Abstract
Background
Thrombosis is reported to occur more often among patients with COVID‐19 than otherwise expected in the setting of viral pneumonia and sepsis. Systemic inflammatory biomarkers may be associated with venous thromboembolism (VTE) risk. The ISTH subcommittee on Predictive and Diagnostic Variables in Thrombotic Disease aimed to report the evidence on prognostic biomarkers for VTE in hospitalized patients with COVID‐19.
Methods
Using a standardized Preferred Reporting Items for Systematic Reviews and Meta‐analysis methodology, we conducted a systematic literature review to identify studies reporting prognostic biomarkers for VTE among hospitalized patients with COVID‐19. Eligible studies included adults hospitalized with COVID‐19 and reported the prognostic associations between any biomarker measured on admission, and the subsequent diagnosis of deep vein thrombosis or pulmonary embolism. Two authors reviewed titles and abstracts, and three authors extracted study data and performed review of bias. Results were displayed descriptively. Meta‐analysis was not possible.
Results
From the initial 196 identified studies, full‐text review was performed for 72 studies. Admission D‐dimer levels were associated with VTE during hospitalization in five studies, and elevated platelet count was associated with VTE during hospitalization in one study. The risk of bias ranged from low to high for included studies. Overall, there was a paucity of high‐quality prognostic studies. Studies on other biomarkers did not meet the systematic review inclusion criteria.
Conclusions
Admission D‐dimer was associated with VTE diagnosis during hospitalization for COVID‐19; however, prospective validation of this finding is needed to identify optimal D‐dimer thresholds to guide VTE prophylaxis measures.
Keywords: COVID‐19, D‐dimer, prognostic biomarkers, venous thromboembolism, VTE
Essentials.
Prognostic biomarkers may predict venous thromboembolism (VTE) in hospitalized patients with COVID‐19.
We performed a systematic review to report prognostic biomarkers for VTE in hospitalized adults.
Few low‐quality studies suggested D‐dimer drawn on admission may predict diagnosis later of VTE.
More research is needed before biomarkers are used to stratify the risk of VTE in these patients.
1. BACKGROUND
The severe acute respiratory syndrome coronavirus 2 infection (COVID‐19) was identified in December 2019, and the World Health Organization declared a pandemic in March 2020. 1 The risk of venous thromboembolism (VTE) in hospitalized patients with COVID‐19 infection is increased compared to other viral pneumonia or acute respiratory distress syndrome illness. 2 , 3 , 4 , 5 , 6 , 7 , 8 Reports of COVID‐19–associated VTE incidence have varied considerably with initial Chinese reports of VTE in up to 40% of hospitalized patients. 9 , 10 Subsequently, it was recognized that the real incidence of VTE is closer to 20% in intensive care unit (ICU) patients 5 , 11 and less than 10% in patients admitted on the medical wards. 12 , 13 The control arms of the randomized trials assessing different anticoagulant dosing regimens have reported VTE rates between 4% and 9% of hospitalized patients. 12 , 14
Because of the perceived high risk of COVID‐19–associated VTE, many centers started to prescribe anticoagulants at higher intensity for VTE prevention in patients hospitalized with acute COVID‐19. Given the bleeding risks associated with anticoagulation, identification of patients at increased risk of VTE could enable targeted anticoagulation for those with the highest benefit–risk ratio.
There have been many reported associations between inflammatory biomarkers and both COVID‐19 mortality 15 , 16 , 17 , 18 , 19 and VTE. 20 , 21 , 22 , 23 Among these biomarkers, D‐dimer, a product of fibrin degradation, is an indirect marker of thrombin generation and is also raised in acute inflammatory states. Many studies have reviewed the association between D‐dimer blood concentration and COVID‐19–associated VTE diagnoses, however, most have been retrospective analyses of administrative data, introducing the potential for confounding.
The ISTH Standardization Subcommittee on Predictive and Diagnostic Variables convened with the aim of performing a systematic review and descriptive analysis to report the evidence on the prognostic biomarkers for identifying hospitalized patients with COVID‐19 at higher risk of developing VTE.
2. METHODS
The methodology of this study was presented at the 2021 ISTH congress, Philadelphia, Pennsylvania. 24 This systematic review and descriptive analysis was performed in adherence with the Preferred Reporting Items for Systematic Reviews and Meta‐analysis statement. 25 The study protocol was published on the international prospective registry of systematic reviews (registered March 25, 2021, CRD42021245249; www.crd.york.ac.uk). We searched for studies reporting the prognostic association between any biomarker measured at hospitalization in patients with COVID‐19 and the subsequent future diagnosis of VTE. We searched PubMed including combinations of MeSH terms and title/abstract terms, which included MEDLINE records, PubMed Central records, and the NCBI Bookshelf for studies in all languages until March 18, 2021. The search strings are available in Appendix S1. This study was approved by uthe Intermountain Healthcare ethics review board. This research was conducted in accordance.
2.1. Study eligibility and review
Inclusion criteria were studies including hospitalized adult patients with a confirmatory laboratory test for COVID‐19, or an agreed‐upon defined clinical diagnosis such as findings characteristic for COVID‐19 on computed tomography (CT) pulmonary angiography in the proper clinical setting. Studies had to report VTE during follow‐up as the primary outcome. To be included, the study had to report proximal lower limb deep vein thrombosis (DVT) and/or pulmonary embolism (PE) by objective testing. Studies had to measure the biomarker of interest on hospital admission. Studies reporting exclusively on unusual‐site thromboses, isolated distal DVT, catheter‐associated DVT, or arterial thrombosis were excluded. We excluded studies with fewer than 50 patients to avoid inclusion of anomalous results. Diagnostic studies where biomarkers were tested as part of investigation for VTE were excluded.
Titles and abstracts were independently reviewed by two authors using the online program Covidence. If either author indicated that the publication should be reviewed further, the publication underwent full‐text review. Three authors performed full‐text review, and disagreements were resolved by discussion and consensus. When relevant information was missing (e.g., the timing of biomarker testing), the paper was excluded.
2.2. Data extraction
Included studies underwent systematic data extraction into a standardized data capture tool by three authors who performed this task independently. Data extraction included first author name, country of origin, ICU versus hospital ward, single hospital versus multiple hospital, follow‐up duration, timing of biomarker acquisition, study design, use of thromboprophylaxis, population age and sex distribution, VTE outcome type (DVT vs. PE vs. both), biomarker (including manufacturer) and the reported study results (extracted as reported in the publication).
2.3. Quality assessment
Risk of bias for selected studies was assessed using the Quality in Prognosis Studies risk‐of‐bias assessment instrument for prognostic factor studies (modified from Hayden et al.). 26 The six risk‐of‐bias domains were study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. Studies were rated for each domain (yes, no, partial, unsure), and ultimately a risk of bias characterized as low, moderate, or high. Each study was independently assessed by three co‐authors for risk of bias, and any disagreement was resolved upon discussion.
2.4. Analysis
While the initial study design included meta‐analysis, an early determination of the number and quality of included studies led to a descriptive analysis only. Results were reported as presented by the study authors, and note was made whether associations were adjusted for other prognostic factors.
3. RESULTS
We identified 196 studies in our search, of which 124 were excluded upon review of the title and abstract. Seventy‐two publications were selected for full‐text review8, 20, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80 including 13 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 identified by experts in the field. Reasons for study exclusions are presented in Figure 1, the most common included being a diagnostic, not prognostic, study (n = 24), biomarker timing not specified (n = 11), and fewer than 50 subjects enrolled (n = 11). Study characteristics of included studies are found in Table 1.
FIGURE 1.
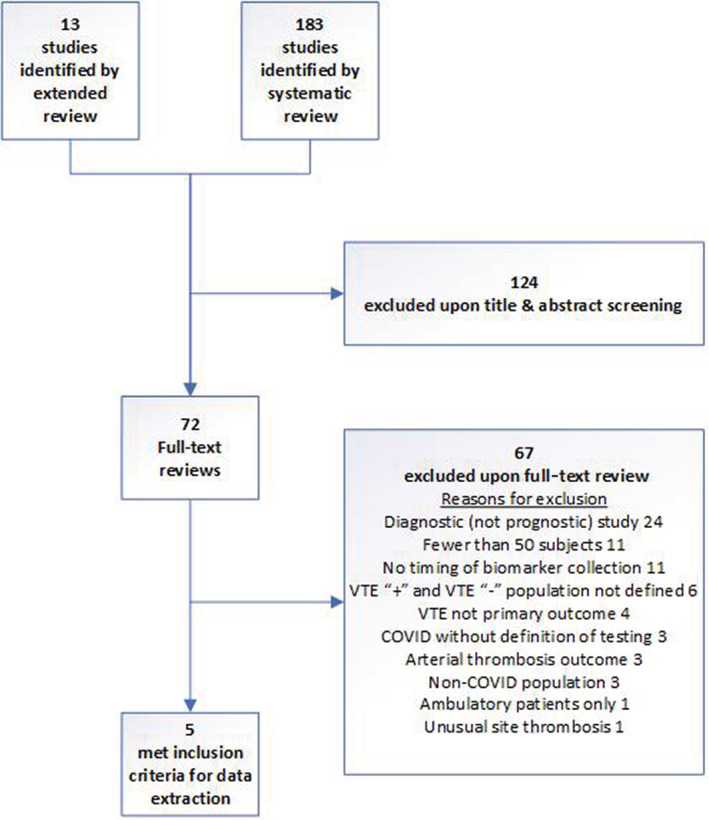
Consort diagram of included studies
TABLE 1.
Included studies and select study characteristics reporting biomarkers predictive of VTE
| Study/Country of origin | Number of subjects | Study dates | Location | Age a , b /%male | Biomarker | Multivariate adjustment? | Increased risk for VTE |
|---|---|---|---|---|---|---|---|
| Artifoni/France | 71 | Mar 25, 2020–Apr 10, 2020 | Wards and ICU | 64 b /61 | D‐dimer | No | Yes |
| Bilalogiu/USA | 3334 | Mar 1, 2020–Apr 17, 2020 | Wards and ICU | 64 a /60 | D‐dimer | Yes | Yes |
| Maatman/USA | 109 | Mar 12, 2020–Mar 31, 2020 | ICU | 61 a /57 | D‐dimer | No | Yes |
| Increased platelets | No | Yes | |||||
| Middeldorp/Netherlands | 198 | Jan 1, 2020–Apr 22, 2020 | Wards and ICU | 61 a /66 | D‐dimer | Yes | Yes |
| Trimaille/France | 289 | Feb 25, 2020–Apr 19, 2020 | Wards | 62 a /59 | D‐dimer | No | Yes |
| Increased WBC | Yes | Yes |
Abbreviations: CRP, C‐reactive protein; ICU, intensive care unit; VTE, venous thromboembolism; WBC, white blood cell count.
Mean.
Median.
Among the studies that met the inclusion criteria, Middeldorp et al. 94 was a single hospital retrospective study in the Netherlands that enrolled adult patients who were COVID‐19 positive, admitted from the onset of the pandemic until April 22, 2020. Pharmacologic VTE chemoprophylaxis was administered to 84%. Patients were followed until the time of death or discharge or April 30, 2020, whichever occurred first. Testing for VTE was initially conducted at the discretion of the treating physician. However, during the study period, screening with proximal lower limb venous compression ultrasonography (CUS) was variably implemented in the ICU. When it was determined that the study would end on April 30, 2020, lower limb proximal CUS was uniformly performed among all hospitalized medical ward patients on April 20, 2020. Among the 198 patients (75 ICU, 123 medical ward), 39 thromboembolic events occurred, including 13 PEs, and 26 DVTs (14 proximal DVTs, 11 distal DVTs, and 1 upper extremity DVT). The VTE event rate was 19.7%, and 6.6% of patients experienced PE. D‐dimer was the only prognostic biomarker that was collected within 72 h of admission. The median D‐dimer value for all patients at baseline was 1.1 mg/L (95% confidence interval [CI], 0.7–2.3) and 2.0 (95% CI, 0.8–8.1) among ICU patients compared with 1.1 (95% CI, 0.7–1.6) among ward patients. D‐dimer association with VTE was reported as a hazard ratio in a competing risk regression model. The median D‐dimer was 2.6 mg/L (95% CI, 1.1–18) among patients with VTE compared to 1.0 mg/L (95% CI, 0.7–1.7) among those without VTE. Following multivariate adjustment for age, sex, and ICU admission, the hazard ratio for VTE was 1.4 (95% CI, 1.1–1.9).
Artifoni et al. 95 reported a multicenter retrospective French study that enrolled adult patients who were COVID‐19 positive or those with a typical COVID‐19 pattern on chest CT admitted to the medical ward in two French hospitals between March 25 and April 10, 2020.
Pharmacologic VTE chemoprophylaxis was administered to 99%. Patients were followed until the time of death or discharge. All patients had a standardized systematic lower‐limb CUS screening for DVT at hospital discharge or earlier if DVT was clinically suspected. The VTE event rate was 22.5%, and 10% of patients experienced PE. Among the 71 patients, 16 thromboembolic events occurred including 7 PEs and 9 DVTs (7 were isolated distal DVT). D‐dimer was drawn upon admission in 65 of the 71 patients. The median D‐dimer for all patients at baseline was 0.79 mg/L (95% CI, 0.48–1.61 mg/L). The median D‐dimer was significantly higher, at 1.63 mg/L (95% CI, 0.86–4.94) among patients with VTE compared to 0.67 mg/L (95% CI, 0.45–1.12) among those without VTE. No multivariable adjustment analysis was performed.
Bilaloglu et al. 92 reported a retrospective study among sputum or nasopharyngeal‐, or oropharyngeal‐swabbed adult patients who were COVID‐19 positive and admitted to a US medical ward or ICU between March 1 and April 17, 2020. “Low‐dose” pharmacologic VTE chemoprophylaxis was administered “in most patients.” Patients were followed until the time of death, discharge, or end of study. VTE assessment occurred at the discretion of the physician during routine clinical care, and natural language processing was used to interrogate the electronic health record to identify thrombotic events with findings confirmed upon manual chart review. Among the 3334 patients, there were 207 VTE events (6.2%), with 106 (3.2%) of patients with PE, and 129 (3.9%) with DVT. Following multivariable adjustment, higher D‐dimer levels at hospital presentation were associated with a VTE.
Trimaille et al. 91 reported a nine‐hospital retrospective study in France among consecutive adults with COVID‐19 with either a positive nasopharyngeal swab or typical findings of COVID‐19 at chest CT admitted to the medical ward or ICU between February 25 and April 19, 2020. Standard preventive thromboprophylaxis was administered to some (however, the percentage was not reported). Patients were followed until the time of death, discharge, or end of study. VTE assessment occurred at the discretion of the physician during routine clinical care. Among the 289 patients, there were 49 VTE events (17.6%), with 42 PEs, 12 DVTs, and 3 cerebral venous sinus thromboses. Following univariable adjustment, D‐dimer at hospital admission was associated with VTE. Following multivariable adjustment, increased leukocyte count was associated with VTE.
Maatman et al. 87 reported a three‐hospital retrospective US study among consecutive adults with laboratory‐confirmed COVID‐19 admitted to the ICU between March 12 and March 31, 2020. One hundred percent of patients received standard chemoprophylaxis. Patients were followed until the time of death, discharge, or May 6, 2020. VTE assessment occurred at the discretion of the physician during routine clinical care. Among the 109 patients, there were 31 VTE events (28%), with 26 having DVT, 4 having DVT/PE, and 1 PE alone. Following univariable adjustment, D‐dimer and elevated platelet count at hospital admission was associated with VTE. Neutrophil : lymphocyte ratio, lactate dehydrogenase, C‐reactive protein, and fibrinogen level were not.
Risk of bias for each study is reported in Table 2.
TABLE 2.
Quality in Prognosis Studies risk‐of‐bias assessment of selected studies rating of risk of bias for included studies
| Artifoni | Middeldorp | Bilalogiu | Maatman | Trimaille | |
|---|---|---|---|---|---|
| Study participation | Moderate | Moderate | Moderate | Moderate | Low |
| Study attrition | High | Moderate | High | High | High |
| Prognostic factor management | High | Low | Moderate | Moderate | Moderate |
| Outcome measurement | Moderate | Low | High | Low | Moderate |
| Study confounding | High | High | High | High | Moderate |
| Statistical analysis and reporting | Moderate | Moderate | Moderate | Moderate | Moderate |
Note: Green: Low risk of bias; Yellow: Moderate risk of bias; Red: High risk of bias.
4. DISCUSSION
We conducted this systematic review to assess the prognostic association between admission biomarkers and subsequent diagnosis of VTE among patients hospitalized for COVID‐19. Despite the high number of studies drawing conclusions on the prognostic value of D‐dimer and other biomarkers for VTE, few met our inclusion criteria. The evidence to date suggests that D‐dimer may be predictive of future VTE diagnosis during hospitalization for COVID‐19, although inadequate evidence exists to refute possible candidate biomarkers, including white blood cell count and platelet count, among others. There are recent international randomized trials comparing the effectiveness of prophylactic‐dose to treatment‐dose anticoagulation among patients who are moderately or severely ill with COVID‐19. 12 , 13 , 14 These trials provide additional indirect evidence that D‐dimer levels are associated with the risk of VTE during hospitalization. For example, the VTE rate was 2.5% in the control arm of one study enrolling patients with COVID‐19 with D‐dimer level above the manufacturer‐recommended cutoff, 12 compared to 15% symptomatic DVT and 8% symptomatic PE in another study enrolling patients with COVID‐19 with D‐dimer more than four times the manufacturer‐recommended cutoff. 14
Our most important finding was a general low quality of evidence among published studies. Of those included studies, only one was prospectively conducted, and risk‐of‐bias scores ranged from low to high risk of bias. All studies had been truncated and terminated data collection within 3 months of the pandemic start. No study followed the entire cohort to a predetermined time end point; rather, each study was stopped at a set date. Inter‐ and intrastudy variability existed in outcome measurement. Two main methodological limitations were observed among the excluded studies. The first limitation was a great heterogeneity among studies regarding the measured VTE outcome and the lack of a clear VTE definition and including diagnostic criteria. The second major limitation was the absence of information on the timing of biomarker measurement in many studies, precluding any conclusion on their prognostic rather than diagnostic usefulness. For prognostic assessment, a biomarker should be measured at a time VTE is neither suspected nor already diagnosed.
The main limitations of our systematic review are the low number of studies that met inclusion criteria and the uncertain heterogeneity of aspects that permitted inclusion (e.g., D‐dimer assay used, interstudy variability in ultrasound technology and technique) that inform outcome measurement. It is unknown whether any biomarkers studied early in the pandemic will be relevant to subsequent variants of the virus that may have different clinical manifestations. Limited data reporting from the individual does not permit analysis of any impact that race, ethnicity, and social determinants of health have on the outcomes. Despite the plenitude of published studies, few met the rigorous inclusion criteria, demonstrating the paucity of high‐quality studies published. We did not contact study authors to obtain further details on inclusion criteria such as biomarker timing and outcome definition. One of the studies that met the inclusion criteria described biomarkers upon admission as being acquired within the first 72 h. However, VTE was reported during those first 72 h such that uncertainty exists that these biomarkers could be interpreted as predictive and not diagnostic. We do not know the timing of D‐dimer measurement in relationship to when VTE chemoprophylaxis was administered. Strengths of this report include that we considered studies for which the diagnosis of COVID‐19 was confirmed according to current widely accepted laboratory and/or imaging criteria. The VTE outcome definition used was restricted to clinically relevant objectively diagnosed DVT and PE. We included studies reporting the timing of biomarker ascertainment so as to identify prognostic rather than diagnostic associations between biomarkers and VTE.
To our knowledge, our work represents the first systematic review reporting the prognostic value of biomarkers for VTE among hospitalized patients with COVID‐19. We concluded that admission D‐dimer may be associated with VTE diagnosis during hospital admission for COVID‐19; however, prospective validation of this finding is needed to identify optimal D‐dimer thresholds to guide VTE prophylaxis measures. Furthermore, at present there is a lack of evidence on alternative prognostic biomarkers for VTE. The quality of the studies performed and the extant literature to date is inadequate for us to endorse any biomarker to be adopted into routine clinical care to predict VTE among patients admitted to a hospital with COVID‐19 at this time.
AUTHOR CONTRIBUTIONS
All authors were involved in conceptualization, data curation, analysis, investigation, and methodology. SCW drafted the manuscript. All authors participated in the writing, review, and editing of later drafts and approval of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in concept, design, drafting of manuscript, critical review, and refinement.
RELATIONSHIP DISCLOSURE
SCW, DC, KDW, CM, JBH, PEM, PLD, and HRE report nothing to disclose. FAK discloses received research support from: Bayer, BMS, Boehringer‐Ingelheim, MSD, Daiichi‐Sankyo, Actelion, the Netherlands Organization for Health Research and Development, the Dutch Thrombosis Association, and the Dutch Heart Foundation.
FUNDING INFORMATION
No funding for this study was provided.
Supporting information
Appendix S1
Woller SC, de Wit K, Robert‐Ebadi H, et al. A systematic review of biomarkers among hospitalized patients with COVID‐19 predictive of venous thromboembolism: A communication from the Predictive and Diagnostic Variables Scientific and Standardization Committee of the ISTH . Res Pract Thromb Haemost. 2022;6:e12786. doi: 10.1002/rth2.12786
Handling Editor: Dr Cihan Ay
Contributor Information
Scott C. Woller, Email: scott.woller@imail.org, @SCwollerMD.
Kerstin de Wit, @kerstindewit.
Camila Masias, @camilamasias3.
Frederikus A. Klok, @erik_Klok_MD.
Paul L. den Exter, @pauldenexter.
Pierre‐Emmanuel Morange, @morange_pierre.
REFERENCES
- 1. WHO Director‐General's opening remarks at the media briefing on COVID‐19. 2020, March 11 [press release]. 2020, March 11.
- 2. Porfidia A, Pola R. Venous thromboembolism in COVID‐19 patients. J Thromb Haemost. 2020;18(6):1516‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Berre A, Marteau V, Emmerich J, Zins M. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID‐19 pneumonia. Diagn Interv Imaging. 2020;101(5):321‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Longchamp A, Longchamp J, Manzocchi‐Besson S, et al. Venous thromboembolism in critically ill patients with COVID‐19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4(5):842‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaptein FHJ, Stals MAM, Grootenboers M, et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID‐19 in the second and first wave. Thromb Res. 2021;199:143‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stals M, Grootenboers M, van Guldener C, et al. Risk of thrombotic complications in influenza versus COVID‐19 hospitalized patients. Res Pract Thromb Haemost. 2021;5(3):412‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan KW, Wong VT, Tang SCW. COVID‐19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese‐Western medicine for the management of 2019 novel coronavirus disease. Am J Chin Med. 2020;48(3):737‐762. [DOI] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mansory EM, Srigunapalan S, Lazo‐Langner A. Venous thromboembolism in hospitalized critical and noncritical COVID‐19 patients: a systematic review and meta‐analysis. TH Open. 2021;5(3):e286‐e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID‐19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic‐dose heparin vs standard prophylactic or intermediate‐dose heparins for thromboprophylaxis in high‐risk hospitalized patients with COVID‐19: the HEP‐COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. REMAP‐CAP Investigators , ACTIV‐4a Investigators , ATTACC Investigators , et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID‐19. N Engl J Med. 2021;385(9):790‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qu R, Ling Y, Zhang YH, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020;92(9):1533‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan C, Huang Y, Shi F, et al. C‐reactive protein correlates with computed tomographic findings and predicts severe COVID‐19 early. J Med Virol. 2020;92(7):856‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 20. Demelo‐Rodriguez P, Cervilla‐Munoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thromb Res. 2020;192:23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu J, Kong J, Wang W, et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D‐dimer in COVID‐19: a retrospective study in Suzhou China. Thromb Res. 2020;192:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu B, Li X, Chen J, et al. Evaluation of variation in D‐dimer levels among COVID‐19 and bacterial pneumonia: a retrospective analysis. J Thromb Thrombolysis. 2020;50(3):548‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thachil J, Longstaff C, Favaloro EJ, et al. The need for accurate D‐dimer reporting in COVID‐19: communication from the ISTH SSC on fibrinolysis. J Thromb Haemost. 2020;18(9):2408‐2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woller SC, De Wit K, Hansen JB, et al. Predictive and diagnostic variables Scientific Standardization Committee podium presentation: biomarkers predictive for VTE in hospitalized COVID‐19 patients: a systematic review paper presented at: International Society of Thrombosis and Haemostasis (ISTH2021); 17 July 2021, 2021; Virtual in Philedelphia.
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427‐437. [DOI] [PubMed] [Google Scholar]
- 27. Baccellieri D, Bertoglio L, Apruzzi L, et al. Incidence of deep venous thrombosis in COVID‐19 hospitalized patients during the first peak of the Italian outbreak. Phlebology. 2021;36(5):375‐383. [DOI] [PubMed] [Google Scholar]
- 28. Bellmunt‐Montoya S, Riera C, Gil D, et al. COVID‐19 infection in critically ill patients carries a high risk of venous thrombo‐embolism. Eur J Vasc Endovasc Surg. 2021;61(4):628‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi JJ, Wehmeyer GT, Li HA, et al. D‐dimer cut‐off points and risk of venous thromboembolism in adult hospitalized patients with COVID‐19. Thromb Res. 2020;196:318‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Cobelli F, Palumbo D, Ciceri F, et al. Pulmonary vascular thrombosis in COVID‐19 pneumonia. J Cardiothorac Vasc Anesth. 2021;35(12):3631‐3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia‐Ortega A, Oscullo G, Calvillo P, et al. Incidence, risk factors, and thrombotic load of pulmonary embolism in patients hospitalized for COVID‐19 infection. J Infect. 2021;82(2):261‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Joncour A, Frere C, Martin‐Toutain I, et al. Antiphospholipid antibodies and thrombotic events in COVID‐19 patients hospitalized in medicine ward. Autoimmun Rev. 2021;20(2):102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leonard‐Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in patients with COVID‐19 at CT angiography and relationship to d‐dimer levels. Radiology. 2020;296(3):E189‐E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loffi M, Regazzoni V, Toselli M, et al. Incidence and characterization of acute pulmonary embolism in patients with SARS‐CoV‐2 pneumonia: a multicenter Italian experience. PloS One. 2021;16(1):e0245565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meiler S, Hamer OW, Schaible J, et al. Computed tomography characterization and outcome evaluation of COVID‐19 pneumonia complicated by venous thromboembolism. PloS One. 2020;15(11):e0242475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mestre‐Gomez B, Lorente‐Ramos RM, Rogado J, et al. Incidence of pulmonary embolism in non‐critically ill COVID‐19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis. 2021;51(1):40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moll M, Zon RL, Sylvester KW, et al. VTE in ICU patients with COVID‐19. Chest. 2020;158(5):2130‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mouhat B, Besutti M, Bouiller K, et al. Elevated D‐dimers and lack of anticoagulation predict PE in severe COVID‐19 patients. Eur Respir J. 2020;56(4):2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mueller‐Peltzer K, Krauss T, Benndorf M, et al. Pulmonary artery thrombi are co‐located with opacifications in SARS‐CoV2 induced ARDS. Respir Med. 2020;172:106135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ooi MWX, Rajai A, Patel R, Gerova N, Godhamgaonkar V, Liong SY. Pulmonary thromboembolic disease in COVID‐19 patients on CT pulmonary angiography ‐ prevalence, pattern of disease and relationship to D‐dimer. Eur J Radiol. 2020;132:109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Planquette B, Le Berre A, Khider L, et al. Prevalence and characteristics of pulmonary embolism in 1042 COVID‐19 patients with respiratory symptoms: a nested case‐control study. Thromb Res. 2021;197:94‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santoliquido A, Porfidia A, Nesci A, et al. Incidence of deep vein thrombosis among non‐ICU patients hospitalized for COVID‐19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020;18(9):2358‐2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ventura‐Diaz S, Quintana‐Perez JV, Gil‐Boronat A, et al. A higher D‐dimer threshold for predicting pulmonary embolism in patients with COVID‐19: a retrospective study. Emerg Radiol. 2020;27(6):679‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Voicu S, Delrue M, Chousterman BG, et al. Imbalance between procoagulant factors and natural coagulation inhibitors contributes to hypercoagulability in the critically ill COVID‐19 patient: clinical implications. Eur Rev Med Pharmacol Sci. 2020;24(17):9161‐9168. [DOI] [PubMed] [Google Scholar]
- 45. Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID‐19. Thromb Res. 2020;195:95‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu T, Zuo Z, Yang D, et al. Venous thromboembolic events in patients with COVID‐19: a systematic review and meta‐analysis. Age Ageing. 2021;50(2):284‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu Y, Tu J, Lei B, et al. Incidence and risk factors of deep vein thrombosis in hospitalized COVID‐19 patients. Clin Appl Thromb Hemost. 2020;26:1076029620953217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID‐19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114‐128. [DOI] [PubMed] [Google Scholar]
- 49. Schiaffino S, Giacomazzi F, Esseridou A, et al. Pulmonary thromboembolism in coronavirus disease 2019 patients undergoing thromboprophylaxis. Medicine (Baltimore). 2021;100(1):e24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rieder M, Goller I, Jeserich M, et al. Rate of venous thromboembolism in a prospective all‐comers cohort with COVID‐19. J Thromb Thrombolysis. 2020;50(3):558‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID‐19 pneumonia. J Thromb Thrombolysis. 2020;50(2):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caro‐Codon J, Rey JR, Buno A, et al. Characterization of NT‐proBNP in a large cohort of COVID‐19 patients. Eur J Heart Fail. 2021;23(3):456‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Griffin DO, Jensen A, Khan M, et al. Pulmonary embolism and increased levels of d‐dimer in patients with coronavirus disease. Emerg Infect Dis. 2020;26(8):1941‐1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alonso‐Fernandez A, Toledo‐Pons N, Cosio BG, et al. Prevalence of pulmonary embolism in patients with COVID‐19 pneumonia and high D‐dimer values: a prospective study. PloS One. 2020;15(8):e0238216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Assimakopoulos SF, Emmanuil A, Tsimeka A, Chalkidi T, Marangos M, Gogos C. Evidence for increased circulating procoagulant phospholipids in patients with COVID‐19 pneumonia and their prognostic role. Clin Chem Lab Med. 2020;59(2):e53‐e55. [DOI] [PubMed] [Google Scholar]
- 56. Atallah B, Sadik ZG, Salem N, et al. The impact of protocol‐based high‐intensity pharmacological thromboprophylaxis on thrombotic events in critically ill COVID‐19 patients. Anaesthesia. 2021;76(3):327‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zerwes S, Hernandez Cancino F, Liebetrau D, et al. Increased risk of deep vein thrombosis in intensive care unit patients with CoViD‐19 infections?‐preliminary data. Chirurg. 2020;91(7):588‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Avruscio G, Camporese G, Campello E, et al. COVID‐19 and venous thromboembolism in intensive care or medical ward. Clin Transl Sci. 2020;13(6):1108‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pizzi R, Gini G, Caiano L, et al. Coagulation parameters and venous thromboembolism in patients with and without COVID‐19 admitted to the emergency department for acute respiratory insufficiency. Thromb Res. 2020;196:209‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lombardi CM, Carubelli V, Iorio A, et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5(11):1274‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martinez Chamorro E, Revilla Ostolaza TY, Perez Nunez M, Borruel Nacenta S, Cruz‐Conde Rodriguez‐Guerra C, Ibanez SL. Pulmonary embolisms in patients with COVID‐19: a prevalence study in a tertiary hospital. Radiologia (Engl ed). 2021;63(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pineton de Chambrun M, Frere C, Miyara M, et al. High frequency of antiphospholipid antibodies in critically ill COVID‐19 patients: a link with hypercoagulability? J Intern Med. 2021;289(3):422‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shah A, Donovan K, McHugh A, et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID‐19: a multicentre observational study. Crit Care. 2020;24(1):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stoneham SM, Milne KM, Nuttall E, et al. Thrombotic risk in COVID‐19: a case series and case‐control study. Clin Med (Lond). 2020;20(4):e76‐e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lazzeri C, Bonizzoli M, Franci A, Socci F, Peris A. Pulmonary embolism and screening for concomitant proximal deep vein thrombosis in noncritically ill hospitalized patients with coronavirus disease 2019. Intern Emerg Med. 2020;15(6):1081‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kho J, Ioannou A, Van den Abbeele K, Mandal AKJ, Missouris CG. Pulmonary embolism in COVID‐19: clinical characteristics and cardiac implications. Am J Emerg Med. 2020;38(10):2142‐2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garcia‐Olive I, Sintes H, Radua J, Abad Capa J, Rosell A. D‐dimer in patients infected with COVID‐19 and suspected pulmonary embolism. Respir Med. 2020;169:106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Giorgi‐Pierfranceschi M, Paoletti O, Pan A, et al. Prevalence of asymptomatic deep vein thrombosis in patients hospitalized with SARS‐CoV‐2 pneumonia: a cross‐sectional study. Intern Emerg Med. 2020;15(8):1425‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stefely JA, Christensen BB, Gogakos T, et al. Marked factor V activity elevation in severe COVID‐19 is associated with venous thromboembolism. Am J Hematol. 2020;95(12):1522‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greco S, Zenunaj G, Bonsi B, et al. SARS‐CoV‐2 and finding of vein thrombosis: can IMPROVE and IMPROVEDD scores predict COVID‐19 outcomes? Eur Rev Med Pharmacol Sci. 2021;25(4):2123‐2130. [DOI] [PubMed] [Google Scholar]
- 72. Bozzani A, Arici V, Tavazzi G, et al. Acute arterial and deep venous thromboembolism in COVID‐19 patients: risk factors and personalized therapy. Surgery. 2020;168(6):987‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID‐19 pneumonia. Eur Respir J. 2020;56(1):2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cerda P, Ribas J, Iriarte A, et al. Blood test dynamics in hospitalized COVID‐19 patients: potential utility of D‐dimer for pulmonary embolism diagnosis. PloS One. 2020;15(12):e0243533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen J, Wang X, Zhang S, et al. Characteristics of acute pulmonary embolism in patients with COVID‐19 associated pneumonia from the City of Wuhan. Clin Appl Thromb Hemost. 2020;26:1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ippolito D, Giandola T, Maino C, et al. Acute pulmonary embolism in hospitalized patients with SARS‐CoV‐2‐related pneumonia: multicentric experience from Italian endemic area. Radiol Med. 2021;126(5):669‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kampouri E, Filippidis P, Viala B, et al. Predicting venous thromboembolic events in patients with coronavirus disease 2019 requiring hospitalization: an observational retrospective study by the COVIDIC initiative in a swiss university hospital. Biomed Res Int. 2020;2020:9126148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Khan MZ, Jamal Y, Sutton B, Rauf F. Venous thromboembolism in patients with COVID‐19 and correlation with D‐dimers: a single‐centre experience. BMJ open Respir Res. 2020;7(1):e000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cho ES, McClelland PH, Cheng O, et al. Utility of d‐dimer for diagnosis of deep vein thrombosis in coronavirus disease‐19 infection. J Vasc Surg Venous Lymphat Disord. 2021;9(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen S, Zhang D, Zheng T, Yu Y, Jiang J. DVT incidence and risk factors in critically ill patients with COVID‐19. J Thromb Thrombolysis. 2021;51(1):33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID‐19 patients: a French multicentre cohort study. Eur Heart J. 2020;41(32):3058‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Grandmaison G, Andrey A, Periard D, et al. Systematic screening for venous thromboembolic events in COVID‐19 pneumonia. TH Open. 2020;4(2):e113‐e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hippensteel JA, Burnham EL, Jolley SE. Prevalence of venous thromboembolism in critically ill patients with COVID‐19. Br J Haematol. 2020;190(3):e134‐e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ierardi AM, Coppola A, Fusco S, et al. Early detection of deep vein thrombosis in patients with coronavirus disease 2019: who to screen and who not to with doppler ultrasound? J Ultrasound. 2021;24(2):165‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Larsen K, Coolen‐Allou N, Masse L, et al. Detection of pulmonary embolism in returning travelers with hypoxemic pneumonia due to COVID‐19 in Reunion Island. Am J Trop Med Hyg. 2020;103(2):844‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maatman TK, Jalali F, Feizpour C, et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48(9):e783‐e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nahum J, Morichau‐Beauchant T, Daviaud F, et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID‐19). JAMA Netw Open. 2020;3(5):e2010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID‐19 in Wuhan. Circulation. 2020;142(2):181‐183. [DOI] [PubMed] [Google Scholar]
- 90. Taccone FS, Gevenois PA, Peluso L, et al. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. 2020;48(11):e1087‐e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Trimaille A, Curtiaud A, Marchandot B, et al. Venous thromboembolism in non‐critically ill patients with COVID‐19 infection. Thromb Res. 2020;193:166‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID‐19 in a new York City health system. JAMA. 2020;324(8):799‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136(4):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID‐19 patients receiving thromboprophylaxis: incidence and role of D‐dimer as predictive factors. J Thromb Thrombolysis. 2020;50(1):211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


