Abstract
Using degenerated primers from conserved regions of previously studied clpX gene products, we cloned the clpX gene of the malolactic bacterium Oenococcus oeni. The clpX gene was sequenced, and the deduced protein of 413 amino acids (predicted molecular mass of 45,650 Da) was highly similar to previously analyzed clpX gene products from other organisms. An open reading frame located upstream of the clpX gene was identified as the tig gene by similarity of its predicted product to other bacterial trigger factors. ClpX was purified by using a maltose binding protein fusion system and was shown to possess an ATPase activity. Northern analyses indicated the presence of two independent 1.6-kb monocistronic clpX and tig mRNAs and also showed an increase in clpX mRNA amount after a temperature shift from 30 to 42°C. The clpX transcript is abundant in the early exponential growth phase and progressively declines to undetectable levels in the stationary phase. Thus, unlike hsp18, the gene encoding one of the major small heat shock proteins of Oenococcus oeni, clpX expression is related to the exponential growth phase and requires de novo protein synthesis. Primer extension analysis identified the 5′ end of clpX mRNA which is located 408 nucleotides upstream of a putative AUA start codon. The putative transcription start site allowed identification of a predicted promoter sequence with a high similarity to the consensus sequence found in the housekeeping gene promoter of gram-positive bacteria as well as Escherichia coli.
The stress response in bacteria involves mechanisms of energy-dependent degradation of denatured proteins. Two ATP-dependent intracellular serine proteases, Lon and Clp, are responsible for the majority of abnormal protein degradation in Escherichia coli (37, 38). Clp proteins consist of two subunits, the ATP-dependent proteolytic component (ClpP) (57) and the ATPase regulatory component (ClpA or ClpX). ClpA possesses two ATPase sites whereas ClpX has only one, resembling the second ATPase site of ClpA (15, 16). It has been demonstrated that the Clp ATPases are not only factors which favor the binding of polypeptide substrates to ClpP (15, 52, 56) but also ClpP-independent molecular chaperones able either to repair proteins during stress conditions or to activate initiation proteins for Mu, λ, or P1 DNA replication (for a review, see reference 54). In Bacillus subtilis, both ClpP and ClpX play a central role. Indeed, a clpP null mutation was shown to have a pleiotropic effect in B. subtilis since ClpP is essential for stationary-phase survival, growth at high temperature, motility, competence development, and sporulation (39). In the same way, ΔClpX or ΔClpP mutants exhibited severely impaired growth under stress conditions such as the presence of 6% (wt/vol) NaCl or 5% (vol/vol) ethanol or after a temperature shift to 50°C (12). In Lactococcus lactis, ClpP plays a major role in the degradation of misfolded proteins accumulated following exposure to stress conditions (11).
In E. coli, clpP and clpX are transcribed together and belong to the ς32 heat shock regulon (15). In B. subtilis, clpP and clpX are located at different loci on the chromosome and are transcribed as monocistronic genes. Many clp genes as clpP and clpC are under control of both ςA- and ςB-dependent promoters (39). Transcription initiation at the ςA promoter is regulated by a CtsR repressor (6, 30). In contrast, a heat-inducible ςA-like promoter controls clpX gene expression (13), which has been reported to be CtsR independent (6). Nevertheless, Krüger and Hecker (30) showed that deletion of ctsR led to a partial effect on the expression of clpX at normal temperature. Thus, clpX gene expression should be regulated by more than one mechanism.
In lactic acid bacteria, several stress genes including groEL and dnaK are regulated by the CIRCE element as is the case for class I genes of B. subtilis (for a review, see reference 2). The target of the CtsR repressor was found in the promoter regions of many clp genes (6) such as clpP from L. lactis (11) and genes encoding small heat shock proteins (Hsps) from lactic acid bacteria (6). The induction of these genes could be CtsR dependent, and this repressor led to the definition of a novel heat shock response system. B. subtilis stress genes subjected to this regulation are referred to as class III genes.
To study the regulation mechanisms controlling heat shock gene expression in lactic acid bacteria, investigations of the molecular and physiological bases of the stress response of Oenococcus oeni (formerly Leuconostoc oenos) have been initiated (20, 22, 27, 28). This bacterium is most often responsible for malolactic fermentation in wine (31), and is able to grow after alcoholic fermentation in an acidic medium and in the presence of a high ethanol concentration. Among the O. oeni stress genes, hsp18 (encoding a small Hsp [27]) and trxA (encoding a thioredoxin [28]) have been characterized. In both cases, the regulation mechanisms remain unclear, although a putative CtsR target sequence has been identified in the promoter region of hsp18 (6).
In some bacteria, the clpX gene belongs to a cluster that also contains the gene encoding the trigger factor (tig). Recent studies have shown that the trigger factor is a peptidyl-prolyl isomerase able to catalyze protein folding in vitro, to associate with nascent polypeptides on ribosomes, and to cooperate with the GroEL chaperone in promoting degradation of some unstable proteins in vivo (for a review, see reference 25).
Here we describe the cloning and sequencing of the tig and clpX genes. The purified ClpX protein exhibited an ATPase activity. Transcription of clpX was examined with respect to temperature and was compared to hsp18 and tig as a function of growth phase. The clpX 5′-end mRNA was also determined.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids.
O. oeni Lo84.13 was obtained from the Oenological Institute of Bordeaux (Bordeaux, France). E. coli TG1 (14) was used as a host for construction of the genomic library and for the subsequent cloning steps. Plasmid pJDC9 (4) was used as a vector for the library. E. coli was grown in Luria-Bertani (LB) broth or agar at 37°C. O. oeni was grown in FT80 medium (pH 5.3) (3) modified by the addition of meat extract instead of Casamino Acids. Erythromycin was used at a final concentration of 250 μg · ml−1.
PCR amplifications.
For amplification of specific probes, each PCR was done in a final volume of 100 μl containing O. oeni genomic DNA (50 ng), deoxyribonucleotide triphosphates (100 μM each), oligonucleotides (400 μM each), 0.5 U of Taq DNA polymerase (Appligene), and the buffer supplied with the enzyme. Amplification was performed with a Hybaid Omn-E thermocycler (Hybaid Ltd., Teddington, United Kingdom) for 35 cycles consisting of 40 s of denaturation at 92°C, 1 min of annealing at 42°C, and 1 min of elongation at 72°C. PCR amplification of clpX was carried out with 5 U of Pfu DNA polymerase (Stratagene, La Jolla, Calif.) for 30 cycles consisting of 45 s of denaturation at 92°C, 45 s of annealing at 45°C, and 4 min of elongation at 72°C.
DNA manipulation, sequencing, and analysis.
The methods described by Sambrook et al. (41) were used for isolation, manipulation of recombinant DNA, and Southern blot analysis. Double-stranded plasmid DNA was purified by using a Qiagen plasmid kit (Tip 100; Qiagen, Hilden, Germany) and was sequenced by the dideoxy-chain termination method (42) with a Thermo Sequenase sequencing kit (Amersham Life Science, Inc., Cleveland, Ohio). Both strands were sequenced with synthetic oligonucleotide primers. Computer analyses of nucleotide and amino acid sequences were carried out with PC/GENE software (Intelligenetics).
Construction and purification of the MBP-ClpX fusion protein.
Construction of the malE-clpX fusion was based on a protein purification system from New England Biolabs (Beverly, Mass.), and plasmid pMAL-c2 was used as the vector. clpX was PCR amplified with the primers CLPXB5′ (5′-ATGGACGTAGGATCCATACAACCG-3′) and CLPXX3′ (5′-GTATAGATATCTAGATTTTTAATT-3′) in order to create 5′-end BamHI and 3′-end XbaI sites. The 1,332-bp product of this amplification was then cleaved with BamHI and XbaI and ligated into BamHI-XbaI-digested pMAL-c2. The resulting plasmid, pMClpX, carries the malE-clpX gene fusion under the control of the tac promoter. The in-frame fusion between malE and clpX was confirmed by sequence analysis. One liter of LB medium containing 2 g of glucose and 100 μg of ampicillin was inoculated with a 10-ml overnight culture of E. coli TG1(pMClpX) or TG1(pMAL-c2) and incubated with vigorous shaking at 37°C. When the culture reached an optical density at 600 nm of 0.5, isopropylthiogalactopyranoside (IPTG) was added to a final concentration of 0.3 mM. After an additional 2 h of incubation, the cells were centrifuged at 4,000 × g for 10 min, washed with column buffer (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA) and resuspended in 10 ml of column buffer containing 0.5 mM phenylmethylsulfonyl fluoride. Cells were broken in a French pressure cell at 1.2 × 105 kPa, and the lysate was centrifuged at 9,000 × g for 20 min at 4°C. The supernatant (crude extract) was diluted with column buffer to obtain a final protein concentration of 2.5 mg · ml−1. The crude extract was applied to a column containing 15 ml of amylose-conjugated agarose (New England Biolabs) and washed with 12 volumes of column buffer at 4°C. Fusion protein was eluted with column buffer supplemented with 10 mM maltose. Purified maltose binding protein (MBP)-ClpX fusion was cleaved with factor Xa (0.5 mg of factor Xa/100 mg of fusion protein) at 4°C. Cleavage was visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10.5% polyacrylamide) as described by Laemmli (34).
ATPase activity.
ATPase activities were measured in 100 μl of reaction buffer containing 50 mM 2-(N-morpholino)ethanesulfonic acid (MES)-KOH (pH 6), 10 mM MgCl2, and 5 mM Na2ATP. Solutions were incubated for 20 min at 37°C, and the reactions were stopped by addition of 300 μl of 1% SDS. Inorganic phosphate was measured with the colorimetric method described by Dufour et al. (8).
Isolation of total RNA and Northern blot analysis.
O. oeni total RNA was purified as previously described (27). RNAs (30 μg) were separated in 1.2% (wt/vol) agarose–6.6% (vol/vol) formaldehyde gels and then transferred to Hybond-N+ membranes (Amersham). Probes A for tig and B for clpX were obtained from pGID552 HpaII and SspI DNA fragments, respectively, by PCR amplification with appropriate primers; the hsp18 probe was obtained as previously described (27). The probes were radiolabeled with [α-32P]dATP, with a random primers DNA labeling kit (Gibco-BRL, Gaithersburg, Md.). Northern blot analyses were performed at least two times.
Reverse transcription-PCR (RT-PCR).
O. oeni RNA (30 μg) was treated with DNase (RNase free; Sigma, St. Louis, Mo.) as recommended by the manufacturer. DNase was removed with one phenol-chloroform extraction. Specific cDNA was synthesized as described for primer extension analysis (see below) and was amplified by PCR.
Primer extension analysis.
Oligonucleotides PE1 (5′-AAATTAGTCTTAACCCGGTTGGT-3′), PE2 (5′-GCCGGCAGAAACCGATTTCTC-3′), and PE3 (5′-TTTCAACTGCTTGAGCACTGT-3′) (Fig. 1B) were used to map the 5′ terminus of clpX mRNA in O. oeni. RNA (5 μg) isolated from O. oeni control cells (grown at 30°C) or heat-shocked (30 min at 42°C) cells was mixed with 2 pmol of each primer. After a 5-min denaturation step at 100°C, the mixture was cooled in ice for annealing. Reverse transcription was carried out with the Superscript II RNase H− reverse transcriptase (Gibco-BRL) as recommended by the manufacturer. Analyses of the extension products were performed as previously described (27).
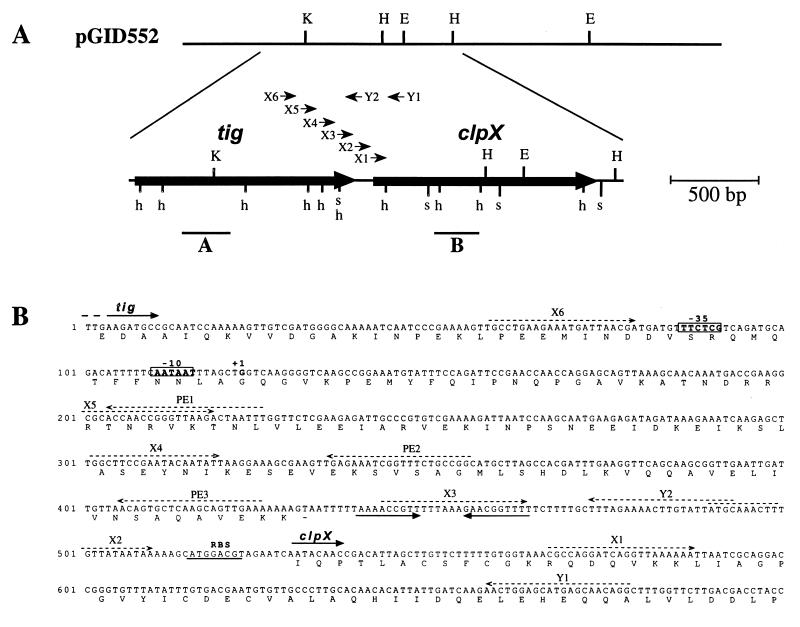
FIG. 1.
(A) Restriction map of the insert of recombinant plasmid pGID552 and of the O. oeni clpX sequenced cluster. Restriction sites for EcoRI (E), HindIII (H), KpnI (K), HpaII (h), and SspI (s) are shown. The large arrows indicate the positions of tig and clpX genes and their directions of transcription. Bold lines under the map indicate the probes used for Southern (B) or Northern (A and B) analyses. Arrowheads indicate oligonucleotides (X1 to X6, Y1, and Y2) used in RT-PCR experiments. (B) Partial nucleotide sequences of the O. oeni tig and clpX genes and deduced amino acid sequences of their products. The RBSs are underlined. The transcription start site (+1) is indicated, and the boxes represent the putative −35 and −10 promoter sequences. Convergent arrows indicate a putative rho-independent terminator. Dashed arrows indicate the oligonucleotides used for primer extension, reverse transcription, and RT-PCR experiments.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. Y15953.
RESULTS
Cloning of the clpX gene from O. oeni.
Oligonucleotide primers with opposite orientations were designed from two conserved regions of known clpX genes: the ATP binding motif 2 (YIDEIDK) and the consensus sequence (GEGVQQ) (13, 15). The two degenerated primers were 5′-TAYATHGAYGARATHGAYAAR-3′ and 5′-YTGYTGNACNCCYTCNCC-3′ (R = A or G; Y = C or T; H = A, C, or T; N = A, G, C, or T). A 0.1-kb probe was amplified from the genomic DNA of O. oeni and was used to screen a genomic library of O. oeni obtained as previously described (33). Of the 2,880 clones in the genomic library, 5 hybridized with the probe. Restriction profiles of plasmid DNA prepared from these five clones indicated that they carried overlapping inserts (data not shown). One of these clones containing a 6.2-kb DNA fragment from O. oeni was designated TG1(pGID552) (Fig. 1A) and was retained for further characterization.
Southern blot analysis was performed with probe A (obtained as described in Materials and Methods) against BamHI, EcoRI, BglII and BamHI-EcoRI digests of total O. oeni DNA. Hybridization yielded single fragments (data not shown), suggesting that a single copy of the cloned gene was present in the O. oeni genome.
Sequence analysis.
The insert in recombinant plasmid pGID552 was partially sequenced. The 2,800-bp nucleotide sequence contains two open reading frames (ORFs). The first ORF (designated tig) is 1,316 nucleotides long starting at a putative AUG start codon. A putative ribosome binding site (RBS) 5′-GGAGG-3′ was complementary to the 3′ end of Leuconostoc mesenteroides 16S rRNA (5′-CACCUCCUUU-3′) (complementary nucleotides are underlined) (58). L. mesenteroides 16S rRNA was taken as a reference since the 3′ end of O. oeni 16S rRNA has not yet been described. This putative RBS is fairly similar to the one described previously for the O. oeni hsp18 gene (27). Inspection of the 3′ noncoding sequence shows an inverted repeat sequence followed by a cluster of T’s that could form a stem-and-loop structure in mRNA with a calculated ΔGf of −8.6 kcal · mol−1 (−36 kJ · mol−1) (53). This structure is likely to function as a rho-independent transcriptional terminator. Two putative start codons could be proposed for the second ORF (clpX). The first one is an usual AUG start codon (nucleotide 516), but no sequence similar to a putative RBS could be found upstream this codon (Fig. 1B). Another start codon could be AUA (nucleotide 531) encoding an isoleucine residue. A putative RBS (5′-AUGGACGU-3′) quite complementary to the 3′ end of L. mesenteroides 16S rRNA (5′-CACCUCCUUU-3′) (complementary nucleotides are underlined) was found seven nucleotides upstream the AUA codon. The sequence of this region was confirmed by sequencing a PCR fragment amplified from O. oeni genomic DNA.
The protein deduced from the tig sequence is composed of 438 residues and has a calculated Mr of 48,176 with a predicted pI of 4.64. The amino acid sequence exhibits similarity to other trigger factors available in protein data banks. The higher identities were observed with B. subtilis (44% identity) (accession no. P97173), Haemophilus influenzae (29%) (10), and E. coli (28%) (19) trigger factors. The 413-amino-acid protein deduced from the nucleotide sequence of clpX has a predicted Mr of 45,650 and a pI of 5.78. A comparison of the sequence with database entries showed a high degree of identity with previously published ClpX proteins (Fig. 2). Identities of 58.6% were found with B. subtilis (13), 57.2% with E. coli (15), 55% with H. influenzae (10), and 44.6% with Azotobacter vinelandii (26) ClpX proteins. Lower identity degrees were shared with bacterial ClpY proteins (also called HslU) (54).
FIG. 2.
Comparison of the O. oeni ClpX predicted amino acid sequence (CLPX_OOENI) with those of B. subtilis (CLPX_BACSU) (13), E. coli (CLPX_ECOLI) (15), H. influenzae (CLPX_HAEIN) (10), and A. vinelandii (CLPX_AZOVI) (26). The sequences were aligned with the CLUSTAL program. Asterisks indicate identical amino acids. The highly conserved regions within the ClpX sequences (zinc finger motif, ATP binding sites 1 and 2, central consensus motif, and C-terminal motifs 1 and 2) are enclosed in boxes. Numbers to the right refer to amino acid positions.
Purification of ClpX protein and ATPase activity.
The vector pMAL-c2 was used to fuse clpX to the E. coli malE gene, which encodes the MBP, resulting in plasmid pMClpX. The MBP part allows purification of the fusion protein (MBP-ClpX) by affinity chromatography. A cleavage site for factor Xa, adjacent to the N-terminal part of ClpX, allows the release of a native-size ClpX protein from this fusion. Analysis of extracts of IPTG-induced E. coli TG1(pMClpX) cells by SDS-PAGE showed that MBP-ClpX was highly produced (data not shown). Subsequently, the MBP-ClpX fusion protein was purified by amylose affinity chromatography, yielding about 23 mg of at least 95% pure fusion protein. An aliquot of 0.5 U of factor Xa was added per 100 μg of purified MBP-ClpX fusion protein, and the mixture was incubated at 4°C. Cleavage was monitored by SDS-PAGE and Western blotting using anti-MBP antibodies (diluted 1:10,000) as previously described (27). Complete cleavage of the fusion occurred after 36 h of treatment with factor Xa. The MBP and ClpX protein mixture was used to test the ATPase activity of ClpX.
While no ATPase activity was measured on MBP-ClpX fusion protein, an activity was detected after cleavage by factor Xa and release of ClpX from the fusion protein. As a control experiment, no ATPase activity was detected on purified MBP. The ATPase activity of purified ClpX was ∼0.2 μmol/min/mg. The weak ATPase activity observed is consistent with values reported for E. coli ClpX (18, 55).
Northern analysis of tig and clpX mRNA.
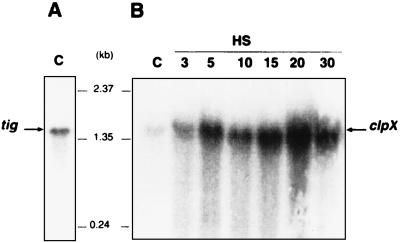
The in vivo transcripts of the clpX and tig genes were detected by Northern analyses with total RNA prepared either from cells of O. oeni in exponential growth or taken at different times after bacteria were transferred to 42°C. Hybridizations with probe A for tig and probe B for clpX revealed two transcripts with sizes of around 1.6 kb each (Fig. 3).
FIG. 3.
Northern blot analysis. Total RNAs were prepared from O. oeni cells grown at 30°C or submitted to heat shock at 42°C for 3, 5, 10, 15, 20, or 30 min. Samples were electrophoresed through an agarose-formaldehyde gel, blotted onto membranes, and hybridized with α-32P-labeled probe A for tig or probe B for clpX (Fig. 1A). The resulting autoradiograms are shown in panels A and B, respectively. Arrows indicate the positions of the detected transcripts. The central numbers represent the molecular size markers.
The temperature dependence of clpX transcription is shown in Fig. 3B. An increase in clpX-specific mRNA was observed after 3 min of heat shock at 42°C. The maximum amount of clpX mRNA was obtained after 5 min of heat shock and remained at the same level afterwards. In contrast, heat shock conditions had no influence on the amount of tig mRNA detected (data not shown). These findings suggest that clpX gene transcription is induced by the rise of temperature.
Comparison of clpX, tig, and hsp18 expression as a function of growth phase.
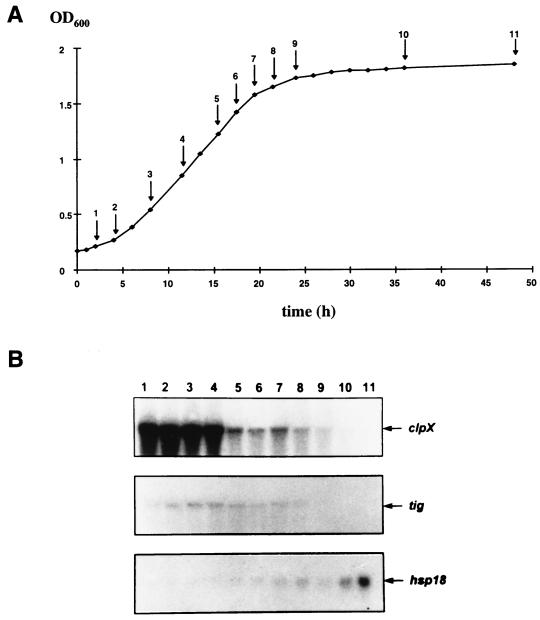
Northern blot analysis was performed on RNA samples from O. oeni cells taken at different growing stages (Fig. 4A) with the clpX, tig, or hsp18 probe. The clpX mRNA was strongly detected in the course of the exponential growth phase (Fig. 4B, lanes 1 to 4). Then the amount of transcript decreased during the early stationary growth phase (Fig. 4B, lanes 5 to 9) and was undetectable at the late stationary growth phase (Fig. 4B, lanes 10 and 11). The abundance of detected tig mRNA decreased from the late growth phase to the stationary phase (Fig. 4B). Furthermore, unlike clpX and tig, an increase in the amount of hsp18 mRNA began in late exponential phase and reached a maximum in late stationary phase (Fig. 4B, lanes 6 to 11).
FIG. 4.
Expression of clpX, tig, and hsp18 as a function of growth phase. (A) Growth curve of O. oeni. Arrows and numbers indicate when cells were sampled and total RNAs were extracted. (B) Northern blot analysis of the total RNAs for detection of clpX, tig, and hsp18 transcripts at each of the 11 sampling times indicated in panel A. OD600, optical density at 600 nm.
Effect of chloramphenicol on clpX, tig, and hsp18 expression.
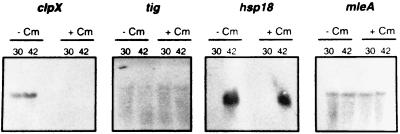
To determine whether de novo protein synthesis is required for clpX, tig, and hsp18 expression, their mRNAs were monitored in the absence of protein synthesis. Fifteen minutes after addition of chloramphenicol (100 mg/ml), cells were or were not submitted to heat shock at 42°C for 5 min and total RNAs were prepared. As monitored by [35S]methionine labeling experiments, protein synthesis was totally abolished after addition of chloramphenicol (data not shown). No clpX mRNA was detected in control cells or heat-shocked cells in the presence of chloramphenicol (Fig. 5). In contrast, the addition of chloramphenicol had no effect on tig expression and on hsp18 heat shock induction (Fig. 5). As a control experiment, we showed that mleA, encoding the malolactic enzyme of O. oeni (32), was expressed at the same level in presence or absence of chloramphenicol (Fig. 5). These results indicate that expression of hsp18 and that of tig do not require de novo protein synthesis, whereas clpX expression seems to require it.
FIG. 5.
Effect of chloramphenicol on clpX, tig, hsp18, and mleA expression. Cells were incubated for 15 min at 30°C after the addition (lanes + Cm) or not (lanes − Cm) of chloramphenicol (100 μg/ml). After a 5-min incubation at 42 or 30°C, total RNAs were extracted, separated on a 1.2% agarose gel, and transferred to membranes. The RNAs were then hybridized with clpX and tig probes (see Materials and Methods) or hsp18 and mleA probes obtained as previously described (27, 32).
Determination of clpX 5′-end mRNA.
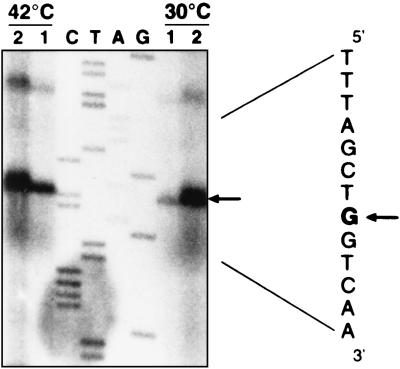
No transcription start point could be identified in the nearby region upstream clpX ORF. Thus, we tried to map the 5′ end of the transcript by using an RT-PCR approach. Total O. oeni RNA was treated with DNase to remove any trace of DNA. Reverse transcription was done with oligonucleotide Y1, complementary to the 5′ end of clpX (Fig. 1). Then PCR was carried out with Y1 and six other antisense oligonucleotides (X1 to X6), located upstream clpX (Fig. 1). The experiments carried out with X1 to X5 led to amplification of DNA that had the expected sizes, whereas X6 led to no amplification (data not shown). A second reverse transcription with oligonucleotide Y2 gave the same results after PCR amplification. As a control experiment, no PCR amplification was observed when no reverse transcription was conducted on RNA. These results suggest that the 5′ end of the clpX transcript was located between X6 and X5, i.e., between nucleotides 59 and 219 (Fig. 1B). The RNAs isolated from O. oeni control cells and heat-shocked cells (30 min at 42°C) were subjected to primer extension analysis to determine the 5′ end of the clpX transcript. Reverse transcription experiments were done with oligonucleotides PE1, PE2, and PE3, complementary to the 5′ noncoding region of clpX mRNA (Fig. 1B). Among nonspecific products of the reverse transcription reaction, one common signal was obtained with each of the oligonucleotides (Fig. 6), allowing the location of the 5′ end of clpX to be 408 nucleotides upstream the putative AUA start codon (Fig. 1B). The 5′ end of clpX-specific mRNA detected during growth at 30°C or in heat-shocked cells was located at the same site. The signal obtained increased with the temperature shift from 30 to 42°C. This transcription start point allowed location of a putative promoter at the sequence TTctCg-19-aATAAT (Fig. 1B). The clpX promoter showed a high similarity (uppercase letters) to the consensus found in the promoter for housekeeping functions of gram-positive bacteria as well as E. coli, such as has already been observed for hsp18 and mleA promoters from O. oeni (27, 32). A direct heptanucleotide repeat (GGTCAAG) resembling the CtsR consensus recognition sequence (6) was found with the first base overlapping the clpX transcriptional start site. However, the repeats are separated by only one to three nucleotides for the CtsR consensus targets. Experiments need to be performed to determine whether this sequence could contribute to the regulation of clpX.
FIG. 6.
Primer extension mapping of the 5′ end of the clpX transcript with oligonucleotide PE1 (see Fig. 1B). Total RNA extracted from O. oeni cells grown at 30°C or heat shocked 30 min at 42°C were used. Different amounts of primer extension products (lanes 1 and 2) were electrophoresed in parallel with the products of sequencing reactions on plasmid pGID552 with the same oligonucleotide as the primer (lanes C, T, A, and G). The sequence shown on the right is the complementary strand, and the arrows point to the base representing the 5′ end of clpX mRNA.
DISCUSSION
Using the high degree of similarity existing between the various clpX genes products known so far, we succeeded in cloning the clpX gene from O. oeni. Sequence analysis revealed that the O. oeni ClpX protein possessed the highly conserved regions of other ClpX: (i) an N-terminal zinc finger motif that could be involved in binding to the denaturated protein substrates (50), (ii) the two potential ATP binding sites, (iii) a central consensus motif located downstream from the second ATP binding site also found in bacterial ClpY, and (iv) two conserved short amino acid clusters separated by 63 amino acids (-PEF- and -GAR-) present in the C-terminal part of the ClpX proteins. It was suggested that these two conserved short sequences could be involved in the interaction of the regulatory subunit ClpX with the proteolytic component ClpP (15).
We took advantage of an MBP fusion system to purify the ClpX protein. Purified ClpX exhibited an in vitro ATPase activity consistent with the activity of E. coli ClpX (18, 55). The high percentages of identity with known ClpX, the presence of these specific regions, and the ATPase activity of the purified protein allowed us to conclude that we have cloned the gene encoding the ClpX homologue of O. oeni. The disruption of the clpX gene in O. oeni would be a means to elucidate the physiological function of the ClpX protein during the growth of O. oeni in diverse environments. Unfortunately, no DNA transfer techniques are available for such a genetic approach in O. oeni.
With respect to clpX genes studied in prokaryotes (Table 1), it appears that the cluster to which clpX belongs includes various genes depending on the microorganism. As in B. subtilis, tig is located upstream of clpX and no clpP was found between the two genes (13). We were not able to identify of a classical start codon for the clpX mRNA. Cases of start codon others than AUG being used to initiate protein translation are well documented and include GUG, CUG, and UUG (45). In addition, AUA has been reported as an in vivo initiation codon for some genes in lactic acid bacteria (44) or in E. coli (49). Taking these data into account, the use of an atypical AUA start codon in the O. oeni clpX mRNA might be considered a possibility.
TABLE 1.
Genetic organization of bacterial clpX clusters
Few transcriptional analyses of bacterial clpX genes have been reported previously. The two examples concern clpX genes from E. coli and B. subtilis. In E. coli, clpX is organized in an operon with clpP (15); in B. subtilis, clpX transcription is monocistronic (13). Analyses of the clpX and tig genes from O. oeni have revealed two mRNA transcripts of approximately the same size (1.6 kb). Two alternative hypotheses can be suggested: (i) tig and clpX are transcribed from two different promoters, yielding monocistronic mRNAs independently expressed with respect to growth phase or heat shock; (ii) transcription is initiated from a single promoter located upstream of tig and generates either the monocistronic 1.6-kb tig product, stopped at the putative transcription terminator, or a short-lived bicistronic tig-clpX mRNA. This large message, undetected in our Northern blot analyses, would be rapidly processed at the 5′ end of clpX mRNA, as determined in RT-PCR and primer experiments, generating the monocistronic clpX messages. The differential tig and clpX expression observed during heat shock or in exponential growth phase may involve a greater stability of clpX mRNA under these conditions, presumably due to its 5′ untranslated region (5′-UTR). At this stage of knowledge, both hypotheses can be considered, but we cannot exclude the possibility that tig and clpX regulation involves elements of both.
In any case, it must be assumed that the putative transcription terminator, located between tig and clpX coding sequences, allows read-through transcription. A growing number of genetic systems have been shown to be controlled by a variety of molecular mechanisms that modulate the termination activity of RNA polymerase (for a review, see reference 24). The stem-loop formed by the terminator is A-T rich with a calculated feature of moderately stable structure. One hypothesis is that formation of the tig terminator is regulated and then would modulate clpX transcription. Based on our detection of tig and clpX mRNA in the absence of protein synthesis, we can suggest the existence of an antitermination event. Binding of a protein may stabilize an antiterminator form of the mRNA, preventing formation of the terminator structure. The chloramphenicol may modulate the cellular level of the protein and then favor a premature termination of clpX transcription.
Generally, genes encoding for Hsps are induced during transition into stationary growth phase (36). In E. coli, a slight increase (1.5-fold) in the level of ClpX and ClpP was observed during transition to the stationary growth phase (48). In O. oeni, clpX gene expression is increased by heat shock, and thus clpX belongs to the heat shock gene family. Considering the growth conditions in wine, we performed RNA extraction from cells subjected to acidic or ethanolic shocks. Unfortunately, these conditions led to RNA degradation. Surprisingly, the clpX transcript is more abundant during the exponential growth phase and decreases in amount during the later stages of growth. This behavior is different from those of hsp18 and trxA, the only stress genes well characterized in O. oeni (27, 28). The trxA gene is constitutively expressed during all growth stages (28). The Lo18 protein encoded by hsp18 was not detected in exponential-growth cells, but the transition to stationary growth phase induces its expression (21). This result was confirmed by Northern analysis, with the detection of hsp18 transcript from the entrance in the stationary growth phase up to at least 24 h afterward. The high expression of the clpX gene in exponential growth may suggest a biological role of the ClpX protein in early growth stages of O. oeni. This observation is in agreement with a previous report showing that a clpX mutant of B. subtilis displayed an extended lag phase (12).
Northern blot analysis did not allow discrimination of whether growth phase regulation is exerted at a transcriptional level or on clpX mRNA stability. However, the 5′-UTR of the O. oeni clpX message is very long (408 bp), as described for other genes for which a secondary structure has been shown to stabilize mRNA in a growth-dependent manner (5, 9, 35). The effect of the mRNA 5′-UTR on clpX expression remains to be elucidated.
Regulation of the heat shock response has been extensively studied in E. coli, where induction of the major heat shock regulon is triggered by the accumulation of denatured or misfolded proteins in the cytoplasm and is governed by the alternative sigma factor ς32 (for a recent review, see reference 57).
In B. subtilis, at least four classes of heat shock genes can be distinguished. Class I genes (operons dnaKJ and groESL) are transcribed from vegetative promoters and are subjected to negative control by the HrcA repressor that interacts with the CIRCE operator (46, 59, 60). Most of the B. subtilis stress genes belong to class II and define the general stress regulon. This regulon is dependent on the alternative sigma factor ςB (23). Class III is composed of clpP, clpC, and clpE, which encode for subunits of the Clp ATP-dependent protease. It has recently been shown that CtsR acts as a repressor of these genes by interacting with their promoter regions (6, 30). This heat shock regulation by CtsR is a highly conserved mechanism in gram-positive bacteria (6, 30). Stress genes for which induction mechanisms have not been completely characterized (clpX, trxA, lonA, ftsH, htpG, and ahpC) are grouped in class IV (1, 7, 13, 40, 43, 47).
In O. oeni, the hsp18 promoter region harbors two directly repeated sequences that overlap the −35 and −10 housekeeping gene promoter sequences. These repeated heptanucleotide sequences have been identified as potential CtsR binding sites (6). We showed that complete inhibition of protein synthesis by chloramphenicol did not prevent hsp18 expression in heat shock conditions. This result indicates that de novo protein synthesis is not necessary for induction of hsp18 transcription and is consistent with the hypothesis of a repression mechanism that could be mediated by a CtsR-like protein. Conversely, clpX mRNA was not detected after chloramphenicol addition under either normal or heat shock conditions. Thus, clpX expression seems to require de novo protein synthesis. The data presented here suggest a complex regulation that could also involve other mechanisms such as regulation at the incomplete CtsR-like recognition sequence, termination-antitermination, or posttranscriptional regulation occurring at the long 5′-UTR of clpX mRNA.
These results provide evidence for the existence of at least two different regulation mechanisms of heat shock gene expression in O. oeni. hsp18 regulation could be similar to the CtsR-mediated repression described for the class III stress genes of B. subtilis, whereas clpX could involve several unknown regulation systems.
ACKNOWLEDGMENTS
This study was supported by the CERQUAVAL and by a grant from the Conseil Régional de Bourgogne.
We are grateful to F. Pierre for assistance in the cloning step and to C. E. Morris and L.-C. Fortier for careful proofreading of the manuscript.
REFERENCES
- 1.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutibonnes P. Les protéines de choc thermique chez Lactococcus lactis: synthèse et régulation; thermotolérance. Lait. 1996;76:111–128. [Google Scholar]
- 3.Cavin J-F, Prevost H, Lin J, Schmitt P, Diviès C. Medium for screening Leuconostoc oenos strains defective in malolactic fermentation. Appl Environ Microbiol. 1989;55:751–753. doi: 10.1128/aem.55.3.751-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J D, Morrisson D A. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene. 1988;64:155–164. doi: 10.1016/0378-1119(88)90489-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen L-H, Emory S A, Bricker A L, Bouvet P, Belasco J G. Structure and function of a bacterial mRNA stabilizer: analysis of the 5′ untranslated region of ompA mRNA. J Bacteriol. 1991;173:4578–4586. doi: 10.1128/jb.173.15.4578-4586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derré I, Rapoport G, Msadek T. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in Gram-positive bacteria. Mol Microbiol. 1999;31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 7.Deuerling E, Mogk A, Richter C, Purucker M, Schumann W. The ftsH gene of Bacillus subtilis is involved in major cellular processes such as sporulation, stress adaptation and secretion. Mol Microbiol. 1997;23:921–933. doi: 10.1046/j.1365-2958.1997.2721636.x. [DOI] [PubMed] [Google Scholar]
- 8.Dufour S A, Amory A, Goffeau A. Plasma membrane ATPase from the yeast Schizosaccharomyces pombe. Methods Enzymol. 1998;157:513–528. doi: 10.1016/0076-6879(88)57100-8. [DOI] [PubMed] [Google Scholar]
- 9.Emory S A, Belasco J G. The ompA 5′ untranslated RNA segment functions in Escherichia coli as a growth-rate-regulated mRNA stabilizer whose activity is unrelated to translational efficiency. J Bacteriol. 1990;172:4472–4481. doi: 10.1128/jb.172.8.4472-4481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, Fitzugh W, Field C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S N, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Frees D, Ingmer H. ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol Microbiol. 1999;31:79–87. doi: 10.1046/j.1365-2958.1999.01149.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerth U, Krüger E, Derré I, Msadek T, Hecker M. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol Microbiol. 1998;28:787–802. doi: 10.1046/j.1365-2958.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerth U, Wipat A, Harwood C R, Carter N, Emmerson P T, Hecker M. Sequence and transcriptional analysis of clpX, a class-III heat-shock gene of Bacillus subtilis. Gene. 1996;181:77–83. doi: 10.1016/s0378-1119(96)00467-2. [DOI] [PubMed] [Google Scholar]
- 14.Gibson T J. Studies on the Epstein-Barr virus genome. Ph.D. thesis. Cambridge, England: Cambridge University; 1984. [Google Scholar]
- 15.Gottesman S, Clark W P, de Crecy-Lagard V, Maurizi M. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1993;268:22618–22626. [PubMed] [Google Scholar]
- 16.Gottesman S, Squires C, Pichersky E, Carrington M, Hobbs M, Mattick J S, Dalrymple B, Kuramitsu H, Shiroza T, Foster T, Clark W P, Ross B, Squires C L, Maurizi M R. Conservation of the regulatory subunit for the ATP-dependent protease in prokaryotes and eukaryotes. Proc Natl Acad Sci USA. 1990;87:3513–3517. doi: 10.1073/pnas.87.9.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths P, Park R, Connerton I. The gene for Campylobacter trigger factor: evidence for multiple transcription start sites and protein products. Microbiology. 1995;141:1359–1367. doi: 10.1099/13500872-141-6-1359. [DOI] [PubMed] [Google Scholar]
- 18.Grimaud R, Kessel M, Beuron F, Steven A C, Maurizi M R. Enzymatic and structural similarities between the Escherichia coli ATP-dependent proteases, ClpXP and ClpAP. J Biol Chem. 1998;273:12476–12481. doi: 10.1074/jbc.273.20.12476. [DOI] [PubMed] [Google Scholar]
- 19.Guthrie B, Wickner W. Trigger factor depletion or overproduction causes defective cell division but does not block protein export. J Bacteriol. 1990;172:5555–5562. doi: 10.1128/jb.172.10.5555-5562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzzo J, Cavin J-F, Diviès C. Induction of stress proteins in Leuconostoc oenos to perform direct inoculation of wine. Biotechnol Lett. 1994;16:1189–1194. [Google Scholar]
- 21.Guzzo J, Delmas F, Pierre F, Jobin M-P, Samyn B, Van Beeumen J, Cavin J-F, Diviès C. A small heat shock protein from Leuconostoc oenos induced by multiple stresses and during stationary growth phase. Lett Appl Microbiol. 1997;24:393–396. doi: 10.1046/j.1472-765x.1997.00042.x. [DOI] [PubMed] [Google Scholar]
- 22.Guzzo J, Jobin M-P, Diviès C. Increase of sulfite tolerance in Oenococcus oeni by means of acidic adaptation. FEMS Microbiol Lett. 1998;160:43–47. [Google Scholar]
- 23.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 24.Henkin T M. Control of transcription termination in prokaryotes. Annu Rev Genet. 1996;30:35–57. doi: 10.1146/annurev.genet.30.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Hesterkamp T, Bukau B. The Escherichia coli trigger factor. FEBS Lett. 1996;389:32–34. doi: 10.1016/0014-5793(96)00582-0. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson M R, Brigle K E, Bennett L T, Setterquist R A, Wilson M S, Cash V L, Beynon J, Newton W E, Dean D R. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J Bacteriol. 1989;171:1017–1027. doi: 10.1128/jb.171.2.1017-1027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jobin M-P, Delmas F, Garmyn D, Diviès C, Guzzo J. Molecular characterization of the gene encoding an 18-kilodalton small heat shock protein associated with the membrane of Leuconostoc oenos. Appl Environ Microbiol. 1997;63:609–614. doi: 10.1128/aem.63.2.609-614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jobin M-P, Garmyn D, Diviès C, Guzzo J. Expression of the Oenococcus oeni trxA gene is induced by hydrogen peroxide and heat shock. Microbiology. 1999;145:1245–1251. doi: 10.1099/13500872-145-5-1245. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions (supplement) DNA Res. 1996;3:185–209. doi: 10.1093/dnares/3.3.185. [DOI] [PubMed] [Google Scholar]
- 30.Krüger E, Hecker M. The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes. J Bacteriol. 1998;180:6681–6688. doi: 10.1128/jb.180.24.6681-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunkee R E. Some roles of malic acid in the malolactic fermentation in wine making. FEMS Microbiol Rev. 1991;88:55–72. [Google Scholar]
- 32.Labarre C, Diviès C, Guzzo J. Genetic organization of the mle locus and identification of a mleR-like gene from Leuconostoc oenos. Appl Environ Microbiol. 1996;62:4493–4498. doi: 10.1128/aem.62.12.4493-4498.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labarre C, Guzzo J, Cavin J-F, Diviès C. Cloning and characterization of the genes encoding the malolactic enzyme and the malate permease of Leuconostoc oenos. Appl Environ Microbiol. 1996;62:1274–1282. doi: 10.1128/aem.62.4.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lundberg U, von Gabain A, Melefors Ö. Cleavages in the 5′ region of the ompA and bla mRNA control stability: studies with an E. coli mutant altering mRNA stability and a novel endoribonuclease. EMBO J. 1990;9:2731–2741. doi: 10.1002/j.1460-2075.1990.tb07460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matin A. The molecular basis of a carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991;5:3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 37.Maurizi M R. ATP-dependent proteases. In: Bond J S, Barrett A J, editors. Proteolysis and protein turnover. Portland Press Proceedings Series. Vol. 6. Ewing, N.J: California/Princeton Fulfillment Services; 1993. pp. 65–71. [Google Scholar]
- 38.Maurizi M R, Clark W P, Katayama Y, Rudikoff Z S, Pumphrey J, Bowers B, Gottesman S. Sequence and structure of ClpP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990;265:12536–12545. [PubMed] [Google Scholar]
- 39.Msadek T, Dartois V, Kunst F, Herbaud M L, Denizot F, Rapoport G. ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol Microbiol. 1998;27:899–914. doi: 10.1046/j.1365-2958.1998.00735.x. [DOI] [PubMed] [Google Scholar]
- 40.Riethdorf S, Völker U, Gerth U, Winkler A, Engelman S, Hecker M. Cloning, nucleotide sequence, and expression of the Bacillus subtilis lon gene. J Bacteriol. 1994;176:6518–6527. doi: 10.1128/jb.176.21.6518-6527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scharf C, Riethdorf S, Ernst H, Engelmann S, Völker U, Hecker M. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J Bacteriol. 1998;180:1869–1877. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schearman C A, Jury K L, Gasson M J. Controlled expression and structural organization of a Lactococcus lactis bacteriophage lysin encoded by two overlapping genes. Appl Environ Microbiol. 1994;60:3063–3073. doi: 10.1128/aem.60.9.3063-3073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider T D, Stormo G D, Gold L. Information content of binding sites on nucleotide sequences. J Mol Biol. 1986;188:415–431. doi: 10.1016/0022-2836(86)90165-8. [DOI] [PubMed] [Google Scholar]
- 46.Schulz A, Schumann W. hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat shock genes. J Bacteriol. 1996;178:1088–1093. doi: 10.1128/jb.178.4.1088-1093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz A, Schwab S, Homuth G, Versteeg S, Schumann W. The htpG gene of Bacillus subtilis belongs to class III heat shock genes and is under negative control. J Bacteriol. 1997;179:3103–3109. doi: 10.1128/jb.179.10.3103-3109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweder T, Lee K-H, Lomovskaya O, Matin A. Regulation of Escherichia coli starvation sigma factor (ςS) by ClpXP protease. J Bacteriol. 1996;178:470–476. doi: 10.1128/jb.178.2.470-476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sussman J K, Simons E L, Simons R W. Escherichia coli translation initiation factor 3 discriminates the initiation codon in vivo. Mol Microbiol. 1996;21:347–360. doi: 10.1046/j.1365-2958.1996.6371354.x. [DOI] [PubMed] [Google Scholar]
- 50.Szabo A, Korszum R, Hartl F U, Flanagan J. A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. EMBO J. 1996;15:408–417. [PMC free article] [PubMed] [Google Scholar]
- 51.Tatsuta T, Tomoyasu T, Bukau B, Kitagawa M, Mori H, Karata K, Ogura T. Heat shock regulation in the ftsH null mutant of Escherichia coli: dissection of stability and activity control mechanisms of sigma32 in vivo. Mol Microbiol. 1998;30:583–593. doi: 10.1046/j.1365-2958.1998.01091.x. [DOI] [PubMed] [Google Scholar]
- 52.Thompson M W, Maurizi M R. Activity and specificity of Escherichia coli ClpAP protease in cleaving model peptide substrates. J Biol Chem. 1994;269:18201–18208. [PubMed] [Google Scholar]
- 53.Tinoco I, Jr, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 54.Wawrzynow A, Banecki B, Zylicz M. The Clp ATPases define a novel class of molecular chaperones. Mol Microbiol. 1996;21:895–899. doi: 10.1046/j.1365-2958.1996.421404.x. [DOI] [PubMed] [Google Scholar]
- 55.Wawrzynow A, Wojtkowiak D, Marszalek J, Banecki B, Jonsen M, Graves B, Georgopoulous C, Zylicz M. The ClpX heat-shock protein of Escherichia coli, the ATP-dependent substrate specificity component of the ClpP-ClpX protease, is a novel molecular chaperone. EMBO J. 1995;14:1867–1877. doi: 10.1002/j.1460-2075.1995.tb07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wojtkowiak D, Georgopoulos C, Zylicz M. Isolation and characterization of ClpX, a new ATP-dependent specificity component of the Clp protease of Escherichia coli. J Biol Chem. 1993;268:22609–22617. [PubMed] [Google Scholar]
- 57.Woo K M, Chung W J, Ha D B, Goldberg A L, Chung C H. Protease Ti from Escherichia coli requires ATP hydrolysis for protein breakdown but not for hydrolysis of small peptides. J Biol Chem. 1989;264:2088–2091. [PubMed] [Google Scholar]
- 58.Yang D, Woese C R. Phylogenetic structure of the Leuconostoc: an interesting case of a rapidly evolving organism. Syst Appl Microbiol. 1989;12:145–149. [Google Scholar]
- 59.Yuan G, Wong S L. Regulation of groE expression in Bacillus subtilis: the involvement of the sigma A-like promoter and the roles of the inverted repeat sequence (CIRCE) J Bacteriol. 1995;177:5427–5433. doi: 10.1128/jb.177.19.5427-5433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]