Abstract
Background/Objectives
The aim of this post-hoc analysis was to evaluate if epicardial adipose tissue (EAT) quantity and quality, as evaluated by computed tomography (CT), have a different role in the risk of mortality and pulmonary embolism in critically ill COVID-19 patients admitted to an intensive care unit (ICU).
Subjects/Methods
CT derived EAT volume and density, as well as anthropometric and blood biomarkers, were evaluated in a sample of 138 subjects, 109 men and 29 women, for whom CT images and information on pulmonary embolism were available from a total of 313 subjects who were consecutively admitted to the ICU for COVID-19 from the REINSURE-ARDS prospective registry.
Results
A total of 28 patients (20.3%) died during the first 28 days after ICU admission. 26 subjects out of 138 had pulmonary embolism (18.8%). Age, weight, BMI, IL-6 levels and pulmonary embolism prevalence were significantly higher across EAT volume tertiles. Subjects who died in the first 28 days from ICU admission were older, had higher EAT volume, D-dimer, LDH and IL-6 level.
After adjustment for age and gender, participants in tertile 3 of EAT volume had lower survival at 28 days from ICU admission as compared to subjects in the tertile 1, HR 2.95 (95% C.I. 1.02–8.49), but after adjusting for potential confounders the relation was no longer significant. No relation between EAT density and mortality was observed.
From a binary logistic regression, subjects in tertile 3 of EAT volume and in tertile 1 of EAT density showed a 4 times and 3.6 times increased risk of pulmonary embolism, respectively.
Conclusions
ICU subjects affected by severe COVID-19 with higher EAT volume and low EAT density should be carefully monitored and managed with a prompt and aggressive approach, to prevent serious and life-threatening consequences and the increase of hospital treatment costs.
Subject terms: Risk factors, Obesity
Introduction
Previous studies have shown that COVID-19 morbidity and mortality may be related to epicardial adipose tissue (EAT) [1] and visceral adipose tissue (VAT) [2], which may act as SARS-CoV-2 reservoir, thereby prolonging the viral spread inside the thorax [3].
Previous studies identified EAT as a source of IL-1, PAI-1, IL-6 and TNF-alfa [4], all of which are proinflammatory cytokines involved in critically ill COVID-19 patients [5]. Previous studies have shown that PAI-1 may suppress clot dissolution in a pulmonary embolism animal model by inhibiting the tissue-type Plasminogen Activator, a key factor in fibrinolysis pathway [6]. High amounts of EAT have also been related to idiopathic deep vein thrombosis [7].
The abdominal visceral adipose tissue area as evaluated by CT is associated with clinical severity in patients with COVID-19 [2, 8]. Also, EAT seems to influence COVID-19 severity in non-ICU patients as reported by Iacobellis et al. [9]., but the role of this peculiar fat depot in intubated critically ill COVID-19 patients has not been previously investigated.
Similarly, Grodecki K et al. showed in an international multicenter study, involving 109 patients aged 64 ± 16 years, that EAT volume and EAT attenuation were independently associated with clinical deterioration or death in COVID-19 patients [10].
Multiorgan thrombosis is a prominent feature in COVID-19. In autopsies reports megakaryocytes and platelet-rich thrombi have been observed in the pulmonary arteries, arterioles and microvessels, suggesting a role in COVID-19 associated lung thrombosis. A high prevalence of pulmonary arterial thrombi has been observed in the COVID-19 setting, also in absence of coexisting deep venous thrombosis. Mild epicardial inflammation with focal acute lymphocytic epimyocarditis has been observed in COVID-19 patients [11]. Previous studies reported high pulmonary embolism prevalence in COVID-19 patients, with up to 50% in those admitted to ICU [12].
The aim of the present study was to evaluate if EAT quantity and quality, as evaluated by computer tomography (CT), are associated with the risk of mortality and pulmonary embolism in critically ill COVID-19 patients admitted to our intensive care unit (ICU).
Materials/subjects and methods
Study population
The main study population consisted of 313 COVID-19 patients from the REINSURE-ARDS registry, consecutively admitted to the ICU of the University Hospital Integrated Trust of Verona between March 8th 2020 and January 31th, 2021. All subjects belonged to the REINSURE-ARDS registry, a prospective registry of patients requiring ICU admission for respiratory failure (Prog 1946CESC, Prot 72485 12/11/2018). All patients had microbiologic confirmation of COVID-19 diagnoses by sampling of oral/nasopharyngeal swab. Upper respiratory specimens were obtained following WHO indications [13].
This study was approved by the ethics board of the University of Verona (Prog 2776CESC Prot 33108 16/06/2020) as a post-hoc analysis of a prospective registry. Patient identification remained anonymous, all participants (and/or initially their families) provided informed consent before inclusion in the REINSURE-ARDS Registry and for the use of their clinical and biological data. The ICU admission criteria and treatment decisions, including the determination of the need for intubation and type of administered antibiotic and antiviral therapy, were not standardized and were made by the attending medical team.
The present analysis was performed in a subsample of 138 subjects for whom CT images and information on pulmonary embolism were available (Fig. 1).
Fig. 1. Flow chart of participant recruitment, screening and assessment.
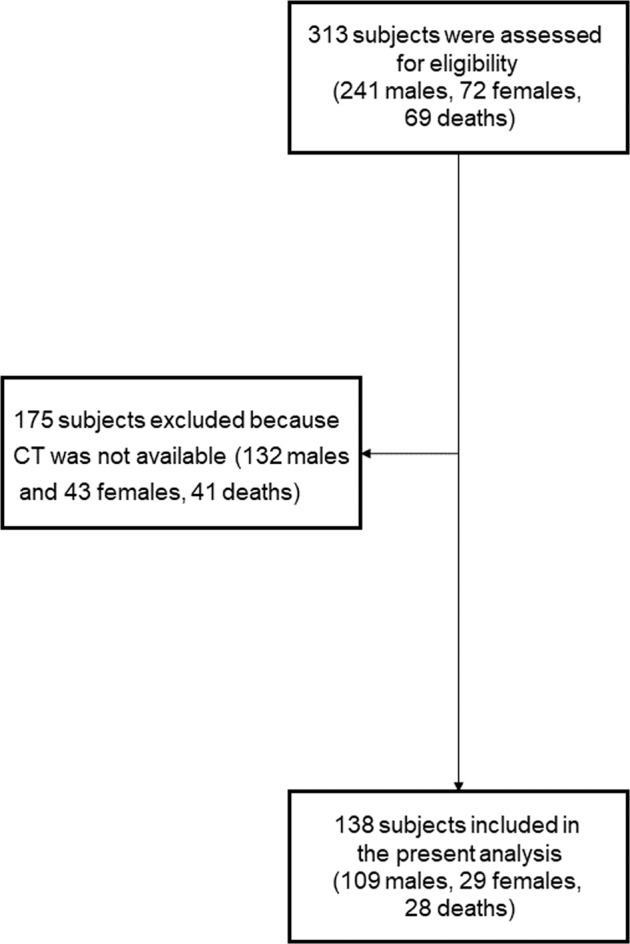
CT Computer tomography.
Patients’ baseline characteristics were recorded along with comorbidities and usual therapy. Height and weight were recorded at the beginning of hospitalization as previously reported [14]. Body mass index (BMI) was calculated as the ratio between weight and height squared (kg/m2).
Patients’ severity was graded from ICU admission. The APACHE II (Acute Physiology and Chronic Health Evaluation II) score was applied within the first 24 h of ICU admission; this score is computed on several measurements calculated from 12 acute physiologic variables, patient age and chronic health status [15].
The SOFA (Sepsis-related Organ Failure Assessment) score was computed daily; when first described, it was designed as a sepsis-related organ failure assessment, to describe a sequence of complications of critical illness and not to predict outcome, although the Authors acknowledged that any functional morbidity score must also be associated with mortality. Being rapidly recognized as useful for the assessment of acute morbidity in a range of critical illnesses, its title changed. SOFA is computed based on six different systems, respiratory, cardiovascular, hepatic, coagulation, renal and neurological, each scored from 0 to 4 with an increasing score reflecting worsening organ dysfunction [16].
Patient involvement statement
Patients and relatives were involved in the conduct of this research. Once the study has been published, participants will be informed of the results through a dedicated website (https://www.univr.it/it/comunicati-stampa) and will be sent details of the results in a study newsletter suitable for a non-specialist audience.
Biochemical measurements
All blood tests were performed according to established routine patient care and were performed upon ICU admission and/or within 24 h from ICU admission for those tests which are not available at night and on Sundays (e.g., IL-6), and, thereafter, on a daily base. Venous blood samples for C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin-6 (IL-6) and creatin phosphokinase (CPK) levels were obtained after overnight fasting as previously reported [17].
CT scan protocol and image analysis
All CT scans were performed within 48 h from ICU admission, except for those patients who had already had a CT scan during the 48 h preceding patient’s ICU admission. All CT scans were performed using multi-detector CT scanners (Brilliance 64, Philips, Best, The Netherlands and SOMATOM Definition Edge, Siemens, Erlangen, Germany), with dedicated protocols for the evaluation of pulmonary arteries, after intravenous administration of high-concentration iodinated contrast agents. All the scans were evaluated for the presence of pulmonary embolism by the same operator (GZ). Another trained operator (APR), blinded to patient outcomes, measured the EAT volume with a dedicated workstation using Sliceomatic software (version 4.2; Tomovision, Montreal) [18]. EAT volume was calculated considering density values in the range between -30 and -190 Hounsfield units (HU) for adipose tissue and respecting as anatomical limits the pulmonary artery bifurcation, the left atrium and the aortic root as the upper limit and the diaphragm and the left ventricle apex as the lower limit; mean density in HU was also calculated [19].
Outcome measures
Pulmonary embolism was clinically suspected on the basis of either worsening hypoxia despite adequate treatment or acute cor pulmonale not being explained by the actual patient-ventilator interactions [20, 21]. Mortality during the first 28 days of hospitalization was also recorded.
Patient management
Patients were recruited during the COVID-19 first wave from March 8th 2020 to July 16th 2020 and during the second wave from October 1th, 2020 to January 31th, 2021
All patients were managed according to our local ICU management protocol which foresaw:
-Endotracheal intubation when P/F ratio was inferior to 100 or <150 with tachy-dyspnoea; mechanical ventilation was initially settled on controlled volume mode with a tidal volume of 6 ml/kg on predicted body weight, respiratory rate 16–18, PEEP according to the best PEEP measured, and FiO2 so as to achieve SpO2 92–95%;
-All intubated patients with P/F ratio <150 were submitted to at least one prone/supine cycle (16/8 h);
-Continuous intravenous analgo-sedation with fentanyl or remifentanil and midazolam or propofol according to patients’ haemodynamic profiles;
-Continuous infusion of neuromuscular blocking agents (rocuronium or cisatracurium in the case of kidney failure) during the acute phase of invasive ventilation, during pronation cycles and when performing pulmonary mechanical measurements;
-Continuous enteral nutrition via nasogastric tube (25–30 kCal/kg/day comprehensive of a protein support of 1.2–2 g/kg/day);
-Multivitamin (Cernevit, 1 vial/day iv), electrolyte (Olitrace, 1 vial/day iv), amino acid (Aminotrofic, 1 dose tid via nasogastric tube), vitamin D (Gelenasi, 800 IU tid via nasogastric tube so as to maintain plasmatic vitamin D levels at 10–20 ng/mL) daily supplement;
-Gastric ulcer prophylaxis with pantoprazole 40 mg/day;
-Graduated compression elastic stockings or foot pumps;
-Glycemic control with continuous insulin infusion when indicated
-Antibiotics only if bacterial co-infection is suspected or documented
Furthermore, during the first wave, patients were treated with:
-Lopinavir/Ritonavir 200/50 mg bid for 10 days;
-Hydroxychloroquine 200 mg/day for 10 days;
-Enoxaparin 100 IU/kg/day or other LMWH in the case of kidney failure; if pulmonary embolism and/or deep venous thrombosis were diagnosed, patients were submitted to full anticoagulation protocol (e.g. Enoxaparin 100 IU/kg bid or equivalent);
-Vitamin C 35 mg/kg tid for 4 days;
-Hydrocortisone 100 mg bid in case of septic shock;
-Methylprednisolone 1 mg/kg/die as rescue therapy for proliferative ARDS.
During the second wave patients were treated with:
-Enoxaparin 100 IU/kg/day bid or equivalent in the case of kidney failure; treatment did not change in the case of diagnosed pulmonary embolism;
-Dexamethasone 6 mg/day;
-Intravenous Vitamin D 200000 IU bid the first day of ICU admission, the 100000 IU bid for 7 days (contraindicated in the case of hypercalcemia), to be repeated for another 7 days in the case of low plasmatic levels, to be substituted with oral administration of 10,000 IU/day in the case of plasmatic vitamin D levels 10–20 ng/ml;
-Vitamin C 12 g bid for 3 days, then 4 g bid for 7 days;
-Thiamine 100 mg tid.
Statistical analysis
Our primary aim was to assess if EAT volume and EAT density were associated with mortality. Our secondary aim was to test the hypothesis that pulmonary embolism was associated with EAT quantity and density.
The study population was divided into subjects with and without a diagnosis of pulmonary embolism during ICU stay. Furthermore, the study population was divided into EAT volume and density tertiles.
Differences between groups were assessed using the chi-squared test and Fisher’s exact test for categorical variables and the Student’s t-test or Mann-Whitney U test for continuous variables.
Differences in mortality rates across EAT volume and density tertiles were preliminarily evaluated by fitting Kaplan-Meier survival curves. Cox proportional hazard models were used to assess the risk of death and Hazard Ratio (HR), and 95% Confidence Intervals (95% C.I.) were estimated and reported. Four models were fitted for each outcome: unadjusted, age and gender-adjusted, age, gender and BMI-adjusted and then they were further adjusted for smoking status and comorbidity (smoking status, coronary heart disease, congestive heart failure, hypertension, diabetes, neurological pathology, chronic obstructive pulmonary disease, chronic renal failure, immunodepression and cancer).
A binary logistic regression model was used that considered the occurrence of pulmonary embolism as a dependent variable, while age, gender, BMI, smoking status, comorbidity (smoking status, coronary heart disease, congestive heart failure, hypertension, diabetes, neurological pathology, chronic obstructive pulmonary disease, chronic renal failure, immunodepression and cancer) and EAT volume or density tertiles (3 vs 1) were considered as independent variables.
A p-value of 0.05 or less was considered to be statistically significant. Data analysis was conducted using SPSS 22.0 software (Chicago, IL, USA).
Results
138 subjects, 109 men and 29 women, (21.0%) were included in the study. No differences were observed in age, sex distribution, BMI, weight, height and APACHE II score between included and excluded subjects.
Mean delay between the onset of symptoms and hospital admission was 3.79 ± 2.1 days, mean length of hospital stay pre-ICU admission was 4.2 ± 6.8 days and 28 out of 138 patients were directly admitted to ICU from Emergency Room. Mean length of ICU hospitalization was 19.59 ± 15.41 days and mean hospital stay was 35.39 ± 18.16. Subjects who died in ICU were excluded using length of stay calculation.
The mean APACHE II score was 19.0 ± 8.7 and the mean SOFA score pre-intubation was 5.58 ± 2.32. All subjects required invasive mechanical ventilation. The most common comorbidities were hypertension (57.2%), followed by dyslipidaemias (17.4%), diabetes (20.3%) and heart disease (9.4%).
Tables 1 and 2 show the main study characteristics dividing the study population according to EAT volume and density tertiles. Age, weight, BMI, IL-6 levels and pulmonary embolism prevalence were significantly higher across EAT volume tertiles. No differences were observed in LDH, CRP and D-dimer.
Table 1.
Characteristics of the study population by EAT volume tertile.
| Tertile 1 (SD) (n = 46) | Min–Max | Tertile 2 (SD) (n = 46) | Min–Max | Tertile 3 (SD) (n = 46) | Min–Max | p | |
|---|---|---|---|---|---|---|---|
| Age (years) | 61.4 (12.2) | 29–77 | 65.4 (8.6) | 44-82 | 67.2 (10.0) | 47-86 | 0.025 |
| Sex (M) n (%) | 34 (73.9%) | 57 (80.4%) | 38 (82.6%) | 0.567 | |||
| Height (m) | 1.7 (0.1) | 1.40–1.96 | 1.7 (0.1) | 1.60–1.94 | 1.7 (0.1) | 1.55–1.90 | 0.810 |
| Weight (Kg) | 81.9 (12.9) | 46–112 | 89.5 (18.7) | 63–157 | 89.7 (13.5) | 68–127 | 0.023 |
| BMI (Kg/m2) | 27.7 (3.1) | 20.4–34.3 | 29.9 (5.3) | 22.5–51.9 | 30.1 (4.4) | 24.2–41.5 | 0.014 |
| Epicardial fat density (HU) | −72.8 (8.1) | −93.0–53.2 | −76.2 (6.6) | −93.9–57.3 | −83.6 (9.1) | −101.6–55.9 | <0.001 |
| APACHE II | 17.1 (7.8) | 4–33 | 19.5 (8.3) | 6–36 | 20.6 (9.6) | 4–53 | 0.138 |
| CRP (mg/dL) | 130.8 (94.1) | 4–410 | 122.6 (77.7) | 21–348 | 112.8 (71.7) | 9–259 | 0.572 |
| D-Dimer (ng/mL) | 1927.4 (2584.4) | 250–10,000 | 2284.3 (2589.2) | 150–10,000 | 2538.7 (3147.5) | 120-10,000 | 0.573 |
| LDH (U/L) | 368.7 (130.1) | 55.0–729.0 | 407.9 (260.4) | 11.0–1544.0 | 184.3 (146.8) | 60.0–776.0 | 0.604 |
| IL-6 (pg/mL) | 23.9 (36.4) | 6.7–168.1 | 66.2 (81.2) | 7.8–367.0 | 142.9 (163.3) | 7.8–500.0 | <0.001 |
| Hypertension | 27 (58.7%) | 26 (56.5%) | 26 (56.5%) | 0.971 | |||
| Hearth failure | 1 (2.2%) | 2 (4.3%) | 1 (2.2%) | 0.773 | |||
| Ischemic cardiopathy | 3 (6.5%) | 3 (6.5%) | 3 (6.5%) | 1.000 | |||
| Neurological disorder | 1 (2.2%) | 4 (8.7%) | 5 (10.9%) | 0.246 | |||
| Type 2 Diabetes | 8 (17.4%) | 8 (17.4%) | 12 (26.1%) | 0.488 | |||
| Thyroid | 4 (8.7%) | 10 (21.7%) | 3 (6.5%) | 1.000 | |||
| Dyslipidemia | 6 (13.0%) | 10 (21.7%) | 8 (17.4%) | 0.546 | |||
| Immunodepression | 3 (6.5%) | 3 (6.5%) | 5 (10.9%) | 0.674 | |||
| Smoke habit | 9 (19.6%) | 3 (6.5%) | 8 (17.4%) | 0.163 | |||
| Chronic renal failure | 2 (4.3%) | 5 (10.9%) | 6 (13.0%) | 0.332 | |||
| Cancer | 3 (6.5%) | 6 (13.0%) | 6 (13.0%) | 0.510 | |||
| Embolia | 3 (6.5%) | 10 (21.7%) | 13 (28.3%) | 0.024 | |||
| 28 Daysa Mortality | 6 (13.0%) | 9 (19.6%) | 13 (28.3%) | 0.191 |
EAT Epicardial Adipose Tissue, SD standard deviation, BMI body mass index, HU Hounsfield units, CRP C reactive protein, CPK creatin phosphokinase, LDH lactate dehydrogenase, aSince Intensive Care Unit admission.
Table 2.
Characteristics of the study population by EAT density tertile.
| Tertile 1 (SD) (n = 46) | Min–Max | Tertile 2 (SD) (n = 46) | Min-Max | Tertile 3 (SD) (n = 46) | Min–Max | p | |
|---|---|---|---|---|---|---|---|
| Age (years) | 66.2 (11.1) | 29–86 | 64.9 (10.2) | 36-82 | 62.8 (10.4) | 35–79 | 0.302 |
| Sex (M) n (%) | 35 (76.1%) | 36 (78.3%) | 38 (82.6%) | 0.737 | |||
| Height (m) | 1.7 (0.1) | 1.40–1.96 | 1.7 (0.1) | 1.63–1.86 | 1.7 (0.1) | 1.55–1.94 | 0.685 |
| Weight (Kg) | 86.0 (16.4) | 46-157 | 88.1 (11.2) | 68-113 | 87.0 (18.5) | 63-157 | 0.802 |
| BMI (Kg/m2) | 29.1 (4.3) | 20.4–40.5 | 29.4 (3.6) | 23.4–41.5 | 29.1 (5.4) | 21.6–51.9 | 0.941 |
| Epicardial fat volume (cm3) | 140.2 (100.8) | 1.9–516.5 | 84.7 (72.5) | 26.0–406.4 | 54.8 (27.3) | 11.3–132.2 | <0.001 |
| APACHE II | 20.8 (9.0) | 4–53 | 18.2 (8.4) | 4–33 | 18.2 (8.5) | 4–36 | 0.239 |
| CRP (mg/dL) | 103.8 (70.3) | 19–259 | 128.2 (85.6) | 9-410 | 134.2 (86.1) | 4-348 | 0.165 |
| D-Dimer (ng/mL) | 2951.0 (3400.7) | 120–10000 | 1727.4 (2268.4) | 150–10,000 | 2072.0 (2441.0) | 200–10,000 | 0.092 |
| LDH (U/L) | 402.8 (223.4) | 11.0–1188.0 | 389.8 (207.3) | 77.0–1544.0 | 368.3 (116.3) | 140.0–729.0 | 0.675 |
| IL-6 (pg/mL) | 103.4 (137.7) | 7.0–500.0 | 70.9 (116.3) | 6.7–487.0 | 58.7 (91.9) | 6.7–345.0 | 0.170 |
| Hypertension | 20 (40.3%) | 32 (69.6%) | 27 (58.7%) | 0.040 | |||
| Hearth failure | 1 (2.2%) | 0 (0.0%) | 3 (6.5%) | 0.165 | |||
| Ischemic cardiopathy | 1 (2.2%) | 3 (6.5%) | 5 (10.9%) | 0.240 | |||
| Neurological disorder | 4 (8.7 %) | 2 (4.3%) | 4 (8.7%) | 0.650 | |||
| Type 2 Diabetes | 10 (21.7%) | 8 (17.4%) | 10 (21.7%) | 0.836 | |||
| Thyroid | 7 (15.2%) | 7 (15.2%) | 3 (6.5%) | 0.342 | |||
| Dyslipidemia | 4 (8.7%) | 8 (17.4%) | 12 (26.1%) | 0.089 | |||
| Immunodepression | 5 (10.9%) | 2 (4.3%) | 4 (8.7%) | 0.501 | |||
| Smoke habit | 4 (8.7%) | 9 (19.6%) | 7 (15.2%) | 0.329 | |||
| Chronic renal failure | 5 (10.9%) | 3 (6.5%) | 5 (10.9%) | 0.712 | |||
| Cancer | 4 (8.7%) | 8 (17.4%) | 3 (6.5%) | 0.208 | |||
| Embolia | 13 (28.3%) | 8 (17.4%) | 5 (10.9%) | 0.098 | |||
| 28 Daysa Mortality | 12 (26.1%) | 10 (21.7%) | 6 (13.0%) | 0.285 |
EAT Epicardial Adipose Tissue, SD standard deviation, BMI body mass index, HU Hounsfield units, CRP C reactive protein, CPK creatin phosphokinase, LDH lactate dehydrogenase.
aSince Intensive Care Unit admission.
A correlation matrix considering main study variables is shown in Table 3. A positive correlation between EAT volume and age, BMI and IL-6, whilst an inverse relationship with EAT density was observed. 28 patients (20.3%) died during the first 28 days from ICU admission. Subjects who died in the first 28 days from ICU admission were older, had higher EAT volume, D-dimer, LDH and IL-6 levels.
Table 3.
Main correlation between main study variablesa.
| EAT Volume | EAT Density | |
|---|---|---|
| EAT Volume | 1 | |
| EAT Density | −0.526* | 1 |
| Age | 0.244** | −0.128 |
| Weight | 0.201*** | −0.004 |
| BMI | 0.228** | −0.038 |
| CRP | 0.014 | 0.109 |
| D-dimer | 0.165 | −0.133 |
| LDH | 0.060 | −0.076 |
| IL-6 | 0.348* | −0.126 |
| Apache II score | 0.087 | −0.135 |
All values are Pearson’s correlation (r).
EAT Epicardial Adipose Tissue, BMI Body Mass Index, CRP C-reactive protein, LDH Lactate Dehydrogenase.
*P < 0.001; **P < 0.01; ***P < 0.05.
an=138.
Figure 2 shows that participants tertile 3 of EAT volume (those with the highest volume of EAT) had lower survival at 28 days from ICU admission as compared to subjects in tertile 1. Estimates derived from the Cox proportional hazard unadjusted model confirmed the results of the survival analysis for tertile 3, compared to tertile 1 (HR 3.04, 95% C.I. 1.08–8.52). On the contrary subjects tertile 2 did not show an increased risk compared to tertile 1. After adjustment for age, gender and BMI, the relationship remained significant with 2.95 times (95% C.I. 1.02–8.49) increased risk. In the full model, after adjusting for age, gender, BMI and comorbidity, the relation was no longer significant and only age was independently associated with mortality (HR 1.08, 95% C.I. 1.03–1.13).
Fig. 2. Kaplan–Meier survival curves for all-cause mortality according to EAT volume tertiles.
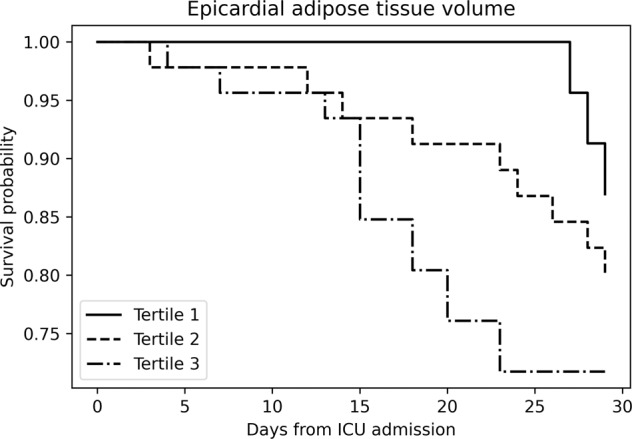
EAT epicardial adipose tissue, ICU intensive care unit.
In a similar analysis (Fig. 3), when the study population was divided into groups upon EAT density tertiles, no significant differences in survival at 28 days between the groups were observed.
Fig. 3. Kaplan–Meier survival curves for all-cause mortality according to EAT density tertiles.
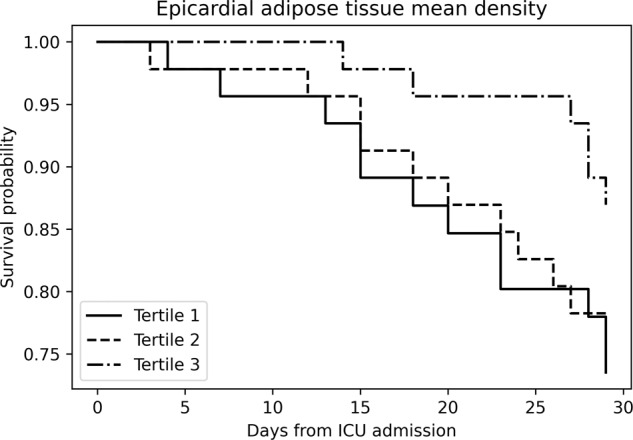
EAT epicardial adipose tissue, ICU intensive care unit.
Moreover, only age was independently associated with mortality in the full adjusted model (HR 1.03, 95% C.I. 1.01–1.12).
26 subjects out of 138 had pulmonary embolism (18.8%). Subjects with pulmonary embolism showed higher weight, BMI, EAT volume, D-dimer and IL-6 levels and lower EAT density (data not show in Table). There was a higher incidence of cancer in those with pulmonary embolism compared to those without pulmonary embolism, but no difference in other important co-morbidities.
From a binary logistic regression that considered pulmonary embolism diagnosis as a dependent variable and EAT volume tertiles, age, sex, BMI and comorbidities as independent variables, subjects in tertile 3 of EAT volume, compared to tertile 1, showed a 4 times increased risk of pulmonary embolism (95% C.I. 1.02–16.12).
Considering EAT density tertiles, age, sex, BMI and comorbidities as independent variables, subjects in tertile 1 of EAT density (those with the lowest density of EAT) showed a 3.6 times increased risk of pulmonary embolism (95% C.I. 1.06–12.17). BMI was independently related to an increased risk of pulmonary embolism (OR 1.13, 95% C.I. 1.02–1.25).
Discussion
Our study shows that in COVID-19 intubated critically ill, subjects with a higher amount of EAT are at higher risk of pulmonary embolism in ICU.
EAT volume, and not EAT density, was associated with increased mortality in our population of critically ill COVID-19 subjects, but after adjustment for potential confounders this relationship was no longer significant.
Previous studies showed that the abdominal visceral fat area, as evaluated by CT, is associated with unfavorable health outcomes and mortality in COVID-19 subjects [2, 8].
Our finding is partially in line with the previous reports from Mehta et al. and Grodecki et al. [1, 10]. and this is not surprising considering that SARS-CoV-2 enters into cells using the ACE2 receptor, which is highly expressed in EAT and other visceral adipose depots [22]. However, when age was included with comorbidities in the final model it was the only significant predictor of mortality.
The role of EAT density as a risk factor for severe COVID-19 is under debate. Lower VAT density, as evaluated with CT, is associated with higher adipocyte weight and diameter and consequent adipose tissue inflammation [23, 24]. In a post-hoc analysis, Conte et al. showed that in a population of subjects admitted to San Raffaele University Hospital emergency department EAT attenuation was predictive of critical illness, while EAT volume was not [25]. Similarly, Iacobellis et al., in a sample of 41 patients admitted for COVID-19, showed that patients with severe and critical COVID-19 had significantly greater EAT attenuation as compared to subjects with mild and moderate COVID-19 [9].
However important differences with those studies should be noted and could partially justify discrepancies. In fact, our population was limited only to critically ill subjects in ICU and not unselected COVID-19 positive subjects admitted to the emergency department. Conte et al. found that EAT attenuation was significantly, although weakly, related with systemic inflammation as evaluated by CRP. On the contrary, in our population, IL-6 was not related to EAT density, whilst a relation with EAT volume was observed, partially explaining higher mortality observed in the highest EAT volume tertile. This is in line with Abrishami et al., who, in a population of 100 subjects, observed an association between EAT volume, CRP and more severe COVID-19 course in obese subjects [26]. Moreover, according to with previous reports [27], we found that EAT density was inversely related to age, which partially explains the lack of association with mortality observed in our population.
Even after adjustment for potential confounders, subjects in the highest tertile of EAT volume showed a 4 times increased risk of pulmonary embolism. 13 subjects out of 46 in the highest tertile of EAT volume presented pulmonary embolism, corresponding to 28.2% and an association between the quantity of this adipose depot and increased thromboembolic risk can be hypothesized. This percentage decreased to 21.7% and 6.5% in subjects in the second and first tertile, respectively. A study by Mazzocolli et al. [7]. carried out on 77 patients showed that patients with a higher amount of EAT had an increased risk of developing idiopathic deep vein thrombosis compared to controls.
In our population, we observed higher IL-6 in subjects in the highest tertile of EAT volume. EAT should be considered to all effects as intrathoracic VAT [28]. Similar to abdominal visceral adipose tissue, EAT produces high amounts of inflammatory cytokines which, in patients affected by COVID-19, can determine an increased prothrombotic risk and facilitate the onset of pulmonary embolism.
The increased expression of Angiotensin-converting enzyme 2 (ACE2) observed in VAT, including EAT, make this extrapulmonary depot a susceptible point for SARS-CoV-2 infection within lung tissue.
COVID-19 is commonly complicated by coagulopathy, disseminated intravascular coagulation and pulmonary embolism associated with a severe inflammatory state [29] leading to higher mortality. Likewise, obesity is highly related with a hypercoagulopathy status.
VAT depots, including EAT, are characterized by systemic oxidative stress, leading to the loss of the antithrombotic properties of the endothelium, increased platelet activation and decreased fibrinolysis [30]. Viral infection and proinflammatory cytokines production from VAT depots synergistically contribute to vascular endothelium, platelets and other circulating vascular cells stimulation, thereby promoting the upregulation of procoagulant factors and adhesion molecules and concomitant downregulation of anticoagulant regulatory proteins, increased thrombin generation and enhanced platelet activation [31].
We found that subjects with low EAT density were at higher risk of pulmonary embolism. This finding can be explained by the fact that CT measured AT density is related to adipocyte hypertrophy and hyperplasia following excess lipid accumulation. Increased adipocytes weight and size is in fact associated with higher inflammation, increased cardiometabolic and prothrombotic risk.
Moreover EAT is anatomically close to the pulmonary artery and may potentially enable the diffusion of proinflammatory cytokines into pulmonary circulation with paracrine and vasocrine consequences on lung tissue and circulation [32]. We can therefore hypothesize that EAT may act as an important SARS-CoV-2 reservoir [3] and promote local viral shedding in the thoracic region, thereby facilitating pulmonary damage and also pulmonary embolism [33], and resulting in an additional increase of mortality in subjects with severe COVID-19.
Some limitations need to be mentioned. Firstly, this is a single-center observational prospective study with relatively small number of subjects and we used arbitrary categorization in tertiles that leads to a loss of power when examining predictor-outcome associations. Therefore, the threshold that we obtained dividing the study sample by tertiles cannot be generalized to other populations. Secondly, patients were treated with different protocols, influencing mortality rates. Thirdly, the study sample size precludes meaningful exploration of the association of wasting with specific disease entities and comorbidities. Finally, our analysis was limited to the population in which pulmonary embolism was clinically suspected and CT was performed on the basis of either worsening hypoxia or acute cor pulmonale, which may have resulted in selection bias.
As the SARS-CoV-2 may continue to spread worldwide, clinicians should identify subjects at higher risk for unfavorable health outcomes. Our results cannot be generalized and must be considered with caution, but if confirmed in wider populations, moderately to severely ill hospitalized COVID-19 patients with high EAT volume and lower EAT density could benefit from prophylactic or therapeutic heparin [34]. Moreover, considering that multiple studies of post-discharge patients with COVID-19 show incidences of symptomatic venous thromboembolism ranging from
below 1% to 2.5%, extended short-term thrombophylaxis beyond hospitalization should also be considered in high-risk population [35].
The use of easily available EAT parameters in critically ill subjects undergoing standard thoracic CT could help to identify, monitor and treat subjects carefully at higher pulmonary embolism and mortality risk, in order to prevent serious life-threatening consequences and the increase of related hospital costs.
In conclusion, EAT volume, and not EAT density, was associated with mortality in subjects admitted to the ICU for severe COVID-19, independently of general obesity, but when age was included in the model the relationship was no longer significant.
Subjects in the highest tertile of EAT volume and in the lowest tertile of EAT density showed respectively a 4- and 3.6-times increased risk of pulmonary embolism after adjustment for potential confounders.
Acknowledgements
The paper was revised by Prof Mark Newman.
Author contributions
The authors’ responsibilities were as follows: AR, LG, EP, and KD designed the research. EP, LG, KD, VS, MZ, and ZDV conducted research. AR and LG analyzed the data or performed statistical analysis. GZ collected CT images. AR analyzed CT images. AR and KD had primary responsibility for final content. AR, LG, KD, EP, ZDV, MZ, and RN wrote the paper.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available since this option was not included in the originally signed consent. Data are however available from authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andrea P. Rossi, Katia Donadello.
Change history
11/28/2022
The author Zeno Dalla Valle has been tagged incorrectly in the HTML version of the original article.
References
- 1.Mehta R, Bello-Chavolla OY, Mancillas-Adame L, Rodriguez-Flores M, Pedraza NR, Encinas BR, et al. Epicardial adipose tissue thickness is associated with increased severity and mortality related to SARS-CoV-2 infection. Int J Obes. 2022;11:1–8. doi: 10.1038/s41366-021-01050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metab Clin Exp. 2020;111:154319. [DOI] [PMC free article] [PubMed]
- 3.Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28:1191–4. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: From the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 201; 43: 1651–4. [DOI] [PubMed]
- 5.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–9. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilly CF, Fujita T, Hutzelmann JE, Mayer EJ, Shebuski RJ. Plasminogen activator inhibitor-1 suppresses endogenous fibrinolysis in a canine model of pulmonary embolism. Circulation. 1991;84:287–92. doi: 10.1161/01.CIR.84.1.287. [DOI] [PubMed] [Google Scholar]
- 7.Mazzoccoli G, Copetti M, Dagostino MP, Grilli M, Fontana A, Pellegrini F, et al. Epicardial adipose tissue and idiopathic deep venous thrombosis: An association study. Atherosclerosis. 2012;223:378–83. doi: 10.1016/j.atherosclerosis.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Petersen A, Bressem K, Albrecht J, Thieß H-M, Vahldiek J, Hamm B, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metab - Clin Exp. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacobellis G, Secchi F, Capitanio G, Basilico S, Schiaffino S, Boveri S, et al. Epicardial Fat Inflammation in Severe COVID-19. Obesity. 2020;28:2260–2. doi: 10.1002/oby.23019. [DOI] [PubMed] [Google Scholar]
- 10.Grodecki K, Lin A, Razipour A, Cadet S, McElhinney PA, Chan C, et al. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism. 2021;115:15443611. doi: 10.1016/j.metabol.2020.154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. E Clinical Medicine. 2020;24:10043412. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56:2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020. World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/331501.
- 14.Rossi AP, Zanandrea V, Zoico E, Zanardo M, Caliari C, Confente S, et al. Inflammation and nutritional status as predictors of physical performance and strength loss during hospitalization. Eur J Clin Nutr. 2016;70:1439–42. doi: 10.1038/ejcn.2016.159. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. “APACHE II: a severity of disease classification system”. Crit Care Med. 1985;13:818–29. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, Willatts S, Mendonca A, Bruining H. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Rossi AP, Gottin L, Donadello K, Schweiger V, Nocini R, Taiana M, et al. Obesity as a risk factor for unfavourable outcomes in critically ill patients affected by Covid 19. Nutr Metab Cardiovasc Dis. 2021;31:762–8. doi: 10.1016/j.numecd.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shmilovich H, Dey D, Cheng VY, Rajani R, Nakazato R, Otaki Y, et al. Threshold for the upper normal limit of indexed epicardial dat volume: derivation in a healthy population and validation in an outcome-based study. Am J Cardiol. 2011;108:1680–5. doi: 10.1016/j.amjcard.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seabolt LA, Welch EB, Silver HJ. Imaging methods for analyzing body composition in human obesity and cardiometabolic disease. Ann N.Y Acad Sci. 2015;1353:41–59. doi: 10.1111/nyas.12842. [DOI] [PubMed] [Google Scholar]
- 20.Vieillard-Baron A, Naeije R, Haddad F, Bogaard HJ, Bull TM, Fletcher N, et al. Diagnostic workup, etiologies and management of acute right ventricle failure: a state-of-the-art paper. Intensive Care Med. 2018;44:774–90. doi: 10.1007/s00134-018-5172-2. [DOI] [PubMed] [Google Scholar]
- 21.Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–58. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couselo-Seijas M, Almengló C, Agra-Bermejo M, Luis Fernandez R, Alvarez Á. E, R González-Juanatey J, et al. Higher ACE2 expression levels in epicardial cells than subcutaneous stromal cells from patients with cardiovascular disease: Diabetes and obesity as possible enhancer. Eur J Clin Invest. 2021;51:e13463. doi: 10.1111/eci.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Côté JA, Nazare J-A, Nadeau M, Leboeuf M, Blackburn L, Després J-P, et al. Computed tomography-measured adipose tissue attenuation and area both predict adipocyte size and cardiometabolic risk in women. Adipocyte. 2015;5:35–42. doi: 10.1080/21623945.2015.1106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy RA, Register TC, Shively CA, Carr JJ, Ge Y, Heilbrun ME, et al. Adipose Tissue Density, a Novel Biomarker Predicting Mortality Risk in Older Adults. J Gerontol A Biol Sci Med Sci. 2014;69:109–17. doi: 10.1093/gerona/glt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conte C, Esposito A, De Lorenzo R, Di Filippo L, Palmisano A, Vignale D, et al. Epicardial adipose tissue characteristics, obesity and clinical outcomes in COVID-19: A post-hoc analysis of a prospective cohort study. Nutr Metab Cardiovasc Dis. 2021;31:2156–64. doi: 10.1016/j.numecd.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrishami A, Eslami V, Baharvand Z, Khalili N, Saghamanesh S, Zarei E, et al. Epicardial adipose tissue, inflammatory biomarkers and COVID-19: Is there a possible relationship? Int Immunopharmacol. 2021;90:107174. doi: 10.1016/j.intimp.2020.107174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nerlekar N, Thakur U, Lin A, Koh JQS, Potter E, Liu D, et al. The Natural history of Epicardial Adipose Tissue Volume and Attenuation: A long-term prospective cohort follow-up study. Sci Rep. 2020;10:710928. doi: 10.1038/s41598-020-63135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–71. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 29.Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos I, de O, Kobinger GP, et al. Hypercoagulopathy and Adipose Tissue Exacerbated Inflammation May Explain Higher Mortality in COVID-19 Patients With Obesity. Front Endocrinol. 2020;11:530. doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anfossi G, Russo I, Trovati M. Platelet dysfunction in central obesity. Nutr Metab Cardiovasc Dis. 2009;19:440–9. doi: 10.1016/j.numecd.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Levi M, van der Poll T, Schultz M. Infection and inflammation as risk factors for thrombosis and atherosclerosis. Semin Thromb Hemost. 2012;38:506–14. doi: 10.1055/s-0032-1305782. [DOI] [PubMed] [Google Scholar]
- 32.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pr Cardiovasc Med. 2005;2:536–43. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 33.Maier HE, Lopez R, Sanchez N, Ng S, Gresh L, Ojeda S, et al. Obesity Increases the Duration of Influenza A Virus Shedding in Adults. J Infect Dis. 2018;218:1378–82. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sholzberg M, da Costa BR, Tang GH, Rahhal H, AlHamzah M, Baumann Kreuziger L. Net al; RAPID Trial Investigators. Randomized trials of therapeutic heparin for COVID-19: A meta-analysis. Res Pract. Thromb Haemost. 2021;5:e12638. doi: 10.1002/rth2.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannis D, Allen SL, Tsang J, Flint S, Pinhasov T, Williams S, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–47. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available since this option was not included in the originally signed consent. Data are however available from authors upon reasonable request.


