Abstract
Background
Awake prone positioning (APP) has been considered as a feasible treatment for patients with acute hypoxemic respiratory failure in non-intubated coronavirus disease 2019 (COVID-19). However, the efficacy and safety of APP remain uncertain. This meta-analysis aims to assess the effect of APP on intubation rate and mortality in COVID-19 patients with acute respiratory failure.
Methods
Relevant studies published from January 1, 2020, to June 17, 2022, were systematically searched. The primary outcomes were the intubation rate and mortality; the secondary outcome was the incidence of adverse events.
Results
Of 5746 identified publications, 22 were eligible for inclusion in the meta-analysis (N = 5146 patients). In comparison to the non-APP group, APP could decrease the intubation rates (OR 0.64; 95% CI 0.48-0.83; P = .001), particularly in the subgroup of the daily median duration of APP > 8 h and in the subgroup of receiving high flow nasal cannula (HFNC) or non-invasive ventilation (NIV). Patients treated with APP were associated with lower mortality rates (OR 0.61; 95% CI 0.45-0.81; P = .0008), but no mortality benefit was found in the APP group in the subgroup of randomized controlled trials (RCTs). No significant difference was found in the incidence of adverse events between the groups (OR 1.13; 95% CI 0.75-1.71; P = .56).
Conclusion
Our results demonstrated that APP could be an effective strategy to avoid intubation without detrimental effects in non-intubated patients with COVID-19, especially for patients requiring HFNC or NIV, and the daily APP duration with the target of minimally eight hours was suggested. In the subgroup of RCTs, the pooled results did not demonstrate any benefit of APP on mortality. Given the limited number of RCTs, further high-quality RCTs are needed to confirm the results.
INPLASY registration number
INPLASY2021110037.
Keywords: awake prone positioning, COVID-19, acute respiratory failure, intubation rate, mortality
Introduction
Since December 2019, the coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread quickly and has become a global pandemic. The COVID-19 disease severity ranges from mild to critical disease. Approximately 15% of COVID-19 patients are severe cases requiring oxygen support, and 5% develop the critically ill disease with respiratory failure, acute respiratory distress syndrome (ARDS), or other complications and even need to be admitted to the intensive care unit (ICU) for mechanical ventilation.1,2 Some studies found that the mortality rates of COVID-19 patients admitted to ICU were between 26% and 53.8%.3,4 Patients receiving invasive mechanical ventilation (IMV) have a higher mortality rate, ranging from 41% to 97%.5–7 The best treatment to improve the outcomes of these patients remains unclear.
Prone position ventilation has several beneficial physiologic effects, including promoting the re-opening of collapsed lung alveolar, improving respiratory compliance, ameliorating the ventilation/perfusion ratio, facilitating the sputum drainage, and improving the function of the right heart.8–10 So, prone position ventilation is considered one of the essential treatments for patients with moderate to severe ARDS. Previous studies have shown that prone positioning improves oxygenation and reduces mortality in intubated ARDS patients.11–13 In non-intubated patients with acute hypoxemic respiratory failure or ARDS, some trials suggested that awake prone positioning (APP) may improve oxygenation and help avoid intubation.14,15
Given the high mortality rate among IMV patients, the risk of virus transmission of endotracheal intubation, and the limited medical resources during the pandemic, it is crucial to find a feasible and effective non-invasive method to stabilize respiratory status and avoid intubation in patients with COVID-19. The APP has been increasingly concerned during the COVID-19 pandemic. Some studies reported that APP effectively ameliorates blood oxygenation in non-intubated COVID-19 patients with acute respiratory failure.16,17 As for the intubation rate and mortality, the results of different studies are inconsistent. A meta-analysis found that APP did not reduce the intubation rate and mortality in COVID-19 patients with acute respiratory failure.18 Some recent meta-analyzes’ findings on the intubation rate were consistent with that meta-analysis, but they found the beneficial effect of APP on mortality.19,20 However, a recent randomized, controlled, meta-trial reported that APP could decrease the incidence of treatment failure and the intubation rate in subjects with hypoxemic respiratory failure due to COVID-19.21 Several latest high-quality studies have also assessed APP’s effect on the outcomes of COVID-19 patients but have produced inconsistent results.22–25 As a result, the use of APP remains controversial. The updated Surviving Sepsis Campaign Guideline also stated that they could not issue a recommendation on using APP in non-intubated COVID-19 adults because of the uncertainty about the effect on patient’s important outcomes.26
It is necessary to solve the controversy regarding APP’s effect on intubation rate and mortality for COVID-19 patients with acute hypoxaemic respiratory failure. Thus, we systematically reviewed the current trials and performed a meta-analysis to assess whether APP can reduce the intubation rate and mortality of COVID-19 adults with acute hypoxemic respiratory failure than standard care. Several new studies are included in this meta-analysis. Moreover, some subgroup analyzes that might influence the results were also considered. The results may help clarify the APP’s effects on patients with COVID-19.
Methods
Search Strategy
Databases of Pubmed, Embase, and Cochrane Library were systematically searched from January 1, 2020, to June 17, 2022. The search terms were as follows: (“COVID-19” or “COVID 19” or “COVID-19 Virus Disease” or “COVID 19 Virus Disease” or “COVID-19 Virus Diseases” or “Disease, COVID-19 Virus” or “Disease 2019, Coronavirus” or “Virus Disease, COVID-19” or “COVID-19 Virus Infection” or “COVID 19 Virus Infection” or “COVID-19 Virus Infections” or “Infection, COVID-19 Virus” or “Virus Infection, COVID-19” or “2019- nCoV” or “2019 nCoV” or “Coronavirus Disease-19” or “Coronavirus Disease 19” or “Coronavirus Disease 2019” or “2019 Novel Coronavirus” or “2019 Novel Coronaviruses” or “COVID19” or “SARS Coronavirus 2” or “Coronavirus 2, SARS” or “SARS CoV 2” or “SARS-CoV-2” or “Severe Acute Respiratory Syndrome Coronavirus 2” or “Coronavirus, 2019 Novel” or “Novel Coronavirus, 2019”) and (“prone position*” or “Pron*”).
In our study, two reviewers (HK, XG) independently searched and screened the study titles and abstracts, and further accessed full texts of eligible studies to select the included studies. No language restrictions were applied. The reference lists of the included studies were manually screened to identify other relevant studies. All disagreements were resolved by a third person (ZT). The INPLASY registration number is INPLASY2021110037.
Study Selection
The inclusion criteria were as follows: (1) adult (≥18 years old) COVID-19 patients with acute hypoxemic respiratory failure or ARDS and in the non-intubated state; (2) studies that compared the experimental group using non-invasive respiratory support in the awake prone position with the control group using non-invasive respiratory support which not in the prone position; (3) studies included required outcomes and the data could be directly extracted or calculated; (4) randomized controlled trials (RCTs) or observational cohort studies.
The exclusion criteria were as follows: (1) reviews, meta-analyzes, case reports, case series, abstract, study protocols, or expert opinions; (2) cross-over trials; (3) duplicates; (4) insufficient data; (5) multiple publications; (6) unpublished studies; (7) not related to COVID-19 patients; (8) studies that enrolled patients younger than 18 years old or animals; (9) experimental group did not receive non-invasive respiratory support combined with APP; 10) control group did not receive non-invasive respiratory support combined with non-APP; 11) studies that only compared pre-APP with post-APP in one cohort/group; 12) not reported the outcomes what we need.
Data Extraction and Risk of Bias Assessment
Two review authors (HK, XG) independently extracted data and evaluated the risk of bias of the included studies. Any disagreement was resolved by a third person (ZT). The following data were extracted from the included studies: first author, publication year, number of centers, study design, clinical setting, characteristics of the patients, respiratory support method, number of patients, description of the intervention group, description of the control group, outcomes. We e-mailed the corresponding authors when any data were insufficient. We judged the risk of bias of the included RCTs using the Cochrane Collaboration Risk of Bias tool27 and rated them as “low,” “unclear,” or “high” in each domain. And we used the Newcastle-Ottawa scale to assess the risk of bias of the included observational cohort studies. An observational study was classified as high, moderate, and low risk of bias when it achieved 1–3, 4–5, and 6–9 points, respectively.28
Outcomes
The primary outcomes included intubation rate and mortality. The secondary outcome was the incidence of adverse events (pain or discomfort, skin breakdown, vomiting, central or arterial line dislodgement, cardiac arrest, etc).
Statistical Analysis
All statistical analyzes were conducted with Review Manager Version 5.4 and Stata version 16.0. Values for dichotomous outcomes were presented as the odds ratios (OR) with 95% confidence intervals (CI). And values for continuous outcomes were reported as the mean (standard deviation). The Mantel-Haenszel random-effects model was used to analyze the results. The results of the meta-analysis were presented in the forest plots. Statistical heterogeneity between studies was assessed by I² testing. I² of more than 50% was regarded as moderate-to-high heterogeneity.29 We used subgroup analyzes and sensitivity analyzes to explore the sources of heterogeneity. For the primary outcomes, subgroup analysis based on RCTs and observational studies was conducted to identify the potential sources of moderate-to-high heterogeneity. Sensitivity analyzes were also performed by excluding studies with a moderate or high risk of bias to find the potential influence. We also performed subgroup analyzes of some other factors that might influence the primary outcomes, such as the daily median duration of APP (<8 h versus >8 h) and the non-invasive respiratory support methods [conventional oxygen therapy (COT) versus high flow nasal cannula (HFNC) or non-invasive ventilation (NIV)] given to patients in APP. The publication bias was evaluated by the funnel plot and Egger tests when more than ten studies were included, and a P value less than .05 was considered a substantial publication bias. The GRADE system was used to evaluate the quality of evidence for the outcomes.
Results
Search Results
We identified 5746 publications, from which 1511 were duplicates (Figure 1). After screening the titles and abstracts, 86 records were included for full-text screening. Then, 64 articles were excluded for reasons described in the flow diagram (Figure 1). Finally, 22 studies with a total of 5146 patients were included in the meta-analysis.21–25,30–46
Figure 1.
Flow chart of the study selection.
Study Characteristics and Risk of Bias
The characteristics of the included studies are shown in Supplemental Table 1. The sample size ranged from 20 to 1121 patients. Seven of these studies were RCTs,21,22,24,25,30–32 of which one was a randomized, controlled, multinational, meta-trial.21 The other fifteen were observational studies.23,33–46 All the studies explored the effect of APP in COVID-19-related acute respiratory failure or ARDS patients without intubation. These patients were treated with different non-invasive respiratory support methods. In the APP group, the median duration of prone position in these included studies varied from 2 h to at least 12 h per day. The assessment of the risk of bias is presented in Supplemental Figures 1, 2, and Supplemental Table 2. Seven RCTs were assessed as low risk of bias because they had a low risk of bias in nearly all domains. For observational cohort studies, ten were considered as low risk of bias with 6–9 points assessed by the Newcastle-Ottawa Scale, and five articles were of moderate risk of bias (5 points).
Intubation Rate
22 studies21–25,30–46 involving 5146 patients reported intubation rates (Supplemental Figure 3). The result found that APP was associated with a significant reduction in intubation rate compared with the control group (OR 0.64; 95% CI 0.48-0.83; P = .001), with moderate heterogeneity among these studies (χ2 = 58.62, I2 = 66%). Subgroup analysis was used to explore the source of potential heterogeneity. The result of the subgroup analysis suggested that APP could reduce intubation rate both in the RCTs subgroup (seven studies; OR 0.72; 95% CI 0.61-0.86; P = .0004; I2 = 0%) and the observational studies subgroup (fifteen studies; OR 0.56; 95% CI 0.36-0.86; P = .008; I2 = 74%), the P-value for subgroup differences was .28 (Figure 2). Then we did sensitivity analysis (Supplemental Figure 4) by excluding five articles with a moderate risk of bias. The result did not change, and the moderate-to-high heterogeneity disappeared (OR 0.66; 95% CI 0.53-0.82; P = .0002; I2 = 36%).
Figure 2.
Forest plot for subgroup analysis of intubation rate according to randomized controlled trials or observational studies.
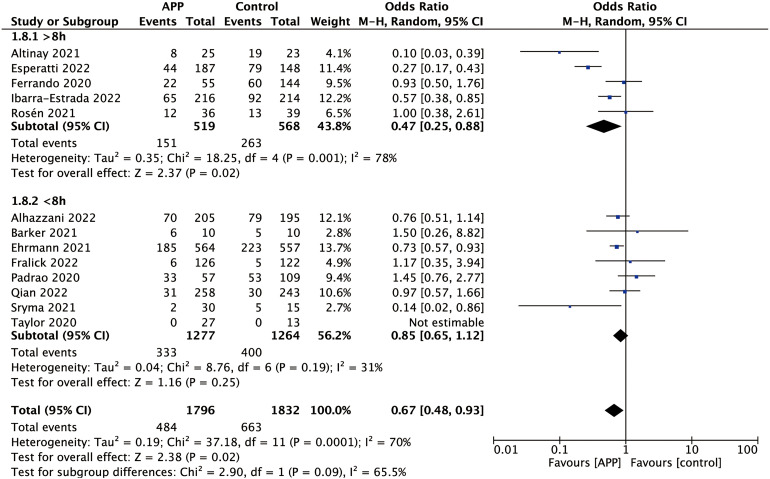
Thirteen of the 22 studies reported the actual daily median duration of APP.21–25,30,36,37,40–43,46 In the subgroup of the daily median duration of APP > 8 h (five studies; OR 0.47; 95% CI 0.25-0.88; P = .02; I2 = 78%), pool analysis showed that the APP reduced the intubation rate in comparison to the control group. However, APP did not appear to reduce the intubation rate in the subgroup of the daily median duration of APP < 8 h (eight studies; OR 0.85; 95% CI 0.65-0.12; P = .25; I2 = 31%). The P-value for subgroup differences was .09. The details are shown in Figure 3.
Figure 3.
Forest plot for subgroup analysis of intubation rate according to the daily median duration of APP.
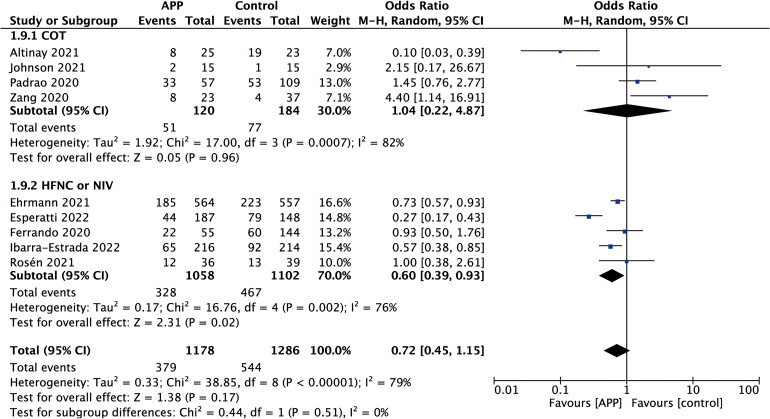
Four of the 22 studies reported that patients were treated with COT in APP.32,34,41,43 In the subgroups of COT, no significant difference in intubation rate was found between the APP group and the control group (four studies; OR 1.04; 95% CI 0.22-4.87; P = .96; I2 = 82%). And five of the 22 studies reported that patients were treated with HFNC or NIV in APP.21,24,30,37,46 The results showed that APP appeared to decrease the intubation rate in the subgroup of HFNC or NIV (five studies; OR 0.60; 95% CI 0.39-0.93; P = .02; I2 = 76%). The P-value for subgroup differences was .51. The result of the subgroup analysis is shown in Figure 4.
Figure 4.
Forest plot for subgroup analysis of intubation rate according to the non-invasive respiratory support methods.
Mortality
20 studies21–25,30–41,43,45,46 estimated the effect of APP on mortality (Supplemental Figure 5). Pooled analysis showed that APP reduced mortality in comparison to the control group (OR 0.61; 95% CI 0.45-0.81; P = .0008), with moderate heterogeneity (χ2 = 47.86, I2 = 60%). The heterogeneity was explored by subgroup analysis (Figure 5). The results indicated that APP was associated with a reduction in mortality in the subgroup of observational studies (thirteen studies; OR 0.44; 95% CI 0.29-0.66; P < .0001; I2 = 61%), but there was no difference between the APP group and non-APP group in the subgroup of RCTs (seven studies; OR 0.89; 95% CI 0.73-1.09; P = .26; I2 = 0%); the P-value for subgroup differences was .002. We also did sensitivity analyzes by excluding articles with a moderate risk of bias (Supplemental Figure 6), and no change was found in the result, which suggested this result was robust.
Figure 5.
Forest plot for subgroup analysis of mortality according to randomized controlled trials or observational studies.
Twelve of the 20 studies reported the daily median duration of APP.21–25,30,36,37,40,41,43,46 APP did not appear to reduce mortality in both subgroups of the daily median duration of APP > 8 h (five studies; OR 0.65; 95% CI 0.31-1.34; P = .24; I2 = 77%) and <8 h (seven studies; OR 0.85; 95% CI 0.65-1.11; P = .24; I2 = 20%) (Supplemental Figure 7). The P-value for subgroup differences was .49. Four studies analyzed mortality among the COT subgroup,32,34,41,43 and the results showed that APP could reduce mortality (OR 0.37; 95% CI 0.17-0.81; P = .01; I2 = 31%); there were five studies in the HFNC or NIV subgroup,21,24,30,37,46 APP did not appear to reduce mortality (OR 0.76; 95% CI 0.46-1.26; P = .29; I2 = 76%); the P-value for subgroup differences was .14. The details are presented in Supplemental Figure 8.
Adverse Events
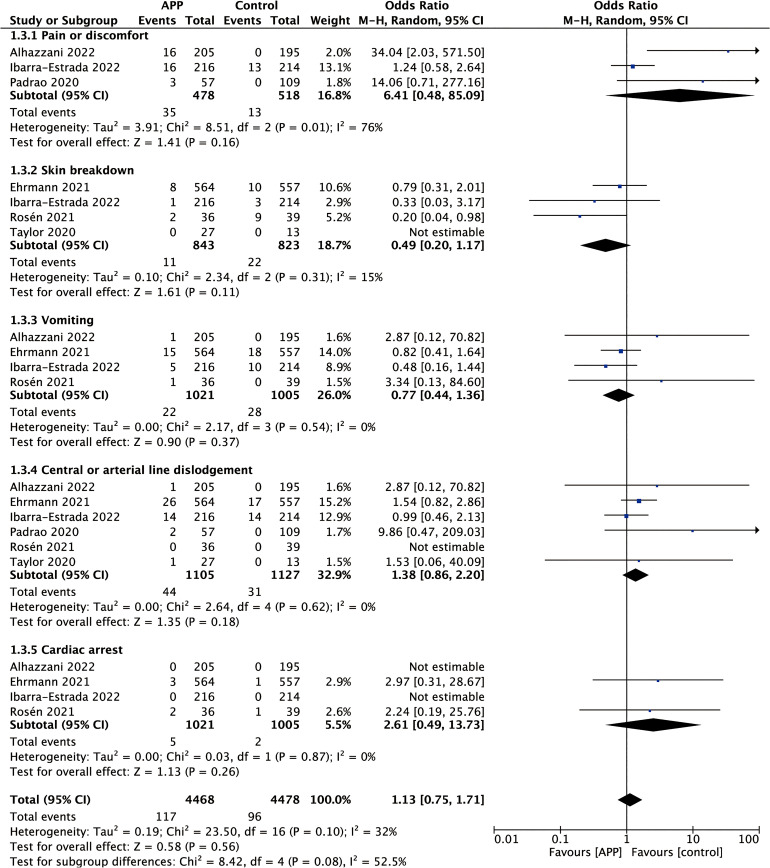
For adverse events (Figure 6), three studies22,24,43 reported the incidence of pain or discomfort, four studies21,24,30,42 reported the incidence of skin breakdown, and six trials21,22,24,30,42,43 reported the incidence of central or arterial line dislodgement, four studies21,22,24,30 reported the incidence of vomiting and cardiac arrest. In general, APP did not increase the incidence of adverse events (OR 1.13; 95% CI 0.75-1.71; P = .56), with mild heterogeneity (χ2 = 23.50, I2 = 32%).
Figure 6.
Forest plot showing the effect of awake prone positioning on the incidence of adverse events of non-intubated COVID-19 patients with acute hypoxemic respiratory failure.
Publication Bias
More than ten studies reported the primary outcomes of intubation rate and mortality, so we used funnel plots (Supplemental Figures 9 and 10) and Egger’s test to assess the publication bias. No significant publication bias was found for the primary outcomes (P values for intubation rate and mortality were .7429 and .3398, respectively).
Quality of the Meta-Analysis Evidence
The GRADE summary of evidence is shown in Supplemental Table 3. For the primary outcomes, the principles of the GRADE system indicated that the quality of evidence for both intubation rate and mortality in the subgroups of RCTs was of high quality. However, the quality of evidence for intubation rate and mortality in the observational studies subgroup was very low.
Discussion
Our systematic review and meta-analysis demonstrated that APP could reduce the incidence of endotracheal intubation without increasing the incidence of adverse events in COVID-19-related acute hypoxemic respiratory failure or ARDS patients requiring non-invasive respiratory support. Overall, the findings support the application of APP to avoid intubation in non-intubated patients with acute hypoxemic respiratory failure or ARDS due to COVID-19. The results differed from another systematic review and meta-analysis,18 which reported that APP was not associated with the reduced intubation rate and mortality of COVID-19 patients with acute respiratory failure.
In the study, we found that APP reduced the intubation rate compared with standard care, contrary to the results of a recent meta-analysis.20 Our result was consistent with a previous study about non-COVID-19 related ARDS patients done by Ding et al,15 which showed that early use of prone position ventilation combined with HFNC might help decrease the rate of intubation in patients with moderate ARDS. Several reasons may account for the lower incidence of intubation rate. First, the ventilation distribution can be more homogeneous in the prone position than in the supine position, thereby promoting ventilation to match perfusion and decreasing the alveolar shunt.47,48 Second, prone positioning is more conducive to the clearance of the secretion on the dorsal lung because of the influence of gravity.9 Last but not least, when prone ventilation strategies are applied in patients, collapsed alveoli in dorsal lung regions tend to re-open.49 In a word, the physiological benefits of the position change might help improve oxygenation, prevent the exacerbation of the disease, and further reduce the intubation rate. Then, the need for IMV may be lower, which could relieve the stress of lacking ICU resources during the pandemic and reduce the risk of disease transmission through aerosols generated during endotracheal intubation. However, it has been reported that delayed intubation was associated with increased mortality in ARDS patients.50 Therefore, we need to closely monitor patients’ responses and the progress of the disease during APP, and if necessary, timely intubation is required to avoid delay.
In the subgroup analysis regarding the daily median duration of APP, we found that APP significantly reduced the intubation rate of COVID-19 patients with acute hypoxaemic respiratory failure, especially among patients in the subgroup with a daily median duration of APP > 8 h. A recent study also demonstrated that the APP duration was associated with APP success, and a longer APP duration with the target of minimally 8 h/day was suggested,24 which was consistent with our subgroup analysis results. However, the goal of minimally 8 h/day was not reached in many studies due to the poor adherence to APP, which may affect the APP’s effectiveness to some extent. Thus, it is crucial to improve the compliance and comfort of APP in clinical practice. In the subgroup analysis regarding the non-invasive respiratory support methods, we found that APP significantly decreased the intubation rate of COVID-19 patients with acute hypoxaemic respiratory failure among patients in the subgroup receiving HFNC or NIV was as same as the latest meta-analysis.51 The reason may be because patients receiving COT had less severe diseases and were less likely to progress to endotracheal intubation than patients receiving HFNC or NIV.
Our meta-analysis suggested that APP was associated with decreased mortality in acute hypoxemic respiratory failure or ARDS patients due to COVID-19. Other meta-analyzes also found evidence of the prone position on reducing mortality rate in non-intubated COVID-19 patients.19,52 The application of APP could help stabilize COVID-19 patients’ respiratory status and avoid intubation, which may partially explain the survival benefit. However, we found that APP did not reduce the mortality rate in the subgroup analysis of the RCTs group. In the subgroup analysis of the observational studies group, we still found that the mortality rate was lower in patients treated with APP. The results of the subgroup analysis suggested that the study design might have a potential impact on patients’ outcomes. More high-quality RCTs are needed to confirm the findings in the future. Besides, we did not find different effects of APP on mortality between subgroups with a duration of APP > 8 h/day and subgroups with APP duration of fewer than 8 h/day. Nevertheless, the results of subgroup analysis regarding the non-invasive respiratory support methods showed that the type of non-invasive respiratory support strategies might affect APP’s effect on patients’ mortality. However, as the number of high-quality RCTs was limited in our included studies, the impact of APP on mortality rate in COVID-19-related acute hypoxemic respiratory failure or ARDS patients remains uncertain. Many factors may influence patients’ mortality. On the one hand, the characteristics of patients can affect the outcomes. For instance, several studies have found that patients’ age,53 the severity of disease,54 and the patient’s response to prone positioning55 were associated with the mortality rate. On the other hand, the strategies of APP in the included studies are inconsistent due to lacking standardized procedures. The duration of APP,56 the timing of APP initiation,57,58 and the type of non-invasive respiratory support methods combined with APP15 have been considered as potential factors affecting mortality in some previous studies. Future studies should focus more on these possible factors influencing patient outcomes.
Complications, such as pressure ulcers, device displacement, vomiting, and hemodynamic instability, can occur in patients in prone positioning.10,59 In our study, we explored the safety of APP. We found that APP did not increase the incidence of adverse events compared with the non-APP group. The possible reason is that patients treated with APP remain conscious and can spontaneously breathe, actively express their discomfort during prone positioning and adequately adjust the body position according to their tolerance. As a result, the risk of adverse events could be reduced. Although two studies reported the occurrence of cardiac arrest,21,30 both mentioned that the cardiac arrest was unrelated to APP.
Moderate heterogeneity could be detected in primary outcomes (intubation rate and mortality). For intubation rate, the subgroup analysis based on RCTs and observational studies could not completely explain the heterogeneity. Although no heterogeneity was found in the subgroup analysis of the RCTs group, the subgroup of observational studies still had potential heterogeneity. Except for five observational studies with a moderate risk of bias, nearly all included studies had a low risk of bias. So, we further did sensitivity analysis by excluding trials with a moderate risk of bias. We found the result was consistent, but the heterogeneity could not be detected. For mortality, subgroup analysis was performed to explore the source of heterogeneity. The results showed that the determined statistical heterogeneity disappeared in the RCTs subgroup. Further sensitivity analyzes were done by excluding studies with a moderate risk of bias. We found that moderate-to-high heterogeneity could not be detected. Hence, we concluded that the included non-RCTs and the risk of bias in some domains potentially impacted the primary outcomes.
To our knowledge, this is the latest meta-analysis focused on the feasibility and safety of APP in non-intubated COVID-19 patients, which includes some recent high-quality RCTs. Another advantage is that our review’s inclusion criteria are more stringent and just included studies that compared APP group with a control group of non-APP, and studies without a control group that only compared pre-APP with post-APP were excluded. Furthermore, we explored more subgroup analyzes and found that APP could reduce the intubation rate in COVID-19 patients, particularly in the subgroup with a median APP duration >8 h/day and those patients treated with HFNC or NIV.
Our study also has some limitations. First, due to the limited number of RCTs, most included studies were observational cohort studies; more high-quality RCTs are required to confirm APP’s effects on COVID-19 patients in the future. Second, there were no standardized strategies for APP in our included studies. The initiation time, the duration of APP, and the combined respiratory support methods varied in these trials, which might affect the outcomes. The potential heterogeneity needs attention, and the optimal APP strategy needs further exploration. Third, given the absence of available patients’ individual data, we couldn’t evaluate the effect of demographic characteristics, severity of disease, and the different response to APP on patients’ outcomes. Future studies should pay more attention to the individual subject’s data that may impact a patient’s prognosis. The appropriate selection of patients for an individualized APP strategy may be more helpful in improving non-intubated COVID-19 patients’ outcomes. Finally, considering the credibility of the results, we only included published studies and not included unpublished grey studies which were not peer-reviewed. Although we evaluated the publication bias of the primary outcomes by the funnel plot and Egger tests, no significant publication bias was found. There is still the potential for publication bias.
Conclusions
In summary, applying APP with close monitoring by professionals may be feasible and safe in non-intubated patients with COVID-19. Our results suggested that APP was associated with reduced intubation rate and mortality of adults with COVID-19-related acute hypoxemic respiratory failure or ARDS without increasing the incidence of adverse events. However, no benefit of APP on mortality was found in the subgroup of RCTs. Because of the limitations of the meta-analysis, more high-quality RCTs should be performed further to confirm the influences of APP on non-intubated COVID-19 patients.
Supplemental Material
Supplemental material, sj-docx-1-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-docx-2-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-3-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-docx-4-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-docx-5-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-7-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-8-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-9-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-10-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-11-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-12-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-13-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-14-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-15-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-16-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Abbreviations
- COVID-19
the coronavirus disease 2019
- ARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- ARDS
acute respiratory distress syndrome
- ICU
intensive care unit
- APP
awake prone positioning
- IMV
invasive mechanical ventilation
- RCTs
randomized controlled trials
- OR
odds ratios
- CI
confidence intervals
- HFNC
high flow nasal cannula
- NIV
non-invasive ventilation
Footnotes
Authors’ Contributions: ZT came up with the idea, HK and XG contributed to the literature search, data collection, study design, and data analysis. HK drafted the manuscript. ZT revised the final manuscript.
Availability of Data and Materials: All data analyzed during this meta-analysis are included in this published article and its supplementary information files.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
ORCID iD: Zhaohui Tong https://orcid.org/0000-0002-5341-6857
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. Jama. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.WHO. COVID-19 Clinical management: living guidance. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1.
- 3.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. Jama. 2020;323(16):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie J, Wu W, Li S, et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med. 2020;46(10):1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua J, Qian C, Luo Z, Li Q, Wang F. Invasive mechanical ventilation in COVID-19 patient management: the experience with 469 patients in Wuhan. Crit Care. 2020;24(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subgroup of Critical Respiratory Diseases. Standardized protocol of prone position ventilation in patients with acute respiratory distress syndrome. Zhonghua Nei Ke Za Zhi 2020;59(10):781–787. [DOI] [PubMed] [Google Scholar]
- 9.Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guérin C, Albert RK, Beitler J, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46(12):2385–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield R, Noble DW, Sudlow A. Prone position for acute respiratory failure in adults. Cochrane Database Syst Rev. 2015;2015(11):Cd008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280–S288. [DOI] [PubMed] [Google Scholar]
- 14.Scaravilli V, Grasselli G, Castagna Let al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390–1394. [DOI] [PubMed] [Google Scholar]
- 15.Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartini C, Tresoldi M, Scarpellini Pet al. et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. Jama. 2020;323(22):2338–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlov I, He H, McNicholas B, et al. Awake Prone Positioning in Non-Intubated Patients With Acute Hypoxemic Respiratory Failure Due to COVID-19. Respir Care. 2022;67(1):102–114. [DOI] [PubMed] [Google Scholar]
- 19.Chua EX, Zahir SMISM, Ng KTet al. et al. Effect of prone versus supine position in COVID-19 patients: a systematic review and meta-analysis. J Clin Anesth. 2021;74:110406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beran A, Mhanna M, Srour O, et al. Effect of prone positioning on clinical outcomes of non-intubated subjects with COVID-19: a comparative systematic review and meta-analysis. Respir Care. 2022;67(4):471–479. [DOI] [PubMed] [Google Scholar]
- 21.Ehrmann S, Li J, Ibarra-Estrada M, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhazzani W, Parhar KKS, Weatherald J, et al. Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: a randomized clinical trial. Jama. 2022;327(21):2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian ET, Gatto CL, Amusina O, et al. Assessment of awake prone positioning in hospitalized adults with COVID-19: a nonrandomized controlled trial. JAMA Intern Med. 2022;182(6):612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibarra-Estrada M, Li J, Pavlov I, et al. Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. Crit Care. 2022;26(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fralick M, Colacci M, Munshi L, et al. Prone positioning of patients with moderate hypoxaemia due to COVID-19: multicentre pragmatic randomised trial (COVID-PRONE). Br Med J. 2022;376:e068585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219–e234. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PCet al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosén J, von Oelreich E, Fors D, et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit Care. 2021;25(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayakumar D, Ramachandran DP, Rabindrarajan DE, Vijayaraghavan MBKT, Ramakrishnan AN, Venkataraman AR. Standard care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection—a multicenter feasibility randomized controlled trial. J Intensive Care Med. 2021;36(8):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson SA, Horton DJ, Fuller MJet al. et al. Patient-directed prone positioning in awake patients with COVID-19 requiring hospitalization (PAPR). Ann Am Thorac Soc. 2021;18(8):1424–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prud’homme E, Trigui Y, Elharrar X, et al. Effect of prone positioning on the respiratory support of nonintubated patients with COVID-19 and acute hypoxemic respiratory failure: a retrospective matching cohort study. Chest. 2021;160(1):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zang X, Wang Q, Zhou H, et al. Efficacy of early prone position for COVID-19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med. 2020;46(10):1927–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonelli R, Pisani L, Tabbì L, et al. Early awake proning in critical and severe COVID-19 patients undergoing noninvasive respiratory support: a retrospective multicenter cohort study. Pulmonology. 2022;28(3):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sryma PB, Mittal S, Mohan A, et al. Effect of proning in patients with COVID-19 acute hypoxemic respiratory failure receiving noninvasive oxygen therapy. Lung India. 2021;38(Supplement):S6–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esperatti M, Busico M, Fuentes NA, et al. Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19-related acute respiratory failure treated with high-flow nasal oxygen: a multicenter cohort study. Crit Care. 2022;26(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Nieto OR, Escarraman-Martinez D, Guerrero-Gutierrez MA, et al. Awake prone positioning and oxygen therapy in patients with COVID-19: the APRONOX study. Eur Respir J. 2022;59(2):2100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jouffroy R, Darmon M, Isnard F, et al. Impact of prone position in non-intubated spontaneously breathing patients admitted to the ICU for severe acute respiratory failure due to COVID-19. J Crit Care. 2021;64:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker J, Pan D, Koeckerling D, Baldwin AJ, West R. Effect of serial awake prone positioning on oxygenation in patients admitted to intensive care with COVID-19. Postgrad Med J. 2022;98(1159):360–364. [DOI] [PubMed] [Google Scholar]
- 41.Altinay M, Sayan I, Turk HSet al. Effect of early awake prone positioning application on prognosis in patients with acute respiratory failure due to COVID-19 pneumonia: a retrospective observational study. Braz J Anesthesiol. 2022;72(2):194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor SP, Bundy H, Smith WM, Skavroneck S, Taylor B, Kowalkowski MA. Awake-prone positioning strategy for non-intubated hypoxic patients with COVID-19: a pilot trial with embedded implementation evaluation. Ann Am Thorac Soc. 2021;18(8):1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padrão EMH, Valente FS, Besen BAMP, et al. Awake prone positioning in COVID-19 hypoxemic respiratory failure: exploratory findings in a single-center retrospective cohort study. Acad Emerg Med. 2020;27(12):1249–1259. [DOI] [PubMed] [Google Scholar]
- 44.Ni Z, Wang K, Wang T, et al. Efficacy of early prone or lateral positioning in patients with severe COVID-19: a single-center prospective cohort. Precis Clin Med. 2020;3(4):260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagan N, Morrow LE, Walters RWet al. et al. The POSITIONED study: prone positioning in nonventilated coronavirus disease 2019 patients-a retrospective analysis. Crit Care Explor. 2020;2(10):e0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrando C, Mellado-Artigas R, Gea A, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020;24(1):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarma A, Calfee CS. Prone positioning in awake, nonintubated patients with COVID-19: necessity is the mother of invention. JAMA Intern Med. 2020;180(11):1539–1540. [DOI] [PubMed] [Google Scholar]
- 48.Touchon F, Trigui Y, Prud’homme E, et al. Awake prone positioning for hypoxaemic respiratory failure: past, COVID-19 and perspectives. Eur Respir Rev. 2021;30(160):210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188(11):1286–1293. [DOI] [PubMed] [Google Scholar]
- 50.Kangelaris KN, Ware LB, Wang CYet al. et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med. 2016;44(1):120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Luo J, Pavlov I, et al. Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Lancet Respir Med. 2022;10(6):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Behesht Aeen F, Pakzad R, Goudarzi Rad M, Abdi F, Zaheri F, Mirzadeh N. Effect of prone position on respiratory parameters, intubation and death rate in COVID-19 patients: systematic review and meta-analysis. Sci Rep. 2021;11(1):14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kao KC, Chang KW, Chan MC, et al. Predictors of survival in patients with influenza pneumonia-related severe acute respiratory distress syndrome treated with prone positioning. Ann Intensive Care. 2018;8(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gattinoni L, Vagginelli F, Carlesso Eet al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med. 2003;31(12):2727–2733. [DOI] [PubMed] [Google Scholar]
- 56.Dardeir A, Marudhai S, Patel M, Ghani MR, Busa V. Factors influencing prone positioning in treating acute respiratory distress syndrome and the effect on mortality rate. Cureus. 2020;12(10):e10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaur R, Vines DL, Mirza S, et al. Early versus late awake prone positioning in non-intubated patients with COVID-19. Crit Care. 2021;25(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173(11):1233–1239. [DOI] [PubMed] [Google Scholar]
- 59.Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. Jama. 2009;302(18):1977–1984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-docx-2-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-3-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-docx-4-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-docx-5-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-7-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-8-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-9-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-10-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-11-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-12-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-13-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-14-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-15-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine
Supplemental material, sj-tif-16-jic-10.1177_08850666221121593 for Effect of Awake Prone Positioning in non-Intubated COVID-19 Patients with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-Analysis by Hanyujie Kang, Xueqing Gu and Zhaohui Tong in Journal of Intensive Care Medicine