Key Points
Question
Is the reported lack of benefit from immune checkpoint inhibition (ICI) in patients with advanced gastroesophageal cancer and absent or low tissue-based expression of programmed death-ligand 1 (PD-L1) only a by-product of the instability inherent in exploratory analyses?
Findings
This systematic review and meta-analysis of 17 phase 3 randomized clinical trials comprising 11 166 patients found that tissue-based PD-L1 expression was the strongest predictor of overall survival benefit from ICI, more than other variables (after microsatellite instability). Benefit from ICI was generally diminished among those with absent or low (vs high) PD-L1.
Meaning
The findings of this review and analysis seem to confirm a reduced benefit from ICI among patients with advanced gastroesophageal cancer and absent or low tissue-based PD-L1 expression.
Abstract
Importance
Approval by the US Food and Drug Administration of immune checkpoint inhibition (ICI) for advanced gastroesophageal cancer (aGEC) irrespective of PD-L1 status has generated controversy. Exploratory analyses from individual trials indicate a lack of meaningful benefit from ICI in patients with absent or low PD-L1 expression; however, analysis of a single variable while ignoring others may not consider the instability inherent in exploratory analyses.
Objective
To systematically examine the predictive value of tissue-based PD-L1 status compared with that of other variables for ICI benefit in aGEC to assess its stability.
Data Sources
MEDLINE, Embase, Scopus, Web of Science, Cochrane Central Register (2000-2022).
Study Selection, Data Extraction, and Synthesis
Randomized clinical trials (RCTs) were included of adults with aGEC (adenocarcinoma [AC] or squamous cell carcinoma [SCC]) randomized to anti−PD-1 or PD-L1−containing treatment vs standard of care (SOC). Study screening, data abstraction, and bias assessment were completed independently by 2 reviewers. Of 5752 records screened, 26 were assessed for eligibility; 17 trials were included in the analysis.
Main Outcomes and Measures
The prespecified primary end point was overall survival. The mean hazard ratio (HR) for ICI vs SOC was calculated (random-effects model). Predictive values were quantified by calculating the ratio of mean HRs between 2 levels of each variable.
Results
In all, 17 RCTs (9 first line, 8 after first line) at low risk of bias and 14 predictive variables were included, totaling 11 166 participants (5067 with SCC, 6099 with ACC; 77.6% were male and 22.4% were female; 59.5% of patients were younger than 65 years, 40.5% were 65 years or older). Among patients with SCCs, PD-L1 tumor proportion score (TPS) was the strongest predictor of ICI benefit (HR, 0.60 [95% CI, 0.53-0.68] for high TPS; and HR, 0.84 [95% CI, 0.75-0.95] for low TPS), yielding a predictive value of 41.0% favoring high TPS (vs ≤16.0% for other variables). Among patients with AC, PD-L1 combined positive score (CPS) was the strongest predictor (after microsatellite instability high status) of ICI benefit (HR, 0.73 [95% CI, 0.66-0.81] for high CPS; and HR, 0.95 [95% CI, 0.84-1.07] for low CPS), yielding a predictive value of 29.4% favoring CPS-high (vs ≤12.9% for other variables). Head-to-head analyses of trials containing both levels of a variable and/or having similar design generally yielded consistent results.
Conclusions and Relevance
Tissue-based PD-L1 expression, more than any variable other than microsatellite instability-high, identified varying degrees of benefit from ICI-containing therapy vs SOC among patients with aGEC in 17 RCTs.
This systematic review and meta-analysis examines the predictive value of tissue-based PD-L1 status compared with other variables for ICI benefit among 17 randomized clinical trials including 11 166 patients with advanced gastroesophageal cancer.
Introduction
Treatment of advanced gastroesophageal carcinoma (aGEC) has been transformed through immune checkpoint inhibition (ICI). Anti−programmed death-ligand 1 (PD-L1) therapy inhibits the interaction of PD-1 with its ligand PD-L1, a marker of T-cell inflammation.1 Clinically, PD-L1 is measured immunohistochemically based on expression on tumor cells (generating a tumor proportion score [TPS]) or tumor and immune cells (generating a combined positive score [CPS]).
The US Food and Drug Administration (FDA) recently approved nivolumab and pembrolizumab as first-line treatments for advanced gastric or gastroesophageal junction adenocarcinoma (AC) and esophageal AC or squamous cell carcinoma (SCC), respectively, irrespective of PD-L1 status. This followed the FDA approval of the second-line treatment of esophageal SCCs (nivolumab irrespective of PD-L1 status, pembrolizumab for CPS ≥10). Consistent with European Medicines Agency guidelines, the US National Comprehensive Cancer Network has given category 1 or 2A recommendation for patients with high PD-L1 status (ie, CPS ≥5 or ≥10 depending on context) and typically 2B approval for those with absent or low expression.
Whether tissue-based PD-L1 testing is needed to select patients for ICI has become an area of debate. Results from exploratory analyses of individual phase 3 trials in aGEC have suggested a lack of meaningful benefit from ICI in patients with absent or low expression of PD-L1. However, reliance on exploratory results is justifiably viewed with extreme caution.2
We performed a systematic review and meta-analysis of randomized clinical trials (RCTs) in aGEC to evaluate overall survival benefit from ICI in patients with high vs absent or low PD-L1 expression. We quantified the predictive value not only of PD-L1 status, but all other reported variables, so that the latter could serve as an informal reference that might reflect the inherent instability of subgroup analyses. The goal of these additional analyses was to help guard against the potentially serious pitfall of denying efficacious therapy to patients based on isolated subgroup analysis of a single variable. This study aimed to determine the predictive value of PD-L1 compared with other baseline variables reported in RCTs evaluating ICI-containing therapy vs standard of care (SOC) in patients with aGEC.
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.3 Institutional review board approval and informed consent were waived because this was not an individual patient-level meta-analysis.
Eligibility Criteria
Randomized clinical trials were included if ICI, alone or combined with chemotherapy, was compared with SOC in adult patients with aGEC. Other criteria included English language, overall survival reported according to at least 1 baseline variable; ICI included anti−PD-1 or PD-L1 therapy; total number was more than 100 patients; and histologic AC and/or SCC.
Data Sources and Search Strategies
A comprehensive literature search was performed for articles or conference abstracts published from January 1, 2000, to July 27, 2021, in MEDLINE, Embase, Scopus, Web of Science, and Cochrane Central Register of Clinical Trials (eMethods in the Supplement). The outcome of interest was overall survival.
Data Extraction, Risk of Bias, Quality of Evidence
Prespecified data elements were extracted from each trial using a structured data abstraction form (eTable 1 in the Supplement). After analysis on August 10, 2021, an updated search was performed (February 2022) with no additional studies added. The Cochrane Collaboration’s tool to assess risk of bias in the trial was used.4
Statistical Analysis
We extracted hazard ratios (HRs) and 95% CIs from included trials. Random-effects models were used to estimate mean HRs (eMethods in the Supplement). The predictive value of a variable was defined as the ratio of mean HRs across each level of that variable. The following analytic approach was prespecified: baseline variables were included if reported in association with overall survival in 3 or more eligible RCTs; definition of high vs low for each variable-defined subpopulation; and primary and sensitivity analyses. Primary analysis comprised all trials. Sensitivity analyses were performed in (1) trials comparing ICI plus chemotherapy vs chemotherapy; (2) ICI vs chemotherapy; and (3) trials amenable to head-to-head analysis (ie, overall survival reported for both levels of each variable).
Results
Study Selection
Of 5781 titles and abstracts identified by the search strategy, 26 full-text articles or conference abstracts with multimedia presentations met the eligibility for assessment (eFigure 1 in the Supplement). Of these, 19 described 17 trials that met inclusion criteria (76%; 13 of 17 full-text articles) and were included in the qualitative and quantitative synthesis (eFigures 1 and 2 in the Supplement).5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23
Study and Participant Characteristics
The 17 trials included comprised 11 166 patients with aGEC, 8273 (77.6%) of which were male, 2383 (22.4%) were female, and 510 (4.6%) were missing data on sex; 5307 (59.5%) were less than 65 years old, and 3616 (40.5%) were 65 years or older (another 1733 had data on age that allowed for predictive analysis, but not for a precise percentage breakdown in the overall population, and 510 were missing data on age). Patient race and ethnicity information were not collected across most of the studies, and therefore, were not included in the analyses. The Table and eTable 1 in the Supplement provide further information on study characteristics. Eight trials enrolled participants in Asia and non-Asian areas, whereas 9 trials focused on Asia only. The control arm was chemotherapy in all trials except placebo in ATTRACTION-2.6 The experimental arm was ICI plus chemotherapy in 9 trials (all first-line) and ICI alone in 10 trials (2 in first-line, 1 as maintenance after first-line, 5 in second-line, 2 in third-line or higher). Two first-line trials (KN-062,15 CM-64810) used 2 experimental arms of ICI plus chemotherapy and ICI alone. Immune checkpoint inhibition targeted PD-1 in most trials (15 of 17) and PD-L1 in 2 trials; ICI was single-agent except anti−PD-1 plus anti−cytotoxic T-lymphocyte-associated protein 4 in CM-648. Overall survival was the primary or co−primary end point in all trials.
Table. Overview of the 17 Phase 3 Randomized Clinical Trials Included.
| Trial | Line | Region | Experimental group(s) | Control group | Patients, No. | ||
|---|---|---|---|---|---|---|---|
| Total | AC | SCC | |||||
| SCC trials | |||||||
| CM-64810 | 1 | Asia and non-Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 970 | 0 | 970 |
| Anti−PD-1 + anti−CTLA-4 (2 arms) | |||||||
| ESCORT-1st12 | 1 | Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 596 | 0 | 596 |
| ORIENT-1521 | 1 | Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 659 | 0 | 659 |
| JUPITER-0622 | 1 | Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 514 | 0 | 514 |
| ATTRACTION-037 | 2 | Asia | Anti−PD-1 | Chemotherapy | 419 | 0 | 419 |
| ESCORT11 | 2 | Asia | Anti−PD-1 | Chemotherapy | 448 | 0 | 448 |
| RATIONALE-30219 | 2 | Asia and non-Asia | Anti−PD-1 | Chemotherapy | 512 | 0 | 512 |
| AC trials | |||||||
| CM-6498,9 | 1 | Asia and non-Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 1581 | 1581 | 0 |
| KN-06215 | 1 | Asia and non-Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 763 | 763 | 0 |
| Anti−PD-1 (2 arms) | |||||||
| ATTRACTION-045 | 1 | Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 724 | 724 | 0 |
| ORIENT-1620 | 1 | Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 650 | 650 | 0 |
| JAVELIN-10013 | MAINT | Asia and non-Asia | Anti−PD-L1 | Chemotherapy | 499 | 499 | 0 |
| KN-06116 | 2 | Asia and non-Asia | Anti−PD-1 | Chemotherapy | 590 | 590 | 0 |
| ATTRACTION-026 | 3 | Asia | Anti−PD-1 | Placebo | 493 | 493 | 0 |
| JAVELIN-30014 | 3 | Asia and non-Asia | Anti−PD-L1 | Chemotherapy | 371 | 371 | 0 |
| Mixed SCC and AC trials | |||||||
| KN-59018 | 1 | Asia and non-Asia | Anti−PD-1 + chemotherapy | Chemotherapy | 749 | 201 | 548 |
| KN-18117 | 2 | Asia and non-Asia | Anti−PD-1 | Chemotherapy | 628 | 227 | 401 |
| Total | NA | NA | NA | NA | 11 166 | 6099 | 5067 |
Abbreviations: AC, adenocarcinoma; CTLA, cytotoxic T-lymphocyte-associated protein 4; MAINT, maintenance; NA, not applicable; PD-L1, programmed death-ligand 1; SCC, squamous cell carcinoma.
Subgroup Variables Included
Thirty-three variables were reported in association with overall survival in 1 or more trials, of which 18 were reported in 3 or more trials (eFigure 3 in the Supplement). Fourteen variables amenable to comparison (including histology) were included, leading to a maximum of 13 variables in each histologic subtype (eMethods in the Supplement).
Meta-analysis and Predictive Values
Squamous Cell Carcinoma vs Adenocarcinoma
A total of 5067 patients with SCC and 6099 patients with AC were enrolled across 7 trials of SCC only, 8 trials of AC only, and 2 trials of both histologies (Table). As shown in Figure 1, ICI was associated with overall survival benefit in SCC overall (9 trials; 10 treatment comparisons) with a pooled mean HR of 0.71 (95% CI, 0.67-0.77). In AC (10 trials; 11 comparisons), the corresponding HR was 0.87 (95% CI, 0.80-0.96). This yielded a predictive value for histology of 22% ([0.87 ÷ 0.71] − 1) that was modestly high, favoring SCC. The predictive value was comparable in trials of ICI plus chemotherapy vs chemotherapy and ICI vs chemotherapy. Predictive values were non-uniform between the 2 trials (KN-181,17 KN-59018), each with different treatment designs, which enrolled both AC and SCC histology (eTable 2 in the Supplement).
Figure 1. Phase 3 Randomized Clinical Trials Evaluating ICI by SCC and AC Histology.
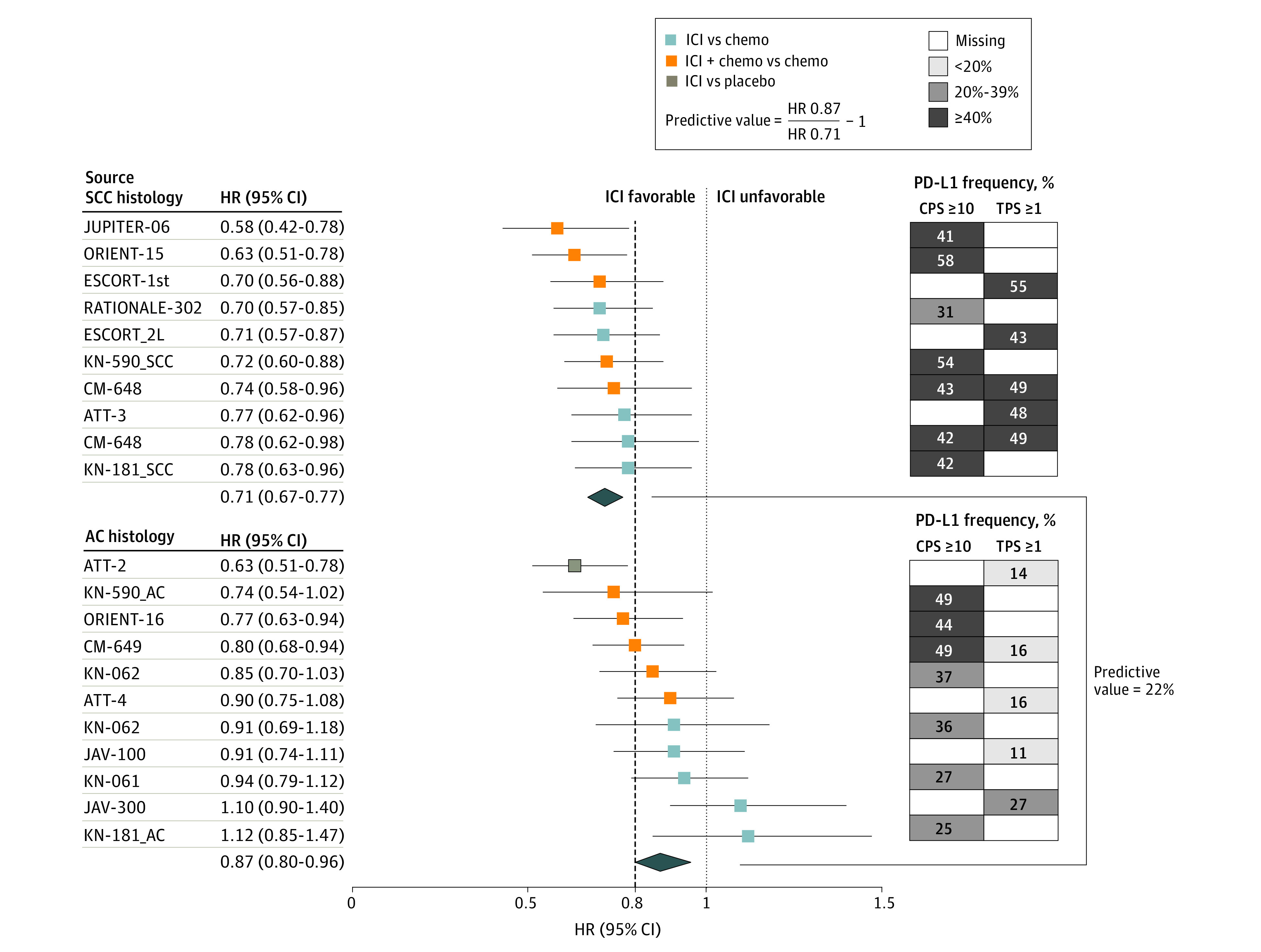
The forest plot shows HRs (95% CI) for OS for each trial (left). The pooled (mean) HR was 0.71 (95% CI, 0.67-0.77) for SCC (9 trials; 10 treatment comparisons) and 0.87 (95% CI, 0.80-0.96) for AC (10 trials; 11 treatment comparisons), yielding a predictive value for histology ([0.87 ÷ 0.71] – 1) of 22% favoring SCC (right). The dashed line denotes the ASCO-recommended HR of 0.80. On the right, the grid shows the frequency of patients in each trial who had positive tissue-based PD-L1 expression (CPS ≥10 or TPS ≥1). The darkest squares are trials most enriched with PD-L1−positive patients. In ORIENT-15, the proportion of tumors with TPS ≥10 was 36%. In the ICI vs chemotherapy comparison for KN-062, the CI was 99.2%. The Table provides the citations and reference numbers for each trial. ASCO indicates the American Society of Clinical Oncology; HRs, hazard ratios; ICI, immune checkpoint inhibition; OS, overall survival; PD-L1, programmed death-ligand 1; SCC, squamous cell carcinoma; and TPS, tumor proportion score.
PD-L1 expression appeared enriched in SCCs rather than ACs (Figure 1). As an example, using an arbitrary cutoff of 40% prevalence, CPS of 10 or greater comprised more than 40% of the trial population in 6 of 7 SCC cohorts vs 3 of 7 AC cohorts that reported CPS data; and TPS 1 or greater comprised more than 40% of patients in 5 of 5 SCC cohorts and 0 of 5 AC cohorts that reported these TPS data.
Squamous Cell Carcinoma
Five variables were excluded from analysis of predictive values in SCC (eMethods in the Supplement): prior surgery (data available in only 1 trial), Lauren classification (not applicable to SCC), chemotherapy (differing variable structure; eMethods in the Supplement), microsatellite instability (MSI), and anatomic location (all esophageal).
The strongest predictive variables (of 8 included) among SCCs were PD-L1 TPS, and less consistently, CPS (Figure 2; eTables 3 and 4 in the Supplement). The TPS-high group (n = 1489) and TPS-low group (n = 1799) were each represented by 5 trials (6 comparisons; eTable 5 in the Supplement). High TPS was defined as TPS of 1 or greater in all trials except ORIENT-1521 (TPS ≥10). In the overall analysis, the HR for an overall survival benefit from ICI was 0.60 (95% CI, 0.53-0.68) in TPS-high and 0.84 (95% CI, 0.75-0.95) in TPS-low, yielding a predictive value of 41.0% favoring high TPS. The predictive value of TPS consistently remained higher than that of non−PD-L1 variables in sensitivity analyses—ie, trials of ICI plus chemotherapy vs chemotherapy (43.9%) or ICI vs chemotherapy (38.4%), or head-to-head comparisons (41.0%; ie, trials that reported overall survival for both levels of each variable—refer to the Methods). In head-to-head analysis of 3 trials comparing ICI plus chemotherapy vs chemotherapy, the predictive value for TPS remained high (43.4%).
Figure 2. Variables Potentially Associated With Benefit From ICI in Phase 3 Trials of SCC Histology.
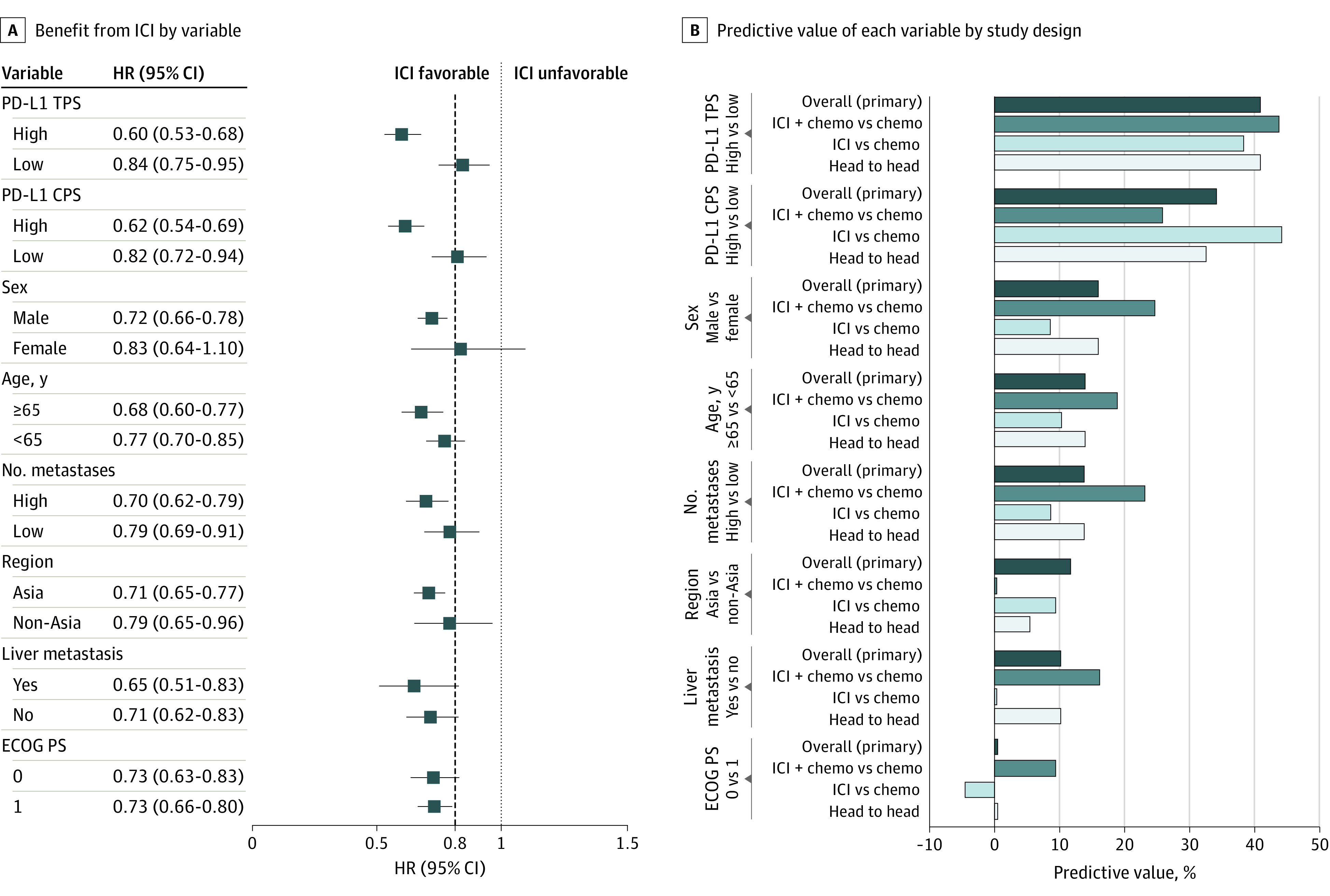
A, A meta-analysis of individual trials with pooled (mean) HRs for OS and 95% CIs shown for each subgroup variable. The dashed line denotes the ASCO-recommended HR of 0.80. B, The bar graphs indicate each variable’s predictive value (ie, ratio of mean HRs across each level of the variable). The predictive value was calculated in all trials (primary analysis) as well as in 3 sensitivity analyses—ie, the subset of trials that compared ICI plus chemotherapy vs chemotherapy and that compared ICI vs chemotherapy, and the subset of trials amenable to head-to-head analysis (those that reported OS outcomes for both levels of each variable). The Table provides the citation and reference numbers for each trial. ASCO indicates the American Society of Clinical Oncology; CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group Performance Status scale; HR, hazard ratio; ICI, immune checkpoint inhibition; OS, overall survival; PD-L1, programmed death-ligand 1; SCC, squamous cell carcinoma; and TPS, tumor proportion score.
Overall, the predictive value for CPS was the second highest among all variables. The CPS-high group (n = 1943) was represented by 6 trials (7 comparisons) and the CPS-low group (n = 1499) by 5 trials (6 comparisons). The cut point for high CPS was 10 or more in all trials except JUPITER-0622 (CPS ≥1). Overall, the HR for an overall survival benefit from ICI was 0.62 (95% CI, 0.54-0.69) for high CPS and 0.82 (95% CI, 0.72-0.94) for low CPS, yielding a predictive value of 34.3% favoring high CPS. The high predictive value of CPS was most evident in trials of ICI vs chemotherapy (44.3% vs ≤10.4% for non−PD-L1 variables). Among trials of ICI plus chemotherapy vs chemotherapy, the predictive value for CPS (26.0%) remained the highest among non−PD-L1 variables but became more comparable to that of multiple non−PD-L1 variables including sex, number of metastases, and age (predictive values 19.0%-24.7%). Further sensitivity analyses in head-to-head trials of ICI plus chemotherapy vs chemotherapy and using the cut point of 10 yielded equivalent results (eTables 5 and 6 in the Supplement). The proportion of studies that used 1 antibody clone vs another to detect PD-L1 immunohistochemically did not appear to differ substantially between these head-to-head analysis groups (eTable 5 in the Supplement).
Only 2 SCC trials reported both TPS and CPS—the CM-64810 and the ORIENT-1521—both evaluating ICI plus chemotherapy vs chemotherapy (further details are available in eTable 7 in the Supplement). The predictive value of TPS appeared to be more consistent than that of CPS.
SCC and TPS Cut Points
As shown in Figure 3, we examined which TPS cut point might be most predictive (eTables 3 and 4 in the Supplement). This included 2 trials (CM-648,10 ESCORT-1st12) comparing ICI plus chemotherapy vs chemotherapy and 2 trials (ATTRACTION-03,7 ESCORT11) comparing ICI vs chemotherapy. Overall, TPS at the cut point of 1 appeared most predictive with an HR of 0.61 (95% CI, 0.53-0.69) in TPS ≥1 and HR of 0.89 (95% CI, 0.78-1.01) in TPS less than 1, yielding a predictive value of 47%. The TPS cut points of 5 or 10 were associated with predictive values of 24% to 26% overall. Results were similar in sensitivity analyses. Only1 SCC study reported data from multiple CPS cut points (eTable 5 in the Supplement).
Figure 3. Predictive Value of PD-L1 TPS at Multiple Cut Points in SCC.
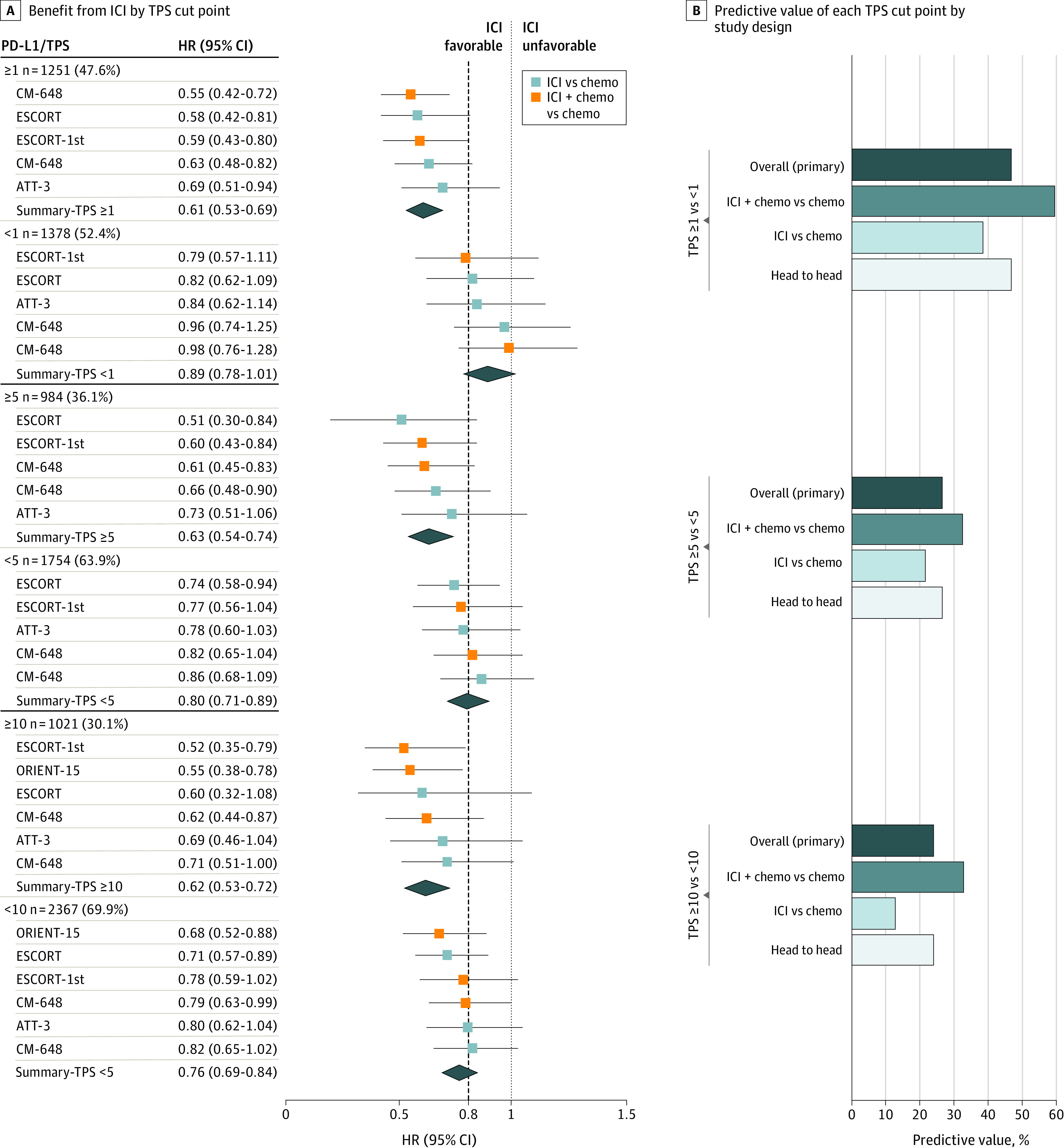
A, A meta-analysis of individual trials with pooled (mean) HRs for OS and 95% CIs shown at each cut point of TPS. The dashed line denotes the ASCO-recommended HR of 0.80. B, The bar graphs indicate each cut point’s predictive value (ie, ratio of mean HRs across each level of the cut point; right). The predictive value was calculated in all trials (primary analysis) as well as in 3 sensitivity analyses—ie, the subset of trials that compared ICI plus chemotherapy vs chemotherapy and that compared ICI vs chemotherapy, and the subset of trials amenable to head-to-head analysis (those that reported OS outcomes for both levels of each variable). The Table provides the citation and reference numbers for each trial. AC indicates adenocarcinoma; ASCO, American Society of Clinical Oncology; CPS, combined positive score; HR, hazard ratio; ICI, immune checkpoint inhibition; OS, overall survival; PD-L1, programmed death-ligand 1; SCC, squamous cell carcinoma; and TPS, tumor proportion score.
Adenocarcinoma
The predictive values of 13 variables were assessed in AC (Figure 4; eTables 8 and 9 in the Supplement). Microsatellite instability exhibited the highest predictive value overall (135.8%; n = 153 MSI-high [4 trials]; n = 1957 non−MSI-high [3 trials]). Results were similar in sensitivity analyses.
Figure 4. Variables Potentially Associated With Benefit From ICI in Phase 3 Randomized Clinical Trials of AC Histology.
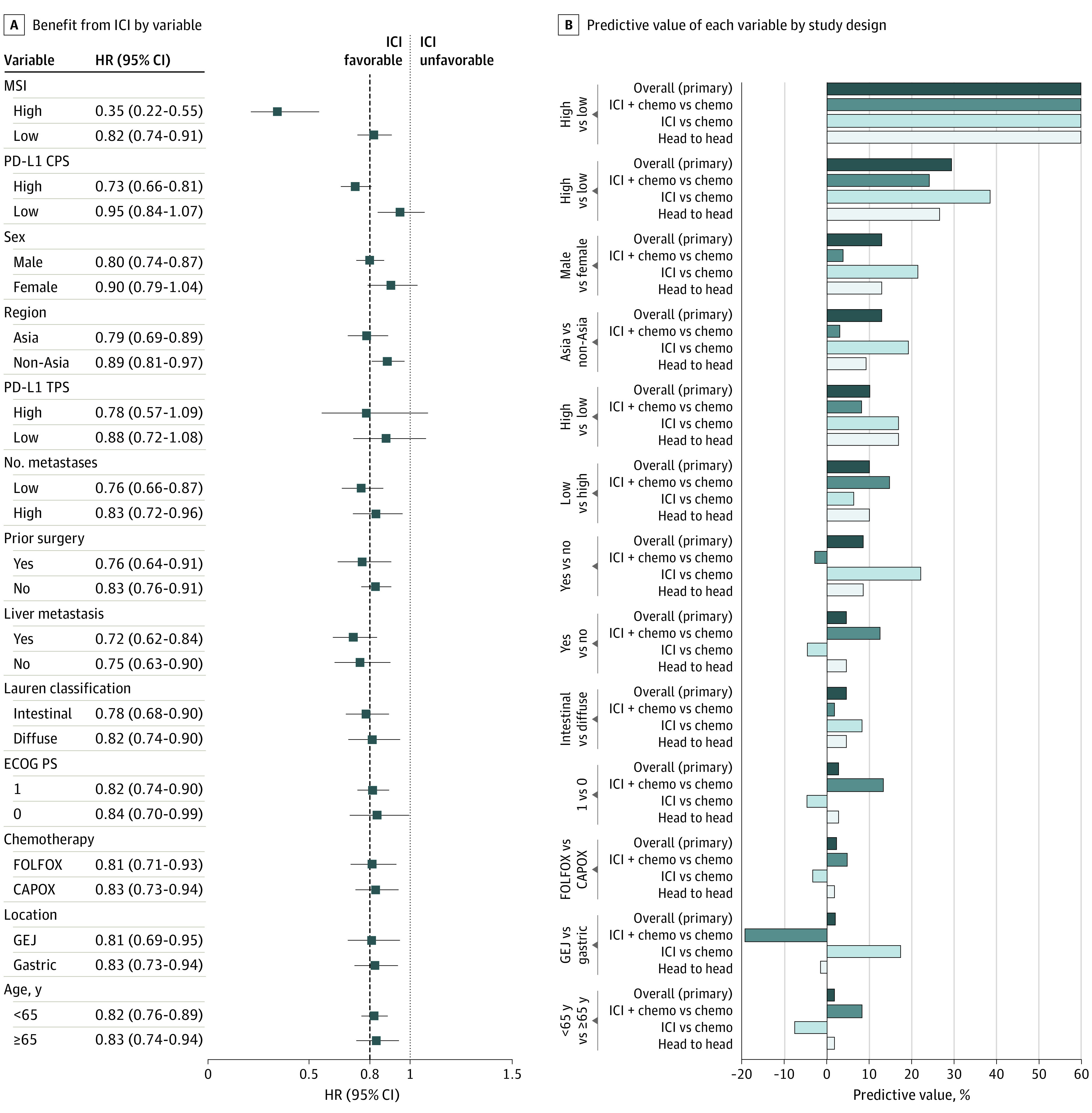
A, A meta-analysis of individual trials with pooled (mean) HRs for OS and 95% CIs shown for each subgroup variable. The dashed line denotes the ASCO-recommended HR of 0.80. B, The bar graphs indicate each variable’s predictive value (ie, ratio of mean HRs across each level of the variable). The predictive value was calculated in all trials (primary analysis) as well as in 3 sensitivity analyses—ie, the subset of trials that compared ICI plus chemotherapy vs chemotherapy and that compared ICI vs chemotherapy, and the subset of trials amenable to head-to-head analysis (those that reported OS outcomes for both levels of each variable): 135.8% overall, 116.2% in ICI plus chemotherapy vs chemotherapy (single trial), 167.0% in ICI vs chemotherapy, and 153.7% in head-to-head analysis. The Table provides the citation and reference numbers for each trial. AC indicates adenocarcinoma; ASCO, American Society of Clinical Oncology; CAPOX, capecitabine and oxaliplatin; CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group Performance Status scale; FOLFOX, 5-fluorouracil and oxaliplatin; GEJ, gastric esophageal junction; HR, hazard ratio; ICI, immune checkpoint inhibition; MSI, microsatellite instability; OS, overall survival; and TPS, tumor proportion score.
The second-strongest predictive variable among ACs was PD-L1 CPS (Figure 4). The frequency of CPS of 10 or greater ranged from 25% to 49% (Figure 1). The CPS-high group (n = 2352) was represented by 6 trials (7 comparisons) and the CPS-low group (n = 1543) by 4 trials (5 comparisons) (eTables 8-10 in the Supplement). For primary analysis (eMethods in the Supplement), CPS-high was defined as CPS of 10 of greater (KN-062,15 KN-59018), CPS of 5 of greater (CM-649,8,9 ORIENT-1620), and CPS of 1 of greater (Javelin-100,13 KN-06116; eTable 10 in the Supplement). Overall, the HR for an overall survival benefit from ICI was HR, 0.73 (95% CI, 0.66-0.81) in CPS-high and 0.95 (95% CI, 0.84-1.07) in CPS-low, yielding a predictive value for CPS of 29.4% (vs ≤12.9% for other variables). Sensitivity analyses consistently showed that the predictive value of CPS exceeded that of other variables (eTables 11-13 in the Supplement). Among head-to-head trials of ICI plus chemotherapy vs chemotherapy (KN-062,15 CM-649,8,9 KN-59018), the predictive value for CPS remained the highest (after MSI-high) among variables studied (predictive value of 18.8% vs ≤14.7% for other variables) but less than had been observed in trials comparing ICI vs chemotherapy (eTable 14 in the Supplement). Results were similar using a uniform CPS cut point of 10 (eTables 15-18 in the Supplement). For head-to-head comparisons limited to the 3 trials of ICI plus chemotherapy vs chemotherapy, the pooled HR for overall survival was 0.74 (95% CI, 0.61-0.88) in CPS of 10 or greater and 0.87 (95% CI, 0.76-0.99) in CPS less than 10 (eTable 19 in the Supplement). The proportion of studies that used 1 antibody clone vs another did not appear to differ substantially between the head-to-head analysis groups (eTable 10 in the Supplement). A single trial (CM-6498,9) reported overall survival data for both high and low levels of CPS at more than a single cut point value, or for both CPS and TPS.
PD-L1 TPS in AC had a predictive value of 10.1% overall and 8.1% to 16.9% in sensitivity analyses (Figure 4). After MSI and PD-L1 CPS, the next highest predictive values for ICI benefit overall were sex (12.9% favoring male over female) and region (12.9% favoring Asia over non-Asia), which were not consistently higher than other variables in sensitivity analyses.
Discussion
Although the addition of ICI to SOC or compared with SOC has improved the therapeutic landscape in aGEC, some patients do not benefit, there is an increased risk of toxic effects and financial costs may be higher.24 A challenge is to identify who will and will not benefit. PD-L1 status has emerged as a potentially important predictive variable based on analysis of individual trials. We performed a meta-analysis of large RCTs to assess the degree of ICI benefit stratified by reported baseline variables. We added a novel step to quantify their predictive values (refer to the introductory section of this article for the rationale). Although, to our knowledge, a statistical test to compare predictive values in meta-analysis without individual patient data is not available, we reasoned that an empirical comparison with other variables could provide a less biased framework to assess the predictive value of PD-L1.
First, we assessed the predictive value of histologic subtype, with the caveat that only 2 trials enrolled both SCC and AC. Overall, ICI was associated with a modestly improved overall survival benefit in SCC vs AC, yielding an overall predictive value of 22% that appeared similar irrespective of treatments being compared (ie, ICI plus chemotherapy vs chemotherapy, or ICI vs chemotherapy). PD-L1 expression, particularly TPS, was enriched in SCCs than ACs, consistent with data from patients across multiple disease stages.25
Then we separated analysis of SCCs and ACs.26 Among SCCs, the strongest predictive variables (of 8 studied) were PD-L1 TPS (generally defined at the cut point of 1) and CPS (generally defined at the cut point of 10), which had overall predictive values of 41.0% and 34.3%, respectively. The other variables had predictive values of 16.0% or less. The predictive value of TPS remained consistently higher than that of other variables irrespective of treatments being compared. However, the predictive value of CPS appeared less consistent, exhibiting higher values in trials comparing ICI vs chemotherapy (44.3%) than those comparing ICI plus chemotherapy vs chemotherapy (26.0%). This pattern remained evident in head-to-head analysis of trials comparing ICI plus chemotherapy vs chemotherapy and in the only 2 SCC trials (both comparing ICI plus chemotherapy vs chemotherapy) that examined both TPS and CPS (each at the cut point of 10). These findings support measurement of TPS (not just CPS) in SCC, as well as research in further understanding the biology of tumor cell vs immune cell expression of PD-L1, which may be regulated by distinct mechanisms that can influence sensitivity to anti−PD-1 or PD-L1 therapy.27,28,29,30
We examined which PD-L1 cut point within SCC might be most predictive, with the caveat that adequate data were available only for TPS from 4 trials. TPS at the cut point of 1 (vs 5 or 10) appeared most predictive (predictive value 47% vs 24%-26%). Results were similar irrespective of treatments being compared. A lower (vs higher) TPS cut point might have been more predictive simply owing to diminished statistical power because TPS 5 or greater or 10 or greater was less frequent (30%-36%) than TPS 1 or greater (48%). In this regard, some data suggest that incremental increases in the values of TPS may be associated with improved response to ICI.31 Regardless, among SCC patients with a TPS less than 1, the pooled HR was 0.89 (95% CI, 0.78-1.01). These findings suggest that, to date, TPS at a cut point of 1 may be ideal to select patients with SCC for ICI-containing therapy, although further research is warranted.
Among ACs, the strongest predictive variable (of 13 variables studied) was MSI-high (predictive value >100%). A limitation in using MSI-high for patient selection is its low frequency (approximately 0% in esophageal, gastroesophageal junction AC, or SCC; approximately 3%-10% in gastric cancers).8,32
The second strongest predictive variable among ACs was PD-L1 CPS (predictive value 29.4% overall vs ≤12.9% for other variables) with CPS 10 or greater showing a frequency of 25% to 49%. The predictive value of CPS, using our primary definition of CPS-high and at the uniform cut point of 10, was consistently the highest in sensitivity analyses vs non−MSI variables. It appeared most predictive in trials comparing ICI vs chemotherapy, and less so in trials comparing ICI plus chemotherapy vs chemotherapy. This pattern was confirmed in head-to-head analyses limited to trials comparing ICI plus chemotherapy vs chemotherapy. In that head-to-head analysis, the pooled HR for overall survival for CPS 10 or less was 0.87 (95% CI, 0.76-0.99). There were insufficient data in AC to identify an ideal CPS cut point through meta-analysis. The only trial to report overall survival data for both high and low levels of CPS at more than a single cut point value (CM-6498,9) suggested that predictive values increase as the cut point increases from 1 to 5 to 10.
In AC, PD-L1 TPS appeared less predictive than CPS. The statistical power for analyzing TPS was lower than that for CPS, given the smaller proportion of patients with TPS 1 or greater vs CPS10 or greater. Only a single AC trial (CM-6498,9) reported outcomes by TPS (at a cut point of 1 only) and CPS, and the predictive value for TPS and CPS at the cut point of 10 was 44.2% and 37.9%, respectively.
Attempts have been made to identify what degree of benefit from an anticancer therapy is clinically meaningful. As an example, an American Society of Clinical Oncology research committee identified an HR of 0.80 or less for overall survival corresponding to an improvement of 2.5 to 6.0 months as a minimum efficacy threshold to represent meaningful benefit in a trial (Figures 1-4).33 This conclusion reflected input from disease experts, patient advocates, biostatisticians, the FDA, and others. Of note, this recommendation was originally designed to be primarily applied to individual trials. A limitation of meta-analyses is the challenge of robustly collecting other parameters that are important for clinical decision-making, such as overall survival rates at fixed time points, quality of life, and drug toxicities.
Limitations and Strengths
Although these study findings support considering PD-L1 status to select patients for ICI, notable limitations in PD-L1 testing exist. These include mixed data on interpathologist agreement especially scoring on a continuous scale or in immune cells; before and after analytic issues; and spatiotemporal heterogeneity.34,35,36,37,38,39,40 Available data in gastroesophageal carcinomas indicate high interobserver agreement for PD-L1 at fixed cut points and high agreement between 22C3 and 28-8 clones.41,42 Although data are limited, the main study findings did not appear to result from differing detection antibody clones used in one study vs another.
Strengths of this study include the recent time window that allows the inclusion of the largest number, to our knowledge, of studies in a meta-analysis of this topic, to date; assessment of multiple PD-L1 cut points; inclusion of both histologic subtypes; and distinction between trial designs.23 A limitation is the lack of IPD and of all variables from all trials, and heterogeneity in the lines of therapy between trials.
Conclusions
Tissue-based PD-L1 expression, more than other variables besides MSI-high, identifies varying degrees of benefit from ICI-containing therapy vs SOC in patients with aGEC. A reduced benefit from ICI was observed among patients with absent or low tissue-based PD-L1 expression. These findings support consideration of tissue-based PD-L1 expression status in selecting patients for ICI treatment.
eTable 1. Baseline characteristics, drug regimens, and efficacy endpoints of 17 phase 3 trials in advanced gastroesophageal carcinoma.
eTable 2. Predictive values of SCC vs AC histology for ICI benefit — sensitivity analyses
eTable 3. Predictive values of baseline variables for ICI benefit in trials of squamous cell carcinoma histology in overall analysis
eTable 4. Overall survival benefit from ICI in individual trials of squamous cell carcinoma histology at each level of every variable (data used to generate pooled hazard ratios in overall analysis)
eTable 5. Availability in squamous cell carcinoma of PD-L1 TPS and CPS data across Phase 3 randomized controlled trials
eTable 6. Predictive values of PD-L1 CPS at the primary cutpoint and cutpoint of 10 for ICI benefit in squamous cell carcinoma histology — head-to-head sensitivity analysis in trials comparing ICI plus chemotherapy vs chemotherapy
eTable 7. Predictive value of PD-L1 for ICI benefit in trials of squamous cell carcinoma that reported overall survival for both PD-L1 TPS and CPS
eTable 8. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — overall primary analysis
eTable 9. Overall survival benefit from ICI in individual trials of adenocarcinoma histology at each level of every variable (data used to generate pooled hazard ratios in overall analysis)
eTable 10. Availability in adenocarcinoma of PD-L1 TPS and CPS data across Phase 3 randomized controlled trials
eTable 11. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — comparing ICI plus chemotherapy vs chemotherapy
eTable 12. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — among trials of ICI vs chemotherapy
eTable 13. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — among head to head trials
eTable 14. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — Head-to-head comparisons among trials evaluating ICI + chemotherapy vs chemotherapy
eTable 15. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology – overall analysis
eTable 16. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology comparing ICI + chemotherapy vs chemotherapy
eTable 17. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology — comparing ICI vs chemotherapy
eTable 18. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology — head-to-head comparisons
eTable 19. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology — head-to-head comparisons of ICI + chemotherapy vs chemotherapy
eFigure 1. Screening and Selection Process
eFigure 2. Risk of Bias
eFigure 3. Variables reported in subgroup analysis in association with overall survival
eMethods
eReferences
References
- 1.Ayers M, Nebozhyn M, Cristescu R, et al. Molecular profiling of cohorts of tumor samples to guide clinical development of pembrolizumab as monotherapy. Clin Cancer Res. 2019;25(5):1564-1573. doi: 10.1158/1078-0432.CCR-18-1316 [DOI] [PubMed] [Google Scholar]
- 2.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355(9209):1064-1069. doi: 10.1016/S0140-6736(00)02039-0 [DOI] [PubMed] [Google Scholar]
- 3.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 4.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang Y-K, Chen L-T, Ryu M-H, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-clinical, phase 3 trial. Lancet Oncol. 2022;23(2):234-247. doi: 10.1016/S1470-2045(21)00692-6 [DOI] [PubMed] [Google Scholar]
- 6.Boku N, Satoh T, Ryu MH, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer. 2021;24(4):946-958. doi: 10.1007/s10120-021-01173-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506-1517. doi: 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 8.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40. doi: 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shitara K, Janjigian YY, Moehler MH, et al. Nivolumab (NIVO) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): expanded efficacy, safety, and subgroup analyses from CheckMate 649. J Clin Oncol. 2022;40(4)(suppl):240. doi: 10.1200/JCO.2022.40.4_suppl.240 [DOI] [Google Scholar]
- 10.Doki Y, Ajani JA, Kato K, et al. ; CheckMate 648 Trial Investigators . Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449-462. doi: 10.1056/NEJMoa2111380 [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Xu J, Chen Y, et al. ; ESCORT Study Group . Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832-842. doi: 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 12.Luo H, Lu J, Bai Y, et al. ; ESCORT-1st Investigators . Effect of Camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916-925. doi: 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moehler M, Dvorkin M, Boku N, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966-977. doi: 10.1200/JCO.20.00892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN gastric 300. Ann Oncol. 2018;29(10):2052-2060. doi: 10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shitara K, Van Cutsem E, Bang Y-J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571-1580. doi: 10.1001/jamaoncol.2020.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shitara K, Özgüroğlu M, Bang YJ, et al. ; KEYNOTE-061 investigators . Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, clinical, phase 3 trial. Lancet. 2018;392(10142):123-133. doi: 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 17.Kojima T, Shah MA, Muro K, et al. ; KEYNOTE-181 Investigators . Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138-4148. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 18.Sun J-M, Shen L, Shah MA, et al. ; KEYNOTE-590 Investigators . Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-clinical, phase 3 study. Lancet. 2021;398(10302):759-771. doi: 10.1016/S0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 19.Shen L, Kato K, Kim S-B, et al. RATIONALE 302: randomized, phase 3 study of tislelizumab versus chemotherapy as second-line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J Clin Oncol. 2021;39(15)(suppl):4012. doi: 10.1200/JCO.2021.39.15_suppl.4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Jiang H, Pan Y, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann Oncol. 2021;32(suppl 5):S1331. doi: 10.1016/j.annonc.2021.08.2133 [DOI] [Google Scholar]
- 21.Shen L, Lu ZH, Wang JY, et al. LBA52 Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: first results of the phase III ORIENT-15 study. Ann Oncol. 2021;32(suppl 5):S1330. doi: 10.1016/j.annonc.2021.08.2132 [DOI] [Google Scholar]
- 22.Xu RH, Wang F, Cui C, et al. JUPITER-06: a randomized, double-blind, phase III study of toripalimab versus placebo in combination with first-line chemotherapy for treatment naive advanced or metastatic esophageal squamous cell carcinoma (ESCC). Ann Oncol. 2021;32:S1041. doi: 10.1016/j.annonc.2021.08.1482 [DOI] [Google Scholar]
- 23.Zhao JJ, Yap DWT, Chan YH, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40(4):392-402. doi: 10.1200/JCO.21.01862 [DOI] [PubMed] [Google Scholar]
- 24.McLouth LE, Nightingale CL, Levine BJ, et al. Unmet care needs and financial hardship in patients with metastatic non-small-cell lung cancer on immunotherapy or chemoimmunotherapy in clinical practice. JCO Oncol Pract. 2021;17(8):e1110-e1119. doi: 10.1200/OP.20.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem ME, Puccini A, Xiu J, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23(11):1319-1327. doi: 10.1634/theoncologist.2018-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency; et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169-175. doi: 10.1038/nature20805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti−PD-1 therapy. Clin Cancer Res. 2014;20(19):5064-5074. doi: 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti−PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi T, Ward JP, Gubin MM, et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res. 2017;5(2):106-117. doi: 10.1158/2326-6066.CIR-16-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Crabill GA, Pritchard TS, et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. 2019;7(1):305. doi: 10.1186/s40425-019-0770-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 32.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis LM, Bernstein DS, Voest EE, et al. American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol. 2014;32(12):1277-1280. doi: 10.1200/JCO.2013.53.8009 [DOI] [PubMed] [Google Scholar]
- 34.Rimm DL, Han G, Taube JM, et al. A Prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3(8):1051-1058. doi: 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Büttner R, Gosney JR, Skov BG, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35(34):3867-3876. doi: 10.1200/JCO.2017.74.7642 [DOI] [PubMed] [Google Scholar]
- 36.Koomen BM, Voorham QJM, Epskamp-Kuijpers CCHJ, et al. Considerable interlaboratory variation in PD-L1 positivity in a nationwide cohort of non-small cell lung cancer patients. Lung Cancer. 2021;159:117-126. doi: 10.1016/j.lungcan.2021.07.012 [DOI] [PubMed] [Google Scholar]
- 37.Cooper WA, Russell PA, Cherian M, et al. Intra- and interobserver reproducibility assessment of PD-L1 biomarker in non-small cell lung cancer. Clin Cancer Res. 2017;23(16):4569-4577. doi: 10.1158/1078-0432.CCR-17-0151 [DOI] [PubMed] [Google Scholar]
- 38.Zhou KI, Peterson B, Serritella A, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res. 2020;26(24):6453-6463. doi: 10.1158/1078-0432.CCR-20-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klempner SJ, Upadhyay V, Chao J. A Space-time continuum for immunotherapy biomarkers in gastroesophageal cancer? Clin Cancer Res. 2020;26(24):6401-6403. doi: 10.1158/1078-0432.CCR-20-3389 [DOI] [PubMed] [Google Scholar]
- 40.Kim R, An M, Lee H, et al. Early Tumor-immune microenvironmental remodeling and response to first-line fluoropyrimidine and platinum chemotherapy in advanced gastric cancer. Cancer Discov. 2022;12(4):984-1001. doi: 10.1158/2159-8290.CD-21-0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulangara K, Zhang N, Corigliano E, et al. Clinical Utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330-337. doi: 10.5858/arpa.2018-0043-OA [DOI] [PubMed] [Google Scholar]
- 42.Ahn S, Kim KM. PD-L1 expression in gastric cancer: interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod Pathol. 2021;34(9):1719-1727. doi: 10.1038/s41379-021-00823-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics, drug regimens, and efficacy endpoints of 17 phase 3 trials in advanced gastroesophageal carcinoma.
eTable 2. Predictive values of SCC vs AC histology for ICI benefit — sensitivity analyses
eTable 3. Predictive values of baseline variables for ICI benefit in trials of squamous cell carcinoma histology in overall analysis
eTable 4. Overall survival benefit from ICI in individual trials of squamous cell carcinoma histology at each level of every variable (data used to generate pooled hazard ratios in overall analysis)
eTable 5. Availability in squamous cell carcinoma of PD-L1 TPS and CPS data across Phase 3 randomized controlled trials
eTable 6. Predictive values of PD-L1 CPS at the primary cutpoint and cutpoint of 10 for ICI benefit in squamous cell carcinoma histology — head-to-head sensitivity analysis in trials comparing ICI plus chemotherapy vs chemotherapy
eTable 7. Predictive value of PD-L1 for ICI benefit in trials of squamous cell carcinoma that reported overall survival for both PD-L1 TPS and CPS
eTable 8. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — overall primary analysis
eTable 9. Overall survival benefit from ICI in individual trials of adenocarcinoma histology at each level of every variable (data used to generate pooled hazard ratios in overall analysis)
eTable 10. Availability in adenocarcinoma of PD-L1 TPS and CPS data across Phase 3 randomized controlled trials
eTable 11. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — comparing ICI plus chemotherapy vs chemotherapy
eTable 12. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — among trials of ICI vs chemotherapy
eTable 13. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — among head to head trials
eTable 14. Predictive values using the primary PD-L1 CPS cutpoint in trials of adenocarcinoma histology — Head-to-head comparisons among trials evaluating ICI + chemotherapy vs chemotherapy
eTable 15. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology – overall analysis
eTable 16. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology comparing ICI + chemotherapy vs chemotherapy
eTable 17. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology — comparing ICI vs chemotherapy
eTable 18. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology — head-to-head comparisons
eTable 19. Predictive values at PD-L1 CPS cutpoint of 10 in trials of adenocarcinoma histology — head-to-head comparisons of ICI + chemotherapy vs chemotherapy
eFigure 1. Screening and Selection Process
eFigure 2. Risk of Bias
eFigure 3. Variables reported in subgroup analysis in association with overall survival
eMethods
eReferences


