Abstract
Objective
We investigated the clinical significance of preoperative pan-immune-inflammation value (PIV) in patients with colorectal cancer (CRC).
Methods
In this retrospective study, 366 cases who underwent surgery for CRC were enrolled. Their clinical data were collected. PIV was calculated with the formula PIV = [neutrophil count (109/L)× platelet count (109/L) × monocyte count (109/L) /lymphocyte count (109/L). Patients were divided into high PIV (> median PIV) and low PIV (< median PIV) groups. The relationship between PIV and clinicopathological features of CRC was investigated. Receiver operating characteristic (ROC) curve was plotted to indicate the value of immune-inflammatory biomarkers (IIBs) in predicting the TNM stage of CRC, and the area under the curve (AUC) was calculated to evaluate the actual clinical value of IIBs. AUC > 0.5 and closer to 1 indicated the better predictive efficacy. The influencing factors of PIV in CRC were analyzed.
Results
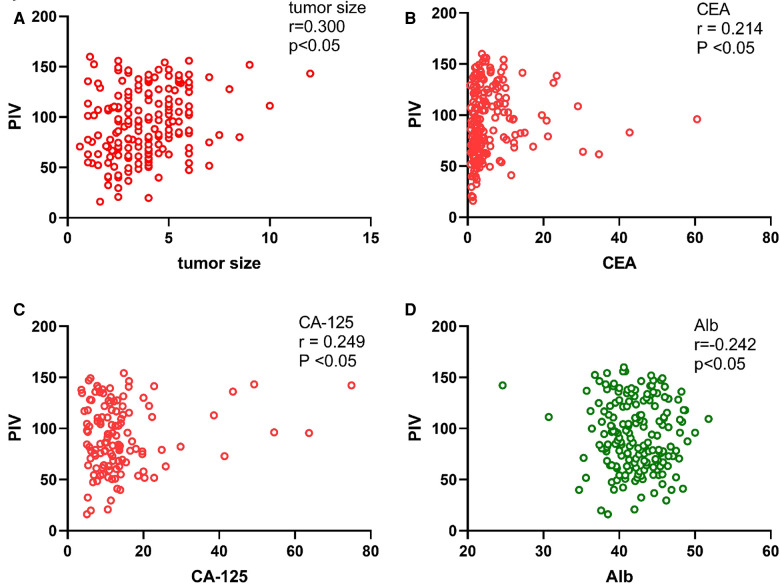
We found that PIV was positively correlated with tumor size (r = 0.300, p < 0.05), carcinoembryonic antigen (CEA) (r = 0.214, p < 0.05) and carbohydrate antigen 125 (CA-125) (r = 0.249, p < 0.05), but negatively correlated with albumin (Alb) (r = −0.242, p < 0.05). PIV was significantly different in patients with different tumor locations (left or right), surgical methods (laparotomy versus laparoscopic surgery) (p < 0.05), and patients with different pathological T stages, N-stage and TNM stages (p < 0.05). ROC curve analysis of IIBs showed the AUC of PIV was greater than other markers when combined with CEA or carbohydrate antigen 19–9 (CA19–9). Multivariate regression analysis identified T stage, CEA, Alb, and tumor size as the independent influential factors of PIV in CRC.
Conclusion
PIV is associated with the tumor stage in patients with CRC, which may be useful in preoperative assessment of CRC.
Keywords: pan-immune-inflammation value, CEA, CA19-9, TNM stage, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common malignancy and its mortality rate ranks second in cancer-related mortality worldwide. The incidence of CRC in developed country is estimated to be 4-fold higher than that in developing country (1). In China, CRC is the third most common cancer, and its mortality rate ranks fifth. The CRC incidence in China continues to increase (2). Due to the lack of early symptoms, a considerable number of CRC patients are diagnosed at an advanced stage, with a poor outcomes (3). Thus, potential biomarkers should be investigated to improve early diagnosis and tumor staging.
As the basis for clinical staging and gold standard for predicting prognosis for CRC patients, TNM staging [local tumor spread (T stage), lymph node spread (N stage) and metastasis (M stage)] plays an important role in preoperative management, treatment selection, and postoperative management of CRC in the clinical practice. Studies have indicated that the TNM staging of CRC is affected by serum tumor markers (TMs) (4), prognostic nutritional index, systemic inflammatory response (5), and the immune microenvironment (6).
Common biomarkers of CRC, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9) of TMs, have the merits of simplicity, availability, and robustness. They are generated and released during tumorigenesis, and can potentially reflect tumor changes at cellular levels (7, 8). However, TMs are mostly tumor-related, and host-related factors as markers are less reported. Recently, new evidence has shown that host-related factors such as inflammation status and immune response play an important role in the occurrence and development of tumor (9, 10). Immune-inflammatory biomarkers (IIBs) can reflect the balance between the status of inflammation and immunity in the host. IIBs such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) show prognostic values in CRC (11, 12), and systemic immune-inflammation index (SII) has also been shown as an effective predictor of CRC (13). Pan-immune-inflammation value (PIV), a new biomarker, has been introduced recently. PIV has been shown as a prognostic marker in HER-2 (+) breast cancer (14). High PIV (> median) was reported to be correlated with poor clinical outcomes in patients with small cell lung cancer (15). Fucà et al reported that, compared with SII, PIV was superior in evaluating progression-free survival and overall survival in patients with metastatic CRC (16).
In the present study, we investigated the association between PIV and clinicopathological features of CRC and compared the predicting values of NLR, SII, and PIV in TNM-stage of CRC patients who received radical surgery.
Materials and methods
Patients
Patients with primary CRC who received radical surgery at the Second Affiliated Hospital of Harbin Medical University between 2017 and 2022 were selected retrospectively. The inclusion criteria were: (a) patients had primary CRC confirmed by colonoscopy or postoperative histopathology; (b) patients underwent radical surgery; (c) patients were >18 years old; and (d) patients had complete and reliable clinical data, such as medical history, surgical records, and pathological results. The exclusion criteria were: (a) patients had history of other malignancies or were concomitant with other primary cancers; (b) patients had clinical evidence of any infection before surgery; (c) patients had hematological system diseases and autoimmune diseases; (d) patients had taken medications that might affect peripheral blood cells counts within 6 months; (e) patients were previously treated with neoadjuvant chemotherapy or radiotherapy; (f) patients underwent emergency surgery due to complications such as bowl obstruction or perforation. Finally, a total of 366 patients were included in this study. Preoperative laboratory data were retrieved from all patients. This study was approved by the Human Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (KY2022–160). The informed consent was waived owing to the retrospective nature of this study. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013).
Data collection
The following variables were collected: sex, age, body mass index (BMI), albumin (Alb), CEA, CA19–9, CA-125, alpha-fetal protein (AFP), tumor location, tumor size, degree of tumor differentiation, surgical approach, duration of operation, T stage, N stage, M stage, and TNM stage. Numbers of lymphocytes, neutrophils, monocytes, and platelets in preoperative blood were retrieved. The NLR, SII, and PIV were calculated correspondingly: NLR = neutrophil count (109/L)/lymphocyte count (109/L); SII = [neutrophil count (109/L)×platelet count (109/L)]/lymphocyte count (109/L); PIV = [neutrophil count (109/L) × platelet count (109/L) × monocyte count (109/L)]/lymphocyte count (109/L). CRC in the cecum, ascending colon, and transverse colon was defined as right-sided CRC. CRC in the descending colon and rectum was defined as left-sided CRC. Tumor staging was performed according to the 7th edition of the Union for International Cancer Control-American Joint Committee on cancer classification for CRC.
Statistical analysis
Data were analyzed using the R language (version 3.5.1). Data with normal distribution were presented as mean ± standard deviation and compared with Student's t test. Qualitative data were presented as absolute numbers or percentages and χ² test was used for comparison. Data with skewed distribution were presented as median (P25, P75) and analyzed with non-parametric rank-sum test. Mann Whitney U non- parametric test was used for ranked data comparison. Variables were also analyzed with Pearson correlation coefficient. The ranked data were analyzed by Wilcoxon or Kruskal-Wallis test and corrected with Bonferroni correction. Receiver operating characteristic (ROC) curve analysis was performed and the area under the ROC curve (AUC) was calculated. A p value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 366 CRC patients, including 215 (59%) males and 151 (41%) females, were included in the present analysis (Table 1). Among them, 217 (59%) patients were older than 60 years. The numbers of patients with right-sided and left-sided CRC were 97 (27%) and 269 (73%), respectively. There were 104 (28%) patients with tumor size larger than 5 cm. Laparotomy was performed in 160 (44%) patients and laparoscopic surgery was performed in 202 (56%) patients. Additionally, 234 (64%) patients had stage I-II CRC, and 132 (36%) had stage III-V CRC. The median value of PIV was 159.95 (93.35, 256.17). Based on this, patients were divided into high PIV (PIV >159.95) and low PIV (PIV ≤159.95) groups.
Table 1.
Patients’ characteristics.
| Variables | n | % |
|---|---|---|
| Age (years) | ||
| ≤60 | 149 | 41 |
| >60 | 217 | 59 |
| Gender | ||
| Female | 151 | 41 |
| Male | 215 | 59 |
| Localization | ||
| Right | 97 | 27 |
| Left | 269 | 73 |
| Size | ||
| ≤5 | 262 | 72 |
| >5 | 104 | 28 |
| Surgical approacha | ||
| Open | 160 | 44 |
| Laparoscopy | 202 | 56 |
| Pathological stage | ||
| Stage I–II | 234 | 64 |
| Stage III–IV | 132 | 36 |
| PIV | ||
| ≤159 | 183 | 50 |
| >159 | 183 | 50 |
Missing in 4 cases.
Relationship between PIV and clinicopathological features in CRC patients
There were statistically significant differences in Alb, CEA, tumor location, tumor size, surgical approach and pathological T-stage, N-stage and TNM-stage between patients with high PIV and low PIV (p < 0.05, Table 2). However, there were no differences in gender, age, CA-125, AFP, CA19–9, BMI, histological type, and pathological M-stage. In addition, cases in the high PIV group were more likely to have low Alb level, larger tumor size, and advanced T stage compared with those in the low PIV group. Patients whose tumors on the right side of the colon seemed to have higher PIV (Table 2). Pearson's correlation coefficient showed that PIV was positively correlated with tumor size (r = 0.300, p < 0.05), CEA (r = 0.214, p < 0.05), and CA-125 (r = 0.249, p < 0.05), but negatively correlated with Alb (r = −0.242, p < 0.05). However, PIV was not correlated with age, CA19–9, or AFP (r = 0.039, 0.096, and −0.062, respectively; p > 0.05) (Table 3A). In addition, we draw a scatter diagram to show the correlation between PIV and the above variables with statistical differences (Figures 1A–D). PIV in patients with left-sided CRC and right-sided CRC was 141.711 (86.210, 237.956) and 220.441 (106.635, 325.176), and in patients undergoing laparotomy and laparoscopic surgery was 188.319 (104.274, 283.652) and 138.109 (84.810, 242.166), respectively. PIV was significantly different in patients with different tumor locations and surgical approach (laparotomy vs laparoscopic surgery) (p < 0.05) (Table 3B). PIV of patients with pathological T-stages (T1, T2, T3, and T4 stage) was 135.645 (70.793, 208.382), 142.129 (95.878, 220.441), 166.963 (98.100, 283.329), and 270.964(207.017, 786.381), respectively. PIV of patients with pathological TNM-stages (I, II, III, and IV stage) was 139.409 (81.332, 214.575), 174.511 (95.970, 289.303), 152.257 (104.278, 263.991), and 203.870 (98.091, 302.717), respectively. There were statistically significant differences in PIV between patients with different pathological T stages and TNM stages (p < 0.05) (Table 3C). Then, we compared PIV of patients with different pathological T stages and TNM stages. When T1 stage was compared with T2, T3, T4, respectively, and when T2 was compared with T3 and T4 patients, the differences were statistically significant (p < 0.05). Pairwise comparison between other pathological T stages showed no significant differences (p > 0.05). There were statistically significant differences in PIV when TNMI stage was compared with II, III and IV stages, and when TNM II was compared with III (p < 0.05). Pairwise comparison of other pathological TNM stages showed no significant differences (p > 0.05) (Table 3D).
Table 2.
Correlation between PIV and clinicopathological features in CRC patients.
| Variables | Low PIV (n = 183) | High PIV (n = 183) | p-value |
|---|---|---|---|
| Age (year, ) | 62 ± 11 | 63 ± 11 | 0.4558 |
| Gender | 0.5955 | ||
| Male | 105 | 110 | |
| Female | 78 | 73 | |
| BMI (kg/m2, )a | 23.5 ± 4.1 | 23.9 ± 3.4 | 0.1643 |
| Alb (g/L, ) | 42.3 ± 3.6 | 41.2 ± 4.3 | 0.01981 |
| CEA | 1.935 (3.300,6.580) | 2.435 (4.680,10.255) | 0.0006496 |
| CA19-9 | 3.40 (7.05,15.92) | 3.940 (9.140,20.615) | 0.1278 |
| CA125b | 8.200 (11.300,19.275) | 8.200 (11.200,18.800) | 0.3022 |
| AFPc | 2.076 (2.660,3.763) | 1.828 (2.545,3.445) | 0.1316 |
| Tumor Location | 0.00645 | ||
| Right | 37 | 60 | |
| Left | 146 | 123 | |
| Tumor Size | 4.003 ± 1.791 | 5.026 ± 1.923 | 0.000000002151 |
| Surgical Approachd | 0.03429 | ||
| Open | 70 | 90 | |
| Laparoscopy | 111 | 91 | |
| Operative Timee | 177.547 ± 57.732 | 179.995 ± 59.988 | 0.6927 |
| Histological Type | 0.1823 | ||
| Low | 19 | 29 | |
| Medium | 157 | 147 | |
| High | 7 | 7 | |
| T stage | 0.00000000000005164 | ||
| T1 | 25 | 3 | |
| T2 | 70 | 11 | |
| T3 | 86 | 158 | |
| T4 | 2 | 11 | |
| N stage | 0.000002474 | ||
| N0 | 150 | 102 | |
| N1 | 23 | 53 | |
| N2 | 10 | 28 | |
| M stage | 0.06237 | ||
| M0 | 172 | 162 | |
| M1 | 11 | 21 | |
| TNM stage | 0.0000000000006989 | ||
| I | 74 | 6 | |
| II | 71 | 83 | |
| III | 27 | 73 | |
| IV | 11 | 21 |
Missing in 25 case.
Missing in 70 case.
Missing in 70 case.
Missing in 4 case.
Missing in 4 case.
Table 3A.
Pearson's correlation coefficient.
| Variables | r | p-value |
|---|---|---|
| Age | 0.03957461 | 0.4504 |
| Alb | −0.2416597 | 0.00001164 |
| Tumor Size | 0.3004814 | 0.00000003592 |
| CEA | 0.2148919 | 0.0000678 |
| CA19-9 | 0.09573459 | 0.08977333 |
| CA125a | 0.2489981 | 0.00003893333 |
| AFPb | −0.06226999 | 0.3264 |
Missing in 70 case.
Missing in 70 case.
Figure 1.
Correlation between PIV and tumor size, CEA, CA-125, Alb. Correlation between PIV and tumor size (A); Correlation between PIV and CEA (B); Correlation between PIV and CA-125 (C); Correlation between PIV and Alb (D).
Table 3B.
Wilcoxon test.
| Variables | Distribution | p-value | |
|---|---|---|---|
| Gender | Male | 160.253 (99.531, 269.980) | 0.1254 |
| Female | 151.951 (80.287, 243.079) | ||
| Tumor Location | Right | 220.441 (106.635, 325.176) | 0.00801 |
| Left | 141.711 (86.211, 237.956) | ||
| Surgical Approacha | Open | 188.320 (104.274, 283.652) | 0.02787 |
| Laparoscopy | 138.109 (84.810, 242.166) |
Missing in 4 case.
Table 3C.
Kruskal-Wallis test for T, N, M and TNM stage.
| Variables | Distribution | p-value | |
|---|---|---|---|
| T stage | T1 | 135.645 (70.793, 208.382) | 0.0000000000000022875 |
| T2 | 142.130 (95.879, 220.441) | ||
| T3 | 166.963 (98.100, 283.329) | ||
| T4 | 270.964 (207.017, 786.381) | ||
| N stage | N0 | 132.556 (80.340, 225.310) | 0.000000008983333 |
| N1 | 220.655 (137.205, 321.471) | ||
| N2 | 249.423 (160.381, 466.006) | ||
| M stage | − | − | 0.06837 |
| TNM stage | I | 139.410 (81.332, 214.575) | 0.0000000000000011 |
| II | 174.511 (95.970, 289.303) | ||
| III | 152.257 (104.278, 263.992) | ||
| IV | 203.870 (98.091, 302.718) |
Table 3D.
Kruskal-Wallis test for different T stages and different TNM stages.
| Variables | p-value | |
|---|---|---|
| T stage | T1/T2 | 0.04236 |
| T1/T3 | 0.0000000489 | |
| T1/T4 | 0.00000378 | |
| T2/T3 | 0.0000000002286 | |
| T2/T4 | 0.00002055 | |
| T3/T4 | 0.06634 | |
| TNM stage | I/II | 0.000000001374 |
| I/III | 0.00000000000000132 | |
| I/IV | 0.0000001648 | |
| II/III | 0.00007425 | |
| II/IV | 0.15504 | |
| III/IV | 0.2614 |
Prediction value of TMs combined with IIBs for TNM staging in CRC
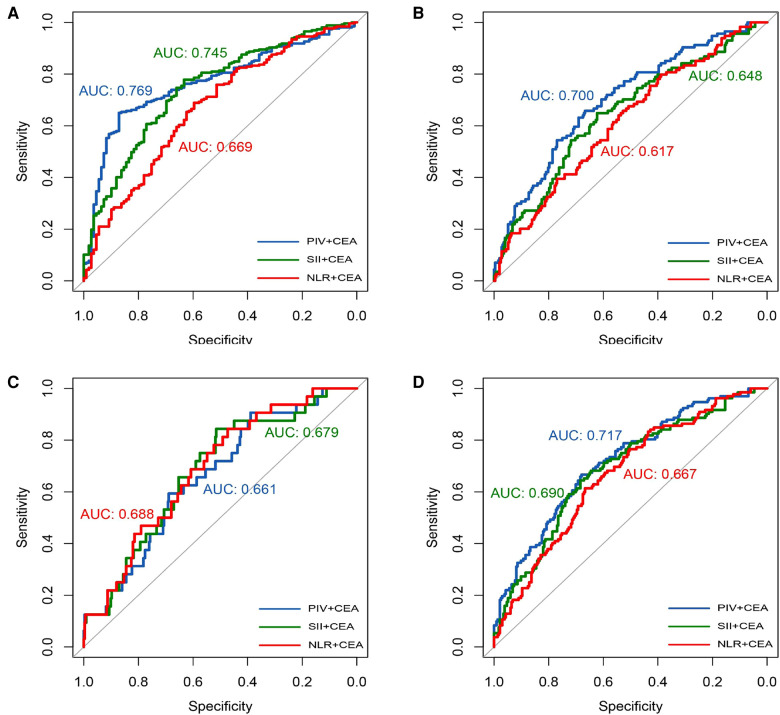
To more comprehensively reflect both the tumor characteristics and host systemic inflammatory status, we evaluated the prediction value of a combination of parameters (TMs and IIBs) for TNM staging in CRC. The AUCs of the NLR, SII, and PIV combined with CEA for pathological T-stage were 0.669, 0.745, and 0.769, respectively (Figure 2A); the AUCs for pathological N-stage were 0.617, 0.648, and 0.700, respectively (Figure 2B); the AUCs for pathological M-stage were 0.688, 0.679, and 0.661, respectively (Figure 2C); and the AUCs for pathological TNM-stage were 0.667, 0.690, and 0.717, respectively (Figure 2D).
Figure 2.
IIBS combined with CEA. Receiver operating curve analysis of T-stage (A); Receiver operating curve analysis of N-stage (B); Receiver operating curve analysis of M-stage (C); Receiver operating curve analysis of TNM-stage (D).
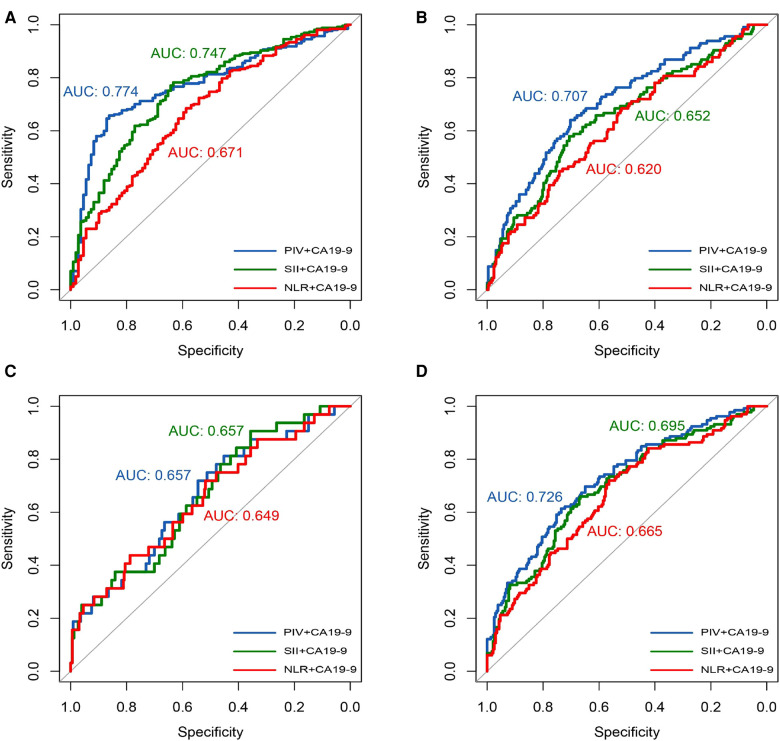
The AUCs of the NLR, SII, and PIV combined with CA19–9 for pathological T-stage were 0.671, 0.747, and 0.774, respectively (Figure 3A); the AUCs for pathological N-stage were 0.620, 0.652, and 0.707, respectively (Figure 3B); the AUCs for pathological M-stage were 0.649, 0.657, and 0.657, respectively (Figure 3C); and the AUCs for pathological TNM-stage were 0.665, 0.695, and 0.726, respectively (Figure 3D). Hence, among the IIBs analyzed in this study, PIV showed a superior prediction value for T, N, and TNM stages of CRC.
Figure 3.
IIBS combined with CA19-9. Receiver operating curve analysis of T-stage (A); Receiver operating curve analysis of N-stage (B); Receiver operating curve analysis of M-stage (C); Receiver operating curve analysis of TNM-stage (D).
Analysis of risk factors of PIV in CRC
To identify the factors that were linked with high PIV in CRC, we conducted univariate regression analysis. Totally, 15 variables were included in the univariate regression analysis. The results showed that BMI, Alb, CEA, CA-125, tumor size, tumor location, histological type, T-stage, N-stage, M-stage and TNM-stage were significantly linked with PIV (p < 0.05) (Table 4A). Then, we performed multivariate regression analysis. The influencing factors identified in univariate regression analysis and some factors that had great effects on PIV in clinical practice were included in the multivariate regression analysis. We found that CEA, CA-125, Alb, tumor size, and T stage were identified as the independent risk factors of PIV (Table 4B).
Table 4A.
Univariate analysis of PIV in patients with CRC.
| Variables | N | Beta | 95% CI | p-value |
|---|---|---|---|---|
| Age | 366 | 0.7568 | −1.206–2.720 | 0.45036 |
| Gender | ||||
| Female | 151 | 1 | − | − |
| Male | 215 | 22.49 | −22.899–67.883 | 0.332 |
| BMI (kg/m2, )a | 341 | −7.098 | −13.238–0.958 | 0.0241 |
| Alb | 366 | −13.21 | −18.656–7.759 | 0.00000291 |
| CEA | 366 | 0.8354 | 0.4453–1.225 | 0.0000339 |
| CA19-9 | 366 | 0.16014 | −0.011–0.331 | 0.0673 |
| CA125b | 296 | 2.175 | 1.208–3.142 | 0.0000146 |
| AFPc | 296 | −10.421 | −29.513–8.671 | 0.286 |
| Localization | ||||
| L | 269 | 1 | − | − |
| R | 97 | 83.06 | 33.085–133.031 | 0.00123 |
| Tumor Size | 366 | 33.622 | 22.658–44.586 | 0.00000000449 |
| Histological type | ||||
| Low | 48 | 1 | − | − |
| Medium | 304 | −88.68 | −154.580–22.778 | 0.00871 |
| High | 14 | −118.71 | −247.592–10.169 | 0.07185 |
| T-stage | ||||
| T1 | 28 | 1 | − | − |
| T2 | 81 | 40.94 | −48.326–130.198 | 0.3693 |
| T3 | 244 | 163.48 | 82.238–244.724 | 0.0000963 |
| T4 | 13 | 294.25 | 157.599–430.904 | 0.0000309 |
| N-stage | ||||
| N0 | 252 | 1 | − | − |
| N1 | 76 | 141.72 | 88.379–195.052 | 0.000000321 |
| N2 | 38 | 153.14 | 82.215–224.067 | 0.0000294 |
| M-stage | ||||
| M0 | 334 | 1 | − | − |
| M1 | 32 | 92.86 | 14.222–171.493 | 0.0212 |
| TNM-stage | ||||
| I | 80 | 1 | − | − |
| II | 154 | 103.43 | 48.831–158.031 | 0.000237 |
| III | 100 | 226.4 | 166.971–285.825 | 0.000000000000619 |
| IV | 32 | 208.33 | 125.464–291.198 | 0.00000127 |
Missing in 25 case.
Missing in 70 case.
Missing in 70 case.
Table 4B.
Multivariate analysis of PIV in patients with CRC.
| Variables | Beta | 95%CI | p-value |
|---|---|---|---|
| BMI (kg/m2, )a | −1.5146 | −7.909–4.879 | 0.64286 |
| Alb | −6.0835 | −11.374–0.793 | 0.024835 |
| Tumor Size | 19.5832 | 7.472–31.694 | 0.001662 |
| Tumor Location | |||
| Left | 1 | − | − |
| Right | 41.8463 | −3.663–87.355 | 0.072365 |
| CEA | 0.7009 | 0.340–1.061 | 0.000164 |
| CA-125 | 1.3451 | 0.287–2.403 | 0.01332 |
| Histological type | |||
| Low | 1 | − | − |
| Medium | −25.5501 | −100.970–49.870 | 0.50731 |
| High | −1.4928 | −167.649–164.663 | 0.98596 |
| T-stage | |||
| T1 | 1 | − | − |
| T2 | −22.6498 | −109.702–64.403 | 0.610403 |
| T3 | 113.6298 | −7.354–234.614 | 0.066487 |
| T4 | 173.9549 | 12.326–335.583 | 0.03561 |
| N-stage | |||
| N0 | 1 | − | − |
| N1 | 60.6297 | −46.635–167.895 | 0.268688 |
| N2 | 17.3333 | −103.814–138.481 | 0.779317 |
| TNM-stage | |||
| I | 1 | − | − |
| II | −86.1616 | −184.690–12.367 | 0.087415 |
| III | 38.2899 | −93.719–170.299 | 0.570059 |
| IV | −9.6966 | −135.886–116.493 | 0.880372 |
Missing in 25 case.
Discussion
The interaction between systemic inflammation and local immune response plays an important role in the initiation, development, and progression of CRC (17, 18). IIBs such as NLR, PLR and SII have positive correlations with the poor outcomes of CRC patients (19, 20). IIBs involved in present study were based on peripheral blood cells. It has been reported that neutorphils act as a key factor in regulating tumor microenvironment (21). Tumor associated neutrophils have two different phenotypes (N1 and N2), owing to the effect of immunosuppression in tumor microenvironment. Tumor associated neutrophils mainly exist as N2 type. N2 type can promote the progression of tumors by expressing vascular endothelial growth factor and a variety of chemokines (CCL2, CCL5, etc.), can inhibit the anti-tumor activity of other immune cells, and can form cell clusters with circulating tumor cells (22–24). Activated platelets can secrete a variety of pro-inflammatory factors that attract circulating white blood cells to the sites of inflammation (25). It has been shown that circulating tumor cells can avoid the cytotoxicity of NK cells through expressing platelet-associated receptors or binding to platelets (26), thus promoting tumor cell metastasis. Tumor infiltrating lymphocytes are derived from lymphocytes in tumors, which play an important role in tumor microenvironment. They can mediate anti-tumor immune response via recognizing and killing tumor cells (27). High NLR and SII may indicate suppressed immune response in the body, which may alter tumor microenvironment and favor cancer initiation, progression, and metastasis. Recently, studies have shown that preoperative elevation of the peripheral absolute monocyte count is associated with prognosis of CRC patients (28). Under external stimulation, monocytes in peripheral blood are recruited into tumor microenvironment and activated as tumor associated macrophages. As the most abundant immune cells in the tumor microenvironment, tumor associated macrophages can promote the proliferation, invasion, migration and angiogenesis of tumor cells by secreting a variety of cytokines. In addition, tumor associated macrophages can also suppress the anti-tumor immune response of T cells by secreting chemokines, leading to an immunosuppressive state (29, 30).
In view of the important role of monocytes in promoting tumor progression, we tested PIV, which is a new type of IIBs based on peripheral neutrophil, platelet, monocyte and lymphocyte counts. The present study revealed interesting correlation between PIV and clinicopathological features in CRC patients. Hypoalbuminemia is a risk factor for poor prognosis in cancer patients (31). In this study, we found that PIV was positively correlated with tumor size and negatively correlated with Alb. This suggests that the patients with high PIV may be in a pro-tumor inflammation state, due to the bigger size of cancer and hypoalbuminemia. Patients with high PIV were more likely to have advanced T-stage compared with those with low PIV, implying a potential predictive value of PIV for T-stage. Tumorigenesis depends on the imbalance of the tumor promoting effects and the anti-tumor responses. Hence, separately using TMs or IIBs has certain limitations, and it is necessary to combine these biomarkers in further investigation.
Herein, we also evaluated the predictive ability of IIBs combined with CEA and CA19–9 for pathological T, N, and M stages in CRC patients. Interestingly, we found that the combination had potential in predicting TNM-stage, which further validates the above hypothesis that compared with other IIBs, PIV is a more comprehensive biomarker for assessing systemic inflammation. Thus, we assume that PIV can facilitate the assessment of inflammation/immune status in CRC patients preoperatively, and guide clinical treatment, which is worthy of further investigation.
Finally, we analyzed the risk factors of PIV. The results showed that the TNM-stage and tumor histological differentiation were the independent risk factors for PIV in CRC patients. We believe that this result emphasizes the distinctive characteristics of PIV in patients with CRC. Early clinical evaluation combined with PIV detection may be helpful to better evaluate the immune status of the body in the early stage of tumorigenesis, which may further guide clinical treatment.
The present study has some limitations. First, this study was a retrospective, single-center study. Therefore, a prospective validation study is needed to validate the results of the present study. Second, the sample size was relatively small. Third, only the patients who received radical surgery were enrolled, thus the results of the present study are not applicable for CRC patients whose tumors could not be surgically treated.
In conclusion, our study identifies PIV as a new type of IIBs strongly associated with the Alb, CEA, tumor location, tumor size, surgical approach, T-stage, N-stage, and TNM-stage of CRC patients. In addition, PIV has a predicting value for TNM-stage of CRC patients, and when compared with other IIBs, PIV is more capable in predicting T-stage, N-stage and TNM-stage. This study imply a clinical application value of PIV in evaluating inflammation/immune status in CRC patients and choosing treatment options preoperatively, which is worthy of further investigation.
Acknowledgments
The authors wish to thank the patients enrolled in this study.
Funding
The present study was funded by the Natural Science Foundation of Hebei Province, China (H2020406008), Technology Innovation Guidance Project-Science and Technology Work Conference of Hebei Provincial Department of Science and Technology, the Key Subjects at Colleges and Universities of Hebei Province.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by H2020406008. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HZ devised concept of article. HZ and WH wrote the manuscript. YH and YN as the corresponding author reviewed the manuscript, made significant revisions. CD, YJ, LP, WL and JY contributed to discussions about the manuscript; XY analyzed the data. WC and JH participated in collecting data. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. (2022) 72(1):7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun. (2020) 40(5):205–10. 10.1002/cac2.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. (2014) 383(9927):1490–502. 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- 4.Sisik A, Kaya M, Bas G, Basak F, Alimoglu O. CEA And CA 19-9 are still valuable markers for the prognosis of colorectal and gastric cancer patients. Asian Pac J Cancer Prev. (2013) 14(7):4289–94. 10.7314/apjcp.2013.14.7.4289 [DOI] [PubMed] [Google Scholar]
- 5.Bai X, Feng L. Correlation between prognostic nutritional index, glasgow prognostic score, systemic inflammatory response, and TNM staging in colorectal cancer patients. Nutr Cancer. (2020) 72(7):1170–7. 10.1080/01635581.2019.1675725 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol. (2014) 31(8):82. 10.1007/s12032-014-0082-9 [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Yu L, Li B, Zhang F, Wang Y, Wang X, et al. Elevated preoperative carcinoembryonic antigen and vascular endothelial growth factor predict shorter survival in patients with sigmoid colon carcinoma. Clin Lab. (2017) 63(3):445–51. 10.7754/Clin.Lab.2016.160720 [DOI] [PubMed] [Google Scholar]
- 8.Peng Y, Zhai Z, Li Z, Wang L, Gu J. Role of blood tumor markers in predicting metastasis and local recurrence after curative resection of colon cancer. Int. J. Clin. Exp. Med. (2015) 8(1):982–90. PMID: [PMC free article] [PubMed] [Google Scholar]
- 9.Murata M. Inflammation and cancer. Environ Health Prev Med. (2018) 23(1):50. 10.1186/s12199-018-0740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. (2021) 54(5):859–74. 10.1016/j.immuni.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: a systematic review and meta-analysis. Cancer Med. (2021) 10(17):5983–97. 10.1002/cam4.4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stojkovic LM, Pavlovic MA, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman VI, et al. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers. (2019):6036979. 10.1155/2019/6036979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yatabe S, Eto K, Haruki K, Shiba H, Kosuge M, Ohkuma M, et al. Signification of Systemic Immune-Inflammation Index for prediction of prognosis after resecting in patients with colorectal cancer. Int J. Colorectal Dis. (2020) 35(8):1549–55. 10.1007/s00384-020-03615-w [DOI] [PubMed] [Google Scholar]
- 14.Ligorio F, Fuca G, Zattarin E, Lobefaro R, Zambelli L, Leporati R, et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers (Basel). (2021) 13(8):1964. 10.3390/cancers13081964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng R, Liu F, Fang C, Yang J, Luo L, Yue P, et al. PIV And PILE score at baseline predict clinical outcome of anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung cancer patients. Front Immunol. (2021) 12:724443. 10.3389/fimmu.2021.724443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuca G, Guarini V, Antoniotti C, Morano F, Moretto R, Corallo S, et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. (2020) 123(3):403–9. 10.1038/s41416-020-0894-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesselink E, Balvers M, Kok DE, Winkels RM, van Zutphen M, Schrauwen R, et al. Levels of inflammation markers are associated with the risk of recurrence and all-cause mortality in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. (2021) 30(6):1089–99. 10.1158/1055-9965.EPI-20-1752 [DOI] [PubMed] [Google Scholar]
- 18.Malka D, Lievre A, Andre T, Taieb J, Ducreux M, Bibeau F. Immune scores in colorectal cancer: where are we? Eur J Cancer. (2020) 140:105–18. 10.1016/j.ejca.2020.08.024 [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Kawada K, Obama K. Inflammation-Related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. (2021) 22(15):8002. 10.3390/ijms22158002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23(34):6261–72. 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol. (2017) 102(2):343–9. 10.1189/jlb.5MR1216-508R [DOI] [PubMed] [Google Scholar]
- 22.Lahoz-Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. (2016) 127(26):3431–8. 10.1182/blood-2016-03-700336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, et al. Houghton: neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. (2017) 8:14381. 10.1038/ncomms14381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. (2019) 566(7745):553–7. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- 25.Maouia A, Rebetz J, Kapur R, Semple JW. The immune nature of platelets revisited. Transfus Med Rev. (2020) 34(4):209–20. 10.1016/j.tmrv.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. (2018) 131(16):1777–89. 10.1182/blood-2017-05-743187 [DOI] [PubMed] [Google Scholar]
- 27.Lin B, Du L, Li H, Zhu X, Cui L, Li X. Tumor-infiltrating lymphocytes: warriors fight against tumors powerfully. Biomed Pharmacother. (2020) 132:110873. 10.1016/j.biopha.2020.110873 [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Xu Z, Huang Y, Zhao R, Cui Y, Zhou Y, et al. The predictive value and the correlation of peripheral absolute monocyte count, tumor-associated macrophage and microvessel density in patients with colon cancer. Medicine (Baltimore). (2018) 97(21):e10759. 10.1097/MD.0000000000010759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol. (2015) 12(1):1–4. 10.1038/cmi.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. (2017) 10(1):58. 10.1186/s13045-017-0430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. (2010) 9:69. 10.1186/1475-2891-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.