Abstract
Objective
To determine if methotrexate or folic acid prescription was associated with differential risk for COVID-19 diagnosis or mortality.
Design
Case–control analysis.
Setting
The population-based UK Biobank (UKBB) cohort.
Participants
Data from 380 380 UKBB participants with general practice prescription data for 2019–2021. Updated medical information was retrieved on 13 December 2021.
Primary and secondary outcome measures
The outcomes of COVID-19 diagnosis and COVID-19-related mortality were analysed by multivariable logistic regression. Exposures evaluated were prescription of folic acid and/or methotrexate. Criteria for COVID-19 diagnosis were (1) a positive SARS-CoV-2 test or (2) ICD-10 code for confirmed COVID-19 (U07.1) or probable COVID-19 (U07.2) in hospital records, or death records. By these criteria, 26 003 individuals were identified with COVID-19 of whom 820 were known to have died from COVID-19. Logistic regression statistical models were adjusted for age sex, ethnicity, Townsend deprivation index, body mass index, smoking status, presence of rheumatoid arthritis, sickle cell disease, use of anticonvulsants, statins and iron supplements.
Results
Compared with people prescribed neither folic acid nor methotrexate, people prescribed folic acid supplementation had increased risk of diagnosis of COVID-19 (OR 1.51 (1.42–1.61)). The prescription of methotrexate with or without folic acid was not associated with COVID-19 diagnosis (p≥0.18). People prescribed folic acid supplementation had positive association with death after a diagnosis of COVID-19 (OR 2.64 (2.15–3.24)) in a fully adjusted model. The prescription of methotrexate in combination with folic acid was not associated with an increased risk for COVID-19-related death (1.07 (0.57–1.98)).
Conclusions
We report an association of increased risk for COVID-19 diagnosis and COVID-19-related death in people prescribed folic acid supplementation. Our results also suggest that methotrexate might attenuate these associations.
Keywords: COVID-19, epidemiology, rheumatology
Strengths and limitations of this study.
A strength of the study is the use of a large population-based cohort with linked data.
Another strength is that the cohort was drawn from a population where food was not fortified with folic acid.
A limitation of the use of prescription data is that it was not possible to assess compliance or to account for over-the-counter supplementation of folic acid.
The findings cannot be generalised outside of the middle-aged (>45 years of age) white European demographic that dominates the UK Biobank cohort.
The observational nature of the data means that causality cannot be inferred from our findings.
Introduction
Folate, a vitamin B, carries out critical roles in the transfer of one-carbon units in intermediary metabolism. Folates exist in various forms depending on the one-carbon substituent attached to the parent molecule and are involved in numerous reactions, including the synthesis of methionine from homocysteine and are also used in purine and pyrimidine metabolism for DNA and RNA synthesis. The oxidised form, folic acid, is presently added to fortified foods in the USA and over 80 other countries. Recently, a decision has been taken in the UK to introduce fortification to prevent neural tube defect pregnancies. Folic acid is also used in dietary supplements to prevent or treat folate deficiency.1 Additionally, folic acid supplementation of up to 5 mg daily is often advised during pregnancy and in women of childbearing age and for other medical conditions (sickle cell anaemia)2 and during treatment with certain anticonvulsants.3
Methotrexate, a structural analogue of folate, has potent antifolate activity and is in widespread use as an antineoplastic agent and as a first-line disease-modifying antirheumatic drug (DMARD) treatment for rheumatoid arthritis (RA).4 Folic acid (at doses commonly ranging from 1 to 2 mg daily) or folinic acid supplementation is often included to lower the toxicity of low-dose methotrexate therapy.5 6
The COVID-19 Global Rheumatology Alliance physician-reported registry has evaluated factors related to death from COVID-19 in individuals with rheumatic diseases.7 Compared with those receiving methotrexate monotherapy, use of rituximab (OR 4.0 (95% CI 2.3 to 7.0)), sulfasalazine (3.6 (1.7 to 7.8)), azathioprine, cyclophosphamide, cyclosporine, mycophenolate and tacrolimus (2.2 (1.4 to 3.4)) or no DMARD (2.1 (1.5 to 3.0)) all had higher risks of death from COVID-19.
In order to generate purines, SARS-CoV-2 post-transcriptionally remodels host folate metabolism. In an in vitro system using African green monkey, kidney cells infected with SARS-CoV-2 intracellular glucose and folate were depleted, and this perturbation was sensitive to folate inhibitors such as methotrexate.8 It is therefore plausible that methotrexate therapy for RA could have a beneficial effect on COVID-19 outcomes given its antifolate activity. However, since folic acid is routinely included with methotrexate to prevent methotrexate-related toxicity, such putative beneficial effect of methotrexate on viral proliferation and hence on COVID-19 outcomes may be negated by folic acid supplementation.
The aim of this study was to determine whether the use of methotrexate and folic acid prescription, together or individually, were associated with a lowered or increased risk, respectively, for COVID-19 diagnosis or mortality in a large population-based cohort.
Methods
Data source
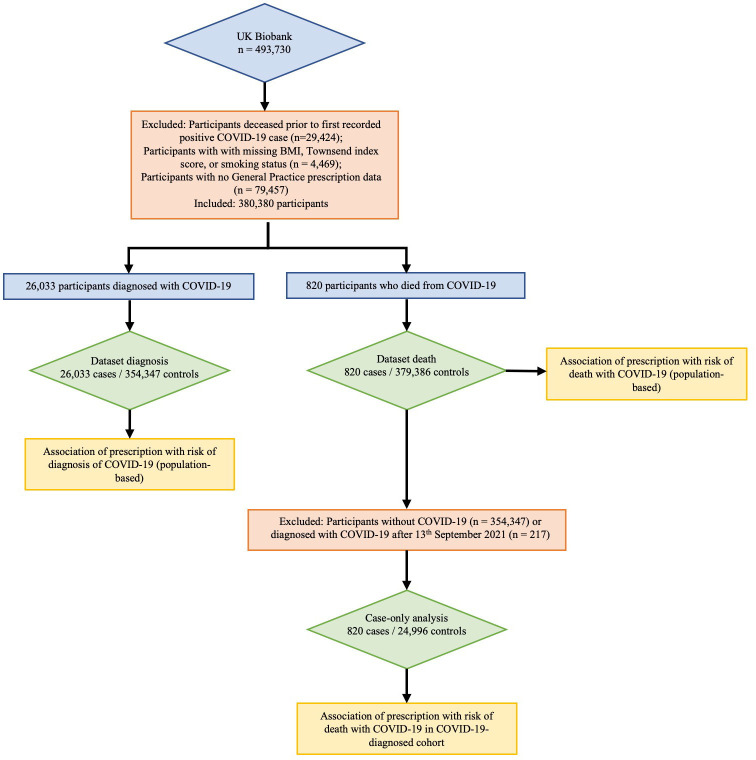
This research was conducted using the UK Biobank (UKBB) Resource (approval number 12611).9 The UKBB is a large resource of volunteers aged 49–86 years at recruitment.10 Recruitment began in 2006 with follow-up intended for at least 30 years. SARS-CoV-2 test information, ICD-10 hospital codes, death records and general practice prescription information were obtained via the UKBB data portal on 13 December 2021. This information covered hospital diagnoses between 18 April 1991 and 30 September 2021, SARS-CoV-2 tests between 13 January 2020 and 18 October 2021, and death records until 12 November 2021. Illustrated in figure 1, there were 464 306 participants, of whom 4469 were removed owing to not having a body mass index (BMI) measure or Townsend index score or smoking status and a further 79 457 were removed owing to lack of prescription data. General practice prescription data from 1 January 2019 to 27 September 2021, available for 380 380 participants, were used to identify people prescribed methotrexate, folic acid, anticonvulsants (phenytoin, carbamazepine, phenobarbital), iron supplements (ferrous fumarate, ferrous sulfate, ferrous gluconate) and coprescribed medications.
Figure 1.
Flow schematic of study design.BMI, body mass index.
Ethics approval statement
The UK Biobank (UKBB) was undertaken with ethical approval from the North West Multi-Centre Research Ethics Committee of the UK. This study was done under this ethical approval; researchers using the UKBB do not require separate ethical approval. The study complies with the Declaration of Helsinki and written informed consent was obtained from all participants.
Patient and public involvement
We did not involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
COVID-19 definitions
The criteria for COVID-19 diagnosis were defined as participants with (1) a positive SARS-CoV-2 PCR test and/or (2) ICD-10 code for confirmed COVID-19 (U07.1) or probable COVID-19 (U07.2) in hospital records, or death records. There were 26 033 cases, of whom 820 died with COVID-19. Figure 2 summarises how cases were diagnosed.
Figure 2.
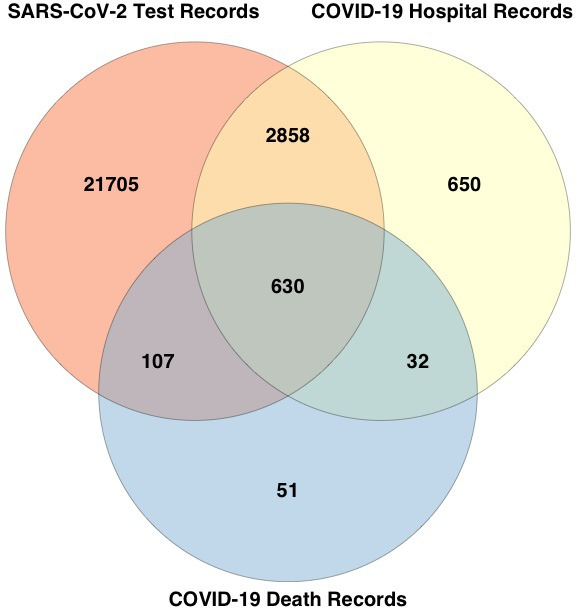
Data sources of COVID-19-diagnosed individuals. Of the 26 033 COVID-19-diagnosed individuals, 25 300 were identified from positive SARS-Cov-2 test results (21 705 unique to this group), 4170 identified from hospital records (650 unique to this group) and 820 identified from death records (51 unique to this group). Overall, 217 diagnosed after 13 October 2021 (30 days before the last recorded death) were removed from the case only analysis in online supplemental table S4 given the unknown outcome.
bmjopen-2022-062945supp001.pdf (160.8KB, pdf)
Ethnicity, age and comorbidity data
Self-reported ethnicity was grouped into white British (British, Irish, white, any other white background), black British (African, white and black African, black or black British, Caribbean, white and black Caribbean, any other black background), Asian British (Asian or Asian British, Chinese, Indian, Pakistani, Bangladeshi, white and Asian, any other Asian background) and other (other ethnic group, mixed, any other mixed background, do not know, prefer not to answer). Age was calculated for 2020 from year of birth. Age groups used in the analysis were<60 years (n=69 849), 60–69 years (n=120 013), 70–74 years (n=90 627) and >74 years (n=99 891). The ICD-10 hospital codes used to determine additional comorbidity status were RA (M05) and sickle cell disease (D57). Demographic characteristics of the study population are presented in table 1.
Table 1.
Study population in the UK Biobank, restricted to those with data on prescriptions
| Demographic | All, N=380 380 |
COVID-19 diagnosis, N=26 033 | No COVID-19 diagnosis, N=354 347 | COVID-19-related death, N=820 | No COVID-19-related death, N=379 560 |
| <60 years of age, n(%) | 69 849 (18.36) | 7937 (30.49) | 61 912 (17.47) | 26 (3.17) | 69 823 (18.4) |
| 60–70 years of age, n(%) | 120 013 (31.55) | 8568 (32.91) | 111 445 (31.45) | 129 (15.73) | 119 884 (31.58) |
| 70–75 years of age, n(%) | 90 627 (23.83) | 4569 (17.55) | 86 058 (24.29) | 171 (20.85) | 90 456 (23.83) |
| >75 years of age, n(%) | 99 891 (26.26) | 4959 (19.05) | 94 932 (26.79) | 494 (60.24) | 99 397 (26.19) |
| Female sex, n(%) | 211 363 (55.57) | 13 802 (53.02) | 197 561 (55.75) | 286 (34.88) | 211 077 (55.61) |
| White British, n(%) | 357 620 (94.02) | 23 807 (91.45) | 333 813 (94.21) | 744 (90.73) | 356 876 (94.02) |
| Black British, n(%) | 9826 (2.58) | 1021 (3.92) | 8805 (2.48) | 36 (4.39) | 9790 (2.58) |
| Asian British, n(%) | 7329 (1.93) | 732 (2.81) | 6597 (1.86) | 25 (3.05) | 7304 (1.92) |
| Other ethnicity, n(%) | 5605 (1.47) | 473 (1.82) | 5132 (1.45) | 15 (1.83) | 5590 (1.47) |
| Prescribed methotrexate only, n(%) | 174 (0.05) | 11 (0.04) | 163 (0.05) | 0 (0) | 174 (0.05) |
| Prescribed folic acid only, n(%) | 12 433 (3.27) | 1273 (4.89) | 11 160 (3.15) | 120 (14.63) | 12 313 (3.24) |
| Prescribed methotrexate and folic acid, n(%) | 3952 (1.04) | 287 (1.1) | 3665 (1.03) | 11 (1.34) | 3941 (1.04) |
| Prescribed neither methotrexate nor folic acid, n(%) | 363 821 (95.65) | 24 462 (93.97) | 339 359 (95.77) | 689 (84.02) | 363 132 (95.67) |
| Rheumatoid arthritis, n(%) | 999 (0.26) | 97 (0.37) | 902 (0.25) | 8 (0.98) | 991 (0.26) |
| Sickle cell disease, n(%) | 517 (0.14) | 51 (0.2) | 466 (0.13) | 2 (0.24) | 515 (0.14) |
| Prescribed anticonvulsant medication, n(%) | 1642 (0.43) | 120 (0.46) | 1522 (0.43) | 10 (1.22) | 1632 (0.43) |
| Prescribed statins, n(%) | 156 064 (41.03) | 10 398 (39.94) | 145 666 (41.11) | 521 (63.54) | 155 543 (40.98) |
| Prescribed iron supplements, n(%) | 18 471 (4.86) | 1661 (6.38) | 16 810 (4.74) | 106 (12.93) | 18 365 (4.84) |
| Body mass index, mean(SD) | 27.41 (4.76) | 28.12 (5.03) | 27.35 (4.74) | 30.21 (5.79) | 27.4 (4.76) |
| Townsend deprivation index, mean (SD) | −1.35 (3.04) | −0.9 (3.18) | −1.39 (3.03) | −0.28 (3.4) | −1.36 (3.04) |
| Never smoked, n(%) | 210 993 (55.47) | 13 990 (53.74) | 197 003 (55.6) | 321 (39.15) | 210 672 (55.5) |
| Current smoker, n(%) | 132 222 (34.76) | 9262 (35.58) | 122 960 (34.7) | 384 (46.83) | 131 838 (34.73) |
| Former smoker, n(%) | 37 165 (9.77) | 2781 (10.68) | 34 384 (9.7) | 115 (14.02) | 37 050 (9.76) |
Association with a diagnosis of COVID-19.
Statistical analysis
All association analyses in this case–control study were done using R V.4.0.2 in RStudio V.1.2.5019. Statistical model 1 was adjusted for age group (four categories), sex, ethnicity, Townsend deprivation index, BMI, smoking status. Model 2 is model 1 plus adjustment for the presence of RA, sickle cell disease (where daily folic acid is prescribed2), prescription of statins, prescription of anticonvulsants (where coprescription of folic acid often occurs11) and iron supplements (supplementary iron has been associated with poorer outcomes of infectious disease, including COVID-1912 13). For methotrexate and folate use, a single variable with four levels was used for statistical modelling (no methotrexate or folate, methotrexate only, folate only, methotrexate and folate). Sex-stratified analyses were done using the same approach to explore any differential association with COVID-19 diagnosis or associated mortality. A p<0.05 threshold indicated nominal evidence for association.
Results
Study population
Demographic characteristics of the study population are presented in table 1. The proportion of those diagnosed with COVID-19 while taking methotrexate was similar to the general study population (1.14% vs 1.09%, respectively) although there was a higher proportion of methotrexate prescriptions in the group that died with COVID-19 (1.34%). There was both a higher proportion of those prescribed folic acid who were diagnosed with COVID-19 (5.99% vs 4.31% in the general population) and those prescribed folic acid in those who died with COVID-19 (15.97% vs 4.31% in the general population). Medications coprescribed with folic acid were investigated (online supplemental table S1). Atorvastatin was coprescribed 23.21% of the time and Simvastatin 9.49% of the time. Due to these high prescription rates and reports describing an association between statin use and reduced mortality from COVID-1914 15 we included statins in model 2.
Association with a diagnosis of COVID-19
Compared with people prescribed neither folic acid nor methotrexate, individuals prescribed folic acid had significantly increased risk of diagnosis of COVID-19 in model 1 (OR 1.60 (1.50–1.70)) (table 2). In model 2, which included a diagnosis of RA, sickle cell disease and prescription of anticonvulsants or statins or iron supplements, this association was not attenuated (OR 1.51 (1.42–1.61)). The prescription of methotrexate without folic acid was uncommon (only 174 people) and did not show an association with COVID-19 diagnosis in either model. The prescription of methotrexate in combination with folic acid was associated with an increased risk for a diagnosis of COVID-19 in model 1 (1.15 (1.02–1.30)) but not in model 2 (1.09 (0.96–1.23)) (table 2 and online supplemental table S2). The risk for COVID-19 diagnosis was associated with similar magnitudes with the prescription of folic acid in men and women in model 2 (OR 1.50 (1.37–1.64) and 1.52 (1.39–1.65), respectively) (online supplemental table S3). The model 2 sex-specific associations were not statistically significant and of similar magnitudes with methotrexate and with methotrexate combined with folic acid.
Table 2.
COVID-19 diagnosis in people prescribed methotrexate and/or folic acid in the UKBB, compared with people not prescribed methotrexate or folic acid
| Unadjusted | Model 1 | Model 2 | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Neither folic acid nor methotrexate, N=363 821 | 1.0 | – | 1.0 | – | 1.0 | – |
| Folic acid only, N=12 433 | 1.58 (1.49 to 1.68) | <0.001 | 1.60 (1.50 to 1.70) | <0.001 | 1.51 (1.42 to 1.61) | <0.001 |
| Methotrexate only, N=174 | 0.94 (0.51 to 1.72) | 0.83 | 0.89 (0.48 to 1.65) | 0.72 | 0.86 (0.47 to 1.6) | 0.64 |
| Methotrexate and folic acid, N=3952 | 1.09 (0.96 to 1.23) | 0.18 | 1.15 (1.02 to 1.30) | 0.021 | 1.09 (0.96 to 1.23) | 0.18 |
Model 1 adjusted for age group, sex, ethnicity, Townsend deprivation index, body mass index, smoking status.
Model 2 is model 1 plus adjustment by the presence of rheumatoid arthritis, sickle cell disease, use of statins, anticonvulsants and iron supplementation.
Association with mortality related to a COVID-19 diagnosis
In the general population, compared with people prescribed neither folic acid nor methotrexate, individuals prescribed folic acid had a significant association with mortality related to COVID-19 in model 1 (OR 2.91 (2.38–3.55)) (table 3 and online supplemental table S4). In model 2, which included a diagnosis of RA, sickle cell disease, prescription of anticonvulsants, statins and iron supplements, this association was maintained (OR 2.64 (2.15–3.24)). Although there was a higher proportion of methotrexate prescriptions in the group that died with COVID-19, there were no deaths reported in individuals diagnosed with COVID-19 who were prescribed only methotrexate (N=11). Moreover, the prescription of methotrexate in combination with folic acid was not associated with an increased odds for death after diagnosis of COVID-19 in model 1 (table 3) (1.26 (0.70–2.30)) or model 2 (1.07 (0.57–1.98)). The risk for mortality after COVID-19 diagnosis was of similar magnitude with the prescription of folic acid in both men and women in model 2 (OR 2.59 (2.00–3.36) and 2.72 (1.93–3.84), respectively) (online supplemental table S5). In both men and women, coprescription of methotrexate attenuated the association.
Table 3.
The association of prescription of methotrexate and folic acid with COVID-19-related death in the UK Biobank
| Unadjusted | Model 1 | Model 2 | ||||
| OR, (95% CI) | P value | OR, (95% CI) | P value | OR, (95% CI) | P value | |
| Neither folic acid nor methotrexate, N=363 821 | 1.0 | – | 1.0 | – | 1.0 | – |
| Folic acid only, N=12 433 | 5.14 (4.23 to 6.24) | <0.001 | 2.91 (2.38 to 3.55) | <0.001 | 2.64 (2.15 to 3.24) | <0.001 |
| Methotrexate and folic acid, N=3952 | 1.47 (0.81 to 2.67) | 0.21 | 1.26 (0.70 to 2.30) | 0.44 | 1.07 (0.57 to 1.98) | 0.84 |
Model 1 adjusted for age group, sex, ethnicity, Townsend deprivation index, body mass index, smoking status.
Model 2 is model 1 plus adjustment by the presence of rheumatoid arthritis, sickle cell disease, use of statins, anticonvulsants and iron supplementation.
*There were no deaths in the group of participants taking only methotrexate without folate.
To account for improvements in outcome of patients with COVID-19, resulting from changes in public health measures and emergence of different SARS-CoV-2 lineages over time16 we tested for association with death in the COVID-19-positive cohort including also a quarterly (3-monthly) categorical time variable for diagnosis of COVID-19 using model 2 (online supplemental table S6). This revealed a similar pattern of association with death—there was association with increased risk of death in patients prescribed folic acid only (OR 1.46 (1.16–1.83)) but not in the group prescribed both folic acid and methotrexate (OR 0.96 (0.50–1.83)).
Discussion
In this population-based analysis, we report association with a 1.5-fold increased risk for COVID-19 diagnosis and 2.6-fold increased risk for COVID-19-related death among those who had been prescribed folic acid supplementation. The prescription of methotrexate was not associated with an increased risk of diagnosis of COVID-19 and we were not able to make an estimate for COVID-19-related death in the small sample of those prescribed methotrexate only. Notably, those prescribed methotrexate and folic acid did not have an increased risk for COVID-19 diagnosis or associated death, indicating that methotrexate might attenuate the increased risk for COVID-19 diagnosis and related death, which were associated with the prescription of folic acid alone.
In the context of SARS-Cov-2 infection, it is established that hijacking of cellular metabolic pathways is important for viral replication.17 Zhang et al described that SARS-CoV-2 remodels host folate and one-carbon metabolism at the post-transcriptional level to support de novo purine synthesis, bypassing viral shutoff of host translation.8 This suggests that viral replication could be sensitive to folate inhibitors, such as methotrexate. Intracellular glucose and folate are depleted in SARS-CoV-2-infected cells, and viral replication is exquisitely sensitive in vitro to inhibitors of folate and one carbon metabolism, notably methotrexate.8 Stegmann et al, based on cell culture experiments, reported that methotrexate alone or in combination with remdesivir limits the replication of SARS-CoV-2.18 With the caveat that our study is observational epidemiology and causality cannot be inferred, our study does support the possibility that external folate supply facilitates the production of large amounts of virus, contributing to clinical infection and mortality. With the same caveat on inference of causality from observational data, our study also supports the notion that SARS-CoV-2 replication is enhanced by folate supply based on our finding that coprescription of an antifolate (methotrexate) attenuated the association of supplementation with folic acid with COVID-19 outcomes.
There is also evidence that inadequate folate status may be harmful in the context of host resistance to infection with SARS-CoV-2. In addition to the well-recognised complication of anaemia, folate deficiency has other detrimental health effects, including suppression of immune function.19 Additional support for the possibility that adequate folate status is important in COVID-19 outcomes is provided by the observation that folate deficiency was associated with poorer outcomes in a cohort of patients with COVID-19.20 It is important to note that it is possible that in the study by Itelman et al,20 if increased folate levels were causal of COVID-19 diagnosis and poor outcomes, that the association with lower folate levels could have been caused by selection (collider) bias.21 Vitamin B12 deficiency has also been proposed as a factor related to poor COVID-19 outcomes, presumed to be through the induction of functional folate deficiency.22 A drug–protein structure interaction analysis raises the possibility that folate blocks the 3CL hydrolase enzyme, which may affect viral entry and replication.23 It is therefore possible that both inadequate and excessive amounts of folate may be detrimental to host resistance to SARS-CoV-2 infection and that there may be an optimal range of physiological folate status related to host resistance to COVID-19 infection and severity.
Data from the COVID-19 Global Rheumatology Alliance describe that a number of immunomodulatory drugs used in rheumatology are associated with an increased risk of infection and death compared with methotrexate.7 Being on no DMARD, therapy was associated with an increased risk of death with COVID-19 (OR 2.11 (1.48–3.01)), which could be interpreted as either a protective effect of methotrexate or an increased risk for death associated with poor rheumatic disease control. The authors of the study additionally noted that people not on DMARD therapy had increased use of glucocorticoids meaning that confounding by indication cannot be ruled out as an explanation.24 Methotrexate was also associated with lower odds for death when compared with sulfasalazine, other immunosuppressants and rituximab. In no case was methotrexate associated with an increased risk for death. The COVID-19 Global Rheumatology Alliance study did not explore the effect of folic acid supplementation in the setting of methotrexate, although it is highly likely that almost all patients on methotrexate also were receiving folic acid supplementation. Considering the widespread use of folic acid supplements and proposals to abandon entirely tolerable upper intake levels for folic acid,25 it would be prudent to monitor the effect of increased folic acid intake at a population level on COVID-19 morbidity and mortality, particularly at the upper end of folic acid intake.
Limitations
Limitations of our analysis are important to note. First, given the small size of the methotrexate-only group and that there were no deaths related to COVID-19 in this group, we could not test a beneficial effect on mortality of methotrexate in isolation. It is uncommon to find patients with methotrexate prescribed without supplemental folic acid as this is the standard of care. Second, over the time period of this study (March 2020–November 2021), COVID-19 outcomes (ie, death) will have been influenced by the development of clinical treatments including antiviral drugs and monoclonal antibodies, changes to public health measures and the appearance of new COVID-19 strains.16 We were unable to account for these factors in the population-based analysis; however, we attempted to account for this in the analysis within the COVID-19-positive group by including a time variable (online supplemental table S4). Third, findings are not necessarily generalisable outside of the middle-aged (>45 years of age) white European cohort that dominates the UKBB. Fourth, the full extent of SARS-CoV-2 infection is not known in the UK population due to incomplete testing rates early in the pandemic. Fifth, prescription data were single script from general practitioners only, and it was not possible to ascertain compliance, dosing or frequency of administration, or whether participants were taking the prescribed medication during the COVID-19 pandemic or continuously during the study period, although we attempted to account for this by restricting our use of prescription information to the years 2019 and 2020 only. Sixth, although we included RA in model 2, we were unable to account for any potential effect of disease activity in RA in people prescribed folic acid. RA disease activity negatively impacts death from COVID-19 outcomes.7 Seventh, while it is a strength of our study that mandatory fortification of the UK diet with folic acid had not been introduced during the period of our study, and thus did not confound our analysis, we were unable to account for the lower-dose over the counter folic acid supplementation available in the UK (400 µg being the most common formulation for over the counter tablets) because there were no self-report information in the UKBB dataset on the use of folic acid supplementation. Finally, residual confounding conferred by the underlying indications for folate prescriptions (besides the ones addressed in our analysis) is a possibility. Finally, given that our study was an observational case–control design, no firm inference can be made for causality of folic acid supplementation on mortality from COVID-19.
Conclusion
In conclusion, and despite the limitations of our study enumerated above, our data support the hypothesis that increased folate resulting from pharmacologic folic acid prescription could contribute to a higher probability of contracting clinically detectable infection with SARS-CoV-2 and to an increase in the risk of death following the infection. The study population was drawn from the >45-year-old segment of the UK population and is predominantly of white European ethnicity, therefore our findings have reduced generalisability to younger people, to other ethnic groups and to other countries. Nevertheless, our findings justify future studies on the influence of folic acid supplementation on COVID-19 outcomes, particularly in pregnant women and people on anticonvulsants requiring supplementary folic acid.
As a final comment, we point out that attention is currently being directed toward establishing whether excessive intake of folate, particularly in the form of folic acid, may have undesirable and potentially deleterious effects.26 The possibility that susceptibility to COVID-19 infection and its serious and even fatal complications may be affected by folic acid intake and folate status should be thoroughly investigated.
Supplementary Material
Footnotes
Twitter: @philipcrobinson, @ALGaffoRheumMD
Contributors: RT, RG, SLM, PR, TM and ALG substantially contributed to study conception and design, to acquisition and analysis of data and interpretation of results. RT, RG, SLM, PR, TM and ALG contributed to drafting the article and critical revision and approved the final version. RT and TM directly accessed and verified the underlying data reported in the manuscript. AG is the author acting as a guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PR reports personal fees from Abbvie, Atom Biosciences, Eli Lilly, Gilead, Janssen, Novartis, UCB, Roche, Pfizer; meeting attendance support from BMS, Pfizer and UCB Pharma and grant funding from Janssen, Novartis, Pfizer and UCB Pharma, all outside the submitted work. ALG reports personal fees from SOBI, Selecta and honoraria from UptoDate, outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The UK Biobank Resource (approval number 12611) was undertaken with ethical approval from the North West Multi-Centre Research Ethics Committee of the UK. This study was done under this ethical approval; researchers using the UK Biobank do not require separate ethical approval. The study complies with the Declaration of Helsinki and written informed consent was obtained from all participants. Participants gave informed consent to participate in the study before taking part.
References
- 1.Department of health and social care UG. Available: https://www.gov.uk/government/news/folic-acid-added-to-flour-to-prevent-spinal-conditions-in-babies [Accessed 01 Nov 2022].
- 2.Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev 2018;3:CD011130. 10.2139/ssrn.3297902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linnebank M, Moskau S, Semmler A, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol 2011;69:352–9. 10.1002/ana.22229 [DOI] [PubMed] [Google Scholar]
- 4.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. 10.1002/art.41752 [DOI] [PubMed] [Google Scholar]
- 5.Morgan SL, Baggott JE, Vaughn WH, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med 1994;121:833–41. 10.7326/0003-4819-121-11-199412010-00002 [DOI] [PubMed] [Google Scholar]
- 6.Morgan SL, Baggott JE, Vaughn WH, et al. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 1990;33:9–18. 10.1002/art.1780330102 [DOI] [PubMed] [Google Scholar]
- 7.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Guo R, Kim SH, et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat Commun 2021;12:1676. 10.1038/s41467-021-21903-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apply for access. Available: https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access [Accessed 22 June 2022].
- 10.Sudlow C, Gallacher J, Allen N, et al. Uk Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore JL. The significance of folic acid for epilepsy patients. Epilepsy Behav 2005;7:172–81. 10.1016/j.yebeh.2005.05.020 [DOI] [PubMed] [Google Scholar]
- 12.Girelli D, Marchi G, Busti F, et al. Iron metabolism in infections: focus on COVID-19. Semin Hematol 2021;58:182–7. 10.1053/j.seminhematol.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonciarz RL, Renslo AR. Emerging role of ferrous iron in bacterial growth and host-pathogen interaction: New tools for chemical (micro)biology and antibacterial therapy. Curr Opin Chem Biol 2021;61:170–8. 10.1016/j.cbpa.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Arocutipa C, Melgar-Talavera B, Alvarado-Yarasca Ángel, et al. Statins reduce mortality in patients with COVID-19: an updated meta-analysis of 147 824 patients. Int J Infect Dis 2021;110:374–81. 10.1016/j.ijid.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollias A, Kyriakoulis KG, Kyriakoulis IG, et al. Statin use and mortality in COVID-19 patients: updated systematic review and meta-analysis. Atherosclerosis 2021;330:114–21. 10.1016/j.atherosclerosis.2021.06.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies NG, Jarvis CI, et al. , CMMID COVID-19 Working Group . Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021;593:270–4. 10.1038/s41586-021-03426-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaker SK, Ch'ng J, Christofk HR. Viral hijacking of cellular metabolism. BMC Biol 2019;17:59. 10.1186/s12915-019-0678-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegmann KM, Dickmanns A, Gerber S, et al. The folate antagonist methotrexate diminishes replication of the coronavirus SARS-CoV-2 and enhances the antiviral efficacy of remdesivir in cell culture models. Virus Res 2021;302:198469. 10.1016/j.virusres.2021.198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green R, Miller JW. Folate deficiency beyond megaloblastic anemia: hyperhomocysteinemia and other manifestations of dysfunctional folate status. Semin Hematol 1999;36:47–64. [PubMed] [Google Scholar]
- 20.Itelman E, Wasserstrum Y, Segev A, et al. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J 2020;22:271–4. [PubMed] [Google Scholar]
- 21.Choi HK, Nguyen U-S, Niu J, et al. Selection bias in rheumatic disease research. Nat Rev Rheumatol 2014;10:403–12. 10.1038/nrrheum.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wee AKH,. COVID-19's toll on the elderly and those with diabetes mellitus - Is vitamin B12 deficiency an accomplice? Med Hypotheses 2021;146:110374. 10.1016/j.mehy.2020.110374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serseg T, Benarous K, Yousfi M. Hispidin and Lepidine E: Two Natural Compounds and Folic Acid as Potential Inhibitors of 2019-novel Coronavirus Main Protease (2019- nCoVMpro), Molecular Docking and SAR Study. Curr Comput Aided Drug Des 2021;17:469–79. 10.2174/1573409916666200422075440 [DOI] [PubMed] [Google Scholar]
- 24.Schäfer M, Strangfeld A, Hyrich KL, et al. Response to: 'Correspondence on 'Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician reported registry'' by Mulhearn et al. Ann Rheum Dis 2021. 10.1136/annrheumdis-2021-220134. [Epub ahead of print: 01 Mar 2021]. [DOI] [PubMed] [Google Scholar]
- 25.Wald NJ, Morris JK, Blakemore C. Public health failure in the prevention of neural tube defects: time to abandon the tolerable upper intake level of folate. Public Health Rev 2018;39:2. 10.1186/s40985-018-0079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruvada P, Stover PJ, Mason JB, et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr 2020;112:1390–403. 10.1093/ajcn/nqaa259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062945supp001.pdf (160.8KB, pdf)
Data Availability Statement
Data are available in a public, open access repository.