Abstract
Objectives: The BNT162b2 mRNA COVID-19 vaccine has been found to be highly effective in preventing COVID-19 but is associated with increased reactogenicity. We aimed to examine the correlation between immunogenicity and reactogenicity of the BNT162b2 vaccine. Methods: Subjects without prior SARS-CoV-2 infection that participated in active surveillance after being vaccinated with the BNT162b2 vaccine were included. Study participants reported adverse drug reactions (ADRs) through questionnaires administered by text message after receiving each dose of the vaccine. A reactogenicity score was developed based on the type and duration of ADRs. In addition, anti-receptor binding domain (RBD) levels and neutralization assays were performed 7–21 and 7–38 days after the first and second vaccine doses, respectively. Associations between ADRs and antibody levels were assessed by Spearman correlations. Multivariable logistic regression analyses were used to identify factors associated with ADRs. Results: A total of 831 health care workers were included. The mean age was 46.5 years (SD = 11.8) and 75.5% were females. 83.4% and 83.3% had at least one local ADR after the first and second doses, respectively. 33% and 83.2% had at least one systemic ADR after the first and second doses, respectively. Multivariate logistic regression analysis found a significant correlation between ADR score and anti-RBD-IgG titers (r = 0.366; p < 0.0001) after adjustment for age, gender, and days after the second vaccination. High anti-RBD-IgG levels, being younger than 55 and being female, were all correlated with increased rates of ADRs. Conclusion: BNT162b2 mRNA COVID-19 vaccine reactogenicity appears to be correlated with higher post-vaccination antibody levels and is independently associated with younger age and female gender.
Keywords: BNT162b2, immunogenicity, reactogenicity, COVID-19, vaccination
1. Introduction
More than 48% of the world population has been vaccinated against COVID-19 as of 22 December 2021, totaling more than 8.8 billion doses. The vaccination rollout with the BNT162b2 mRNA vaccine was initiated in Israel on 19 December 2020 and by 7 September 2021, 60.9% of the eligible Israeli population had received two doses.
In the phase 3 clinical trial on the safety and efficacy of the BNT162b2 vaccine, adverse drug reactions data were collected by registration through an electronic diary (solicited) or by participant report without prompts using an electronic diary (unsolicited). It was found that reactogenicity was common, but mostly mild [1]. ADRs included local as well as systemic reactions, such as fatigue, headache, and fever. Systemic ADRs were more common after the second dose of the vaccine. Severe ADRs were rare.
In comparison to clinical trials, real-world data shows variable rates of ADRs after vaccination with the BNT162b2 mRNA COVID-19 vaccine. In a British prospective observational study, 71.9% and 68.5% of vaccinated people developed local adverse events after the first and second dose, respectively, and 13.5% and 22% developed systemic adverse events after the first and the second dose, respectively [2]. Furthermore, prior infection with SARS-CoV-2 was associated with higher rates of local and systemic ADRs. In a study conducted on 3078 health care workers (HCW) that were vaccinated with the BNT162b2 vaccine, 59.6% and 73.4% developed any ADRs (local and/or systemic) after the first and second dose, respectively. Prior infection with SARS-CoV-2 before vaccination increased the risk of moderate or severe ADRs by at least three-fold [3].
The BNT162b2 vaccine study, conducted on over 2 million people in Israel, found that the vaccine is safe and causes significantly fewer serious ADRs than COVID-19 [4].
Associations between ADRs and immunogenicity of the vaccine have been assessed in several studies. An association between systemic ADRs and higher anti-S protein was found in two Japanese studies that examined factors associated with adverse events among healthcare workers. In the first study [5], a significant positive correlation between higher body temperature and higher anti-SARS-CoV-2 antibody titer was observed 3 months after vaccination but not at 6 months. In the second study [6] it was found that systemic adverse events, specifically muscle and joint pain after the second dose, were associated with elevated anti-S1 protein IgG antibody titer and neutralizing activity.
However, in other studies, only a weak correlation or no correlation at all between immunogenicity and reactogenicity was found [7,8,9].
Given the mixed results of data on this topic, this study aims to evaluate ADRs and antibody titers following vaccination with the BNT162b2 vaccine and determine the correlation between immunogenicity and reactogenicity.
2. Methods
2.1. Study Design and Population
This is a prospective observational study conducted from 21 December 2020 to 15 April 2021 at Sheba Medical Center (SMC) as part of an active surveillance of SMC health care workers (HCWs) vaccinated with the BNT162b2 mRNA vaccine. This study was approved by the Sheba Ethics Committee (SMC 8008-20).
SMC is the largest tertiary medical center in Israel, with 1600 beds and 14,479 HCW, all of which were invited to participate in this study. Vaccinated HCWs were included in the study if they provided written informed consent, completed the digital ADR form, had at least one serological test after the first dose of the vaccine, and had a negative anti-SARS-CoV-2 IgG assay before receiving the first dose. HCWs with a positive SARS-CoV-2 PCR test before vaccination or a confirmed infection with COVID-19 following vaccination were excluded from the study. Immunocompromised HCW and those with an autoimmune disease were also excluded.
Serological tests were performed before administration of the first vaccine dose, 1–2 weeks after the first dose, at week 3 with the administration of the second dose, and 1–2 weeks after the second dose. The time interval between the first and second vaccine doses was 21 days. Seven days after each vaccine dose, study participants received a text message survey on their personal cell phones that queried their response to the vaccine. Solicited ADRs collected included localized and systemic side effects, the duration of these symptoms, and whether the participant required medical care or hospitalization. Unsolicited serious and non-serious adverse events were collected up to a month after the second dose. In addition, demographic data such as age, sex, and underlying comorbidities were collected.
2.2. Reactogenicity
A reactogenicity score was developed to rate the number and duration of systemic adverse events participants had to the vaccine (Table 1), with a higher score indicating more adverse events. Systemic adverse events were selected based on the most common reactions that presented in the phase 3 study of the vaccine [1]. Each event received one or two points according to the assumed association of the reaction with the vaccine. For example, fatigue, which is less typical and may be attributed to other factors, received 1 point for each day reported, whereas fever and myalgia, which are more typically associated with reactogenicity, received 2 points for each day they were reported. Local reactions were not included in the total score as they are usually attributed to mechanical factors (e.g., local damage to the muscle or reaction to ancillary materials such as preservatives, stabilizers, or adjuvants).
Table 1.
Adverse events score system.
| Adverse Event | Number of Points for Each Day of the Event |
|---|---|
| Fatigue | 1 |
| Headache | 1 |
| Myalgia | 2 |
| Fever > 38 °C | 2 |
| Arthralgia | 1 |
| Local Lymphadenopathy | 1 |
| Systemic rash | 1 |
| Pruritus | 1 |
| Facial paresthesia | 1 |
| Non-facial paresthesia | 1 |
| Need for antipyretic or analgesic medication | 2 |
| Total score | Sum of the above |
2.3. Immunogenicity
Blood samples were tested using the SARS-CoV-2 anti-RBD IgG assay (Beckman Coulter, CA, USA). In addition, a SARS-CoV-2 pseudo-virus (psSARS-2) neutralization assay was performed as described [10] to detect SARS-CoV-2 neutralizing antibodies (NA) using a green fluorescent protein (GFP) reporter-based pseudotyped virus with a vesicular stomatitis virus (VSV) backbone coated with the SARS-CoV-2 spike (S) protein [generously provided by Dr. Gert Zimmer (Institute of Virology and Immunology (IVI), Mittelhäusern, Switzerland]. Sera not capable of reducing viral replication by 50% at a 1 to 8 dilution or below were considered non-neutralizing.
2.4. Statistical Methods
Data analysis was performed using SAS 9.4 software (Cary, NC, USA). Descriptive statistics were expressed as percentages for categorical data or mean ± standard deviation (SD) and median (interquartile range) for normally or non-normally distributed continuous data, respectively. Anti-RBP-IgG titers were expressed as geometrical mean titers (GMT). Since none of the variables were normally distributed, Spearman correlation coefficients were used to measure degrees of association between ADR scores as well as individual ADRs and anti-RBP-IgG titers. McNemar’s test and the Wilcoxon Signed Rank test were used to compare statistical differences in the ADRs proportion or ADR score medians between the two time points (after 1st and 2nd vaccinations).
Multivariable logistic regression analysis was used to identify factors associated with ADRs. The variables included gender, age, and RBD-IgG titers. Results were presented as odds ratios (OR), 95% confidence intervals (CI), and p-values. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Participants
Between 20 December 2020 and 15 April 2021, 91% (13,212/14,519) of SMC personnel received two doses of the BNT162b2 vaccine. Of these, 12,582 received a text message asking them to participate in this study. 2240 HCWs responded to the questionnaire of which 124 were excluded due to immunosuppression or autoimmune diseases and 37 were excluded due to incomplete data. There was a higher response rate among women compared to men both after first (18.7% vs. 15.5%, p < 0.0001) and second (14.2% vs. 9.9%, p < 0.0001) doses.
738 HCWs completed the ADR questionnaires and had serological tests after the first or second vaccine, respectively, and were included in the final analysis.
The mean age of study participants was 46.5 ± 11.8 years (range: 20–82.3) and 75.5% (n = 627) were females. 18.5% (n = 154) were physicians, 22.3% (n = 185) nurses, 35% (n = 291) paramedical, and 24.2% (n = 201) administrative workers. Blood for anti-RBD-IgG antibodies was drawn 11 ± 3.6 days and 13.7 ± 5.6 days after the first and the second doses, respectively. Blood for NAs was drawn from 71 participants 14.1 ± 0.4 days after the first dose and from 118 participants 6.9 ± 0.6 days after the second dose.
3.2. Adverse Drug Reactions
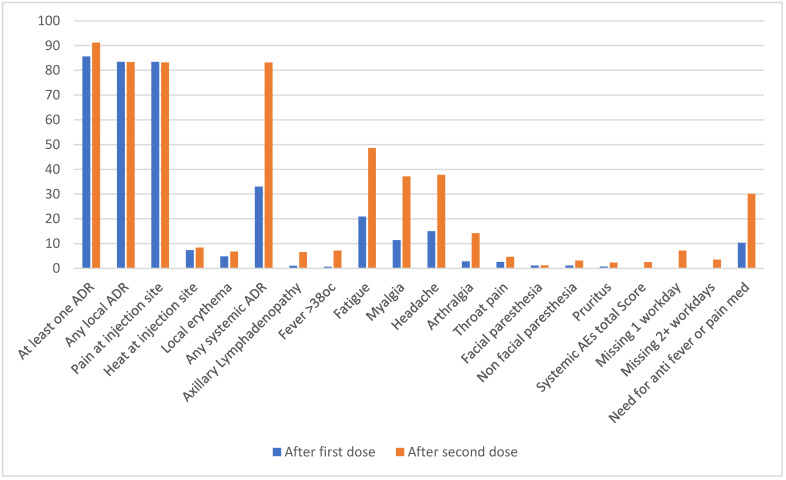
In total, 85.6% and 91.2% had any adverse event after the first and second doses, respectively. Yet, these were mostly local reactions. There were no significant differences in the rates of local ADRs following the first and second vaccines (83.4% and 83.3%, respectively). However, systemic ADRs were significantly more frequent after the second dose (83.2% vs. 33%r): axillary lymphadenopathy (6.5% vs. 1%, p < 0.0001), fever > 38 °C (7.2% vs. 0.6%, p < 0.0001), fatigue (48.6% vs. 20.9%, p < 0.0001), myalgia (37.1% vs. 11.4%, p < 0.0001), headache (37.8% vs. 15%, p < 0.0001), arthralgia (14.2% vs. 2.8%, p < 0.0001), and non-facial paresthesia (3.1% vs. 1.1%, p = 0.001). The median (Q1–Q3) score reflecting all systemic ADRs and their duration was significantly higher after the second dose (2.5 (0–6)) than the first dose (0 (0–2)). Antipyretics were used significantly more after the second dose (30.1% vs. 10.3%, p < 0.0001). Following the second vaccine dose, HCWs missed significantly more workdays than following the first dose (7.2% vs. 0.2%, p < 0.0001 for missing 1 day and 3.5% vs. 0.2%, p < 0.0001 for missing 2 or more days), (Table 2, Figure 1).
Table 2.
Adverse events rate after first and second vaccination.
| Adverse Event | After 1st Dose N = 831 (100%) | After 2nd Dose N = 738 (100%) | p * |
|---|---|---|---|
| At least one adverse event | 711 (85.6) | 673 (91.2) | 0.0003 |
| Any local AE | 693 (83.4) | 615 (83.3) | 0.5807 |
| Pain at injection site | 693 (83.4) | 614 (83.2) | 0.5279 |
| Heat at injection site | 61 (7.3) | 62 (8.4) | 0.4855 |
| Local erythema | 40 (4.8) | 50 (6.8) | 0.1229 |
| Any systemic AE | 274 (33.0) | 614 (83.2) | <0.0001 |
| Axillary Lymphadenopathy | 8 (1.0) | 48 (6.5) | <0.0001 |
| Fever > 38 °C | 5 (0.6) | 53 (7.2) | <0.0001 |
| Fatigue | 174 (20.9) | 359 (48.6) | <0.0001 |
| Myalgia | 95 (11.4) | 274 (37.1) | <0.0001 |
| Headache | 125 (15.0) | 279 (37.8) | <0.0001 |
| Arthralgia | 23 (2.8) | 105 (14.2) | <0.0001 |
| Throat pain | 22 (2.6) | 35 (4.7) | 0.0159 |
| Facial paresthesia | 9 (1.1) | 9 (1.2) | 0.7963 |
| Non-facial paresthesia | 9 (1.1) | 23 (3.1) | 0.0025 |
| Pruritus | 6 (0.7) | 17 (2.3) | 0.0105 |
| Systemic AEs total Score—median (Q1–Q3) | 0 (0–2) | 2.5 (0–6) | <0.0001 |
| Missing 1 workday | 2 (0.2) | 53 (7.2) | <0.0001 |
| Missing 2 + workdays | 2 (0.2) | 26 (3.5) | <0.0001 |
| Need for anti-fever or anti-pain medication | 86 (10.3) | 222 (30.1) | <0.0001 |
* By McNemar’s test.
Figure 1.
Adverse events rate after first and second vaccination (%).
Pain at the injection site was significantly more common in HCWs younger than 55 years versus those older than 55 years (89.8% vs. 64.9%, p < 0.0001 after the first and 87.8% vs. 69.1%, p < 0.0001 after the second dose). Headache and myalgia were more common among HCWs younger than 55 years old (42.5% vs. 23.4%, p < 0.0001 and 41% vs. 24.6%, p < 0.0001, respectively), (Table S1).
After the second vaccine dose, ADRs including pain and warmth at the injection site, axillary lymphadenopathy, fatigue, headache, myalgia, arthralgia, and the need for antipyretics or analgesics were significantly more common in females (Table S2).
3.3. Immunogenicity
Anti-RBD IgG titer increased significantly from a GMT of 0.11 S/CO (95% CI: 0.08–0.14) 11.7 ± 3.6 days after the first dose to a GMT of 32.55 S/CO (95% CI: 31.11–34.05) 13.7 ± 5.6 days after the second vaccination (Table 3). Anti-RBD IgG GMT after the second dose was significantly higher among females versus males: GMT of 33.5 S/CO (95% CI: 33.85–37.2) vs. 26.9 S/CO (95% CI: 24.1–30.1) (p < 0.0001). IgG anti-RBD GMT after the second dose was significantly higher among HCWs younger than 55 years versus older HCWs: 35.62 S/CO (95% CI: 34.1–37.2) vs. 26.62 S/CO (95% CI: 23.44–30.2) (p < 0.0001).
Table 3.
Anti-RBD IgG and neutralizing antibodies titers following first and second vaccine. doses.
| Assay | After 1st Vaccine * | After 2nd Vaccine ** |
|---|---|---|
| Days for Anti-RBD–IgG; mean ± SD (range) | 11.7 ± 3.6 (7–21) | 13.7 ± 5.6 days (7–38) |
| Anti-RBD-IgG (Geometric mean (CI95%)) | 0.11 (0.08–0.14) | 32.55 (31.11–34.05) |
| Days for neutralizing Ab; Mean ± SD (range) | 14.1 ± 0.4 (13–17) | 6.9 ± 0.6 (4–10) |
| Neutralizing Ab (Geometric mean (CI95%)) | 23.41 (17.95–30.54) | 745 (611.5–908.9) |
* N = 227 participants for anti-RBD-IgG and 71 participants for neutralizing Ab. ** N = 823 participants for anti-RBD-IgG and 185 participants for neutralizing Ab.
Neutralizing antibodies GMT increased from 23.41 (95% CI: 17.95–30.54) 14.1 ± 0.4 days after the first dose to a GMT of 745 (95% CI: 611.5–908.9) 6.9 ± 0.6 days after the second vaccination (Table 3). Neutralizing titer after the second dose was significantly higher among females compared to males: 923.6 (95% CI: 734–1161) vs. 362 (95% CI: 153–858), respectively, but did not differ between younger and older HCWs. However, NA was performed in only 54 and 17 HCWs under 55 and over 55 years old, respectively, after the first vaccine and in 78 and 40 HCWs under 55 and over 55 years respectively after the second vaccine.
3.4. Correlation between Adverse Effects, Anti-RBD IgG, and Neutralizing Antibodies GMT
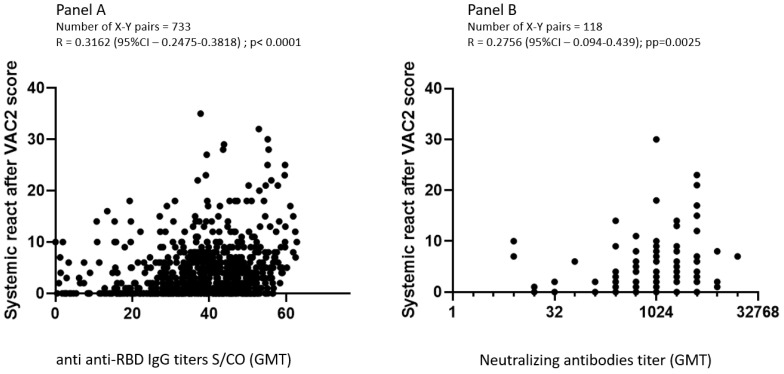
Spearman correlation, adjusted for age, gender, and days after second vaccination, found a significant correlation between systemic ADR score and anti-RBD-IgG titers (R = 0.366; p < 0.0001) and a weaker correlation with neutralization antibodies (R = 0.283; p = 0.005). There was no correlation between ADRs and immunogenicity after the first vaccine dose (Table 4, Figure 2).
Table 4.
Spearman Correlation between adverse events and immunogenicity after the first and the second vaccines adjusted for age, gender, and days after vaccination.
| Anti-IgG-RBD | Neutralizing AB | |||||||
|---|---|---|---|---|---|---|---|---|
| After 1st Vaccination | After 2nd Vaccination | After 1st Vaccination | After 2nd Vaccination | |||||
| N | 233 | 733 | 89 | 118 | ||||
| R | p | R | p | R | p | R | p | |
| Score of systemic AEs | 0.1 | 0.13 | 0.366 | <0.0001 | −0.1 | 0.34 | 0.283 | 0.005 |
| Axillary lymphadenopathy | −0.018 | 0.78 | 0.15 | <0.0001 | NA | 0.1 | 0.28 | |
| Fever | 0.12 | 0.7 | 0.19 | <0.0001 | −0.2 | 0.057 | 0.13 | 0.15 |
| Fatigue | 0.1 | 0.12 | 0.18 | <0.0001 | −0.08 | 0.46 | 0.18 | 0.04 |
| Headache | 0.07 | 0.04 | 0.19 | <0.0001 | 0.08 | 0.45 | 0.12 | 0.18 |
| Myalgia | 0.14 | 0.3 | 0.21 | <0.0001 | 0.017 | 0.87 | 0.2 | 0.03 |
| Arthralgia | 0.09 | 0.15 | 0.135 | <0.05 | 0.003 | 0.97 | 0.03 | 0.7 |
| Need for antipyretic or analgetic medication | 0.078 | 0.24 | 0.197 | <0.0001 | −0.12 | 0.25 | −0.16 | 0.07 |
| Missing workdays | 0.06 | 0.36 | 0.11 | 0.0027 | NA | 0.06 | 0.4 | |
Figure 2.
Correlation between reactogenicity score and immunogenicity as measured with anti-RBD IgG (panel A) and neutralizing antibodies (panel B), Spearman correlation coefficient.
3.5. Factors Associated with Adverse Events following Vaccination Using Multivariate Logistic Regression Analysis
Multivariate logistic regression analysis found that following the first vaccine dose, being female and under 55 years of age were associated with increased risk of any ADRs (OR = 2.38, 95% CI: 1.01–5.55; OR = 3.29, 95% CI:1.47–7.39, respectively), whereas IgG anti-RBD titers did not influence the risk of ADRs. However, after the second vaccine dose, being a female, younger than 55 years old and having an increased IgG anti-RBD GMT were significantly and independently associated with increased risk of any adverse events (OR = 2.86, 95% CI: 1.6–5.1, p = 0.0004; OR = 3.18, 95% CI: 1.83–5.52, p < 0.0001; OR = 1.36, 95% CI: 1.33–1.39, p = 0.0029, respectively), (Table 5).
Table 5.
Factors associated with adverse events following 1st and 2nd vaccination doses using multivariate logistic regression analysis.
| After 1st Vaccination (N = 233) C Statistics = 0.719 |
After 2nd Vaccination (N = 733) C Statistics = 0.751 |
|||
|---|---|---|---|---|
| OR (CI 95%) | p | OR (CI 95%) | p | |
| Female | 2.38 (1.02–5.55) | 0.0441 | 2.86 (1.6–5.1) | 0.0004 |
| Age < 55 years old | 3.29 (1.47–7.39) | 0.0039 | 3.18 (1.83–5.52) | <0.0001 |
| IgG-Anti-RBD | 1.15 (0.88–1.51) | 0.3 | 1.36 (1.33–1.39) | 0.0029 |
| Number of days after second vaccine | 0.96 (0.86–1.08) | 0.5143 | 1.01 (0.96–1.06) | 0.6905 |
4. Discussion
This study found a significant correlation between reactogenicity and immunogenicity after adjusting for age and sex among 831 health care workers vaccinated with the BNT162b2 mRNA vaccine. Systemic ADRs were more common after the second dose of the vaccine. Gender and age independently affected antibody levels and the magnitude of ADRs.
Several studies have looked at correlations between reactogenicity and immunogenicity among recipients of mRNA anti-COVID-19 vaccine and mixed results were found. Our study found a weak but significant association. In two separate studies, Koike and Kobashi in Japan did find a significant correlation of immunogenicity reflected by anti-SARS-CoV-2 S protein IgG antibodies titer and reactogenicity after the second dose of the vaccine. In these studies, the correlation was found to be only with some of the ADRs. In the first study [5], a significant positive correlation was found between higher body temperature and higher antibody titer 3 months but not 6 months after vaccination, and in the second study [6], a significant correlation was found between muscle and joint pain and anti-S1 protein IgG antibody titer and neutralizing activity.
However, in another study Zhang et al. [7] looked for a correlation between neutralizing activity against SARS-CoV-2 after vaccination with BNT162b2 or Coronavac vaccine, which is a whole inactivated virus COVID-19 vaccine, and found only a low correlation between AEs and the BNT162b2 vaccine.
Takeuchi et al. found no correlation between reactogenicity and antibody production in a study of 67 HCWs [8], while Held et al. found that adverse events were weakly correlated with spike protein antibody levels after vaccination with BNT162b2 vaccine in a study of 80 HCWs [9]. Hwang et al. did not find an association between local or systemic reactogenicity and humoral immunogenicity in individuals who received either the BNT162b2 mRNA or the AZD1222 vaccine [11]. However, a weak correlation was found between reactogenicity and immunogenicity following the herpes zoster vaccine [12].
Several studies have demonstrated that individuals who recovered from COVID-19 had increased reactogenicity following vaccination and higher titers of RBD-IgG compared to those who were vaccinated and not infected [2,3], or were infected but had mild disease [13]. As expected, anti-S-RBD IgG as well as neutralizing antibodies titers increased after each vaccination dose. Local ADRs usually result from local resident immune cell activation (such as macrophages, dendritic, and mast cells) by the adjuvant or lipid nanoparticle in mRNA vaccines used to stabilize the mRNA. Systemic reactogenicity results from spillage of inflammatory mediators or products into the circulation or via immune system activation by the protein used as the antigen (e.g., S protein) [13]. The latter may be more profound after provocation with the second dose. This may explain why we found a correlation between immunogenicity and reactogenicity with only systemic ADRs after the second dose, but not the first dose.
Our study found a higher rate of ADRs compared to the rate reported in the drug registration study. Other studies have also reported higher rates [14]. Different modes of reporting ADRs or selection bias could explain these findings as people who experience ADRs are more likely to participate in solicited questionnaires than those with mild or no ADRs.
Similar to other mRNA vaccine studies, ADRs [1,15,16,17] and reactogenicity [18] were less common among older participants, while younger subjects had higher levels of anti-RBD IgG levels after the second dose [19,20]. Immunosenescence and aging may explain these findings [21]. Immunosenescence decreases the ability of both CD4+ and CD8+ cells to function correctly, lowers naïve T cell frequency, expands memory T cells, and shrinks T cell diversity [22]. Aging changes the microenvironment and regulation of developmental checkpoints, resulting in quantitative and qualitative changes in B cell generation [23], as well as impaired replenishment of peripheral B cells, reduction in regenerative B cell capacity and, ultimately, humoral responses. Decreased vaccine effectiveness in the elderly has been demonstrated after vaccination against influenza [24] and PPV23 [25].
Our study also found that females reported more ADRs than males even after adjusting for age and professional sector. This may be related to higher responses to questionnaires among women than men; indeed, we found a higher response rate to text messages in women. Both registration studies of mRNA vaccines and real-life studies showed a higher rate of ADRs among females. Higher reactogenicity in women was also shown in other vaccination studies such as influenza and diphtheria, tetanus and pertussis (DTP) [26,27].
Limitations
Selection bias was a major limitation since only HCWs with high compliance participated in the study, and those responding to the questionnaires may have had increased ADRs. Nevertheless, this should not influence the correlation between reactogenicity and immunogenicity.
In summary, we found that vaccination with the BNT162b2 mRNA vaccine induces expected local and systemic ADRs. In addition, we found independent correlations between reactogenicity and younger age, female sex, and antibody levels. This result does not point to causality, and the mechanism of this association is yet to be shown. Further studies are needed to further understand whether there is causality in either direction or if and what is the indirect association.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines10081220/s1, Table S1: Adverse events following first and second vaccine doses by age; Table S2: Adverse events following first and second vaccine by gender; Table S3. Reactogenicity and immunogenicity following first and second vaccine by gender; Table S4. Reactogenicity and immunogenicity following first and second vaccine by age
Author Contributions
Conceptualization, I.L., G.R. and G.R.-Y.; methodology, I.L., G.R. and G.R.-Y.; validation, G.R. and E.G.L.; formal analysis, L.O. and Y.L.; investigation, all; resources, I.L., R.D., Y.L. and G.R.; data curation, I.L., E.G.L., A.W.-F., V.I., R.D., K.A., R.H., Y.L. and G.R.; writing—original draft preparation, I.L., E.G.L., L.O., G.R.-Y., N.A.-L., A.W.-F., K.H., Y.L. and G.R.; writing—review and editing, I.L., L.O., G.R.-Y., K.H., Y.L. and G.R.; visualization, L.O. and Y.L.; supervision, G.R.; project administration, I.L.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Sheba Medical Center (protocol SMC 812—21, date of approval 2 February 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, itsik.levy@sheba.health.gov.il (I.L.) and galia.rahav@sheba.health.gov.il (G.R.), upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Arminio Monforte A., Tavelli A., Perrone P.M., Za A., Razzini K., Tomasoni D., Bordoni V., Romanò L., Orfeo N., Marchetti G., et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: Data from 3078 health care workers. eClinicalMedicine. 2021;36:100914. doi: 10.1016/j.eclinm.2021.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., Hernán M.A., Lipsitch M., Kohane I., Netzer D., et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koike R., Sawahata M., Nakamura Y., Nomura Y., Katsube O., Hagiwara K., Niho S., Masuda N., Tanaka T., Sugiyama K. Systemic Adverse Effects Induced by the BNT162b2 Vaccine Are Associated with Higher Antibody Titers from 3 to 6 Months after Vaccination. Vaccines. 2022;10:451. doi: 10.3390/vaccines10030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobashi Y., Shimazu Y., Kawamura T., Nishikawa Y., Omata F., Kaneko Y., Kodama T., Tsubokura M. Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine. PLoS ONE. 2022;17:e0269917. doi: 10.1371/journal.pone.0269917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R., Leung K.-Y., Liu D., Fan Y., Lu L., Chan P.-C., To K.K.-W., Chen H., Yuen K.-Y., Chan K.-H., et al. Correlation of Immunogenicity and Reactogenicity of BNT162b2 and CoronaVac SARS-CoV-2 Vaccines. mSphere. 2022;7:e0091521. doi: 10.1128/msphere.00915-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi M., Higa Y., Esaki A., Nabeshima Y., Nakazono A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLoS ONE. 2021;16:e0257668. doi: 10.1371/journal.pone.0257668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Held J., Esse J., Tascilar K., Steininger P., Schober K., Irrgang P., Alsalameh R., Tenbusch M., Seggewies C., Bogdan C. Reactogenicity Correlates Only Weakly with Humoral Immunogenicity after COVID-19 Vaccination with BNT162b2 mRNA (Comirnaty®) Vaccines. 2021;9:1063. doi: 10.3390/vaccines9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lustig Y., Nemet I., Kliker L., Zuckerman N., Yishai R., Alroy-Preis S., Mendelson E., Mandelboim M. Neutralizing Response against Variants after SARS-CoV-2 Infection and One Dose of BNT162b2. N. Engl. J. Med. 2021;384:2453–2454. doi: 10.1056/NEJMc2104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang Y.H., Song K.-H., Choi Y., Go S., Choi S.-J., Jung J., Kang C.K., Choe P.G., Kim N.-J., Park W.B., et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021;36:1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callegaro A., Burny W., Hervé C., Kim J.H., Levin M.J., Zahaf T., Cunningham A.L., Didierlaurent A.M. Association between Immunogenicity and Reactogenicity: A Post Hoc Analysis of 2 Phase 3 Studies with the Adjuvanted Recombinant Zoster Vaccine. J. Infect. Dis. 2021;20:jiab536. doi: 10.1093/infdis/jiab536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebinger J.E., Lan R., Sun N., Wu M., Joung S., Botwin G.J., Botting P., Al-Amili D., Aronow H., Beekley J., et al. Symptomology following mRNA vaccination against SARS-CoV-2. Prev. Med. 2021;153:106860. doi: 10.1016/j.ypmed.2021.106860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hervé C., Laupeze B., Del Giudice G., Didierlaurent A.M., Da Silva F.T. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saita M., Yan Y., Ito K., Sasano H., Seyama K., Naito T. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers in Japan. J. Infect. Chemother. 2022;28:116–119. doi: 10.1016/j.jiac.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M.A., Wieler H.J., Enders P., Buchholz H.-G., Plachter B. Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the BNT162b2 mRNA Vaccine in a Local Vaccination Center in Germany. Vaccines. 2021;9:911. doi: 10.3390/vaccines9080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borobia A.M., Carcas A.J., Pérez-Olmeda M., Castaño L., Bertran M.J., García-Pérez J., Campins M., Portolés A., González-Pérez M., Morales M.T.G., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. Erratum in Lancet 2021, 398, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell A.A., Power L., Westrop S., McOwat K., Campbell H., Simmons R., Ramsay M.E., Brown K., Ladhani S.N., Amirthalingam G. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March−June 2021, England. Eurosurveillance. 2021;26:2100634. doi: 10.2807/1560-7917.ES.2021.26.28.2100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lustig Y., Sapir E., Regev-Yochay G., Cohen C., Fluss R., Olmer L., Indenbaum V., Mandelboim M., Doolman R., Amit S., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrobon A.J., Teixeira F.M.E., Sato M.N. I mmunosenescence and Inflammaging: Risk Factors of Severe COVID-19 in Older People. Front. Immunol. 2020;11:579220. doi: 10.3389/fimmu.2020.579220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C., Fang F., Weyand C.M., Goronzy J.J. The life cycle of a T cell after vaccination—Where does immune ageing strike? Clin. Exp. Immunol. 2017;187:71–81. doi: 10.1111/cei.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancro M.P. Age-Associated B Cells. Annu. Rev. Immunol. 2020;38:315–340. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]
- 24.Jefferson T., Rivetti D., Rivetti A., Rudin M., Di Pietrantonj C., Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: A systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. Erratum in Lancet 2006, 367, 986. [DOI] [PubMed] [Google Scholar]
- 25.Djennad A., Ramsay M.E., Pebody R., Fry N.K., Sheppard C., Ladhani S.N., Andrews N.J. Effectiveness of 23-Valent Polysaccharide Pneumococcal Vaccine and Changes in Invasive Pneumococcal Disease Incidence from 2000 to 2017 in Those Aged 65 and over in England and Wales. eClinicalMedicine. 2019;6:42–50. doi: 10.1016/j.eclinm.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein S.L., Pekosz A. Sex-based Biology and the Rational Design of Influenza Vaccination Strategies. J. Infect. Dis. 2014;209((Suppl. 3)):S114–S119. doi: 10.1093/infdis/jiu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, itsik.levy@sheba.health.gov.il (I.L.) and galia.rahav@sheba.health.gov.il (G.R.), upon reasonable request.