Abstract
Lumpy skin disease is an arthropod-borne bovine disease caused by lumpy skin disease virus. A suspect lumpy skin disease case in a breeding cattle farm on Kinmen Island, Taiwan was reported on July 8, 2020 and later confirmed the first occurrence of lumpy skin disease in the country by molecular biological detections, electron microscopy, and sequence comparison. Implementation of control measures including blanket vaccination on the island effectively ceased the outbreaks. Phylogenetic analyses revealed that the virus discovered in the outbreaks was most similar to those identified in China in 2019. Identifying this virus in the coastal areas in East Asia indicated the rapid eastward spread of lumpy skin disease in Asia.
Keywords: cattle, lumpy skin disease, Taiwan
Lumpy skin disease (LSD) is a poxviral animal disease affecting bovine species (Bos taurus and Bos indicus) and water buffalo (Bubalus bubalis) [25]. The causative agent of LSD is the lumpy skin disease virus (LSDV) which belongs to the genus Capripoxvirus within the family Poxviridae. This disease is characterized by fever, emaciation, enlarged superficial lymph nodes, lacrimation, conjunctivitis, and notable nodules on the skin and mucous membranes of the mouth, respiratory tract, and genitalia [5]. Nodules are frequently observed on the head, neck, udder, scrotum, vulva, and perineum in infected cattle. Edema of the skin may be observed [26].
Lumpy skin disease was first seen in 1929 [9] and had been a disease of Africa [2]. However, in recent years, LSD has been recorded beyond the territory of Africa: Albania [8] in 2016; Greece, Macedonia, and Russia in 2017; Georgia in 2018; Bangladesh, China, India, Israel, Palestine, Russia, and Syria in 2019; Bhutan, China, Syria, Taiwan, Vietnam in 2020; Cambodia, Laos, and Thailand in 2021; and Indonesia in 2022 (World Animal Health Information System, World Organization for Animal Health). These events revealed the transcontinental and eastward expansion of this disease. Prior to the outbreak that was reported in the present article, LSD was not recorded in eastern Asian countries, including Taiwan.
The aim of the present study is to record the outbreaks and to identify and phylogenetically characterize the first LSDV isolate in Taiwan.
MATERIALS AND METHODS
The outbreak and clinical findings
On July 6, 2020, an animal caretaker at the Livestock Research Institute, Kinmen County observed several nodules on the skin of six Brangus oxen and more similar cutaneous lesions were observed on other cattle in the following days. When the disease was noted, 548 cattle were raised in the institute. The herd of Brangus was introduced in 2011 and had inbred since then. Kinmen, a Taiwan’s territory, is a 150-km2 island near the coast of Fujiang Province, China (Fig. 1). After the report by the Livestock Research Institute, Kinmen County on July 8, field investigation, necropsy, and sample collection were conducted on July 9 by veterinarians of the Kinmen County Animal and Plant Disease Control Center, Animal Health Research Institute, and the Bureau of Animal and Plant Health Inspection and Quarantine.
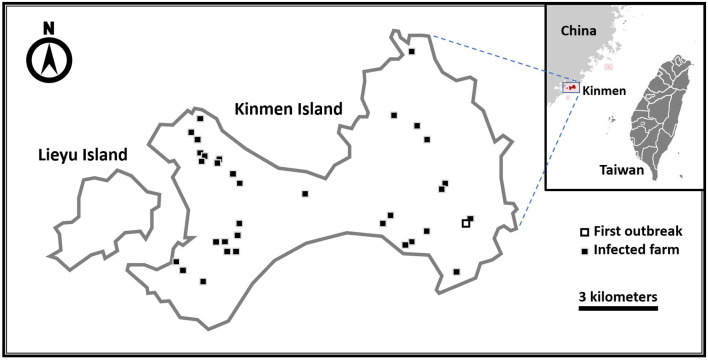
Fig. 1.
Thirty-four lumpy skin disease infected farms in Kinmen Island in 2020. The open square on southeastern Kinmen Island indicates the first identified infected farm and the solid squares indicate all the other infected farms.
Samples
For laboratory diagnosis, anti-coagulated blood, oral and nasal swabs, and tissues of nodular lesions were sampled from each of the twelve diseased cattle (Table 1), including one that died while being led to an isolation pen. Swabs of each cattle were stored in a 15 mL tube with 3 mL of minimum essential medium. Necropsy was performed for the one dead ox (Cattle ID: 104V1296; Table 1) and another severely diseased ox, which was euthanized humanely (Cattle ID: 104V1290; Table 1), and blood, oral and nasal swabs, and tissues from the skin nodules, lung, and abomasun of the two cattle were sampled. These samples were brought back to the Animal Health Research Institute, which is the National Veterinary Laboratory of Taiwan.
Table 1. Detection of lumpy skin disease viral DNA in the samples collected from the diseased cattle at the first infected farm in Kinmen Island, Taiwan during the first outbreak in 2019.
| Cattle ID | Age (year-old) | Samples taken | Samples tested positive |
|---|---|---|---|
| 104V1290 | 5 | Blood, oral and nasal swabs, skin, lung, abomasum | Oral and nasal swabs, skin |
| 104V1296 | 5 | Blood, oral and nasal swabs, skin, lung, abomasum | Skin |
| 102V18 | 7 | Blood, oral and nasal swabs | None |
| 104V369 | 5 | Blood, oral and nasal swabs | None |
| 104V981 | 5 | Blood, oral and nasal swabs | None |
| 104V1288 | 5 | Blood, oral and nasal swabs | None |
| 104V1384 | 5 | Blood, oral and nasal swabs | Oral and nasal swabs |
| 104V1453 | 5 | Blood, oral and nasal swabs | None |
| 104V1461 | 5 | Blood, oral and nasal swabs | None |
| 104V1464 | 5 | Blood, oral and nasal swabs | None |
| 104V1467 | 5 | Blood, oral and nasal swabs | Oral and nasal swabs |
| 105V195 | 4 | Blood, oral and nasal swabs | Oral and nasal swabs |
The samples were tested by conventional polymerase chain reaction (PCR) and real-time PCR recommended by the World Organization for Animal Health. A positive result was interpreted when both PCRs gave positivity.
Additionally, three cattle (Cattle IDs: 20H42-8, 20H42-9, and 20H42-10) at a nearby cattle farm was sampled on August 12, 2020. Skin nodules, blood, and nasal and oral swabs were sampled.
DNA extraction
For the swabs from each cattle, 3 mL of medium was centrifuged at 3,000 rpm and the supernatant was collected and stored at 4°C until use. To prepare 10% (w/v) homogenate, sampled tissues were homogenized, which was followed by the addition of 3 mL of minimum essential medium supplemented with 5% fetal calf serum and 1% antibiotics. The resuspended homogenate was centrifuged at 3,000 rpm and the supernatant was collected and stored at 4°C until use. Anti-coagulated blood along with the supernatants obtained from the swab and tissue samples were subjected to automated nucleic acid extraction with either TANBead Nucleic Acid Extraction kit (Taiwan Advanced Nanotech Inc., Taoyuan City, Taiwan) or MagNA Pure Compact Nucleic Acid Isolation Kit I (Roche Diagnostics, Mannheim, Germany).
Polymerase chain reaction (PCR)
To detect the possible presence of LSDV-specific DNA, the nucleic acid extracted from the samples which is listed in Table 1 was detected by conventional and real-time PCR targeting the envelope protein (P32) gene [6, 24]. For the samples taken from the nearby farm on August 12, 2020, the extracted nucleic acid of those samples was tested by real-time PCR only.
Virus isolation
The skin samples for virus isolation were obtained from two cattle of the Livestock Research Institute, Kinmen County: Cattle IDs 104V1290 and 104V1296 (Table 1) and two from a nearby farm sampled on August 12, 2020: sample numbers 20H42-8 and 20H42-10. Each skin sample was homogenized with minimal essential medium supplemented with penicillin (500 U/mL) and streptomycin (500 mg/mL) to make a 10% w/v suspension. After the suspension was centrifugated, its supernatant was collected, filtrated through a 0.45-micrometer membrane filter, and inoculated onto a monolayer of primary sheep testicle cells in 12-well culture plates. The inoculated cells were then incubated for 1 hr in a humid, 37°C, 5% CO2 atmosphere using minimal essential medium containing Earl’s salts, L‐glutamine, fetal bovine serum (10% v/v) and Antibiotic-Antimycotic (100 U/mL penicillin, 100 µg/mL streptomycin, and 25 µg/mL amphotericin B; Invitrogen, Carlsbad, CA, USA). Followed by the 1-hr incubation, the cells were washed with minimum essential medium containing the aforementioned materials except that the ratio of fetal bovine serum was 2% (v/v). These inoculated cells were cultivated for seven days and observed daily. Whenever cytopathic effects (CPE) were observed, the supernatant of the culture was subjected to electron microscopy and real-time PCR for virus identification. Additional blind passage was performed if the cytopathic effect was not observed in the initial inoculation.
Electron microscopy
The sampled skin nodules from the cattle 104V1290 and 104V1296 were homogenized and subjected to negative staining electron microscopy with standard treatment. To examine the possible presence of virus particles produced by virus isolation, the supernatant of cell culture was also subjected to the electron microscopy as needed.
Nucleotide sequencing
To further characterize the identified virus, the coding sequence of G-protein-coupled chemokine receptor (GPCR), RNA polymerase 30 kDa polypeptide (RPO30), and P32 genes were amplified using the primers listed in Table 2. The PCR mixtures contained 2 µL of extracted viral DNA, 1x Super-Therm Thermostable DNA Polymerase reaction buffer (Bertec Enterprise Co., New Taipei City, Taiwan), 50 µM dNTP, 0.4 µM each forward and reverse primer, and 0.25 U of Super-Therm Thermostable DNA Polymerase (Bertec Enterprise Co.). The amplification program consisted of an initial step of 94°C for 4 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1 min, and a final elongation step at 72°C for 7 min.
Table 2. Oligonucleotide primers used in amplification of the capripoxvirus by conventional polymerase chain reactions.
| Protein coding gene | Oligonucleotides | Positions | Length of amplicon (bp) |
|---|---|---|---|
| GPCR | Forward: ATGAATTATACTCTTAGCACAGTT | 8511–7366 | 1,146 |
| Reverse: TTATCCAATGCTAATACTAC | |||
| RPO30 | Forward: ATGGATGATGATAATACTAATTCA | 28973–28368 | 606 |
| Reverse: TTATTTTTCTACAGCTCTAAAC | |||
| P32 | Forward: ATGGCAGATATTCCATTATATGTT | 65361–66329 | 969 |
| Reverse: CTAAATTATATACGTAAATAACA |
The PCR amplicons were sequenced using the 3700XL DNA analyzer (Applied Biosystems, Life Technologies, Carlsbad, CA, USA) by a commercial sequencing service (Mission Biotech, Taipei City, Taiwan). Each nucleotide position was sequenced at least five times. The nucleotide sequences were assembled by the SeqMan program within the bioinformatic analysis software DNASTAR (Lasergene Inc., Madison, WI, USA).
Sequence analysis
For sequence comparison, the obtained GPCR, RPO30, and P32 sequences were aligned with corresponding gene sequences available in GenBank using multiple alignment fast Fourier transform, or the MAFFT, program version 7 (https://mafft.cbrc.jp/alignment/software/) online service [12].
Phylogenetic relationships of viral isolates were analyzed by the maximum likelihood method with a best-fit substitution model and partition scheme [7, 13] evaluated using ModelFinder based on the Bayesian information criterion implemented in IQ-TREE version 1.6.9 [23], supporting bootstrap values were inferred from 1,000 replicates of ultrafast bootstrap approximation [11]. Phylogeny was visualized by FigTree version 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). The genetic distance was computed using MEGA X software [17].
RESULTS
Clinical and histopathological findings
Well delineated, firm, round, and raised nodules of the diseased cattle distributed on the head, neck, trunk, forelimb, tail, and scrotum (Fig. 2). Some nodules contained a characteristic necrotic center with a cone shape and a flat top. Multifocal poorly demarcated macules were observed on the mucous membrane of the buccal cavity and trachea.
Fig. 2.

Extensive distribution of dermal nodules appeared on the skin of diseased cattle (Cattle ID: 104V1290).
Histopathologically, the necrotizing dermatitis was characterized by vasculitis, perivascular fibroplasia, infarction, and infiltration of macrophages and some lymphocytes and eosinophils. Some skin lesions involved the deep dermis, subcutis, and the underlying muscular tissue. Keratinocytes in the epidermis and hair follicles revealed hydropic degeneration with the presence of eosinophilic intracytoplasmic inclusion bodies (Fig. 3). The inclusion bodies were also found in the macrophages and fibroblasts in the dermis.
Fig. 3.
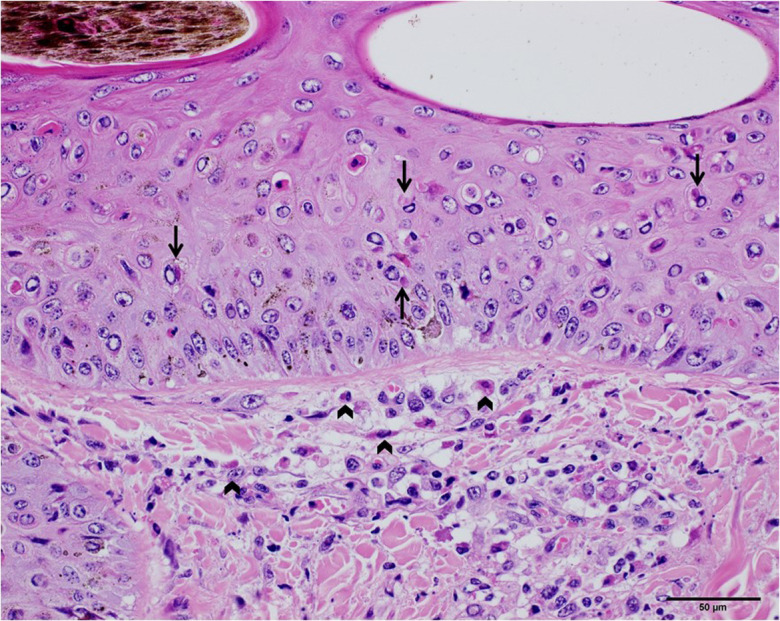
The skin of the diseased cattle (Cattle ID: 104V1290). Hydropic degeneration was observed in the dermis, as showed in the upper half of the photograph. Eosinophilic intracytoplasmic inclusion bodies in the keratinocytes and macrophages were pointed with arrows (scale bar=50 µm). The inclusion bodies in the fibroblasts and macrophages were pointed with wedge symbols.
Detection of LSDV DNA
Amplicons of 195 base pairs in length were obtained by the conventional PCR from the samples (mostly skin nodules) of the five diseased cattle. The nucleotide sequence of the amplicons obtained from one of the cattle, 104V1290 (Table 1), was further determined by DNA sequencing by Sanger sequencing. Comparison of the sequence of the PCR product to those available ones in GenBank demonstrated nucleotide identities of 99.27% to 99.29% with LSDV isolates identified in Zimbabwe (GenBank accession number KX033505; isolate Nya10) [18], Egypt (MN418202; isolate LSDV-EGY-BSU/2012-R2), Saudi Arabia (MN422449; isolate LSDV/KSA4/2016), and China (MN598005; isolate LSDV/Xinjiang/2019). Results obtained from the real-time PCR were consistent with those from the conventional PCR.
Isolation of lumpy skin disease virus
The attempt to isolate etiological virus from the bovine skin samples of the Livestock Research Institute, Kinmen County failed. On the other hand, LSDV was isolated successfully later from the nearby farm. The skin samples taken from one of the two diseased cattle of this farm gave positivity by the virus isolation. Upon the fifth days of the second blind passage, the inoculated sheep testicle cells showed CPE characterized by cell rounding, degeneration, and detachment. After identified by the real-time PCR and electron microscopy, the LSDV isolate was designated as LSDV/KM/Taiwan/2020.
Electron microscopy
Poxvirus-like particles were observed within the homogenate of skin nodules of diseased cattle 104V1290 and 104V1296 by negative stain electron microscopy (magnification × 100,000). The particles were approximately 290–330 × 260–300 nm in size and showed a surface structure of thread-like tubules (Fig. 4).
Fig. 4.
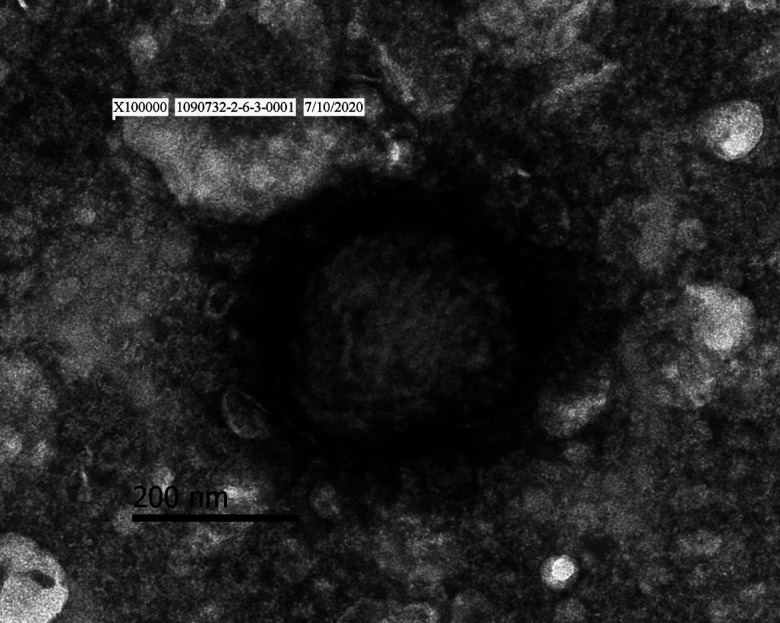
Transmission electron micrograph of poxvirus-like particles with negative staining (magnification × 100,000) in the bovine skin of lumpy skin disease.
Phylogenetic analyses
The obtained sequences of the GPCR (1,146 bp), RPO30 (606 bp), and P32 (969 bp) genes in this study were deposited in GenBank database with the accession numbers MZ934385, MZ934386, and MZ934387, respectively.
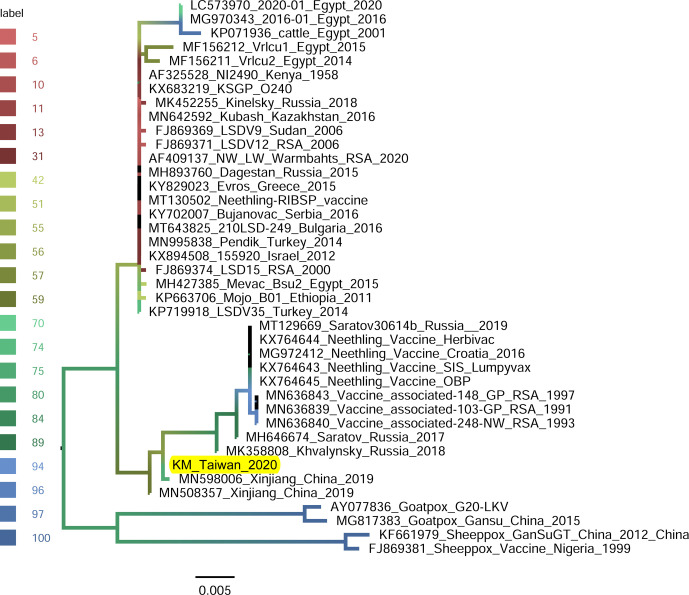
GPCR-gene-based phylogeny: The GPCR gene of LSDV/KM/Taiwan/2020 isolate was analyzed with 39 available sequences: composed of 35 field and vaccine strains of LSDVs, 2 sheeppox viruses and 2 goatpox viruses. Of the sequences analyzed for their phylogenetic relationships, the sheeppox viruses and goatpox viruses were as the outer group. The phylogenetic tree based on the GPCR gene (Fig. 5) presents two distinct groups: one composed of 23 viruses which are mainly field isolates and the other composed of 13 vaccine and vaccine-derived viruses. The LSDV/KM/Taiwan/2020 was grouped with vaccine and vaccine-derived viruses and shared nucleotide identities higher than 98.78% within the group. In this group, the LSDV/KM/Taiwan/2020 isolate was closest to the LSDV identified in Xinjiang Province of China in 2019 (Accession number MN598006) with 99.91% nucleotide identity.
Fig. 5.
Maximum-likelihood phylogenetic tree showing the relationship between the G-protein-coupled chemokine receptor (GPCR) gene of lumpy skin disease virus isolated in Kinmen, 2020 (indicated as “KM_Taiwan_2020”) with the other GPCR gene of the same virus available in GenBank. Sheeppox and Goatpox viruses retrieved from GenBank were employed as outgroup. The branch color represents the value of the ultrafast bootstrap approximation based on 1,000 replications.
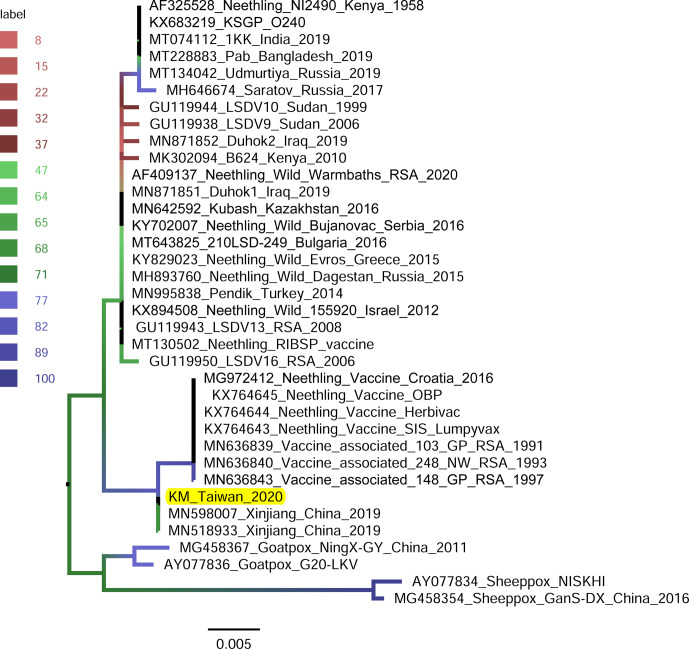
RPO30-gene-based phylogeny: The RPO30 gene of the LSDV/KM/Taiwan/2020 isolate was analyzed with 35 available sequences: composed of 31 field and vaccine LSDVs and 4 aforementioned sheeppox and goatpox viruses. Similar to the GPCR-based tree, the phylogenetic tree based on the RPO30 gene (Fig. 6) shows two groups: one composed of 22 viruses which are mainly field isolates and the other composed of 10 vaccine and vaccine-derived viruses. The LSDV/KM/Taiwan/2020 was grouped with vaccine and vaccine-derived viruses and shared nucleotide identities higher than 99.67% within the group. The RPO30 gene of LSDV/KM/Taiwan/2020 isolate and those of two LSDVs from Xinjiang Province, China in 2019 (Accession numbers MN598007 and MN518933) were 100.0% identical.
Fig. 6.
Maximum-likelihood phylogenetic tree showing the relationship between the RNA polymerase 30 kDa polypeptide (GPO30) gene of lumpy skin disease virus isolated in Kinmen, 2020 (indicated as “KM_Taiwan_2020”) with the other GPO30 gene of the same virus available in GenBank. Sheeppox and Goatpox viruses retrieved from GenBank were employed as outgroup. The branch color represents the value of the ultrafast bootstrap approximation based on 1,000 replications.
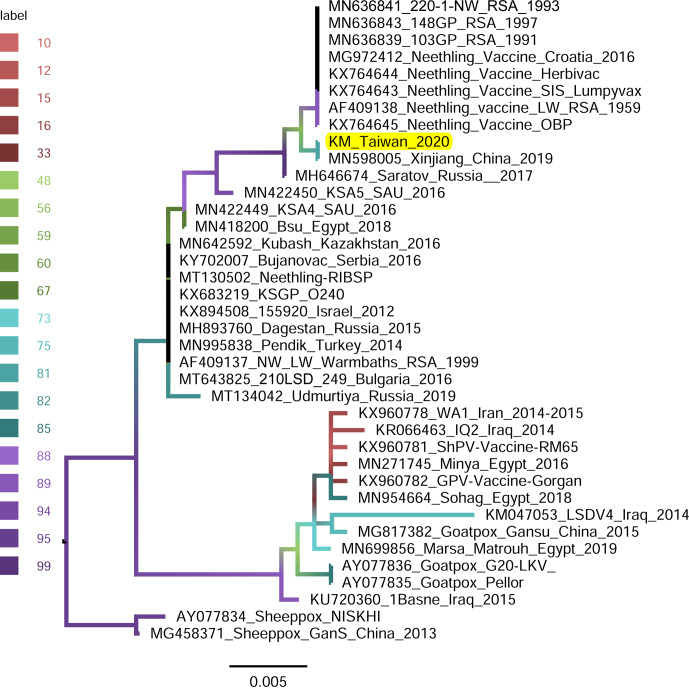
P32-gene-based phylogeny: The P32 gene of LSDV/KM/Taiwan/2020 isolate was analyzed with 37 available sequences: composed of 33 field and vaccine LSDVs and 4 sheeppox and goatpox viruses. The LSDVs in the P32-gene-based phylogenetic tree (Fig. 7) were divided into three groups and each group comprised a mixed population of field isolates and vaccine viruses. The LSDV/KM/Taiwan/2020 was also phylogenetically closest to the isolate from Xinjiang Province, China in 2019 (Accession number MN598005) with a nucleotide identity of 100.0%.
Fig. 7.
Maximum-likelihood phylogenetic tree showing the relationship between the envelope protein (P32) gene of lumpy skin disease virus isolated in Kinmen, 2020 (indicated as “KM_Taiwan_2020”) with the other P32 gene of the same virus available in GenBank. Sheeppox and Goatpox viruses retrieved from GenBank were employed as outgroup. The branch color represents the value of the ultrafast bootstrap approximation based on 1,000 replications.
DISCUSSION
The present article records the first discovery of LSDV in Taiwan’s territory and the finding proved the first appearance of this bovine pathogen around coastal countries of East Asia. Afterward, we performed phylogenetic analysis about the viral sequence to investigate the possible origin of the invading LSDV.
The causative agent of this outbreak, LSDV, was identified based on the findings of electron microscopy and molecular detection. Diagnostic electron microscopy, with its advantages of broad specimen acceptability and rapid sample preparation, is quite suitable for instant identification of infectious agents [10]. As exemplified by this case, visualizing poxvirus-like particles within the skin samples by electron microscopy (Fig. 4) and the molecular detection gave the results within a few hours after the samples arrived laboratories. Histopathological findings (Fig. 3), consistent with previously described LSD lesions [1], and later isolating LSDV with primary cell culture from the skin sampled at the nearby farm further supported that the disease observed in Kinmen was an LSD outbreak.
Following the identification of LSDV, this event was reported to the World Organization for Animal Health on July 10, 2020. Control measures including movement restriction, insect and pest control, intensive field investigation, and humane culling of diseased cattle had been implemented in a bid to control LSD effectively. Emergency vaccination with an imported attenuated LSD vaccine (Onderstepoort Biological Products, Pretoria, South Africa), generously provided by the European Union, was undertaken from July 23 to August 4 and all 6,342 clinical healthy cattle on Kinmen Island were vaccinated. By September 21, 218 infected cattle in 34 farms, as shown on the map of Fig. 1, were identified and humanely euthanized. No additional cases have been confirmed thenceafter.
The invading LSDV was similar to those discovered in China in 2019, evidenced by our phylogenetic analyses. A few viral genomic regions, GPCR gene [20, 22], ORF011, ORF012, ORF036 [16], P32 gene [21], and RPO30 gene [3, 19], have been used in studying phylogeny of LSDV isolates. In the present study, GPCR, RPO30, and P32 genes were employed in generating phylogenetic trees (Figs. 5, 6, and 7, respectively). All the trees illustrated that the isolates closest to the LSDV/KM/Taiwan/2020 isolate were those identified in Xinjiang province, China in 2019.
The definite origin of the LSDV responsible for this outbreak remains unknown. The case documented in the present study was the first identified farm but may not be the first affected. The field investigation conducted soon after the laboratory diagnosis found that a number of cattle in other farms on Kinmen Island were also infected and the infected cattle displayed various stages of dermal lesions, indicating that many farms were suffering from the disease simultaneously. Therefore, it was not possible to depict where the virus forayed into the island and how the virus transmitted between the farms. Although we were unable to locate the source of the causative LSDV, viral sequence similarities and geographical closeness suggested that China might be the suspected source of LSDV. The belated announcement by Ministry of Agriculture and Rural Affairs of the People’s Republic of China on July 15, 2020 reported that several LSD had broken out in five southern Chinese provinces (Fujian, Jiangxi, Guangdong, Anhui, and Zhejiang) from early June to middle July (http://www.xmsyj.moa.gov.cn/yqfb/202007/t20200715_6348686.htm, in simplified Chinese), further implying the chronological relationships between the outbreaks.
These outbreaks highlighted that LSD is being an emerging disease in Asia. Since 2019, recent LSD outbreaks occurring in Bangladesh, China, Syria, Taiwan, and later in Nepal, Vietnam, and Bhutan were all the first occurrences in these countries. Similar to the terrestrial expansion of peste des petits ruminants [4], African swine fever [14], and African horse sickness [15] during the past years, LSD had also left its original habitats, African and the Middle East, and been rapidly expanding eastward and northward.
In conclusion, the introduction of LSD into Kinmen Island is the first occurrence of this disease in the country. Phylogenetic analysis suggested the introduced LSDV may come from China. The outbreaks spotlighted that livestock industries of Asian countries were being threatened by a few emerging infectious viral diseases.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Bureau of Animal and Plant Health Inspection and Quarantine for providing geographic information of the infected farms.
REFERENCES
- 1.Andrews AH, Blowey RW, Boyd H, Eddy RG. 2004. Bovine Medicine: Diseases and Husbandry of Cattle, 2nd ed., Blackwell Publishing, Oxford. [Google Scholar]
- 2.Babiuk S, Bowden TR, Boyle DB, Wallace DB, Kitching RP. 2008. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound Emerg Dis 55: 263–272. doi: 10.1111/j.1865-1682.2008.01043.x [DOI] [PubMed] [Google Scholar]
- 3.Badhy SC, Chowdhury MGA, Settypalli TBK, Cattoli G, Lamien CE, Fakir MAU, Akter S, Osmani MG, Talukdar F, Begum N, Khan IA, Rashid MB, Sadekuzzaman M. 2021. Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains. BMC Vet Res 17: 61. doi: 10.1186/s12917-021-02751-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banyard AC, Wang Z, Parida S. 2014. Peste des petits ruminants virus, eastern Asia. Emerg Infect Dis 20: 2176–2178. doi: 10.3201/eid2012.140907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blowey RW, Weaver AD. 2011. Color Atlas of Diseases and Disorders of Cattle, 3rd ed., Elsevier, St. Louis. [Google Scholar]
- 6.Bowden TR, Babiuk SL, Parkyn GR, Copps JS, Boyle DB. 2008. Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology 371: 380–393. doi: 10.1016/j.virol.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernomor O, von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol 65: 997–1008. doi: 10.1093/sysbio/syw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubbins S, Stegeman A, Klement E, Pite L, Broglia A, Cortiñas Abrahantes J. 2020. Inferences about the transmission of lumpy skin disease virus between herds from outbreaks in Albania in 2016. Prev Vet Med 181: 104602. doi: 10.1016/j.prevetmed.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haig D. 1957. Lumpy skin disease. Bull Epizoot Dis Afr 5: 421–430. [Google Scholar]
- 10.Hazelton PR, Gelderblom HR. 2003. Electron microscopy for rapid diagnosis of infectious agents in emergent situations. Emerg Infect Dis 9: 294–303. doi: 10.3201/eid0903.020327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35: 518–522. doi: 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20: 1160–1166. doi: 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14: 587–589. doi: 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedkovid R, Sirisereewan C, Thanawongnuwech R. 2020. Major swine viral diseases: an Asian perspective after the African swine fever introduction. Porcine Health Manag 6: 20. doi: 10.1186/s40813-020-00159-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King S, Rajko-Nenow P, Ashby M, Frost L, Carpenter S, Batten C. 2020. Outbreak of African horse sickness in Thailand, 2020. Transbound Emerg Dis 67: 1764–1767. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Chander Y, Kumar R, Khandelwal N, Riyesh T, Chaudhary K, Shanmugasundaram K, Kumar S, Kumar A, Gupta MK, Pal Y, Barua S, Tripathi BN. 2021. Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS One 16: e0241022. doi: 10.1371/journal.pone.0241022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35: 1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mafirakureva P, Saidi B, Mbanga J. 2017. Incidence and molecular characterisation of lumpy skin disease virus in Zimbabwe using the P32 gene. Trop Anim Health Prod 49: 47–54. doi: 10.1007/s11250-016-1156-9 [DOI] [PubMed] [Google Scholar]
- 19.Molini U, Aikukutu G, Khaiseb S, Haindongo NN, Lilungwe AC, Cattoli G, Dundon WG, Lamien CE. 2018. Molecular characterization of lumpy skin disease virus in Namibia, 2017. Arch Virol 163: 2525–2529. doi: 10.1007/s00705-018-3891-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochwo S, VanderWaal K, Ndekezi C, Nkamwesiga J, Munsey A, Witto SG, Nantima N, Mayanja F, Okurut ARA, Atuhaire DK, Mwiine FN. 2020. Molecular detection and phylogenetic analysis of lumpy skin disease virus from outbreaks in Uganda 2017–2018. BMC Vet Res 16: 66. doi: 10.1186/s12917-020-02288-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouby SR, Safwat NM, Hussein KH, Abdel-Ra’ouf AM, Madkour BS, Abdel-Moneim AS, Hosein HI. 2021. Lumpy skin disease outbreaks in Egypt during 2017–2018 among sheeppox vaccinated cattle: Epidemiological, pathological, and molecular findings. PLoS One 16: e0258755. doi: 10.1371/journal.pone.0258755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saltykov YV, Kolosova AA, Filonova NN, Chichkin AN, Feodorova VA. 2021. Genetic evidence of multiple introductions of lumpy skin disease virus into Saratov Region, Russia. Pathogens 10: 1616–1624. doi: 10.3390/pathogens10060716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32: 268–274. doi: 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuppurainen ESM, Venter EH, Coetzer JAW. 2005. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort J Vet Res 72: 153–164. doi: 10.4102/ojvr.v72i2.213 [DOI] [PubMed] [Google Scholar]
- 25.Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, Lyons NA, De Clercq K, Upton C, Bowden TR, Babiuk S, Babiuk LA. 2017. Review: capripoxvirus diseases: current status and opportunities for control. Transbound Emerg Dis 64: 729–745. doi: 10.1111/tbed.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Organization for Animal Health.2018. Chapter 3.4.12: Lumpy Skin Disease, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Paris. [Google Scholar]