Abstract
We investigated how inclusion of a “toll-free statement” for reporting side effects to FDA in prescription drug direct-to-consumer (DTC) television ads affects comprehension of product risks and benefits, and comprehension of and memory for the toll-free statement itself. Participants viewed one of nine mock prescription drug television ads that varied elements of the toll-free statement, and then responded to a questionnaire. Presenting the statement in both text and audio resulted in better processing of the statement compared to only text. When shown in text alone, presenting the statement during the entire advertisement or after the statement of risks resulted in better processing of the statement compared to placement before the presentation of risk information. The placement, duration, or prominence of the statement did not affect comprehension of product risk and benefit information. Our findings suggest that the toll-free statement can be added to DTC television ads without significantly affecting comprehension of product risk and benefit information, and that select presentations are preferable for communicating the toll-free statement. The appropriate inclusion of the toll-free statement in DTC television ads may increase the visibility of the adverse event reporting system, without any apparent cost to the understanding of benefits and risks.
Keywords: DTC, prescription drugs, advertisements, drug safety, risk perception, risk comprehension, toll-free statement, Food and Drug Administration Amendments Act
Manufacturers of human drugs, biologics, and medical devices are required to report adverse events to the US Food and Drug Administration (FDA) according to applicable regulations (Code of Federal Regulations 2014a). In 1993, FDA created the Medical Products Reporting Program (MedWatch) to encourage health care professionals to voluntarily report adverse events directly to FDA. MedWatch provides a single portal for reporting adverse events for FDA-regulated human medical products, including serious drug side effects, quality problems, and therapeutic failures. This includes prescription and over-the-counter drugs, medical devices, biologics, dietary supplements, cosmetics, and infant formulas (FDA 2014a). Reports can be submitted either through the website, www.fda.gov/medwatch, or via telephone at 1–800-FDA-1088. The goal of the program is to increase reporting of serious events in order to facilitate earlier identification of medical product problems (Kessler 1993), which is a central tenet of pharmacovigilance (Dal Pan, 2014). MedWatch is also used to inform the public and the healthcare community about adverse drug events and emerging safety information (Liang and Mackey 2011). Safety alerts are posted regularly on FDA’s MedWatch public website (FDA, 2015a). Adverse event reports for drugs and biologics received by FDA are entered into the FDA Adverse Event Reporting System (FAERS); a public version is also available (FDA Adverse Event Reporting System 2014b).
It is notable that direct consumer reporting of adverse events was not a focus of MedWatch in the beginning, as most patient reports are transmitted by pharmaceutical companies (van Grootheest et al. 2003). Interest in consumer/patient reporting of adverse events is increasing both domestically and internationally (van Grootheest and de Jong-van den Berg 2004). FDA has always accepted consumer reports, though it was not until 2002 that direct reporting by consumers was first addressed by law. The Best Pharmaceuticals for Children Act (Public Law 107–109) and subsequent regulations (FDA 2008) require most human drug product labels to include (1) a toll-free number maintained by FDA to receive reports of adverse events, and (2) a statement that the number should be used for reporting purposes only, not to receive medical advice.
There is some evidence that this push to encourage consumers to report adverse events to FDA is having its intended effect. Over time, the balance of reports has shifted from those submitted by medical product manufacturers to those submitted by healthcare professionals and consumers. In 2004, FDA received 21,653 direct reports, of which 3,885 (17.9%) were from consumers. In 2014, that number increased to 34,256, of which 14,446 (42.2%) were from consumers (FDA 2015b). A study by Du et al. (2012) suggested a positive relationship between DTC advertising spend and adverse event reports. According to their calculations, after the enactment of FDAAA, spending on DTC print ads resulted in 0.24 more reports per month per drug, of which approximately 65% could be attributed to the MedWatch number requirement. Thus, while more consumers are reporting adverse events than before, increasing the visibility of the platform will ensure that more consumers are aware and have the ability to report adverse events. One way to increase awareness of the available reporting avenue is through inclusion of this information in DTC prescription drug advertisements.
The Food and Drug Administration Amendments Act of 2007 (FDAAA), which amended the Federal Food, Drug, and Cosmetic Act, specifically addressed the inclusion of this reporting availability in printed prescription drug direct-to-consumer (DTC) advertising. Title IX of FDAAA now requires printed DTC advertisements (ads) for prescription drug products to include the following statement: “You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1–800-FDA-1088.” FDA has authority over the labeling and advertising of prescription drugs.
Title IX also required that a study be conducted to determine (1) if the FDAAA “toll-free statement” is appropriate for inclusion in DTC television ads for prescription drug products, or if its presence would interfere with viewers’ comprehension of risk information in the ads; and (2) if it is appropriate, the length of time the toll-free statement should be displayed in the ads. These requirements prompted the present research, in which we examined how cognitive processing outcomes are impacted by variations in (1) the duration of time the toll-free statement is presented on screen in a DTC television ad, (2) the placement of the toll-free statement within the ad, and (3) the prominence (presentation in audio and text versus just text) of the toll-free statement. The primary outcome measures assessed (1) comprehension of product risk information, (2) comprehension of product benefit information, and (3) comprehension of and memory for the toll-free statement. Comprehension of product risk and benefit information were identified as primary outcome measures to ensure that requiring the addition of a toll-free statement would not interfere with consumers’ ability to understand the potential risks and benefits of DTC advertised drugs. Comprehension of and memory for the toll-free statement was used as a key outcome measure to look at differences in memory and comprehension of the toll-free statement when its placement, duration, or prominence was manipulated. We also examined consumers’ perceived benefit and perceived risk of the product. To ensure that the toll-free statement is clear to consumers, we also measured the clarity and understandability of the toll-free statement.
Research Approach and Theoretical Framework
As our introductory paragraphs suggest, the present research is driven by applied questions: does inclusion of the toll-free statement in DTC television advertising impede comprehension of other important ad elements, and is there a particular presentation that best implements this change? Our research design examines these questions in straightforward, empirical fashion by presenting participants with one of several reasonable presentations of the toll-free statement, and then testing relevant cognitive processing outcomes. Below, we consider potential outcomes that may result based on these varied presentations, with rationale grounded in the existing psychology and marketing literature. Note that these potential outcomes do not necessarily reflect desired outcomes, but rather likely possibilities that warrant consideration if the toll-free statement is to be implemented. Ideally, inclusion of the toll-free statement would not interfere with processing of other ad elements, regardless of its duration, placement, or prominence. The full set of research questions is formally stated in Table 1.
TABLE 1:
FORMAL RESEARCH QUESTIONS AND ASSOCIATED OUTCOMES
| Research Questions | Outcomes |
|---|---|
| 1. Does inclusion of the toll-free statement impact comprehension of drug risk and benefit information? | Toll-free statement not disruptive in any condition |
| Contingent on toll-free statement impacting risk and benefit comprehension: 2. Does placement of the toll-free statement during the whole ad or at the end of the ad versus the beginning of the ad allay any negative impact on drug risk and benefit comprehension? 3. Does whole ad presentation versus shorter presentation allay any negative impact on drug risk and benefit comprehension? | |
| 4. Across designs, do participants who view an ad with the toll-free statement have greater comprehension of and memory for the toll-free statement than participants in a control condition who viewed an ad without the toll-free statement? | Participants who viewed an ad with the toll-free statement demonstrated superior comprehension of and memory for the toll-free statement |
| 5. Among placement conditions, is comprehension of and memory for the toll-free statement best when the statement is presented after information about risks and benefits? | Placement of the toll-free statement after the risk and benefit information did not result in superior comprehension and memory, although there was some evidence that processing is worsened when toll-free statement is presented before the major statement |
| 6. Among duration conditions, do participants in the “whole ad” presentation produce superior comprehension and memory for the toll-free statement? | Toll-free statement comprehension and memory not impacted by duration |
| 7. Among prominence conditions, does addition of audio to the text-only presentation (both full text and short text) improve comprehension of and memory for the toll-free statement? | Dual modality (audio plus text) presentation resulted in superior comprehension of and memory for the toll-free statement |
Effect of Toll-Free Statement on Comprehension of Product Risk and Benefit Information
Ads broadcast through media such as television, radio, or telephone communications systems must disclose the product’s major risks in either the audio or audio and visual parts of the presentation and make adequate provision to disseminate the product’s approved or permitted labeling in connection with the ad (21 CFR 202.1(e)(1)). This presentation of major risks in broadcast ads is sometimes referred to as the major statement (FDA, 1999). In addition to the major statement, broadcast ads communicate drug benefits including the indication, or FDA-approved use of the drug, and other features that might be construed as benefits (e.g., easy or quick administration). Regulations (Code of Federal Regulations, 2014b) require fair balance in how risk and benefit information is communicated, and ideally consumers achieve satisfactory comprehension regarding both types of information. This is an important consideration for our purposes because prescription drug ads are already dense with information, and so adding the toll-free statement may have implications for consumer comprehension of existing risk and benefit information.
Some researchers have argued that there is an optimal amount of information that can be included in an ad. At some point, the amount of information in the ad may cognitively overload the consumer (Anderson, Ciliberto, and Liaukonyte 2012; Jacoby 1984; Pieters, Wedel, and Zhang 2007; Sweller 1988). Thus, it is reasonable to expect that adding the toll-free statement to DTC television advertising could have the unintended consequence of interfering with viewers’ processing of existing risk and benefit information.
Alternatively, one might reasonably expect no impact of the toll-free statement on risk and benefit comprehension. Because prescription drug broadcast ads are dense with information, inclusion of the toll-free statement may not make any incremental difference. Or it may be that the toll-free statement is not sufficiently relevant to most individuals and so they allocate the bulk of their attention to risk and benefit information which is arguably more central in these ads. DTC ads have appeared frequently on television for almost 20 years, and with rare exceptions, they all follow roughly the same pattern: statement of benefit, major statement, and a closing scene with a call to contact a healthcare professional. Consumers have, therefore, had ample opportunity to familiarize themselves with the type of information and the importance of the information in the ads. Although the toll-free statement is important, few would deny that the drug’s risk and benefit information is critical.
The outcome of these varied considerations is that inclusion of the toll-free statement may or may not impact comprehension of drug risk and benefit information. Thus, we pose as Research Question 1: Does inclusion of the toll-free statement impact comprehension of product risk and benefit information?
If comprehension of risk and benefit information is indeed impacted by inclusion of the toll-free statement, it may be possible to mitigate this impact by varying the presentation of the toll-free statement. One potential mitigating strategy involves placement of the toll-free statement during the whole ad or after the statement of risks. Presentation during the whole ad may interfere less with processing of risk and benefit information because participants would have an opportunity to read and process the toll-free statement at any point during the ad. Presentation after the major statement may interfere less with comprehension of risk information because the toll-free statement would not overlap with the risk information, and thus would not require viewers to attend to, and process, competing information (e.g., Gilbert and Hixon 1991).
Another potential mitigating strategy involves increasing the duration of the toll-free statement. This strategy is partially accounted for by the “whole ad” placement strategy described above. Shorter durations of the toll-free statement may interfere with comprehension of risk and benefit information more than a whole ad presentation because attention must shift between the messages in quick succession. Research in the area of dual-task processing suggests that requiring viewers to pay attention to two different simultaneous tasks results in decreased performance on both (e.g., Charlton 2009; Pashler 1994). Whole ad presentation allows viewers opportunity to read the toll-free statement at a time of their choosing, and therefore does not force attention away from risk and benefit messages at any particular point in the ad.
These potential mitigating strategies pose two additional research questions. As Research Question 2, does placement of the toll-free statement during the whole ad or at the end of the ad versus the beginning of the ad allay any negative impact on drug risk and benefit comprehension? As Research Question 3, does whole ad presentation versus shorter presentation allay any negative impact on drug risk and benefit comprehension? Again, Research Questions 2 and 3 are contingent on Research Question 1, namely that inclusion of the toll-free statement impacts comprehension of risk and benefit information at all. If inclusion of the toll-free statement does not impact risk and benefit comprehension, then placement and duration of the toll-free statement should not matter.
Toll-Free Statement Recall and Comprehension
In an effort to inform consumers of their ability to report adverse events, we are also interested in examining differences in comprehension of and memory for the toll-free statement itself based on variation in its placement, duration, and prominence. First, across designs, we expected that participants who viewed an ad with the toll-free statement would have greater comprehension of and memory for the toll-free statement than participants in a control condition who viewed an ad without the toll-free statement (Research Question 4). Although seemingly obvious, this finding would confirm that participants are able to process the toll-free statement when it is presented in a DTC television ad and ensure that toll-free statement findings do not represent floor effects. Previous research has used this approach in DTC print ads (Woloshin and Schwartz, 2009).
With regard to placement, we expected (Research Question 5) that comprehension of and memory for the toll-free statement would be best when it was presented after the major statement of risks, due in part to the common risk-related nature of these statements (Hintzman 2010; Jacoby and Wahlheim 2013). Because participants would hear the risks and side effects of the product before being told how they may report this information, the presentation of risks in this condition might prime the toll-free statement (Traxler 2008). The toll-free statement may be more useful in this condition relative to others, therefore, because viewers have been provided with context. Additionally, presentation after the major statement of risks prevents competing information from interfering with the message. Classic research in the cognitive sciences demonstrates that retroactive (i.e., post-message) interference is a primary cause of forgetting (Waugh and Norman, 1965). Accordingly, individuals are often better able to remember information from the end of a message than the beginning (Stewart, Stewart, Tyson, Vinci and Fioti 2004; Tan and Ward 2000).
For duration, we expected (Research Question 6) that longer presentations of the toll-free statement would result in better comprehension and memory; specifically, participants in the “whole ad” presentation would have the best processing outcomes. Working memory allows individuals to maintain (i.e., keep in mind) perceptual information from their environment. Yet, working memory is limited in its duration (Atkinson and Shiffrin 1968; 1971). Thus, for the toll-free statement to be adequately perceived and understood, we expected that it would need to be presented for a sufficient amount of time. “Whole ad” presentation of the toll-free statement most effectively achieves this requirement. Further, presentation during the entire ad may signal to individuals that the toll-free statement is important, which would likely enhance processing outcomes (Rucker and Petty, 2006).
In terms of prominence, we expected that the addition of audio to the text-only presentation (both full text and short text; Research Question 7) would improve comprehension of and memory for the toll-free statement (Wogalter, Shaver, and Kalsher, 2013). Supporting this expectation, multiple studies have shown that presenting the same information in audio and superimposed visual text (dual-mode processing) improves recall of the communicated information over and above that of using the audio mode alone (see, for example, Morris, Mazis, and Brinberg 1989; Murray, Manrai, and Manrai 1998).
Method
Sample
The sample consisted of 4,961 adults drawn from Knowledge Network’s (now GfK) KnowledgePanel®, a nationally representative online panel of individuals aged 13 or older who have been recruited through a combination of random-digit-dialing and address-based sampling. Zintria, the product presented in our study, is a fictitious prescription drug treatment for high blood pressure (HBP). Consequently, we oversampled participants who reported HBP when they joined the panel to account for increased salience among this population. The study population was restricted to noninstitutionalized adults aged 18 or older with an oversample of respondents with diagnosed HBP. To qualify, participants had to consent to participate in the experiment, be able to and actually view the streaming video ad for the fictitious drug Zintria, and recall that they saw the ad. Table 2 shows the distribution of HBP and non-HBP participants as well as other demographic characteristics.
TABLE 2:
DISTRIBUTION OF DEMOGRAPHIC CHARACTERISTICS, BY TARGET POPULATION
| Category | Overall Sample % (95% C.I.) (n = 4,961) |
High blood pressure % (95% C.I.) (n = 2,942) |
No high blood pressure % (95% C.I.) (n = 2,019) |
|
|---|---|---|---|---|
| Age | Mean: | 46.9 (46.3, 47.6) |
58.7 (57.9, 59.4) |
42.4 (41.6, 43.2) |
| 18–24 | 8.5% (7.3, 9.9) |
.4% (0.2, 1.1) |
11.6% (9.9, 13.5) |
|
| 25–34 | 16.6% (15.0, 18.4) |
3.9% (2.7, 5.7) |
21.5% (19.3, 23.8) |
|
| 35–44 | 22.6% (20.8, 24.5) |
10.9% (9.3, 12.9) |
27.1% (24.7, 29.6) |
|
| 45–54 | 19.3% (17.7, 21.0) |
19.7% (17.6, 22.0) |
19.1% (17.1, 21.4) |
|
| 55–64 | 16.5% (15.1, 17.9) |
30.9% (28.5, 33.3) |
10.9% (9.4, 12.6) |
|
| 65 or older | 16.5% (15.2, 17.9) |
34.2% (31.7, 36.7) |
9.8% (8.3, 11.4) |
|
|
| ||||
| Race/Ethnicity | White, non-Hispanic | 69.7% (67.5, 71.7) |
73.9% (71.2, 76.4) |
68.0% (65.3, 70.7) |
| Black, non-Hispanic | 11.2% (9.8, 12.8) |
13.6% (11.6, 15.8) |
10.3% (8.5, 12.5) |
|
| Hispanic | 13.4% (11.7, 15.2) |
9.0% (7.2, 11.2) |
15.0% (12.9, 17.4) |
|
| Other, non-Hispanic | 5.8% (4.9, 6.8) |
3.6% (2.8, 4.5) |
6.6% (5.4, 8.0) |
|
|
| ||||
| Gender | Male | 48.5% (46.5, 50.6) |
49.3% (46.6, 52.0) |
48.3% (45.6, 51.0) |
| Female | 51.5% (49.4, 53.5) |
50.7% (48.0, 53.4) |
51.8% (49.1, 54.5) |
|
|
| ||||
| Highest level of education | Some high school | 6.7% (5.6, 8.0) |
6.6% (5.1, 8.5) |
6.8% (5.4, 8.5) |
| High school graduate | 36.9% (34.8, 39.0) |
39.3% (36.6, 42.1) |
35.9% (33.3, 38.7) |
|
| Some college | 28.5% (26.8, 30.4) |
29.3% (27.1, 31.7) |
28.2% (26.0, 30.6) |
|
| Bachelor’s or higher | 27.9% (26.2, 29.7) |
24.8% (23.0, 26.8) |
29.1% (26.8, 31.4) |
|
|
| ||||
| Currently taking prescription drug(s) for high blood pressure | Yes, taking prescription drug | 24.5% (23.1, 25.9) |
80.9% (78.1, 83.4) |
2.9%a (2.3, 3.6) |
| No, not taking prescription drug | 75.1% (73.7, 76.5) |
18.6% (16.1, 21.4) |
96.8% (96.0, 97.5) |
|
| Refused | .4% (.2, .7) |
.5% (.3, .9) |
.3% (.1, .9) |
|
Note: C.I. = confidence interval
This discrepancy may be due to the fact that high blood pressure status was measured at an earlier time than prescription drug status.
Study Design
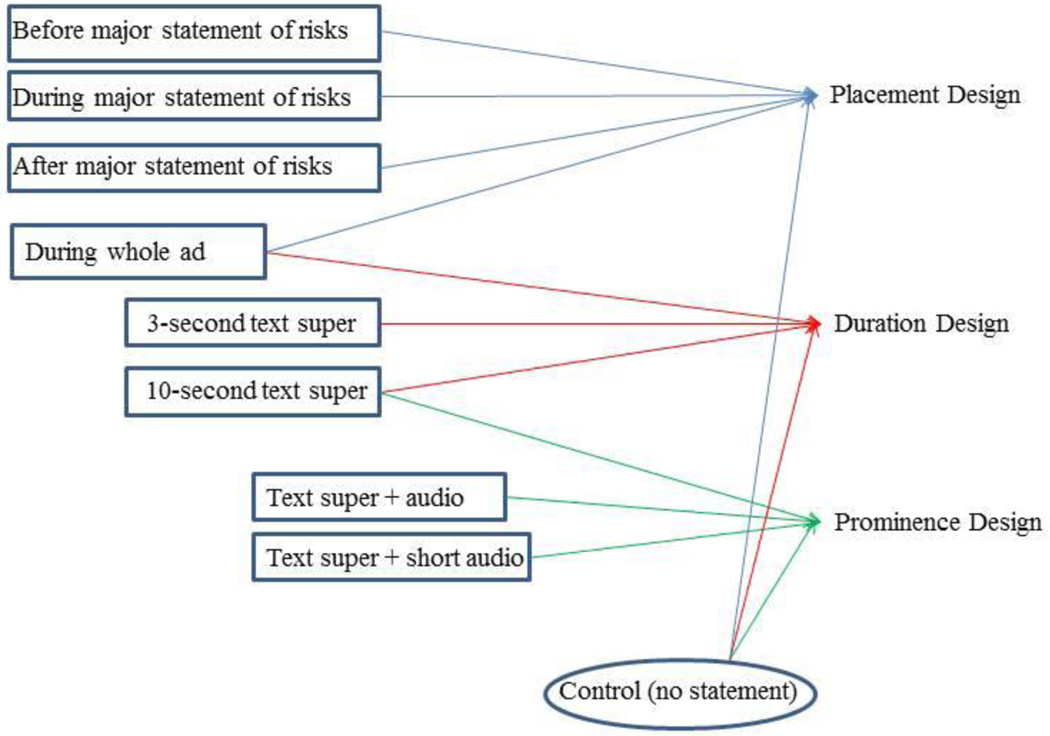
Because we could not fully cross them, we simultaneously examined three independent variables in three separate designs: (1) the placement of the FDAAA toll-free statement, (2) the duration that the toll-free statement was presented on screen, and (3) the prominence of the toll-free statement in the ad (see Figure 1). The toll-free statement read, “You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1–800-FDA-1088.” Of note, we also tested alternative toll-free statement wording from the Best Pharmaceuticals for Children Act in a separate design. The FDAAA toll-free statement was found to be superior in that test (Authors, in press)
Figure 1:
Study Design
Placement was defined as including the toll-free statement before, during, or after the major statement of risks, in text only. The toll-free statement was shown for 10 seconds at the bottom of the screen as white superimposed text (“text super”) in a black box. We also tested a condition that included the toll-free statement for the entirety of the ad.
Duration was defined by text-only presentations of the toll-free statement after the major statement of risks for a length of 3 seconds, 10 seconds, or during the whole ad.
Prominence was defined by combinations of text and audio presentations of the toll-free statement in the ad after the major statement of risks as follows:
Full toll-free statement in text super only,
Full toll-free statement in text super and audio, or
Full toll-free statement in audio and shortened version in text super (www.fda.gov/medwatch, “Report negative side effects, 1–800-FDA-1088”).
To accommodate the additional audio track, the “text super + audio” presentations were 13 seconds longer than the text-only presentation.
Each study design also included a control condition in which no toll-free statement was presented. This one control cell was used across designs (see Figure 1).
Procedure
Participants were randomly assigned to one test condition in one of the designs or to the control condition. All participants were shown a series of ads for four products in this order: iTunes (15 seconds), Nasonex (30 seconds), Tide (15 seconds), and the ad for the fictitious prescription drug Zintria (73 or 86 seconds depending on condition).1 The study ad was longer than the other ads, as is typical of DTC ads (e.g., Frosch, Krueger, Hornik, Cronholm, and Barg, 2007), which often last 60 or even 90 seconds. The other ads did not vary across experimental conditions. To reduce effects that might result from the novelty of the toll-free statement, the Nasonex ad also included the FDAAA toll-free statement matched to the test condition with which it was paired (e.g., 3-second duration after the major statement in both ads). Participants then responded to a series of questions about the ad (Appendix A). At the conclusion of the study, participants were informed that Zintria was not a real prescription drug.
Study Measures
Comprehension of product risks.
Following previous research (e.g., Aikin et al. 2011), participants saw eight statements about the risks of Zintria and indicated whether the information was in the ad (e.g., “If you have a very slow heart rate, you should not take Zintria”; yes/no/don’t know). Four statements were “true” and the other four were “false.” In addition, participants answered three multiple-choice questions about the risks (e.g., “Why should you not stop taking Zintria suddenly?”). We summed the number of correct responses to these 11 items to create one scale of risk comprehension (0–11). This combined measure allowed us to assess both the recognition and application of risk information.
Comprehension of product benefits.
Participants saw seven statements about the benefits of Zintria and indicated whether the information was in the ad (e.g., “Zintria can reduce your risk of having a stroke”; yes/no/don’t know). Four statements were “true” and the other three were “false.” In addition, participants answered two multiple-choice questions about the benefits (e.g., “Why would your doctor prescribe Zintria for you?”). We summed the number of correct responses to these 9 items to create one scale of benefit comprehension (0–9). This combined measure allowed us to assess both the recognition and application of benefit information.
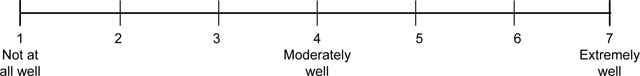
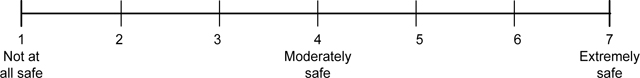
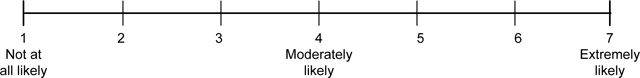
Perceived product risk and benefit.
Perceived benefit was measured with two closed-ended 7-point Likert questions, “How well do you think Zintria would or would not work for you?” (1 = not at all well, 7 = extremely well) and “How likely or not likely would you be to lower your blood pressure if you took Zintria?” (1 = not at all likely, 7 = extremely likely). Similarly, perceived risk of the product was measured with two closed-ended, 7-point Likert questions, “How safe or not safe do you think Zintria is?” (1 = not at all safe, 7 = extremely safe) and “How risky or not risky do you think Zintria is?” (1 = not at all risky, 7 = extremely risky). These items, which were the same as or similar to those used in previous research (e.g., O’Donoghue et al. 2014), did not reach a threshold of Cronbach’s alpha ≥ .80 (Bland and Altman 1997) when combined for either measure (benefit: Cronbach’s alpha = .64; risk: Cronbach’s alpha = .75) and thus were analyzed separately.
Memory and comprehension of the toll-free statement.
We used a series of closed and open-ended questions to assess memory and comprehension of the toll-free statement. Participants were asked three closed-ended questions: whether the ad listed any websites, whether the ad listed any phone numbers, and whether they noticed a statement in the ad about FDA. Participants who reported noticing a statement about FDA were asked two additional questions: what the statement said (open-ended) and why you should contact FDA according to the statement (closed-ended). Participants who did not remember a statement about FDA were asked if they remembered a statement about reporting side effects. Finally, all participants were shown six statements and asked to select which appeared in the ad. Wording of and correct responses to each of these questions appear in Tables 3, 4, and 5.
TABLE 3.
MEAN COMPREHENSION OF PRODUCT RISKS AND BENEFITS, BY CONDITION
| Design | Condition | Comprehension of Product Risks M (95% C.I.) |
Comprehension of Product Benefits M (95% C.I.) |
|---|---|---|---|
| Placement, duration, prominence | Control | 5.3 (4.8, 5.7) |
4.8 (4.4, 5.1) |
| Placement | Before risk | 5.2 (4.8, 5.7) |
4.9 (4.6, 5.2) |
| Placement | During risk | 5.3 (4.9, 5.7) |
4.5 (4.2, 4.7) |
| Placement | After risk | 5.4 (5.0, 5.9) |
4.4 (4.1, 4.7) |
| Placement, duration | Whole ad | 4.9 (4.4, 5.4) |
4.3 (4.0, 4.7) |
| Duration | 3-second text super | 5.5 (5.0, 5.9) |
4.4 (4.1, 4.8) |
| Duration, prominence | 10-second text super | 5.4 (5.0, 5.9) |
4.4 (4.1, 4.7) |
| Prominence | Text super + short audio | 5.3 (4.9, 5.7) |
4.6 (4.3, 4.9) |
| Prominence | Text super + audio | 5.5 (5.1, 5.9) |
4.6 (4.3, 4.9) |
Note. The range for the risk index was 0 to 11 and the range for the benefit index was 0 to 9.
TABLE 4:
WEIGHTED PERCENTAGE OF PARTICIPANTS REMEMBERING THE TOLL-FREE STATEMENT, BY PLACEMENT
| Condition | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable | Response | Control % (95% C.I.) |
Before risk % (95% C.I.) |
During risk % (95% C.I.) |
After risk % (95% C.I.) |
During the Whole Ad % (95% C.I.) |
| Did the ad list any web sites? | % who said yes (correct answer for all conditions) | 39.9 a (32.9, 47.4) |
40.4 a (33.6, 47.6) |
42.4 a
(35.3, 49.8) |
43.2 a
(36.4, 50.3) |
47.5 a (39.7, 55.4) |
| Did the ad list any telephone numbers? | % who said yes (correct answer for all conditions) | 34.5 a (27.8, 41.8) |
39.0 a (32.3, 46.2) |
36.5 a (29.7, 43.8) |
44.1 a (37.1, 51.2) |
38.2 a (31.0, 46.0) |
| Did you notice a statement about FDA in the ad for Zintria? | % who said yes (incorrect answer for control condition, correct answer for all other conditions) | 26.4 a (29.3, 33.5) |
37.2 a,b (30.9, 44.0) |
48.8 b,c
(41.5, 56.1) |
50.5 b,c (43.5, 57.6) |
63.3 c (55.4, 70.6) |
| What did the statement say?1 | % whose open-ended responses were coded as “to report side effects” or “call FDA” (guessing for control condition, correct answer for all other conditions) | 0 a | 27.6 b (19.5, 37.6) |
37.9 b (28.2, 48.8) |
34.9 b (26.5, 44.3) |
22.0 b (15.8, 29.9) |
| Why should you contact FDA, according to the statement?1 | % who selected “to report side effects” (guessing for control condition, correct answer for all other conditions) | 31.9 a (19.1, 48.1) |
42.1 a (32.4, 52.4) |
54.3 a (43.7, 64.6) |
49.5 a (40.0, 59.0) |
52.7 a (42.9, 62.4) |
| Did you notice a statement about reporting side effects?2 | % who said yes (incorrect answer for control condition, correct answer for all other conditions) | 70.3 a (61.8, 77.5) |
70.9 a (61.7, 78.7) |
65.7 a (54.9, 75.1) |
78.9 a (69.8, 85,9) |
68.6 a (53.9, 80.3) |
| Which, if any, of the following appeared in the ad? | % who selected the toll-free statement (incorrect answer for control condition, correct answer for all other conditions) | 18.0 a (12.9, 24.5) |
35.0 b (28.9, 41.6) |
40.8 b, c (33.9, 48.0) |
50.9 c (43.8, 57.9) |
51.0 c (43.1, 58.8) |
Note. Means sharing a superscript were not significantly different at the Bonferroni-adjusted p < .005.
These questions were asked only of participants who responded “yes” when asked whether they noticed a statement about FDA in the ad.
This question was asked only of participants who responded “no” when asked whether they noticed a statement about FDA in the ad.
TABLE 5:
WEIGHTED PERCENTAGE OF PARTICIPANTS REMEMBERING THE TOLL-FREE STATEMENT, BY DURATION
| Condition | |||||
|---|---|---|---|---|---|
| Dependent Variable | Response | Control % (95% C.I.) |
3 Seconds % (95% C.I.) |
10 Seconds % (95% C.I.) |
During the Whole Ad % (95% C.I.) |
| Did the ad list any web sites? | % who said yes (correct answer for all conditions) | 39.9 a (32.9, 47.4) |
38.7 a (31.9, 46.0) |
43.2 a
(36.4, 50.3) |
47.5 a (39.7, 55.4) |
| Did the ad list any telephone numbers? | % who said yes (correct answer for all conditions) | 34.5 a (27.8, 41.8) |
33.8 a (27.5, 40.9) |
44.1 a (37.1, 51.2) |
38.2 a (31.0, 46.0) |
| Did you notice a statement about FDA in the ad for Zintria? | % who said yes (incorrect answer for control condition, correct answer for all other conditions) | 26.4 a (29.3, 33.5) |
51.2 b (43.9, 58.5) |
50.5 b (43.5, 57.6) |
63.3 b (55.4, 70.6) |
| What did the statement say? 1 | % whose open-ended responses were coded as “to report side effects” or “call FDA” (guessing for control condition, correct answer for all other conditions) | 0 a | 35.6 b (26.3, 46.1) |
34.9 b (26.5, 44.3) |
22.0 b (15.8, 29.9) |
| Why should you contact FDA, according to the statement? 1 | % who selected “to report side effects” (guessing for control condition, correct answer for all other conditions) | 31.9 a (19.1, 48.1) |
54.3 a (43.9, 64.3) |
49.5 a (40.0, 59.0) |
52.7 a (42.9, 62.4) |
| Did you notice a statement about reporting side effects?2 | % who said yes (incorrect answer for control condition, correct answer for all other conditions) | 70.3 a (61.8, 77.5) |
73.5 a (64.1, 81.1) |
78.9 a (69.8, 85.9) |
68.6 a (53.9, 80.3) |
| Which, if any, of the following appeared in the ad? | % who selected the toll-free statement (incorrect answer for control condition, correct answer for all other conditions) | 18.0 a (12.9, 24.5) |
45.0 b (37.9, 52.4) |
50.9 b (43.8, 57.9) |
51.0 b (43.1, 58.8) |
Note. Means sharing a superscript were not significantly different at the Bonferroni-adjusted p < .008.
These questions were asked only of participants who responded “yes” when asked whether they noticed a statement about FDA in the ad.
This question was asked only of participants who responded “no” when asked whether they noticed a statement about FDA in the ad.
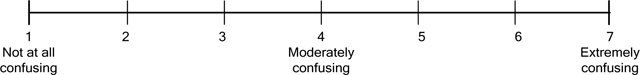
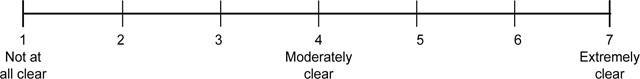
Clarity of toll-free statement.
After completing the toll-free memory and comprehension questions, all participants were shown the toll-free statement and asked three closed-ended questions to assess perceived toll-free statement clarity: “How understandable is this statement?” (1 = not at all understandable, 7 = extremely understandable), “How confusing is this statement?” (1 = extremely confusing, 7 = not at all confusing), and “How clear is this statement?” (1 = not at all clear, 7 = extremely clear) (Cronbach’s alpha = .88). Because the distribution of responses was highly skewed (more than 60% of participants indicated “7” for all three questions), this scale was converted to a binary measure in which a code of “1” was assigned for all participants that indicated “Extremely understandable,” “Not at all confusing,” and “Extremely clear” to each respective question. With the exception of 44 observations for which no summary variable was calculated because of missing data (due to participants not answering a question), all other response patterns were coded as a “0.”
Analyses
We suspected that effects on comprehension of risk and benefit information as well as comprehension of and memory for the toll-free statement might be stronger among HBP participants than among participants without diagnosed HBP, as HBP sufferers would likely have higher involvement with the medical condition presented in the Zintria ad and be more motivated to pay attention to a potential treatment for their condition (McLean, Hoek and Hedderley, 2012). Nonetheless, our analyses revealed few differences between the HBP and non-HBP participants, and so the results presented here reflect estimates from the population-weighted data representing the general population, including both HBP participants and non-HBP participants.
We performed bivariate analyses to test for significant differences between conditions in each design. We report weighted estimates with 95% confidence intervals and significance level determined by Student’s t-tests. Due to multiple comparisons among conditions, we used Bonferroni-adjusted p values as the significance threshold in the placement (p < .005 = .05/10 comparisons) and duration and prominence designs (p < .008 = .05/6 comparisons). In addition, multivariate analyses were performed using gender, age, race/ethnicity, education, income, marital status, HBP status, and HBP medication use as covariates. Because the multivariate analyses provided similar conclusions as the bivariate analyses, even though the HBP group was generally older than the non-HBP group, we report only the results from the bivariate analyses.
Results
Comprehension of Product Risks and Benefits
There were no statistically significant differences in comprehension of product risks and benefits by placement, duration, or prominence (all ps > .008; Table 3). Approximately half of all statements were correctly identified. These findings address Research Question 1, 2 and 3, and lend support to the notion that inclusion of the toll-free statement does not impact comprehension of drug risks and benefits.
Perceived Product Risk and Benefit
The presence of the toll-free statement did not affect participants’ ratings of the product’s benefit or risk. There were no differences in participants’ ratings of Zintria’s likeliness to work, likeliness to lower blood pressure, safety, and riskiness by placement, duration or prominence (all ps > .008).
Memory and Comprehension of FDAAA Toll-Free Statement
In general, across all designs, participants had better memory of and comprehension for the toll-free statement in the test conditions than in the control condition (see Tables 3–5). These findings address Research Question 4 and suggest that participants were able to process the toll-free statement when it was presented in a DTC television ad.
We predicted that processing of the toll-free statement would be best when it was placed after the major statement (Research Question 5). Participants in the “After Major Statement” condition did not significantly differ from those in the “During Major Statement,” or “During Whole Ad” conditions. They significantly differed from the “Before Major Statement” condition participants on one measure: more participants in the “After Major Statement” condition recognized the toll-free statement when asked which statements appeared in the ad than in the “Before Major Statement” condition (p < .001; Table 3). There were two other significant findings for the placement conditions. Participants in the “Before Major Statement” condition significantly differed from the “During Whole Ad” condition participants on two measures: they were less likely to notice the FDA toll-free statement and they were less likely to recognize the toll-free statement when asked which statements appeared in the ad (both ps < .001). Thus, although processing of the toll-free statement was not significantly improved by placing the toll-free statement after the major statement, there is some evidence that it was worsened by placing it before the major statement.
There were no significant differences among duration conditions (Table 5). Research Question 6, which examined whether comprehension of and memory for the toll-free statement would be best when it was presented during the whole ad, did not receive affirmative support.
Table 6 shows the results for the prominence design. Compared with participants in the low prominence condition (“text super” only), participants in the high prominence conditions (“text super + audio” and “text super + short audio”) were more likely to: remember that the ad listed any websites, remember that the ad listed any phone numbers, notice the FDA toll-free statement, know what the toll-free statement said, know why you should contact FDA according to the toll-free statement, and recognize the toll-free statement when asked which statements appeared in the ad (all ps < .001). These results address Research Question 7.
TABLE 6:
WEIGHTED PERCENTAGE OF PARTICIPANTS REMEMBERING THE TOLL-FREE STATEMENT, BY PROMINENCE
| Condition | |||||
|---|---|---|---|---|---|
| Dependent Variable | Response | Control % (95% C.I.) |
Text super only % (95% C.I.) |
Text super + audio % (95% C.I.) |
Text super + short audio % (95% C.I.) |
| Did the ad list any web sites? | % who said yes (correct answer for all conditions) | 39.9 a (32.9, 47.4) |
43.2 a (36.4, 50.3) |
66.9 b (60.0, 73.2) |
67.3 b (60.4, 73.6) |
| Did the ad list any telephone numbers? | % who said yes (correct answer for all conditions) | 34.5 a (27.8, 41.8) |
44.1 a (37.1, 51.2) |
63.2 b (56.0, 69.8) |
64.6 b (57.9, 70.8) |
| Did you notice a statement about FDA in the ad for Zintria? | % who said yes (incorrect answer for control condition, correct answer for all other conditions) | 26.4 a (29.3, 33.5) |
50.5 b (43.5, 57.6) |
73.3 c (66.5, 79.2) |
77.0 c (70.5, 82.4) |
| What did the statement say? 1 | % whose open-ended responses were coded as “to report side effects” or “call FDA” (guessing for control condition, correct answer for all other conditions) | 0 a | 34.9 b (26.5, 44.3) |
55.9 c (47.5, 64.0) |
65.6 c 57.9, 72.6) |
| Why should you contact FDA, according to the statement? 1 | % who selected “to report side effects” (guessing for control condition, correct answer for all other conditions) | 31.9 a (19.1, 48.1) |
49.5 a (40.0, 59.0) |
71.2 b (62.5, 78.6) |
77.8 b (70.3, 83.9) |
| Did you notice a statement about reporting side effects? 2 | % who said yes (incorrect answer for control condition, correct answer for all other conditions) | 70.3 a (61.8, 77.5) |
74.5 a (60.9, 84.5) |
79.5 a (65.5, 88.7) |
78.9 a (69.8, 85.9) |
| Which, if any, of the following appeared in the ad? | % who selected the toll-free statement (incorrect answer for control condition, correct answer for all other conditions) | 18.0 a (12.9, 24.5) |
50.9 b (43.8, 57.9) |
73.9 c (67.1, 79.8) |
76.6 c (69.8, 82.3) |
Note. Means sharing a superscript were not significantly different at the Bonferroni-adjusted p < .008.
These questions were asked only of participants who responded “yes” when asked whether they noticed a statement about FDA in the ad.
This question was asked only of participants who responded “no” when asked whether they noticed a statement about FDA in the ad.
Clarity of Toll-Free Statement
As noted above, over 60% of participants in all conditions rated the toll-free statement as extremely clear. There were no differences in ratings of toll-free statement clarity by placement, duration, or prominence (all ps > .30).
Discussion
This study examined effects of placement, duration, and prominence of the FDAAA toll-free statement presented in a direct-to-consumer prescription drug television ad. The analysis focused on three primary sets of outcomes: (1) comprehension of the risk and benefit information detailed in the ad, (2) the ability to remember the presence and content of the toll-free statement, and (3) an adequate understanding of the toll-free statement (i.e., that the toll-free statement encourages consumers to contact the FDA to report side effects).
Based on the results of this study, the presence of the toll-free statement appears unlikely to adversely affect consumers’ processing of drug risk and benefit information in television ads. Specifically, the presence of the toll-free statement was not found to interfere with comprehension of the drug risk and benefit information in any experimental group in any design, regardless of placement, duration, or prominence. On the one hand, this is reassuring because the risk and benefit information are the key pieces of information in DTC ads and anything that interfered with their processing would be ill-advised. On the other hand, the fact that we showed no differences in the processing of risk information even when the toll-free statement was displayed during the major statement is curious. We explored some reasons why this may occur in our research approach section. To reiterate, it is possible that the content of the toll-free statement is not sufficiently relevant to most individuals, particularly those encountering a prescription drug that is new to them. This contention is supported by our finding that only about 30% of participants across experimental groups accurately recalled the content of the toll-free statement. Individuals who have experienced a negative side effect, particularly one from the advertised drug in question, may pay more attention to such a directive (Rucker and Petty, 2006). Additionally, the majority of people may have realized that the list of risks and side effects is more important than the toll-free statement and chose to focus their attention on those risks and side effects.
Another possibility is that DTC ads in general are already densely packed with information and the addition of another piece of information is not likely to have an additive effect. FDA regulations require that advertising for prescription drugs include fair balance of both the risks and benefits, in terms of both content and presentation (Code of Federal Regulations, 2014b). In order to maintain this balance, more information is present in DTC ads than in other product categories. Additionally, this information is densely medical in nature, making it more difficult to process and increasing cognitive load. FDA encourages pharmaceutical companies to present information in consumer-friendly language, but it can be admittedly difficult to translate some complex, although important, concepts. Given the density of DTC ads without the toll-free statement, perhaps it is not surprising that an additional concept does not interfere with the risk and benefit information. Our findings provide some assurance that incorporating additional information into a dense ad will not cause more difficulty with the risk information.
One important purpose of the study was to determine whether the toll-free statement would interfere with risk and benefit information. Another key purpose was to determine what people absorbed from the toll-free statement itself. Overall, approximately 30% of participants were able to recall and comprehend the purpose of the toll-free statement, but this varied by experimental condition. Placement and prominence of the toll-free statement affected consumers’ comprehension of and memory for the toll-free statement. Placement of the toll-free statement before the major statement resulted in worse processing outcomes than placement during the major statement of drug risks, after the major statement, and during the whole ad. Increasing the prominence of the toll-free statement through the addition of reinforcing audio to the on-screen text (“text super + short audio” and “text super + audio”) resulted in better processing outcomes as compared with text-only and control conditions. This is consistent with other work showing that presenting the same information in audio and superimposed visual text (dual-mode processing) improves recall of the communicated information over and above that of audio mode alone (see, for example, Morris, Mazis, and Brinberg 1989; Murray, Manrai, and Manrai 1998). Duration of the toll-free statement did not impact memory or comprehension outcomes. It is unclear why no effects were shown in this design between the whole ad condition and the other conditions, which mimicked those in the placement design.
Interestingly, we found very few differences in our study between those who have been diagnosed with HBP, the medical condition Zintria purports to treat, and those who have not been diagnosed. It is possible that some participants misreported their medical condition, leading to a dilution of any effects we may have found. Previous research suggests that consumers, depending on their motivations, respond to DTC advertising in a variety of ways (Arney, Street, and Naik 2013). We suspected that people who have personal experience with a topic will be motivated to process information about it more avidly. In this case, HBP, a primarily asymptomatic condition, did not appear to increase the motivation of participants across the board, consistent with previous research (McLean et al., 2012). Despite being diagnosed, people may not identify as sufferers of HBP as they might identify as someone who has diabetes or has survived cancer because HBP is silent and results in long term rather than immediate effects. It is also possible that participants with HBP were more motivated in our study but that motivation did not influence the outcomes we measured. Finally, previous research has shown that involvement is important for processing print advertising (Greenwald and Leavitt 1984) but Buchholz and Smith (1991) suggest that involvement may not be as important a factor in processing television ads.
Limitations
Our study has a number of limitations which should be considered. First, although participants were randomly assigned to conditions, we performed separate analyses within placement, duration, and prominence. Because some combinations of the factors would not have been possible (e.g., audio presentations cannot be presented for all placement conditions), we chose to examine factors independently. However, this eliminated the ability to examine interaction effects. Second, within the prominence design, the ads with the toll-free statement in the audio were slightly longer than the ads without the toll-free statement in the audio. Thus, participants in the “text super + audio” conditions were exposed to the text for a longer period of time, and thus outcomes may not be solely a result of dual modality. Future investigations that included another text only condition matched to the time spent in the “text super + audio” condition would allow us to tease out the effects of duration from dual modality. Nonetheless, we did not find effects of duration itself, reducing the likelihood that this caused the prominence effects. Third, this study only tested one ad for one medical condition (i.e., HBP). Examination of additional ads for varying medical conditions would provide confidence in the generalizability of our findings. Fourth, the structured presentation of ads in the experimental procedure does not reflect the way people generally are exposed to DTC ads (i.e., during unstructured television viewing). We presented the test conditions as realistically as possible by showing the ad in conjunction with three other ads, including one real DTC ad. Finally, due to the bandwidth required to view the videos, only panel participants with broadband internet service were eligible to participate. This may have introduced bias in that participants with broadband internet service may be different demographically or in other ways that impact the results. We attempted to correct for this through the random assignment inherent in an experimental design. Further, weighting was used to ensure that the study data more accurately represented the target population and helped account for nonresponse, noncoverage, underrepresentation of minority groups, and other types of sampling and survey error.
Although not a strict limitation of the study, we note that 18% of participants in the control condition answered “yes” when asked if they had seen a statement about FDA in the ad. Although this could be simple confusion about the particular statement inquired about, this could also represent “yea-saying” on the part of participants; that is, the tendency to say “yes” or agree in response to questions, regardless of the participants’ actual feelings or knowledge (Hurd and Kapteyn 2000). Individuals may “yea-say” for a number of reasons, including social desirability (Leggett et al. 2003) and expediency (Knowles and Condon 1999). Therefore, erring on the side of caution, there may be an 18% overstatement of recognition of the toll-free statement.
Implications for Policy
The results of our study indicate that use of the toll-free statement in DTC television ads will not adversely impact comprehension of product risk and benefit information. Moreover, our data suggest that the toll-free statement itself is well understood. Participants found the toll-free statement understandable and clearly recognized its intent to encourage reporting of side effects to FDA. These findings suggest that consideration should be given to the inclusion of the toll-free statement in television DTC ads. Doing so would reach a larger audience that would then have a more accessible route for reporting adverse events, helping FDA make informed decisions about prescription drugs on the market.
From an industry standpoint, PhRMA, the Pharmaceutical Research and Manufacturers of America, has incorporated the FDAAA MedWatch recommendations into their DTC guidelines (PhRMA 2008). The updated principles took effect on March 2, 2009 (PhRMA 2013). As of April 22, 2014, 26 companies had committed to abide by the principles, representing approximately 54% of the PhRMA members. As mandated by law, the MedWatch number appears in DTC print ads, though its use in DTC TV ads is rare, which may represent discomfort on the part of PhRMA members to proactively include this toll-free statement in TV ads in the absence of specific FDA guidance on this issue.
Although FDAAA does not provide specific parameters for how the toll-free statement should be displayed, it is useful to look to other agencies for guidance. The Federal Trade Commission (FTC) regulates the advertising of foods, dietary supplements, and over-the-counter drugs. Because FDA and FTC operate under different regulations, the advertisements (ads) for these various products are subject to different regulatory requirements. In its “Clear and Conspicuous” Standard, the Federal Trade Commission (FTC 1970, 1983) states that for disclosures to be effective, they should be presented simultaneously in both an ad’s audio and video. Our findings regarding the prominence of the toll-free statement confirm prior research and reinforce the approach advocated by the FTC for disclosures. Presenting the toll-free statement in both text and audio leads to better comprehension and memory for the toll-free statement.
Our findings also suggest specific strategies to enhance comprehension of and memory for the toll-free statement without threatening processing of other important product messages. The results indicate that placement of the toll-free statement during or after the major statement of risks, or placement of the toll-free statement during the entire ad, will lead to better comprehension of and memory for the toll-free statement in television ads. The implementation of these results must be balanced with other regulatory requirements. Specifically, Section 901(d)(3)(A) of FDAAA (Public Law No. 110–85) requires “…television and radio advertisements for human prescription drugs to present the major statement (i.e., the disclosure of the major side effects and contraindications of the drug) in a clear, conspicuous, and neutral manner, regardless of the manner in which effectiveness information is presented in the advertisement.” (FDA, 2010). As part of its proposed rule meeting this requirement, FDA has proposed that information in the major statement be presented in both the audio and text portion of the ad. Further, the major statement must not be presented with other elements that might distract from or otherwise interfere with the risk information. Therefore, consideration should be given to the placement of the toll-free statement so that it does not occur during the major statement, avoiding potential interference.
Overall, our study demonstrates that implementing the television presence of the toll-free statement is not likely to adversely affect the comprehension of the risks and may result in greater awareness of the vehicle for reporting adverse events. Although only 15% of participants recalled the content of the toll-free statement, television DTC ads are widely dispersed. Thus, this percentage could translate to thousands of extra eyes and increased usage of the MedWatch system. Creating awareness of this outlet for reporting negative side effects of prescription drugs may empower and encourage consumers to contribute their voice in ensuring the safe use of prescription drugs.
Appendix A
Excerpts from the Questionnaire
This study is about advertising for new products. You will see four ads and then answer questions about what you’ve seen. The study will take about 20 minutes.
[PROGRAMMER: Show ads in the following order:
15-second non-DTC filler ad 1
30-second unrelated DTC ad (Nasonex®)
15-second non-DTC filler ad 2
~60-second appropriate Zintria ad
Please answer the following questions.
Q1b. Did the ad for Zintria list any Web sites?
- Yes
- No
- Don’t know
Q1c. Did the ad for Zintria list any phone numbers?
- Yes
- No
- Don’t know
Q1d. Did you notice a statement about FDA in the ad for Zintria?
- Yes (ask Q1e and Q1f, do not ask Q1g)
- No (skip to Q1g)
- Don’t know (skip to Q1g)
Q1e. What did the statement say? (open-ended: codes below)
- Participate in a study/clinical trial
- Seek medical advice
- Get more information
- Report side effects
- Contact drug company
- Call FDA
- Other
Q1f. Why should you contact FDA, according to the statement?
- To participate in a clinical trial
- To seek medical advice
- To get more information about the drug
- To report side effects
- None of the above
- Don’t know
Q1g. Did you notice a statement about reporting side effects in the Zintria ad?
- Yes
- No
- Don’t know
-
Q1h. Which, if any, of the following statements appeared in the ad? You may select more than one.
[PROGRAMMER: randomize]
-
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088. [FDAAA]ORCall your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. [TNFR]
- 800–555-ZINT
- Clinical trials involved men and women over the age of 18 and were conducted up to 12 months.
- For more information on reduced cost prescription drug programs, contact FDA at 1-800-FDA-1088.
- Individual results may vary.
-
Q2. How well do you think Zintria would or would not work for you?
Q3. How safe or not safe do you think Zintria is?
Q4. How likely or not likely would you be to lower your blood pressure if you took Zintria?
Q5. How risky or not risky do you think Zintria is?
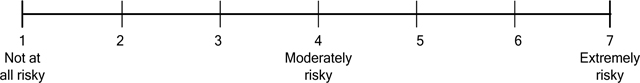
-
Q7a. (Recall of Risks) Based on the ad for Zintria that you saw, please indicate whether each of the following topics was included in the ad:
[PROGRAMMER: randomize]
Yes No Don’t Know a. Taking some kinds of over-the-counter cough medicines at the same time as taking Zintria increases your risk of having a heart attack. b. Zintria may cause excitability. c. If you have a very slow heart rate, you should not take Zintria. d. Zintria may cause you to have blurry vision. e. One of the most common side effects of Zintria is tiredness. f. Antibiotics may not work as well if you use Zintria at the same time. g. You should have regular eye exams when you take Zintria. h. One of the most common side effects of Zintria is nausea. -
Q7b. (Comprehension of Risks)
Please answer the following questions based on the information in the ad for Zintria:
[PROGRAMMER: randomize items and item responses]
- Why should you NOT stop taking Zintria suddenly?
- You may have unusual changes in behavior.
- Your eyes will have trouble adjusting to the change in pressure.
- You may have a temporary loss of coordination.
- You may experience chest pain.
- Don’t know.
- When you first take Zintria, why should you avoid activities that require you to be alert?
- You may have a temporary loss of coordination.
- A common side effect of Zintria is dizziness.
- A common side effect of Zintria is nervousness.
- You may faint.
- Don’t know.
- Why might you have blurry vision when taking Zintria?
- Zintria lowers the pressure in the eye.
- Zintria increases the chance of chronic dry eye.
- Zintria lowers the concentration of red blood cells in the eye.
- Zintria increases sensitivity to light.
- Don’t know.
-
Q8a. (Recall of Benefits) Based on the ad for Zintria that you saw, please indicate whether each of the following topics was included in the ad:
[PROGRAMMER: randomize]
Yes No Don’t Know a. It is safe to take Zintria with aspirin or other pain relievers. b. Zintria helps lower your blood pressure. c. You take Zintria only once a month. d. Zintria can be taken with or without food. e. Zintria can reduce your risk of having a stroke. f. Zintria is the only high blood pressure medication approved to treat children. g. Zintria is proven to help prevent heart attacks. Q8b. (Comprehension of Benefits) Please choose a response based on the information you learned in the ad. [PROGRAMMER: randomize items and item responses]
-
- What advantage does Zintria have over other treatments for this condition?
- Zintria is taken only once a month.
- Zintria is approved to treat more than one type of high blood pressure.
- Zintria helps lower cholesterol.
- Zintria helps you lose weight.
- Don’t know.
- Why would your doctor prescribe Zintria for you?
- To increase my blood circulation
- To reduce the risk of liver damage
- To reduce the risk of stroke
- To decrease my joint pain
- Don’t know
[PROGRAMMER: show the correct statement while Questions 9–12 appear]
Q10. How understandable is this statement?

Q11. How confusing is this statement?
Q12. How clear is this statement?
Footnotes
Note that use of brand name products in this study does not imply endorsement by FDA.
References
- Authors. (in press). Who Said It Better? A Test of Wording Differences in the MedWatch “Toll-Free Statement” for Consumer Reporting of Side Effects in Direct-To-Consumer Television Advertisements. Therapeutic Innovation and Regulatory Science. [DOI] [PMC free article] [PubMed]
- Aikin Kathryn, J., O’Donoghue Amie C., Swasy John L., and Sullivan Helen W. (2011), “Randomized Trial of Risk Information Formats in Direct-to-Consumer Prescription Drug Advertisements,” Medical Decision Making, 31, E23–E33. [DOI] [PubMed] [Google Scholar]
- Anderson Simon P., Ciliberto Frederico, and Liaukonyte Jura (2013), “Information Content of Advertising: Empirical Evidence From the OTC Analgesic Industry,” International Journal of Industrial Organization, 31, 355–367. [Google Scholar]
- American Association for Public Opinion Research Response (2011), “Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys,” (accessed June 3, 2014), [available at http://aapor.org/Content/NavigationMenu/AboutAAPOR/StandardsampEthics/StandardDefinitions/StandardDefinitions2011.pdf].
- Arney Jennifer, Street Richard L., and Naik Aanand D. (2013), “Consumers’ Various and Surprising Responses to Direct-to-Consumer Advertisements in Magazine Print,” Patient Preference and Adherence, 7, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson Richard C., and Shiffrin Richard M. (1968), “Human Memory: A Proposed System and its Control Processes,” In Spence KW and Spence JT (Eds.), The Psychology of Learning and Motivation (Vol. 2, pp. 89–195). Orlando, FL: Academic Press. [Google Scholar]
- Atkinson Richard C. and Shiffrin Richard M. (1971), “The Control of Short-Term Memory,” Scientific American, 225, 82–90. [DOI] [PubMed] [Google Scholar]
- Bland Martin J. and Altman Douglas G. (1997), “Statistics Notes: Cronbach’s Alpha,” British Medical Journal, 314, 572.9055718 [Google Scholar]
- Buchholz Laura M. and Smith Robert E. (1991), “The Role of Consumer Involvement in Determining Cognitive Response to Broadcast Advertising,” Journal of Advertising, 20(1), 4–17. [Google Scholar]
- Charlton Samuel G. (2009), “Driving While Conversing: Cell Phones That Distract and Passengers Who React,” Accident Analysis and Prevention, 41, 160–173. [DOI] [PubMed] [Google Scholar]
- Code of Federal Regulations (2014a), 21 CFR 310.305 (drugs); 21 CFR 600.80 (biologics); 21 CFR 803 (devices).
- Code of Federal Regulations (2014b), 21 CFR 202.1(e)(6).
- Dal Pan J. Gerald (2014). Ongoing challenges in pharmacovigilance. Drug Safety, 37, 1–8. [DOI] [PubMed] [Google Scholar]
- Du Dongyi Tony John Goldsmith, Aikin Kathryn J., Encinosa William E., and Nardinelli Clark. (2012), “Despite 2007 Law Requiring FDA Hotline to be Included in Print Drug Ads, Reporting of Adverse Events by Consumers Still Low,” Health Affairs, 31(5), 1022–1029. [DOI] [PubMed] [Google Scholar]
- Federal Trade Commission (1970), “Statement of enforcement policy (October 21), CCH Trade Regulation Reporter, 7569.09.
- Federal Trade Commission (1983), “Federal Trade Commission Policy Statement on Deception, Appended to Cliffdale Associates, Inc., 103 F.T.C. 110 (1984),” (accessed July 2, 2014). [available at http://www.ftc.gov/public-statements/1983/10/ftc-policy-statement-deception].
- Food and Drug Administration (1999), “Consumer-Directed Broadcast Advertisements,” (accessed June 3, 2014), [available at HTTP://WWW.FDA.GOV/DOWNLOADS/DRUGS/GUIDANCECOMPLIANCEREGULATORYINFORMATION/GUIDANCES/UCM070065.PDF].
- Food and Drug Administration (2008), “Toll-Free Number for Reporting Adverse Events on Labeling for Human Drug Products: Final Rule,” 73 FR 209, 63886–63987. [PubMed]
- Food and Drug Administration (2010), “Direct-to-Consumer Prescription Drug Advertisements; Presentation of the Major Statement in Television and Radio Advertisements in a Clear, Conspicuous, and Neutral Manner,” 75 FR 59, 15376–15387.
- Food and Drug Administration (2014a), MedWatch: The FDA Safety Information and Adverse Event Reporting Program. Retrieved from http://www.fda.gov/safety/medwatch/default.htm, October 6, 2014.
- Food and Drug Administration (2014b), FDA Adverse Event Reporting System (FAERS). Retrieved from http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/, February 10, 2015.
- Food and Drug Administration (2015a). MedWatch What’s New Archive, 2015. Retrieved from http://www.fda.gov/Safety/MedWatch/ucm429847.htm, June 11, 2015.
- Food and Drug Administration (2015b), FAERS Reporting by Healthcare Providers and Consumers by Year. Unpublished data.
- Frosch Dominick L., Krueger Patrick M., Hornik Robert C., Cronholm Peter F., and Barg Frances K. (2007), “Creating Demand for Prescription Drugs: A Content Analysis of Television Direct-to-Consumer Advertising,” Annals of Family Medicine, 5(1), 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert Daniel T. and Hixon J. Gregory (1991), “The Trouble of Thinking: Activation and Application of Stereotypic Beliefs,” Journal of Personality and Social Psychology, 60(4), 509–517. [Google Scholar]
- Greenwald Anthony G. and Leavitt Clark (1984), “Audience Involvement in Advertising: Four levels,” Journal of Consumer Research, 11, 581–592. [Google Scholar]
- Hicks Charles R. and Turner Kenneth V. Jr. (1999), Fundamental Concepts in the Design of Experiments, 5th ed. New York, NY: Oxford University Press. [Google Scholar]
- Hintzman Douglas L. (2010), “How Does Repetition Affect Memory? Evidence from Judgments of Recency,” Memory & Cognition, 38(1), 102–115. [DOI] [PubMed] [Google Scholar]
- Hurd Michael D. and Kapteyn Arie (2000), “Anchoring and Acquiescence Bias in Measuring Assets in Household Surveys,” Netherlands: Springer. [Google Scholar]
- Jacoby Jacob (1984), “Perspectives on Information Overload,” Journal of Consumer Research,10(4), 432–435. [Google Scholar]
- Jacob Larry L., and Wahlheim Christopher N. (2013), “On the Importance of Looking Back: The Role of Recursive Remindings in Recency Judgments and Cued Recall,” Memory & Cognition, 41, 625–637. [DOI] [PubMed] [Google Scholar]
- Kessler David A. (1993), “Introducing MEDWatch: A new approach to reporting medication and device adverse effects and product problems,” JAMA, 21(269), 2765–2768. [DOI] [PubMed] [Google Scholar]
- Knowles Eric S. and Condon Christopher A. (1999), “Why People Say ‘Yes’: A Dual-Process Theory of Acquiescence,” Journal of Personality and Social Psychology, 77(2), 379–386. [Google Scholar]
- Leggett Christopher G., et al. (2003), “Social Desirability Bias in Contingent Valuation Surveys Administered Through In-Person Interviews,” Land Economics, 79(4), 561–575. [Google Scholar]
- Liang Bryan A. and Mackey Tim (2011), “Reforming Direct-to-Consumer Advertising,” Nature Biotechnology, 29(5), 397–400. [DOI] [PubMed] [Google Scholar]
- McLean Rachael, Hoek Janet, and Hedderley Duncan (2012), “Effects of Alternative Label Formats on Choice of High- and Low-Sodium Products in a New Zealand Population Sample,” Public Health Nutrition, 15(5), 783–791. [DOI] [PubMed] [Google Scholar]
- Morris Louis A., Mazis Michael B., and Brinberg David (1989), “Risk Disclosures in Televised Prescription Drug Advertising to Consumers,” Journal of Public Policy and Marketing, 8, 64–80. [Google Scholar]
- Murray Noel M., Manrai Lalita A., and Manrai Ajay K. (1998), “How Super are Video Supers? A Test of Communication Efficacy,” Journal of Public Policy and Marketing, 17(1), 24–34. [Google Scholar]
- O’Donoghue Amie C., Sullivan Helen W., Aikin Kathryn J., Chowdhury Dhuly, Moultrie Remecca R., and Rupert Douglas J. (2014), “Presenting Efficacy Information in Direct-to-Consumer Prescription Drug Advertisements,” Patient Education and Counseling, 95, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler Harold (1994), “Dual-Task Interference in Simple Tasks: Data and Theory,” Psychological Bulletin, 116(2), 220–244. [DOI] [PubMed] [Google Scholar]
- PhRMA (2008), “PhRMA Guiding Principles: Direct to Consumer Advertisements About Prescription Medicines,” Retrieved from http://www.phrma.org/sites/default/files/pdf/phrmaguidingprinciplesdec08final.pdf (10/7/14).
- PhRMA (2013), “Providing Accurate Information about Disease and Treatment Options,” Retrieved from http://www.phrma.org/direct-to-consumer-advertising (10/7/14).
- Pieters Rik, Wedel Michel, and Zhang Jie (2007), “Optimal Feature Advertising Design Under Competitive Clutter,” Management Science, 53(11), 1815–1828. [Google Scholar]
- Rucker Derek D., and Petty Richard E. (2006), “Increasing the Effectiveness of Communications to Consumers: Recommendations Based on Elaboration Likelihood and Attitude Certainty Perspectives,” Journal of Public Policy & Marketing, 25(1), 39–52. [Google Scholar]
- Stewart Dennis D., Stewart Cheryl B., Tyson Clare, Vinci Gail, and Fioti Tom (2004), “Serial Position Effects and the Picture-Superiority Effect in the Group Recall of Unshared Information,” Group Dynamics: Theory, Research and Practice, 8(3), 166–181. [Google Scholar]
- Sweller John (1988), “Cognitive Load During Problem Solving: Effects on Learning,” Cognitive Science, 12, 257–85. [Google Scholar]
- Tan Lydia and Ward Geoff (2000), “A Recency-Based Account of the Primacy Effect in Free Recall,” Journal of Experimental Psychology: Learning, Memory and Cognition, 26(6), 1589–1625. [DOI] [PubMed] [Google Scholar]
- Traxler Matthew J. (2008), “Lexically Independent Priming in Online Sentence Comprehension,” Psychonomic Bulletin and Review, 15(1), 149–155. [DOI] [PubMed] [Google Scholar]
- van Grootheest Kees, Linda de Graaf, and Lolkje TW. de Jong-van den Berg (2003), “Consumer Adverse Drug Reaction Reporting: A New Step in Pharmacovigilance?” Drug Safety, 26(4), 211–217. [DOI] [PubMed] [Google Scholar]
- van Grootheest Kees and Lolkje TW. de Jong-van den Berg (2004), “Patients’ Role in Reporting Adverse Drug Reactions,” Expert Opinion on Drug Safety, 3(4), 363–368. [DOI] [PubMed] [Google Scholar]
- Waugh Nancy C., and Norman Donald A. (1965), “Primary Memory,” Psychological Review, 72, 89–104. [DOI] [PubMed] [Google Scholar]
- Wogalter Michael S., Shaver Erik F., and Kalsher Michael J. (2014), “Effect of Presentation Modality in Direct-to-Consumer (DTC) Prescription Drug Television Advertisements,” Applied Ergonomics, 45(5), 1330–1336. [DOI] [PubMed] [Google Scholar]
- Woloshin Steven, Schwartz Lisa M., and Welch Gilbert H. (2009), “Using a Drug Facts Box to Communicate Drug Benefits and Harms: Two Randomized Trials,” Annals of Internal Medicine, 150, 516–527. [DOI] [PubMed] [Google Scholar]