Abstract
Sweet Birch (Betula lenta) has several economic and medicinal uses. Very little is known about the chemical composition of B. lenta. In this study, the volatile compositions of the bark of B. lenta from authentic and commercial sources were assessed by gas chromatography-mass spectrometry (GC–MS) and gas chromatography–flame ionization detection (GC–FID). Overall, more than 60 compounds were identified in natural sweet birch EO obtained by hydro-distillation. The oil was dominated by methyl salicylate (93.24–99.84%). A good approach to distinguishing wintergreen and birch oils would be biomarker-based analysis. The biomarkers are selected based upon three main criteria: (1) the marker should be commercially unavailable or too expensive which renders the adulteration process very costly, (2) The marker should be detected consistently in all the tested authentic EO samples, and (3) A birch EO marker should be found exclusively in birch EO, not in wintergreen and vice versa. The minor components o-guaiacol, veratrole, 2-E-4-Z-decadienal, and 2-E-4-E-decadienal were identified as natural marker compounds for authentic sweet birch oil. Surprisingly, none of the tested 27 commercial samples contained any of the identified birch markers. The detection of wintergreen markers such as vitispirane and β-dehydroelsholtzia ketone, the synthetic marker dimethyl-2-hydroxyterephthalate, and ricenalidic acid lactone suggest the addition of wintergreen, synthetic methyl salicylate, and castor oil, respectively. This is the first report to identify birch biomarkers to the best of our knowledge.
Keywords: sweet birch, Betula lenta, essential oil, o-guaiacol, veratrole, 2-E-4-Z-decadienal, 2-E-4-E-decadienal, dimethyl 2-hydroxyterephthalate
1. Introduction
Betula species (Betulaceae) are largely found in regions north of the equator. Commonly known as birch, they have been used for centuries in various economic and medicinal applications [1]. Betula species are beneficial in cases of inflammation, infections, stomachache, urinary tract disorders, and skin problems [1,2]. Betula species contain flavonoids, glycosides, phenolics, saponins, sterols, tannins, and terpenes [3]. Being a rich source of antimicrobial compounds supports their traditional use to treat infections [4]. All parts of the tree, especially the wood, are used as raw materials in the paper and furniture industries in addition to charcoal production, dietary supplements, and cosmetics [5,6].
Betula lenta L., commonly known as sweet birch, black birch, mahogany birch, cherry birch, and southern birch, is one of the dark-barked birch species. B. lenta is a deciduous tree native to the eastern portion of North America. It is frequently found in northern forest ecosystems in the Appalachian Mountains. In the USA, it is found in Connecticut, Georgia, Kentucky, Maine, Maryland, Massachusetts, New Hampshire, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Vermont, Virginia, and West Virginia [7]. The tree can reach up to 30 m tall. B. lenta is a pharmacologically important tree. Traditionally, infusions and teas made of B. lenta bark have been taken for stomachache and lung diseases, while teas from bark and twigs are beneficial for fevers. B. lenta EO is an anti-inflammatory agent and is suitable for skin problems, rheumatism, and bladder infections. The young sweet birch tree has smooth bark with distinctive horizontal lenticels like other birch species. The mature tree has rough dark brown bark with irregular vertical cracks [8]. Birch bark is one of the abundant, underutilized natural resources [9]. It is the primary waste product of birch processing (about 10–15% of birch mass) [5]. The outer bark of white-barked birches is a good source of pentacyclic triterpenoids especially lupeol and betulin [9]. Several triterpenoids were identified in the outer bark of B. lenta, including betulin, betulone, betulinic acid, betulin-3-caffeate, lupeol, lupenone, lup-20(29)-ene-3ß,30-diol, lup-20(29)-ene-3ß-ol-30-al, lup-20(29)-ene-3ß,28-diol-30-al, and lupan-3ß,20diol [9]. Betula essential oils are generally produced by hydro-distillation. The bark of B. lenta is a known source of methyl salicylate and ethyl salicylate [10,11]. A significant amount of attention has been given to the authentication of wintergreen (Gaultheria spp.) EO. The authenticity of the essential oils of B. lenta offered in the market must be regarded with a similar attention. Unlike birch EO, the authentication of wintergreen EO has been the subject of several studies since 1986 [12]. Wintergreen and sweet birch EOs have a very similar chemical composition, dominated by methyl salicylate (about 99%). To authenticate the naturalness of this ester, it is easy to detect by GC-MS, preferably in SIM mode, the impurities that accompany the synthetic products acting as markers of the synthetic origins [12,13,14,15]. The efficiency of this task is enhanced using 13C, and 2H isotopic ratio measurements [14,15], and quantitative 2H-ERETIC-NMR [16]. Ultimately, 14C dating helps to ensure the contemporary origin of methyl salicylate [14]. Due to the high cost of natural birch oil, commercial birch oils are heavily adulterated [17]. Birch oil can be adulterated by adding a cheaper oil or synthetic methyl salicylate to increase the profit or by completely replacing the natural methyl salicylate with the inexpensive synthetic option. Therefore, the current study explores the volatile composition of the EO extracted from the bark of B. lenta obtained from trusted sources from Pennsylvania, USA, and compares the composition to the oils available in the US market. To the best of our knowledge, the EO composition of the bark of B. lenta has not been previously reported. Moreover, the essential oils of B. lenta were examined to identify unique chemical markers to help with authentication or adulteration detection.
2. Materials and Methods
2.1. Essential Oils
B. lenta bark samples were collected from McKean County, Pennsylvania, USA. Sweet birch grows in this area along with red maple (Acer rubrum L.), sugar maple (Acer saccharum Marshall), black cherry (Prunus serotina Ehrh), American beech (Fagus grandifolia Ehrh), Eastern hemlock (Tsuga canadensis (L.) Carriere), and yellow birch (Betula alleghaniensis Britt). B. lenta essential oils from authentic sources (hydro-distilled in both lab and industrial settings) and commercial suppliers were acquired from the EO collection of the Aromatic Plant Research Center (APRC, Lehi, UT, USA). Samples A1-A18 were distilled in industrial distillers while A19-A21 were extracted in the laboratory. The distillation conditions have been optimized in our previous work on wintergreen essential oil [13]. Birch bark samples were air-dried and then fermented in water for 8–10 h at 50 °C prior to distillation. Fermentation was over when the pH reached 3–4 and held this number for more than two hours. In the lab, the plant material was distilled for 6–10 h in a Clevenger-type apparatus. In the industrial setting, the plant material was distilled for 6–10 h at 600 kPa in a 5.5 m3 distiller, starting slowly for 2 h and then stabilizing around a flow rate of 2 L/min. Twenty-seven commercial birch essential oil products were purchased online. The product labels of most of these samples were labeled as “100% Pure Essential oil” or “100% pure Therapeutic Grade Essential oil” (Table 1).
Table 1.
Available information on commercial sweet birch samples.
| Sample ID | Name | Sample Description | Botanical Name |
|---|---|---|---|
| C1 | Birch | 100% Pure Essential oil | Betula sp. |
| C2 | Birch Sweet | 100% Pure Essential oil | Betula lenta |
| C3 | Birch | 100% pure and natural Therapeutic Grade Essential oil | Betula lenta |
| C4 | Birch | 100% pure Essential oil | Betula lenta |
| C5 | Birch | 100% Pure Essential oil | Betula lenta |
| C6 | Birch | 100% pure Therapeutic Grade Essential oil | Betula lenta |
| C7 | Birch | 100% pure Therapeutic Grade Essential oil | Betula lenta |
| C8 | Birch | Therapeutic Grade Essential oil | NA |
| C9 | Birch | 100% pure Therapeutic Grade Essential oil | Betula lenta |
| C10 | Birch | 100% pure Essential oil, Therapeutic Grade | Betula lenta |
| C11 | Birch Sweet | 100% pure Therapeutic Grade Essential oil | Betula lenta |
| C12 | Birch | 100% pure Essential oil | Betula lenta |
| C13 | Birch Sweet | Pure Essential oil | Betula lenta |
| C14 | Sweet Birch | 100% pure Therapeutic Grade Essential oil | Betula lenta |
| C15 | Birch Sweet | 100% pure Therapeutic Grade Essential oil | Betula lenta |
| C16 | Birch | 100% Pure Essential oil | Betula lenta |
| C17 | Birch premium | 100% pure Therapeutic Grade Essential oil | Betula lenta |
| C18 | Birch | 100% Pure Essential oil | Betula lenta |
| C19 | Birch | 100% pure Essential oil | Betula lenta |
| C20 | Birch | 100% pure Essential oil | Betula lenta |
| C21 | Birch | 100% Pure Essential oil | Betula sp. |
| C22 | Birch | 100% Pure Essential oil | Betula lenta |
| C23 | Birch | 100% Pure Essential oil | Betula lenta |
| C24 | Birch | 100% Pure Essential oil | Betula lenta |
| C25 | Birch | 100% pure Essential oil | Betula lenta |
| C26 | Birch | 100% pure Essential oil | Betula lenta |
| C27 | Birch | 100% pure Essential oil | Betula lenta |
2.2. Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
Natural and commercial birch oil samples were analyzed using a gas chromatograph coupled to a mass spectrometer QP2010 Ultra (Shimadzu Scientific Instruments, Columbia, MD, USA) with electron impact (EI) mode with 70 eV. The separation of the analytes was carried out by using a ZB5MS GC capillary column, using 40–400 m/z range scans with a scan rate of 3.0 scan/s. The column temperature was set at 50 °C for 2 min and then increased at 2 °C/min to the temperature of 260 °C. The carrier gas was helium with a constant flow rate of 1.37 mL/min. The injector temperature was kept at 260 °C. For each essential oil sample, 1:10 v/v solution in dichloromethane (DCM) was prepared, and 0.3 μL was injected using a split ratio of 1:30. The essential oil components were identified by comparing mass spectral fragmentation patterns (over 80% similarity match) and retention indices (RI) based on a series of homologous C8–C20 n-alkanes with those reported in databases (NIST database, and our in-house library) using the Lab Solutions GCMS post-run analysis software version 4.45 (Shimadzu Scientific Instruments, Columbia, MD, USA) [18].
2.3. Gas Chromatography–Flame Ionization Detection (GC–FID) Analysis
Analysis of natural birch essential oil was carried out using a Shimadzu GC 2010 equipped with a flame ionization detector (Shimadzu Scientific Instruments, Columbia, MD, USA), as previously described [18] with a ZB-5 capillary column (Phenomenex, Torrance, CA, USA).
3. Results and Discussion
3.1. Authentic B. lenta Oil
Sweet birch samples were hydro-distilled both in the lab and in industrial distillers by trusted sources. Thus, there is no question about authenticity. The EO yield was in the range of 0.07–0.1%. The odor can be described as papery, lightly weak wintergreen, sweet, and lightly woody. There were no significant differences between the lab-distilled oils vs. industrially distilled ones. The chemical compositions of authentic birch EO (21 samples) are summarized in Table 2. Overall, more than 60 compounds were identified in natural sweet birch EOs. The oils are dominated by methyl salicylate (93.24–99.84%). Methyl salicylate is an analgesic phenolic ester [19] that dominates wintergreen [17] and sweet birch [11] oils. During the hydro-distillation of wintergreen (Gaultheria fragrantissima Wall), the glycoside gaultherin is converted to methyl salicylate, which is the major compound of the oil (above 98%) [17]. Some variations were observed in some of the minor components. o-Guaiacol, veratrole, ethyl salicylate, 2-E-4-Z-decadienal, 2-E-4-E-decadienal, and methyl o-anisate were consistently detected in all the authentic samples. The composition of B. lenta bark in the current study is quite different from that of other birch bark oils. The EO obtained from B. nigra bark was dominated by fatty acids and fatty acid-derived compounds (51.2–80.4%) and saturated normal alkanes (4.5–29.8%) [2,20,21]. The inner bark of B. pendula yielded an EO made of methyl salicylic, palmic, phenic, and behenic acids, and sesquiterpenes [22]. In another study from New Zealand, the EO of the inner bark of B. pendula had E-α-bergamotene (31%) and α-santalene (19%) as the main components. In comparison, the major components of B. papyrifera inner bark oil were E-α-bergamotene (18%), ar-curcumene (12%), E-β-farnesene (12%), Z-β-farnesene (10%) and Z-α-bergamotene (8%) [23]. The main constituents of the EO obtained from the bark of B. pubescens from Russia were α-santalene (2.0%), E-α-bergamotene (3.5%), E-β-bergamotene (0.8%), α-epoxysantalene (0.3%), E-α-epoxybergamotene (0.3%) and E-β-epoxybergamotene (0.4%) [24].
Table 2.
Chemical composition of authentic (A) sweet birch EO samples.
| RIcalc | RIexp | Compounds | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 | A12 | A13 | A14 | A15 | A16 | A17 | A18 | A19 | A20 | A21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 801 | 802 | Hexanal | - | - | 0.01 | 0.01 | 0.01 | - | - | - | - | - | - | 0.01 | 0.01 | 0.02 | tr | 0.02 | 0.05 | 0.05 | 0.06 | 0.01 | 0.01 |
| 846 | 846 | 2E-Hexenal | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.13 | 0.02 | - | - | - | - | - | - |
| 862 | 862 | n-Hexanol | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | 0.01 | 0.02 | 0.01 | - | - | |
| 934 | 931 | α-Pinene | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | tr | - | - | - | - | 0.01 | - | - | - | - |
| 970 | 971 | Sabinene | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - |
| 975 | 974 | β-Pinene | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | 0.01 | tr | tr | - | - | - | - | - | - | - |
| 977 | 977 | Phenol | - | - | - | - | - | - | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | - | - | - | - |
| 988 | 984 | 2-Pentyl furan | - | 0.01 | - | 0.01 | 0.02 | tr | - | - | - | tr | - | 0.01 | 0.02 | - | - | 0.04 | 0.01 | 0.01 | - | - | - |
| 1009 | 1009 | o-Methyl anisole | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | tr | - | - | - | - | - | - | - | - | - |
| 1024 | 1020 | p-Cymene | - | - | - | - | -- | - | - | - | - | -- | - | - | - | tr | - | 0.02 | - | 0.01 | - | - | - |
| 1044 | 1044 | E-β-Ocimene | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - |
| 1050 | 1048 | o-Cresol | - | - | - | - | - | - | - | tr | - | - | tr | - | - | tr | - | - | 0.01 | 0.01 | 0.03 | - | - |
| 1068 | 1070 | n-Octanol | - | - | - | - | - | - | - | tr | - | - | - | - | - | - | - | - | 0.08 | 0.04 | 0.03 | - | - |
| 1087 | 1087 | o-Guaiacol | tr | tr | tr | tr | tr | 0.02 | tr | 0.04 | 0.04 | 0.06 | 0.10 | 0.01 | tr | 0.04 | 0.05 | tr | 0.47 | 0.30 | 0.39 | 0.02 | 0.02 |
| 1095 | 1098 | Linalool | - | - | - | - | - | - | - | tr | - | - | - | - | - | 0.01 | tr | - | 0.13 | 0.01 | 0.04 | - | - |
| 1100 | 1103 | n-Nonanal | - | 0.02 | 0.02 | 0.02 | 0.02 | - | - | tr | - | - | - | 0.01 | 0.01 | 0.02 | 0.01 | - | - | 0.01 | - | 0.01 | 0.01 |
| 1141 | 1142 | Veratrole | tr | tr | tr | 0.01 | 0.01 | tr | tr | 0.01 | tr | 0.01 | 0.02 | 0.01 | tr | 0.01 | 0.01 | 0.02 | tr | tr | tr | tr | 0.01 |
| 1163 | 1160 | 2-E-Nonenal | - | 0.01 | - | 0.01 | 0.01 | - | - | - | - | tr | - | - | tr | - | - | - | - | - | - | 0.03 | 0.01 |
| 1177 | 1180 | Terpinen-4-ol | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | 0.02 | 0.02 | - | 0.01 | - | - |
| 1191 | 1191 | Methyl salicylate | 98.97 | 97.37 | 98.96 | 98.30 | 98.22 | 99.54 | 99.78 | 99.77 | 99.84 | 98.70 | 99.11 | 98.38 | 97.93 | 99.48 | 99.67 | 93.24 | 97.74 | 99.20 | 98.82 | 97.87 | 98.49 |
| 1195 | 1195 | Methyl chavicol | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.12 | - | - | - | - |
| 1201 | 1201 | n-Decanal | - | 0.01 | 0.01 | 0.01 | 0.01 | - | - | tr | - | - | - | 0.01 | 0.01 | tr | tr | - | - | - | - | 0.01 | - |
| 1239 | 1236 | o-Anisaldehyde | - | - | - | 0.01 | 0.01 | - | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - | - | - | - | - | - |
| 1260 | 1260 | 2-E-Decenal | - | - | - | - | tr | - | - | - | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | tr | - |
| 1266 | 1266 | Ethyl salicylate | 0.58 | 0.76 | 0.49 | 0.61 | 0.61 | 0.06 | 0.05 | 0.04 | 0.03 | 0.63 | 0.33 | 0.60 | 0.59 | 0.03 | 0.03 | 6.41 | 0.74 | 0.08 | 0.40 | 1.7 | 0.84 |
| 1283 | 1282 | Bornyl acetate | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - |
| 1292 | 1291 | 2-E-4-Z-Decadienal | 0.04 | 0.10 | 0.10 | 0.05 | 0.06 | 0.02 | tr | tr | tr | tr | tr | 0.05 | 0.09 | tr | tr | tr | tr | tr | tr | 0.01 | 0.01 |
| 1319 | 1315 | 2-E-4-E-Decadienal | 0.06 | 0.21 | 0.15 | 0.12 | 0.12 | 0.06 | 0.01 | tr | tr | 0.02 | tr | 0.11 | 0.19 | 0.01 | tr | tr | tr | tr | tr | 0.02 | 0.02 |
| 1322 | 1319 | Methyl geranate | - | - | - | - | - | - | - | - | - | - | - | 0 | 0.01 | - | - | - | - | - | - | - | - |
| 1332 | 1332 | Methyl o-anisate | 0.29 | 0.40 | 0.15 | 0.53 | 0.45 | 0.26 | 0.13 | 0.04 | 0.04 | 0.54 | 0.39 | 0.48 | 0.34 | 0.04 | 0.08 | 0.09 | 0.44 | 0.08 | 0.14 | 0.12 | 0.19 |
| 1356 | 1345 | Eugenol | - | - | - | - | - | - | 0.01 | 0.03 | 0.02 | tr | 0.01 | - | - | 0.03 | 0.02 | - | 0.01 | 0.04 | 0.02 | tr | - |
| 1365 | 1365 | Z-8-Undecenal | - | 0.01 | 0.02 | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - |
| 1382 | 1382 | Hexyl hexanoate | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - |
| 1417 | 1417 | β-Caryophyllene | - | 0.01 | - | - | 0.01 | - | - | tr | - | - | - | tr | 0 | 0.01 | 0.01 | - | - | - | - | - | - |
| 1423 | 1423 | Isobutyl salicylate | - | 0.04 | - | 0.01 | 0.02 | - | - | - | - | - | - | 0.01 | 0.04 | - | - | - | - | - | - | 0.03 | 0.01 |
| 1453 | 1445 | Geranyl acetone | - | 0.01 | - | tr | 0.01 | - | - | - | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | - | 0.01 |
| 1452 | 1453 | α-Humulene | - | 0.03 | - | 0.02 | 0.02 | - | - | tr | - | - | - | 0.02 | 0.02 | tr | 0.01 | - | - | - | - | - | - |
| 1432 | 1480 | E-β-Bergamotene | - | - | - | - | - | - | - | - | - | - | - | - | tr | - | - | - | - | - | - | - | - |
| 1500 | 1495 | α-Muurolene | - | 0.02 | 0.01 | - | - | - | - | - | - | - | - | tr | 0.01 | - | - | - | - | - | - | - | - |
| 1505 | 1502 | E-E-α-Farnesene | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - |
| 1507 | Unidentified | - | - | - | - | 0.02 | 0.01 | - | - | - | - | - | 0.02 | - | - | 0.09 | - | - | - | - | - | - | |
| 1535 | 1528 | Isopentyl salicylate | 0.01 | 0.04 | 0.01 | 0.05 | 0.09 | - | - | - | - | - | - | 0.06 | 0.03 | - | - | 0.12 | - | - | - | - | 0.01 |
| 1568 | 1568 | 3-E-Hexenyl benzoate | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.02 | 0.01 | - | - | - | - | - | - |
| 1574 | 1574 | Pentyl salicylate | - | - | - | - | - | - | - | - | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | - | - |
| 1579 | 1575 | n-Hexyl benzoate | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - |
| 1577 | 1577 | Caryophyllene oxide | - | - | - | - | - | - | - | tr | - | - | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - |
| 1679 | 1679 | Hexyl salicylate | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - |
| 1697 | 1696 | 2-Pentadecanone | - | - | - | - | - | - | - | - | - | - | - | - | tr | - | - | - | - | - | - | - | - |
| 1700 | 1699 | Heptadecane | - | - | - | - | 0.01 | - | - | - | - | - | - | tr | 0.01 | tr | - | - | - | - | - | - | - |
| 1714 | 1714 | Pentadecanal | - | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | tr | - | - | 0.01 | 0.01 | 0.01 | 0.02 | - | - | - | tr | - | - | - | - |
| 1800 | 1799 | Octadecane | - | 0.01 | tr | tr | 0.01 | - | - | - | - | - | - | 0.01 | 0.01 | tr | tr | - | - | 0.01 | - | tr | - |
| 1864 | 1864 | Benzyl salicylate | - | 0.02 | - | 0.01 | 0.01 | - | tr | 0.01 | tr | tr | - | 0.01 | 0.03 | - | - | - | - | - | - | 0.01 | 0.01 |
| 1900 | 1899 | Nonadecane | - | 0.01 | 0.01 | 0.01 | 0.01 | - | - | tr | - | - | - | 0.01 | 0.01 | tr | tr | - | - | 0.01 | - | 0.01 | - |
| 1903 | 1903 | E-E,-5,9,13-Pentadecatrien-2-one, 6,10,14-trimethyl | - | - | - | - | 0.01 | - | - | - | - | - | - | 0 | 0.01 | - | - | - | - | - | - | - | - |
| 2000 | 2000 | Eicosane | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | - | tr | tr | tr | - | - | 0.02 | 0.02 | - | tr | - | - | 0.01 | - | - | 0.01 |
| 2061 | 2061 | Phenyl ethyl alcohol dimer | - | - | - | - | - | - | - | tr | - | - | - | tr | - | tr | - | - | - | - | - | 0.01 | - |
| 2100 | 2100 | Heneicosane | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | - | 0.01 | 0.01 | 0.01 | tr | - | 0.02 | 0.02 | tr | 0.01 | - | - | 0.02 | - | 0.01 | tr |
| 2138 | 2138 | Oxacycloheptadecenone | - | - | - | - | - | - | - | - | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | - | - |
| 2200 | 2199 | Docosane | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - |
| 2300 | 2300 | n-Tricosane | - | - | - | 0.01 | - | - | - | tr | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - |
| 2500 | 2498 | Pentacosane | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - |
| 2700 | 2698 | Heptacosane | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - |
| Yield (%) | 0.07 | 0.1 | 0.08 | 0.07 | 0.09 | 0.09 | 0.07 | 0.07 | 0.07 | 0.1 | 0.07 | 0.07 | 0.08 | 0.1 | 0.08 | 0.1 | 0.1 | 0.08 | 0.07 | 0.07 | 0.1 |
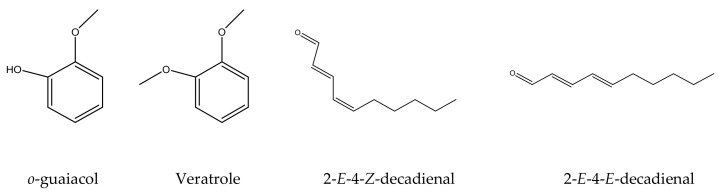
Enantiomeric distribution is one of the popular methods of detecting adulteration in essential oils [25]. However, it was not possible to perform chiral analysis on B. lenta bark oil since methyl salicylate lacks a chiral core, and the minor chiral components are present in very low quantities. It is easy to confuse wintergreen and sweet birch oils due to their high methyl salicylate content. A good approach to distinguishing wintergreen and birch oils would be biomarker-based analysis. The biomarkers are selected based upon three main criteria: (1) the marker should be commercially unavailable or too expensive which renders the adulteration process very costly, (2) The marker should be detected consistently in all the tested authentic EO samples, and (3) A birch EO marker should be found exclusively in birch EO, not in wintergreen and vice versa. Table 3 shows a comparison between authentic sweet birch and wintergreen oil compositions. A total of 18 natural compounds are common in both oils. Ethyl salicylate and methyl o-anisate are common markers in both oils; however, they are present in higher amounts in sweet birch EO. Therefore, we can identify o-guaiacol, veratrole, 2-E-4-Z-decadienal, and 2-E-4-E-decadienal as natural marker compounds for authentic B. lenta oil (Figure 1).
Table 3.
Comparison of authentic wintergreen EOs and authentic birch EOs.
| Compound Name | Wintergreen (34 Samples) | Birch (21 Samples) | ||||
|---|---|---|---|---|---|---|
| Range (%) | Mean | SD | Range (%) | Mean | SD | |
| 1,8-Cineole | tr–0.05 | 0.01 | 0.01 | - | - | - |
| 2-Pentyl furan | - | - | - | 0–0.04 | 0.02 | 0.01 |
| 2-E-4-Z-Decadienal | - | - | - | tr–0.10 | 0.08 | 0.03 |
| 2-E-4-E-Decadienal | - | - | - | tr–0.21 | 0.05 | 0.07 |
| 2-E-Decenal | - | - | - | 0–0.01 | 0.01 | 0 |
| 2E-Hexenal | - | - | - | 0–0.13 | 0.08 | 0.08 |
| 2-E-Nonenal | - | - | - | 0–0.01 | 0.01 | 0.01 |
| 3-Z-Hexenol | 0.01–0.03 | 0.01 | 0.01 | - | - | - |
| 3-Z-Hexenyl 3-methyl butanoate | tr | tr | 0 | - | - | - |
| 3-Z-Hexenyl butanoate | 0.01 | 0.01 | 0 | - | - | - |
| 3-Methyl-1,2-cyclopentanedione | tr–0.01 | 0.01 | 0 | - | - | - |
| 3-E-Hexenyl benzoate | - | - | - | 0–0.02 | 0.02 | 0.01 |
| allo-Ocimene | 0.01 | 0.01 | 0 | - | - | - |
| α-Humulene | tr | tr | 0 | 0–0.03 | 0.02 | 0.01 |
| α-Muurolene | - | - | 0–0.02 | 0.02 | 0.01 | |
| α-Phellandrene | tr–0.01 | tr | 0.01 | - | 0.01 | - |
| α-Pinene | tr–0.09 | 0.02 | 0.02 | 0–0.01 | 0.01 | 0 |
| α-Thujene | tr | tr | 0 | - | - | - |
| Artemisia alcohol | tr | tr | 0 | - | - | - |
| Benzaldehyde | tr–0.01 | 0.01 | 0.01 | - | - | - |
| Benzyl alcohol | tr–0.02 | 0.01 | 0.01 | - | - | - |
| Benzyl salicylate | - | - | - | 0–0.03 | 0.01 | 0.01 |
| β-Caryophyllene | tr–0.02 | 0.01 | 0 | 0–0.01 | 0.01 | 0 |
| β-Dehydro elsholtzia ketone | tr–0.01 | 0.01 | 0 | - | - | - |
| β-Pinene | tr–0.05 | 0.02 | 0.01 | 0–0.01 | 0.01 | 0 |
| Bornyl acetate | tr–0.01 | 0.01 | 0 | 0–0.01 | 0.01 | 0 |
| Camphene | tr–0.01 | 0.01 | 0.01 | - | - | - |
| Caryophyllene oxide | - | - | - | 0–0.01 | 0.01 | 0 |
| Z-8-Undecenal | - | - | - | 0–0.02 | 0.01 | 0.01 |
| δ-Cadinene | tr | tr | 0 | - | - | - |
| Docosane | - | - | - | 0–0.01 | 0.01 | 0 |
| Eicosane | - | - | - | 0–0.03 | 0.02 | 0.01 |
| Ethyl benzoate | tr–0.01 | 0.01 | 0 | - | - | - |
| Ethyl salicylate | tr–0.33 | 0.1 | 0.07 | 0.03–6.41 | 0.74 | 1.36 |
| Eugenol | 0.01–0.14 | 0.05 | 0.03 | 0–0.04 | 0.03 | 0.01 |
| Geraniol | tr–0.01 | 0.01 | 0 | - | - | - |
| Geranyl acetone | - | - | - | 0–0.01 | tr | 0 |
| Germacrene D | tr | tr | 0 | - | - | - |
| Heneicosane | - | - | - | 0 -0.03 | 0.02 | 0.01 |
| Heptacosane | - | - | - | 0–0.01 | 0.01 | 0 |
| Heptadecane | - | - | - | 0–0.01 | 0.01 | 0 |
| Hexanal | - | - | - | 0–0.06 | 0.02 | 0.02 |
| Hexenyl acetate | 0.01 | 0.01 | 0 | - | - | - |
| Hexyl hexanoate | - | - | - | 0–0.01 | 0.01 | 0 |
| Hexyl salicylate | - | - | - | 0–0.01 | 0.01 | 0 |
| Hotrienol | tr | tr | 0 | - | - | - |
| Isobutyl salicylate | - | - | - | 0–0.04 | 0.01 | 0.01 |
| Isopentyl salicylate | - | - | - | 0–0.09 | 0.05 | 0.04 |
| Isothymol | tr–0.01 | 0.01 | 0 | - | - | - |
| Limonene | tr–0.02 | 0.01 | 0.01 | - | - | - |
| Linalool | 0–0.06 | 0.03 | 0.01 | 0–0.13 | 0.05 | 0.06 |
| Menthone | tr–0.02 | 0.02 | 0 | - | - | - |
| Methyl chavicol | - | - | - | 0–0.12 | 0.10 | 0 |
| Methyl geranate | - | - | - | 0–0.01 | 0.01 | 0.01 |
| Methyl o-anisate | tr–0.01 | 0.01 | 0 | 0.04–0.54 | 0.25 | 0.18 |
| Methyl salicylate | 99.47–100 | 99.77 | 0.13 | 93.24–99.84 | 98.54 | 1.42 |
| n-Decanal | - | - | - | 0–0.01 | 0.01 | 0 |
| n-Hexanol | tr–0.01 | 0.01 | 0 | 0–0.02 | 0.01 | 0 |
| n-Hexyl benzoate | - | - | - | 0–0.01 | 0.01 | 0 |
| n-Nonanal | tr–0.01 | tr | 0.01 | 0–0.02 | 0.01 | 0 |
| n-Octane | tr | tr | 0 | - | - | - |
| n-Octanol | tr–0.01 | 0.01 | 0.01 | 0–0.08 | 0.05 | 0.03 |
| Nonadecane | - | - | - | 0–0.01 | 0.01 | 0 |
| n-Tricosane | - | - | - | 0–0.01 | 0.01 | 0 |
| Octadecane | - | - | - | 0–0.01 | 0.01 | 0 |
| o-Anisaldehyde | - | - | - | 0–0.01 | 0.01 | 0 |
| o-Cresol | - | - | - | 0–0.03 | 0.01 | 0.01 |
| o-Cymene | tr | tr | 0 | - | - | - |
| o-Guaiacol | - | - | - | tr–0.47 | 0.12 | 0.16 |
| o-Methyl anisole | - | - | - | 0–0.01 | 0.01 | 0 |
| Oxacycloheptadecenone | - | - | - | 0–0.01 | 0.01 | 0 |
| p-Mentha-1(7),8(10)-dien-9-ol | tr–0.01 | 0.01 | 0 | - | - | - |
| p-Xylene | tr | tr | 0 | - | - | - |
| p-Cymene | tr–0.01 | 0.01 | 0.01 | 0–0.02 | 0.02 | 0.01 |
| Pentacosane | - | - | - | 0–0.01 | 0.01 | 0 |
| Pentadecanal | - | - | - | 0–0.03 | 0.01 | 0.01 |
| Pentyl salicylate | - | - | - | 0–0.01 | 0.01 | 0 |
| Phenol | tr–0.01 | 0.01 | 0 | 0–0.01 | 0.01 | 0 |
| Phenyl ethyl alcohol dimer | tr | tr | 0 | - | - | - |
| Pulegone | tr–0.01 | 0.01 | 0 | - | - | - |
| Sabinene | - | - | - | 0–0.01 | 0.01 | 0 |
| Terpinen-4-ol | tr | tr | 0 | 0–0.02 | 0.02 | 0.01 |
| Thymol | tr–0.01 | 0.01 | 0 | - | - | - |
| Toluene | tr–0.01 | 0.01 | 0.01 | - | - | - |
| E-β-Ocimene | - | - | - | 0–0.01 | 0 | |
| E-Caryophyllene | tr–0.01 | 0.01 | 0 | - | - | - |
| E-E,-5,9,13-Pentadecatrien-2-one, 6,10,14-trimethyl | - | - | - | 0–0.01 | 0.01 | 0 |
| E-E-α-Farnesene | - | - | - | 0–0.03 | 0.02 | 0 |
| Veratrole | - | - | - | tr–0.02 | 0.01 | 0 |
| Vitispirane | tr–0.02 | 0.01 | 0.01 | - | - | - |
| Wintergreen sesquiterpenoid 1 | tr–0.01 | 0.01 | 0 | - | - | - |
| Xylene isomer | tr | tr | 0 | - | - | - |
Figure 1.
Chemical structures of the identified birch biomarkers.
3.2. Commercial Sweet Birch Oil
Twenty-seven commercial birch oil samples were obtained from commercial vendors available in the US market. The chemical compositions of commercial birch EOs are shown in Table 4, with more than 100 identified components. Similar to the natural EO, commercial sweet birch EOs were dominated by methyl salicylate (56.71–99.9%). Minor components varied greatly between commercial samples. Woods and colleagues analyzed a commercial EO sample that was exclusively made of methyl salicylate [2,20]. Sweet birch markers (o-guaiacol, veratrole, 2-E-4-Z-decadienal, and 2-E-4-E-decadienal) were not detected in any of the tested commercial samples. Ethyl salicylate was absent from 11 samples. Interestingly, wintergreen markers such as vitispirane (10 occurrences) and β-dehydroelsholtzia ketone (3 occurrences) were detected in the tested samples. This finding suggests that wintergreen oil is available in the market as birch EO or the addition of wintergreen oil to natural birch oil [13]. Moreover, synthetic markers such as dimethyl-2-hydroxyterephthalate (6 occurrences) were also detected, which suggests the addition of synthetic methyl salicylate [17]. Ricenalidic acid lactone, a marker of the addition of castor oil, was detected in one sample.
Table 4.
Chemical composition of commercial (C) birch EO samples.
| RIcalc | RIexp | Compounds | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | C15 | C16 | C17 | C18 | C19 | C20 | C21 | C22 | C23 | C24 | C25 | C26 | C27 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 848 | 848 | 3-Z-Hexenol | - | - | 0.02 | 0.03 | - | - | - | - | 0.05 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 862 | 862 | n-Hexanol | - | - | tr | 0.01 | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 895 | 895 | o-Xylene | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.36 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 900 | 900 | n-Nonane | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 920 | 919 | Hashishene | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 921 | Tricyclene + Hexylene glycol | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.14 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 921 | 921 | Tricyclene | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.04 | - | - | - |
| 924 | 924 | α-Thujene | - | - | - | tr | - | - | - | - | - | 2.93 | - | - | 0.01 | 0.06 | - | - | - | - | - | - | - | - | - | - | 0.27 | - | - |
| 934 | 931 | α-Pinene | - | - | 0.01 | 0.04 | - | - | 0.01 | 0.28 | 0.02 | 0.21 | - | - | 0.19 | 6.11 | - | 0.18 | 2.30 | - | - | - | - | 0.01 | 1.86 | 0.93 | 0.01 | - | |
| 942 | 942 | Thujadiene | - | - | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 993 | 945 | α-Fenchene | - | - | - | - | - | - | - | 0.04 | - | - | - | - | - | 0.05 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 949 | 948 | Camphene | - | - | - | - | - | - | - | 0.14 | - | - | - | - | 0.02 | 0.36 | - | 0.03 | 0.50 | - | - | - | - | - | - | 0.65 | - | - | - |
| 969 | 970 | 3,7,7-Trimethyl-1,3,5-cycloheptatriene | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 970 | 971 | Sabinene | - | - | - | - | - | - | - | - | - | 0.18 | - | - | 0.01 | 0.10 | - | - | - | - | - | - | - | - | - | 1.31 | - | - | - |
| 975 | 974 | β-Pinene | - | - | 0.01 | 0.02 | - | - | tr | 0.03 | - | 0.01 | - | - | 0.07 | 0.17 | - | 0.02 | 2.28 | 0.03 | - | - | - | tr | - | - | - | - | - |
| 988 | 988 | Myrcene | - | - | - | tr | - | - | tr | 0.06 | - | 0.02 | - | - | 0.02 | 1.66 | - | tr | - | - | - | - | - | - | - | - | - | - | - |
| 1001 | 999 | δ-2-Carene | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | 0.19 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1006 | 1006 | α-Phellandrene | - | - | - | - | - | - | - | 0.01 | - | 0.04 | - | - | tr | 1.94 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1008 | 1008 | δ-3-Carene | - | - | - | 0.01 | - | - | - | 1.92 | - | 0.13 | - | - | - | 8.42 | - | - | 0.24 | 0.04 | - | - | - | - | - | 1.36 | - | - | - |
| 1012 | 1012 | 1,4-Cineole | - | - | - | - | - | - | - | 0.14 | - | - | - | - | - | 0.30 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1014 | 1014 | α-Terpinene | - | - | - | - | - | - | - | 0.04 | - | 0.01 | - | - | 0.09 | 2.57 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1020 | 1020 | p-Cymene | - | - | 0.01 | 0.01 | - | - | - | 0.05 | - | 0.05 | - | - | 0.12 | 0.68 | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - |
| 1026 | 1026 | Acetyl methyl furan | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1024 | 1027 | Limonene | - | - | 0.01 | 0.01 | 0.05 | 0.06 | 0.02 | 0.28 | 0.01 | 0.06 | - | - | 0.26 | 1.39 | - | 0.04 | 0.55 | 0.09 | 0.02 | - | - | 0.01 | - | 0.47 | 0.27 | 0.01 | - |
| 1025 | 1029 | β-Phellandrene | - | - | - | 0.01 | - | - | tr | - | - | 0.02 | - | - | - | 0.19 | - | - | - | - | - | - | - | - | - | 0.15 | - | - | - |
| 1026 | 1031 | 1,8-Cineole | - | - | 0.01 | 0.02 | - | - | 0.01 | 0.11 | 0.01 | - | - | - | 0.69 | 2.30 | - | 0.33 | - | - | - | 0.06 | - | 0.02 | - | - | - | 0.02 | 0.06 |
| 1032 | 1033 | Z-β-Ocimene | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | 0.12 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1041 | Unidentified | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.48 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 1044 | 1044 | E-β-Ocimene | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.24 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1055 | Unidentified | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.25 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 1054 | 1058 | γ-Terpinene | - | - | - | - | - | - | - | 0.02 | - | 0.02 | - | - | 0.23 | 0.27 | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - |
| 1063 | 1070 | n-Octanol | - | - | - | - | 0.01 | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1086 | 1088 | Terpinolene | - | - | - | - | - | - | - | 0.11 | - | 0.01 | - | - | 0.04 | 1.67 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1086 | 1092 | Fenchone | - | - | - | - | - | - | tr | - | - | - | - | - | - | 0.04 | - | - | - | - | - | - | - | tr | - | - | - | - | - |
| 1095 | 1098 | Linalool | - | 0.03 | 0.03 | 0.04 | 0.01 | 0.02 | 0.03 | - | 0.05 | - | - | 0.02 | 0.08 | - | - | 0.03 | - | 0.04 | 0.03 | 0.07 | 0.04 | 0.03 | 0.03 | - | - | 0.03 | 0.06 |
| 1106 | 1106 | Isocamphone | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.33 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1106 | 1114 | Z-Rose oxide | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1112 | 1119 | E-Thujone | - | - | - | - | - | - | 0.01 | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - |
| 1118 | 1121 | Isophorone | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.96 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1130 | 1131 | Terpin-3-en-1-ol | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.22 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1141 | 1145 | Camphor | - | tr | - | - | - | - | - | - | - | - | - | 0.03 | 4.54 | - | 0.09 | 0.54 | - | - | - | - | - | - | - | - | - | - | |
| 1143 | 1150 | Z-β-Terpineol | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.13 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1148 | 1152 | Citronellal | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1148 | 1157 | Menthone | - | - | - | - | - | - | - | - | - | 0.01 | - | - | 0.01 | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1155 | 1162 | Isoborneol | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1157 | 1163 | Benzyl acetate | - | 0.02 | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1166 | 1166 | iso-Menthone | - | - | - | - | - | - | - | - | - | - | - | - | 0.05 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1169 | 1170 | Borneol | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.14 | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - |
| 1176 | 1175 | Menthol | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1177 | 1180 | Terpinen-4-ol | - | - | - | - | 0.01 | 0.01 | - | - | - | 0.01 | - | - | 0.47 | 0.12 | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - |
| 1191 | 1191 | Methyl salicylate | 99.99 | 99.90 | 99.80 | 99.64 | 99.38 | 99.29 | 99.78 | 95.18 | 99.34 | 95.64 | 99.68 | 99.91 | 96.67 | 56.71 | 99.95 | 98.89 | 93.37 | 99.68 | 99.95 | 99.82 | 99.87 | 99.85 | 99.96 | 93.83 | 97.58 | 99.74 | 99.78 |
| 1195 | 1195 | Methyl chavicol | - | - | - | - | - | - | - | - | - | 0.21 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1196 | 1196 | Isocamphenone | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.26 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1199 | 1197 | γ-Terpineol | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.35 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1209 | 1209 | Octyl acetate | - | - | - | - | 0.01 | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1249 | 1250 | Geraniol | - | - | - | - | tr | 0.01 | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | - |
| 1252 | 1252 | Linalyl acetate | - | - | - | 0.01 | - | - | - | - | - | - | - | - | 0.20 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1266 | 1266 | Ethyl salicylate | 0.01 | 0.04 | 0.05 | 0.08 | 0.50 | 0.55 | 0.07 | - | 0.07 | - | 0.26 | 0.03 | - | - | - | 0.15 | - | 0.05 | - | 0.04 | 0.04 | 0.04 | - | - | - | 0.11 | 0.08 |
| 1271 | 1271 | Citronellyl formate | - | - | - | - | - | - | - | - | - | - | - | - | 0.08 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1281 | 1281 | Vitispirane | - | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | - | 0.03 | - | - | 0.01 | - | - | - | 0.01 | - | - | - | - | - | 0.01 | - | - | - | - | - |
| 1283 | 1282 | Bornyl acetate | - | - | - | - | - | - | - | - | - | - | - | - | 0.02 | 0.65 | - | 0.08 | 0.20 | 0.07 | - | - | - | Tr | - | 0.15 | - | - | - |
| 1293 | 1293 | Methyl naphthalene | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1298 | 1297 | Geranyl formate | - | - | - | - | - | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1299 | 1299 | β-Dehydro elsholtzia ketone | - | - | - | 0.01 | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - |
| 1346 | 1344 | α-Terpinyl acetate | - | - | - | - | - | - | - | - | - | - | - | - | 0.02 | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - |
| 1356 | 1345 | Eugenol | - | - | 0.01 | 0.03 | - | - | 0.01 | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1371 | 1371 | Longicyclene | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1379 | 1376 | Geranyl acetate | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1387 | 1384 | β-Bourbonene | - | - | - | - | - | - | - | - | - | 0.01 | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1407 | 1407 | Longifolene | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.15 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1417 | 1417 | β-Caryophyllene | - | 0.01 | tr | 0.01 | 0.01 | 0.01 | - | 0.02 | 0.01 | - | - | 0.01 | 0.05 | - | - | 0.01 | - | - | - | - | - | - | - | - | - | 0.01 | - |
| 1439 | 1434 | Aromadendrene | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | - |
| 1452 | 1453 | α-Humulene | - | - | - | - | - | - | - | 0.17 | - | - | - | - | 0.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1458 | 1457 | allo-Aromadendrene | - | - | - | - | - | - | 0.01 | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - |
| 1461 | 1459 | Z-Cadina-1(6),4-diene | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1475 | 1470 | E-Cadina-1(6),4-diene | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1483 | 1476 | α-Amorphene | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1489 | 1487 | β-Selinene | - | - | - | - | - | - | - | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1496 | 1490 | Viridiflorene | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1489 | 1492 | α-Selinene | - | - | - | - | - | - | - | 0.06 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1500 | 1493 | Bicyclogermacrene | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1500 | 1495 | α-Muurolene | - | - | - | - | - | - | - | 0.05 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1509 | 1501 | α-Bulnesene | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1514 | Unidentified | - | - | - | - | - | - | - | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 1522 | 1517 | δ-Cadinene | - | - | - | - | - | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1521 | 1518 | E-Calamenene | - | - | - | - | - | - | - | 0.11 | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1523 | Unidentified | - | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 1533 | 1531 | E-Cadine-1,4-diene | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1544 | 1539 | α-Calacorene | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1548 | 1548 | Isocaryophyllene oxide | - | - | - | - | - | - | - | 0.08 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1570 | 1570 | Caryophyllenyl alcohol | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1577 | 1577 | Caryophyllene oxide | - | - | - | - | - | - | - | 0.12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1610 | 1610 | Dimethyl hydroxy terephthalate | - | - | - | - | - | - | - | 0.33 | 0.01 | 0.35 | - | - | 0.39 | 0.09 | 0.05 | - | - | - | - | - | - | - | - | - | - | - | - |
| 2057 | 2057 | Ricenalidic acid lactone | - | - | - | - | - | - | - | 0.14 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1282 | 1290 | E-Anethole | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.03 | - | - | - | - | - | - |
| 1298 | 1300 | Carvacrol | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.02 | - | - | - | - | - | - |
| 905 | 905 | Cyclofenchene | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - |
| 884 | 907 | Santene | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.18 | - | - | - |
| 1135 | 1123 | Neral | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.02 |
| 1800 | 1800 | Octadecane | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
4. Conclusions
Hydro-distilled Betula lenta L. essential oils showed almost similar chemical compositions, with methyl salicylate as the main component (93.24–99.84%). Four biomarkers, namely, o-guaiacol, veratrole, 2-E-4-Z-decadienal, and 2-E-4-E-decadienal were identified for the natural B. lenta oil. These markers can be used to distinguish between sweet birch and wintergreen oils and may be used in sweet birch oil authentication and adulteration detection. Interestingly, none of the tested commercial samples contained any of the identified birch EO markers. The detection of wintergreen markers such as vitispirane and β-dehydroelsholtzia ketone, the synthetic marker dimethyl-2-hydroxyterephthalate, and ricenalidic acid lactone suggest the addition of wintergreen, synthetic methyl salicylate, and castor oil, respectively. Further investigations on the evaluation of biological activities of B. lenta essential oil are required.
Acknowledgments
The authors would like to thank Lindsey Novosel, Albert McGarity, and Plamen Nikolov for kindly providing authentic birch samples. Special thanks to Casera Wootton, Sushant Sharma Banjara, and Sumitra Dahal for their help with sample preparation. We would also like to thank Russel Osguthorpe for supporting this research project.
Author Contributions
Conceptualization, N.S.D. and P.S.; methodology, A.P.; validation, P.S. and A.P.; formal analysis, A.P.; investigation, N.S.D. and P.S.; data curation, P.S.; writing—original draft preparation, N.S.D.; writing—review and editing, N.S.D., A.P. and P.S.; supervision, P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hua Y., Bentley M.D., Cole B.J.W., Murray K.D., Alford A.R. Triterpenes from the Outer Bark of Betula nigra. J. Wood Chem. Technol. 1991;11:503–516. doi: 10.1080/02773819108051090. [DOI] [Google Scholar]
- 2.Rastogi S., Pandey M.M., Kumar Singh Rawat A. Medicinal Plants of the Genus Betula—Traditional Uses and a Phytochemical–Pharmacological Review. J. Ethnopharmacol. 2015;159:62–83. doi: 10.1016/j.jep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demirci B., Paper D.H., Demirci F., Can Başer K.H., Franz G. Essential Oil of Betula Pendula Roth. Buds. Evid. Based Complement. Altern. Med. 2004;1:301–303. doi: 10.1093/ecam/neh041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blondeau D., St-Pierre A., Bourdeau N., Bley J., Lajeunesse A., Desgagné-Penix I. Antimicrobial Activity and Chemical Composition of White Birch (Betula papyrifera Marshall) Bark Extracts. Microbiologyopen. 2020;9:e00944. doi: 10.1002/mbo3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julkunen-Tiitto R., Rousi M., Bryant J., Sorsa S., Keinänen M., Sikanen H. Chemical Diversity of Several Betulaceae Species: Comparison of Phenolics and Terpenoids in Northern Birch Stems. Trees. 1996;11:16–22. doi: 10.1007/s004680050053. [DOI] [Google Scholar]
- 6.Foster S., Duke J.A. Medicinal Plants. Houghton Mifflin Company; Boston, MA, USA: 1990. [Google Scholar]
- 7.Stritch L. Betula lenta. Botanic Gardens Conservation International; Richmond, UK: 2014. The IUCN Red List of Threatened Species 2014: E.T194483A2340770. [Google Scholar]
- 8.Elias T.S., Dykeman P.A. Edible Wild Plants: A North American Field Guide to Over 200 Natural Foods. Sterling; New York, NY, USA: 2009. [Google Scholar]
- 9.Cole B.J., Bentley M., Hua Y. Triterpenoid Extractives in the Outer Bark of Betula lenta (Black Birch) Holzforschung. 1991;45:265–268. doi: 10.1515/hfsg.1991.45.4.265. [DOI] [Google Scholar]
- 10.Van Wyk B.-E., Wink M. Medicinal Plants of the World: An Illustrated Scientific Guide to Important Medicinal Plants and Their Uses. 2nd ed. CABI; Wallingford, UK: 2017. [Google Scholar]
- 11.Dosoky N.S., Setzer W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021;22:2380. doi: 10.3390/ijms22052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey C. Detection of Synthetic Flavorant Addition to Some Essential Oils by Selected Ion Monitoring GC/MS. In: Lawrence B.M., Mookherjee B.D., Willis B.J., editors. Flavors and Fragrances: A World Perspective, Proceedings of the 10th International Congress of Essential Oils, Fragrances and Flavors, Washington, DC, USA, 16–20 November 1986. Elsevier; Amsterdam, The Netherlands: 1988. [Google Scholar]
- 13.Ojha P.K., Poudel D.K., Dangol S., Rokaya A., Timsina S., Satyal P., Setzer W.N. Volatile Constituent Analysis of Wintergreen Essential Oil and Comparison with Synthetic Methyl Salicylate for Authentication. Plants. 2022;11:1090. doi: 10.3390/plants11081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuchet A., Jame P., Anchisi A., Schiets F., Oberlin C., Lefèvre J.-C., Carénini E., Casabianca H. Authentication of the Naturalness of Wintergreen (Gaultheria Genus) Essential Oils by Gas Chromatography, Isotope Ratio Mass Spectrometry and Radiocarbon Assessment. Ind. Crops Prod. 2019;142:111873. doi: 10.1016/j.indcrop.2019.111873. [DOI] [Google Scholar]
- 15.Murphy B.J., Carlson R.E., Howa J.D., Wilson T.M., Buch R.M. Determining the Authenticity of Methyl Salicylate in Gaultheria procumbens L. and Betula lenta L. Essential Oils Using Isotope Ratio Mass Spectrometry. J. Essent. Oil Res. 2021;33:442–451. doi: 10.1080/10412905.2021.1925362. [DOI] [Google Scholar]
- 16.Le Grand F., George G., Akoka S. Natural Abundance 2H-ERETIC-NMR Authentication of the Origin of Methyl Salicylate. J. Agric. Food Chem. 2005;53:5125–5129. doi: 10.1021/jf050385a. [DOI] [PubMed] [Google Scholar]
- 17.Nikolić M., Marković T., Mojović M., Pejin B., Savić A., Perić T., Marković D., Stević T., Soković M. Chemical Composition and Biological Activity of Gaultheria procumbens L. Essential Oil. Ind. Crops Prod. 2013;49:561–567. doi: 10.1016/j.indcrop.2013.06.002. [DOI] [Google Scholar]
- 18.Decarlo A., Johnson S., Ouédraogo A., Dosoky N.S., Setzer W.N. Chemical Composition of the Oleogum Resin Essential Oils of Boswellia dalzielii from Burkina Faso. Plants. 2019;8:223. doi: 10.3390/plants8070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel P., Owczarek A., Matczak M., Kosno M., Szymański P., Mikiciuk-Olasik E., Kilanowicz A., Wesołowski W., Olszewska M. Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity. Molecules. 2017;22:412. doi: 10.3390/molecules22030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods K.E., Chhetri B.K., Jones C.D., Goel N., Setzer W.N. Bioactivities and Compositions of Betula nigra Essential Oils. J. Med. Act. Plants. 2013;2:1–9. [Google Scholar]
- 21.Klika K.D., Demirci B., Salminen J.-P., Ovcharenko V.V., Vuorela S., Can Başer K.H., Pihlaja K. New, Sesquiterpenoid-Type Bicyclic Compounds from the Buds of Betula pubescens−Ring-Contracted Products Of β-Caryophyllene? Eur. J. Org. Chem. 2004;2004:2627–2635. doi: 10.1002/ejoc.200300808. [DOI] [Google Scholar]
- 22.Zyryanova O.A., Terazawa M., Koike T., Zyryanov V.I. White Birch Trees as Resource Species of Russia: Their Distribution, Ecophysiological Features, Multiple Utilizations. Eurasian J. For. Res. 2010;13:25–40. [Google Scholar]
- 23.Weston R.J., Smith G.J. Sesquiterpenes from the Inner Bark of the Silver Birch and the Paper Birch. Nat. Prod. Commun. 2012;7:1934578X1200700. doi: 10.1177/1934578X1200700202. [DOI] [PubMed] [Google Scholar]
- 24.Abyshev A.Z., Agaev E.M., Guseinov A.B. Studies of the Chemical Composition of Birch Bark Extracts (Cortex betula) from the Betulaceae Family. Pharm. Chem. J. 2007;41:419–423. doi: 10.1007/s11094-007-0091-5. [DOI] [Google Scholar]
- 25.Do T.K.T., Hadji-Minaglou F., Antoniotti S., Fernandez X. Authenticity of Essential Oils. TrAC Trends Anal. Chem. 2015;66:146–157. doi: 10.1016/j.trac.2014.10.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.