Abstract
Objectives
As emerging adoptive immunotherapy after CAR-T cell therapy, CAR-NK cell therapy has been developing rapidly in recent years. Presently, the research on CAR-NK cells has become a hotspot in the field of tumor immunotherapy.
Methods
In this descriptive study, CtieSpace and VOSviewer were used to perform the bibliometric and scientific knowledge-map analysis of articles and reviews related to CAR-NK cells.
Results
5371 authors from 715 institutions in 65 countries published 1028 papers about CAR-NK cells in 346 journals. The number of publications related to CAR-NK cells was increasing overall, especially from 2018 to 2021. The United States was in a leading position. The most active institution was Univ Texas, MD Anderson Cancer Center (USA). The journal with the most publications was Frontiers in immunology, and the most co-cited journal was Blood. The researcher with the most published papers was Winfried S. Wels, while the most co-cited researcher was Shannon L Maude. The research of CAR-NK cells in hematological malignancies and solid tumors (especially the selection of targets and the evaluation of efficacy and safety) was a research hotspot in this field. The emerging topics mainly included three aspects. First, further improve the proliferation and persistence of NK cells in vivo. Secondly, optimizing and improving the CAR structure for NK cells to improve the anti-tumor ability of CAR-NK cells. Thirdly, the related research of CRISPR/Cas9 gene-editing technology in constructing engineered immune cells.
Conclusion
In this study, a bibliometric and scientific knowledge-map study provided a unique and objective perspective for the CAR-NK cell field. This information would provide a helpful reference for researchers interested in this field.
Keywords: CAR-NK cells, CiteSpace, VOSviewer, bibliometric, knowledge-map
Introduction
Chimeric antigen receptor (CAR)-T cell therapy has made a tremendous breakthrough in the research and treatment of hematological malignancies, bringing new hope to patients. Five CAR-T cell products have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of hematological tumors. The success of CAR-T cell therapy aroused researchers’ interest in this adoptive immunotherapy. With the development of genetic modification technology, NK cells have been further customized, including introducing CAR and knocking out suppressor genes (1). Therefore, NK cells, which are thought to have the same potential to enhance the anti-tumor ability through CAR modification (2), gradually come into the researchers’ field of vision.
As innate immune effector cells, NK cells are essential members of the immune system and have broad-spectrum anti-tumor effects. NK cells can rapidly kill abnormal cells without pre-sensitization and MHC restriction (3). Moreover, NK cells play a killing role in four ways (4–7), including perforin/granzyme pathway, Fas/FasL pathway, cytokine pathway, and antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by cluster of differentiation-16 (CD16).
Compared with CAR-T cells, CAR-NK cells have some disadvantages, including low persistence (8), proliferation in vivo only in the presence of specific cytokines (such as IL-2 and IL-15) (9), and lack effective CAR gene transfer methods (9). CAR-NK cells also have some advantages, including 1. There are many sources of NK cells (10–12), such as NK cell lines, peripheral blood, cord blood and induced pluripotent stem cells (iPSCs), especially NK92 cells (13–15); 2. Higher security. Graft-versus-host disease (GVHD) hardly occurs, and the incidence of cytokine release syndrome (CRS) and neurotoxicity is also very low (16); 3. In addition to the CAR-dependent mechanism, the anti-tumor mechanism of CAR-NK cells also includes a CAR-independent (NK cell receptor-dependent) mechanism (17–19). It is these advantages that make the research on CAR-NK cells become the research focus in the field of tumor immunotherapy (20, 21).
As a hot field, research and publications related to CAR-NK cells are increasing year by year. For those researchers who are initially involved in CAR-NK cell research, especially non-professionals interested in this field, it is difficult to quickly grasp and summarize the relevant knowledge in a short time. The bibliometric analysis can well summarize and analyze these numerous documents and complicated data. However, currently, there are no bibliometric studies on CAR-NK cells. This study is the first time to comprehensively and objectively analyze the evolution process, development trend, important knowledge, research hotspots and emerging topics in this field by combining bibliometrics analysis with knowledge-map analysis.
Bibliometrics analyzes literature quantitatively and objectively through mathematical and statistical methods. Through bibliometric analysis of literature on a particular topic, this field’s development and change trend and discover the research hotspots and the latest research topics can be understand quickly (22, 23). In addition, it can also help us obtain some critical information, including the contributions and cooperation of countries, institutions and authors, as well as the contributions and the topic distribution of journals. In this study, the bibliometric analysis of the CAR-NK cell field was carried out, and relevant scientific knowledge maps were constructed. It aimed to explore the research overview and development trend in the CAR-NK cell field and seek research hotspots and emerging topics. We hope this study can provide new clues and ideas for researchers in the CAR-NK cell field, and provide convenience and help for researchers who are initially involved in this field.
Materials and methods
Data collection
The relevant literature was retrieved and downloaded from WoSCC at 21:36 on May 2nd, 2022. The retrieval formula was shown in Annexes 1. 1028 papers were obtained, including 454 articles and 574 reviews (Annexes 2).
Data analysis and visualization
As a citation visualization software, CtieSpace is good at mining and analyzing potential information in literature, including citation and co-citation times, subject distribution, contributions and cooperation of countries and institutions, contributions and cooperation of authors, research hotspots, and emerging topics (24). VOSviewer, a software for bibliometric mapping, has a strong capability of bibliometric network analysis and is better at constructing the visual network maps of scientific knowledge (25).
The acquired data was processed and visualized by Microsoft Office Excel 2010, CtieSpace (version 5.8.R3) and VOSviewer (version 1.6.17). All three researchers involved in this study were able to use the above software to process data proficiently. Two researchers (JZ and LM) independently analyzed and extracted the following information (1): the number of papers published each year; (2) contribution degree, centrality and cited times of countries/institutions; (3) contribution and citation times of authors; (4) contribution degree, impact factor and journal citation reports (JCR) region of journals; (5) Discipline distribution; (6) the frequency of keywords; and (7) co-citation times and centrality of references. Two people used Microsoft Excel to store and analyze data. Disagreements were resolved through discussion with the third reviewer (PC). In this descriptive study, variables were presented as numbers and percentages. No comparisons were made, therefore, no P values was set.
Results
The annual growth trend of publications
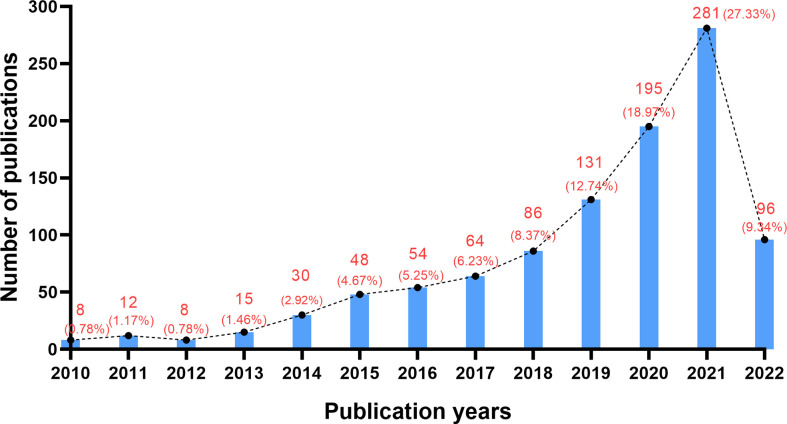
By counting the number of papers related to the CAR-NK cell field each year, we can know the development speed and trend of the field. According to the above retrieval strategy, 1028 papers about CAR-NK cells were obtained. Figure 1 shows that the number of papers in this field was rising (2010-2021). This trend could be divided into the stagnation period (2010-2012), the slow growth period (2013-2017) and the rapid growth period (2018-2021). In the stagnation period, less than 15 papers were published each year. This situation lasted for about 3 years, with 57 papers (only 2.7% of the total). However, from 2018 to 2021, a total of 693 papers were published (accounting for 67.4% of the total), far exceeding the sum of the past eight years.
Figure 1.
The trend of publication outputs about CAR-NK cells.
Countries and institutions
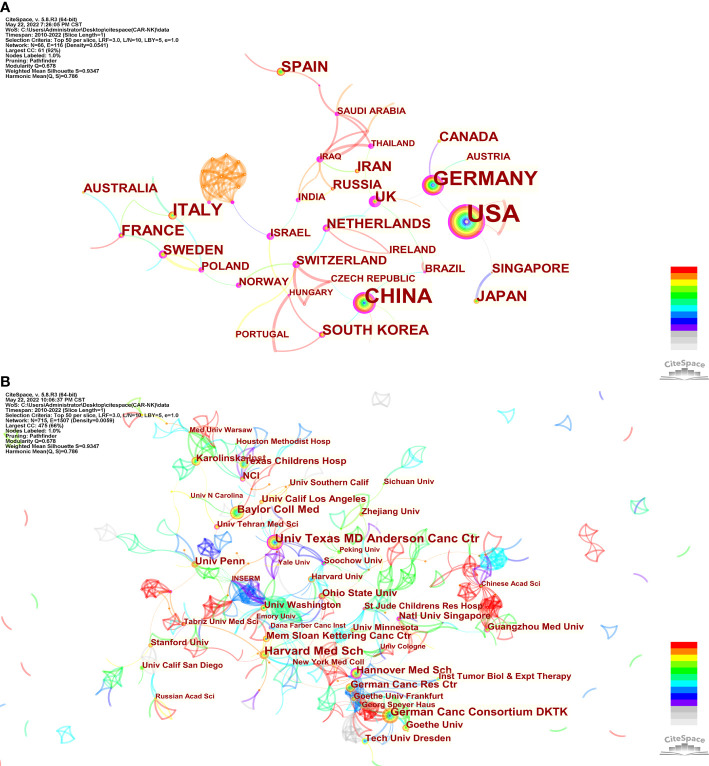
715 institutions from 65 countries co-authored 1028 papers (as an article might be completed by cooperation of several countries or organizations, it would be counted many times). Table 1 shows that the USA published the most papers (n = 461, 33.50%) in the field related to CAR-NK cells, followed by China (n = 184, 13.37%) and Germany (n = 137, 9.96%), and only these three countries published more than 100 papers. The country with the most citations was still USA (n = 16250), with an average of 35.2 citations per paper. Moreover, among the top 10 countries, UK had the highest centrality (0.7), followed by Germany (0.37), the China (0.25) and the USA (0.22). It means that these countries, especially UK, have played an important role as a bridge in this field. Additionally, 90% of the top10 countries were developed countries. From Figure 2A , we can intuitively see the contribution of these countries to this field (the size of the circle), the cooperation between countries (the lines between the circles), and the countries with high centrality (with purple circles).
Table 1.
The top 10 countries and institutions involved in CAR-NK cells.
| Rank | country | Count | Centrality | Total Citations | Institution | Count | Centrality | Total Citations |
|---|---|---|---|---|---|---|---|---|
| 1 | USA | 461 (33.50%) | 0.22 | 16250 | Univ Texas MD Anderson Canc Ctr (USA) | 43 (2.53%) | 0.28 | 2726 |
| 2 | CHINA | 184 (13.37%) | 0.25 | 4929 | Harvard Med Sch (USA) | 37 (2.18%) | 0.05 | 725 |
| 3 | GERMANY | 137 (9.96%) | 0.37 | 4725 | German Canc Consortium DKTK (Germany) | 30 (1.76%) | 0.02 | 1240 |
| 4 | ITALY | 67 (4.87%) | 0.09 | 1029 | Baylor Coll Med (USA) | 26 (1.53%) | 0.03 | 1590 |
| 5 | SPAIN | 38 (2.76%) | 0.06 | 957 | Hannover Med Sch (Germany) | 24 (1.41%) | 0.21 | 861 |
| 6 | FRANCE | 36 (2.62%) | 0.13 | 1072 | German Canc Res Ctr (Germany) | 22 (1.29%) | 0.07 | 755 |
| 7 | JAPAN | 34 (2.47%) | 0 | 626 | Univ Penn (USA) | 21 (1.23%) | 0.06 | 594 |
| 8 | UK | 32 (2.33%) | 0.7 | 756 | Mem Sloan Kettering Canc Ctr (USA) | 20 (1.18%) | 0.02 | 692 |
| 9 | IRAN | 29 (2.11%) | 0 | 228 | Ohio State Univ (USA) | 19 (1.12%) | 0.08 | 1092 |
| 10 | SOUTH KOREA | 26 (1.89%) | 0.13 | 330 | Goethe Univ (Germany) | 18 (1.06%) | 0 | 452 |
Figure 2.
The co-occurrence map of countries (A) and institutions (B) in the CAR-NK cell field.
The institution with the most papers published was Univ Texas, MD Anderson Cancer Center (n = 43, 2.53%), followed by Harvard Med Sch (n = 37, 2.18%) and German Canc Consortium DKTK (n = 30, 1.76%). Furthermore, the institution with the most citations was still Univ Texas MD Anderson Cancctr (n = 2726), with an average of 63.4 citations per paper. Among the top10 institutions, 60% and 40% were from USA and Germany respectively ( Table 1 ). Figure 2B shows intensive cooperation among institutions, and this cooperation is mainly concentrated from 2016 to 2021.
Journals and co-cited journals
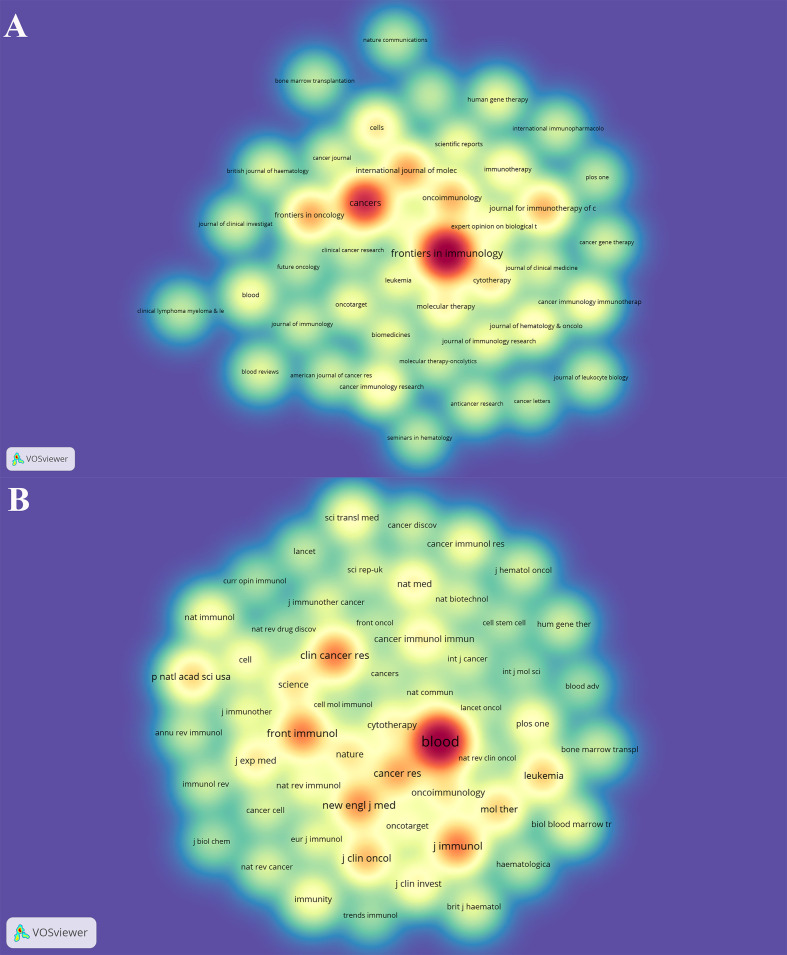
1028 CAR-NK cell-related publications were published in 346 academic journals. Table 2 shows the top 10 journals with published papers and the top 10 journals with co-citations. The journal with the most publications was Frontiers in Immunology (n = 95, 9.24%), followed by Cancers (n = 60, 5.84%) and International Journal of Molecular Sciences (n = 27, 2.63%). There were 6 journals with at least 20 publications. Figure 3A can intuitively display those journals with more publications (the darker the color, the more publications).
Table 2.
The top 10 journals and co-cited journals related to CAR-NK cells.
| Rank | Journal | Counts | IF(2020) | JCR(2020) | Co-cited journal | Coitations | IF(2020) | JCR(2020) |
|---|---|---|---|---|---|---|---|---|
| 1 | Frontiers in Immunology | 95 (9.24%) | 7.561 | Q2 | Blood | 9612 (8.91%) | 23.629 | Q1 |
| 2 | Cancers | 60 (5.84%) | 6.639 | Q2 | Journal of Immunology | 3389 (3.14%) | 5.422 | Q1 |
| 3 | International Journal of Molecular Sciences | 27 (2.63%) | 5.924 | Q3 | Clinical Cancer Research | 3346 (3.10%) | 12.531 | Q1 |
| 4 | Frontiers in Oncology | 26 (2.53%) | 6.244 | Q2 | Frontiers in immunology | 3325 (3.08%) | 7.561 | Q2 |
| 5 | Oncoimmunology | 24 (2.33%) | 8.110 | Q1 | New England Journal of Medicine | 2963 (2.75%) | 91.253 | Q1 |
| 6 | Journal for Immunotherapy of Cancer | 24 (2.33%) | 13.751 | Q1 | Cancer Research | 2594 (2.41%) | 12.701 | Q1 |
| 7 | Cytotherapy | 17 (1.65%) | 5.414 | Q1 | Journal Clinical Oncology | 2320 (2.15%) | 44.544 | Q1 |
| 8 | Cells | 16 (1.56%) | 6.600 | Q3 | leukemia | 2082 (1.93%) | 11.528 | Q1 |
| 9 | molecular therapy | 14 (1.36%) | 11.454 | Q1 | molecular therapy | 1976 (1.83%) | 11.454 | Q1 |
| 10 | Immunotherapy cancer immunology research journal of hematology & oncology |
13 (1.26%) 13 (1.26%) 13 (1.26%) |
4.196 11.151 17.388 |
Q3 Q1 Q1 |
Proceedings of the National Academy of Sciences of the United States of America | 1948 (1.81%) | 11.205 | Q1 |
IF: Impact Factor. JCR: Journal citation reports.
Figure 3.
The density map of journals (A) and co-cited journals (B) in the CAR-NK cell field. Figure A shows journals with a number of publications ≥5; Figure B shows the journals with citations ≥100.
It can also be seen from Table 2 that Blood (n = 9612, 8.91%) is the most co-cited journal, far exceeding other journals, followed by Journal of Immunology (n = 3389, 3.14%) and Clinical Cancer Research (n = 3346, 3.10%). Furthermore, 10 journals were co-cited more than 1,900 times, and 8 journals had an impact factor (IF) more than 10. Figure 3B intuitively displays those journals with more co-citations (the darker the color, the more co-citations).
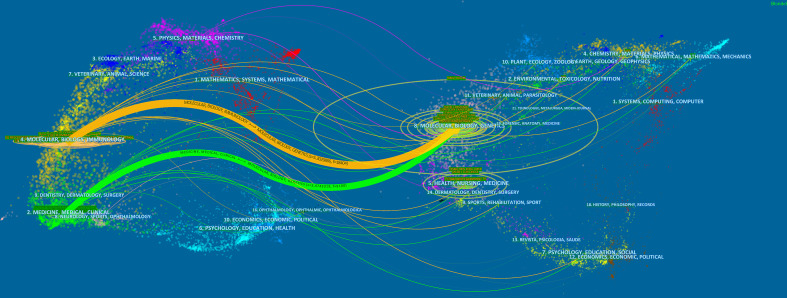
Figure 4 shows the distribution of journal topics and the citation relationship between journals. Additionally, there were two main reference paths (one orange and one green) in it. It suggests that the papers published in “Molecular/Biology/Genetics” journals were often cited by the papers published in “Molecular/Biology/Immunology” journals and “Medicine/Medical/Clinical” journals.
Figure 4.
The dual-map overlay of journals on CAR-NK cells. Image parameter: a: 3; Source Circle Size: 200; Target Circle Size: 8; Snap to centroids (< Radius): 0.
Authors and co-cited authors
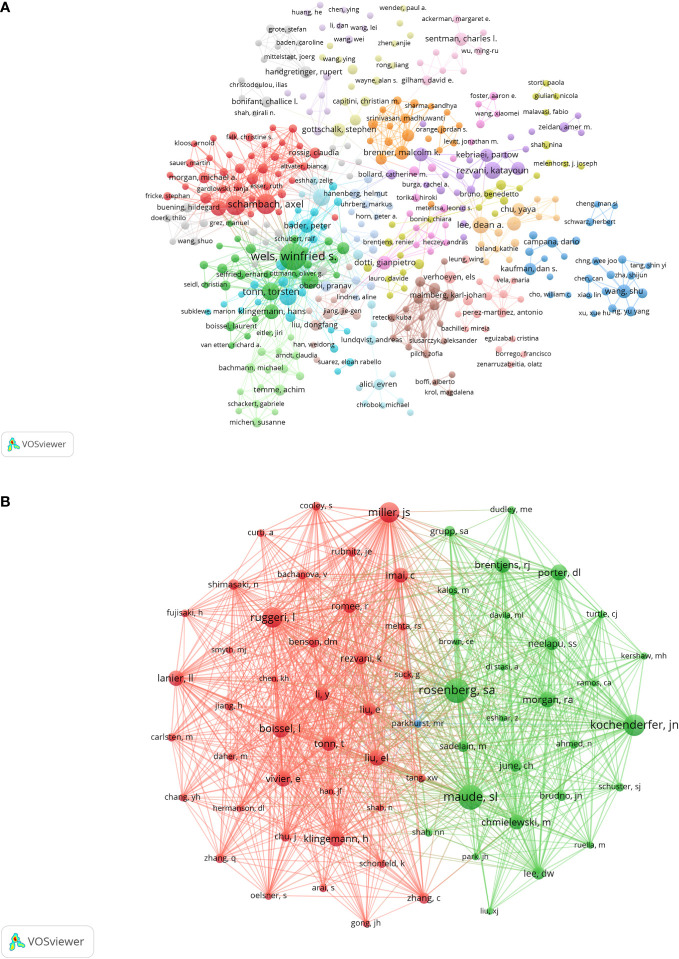
A total of 5,371 researchers participated in the publication of these papers. The top 10 researchers had published at least 10 papers ( Table 3 ). Winfried S. Wels (n = 30) published the most papers, followed by Axel Schambach (n = 17), Ulrike Koehl (n = 16) and Torsten Tonn (n = 13). We screened authors who had published at least 2 papers to construct the corresponding collaboration network map ( Figure 5A ). It shows 18 clusters of different colors, and the authors in the same cluster have active cooperation.
Table 3.
The top 10 authors and co-cited authors of CAR-NK cell researches.
| Rank | Author | Counts | Citations | Rank | Co-cited author | Citations |
|---|---|---|---|---|---|---|
| 1 | Winfried S. Wels | 30 | 2127 | 1 | Maude SL | 365 |
| 2 | Axel Schambach | 17 | 475 | 2 | Rosenberg SA | 362 |
| 3 | Ulrike Koehl | 16 | 811 | 3 | Kochenderfer JN | 313 |
| 4 | Torsten Tonn | 13 | 1280 | 4 | Miller JS | 301 |
| 5 | Hinrich Abken | 12 | 487 | 5 | Ruggeri L | 299 |
| 6 | Katayoun Rezvani | 12 | 790 | 6 | Boissel L | 240 |
| 7 | Evelyn Ullrich | 12 | 349 | 7 | Klingemann H | 235 |
| 8 | Congcong Zhang | 12 | 911 | 8 | Vivier E | 232 |
| 9 | Dean a. Lee | 11 | 521 | 9 | Liu EL | 231 |
| 10 | Stephan Kloess | 10 | 640 | 10 | Morgan RA Porter DL |
229 229 |
Figure 5.
The visualization map of authors (A) co-cited authors (B) involved in CAR-NK cells. Minimum number of documents of an aduthor ≥2; Note: Minimum number of citations of an aduthor ≥100.
68 authors co-cited more than 100 times in this study. The most frequently co-cited researchers were Maude SL (n = 365), followed by Rosenberg SA (n = 362), Kochendorfer JN (n = 313) and Miller JS (n = 301). These 68 authors were included to construct a co-citation network map ( Figure 5B ). Figure 5B shows these co-cited authors and co-cited relationships more intuitively.
Co-occurrence, clusters, and evolution of keywords
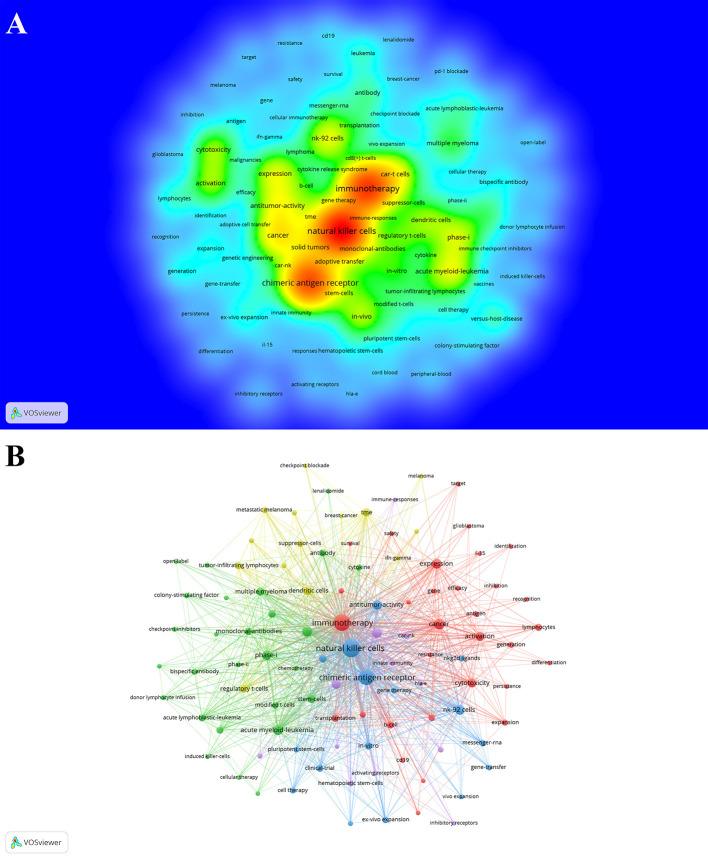
Keywords generally reflect the theme and research content of a paper. We can rapidly understand one certain field’s research focus and direction through the co-occurrence analysis of keywords. After merging some duplicate keywords, a total of 3908 keywords were obtained. Table 4 shows the top 20 keywords. The most frequently occurring keywords were natural killer cells (n = 735), followed by immunotherapy (n = 574), chimeric antigen receptor (n = 504), and cancer (n = 178). A total of 100 keywords with at least 15 occurrences were included, and a density map of keywords (Figure 6A) was constructed with these keywords. Those high-frequency keywords can be found more easily in Figure 6A.
Table 4.
Top 20 keywords related to CAR-NK cells.
| Rank | Keyword | Count | Rank | Keyword | Count |
|---|---|---|---|---|---|
| 1 | natural killer cells | 735 | 11 | solid tumors | 117 |
| 2 | immunotherapy | 574 | 12 | in-vivo | 110 |
| 3 | chimeric antigen receptor | 504 | 13 | cytotoxicity | 108 |
| 4 | cancer | 178 | 14 | activation | 107 |
| 5 | car-t cells | 143 | 15 | dendritic cells | 96 |
| 6 | acute myeloid-leukemia | 141 | 16 | tme | 78 |
| 7 | nk-92 cells | 138 | 17 | multiple myeloma | 77 |
| 8 | expression | 132 | 18 | regulatory t-cells | 72 |
| 9 | antitumor-activity | 125 | 19 | stem-cells | 70 |
| 10 | phase-i | 123 | 20 | monoclonal-antibodies | 67 |
Figure 6.
The co-occurrence density map (A) and network (B) of keywords involved in CAR-NK cells. Minimum number of occurrences of keywords ≥15.
Keywords with at least 15 occurrences were included, and a network cluster analysis of these keywords (Figure 6B) was constructed. A total of 5 different clusters were obtained. There were 31 keywords in cluster 1 (red), including immunotherapy, cancer, leukemia, glioblastoma, efficacy, resistance, CRS, cytotoxicity, and safety. There were 27 keywords in cluster 2 (green), including CAR-T cells, acute myeloid-leukemia(AML), acute lymphoblastic-leukemia(ALL), multiple myeloma (MM), phase-I, phase-II. There were 16 keywords in cluster 3 (blue), including CAR, NK cells, antitumor-activity, in-vitro, clinical-trial, NKG2D ligands, NK-92 cells, cord blood, and pluripotent stem-cells. There were 14 keywords in cluster 4 (yellow), including dendritic cells(DC), CD8+ T cells, regulatory T-cells, suppressor-cells, tumor-associated macrophages, tumor-infiltrating lymphocytes, and TME. There were 12 keywords in cluster 5 (purple), including CAR-NK, solid tumors, activating receptors, inhibitory receptors, and immune-responses.
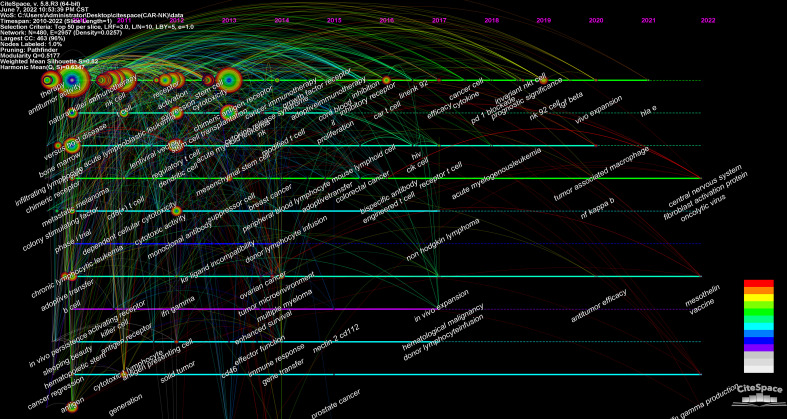
The Timeline viewer of keywords adds a time factor to the clustering of these keywords. It can help us explore the evolution track of these keywords in different topics. From Figure 7 , we can intuitively see the evolution track of keywords in this field and focused keywords in each stage.
Figure 7.
The timeline viewer of keywords involved in CAR-NK cells.
Co-cited references and reference burst
Table 5 consisted of two parts. The first part showed the top 10 co-cited articles about the field. The publication time was from 2014 to 2020. The top 3 articles were co-cited more than 150 times. The co-cited article the most co-citations was “Use of CAR-transferred natural killer cells in CD19-positive lymphoid tumors” (n = 214), published by Liu et al. (16) in 2020. The second part showed the top 10 co-cited reviews related to CAR-NK cells. The publication time was from 2015 to 2020. The most frequently co-cited review was “Engineering Natural Killer Cells for Cancer Immunotherapy” (n = 89) by Rezvani et al. (34) in 2017.
Table 5.
The top 10 co-cited references related to CAR-NK cells. .
| Top 10 co-cited references (only including articles) related to CAR-NK cells | ||||||
|---|---|---|---|---|---|---|
| Rank | Year | Author | Title | Journal | Co-citation | Centrality |
| 1 | 2020 | Liu EL et al. (16) | Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors | N Engl J Med | 214 | 0.01 |
| 2 | 2018 | Liu EL et al. (26) | Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent anti-tumor activity | Leukemia | 173 | 0.04 |
| 3 | 2018 | Li Y et al. (27) | Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity | Cell Stem Cell | 153 | 0.01 |
| 4 | 2018 | Tang XW et al. (28) | First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia | Am J Cancer Res | 112 | 0.01 |
| 5 | 2017 | Zhang CC et al. (20) | Chimeric Antigen Receptor-Engineered NK-92 Cells: An Off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity | Front Immunol | 87 | 0.01 |
| 6 | 2015 | Schönfeld K et al. (29) | Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor | Mol Ther | 86 | 0.01 |
| 7 | 2014 | Chu J et al. (30) | CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma | Leukemia | 83 | 0.02 |
| 8 | 2016 | Romanski A et al. (31) | CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies | J Cell Mol Med | 70 | 0.01 |
| 9 | 2015 | Zhang CC et al. (32) | ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma | J Natl Cancer Inst | 64 | 0.01 |
| 10 | 2017 | Oelsner S et al. (33) | Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma | Cytotherapy | 63 | 0 |
| Top 10 co-cited references (only including reviews) related to CAR-NK cells | ||||||
| 1 | 2017 | Rezvani K et al. (34) | Engineering Natural Killer Cells for Cancer Immunotherapy | Mol Ther | 89 | 0 |
| 2 | 2016 | Suck G et al. (35) | NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy | Cancer Immunol Immunother | 84 | 0.01 |
| 3 | 2016 | Klingemann H et al. (36) | Natural Killer Cells for Immunotherapy - Advantages of the NK-92 Cell Line over Blood NK Cells | Front Immunol | 80 | 0 |
| 4 | 2018 | Mehta RS et al. (37) | Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer | Front Immunol | 75 | 0 |
| 5 | 2015 | Glienke W et al. (38) | Advantages and applications of CAR-expressing natural killer cells | Front Pharmacol | 63 | 0.01 |
| 6 | 2020 | Xie GZ et al. (39) | CAR-NK cells: A promising cellular immunotherapy for cancer | EBioMedicine | 33 | 0 |
| 7 | 2016 | Guillerey C et al. (40) | Targeting natural killer cells in cancer immunotherapy | Nat Immunol | 49 | 0 |
| 8 | 2018 | Hu Y et al. (41) | Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy | Acta Pharmacol Sin | 49 | 0 |
| 9 | 2019 | Hu WL et al. (42) | Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities | Front Immunol | 35 | 0 |
| 10 | 2015 | Rezvani K et al. (43) | The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer | Front Immunol | 29 | 0 |
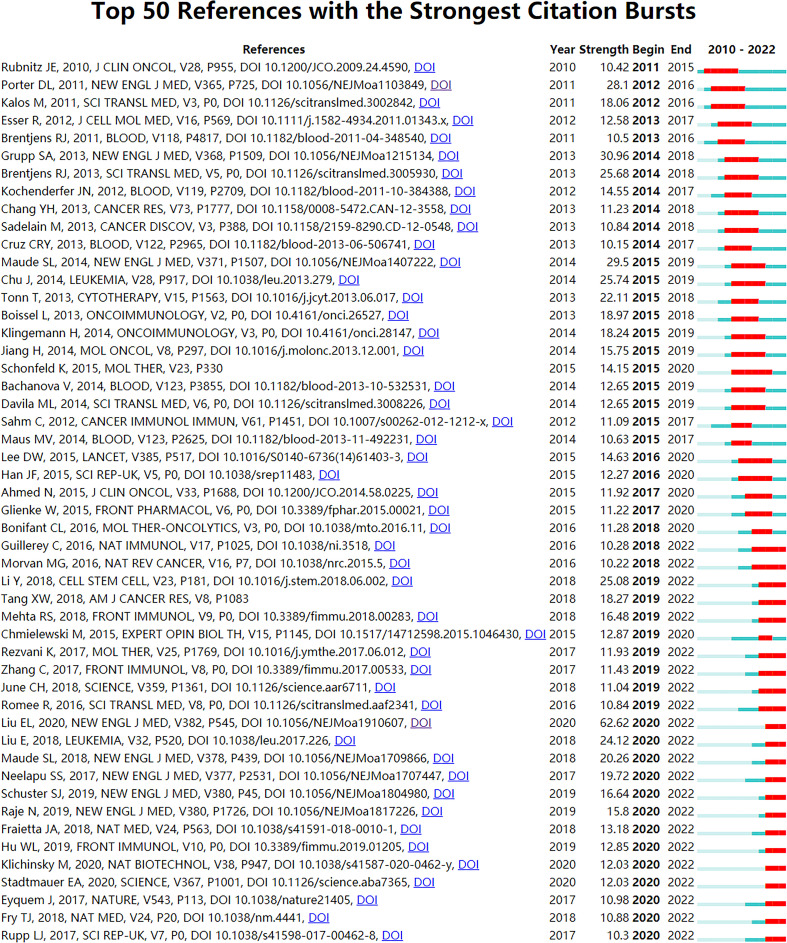
References with citation bursts refer to references whose citations suddenly increase in a certain period. 255 references with the strongest citation bursts were obtained through CiteSpace (selection criteria: top 100 per slice; minimum duration: 2). We chose the top 50 (Figure 8). The reference with the strongest burstness (strength = 62.62) was “Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors (16)” (publication year: 2020). Among these 50 references, 46 were published from 2012 to 2022, and 19 (38%) were published from 2017 to 2022. It indicates that these papers were frequently cited within 10 years and 5 years. More importantly, 22 (44%) of these 50 papers are currently in citation burstness. All these mean that the research about CAR-NK cells will continue to receive attention in the future. Furthermore, 10 of the 22 papers were related to CAR-T cells, which indicates that the CAR-T cell field has a significant influence on the research field of CAR-NK cells.
Figure 8.
The top 50 references with the strongest citation bursts involved in CAR-NK cells. The Blue bars mean the reference had been published; the red bars mean citation burstness.
Discussion
CAR-T cell therapies have made breakthroughs in the treatment of hematological malignancies, and five CAR-T cell therapies have been approved for marketing. However, there is still no CAR-T cell therapy for solid tumors on the market. This is because, compared with hematological malignancies, CAR-T cells face greater limitations in solid tumors, such as the homing obstacle of CAR-T cells and the inhibition of tumor microenvironment (TME). As a new anti-tumor immunotherapy, CAR-NK cell therapy has great potential. Compared with CAR-T cells, the sources of NK cells used to construct CAR-NK cells are extremely wide (10–12), CAR-NK does not cause GVHD, and the incidence of CRS and neurotoxicity is extremely low (16). Moreover, NK cells themselves have multiple anti-tumor mechanisms (17–19).
So far, over 30 registered CAR-NK cell-related clinical trials have been retrieved (clinicaltrials.gov). Although CAR-NK cell therapy has some unique advantages, it also has some limitations that cannot be ignored. These limitations are similar to those of CAR-T cell therapy, including lack of specific target antigens, antigen heterogeneity and challenges after infusion (44). Furthermore, CAR-NK cells also face many obstacles in solid tumors, such as homing obstacle of CAR-NK cells and inhibition of TME. Thus, there are still many problems and obstacles to be solved and broken through in this field.
In recent years, the field of CAR-NK cells has been developing rapidly, and new knowledge has been generated like a blowout. It is very difficult to quickly grasp the core knowledge in a huge knowledge base. The bibliometric analysis can well summarize and analyze these numerous documents and complicated data. This study obtained some valuable results in the CAR-NK cell field through bibliometric analysis and knowledge-map analysis, including contributions and cooperation of countries/institutions/authors, contributions and topic distribution of journals, development trends, important knowledge, research hotspots, and emerging topics. In this study, we expounded and discussed the general information, knowledge base, hotspot evolution, emerging topics related to CAR-NK cells, and the limitations of our research.
General information
The number of papers was on the rise, which was divided into the stagnation period (2010-2012), the slow growth period (2013-2017) and the rapid growth period (2018-2021) (Figure 1). In the stagnant period, the research on CAR-NK cells was inactive, and the number of papers published each year was less than 5. During the slow growth period, the number of CAR-NK cell-related papers increased slowly, indicating that this field was attracting attention. Interestingly, there was an obvious turning point in this stage; after 2012, the number of publications has increased substantially. This phenomenon may be related to an important event. Emily Whitehead, 7-year-old with ALL, achieved complete remission after receiving CD19-CAR-T cell therapy (45). This event made CAR-T cells begin to attract widespread attention. The progress of CAR-T cell research has also promoted research in the field of CAR-NK cells (20). In the period of rapid growth, the field has developed rapidly, which indicates that the research on CAR-NK cells has aroused great interest of researchers. It should be mentioned that 693 papers (accounting for 67.4% of the total) were published in this stage, exceeding the sum of the past 8 years. According to the current trends, the number of publications in this field may continue to show positive growth.
The USA (n = 461, accounting for 33.50% of the total) published the most papers in the CAR-NK cell field, far exceeding other countries. UK had the highest centrality (0.7), which means that UK played an important role as a bridge in this field. Besides, 90% of the top10 countries were developed countries. It may be because the research and development of CAR-NK cells need much financial support. Among the top10 institutions, 60% and 40% were from USA and Germany respectively. The institution with the most papers published the most citations was Univ Texas, MD Anderson Cancer Center from the USA. Besides, Figure 2B shows intensive cooperation among institutions, and this cooperation is mainly concentrated from 2016 to 2021.
Frontiers in Immunology published the most papers (n = 95, 9.24%), while Blood was the most co-cited journal (n = 9612, 8.91%). Figure 4 suggests that the research on CAR-NK cells mainly focuses on two aspects, including basic studies and translational medicine.
Through the authors and co-cited authors analysis, we could find the authors with the most published papers and the most co-cited authors in the CAR-NK cell field. In our analysis, Winfried S. Wels published the most papers (n = 30), while Shannon L Maude had the most co-citations (n = 365). Moreover, there was intensive cooperation among researchers in the same cluster and active cooperation among different clusters (Figures 5A).
The number of publications related to CAR-NK cells is increasing year by year, especially in recent years. As a hot field, CAR-NK cell-related research is attracting more and more researchers. By reading the relevant papers of experts (including those listed in Table 3 ) in this field, it will undoubtedly help readers to understand and master the research status and focus of the CAR-NK cell field. From the general information, we also obtain some important information, including: 1. In the field of CAR-NK cells, the United States is in an absolute leading position; 2. Journals in the field of tumor and immunity are interested in CAR-NK cell-related research. Overall, the general information can help readers quickly understand the general situation of this field.
Knowledge base
The knowledge base is a collection of co-cited references (46). The 10 most co-cited papers in CAR-NK cell-related fields (the first part of Table 5 ) were as follows:
The most co-cited paper (n = 159) was “Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors” by Liu et al. (16) in 2020. In this phase 1 and 2 trial, 11 patients with CD19+ non-Hodgkin lymphoma or CLL received CD19-CAR-NK cells (derived from cord blood). The experimental results showed that about 73% of patients responded to the treatment with high safety, and no CRS or ICANS appeared in all patients. The second-ranked paper was published in 2018 (26). This study was a preclinical experiment of the previous study. The third-ranked paper was published in 2018 (27). Researchers used human iPSC-derived NK cells to construct CAR-NK cells targeting mesothelin to treat ovarian cancer. The CAR consisted of the natural killer group 2 member D (NKG2D) transmembrane domain, 2B4 costimulatory domain and CD3ζ signaling domain (2nd generation CAR, 2B4). The results showed that mesothelin-CAR-NK cells exhibited similar anti-tumor activity to CAR-T cells in vivo, and the toxicity was lower. The fourth-ranked paper was published in 2018 (28). It was the first human clinical trial of CAR NK-92 cells (phase 1). 3 patients with RR-AML received CD33-CAR NK-92 cells (3rd generation CAR; CD28 and 4-1BB). The experimental results showed that although CD33-CAR NK-92 cells did not show apparent clinical efficacy in RR-AML, the experiment proved that this therapy was safe in these patients. The fifth-ranked paper was published in 2017 (20). Researchers introduced the research progress of HER2-CAR NK -92 cells in treating of HER2+ malignant tumors. Combined with their research, they proved that HER-CAR NK -92 cells (2nd generation CAR, CD28) could effectively control the tumor progression in the mouse models of HER2+ glioblastoma. The sixth-ranked paper was published in 2015 (29). This study showed that HER2-CAR NK-92 cells (2nd generation CAR, CD28) could recognize and kill HER2+ tumor cells. The seventh -ranked paper was published in 2014 (30). This study showed that CS1-CAR NK-92 cells (2nd generation CAR, CD28) could effectively inhibit tumor growth in the multiple myeloma (MM) xenograft mouse models. The eighth-ranked paper was published in 2016 (31). In vitro experiments showed that CD19-CAR NK -92 cells (1st generation CAR) could specifically and effectively kill CD19+ B-ALL cells. The ninth-ranked paper was published in 2015 (32). The research showed that in the mouse models, HER2-CAR NK -92 cells (2nd generation CAR, CD28) could specifically recognize and kill HER2+ glioblastoma cells and induce endogenous anti-tumor immunity. The tenth-ranked paper was published in 2017 (31). Researchers constructed three types of CD19-CAR NK-92 cells for the study of B-cell leukemia and lymphoma, including NK-92/63.z cells (1st generation CAR), NK-92/63.28.z cells (2nd CAR, CD28) and NK-92/63.137.z cells (2nd CAR, CD137). The study showed that all three CAR-NK cells could kill CD19+ B-cell leukemia and lymphoma cells, and the first two CAR-NK cells were more potent than the third one in cell killing and cytokine production.
Generally speaking, from these 10 most co-cited articles, we can obtain some vital information about CAR-NK cells, as follows:
Currently, most research about CAR-NK cells is still in the basic research stage;
CAR-NK cells are mainly used in the research about hematological malignancies and solid tumors, including anti-tumor effects and safety;
The hematological malignancies and targets involved in these studies include non-Hodgkin lymphoma (CD19), CLL(CD19), ALL(CD19), AML(CD33) and MM (CS1);
The solid tumors and targets involved in these studies mainly include glioblastoma (HER2), breast cancer (HER2) and ovarian cancer (mesothelin), especially glioblastoma. Beyond that, some recent studies on CAR-NK cells in solid tumors, such as lung cancer (DLL3) (47), gastric cancer (mesothelin) (48), pancreatic cancer (PSCA) (49), and prostate cancer (PSMA) (50).
The main source for constructing CAR-NK cells is NK92 cells. It is mainly because 1. NK92 cells can expand indefinitely in vitro (51); 2. Reduced manufacturing time and cost of CAR-NK cells (39); 3. Reduced the sensitivity to freeze-thaw cycles (8). However, NK92 cells also have inherent disadvantages, including the risk of tumorigenesis, lack of CD16 (inability to trigger ADCC) (52), and requiring irradiation prior to infusion (loss of in vivo proliferative capacity) (29, 52).
The second part of Table 5 is the 10 most co-cited reviews in the CAR-NK cell-related field. These reviews introduce and summarize the CAR-NK cell field from many aspects, which can help researchers understand the general situation, research focus and development trend.
Like CAR-T cell therapy, CAR-NK cell therapy also belongs to clinical application research, and its ultimate goal is to be used for clinical treatment. Up to now, only one clinical trial article (16) related to CAR-NK cells can be retrieved from Pubmed. Most research on CAR-NK cells is still in basic research and preclinical research stage. CAR-NK cells are mainly used in the research of hematological malignancies and solid tumors (9, 53, 54), and their anti-tumor effect and safety are the focus of attention, because these two aspects can affect the clinical promotion of this therapy. Additionally, because of some advantages of NK92 cells, they have become the main source of constructing CAR-NK cells, and have been approved for clinical applications (55).
The analysis of hotspots and emerging topics
Figure 7 shows the evolutionary trajectories of keywords and the topics focused in each stage in CAR-NK cell-related fields. During the stasis period (2010-2012), the keywords in this stage indicated that the relevant research mainly focused on the basic research or phase 1 clinical research (including the evaluation of efficacy, toxicity reaction and safety) of CAR-NK cells in hematological malignancies (mainly) and solid tumors. During the slow growth period (2013-2017), the research of CAR-NK cells in solid tumors gradually increased, for example, breast cancer, ovarian cancer, prostate cancer and colorectal cancer. In the period of rapid growth (2018-2021), it was the main research topic to explore further some intrinsic mechanisms (including immune checkpoints, TGF-β signaling pathway, NF-kB signaling pathway, and tumor-associated macrophages) to improve the antitumor efficacy of CAR-NK cells.
Keywords can generally reflect the theme and research content of a paper. Table 4 shows the top 20 keywords. Some critical information in this field can be summarized through these keywords: 1. CAR-NK cell therapy is anti-tumor immunotherapy; 2. It is currently mainly used to study hematological malignancies (9, 56–58)and solid tumors (54, 59); 3. The anti-tumor effect of this therapy is the focus of attention; 4. Presently, CAR-NK cell research is mainly in basic research, while clinical trials are primarily in phase 1.
By summarizing the keywords of each cluster ( Figure 6B ), we can understand the scope and direction of research in this field. Cluster 1 keywords are mainly related to the research of immunotherapy in malignant tumors, including efficacy, safety and toxicity. Cluster 2 keywords are mainly related research of CAR-T cells in hematological malignancies. Cluster 3 keywords may be about the research of CAR-NK cells in tumors, and NK cells used in the experimental research are mostly derived from NK92 cells, pluripotent stem-cells and cord blood. Cluster 4 keywords are mainly related to tumor microenvironment. Cluster 5 keywords may be about the basic research of CAR-NK cells in solid tumors.
We can find emerging topics in a field by analyzing those references with citation bursts (60). Of these 50 papers, 22 (44%) were in the state of citation burstness ( Figure 8 ). It suggests that the research about CAR-NK cells will continue to receive attention in the future. From these 22 papers, we screened 14 papers (9 articles and 5 reviews) related to CAR-NK cells or NK cells and arranged them according to their strength ( Table 6 ). These 9 articles may represent emerging topics in the field. Table 6 and Table 5 show that 5 articles appear in both tables at the same time. These 5 articles (16, 20, 26–28) may be crucial references in CAR-NK cells (already introduced in the knowledge base). The remaining 4 articles in Table 6 are introduced as follows:
Table 6.
The references (in the state of citation burstness) related to CAR-NK cells or NK cells. .
| NO. | Year | Author | Article Type | Targets | Associated Tumor | Title | Strength |
|---|---|---|---|---|---|---|---|
| 1 | 2020 | Liu E et al. (16) | article | CD19 | Hodgkin’s lymphoma, chronic lymphocytic leukemia (CLL) | Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors | 60.9 |
| 2 | 2018 | Li Y et al. (27) | article | mesothelin | ovarian cancer | Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity | 25.08 |
| 3 | 2018 | Liu E et al. (26) | article | CD19 | hematological malignancies | Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity | 24.12 |
| 4 | 2018 | Tang XW et al. (28) | article | CD33 | acute myeloid leukemia (AML) | First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia | 18.27 |
| 5 | 2018 | Mehta RS et al. (37) | review | Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer | 16.48 | ||
| 6 | 2019 | Hu WL et al. ( 42) | review | Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities | 12.85 | ||
| 7 | 2020 | Stadtmauer EA et al. (61) | Article | CRISPR-engineered T cells in patients with refractory cancer | 12.03 | ||
| 8 | 2017 | Rezvani K et al. (34) | review | Engineering Natural Killer Cells for Cancer Immunotherapy | 11.93 | ||
| 9 | 2017 | Zhang C et al. (20) | article | HER2 | solid tumors | Chimeric Antigen Receptor-Engineered NK-92 Cells: An Off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity | 11.43 |
| 10 | 2017 | Eyquem J et al. (62) | article | Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection | 10.98 | ||
| 11 | 2016 | Romee R et al. (63) | article | acute myeloid leukemia (AML) | Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia | 10.84 | |
| 12 | 2017 | Rupp LJ et al. (64) | article | CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells | 10.3 | ||
| 13 | 2016 | Guillerey C et al. (40) | review | Targeting natural killer cells in cancer immunotherapy | 10.28 | ||
| 14 | 2016 | Morvan MG et al. (65) | review | NK cells and cancer: you can teach innate cells new tricks | 10.22 |
The articles ranked 7th (strength: 12.03) (61), 10th (strength: 10.98) (62) and 12th (strength: 10.3) (64) in strength were all related research on CRISPR/Cas9 gene-editing technology in constructing engineered immune cells. The 11th-ranked article (strength: 10.84) published by Romee et al. (63) in 2016. Romee et al. proved that primary human NK cells could differentiate into memory-like NK cells after short-term pre-stimulation with IL-12, IL-15 and IL-18. In phase 1 clinical trial, these memory-like NK cells could proliferate in large numbers in AML patients, showing more vital anti-tumor ability.
By analyzing and summarizing the 9 articles (16, 20, 26–28, 61, 62, 64) that are in the state of citation burst, we can obtain the current emerging topics about the CAR-NK cell field:
The basic research and clinical experimental research of CAR-NK cells in hematological malignancies involve targets including CD19 and CD33.
The basic studies of CAR-NK cells in solid tumors involves tumors and targets, including ovarian cancer (mesothelin) and glioblastoma (HER2).
Evaluating the efficacy and safety of CAR-NK cells in clinical trials.
How to further improve the proliferation and persistence of NK cells in vivo (66). For example, promoting the differentiation of NK cells into memory-like NK cells.
Optimizing and improving the CAR structure for NK cells to improve the anti-tumor ability of CAR-NK cells, such as taking NKG2D as transmembrane domain, and using costimulatory molecules (2B4, CD28, 4-1BB).
The related research of CRISPR/Cas9 gene-editing technology in constructing engineered immune cells.
Combined with Knowledge Base, it can be found that the research of CAR-NK cells in hematological malignancies and solid tumors, and the evaluation of their efficacy and safety are the research focuses. In optimizing the structure of CAR, adding costimulatory molecules is an important strategy. Currently, CD28 and 4-1BB are commonly used. The combination of them can further enhance the anti-tumor effect of CAR-T cells (67). CD28 and 4-1BB can improve the function of CAR-T cells, which is also applicable to CAR-NK cells (68). In addition, researchers used NKG2D as transmembrane domain and added costimulatory molecule 2B4 to construct mesothelin-CAR-NK cells. The results showed that the anti-tumor effect of mesothelin-CAR-NK cells was similar to that of mesothelin-CAR-T cells (27). Interestingly, NKG2D can not only be used as a transmembrane domain of CAR, but also be used to construct single chain variable fragment (scFv) of CAR. Some studies showed that NKG2D-CAR-NK cells could effectively kill tumor cells expressing NKG2D ligands (69, 70). Furthermore, as a widely used gene editing technology, CRISPR/Cas9 technology is often applied to the construction of CAR-T cells and CAR-NK cells to enhance their anti-tumor effects (71, 72). Generally speaking, this section shows rich contents related to CAR-NK cells.
Limitation
This study also has some limitations. First of all, all the data in this study came from the WoSCC database. Although the database contained most of the literature, there were still a few pieces of literature not included. Secondly, the quality of the included literature was uneven, which may lead to some degree of deviation in the analysis. Nevertheless, quantitative and visual analysis based on the literature data can help researchers quickly and intuitively understand the research trends, research directions, research hotspots and emerging topics in CAR-NK cell-related fields.
Conclusion
CAR-NK cell therapy is a promising adoptive cell therapy (73). This study is the first time comprehensively and objectively analysing the CAR-NK cell field by combining bibliometrics with scientific knowledge maps. The number of publications related to CAR-NK cells was on the rise, especially from 2018 to 2021. In this field, the United States was in a leading position. The most active scientific research institution was Univ Texas, MD Anderson Cancer Center (USA). The journal with the most published papers was Frontiers in immunology, while the most co-cited was Blood. The researcher with the most published papers was Winfried S. Wels, while the most co-cited was Shannon L Maudeare. The research of CAR-NK cells in hematological malignancies and solid tumors (especially the selection of targets and the evaluation of efficacy and safety) was a research hotspot in this field. The emerging topics mainly included three aspects. First, further improve the proliferation and persistence of NK cells in vivo. Secondly, optimizing and improving the CAR structure for NK cells to improve the anti-tumor ability of CAR-NK cells. Thirdly, the related research of CRISPR/Cas9 gene-editing technology in constructing engineered immune cells.
In conclusion, this study provides a unique and objective perspective for the field of CAR-NK cells. We believe this study will provide a helpful reference for researchers interested in this field.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JZ: Writing-original draft preparation, manuscript, investigation, and figure preparation. PC: Investigation, methodology, supervision. LM: Conceptualization, methodology, supervision, manuscript, figure preparation. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.969196/full#supplementary-material
References
- 1. Huang RS, Lai MC, Shih HA, Lin S. A robust platform for expansion and genome editing of primary human natural killer cells. J Exp Med (2021) 218(3):e20201529. doi: 10.1084/jem.20201529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chu J, Gao F, Yan M, Zhao S, Yan Z, Shi B, et al. Natural killer cells: a promising immunotherapy for cancer. J Trans Med (2022) 20(1):240. doi: 10.1186/s12967-022-03437-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fang F, Xiao W, Tian Z. Challenges of NK cell-based immunotherapy in the new era. Front Med (2018) 12(4):440–50. doi: 10.1007/s11684-018-0653-9 [DOI] [PubMed] [Google Scholar]
- 4. Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol (2006) 6(12):940–52. doi: 10.1038/nri1983 [DOI] [PubMed] [Google Scholar]
- 5. Screpanti V, Wallin RP, Grandien A, Ljunggren HG. Impact of FASL-induced apoptosis in the elimination of tumor cells by NK cells. Mol Immunol (2005) 42(4):495–9. doi: 10.1016/j.molimm.2004.07.033 [DOI] [PubMed] [Google Scholar]
- 6. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol (2008) 9(5):503–10. doi: 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 7. Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer (2020) 19(1):120. doi: 10.1186/s12943-020-01238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daher M, Rezvani K. Outlook for new car-based therapies with a focus on car nk cells: what lies beyond car-engineered t cells in the race against cancer. Cancer Discovery (2021) 11(1):45–58. doi: 10.1158/2159-8290.Cd-20-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marofi F, Saleh MM, Rahman HS, Suksatan W, Al-Gazally ME, Abdelbasset WK, et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies. Stem Cell Res Ther (2021) 12(1):374. doi: 10.1186/s13287-021-02462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discovery (2020) 19(3):200–18. doi: 10.1038/s41573-019-0052-1 [DOI] [PubMed] [Google Scholar]
- 11. Tanaka J. Recent advances in chimeric antigen receptor natural killer cell therapy for overcoming intractable hematological malignancies. Hematological Oncol (2021) 39(1):11–9. doi: 10.1002/hon.2802 [DOI] [PubMed] [Google Scholar]
- 12. Arias J, Yu J, Varshney M, Inzunza J, Nalvarte I. Hematopoietic stem cell- and induced pluripotent stem cell-derived CAR-NK cells as reliable cell-based therapy solutions. Stem Cells Trans Med (2021) 10(7):987–95. doi: 10.1002/sctm.20-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fabian KP, Hodge JW. The emerging role of off-the-shelf engineered natural killer cells in targeted cancer immunotherapy. Mol Ther oncolytics (2021) 23:266–76. doi: 10.1016/j.omto.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roex G, Campillo-Davo D, Flumens D, Shaw PAG, Krekelbergh L, De Reu H, et al. Two for one: targeting BCMA and CD19 in b-cell malignancies with off-the-shelf dual-CAR NK-92 cells. J Trans Med (2022) 20(1):124. doi: 10.1186/s12967-022-03326-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grote S, Mittelstaet J, Baden C, Chan KC, Seitz C, Schlegel P, et al. Adapter chimeric antigen receptor (AdCAR)-engineered NK-92 cells: an off-the-shelf cellular therapeutic for universal tumor targeting. Oncoimmunology (2020) 9(1):1825177. doi: 10.1080/2162402x.2020.1825177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in cd19-positive lymphoid tumors. New Engl J Med (2020) 382(6):545–53. doi: 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oei VYS, Siernicka M, Graczyk-Jarzynka A, Hoel HJ, Yang W, Palacios D, et al. Intrinsic functional potential of nk-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol Res (2018) 6(4):467–80. doi: 10.1158/2326-6066.Cir-17-0207 [DOI] [PubMed] [Google Scholar]
- 18. Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol (2015) 6:368. doi: 10.3389/fimmu.2015.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snyder KM, Hullsiek R, Mishra HK, Mendez DC, Li Y, Rogich A, et al. Expression of a recombinant high affinity igg fc receptor by engineered nk cells as a docking platform for therapeutic mabs to target cancer cells. Front Immunol (2018) 9:2873. doi: 10.3389/fimmu.2018.02873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang C, Oberoi P, Oelsner S, Waldmann A, Lindner A, Tonn T, et al. Chimeric antigen receptor-engineered nk-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front Immunol (2017) 8:533. doi: 10.3389/fimmu.2017.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebrahimiyan H, Tamimi A, Shokoohian B, Minaei N, Memarnejadian A, Hossein-Khannazer N, et al. Novel insights in CAR-NK cells beyond CAR-T cell technology; promising advantages. Int Immunopharmacol (2022) 106:108587. doi: 10.1016/j.intimp.2022.108587 [DOI] [PubMed] [Google Scholar]
- 22. Cooper ID. Bibliometrics basics. J Med Library Association: JMLA (2015) 103(4):217–8. doi: 10.3163/1536-5050.103.4.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PloS One (2019) 14(10):e0223994. doi: 10.1371/journal.pone.0223994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci United States America (2004) 101 Suppl 1(Suppl 1):5303–10. doi: 10.1073/pnas.0307513100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia (2018) 32(2):520–31. doi: 10.1038/leu.2017.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell (2018) 23(2):181–92.e5. doi: 10.1016/j.stem.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res (2018) 8(6):1083–9. [PMC free article] [PubMed] [Google Scholar]
- 29. Schönfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol therapy: J Am Soc Gene Ther (2015) 23(2):330–8. doi: 10.1038/mt.2014.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia (2014) 28(4):917–27. doi: 10.1038/leu.2013.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in b-cell malignancies. J Cell Mol Med (2016) 20(7):1287–94. doi: 10.1111/jcmm.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang C, Burger MC, Jennewein L, Genßler S, Schönfeld K, Zeiner P, et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst (2016) 108(5). doi: 10.1093/jnci/djv375 [DOI] [PubMed] [Google Scholar]
- 33. Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against b-cell leukemia and lymphoma. Cytotherapy (2017) 19(2):235–49. doi: 10.1016/j.jcyt.2016.10.00 [DOI] [PubMed] [Google Scholar]
- 34. Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther (2017) 25(8):1769–81. doi: 10.1016/j.ymthe.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, et al. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer immunol immunother: CII (2016) 65(4):485–92. doi: 10.1007/s00262-015-1761-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the nk-92 cell line over blood nk cells. Front Immunol (2016) 7:91. doi: 10.3389/fimmu.2016.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol (2018) 9:283. doi: 10.3389/fimmu.2018.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, et al. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol (2015) 6:21. doi: 10.3389/fphar.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine (2020) 59:102975. doi: 10.1016/j.ebiom.2020.102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol (2016) 17(9):1025–36. doi: 10.1038/ni.3518 [DOI] [PubMed] [Google Scholar]
- 41. Hu Y, Tian ZG, Zhang C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta pharmacol Sin (2018) 39(2):167–76. doi: 10.1038/aps.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol (2019) 10:1205. doi: 10.3389/fimmu.2019.01205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol (2015) 6:578. doi: 10.3389/fimmu.2015.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pan K, Farrukh H, Chittepu V, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer research: CR (2022) 41(1):119. doi: 10.1186/s13046-022-02327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New Engl J Med (2013) 368(16):1509–18. doi: 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen CM. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Of Am Soc For Inf Sci And Technol (2006) 57(3):359–77. doi: 10.1002/asi.20317 [DOI] [Google Scholar]
- 47. Liu M, Huang W, Guo Y, Zhou Y, Zhi C, Chen J, et al. CAR NK-92 cells targeting DLL3 kill effectively small cell lung cancer cells in vitro and in vivo . J leukoc Biol (2022). doi: 10.1002/jlb.5ma0122-467r [DOI] [PubMed] [Google Scholar]
- 48. Cao B, Liu M, Huang J, Zhou J, Li J, Lian H, et al. Development of mesothelin-specific CAR NK-92 cells for the treatment of gastric cancer. Int J Biol Sci (2021) 17(14):3850–61. doi: 10.7150/ijbs.64630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teng KY, Mansour AG, Zhu Z, Li Z, Tian L, Ma S, et al. Off-the-shelf prostate stem cell antigen-directed chimeric antigen receptor natural killer cell therapy to treat pancreatic cancer. Gastroenterology (2022) 162(4):1319–33. doi: 10.1053/j.gastro.2021.12.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Montagner IM, Penna A, Fracasso G, Carpanese D, Dalla Pietà A, Barbieri V, et al. Anti-PSMA CAR-engineered NK-92 cells: An off-the-shelf cell therapy for prostate cancer. Cells (2020) 9(6):1382. doi: 10.3390/cells9061382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia (1994) 8(4):652–8. [PubMed] [Google Scholar]
- 52. Matosevic S. Viral and nonviral engineering of natural killer cells as emerging adoptive cancer immunotherapies. J Immunol Res (2018) 2018:4054815. doi: 10.1155/2018/4054815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baghery Saghchy Khorasani A, Yousefi AM, Bashash D. CAR NK cell therapy in hematologic malignancies and solid tumors; obstacles and strategies to overcome the challenges. Int Immunopharmacol (2022) 110:109041. doi: 10.1016/j.intimp.2022.109041 [DOI] [PubMed] [Google Scholar]
- 54. Wrona E, Borowiec M, Potemski P. CAR-NK cells in the treatment of solid tumors. Int J Mol Sci (2021) 22(11):5899. doi: 10.3390/ijms22115899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J, Zheng H, Diao Y. Natural killer cells and current applications of chimeric antigen receptor-modified nk-92 cells in tumor immunotherapy. Int J Mol Sci (2019) 20(2):317. doi: 10.3390/ijms20020317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marofi F, Abdul-Rasheed OF, Rahman HS, Budi HS, Jalil AT, Yumashev AV, et al. CAR-NK cell in cancer immunotherapy; a promising frontier. Cancer Sci (2021) 112(9):3427–36. doi: 10.1111/cas.14993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lu H, Zhao X, Li Z, Hu Y, Wang H. From CAR-T cells to CAR-NK cells: A developing immunotherapy method for hematological malignancies. Front Oncol (2021) 11:720501. doi: 10.3389/fonc.2021.720501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Albinger N, Pfeifer R, Nitsche M, Mertlitz S, Campe J, Stein K, et al. Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia. Blood Cancer J (2022) 12(4):61. doi: 10.1038/s41408-022-00660-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marofi F, Al-Awad AS, Sulaiman Rahman H, Markov A, Abdelbasset WK, Ivanovna Enina Y, et al. CAR-NK cell: A new paradigm in tumor immunotherapy. Front Oncol (2021) 11:673276. doi: 10.3389/fonc.2021.673276 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Chen C. Science mapping: A systematic review of the literature. J Data Inf Sci (2017) 2(2):1–40. doi: 10.1515/jdis-2017-0006 [DOI] [Google Scholar]
- 61. Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Sci (New York NY) (2020) 367(6481):eaba7365. doi: 10.1126/science.aba7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature (2017) 543(7643):113–7. doi: 10.1038/nature21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Trans Med (2016) 8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep (2017) 7(1):737. doi: 10.1038/s41598-017-00462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer (2016) 16(1):7–19. doi: 10.1038/nrc.2015.5 [DOI] [PubMed] [Google Scholar]
- 66. Ruppel KE, Fricke S, Köhl U, Schmiedel D. Taking lessons from car-t cells and going beyond: tailoring design and signaling for car-nk cells in cancer therapy. Front Immunol (2022) 13:822298. doi: 10.3389/fimmu.2022.822298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Drent E, Poels R, Ruiter R, van de Donk N, Zweegman S, Yuan H, et al. Combined cd28 and 4-1bb costimulation potentiates affinity-tuned chimeric antigen receptor-engineered t cells. Clin Cancer Res (2019) 25(13):4014–25. doi: 10.1158/1078-0432.Ccr-18-2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Luanpitpong S, Poohadsuan J, Klaihmon P, Issaragrisil S. Selective cytotoxicity of single and dual anti-cd19 and anti-cd138 chimeric antigen receptor-natural killer cells against hematologic malignancies. J Immunol Res (2021) 2021:5562630. doi: 10.1155/2021/5562630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leivas A, Valeri A, Córdoba L, García-Ortiz A, Ortiz A, Sánchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J (2021) 11(8):146. doi: 10.1038/s41408-021-00537-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Du Z, Ng YY, Zha S, Wang S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol Ther Methods Clin Dev (2021) 23:582–96. doi: 10.1016/j.omtm.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gurney M, Stikvoort A, Nolan E, Kirkham-McCarthy L, Khoruzhenko S, Shivakumar R, et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica (2022) 107(2):437–45. doi: 10.3324/haematol.2020.271908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guo Y, Tong C, Su L, Zhang W, Jia H, Liu Y, et al. CRISPR/Cas9 genome-edited universal CAR T cells in patients with relapsed/refractory lymphoma. Blood Adv (2022) 6(8):2695–9. doi: 10.1182/bloodadvances.2021006232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Karvouni M, Vidal-Manrique M, Lundqvist A, Alici E. Engineered nk cells against cancer and their potential applications beyond. Front Immunol (2022) 13:825979. doi: 10.3389/fimmu.2022.825979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.