Abstract
Pancreatic cancer (PC) is one of the most common causes of cancer-associated death worldwide, with a low rate of 5-year survival. Currently, the pathogenesis of PC is complicated, with no efficient therapy. Coronavirus disease 2019 (COVID-19) disease caused by severe acute respiratory syndrome coronavirus 2 further exacerbates the challenge of patients with PC. The alteration of gut microbiota caused by COVID-19 infection may impact PC progression in patients via immune regulation. The expression of inflammatory immune mediators such as interleukin (IL)-6, IL-8, and IL-10 has been found to increase in both PC and COVID-19 patients, which is associated with the disease severity and prognostic outcome. Gut microbiome serves as a critical connector between viral infection and PC. It can regulate host systemic immune response and impact the efficacy of immunotherapy. Here, we first demonstrated the features of inflammatory cytokines in both diseases and their impact on disease outcomes. Then, we demonstrated the importance of immunotherapeutic strategies. This includes the immune modulation that targets a single or dual receptors using a single agent or their combinations for the treatment of PC in patients who get infected with COVID-19. Additionally, we explored the possibility of managing the disease by regulating gut microbiome. Overall, modulation of the lung-gut-pancreases axis can boost anti-cancer immunotherapy and reduce adverse prognostic outcomes.
Keywords: COVID-19, SARS-CoV-2, Gut microbiota, Pancreatic cancer, Interleukin-6, Interleukin-8, Interleukin-10, Monoclonal antibodies, Modulatory treatment
Core Tip: Pancreatic cancer (PC) is a leading cause of cancer-associated death worldwide. Currently, the pathogenesis of this disease is complicated without efficient therapy. Coronavirus disease 2019 (COVID-19) disease exacerbates the challenge of PC patients. The gut microbiome serves as a critical connector between viral infection and PC through the regulation of host systemic immune response. Therefore, by targeting the lung-gut-pancreases axis, we can modulate both cytokine storm and inflammation in patients with PC and COVID-19 infection.
INTRODUCTION
Pancreatic cancer (PC) is the leading cause of cancer-associated death globally, only about 9% of patients can survive more than 5 years according to the American Cancer Society's report (February 2021)[1,2]. The major type of PC is pancreatic ductal adenocarcinoma (PDAC), about 90% of all PC cases[3], which is caused by tumor growth of the cell that lines in the pancreatic ducts[4,5]. The pancreatic ducts play a key role in the transportation of pancreas-produced digestive enzymes to the duodenum (the proximal part of the small intestine). This process is critical for digestion[6,7]. Although the pathogenesis of PC is still under intensive investigation, there is a lot of progress has been made. Several factors such as smoking, diabetes, alcohol abuse, and dietary factors have been identified as contributors. They are closely associated with cancer development. Those are the potential factors that contribute to the higher risk of PC development[8-11].
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus infection may worsen the disease progression in patients with PC. Here, we summarize the role of gut microbiota, which functions as an important connector for COVID-19 and PC.
PC AND ITS CLOSE ASSOCIATION WITH GUT MICROBIOME
Accumulating studies showed that gut microbiome plays a vital role in pancreatic diseases, including gut microbiota and their components such as CpG-rich DNAs. For example, dysbiosis of gut microbiota can accelerate the severity of chronic pancreatitis. Chronic pancreatitis is considered as one of the contributing factors that cause PC[12,13]. In addition, the profiles of gut microbiota have been shown to be altered in PC patients compared to that in the cohort controls. However, several studies showed that the treatment with probiotics[14] or synobiotics[15] did not show a significant effect on patients with acute pancreatitis. Recently, a remarkable finding was made by Riquelme et al[16], which showed the diversity and composition of gut microbiome were associated with the survival time of patients with PDAC. PDAC patients who survived more than 5 years showed a higher diversity of gut microbiome. In addition, they found the microbiome contains an intra-tumoral unique microbiome component, including Pseudoxanthomonas, Streptomyces, Saccharopolyspora, and Bacillus clausii, compared to the cohorts who survived less than 5 years[16]. This study also shows that the associated immune signatures are different between the two cohorts. There was a significant positive correlation between CD3+, CD8+, and GzmB+ cells tissue densities and the overall survival of PDAC patients. The causation role of gut microbiome in the survival of PC patients was further verified. The corresponding microbiome from the patient was colonized into the tumor-bearing germ-free mice, respectively. The result showed a similar pattern of survival between the colonized mice and the clinical patient. In detail, the tumor-bearing germ-free mice that were colonized with the microbiome originated from the long-term survival patients had long-term survival. The mice received microbiome that from the short-term survival patients displayed a short-term survival[16]. In addition, tumor-bearing mice who received fecal microbial transplantation (FMT) from long survival patients have a higher number of CD8+ T cells CD8+ T cells, specifically activated T cells (CD8+/IFNγ+ T cells) in the tumor environment, whereas mice that received FMT from short term survival patients had increased infiltration of CD4+FOXP3+ regulatory T cells (Tregs) and myeloid-derived suppressor cells in the tumor. In summary, the abovementioned examples demonstrate that gut microbiome contributes a significant role in the pathogenesis of PC and tumor progression through the mechanism of microbial components modulation and associated change of immune activation. Therefore, alteration of gut microbiome could affect the severity and prognostic outcome of PC.
COIVD-19 INFECTION ALTERNATED GUT MICROBIOME
In the pandemic era of COVID-19, the situation may be even worse for PC patients who get infected by SARS-CoV-2. Besides the major respiratory syndrome, gastrointestinal symptoms such as diarrhea and abdominal pain were also observed, reported, and identified in patients infected with COVID-19[17,18]. In addition, the isolation and detection of SARS-CoV-2 viruses from the gut enterocytes and fecal samples in COVID-19 patients indicated that infection of viruses influences the intestine system. The viruses in the intestine could impact gut microbiome[19]. Notably, angiotensin-converting enzyme II (ACE2) is the important binding receptor for SARS-CoV-2 viruses binding to the host. The ACE2 is broadly expressed in the epithelial cells in the lung, gastrointestinal, vascular endothelial cells, brain, etc. The presence of those ACE2 receptors increases the susceptibility of the abovementioned cells to the virus infection[20]. Meanwhile, the other critical enzyme for viral binding and entrying into the cell is transmembrane protease serine 2 (TMRPSS2), which is also expressed in the small intestinal epithelial cells[18]. Thus, the presence and expression of ACE2 and TMRPSS2 in gastrointestinal epithelial cells provide a physiologic foundation for the interaction between COVID-19 and gut microbiome. What’s more, studies have demonstrated that the components of gut microbiota are closely associated with the expression level of ACE2. For example, some Bacteroides species, such as Bacteroides dorei and Bacteroides thetaiotaomicron, have the properties of downregulating the ACE2 expression in the murine model[21]. This indicates that gut microbiome plays an important role in the expression level of ACE2. Because of that, gut microbiome is important to host susceptibility and immunity during the COVID-19 infection.
In addition, serving as the binding receptor of coronavirus, ACE2 also plays an essential role in the expression of neutral amino acid transporters. Those transporters can be found in the intestine and the compositions of the gut[22]. The alteration of gut microbiome in COVID-19 patients has been investigated by several studies[23-25]. The results from those studies showed that there were an increased level of opportunistic pathogens and a decreased level of commensal symbionts in the gut of COVID-19 patients. Those commensal symbionts possess the properties of the immunomodulatory function. Butyrate-producing microbiota such as Faecalibacterium prausnitzii (F. prausnitzii) (phylum Firmicutes), Eubacterium rectale (phylum Firmicutes), and Bifidobacterium adolescentis (phylum Actinobacteria) are well-known as immunomodulators. They play important role in maintaining intestinal health with anti-inflammatory function[24]. For example, the F. prausnitzii has been demonstrated to display anti-inflammatory function and induce polarization of dendritic cells and the priming of interleukin (IL)-10-producing T cells in the human colon. A study showed a significant association between the decreased level of F. prausnitzii and the severity of COVID-19 disease in the patients[23,24].
Taken all together, the presence of ACE2 and TMRPSS2 in intestinal epithelium cells is the physiological foundation. The impact of gut microbiome on the expression level of ACE2 provided evidence of their association. Plus, the alteration of gut microbiome happened during COVID-19 occurrence. Additionally, the severity of the COVID-19 was shown to be associated with the level of a certain microbiome. All those above-mentioned aspects illustrate that the gut microbiome is closely associated with COVID-19. Gut microbiome could be the connection for the pancreatic patients infected with SARS-CoV-2[26], through the gut-pancreas axis.
PANCREATIC INJURY AND ABNORMALITIES IN COVID-19 PATIENTS
Interestingly, pancreatic injury and abnormalities have been reported in SARS-CoV-2 infected patients. However, the mechanism including the cause-effect needs to be further investigated[27]. The statistical analysis was performed for 1378 SARS-CoV-2 infected patients (including both males and females) ranging from mild to severe infection. The result showed that the increased levels of enzyme amylase in serum were significantly related to the COVID-19 severity and the prognosis of infection[28]. Elevated serum enzyme amylase level is also known as an indicator of pancreatic-associated diseases, such as acute pancreatitis and pancreas inflammation[29]. The serum amylase comes from both salivary amylase and pancreatic amylase. The gut serves as a linkage. Through the gut-blood barrier and peritoneal blood barrier, the salivary amylase and the pancreatic amylase are absorbed into the blood vessel. Therefore, both pancreatic inflammation and leakage or damage of gut epithelium integrity can cause an increase in serum amylase[30,31]. Previous analysis of 351 metastatic PC patients showed that there was a positive association between the increased plasma amylase level and negative prognostic outcomes for PC[32]. Thus, the observation of elevated serum amylase levels from the COVID-19 patients highlights the importance to investigate the crosstalk between the SARS-CoV-2 infection, the pancreatic-associated inflammation, and the gut-associated inflammation.
Another analytical study was conducted by a group using the COVID-19 family database (SARS-CoV, SARS-dORF6, SARS-BatSRBD, and influenza A virus subtype H1N1 included) due to the lack of COVID-19 patient databases. They found an upregulated expression level of several genes, such as CREB1, PTEN, SMAD3, and CASP3 genes in COVID-19 patients. Meanwhile, those genes were also highly expressed in PC. Scientists proposed that there was a potential risk of development of pancreatic severity followed by the SARS-CoV-2 infection[33]. In addition to the data analysis, oncological treatment procedures should be optimized to provide better outcomes for pancreatic patients in the COVID-19 pandemic era, minimizing morbidity and mortality[34,35].
LUNG-GUT-PANCREAS AXIS
Gut microbiota plays an essential role in host health and disease through various mechanisms[36,37]. (1) Gut microbiota serves as an extensive metabolic repertoire to help the absorption of nutrition and to provide an energy source to maintain the host homeostasis and health; (2) Gut microbiota plays a crucial role in drug metabolism under the disease condition to facilitate the drug uptake, distribution, absorption, metabolism, excretion, and toxicity modulation; (3) Gut microbiota plays an important role in fighting against infection from bacteria and viruses; and (4) Gut microbiome contributes to maintaining homeostasis and reducing the dysbiosis caused by variable factors from both the endogenous and exogenous antigens. The gut microbiome plays the aforementioned functions through colonization resistance, immunomodulation, and metabolism.
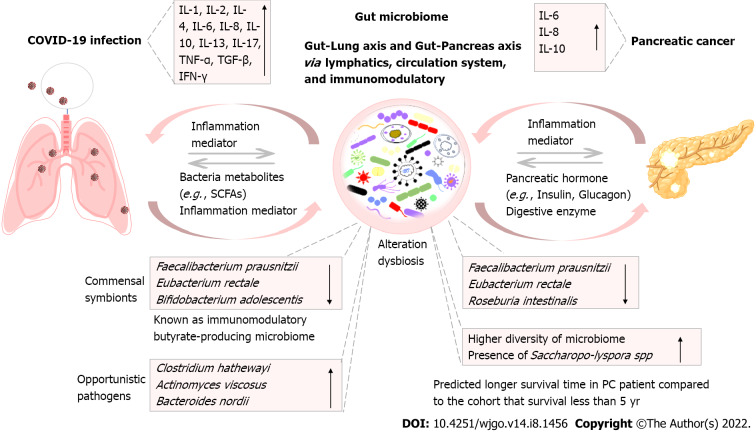
Gut microbiota serves as a central connection between different organs to maintain the balance of the host system[38-40]. The gut-lung axis and gut-pancreas axis are related to each other via lymphatics, circulation system, immunomodulatory, etc. (Figure 1). Diseases such as SARS-CoV-2 virus infection can cause dysbiosis or gut microbiota alteration through inflammation mediators; meanwhile, pancreas diseases such as PC also can lead to the dysbiosis of gut microbiota. That was mediated by pancreatic hormone (e.g., insulin, glucagon) and digestive enzyme. Similarly, the change or disruption of the stability or equilibrium of gut microbiota also can lead to various severity of the disease. This is contributed by the immunomodulators (e.g., inflammatory cytokines) or bacterial metabolites [e.g., short-chain fatty acids (SCFAs)]. As demonstrated and highlighted by the above-mentioned paragraphs, the alteration of gut microbiota in both COVID-19 and PC patients showed a decreased level of F. prausnitzii and E. rectale. Both are known as commensal symbionts. They are also known as butyrate-producing microbiota with important immunomodulatory properties in the host[41,42].
Figure 1.
The gut-lung axis and gut-pancreas axis connect the interaction of lung infection with pancreatic cancer, via altering gut microbiota, systemic inflammation, and immune responses. COVID-19: Coronavirus disease 2019; IL: Interleukin; TGF-β: Transforming growth factor-β; IFN: Interferon; TNF: Tumor necrosis factor; SCFA: Short-chain fatty acid; PC: Pancreatic cancer.
In summary, the host acts as a whole system to fight against disease and to maintain homeostasis and health condition. Therefore, it is essential to better understand the disease features such as the underlying mechanism, the immune response, the outcome of prognosis, and their associations with each other. For example, the alteration of some factors in one disease may complicate the another newly occurred disease. The altered microenvironment may cause an adverse influence on the therapeutic efficacy. Especially, caution should be taken, when it is needed to treat both the initial disease and a newly emerging disease in the same patient.
Here, we focus on the case of SARS-CoV-2 infection in PC patients. By investigating the association, correlation, and underlying mechanism, an optimized therapeutic option could be developed to better facilitate the prevention of both diseases. For PC and COVID-19, an immune response is a critical factor that influences the severity of the disease and prognostic outcome. The microenvironment in PC and the change of associated immune profile may positively/negatively influence the severity of COVID-19 in patients, and vice versa. Gut microbiome, as a mediator between those two diseases, needs to be further explored. This exploration could be considered from the perspective of improving the host systemic immune response and promoting treatment efficacy for both diseases. In the following discussion, we will focus on the commonality of the immune mediator in both disease and immunotherapy treatment strategies. That includes the single target and dual targets of immune mediators using a single agent or combination therapy.
COVID-19 INFECTION INFLUENCES THE SEVERITY OF PC VIA IMMUNE MODULATION
The change of immune profile due to the SARS-CoV-2 infection could impact the severity of PC patients. A study has demonstrated that the increased levels of inflammatory cytokines are detected in the serum of COVID-19 patients compared with that in normal controls, such as increased levels of ILs (IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17), tumor necrosis factor-α (TNF-α), transforming growth factor-β, interferon-gamma (IFN-γ)[43-46]. In PC, higher serum levels of IL-6, IL-8, and IL-10 are strongly associated with the progression of cancer. They are the prediction of poor prognostic outcomes of PC[47]. Thus, the increased levels of IL-6, IL-8, and IL-10 derived from SARS-CoV-2 infection may further complicate the tumor microenvironment of PC patients.
IL-6 was found as an essential factor that promotes the progression of PC. One study illustrated that the depletion of IL-6 abrogated PC progression regardless of the existence of oncogenic Kras (Kirsten rat sarcoma 2 viral oncogene homolog). The study showed that IL-6 is necessary for activation of the reactive oxygen species detoxification program during PC progression[48]. In addition, IL-6 regulates inflammatory response and results in carcinogenesis. Thus, the increasing level of IL-6 in COVID-19 patients has a negative influence on the disease severity of PC patients. Notably, Il-6 serves as a biomarker for predicting the overall severity of the COVID-19 disease[49]. Taken together, the coronavirus infection may cause an even worse situation or poor prognostic outcome for PC patients due to the increased level of IL-6.
IL-8, a neutrophil chemoattractant cytokine with pro-inflammatory function, is broadly produced by monocytes/macrophages[50], smooth muscle cells[51], epithelial cell[52], endothelial cells[53], and other cell types[54]. A study showed that a high serum level of IL-8 was detected in PC patients. That was strongly associated with a higher level of IL-6. Additionally, a higher level of IL-8 showed a significant correlation with a shorter survival time of PC patients (P < 0.001, correlation coefficient value -0.414)[47], which indicated that IL-8 could be one of the important biomarkers for the prediction of prognostic outcome in patients with PC. In vivo study showed that nude mice implanted with tumor tissues from the PC patients with higher serum levels of IL-8 grow tumors faster than the mice implanted with the tumor tissues from the patients with a lower level of serum IL-8[55]. Thus, a higher level of IL-8 serves as a predictor of the worse prognostic outcome of PC. Notably, during the SARS-CoV-2 infection, a remarkably higher level of serum IL-8 was also confirmed by several studies from COVID-9 patients[44,56]. In a study that includes 40 COVID-19 patients, the result showed there was a significantly higher level of IL-8 in non-survival patients compared with that in survival patients. This result suggested an association between IL-8 Levels and the fatal outcome of COVID-19 disease[57]. Another study showed that the IL-8 displayed a better correlation with the clinical score of COVID-19 progression compared to IL-6. The study compared the IL-8 and the IL-6 at different time points. This indicated a possibility of using IL-8 as a biomarker to define disease status[56]. Therefore, IL-8 plays a pivotal role in both PC and COVID-19, especially for PC patients infected with SARS-CoV-2.
IL-10, a controversial immunoregulatory cytokine. Up to date, studies have reported that IL-10 displays both tumor-promoting and anti-tumor functions in cancer. Meanwhile, IL-10 also plays a complicated role in viral infection[58-60]. The elevated IL-10 in the serum of COVID-19 patients has been identified and it showed a close association with the severity of the COVID-19 disease[43,61,62].
Overall, the increased levels of inflammatory cytokines IL-6, IL-8, and IL-10 that resulted from the COIVD-19 infection can facilitate the progression of acute pancreatitis. It further promotes PC progression in the patients[43,63]. Thus, it is important to consider immunotherapy as one of the treatment strategies for PC patients who encounter viral infections. Due to the complicated immune response and the commonality of the elevated levels of IL-6, IL-8, and IL-10 in both patients with COVID-19 or PC, or both, the immune mediators for a single target or dual targets could be used as a therapeutic treatment. The treatment agents could also include a single agent and the combination treatments to better improve therapeutic outcomes.
CLINICAL TREATMENT FOR COVID-19 BY BLOCKADE OF IL-6 AND/OR IL-8 SIGNALING
An anti-inflammatory therapeutic strategy plays an important role in combating viral infections including SARS-CoV-2 infection. Targeting pro-inflammatory cytokines or non-cytokines can be chosen based on their highly elevated levels that are associated with the severity of the disease in patients, as well as the association with prognostic results[64,65]. For instance, targeting IL-6 is an attractive therapeutic option due to its critical role in COVID-19. That has been investigated by multiple studies and was mentioned above[66,67]. Treatment options for blocking IL-6/IL-6 receptors in COVID-19 include monoclonal antibodies and small molecules. Based on the mechanism, they can be divided into three categories: (1) Anti-IL-6 receptor monoclonal antibodies such as Tocilizumab and Sarilumab; (2) Anti-IL-6 monoclonal antibodies such as Siltuximab; and (3) Small molecules such as Furosemide[68-70].
Anti-IL-6 monoclonal antibodies
Multiple clinical trials have been conducted to date with the status of either completed or in progress at different phases. Here, we selected some examples and summarized them in detail in a table (Table 1).
Table 1.
Clinical and pre-clinical studies in coronavirus disease 2019 and pancreatic cancer
|
Disease
|
Antibody/drug
|
Target
|
Title
|
ClinicalTrials.gov identifier
|
Ref.
|
| COVID-19 | Tocilizumab | IL-6 receptor | Efficacy of Tocilizumab on Patients With COVID-19 | NCT04356937 | [75] |
| COVID-19 | Tocilizumab | IL-6 receptor | A Study to Investigate Intravenous Tocilizumab in Participants with Moderate to Severe COVID-19 Pneumonia | NCT04363736 | [76,77] |
| COVID-19 | Tocilizumab | IL-6 receptor | RECOVERY Trial: Open-Label RCT of Tocilizumab and Usual Care in Hospitalized Patients With COVID-19 | NCT04381936 | [78,79] |
| COVID-19 | Sarilumab | IL-6 receptor | Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | NCT04315298 | [80,81] |
| COVID-19 | Sarilumab | IL-6 receptor | Sarilumab COVID-19 | NCT04327388 | [82] |
| COVID-19 | Siltuximab | IL-6 | An Observational Study of the Use of Siltuximab (SYLVANT) in Patients Diagnosed With COVID-19 Infection Who Have Developed Serious Respiratory Complications (SISCO) | NCT04322188 | [83] |
| COVID-19 | Siltuximab | IL-6 | Treatment of COVID-19 Patients with Anti-interleukin Drugs (COV-AID) | NCT04330638 | [84] |
| COVID-19 | Clazakizumab | IL-6 | Study for the Use of the IL-6 Inhibitor Clazakizumab in Patients with Life-threatening COVID-19 Infection | NCT04381052 | None |
| COVID-19 | Clazakizumab | IL-6 | Clazakizumab (Anti-IL-6 Monoclonal) Compared to Placebo for COVID-19 | NCT04348500 | None |
| COVID-19 | Clazakizumab | IL-6 | A Randomized Placebo-controlled Safety and Dose-finding Study for the Use of the IL-6 Inhibitor Clazakizumab in Patients with Life-threatening COVID-19 Infection | NCT04343989 | None |
| COVID-19 | Furosemide | IL-6 and TNF-α | Furosemide as Supportive Therapy for COVID-19 Respiratory Failure | NCT04588792 | [72] |
| COVID-19 | BMS-986253 | IL-8 | Anti-IL-8 for Patients With COVID-19 | NCT04347226 | None |
| Pancreatic cancer | Siltuximab | IL-6 | Siltuximab and Spartalizumab in Patients with Metastatic Pancreatic Cancer | NCT04191421 | None |
| Pancreatic cancer | Bazedoxifene | IL-6 | Bazedoxifene as a Concomitant Treatment of Patients with Metastatic Pancreatic Adenocarcinoma (BAZE) | NCT04812808 | None |
| Pancreatic cancer | Bazedoxifene and Navarixin (SCH527123) | IL-6 and IL-8 | Blocking IL-6 and IL-8 Signaling Inhibits Cell Viability, Colony-forming Activity, and Cell Migration in Human Triple-negative Breast Cancer and Pancreatic Cancer Cells | Pre-clinical research | None |
| Pancreatic cancer | Antibody | IL-6 and PD-L1 | IL-6 and PD-L1 antibody blockade combination therapy reduces tumor progression in murine models of pancreatic cancer | Pre-clinical research | None |
| Pancreatic cancer | Oncolytic vaccinia virus armed with IL-10 | IL-10 | A new role of IL-10 in enhancing the antitumor efficacy of oncolytic vaccinia virus for the treatment of pancreatic cancer | Pre-clinical research | None |
COVID-19: Coronavirus disease 2019; IL: Interleukin; TNF: Tumor necrosis factor; PD-L1: Programmed death 1.
Small molecules are targeted to inhibit IL-6 and TNF-α
Compared to the IL-6 monoclonal antibody that specifically targeted the inflammatory cytokine IL-6, a small molecule has the potential advantage of expanding the targeting range. The treatment targets of the small molecule can be expanded to a broad range for therapeutic efficacy. A preclinical study that aimed to explore the treatment of using small molecules for SARS-CoV-2 infection, was conducted using in silico screening method and molecular simulation. As a result, a potential small molecule, Furosemide, was found to have the function of inhibiting both IL-6 and TNF-α. In addition, this inhibiting function was verified by in vitro experiment assay. Encouragingly, more investigation and evaluation are needed to screen the small molecules with the properties of dual targets such as Furosemide for COVID-19 treatment[71,72].
IL-8 neutralization
The clinical trial of investigation on the effect of using BMS-986253 (neutralization of inflammatory cytokine IL-8) to treat the COVID-19 patients has been approved for recruiting. The investigation is currently ongoing (Phase 2, NCT04347226).
Treatment for PC by blockage of IL-6 and/or IL-8 signaling
For PC, anti-inflammatory therapy has also been investigated in many studies, including both monotherapy and combinational treatments to improve the efficacy. For instance, an in vitro study showed that combinational treatment by blocking both IL-6 (Bazedoxifene) and IL-8 (SCH527123) signaling pathways displayed an enhanced effect on the reduction of cell viability and migration of PC cells[73]. Clinical trials are ongoing to evaluate the treatment efficacy of siltuximab and spartalizumab, such as trials NCT04191421 and NCT04812808 (https://clinicaltrials.gov, accessed on 03/10/2022). The combinational treatment with anti-IL-6R and anti-programmed death 1 (PD-L1)-blocking antibodies showed significant antitumor activity at in vitro cell culture. In vivo study, this combinational treatment improved therapeutic results and extended the survival time of mice with PC compared to controls[74].
It is worthy to point out that there are some treatments in pre-clinical and clinical studies, such as Tocilizumab[75-79], Sarilumab[80-82], Siltuximab[83,84], and others (Table 1). These above-mentioned treatments are either specifically for PC patients or specifically for COVID-19 patients. Less data is available related to the investigation of the treatment efficacy in SARS-CoC-2-infected PC patients. This shed light on the importance of investigating or documenting the clinical data in the field related to COVID-19 treatment options or strategies in PC patients.
FURTHER EXPLORATION OF THE IMPACT OF GUT MICROBIOME ON CYTOKINE SECRETION TO ENHANCE THE TREATMENT EFFICACY
Cytokine storm in COVID-19 and inflammatory cytokines in the pancreatic tumor microenvironment are important factors that exacerbate the disease severity. In addition to directly targeting viruses and tumor cells, the exploration of clinical treatments to reduce the inflammation by targeting interleukins such as IL-6 and IL-8 is also required.
Meanwhile, as illustrated early in this paper, gut microbiome reciprocally impacts the severity of PC and SARS-CoV-2 infection. On one hand, the alteration of gut microbiota in COVID-19 may increase the severity of PC. On the other hand, the alteration of gut microbiota resulting from the PC disease could exacerbate the COVID-19 symptoms, increase the susceptibility to the infection, and influence the recovery process due to the weakened immune response. The reciprocal influence of COVID-19 and PC via the lung-gut-pancreas axis might be mediated by metabolites and immune modulators. Therefore, the modulation of the gut microbiome could provide a better microenvironment. The enhanced microenvironment is beneficial to promote the treatment efficacy through the modulation of microbiota-associated immunity. There are several strategies to modulate the microbiome. For instance, (1) Supplementing with beneficial microbiota such as butyrate-producing bacteria F. prausnitzii with anti-inflammatory and immunoregulatory functions. The decreased abundance of F. prausnitzii was found to be associated with a negative prognosis in both COVID-19 and PC patients; and (2) Modulating the gut microbiota to improve the colonization resistance via immune modulator or metabolism. This could assist to boost the systemic immune resilience and reduce microbial dysbiosis-induced inflammation. For example, commensal bacteria Bifidobacterium longum displayed protective properties against the influenza viruses in a mouse model[85]. Using the fecal microbiota transfer method, scientists transferred the antigen-experienced microbiota from wild mice into germ-free mice. The result showed the enhanced resistance to lethal influenza A virus infection and increased survival in a mouse model[86]. Those studies demonstrated the important roles of gut microbiota conferred against viral infection. Therefore, more investigation is needed to explore and improve host resistance to viruses. In particular, it is necessary to explore the strategy from the perspective of creating a favorited gut microbial environment that is beneficial to the host immune response during viral clarence, disease progression, and treatment efficacy.
From the clinical perspective, accumulating studies and clinical outcomes demonstrated that the gut microbiome influences the response of immune therapy in cancer patients[87-89]. A previous study found that the microbiome, such as Faecalibacterium and Ruminococcaceae, positively correlated with the better outcome of the anti-PD-1 treatment for melanoma cancer[90]. The gut microbiome also influences the efficacy of PD-1 blockade immunotherapy in epithelial tumors. The low level of commensal bacteria Akkermansia muciniphila (A. muciniphila) was identified in the non-response patient. Supplemented with A. muciniphila could alter the nonresponse response to PD-1 blockade treatment. The underlying mechanism is through the modulation of IL-12[91]. The enriched commensal bacteria F. prausnitzii showed close association with a better response to immune therapy. The underlying mechanism is related to the metabolite, SCFA butyrate. F. prausnitzii could produce butyrate through metabolism. The concentration of butyrate (high or low) could modulate the production of IFN-γ and IL-10[92,93], respectively. Most recently, a report showed that gut microbiome Bacteroides, Ruminococcus, and Faecalibacterium were associated with the clinical outcome of anti-CD19 CAR T cell treatment[94]. The above-mentioned examples better illustrated that the microbiome has an impact on the clinical treatment efficacy in cancer patients.
It is worth noticing that the clinical data on PC treatment and the influence of the gut microbiome is limited. However, regardless of what kind of cancer, there are commonalities in immunotherapy between cancers. Plus, there are some shared similarities in the underlying mechanism between cancers. Thereby, the clinical investigation of microbiome influence on the response of PC is urgently needed.
Currently, there is limited clinical data on the relationship between treatment efficacy of COVID-19 and gut microbiome. One reason is that only the infected patients who have critical emergency conditions can be hospitalized due to the pandemic. At this critical stage, life-saving medical care is needed. Another reason is that the medicine for COVID-19 is under development. For clinical trials, most efforts were focused on the evaluation of the effectiveness on a large scale. The effort is limited, especially, for further examining the influence of the associated factors on treatment efficacy.
However, there is accumulating data on the association between the COVID-19 vaccination and gut microbiome. Several clinical trials are ongoing. For example, some clinical trials (NCT04884776 and NCT04798677; Clinicaltrials.gov) are focusing on the investigation of gut microbiome influence on COVID-19 vaccination efficacy[95]. In addition, most recently, a report better demonstrated the association between the gut microbiome and clinical vaccination efficacy. This investigation was performed using shotgun metagenomic sequencing in the vaccinated population. They discovered that a gut microbiome community that facilitates the carbohydrate metabolism is beneficial to the efficacy of COVID-19 vaccination. In people with a higher richness of Bifidobacterium adolescentis, a higher level of neutralizing antibodies was produced when vaccinated with CoronaVac. People with enriched microbiome such as Roseburia faecis showed close association with the BNT162b2 vaccination efficacy[96]. Therefore, the commensal microbiome was correlated with the vaccine-induced neutralization effect. Collectively, gut microbiome plays an important role in host response to the virus (vaccination or treatment). More clinical studies are desired.
The cancer treatment normally causes a weakened immune system in patients. This increases the patient risk and susceptibility to virus infection. Upon the infection, the disease severity could dramatically increase. The management of the clinical care and treatment strategy is a big challenge[97]. Moreover, the application of the COVID-19 vaccine to a cancer patient is another big challenge. The efficacy and safety need to be well-evaluated. Recently, the first safety-related clinical case was reported. The case showed that a cancer patient got the Vaccine-induced thrombotic thrombocytopenia after mRNA-1273 vaccination[98]. In summary, strategies need to be explored to enhance the clinical treatment efficacy for cancer. Meanwhile, exploration should be made to improve the vaccination and treatment efficacy for virus infection. Gut microbiome, serve as an important component in both cancer treatment outcome and vaccination response. Modulation of the gut microbiome could be a potential option to be investigated. The change of microbial environment in the initial disease should be taken into consideration. That consideration helps develop the best options for health care and treatment.
CONCLUSION
Collectively, the reciprocal influence between COVID-19 and PC disease through the cross-link of gut microbiota may pave the way for the exploration of therapeutic options. For instance, the options include the modulation of gut microbiota via dietary intervention, the supplementation of beneficial bacteria, or intake of favored metabolites. Those options can be used to enhance the systemic immune response to battle against both viruses and tumors. The connection of diseases such as COVID-19 and PC through gut microbiota should be investigated to better prepare for a newly emerged disease in the future. Additionally, the efficacy of using synergistic treatment also needs to be explored and evaluated. For instance, it is important to explore the treatment efficacy of using dual agents compared to a single agent. The treatment strategy that aims to target multiple factors in the disease is also favored. For example, in addition to directly controlling the pathogen (e.g. virus), it is also critical to control the inflammation-caused damage (e.g. Cytokine storm). Therefore, the exploitation of diverse treatment strategies is urgently needed, especially, for patients with complex disease situations.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 20, 2022
First decision: April 17, 2022
Article in press: July 5, 2022
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mrzljak A, Croatia; Tang D, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Chun-Ye Zhang, Department of Veterinary Pathobiology, University of Missouri, Columbia, MO 65211, United States.
Shuai Liu, The First Affiliated Hospital, Zhejiang University, Hangzhou 310006, Zhejiang Province, China.
Ming Yang, Department of Surgery, University of Missouri, Columbia, MO 65211, United States. yangmin@health.missouri.edu.
References
- 1.Lam F, Colombet M, Mery L, Pineros M, Znaor A, Soerjomataram I, Bray F, Ferlay J, Ervik M. Global cancer observatory: cancer today. Inter Age Resear Cancer. 2018 [Google Scholar]
- 2.Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang M, Zhang CY. Diagnostic biomarkers for pancreatic cancer: An update. World J Gastroenterol. 2021;27:7862–7865. doi: 10.3748/wjg.v27.i45.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pishvaian MJ, Brody JR. Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer. Oncology (Williston Park) 2017;31:159–166, 168. [PubMed] [Google Scholar]
- 5.Gupta N, Yelamanchi R. Pancreatic adenocarcinoma: A review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World J Gastroenterol. 2021;27:3158–3181. doi: 10.3748/wjg.v27.i23.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk N, Lacy B. Anatomy and Physiology of the Small Bowel. Gastrointest Endosc Clin N Am. 2017;27:1–13. doi: 10.1016/j.giec.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson MA, Campbell-Thompson M, Kusmartseva I, Kaestner KH. Organisation of the human pancreas in health and in diabetes. Diabetologia. 2020;63:1966–1973. doi: 10.1007/s00125-020-05203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzmickiene I, Everatt R, Virviciute D, Tamosiunas A, Radisauskas R, Reklaitiene R, Milinaviciene E. Smoking and other risk factors for pancreatic cancer: a cohort study in men in Lithuania. Cancer Epidemiol. 2013;37:133–139. doi: 10.1016/j.canep.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno S, Nakai Y, Isayama H, Kawahata S, Saito T, Takagi K, Watanabe T, Uchino R, Hamada T, Miyabayashi K, Kogure H, Sasaki T, Yamamoto N, Sasahira N, Hirano K, Tsujino T, Ijichi H, Tateishi K, Tada M, Koike K. Smoking, family history of cancer, and diabetes mellitus are associated with the age of onset of pancreatic cancer in Japanese patients. Pancreas. 2014;43:1014–1017. doi: 10.1097/MPA.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 10.Haugvik SP, Hedenström P, Korsæth E, Valente R, Hayes A, Siuka D, Maisonneuve P, Gladhaug IP, Lindkvist B, Capurso G. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology. 2015;101:133–142. doi: 10.1159/000375164. [DOI] [PubMed] [Google Scholar]
- 11.Paluszkiewicz P, Smolińska K, Dębińska I, Turski WA. Main dietary compounds and pancreatic cancer risk. The quantitative analysis of case-control and cohort studies. Cancer Epidemiol. 2012;36:60–67. doi: 10.1016/j.canep.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 13.Chang JS, Tsai CR, Chen LT, Shan YS. Investigating the Association Between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas. 2016;45:134–141. doi: 10.1097/MPA.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 14.Gou S, Yang Z, Liu T, Wu H, Wang C. Use of probiotics in the treatment of severe acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18:R57. doi: 10.1186/cc13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang MM, Cheng JQ, Lu YR, Yi ZH, Yang P, Wu XT. Use of pre-, pro- and synbiotics in patients with acute pancreatitis: a meta-analysis. World J Gastroenterol. 2010;16:3970–3978. doi: 10.3748/wjg.v16.i31.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, Scheet P, Xu H, Hanash SM, Feng L, Burks JK, Do KA, Peterson CB, Nejman D, Tzeng CD, Kim MP, Sears CL, Ajami N, Petrosino J, Wood LD, Maitra A, Straussman R, Katz M, White JR, Jenq R, Wargo J, McAllister F. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178:795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fändriks L. The angiotensin II type 2 receptor and the gastrointestinal tract. J Renin Angiotensin Aldosterone Syst. 2010;11:43–48. doi: 10.1177/1470320309347788. [DOI] [PubMed] [Google Scholar]
- 21.Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, Benoist C, Kasper DL. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell. 2017;168:928–943.e11. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlot T, Penninger JM. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866–873. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Shi X, Fu W, Xiang F, He X, Yang B, Wang X, Ma WL. Gut Microbiota Dysbiosis Correlates with Abnormal Immune Response in Moderate COVID-19 Patients with Fever. J Inflamm Res. 2021;14:2619–2631. doi: 10.2147/JIR.S311518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samanta J, Gupta R, Singh MP, Patnaik I, Kumar A, Kochhar R. Coronavirus disease 2019 and the pancreas. Pancreatology. 2020;20:1567–1575. doi: 10.1016/j.pan.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacaksız F, Ebik B, Ekin N, Kılıc J. Pancreatic damage in COVID-19: Why? Int J Clin Pract. 2021;75:e14692. doi: 10.1111/ijcp.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YS, Chang JH, Kim TH, Kim CW, Kim JK, Han SW. Prolonged hyperamylasemia in patients with acute pancreatitis is associated with recurrence of acute pancreatitis. Medicine (Baltimore) 2020;99:e18861. doi: 10.1097/MD.0000000000018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su YR, Hong YP, Mei FC, Wang CY, Li M, Zhou Y, Zhao KL, Yu J, Wang WX. High-Fat Diet Aggravates the Intestinal Barrier Injury via TLR4-RIP3 Pathway in a Rat Model of Severe Acute Pancreatitis. Mediators Inflamm. 2019;2019:2512687. doi: 10.1155/2019/2512687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan LY, Chen YF, Li HC, Bi LM, Sun WJ, Sun GF, Zhang XF, Xu K, Feng DX. Dachengqi Decoction Attenuates Intestinal Vascular Endothelial Injury in Severe Acute Pancreatitis in Vitro and in Vivo. Cell Physiol Biochem. 2017;44:2395–2406. doi: 10.1159/000486155. [DOI] [PubMed] [Google Scholar]
- 32.Asamer E, Szkandera J, Gibiser P, Lembeck AL, Stojakovic T, Kornprat P, Lackner C, Winder T, Schlick K, Stöger H, Gerger A, Pichler M, Stotz M. Elevated amylase in plasma represents an adverse prognostic marker in patients with metastatic pancreatic cancer : A retrospective analysis. Wien Klin Wochenschr. 2018;130:569–574. doi: 10.1007/s00508-018-1383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahimi Sadrabadi A, Bereimipour A, Jalili A, Gholipurmalekabadi M, Farhadihosseinabadi B, Seifalian AM. The risk of pancreatic adenocarcinoma following SARS-CoV family infection. Sci Rep. 2021;11:12948. doi: 10.1038/s41598-021-92068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moslim MA, Hall MJ, Meyer JE, Reddy SS. Pancreatic cancer in the era of COVID-19 pandemic: Which one is the lesser of two evils? World J Clin Oncol. 2021;12:54–60. doi: 10.5306/wjco.v12.i2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casolino R, Biankin AV PanCaCovid-19 Study Group. Impact of COVID-19 on Pancreatic Cancer Research and the Path Forward. Gastroenterology. 2021;161:1758–1763. doi: 10.1053/j.gastro.2021.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SM, Chieng WW, Huang SW, Hsu LJ, Jan MS. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci Rep. 2020;10:20319. doi: 10.1038/s41598-020-77322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Yang M. The Emerging Factors and Treatment Options for NAFLD-Related Hepatocellular Carcinoma. Cancers (Basel) 2021;13 doi: 10.3390/cancers13153740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front Immunol. 2021;12:635471. doi: 10.3389/fimmu.2021.635471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allali I, Bakri Y, Amzazi S, Ghazal H. Gut-Lung Axis in COVID-19. Interdiscip Perspect Infect Dis. 2021;2021:6655380. doi: 10.1155/2021/6655380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang M, Zhang CY. G protein-coupled receptors as potential targets for nonalcoholic fatty liver disease treatment. World J Gastroenterol. 2021;27:677–691. doi: 10.3748/wjg.v27.i8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siddiqui MT, Cresci GAM. The Immunomodulatory Functions of Butyrate. J Inflamm Res. 2021;14:6025–6041. doi: 10.2147/JIR.S300989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donlan AN, Sutherland TE, Marie C, Preissner S, Bradley BT, Carpenter RM, Sturek JM, Ma JZ, Moreau GB, Donowitz JR, Buck GA, Serrano MG, Burgess SL, Abhyankar MM, Mura C, Bourne PE, Preissner R, Young MK, Lyons GR, Loomba JJ, Ratcliffe SJ, Poulter MD, Mathers AJ, Day AJ, Mann BJ, Allen JE, Petri WA Jr. IL-13 is a driver of COVID-19 severity. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng Z, Liu L. Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J Int Med Res. 2018;46:5228–5236. doi: 10.1177/0300060518800588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD, di Magliano MP. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359–6374. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerri C, Chimenti D, Conti I, Neri T, Paggiaro P, Celi A. Monocyte/macrophage-derived microparticles up-regulate inflammatory mediator synthesis by human airway epithelial cells. J Immunol. 2006;177:1975–1980. doi: 10.4049/jimmunol.177.3.1975. [DOI] [PubMed] [Google Scholar]
- 51.Govindaraju V, Michoud MC, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol. 2006;291:C957–C965. doi: 10.1152/ajpcell.00451.2005. [DOI] [PubMed] [Google Scholar]
- 52.Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX. Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1289–L1296. doi: 10.1152/ajplung.00356.2006. [DOI] [PubMed] [Google Scholar]
- 53.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 54.Pekalski ML, García AR, Ferreira RC, Rainbow DB, Smyth DJ, Mashar M, Brady J, Savinykh N, Dopico XC, Mahmood S, Duley S, Stevens HE, Walker NM, Cutler AJ, Waldron-Lynch F, Dunger DB, Shannon-Lowe C, Coles AJ, Jones JL, Wallace C, Todd JA, Wicker LS. Neonatal and adult recent thymic emigrants produce IL-8 and express complement receptors CR1 and CR2. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Shi M, Yu GZ, Qin XR, Jin G, Chen P, Zhu MH. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol. 2012;18:1123–1129. doi: 10.3748/wjg.v18.i10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Li J, Gao M, Fan H, Wang Y, Xu X, Chen C, Liu J, Kim J, Aliyari R, Zhang J, Jin Y, Li X, Ma F, Shi M, Cheng G, Yang H. Interleukin-8 as a Biomarker for Disease Prognosis of Coronavirus Disease-2019 Patients. Front Immunol. 2020;11:602395. doi: 10.3389/fimmu.2020.602395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Rong L, Cui R, Feng J, Jin Y, Chen X, Xu R. Dynamic changes in serum IL-6, IL-8, and IL-10 predict the outcome of ICU patients with severe COVID-19. Ann Palliat Med. 2021;10:3706–3714. doi: 10.21037/apm-20-2134. [DOI] [PubMed] [Google Scholar]
- 58.Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2:194–199. doi: 10.1158/2326-6066.CIR-13-0214. [DOI] [PubMed] [Google Scholar]
- 59.Rallis KS, Corrigan AE, Dadah H, George AM, Keshwara SM, Sideris M, Szabados B. Cytokine-based Cancer Immunotherapy: Challenges and Opportunities for IL-10. Anticancer Res. 2021;41:3247–3252. doi: 10.21873/anticanres.15110. [DOI] [PubMed] [Google Scholar]
- 60.Vicari AP, Trinchieri G. Interleukin-10 in viral diseases and cancer: exiting the labyrinth? Immunol Rev. 2004;202:223–236. doi: 10.1111/j.0105-2896.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J, Xu B, Dai Y, Li X, Zhang C, Peng Y, Feng Y, Li A, Hu Z, Xiang H, Ogg G, Ho LP, McMichael A, Jin R, Knight JC, Dong T, Zhang Y. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, Liu W, Zhu Y, Lin Q, Mao L, Fang M, Zhang H, Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goyal H, Kopel J, Ristić B, Perisetti A, Anastasiou J, Chandan S, Tharian B, Inamdar S. The pancreas and COVID-19: a clinical conundrum. Am J Transl Res. 2021;13:11004–11013. [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshikawa T, Hill T, Li K, Peters CJ, Tseng CT. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quartuccio L, Semerano L, Benucci M, Boissier MC, De Vita S. Urgent avenues in the treatment of COVID-19: Targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine. 2020;87:191–193. doi: 10.1016/j.jbspin.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones SA, Hunter CA. Is IL-6 a key cytokine target for therapy in COVID-19? Nat Rev Immunol. 2021;21:337–339. doi: 10.1038/s41577-021-00553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shekhawat J, Gauba K, Gupta S, Purohit P, Mitra P, Garg M, Misra S, Sharma P, Banerjee M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J Clin Biochem. 2021:1–11. doi: 10.1007/s12291-021-00989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 69.Investigators TRC, Derde LPG. Effectiveness of Tocilizumab, Sarilumab, and Anakinra for critically ill patients with COVID-19 The REMAP-CAP COVID-19 Immune Modulation Therapy Domain Randomized Clinical Trial. 2021 Preprint. Available from: medRxiv: 2021.2006.2018.21259133.
- 70.Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs. 2020;80:1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brennecke A, Villar L, Wang Z, Doyle LM, Meek A, Reed M, Barden C, Weaver DF. Is Inhaled Furosemide a Potential Therapeutic for COVID-19? Am J Med Sci. 2020;360:216–221. doi: 10.1016/j.amjms.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, Wang Y, Vilekar P, Yang SP, Gupta M, Oh MI, Meek A, Doyle L, Villar L, Brennecke A, Liyanage I, Reed M, Barden C, Weaver DF. Small molecule therapeutics for COVID-19: repurposing of inhaled furosemide. PeerJ. 2020;8:e9533. doi: 10.7717/peerj.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu S, Lin J. Blocking Interleukin-6 and Interleukin-8 Signaling Inhibits Cell Viability, Colony-forming Activity, and Cell Migration in Human Triple-negative Breast Cancer and Pancreatic Cancer Cells. Anticancer Res. 2018;38:6271–6279. doi: 10.21873/anticanres.12983. [DOI] [PubMed] [Google Scholar]
- 74.Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, Zhong X, Zimmers TA, Ostrowski MC, Ludwig T, Bloomston M, Bekaii-Saab T, Lesinski GB. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67:320–332. doi: 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tom J, Bao M, Tsai L, Qamra A, Summers D, Carrasco-Triguero M, McBride J, Rosenberger CM, Lin CJF, Stubbings W, Blyth KG, Carratalà J, François B, Benfield T, Haslem D, Bonfanti P, van der Leest CH, Rohatgi N, Wiese L, Luyt CE, Kheradmand F, Rosas IO, Cai F. Prognostic and Predictive Biomarkers in Patients With Coronavirus Disease 2019 Treated With Tocilizumab in a Randomized Controlled Trial. Crit Care Med. 2022;50:398–409. doi: 10.1097/CCM.0000000000005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumar PN, Hernández-Sánchez J, Nagel S, Feng Y, Cai F, Rabin J, Morse CG, Nadig NR, Ashraf O, Gotur DB, McComsey GA, Gafoor K, Perin P, Thornton SC, Stubbings W, Lin CJF, Tsai L. Safety and Efficacy of Tocilizumab 4 or 8 mg/kg in Hospitalized Patients With Moderate to Severe Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial. Open Forum Infect Dis. 2022;9:ofab608. doi: 10.1093/ofid/ofab608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sivapalasingam S, Lederer DJ, Bhore R, Hajizadeh N, Criner G, Hosain R, Mahmood A, Giannelou A, Somersan-Karakaya S, O'Brien MP, Boyapati A, Parrino J, Musser BJ, Labriola-Tompkins E, Ramesh D, Purcell LA, Gulabani D, Kampman W, Waldron A, Gong MN, Saggar S, Sperber SJ, Menon V, Stein DK, Sobieszczyk ME, Park W, Aberg JA, Brown SM, Kosmicki JA, Horowitz JE, Ferreira MA, Baras A, Kowal B, DiCioccio AT, Akinlade B, Nivens MC, Braunstein N, Herman GA, Yancopoulos GD, Weinreich DM Sarilumab-COVID-19 Study Team. Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roumier M, Paule R, Vallée A, Rohmer J, Ballester M, Brun AL, Cerf C, Chabi ML, Chinet T, Colombier MA, Farfour E, Fourn E, Géri G, Khau D, Marroun I, Ponsoye M, Roux A, Salvator H, Schoindre Y, Si Larbi AG, Tchérakian C, Vasse M, Verrat A, Zuber B, Couderc LJ, Kahn JE, Groh M, Ackermann F Foch COVID-19 Study Group. Tocilizumab for Severe Worsening COVID-19 Pneumonia: a Propensity Score Analysis. J Clin Immunol. 2021;41:303–314. doi: 10.1007/s10875-020-00911-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, Patel N, Hagino O Sarilumab COVID-19 Global Study Group. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395:e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maes B, Bosteels C, De Leeuw E, Declercq J, Van Damme K, Delporte A, Demeyere B, Vermeersch S, Vuylsteke M, Willaert J, Bollé L, Vanbiervliet Y, Decuypere J, Libeer F, Vandecasteele S, Peene I, Lambrecht B. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:468. doi: 10.1186/s13063-020-04453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iwabuchi N, Xiao JZ, Yaeshima T, Iwatsuki K. Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol Pharm Bull. 2011;34:1352–1355. doi: 10.1248/bpb.34.1352. [DOI] [PubMed] [Google Scholar]
- 86.Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA, Badger JH, Ajami NJ, Trinchieri G, Pardo-Manuel de Villena F, Yewdell JW, Rehermann B. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell. 2017;171:1015–1028.e13. doi: 10.1016/j.cell.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 92.Kespohl M, Vachharajani N, Luu M, Harb H, Pautz S, Wolff S, Sillner N, Walker A, Schmitt-Kopplin P, Boettger T, Renz H, Offermanns S, Steinhoff U, Visekruna A. The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4+ T Cells. Front Immunol. 2017;8:1036. doi: 10.3389/fimmu.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, Slingerland JB, Beghi S, Herrera PS, Giardina P, Clurman A, Dwomoh E, Armijo G, Gomes ALC, Littmann ER, Schluter J, Fontana E, Taur Y, Park JH, Palomba ML, Halton E, Ruiz J, Jain T, Pennisi M, Afuye AO, Perales MA, Freyer CW, Garfall A, Gier S, Nasta S, Landsburg D, Gerson J, Svoboda J, Cross J, Chong EA, Giralt S, Gill SI, Riviere I, Porter DL, Schuster SJ, Sadelain M, Frey N, Brentjens RJ, June CH, Pamer EG, Peled JU, Facciabene A, van den Brink MRM, Ruella M. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28:713–723. doi: 10.1038/s41591-022-01702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen J, Vitetta L, Henson JD, Hall S. The intestinal microbiota and improving the efficacy of COVID-19 vaccinations. J Funct Foods. 2021;87:104850. doi: 10.1016/j.jff.2021.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng SC, Peng Y, Zhang L, Mok CK, Zhao S, Li A, Ching JY, Liu Y, Yan S, Chan DLS, Zhu J, Chen C, Fung AC, Wong KK, Hui DS, Chan FK, Tun HM. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut. 2022;71:1106–1116. doi: 10.1136/gutjnl-2021-326563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G Jr, Grivas P, Painter CA, Peters S, Thompson MA, Bakouny Z, Batist G, Bekaii-Saab T, Bilen MA, Bouganim N, Larroya MB, Castellano D, Del Prete SA, Doroshow DB, Egan PC, Elkrief A, Farmakiotis D, Flora D, Galsky MD, Glover MJ, Griffiths EA, Gulati AP, Gupta S, Hafez N, Halfdanarson TR, Hawley JE, Hsu E, Kasi A, Khaki AR, Lemmon CA, Lewis C, Logan B, Masters T, McKay RR, Mesa RA, Morgans AK, Mulcahy MF, Panagiotou OA, Peddi P, Pennell NA, Reynolds K, Rosen LR, Rosovsky R, Salazar M, Schmidt A, Shah SA, Shaya JA, Steinharter J, Stockerl-Goldstein KE, Subbiah S, Vinh DC, Wehbe FH, Weissmann LB, Wu JT, Wulff-Burchfield E, Xie Z, Yeh A, Yu PP, Zhou AY, Zubiri L, Mishra S, Lyman GH, Rini BI, Warner JL COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su PH, Yu YC, Chen WH, Lin HC, Chen YT, Cheng MH, Huang YM. Case Report: Vaccine-Induced Immune Thrombotic Thrombocytopenia in a Pancreatic Cancer Patient After Vaccination With Messenger RNA-1273. Front Med (Lausanne) 2021;8:772424. doi: 10.3389/fmed.2021.772424. [DOI] [PMC free article] [PubMed] [Google Scholar]