Abstract
AIM
To perform an overview of systematic reviews and more recent randomized controlled trials (RCTs) on early motor interventions in infants aged 0 to 3 years with or at risk of cerebral palsy to inform current clinical and research efforts and provide a benchmark to assess future interventions ideally initiated within the first 6 months.
METHOD
Standardized searches of the PubMed, Embase, Scopus, and Web of Science databases were conducted for systematic reviews (2009–2020) and RCTs (2015–2020).
RESULTS
From 840 unique records, 31 full texts were reviewed, yielding three systematic reviews encompassing 46 studies, 16 with comparison groups, and six additional RCTs that met the criteria. Two enrichment- and activity-based approaches had medium effect sizes on motor development, only one with low risk of bias; two others had large task-specific effect sizes but some bias concerns; and three enriched environment studies with some bias concerns had medium effect sizes on cognitive development. Most had small or no effect sizes, bias concerns, and uncertain diagnostic determinations.
INTERPRETATION
Data synthesis revealed limited data quantity and quality, and suggest, although not yet confirmed, greater benefit from early versus later intervention. Research efforts with greater early diagnostic precision and earlier intervention are accelerating, which may transform future outcomes and practices.
Abstract
OBJETIVO
Realizar un resumen de revisiones sistemáticas y ensayos controlados aleatorios (ECA) más recientes sobre intervenciones motoras tempranas en bebés de 0 a 3 años con o en riesgo de parálisis cerebral para informar los esfuerzos clínicos y de investigación actuales y proporcionar un punto de referencia para evaluar las intervenciones futuras de manera ideal iniciado dentro de los primeros 6 meses.
MÉTODO
Se realizaron búsquedas estandarizadas en las bases de datos PubMed, Embase, Scopus y Web of Science para revisiones sistemáticas (2009-2020) y ECA (2015-2020).
RESULTADOS
De 840 registros únicos, se revisaron 31 textos completos, lo que arrojó tres revisiones sistemáticas que abarcaron 46 estudios, 16 con grupos de comparación y seis ECA adicionales que cumplieron con los criterios. Dos enfoques basados en el enriquecimiento y la actividad tuvieron tamaños de efecto medios sobre el desarrollo motor, solo uno con bajo riesgo de sesgo; otros dos tenían grandes tamaños de efecto específicos de la tarea, pero algunos problemas de sesgo; y tres estudios de entornos enriquecidos con algunas preocupaciones sobre el sesgo tuvieron tamaños de efecto medios sobre el desarrollo cognitivo. La mayoría tenía tamaños de efecto pequeños o nulos, problemas de sesgo y determinaciones diagnósticas inciertas.
INTERPRETACIÓN
La síntesis de datos reveló una cantidad y calidad de datos limitadas, y sugiere, aunque aún no se ha confirmado, un mayor beneficio de la intervención temprana frente a la posterior. Se están acelerando los esfuerzos de investigación con una mayor precisión de diagnóstico temprano y una intervención más temprana, lo que puede transformar los resultados y las prácticas futuras.
Abstract
OBJETIVO
Realizar uma revisão de revisões sistemáticas e estudos clínicos randomizados (ECRs) sobre intervenções precoces motoras em lactentes de 0 a 3 anos com paralisia cerebral ou de risco, para informar esforços clínicos e de pesquisas atuais e para fornecer uma base para avaliar futuras intervenções idealmente iniciadas nos primeiros 6 meses.
MÉTODO
Buscas padronizadas nas bases de dados PubMed, Embase, Scopus, e Web of Science foram realizadas quanto a revisões sistemáticas (2009–2020) e ECRs (2015–2020).
RESULTADOS
De 840 registros únicos, 31 textos completos foram revisados, reultado em 3 revisões sistemáticas que envolveram 46 estudos, 16 com grupo de comparação, e seis ECRs adicionais que atenderam aos critérios. Duas abordagens de enriquecimento e baseadas em atividades tiveram tamanhos de efeito médios para o desenvolvimento motor, apenas uma com baixo risco de viés; duas outras tiveram grandes tamanhos de efeito tarefa-específicos mas algumas preocupações com viés; e três estudos com ambientes enriquecidos com algumas preocupações relativas ao viés tiveram tamanhos de efeito médios para o desenvolvimento cognitivo. A maioria tinha tamanhos de efeito pequenos ou inexistentes, preocupações com viés, e determinações diagnósticas incertas.
INTERPRETAÇÃO
A síntese de dados revelou limitada quantidade e qualidade de dados e sugere, embora ainda não confirmado, maior benefício da intervenção precoce versus tardia. Esforços de pesquisas com maior precisão diagnóstica precoce e intervenção mais precoce têm se acelerado, o que pode transformar os resultados e práticas futuros.
Cerebral palsy (CP) is a group of disorders characterized by injuries to sensorimotor regions of the brain early in development. CP is characterized initially by delayed acquisition of infant motor milestones and impairments in motor functioning that persist throughout the lifespan. It is the most prevalent motor disability originating in early childhood with a reported incidence of 2 per 1000 live births1 in higher resource countries, where early intervention is typically recommended when the diagnosis is made or suspected. The prevalence of CP in developing countries is less well known, although more accurate reports are starting to emerge, and the extent to which early intervention is available in these settings is often limited.
Early intervention generally refers to a system of services available for infants or toddlers from birth until 3 years of age who are identified with a specific developmental disorder or as being at risk for delays in one or more aspect of functioning and their families or others who care for them. The primary goal of early intervention is to support the child’s development, functioning, and participation within the context of the family and community, which is consistent with the framework of the International Classification of Functioning, Disability and Health. Early intervention services have been available in many countries for decades based on the premise that supporting the child and family early during development leads to better long-term outcomes for the child and improves the well-being of the family. However, a major impact on developmental outcomes has somewhat surprisingly not been consistently demonstrated in the literature.2 Potential explanations for the limited effectiveness of early intervention on child outcomes include the types of interventions provided, insufficient intervention doses, and/or more recently the concern that the intervention was not delivered early enough to significantly alter the developmental trajectory. Basic neuroscience evidence from animal models of perinatal brain injury suggests that very early motor training may facilitate recovery of the brain lesion and improve the developmental outcome and motor prognosis.3,4 However, a major challenge in providing earlier intervention has been the creation and large-scale implementation of more precise earlier identification strategies for children with specific neurodevelopmental disorders. Significant strides have been made in recent years in reducing the mean age at which infants receive a diagnosis of CP.5,6 Consequently, several studies that focus on earlier identification and intervention are now emerging that may enable the field to determine whether outcomes from early intervention in CP can be improved beyond those reported historically in older children and/or cohorts that included individuals who did not later receive a CP diagnosis.
Perhaps not coincidentally, several systematic reviews have also been published in recent years evaluating the effectiveness of early intervention therapies, some of which specifically evaluated those provided within the first year or two of life. Systematic reviews became more prevalent after study numbers increased; thus, it is logical that reviews at the next level are warranted since the numbers of systematic reviews have increased, a step forward that has been adopted by Cochrane and others.7 Therefore, we chose to conduct an overview of systematic reviews, rather than conduct yet another systematic review of individual studies, on early intervention in individuals with CP with the following as our primary PICO (participants, interventions, comparators and outcomes) question: for children aged 0 to 3 years explicitly identified with or at high risk of CP, which therapeutic motor interventions demonstrate evidence of greater efficacy or effectiveness for enhancing developmental outcomes compared to a control group? With our aim to summarize and synthesize the recent literature, we performed an overview of systematic reviews published within the past decade on early intervention for very young children with or at high risk of CP. We intentionally did not include systematic reviews on groups of infants with known risk factors for CP (e.g. preterm birth), many of whom may not ultimately receive a diagnosis of CP, or at risk of neurodevelopmental disorders in general that did not explicitly state that the intervention was intended for those at risk of or having CP. Given that neuroscience evidence suggests that earlier intervention can have a large positive effect on infant development across multiple domains, all evaluated developmental outcomes were of interest in this review. We further wanted to reflect the interdisciplinary approach in early intervention whereby many aspects of development are addressed concurrently and emphasize that while delayed or atypical motor development is a defining characteristic of CP, many aspects of functioning are often affected.8
Cochrane provides extensive guidance on how to perform overviews of reviews aimed at summarizing current evidence on a specific topic.9 The optimal decision criteria for performing an overview as outlined by Ballard and Montgomery10 are: (1) limited overlap in the primary studies across reviews; (2) similar and typically more narrow scope in the systematic reviews than in the overview PICO question; (3) the presence of high-quality data for the synthesis; and (4) that the reviews be up to date. While these may not be fully known until after the search process is complete, we suspected some data overlap, so we decided a priori that overlap would be specifically evaluated and key details on primary studies extracted. Similarly, we determined that scope, quality, and timeliness would be evaluated once searches were complete and addressed at that time as needed. Beyond reporting similarities or differences in conclusions across reviews, we aimed primarily to explore the contributions and quality of all included papers with comparison or control groups to better understand the sources of provided recommendations and avoid repetition or inflation of any one finding due to overlap. Our ultimate aims were to inform current research and clinical practice and provide a baseline from which to compare future research on children with CP diagnosed at earlier mean ages and interventions implemented far sooner within the first years or ideally months of life.
METHOD
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in performing this review, as well as guidance from recent publications describing the overview methodology.11–13
Search strategy
In consultation with a medical librarian at the National Institutes of Health, the following four scientific databases were searched: PubMed, Embase, Scopus, and Web of Science. The search strategies individualized for each source are listed in Appendix S1 (online supporting information). The search was restricted to the English language and publication for the years 2009 to 2020 to summarize the most up-to-date evidence available, with 12th November 2020 as the final search date.
Selection criteria
Duplicates were removed from the initial combined list of citations and the titles and abstracts of all remaining articles were reviewed separately by the two authors. The lists of the retained articles were then compared and any differences resolved through discussion to determine which papers to include for full-text review.
Types of studies
We included all reviews or meta-analyses of randomized trials only or both randomized and non-randomized trials that compared two groups, at least one of which received an intervention and used systematic methods to minimize bias.
Participant characteristics
The population of interest was children specifically stated as with or at high risk of CP who were less than 3 years of age; if not, data for children aged less than 3 years were discussed separately. Studies that included children in risk categories for CP but who were not explicitly described as with or at high risk of CP (e.g. infants born preterm) or studies that included mixed risk or diagnostic groups and did not describe outcomes for those with or at high risk of CP separately were excluded.
Intervention type
Intervention was restricted to therapeutic or educational motor training approaches (not medical or surgical) as the major focus of the intervention.
Outcomes
Outcomes of interest were those related to motor development or function and other areas of child development. Outcomes related to parents or caregivers were not included.
Final selection and evaluation
The full-text review was completed separately by each author and the final selection of papers was determined similarly through comparison between authors with discussion to resolve any differences. A third person with similar expertise was available in case of unresolved differences. A data extraction spreadsheet was designed by each author. A decision was made a priori to include relevant participant and intervention details for all research studies with comparison or control groups that comprised each review so that we could more fully evaluate and interpret potential similarities and differences across reviews. Data extracted included: all research studies included in the review listed by first author and year with the number of participants in each; the type of study design included in each review; interventions and comparators with dosage for each where present in the included studies; the main results from each study as well as the results of any meta-analysis. One author completed the initial drafts of the data summary tables, which were reviewed and revised by the other author with agreement from both on the final versions.
A risk of bias assessment was performed using the tool recommended by Cochrane for the evaluation of systematic reviews, AMSTAR-2, with this second version adapted for measurement of bias when observational (non-randomized) studies are included.14 This was done independently by each author with disagreements resolved through discussion. If provided in the review, the overall quality of evidence for or against an intervention as assessed with Grading of Recommendations, Assessment, Development and Evaluation (GRADE)15 aimed at improving outcomes in young children with CP was reported; if not, this was determined by the authors. Finally, the risk of bias was recorded or assessed for all primary studies with comparison or control interventions.
Registration
This systematic review was not registered.
RESULTS
Figure S1 (online supporting information) shows the PRISMA flow diagram; the completed PRISMA checklist is provided in Appendix S2 (online supporting information). The database searches yielded a combined total of 816 citations from each of the four databases searched. After all duplicates were removed, 485 articles remained. After review of the titles and abstracts by both authors and discussion to resolve any differences, 21 papers were selected for full-text review. After independent full-text review and further discussion, three systematic reviews remained for data extraction and interpretation that focused on interventions primarily aimed at improving motor function that met the inclusion criteria and were within the scope of our PICO question.16–18 Two reviews were eliminated because they were solely on medical interventions without any therapy recommendations. One was a review of tone management in children aged 0 to 2 years19 and one addressed the use of botulinum neurotoxin A in young infants.20 Other reasons for exclusion were: four were abstracts summarizing the results of a systematic review but no full review had yet been published and three were focused on infants born preterm21–23 and not specifically on CP or risk of CP. One reviewed the extent to which families were involved in early intervention but did not evaluate the effectiveness of the interventions themselves,24 one reviewed coaching practices but did not consider child outcomes,25 and one focused solely on parent–child interactions, not development.26 One was on older children,27 two included mixed or other diagnoses,28,29 and three did not involve younger children and/or were not about CP exclusively.30–32 Table 1 summarizes the data from each of the systematic reviews, including details from each of the included studies and indicating overlap where present.
Table 1:
Summary of the identified systematic reviews (when repeated, citations are in bold and not summarized again)
| Study | Studies included | Study type | Interventions/comparisons | Results |
|---|---|---|---|---|
| Morgan et al.16 | 7 studies (n=366) Badr et al.46 (n=62); Law et al.48 (n=128); Nelson et al.81 (n=37); Ohgi et al.80 (n=23); Palmer et al.49 (n=48); Taub et al.83 (n=18); Wallen et al.47 (n=50) |
RCT | Enriched intensive stimulation using CAMS vs standard care46; 6mo child vs context-focused approaches48; enrichment via multisensory stimulation+standard care vs standard care81; enrichment via training of mother–infant interaction+handling and developmental support using NDT vs standard care80; infant stimulation followed by 6mo of physical therapy vs 12mo of NDT49; CIMT (6h for 21d) vs standard physiotherapy and/or occupational therapy (mean of 2.2h/wk)83; enriched intensive modified CIMT+home program vs enriched intensive occupational therapy+home program47 | No group differences46; child-focused outcomes similar to context-focused48; no group difference on Bayley at 12mo81; no group differences on motor outcomes at 6mo80; significant group differences favoring enrichment at both 6 and 12mo49; CIMT improved more in motor function and new motor patterns83; no group differences in motor outcomes47 Meta-analysis: favorable benefit from enriched environment (environmental enrichment) interventions on motor outcomes; not possible to draw conclusions about individual components of environmental enrichment GRADE quality: moderate |
| Hadders-Algra et al.18 | 3/7 new studies (n=155) Mayo87 (n=29); Weindling et al.50 (n=105); Nelson et al.81 (n=21); Badr et al.46 (n=62); Hielkema et al.86 (n=46) Blauw-Hospers et al.99 (n=46); Morgan et al.45 (n=13); Ohgi et al.80 (n=23) |
RCT | Weekly vs monthly NDT87; NDT vs standard care50; COPCA vs standard physiotherapy86; GAME vs standard care45 | Greater percentage change in motor development for intensive group87; no group differences50; no group differences; NDT hands-on techniques associated with worse mobility at 18mo86; GAS and COPM scores not different; PDMS scores better in the GAME group45 General conclusion: developmental stimulation may improve cognitive outcomes. No convincing evidence that NDT or developmental stimulation improves motor outcomes GRADE quality: low |
| Morgan et al.17 | 28/34 new studies (n=260); 6/12 new studies with control groups (n=151) Batra et al.40 (n=15); Campbell et al.85 (n=46); Palmer et al.100 (n=48); Park et al.84 (n=26); Shamir et al.41 (n=10); Mahoney et al.43 (n=6) No control group (n=109) Coker57 (n=1); Dickerson58 (n=1); Horn et al.59 (n=4); Kanda et al.60 (n=8); Kinghorn61 (n=1); Lowes et al.62 (n=5); Naylor et al.63 (n=9); Nordstrand et al.64 (n=31); Prosser et al.65 (n=5); Richards et al.66 (n=4); Smelt67 (n=1); Trahan et al.68 (n=5); Ustad et al.69 (n=5); Bodkin et al.70 (n=1); Bollea et al.71 (n=1); Cope et al.72 (n=1); DeLuca73 (n=1); Fergus et al.74 (n=1); Glover et al.75 (n=6); Heathcock et al.76 (n=2); Huang et al.77 (n=1); Kanda et al.78 (n=10); Yang et al.79 (n=5); Badr et al.46 (n=62); Hielkema et al.86 (n=46); Blauw-Hospers et al.99 (n=46); Morgan et al.45 (n=13); Ohgi et al.80 (n=23); Palmer et al.49 (n=48); Weindling et al.50 (n=105) |
RCT; cohort with concurrent control; single subject research design; cohort; cohort with historical control; case study; case series | CAMS vs standard care40; kicking/stepping program vs standard care85; electrical stimulation+NDT vs NDT only84; intensive vs regular NDT41; developmental skills program+parent recommendations vs NDT43 No control group: CIMT57; CIMT+occupational therapy: orthoses58; developmental program59; early vs late Vojta therapy60; weight-bearing splint (upper limb)+NDT61; cast+CIMT+bimanual training62; CIMT63; baby-CIMT vs standard care64; mobility training65; treadmill training66; upper limb inhibitive cast+NDT67; NDT+occupational therapy: fine motor, perception68; physiotherapy69; physiotherapy+treadmill training57; CIMT71–75; physiotherapy motor learning76; assistive technology car77; Vojta therapy78; walking program79 |
No group differences40,43,85; electrical stimulation+NDT group had better kyphotic angle and GMFM-Sitting score84; intensive group improved more41 No control group: positive motor changes after modified CIMT57 and CIMT with splints58; neurobehavioral intervention improved motor skills59; early Vojta improved walking skills but had more severe complications60; fine and gross motor skills improved during CIMT and were maintained at the 1-mo follow-up62; improved hand function during and after treatment63; baby-CIMT group six times more likely to have a high functional level at 2y than the group without baby-CIMT64; greater gains in gross motor function during intervention65; no change in gait spatiotemporal parameters or GMFM total score66; gains in passive range of motion67; NDT+occupational therapy improved motor function68; effect of physiotherapy inconclusive69; treadmill training feasible for high-risk infants70; gains in upper arm function observed71–75; improved motor function, language, and cognitive skills found76; gains in mobility and vocalization during intervention77; 4 out of 5 could stand for 5s or walk at 52mo of therapy78; walking improved79 General conclusion: the two interventions with the largest effect sizes had common elements of child-initiated movement, environment modification/enrichment, and task-specific training GRADE quality: low |
RCT, randomized controlled trial; CAMS, Curriculum and Monitoring System; NDT, neurodevelopmental treatment; CIMT, constraint-induced movement therapy; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; COPCA, Coping with and Caring for Infants with Special Needs; GAME, Goals-Activity-Motor Enrichment; GAS, Goal Attainment Scale; COPM, Canadian Occupational Performance Measure; PDMS, Peabody Developmental Motor Scale; GMFM, Gross Motor Function Measure.
To further ensure that this overview was comprehensive and current, since the most recent review was from 2017 and the most comprehensive one was from 2016, we subsequently decided to supplement systematic review data with those from randomized controlled trials (RCTs) published since 2015 so as not to miss any relevant studies while limiting duplication with the reviews. The search strategy, inclusion criteria, and evaluation processes were identical except for seeking RCTs instead of systematic reviews or meta-analyses and being restricted to the years 2015 to 2020 (last search date of 12th November 2020). A total of 428 records were identified, with 355 remaining after 73 duplicates were removed. After title and abstract review by both authors and once consensus was obtained, 10 full texts were retrieved. Five were retained with the rest excluded for the following reasons: one included children with different neurodisabilities and data on children with CP were not analysed separately;33 in one 50% or more participants were described as born preterm;34 one evaluated feasibility only;35 one provided a nutritional versus therapeutic intervention;36 and one was a secondary video analysis of parent handling behaviors.37 The full text of one additional RCT by the first author of an earlier study included in the systematic reviews that had not been identified in the literature search, was retrieved when evaluating the former and met the inclusion criteria.38 Table 2 summarizes the data elements from the included RCTs similar to those from the primary studies in the reviews, with additional details related to dose in each study arm.
Table 2:
Relevant study details from the randomized controlled trials not included in the systematic reviews
| Study | Participants and inclusion criteria | Intervention/control or comparison | Child outcome measures | Results |
|---|---|---|---|---|
| Morgan et al.39 |
n=30; intervention group, n=15 (53% male; median age=15.7wks at enrollment); control group, n=15 (60% male; median age=20.1wks at enrollment) Inclusion criteria: corrected age 3–4mo+absent fidgety on GMA; or 5–6mo with CP diagnosis; or abnormal neuroimaging |
GAME (216h)/standard care (164h) | PDMS-2, COPM, BSID-III, GMFM-66 | Significant between-group differences in favor of GAME on PDMS-2 raw scores but not Total Motor Quotient at 16wks. Both significantly higher for GAME at 12mo as were GMFM-66, BSID-III, and COPM satisfaction scores. No group differences between doses |
| Eliasson et al.51 |
n=37; intervention group, n=19 (44.4% male; median age=34wks at enrollment); control group, n=18 (61.5% male; median age=34wks at enrollment) Inclusion criteria: corrected age 3–8mo+≥15% difference between hands-on HAI; high risk of unilateral CP (known event affecting the brain and/or clinical signs, e.g. from AIMS or HINE) |
Baby-CIMT (30min/6d/wks/12wks); massage (5–30min/6d/wks/12wks) | HAI, AHA | Affected hand score improved more for baby-CIMT group with high effect size. Other hand score improved with no significant group difference. Both hand score improved by 10 HAI units (median) for baby-CIMT, 0 for massage, but difference was not significant. Dose/adherence less in control group |
| Stark et al.53 |
n=24; group A=12 (50% male, median age=18.6mo at enrollment); group B=12 (58.3% male, median age=19.4mo at enrollment) Inclusion criteria: corrected age 12–24mo with or highly suspected CP diagnosis and in GMFCS levels II–IV |
Home-based sWBV (T0–T1), no sWBV (T1–T2)/no sWBV (T0–T2), sWBV (T1–T2). Ten 9-min sessions per week. All had standard care | GMFM-66, PEDI, BSID-II | No significant differences between with and without sWBV at T1 or between early and late onset of sWBV at T2 |
| Chamudot et al.52 |
n=33; intervention group, n=17 (47% male; median corrected age=11.4mo); control group, n=16 (69% male; median corrected age=10.9mo) Inclusion criteria: spastic unilateral CP; 8–16mo (18mo at end of treatment); ability to follow simple age-appropriate instructions |
Modified CIMT/bimanual; 1h play/7d/8wks in-home for both | Mini-AHA, Functional Inventory | Significantly improved hand function in both groups on Mini-AHA and Functional Inventory. No group differences |
| Mattern-Baxter et al.54 |
n=19; group 1, n=10 (30% male; median age=20.4mo); group 2, n=9 (44.4% male; median age=20.7mo) Inclusion criteria: (1) high risk of spastic CP; (2) <3y; (3) in GMFCS levels I and II; and (4) signs of walking readiness |
Low-intensity treadmill training (2×/week/6wks)/ high-intensity treadmill training (10×/week/6wks); up to 20min per session; (low:183min; high:782min) | GMFM-D&E, PDMS-2, PEDI | No significant difference between groups, although both improved significantly at each time point |
| Hielkema et al.55 |
n=43; intervention group, n=23 (65.2% male; median gestational age=32wks); control group, n=20 (55% male; median gestational age=29wks) Inclusion criteria: (1) cystic periventricular leukomalacia; (2) parenchymal lesions as the result of infarction or hemorrhage; (3) severe asphyxia with brain lesions on magnetic resonance imaging; (4) clinical dysfunction suspicious for development of CP |
COPCA/typical infant physical therapy for 1y; once per week. Mean of 3.0 COPCA and 2.5 TIP (6 more sessions in COPCA) | IMP, AIMS, TINE, BSID-II, GMFM-66, CBCL | Neuromotor, cognitive, and behavioral outcomes between groups not significantly different at all time points |
GMA, General Movement Assessment; CP, cerebral palsy; GAME, Goals-Activity-Motor Enrichment; PDMS-2, Peabody Developmental Motor Scale, Second Edition; COPM, Canadian Occupational Performance Measure; BSID-III, Bayley Scales of Infant and Toddler Development, Third Edition; GMFM-66, Gross Motor Function Measure-66 items; AIMS, Alberta Infant Motor Scale; HINE, Hammersmith Infant Neurological Examination; CIMT, constraint-induced movement therapy; HAI, Hand Assessment of Infants; AHA, Assisting Hand Assessment; GMFCS, Gross Motor Function Classification System; sWBV, side-alternating, whole-body vibration; PEDI, Pediatric Evaluation of Development Inventory; BSID-II, Bayley Scales of Infant and Toddler Development, Second Edition; COPCA, Coping with and Caring for infant with special needs; TIP, traditional infant physical therapy; IMP, Infant Motor Profile; TINE, Touwen Infant Neurological Examination; CBCL, Child Behavior Checklist.
Participants either ranged in age from 0 to 3 years across the studies included, or the reported mean age was no more than 3 years; all were identified as having CP or as being at risk of CP. The total number of participants across the motor studies with comparison groups, after duplicates were removed, was 858, including 672 from the studies included from the reviews and an additional 186 from the RCTs.
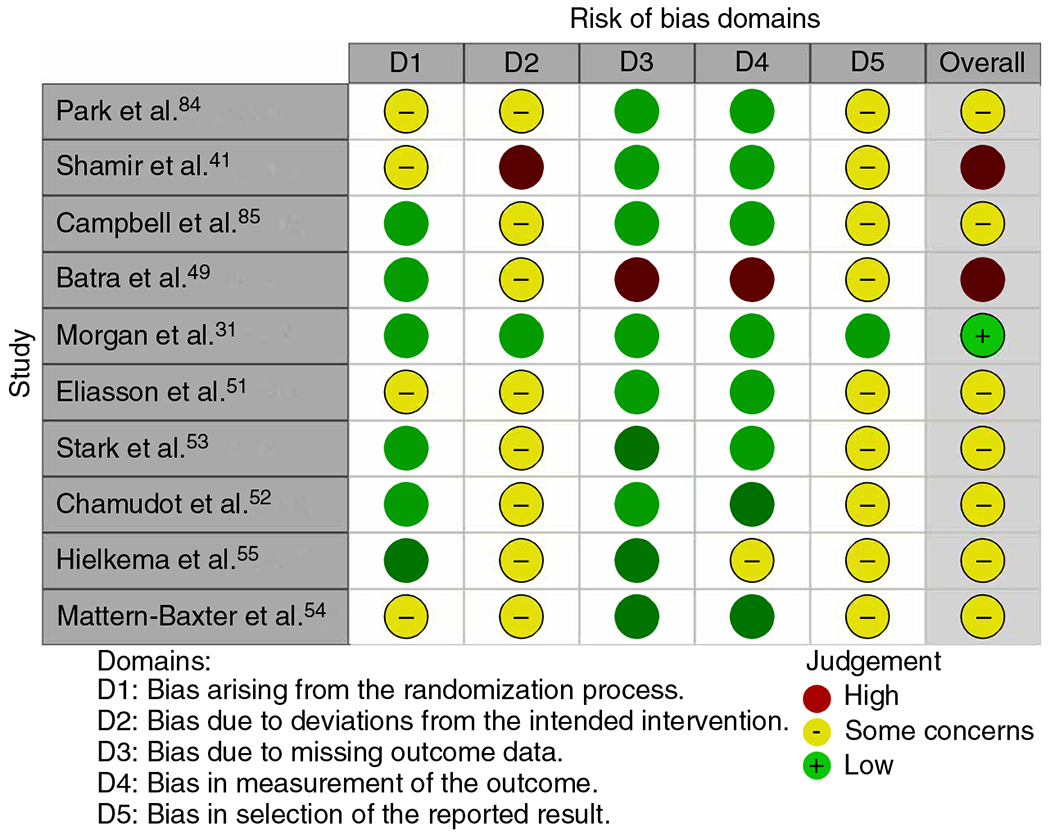
Figure 1 shows the Risk of Bias 2 in randomized trials38 scores for the additional RCTs as well as for within the RCTs in the Morgan et al.17 review that had not been evaluated for risk of bias. Only one study39 had a low risk of bias and two of the 11 studies demonstrated a high risk of bias,40,41 with the remaining studies assessed as having some concerns. The Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool42 was used for the only study included from Morgan et al.17 that had a comparison group but was not an RCT;43 some concerns were identified for nearly all categories. The other two reviews utilized an earlier risk of bias tool44 and these determinations are listed in Table 3. For the studies that were replicated, we included the determinations made by each review since they were not always consistent. In summarizing these data, we utilized the more critical of the two scores; however, in all cases the overall determination was the same. If any one item for a study was judged as having a ‘high’ risk of bias, with the exception of items 3 and 7 which are not specifically represented in risk of bias for randomized trials, that study was considered as being at ‘high’ risk of bias in the data summary, which was the case for five of the 11. Four were judged as low-risk45–48 and two were unclear.49,50
Figure 1:
Risk of Bias 2 assessment for the randomized controlled trials not evaluated within the systematic reviews and those identified in a separate systematic search from 2015 to 2020.
Table 3:
Cochrane risk of bias assessments as reported in the reviews and ROBINS-I determination for one non-randomized study
| Study/dimensions of Cochrane risk of bias assessment | D1 | D1 | D3 | D4 | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| Weindling et al.50 | Unclear | Low | High | Low | Low | Low | High |
| Ohgi et al80, a | Low | Unclear | High | Low | High | Low | High |
| Ohgi et al.80, b | Low | High | High | Low | Low | Low | Low |
| Badr et al.46, a | Low | Low | High | Low | High | Low | High |
| Badr et al.46, b | Unclear | Unclear | High | Low | High | Low | High |
| Hielkema et al.86 | Low | Low | High | Low | Low | Low | High |
| Morgan et al.39 | Low | Low | High | Low | Low | Low | High |
| Mayo87 | Low | Low | High | High | Low | High | High |
| Palmer et al.49 | Unclear | Unclear | High | Low | Low | Low | Low |
| Nelson et al.81, a | Low | Low | High | Low | High | Low | High |
| Nelson et al.81, b | Unclear | Unclear | High | High | High | Low | Low |
| Law et al.48 | Low | Low | High | Low | Low | Low | Low |
| Taub et al.83 | Unclear | Low | High | High | High | High | High |
| Wallen et al.47 | Low | Low | High | Low | Low | Low | Low |
|
| |||||||
| Study/dimensions of ROBINS-I | D1 | D1 | D3 | D4 | D5 | D6 | D7 |
| Mahoney et al.43 | Serious | Serious | Low | Serious | Serious | Low | Serious |
One of the six RCTs was a larger trial on the Goals-Activity-Motor Enrichment (GAME) intervention, similar in design and outcomes to the already reported pilot study,45 enrolling infants aged 3 to 5 months at high risk of CP for a 16-week intervention period.39 However, unlike the earlier trial, the dose of intervention was the same across groups. Mean therapist and parent intervention time was greater in the GAME group, although the differences were not statistically significant. A medium positive effect was demonstrated for GAME on the Peabody Developmental Motor Scale, Second Edition (PDMS-2) Total Motor Quotient and raw scores at 12 months compared to standard care. Two other RCTs also demonstrated significant positive results for the primary outcomes, favoring the experimental groups. One was an in-home parent-administered constraint-induced movement therapy (CIMT) program compared to an infant massage control group that enrolled infants aged from 3 to 8 months at risk of unilateral CP.51 The Hand Assessment of Infants revealed significant group differences, favoring CIMT with no adverse developmental effects on the hand being constrained. Although mean change in the Assisting Hand Assessment was greater in the CIMT group, it was not significantly better, which may be explained by the fact that training was unilateral. However, another RCT comparing bilateral to unilateral training in a group of slightly older infants with a mean age of 11.1 months demonstrated equivalent large positive changes in the mini-Assisting Hand Assessment in both groups.52 Although upper extremity results are similar to those found in older children with CP, effect sizes in infant studies may be larger than in older cohorts.51 In contrast, three other recent RCTs failed to support the superiority of the experimental or higher-intensity group on the primary outcomes. One RCT enrolled toddlers from 12 to 24 months with CP randomized to 14 weeks with or without treatment with whole-body vibration accompanied by some active exercises; however, no significant effect on motor function was found.53 Another RCT compared in-home treadmill training at two intensities, 2 versus 10 times per week for 6 weeks, in toddlers less than 3 years of age (mean of 20mo) with no significant group differences in the Gross Motor Function Measure standing and walking domains or the PDMS-2, although both groups progressed over the intervention period.54 A final RCT with all 43 infants enrolled within the first year of life had similar results as an earlier trial comparing the Coping with and Caring for Infants with Special Needs intervention to traditional physiotherapy; however, this study included infants with a very high and more definitive risk of CP and the intervention period was 1 year instead of 3 months.55 No between-group differences in neuromotor, cognitive, or behavioral outcomes were found across the two equally dosed arms, although therapist behaviors promoting more active infant responses again correlated with better outcomes.
We reviewed all developmental outcomes in the 16 unique motor intervention studies across reviews that were levels I to III and the six RCTs (listed by study in Table S1 and Table 2 respectively), and found a diverse list of measures across studies, with the Bayley Scales of Infant Development (BSID) and the Gross Motor Function Measure used most commonly (n=9 and n=7 respectively), sometimes in the same study. The PDMS-2 was used in four studies, and the Alberta Infant Motor Scale, the Pediatric Evaluation of Disability Inventory, and the Canadian Occupational Performance Measure and/or Goal Attainment Scale in three studies. The remaining developmental outcome measures identified were used in only one or two studies. Given the variability in outcome measures across studies and reviews, meta-analyses were not performed.
The final evidence summary that emerged from all studies included in this overview is presented in Table 4. Effect sizes were largely negligible or low; however, medium effect sizes as judged by Cohen’s d56 (>0.50) were seen for several studies, mainly for the BSID mental and physical development scores and the PDMS-2 Total Motor Quotient.
Table 4:
Final summary table listing the results by outcomes for all studies for which data were available to calculate effect sizesa
| Outcome | Study | Comparison | Total n | Cohen’s d | Effect size |
|---|---|---|---|---|---|
| Motor | |||||
| BSID PDI | Ohgi et al.;80 Morgan et al.;17 Palmer et al.;49 Nelson et al.81 | Enriched NDT vs standard care; GAME vs standard care; NDT vs developmental stimulation; multisensory+standard care vs standard care | 148 | 0.40; 0.42; −0.63; 0.43 | 0.19; 0.40; −0.30; 0.12 |
| GMFM | Stark et al.;53 Morgan et al.;17 Law et al.48 | sWBV+standard care vs standard care; GAME vs standard care; child- vs context-focused | 191 | 0.13; 0.20; 0.11 | 0.06; 0.10; 0.05 |
| GMFM-D | Mattern-Baxter et al.54 | High- vs low-intensity treadmill training | 19 | 0.36 | 0.17 |
| GMFM-E | Mattern-Baxter et al.54 | High- vs low-intensity treadmill training | 19 | 0.26 | 0.13 |
| GMFM-Sitting | Park et al.84 | Electrical stimulation+NDT vs NDT |
26 | 1.27 | 0.53 |
| PDMS-2 | Morgan et al.;17 Mahoney et al.;43 Mattern-Baxter et al.54 | GAME vs standard care equal dose; NDT vs developmental skills; high- vs low-intensity treadmill training | 55 | 0.57; −0.12; −0.29 | 0.27; −0.16; −0.14 |
| PMAL-amount | Taub et al.;83 Wallen et al.47 | CIMT vs standard care; CIMT vs intensive occupational therapy | 68 | 1.61; 0.32 | 0.62; 0.15 |
| PMAL-quality | Taub et al.;83 Wallen et al.47 | CIMT vs standard care; CIMT vs intensive occupational therapy | 68 | 0.75; 0.38 | 0.35; 0.18 |
| Daily total steps | Mattern-Baxter et al.54 | High- vs low-intensity treadmill training | 19 | −0.40 | −0.19 |
| AHA | Wallen et al.47 | CIMT vs intensive occupational therapy | 50 | 0.37 | 0.18 |
| PEDI-Mobility | Mattern-Baxter et al.54 | High- vs low-intensity treadmill training | 19 | −0.40 | −0.20 |
| PEDI-Mobility FSS | Law et al.48 | Child- vs context-focused | 128 | 0.04 | 0.02 |
| PEDI-Mobility CAS | Law et al.48 | Child- vs context-focused | 128 | 0.01 | 0.00 |
| TIME | Mahoney et al.43 | Developmental skills vs NDT | 6 | −0.30 | −0.15 |
| Cognitive | |||||
| BSID MDI | Ohgi et al.;80 Palmer et al.;49 Nelson et al.81 | Enriched NDT vs standard care; NDT vs developmental stimulation; multisensory+standard care vs standard care | 108 | 0.55; −0.57; 0.59 | 0.26; −0.27; 0.28 |
| Other/general development | |||||
| Vineland-Social | Palmer et al.49 | NDT vs developmental stimulation | 48 | −0.25 | −0.12 |
| PEDI-Self-care | Stark et al.53 | WBV+standard care vs standard care |
33 | 0.23 | 0.11 |
| PEDI-Self-care | Law et al.48 | Child- vs context-focused | 128 | 0.14 | 0.07 |
| FSS | |||||
| PEDI-Self-care | Law et al.48 | Child- vs context-focused | 128 | −0,02 | −0.01 |
| CAS | |||||
| COPM | Morgan et al.;17 Wallen et al.47 | GAME vs standard care; CIMT vs intensive occupational therapy | 80 | 0.25; 0.04 | 0.12; 0.02 |
| BSID | Morgan et al.17 | GAME vs standard care | 30 | 0.42 | 0.20 |
| EBS | Taub et al.83 | CIMT vs standard care | 18 | 1.27 | 0.53 |
No information is available from nine studies.40,41,46,50–52,55,85,86 The mean and standard deviation were used to calculate the effect size.
BSID PDI, Bayley Scales of Infant Development Psychomotor Developmental Index; NDT, neurodevelopmental treatment; GAME, Goals-Activity-Motor Enrichment; GMFM, Gross Motor Function Measure; sWVB, side-alternating, whole-body vibration; PDMS-2, Peabody Developmental Motor Scale, Second Edition; PMAL, Pediatric Motor Activity Log; CIMT, constraint-induced movement therapy; AHA, Assisting Hand Assessment; PEDI, Pediatric Evaluation of Development Inventory; FSS, Functional Skills Scales; CAS, Caregiver Assistance Scales; TIME, Toddler Infant Motor Evaluation; COPM, Canadian Occupational Performance Measure; EBS, Emerging Behaviors Scale.
The reviews varied in the extent to which sources of bias were controlled for or addressed. Results from the AMSTAR-2 tool are summarized in Table 5 with the items recommended as critical in bold. It is not appropriate to provide a summary score for this measure. Instead, developers recommend rating the extent to which suggested critical flaws and non-critical weaknesses were present using the following scale: high, no or one non-critical weakness; moderate, more than one non-critical weakness; low, one critical flaw with or without non-critical weaknesses; critically low, more than one critical flaw with or without non-critical weaknesses. While each of the reviews employed systematic searches, all excluded non-English studies and failed to provide a justification for doing this, so each was partially downgraded due to this on item 4. The Hadders-Algra et al.18 and Morgan et al.16 reviews were rated as low because both had partial flaws in two critical elements and one or more non-critical weaknesses; the Morgan et al.17 review was judged as critically low.
Table 5:
Assessing the risk of bias in each review using the AMSTAR-2 criteria
| AMSTAR-2 criteria | Hadders-Algra et al.18 | Morgan et al.16 | Morgan et al.17 |
|---|---|---|---|
| 1. Did the research questions and inclusion criteria include the components of PICO? | + | + | + |
| 2. Did the review contain an explicit statement that the methods were decided before the review, justifying any significant deviations? | +/− | +/− | +/− |
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? | + | − | − |
| 4. Did the review authors use a comprehensive literature search strategy? | +/− | +/− | +/− |
| 5. Did the review authors perform study selection in duplicate? | + | + | + |
| 6. Did the review authors perform data extraction in duplicate? | + | + | + |
| 7. Did the review authors provide a list of excluded studies and reasons why they were excluded? | + | + | − |
| 8. Did the review authors describe the included studies in adequate detail? | + | + | + |
| 9a. Did the review authors use an adequate technique to assess the risk of bias in individual studies included in the review (RCTs)? | + | + | − |
| 9b. Did the review authors use an adequate technique to assess the risk of bias in individual studies included in the review (NRSI)? | NA | NA | − |
| 10. Did the review authors report on the sources of funding for the studies included in the review? | − | − | − |
| 11a. If a meta-analysis was performed, did the review authors use appropriate methods for statistical combination of results (RCTs)? | NA | + | NA |
| 11b. If a meta-analysis was performed, did the review authors use appropriate methods for statistical combination of results (NRSI)? | NA | NA | NA |
| 12. If a meta-analysis was performed, did the review authors assess the potential impact of risk of bias in individual studies included on the results of the meta-analysis or other evidence synthesis? | NA | + | NA |
| 13. Did the review authors account for risk of bias in individual studies when discussing/interpreting the results of the review? | + | + | + |
| 14. Did the review authors provide a satisfactory explanation, and discussion of, any heterogeneity observed in the results of the review? | + | + | + |
| 15. If they performed quantitative synthesis, did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? | NA | + | NA |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | + | + | + |
PICO, participants, interventions, comparators, and outcomes; RCT, randomized controlled trial; NRSI, non-randomized studies of interventions; NA, not applicable.
Two reviews used GRADE to evaluate the cumulative evidence.16,17 Morgan et al.17 rated the overall quality of evidence as low, despite the fact that 10 RCTs were included; however, they stated that these had many methodological flaws. The body of evidence supporting environmental enrichment to improve motor outcomes was rated as moderate.16 Although GRADE was not used, Hadders-Algra et al.18 employed a methodological quality assessment a priori to choose only those studies with strong-to-moderate quality for detailed review, with seven of the 13 studies identified by them as meeting these criteria, but only one of the seven having a strong rating. These seven were each further assessed with the Mallen score and with the Cochrane risk of bias criteria, each of which showed considerable variation across studies. Specifically, small sample size and non-blinding of participants were the most consistent weaknesses found. On evaluation by the authors, it was assigned a GRADE quality rating of low.
For the one review17 which included studies without comparison groups that failed to meet inclusion criteria for the overview, those additional studies57–79 are cited and summarized briefly in Table 1.
DISCUSSION
Several general and mostly tentative conclusions can be gleaned from this overview of the three systematic reviews and six RCTs published several years after the most recent reviews, as based on the authors’ summaries of the evidence and judgements of study quality. Emerging positive evidence was found supporting training that encourages children who are not using one arm or hand as much as the other to incorporate that hand more during everyday activities16,51 rather than depending disproportionately or exclusively on the opposite extremity. Weakly positive evidence supported several different intervention components or training principles. The review on environmental enrichment16 demonstrated weakly positive evidence of moderate quality. The aim of environmental enrichment is to provide a stimulating motor and cognitive environment that challenges the child to explore different movement opportunities and other learning activities, which are progressed and changed over time. However, data were deemed insufficient to determine the effectiveness or relative effectiveness of the multiple individual components that may comprise environmental enrichment. Regarding other developmental outcomes, three studies showed a medium effect size on cognitive development outcomes as measured by the BSID for the intervention deemed in each as the ‘enriched environment’ one.49,80,81 Weakly positive evidence further supported motor activities that are engaging16,17 to the child and include self-initiated and directed exploration17 and the use of functionally relevant, task-specific, and goal-directed training.17,51
Some approaches may have negative outcomes or be less beneficial than alternatives, for example, the use of excessive external facilitation or physical guidance17 while the child is learning to move is not recommended and handling techniques used to inhibit muscle tone are not effective14 and should not be used (e.g. reflex-inhibiting postures). Evidence supports that moving the child’s legs or arms passively17 or utilizing a whole-body vibration plate53 will not help them learn to move and that passive stretching of muscles is insufficient to keep muscles from getting tight.17 Motor learning principles support that the child needs to produce and react to their own sensory feedback from their movements to be able to learn how to do these better; excessive external sensory input may disrupt this process.82 Evidence does not support the effectiveness of generalized (non-adapted) developmental stimulation approaches for improving motor outcomes in children with neurological disorders compared to other groups of high-risk infants (stated to ‘lack the potency’ for children with specific disorders).18 However, limited data suggest that these may help to improve a child’s cognitive outcomes.16
Nonetheless, closer examination of the studies included shows how tenuous the support for the efficacy of early intervention is in significantly improving motor outcomes. Significant differences between interventions and comparisons were reported in only four of the 16 studies included in the reviews, each with a different intervention: GAME;45 CIMT;83 a developmental stimulation/active learning approach;49 and electrical stimulation to the trunk muscles.84 Three showed medium effect sizes on motor outcomes as seen in Table 3 with no data available to calculate this in the fourth.45 Two of the six additional RCTs reinforced the efficacy of both GAME39 – now shown with equivalent doses across groups and a medium effect size on the PDMS-2 – and CIMT,51 with no other new interventions showing superiority. Furthermore, it is important to note that not all interventions focusing on environmental enrichment principles81 or active task-specific motor training (e.g. kicking practice85) or designed to incorporate parent coaching and more child-directed activities86 showed developmental benefit over a control intervention. A second RCT on the Coping with and Caring for Infants with Special Needs intervention years later also failed to demonstrate superiority to neurodevelopmental treatment (NDT),55 although greater promotion of active versus passive activities was again associated with positive effects seen in both groups. CIMT, while shown to be more effective than standard care, was not superior when compared to similarly intensive occupational therapy47 or when compared to bimanual training,52 although there were some task-specific differences between groups in the less affected hand.
Nine40,45,46,50,80,81,83,85,86 of 16 studies included in the reviews had a usual care control group, with only the aforementioned GAME45 and CIMT83 approaches showing significantly greater efficacy in comparison. Two studies53,81 included standard care in both arms and one included NDT84 in both arms. This design can provide greater internal validity because any significant differences between groups is likely due to the intervention. Of those three, only the study with additive electrical stimulation84 produced a relatively greater effect on motor function, specifically on the Sitting Dimension of the Gross Motor Function Measure. Several other studies included NDT as the control or intervention group. One compared NDT as the experimental group to standard care with no between-group differences in outcomes,80 another compared NDT to a developmental stimulation program that included the components of enriched environmental, with the latter showing greater benefit;49 the third non-randomized study compared sites purported to have a developmental skills versus NDT focus and found no differences.43 Several studies compared two intensities of the same intervention: two41,87 showed that more intensive NDT demonstrated greater benefit. In contrast, a recent study comparing two intensities of treadmill training54 found no difference in outcomes, leading the authors to advocate for the less intensive program. However, while both groups improved significantly in the 3-month trial, these changes may have been due to maturation rather than intervention effects; further study is needed.
The literature about early intervention in CP has been limited because many studies have included groups receiving broad intervention categories (e.g. NDT) or comparators including ‘standard practice’ and ‘usual care’ without defining the components of each, including the specific types and/or the amount of training. This makes interpretation of the results within and across studies problematic. As an example, a recent study comparing a novel active training early intervention approach that failed to meet inclusion criteria because the analyses combined children with CP and Down syndrome,33 found no benefit over standard care. These authors interestingly suggested that this result may perhaps reflect how much standard care has improved in recent years.
No study included in this overview had a control group where participants received no intervention, which reveals the prevalent belief or bias that it is not ethical to do nothing; therefore, the effects of maturation alone are not known for any of the studies. Measures such as the BSID can track rate of development, but children with CP often have slower than normal developmental rates compared to their peers with considerable variation across individuals, so the amount of expected progress in the absence of an intervention cannot be reliably estimated in this population.
Another limitation in each of the reviews was that the preponderance of evidence included was more than a decade old. Study quality was largely poorer for less recent studies; interventions conducted in the older studies may have been based on outdated knowledge or assumptions about brain development or motor control. Recent neuroscience evidence has reinvigorated the field of early intervention with the prospect that recovery from early difficulties, disturbances, or trauma may be possible if addressed in a timely manner,88,89 which will hopefully stimulate more innovative and higher-quality research. Providing earlier intervention requires earlier identification of increased developmental risk, which is possible in some cases (e.g. through prenatal genetic screening, preterm birth, or other adverse birth history), or where developmental screening is conducted more broadly and then conducting the most sensitive and specific assessments to improve CP diagnostic accuracy. Now that earlier identification of CP is possible,5,90 earlier intervention in practice and research can be delivered to infants far more likely to have CP than in the past when a significant proportion of included infants did not go on to receive a CP diagnosis. Only a handful of studies included in the systematic reviews or in the more recent RCTs adhered to the current standards for diagnosing CP risk; therefore, conclusions from this review lack the specificity that might be possible moving forward.
This overview revealed the paucity of both quantity and quality of research on early intervention in children with CP. This is in stark contrast to a recent systematic review of systematic reviews on interventions for children with CP of all ages,91 which reported a far larger number of studies on interventions shown to be efficacious for improving motor function. In their review, shown to a lesser extent in this article, commonalities across more or less effective therapy interventions are emerging (e.g. activity-based vs more passive therapies respectively) and could be a source of inspiration for potentially impactful interventions that could be initiated earlier in development.
While the type of intervention is predominant in whether or not it may be efficacious, the dose of the intervention92 is now recognized as another major factor; however, details on the dose of the intervention or dose comparison were lacking in many of the earlier studies. More recent RCTs provided explicit details describing the intervention including, in some cases, the intended and actual doses in both study arms. Dose comparisons are important for determining the minimum effective dose and for allocating scarce resources. The feasibility and durability of the effects of training programs also warrant closer examination, especially for those that place a larger burden on families. It is a well-established motor learning principle that practice is important for improving motor skills, which is why incorporating motor activity in everyday family routines is an effective way to ensure adequate practice or dose. The activities provided should aim for an optimal balance of difficulty and success (‘just right’ challenge). Task-specific and goal-directed training are well-established motor learning principles regardless of age or condition93 and help explain why non-specific play may have less of an effect on developmental outcomes.94 While motor learning principles may apply regardless of age, the importance of the learning environment that must provide both opportunities and encouraging interactions with caretakers and/or other children may be especially prominent in early development since environmental experiences are largely not under the infant’s control.
While not discussed explicitly in this article, it is also reasonable to expect that interventions provided during the emergence of skills earlier in life rather than modifying already learned behaviors later on would likely lead to better outcomes. However, this and several other proposed advantages of intervening earlier have yet to be conclusively supported by evidence in human infants.
This overview has several limitations. One is that it was not registered; however, PRISMA guidelines were adhered to and risk of bias instruments as recommended by Cochrane were reported or utilized. A second limitation was that few systematic reviews were found with the most recent one from 2017;14 therefore, data were not as current as a review of individual studies, which is often the case when reviewing systematic reviews or guidelines. To address this issue in this study, we subsequently searched for RCTs published since these reviews using the same process and inclusion criteria, except for seeking RCTs instead of reviews. Another limitation was that while recent guidance from Cochrane and others advocate for the use of overview methodology for PICO questions comparing interventions for a specific population, the resultant reviews identified from the search process did not meet optimal criteria10 for an overview. Limited numbers of reviews were identified, with considerable overlap, with two of lower quality17,18 and one of moderate quality.16 We addressed this by providing detail at the level of the primary studies to enhance accuracy and minimize the effects of duplication; we used a standardized tool to evaluate the risk of bias for all studies in addition to evaluating the reviews themselves. The quality of the data retrieved from a search is measured retrospectively, so it is difficult to anticipate this before deciding to perform an overview. Recognition of the limitations of data supporting early intervention for children with CP should be a clarion call to our field to strive for greater rigor and accountability.
A final limitation of this review was its focus on child outcomes and more child-specific therapy principles that did not expressly include other key components that may affect outcomes in CP and other neurodevelopmental disabilities as presented by Hutchon et al.95 in their recent narrative review. These include supporting parents’ mental health, promoting positive parenting, and addressing the capability of the infant to be attentive, among others that should also be integral parts of early intervention programs.
In summary, the evidence reviewed in this study supports to a limited degree several key principles for promoting early motor development and leveraging the purportedly greater potential and brain plasticity4 early in development. Evidence-informed early intervention principles support active infant exploration in an enriched environment, along with greater intensity and task specificity. For implementation, these broad concepts must be developed further with more specific suggestions when coaching caregivers on how to incorporate these into play and other daily routines, such as eating, dressing, and diaper (nappy) changes, among others. Important areas for future research include the further development and testing of innovative strategies that optimally incorporate these principles, including perhaps the investigation of the role of ‘smart’ devices to encourage greater movement initiation and progression in infants with CP and address muscle as well as brain development.65 Research on more specific questions, such as how early can or should an intervention be introduced, whether there are harmful effects from starting too early, and what interventions or intensities are appropriate for young infants, among others, are also needed. The term ‘at risk’ for CP is inconsistently defined across studies and a requirement for researchers to utilize the established diagnostic guidelines for the early identification of CP risk would greatly improve comparability across studies. Published protocols on several clinical trials which are underway demonstrate the recent impetus for more precise earlier identification and intervention in CP.96–98 A major unanswered question is whether motor interventions alone, or in combination with other therapies, can promote neural recovery and/or alter the developmental prognosis for infants with CP, as has been provocatively demonstrated in animal studies.
Supplementary Material
Table S1: Developmental outcome measures for included studies within the systematic reviews
Figure S1: PRISMA flow chart for the process for and results of the search for systematic reviews from 2009 to 2020.
Appendix S2: PRISMA checklist.
Appendix S1: Search strategies.
What this paper adds.
For over 50% of trials within the reviews, the intervention was compared to standard care with only two showing efficacy.
Similar to results in older children, constraint-induced movement therapy (CIMT) emerged as efficacious with high effect sizes.
CIMT was not superior to similarly intense bimanual training or occupational therapy.
Goals-Activity-Motor Enrichment intervention initiated before 5 months of age was superior to equally intense standard care.
Several other enriched environment strategies promoted cognitive and/or motor development.
ABBREVIATIONS
- BSID
Bayley Scales of Infant Development
- CIMT
Constraint-induced movement therapy
- GAME
Goals-Activity-Motor Enrichment
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- NDT
Neurodevelopmental treatment
- PDMS-2
Peabody Developmental Motor Scale, Second Edition
- PICO
Participants, interventions, comparators and outcomes
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
Footnotes
SUPPORTING INFORMATION
The following additional material may be found online:
DATA AVAILABILITY STATEMENT
Data available by request from the authors.
REFERENCES
- 1.Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers 2016; 2: 15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadders-Algra M Challenges and limitations in early intervention. Dev Med Child Neurol 2011; 53(Suppl. 4): 52–5. [DOI] [PubMed] [Google Scholar]
- 3.Herskind A, Greisen G, Nielsen J. Early identification and intervention in cerebral palsy. Dev Med Child Neurol 2015; 57: 29–36. [DOI] [PubMed] [Google Scholar]
- 4.Kolb B, Muhammad A. Harnessing the power of neuroplasticity for intervention. Front Hum Neurosci 2014; 8: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novak I, Morgan C, Adde L, et al. Early accurate diagnosis and early intervention in CP: advances in diagnosis and treatment. JAMA Pediatr 2017; 171: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne R, Noritz G, Maitre NL, NCH Early Developmental Group. Implementation of early diagnosis and intervention guidelines for cerebral palsy in a high-risk infant follow-up clinic. Pediatr Neurol 2017; 76: 66–71. [DOI] [PubMed] [Google Scholar]
- 7.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011; 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol 2005; 47: 571–6. [DOI] [PubMed] [Google Scholar]
- 9.Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: overviews of reviews. In Higgins JPT, Thomas J, Chandler J et al. , editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Hoboken, NJ: Wiley-Blackwell, 2020. www.training.cochrane.org/handbook. [Google Scholar]
- 10.Ballard M, Montgomery P. Risk of bias in overviews of reviews: a scoping review of methodological guidance and four-item checklist. Res Synth Methods 2017; 8: 92–108. [DOI] [PubMed] [Google Scholar]
- 11.Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syr Rev 2017; 6: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 2-risk of bias assessment; synthesis, presentation and summary of the findings; and assessment of the certainty of the evidence. Syst Rev 2018; 7: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock M, Fernandes RM, Pieper D, et al. Preferred Reporting Items for Overviews of Reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev 2019; 8: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction–GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94. [DOI] [PubMed] [Google Scholar]
- 16.Morgan C, Novak I, Badawi N. Enriched environments and motor outcomes in CP: systematic review and meta-analyses. Pediatrics 2013; 132: e735–44. [DOI] [PubMed] [Google Scholar]
- 17.Morgan C, Darrah J, Gordon AM, et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol 2016; 58: 900–9. [DOI] [PubMed] [Google Scholar]
- 18.Hadders-Algra M, Boxum AG, Hielkema T, Hamer EG. Effect of early intervention in infants at very high risk of CP: a systematic review. Dev Med Child Neurol 2017; 59: 246–58. [DOI] [PubMed] [Google Scholar]
- 19.Ward R, Reynolds JE, Bear N, Elliott C, Valentine J. What is the evidence for managing tone in young children with, or at risk of developing, cerebral palsy: a systematic review. Disabil Rehabil 2017; 39: 619–30. [DOI] [PubMed] [Google Scholar]
- 20.Druschel C, Althuizes HC, Funk JF, Placzek R. Off label use of botulinum toxin in children under two years of age: a systematic review. Toxins 2013; 5: 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan SH, Johnson MJ, Leaf AA, Vollmer B. Nutrition and neurodevelopmental outcomes in preterm infants: a systematic review. Acta Paediatr 2016; 105: 587–99. [DOI] [PubMed] [Google Scholar]
- 22.Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev 2015: CD005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khurana S, Kane AE, Brown SE, Tarver T, Dusing SC. Effect of neonatal therapy on the motor, cognitive, and behavioral development of infants born preterm: a systematic review. Dev Med Child Neurol 2020; 62: 684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirks T, Hadders-Algra M. The role of the family in intervention of infants at high risk of cerebral palsy: a systematic analysis. Dev Med Child Neurol 2011; 53 (Suppl. 4): 62–7. [DOI] [PubMed] [Google Scholar]
- 25.Ward R, Reynolds JE, Pieterse B, Elliott C, Boyd R, Miller L. Utilisation of coaching practices in early interventions in children at risk of developmental disability/delay: a systematic review. Disabil Rehabil 2020; 42: 2846–67. [DOI] [PubMed] [Google Scholar]
- 26.Festante F, Antonelli C, Chorna O, Corsi G, Guzzetta A. Parent-infant interaction during the first year of life in infants at high risk for cerebral palsy: a systematic review of the literature. Neural Plast 2019; 2019: 5759694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelhaleem N, Taher S, Mahmoud M, et al. Effect of action observation therapy on motor function in children with cerebral palsy: a systematic review of randomized trials with meta-analysis. Clin Rehabil 2021; 35: 51–63. [DOI] [PubMed] [Google Scholar]
- 28.Clark GF, Kingsley KL. occupational therapy practice guidelines for early childhood: birth-5 years. Am J Occup Ther 2020; 74: 7403397010p1–7403397010p42. [DOI] [PubMed] [Google Scholar]
- 29.Khamis A, Novak I, Morgan C, et al. Motor learning feeding interventions for infants at risk of cerebral palsy: a systematic review. Dysphagia 2020; 35: 1–17. [DOI] [PubMed] [Google Scholar]
- 30.Cameron D, Craig T, Edwards B, Missiuna C, Schwellnus H, Polatajko HJ. Cognitive Orientation to daily Occupational Performance (CO-OP): a new approach for children with cerebral palsy. Phys Occup Ther Pediatr 2017; 37: 183–98. [DOI] [PubMed] [Google Scholar]
- 31.Case-Smith J, Frolek Clark GJ, Schlabach TL. Systematic review of interventions used in occupational therapy to promote motor performance for children ages birth-5 years. Am J Occup Ther 2013; 67: 413–24. [DOI] [PubMed] [Google Scholar]
- 32.Tanner K, Schmidt E, Martin K, Bassi M. Interventions within the scope of occupational therapy practice to improve motor performance for children ages 0–5 years: a systematic review. Am J Occup Ther 2020; 74: 7402180060p1–7402180060p40. [DOI] [PubMed] [Google Scholar]
- 33.Holmström L, Eliasson A-C, Almeida R, et al. Efficacy of the Small Step Program in a randomized controlled trial for infants under 12 months old at risk of cerebral palsy (CP) and other neurological disorders. J Clin Med 2019; 8: 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dusing SC, Tripathi T, Marcinowski EC, Thacker LR, Brown LF, Hendricks-Muñoz KD. Supporting play exploration and early developmental intervention versus usual care to enhance development outcomes during the transition from the neonatal intensive care unit to home: a pilot randomized controlled trial. BMC Pediatr 2018; 18: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu AP, Pearse J, Watson R, et al. Feasibility trial of an early therapy in perinatal stroke (eTIPS). BMC Neurol 2018; 18: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrew MJ, Parr JR, Montague-Johnson C, et al. Nutritional intervention and neurodevelopmental outcome in infants with suspected cerebral palsy: the Dolphin infant double-blind randomized controlled trial. Dev Med Child Neurol 2018; 60: 906–13. [DOI] [PubMed] [Google Scholar]
- 37.Dirks T, Hielkema T, Hamer EG, Reinders-Messelink HA, Hadders-Algra M. Infant positioning in daily life may mediate associations between physiotherapy and child development-video-analysis of an early intervention RCT. Res Dev Disabil 2016; 53–54: 147–57. [DOI] [PubMed] [Google Scholar]
- 38.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 39.Morgan C, Novak I, Dale RC, Guzzetta A, Badawi N. Single blind randomised controlled trial of GAME (Goals-Activity-Motor Enrichment) in infants at high risk of cerebral palsy. Res Dev Disabil 2016; 55: 256–67. [DOI] [PubMed] [Google Scholar]
- 40.Batra M, Sharma VP, Batra V, Malik GK, Pandey RM. Neurofacilitation of Developmental Reaction (NFDR) approach: a practice framework for integration/modification of early motor behavior (primitive reflexes) in cerebral palsy. Indian J Pediatr 2012; 79: 659–63. [DOI] [PubMed] [Google Scholar]
- 41.Shamir M, Dickstein R, Tirosh E. Intensive intermittent physical therapy in infants with cerebral palsy: a randomized controlled pilot study. Isr Med Assoc J 2012; 14: 737–41. [PubMed] [Google Scholar]
- 42.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahoney G, Robinson C, Fewell RR. The effects of early motor intervention on children with Down syndrome or cerebral palsy: a field-based study. J Dev Behav Pediatr 2001; 22: 153–62. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JTP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5.0.0. Hoboken, NJ: Wiley-Blackwell, 2008. [Google Scholar]
- 45.Morgan C, Novak I, Dale RC, Badawi N. Optimising motor learning in infants at high risk of cerebral palsy: a pilot study. BMC Pediatr 2015; 15: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badr LK, Garg M, Kamath M. Intervention for infants with brain injury: results of a randomized controlled study. Infant Behav Dev 2006; 29: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallen M, Ziviani J, Naylor O, Evans R, Novak I, Herbert RD. Modified constraint-induced therapy for children with hemiplegic cerebral palsy: a randomized trial. Dev Med Child Neurol 2011; 53: 1091–9. [DOI] [PubMed] [Google Scholar]
- 48.Law MC, Darrah J, Pollock N, et al. Focus on function: a cluster, randomized controlled trial comparing child- versus context-focused intervention for young children with cerebral palsy. Dev Med Child Neurol 2011; 53: 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer FB, Shapiro BK, Wachtel RC, et al. The effects of physical therapy on cerebral palsy. A controlled trial in infants with spastic diplegia. N Engl J Med 1988; 318: 803–8. [DOI] [PubMed] [Google Scholar]
- 50.Weindling AM, Hallam P, Gregg J, Klenka H, Rosenbloom L, Hutton J. A randomized controlled trial of early physiotherapy for high-risk infants. Acta Paediatr 1996; 85: 1107–11. [DOI] [PubMed] [Google Scholar]
- 51.Eliasson A-C, Nordstrand L, Ek L, et al. The effectiveness of baby-CIMT in infants younger than 12 months with clinical signs of unilateral-cerebral palsy; an explorative study with randomized design. Res Dev Disabil 2018; 72: 191–201. [DOI] [PubMed] [Google Scholar]
- 52.Chamudot R, Parush S, Rigbi A, Horovitz R, Gross-Tsur V. Effectiveness of modified constraint-induced movement therapy compared with bimanual therapy home programs for infants with hemiplegia: a randomized controlled trial. Am J Occup Ther 2018; 72: 1–9. [DOI] [PubMed] [Google Scholar]
- 53.Stark C, Herkenrath P, Hollmann H, et al. Early vibration assisted physiotherapy in toddlers with cerebral palsy: a randomized controlled pilot trial. J Musculoskelet Neuronal Interact 2016; 16: 183–92. [PMC free article] [PubMed] [Google Scholar]
- 54.Mattern-Baxter K, Looper J, Zhou C, Bjornson K. Low-intensity vs high-intensity home-based treadmill training and walking attainment in young children with spastic diplegic cerebral palsy. Arch Phys Med Rehabil 2020; 101: 204–12. [DOI] [PubMed] [Google Scholar]
- 55.Hielkema T, Boxum AG, Hamer EG, et al. LEARN2-MOVE 0–2 years, a randomized early intervention trial for infants at very high risk of cerebral palsy: neuromotor, cognitive, and behavioral outcome. Disabil Rehabil 2020; 42: 3752–61. [DOI] [PubMed] [Google Scholar]
- 56.Cohen J A power primer. Psychol Bull 1992; 112: 155–9. [DOI] [PubMed] [Google Scholar]
- 57.Coker P, Lebkicher C, Harris L, Snape J. The effects of constraint-induced movement therapy for a child less than one year of age. NeuroRehabilitation 2009; 24: 199–208. [DOI] [PubMed] [Google Scholar]
- 58.Dickerson A, Brown L. Pediatric constraint-induced movement therapy in a young child with minimal active arm movement. Am J Occ Ther 2007; 61: 563–73. [DOI] [PubMed] [Google Scholar]
- 59.Horn E, Warren S, Jones H. An experimental analysis of a neurobehavioral motor intervention. Dev Med Child Neurol 1995; 37: 697–714. [DOI] [PubMed] [Google Scholar]
- 60.Kanda T, Yuge M, Yamori Y, Suzuki J, Fukase H. Early physiotherapy in the treatment of spastic diplegia. Dev Med Child Neurol 1984; 26: 438–44. [DOI] [PubMed] [Google Scholar]
- 61.Kinghorn J, Roberts G. The effect of an inhibitive weight-bearing splint on tone and function: a single-case study. Am J Occ Ther 1996; 50: 807–15. [DOI] [PubMed] [Google Scholar]
- 62.Lowes L, Mayhan M, Orr T, et al. Pilot study of the efficacy of constraint-induced movement therapy for infants and toddlers with cerebral palsy. Phys Occup Ther Pediatr 2014; 34: 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naylor C, Bower E. Modified constraint-induced movement therapy for young children with hemiplegic cerebral palsy: a pilot study. Dev Med Child Neurol 2005; 47: 365–69. [DOI] [PubMed] [Google Scholar]
- 64.Nordstrand L, Holmefur M, Kits A, Eliasson A. Improvements in bimanual hand function after baby-CIMT in two-year-old children with unilateral cerebral palsy: a retrospective study. Res Dev Disabil 2015; 41: 86–93. [DOI] [PubMed] [Google Scholar]
- 65.Prosser L, Ohlrich L, Curatalo L, Alter K, Damiano D. Feasibility and preliminary effectiveness of a novel mobility training intervention in infants and toddlers with cerebral palsy. Dev Neurorehabil 2012; 15: 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richards C, Malouin F, Dumas F, Marcoux S, Lepage C, Menier C. Early and intensive treadmill locomotor training for young children with cerebral palsy: a feasibility study. Pediatr Phys Ther 1997; 9: 158–65. [Google Scholar]
- 67.Smelt H Effect of an inhibitive weight-bearing mitt on tone reduction and functional performance in a child with cerebral palsy. Phys Occ Ther Pediatr 1989; 9: 53–80. [PubMed] [Google Scholar]
- 68.Trahan J, Malouin F. Intermittent intensive physiotherapy in children with cerebral palsy: a pilot study. Dev Med Child Neurol 2002; 44: 233–39. [DOI] [PubMed] [Google Scholar]
- 69.Ustad T, Sorsdahl A, Ljunggren A. Effects of intensive physiotherapy in infants newly diagnosed with cerebral palsy. Pediatr Phys Ther 2009; 21: 140–8. [DOI] [PubMed] [Google Scholar]
- 70.Bodkin A, Baxter R, Heriza C. Treadmill training for an infant born preterm with a grade III intraventricular hemorrhage. Phys Ther 2003; 83: 1107–18. [PubMed] [Google Scholar]
- 71.Bollea L, Di RG, Gisondi A. Recovery from hemiparesis and unilateral spatial neglect after neonatal stroke. Case report and rehabilitation of an infant. Brain Inj 2007; 21: 81–91. [DOI] [PubMed] [Google Scholar]
- 72.Cope S, Forst H, Bibis D, Liu X. Modified constraint-induced movement therapy for a 12-month-old child with hemiplegia: a case report. Am J Occup Ther 2008; 62: 430–7. [DOI] [PubMed] [Google Scholar]
- 73.DeLuca S, Echols K, Ramey S, Taub E. Pediatric constraint-induced movement therapy for a young child with cerebral palsy: two episodes of care. Phys Ther 2003; 83: 1003–13. [PubMed] [Google Scholar]
- 74.Fergus A, Buckler J, Farrell J, Isley M, McFarland M, Riley B. Constraint-induced movement therapy for a child with hemiparesis: a case report. Pediatr Phys Ther 2008; 20: 271–83. [DOI] [PubMed] [Google Scholar]
- 75.Glover J, Mateer C, Yoell C, Speed S. The effectiveness of constraint induced movement therapy in two young children with hemiplegia. Pediatr Rehabil 2002; 5: 125–31. [DOI] [PubMed] [Google Scholar]
- 76.Heathcock J, Baranet K, Ferrante R, Hendershot S. Daily intervention for young children with cerebral palsy in GMFCS level V: a case series. Phys Ther 2015; 27: 285–92. [DOI] [PubMed] [Google Scholar]
- 77.Huang H, Ragonesi C, Stoner T, Peffley T, Galloway J. Modified toy cars for mobility and socialization: case report of a child with cerebral palsy. Pediatr Phys Ther 2014; 26: 76–84. [DOI] [PubMed] [Google Scholar]
- 78.Kanda T, Pidcock F, Hayakawa K, Yamori Y, Shikata Y. Motor outcome differences between two groups of children with spastic diplegia who received different intensities of early onset physiotherapy followed for 5 years. Brain Dev 2004; 26: 118–26. [DOI] [PubMed] [Google Scholar]
- 79.Yang J, Livingstone D, Brunton K, et al. Training to enhance walking in children with cerebral palsy: are we missing the window of opportunity? Semin Pediatr Neurol 2013; 20: 106–15. [DOI] [PubMed] [Google Scholar]
- 80.Ohgi S, Fukuda M, Akiyama T, Gima H. Effect of an early intervention programme on low birthweight infants with cerebral injuries. J Paediatr Child Health 2004; 40: 689–95. [DOI] [PubMed] [Google Scholar]
- 81.Nelson MN, White-Traut RC, Vasan U, et al. One-year outcome of auditory-tactile-visual vestibular intervention in the neonatal intensive care unit: effects of severe prematurity and central nervous system injury. J Child Neurol 2001; 16: 493–8. [DOI] [PubMed] [Google Scholar]
- 82.Zwicker JG, Missiuna C, Harris SR, Boyd LA. Developmental coordination disorder: a review and update. Eur J Paediatr Neurol 2012; 16: 573–81. [DOI] [PubMed] [Google Scholar]
- 83.Taub E, Ramey SL, DeLuca S, Echols K. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics 2004; 113: 305–12. [DOI] [PubMed] [Google Scholar]
- 84.Park ES, Park CI, Lee HJ, Cho YS. The effect of electrical stimulation on the trunk control in young children with spastic diplegic cerebral palsy. J Korean Med Sci 2001; 16: 347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell S, Gaebler-Spira D, Zawacki L, et al. Effects on motor development of kicking and stepping exercise in preterm infants with periventricular brain injury: a pilot study. J Pediatr Rehabil Med 2012; 5: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hielkema T, Blauw-Hospers C, Dirks T, Drijver-Messelink M, Bos AF, Hadders-Algra M. Does physiotherapeutic intervention affect motor outcome in high-risk infants? An approach combining a randomized controlled trial and process evaluation. Dev Med Child Neurol 2011; 53: e8–15. [DOI] [PubMed] [Google Scholar]
- 87.Mayo NE. The effect of physical therapy for children with motor delay and cerebral palsy. A randomized clinical trial. Am J Phys Med Rehabil 1991; 70: 258–67. [DOI] [PubMed] [Google Scholar]
- 88.Friel KM, Chakrabarty S, Martin JH. Pathophysiological mechanisms of impaired limb use and repair strategies for motor systems after unilateral injury of the developing brain. Dev Med Child Neurol 2013; 55 (Suppl. 4): 27–31. [DOI] [PubMed] [Google Scholar]
- 89.Kolb B, Gibb R. Tactile stimulation after frontal or parietal cortical injury in infant rats facilitates functional recovery and produces synaptic changes in adjacent cortex. Behav Brain Res 2010; 214: 115–20. [DOI] [PubMed] [Google Scholar]
- 90.Morgan C, Fahey M, Roy B, Novak I. Diagnosing cerebral palsy in full-term infants. J Paediatr Child Health 2018; 54: 1159–64. [DOI] [PubMed] [Google Scholar]
- 91.Novak I, Morgan C, Fahey M, et al. State of the Evidence Traffic Lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep 2020; 20: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolobe TH, Christy JB, Gannotti ME, et al. Research summit III proceedings on dosing in children with an injured brain or cerebral palsy: executive summary. Phys Ther 2014; 94: 907–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shepherd RB, Carr JH. Neurological rehabilitation. Disabil Rehabil 2006; 28: 811–2. [DOI] [PubMed] [Google Scholar]
- 94.Will EA, Caravella KE, Hahn LJ, Fidler DJ, Roberts JE. Adaptive behavior in infants and toddlers with Down syndrome and fragile X syndrome. Am J Med Genet B Neuropsychiatr Genet 2018; 177: 358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hutchon B, Gibbs D, Harniess P, et al. Early intervention programmes for infants at high risk of atypical neurodevelopmental outcome. Dev Med Child Neurol 2019; 61: 1362–7. [DOI] [PubMed] [Google Scholar]
- 96.Boyd RN, Ziviani J, Sakzewski L, et al. REACH: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia. BMJ Open 2017; 7: e017204.protocol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benfer KA, Novak I, Morgan C, et al. Community-based parent-delivered early detection and intervention programme for infants at high risk of cerebral palsy in a low-resource country (Learning through Everyday Activities with Parents (LEAP-CP): protocol for a randomised controlled trial. BMJ Open 2018; 8: e021186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sgandurra G, Beani E, Giampietri M, et al. Early intervention at home in infants with congenital brain lesion with CareToy revised: a RCT protocol. BMC Pediatr 2018; 18: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blauw-Hospers C, Dirks T, Hulshof LJ, Bos AF, Hadders-Algra M. Pediatric physical therapy in infancy: from nightmare to dream? A two-arm randomized trial. Phys Ther 2011; 91: 1323–38. [DOI] [PubMed] [Google Scholar]
- 100.Palmer FB, Shapiro BK, Allen MC, et al. Infant stimulation curriculum for infants with cerebral palsy: effects on infant temperament parent-infant interaction and home environment. Pediatric 1990; 85: 411–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Developmental outcome measures for included studies within the systematic reviews
Figure S1: PRISMA flow chart for the process for and results of the search for systematic reviews from 2009 to 2020.
Appendix S2: PRISMA checklist.
Appendix S1: Search strategies.
Data Availability Statement
Data available by request from the authors.


