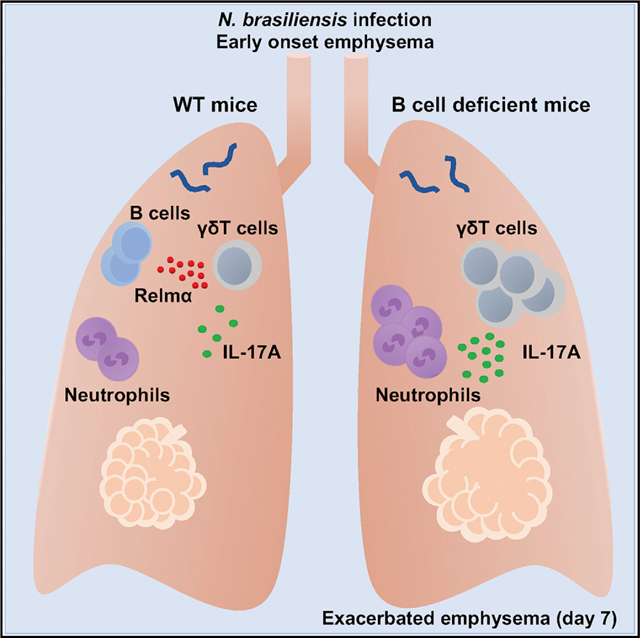
SUMMARY
Emphysema results in destruction of alveolar walls and enlargement of lung airspaces and has been shown to develop during helminth infections through IL-4R-independent mechanisms. We examined whether interleukin 17A (IL-17A) may instead modulate development of emphysematous pathology in mice infected with the helminth parasite Nippostrongylus brasiliensis. We found that transient elevations in IL-17A shortly after helminth infection triggered sub-sequent emphysema that destroyed alveolar structures. Furthermore, lung B cells, activated through IL-4R signaling, inhibited early onset of emphysematous pathology. IL-10 and other regulatory cytokines typically associated with B regulatory cell function did not play a major role in this response. Instead, at early stages of the response, B cells produced high levels of the tissue-protective protein, Resistin-like molecule α (RELMα), which then downregulated IL-17A expression. These studies show that transient elevations in IL-17A trigger emphysema and reveal a helminth-induced immune regulatory mechanism that controls IL-17A and the severity of emphysema.
In Brief
Emphysema causes pathology that can compromise lung function, and mechanisms for reducing disease severity remain unclear. Using a helminth model, Chen et al. show that type 2 immune response triggers lung B cells to produce RELMα, which then downregulates IL-17 production in the lung to limit emphysema.
Graphical Abstract
INTRODUCTION
Type 2 immunity includes both resistance and tolerance responses that together mediate host protection against helminths. Tolerance mechanisms reduce harmful effects of the pathogen without affecting the parasite burden, and disease tolerance is recognized as a significant host-defense strategy (Medzhitov et al., 2012; Soares et al., 2017). Control of tissue injury during helminth infection is particularly important, because these large multicellular parasites can cause considerable damage as they traffic through host tissues (Gause et al., 2013). Previous studies have shown that the rodent helminth, Nippostrongylus brasiliensis, which has a life cycle similar to that of human hookworms, can cause acute lung injury (ALI) shortly after invasion of the lung (Chen et al., 2012; Harvie et al., 2010). ALI is observed as early as day 2 after N. brasiliensis inoculation and includes both neutrophil inflammation and hemorrhaging, which are triggered partly by early elevations in interleukin (IL)-17 produced by γδ T cells activated by endogenous danger signals (Sutherland et al., 2014). However, by several days after inoculation, a type 2 immune response develops that mitigates ALI, making this a useful model for interrogating mechanisms of disease tolerance. ALI is controlled through interleukin-4 receptor (IL-4R)-dependent mechanisms, which include downregulation of harmful inflammation and activation of alternatively activated (M2) macrophages and other cell types to produce factors that directly promote tissue repair (Bosurgi et al., 2017; Chen et al., 2012). This is consistent with studies suggesting type 2 responses can promote tissue regeneration in the liver (Goh et al., 2013) and skeletal muscle (Heredia et al., 2013) and fibrosis and scarring in skin, the latter partly mediated by Resistin-like molecule (RELM) α produced by M2 macrophages (Knipper et al., 2015).
Although the type 2 immune response reduces the severity and duration of ALI, studies indicate that by day 30 after inoculation, severe emphysematous pathology develops in the lung, compromising lung function (Marsland et al., 2008). This emphysematous pathology is similar to human emphysema and includes characteristic loss of alveolar septal structures, resulting in irreversible lung damage associated with destructive enlargement of air-spaces and reduced air flow (Craig et al., 2017; Marsland et al., 2008). How parasite invasion of the lung triggers emphysematous pathology is as yet unknown. Although chronic fibrosis is blocked in N. brasiliensis-inoculated IL-4R-deficient mice, the development and severity of emphysematous pathology is not reduced (Marsland et al., 2008), indicating that emphysema develops independently of IL-4R-dependent type 2 immunity and associated fibrosis. Studies have shown that IL-17A, produced by γδ T cells, is elevated between days 2 and 3 after N. brasiliensis inoculation, after which it rapidly decreases (Allen et al., 2015; Chen et al., 2012; Sutherland et al., 2014). IL-17A has also been implicated in the development of emphysema in experimental models and in humans (Fujii et al., 2016; Lu et al., 2015; You et al., 2015), raising the possibility that the early and transient IL-4R independent elevations in IL-17A after N. brasiliensis infection may trigger subsequent development of chronic emphysematous pathology. The delayed development of emphysema also raises the possibility of regulatory mechanisms preventing its acute onset. Immune response modulation following helminth infection has been shown to control several inflammatory diseases, including asthma, diabetes, metabolic disorders, and inflammatory bowel disease (IBD) (Allen and Maizels, 2011; Mishra et al., 2014). In some cases, IL-10 produced by T regulatory (Treg) cell populations has been implicated (Wilson et al., 2005). However, in other cases, immune regulatory effects triggered by helminths can be IL-10 independent, although the actual cell types and/or regulatory molecules involved remain uncertain (Allen and Maizels, 2011; Mishra et al., 2013, 2014; Wilson et al., 2010). Thus, additional helminth-induced immune regulatory mechanisms may yet be revealed. As such, studies of emphysema in the N. brasiliensis model provide an opportunity to investigate potential helminth-induced regulatory mechanisms that contribute to disease tolerance and that specifically mitigate this characteristic type of lung damage.
Here we examined the development and control of emphysematous pathology during helminth infection. Our findings show that transient elevations in IL-17A shortly after helminth infection trigger subsequent emphysema that can mediate destruction of alveolar septa. We further show that lung B cells activated in the context of helminth infection produce RELMα, which then plays an essential role in controlling emphysema through downregulation of IL-17A shortly after infection. These studies thus reveal how emphysema develops independently of IL-4Rα signaling during helminth function and provides mechanisms of helminth-induced B cell control of this pathology.
RESULTS
IL-17 Promotes Emphysema after N. brasiliensis Inoculation
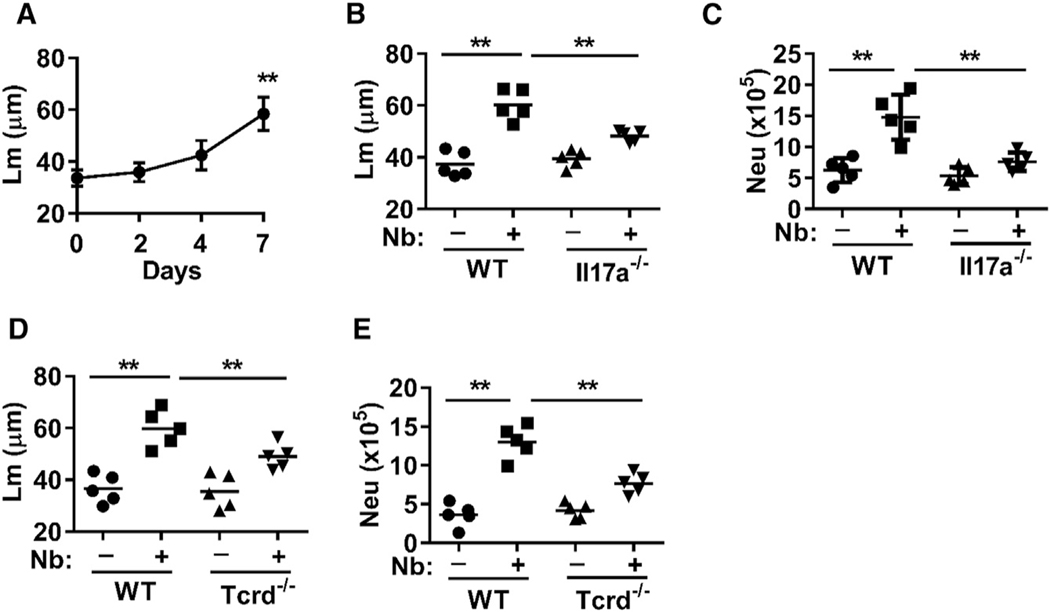
We investigated the development of emphysematous pathology after N. brasiliensis infection. As previously published (Marsland et al., 2008), we observed, through histological analyses, severe emphysema at 30 days post-infection (data not shown) and further showed initial development of emphysema as early as 7 days post-infection, as assessed by mean linear intercept length (Lm), a measure of interalveolar septal wall distance and a standard quantitative measure of emphysematous pathology (Hautamaki et al., 1997) (Figure 1A; Figure S1A). The changes in the lung environment that mediate the development of emphysema remain unclear, particularly because emphysematous pathology still develops in Il4ra−/− mice (Marsland et al., 2008). Studies show a transient but pronounced increase in IL-17A by day 2 after inoculation, which nonetheless promotes neutrophil infiltration and ALI, both of which are largely resolved by day 5 after inoculation (Chen et al., 2012). To examine whether these early increases in IL-17A contribute to the subsequent development of emphysematous pathology, Il17a−/− mice were inoculated with N. brasiliensis. IL-17A deficiency resulted in reduced emphysematous pathology (Figure 1B; Figure S1B) and less infiltration of neutrophils (Figure 1C). Our results are consistent with studies showing that blocking IL-17A inhibits porcine pancreatic elastase (PPE)-induced emphysema (Fujii et al., 2016), indicating a common role for IL-17A across experimental emphysema models. Because IL-17A-producing γδ T cells are known to play a dominant role in the recruitment of neutrophils to the lung through their production of IL-17 during N. brasiliensis infection (Sutherland et al., 2014), we also infected Tcrd−/− mice, which are deficient in γδ T cells (Cai et al., 2011). Similar to Il17a−/− mice, we observed reduced emphysematous pathology (Figure 1D; Figure S1C) and less infiltration of neutrophils (Figure 1E). Altogether, these data indicate that γδ T cells and associated transient IL-17A signaling promote the subsequent development of emphysematous pathology after N. brasiliensis inoculation.
Figure 1. Emphysematous Pathology Develops by 7 Days after Nb Inoculation, and Its Severity Is Reduced in Il17a−/− and Tcrd−/− Mice.
(A) Emphysematous pathology was determined by digital imaging analysis of mean linear intercept measurements (Lm) of alveolar spaces at days 2, 4, and 7 after N. brasiliensis (Nb) inoculation of WT BALB/c mice.
(B–E) Il17a−/− and WT BL/6 control mice or Tcrd−/− and WT BL/6 control mice were inoculated with Nb. Then 8 days later, emphysematous pathology was assessed by Lm (B and D) and the total number of CD11b+Ly6G+ neutrophils at day 2 was determined by flow cytometry (C and E).
Each symbol represents an individual mouse, and horizontal lines indicate the mean. Data shown are the mean and SEM from at least five individual mice per group and are representative of at least two independent experiments (**p < 0.01). See also Figure S1.
B Cells Mitigate Severity of Emphysema by Downregulating IL-17A
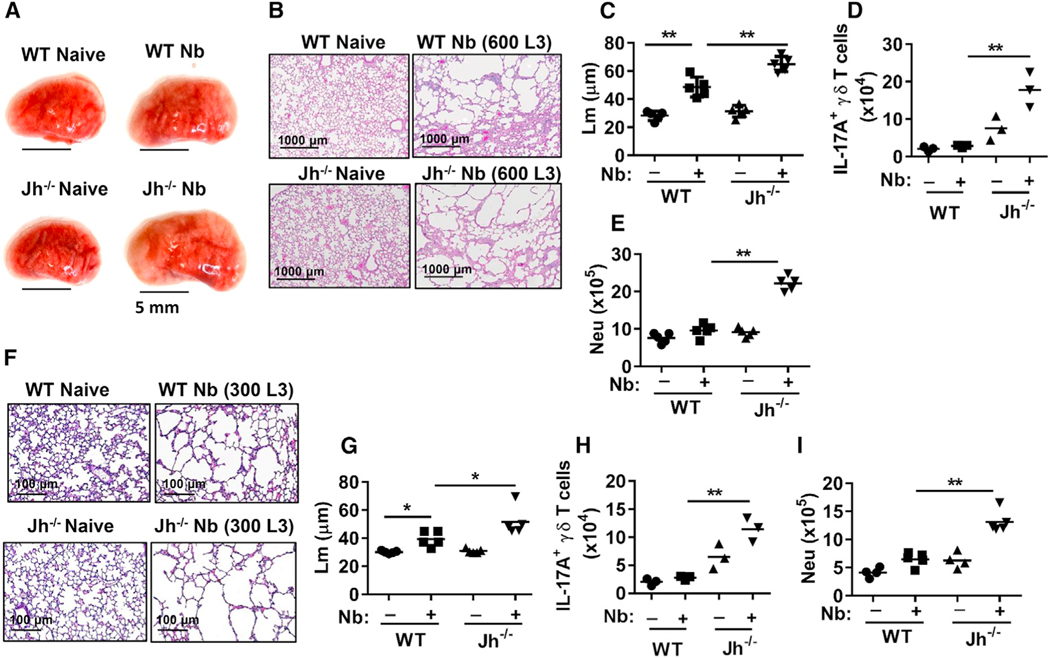
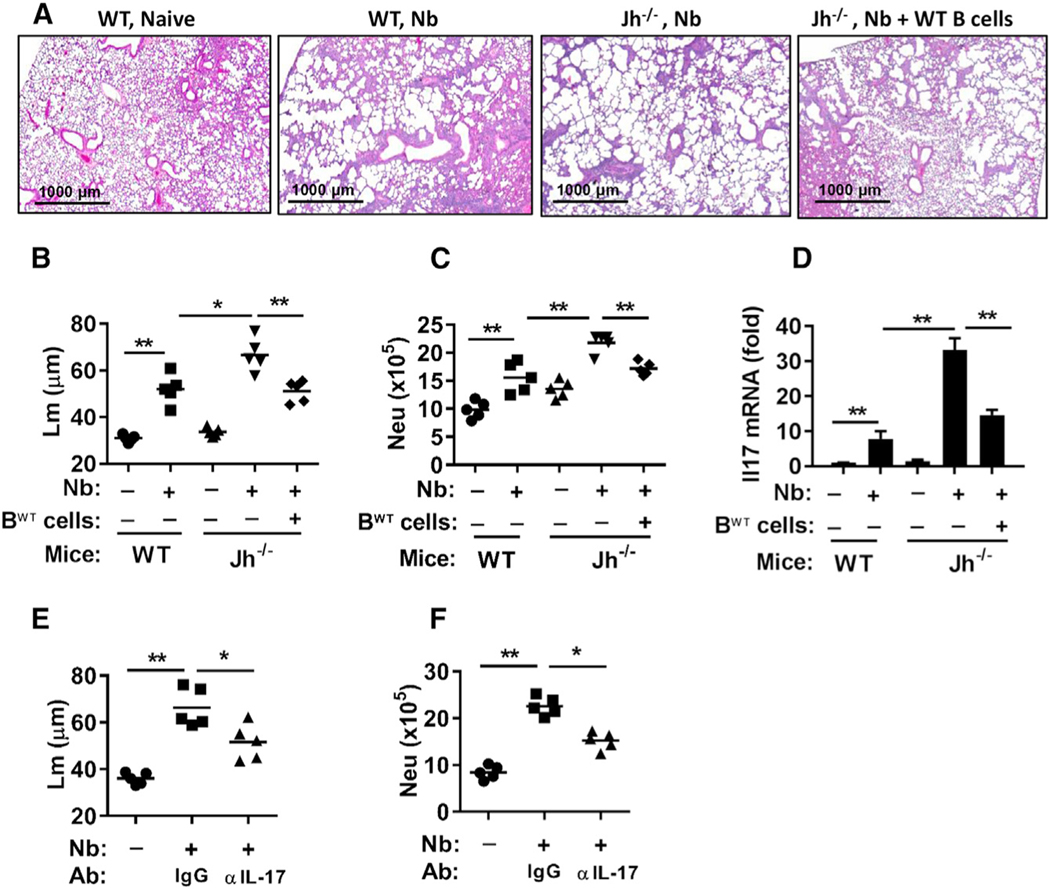
In further studies, we unexpectedly found that emphysematous pathology-associated lung size was markedly increased in B cell-deficient Jh−/− mice, with approximately 30% increases in Lm as early as day 7 after N. brasiliensis inoculation (600 larval stage 3 [L3] larvae) (Figures 2A–2C) and associated marked increases in IL-17A+ γδ T cells and neutrophils in the lungs of Jh−/− mice (Figures 2D and 2E). This suggests that B cells controlled the development of emphysematous pathology. We also extended analysis of effects of B cell deficiency to 30 days after inoculation. Although emphysematous pathology was severe and comparable in wild-type (WT) and Jh−/− mice after inoculation with 600 L3 larvae (data not shown), inoculation with 300 L3 larvae resulted in marked increases in emphysematous pathology and associated marked increases in IL-17A+ γδ T cells and neutrophil infiltration in Jh−/− compared to WT mice (Figures 2F–2I). At the higher dose, the resultant progression of emphysema is apparently sufficiently severe by day 30 that the presence of B cells cannot mitigate the damage. To examine whether WT B cells could rescue control of emphysema in the Jh−/− mice, WT naive B cells were transferred to Jh−/− mice before N. brasiliensis inoculation. B cell transfers markedly reduced emphysema, neutrophils, and Il17a mRNA in Jh−/− mice at day 7 after N. brasiliensis inoculation (Figures 3A–3D). Donor CD45.1 B cells were readily detected in recipient lung and lymphoid tissues of Jh−/− mice (Figure S2). To investigate whether IL-17A signaling contributes to exacerbated emphysematous pathology in Jh−/− mice, neutralizing anti-IL-17A antibody was administered to N. brasiliensis-infected Jh−/− mice. IL-17A blockade resulted in decreased emphysematous pathology and less infiltration of neutrophils (Figures 3E and 3F; Figure S1D). These data thus indicate that B cells play a significant role in controlling the development of IL-17A-dependent emphysema by inhibiting IL-17A expression and associated neutrophil recruitment and activation.
Figure 2. B Cell Deficiency Exacerbates Emphysematous Pathology after Nb Inoculation.
(A–E) BALB/c WT and Jh−/− mice were inoculated with 600 L3 and, 7 days later, were analyzed for emphysematous pathology and IL-17 expression.
(A) Left lobe of lung was collected and photographed (scale bar, 5 mm).
(B) H&E staining of representative formalin-fixed lung sections of mice.
(C) Digital imaging analysis of mean linear intercept measurements (Lm) of alveolar spaces.
(D) Number of lung IL-17A+ γδ T cells.
(E) Number of neutrophils (CD11b+Ly6G+), analyzed by FACS.
(F–I) WT and Jh−/− mice were inoculated with 300 L3 and, 30 days later, were analyzed for emphysematous pathology. H&E staining (F) and Lm analyses (G) were assessed as described earlier, and the number of lung IL-17A+ γδ T cells (H) and lung neutrophils (CD11b+Ly6G+) (I) was determined by flow cytometry.
Each symbol represents an individual mouse, and horizontal lines indicate the mean. Data shown are representative of at least two independent experiments (*p < 0.05, **p < 0.01).
Figure 3. Transfer of WT B Cells or Administration of Neutralizing Anti-IL-17A Antibody Restored Control of Emphysematous Pathology in B Cell-Deficient Nb-Inoculated Mice.
(A–D) BALB/c WT, B cell-deficient Jh−/−, and Jh−/− recipient mice with transferred BABL/c WT B cells were all assessed for severity of emphysematous pathology at day 7 and for neutrophils and Il17 gene expression at day 2 after Nb inoculation.
(A) H&E staining of representative sections is shown.
(B) Emphysematous pathology was measured using digital imaging analysis of mean linear intercept measurements (Lm) of alveolar spaces.
(C) The number of lung neutrophils (CD11b+Ly6G+) was determined by flow cytometry at day 2.
(D) Whole-lung Il17a gene expression by qPCR is presented as the fold increase over naive WT mice after normalization to 18s ribosomal RNA (18srRNA).
(E and F) Jh−/− mice were administered blocking anti-IL-17A Ab and inoculated with Nb. Then 7 days later, they were assessed for emphysematous pathology through Lm analysis (E), and 2 days later, they were analyzed for number of lung neutrophils (F); results are presented as described earlier.
Each symbol represents an individual mouse. Horizontal lines indicate the mean (B, C, E, and F), or data shown are the mean and SEM from at least five individual mice per group (D). Data shown are representative of at least two independent experiments (*p < 0.05, **p < 0.01). See also Figure S2.
Type 2 Immunity Controls ALI in B Cell-Deficient Mice and Drives Development of B Regulatory Cells
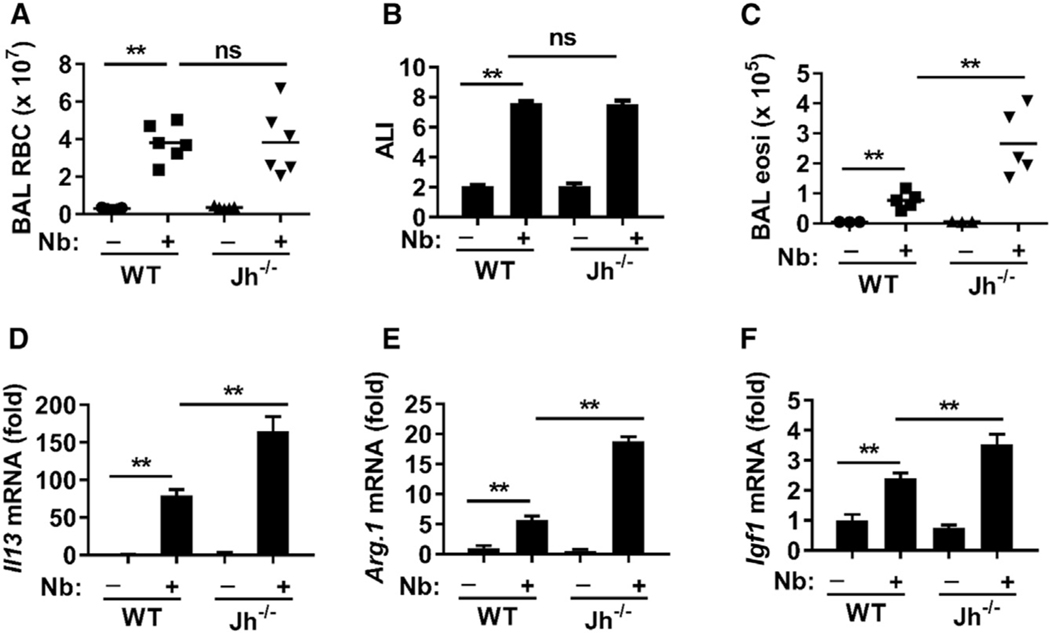
Effects of B cell deficiency on the development of ALI were next examined. At day 4 after N. brasiliensis inoculation, markers used to assess ALI (as previously published by Chen et al., 2012), including bronchoalveolar lavage (BAL) red blood cells (RBCs) and the histological ALI score, were not enhanced between WT and Jh−/− mice (Figures 4A and 4B). However, indicators of the associated type 2 response, including BAL eosinophil numbers (Figure 4C) and lung tissue Il13 mRNA levels (Figure 4D), were significantly increased in Jh−/− mice compared to WT mice, indicating that B cells are not required for, and limit, type 2 immunity. The increased type 2 immunity in Jh−/− mice may compensate for IL-17-related ALI. mRNA levels of Arg1 and Igf1, factors that promote lung wound repair (Chen et al., 2012), were expressed at significantly higher levels in the lungs of Jh−/− mice after N. brasiliensis infection (Figures 4E and 4F). These findings indicate that the development of pathology associated with ALI is separable from the development of subsequent emphysematous pathology. Previous studies have shown that B cells can have potent regulatory functions in controlling harmful inflammation during autoimmune and infectious diseases and during allergic responses, in many cases through IL-10-dependent mechanisms (Mauri and Menon, 2015). However, B regulatory (Breg) cell effects induced by the helminth H. polygyrus can control autoimmune and allergic responses through IL-10-independent effects, although their regulatory mechanism of action remains unknown (Wilson et al., 2010). To examine the role of IL-10, produced by B cells, in controlling the development of emphysematous pathology, B cells from naive WT or Il10−/− mice were transferred to Jh−/− recipient mice before infection. WT and IL-10−/− B cells similarly restored control of emphysematous pathology 7 days after N. brasiliensis inoculation (Figures S3A–S3C), demonstrating that Breg cell function controlling emphysema severity was IL-10 independent. To examine whether IL-4R signaling promotes the development of B cells that downregulate emphysematous pathology, B cells from WT and Il4ra−/− mice were transferred to Jh−/− recipient mice before infection. WT B cells, but not Il4ra−/− donor B cells, effectively controlled exacerbation of emphysematous pathology and associated inflammatory responses, including infiltration of neutrophils into the lung and increases in Il17a mRNA levels (Figure 5). There is no effect on N. brasiliensis transit to the small intestine in Il4ra−/− mice, Il17a−/− mice, Tcrd−/− mice, Il10−/− mice, and Jh−/− mice after primary inoculation (Chen et al., 2014, and data not shown). These findings thus indicate that control of ALI is sustained in B cell-deficient mice and that the Breg cell function mitigating emphysema is independent of IL-10 and dependent on IL-4R signaling.
Figure 4. B Cell Deficiency Did Not Exacerbate Acute Lung Injury.
WT and Jh−/− mice were inoculated with Nb and analyzed for acute lung injury (ALI) and type 2 immunity at day 4.
(A–C) BAL red blood cell (RBC) count (A), ALI pathology scores (B), and BAL eosinophil count (C).
(D–F) Lung tissues were examined for the expression of Il13 (D), Arginase 1 (Arg1) (E), and IGF1 mRNA (F) by qPCR. Gene expression is presented as the fold increase over naive WT mice after normalization to 18sRNA.
Each symbol represents an individual mouse. Horizontal lines indicate the mean (A and C), or data shown are the mean and SEM from five individual mice per group (B–F). Data shown are representative of at least two independent experiments (**p < 0.01).
Figure 5. IL-4R Signaling Promotes B Cell-Mediated Control of Emphysema.
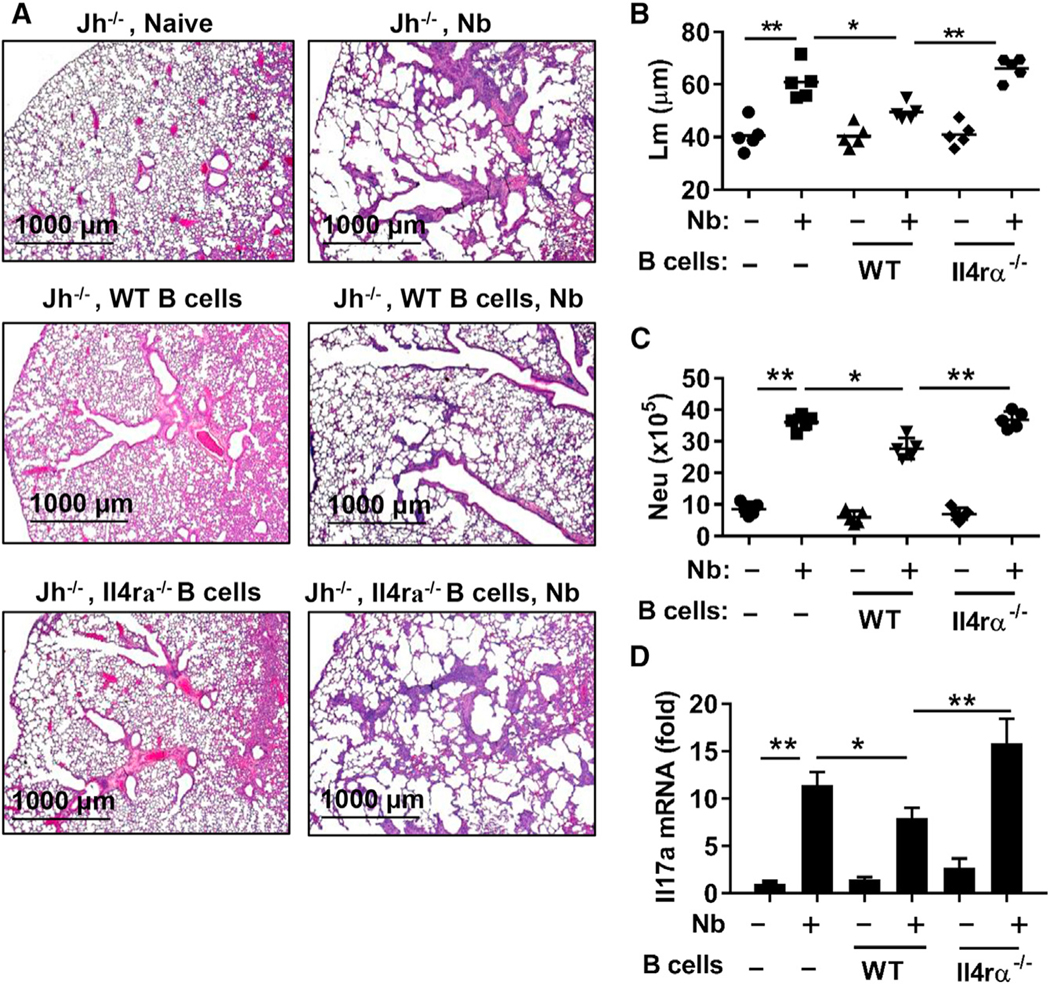
B cells from WT and Il4ra−/− mice were transferred to Jh−/− recipients at days −3, 0, and +1 during N. brasiliensis inoculation. Lungs were collected for analysis 7 days after inoculation.
(A) H&E staining of formalin-fixed lung sections.
(B) Emphysematous pathology was quantitated by digital imaging analysis of mean linear intercept measurements (Lm) of alveolar spaces.
(C) Number of lung neutrophils (CD11b+Ly6G+) was determined by flow cytometry. Each symbol represents an individual mouse, and horizontal lines indicate the mean.
(D) Lung tissues were examined for the expression of Il17a by qPCR. Gene expression is presented as the fold increase over naive WT mice after normalization to 18sRNA and expressed as the mean and SEM from five individual mice per group. Data shown are representative of at least two independent experiments (*p < 0.05, **p < 0.01). See also Figure S3.
RELMα Mediates Immunoregulatory Effects of B Cells
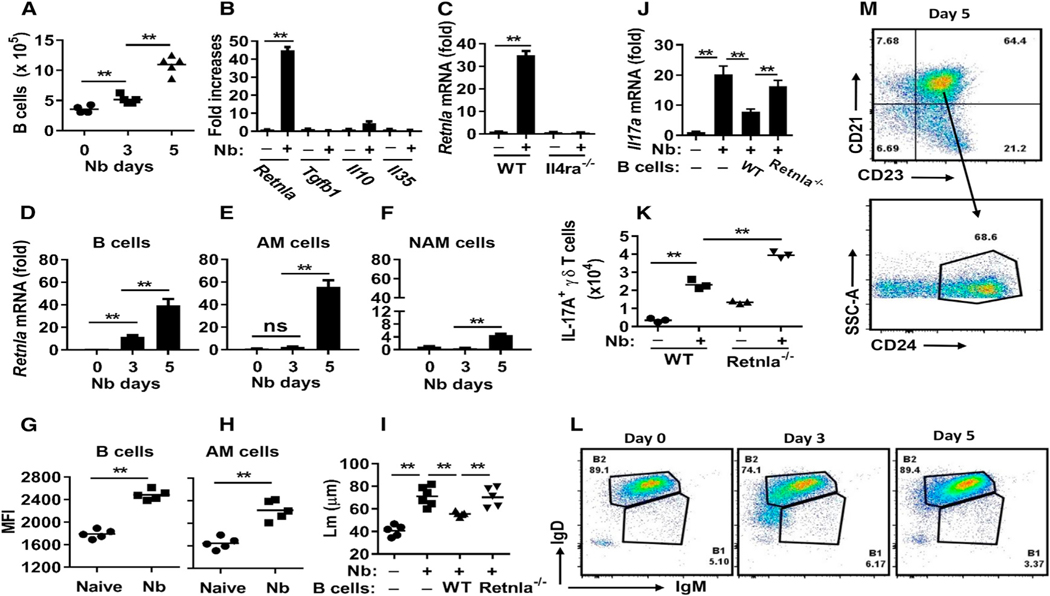
Because the control of emphysematous pathology in the context of helminth infection is little studied, it was possible that immune regulatory mechanisms could be identified. To examine lung B cells during helminth infection, we first showed that they were increased in the lung as early as day 3 after N. brasiliensis inoculation (Figure 6A). We next investigated the expression of potential regulatory molecules known to mediate Breg cell function and observed little change in Tgfb1 mRNA (Lee et al., 2014) and Il35 mRNA (Wang et al., 2014) and a modest increase in Il10 mRNA (Figure 6B). We also assessed markers characteristic of type 2 immune responses generally. Surprisingly, we detected marked increases in Retnla mRNA (Figure 6B). Although not previously shown to be produced by B cells, RELMα is expressed at high levels by alternatively activated (M2) macrophages during helminth infection (Chen et al., 2014). Studies have shown that RELMα has potent and pleiotropic effects, including contributions to tissue repair (Knipper et al., 2015), downregulation of helminth-induced type 2 immune responses (Nair et al., 2009), and most recently, enhancement of positive energy balance (Kumamoto et al., 2016). Increased B cell expression of Retnla depended on IL-4Rα signaling, because expression was blocked in N. brasiliensis-inoculated Il4ra−/− mice (Figure 6C). Pronounced increases in Retnla mRNA were observed as early as day 3 in B cells, with further increases on day 5 (Figure 6D). As a comparison, Retnla was not significantly elevated in macrophages until day 5 and was expressed at higher levels in alveolar macrophages compared to nonalveolar macrophages (Figures 6E and 6F). At day 5, alveolar macrophages and B cells were cytoplasmically stained for RELMα and analyzed by fluorescence-activated cell sorting (FACS); mean fluorescence intensities (MFIs) were comparable (Figures 6G and 6H). Increases in RELMα protein were observed on cytospins as early as day 1, with pronounced increases by day 5 in lung B cells (Figure S3D). To examine whether RELMα expression by B cells affected Breg function, B cells from WT and RELMα-deficient (Retnla−/−) mice were transferred to Jh−/− mice before infection. Although WT donor B cells restored control of IL-17A, emphysematous pathology, and associated inflammation, donor B cells from Retnla−/− mice had little effect (Figures 6I and 6J; Figures S4D and S4E). Furthermore, we observed significantly higher numbers of IL-17A+ γδ T cells in the lungs of Retnla−/− mice compared to WT mice after N. brasiliensis inoculation at day 2 (Figure 6K) and more severe emphysematous pathology, increased numbers of neutrophils, and increased Il17a gene expression in the lungs of Retnla−/− mice after N. brasiliensis inoculation at day 7 (Figures S4A–S4C). Retnla mRNA levels were higher in the lung of WT mice compared to Jh−/− mice after N. brasiliensis inoculation at days 2 and 3 (Figure S4F). These data indicated that IL-17A production was controlled by RELMα. These results thus show that B cell-derived RELMα mediates downregulation of IL-17 after its initial surge in the lung shortly after N. brasiliensis inoculation.
Figure 6. RELMα Expression by B Cells Is Required for Limiting Nb-Induced Emphysematous Pathology.
(A) B cell numbers in the lung at different time points after N. brasiliensis inoculation were assessed by flow cytometry.
(B) At day 5 after Nb inoculation, sort-purified B cells were analyzed for mRNA expression of candidate regulatory factors.
(C) Sort-purified B cells from Il4ra−/− and WT BALB/c mice were analyzed for Retnla mRNA expression at day 5 after Nb inoculation.
(D–F) Sort-purified B cells (D), alveolar macrophages (E), or non-alveolar macrophages (F) at different time points after Nb infection were analyzed for Retnla mRNA expression.
(G and H) RELMα mean fluorescence intensity (MFI) of lung B cells (CD19+) (G) and alveolar macrophages (H) was determined by cytoplasmic staining and FACS at day 5 after N. brasiliensis inoculation.
(I and J) Sort-purified B cells from either untreated Retnla−/− or WT mice were transferred to recipient Jh−/− mice at days −3, 0, and +1 after N. brasiliensis inoculation. Lungs were collected for analysis 7 days after inoculation, emphysematous pathology was digitally imaged as described in Figure 1 (I), and lung tissues were analyzed for the expression of Il17a by qPCR (J).
(K) Retnla−/− and WT mice were inoculated with Nb, and 2 days later, γδ T cells were assessed for IL-17A production by intracellular staining and flow cytometric analyses.
(L and M) FACS analysis of lung B cell subsets at day 5 after N. brasiliensis inoculation, showing expression of IgM and IgD (L) and CD21, CD23, and CD24 (M) on gated CD19+ lymphocytes.
Each symbol represents an individual mouse. Small horizontal lines indicate the mean (A and D–I), or data shown are the mean and SEM from five individual mice per group (B–F and J) or a pool of five mice per group (K and L). Data shown are representative of at least two independent experiments (**p < 0.01). See also Figures S4 and S5.
Phenotyping of Lung B Cells Shows that B2 B Cells Express RELMα Shortly after N. brasiliensis Inoculation
Studies indicate that Breg cells can develop from various B cell lineages, with no specific transcription factors associated with the Breg phenotype (Rosser and Mauri, 2015). Most B cells in the lungs of N. brasiliensis-inoculated mice showed a characteristic B2 phenotype, with high expression of CD19 and immunoglobulin (Ig) D and intermediate expression of IgM (Figure 6L) (Kushnir et al., 2001). These cells also showed high expression of CD21, CD23, and CD24, characteristic of the T2-MZP B2 subpopulation (Figure 6M) (Rosser and Mauri, 2015). B10 cells (CD19+CD5+CD1hi), plasma cells (B220+CD138+MHC-IIlow), and plasmablasts (CD138+CD44hi) constituted less than 1% of lung B cells in N. brasiliensis-infected mice (data not shown). There was the possibility that B cells in the Retnla−/− mice may not have developed normally, potentially affecting their ability to secrete antibody (Ab). Analysis of lung B cell populations showed similar phenotypes in WT and Retnla−/− mice (Figure S5A). To test whether B cell Ab production was altered by RELMα deficiency, WT and Retnla−/− mice were inoculated with N. brasiliensis for 3, 6, or 7 days (primary [1°]), or at 20 days after primary inoculation, they were challenged again with N. brasiliensis and assessed after 7 days (secondary [2°]). Changes in total (Figure S5B) and antigen (Ag)-specific IgG1, IgE (Figure 5C), and IgM (Figure 5D) were similar in WT and Retnla−/− mice after primary or secondary inoculations. Previous studies have shown that although resistance is intact in B cell-deficient mice, transfer of serum Abs from N. brasiliensis-inoculated WT mice to naive mice accelerates expulsion (Liu et al., 2010). As shown in Figure S5D, transfer of immune serum from either N. brasiliensis-inoculated WT or Retnla−/− mice comparably accelerated worm expulsion. Altogether, these findings indicate that B2 B cells are the primary source of RELMα and that a robust B cell and associated Ab response is sustained in Retnla−/− mice.
DISCUSSION
Our studies reveal that the IL-4Rα-independent development of emphysematous pathology is caused by transient elevations in IL-17A at day 2 after N. brasiliensis inoculation. Our findings further show that after day 2, IL-4Rα-dependent activation of a Breg cell population in the lung, characterized by its production of RELMα, is required for reducing IL-17A elevations, neutrophil infiltration, and associated emphysematous pathology.
Helminth infection has previously been shown to play a significant role in controlling inflammatory diseases, including type 1 diabetes and IBD. In these diseases, Treg and Breg cells have both been implicated through their production of IL-10 (Mangan et al., 2004; Mishra et al., 2013; Setiawan et al., 2007). However, inflammatory conditions including airway inflammation and experimental autoimmune encephalitis can also be controlled by helminth infection through as-yet-unidentified IL-10-independent mechanisms (Wilson et al., 2010). Our findings reveal a role for helminth-induced B cells in controlling IL-17A expression through their production of RELMα. Although RELMα has not previously been shown to downregulate IL-17A, previous studies have indicated that it can suppress CD4 T helper 2 (Th2) cell cytokine responses (Nair et al., 2009), consistent with our findings that upregulated type 2 immunity in N. brasiliensis-inoculated B cell-deficient mice was controlled by transfer of WT B cells, but not RELMα−/− B cells.
Transient IL-17A elevations, within 2–3 days after N. brasiliensis inoculation, contribute to acute lung hemorrhaging, partly through neutrophil recruitment. However, by day 4, ALI is mitigated through IL-4Rα-dependent mechanisms, which include downregulation of IL-17A, associated harmful neutrophil inflammation, and direct enhancement of tissue repair mechanisms (Allen et al., 2015; Chen et al., 2012). Our findings demonstrate that the acute 2 day increase in IL-17A, triggered by parasite migration through the lung at the initiation of the response, also contributes to the development of severe emphysema, because IL-17A deficiency reduced emphysematous pathology. Apparently, the increased IL-17A during this brief interval alters the lung milieu sufficiently to support chronic destruction of elastin walls forming the alveoli. These findings are consistent with the development of emphysema in humans long after smoking cessation (Taraseviciene-Stewart and Voelkel, 2008). Alternatively, there may be other factors contributing to the chronic emphysematous pathology, including potential residual parasite products. The more prolonged elevations in IL-17A and associated accelerated onset of emphysematous pathology in the absence of B cell-derived RELMα indicates an essential role for B cells and RELMα in downregulating IL-17A and emphysema at early stages of the response. When lower-dose inoculums of N. brasiliensis were administered, emphysematous pathology was more pronounced as late as 30 days after inoculation in B cell-deficient mice, indicating B cells can also mitigate chronic emphysema. It would be of interest to examine whether the severity of emphysematous pathology progressively increases in mice with age, as observed in humans, and whether control by RELMα is sustained at these later time points. In contrast, control of ALI was not impaired by B cell deficiency. This is consistent with our observation that the type 2 response, including M2 macrophage-derived factors like insulin growth factor 1 (IGF-1) and ARG1, which mediate essential acute tissue repair (Chen et al., 2012), was sustained and even increased. Apparently, this potent wound-healing type 2 response was sufficient to control acute tissue damage from additional inflammation associated with more pronounced IL-17A elevations in B cell-deficient mice. Emphysematous pathology was still exacerbated, which suggests that the process leading to destruction of septal walls is sustained. It will be interesting in future studies to elucidate the specific cell populations and factors involved in the exacerbation of the emphysematous pathology following helminth infection.
Previous studies have shown that Retnla, which encodes a small, cysteine-rich secreted molecule homologous to human RELMβ (Holcomb et al., 2000), is expressed in myeloid cells in which it is a characteristic marker for alternatively activated macrophages and neutrophils in the context of a type 2 immune response (Chen et al., 2014; Nair et al., 2006). Our findings that lymphocytes, specifically B cells, can also express RELMα indicates that Breg cells provide an additional significant source for this cytokine and associated functions formerly ascribed to myeloid cells. Our findings that RELMα production by B cells reaches high levels by 3 days following N. brasiliensis inoculation, while macrophage RELMα expression is not high until 5 days, is consistent with this Breg cell population being essential in rapidly downregulating early increases in IL-17A and associated neutrophil recruitment. Several distinct subsets of B cells have been shown to have regulatory properties, with no specific marker or transcription factor characterizing their regulatory function (Rosser and Mauri, 2015). Lung B cells after N. brasiliensis infection predominantly showed a transitional stage 2 (T2)-like B2 cell phenotype, with high expression of IgD, CD21, CD23, and CD24 and intermediate expression of IgM (Kushnir et al., 2001). Previously, this subset has been shown to have Breg properties, including expression of IL-10 (Rosser and Mauri, 2015). Our studies show that in the context of N. brasiliensis infection, they instead express high levels of RELMα, which mediates its Breg function. Previous studies of Breg cell function during helminth infection also identified a B2 B cell phenotype with IL-10-independent immune regulatory functions (Wilson et al., 2010). Our findings of little IL-10 expression by B cells is consistent with previous findings that IL-10 is not expressed at high levels in the lung until day 5 after N. brasiliensis inoculation, with the major source being CD4+ T cells (Chen et al., 2012; Thomas et al., 2012).
Studies have shown that RELMα is a multifunctional cytokine that can promote tissue fibrosis during wound healing (Knipper et al., 2015), favor normoglycemia in lean and obese animals (Kumamoto et al., 2016), and downregulate type 2 immune responses (Nair et al., 2009). Our findings that RELMα also downregulates IL-17A extends its known activities and provides a potential target for control of IL-17A-mediated inflammation. More specifically, RELMα contributes to the rapid downregulation of IL-17 after its peak at day 2 following N. brasiliensis inoculation, thus limiting the duration of the elevated IL-17 response. Our findings may also explain previous findings in these other model systems. For example, studies suggest that IL-17A may contribute to decreased insulin resistance (Ohshima et al., 2012), suggesting that effects of RELMα on insulin resistance may result partly from its counter-regulation of IL-17A. More studies are needed to elucidate the mechanism of RELMα function and associated control of IL-17A expression under these different conditions.
It is increasingly recognized that the immune system may play a causative role in the development of emphysema (Grumelli et al., 2004; Taraseviciene-Stewart and Voelkel, 2008) and that IL-17A may be particularly important in driving the destruction of elastin-containing alveolar walls (Lu et al., 2015; Shan et al., 2012; You et al., 2015). Our studies of emphysema development during helminth infection reveal a critical role for IL-17A, in this case produced by lung γδ T cells, in driving this type of pathology. Our finding that RELMα, produced by B cells in the context of the type 2 immune response, can control type 17-mediated inflammation provides an additional potent negative regulatory mechanism elicited by the helminth-induced immune response. Our findings that B cell subpopulations and Ab production are comparable in WT and Retnla−/− mice after N. brasiliensis inoculation suggests that this requirement for B cell-derived resistin-like molecule alpha (RELMΑα) is not due to impaired B cell development that might compromise Ab production. It will be important in future studies to interrogate how this intrinsic regulator of this response interacts with other negative regulators, such as T reg cells or TAM (Tyr03,Axl1,MerTK) receptor interactions (Chan et al., 2016; de Kouchkovsky et al., 2017). Potentially in combination with other negative regulators, RELMα provides a target that may be useful in the development of future therapies for control of tissue-damaging type 17 responses, although more studies are needed to evaluate its general significance. More specifically, B cell-derived RELMα, and its human homologue, may provide a useful target for specific immune-based therapies developed to control the progression of emphysematous pathology.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, William C. Gause (gausewc@rutgers.njms.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Female 5–8 week old BALB/c or BL/6 WT mice, Tcrd−/− (BL/6), and Il4ra−/− mice (BALB/c) were purchased from The Jackson Laboratory (Bar Harbor, ME). BALB/c Jh−/− mice (BALB/c), and Il10−/− mice (BALB/c) were purchased from Taconic Biosciences (Albany, NY). Retnla−/− mice (BALB/c) were generously provided by Dr. Marc Rothenberg (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH). Il17a−/− (BL/6) mice were generously provided by Dr. Karen Edelblum (Rutgers-NJMS). All mice were maintained in a specific pathogen-free, virus Ab-free facility during the experiments. Healthy mice were selected for treatment groups from purchased or bred colonies, without using specific randomization methods or specific blinding methods. The studies have been reviewed and approved by the Institutional Animal Care and Use Committee at Rutgers-the State University of New Jersey. The experiments herein were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals, Institute of Animal Resources, National Research Council, Department of Health, Education and Welfare (US National Institutes of Health).
Parasites
N. brasiliensis L3 larvae were maintained in Petri dish culture containing charcoal and sphagnum moss. Mice were inoculated subcutaneously with a 40μl suspension of 600 N. brasiliensis L3 isolated from cultures using a modified Baermann apparatus. In one experiment, F2f-I, we inoculated 300 L3. Worm expulsion was detected on day 7. For secondary N. brasiliensis inoculation, mice were inoculated with 600 L3, rested 20 days, secondarily inoculated with 600 L3. At day 7, mice serum were collected.
METHOD DETAILS
Parasite inoculation and serum collection
N. brasiliensis L3 larvae were maintained in Petri dish culture containing charcoal and sphagnum moss. Mice were inoculated subcutaneously with a 40μl suspension of 600 N. brasiliensis L3 isolated from cultures using a modified Baermann apparatus. In one experiment, F2f-I, we inoculated 300 L3. Worm expulsion was detected on day 7. For secondary N. brasiliensis inoculation, mice were inoculated with 600 L3, rested 20 days, secondarily inoculated with 600 L3. At day 7, mice serum were collected. For serum transfer, serum was administered i.p. one day prior and the same day (−1, 0) to N. brasiliensis inoculation at a dose of 0.25 mL respectively.
Flow cytometry, cell sorting, adoptive transfer
Lung tissue was incubated with stirring at 37°C for 30 min in Hank’s balanced salt solution (HBSS) with 1.3 mM EDTA (Invitrogen), then minced and followed by treatment at 37°C for 45 min. with collagenase (1 mg / ml; Sigma) in RPMI with 5% fetal calf serum (FCS) and with 100 μg / ml of DNase for 10 min. Cells were lysed with ACK (Lonza, Walkersville, MD) to remove erythrocytes. Cells were blocked with Fc Block (BD Biosciences, San Jose, CA), directly stained with fluorochrome-conjugated Abs against Ly6G (1A8), MHCII, CD11c, Siglec-F, F4/80, CD19, B220 (BD Biosciences), CD21, CD23, CD24, CD5, Cd1, CD138 (Biolegend), RELMα (Biorbyt), and analyzed by flow cytometry. Neutrophils were identified as CD11b+Ly6G+, Eosinophils as F4/80+CD11c-Siglec-F+, Alveolar macrophages as F4/80+CD11cvarSiglec-F+, nonalveolar macrophages as F4/80+CD11cvarSiglec-F-. In some experiments, B cells were sort purified (> 98%) for adoptive transfer. For B lymphocyte transfers, B cells purified from lung tissue and MLN of donor mice were transferred i.t. into recipient mice. B cells purified from spleens of donor mice were transferred intra-peritoneally (i.p.) into recipient mice. A total of three cell transfers were performed (day −3, 0, and +1) during N. brasiliensis inoculation.
Histology
Lungs were fixed by tracheal instillation of 10% neutral buffered formalin at a pressure of 25 cm water for 5 min followed by immersion in 10% neutral buffered formalin overnight. Lungs were embedded in paraffin, and 5-μm sections were cut and stained with hematoxylin and eosin (H&E). 5 sections per mouse were collected with 20 layers of separation between each one. Digital images were obtained with a Zeiss Axioskop 2 microscope and Zeiss Axiovision software (Carl Zeiss Microscopy, LLC, Thornwood, NY). The measurement of alveolar space area and mean linear intercept of alveolar spaces (Lm) were imaged at 200X. Five histological fields for each section were analyzed with Image Pro Plus software (Media Cybernetics, Rockville, MD).
Cytospin and Immunofluorescent staining
Sorted B cells (2 ×105) from lung tissue were suspended in 200 μL of 1X PBS with 2.5% FCS. The sorted cell suspensions were loaded into a Shandon Cytospin 4 (Thermo Electron Corporation, Waltham, MA), spun at 800–1000 rpm for 5 min and stored at −80°C. Frozen cytospin slides were thawed at room temperature for 30 min, fixed in 4% PFA for 15 min, and stained with an AlexaFluor488-conjugated Ab specific to RELMα (Bioss Inc., Woburn, MA). Coverslips were applied to the slides using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) with DAPI. Images were taken using a Leica DM6000B fluorescent microscope, Orca Flash 4.0 mounted digital camera (Hamamatsu Photonics K.K., Japan) and LAS Advanced Fluorescence software (Leica Microsystems, Buffalo Grove, IL). Fluorescent channels were photographed separately and then merged. Exposure times and fluorescence intensities were normalized to appropriate control images.
Quantitative levels of serum immunoglobulin
For serum collection, mice were anesthetized with 0.1ml / 20 g body weight of Ketamine/xylazine cocktail (Ketamine, 20 mg / ml, xylazine, 2.5 mg/ ml), blood was withdraw by cardiac puncture, and was kept at room temperature for 2 – 3 hours. The blood was then centrifuged at 14000 rpm for 15 min at 4°C, serum was collected and stored at −80°C. Serum total IgG1and IgE levels were detected by ELISA using IgE and IgG1 ELISA kit from Biolegend. N. brasilensis excretory / secretory product (NES) was coated on 96 well ELISA plate overnight (2.5 μg/μl, 100 μl) and then incubated with serial dilutions of serum from N. brasiliensis inoculated mice or naive control mice and then processed using commercial ELISA kit.
Cytokine gene expression by RT-PCR
For qPCR, RNA was extracted from lung tissue or sorted macrophages or B lymphocytes and reverse transcribed to cDNA. qPCR was done with Taqman (Life Technologies Corporation, Carlsbad, CA) kits and the Applied Biosystems 7500 Real-Time PCR System. All data were normalized to 18S ribosomal RNA, and the quantification of differences between treatment groups was calculated according to the manufacturer’s instructions. Gene expression is presented as the fold increase over naive WT controls.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data were analyzed using the statistical software program Prism (GraphPad Software, Inc., La Jolla, CA) and are reported as means (±SEM). Differences between two groups were assessed by Student’s t test, differences among multiple groups were assessed by one way ANOVA and individual comparisons were analyzed using Holm-Sidak test. Differences of p < 0.05 were considered statistically significant.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Anti-Ly6G (1A8) | BD Bioscience | Cat#551461, RRID: AB_394208 |
| Anti-MHCII | eBioscience | Cat# 11–5320-80, RRID: AB_2572500 |
| Anti-CD11b | BD Bioscience | CaT#563553, RRID: AB_2738276 |
| Anti-CD11c | BD Bioscience | Cat#564080, RRID: AB_2738580 |
| Anti-Siglec F | BD Bioscience | Cat# 562681, RRID: AB_2722581 |
| Anti-F4/80 | Biolegend | Cat# 123116, RRID: AB_893481 |
| Anti-CD19 | BD Bioscience | Cat# 553786, RRID: AB_395050 |
| Anti-B220 | Biolegend | Cat#103225, RRID: AB_389308 |
| Anti-CD21 | Biolegend | Cat# 354903, RRID: AB_2561406 |
| Anti-CD23 | Biolegend | Cat# 101607, RRID: AB_312832 |
| Anti-CD24 | Biolegend | Cat# 101807, RRID: AB_312840 |
| Anti-CD5 | Biolegend | Cat# 100610, RRID: AB_312739 |
| Anti-CD1 | eBioscience | Cat# 12–0011-81, RRID: AB_465482 |
| Anti-CD138 | Biolegend | Cat# 142505, RRID: AB_10960141 |
| Anti-Relma | Biorbyt | Cat# orb14229, RRID: AB_10735625 |
| Anti-IL-17A | R&D systems | Cat# MAB421, RRID: AB_2125018 |
| Rat IgG2a isotype control (2A3) | BioXcell | Cat# BE0089, RRID: AB_1107769 |
|
| ||
| Critical Commercial Assays | ||
|
| ||
| Serum IgG1, IgE ELISA kit | Biolegend | 432405 |
|
| ||
| Experimental Models: Organisms/Strains | ||
|
| ||
| N. brasiliensis | Our Laboratory | N/A |
| WT BALB/c | Jackson Laboratory | JAX:000651 |
| Il10−/− BALB/c | Taconic Bioscience | Taconic 15660 |
| Il4ra−/− BALB/c | Jackson Laboratory | JAX:003514 |
| Jh/ BALB/c | Taconic Bioscience | Taconic:1147 |
| Retnla−/− BALB/c | Provided by Dr. Marc Rothenberg | Cincinnati Children’s hospital Medical Center |
| Il17a−/− BL/6 | Provided by Dr. Karen Edelblum | Rutgers-NJMS |
| Tcrd−/− BL/6 | Jackson Laboratory | JAX 002120 |
| WT BL/6 | Jackson Laboratory | JAX:000664 |
|
| ||
| Oligonucleotides | ||
|
| ||
| PCR primer for Il4 | Thermo Scientific | Mm00445259_m1 |
| PCR primer for Il5 | Thermo Scientific | Mm00439646_m1 |
| PCR primer for Il13 | Thermo Scientific | Mm99999190_m1 |
| PCR primer for Il10 | Thermo Scientific | Mm00439614_m1 |
| PCR primer for TGFβ | Thermo Scientific | Mm01178820 |
| PCR primer for Il35 | Thermo Scientific | Mm00469294-m1 |
| PCR primer for arginase 1 | Thermo Scientific | Mm00475988_m1 |
|
| ||
| Software and Algorithms | ||
|
| ||
| Statistical software program Prism | GraphPad Software | https://www.graphpad.com/ |
Highlights.
Helminth-induced emphysema is IL-17-dependent and exacerbated in B cell-deficient mice
IL-4R signaling triggers activation of B cells that mitigate emphysematous pathology
These B cells express RELMα, which inhibits IL-17-producing γδ T cells
ACKNOWLEDGMENTS
This research was supported by NIH grant R01 AI131634-01A1.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.11.038.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Allen JE, and Maizels RM (2011). Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11, 375–388. [DOI] [PubMed] [Google Scholar]
- Allen JE, Sutherland TE, and Rückerl D. (2015). IL-17 and neutrophils: unexpected players in the type 2 immune response. Curr. Opin. Immunol. 34, 99–106. [DOI] [PubMed] [Google Scholar]
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, et al. (2017). Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, and Yan J. (2011). Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PY, Carrera Silva EA, De Kouchkovsky D, Joannas LD, Hao L, Hu D, Huntsman S, Eng C, Licona-Limón P, Weinstein JS, et al. (2016). The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science 352, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF Jr., Wynn TA, and Gause WC (2012). An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 18, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, Boucher JL, Urban JF Jr., Kim CC, and Gause WC (2014). Neutrophils prime along-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 15, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JM, Scott AL, and Mitzner W. (2017). Immune-mediated inflammation in the pathogenesis of emphysema: insights from mouse models. Cell Tissue Res. 367, 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kouchkovsky DA, Ghosh S, and Rothlin CV (2017). Negative regulation of type 2 immunity. Trends Immunol. 38, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii U, Miyahara N, Taniguchi A, Waseda K, Morichika D, Kurimoto E, Koga H, Kataoka M, Gelfand EW, Cua DJ, et al. (2016). IL-23 is essential for the development of elastase-induced pulmonary inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 55, 697–707. [DOI] [PubMed] [Google Scholar]
- Gause WC, Wynn TA, and Allen JE (2013). Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. 13, 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, and Chawla A. (2013). Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA 110, 9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, and Kheradmand F. (2004). An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 1, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Camberis M, Tang SC, Delahunt B, Paul W, and Le Gros G. (2010). The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect. Immun. 78, 3753–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, and Shapiro SD (1997). Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, and Chawla A. (2013). Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, et al. (2000). FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 19, 4046–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maaß T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg ME, et al. (2015). Interleukin-4 receptor α signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 43, 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto Y, Camporez JPG, Jurczak MJ, Shanabrough M, Horvath T, Shulman GI, and Iwasaki A. (2016). CD301b(+) mononuclear phagocytes maintain positive energy balance through secretion of resistin-like molecule alpha. Immunity 45, 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir N, Bos NA, Zuercher AW, Coffin SE, Moser CA, Offit PA, and Cebra JJ (2001). B2 but not B1 cells can contribute to CD4+ T-cell-mediated clearance of rotavirus in SCID mice. J. Virol. 75, 5482–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, Fitzgerald L, Shi S, Akrawi E, Lei J, et al. (2014). TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur. J. Immunol. 44, 1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kreider T, Bowdridge S, Liu Z, Song Y, Gaydo AG, Urban JF Jr., and Gause WC (2010). B cells have distinct roles in host protection against different nematode parasites. J. Immunol. 184, 5213–5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, You R, Yuan X, Yang T, Samuel EL, Marcano DC, Sikkema WK, Tour JM, Rodriguez A, Kheradmand F, and Corry DB (2015). The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nat. Immunol. 16, 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, and Fallon PG (2004). Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 173, 6346–6356. [DOI] [PubMed] [Google Scholar]
- Marsland BJ, Kurrer M, Reissmann R, Harris NL, and Kopf M. (2008). Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur. J. Immunol. 38, 479–488. [DOI] [PubMed] [Google Scholar]
- Mauri C, and Menon M. (2015). The expanding family of regulatory B cells. Int. Immunol. 27, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, and Soares MP (2012). Disease tolerance as a defense strategy. Science 335, 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Patel N, Wu W, Bleich D, and Gause WC (2013). Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 6, 297–308. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Palma M, Bleich D, Loke P, and Gause WC (2014). Systemic impact of intestinal helminth infections. Mucosal Immunol. 7, 753–762. [DOI] [PubMed] [Google Scholar]
- Nair MG, Guild KJ, and Artis D. (2006). Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J. Immunol. 177, 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, et al. (2009). Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206, 937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima K, Mogi M, Jing F, Iwanami J, Tsukuda K, Min LJ, Higaki J, and Horiuchi M. (2012). Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension 59, 493–499. [DOI] [PubMed] [Google Scholar]
- Rosser EC, and Mauri C. (2015). Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612. [DOI] [PubMed] [Google Scholar]
- Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF Jr., Elliott DE, and Weinstock JV (2007). Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 75, 4655–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C, et al. (2012). Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Sci. Transl. Med. 4, 117ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MP, Teixeira L, and Moita LF (2017). Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 17, 83–96. [DOI] [PubMed] [Google Scholar]
- Sutherland TE, Logan N, Rückerl D, Humbles AA, Allan SM, Papayannopoulos V, Stockinger B, Maizels RM, and Allen JE (2014). Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat. Immunol. 15, 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraseviciene-Stewart L, and Voelkel NF (2008). Molecular pathogenesis of emphysema. J. Clin. Invest. 118, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Rückerl D, Maskrey BH, Whitfield PD, Blaxter ML, and Allen JE (2012). The biology of nematode- and IL4Rα-dependent murine macrophage polarization in vivo as defined by RNA-seq and targeted lipidomics. Blood 120, e93–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, and Egwuagu CE (2014). Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, and Maizels RM (2005). Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202, 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Taylor MD, O’Gorman MT, Balic A, Barr TA, Filbey K, Anderton SM, and Maizels RM (2010). Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur. J. Immunol. 40, 1682–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You R, Lu W, Shan M, Berlin JM, Samuel EL, Marcano DC, Sun Z, Sikkema WK, Yuan X, Song L, et al. (2015). Nanoparticulate carbon black in cigarette smoke induces DNA cleavage and Th17-mediated emphysema. eLife 4, e09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.