10.3390/genes11111328. [Epub ahead of print: 11 11 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang G, Fan R, Ji R, Zou W, Penny DJ, Varghese NP, Fan Y. Novel homozygous BMP9 nonsense mutation causes pulmonary arterial hypertension: a case report. BMC Pulm Med 2016;16:17. 10.1186/s12890-016-0183-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navas Tejedor P, Tenorio Castaño J, Palomino Doza J, Arias Lajara P, Gordo Trujillo G, López Meseguer M, Román Broto A, Lapunzina Abadía P, Escribano Subía P. An homozygous mutation in KCNK3 is associated with an aggressive form of hereditary pulmonary arterial hypertension. Clin Genet 2017;91:453–7. 10.1111/cge.12869 [DOI] [PubMed] [Google Scholar]
- 13. Turro E, Astle WJ, Megy K, Gräf S, Greene D, Shamardina O, Allen HL, Sanchis-Juan A, Frontini M, Thys C, Stephens J, Mapeta R, Burren OS, Downes K, Haimel M, Tuna S, Deevi SVV, Aitman TJ, Bennett DL, Calleja P, Carss K, Caulfield MJ, Chinnery PF, Dixon PH, Gale DP, James R, Koziell A, Laffan MA, Levine AP, Maher ER, Markus HS, Morales J, Morrell NW, Mumford AD, Ormondroyd E, Rankin S, Rendon A, Richardson S, Roberts I, Roy NBA, Saleem MA, Smith KGC, Stark H, Tan RYY, Themistocleous AC, Thrasher AJ, Watkins H, Webster AR, Wilkins MR, Williamson C, Whitworth J, Humphray S, Bentley DR, Kingston N, Walker N, Bradley JR, Ashford S, Penkett CJ, Freson K, Stirrups KE, Raymond FL, Ouwehand WH, NIHR BioResource for the 100,000 Genomes Project . Whole-Genome sequencing of patients with rare diseases in a national health system. Nature 2020;583:96–102. 10.1038/s41586-020-2434-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barozzi C, Galletti M, Tomasi L, De Fanti S, Palazzini M, Manes A, Sazzini M, Galiè N. A combined targeted and whole exome sequencing approach identified novel candidate genes involved in heritable pulmonary arterial hypertension. Sci Rep 2019;9:753. 10.1038/s41598-018-37277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Retterer K, Juusola J, Cho MT, Vitazka P, Millan F, Gibellini F, Vertino-Bell A, Smaoui N, Neidich J, Monaghan KG, McKnight D, Bai R, Suchy S, Friedman B, Tahiliani J, Pineda-Alvarez D, Richard G, Brandt T, Haverfield E, Chung WK, Bale S. Clinical application of whole-exome sequencing across clinical indications. Genet Med 2016;18:696–704. 10.1038/gim.2015.148 [DOI] [PubMed] [Google Scholar]
- 16. Dong C, Wei P, Jian X, Gibbs R, Boerwinkle E, Wang K, Liu X. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet 2015;24:2125–37. 10.1093/hmg/ddu733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palmgren MG, Nissen P. P-Type ATPases. Annu Rev Biophys 2011;40:243–66. 10.1146/annurev.biophys.093008.131331 [DOI] [PubMed] [Google Scholar]
- 18. Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, Gauthier LD, Brand H, Solomonson M, Watts NA, Rhodes D, Singer-Berk M, England EM, Seaby EG, Kosmicki JA, Walters RK, Tashman K, Farjoun Y, Banks E, Poterba T, Wang A, Seed C, Whiffin N, Chong JX, Samocha KE, Pierce-Hoffman E, Zappala Z, O'Donnell-Luria AH, Minikel EV, Weisburd B, Lek M, Ware JS, Vittal C, Armean IM, Bergelson L, Cibulskis K, Connolly KM, Covarrubias M, Donnelly S, Ferriera S, Gabriel S, Gentry J, Gupta N, Jeandet T, Kaplan D, Llanwarne C, Munshi R, Novod S, Petrillo N, Roazen D, Ruano-Rubio V, Saltzman A, Schleicher M, Soto J, Tibbetts K, Tolonen C, Wade G, Talkowski ME, Neale BM, Daly MJ, MacArthur DG, Genome Aggregation Database Consortium . The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581:434–43. 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindeboom RGH, Supek F, Lehner B. The rules and impact of nonsense-mediated mRNA decay in human cancers. Nat Genet 2016;48:1112–8. 10.1038/ng.3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, Bleda M, Haimel M, Coghlan G, Corris PA, Howard LS, Kiely DG, Peacock AJ, Pepke-Zaba J, Toshner MR, Wort SJ, Gibbs JSR, Lawrie A, Gräf S, Morrell NW, Wilkins MR. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017;135:460–75. 10.1161/CIRCULATIONAHA.116.024602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He Y-Y, Yan Y, Jiang X, Zhao J-H, Wang Z, Wu T, Wang Y, Guo S-S, Ye J, Lian T-Y, Xu X-Q, Zhang J-L, Sun K, Peng F-H, Zhou Y-P, Mao Y-M, Zhang X, Chen J-W, Zhang S-Y, Jing Z-C. Spermine promotes pulmonary vascular remodelling and its synthase is a therapeutic target for pulmonary arterial hypertension. Eur Respir J 2020;56. 10.1183/13993003.00522-2020. [Epub ahead of print: 05 11 2020]. [DOI] [PubMed] [Google Scholar]
- 22. Sørensen DM, Holemans T, van Veen S, Martin S, Arslan T, Haagendahl IW, Holen HW, Hamouda NN, Eggermont J, Palmgren M, Vangheluwe P. Parkinson disease related ATP13A2 evolved early in animal evolution. PLoS One 2018;13:e0193228. 10.1371/journal.pone.0193228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gamble LD, Purgato S, Murray J, Xiao L, Yu DMT, Hanssen KM, Giorgi FM, Carter DR, Gifford AJ, Valli E, Milazzo G, Kamili A, Mayoh C, Liu B, Eden G, Sarraf S, Allan S, Di Giacomo S, Flemming CL, Russell AJ, Cheung BB, Oberthuer A, London WB, Fischer M, Trahair TN, Fletcher JI, Marshall GM, Ziegler DS, Hogarty MD, Burns MR, Perini G, Norris MD, Haber M. Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci Transl Med 2019;11:477. 10.1126/scitranslmed.aau1099 [DOI] [PubMed] [Google Scholar]