Abstract
In the last decades, a significant increase in the incidence of diabetic kidney disease (DKD) was observed concomitant with rising diabetes mellitus (DM) incidence. Kidney disease associated with DM in children and adolescents is represented by persistent albuminuria, arterial hypertension, progressive decline in estimated glomerular filtration rate to end-stage renal disease and increased cardiovascular and all-cause morbidity and mortality of these conditions. In medical practice, the common and still the “gold standard” marker for prediction and detection of diabetic kidney involvement in pediatric diabetes is represented by microalbuminuria screening even if it has low specificity to detect early stages of DKD. There are some known limitations in albuminuria value as a predictor biomarker for DKD, as not all diabetic children with microalbuminuria or macroalbuminuria will develop end-stage renal disease. As tubular damage occurs before the glomerular injury, tubular biomarkers are superior to the glomerular ones. Therefore, they may serve for early detection of DKD in both type 1 DM and type 2 DM. Conventional and new biomarkers to identify diabetic children and adolescents at risk of renal complications at an early stage as well as renoprotective strategies are necessary to delay the progression of kidney disease to end-stage kidney disease. New biomarkers and therapeutic strategies are discussed as timely diagnosis and therapy are critical in the pediatric diabetic population.
Keywords: Diabetes, Kidney disease, Biomarkers, Microalbuminuria, Therapy, Children
Core Tip: Several reviews in the literature contributed to the pathophysiology, diagnostics and therapeutic options for diabetic kidney disease in pediatric patients. In this review, we reported the latest data regarding novel biomarkers and methods to identify diabetic children and adolescents at risk of renal complications at an early stage as well as renoprotective strategies to delay the progression of kidney disease to end-stage kidney disease.
INTRODUCTION
Diabetes mellitus (DM), a chronic metabolic condition, is characterized by complete or insufficient insulin production. The main form of DM in childhood and adolescence is type 1 DM (T1DM) compared to type 2 DM (T2DM), which is more frequent in adulthood. Within the last 20 years, DM prevalence increased significantly worldwide. In the last decades, we have also assisted in an ascending trend in the prevalence of T2DM in childhood and youth because of the outbreak in juvenile obesity prevalence[1]. T1DM and T2DM have similar symptoms upon diagnosis, and both include polyuria, polydipsia and polyphagia. While obesity and insulin resistance signs (acanthosis nigricans and polycystic ovarian syndrome) are typical hallmarks of T2DM, loss of weight may be present in both types of DM[1].
Both T1DM and T2DM, with lasting inadequate glycemic control, are associated with long-term vascular complications[2] and a significant increase in mortality, especially in those who develop kidney disease[3]. While DM represents the main worldwide cause of end-stage kidney disease in adults, this is uncommon during childhood[2,3].
Although specific kidney structural changes in DM patients, namely thickening of the glomerular basement membrane and mesangial expansion, appear soon after DM onset (1.5 years to 5.0 years), they are in a clinically silent phase[4]. These structural changes of diabetic kidney injury progress at different rates among T1DM patients, and this is more evident in T2DM cases[4]. Clinical and biological abnormalities (micro/macroalbuminuria) and glomerular filtration rate (GFR) decline will develop over a longer period (10 years to 25 years)[3]. This emphasizes that diabetic kidney disease (DKD) starts early. Therefore, an early diagnosis, intensive monitoring and therapeutic interventions are necessary. Albuminuria and changes in GFR, which are late biomarkers, are the most used tools to assess kidney involvement. Diagnostic strategies for early diagnosis of kidney involvement are necessary.
There are several reviews in the literature that contributed to the pathophysiology, diagnostics and therapeutic options for DKD in pediatric patients. In this work, the state-of-the-art novel biomarkers and methods to identify diabetic children and adolescents at risk of renal complications at an early stage as well as renoprotective strategies to delay the progression of kidney disease to end-stage kidney disease was carried out.
EPIDEMIOLOGY OF DM IN CHILDREN
From 2002 to 2015 the Centers for Disease Control and Prevention reported a 4.8% increase per year for T1DM and a 1.9% increase per year for T1DM in youths aged < 20 years[5]. A very recent study, comprising six areas of the United States from 2001 to 2017, reported an important increase in estimated prevalence for both T1DM and T2DM (T1DM from 1.48 to 2.15 per 1000 youths < 19 years and T2DM from 0.34 to 0.67 per 1000 youths among those aged 10-19 years)[6]. Up-to-date research that included a large cohort of Hungarian children and teenagers during the period 2001 to 2016 (covering 16 years), showed that T1DM is still the most common type, and its prevalence is rising, with a significant male predominance (male/female ratio: 1.25). Also, there is a high prevalence of T2DM, affecting more females every year (female/male ratio: 2.86)[7]. A Danish study showed no increase in T2DM prevalence in children and adolescents[8], while in the United Kingdom a rising incidence and prevalence of T2DM have been observed in youths, especially in some ethnicities[9].
Contributing risk factors to this major increase in incidence are obesity, race, ethnicity, exposure to maternal obesity and diabetes as well as exposure to environmental contaminants[6]. There is an increased morbidity and mortality rate, mainly in T1DM and in those with early T2DM onset. According to Rhodes et al[10], a considerably lower life expectancy (approximately 15 years) was observed in the diabetic group compared to the general population of children without diabetes[10]. A significantly shorter life expectancy was reported in children developing T1DM before 10 years of age (loss of 17.7 years for females vs 14.0 years for males) compared with those diagnosed at 25-30 years (loss of 10.0 years for females and 9.4 years for males)[11]. There is a double cardiovascular risk in pediatric diabetes that triggers early cardiovascular mortality and a four-fold higher mortality rate for all causes in youth[12]. In a nationwide Swedish study of patients with T1DM, age before 10 years at diabetes onset, was the most important risk factor for survival and cardiovascular disease (coronary heart disease and acute myocardial infarction) in their early adult years, especially in females (2-3-fold higher vs males)[13].
DM represents the main cause of end-stage renal disease (ESRD) worldwide in adults[14]. Diabetic nephropathy affects 20% (1 in 5) of adults with diabetes[15]. Within the pediatric population, a significant increase in the incidence of DKD was also observed, the prevalence rate being three times higher in 2013 compared to 2002 (1.16% to 3.44%)[16].
A 4-fold higher risk of kidney failure was found in a large cohort of youth with T2DM vs those with T1DM[17]. Also, compared with the control group, those with youth-onset T2DM had a 16-fold higher risk of a kidney disorder, a 23-fold higher risk of severe renal injury and a 39-fold increased risk of ESRD[17]. A multicenter study reported that more than a quarter (28%) of T2DM youth aged under 20 years developed microalbuminuria[18].
PATHOPHYSIOLOGY
Chronic hyperglycemia leads to the occurrence of diabetic nephropathy, retinopathy and neuropathy as well as macrovascular complications (cardiovascular disease: Stroke, coronary artery disease, peripheral vascular disease)[1,19,20]. DKD recognizes four major pathogenic mechanisms: Glomerular damage, tubular injury, inflammation and oxidative stress[21] (Figure 1). In DKD patients there are important alterations in tubules as well as in the interstitium. These findings may pave the way, or they may appear concomitant with glomerular changes[22].
Figure 1.
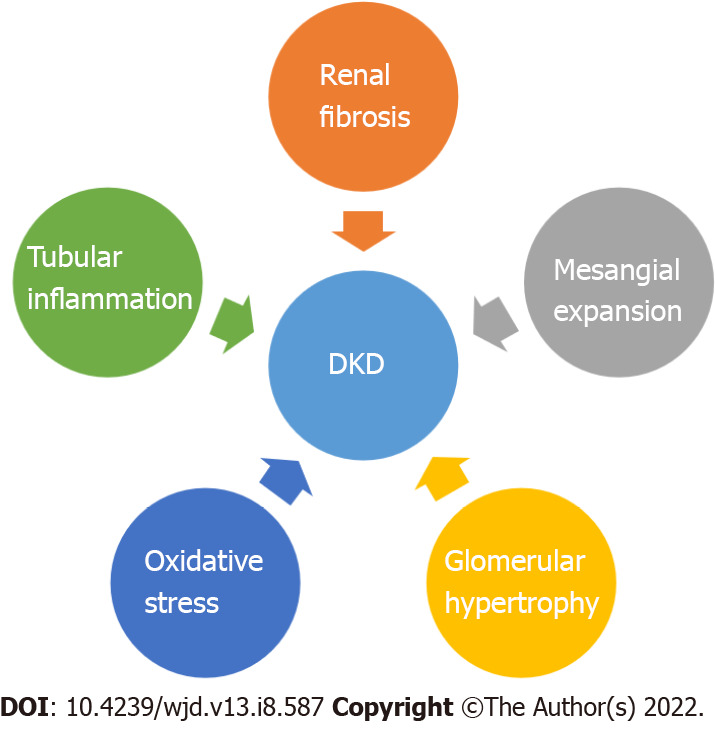
Pathogenesis in diabetic kidney disease. DKD: Diabetic kidney disease.
This is sustained by tubular hypertrophy observed in the immediate future of hyperglycemia. Also, an increase in tubular basement membrane thickening was found even among diabetic patients with normoalbuminuria. Tubular basement membrane is one of the location of the earliest structural changes. Therefore, it may represent a better severity marker of DKD than glomerular basement membrane alteration[22]. Pathological glomerular changes in DKD are typical and consist of glomerular basement membrane thickening, podocyte foot process widening, expansion of the mesangial matrix and loss of endothelial fenestrations[23].
There is a greater risk for complication occurrence in youths with T2DM vs adults with T1DM and T2DM[1]. The main microvascular complication of diabetes is represented by DKD and later by diabetic nephropathy, which finally leads to ESRD. In time, with diabetes evolution, clinical and biological changes will be observed (Figure 2). DKD, one of the most important and frequent complications of DM, recognizes a wide spectrum of risk factors, some of which are modifiable. Therefore, DKD occurrence or evolution may be considerably influenced by strict control of these factors that are listed in Table 1. Children with T1DM may have damaged renal function at the disease onset as acute complications through acute kidney injury (AKI) and renal tubular damage as well as chronic complications by diabetic nephropathy development[24].
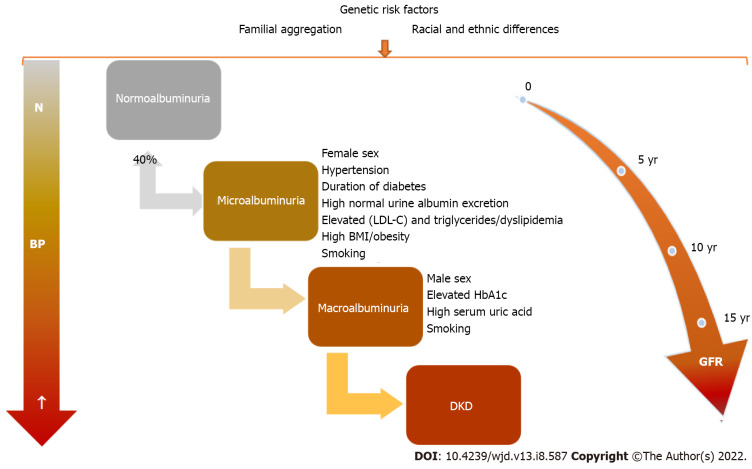
Figure 2.
Changes in diabetic kidney disease: Blood pressure evolution and glomerular filtration rate decline along with albuminuria level. Influence of factors involved in diabetic kidney disease occurrence and progression. N: Normal; DKD: Diabetic kidney disease; BP: Blood pressure; GFR: Glomerular filtration rate; LDL-C: Low-density lipoprotein cholesterol; BMI: Body mass index; HbA1c: Glycated hemoglobin.
Table 1.
Risk factors for diabetic kidney disease development
|
Non-modifiable
|
Modifiable
|
| Small/young age at DM onset | Poor glycemic control |
| Diabetes duration | Glucose variability: Hypo/hyperglycemia |
| Puberty | Overweight/obesity |
| Family history of diabetic complications and insulin resistance | Dyslipidemia |
| Genetic factors | High blood pressure |
| Race/ethnicity | Microalbuminuria |
| Smoking, alcohol | |
| Intrauterine exposure (maternal diabetes, obesity) | |
| Low birth weight |
DM: Diabetes mellitus.
Genetic aspects
DKD is a multifactorial disorder and is influenced by genetic susceptibility, epigenetics and environmental factors (such as lifestyle, diet and medication). Also, oxidative stress, metabolic disturbance, activation of the renin-angiotensin-aldosterone system and production of inflammatory factors are involved in the development and progression of DKD[25]. Genetic and epigenetic studies were performed to understand the pathogenesis of the DKD and to identify genes that confer susceptibility to disease. Genetic studies of DKD investigated mainly the association between genomic DNA variants (for example, single nucleotide polymorphisms, copy number variants, etc) and clinical phenotypes of DKD in both T1DM and T2DM[26]. Epigenetic modifications (histone modifications and DNA methylation) may play a critical role in DKD as it was shown that histone acetylation and methylation are involved in the regulation of inflammation and fibrosis in DKD[27]. Epigenetics studies of DKD investigated the potentially inherited changes in gene expression that occur without changing the DNA nucleotide sequence.
Candidate gene association studies, genome-wide association studies (GWAS) and epigenome-wide association studies were performed in DKD patients[28]. A large meta-analysis study conducted by Mooyaart[29] identified 24 genetic variants in 16 genes (EPO, APOE, APOC1, ACE, ALR2, eNOS, HSPG2, VEGF, FRMD3, GREM1, ELMO1, CCR5 and CNDP1, CARS, UNC13B and CPVL/CHN2), which are the most likely to be associated with diabetic nephropathy[29]. Recently, Tziastoudi et al[30] conducted a systematic review and meta-analysis of genetic association studies in diabetic nephropathy in order to elucidate the contribution of genetic background in the development of this disease and observed an association with the genes revealed by Mooyaart[29] and some additional genes (ACACB, ADIPOQ, AGT, AGTR1, AKR1B1, ATP1B2, ATP2A3, CGNL1, CNDP1, CYGB-PRCD, EDN1, ENPP1, FLT4, FTO, GLO1, HMGA2, IGF2/INS/TH cluster, interleukin genes (IL1B, IL8, IL10), KCNQ1, KNG, LOC101927627, MTHFR, NOS3, SETD7, SIRT1, SLC2A1, SLC2A2, SLC12A3, SLC19A3, TCF7L2, TGFB1, TIMP1, TTC39C, UNC13B, VEGFA, WTAPP1, WWC1, XYLT1)[30].
Genome-wide association studies identified about 30 genes associated with DKD (for example ELMO1, CNDP1, FRMD3, MMP9, UMOD, SLC12A3, etc)[25]. Epigenome-wide association studies identified several genes (for example TRPM6, AQP9, SLC22A12, HP, HYAL2, AGTX) that have epigenetic effects on DKD[25]. The data presented above provide further evidence for the contribution of genetic factors in DKD offering new perspectives in the discovery of new therapies for personalized medicine.
DIAGNOSIS
GFR abnormalities
Hyperfiltration, defined as an increase in GFR with more than 2 standard deviations than the mean GFR value, is related to an early increase in renal blood flow and high intraglomerular pressure[31]. In the first phases of DKD, hyperfiltration is observed in up to 40% of diabetic patients[32]. In both T1DM and T2DM, hyperfiltration has been linked to GFR loss[33,34]. Hyperfiltration was noticed more frequently in females vs males in both T1DM and T2DM[32,35]. The estimated GFR (eGFR) in children and adolescents with T1DM or T2DM should be screened at diagnosis and then annually[36]. These ongoing changes help us to assess DKD stages, which are presented in Table 2[20,21,37]. Normal GFR values according to child age are listed in Table 3.
Table 2.
Diabetic kidney disease stages
|
Stage
|
Estimated period
|
Characteristics
|
GFR
|
BP
|
Biomarker-albuminuria
|
Biomarker UACR mg/mmoL
|
| 1 = hyperfiltration | From diabetes onset to 5 yr | Glomerular hyperfiltration and hypertrophy. No ultrastructure abnormality. A 20% increase in renal size. ↑Renal plasma flow | N/increased | N | Normoalbuminuria < 30 mg/g | < 2 |
| 2 = silent | From 2 yr after onset | Mild GBM thickening and interstitial expansion | N | N | Normoalbuminuria < 30 mg/g | < 3 |
| 3 = incipient | 5–10 yr after onset | More significant changes vs stage 2. Moderate tubular and GBM thickening and variable focal mesangial sclerosis | GFR–N or mild decreased | Increasing BP; +/- hypertension | Microalbuminuria appears Albuminuria 30-300 mg/g | 2-20 |
| 4 = overt | 10-15 yr after onset | Marked GBM thickening and variable focal mesangial sclerosis | GFR-decreased < 60 mL/min/1.73 m2 | BP↑ | Macroalbuminuria > 300 mg/g | > 20 |
| 5 = uremic | Diffuse glomerulosclerosis, ESRD | GFR-marked decreased < 15 mL/min/1.73 m2 | BP↑ | Decreasing albuminuria |
UACR: Urinary albumin to creatinine ratio; GBM: Glomerular basement membrane; GFR: Glomerular filtration rate; BP: Blood pressure; ESRD: End-stage renal disease; ↑: Increase; N: Normal.
Table 3.
Normal glomerular filtration rate limit at different ages according to KDOQI Guidelines[66] and Hogg et al[67]
|
Age
|
Gender
|
Normal GFR
|
| 1 wk | Males and females | 41 ± 15 mL/min/1.73 m2 |
| 2–8 wk | Males and females | 66 ± 25 mL/min/1.73m2 |
| > 8 wk | Males and females | 96 ± 22 mL/min/1.73 m2 |
| 2–12 yr | Males and females | 133 ± 27 mL/min/1.73 m2 |
| 13–21 yr | Males | 140 ± 30 mL/min/1.73m2 |
| 13–21 yr | Females | 126 ± 22 mL/min/1.73m2 |
GFR: Glomerular filtration rate.
Seric and urinary biomarkers for DKD
Common markers for kidney injury are creatinine, albuminuria, cystatin C, neutrophil gelatinase-associated lipocalin and alfa-1-microglobulin in plasma and urine. Kidney function in pediatrics is assessed mainly by eGFR according to updated/bedside Schwarts equation eGFR = k × height (cm)/serum creatinine (mg/dL), k = 0.413[38].
In a recent study, 11.5% of Romanian children with T1DM had DKD, manifested as transitory microalbuminuria (7.7%) and incipient diabetic nephropathy (3.8%)[39]. In another research study, T1DM patients were found to have microalbuminuria in 30% of cases, representing the most common microvascular complication. In T1DM children the occurrence of microvascular complications was correlated with metabolic control, higher glycated hemoglobin, albuminuria, systolic blood pressure (SBP), triglycerides and total cholesterol[40].
Microvascular as well as macrovascular complications can lead to serious morbidity and mortality. Nephropathy (which is preceded by microalbuminuria), retinopathy and neuropathy represent diabetic microvascular complications[2,41]. According to the International Society for Pediatric and Adolescent Diabetes guidelines, annual microalbuminuria or urinary protein screening should start from the age of 11 years and after 2 years of diabetes evolution and then annually. It was demonstrated that persistent microalbuminuria predicts the progression to ESRD and is linked with an increased risk of macrovascular complications occurrence[41].
In T1DM pediatric patients, urine microalbumin to creatinine ratio (UACR) monitoring should start at puberty or 10 years of age (whichever is earlier), and when the child has had DM for 5 years this parameter should be checked annually. In T2DM the UACR should be checked at diagnosis and every year thereafter[36].
In medical practice, the common and still the “gold standard” marker for prediction and detection of diabetic kidney involvement in pediatric diabetes is represented by the microalbuminuria screening[21], even if it has a low specificity and sensitivity to detect early stages of DKD. Microalbuminuria screening should be done annually by timed overnight or 24-h urine collections (albumin excretion rate) or first-morning UACR[41].
Definitions of albuminuria and its abnormalities are based on the International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines[37,41]. Normoalbuminuria is defined as a urine albumin level of ≤ 30 mg/L in all first-morning urine specimens, while microalbuminuria is characterized by the presence of an albumin limit of 30–300 mg or 20–200 μg/min in 24-h urine collection or a value of 30-300 mg/L in at least 2 of 3 first-morning urine specimens. Another parameter, namely UACR of 2.5–25.0 mg/mmol in males or 3.5–25.0 mg/mmol in females in at least 2 of 3 first-morning urine specimens quantifies microalbuminuria. Macroalbuminuria is defined as the presence of > 300 mg/L albumins in at least two first-morning urine specimens[37,41].
There are some limitations in albuminuria value as a biomarker for the prediction and detection of DKD, as not all diabetic children with micro- or macroalbuminuria will present a decrease in kidney function. Also, there are a lot of factors that may influence albuminuria level, UACR and eGFR: Fever, infection, diet, hydration status, hemodynamics, stress, physical activity, periods and hyperglycemia. Furthermore, a significant proportion of cases with microalbuminuria (up to 40%) may return to normoalbuminuria with strict glycemic and blood pressure (BP) control. Therefore, microalbuminuria can be transitory[21].
Microalbuminuria incidence in children with T1DM spans between 3% to 30%[37]. A cross-sectional study that involved children with T1DM reported a 25.0% frequency for microalbuminuria, while macroalbuminuria was found in 3.5% of these cases. The results of the cited study revealed a significantly higher (3 times) prevalence of microalbuminuria in T2DM (68%) compared to T1DM (24%) patients[37]. This is of particular interest given that children with T1DM are already at risk for renal complications secondary to DKD over the long term. A recent study reported early occurrence of microalbuminuria within 2 years of diagnosis of DM in 3.5% (7 of 199) of patients, whereas in 2 of those with microalbuminuria it appeared within the 1st year of diagnosis (in 7 mo)[37].
In a recent study, Hursh et al[24] showed that more than 64% of children hospitalized for DKD developed AKI. The same authors showed that a decreased serum bicarbonate level (< 10 mEq/L) and an increased heart rate are associated with a higher risk of severe AKI[24]. Higher morbidity and mortality rate is encountered in diabetic children that developed AKI along with a higher risk of chronic kidney disease, a finding that is particularly important in these patients who are already at risk for DKD[24].
It is already known that patients with DM may present with kidney damage (decrease in GFR) but without micro- or macroalbuminuria[42]. Therefore, other biomarkers that precede albuminuria should be considered more reliable to predict renal lesions, especially in the early stages. However, most of these biomarkers still need validation in clinical practice[43].
As tubular damage occurs before the glomerular injury, tubular biomarkers are superior to the glomerular ones, namely microalbuminuria. Therefore, they may serve for early detection of DKD in both T1DM and T2DM[44]. Tubulointerstitial damage may be suggested by the urinary albumin-to-creatinine to total protein-to-creatinine ratio of 0.40, with high sensitivity and specificity[45].
In patients without glomerular involvement, low-molecular-weight (LMW) proteinuria or non-albumin proteinuria represents an adequate marker of tubular dysfunction[46]. Urinary LMW proteins are absorbed in the proximal tubules so healthy individuals excrete up to 20 mg of LMW proteins/d in urine[46]. Alpha1 microglobulin, beta-2 microglobulins, immunoglobulin light chains, retinol-binding protein, cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-D-glucosaminidase, kidney injury molecule 1 and liver-type fatty acid-binding protein, etc are included in the LMW protein group[46]. In the early period of diabetes, an increase in urinary tubular biomarkers suggests that kidney injury is present[47].
A recent study showed the association of proximal tubule (alpha-1 microglobulin and kidney injury molecule 1) and podocyte (nephrin, vascular endothelial growth factor) damage biomarkers in T2DM even in the normoalbuminuric stage, indicating they may serve for early DKD diagnosis[47].
Urinary NGAL level increases before the onset of microalbuminuria in the very early phase of the kidney disease[48]. Alongside urinary biomarkers of tubular health (NGAL), the oxidative stress biomarker (pentosidine) may be used in the early detection of diabetic nephropathy before the microalbuminuric phase, as they correlate with albumin excretion and loss of nocturnal dipping of SBP and mean arterial BP[49].
Klotho, a transmembrane protein, is composed of a large extracellular and a small intracellular domain. Klotho is highly expressed in the renal tissue, especially in the distal tubules. The extracellular domain is cleaved by membrane proteases and discharged into the bloodstream, urine and cerebrospinal fluid as soluble klotho (s-klotho)[50,51]. A faster decline in eGFR was observed in DKD patients with low levels of serum s-klotho concentrations[52], which was opposite to the results of another study where s-klotho levels did not correlate with eGFR[50]. Bob et al[50] found a direct correlation of s-klotho levels with the rate of eGFR decline and with the serum levels of tubular injury marker kidney injury molecule 1[50]. A recent study found an inverse correlation between the klotho and glycated hemoglobin levels in T1DM children suggesting its possible role in chronic complications of diabetes occurrence[53].
Early stage prediction and recognition of DKD before microalbuminuria occurrence have a pivotal role in providing timely management. In this process, the assessment of more sensitive and specific biomarkers is essential. A new study showed that serum cystatin C may be used as a biomarker for DKD at an early stage in T1DM children with disease duration not exceeding 5 years before albuminuria detection[21]. The same study found a significantly lower eGFR-cystatin C value in diabetic children compared to controls. Also, significantly higher urinary cyclophilin A (CypA) and urinary CypA/ creatinine ratios were found in T1DM children with microalbuminuria compared to the control group or normoalbuminuric subjects[21].
Salem et al[21] observed a better diagnostic value with the highest sensitivity (93.5%), specificity (71.4%) and accuracy (86.7%) to predict microalbuminuria in T1DM children by the combined use of serum cystatin C and urinary CypA than that of urinary CypA alone[21]. CypA, an endogenous cytosolic protein, is expressed mainly by the proximal tubular epithelial cells. A kidney injury is followed by an increase in urinary CypA concentration[21]. CypA level proved an encouraging biomarker for the early stage of diabetic nephropathy in adults with T2DM, and it correlates with the progression of diabetic nephropathy[54-56]. Novel biomarkers (Table 4) were proposed as early predictors of DKD[21,43].
Table 4.
|
Biomarkers
|
|
|||
| Traditional biomarkers | Traditional biomarkers of glomerular injury | Albumin/creatinine ratio eGFR | Lack of specificity and sensitivity | (1) Predict the late stages of DKD; (2) Daily variation in urine albumin/creatinine ratio; and (3) eGFR values may be affected by the patient’s hemodynamics, diet and hydration status |
| Novel biomarkers | Glomerular biomarkers | NF-α, transferrin, Type IV collagen, L-PGDS, IgG, ceruloplasmin, laminin, GAGs, fibronectin, podocalyxin, VEGF | Appear before microalbuminuria | Early predictor of DKD |
| Tubular biomarkers | α-1-microglobulin CysC; KIM-1; NGAL; nephrin; NAG; L-FABP; VDBP; CypA; s-Klotho | Appear before/precede microalbuminuria | (1) Are more sensitive vs new glomerular biomarkers; (2) Early predictors of DKD; and (3) Predictor of DKD progression | |
| Biomarkers of inflammation | Cytokines: TNF-α, IL-1β, IL-18, interferon gamma-IP-10, MCP-1, adiponectin, G-CSF, eotaxins, RANTES or CCL-5, orosomucoid | (1) Precede a significantly increased albuminuria; (2) Correlate positively with albumin excretion rate and intima-media thickness; and (3) May trigger direct renal injury | Predictor of DKD progression | |
| Biomarkers of oxidative stress | Urinary 8oHdG Pentosidine | Predict the development of DKD | ||
L-PGDS: Lipocalin-type prostaglandin D synthase; IgG: Immunoglobulin G; GAGs: Glycosaminoglycans; CysC: Cystatin C; KIM-1: Kidney injury molecule 1; 8oHdG: 8-oxo-7,8-dihydro-2-deoxyguanosine; RANTES: Regulated on activation, normal T cell expressed and secreted; G-CSF: Granulocyte colony-stimulating factor; MCP-1: Monocyte chemoattractant protein 1; IP-10: Induced protein-10; TNF-α: Tumor necrosis factor α; IL: Interleukin; CypA: Cyclophilin A; VDBP: Vitamin D-binding protein; L-FABP: Liver-type fatty-acid binding protein; NAG: N-acetyl-β-D-glucosaminidase; NGAL: Neutrophil gelatinase-associated lipocalin; DKD: Diabetic kidney disease; eGFR: Estimated glomerular filtration rate; VEGF: Vascular endothelial growth factor; CCL-5: Chemokine ligand 5T.
Urinary biomarkers in DKD are crucial as they can indicate the site of injury within the nephron (structural biomarkers) as well as the loss of/reduced function of the nephron (functional biomarkers) and the main pathophysiological pathways (pathophysiological biomarkers)[57]. The proposed functional and/or structural tubular biomarkers might be valuable in the timely detection of DKD[57].
BP in diabetic children
Another important sign of diabetes-related nephropathy is BP measurement. In pediatric T2DM the guidelines recommend BP and UACR evaluation at diagnosis and annually thereafter[58].
An important and modifiable risk factor for the development of DKD is hypertension[59]. Arterial hypertension is an important and frequent risk factor for the appearance of cardiovascular disease in T1DM patients. High BP triggers the development and progression of microvascular complications, namely nephropathy, and retinopathy.
Ambulatory blood pressure measurement is superior to office BP measurements in predicting future cardiovascular events and targeting organ damage[60]. In their study, Shalaby and Shalaby[60] showed an abnormal BP profile for systolic and diastolic BP, with significant loss of nocturnal dipping. A significantly higher frequency of non-dipping patterns was observed in T1DM patients with microalbuminuria[60].
A recent study that comprises 3529 children and adolescents with T1DM revealed impaired BP regulation with elevated systolic BP, nocturnal diastolic BP, mean arterial pressure and diastolic dipping but lower nocturnal systolic dipping[61]. Lurbe et al[62] showed that an increase in nocturnal SBP precedes microalbuminuria occurrence within T1DM children[62].
The non-dipper pattern for SBD is one of the earliest abnormalities in the BP profile detected for children with T1DM. Also, non-dipping status has been associated with kidney damage (renal morphological changes) and hyperfiltration in adolescents with T1DM[63]. Also, the non-dipping status seems to be an early predictor of later nephropathy[63].
Teenagers with T1DM are at risk for hyperfiltration and higher UACR (urinary albumin-to-creatinine ratio), which are biomarkers for early/ incipient nephropathy[35]. A recent meta-analysis found that almost 25% of T2DM patients have arterial hypertension, the male sex being more frequently affected, and that 1 in 4 or 5 children have albuminuria[58].
Mamilly et al[49] found an increased urinary NGAL/creatinine (a marker of tubular injury) and pentosidine/creatinine (a marker of oxidative stress) in subjects with T1DM compared to controls even in the absence of microalbuminuria[49]. The same study reported a high incidence of abnormal BP dipping, which is important because dipping abnormalities may serve as a predictor for vascular complications, especially kidney injury in diabetic individuals[49]. The same study proved that urine NGAL correlates with loss of nocturnal dipping of SBP[49].
Based on these data, ambulatory blood pressure measurement represents the gold standard to assess BP regulation and should be used in all diabetic patients for timely therapeutic intervention to prevent renal and cardiovascular diabetic complications later in life.
PROPHYLACTIC AND THERAPEUTIC STRATEGIES FOR DKD
The well-known strategies, namely rigorous glycemic control, strict BP control and modulation of obesity, still represent the most important tools to prevent and slow down the progression of diabetic nephropathy/the deterioration of renal function. These therapies proved to be effective mainly by targeting the modifiable risk factors for diabetic nephropathy, which are listed in Table 1.
A recent systematic review confirmed that early high doses of vitamin D supplementation in combination with renin-angiotensin-aldosterone system blockers may slow the onset or progression of DKD[64]. Standard and some novel proposed therapies in early-stage or late-stage development of diabetic nephropathy are presented in Table 5[20,64,65].
Table 5.
Common and new therapeutic strategies in diabetic kidney disease
|
Therapy
|
Drug class
|
Aim
|
Mechanism of action
|
DKD result/effect
|
Dose adjustment to eGFR (mL/min/1.73 m2)
|
| Conventional therapies | |||||
| Strict glycemic control (Insulin) | - | HbA1c < 7% | (1) Reduces the risk of microalbuminuria; and (2) Reduces progression of microalbuminuria to macroalbuminuria | Delay DKD progression/risk | GFR = 10–50: Reduce the dose to 75%; GFR < 10: Reduce dose to 50% |
| Dietary protein/phosphate restriction | - | ↓High protein intake | (1) Reduces hyperfiltration; and (2) Slows down/delays the loss of function or progression of diabetic nephropathy in T1DM and T2DM | Lower DKD risk | No restriction. CKD stage 3: 100%-140% of the DRI. CKD stage 4-5: 100%-120% of the DRI |
| Weight loss, increased physical activity | - | (1) Reduces hyperfiltration; and (2) Reduces albuminuria, especially in moderate/severe obesity | Lower DKD risk | No | |
| Antihypertensive therapy | (1) ACEI/ARB/calcium-channel blockers; and (2) ACEI/ARB + calcium-channel blockers | Control of BP | (1) Reduces albuminuria and delays the onset of DN; (2) Prevents progression of DN in microalbuminuric patients; and (3) Reduces the frequency of microalbuminuria in hypertensive normoalbuminuric cases | Delay DKD progression | ARB, calcium channel blockers: No adjustment ACEI: GFR 30-60: Reduce dose to 50%; GFR < 30: Stop |
| Treatment of Dyslipidaemia | (1) Atorvastatin; (2) Fluvastatin; and (3) Osuvastatin | Reduce LDL-C | Reduce albuminuria in patients with DKD receiving RAAS blockers | Reduces CV disease/risk | No |
| Psychological Intervention | (1) Family therapy; (2) Cognitive behavioral therapy; (3) Motivational interviewing; (4) Counselling; (5) Mentoring; and (6) Peer support | Reduce depression | Follow lifestyle adjustment regimens and achieve optimal glucose levels | Delay DKD progression | No |
| Novel therapies | |||||
| Vitamin D analogues | Paricalcitol. Calcitriol | (1) Ameliorates nephropathy by reducing the albuminuria; and (2) Prevent glomerulosclerosis | Delay DKD progression | No | |
| Vitamin D metabolites | Inhibit RAAS and prevent glomerulosclerosis | Delay DKD progression/risk | No | ||
| Uric acid antagonist | Allopurinol | Uric acid antagonist/xanthine oxidase inhibitor | (1) Reduces urinary TGF-β1 in diabetic nephropathy; (2) Reduces albuminuria in T2DM; and (3) Improves endothelial dysfunction | Delay DKD risk/progression | GFR > 50: No adjustment. GFR 30-50: Reduce dose by 50%. GFR < 10: Reduce dose to 30%, longer interval |
| Renin inhibitor | Aliskiren | Block RAAS cascade | Reduces albuminuria and serves as an antihypertensive in T2DM | Delay DKD progression | No |
| Endothelin antagonist or I inhibitor ETA receptor antagonist | Atransetan, avosentan, sparsentan (irbesartan + ETA) | (1) Reduces residual albuminuria in type 2 diabetic nephropathy; (2) Reduces proteinuria in T2DM patients and nephropathy; and (3) Significant proteinuria reduction | Delay/slow DKD progression | Yes | |
| MRA Mineralocorticoid Receptor Antagonists | Spironolactone = nonselective MRA. Eplerenone | ↑Natriuresis | Reduce albuminuria and blood pressure in patients with DN when added to a RAAS inhibitor | Delay DKD risk/progression | GFR > 50: No dose adjustment. GFR 30-50: Reduce dose to 25%, once daily. GFR < 10: No use |
| SGLT2 inhibitors | Empagliflozin, canagliflozin | Glucose-lowering | (1) Improves glycaemic control, reduces fasting blood glucose and HbA1c by increasing urinary glucose excretion; and (2) Reduces the reabsorption of sodium | Delay DKD progression, reduces blood pressure | No |
| GLP-1 agonist | Liraglutide, semaglutide | Stimulates insulin secretion, ↑satiety | Improves glycaemic control | Delay DKD risk/progression | No |
| Exenatide, lixisenatide | Stimulates insulin secretion | Improves glycaemic control | Delay DKD risk/progression | Caution in CrCl < 50 mL/min | |
| DDP-4 inhibitors | Linagliptin, saxagliptin, vildagliptin | Glucose-lowering-preserve the glucagon-like peptide effect | Reduce albuminuria in macroalbuminuric T2DM patients | Delay DKD risk/progression | eGFR < 50 mL/min: Reduce dose by 50%; eGFR < 30 mL/min: Reduce dose by 75% |
| TZD Thiazolidinediones | Rosiglitazone. Pioglitazone | ↓Hepatic glucose production activate peroxisome proliferator-activated receptor-γ to increase tissue insulin sensitivity | (1) Reduce albuminuria in macroalbuminuric T2DM patients; and (2) Lower microalbuminuria and proteinuria | Delay DKD risk/progression | No |
| Aldosterone synthase (CYP11B2) inhibition | Decrease in plasma aldosterone levels | Delay DKD risk/progression | NL | ||
| Anti-inflammatory Compounds | |||||
| CCR2 Antagonists | Emapticap pegol (NOX-E36), CCX-140 | Reduces UACR and HbA1c | In T2DM-delay DKD, DN risk/progression | NL | |
| VAP-1 inhibitors | An adhesion molecule for lymphocytes, regulating leukocyte migration into inflamed tissue | ASP-8232 | Reduces albuminuria in T2DM in CKD | Delay DKD risk/progression | NL |
ETA: Endothelin type A; T2DM: Type 2 diabetes mellitus; DKD: Diabetic kidney disease; UACR: Urine microalbumin to creatinine ratio; HbA1c: Glycated hemoglobin; GFR: Glomerular filtration rate; RAAS: Renin-angiotensin-aldosterone system; eGFR: Estimated glomerular filtration rate; ↓: Decreased; T1DM: Type 1 diabetes mellitus; CKD: Chronic kidney disease; DRI: Dietary reference intake; ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin II receptor blocker; BP: Blood pressure; DN: Diabetic nephropathy; LDL-C: Low-density lipoprotein cholesterol; CV: Cardiovascular; TGF-1: Transforming growth factor 1; MRA: Mineralocorticoid receptor antagonists; SGLT-2: Sodium-glucose cotransporter-2; GLP-1: Glucagon-like peptide 1; CrCl: Creatinine clearance; DPP-4: Dipeptidyl peptidase 4; TZD: Thiazolidinediones; NL: Not listed; CCR2: Chemokine receptor 2; VAP-1: Vascular adhesion protein 1.
CONCLUSION
DKD, the most significant and frequent burden of this metabolic disorder, is still discovered late as microalbuminuria is the most used biomarker for predicting kidney involvement. Novel biomarkers are valuable tools in the detection of kidney damage in the early phases as well as reliable predictors for DKD progression. Therefore, effective therapies may be proposed. Early stage prediction and recognition of DKD in children and adolescents before microalbuminuria occurrence have a pivotal role in preventing the development of and/or progression to irreversible kidney damage and to provide timely management and appropriate treatment by using conventional and novel therapies that may slow the onset or progression of DKD.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 27, 2022
First decision: May 30, 2022
Article in press: July 11, 2022
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He Z, China; Zavaleta MJC, Peru S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
Contributor Information
Carmen Muntean, Department of Pediatrics I, “George Emil Palade” University of Medicine, Pharmacy, Sciences and Technology of Târgu Mures, Târgu Mures 540142, Romania. duicucarmen@yahoo.com.
Iuliana Magdalena Starcea, Department of IVth Pediatrics, University of Medicine and Pharmacy “Grigore T. Popa”, Iasi 700115, Romania.
Claudia Banescu, Center for Advanced Medical and Pharmaceutical Research, University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureș, Mureș, Târgu Mures 540142, Romania.
References
- 1.Zhao L, Long T, Hui A, Zhao R, Long S, Peng W. Type 2 Diabetes Mellitus in Children and Adolescents: Early Prevention and Non-Drug Therapy. J Dia Mell. 2017;7:121–141. [Google Scholar]
- 2.Stoian A, Bacârea A, Moţăţăianu A, Stoian M, Gliga F, Bacârea V, Duicu C, Bănescu C. Vascular Endothelial Growth Factor Insertion/Deletion gene polymorphism in patients with type 2 diabetes and diabetic peripheral polyneuropathy. Rev Romana Med Lab. 2014;22:165–172. [Google Scholar]
- 3.Afkarian M. Diabetic kidney disease in children and adolescents. Pediatr Nephrol. 2015;30:65–74; quiz 70. doi: 10.1007/s00467-014-2796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall CB. Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic? Am J Physiol Renal Physiol. 2016;311:F831–F843. doi: 10.1152/ajprenal.00313.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divers J, Mayer-Davis EJ, Lawrence JM, Isom S, Dabelea D, Dolan L, Imperatore G, Marcovina S, Pettitt DJ, Pihoker C, Hamman RF, Saydah S, Wagenknecht LE. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths - Selected Counties and Indian Reservations, United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2020;69:161–165. doi: 10.15585/mmwr.mm6906a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence JM, Divers J, Isom S, Saydah S, Imperatore G, Pihoker C, Marcovina SM, Mayer-Davis EJ, Hamman RF, Dolan L, Dabelea D, Pettitt DJ, Liese AD SEARCH for Diabetes in Youth Study Group. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017. JAMA. 2021;326:717–727. doi: 10.1001/jama.2021.11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkai L, Kiss Z, Rokszin G, Abonyi-Tóth Z, Jermendy G, Wittmann I, Kempler P. Changes in the incidence and prevalence of type 1 and type 2 diabetes among 2 million children and adolescents in Hungary between 2001 and 2016 - a nationwide population-based study. Arch Med Sci. 2020;16:34–41. doi: 10.5114/aoms.2019.88406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oester IM, Kloppenborg JT, Olsen BS, Johannesen J. Type 2 diabetes mellitus in Danish children and adolescents in 2014. Pediatr Diabetes. 2016;17:368–373. doi: 10.1111/pedi.12291. [DOI] [PubMed] [Google Scholar]
- 9.Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet Med. 2018;35:737–744. doi: 10.1111/dme.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med. 2012;29:453–463. doi: 10.1111/j.1464-5491.2011.03542.x. [DOI] [PubMed] [Google Scholar]
- 11.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 12.Pastore I, Bolla AM, Montefusco L, Lunati ME, Rossi A, Assi E, Zuccotti GV, Fiorina P. The Impact of Diabetes Mellitus on Cardiovascular Risk Onset in Children and Adolescents. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21144928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjörnsdottir S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392:477–486. doi: 10.1016/S0140-6736(18)31506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narres M, Claessen H, Droste S, Kvitkina T, Koch M, Kuss O, Icks A. The Incidence of End-Stage Renal Disease in the Diabetic (Compared to the Non-Diabetic) Population: A Systematic Review. PLoS One. 2016;11:e0147329. doi: 10.1371/journal.pone.0147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med. 2016;165:473–481. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Jick S, Breitenstein S, Michel A. Prevalence of Diabetes and Diabetic Nephropathy in a Large U.S. Commercially Insured Pediatric Population, 2002-2013. Diabetes Care. 2016;39:278–284. doi: 10.2337/dc15-1710. [DOI] [PubMed] [Google Scholar]
- 17.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35:1265–1271. doi: 10.2337/dc11-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Bell RA, Wadwa RP, Palla SL, Liu LL, Kershnar A, Daniels SR, Linder B. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2006;29:1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 19.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117:662–675. doi: 10.1016/j.jfma.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Uwaezuoke SN, Ayuk AC. Diabetic Kidney Disease in Childhood and Adolescence: Conventional and Novel Renoprotective Strategies. EMJ Nephrol. 2020;8:68–77. [Google Scholar]
- 21.Salem NA, El Helaly RM, Ali IM, Ebrahim HAA, Alayooti MM, El Domiaty HA, Aboelenin HM. Urinary Cyclophilin A and serum Cystatin C as biomarkers for diabetic nephropathy in children with type 1 diabetes. Pediatr Diabetes. 2020;21:846–855. doi: 10.1111/pedi.13019. [DOI] [PubMed] [Google Scholar]
- 22.Fu H, Liu S, Bastacky SI, Wang X, Tian XJ, Zhou D. Diabetic kidney diseases revisited: A new perspective for a new era. Mol Metab. 2019;30:250–263. doi: 10.1016/j.molmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hursh BE, Ronsley R, Islam N, Mammen C, Panagiotopoulos C. Acute Kidney Injury in Children With Type 1 Diabetes Hospitalized for Diabetic Ketoacidosis. JAMA Pediatr. 2017;171:e170020. doi: 10.1001/jamapediatrics.2017.0020. [DOI] [PubMed] [Google Scholar]
- 25.Gu HF. Genetic and Epigenetic Studies in Diabetic Kidney Disease. Front Genet. 2019;10:507. doi: 10.3389/fgene.2019.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florez JC. Genetics of Diabetic Kidney Disease. Semin Nephrol. 2016;36:474–480. doi: 10.1016/j.semnephrol.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Lu HC, Dai WN, He LY. Epigenetic Histone Modifications in the Pathogenesis of Diabetic Kidney Disease. Diabetes Metab Syndr Obes. 2021;14:329–344. doi: 10.2147/DMSO.S288500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Lee K, He JC. Genetics and Epigenetics of Diabetic Nephropathy. Kidney Dis (Basel) 2015;1:42–51. doi: 10.1159/000381796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mooyaart AL. Genetic associations in diabetic nephropathy. Clin Exp Nephrol. 2014;18:197–200. doi: 10.1007/s10157-013-0874-9. [DOI] [PubMed] [Google Scholar]
- 30.Tziastoudi M, Stefanidis I, Zintzaras E. The genetic map of diabetic nephropathy: evidence from a systematic review and meta-analysis of genetic association studies. Clin Kidney J. 2020;13:768–781. doi: 10.1093/ckj/sfaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, Joles JA. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol. 2017;28:1023–1039. doi: 10.1681/ASN.2016060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjornstad P, Nehus E, El Ghormli L, Bacha F, Libman IM, McKay S, Willi SM, Laffel L, Arslanian S, Nadeau KJ TODAY Study Group. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am J Kidney Dis. 2018;71:65–74. doi: 10.1053/j.ajkd.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant. 2015;30:1706–1711. doi: 10.1093/ndt/gfv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Dodesini AR, Trevisan R, Bossi A, Zaletel J, Remuzzi G GFR Study Investigators. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35:2061–2068. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovshin JA, Škrtić M, Bjornstad P, Moineddin R, Daneman D, Dunger D, Reich HN, Mahmud F, Scholey J, Cherney DZI, Sochett E. Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with Type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2018;314:F667–F674. doi: 10.1152/ajprenal.00400.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez LN, Wang W, Loomba L, Afkarian M, Butani L. Diabetic kidney disease in children and adolescents: an update. Pediatr Nephrol. 2021 doi: 10.1007/s00467-021-05347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabeen B, Nahar J, Islam N, Azad K, Donaghue K. Risk Factors Associated with Microalbuminuria in Children and Adolescents with Diabetes in Bangladesh. Indian J Endocrinol Metab. 2018;22:85–88. doi: 10.4103/ijem.IJEM_269_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo CE, Man OI, Istrate A, Kiss E, Catana A, Creț V, Șerban RS, Pop IV. Role of Adiponectin and Tumor Necrosis Factor-Alpha in the Pathogenesis and Evolution of Type 1 Diabetes Mellitus in Children and Adolescents. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Samahy MH, Adly AA, Ismail EA, Salah NY. Regulatory T cells with CD62L or TNFR2 expression in young type 1 diabetic patients: relation to inflammation, glycemic control and micro-vascular complications. J Diabetes Complications. 2015;29:120–126. doi: 10.1016/j.jdiacomp.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 41.International Diabetes Federation, 2011. ISPAD. [cited 10 March 2022]. Available from: https://cdn.ymaws.com/www.ispad.org/resource/resmgr/Docs/idf-ispad_guidelines_2011_0.pdf .
- 42.Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: Present and future. World J Diabetes. 2014;5:763–776. doi: 10.4239/wjd.v5.i6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uwaezuoke SN. The role of novel biomarkers in predicting diabetic nephropathy: a review. Int J Nephrol Renovasc Dis. 2017;10:221–231. doi: 10.2147/IJNRD.S143186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uwaezuoke SN, Muoneke VU, Mbanefo NR. Tubular Biomarkers as Diagnostic Tools in Diabetic Kidney Disease: A Review of Published Evidence. Int J Nephrol Kidney Fail. 2018:4. [Google Scholar]
- 45.Smith ER, Cai MM, McMahon LP, Wright DA, Holt SG. The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients. Nephrol Dial Transplant. 2012;27:1534–1541. doi: 10.1093/ndt/gfr708. [DOI] [PubMed] [Google Scholar]
- 46.Thethi TK, Batuman V. Challenging the conventional wisdom on diabetic nephropathy: Is microalbuminuria the earliest event? J Diabetes Complications. 2019;33:191–192. doi: 10.1016/j.jdiacomp.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Petrica L, Vlad A, Gluhovschi G, Gadalean F, Dumitrascu V, Gluhovschi C, Velciov S, Bob F, Vlad D, Popescu R, Milas O, Ursoniu S. Proximal tubule dysfunction is associated with podocyte damage biomarkers nephrin and vascular endothelial growth factor in type 2 diabetes mellitus patients: a cross-sectional study. PLoS One. 2014;9:e112538. doi: 10.1371/journal.pone.0112538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yürük Yıldırım Z, Nayır A, Yılmaz A, Gedikbaşı A, Bundak R. Neutrophil Gelatinase-Associated Lipocalin as an Early Sign of Diabetic Kidney Injury in Children. J Clin Res Pediatr Endocrinol. 2015;7:274–279. doi: 10.4274/jcrpe.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mamilly L, Mastrandrea LD, Mosquera Vasquez C, Klamer B, Kallash M, Aldughiem A. Evidence of Early Diabetic Nephropathy in Pediatric Type 1 Diabetes. Front Endocrinol (Lausanne) 2021;12:669954. doi: 10.3389/fendo.2021.669954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bob F, Schiller A, Timar R, Lighezan D, Schiller O, Timar B, Bujor CG, Munteanu M, Gadalean F, Mihaescu A, Grosu I, Hategan A, Chisavu L, Pusztai AM, Covic A. Rapid decline of kidney function in diabetic kidney disease is associated with high soluble Klotho levels. Nefrologia (Engl Ed) 2019;39:250–257. doi: 10.1016/j.nefro.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological Role of Anti-aging Protein Klotho. J Lifestyle Med. 2015;5:1–6. doi: 10.15280/jlm.2015.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013;28:352–359. doi: 10.1093/ndt/gfs460. [DOI] [PubMed] [Google Scholar]
- 53.Zubkiewicz-Kucharska A, Wikiera B, Noczyńska A. Soluble Klotho Is Decreased in Children With Type 1 Diabetes and Correlated With Metabolic Control. Front Endocrinol (Lausanne) 2021;12:709564. doi: 10.3389/fendo.2021.709564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Saeed GK, El-Deen WAS, Montasr BA, Omar TA, Mohamed DS. Urinary podocalyxin and cyclophilin A: markers for early detection of type 2 diabetic nephropathy. Menoufia Med J. 2019;32:996–1003. [Google Scholar]
- 55.Harun H, Lunesia R, Azmi S. Correlation between urinary Cyclophilin A and urinary albumin levels on diabetic kidney disease. Indones J Kidney Hypertension. 2019;2:10–16. [Google Scholar]
- 56.Amer HMA, Sabry IM, Bekhet MMM, Mohammed RNS. The role of urinary cyclophilin A as a new marker for diabetic nephropathy. Egypt J Hosp Med. 2018;70:1431–1439. [Google Scholar]
- 57.Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrol. 2017;30:701–717. doi: 10.1007/s40620-017-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cioana M, Deng J, Hou M, Nadarajah A, Qiu Y, Chen SSJ, Rivas A, Banfield L, Chanchlani R, Dart A, Wicklow B, Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. Prevalence of Hypertension and Albuminuria in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e216069. doi: 10.1001/jamanetworkopen.2021.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rohani F, Hooman N, Moradi S, Mobarra M, Najafizadeh M, Tatarpoor P. The Prevalence of Pre-hypertension in Children with Type 1 Diabetes Mellitus. Int J Prev Med. 2014;5:S44–S49. [PMC free article] [PubMed] [Google Scholar]
- 60.Shalaby NM, Shalaby NM. Study of ambulatory blood pressure in diabetic children: prediction of early renal insult. Ther Clin Risk Manag. 2015;11:1531–1537. doi: 10.2147/TCRM.S87751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dost A, Bechtold-Dalla Pozza S, Bollow E, Kovacic R, Vogel P, Feldhahn L, Schwab KO, Holl RW Initiative DPV. Blood pressure regulation determined by ambulatory blood pressure profiles in children and adolescents with type 1 diabetes mellitus: Impact on diabetic complications. Pediatr Diabetes. 2017;18:874–882. doi: 10.1111/pedi.12502. [DOI] [PubMed] [Google Scholar]
- 62.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 63.Torbjörnsdotter TB, Jaremko GA, Berg UB. Nondipping and its relation to glomerulopathy and hyperfiltration in adolescents with type 1 diabetes. Diabetes Care. 2004;27:510–516. doi: 10.2337/diacare.27.2.510. [DOI] [PubMed] [Google Scholar]
- 64.Uwaezuoke SN. Vitamin D Analogs Can Retard the Onset or Progression of Diabetic Kidney Disease: A Systematic Review. Front Clin Dia Heal. 2021;2:763844. doi: 10.3389/fcdhc.2021.763844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Idzerda NMA, Pena MJ, de Zeeuw D, Heerspink HJL. Future and Novel Compounds in the Treatment of Diabetic Nephropathy. Springer . 2019 [Google Scholar]
- 66.KDOQI KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS National Kidney Foundation's Kidney Disease Outcomes Quality Initiative. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111:1416–1421. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]