Abstract
BACKGROUND
Metformin is arguably the most commonly prescribed oral hypoglycemic agent for the management of diabetes. Due to the lack of randomized control trials, most of the data pertaining to the clinical course, therapeutic interventions and outcomes of patients with metformin induced toxicity has come from case reports or series.
AIM
To analyse the symptomology, clinical interventions and outcomes of patients presenting with severe metformin toxicity by reviewing the published case reports and series.
METHODS
We performed a systematic search from PubMed, Science Direct, Reference Citation Analysis (https://www.referencecitationanalysis.com/) and Google Scholar databases using the terms “metformin” AND “toxicity” OR “overdose” OR “lactic acidosis” OR “hyperlactatemia”. The inclusion criteria were: (1) Case reports or case series with individual patient details; and (2) Reported toxicity or overdose of metformin in adults, published in the English language. Data regarding baseline demographics, clinical presentation, therapeutic interventions, intensive care unit course and overall outcome were collected.
RESULTS
Two hundred forty-two individual cases were analysed, from 158 case reports and 26 case series, with a cumulative mortality of 19.8%. 214 (88.4%) patients were diabetics on metformin. 57 (23.6%) had acute ingestion, but a great majority (76.4%) were on metformin in therapeutic doses when they developed toxicity. Metformin associated lactic acidosis (MALA) was the most commonly reported adverse effect present in 224 (92.6%) patients. Most of the patients presented with gastrointestinal and neurological symptoms and a significant number of patients had severe metabolic acidosis and hyperlactatemia. The organ support used was renal replacement therapy (RRT) (68.6%), vaso-pressors (58.7%) and invasive mechanical ventilation (52.9%). A majority of patients (68.6%) received RRT for toxin removal, renal dysfunction and correction of MALA. Patients with lowest pH and highest serum lactate and metformin levels also had favourable outcomes with use of RRT.
CONCLUSION
Most of the reported cases were on therapeutic doses of metformin but developed toxicity after an acute deterioration in renal functions. These patients may develop severe lactic acidosis, leading to significant morbidity and need for organ support. Despite severe MALA and the need for multiple organ support, they may have good outcomes, especially when RRT is used. The dose of metformin, serum pH, lactate and metformin levels may indicate the severity of toxicity and the need for aggressive therapeutic measures but may not necessarily indicate poor outcomes.
Keywords: Extracorporeal toxin removal, Haemodialysis, Metformin associated lactic acidosis, Metformin overdose, Renal replacement therapy
Core Tip: Metformin may be associated with significant toxicity, even when used in therapeutic doses, of which metformin associated lactic acidosis is the most commonly reported toxicity. These patients may have favourable outcomes in spite of consumption of high doses, severe acidosis, and high serum lactate and metformin concentrations. Early aggressive supportive care, use of renal replacement therapy for toxin removal and organ support may help in improving outcomes.
INTRODUCTION
Metformin is arguably the most commonly prescribed oral hypoglycemic agent (OHA) for the management of diabetes mellitus (DM). In addition to its hypoglycemic properties, it has the potential to reduce micro and macro vascular complications associated with DM and have a clinically beneficial role in reducing serum lipids levels and body weight[1,2]. Use of metformin in the management of type II DM has been shown to reduce all-cause mortality and risk of cardiovascular complications[3].
The primary mode of action of metformin is to reduce hepatic glucose production. In addition, it also exerts hypoglycemic effect through the neuroendocrine axis, enhancing cellular uptake of glucose and reducing insulin resistance[4]. It is considered a very safe drug and is generally not associated with hypoglycemia. Rarely patients may develop toxicity related to its use. Metformin associated lactic acidosis (MALA) has been defined as serum lactate levels above 5 mmol/L and arterial pH below 7.35 in association with metformin exposure[5]. It is a rare complication with a reported incidence of 1-30 cases per 100000 patient years but is associated with a high mortality rate of 25%-50%[6,7].
Despite severe acidosis, patients with MALA may have good clinical outcomes if it is recognised early and aggressive resuscitative measures are initiated[8]. In addition, certain therapeutic interventions like extracorporeal toxin removal (ECTR), if instituted timely, may improve survival in select patient subgroups and hence, it is currently recommended in patients with severe metformin toxicity[9]. Even though MALA remains the most dreaded complication associated with metformin use, other complications have also been reported that may require hospitalisation. Due to the lack of randomised control trials, most of the data pertaining to the clinical course, therapeutic interventions and outcomes of these patients have come from case reports and case series. Hence, we conducted this scoping review of case reports and series to analyse the symptomology, clinical interventions and outcomes of patients presenting with severe metformin toxicity requiring hospitalisation and acute intervention.
MATERIALS AND METHODS
We performed a systematic search for this review from PubMed, Science Direct, Reference Citation Analysis (https://www.referencecitationanalysis.com/) and Google Scholar databases from January 1, 1975 till December 31, 2021. The search terms used were “metformin” AND “toxicity” OR “overdose” OR “lactic acidosis” OR “hyperlactatemia”. The inclusion criteria were: (1) Case reports or case series with individual patient details; and (2) Reported toxicity or overdose of metformin. Further, it was filtered for the literature published in the English language and on adult (> 18 years) humans. We excluded: (1) Conference abstracts; and (2) Case reports or series which did not have individual biochemical data. The authors screened all the search results to include only the relevant literature for metformin toxicity. Duplicate articles from different search databases were excluded.
All the case reports and case series were evaluated, and the data were extracted for patient demographics, clinical symptomatology, clinical interventions including extracorporeal therapies (ECT), intensive care unit (ICU) course, need for organ support and outcomes. Concomitant use of nephrotoxic drugs including nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting-enzyme inhibitors (ACE-Is), angiotensin II receptor blockers (ARBs), aminoglycoside antibiotics and diuretics was also made. A datasheet for evaluation was further prepared.
Statistical analysis
The prepared datasheet was evaluated by Excel, Microsoft office 2019. Categorical variables were presented as frequency and percentage. Median (interquartile range) or mean ± SD was used for continuous variables. Qualitative correlation statistics were analysed by Chi-square test and Fisher's exact test. A P value of < 0.05 was deemed significant. Unless otherwise indicated, all the statistical analyses were done using SPSS (version 25.0, IBM SPSS Inc., Chicago, IL, United States). Tabulation and final documentation were done using MS Office software (MS office 2019, Microsoft Corp, WA, United States).
RESULTS
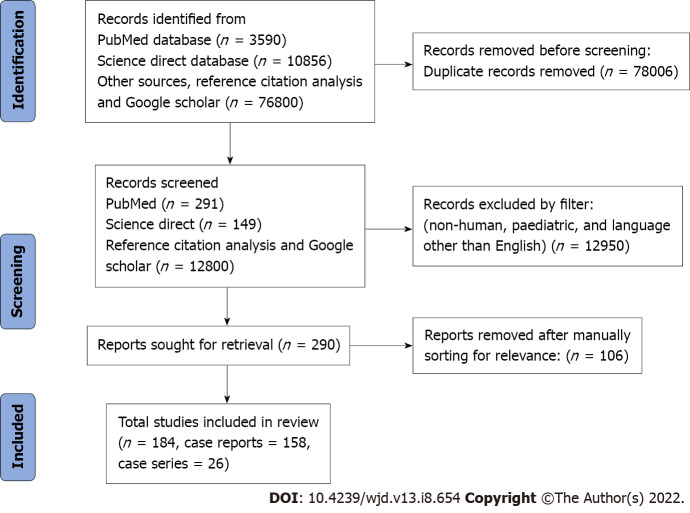
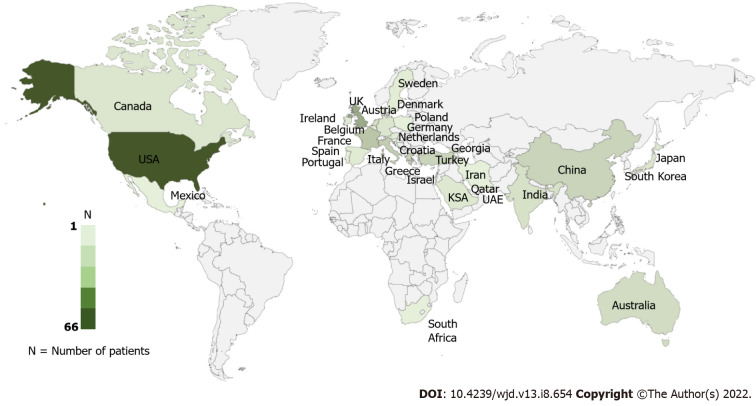
This review was performed using PRISMA 2009 checklist (Figure 1). Finally, 184 studies with 242 unique patients that met all the inclusion criteria were included (Supplementary material). Most of the included patients were from the United States of America (66, 27.3%) and the United Kingdom (30, 12.4%) (Figure 2). 185 (76.4%) patients developed toxicity while on chronic therapeutic doses of metformin while 57 (23.6%) patients developed toxicity after an acute overdose of the drug.
Figure 1.
PRISMA flow diagram of the selected literature for this Meta summary. The inclusion criteria were (1) Case reports or case series with individual patient details; and (2) Reported toxicity or overdose of metformin.
Figure 2.
Geographical distribution of the patients reported with metformin toxicity.
The commonly reported symptoms were gastrointestinal (vomiting 52.5%, abdominal pain 40%) and neurological (altered mental status 36%, loss of consciousness 11.6%). Two hundred fourteen (88.4%) patients had underlying diabetes and were on metformin (Table 1).
Table 1.
The commonly reported symptoms (mean ± SD)
|
Parameter
|
Number of patients (n = 242)
|
| Age | 59.3 (16) yr |
| Gender, n (%) | Females, 126 (52.1) |
| Males, 115 (47.5) | |
| Not mentioned, 1 (0.4) | |
| Clinical presentation, n (%) | Vomiting, 127 (52.5) |
| Abdominal pain, 96 (40) | |
| Altered mental status, 87 (36) | |
| Shock, 43 (17.8) | |
| Breathlessness, 41 (16.9) | |
| Loss of consciousness, 28 (11.6) | |
| Anuria, 22 (8.3) | |
| Cardiac arrest, 5 (2) | |
| Others, 15 (6.2) | |
| Comorbidities, n (%) | Diabetes, 214 (88.4) |
| Hypertension, 94 (38.8) | |
| Coronary artery disease, 34 (14.1) | |
| Chronic kidney disease, 41 (16.9) | |
| Chronic liver disease, 6 (2.5) | |
| Others, 24 (9.9) | |
| None, 22 (9.9) | |
| Not mentioned, 2 (0.8) | |
| History of psychiatric illness, n (%) | 30 (12.4) |
| History of metformin use, n (%) | 214 (88.4) |
| Type of ingestion, n (%) | Chronic use, 185 (76.4) |
| Suicidal, 52 (21.5) | |
| Accidental, 3 (1.2) | |
| Unclear, 1 (0.4) | |
| Not mentioned, 1 (0.4) | |
| Urine toxicology screen, n (%) | 15 (6.2) |
| Time to presentation after acute intoxication (h) | 10.9 ± 13.8 |
| Hypoglycemia, n (%) | 59 (24.4) |
| Therapies to reduce absorption, n (%) | Activated charcoal, 16 (6.6) |
| Gastric lavage, 14 (5.8) | |
| Whole bowel irrigation, 1 (0.4) | |
| Need for organ support, n (%) | RRT, 166 (68.6) |
| Vasopressors, 142 (58.7) | |
| Invasive mechanical ventilation, 128 (52.9) | |
| Extracorporeal membrane oxygenation, 2 (0.8) | |
| Type of RRT, n (%) | Haemodialysis, 83 (34.3) |
| Continuous RRT, 60 (24.8) | |
| Slow low-efficiency dialysis, 13 (5.4) | |
| Peritoneal dialysis, 6 (2.5) | |
| Haem-adsorption columns, 3 (1.2) | |
| Plasmapheresis, 1 (0.4) | |
| Other treatments given, n (%) | Sodium bicarbonate, 158 (65.3) |
| Glucose/insulin, 15 (6.2) | |
| Methylene blue, 2 (0.8) | |
| ECMO, 2 (0.8) | |
| L-carnitine, 1 (0.4) | |
| High dose vitamin C, 1 (0.4) | |
| Development of organ failure, n (%) | Renal, 179 (74) |
| Cardiac, 144 (59.5) | |
| Pulmonary, 114 (47.1) | |
| Neurological, 88 (36.4) | |
| Liver, 18 (17.4) | |
| Haematological, 2 (0.8) | |
| Days on RRT | 3.1 ± 6.7 |
| Days on IMV | 2.2 ± 5.1 |
| Number of sessions of RRT | 2 ± 2.6 |
| Time of initiation of RRT after presentation (h) | 6.3 ± 12.7 |
| Days in hospital | 7.3 ± 11.4 |
| Days in ICU | 4.1 ± 6.6 |
| Outcome, n (%) | Alive, 192 (79.3) |
| Death, 48 (19.8) | |
| Not mentioned, 2 (0.8) |
RRT: Renal replacement therapy; IMV: Invasive mechanical ventilation; ICU: Intensive care unit.
MALA was the most commonly reported adverse effect in 224 (92.6%) patients. Other reported isolated adverse effects were encephalopathy in 6 (2.5%) patients, hepatitis or acute liver failure in 5 (2.1%) patients, hypoglycemia in 4 (1.6%) patients, and psychosis, vitamin B12 deficiency and acute pancreatitis in 1 (0.4%) patient each.
Overall, 57 (23.6%) patients had acute ingestion, out of which 52 were suicidal, three were reported as accidental and in two cases the cause was not reported or was unclear. The reported median dose consumed by these patients was 42.5 gms (interquartile range 24.8–61.5 gms). Out of these 57 cases, there were 11 deaths with a cumulative mortality rate of 19.3%. Sixteen patients with acute intoxications had a history of co-ingestion of other drugs.
The mean duration of presentation after acute intoxication was 10.9 (± 13.8) hours. Activated charcoal (6.6%) and gastric lavage (5.8%) was rarely employed to reduce the absorption of metformin in patients with acute intoxication. Intravenous soda-bicarbonate was most commonly used among the other therapies employed in 65.3% of patients.
Arterial blood gas at presentation was available in 214 (88.4%) patients and serum metformin concentration was measured only in 58 (24%) cases (Table 2).
Table 2.
Arterial blood gas parameters
|
Parameter
|
Mean
|
Standard deviation
|
Range
|
| pH, at presentation | 7.00 | 0.11 | 6.38-7.5 |
| Lactates, at presentation (mmol/L) | 15.7 | 7.6 | 2.1-40.2 |
| Bicarbonate, at presentation (mmol/L) | 7.7 | 6 | 1–23.7 |
| Anion Gap, at presentation | 32 | 10.8 | 10-61 |
| Lowest pH reported | 6.97 | 0.22 | 6.28–7.5 |
| Highest lactates reported (mmol/L) | 18 | 8.6 | 2.4-48 |
| Serum metformin concentration (mcg/mL) | 108.7 | 280 | 0.9-2020 |
Overall, 185 (76.4%) patients were on long-term therapeutic doses of metformin when they developed metformin toxicity. These patients were on metformin doses ranging from 250–3000 mg/day (median 1625 mg/day). The cumulative mortality was 37/185 (20%) in this group of patients. Out of these 185 patients, 38 patients had underlying chronic kidney disease (CKD) and 73 patients had documented reasons, which may have caused acute renal dysfunction precipitating metformin toxicity. These reasons included acute gastroenteritis leading to dehydration (36 patients), ACE-I inhibitors (22 patients), NSAIDs (18 patients), diuretics (16 patients), ARBs (8 patients), IV contrast (7 patients), post-operative (5 patients), acute urinary tract infection (5 patients), anti-retroviral drugs like tenofovir (4 patients), aminoglycosides (2 patients), and obstructive uropathy (1 patient). Several patients had multiple risk factors for acute kidney injury.
Renal dysfunction was the most common organ dysfunction (74%), followed by cardiac (59.5%) and pulmonary (47.1%). One hundred sixty-six (68.6%) patients underwent renal replacement therapy (RRT) for underlying renal dysfunction, metabolic acidosis correction, or metformin removal. Intermittent haemodialysis (IHD, 34.3%) was the most commonly employed method of RRT followed by continuous RRT (CRRT, 24.8%), which included both continuous veno-venous hemofiltration and continuous veno-venous hemodiafiltration. Overall, 41 (16.9%) patients had underlying CKD and were already on dialysis support. Only 17 survivors, who did not have pre-existing CKD, required RRT after hospital discharge, rest all showed complete recovery of renal function.
DISCUSSION
This review evaluated data of 242 individual patients from 158 case reports and 26 case series. Most of the patients had gastrointestinal or neurological symptoms at presentation. A great majority of patients (76.4%) developed metformin toxicity on chronic therapeutic doses. The most commonly reported side effect was MALA (92.6%). These patients had severe metabolic acidosis with hyperlactatemia and required multi organ support. RRT was employed in 68.6% of patients, and the cumulative mortality rate was 19.8%.
Several other isolated serious complications, in the absence of MALA, were also reported in many case reports. These included encephalopathy[10-14], psychosis[15], vitamin B12 deficiency[16], acute pancreatitis[17] and acute liver failure[18-22]. In our analysis, many patients (24.4%) developed hypoglycemia, attributed to sulphonylureas or other OHA that the patients were co-prescribed. However, in a few case reports no other cause could be ascertained and hypoglycemia was attributed to metformin toxicity[23-26]. The reported incidence for development of moderate to severe hypoglycemia is 60 per 100000 for patients on metformin therapy, with an odds ratio of 1.42[27].
As metformin is primarily excreted by the kidneys, it is generally recommended not to use metformin in patients with underlying renal dysfunction[28]. Nonetheless, in our review we observed that 16.9% patients who developed metformin related side effects had underlying CKD. However, emerging literature supports the use of metformin in patients with mild to moderate renal dysfunction, when used in reduced dosage and regular monitoring[29].
Even though renal dysfunction is a major contributing factor for metformin toxicity, other factors like hypotension, dehydration, sepsis, ischemia, and liver impairment, which lead to increased production or impaired clearance of lactates, may also contribute to lactic acidosis. In our analysis, most of the patients on therapeutic doses of metformin who developed toxicity had some insult causing acute renal dysfunction which could have precipitated metformin toxicity. This acute insult included concomitant use of nephrotoxic drugs, acute infection, post-operative state or dehydration secondary to severe diarrhoea. Hence, it may be suggested that patients on long-term metformin should be closely monitored for the development of any side-effects in case of any acute renal insult, and concomitant use of nephrotoxic drugs should be avoided in these patients.
Patients with MALA had severe metabolic acidosis and hyperlactatemia, with the reported nadir of pH being 6.28[30]. The mainstay of therapy for MALA remains early aggressive resuscitation and organ support, as there is no specific antidote. In our analysis, intravenous soda-bicarbonate was used in a large proportion of patients (65.3%). Even though it may help in the correction of acidosis, it may lead to electrolyte imbalance and fluid overload. In addition, it does not help in the correction of the underlying cause. However, it may be reasonable to use intravenous bicarbonate in patients with severe acidemia and patients with an arterial pH less than 7.20 in the presence of underlying cardiovascular disease or hemodynamic compromise[31].
Low serum pH levels, and high lactate and metformin concentration have been associated with severe toxicity and higher mortality[32]. However, in our review, the patients with the lowest pH and highest serum lactate and metformin levels survived. All three patients with acute ingestion of massive doses of metformin (more than 100 g) survived after the institution of ECTR with complete recovery of renal functions[33-35]. In addition, patients with the highest serum metformin levels (2020 and 678 mcg/mL)[36,37] and patients with the highest presenting lactate levels (40.2 and 35.3 mmol/L)[38,39] also had favourable outcomes. The lowest pH reported was 6.28, in a post-operative diabetic CKD patient on prolonged metformin therapy, who survived after aggressive intensive care[30]. Similar findings have also been reported by other authors who found that blood pH, lactate and metformin levels were poor predictors of mortality in patients with MALA[40].
Even though metformin has a relatively large volume of distribution (1-5 L/kg), but its small molecular size (165 Da) and lack of protein binding makes it amenable for removal through ECT and use of ECTR is recommended in the management of patients with severe toxicity[9,41]. The currently recommended indications of ECTR include lactate levels above 20 mmol/L, pH less than 7.0, presence of shock, reduced consciousness or in patients with failure of standard supportive measures. The current recommendations suggest discontinuing ECTR when serum lactate levels fall below 3 mmol/L and the pH becomes more than 7.35[9].
Intermittent HD has been recommended as the RRT modality of choice for ECTR in patients with metformin toxicity as it provides rapid and superior correction of acidemia and removal of metformin and lactates[9]. Lactate clearance may also be enhanced with use of higher effluent rates and high-flux/high-efficiency dialyzers[33,42]. In addition, IHD has wider availability, lesser costs and a better safety profile. Hence, it was the most commonly used mode of ECTR as observed in our review.
CRRT may be used as the second line therapy in patients with haemodynamic instability who cannot tolerate IHD[9]. As many patients in our analysis had haemodynamic instability requiring vasopressor support, CRRT was the second most common mode of RRT, employed in 24.8% patients.
Slow low efficiency dialysis (SLED) is increasingly becoming a popular RRT option, especially in ICU patients as it can achieve rapid and efficient solute clearance while offering good haemodynamic tolerability. This fact was evidenced in our review, where SLED was used in a few patients (5.4%) for initial RRT. There are a few reports of effective use of resin or charcoal based haem-adsorbent filters in managing patients with severe metformin toxicity[43,44]. However, lack of widespread availability, higher cost, limited data regarding their efficacy and risk of complications especially haemolysis, precludes haemoperfusion (HP) using haemadsorption filters from becoming the modality of choice for ECTR. Additionally, as metformin is not protein-bound, HP and plasmapheresis do not offer any advantage over IHD. Peritoneal dialysis is rarely used for ECTR because of inefficient and slow correction of hyperlactatemia and acidosis[9].
A similar meta-summary included 253 cases and reported a cumulative mortality of 17.2% in patients with MALA[45]. The authors reported that non-survivors had significantly higher levels of lactates and metformin. Additionally, lactate levels above 20 mmol/L were significantly associated with mortality. Even though the cumulative mortality rate in our review was 19.8%, which is close to that reported by Yeh et al[45], our review has significant differences. The previous meta-summary had included patients only up to September 2014, so must have missed the recent changes in clinical practices which might have happened after EXtracorporeal TReatments In Poisoning guidelines, released in 2015, recommending ECTR for metformin toxicity[9]. Yeh et al[45], also included conference abstracts from the EMBASE database and included publications in all languages, explaining their relatively higher case numbers[45]. On the other hand, we included only English language papers and excluded conference abstracts. In addition, we also included all patients with metformin toxicity and even those in whom ECTR was not employed.
Strength and limitations
This review compiled 184 global studies involving 242 unique patients who had developed metformin toxicity. In addition, we included only studies with individual patient’s details to compare demographics, therapeutic interventions and outcomes.
The included studies were only case reports and case series without a control arm. Hence, the efficacy and cost-benefit analysis of ECTR compared to pharmacological therapy could not be performed. The studies were heterogeneous, with a high risk of bias and missing data, which may impact the generalisability of the results. As we excluded case reports or series which did not have individual biochemical data, we might have missed some relevant case reports or series.
CONCLUSION
Metformin is associated with significant toxicity, of which MALA is most commonly reported. Most of the reported cases were on therapeutic doses of metformin but developed toxicity after an acute deterioration in renal function. These patients may develop severe lactic acidosis, leading to significant morbidity and need for organ support. However, in spite of severe lactic acidosis and need for multiple organ support they may have good outcomes, especially when RRT is used for toxin removal. The dose of metformin, serum pH, lactate and metformin levels may indicate the severity of toxicity and the need for aggressive therapeutic measures but may not necessarily indicate poor outcomes.
ARTICLE HIGHLIGHTS
Research background
Metformin is arguably the most commonly prescribed oral hypoglycemic agent for the management of diabetes. Due to the lack of randomized control trials, most of the data pertaining to the clinical course, therapeutic interventions and outcomes of patients with metformin induced toxicity has come from case reports or series.
Research motivation
Despite severe acidosis, patients with metformin associated lactic acidosis (MALA) may have good clinical outcomes, if it is recognized early and aggressive resuscitation measures are initiated.
Research objectives
This study aimed to analyse the symptomology, clinical interventions and outcomes of patients presenting with severe metformin toxicity by reviewing the published case reports and series.
Research methods
We performed a systematic search from PubMed, Science Direct, Reference Citation Analysis (https:// www.referencecitationanalysis.com/) and Google Scholar databases using the terms “metformin” AND “toxicity” OR “overdose” OR “lactic acidosis” OR “hyperlactatemia”. The inclusion criteria were case reports or case series with individual patient details; and reported toxicity or overdose of metformin in adults, published in the English language. Data regarding baseline demographics, clinical presentation, therapeutic interventions, intensive care unit course and overall outcome were collected.
Research results
Two hundred forty-two individual cases were analyzed, from 158 case reports and 26 case series, with a cumulative mortality of 19.8%. 214 (88.4%) patients were diabetics on metformin. 57 (23.6%) had acute ingestion, but 76.4% were on metformin in therapeutic doses when they developed toxicity. MALA was the most commonly reported adverse effect present in 224 (92.6%) patients. Patients with lowest pH and highest serum lactate and metformin levels also had favorable outcomes with use of renal replacement therapy.
Research conclusions
Most of the reported cases were on therapeutic doses of metformin but developed toxicity after an acute deterioration in renal function. These patients may develop severe lactic acidosis, leading to significant morbidity and need for organ support. Despite severe MALA and the need for multiple organ support, they may have good outcomes, especially when renal replacement therapy is used. The dose of metformin, serum pH, lactate and metformin levels may indicate the severity of toxicity and the need for aggressive therapeutic measures but may not necessarily indicate poor outcomes.
Research perspectives
Larger trials may be required to identify the risk factors associated with poor outcomes in patients with MALA.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 22, 2022
First decision: May 11, 2022
Article in press: July 25, 2022
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gheshlaghi F, Iran; Neagu TP, Romania; Shen F, China; Swai J, Canada S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Deven Juneja, Institute of Critical Care Medicine, Max Super Speciality Hospital, Saket, New Delhi 110017, India. devenjuneja@gmail.com.
Prashant Nasa, Department of Critical Care Medicine, NMC Specialty Hospital, Dubai 7832, United Arab Emirates.
Ravi Jain, Department of Critical Care Medicine, Mahatma Gandhi Medical College and Hospital, Jaipur 302022, India.
References
- 1.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–827. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 4.Cho YM, Kieffer TJ. New aspects of an old drug: metformin as a glucagon-like peptide 1 (GLP-1) enhancer and sensitiser. Diabetologia. 2011;54:219–222. doi: 10.1007/s00125-010-1986-3. [DOI] [PubMed] [Google Scholar]
- 5.Luft D, Deichsel G, Schmülling RM, Stein W, Eggstein M. Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol. 1983;80:484–489. doi: 10.1093/ajcp/80.4.484. [DOI] [PubMed] [Google Scholar]
- 6.Finkle SN. Should dialysis be offered in all cases of metformin-associated lactic acidosis? Crit Care. 2009;13:110. doi: 10.1186/cc7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajbaf F, Lalau JD. Mortality rate in so-called "metformin-associated lactic acidosis": a review of the data since the 1960s. Pharmacoepidemiol Drug Saf. 2014;23:1123–1127. doi: 10.1002/pds.3689. [DOI] [PubMed] [Google Scholar]
- 8.Friesecke S, Abel P, Roser M, Felix SB, Runge S. Outcome of severe lactic acidosis associated with metformin accumulation. Crit Care. 2010;14:R226. doi: 10.1186/cc9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calello DP, Liu KD, Wiegand TJ, Roberts DM, Lavergne V, Gosselin S, Hoffman RS, Nolin TD, Ghannoum M Extracorporeal Treatments in Poisoning Workgroup. Extracorporeal Treatment for Metformin Poisoning: Systematic Review and Recommendations From the Extracorporeal Treatments in Poisoning Workgroup. Crit Care Med. 2015;43:1716–1730. doi: 10.1097/CCM.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 10.Jung EY, Cho HS, Seo JW, Kim DW, Kim HJ, Chang SH, Park DJ. Metformin-induced encephalopathy without lactic acidosis in a patient with contraindication for metformin. Hemodial Int. 2009;13:172–175. doi: 10.1111/j.1542-4758.2009.00358.x. [DOI] [PubMed] [Google Scholar]
- 11.Kang YJ, Bae EJ, Seo JW, Jeon DH, Cho HS, Kim HJ, Chang SH, Park DJ. Two additional cases of metformin-associated encephalopathy in patients with end-stage renal disease undergoing hemodialysis. Hemodial Int. 2013;17:111–115. doi: 10.1111/j.1542-4758.2012.00698.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanazono A, Takahashi Y, Sanpei Y, Kamada S, Sugawara M. Focal brain lactate accumulation in metformin-induced encephalopathy without systemic lactic acidosis: A case report suggesting mitochondrial vulnerability in lentiform fork sign. eNeurologicalSci. 2021;25:100383. doi: 10.1016/j.ensci.2021.100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon SP, Thomas J. Metformin-associated Encephalopathy in Hemodialysis. Indian J Nephrol. 2019;29:194–196. doi: 10.4103/ijn.IJN_257_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander T, Hallevy H, Ifergane G, Herishanu YO. Metformin-induced encephalopathy without lactic acidosis. Diabet Med. 2004;21:194–195. doi: 10.1046/j.1464-5491.2003.01087.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheng HY, Ko KT, Tzang RF. Metformin-induced first-episode psychosis in patient with polycystic ovary syndrome using drospirenone. Psychiatry Clin Neurosci. 2019;73:196. doi: 10.1111/pcn.12825. [DOI] [PubMed] [Google Scholar]
- 16.Tung ML, Tan LK. Long term use of metformin leading to vitamin B 12 deficiency. Diabetes Res Clin Pract. 2014;104:e75–e76. doi: 10.1016/j.diabres.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 17.Mallick S. Metformin induced acute pancreatitis precipitated by renal failure. Postgrad Med J. 2004;80:239–240. doi: 10.1136/pgmj.2003.011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashmi T. Probable hepatotoxicity associated with the use of metformin in type 2 diabetes. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.04.2011.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nammour FE, Fayad NF, Peikin SR. Metformin-induced cholestatic hepatitis. Endocr Pract. 2003;9:307–309. doi: 10.4158/EP.9.4.307. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L. Metformin as a Rare Cause of Drug-Induced Liver Injury, a Case Report and Literature Review. Am J Ther. 2016;23:e315–e317. doi: 10.1097/MJT.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 21.Cone CJ, Bachyrycz AM, Murata GH. Hepatotoxicity associated with metformin therapy in treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Ann Pharmacother. 2010;44:1655–1659. doi: 10.1345/aph.1P099. [DOI] [PubMed] [Google Scholar]
- 22.Babich MM, Pike I, Shiffman ML. Metformin-induced acute hepatitis. Am J Med. 1998;104:490–492. doi: 10.1016/s0002-9343(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 23.Aldobeaban S, Mzahim B, Alshehri AA. Recurrent hypoglycemia secondary to metformin toxicity in the absence of co-ingestions: a case report. J Med Case Rep. 2018;12:223. doi: 10.1186/s13256-018-1758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Abri SA, Hayashi S, Thoren KL, Olson KR. Metformin overdose-induced hypoglycemia in the absence of other antidiabetic drugs. Clin Toxicol (Phila) 2013;51:444–447. doi: 10.3109/15563650.2013.784774. [DOI] [PubMed] [Google Scholar]
- 25.Joseph CMC. Symptomatic Hypoglycemia During Treatment with a Therapeutic Dose of Metformin. Am J Case Rep. 2021;22:e931311. doi: 10.12659/AJCR.931311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma MP, Kar SK. Surreptitious metformin abuse in anorexia nervosa presenting as periodic hypoglycaemia. Aust N Z J Psychiatry. 2015;49:851–852. doi: 10.1177/0004867415584642. [DOI] [PubMed] [Google Scholar]
- 27.Zitzmann S, Reimann IR, Schmechel H. Severe hypoglycemia in an elderly patient treated with metformin. Int J Clin Pharmacol Ther. 2002;40:108–110. doi: 10.5414/cpp40108. [DOI] [PubMed] [Google Scholar]
- 28.Glucophage Metformin hydrochloride [final printed labelling]. [cited 17 March 2022]. Available from: http://packageinserts.bms.com/pi/pi_glucophage.pdf .
- 29.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonçalves BM, Coelho D. Metformin-associated lactic acidosis: A case reporting a serious complication in the perioperative period. Rev Esp Anestesiol Reanim (Engl Ed) 2019;66:483–486. doi: 10.1016/j.redar.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Sabatini S, Kurtzman NA. Bicarbonate therapy in severe metabolic acidosis. J Am Soc Nephrol. 2009;20:692–695. doi: 10.1681/ASN.2007121329. [DOI] [PubMed] [Google Scholar]
- 32.Dell'Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54:818–823. doi: 10.1016/j.annemergmed.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Akoglu H, Akan B, Piskinpasa S, Karaca O, Dede F, Erdem D, Albayrak MD, Odabas AR. Metformin-associated lactic acidosis treated with prolonged hemodialysis. Am J Emerg Med. 2011;29:575.e3–575.e5. doi: 10.1016/j.ajem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Dell'Aglio DM, Perino LJ, Todino JD, Algren DA, Morgan BW. Metformin overdose with a resultant serum pH of 6.59: survival without sequalae. J Emerg Med. 2010;39:e77–e80. doi: 10.1016/j.jemermed.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 35.Chiew AL, Wright DFB, Dobos NM, McArdle K, Mostafa AA, Newth A, Roberts MS, Isbister GK. 'Massive' metformin overdose. Br J Clin Pharmacol. 2018;84:2923–2927. doi: 10.1111/bcp.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almaleki N, Ashraf M, Hussein MM, Mohiuddin SA. Metformin-associated lactic acidosis in a peritoneal dialysis patient. Saudi J Kidney Dis Transpl. 2015;26:325–328. doi: 10.4103/1319-2442.152498. [DOI] [PubMed] [Google Scholar]
- 37.Ives Tallman C, Zhang Y, Black N, Lynch K, Fayed M, Armenian P. Refractory vasodilatory shock secondary to metformin overdose supported with VA ECMO. Toxicol Rep. 2022;9:64–67. doi: 10.1016/j.toxrep.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avcı D, Çetinkaya A, Karahan S, Oğuzhan N, Karagöz H, Başak M, Erden A. Suicide commitment with metformin: our experience with five cases. Ren Fail. 2013;35:863–865. doi: 10.3109/0886022X.2013.801299. [DOI] [PubMed] [Google Scholar]
- 39.Friesecke S, Abel P, Kraft M, Gerner A, Runge S. Combined renal replacement therapy for severe metformin-induced lactic acidosis. Nephrol Dial Transplant. 2006;21:2038–2039. doi: 10.1093/ndt/gfl011. [DOI] [PubMed] [Google Scholar]
- 40.Kajbaf F, Lalau JD. The prognostic value of blood pH and lactate and metformin concentrations in severe metformin-associated lactic acidosis. BMC Pharmacol Toxicol. 2013;14:22. doi: 10.1186/2050-6511-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juneja D, Singh O. Extracorporeal Therapies: Specific Poisons. In: Singh O, Juneja D. Principles and Practice of Critical Care Toxicology. New Delhi: Jaypee Brothers Medical Publishers, 2019: 274-287. [Google Scholar]
- 42.Liu Y, Ouyang B, Chen J, Chen M, Ma J, Wu J, Huang S, Li L, Liu Z, Guan X. Effects of different doses in continuous veno-venous hemofiltration on plasma lactate in critically ill patients. Chin Med J (Engl) 2014;127:1827–1832. [PubMed] [Google Scholar]
- 43.Liu S, Xu L, Ma J, Huang R, Lin T, Li Z, Liang H, Li S, Li R, Zhang L, Tao Y, Chen Y, Ye Z, Zhang B, Wang W, Xiao H, Liang X, Shi W. High-volume continuous venovenous hemodiafiltration plus resin hemoperfusion improves severe metformin-associated toxicity. J Diabetes Investig. 2018;9:975–978. doi: 10.1111/jdi.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo PY, Storsley LJ, Finkle SN. Severe lactic acidosis treated with prolonged hemodialysis: recovery after massive overdoses of metformin. Semin Dial. 2006;19:80–83. doi: 10.1111/j.1525-139X.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 45.Yeh HC, Ting IW, Tsai CW, Wu JY, Kuo CC. Serum lactate level and mortality in metformin-associated lactic acidosis requiring renal replacement therapy: a systematic review of case reports and case series. BMC Nephrol. 2017;18:229. doi: 10.1186/s12882-017-0640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]