Abstract
Melanopsin ganglion cells have defied convention since their discovery almost 20 years ago. In the years following, many types of these intrinsically photosensitive retinal ganglion cells (ipRGCs) have emerged. In the mouse retina, there are currently six known types (M1–M6) of melanopsin ganglion cells, each with unique morphology, mosaics, connections, physiology, projections, and functions. While melanopsin-expressing cells are usually associated with behaviors like circadian photoentrainment and the pupillary light reflex, the characterization of multiple types has demonstrated a reach that may extend far beyond non-image-forming vision. In fact, studies have shown that individual types of melanopsin ganglion cells have the potential to impact image-forming functions like contrast sensitivity and color opponency. Thus, the goal of this review is to summarize the morphological and functional aspects of the six known types of melanopsin ganglion cells in the mouse retina and to highlight their respective roles in non-image-forming and image-forming vision. Although many melanopsin ganglion cell types do project to image-forming brain targets, it is important to note that this is only the first step in determining their influence on image-forming vision. Even so, the visual system has canonically been divided into these two functional realms and melanopsin ganglion cells have begun to challenge the boundary between them, providing an overlap of visual information that is complementary rather than redundant. Further studies on these ganglion cell photoreceptors will no doubt continue to illustrate an ever-expanding role for melanopsin ganglion cells in image-forming vision.
Keywords: image-forming vision, ipRGCs, melanopsin ganglion cells, melanopsin, mouse, non-image-forming vision, photosensitive ganglion cells, retina
1 |. INTRODUCTION
1.1 |. The two functional realms of the visual system
Image-forming vision begins with light activation of rods and cones in the retina, a thin tissue layer on the back of the eye that sends information about the color, contrast, and motion of objects to the brain. These rod and cone light responses, along with the retinal encoding that follows, constitute the initial steps of pattern vision. This visual information is then sent to image-forming brain targets like the lateral geniculate nucleus (LGN) and superior colliculus (SC), and finally to the primary visual cortex. Hierarchical integration of this visual information in multiple visual cortices ultimately results in image-forming vision, allowing the animal to navigate through and interact with its environment.
However, the retina also encodes global luminance levels in order to align the biological clock with cyclical changes in environmental light. The ability to do so provides a distinct evolutionary advantage by allowing an organism to anticipate environmental changes and subsequently modulate their own behavior (Hastings, Reddy, & Maywood, 2003; Lazzerini Ospri, Prusky, & Hattar, 2017). As such, a distinct visual pathway exists to regulate certain behaviors in accordance with the amount of light present at any given time (Sonoda & Schmidt, 2016).
In this pathway, light information is instead relayed from the retina to the superchiasmatic nucleus (SCN) via the retinohypothalamic tract in order to modulate circadian rhythms (Foster & Hankins, 2002; Gooley, Lu, Chou, Scammell, & Saper, 2001; Hannibal et al., 2004; Moore & Lenn, 1972; Ralph, Foster, Davis, & Menaker, 1990). Similarly, light information is sent from the retina to the olivary pretectal nucleus (OPN) to regulate the size of the pupil, culminating in the pupillary light reflex (Foster & Hankins, 2002; M. J. Young & Lund, 1994). Many other behaviors are tied to the amount of light levels in the environment, including regulation of mood (Fernandez et al., 2018; Lazzerini Ospri et al., 2017; LeGates et al., 2012; LeGates, Fernandez, & Hattar, 2014), learning (LeGates et al., 2012; LeGates et al., 2014; Tam et al., 2016), body temperature (Rupp et al., 2019), induction of sleep and arousal (Altimus et al., 2008; Chellappa et al., 2011; Fisk et al., 2018; LeGates et al., 2014; Lockley & Gooley, 2006; Lupi, Oster, Thompson, & Foster, 2008; Morin, 2015; Muindi, Zeitzer, Colas, & Heller, 2013; Rupp et al., 2019; Tsai et al., 2009), masking and phototaxis (Delwig et al., 2013; J. Johnson et al., 2010), as well as exacerbating migraines and photophobia (Noseda et al., 2010; Noseda, Copenhagen, & Burstein, 2018).
Because these non-image-forming behaviors begin with light detection in the eye, there is a distinct branch of the visual system that uses light to prime certain behaviors for optimal function in daylight (Lucas et al., 2014), and this pathway is distinct from the one that encodes light information via cortical visual pathways to form visual representations of the surrounding environment. Thus, the visual system has canonically been divided into two branches: image-forming vision and non-image-forming vision.
1.2 |. Discovery of the third photoreceptor
Historically, as image-forming visual circuits were discovered and dissected, the mechanism responsible for non-image-forming vision remained poorly understood. One of the pioneering studies that provided evidence for a visual pathway independent of outer retinal photoreception was done by Clyde Keeler, who found that a blind mouse lacking rods still displayed pupil constriction in response to light exposure (Keeler, 1927). At the end of the 20th century, multiple reports provided further evidence for the existence of such a pathway, since circadian photoentrainment, melatonin suppression, the pupillary light reflex, and masking behaviors were found to be intact in mice without functional rods and cones (Foster & Hankins, 2002; Freedman et al., 1999; Lucas, Douglas, et al., 2001; Lucas, Freedman, et al., 2001; Lucas, Freedman, Munoz, Garcia-Fernandez, & Foster, 1999; Mrosovsky & Hattar, 2003; Mrosovsky, Lucas, & Foster, 2001; Panda et al., 2003; Semo et al., 2003; Yoshimura & Ebihara, 1996). Circadian photoentrainment was also preserved in mice with significant outer retinal degeneration, but was abolished upon eye removal (Foster et al., 1991; Freedman et al., 1999), suggesting that rod and cone independent mechanisms for this non-image-forming behavior must exist somewhere in the eye. Furthermore, in humans, some blind individuals remain able to suppress the production of melatonin in response to light, indicating that a type of blindness exists in which light information is still relayed to the circadian system in spite of loss of conventional vision (Czeisler et al., 1995; Hull, Czeisler, & Lockley, 2018).
The search reached a tipping point when Provencio and colleagues discovered the light responsive pigment melanopsin in the skin, brain, and eye of Xenopus laevis (Provencio, Jiang, De Grip, Hayes, & Rollag, 1998; Provencio et al., 2000). Upon further examination, melanopsin was found to be expressed in the retinohypothalamic tract and the SCN, which are the principle conduits for circadian rhythm regulation (Hannibal & Fahrenkrug, 2002; Hattar, Liao, Takao, Berson, & Yau, 2002; Provencio et al., 1998; Provencio, Rollag, & Castrucci, 2002). The identity of the elusive third photoreceptor was finally unearthed when a subpopulation of retinal ganglion cells (RGCs) were found to express melanopsin and electrically respond to light independently of rods and cones, making them intrinsically photosensitive (Berson, Dunn, & Takao, 2002; Hattar et al., 2002). These neurons were named ipRGCs, although they are also commonly referred to as photosensitive ganglion cells or melanopsin ganglion cells, as in this review.
The melanopsin protein, which is encoded by the gene Opn4, has a peak sensitivity to roughly 480 nm light (Berson et al., 2002; Hattar et al., 2002; Lucas, Douglas, et al., 2001; Yoshimura & Ebihara, 1996). One primary function of the ganglion cells that express it is to encode irradiance, resulting in the ability to track changes in global luminance levels over the course of an entire day (Berson, Castrucci, & Provencio, 2010; Wong, 2012). The responses recorded from these cells align with this primary function, as they exhibit a sustained ON response to light (Berson et al., 2002; Hattar et al., 2002; Hu, Hill, & Wong, 2013; Schmidt & Kofuji, 2009). Although melanopsin ganglion cells do not require rod and cone input to respond to light, they do receive information from retinal interneurons carrying rod and cone information (Do & Yau, 2010; Schmidt, Chen, & Hattar, 2011; Schmidt & Kofuji, 2010; Weng, Estevez, & Berson, 2013; Wong, 2012; Wong, Dunn, Graham, & Berson, 2007; Zhao, Stafford, Godin, King, & Wong, 2014). As such, their responses have both an intrinsic component (melanopsin-mediated) and an extrinsic (rod and conemediated) component, making melanopsin ganglion cells a unique inner-retinal photoreceptor that can align its intrinsic light response with the information being received from the outer retina in order to encode irradiance (Brown et al., 2010).
The role of this ganglion cell photoreceptor in non-image-forming vision was confirmed with the knockout of the melanopsin protein resulted in attenuation of circadian photoentrainment and a reduced pupillary light reflex at high irradiances (Lucas et al., 2003; Panda et al., 2002; Ruby et al., 2002). This phenomenon also affects masking, as the knock-out of the melanopsin protein results in impaired negative masking behavior when mice are exposed to bright light (Mrosovsky & Hattar, 2003). Since the absence of the melanopsin protein does not completely abolish these behaviors, it seems that rods and cones work in tandem with melanopsin ganglion cells to maintain non-image-forming vision (Altimus et al., 2010; Gooley et al., 2012; Lall et al., 2010). Moreover, genetic ablation of melanopsin ganglion cells themselves results in a complete loss of these non-image-forming functions, indicating that melanopsin ganglion cells are the necessary and principle conduit for rod- and cone-mediated component of non-image-forming vision (Goz et al., 2008; Guler et al., 2008; Hatori et al., 2008; J. Zhang, Wang, Wu, Liu, & Wang, 2017).
Because the origin of these ganglion cell photoreceptors is rooted in behaviors like the pupillary light reflex and circadian rhythms, melanopsin ganglion cells are traditionally and often associated with the non-image-forming branch of the visual system. But it has become apparent that there is more to these ganglion cell photoreceptors than meets the eye.
1.3 |. Emergence of melanopsin ganglion cell types
Since the discovery of melanopsin, studies have revealed six types of melanopsin ganglion cells (M1–M6), each with a unique set of morphology, mosaics, connections, physiology, projections, and functions. Many different mouse lines have been used to characterize melanopsin ganglion cells, including C57BL/6J (WT), Opn4-EGFP, Opn4−/−, Opn4-tdTomato, Opn4Cre/+ Z/EG, Gnat−/ Cnga3−/−, Gnat−/ Cnga3−/ Opn4−/−, and Cdh3-GFP (Table 1). Documenting and characterizing the six types in these mouse lines has resulted in further understanding of the many roles of melanopsin ganglion cells in both image-forming and non-image-forming vision.
TABLE 1.
Morphological characteristics of melanopsin ganglion cell types reported for distinct mouse ages and genotypes
| Type | Soma size (μm; mean ± SEM) | Dendritic field size (μm; mean ± SEM) | Branch points (mean ± SEM) | Age | Genotype | Sample size (n) | References |
|---|---|---|---|---|---|---|---|
| M1 | 17.0 ± 0.4 | 313.6 ± 17.3 | - | P22–P40 | Opn4+/− EGFP+/− | 16 | Schmidt and Kofuji (2009) |
| 12.8 ± 0.1 | 275 ± 6.9 | - | Adult | C57BL/6J | 142 | Berson et al. (2010) | |
| 15.6 ± 0.9 | 350 ± 25.1 | - | 2–4 months | Opn4Cre/+ Z/EG | 7 (s), 12 (d) | Ecker et al. (2010) | |
| 16.7 ± 0.7 | 377 ± 15.6 | - | >2 months | Opn4+/− tdTomato+/− | 27 | Muller, Do, Yau, He, and Baldridge (2010) | |
| 16.8 ± 0.6 | 421.1 ± 35.8 | - | P25–P40 | Opn4−/− | 9 | Schmidt and Kofuji (2010) | |
| 16.9 ± 0.7 | 391.2 ± 24.5 | - | P25–P40 | C57BL/6 WT | 12 | Schmidt and Kofuji (2010) | |
| 15.7 ± 0.4 | 365 ± 16.3 | - | P22–P40 | Opn4+/− EGFP+/− | 24 | Schmidt and Kofuji (2011) | |
| 13.9 ± 0.5 | 290.1 ± 16.5 | 10.2 ± 1.6 | 6 weeks–4 months | Opn4Cre/+ Z/EG+/− | 17 | Estevez et al. (2012) | |
| M2 | 21.8 ± 0.8 | 422.9 ± 23.5 | - | P22–P40 | Opn4+/− EGFP+/− | 13 | Schmidt and Kofuji (2009) |
| 14.8 ± 0.1 | 314 ± 7.5 | 14.3 ± 4.4 | Adult | C57BL/6J | 102 | Berson et al. (2010) | |
| 17.4 ± 0.8 | 324 ± 15 | - | 2–4 months | Opn4Cre/+ Z/EG | 5 (s), 4 (d) | Ecker et al. (2010) | |
| 18.6 ± 1.2 | 403 ± 34.5 | - | >2 months | Opn4+/− tdTomato+/− | 10 | Muller et al. (2010) | |
| 20.8 ± 0.6 | 486.5 ± 22.1 | - | P25–P40 | Opn4−/− | 11 | Schmidt and Kofuji (2010) | |
| 20.0 ± 0.6 | 445.4 ± 18.8 | - | P25–P40 | C57BL/6 WT | 7 | Schmidt and Kofuji (2010) | |
| 18.9 ± 0.6 | 425 ± 13.4 | - | P22–P40 | Opn4+/− EGFP+/− | 15 | Schmidt and Kofuji (2011) | |
| 15.7 ± 0.4 | 316.6 ± 13.8 | 24.4 ± 1.5 | 6 weeks–4 months | Opn4Cre/+ Z/EG+/− | 20 | Estevez et al. (2012) | |
| 15.8 ± 0.4 | 316.6 ± 13.8 | - | 1–3 months | Opn4Cre/+ Z/EG+/− | 20 | Stabio et al. (2018) | |
| M3 | 14.5 ±? | - | - | Adult | C57BL/6J | 6 | Berson et al. (2010) |
| 16.7 ± 1.8 | 449 ± 34.6 | - | >2 months | Opn4+/− tdTomato+/− | 3 | Muller et al. (2010) | |
| 17.8 ± 0.6 | 477.4 ± 20.1 | - | P22–P40 | Opn4+/− EGFP+/− | 10 | Schmidt and Kofuji (2011) | |
| M4 | 17.1–22.3 | 302–444 | 37.8 ± 5.5 | 2–4 months | Opn4Cre/+ Z/EG | 3 | Ecker et al. (2010) |
| 21.0 ± 0.4 | 359.6 ± 12.8 | 38.2 ± 1.6 | 6 weeks – 4 months | Opn4Cre+/− ZEG+/− | 27 | Estevez et al. (2012) | |
| 31.5 ± 3.7 | 397.7 ± 51.8 | - | 1–3 months | C57BL/6 WT | 8 | Schmidt et al. (2014) | |
| 21.1 ± 0.4 | 359.6 ± 12.8 | - | 1–3 months | Opn4Cre/+ Z/EG+/− | 27 | Stabio et al. (2018) | |
| M4 (n) | 23.1 ± 0.6 | 370.8 ± 13.5 | 27.4 ± 1.2 | 1–2.5 months | Opn4Cre/+ Z/EG | 16 | Sonoda, Okabe, and Schmidt (2019) |
| M4 (t) | 21.3 ± 0.7 | 207.6 ± 6.9 | 30.4 ± 1.5 | 1–2.5 months | Opn4Cre/+ Z/EG | 18 | Sonoda et al. (2019) |
| M5 | - | 149–217 | - | 2–4 months | Opn4Cre/+ Z/EG | 3 | Ecker et al. (2010) |
| 14.2 ± 0.4 | 224 ± 6.6 | 52.1 ± 1.9 | 1–3 months | Opn4Cre/+ Z/EG+/− | 44 | Stabio et al. (2018) | |
| M6 | 12.7 ± 0.3 | 216 ± 5.1 | 100 ± 4.6 | P25–P43 | Cdh3-GFP | 34 | Quattrochi et al. (2019) |
Note: Each row corresponds to a set of morphological characteristics, age, genotype, and sample size obtained from the reference adjacent to it (right). In the sample size column, (s) indicates the sample size used to calculate the soma size, and (d) indicates the sample size used to calculate the dendritic field size where applicable. For the M4 type, (n) indicates values obtained from M4 cells in the nasal retina, while (t) indicates values obtained from M4 cells in the temporal retina. Values are mean ± SEM.
However, it is important to note that some of the evidence for the involvement of individual types of melanopsin ganglion cells in image-forming vision is only in the form of axonal projections to image-forming brain targets. When this is the case, more studies are needed to further understand the influence of a melanopsin ganglion cell type on a particular image-forming behavior. So although some melanopsin ganglion cell types project to the dorsal lateral geniculate nucleus (dLGN) and exhibit physiologies that have the potential to provide input to the image-forming visual system, their precise impact on image-forming behaviors remains undetermined. Even so, there is an abundance of evidence for melanopsin ganglion cell involvement in image-forming vision, including extensive roles in brightness detection and the enhancement of environmental scenes. As these influences on image-forming vision have not been attributed to a particular type, they are considered at length in the discussion. Thus, the goal of this review is to summarize the morphological and functional aspects of the six known types of melanopsin ganglion cells in the mouse retina, to highlight their respective roles in image-forming and non-image-forming vision, and to call attention to what is not yet known about these ganglion cell photoreceptors.
2 |. THE M1 TYPE
2.1 |. Morphology and mosaics of the M1 type
The M1 was the first melanopsin ganglion cell type to be discovered, as it is exhibits robust melanopsin immunoreactivity and is easily detected with conventional melanopsin immunohistochemistry (Figure 1a; Baver, Pickard, Sollars, & Pickard, 2008; Berson et al., 2002, 2010; Hattar et al., 2006; Hattar et al., 2002; Viney et al., 2007). However, there are two isoforms of melanopsin in the mouse: Opn4S and Opn4L (Pires et al., 2009). Expression of the Opn4S isoform appears to be a feature of the M1 type (Pires et al., 2009). M1 cells have a relatively small and spherical soma, with an average diameter of 15.7 ± 0.5 μm (mean ± SEM; n = 254; Figure 1b; Tables 1 and 2; Berson et al., 2010; Ecker et al., 2010; Estevez et al., 2012; Hattar et al., 2002; Muller, Do, Yau, He, & Baldridge, 2010). As the somas of these cells can be in either the ganglion cell layer (GCL) or displaced to the inner nuclear layer (INL), the M1 type can be further subdivided into displaced (M1d), and conventional (M1) cells (Berson et al., 2002; Hattar et al., 2002). A recent study also discovered a set of melanopsin interneurons that have somas in the INL but do not send axons to the optic nerve (Valiente-Soriano et al., 2014), but their function remains unclear.
FIGURE 1.
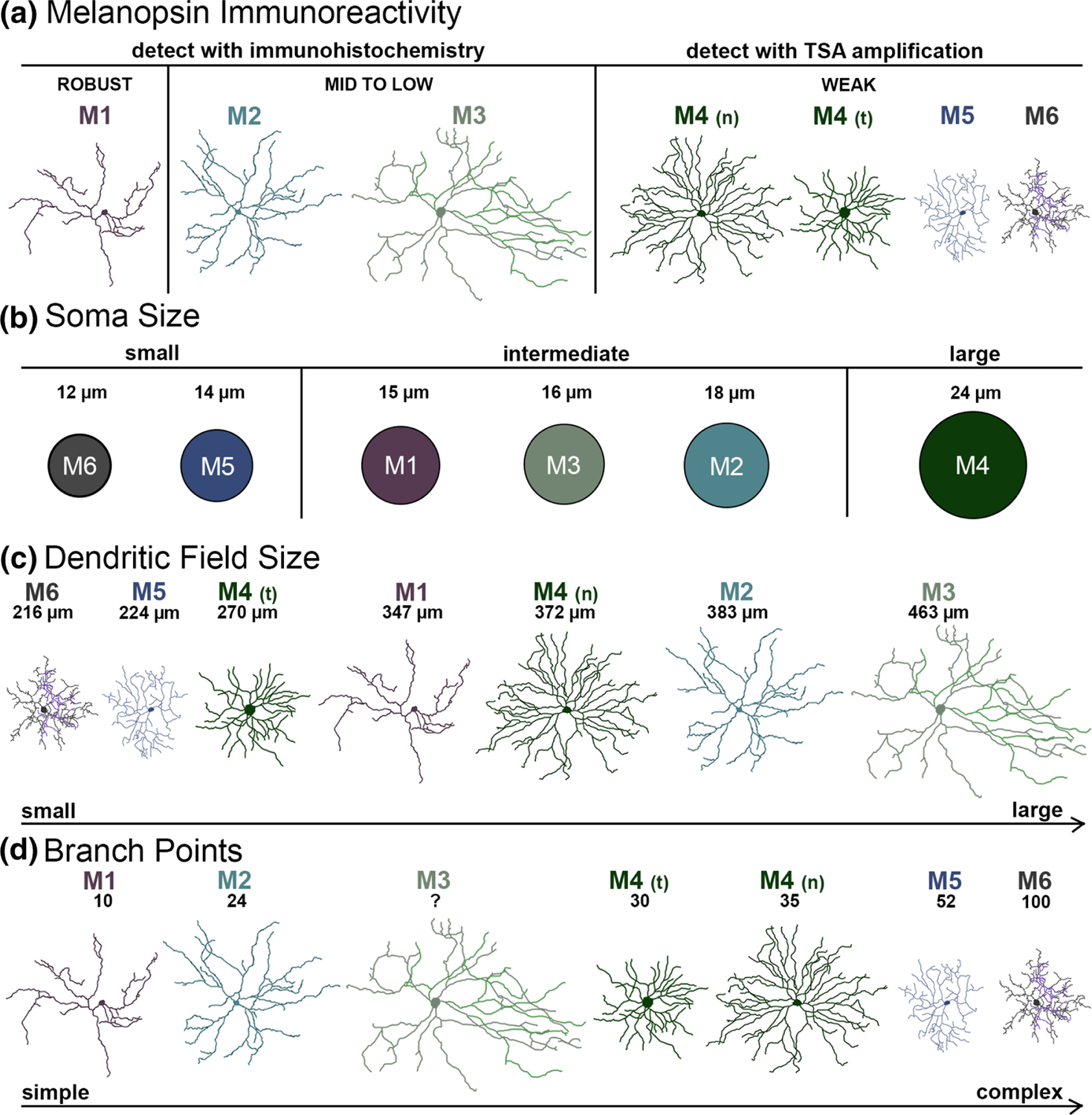
Morphological characteristics of melanopsin ganglion cell types in the mouse retina. (a) The level of melanopsin expression based on melanopsin immunoreactivity of each type arranged from strong (left) to weak (right). M1 cells have the highest expression of melanopsin, and as such exhibit bright dendrites and somas with conventional immunohistochemistry. M2 and M3 cells have mid to low melanopsin expression, showing up faintly with conventional immunohistochemistry. M4, M5, and M6 types have very weak expression of melanopsin and are only detectable using TSA amplification. (b) Circles represent approximate soma size. Type soma diameter sizes arranged from small (left) to large (right). M5 and M6 cells have small soma diameters, M1, M2, and M3 cells have intermediate soma diameters, and M4 cells have large soma diameters. M4 cells, which are equivalent to ON-α ganglion cells, have the largest somas of any ganglion cell type (Schmidt et al., 2014). However, it is important to note that types of melanopsin ganglion cells cannot be distinguished exclusively by soma diameter, as this is a morphological characteristic that can vary widely, even within an individual type. Values are calculated as mean ± SEM and then rounded for simplification (Tables 1 and 2). (c) The dendritic field size of each type arranged from smallest (left) to largest (right). M3 and M4 cells have the largest dendritic field sizes, while M5 and M6 cells have the smallest. A recent publication by Sonoda et al. (2019) demonstrated that there is a dendritic field size gradient for M4 cells: Those in the temporal retina—designated here as M4 (t)—have smaller dendritic field sizes, and those in the nasal retina—designated here as M4 (n)—have larger dendritic field sizes (Sonoda et al., 2019). As such, the dendritic field size of the M4 differs based on retinal location. It is also important to note that melanopsin ganglion cell types cannot be distinguished exclusively by dendritic field size, as this is a morphological characteristic that can vary even within an individual type (Lee & Schmidt, 2018). Values are calculated as mean ± SEM and then rounded for simplification (Tables 1 and 2). (d) The number of dendritic branch points for each type arranged from fewest (left) to most (right). M6 cells have the most complex and highly branched dendritic arbors, while M1 cells have the simplest (Tables 1 and 2). Reproduced tracings: M1 and M2: (Berson et al., 2010); M3: (Schmidt & Kofuji, 2011); M4 (temporal): (Sonoda et al., 2019); M4 (nasal): (Estevez et al., 2012) M5: (Stabio, Sabbah, et al., 2018); M6: (Quattrochi et al., 2019). Summarized information from the following: (Baver, Pickard, Sollars, & Pickard, 2008; Belenky, Smeraski, Provencio, Sollars, & Pickard, 2003; Berson et al., 2002, 2010; Ecker et al., 2010; Estevez et al., 2012; Hattar et al., 2002; Hattar et al., 2006; Muller et al., 2010; Pu, 1999; Quattrochi et al., 2019; Schmidt et al., 2014; Schmidt & Kofuji, 2009, 2010, 2011; Schmidt, Taniguchi, & Kofuji, 2008; Sonoda et al., 2019; Sonoda, Lee, Birnbaumer, & Schmidt, 2018; Stabio, Sabbah, et al., 2018; Warren et al., 2003)
TABLE 2.
A summary of the morphological characteristics of melanopsin ganglion cell types in the mouse retina
| Type | Soma size (μm; mean ± SEM) | Dendritic field size (μm; mean ± SEM)a | Branch points (mean ± SEM) | Sample size (n =?) |
|---|---|---|---|---|
| M1 | 15.7 ± 0.5 | 347.9 ± 19.7 | 10.2 ± 1.6 | 254 (s), 259 (d) |
| M2 | 18.2 ± 0.6 | 383.8 ± 18.0 | 19.4 ± 1.9 | 183 (s), 182 (d) |
| M3 | 16.3 ± 1.2 | 463.2 ± 27.4 | Undetermined | 19 |
| M4 (n) | 24.2 ± 1.5 | 371.9 ± 22.7 | 34.5 ± 2.8 | 54 |
| M4 (t) | 21.3 ± 0.7 | 207.6 ± 6.9 | 30.4 ± 1.5 | 18 |
| M5 | 14.2 ± 0.4 | 224 ± 6.6 | 52.1 ± 1.9 | 47 |
| M6 | 12.7 ± 0.3 | 216 ± 5.1 | 100 ± 4.6 | 34 |
Note: Values are mean ± SEM and were calculated using reported values from the papers in Table 1 (Berson et al., 2010; Ecker et al., 2010; Estevez et al., 2012; Muller et al., 2010; Quattrochi et al., 2019; Schmidt et al., 2014; Schmidt & Kofuji, 2009, 2011; Sonoda et al., 2019; Stabio, Sabbah, et al., 2018).
It should be noted that the dendritic field size for M4 cells differs based on retinal location, with M4 cells located in the nasal retina having a larger dendritic field than M4 cells located in the temporal retina (Bleckert, Schwartz, Turner, Rieke, & Wong, 2014; Sonoda et al., 2019). M4 (n) = M4 cells located in the nasal retina; M4 (t) = M4 cells located in the temporal retina.
There are about 900 M1 and M1d melanopsin ganglion cells in the mouse retina (Berson et al., 2010). Conventional, nondisplaced M1 cells make up roughly 71% of this population, eliminating M1d cells as an independent type as they do not tile the retina (Berson et al., 2010). However, the number of M1 cells varies throughout development: One group has reported roughly 300 M1 melanopsin ganglion cells in developing retinas from the C57Bl/6N mouse line (Sondereker, Onyak, Islam, Ross, & Renna, 2017) and another has reported 920 M1 cells in mature retinas from C57/Bl6 mice (Hughes, Watson, Foster, Peirson, & Hankins, 2013). Thus, it is important to consider both the age, genotype, and genetic background of mice when quantifying characteristics of melanopsin ganglion cells.
With an average dendritic field size of 347.9 ± 19.7 μm (mean ± SEM; n = 259; Figure 1c; Tables 1 and 2), the dendrites of M1 cells typically form relatively sparse arbors, branching only 10 times on average (Figure 1d; Tables 1 and 2; Ecker et al., 2010; Estevez et al., 2012; Muller et al., 2010; Schmidt & Kofuji, 2009). However, both M1 and M1d cells have dendrites that stratify exclusively in the OFF sublamina of the inner plexiform layer (IPL), making them outer-stratifying melanopsin ganglion cells (Figure 2; Berson et al., 2002, 2010; Hattar et al., 2002). Although M1 cells do tile the retina, they are asymmetrically distributed to the dorsal retina, where m-opsin is dominantly expressed (Figure 3; Applebury et al., 2000; Hughes et al., 2013; Stabio, Sondereker, et al., 2018).
FIGURE 2.
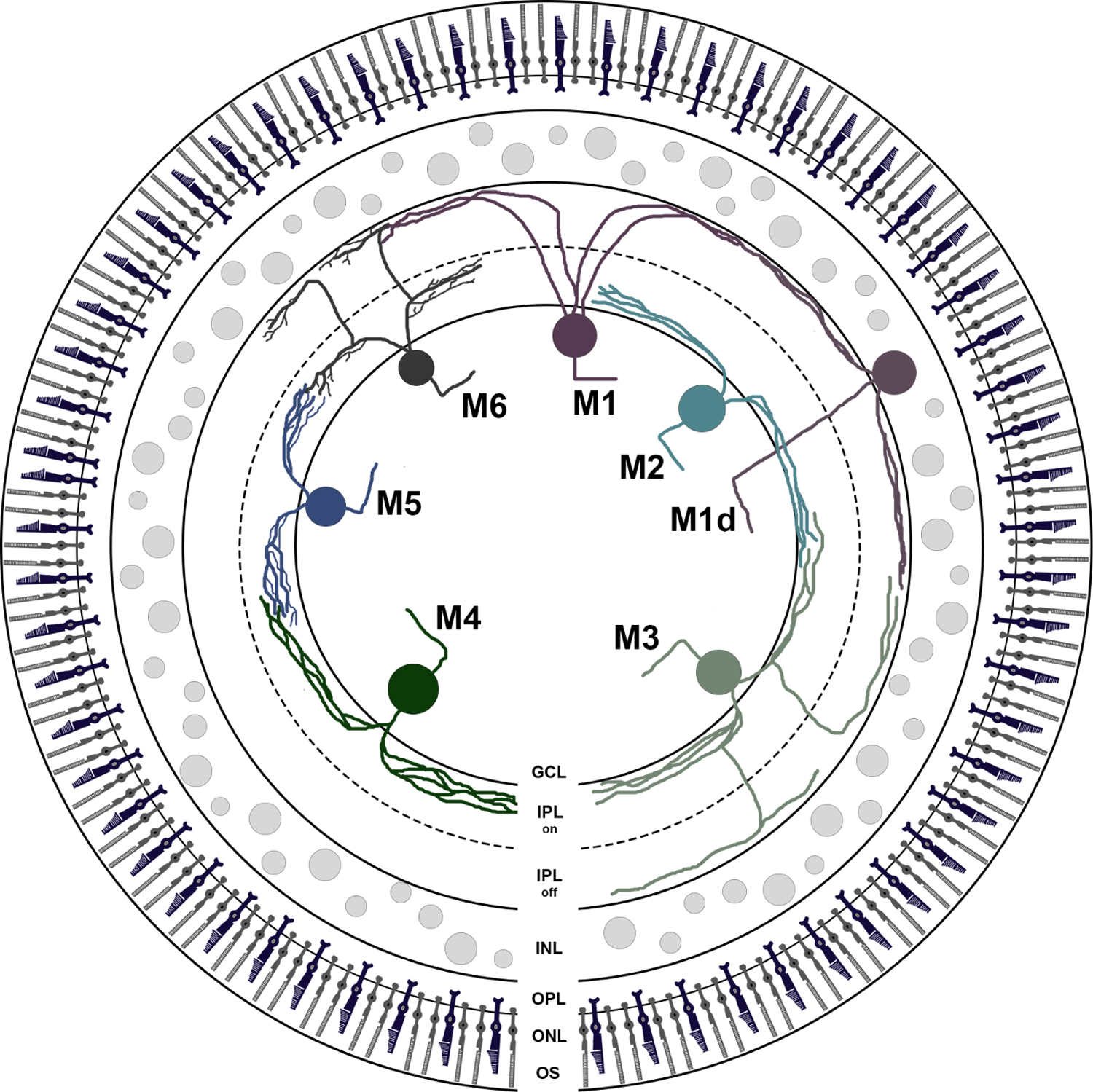
A retinal schematic displaying IPL stratification for each melanopsin ganglion cell type in the mouse retina. Melanopsin ganglion cell types are most easily identified by stratification in the IPL (Lee & Schmidt, 2018). M1 and M1d cells stratify only in the OFF sublamina of the IPL, forming ectopic synapses with Type 6 ON bipolar cells. In contrast, M2, and M4 cells stratify only in the ON sublamina of the IPL, forming synapses with Type 8 and Type 7 ON cone bipolar cells, respectively. Although both M2 and M4 cells stratify in the ON sublamina, M4 cells stratify slightly above the dendrites of M2 cells but below the ON ChAT band. M5 cells also stratify just below the ON ChAT band in the ON sublamina of the IPL, receiving excitatory input from s-cones via Type 9 ON cone bipolar cells and mixed input from Types 6, 7, 8, and 9 ON cone bipolar cells. M3 and M6 cells are bistratified, with dendrites in both the ON and OFF sublamina of the IPL. Dendrites of M6 cells can also be seen diving between the ON and OFF sublamina. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; OS, outer segments. Summarized information from the following: (Baver et al., 2008; Belenky et al., 2003; Berson et al., 2002, 2010; Dumitrescu, Pucci, Wong, & Berson, 2009; Ecker et al., 2010; Estevez et al., 2012; Hattar et al., 2002, 2006; Muller et al., 2010; Pu, 1999; Quattrochi et al., 2019; Schmidt et al., 2008; Schmidt et al., 2014; Schmidt & Kofuji, 2009, 2010, 2011; Sonoda et al., 2018; Stabio, Sabbah, et al., 2018; Viney et al., 2007; Warren et al., 2003; Wassle et al., 2009)
FIGURE 3.
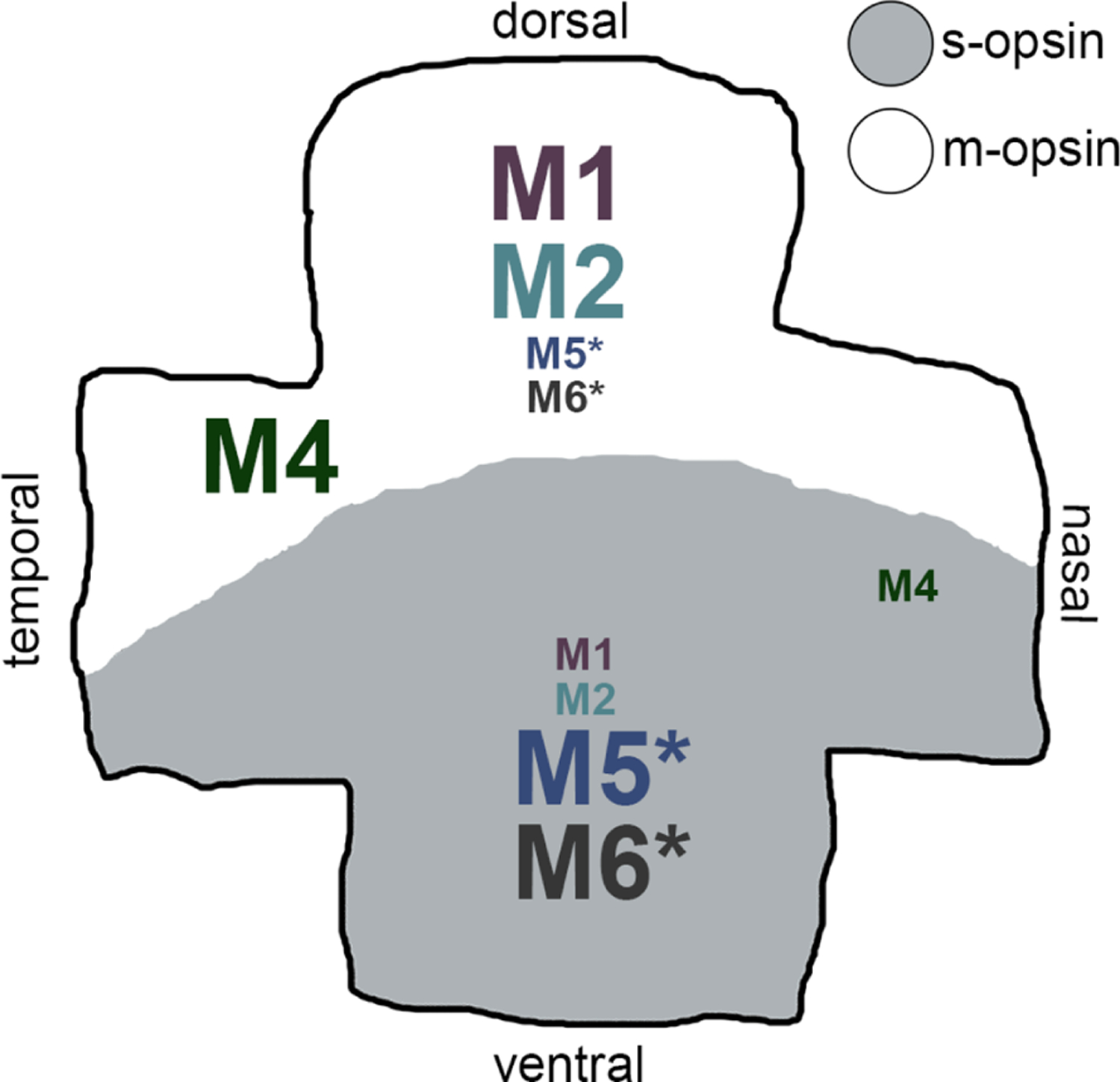
Retinal distribution of melanopsin ganglion cell types in the mouse retina. Like cone opsins, melanopsin ganglion cells are not distributed evenly across the retina. M1 and M2 cells are more prevalent in the dorsal retina (large font) than in the ventral retina (small font) (Hughes, Watson, Foster, Peirson, & Hankins, 2013), where m-opsin (white) is predominantly expressed (Applebury et al., 2000). Interestingly, M4 cells have a unique retinal distribution with more M4 cells in the temporal-dorsal retina (large font) than in the nasal-ventral retina (small font) (Bleckert et al., 2014). Additionally, M4 cells exhibit a dendritic field size gradient across the retina: M4 cells in the temporal retina have a smaller dendritic field size than M4 cells in the nasal retina (Bleckert et al., 2014; Sonoda et al., 2019). *Preliminary data suggests that M5 and M6 cells are asymmetrically distributed to the ventral retina (large font) where s-opsin is predominantly expressed (Applebury et al., 2000; Hughes et al., 2013; Quattrochi et al., 2019; Stabio, Sabbah, et al., 2018), but further studies of the distribution of these types is required. As the M3 type is rare, its distribution is unknown and it was not included in this diagram
2.2 |. Retinal influences and connections of the M1 type
At first glance, M1 cells appear to be somewhat of a paradox: they exhibit sustained ON responses to light (Berson et al., 2002; Hattar et al., 2002; Hu, Hill, & Wong, 2013; Schmidt & Kofuji, 2009), but they stratify in the OFF sublamina of the IPL (Figure 2). Interestingly, M1 cells defy the conventional organization of the IPL by forming en passant synapses with Type 6 ON bipolar cells in the OFF sublamina (Dumitrescu, Pucci, Wong, & Berson, 2009). This uniquely located synapse allows M1 cells to respond to light onset in a layer of the IPL that traditionally contains only light OFF information (Dumitrescu et al., 2009), essentially upending the neuroanatomy dogma of IPL stratification.
M1 cells have the highest membrane resistance but the lowest spiking frequency of any type and are therefore physiologically distinct from all other types (Table 3; Hu et al., 2013; Zhao et al., 2014). While both s-cone and m-cone responses contribute to the small and sustained extrinsic ON responses in M1 melanopsin ganglion cells, they also receive substantial input from rods (Table 3; Hu et al., 2013; Schmidt & Kofuji, 2010; Weng, Estevez, & Berson, 2013; Wong et al., 2007; Zhao et al., 2014). Thus, M1 cells respond to light onset extrinsically via rods and cones and intrinsically via the melanopsin light response. Even so, M1 physiology is dominated by the intrinsic melanopsin light response due to the high level of melanopsin expression in M1 cells (Figure 4; Table 3) (Schmidt & Kofuji, 2010).
TABLE 3.
Physiological characteristics of melanopsin ganglion cells in the mouse
| Type | Intrinsic light response | Extrinsic light response | Membrane properties |
|---|---|---|---|
| M1 | Large; highest peak amplitude | ON; small and sustainedb | High membrane resistance; low spiking frequency |
| M2 | Intermediate | ON; large and sustained; surround antagonism | Intermediate membrane resistance; intermediate spiking frequency |
| M3 | Intermediate | ON; large and sustained; surround antagonism | Intermediate membrane resistance; intermediate spiking frequency |
| M4 | Weak in light-adapted retinas; large in dark-adapted retinasa | ON; large and sustained; surround antagonism; chromatic opponency | Low membrane resistance; high spiking frequency |
| M5 | Weak | ON; large and sustained; surround antagonism; chromatic opponency | Low membrane resistance; high spiking frequency |
| M6 | Weak | ON; large and sustained; surround antagonism | Low membrane resistance; high spiking frequency |
Note: All types are dominated by their extrinsic ON response except the M1 type, which is dominated by its intrinsic melanopsin response.
Although M4 cells have a very low level of melanopsin immunoreactivity, the intrinsic melanopsin response of M4 cells differs between light-adapted and dark-adapted retinas. In light-adapted retinas, M4 cells exhibit a very weak intrinsic light response that is smaller in amplitude than those of both M1, M2, and M3 cells (Ecker et al., 2010; Estevez et al., 2012; Hu et al., 2013; Schmidt et al., 2014). In contrast, the intrinsic response of M4 cells in dark-adapted retinas is actually fairly large and comparable to that of M1 cells (Schroeder et al., 2018; Zhao et al., 2014). Thus, during the daytime, the large and sustained ON responses of M4 cells come from extrinsic excitatory input via the ON pathway.
It should be noted that the small and sustained ON light response of M1 cells is from light-adapted M1 cells (Estevez et al., 2012; Schmidt & Kofuji, 2010; Wong et al., 2007) and that dark-adapted M1 cells exhibit an extrinsic light response similar to the remaining types (Zhao et al., 2014). Summarized information from the following: (Bleckert et al., 2014; Hu et al., 2013; Quattrochi et al., 2019; Schmidt et al., 2014; Schmidt & Kofuji, 2010, 2011; Schroeder et al., 2018; Sonoda et al., 2018; Sonoda & Schmidt, 2016; Stabio, Sabbah, et al., 2018; Wong et al., 2007; Zhao et al., 2014).
FIGURE 4.
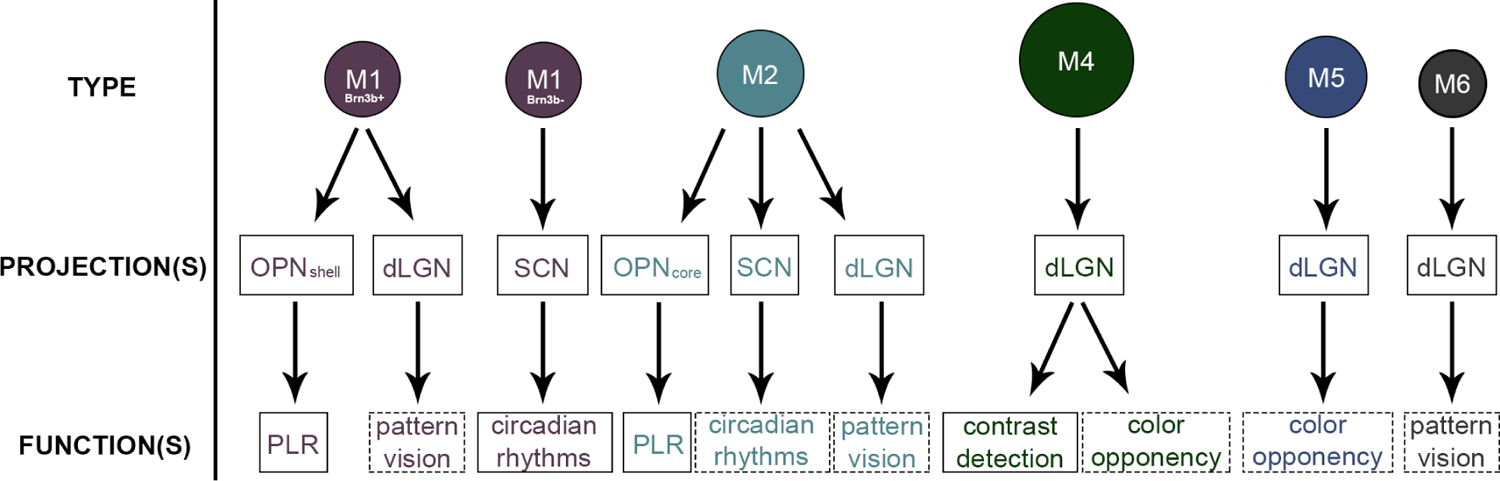
The major projections and functional roles of melanopsin ganglion cells in the mouse. While melanopsin ganglion cells have a large role in non-image-forming behaviors like circadian rhythms and the pupillary light reflex, many types project to the dLGN and are also involved in image-forming vision. It is important to note that determining the brain projections of melanopsin ganglion cells is only the first step in elucidating the function of a particular type, and that behavioral studies are necessary for further understanding of a melanopsin ganglion cell type’s particular impact on behavior. So although some melanopsin ganglion cell types project to the dLGN and exhibit physiologies like color opponency, further examination of their exact influence on color discrimination must be done before solidifying their role in such image-forming behaviors. A dashed box indicates those types whose functions are supported only by axonal projections to image-forming brain targets and require further study. dLGN, dorsal lateral geniculate nucleus; OPN, olivary pretectal nucleus; PLR, pupillary light reflex; SCN, superchiasmatic nucleus. Summarized information from the following: (Baver et al., 2008; Belenky et al., 2003; Berson et al., 2002, 2010; Brown et al., 2010; Chen, Badea, & Hattar, 2011; Ecker et al., 2010; Estevez et al., 2012; Gooley et al., 2001; Gooley, Lu, Fischer, & Saper, 2003; Guler et al., 2008; Hatori et al., 2008; Hattar et al., 2002; Hattar et al., 2006; Jain, Ravindran, & Dhingra, 2012; Li & Schmidt, 2018; Muller et al., 2010; Pu, 1999; Quattrochi et al., 2019; Schmidt et al., 2008, 2014; Schmidt & Kofuji, 2009, 2010, 2011; Schroeder et al., 2018; Sollars et al., 2003; Sonoda et al., 2018; Sonoda & Schmidt, 2016; Stabio, Sabbah, et al., 2018; Warren et al., 2003; Zhao et al., 2014)
Although all M1 cells consistently stratify in the OFF sublamina of the IPL, recent studies have found that individual M1 cells can vary widely in their physiology and morphology (Emanuel, Kapur, & Do, 2017). In fact, even M1 cells that project to the same area of the brain show little to no correlation in their biophysical parameters (Emanuel et al., 2017). Thus, the output of individual M1 cells to downstream targets appears to vary within the M1 population, even among M1 cells that lie in close proximity to one another (Emanuel et al., 2017). Consistent with these findings is evidence for variation in M1 melanopsin ganglion cell responses to dim light intensities. This variation appears to be due in part to the amount of input from the rod pathway onto individual M1 cells (Lee, Sonoda, & Schmidt, 2019). M1 cells with more complex morphologies—those with larger dendritic fields and longer total dendritic lengths—tend to receive more input from the rod pathway than those with simpler ones (Lee et al., 2019). Even more interesting is the fact that some M1 cells rely only on their intrinsic photosensitivity to respond to dim light, while others exhibit no scotopic light responses at all (Lee et al., 2019). As such, the variations in M1 responses indicate a ganglion cell type that is uncharacteristically diverse, a quality that should be taken into account when attempting to tease out functions of the M1 population both behaviorally and within the retina.
The physiology of M1 melanopsin ganglion cells is also influenced by dopamine amacrine cells (DACs), as they form extensive networks with these dopamine-releasing interneurons (Dkhissi-Benyahya et al., 2013; Joo, Peterson, Dacey, Hattar, & Chen, 2013; Prigge et al., 2016; Van Hook, Wong, & Berson, 2012; Vugler, Redgrave, Hewson-Stoate, Greenwood, & Coffey, 2007; D. Q. Zhang, Belenky, Sollars, Pickard, & McMahon, 2012; D. Q. Zhang et al., 2008). Dopamine synthesis and release, which is under the influence of the retinal circadian clock and is released in highest concentration in the early hours of the morning, (Doyle, Grace, et al., 2002; Doyle, McIvor, et al., 2002; Nir, Haque, & Iuvone, 2000) has a profound effect on M1 cells via the dopamine receptor D1 (Van Hook et al., 2012). During the day, activation of D1 receptors on M1 cells facilitates light adaptation when dopamine levels are high (Van Hook et al., 2012). However, the intrinsic M1 light response in turn drives excitation of DACs, potentially through glutamatergic M1 axon collaterals that reach into both the ON and OFF sublamina of the IPL (Joo et al., 2013; Prigge et al., 2016; D. Q. Zhang et al., 2012; D. Q. Zhang et al., 2008). This creates a positive feedback loop in which M1 cells facilitate their own light adaptation and align their responses with the time of day (Dumitrescu et al., 2009; Van Hook et al., 2012; Viney et al., 2007). While M1 melanopsin ganglion cells excite DACs in both the dorsal and ventral mouse retina, their influence only occurs under intermediate and bright light conditions (Zhao, Wong, & Zhang, 2017).
Because M1 cells relay light information to DACs, they have the potential to influence the setting of the retinal circadian clock (Barnard, Hattar, Hankins, & Lucas, 2006; Dkhissi-Benyahya et al., 2013; Storch et al., 2007). The retinal circadian clock is entrained independently of the SCN because it is mainly controlled by clock gene expression within the retina (Ko & Takahashi, 2006; Reppert & Weaver, 2001; Storch et al., 2007). Dopamine is a potent neuromodulator, particularly for regulating the expression of clock genes, and, by extension, for regulating the retinal circadian clock (Yujnovsky, Hirayama, Doi, Borrelli, & Sassone-Corsi, 2006). M1 melanopsin ganglion cells appear to be involved in this pathway, as the knockout of melanopsin abolishes the rhythmic expression of clock genes and light no longer initiates the expression of Period clock genes in the outer retina (Dkhissi-Benyahya et al., 2013). This effect is the result of M1 influence on DACs: When melanopsin is knocked out, the levels of dopamine and the mRNA of its precursor, tyrosine hydroxylase (TH), do not increase during the day as they do when melanopsin is present (Dkhissi-Benyahya et al., 2013). This in turn prevents the expression of necessary clock genes, altering the rhythm of the retinal clock. While the role of melanopsin ganglion cells in retinal clock regulation is still controversial, this evidence suggests that melanopsin ganglion cells are an important component for setting the retinal circadian clock by controlling dopamine release, and subsequently the expression of clock genes, via their networks with DACs (Dkhissi-Benyahya et al., 2013).
With their intrinsic photosensitivity, melanopsin ganglion cells have a unique opportunity to use ambient light levels to influence the physiology of additional cell types within the retina (Reifler, Chervenak, Dolikian, Benenati, Li, et al., 2015a). As most ganglion cells are coupled to each other via Connexin36 (Cx36; Bloomfield & Volgyi, 2009; Pan, Paul, Bloomfield, & Volgyi, 2010), it is likely that Cx36 is also the primary connexin at M1 melanopsin ganglion cell gap junctions. However, a recent study suggests that M1 cells may utilize a heteromeric gap junction containing both Cx36 and Connexin30.2 (Cx30.2) for gap junctional coupling (Meyer et al., 2016). Either way, M1 cells are gap junctionally coupled with displaced GABAergic wide-field amacrine cells, allowing for excitation of these interneurons and regulation of the neuromodulators that they release. Taken together with the M1 network with DACs, the M1 type uses its intrinsic photosensitivity to align retinal function with the flow and ebb of environmental light levels.
It is worthwhile to note that a subset of M1 and M1d cells extend dendrites into the outer retina during development (Renna, Chellappa, Ross, Stabio, & Berson, 2015; Sondereker et al., 2017). Melanopsin ganglion cell dendrites were first seen reaching into the INL in rat retina (Hattar et al., 2002), but have also been noted in macaque retina (Liao et al., 2016) and have been extensively documented in both the INL and OPL of the mouse retina (Renna et al., 2015; Sondereker et al., 2017). These outer retinal dendrites (ORDs) lie in close proximity to cone photoreceptor terminals and are present in greatest number during development, peaking at postnatal day 12 (P12; Renna et al., 2015; Sondereker et al., 2017). ORDs colocalize with the presynaptic marker vGlut1, suggesting the possibility of a direct connection between a ganglion cell and a cone photoreceptor (Renna et al., 2015; Sondereker et al., 2017). Interestingly, at all stages of development, ORDs are asymmetrically distributed to the dorsal retina, where M1 and M1d cells and m-opsin cones are in highest concentration (Applebury et al., 2000; Hughes et al., 2013; Sondereker et al., 2017). As the number of ORDs decreases significantly by P30, this phenomenon seems to be mostly developmental in nature and thus most likely has a role early in development that precedes eye-opening (Renna et al., 2015; Sondereker et al., 2017). One such role might be the regulation of s-cone lamination by ORDs working in conjunction with DACs, as ablation of melanopsin ganglion cells causes a higher occurrence of displaced s-cones (Tufford et al., 2018). While this may not be their only function, ORDs appear to play a role in organizing retinal lamination early in development, perhaps through a direct cone-toganglion cell connection.
2.3 |. Projections and functions of the M1 type
The ablation of M1 melanopsin ganglion cells results in the loss of non-image-forming behaviors, indicating that the M1 type plays a primary role in both circadian rhythm regulation and the pupillary light reflex (Goz et al., 2008; Guler et al., 2008; Hatori et al., 2008; Lucas et al., 2003; Panda et al., 2002; Ruby et al., 2002; J. Zhang et al., 2017). This is further supported in that M1 cells express the Opn4S isoform of melanopsin, which has been shown to be associated with both circadian photoentrainment and pupillary light reflex behaviors (Jagannath et al., 2015; Pires et al., 2009) It is therefore unsurprising that within the M1 type exists a subpopulation: M1 cells that express the transcription factor Brn3b (Brn3b+) and M1 cells that do not (Brn3b−; Chen, Badea, & Hattar, 2011; Jain, Ravindran, & Dhingra, 2012). These two populations send axonal projections to distinct areas of the brain and therefore have different functions (Table 4; Figure 4; Chen et al., 2011; Jain et al., 2012; Li & Schmidt, 2018).
TABLE 4.
A summary of the known brain targets of melanopsin ganglion cell types in the mouse retina
| Brain target | Function | IF or NIF vision | M1 Brn3b+ | M1 Brn3b− | M2 | M3 | M4 | M5 | M6 |
|---|---|---|---|---|---|---|---|---|---|
| SCN | Regulation of circadian rhythms; arousal from sleep | NIF | |||||||
| VLPO | Regulation of circadian rhythms; sleep induction | NIF | |||||||
| OPNshell | Pupillary light reflex | NIF | |||||||
| OPNcore | Pupillary light reflex | NIF | ? | ? | |||||
| PPN | Pupillary light reflex | NIF | ? | ? | |||||
| PHb | Regulation of mood | NIF | |||||||
| IGL | Circadian rhythm period lengthening | NIF | ? | ? | |||||
| vLGN | Circadian rhythm period lengthening | NIF | ? | ? | |||||
| VMH | Regulation of mood, body temperature, and feeding | NIF | |||||||
| pSON | Regulation of mood, body temperature, and feeding | NIF | |||||||
| dLGN | Pattern vision | IF | |||||||
| SC | Orienting eyes toward movement | IF | ? |
Note: Shaded blocks indicate that a particular type sends axons to that area of the brain. Gray shaded rectangles with question marks indicate that there is a possibility that the type sends axons to that brain target, but it has not been definitively shown. Specifically for the M5 and M6 types, the uncertainty comes from the use of the Cdh3-GFP mouse line, which labels both M5 and M6 cells, making the determination of axonal projections difficult. dLGN, dorsal lateral geniculate nucleus; IF, image-forming vision; IGL, intergeniculate leaflet; NIF, non-image-forming vision; OPN, olivary pretectal nucleus; PPN, posterior pretectal nucleus; PHb, perihabenular nucleus; pSON, perisupraoptic nucleus; SC, superior colliculus; SCN, superchiasmatic nucleus; vLGN, ventral lateral geniculate nucleus; VLPO, ventrolateral preoptic nucleus; VMN, ventromedial nucleus. Summarized information from the following: (Baver et al., 2008; Belenky et al., 2003; Berson et al., 2010; Berson et al., 2002; Brown et al., 2010; Chen et al., 2011; Ecker et al., 2010; Estevez et al., 2012; Fernandez et al., 2018; Gooley et al., 2001; Gooley, Lu, Fischer, & Saper, 2003; Guler et al., 2008; Hatori et al., 2008; Hattar et al., 2006; Hattar et al., 2002; Jain et al., 2012; Li & Schmidt, 2018; Muller et al., 2010; Pu, 1999; Quattrochi et al., 2019; Schmidt et al., 2014; Schmidt & Kofuji, 2009, 2010, 2011; Schmidt et al., 2008; Schroeder et al., 2018; Sollars et al., 2003; Sonoda et al., 2018; Sonoda & Schmidt, 2016; Stabio, Sabbah, et al., 2018; Warren et al., 2003; Zhao et al., 2014).
The majority of M1 cells are Brn3b+ and project to the shell of the OPN, indicating a role in regulating the pupillary light reflex (Table 4) (Chen et al., 2011; Jain et al., 2012; Li & Schmidt, 2018). Interestingly, mice with outer retinal degeneration (rd/rd) have a pupillary light response that is attenuated at low irradiances but normal at high irradiances. This phenomenon has been attributed to an increase in the number of Brn3b+ M1 melanopsin ganglion cells in this mouse model, presumably in order to compensate for the loss of outer retinal photoreceptors (Jain et al., 2016). Brain targets important for regulating mood, body temperature, and feeding behavior like the ventral medial hypothalamus (VMH), the peri-supraoptic nucleus (pSON), and the perihabenular nucleus (PHb) are innervated by Brn3b+ M1 cells, suggesting alternative non-image-forming roles for this population of cells (Fernandez et al., 2018; Li & Schmidt, 2018). In fact, the influence of light on mood is largely accomplished by Brn3b+ M1 innervation of the PHb (Fernandez et al., 2018).
Brn3b+ M1 cells are also involved in inducing sleep, as they project to the ventrolateral preoptic nucleus (VLPO; Table 4; Li & Schmidt, 2018). In contrast, Brn3b− cells induce arousal from sleep, projecting to the SCN of the hypothalamus to regulate circadian photoentrainment and composing only 10% of the M1 population (Table 4; Chen et al., 2011; Jain et al., 2012; Li & Schmidt, 2018). In this respect, it appears that Brn3b+ and Brn3b− M1 cells have opposing functions. However, both Brn3b+ and Brn3b− cells send axons to the intergeniculate leaflet (IGL) and ventral lateral geniculate nucleus (vLGN) of the thalamus, two areas of the brain involved in lengthening the period of circadian rhythms (Table 4; Li & Schmidt, 2018).
Because of their intrinsic photosensitivity, melanopsin ganglion cells are also uniquely situated to influence visual system development. Interestingly, both mice without melanopsin (Opn4−/−) and dark-reared mice have substantial overgrowth of hyaloid vasculature as early as P8, indicating a role for melanopsin in blood vessel development (Rao et al., 2013). Additionally, during retinal development, waves of activity sweep across the retina to ensure correct wiring of the retina and to promote the wiring of axons to appropriate brain targets (Renna, Weng, & Berson, 2011). Light activation of melanopsin ganglion cells during this critical time period refines the formation of these connections, making them essential for accurate wiring of the visual system (Chew et al., 2017; Renna et al., 2011). Interestingly, the cells responsible for normal development of both circadian rhythm circuits and image-forming visual circuits is the rather small population of Brn3b− M1 melanopsin ganglion cells (Chew et al., 2017), revealing yet another overlap, albeit an indirect one, between melanopsin ganglion cells and image-forming vision.
A recent study has shown that the projections, and therefore also the functions, of Brn3b+ M1 cells are more diverse than previously thought, and even stretch beyond non-image-forming vision (Table 4; Figure 4; Li & Schmidt, 2018). Surprisingly, Brn3b+ M1 cells innervate the SC and the dLGN, which are both involved in image-forming visual functions like pattern vision (Brown et al., 2010; Li & Schmidt, 2018). In fact, melanopsin ganglion cells are capable of modulating the activity of neurons in the SC (Dasilva, Storchi, Davis, Grieve, & Lucas, 2016) and can regulate the baseline firing of the dLGN (Brown et al., 2010; Davis, Eleftheriou, Allen, Procyk, & Lucas, 2015; Storchi et al., 2015). While these influences have not been attributed to a particular type, the fact that M1 cells project to image-forming brain targets is an exciting area for future study. Even so, behavioral and functional studies are needed to characterize their influence on image-forming visual behaviors.
3 |. THE M2 TYPE
3.1 |. Morphology and mosaics of the M2 type
The M2 type was discovered shortly after the M1 and has vastly different characteristics. M2 cells have significantly less melanopsin protein expression than the M1, their dendrites and somas staining only faintly with typical immunohistochemistry techniques (Figure 1a) (Baver et al., 2008; Hattar et al., 2006). Whereas M1 cells express Opn4S, M2 cells express the Opn4L isoform of melanopsin (Pires et al., 2009). The somas of M2 cells are also slightly larger than those of M1 cells, with an average diameter of 18.2 ± 0.6 μm (mean ± SEM; n = 183; Tables 1 and 2; Figure 1b; Berson et al., 2010; Ecker et al., 2010; Estevez et al., 2012). The dendritic field size of M2 cells follows this trend, with an average diameter of 383.8 ± 18.0 μm, slightly surpassing that of M1 cells (mean ± SEM; n = 182; Table 2; Figure 1c; Ecker et al., 2010; Estevez et al., 2012; Schmidt & Kofuji, 2009). M2 cells also have dendritic arbors that are more complex than those of M1 cells, branching an average of 24 times (Tables 1 and 2; Figure 1d). As such, M2 cells have a morphology that is distinct from the M1 type (Ecker et al., 2010; Estevez et al., 2012; Schmidt & Kofuji, 2009).
M1 and M2 cells appear in roughly equal numbers, as there are approximately 830 M2 cells in the mouse retina (Berson et al., 2010). However, M2 cells stratify in the ON sublamina of the IPL, making them inner-stratifying melanopsin ganglion cells (Figure 2; Belenky, Smeraski, Provencio, Sollars, & Pickard, 2003; Berson et al., 2010; Ecker et al., 2010; Estevez et al., 2012; Hattar et al., 2002; Schmidt & Kofuji, 2009; Viney et al., 2007). The dendritic arbors of M2 cells overlap with those from M1 cells and are also asymmetrically distributed to the dorsal retina, where m-opsin is dominantly expressed (Figure 3; Applebury et al., 2000; Hughes et al., 2013; Stabio, Sondereker, et al., 2018).
3.2 |. Retinal connections of the M2 type
Because M2 cells stratify in the ON sublamina of the IPL, they respond to light onset without the use of the ectopic synapses utilized by M1 cells (Berson et al., 2010; Dumitrescu et al., 2009). Instead, M2 cells form a synaptic triad with Type 8 ON cone bipolar cells and inhibitory monostratified amacrine cells stratifying in the S4–5 plexus of the IPL (Viney et al., 2007; Wassle, Puller, Muller, & Haverkamp, 2009). Although M2 cells are intrinsically photosensitive (Schmidt, Taniguchi, & Kofuji, 2008), they exhibit significantly weaker melanopsin immunoreactivity than M1 cells and are roughly 10 times less sensitive to 480 nm light (Schmidt & Kofuji, 2010). With a low membrane resistance and high spiking frequency, M2 cells rely heavily on extrinsic input from rods and cones (Table 3; Figure 4) (Schmidt & Kofuji, 2009, 2010; Zhao et al., 2014).
Like M1 cells, M2 cells form gap junctions with select neighboring cells in the retina (Muller et al., 2010; Sekaran, Foster, Lucas, & Hankins, 2003; Viney et al., 2007), and can influence retinal processing. M2 cells are connected to displaced wide-field GABAergic amacrine cells via Cx30.2-mediated gap junctions, since RGA1 cells, which have been shown to be synonymous with M2 cells (Sun, Li, & He, 2002), are no longer gap junctionally coupled to other cells when Cx30.2 is knocked out (Meyer et al., 2016; Muller et al., 2010; Pan et al., 2010; Sekaran et al., 2003). While the function of this connection is not entirely clear, it likely allows M2 melanopsin ganglion cells to excite GABAergic wide-field amacrine cells, thus exerting influence over the retinal neuromodulators that are released by these interneurons (Reifler, Chervenak, Dolikian, Benenati, Li, et al., 2015a).
3.3 |. Projections and functions of the M2
M2 cells project to a variety of brain regions, revealing the potential for roles in both image-forming and non-image-forming vision (Table 4; Figure 4). Because the Opn4L isoform of melanopsin is associated with negative masking and circadian photoentrainment, it stands to reason that M2 cells may be involved in the regulation of these behaviors (Jagannath et al., 2015; Pires et al., 2009). Although innervation of the SCN is dominated by M1 cells (80%), M2 cells make up the remainder of SCN innervation and therefore may provide light information to the SCN for some level of circadian rhythm regulation, although this has not been directly shown (Table 4, Figure 4; Baver et al., 2008). In contrast, M2 cells make up the majority of OPN innervation (55%), and send axons specifically to its core (Baver et al., 2008). M2 cells also send axons to image-forming regions of the brain like the dLGN, suggesting a role for these cells in cortical visual pathways (Table 4; Figure 4; Ecker et al., 2010; Estevez et al., 2012; Sonoda & Schmidt, 2016). Although the potential exists, functional studies that specifically tease out the influence of the M2 type in image-forming visual behaviors is an area of research that can be expanded upon.
4 |. THE M3 TYPE
4.1 |. Morphology and mosaics of the M3 type
Multiple studies have documented the existence of M3 cells, but their classification as a true type has remained controversial due to the fact that they are relatively rare and do not tile the retina (Berson et al., 2010). As such, it is estimated that M3 cells make up less than 10% of the melanopsin ganglion cell population (Berson et al., 2010). Because of their rare nature, the retinal distribution and exact number of M3 cells in the retina has not been documented (Figure 3; Hughes et al., 2013). M3 melanopsin ganglion cells exhibit characteristics that are in some ways chimeric; a hybrid cross between the M1 and M2 type. The somas of M3 cells are similar in size to those of M2 cells with an average diameter of 16.3 ± 1.2 μm (mean ± SEM; n = 19; Tables 1 and 2; Figure 1b; Schmidt & Kofuji, 2011), but are located in either the GCL or the INL, much like the somas of the M1 type (Berson et al., 2010; Schmidt & Kofuji, 2011).
M3 cells are bistratified, with dendritic arbors in both the ON and OFF sublamina of the IPL (Figure 2; Berson et al., 2010; Estevez et al., 2012; Muller et al., 2010; Pu, 1999; Schmidt & Kofuji, 2009, 2011; Schmidt et al., 2008; Viney et al., 2007; Warren, Allen, Brown, & Robinson, 2003). Although most M3 cells stratify equally in both the ON and OFF sublamina, there are rare instances in which the arbors are biased to the ON portion of the IPL (Schmidt & Kofuji, 2011). The dendrites of M3 cells form branched arbors similar to those of M2 cells, but they cover a larger area of the retina with an average field diameter of 463.2 ± 27.4 μm (mean ± SEM; n = 19; Tables 1 and 2; Figure 1c; Muller et al., 2010; Schmidt & Kofuji, 2011).
4.2 |. Retinal connections of the M3 type
M3 cells exhibit an intrinsic light response to 480 nm light that is similar in amplitude to the M2 response (Table 3; Schmidt & Kofuji, 2011). Unsurprisingly, the M3 light response is dominated by its extrinsic synaptic input from the associated ON cone pathway (Table 4; Figure 4; Schmidt & Kofuji, 2011). Because M3 cells have dendrites stratifying in the OFF sublamina of the IPL, it is reasonable to assume that they might also form en passant synapses with Type 6 ON bipolar cells as M1 cells do, but this has not been definitively shown (Dumitrescu et al., 2009). Like the M1 and M2 types, M3 cells are likely gap junctionally coupled to wide-field amacrine cells that are presumably GABAergic, most likely through Cx30.2 but perhaps via a heteromeric gap junction containing both Cx30.2 and Cx45 (Gemel, Lin, Collins, Veenstra, & Beyer, 2008; Meyer et al., 2016; Schubert, Maxeiner, Kruger, Willecke, & Weiler, 2005). As such, they might exert a similar influence on retinal processing (Muller et al., 2010; Reifler, Chervenak, Dolikian, Benenati, Li, et al., 2015a).
Because M3 cells are so rare and do not tile the retina, there is some controversy as to whether they form a distinct type. Some groups have found that M3 cells display a consistent and markedly different physiology from other types, defining them as a distinct cell population (Schmidt & Kofuji, 2011). However, other studies have reported almost no notable or significant differences in physiology between the M2 and M3 types, and as such, this remains controversial (Hu et al., 2013).
4.3 |. Projections and functions of the M3 type
The sparse nature of M3 cells makes it difficult to determine their axonal brain targets (Schmidt & Kofuji, 2011). It is unclear whether M3 cells project to the SCN and OPN, but there has been some evidence that M3 cells may project to the SC (Zhao et al., 2014). Because this area of the brain is involved in image-forming vision, it is possible that the M3 type contributes to pattern vision, but the function of this type is still poorly understood (Table 4; Estevez et al., 2012). Additionally, because some studies have shown no significant differences between M2 and M3 physiologies, it is possible that the projections and functions of M3 cells parallel those of the M2 type (Hu et al., 2013).
5 |. THE M4 TYPE
5.1 |. Morphology and mosaics of the M4 type
The M4 type, which has recently been proved synonymous with the mouse ON-α sustained RGC, went long undetected as a melanopsin ganglion cell type because M4 cells exhibit very weak melanopsin immunoreactivity (Figure 1a; Berson et al., 2010; Ecker et al., 2010; Estevez et al., 2012; Schmidt et al., 2014; Sonoda, Okabe, & Schmidt, 2019). In fact, M4 melanopsin immunostaining is only detectable with amplification methods (Estevez et al., 2012), even though all ON-α RGCs are intrinsically photosensitive (Schmidt et al., 2014). Immunostaining for the marker SMI-32 can also be used to selectively detect M4 cells (Lee & Schmidt, 2018; Schmidt et al., 2014; Sonoda et al., 2019).
The somas of M4 cells are the largest of any mouse ganglion cell type, with an average of 24.2 ± 1.5 μm (mean ± SEM; n = 38; Tables 1 and 2; Figure 1b; Ecker et al., 2010; Estevez et al., 2012; Schmidt et al., 2014; Stabio, Sabbah, et al., 2018). M4 dendrites stratify in the ON sublamina of the IPL, slightly above the dendrites of M2 cells but below the ON choline acetyl transferase (ChAT) band (Figure 2; Berson et al., 2010;Ecker et al., 2010; Estevez et al., 2012; Schmidt et al., 2014). Dendritic arbors from the M4 type are also more radiate, and they branch more frequently than those of M2 cells with an average of 35 branch points Tables 1 and 2; Figure 1d) (Ecker et al., 2010; Estevez et al., 2012; Schmidt et al., 2014).
While M4 cells tile the retina and can be found in both the dorsal and ventral retina, studies of ON-α cell mosaics show they are asymmetrically distributed to the temporal-dorsal retina (Figure 3; Bleckert, Schwartz, Turner, Rieke, & Wong, 2014). Using SMI-32 staining, this finding has been recapitulated and M4 cells have been definitively equated with ON-α RGCs (Sonoda et al., 2019). As such, M4 cells in the mouse retina have a temporal to nasal dendritic field gradient, with smaller dendritic fields in the temporal retina (207.6 ± 6.9 μm; mean ± SEM; n = 18; Table 1) than in the nasal retina (371.9 ± 22.7 μm; mean ± SEM; n = 54; Table 1) (Bleckert et al., 2014; Lee & Schmidt, 2018; Schmidt et al., 2014). Other characteristics, including soma size and number of branch points, do not differ based on retinal location (Sonoda et al., 2019).
5.2 |. Retinal connections of the M4 type
Although M4 cells have a very low level of melanopsin immunoreactivity, the intrinsic melanopsin response of M4 cells differs between light-adapted and dark-adapted retinas. In light-adapted retinas, M4 cells exhibit a very weak intrinsic light response that is smaller in amplitude than those of both M1, M2, and M3 cells (Table 3; Ecker et al., 2010; Estevez et al., 2012; Hu et al., 2013; Schmidt et al., 2014). In contrast, the intrinsic response of M4 cells in dark-adapted retinas is actually fairly large and comparable to that of M1 cells (Schroeder et al., 2018; Zhao et al., 2014). Thus, during the daytime, the large and sustained ON responses of M4 cells come from extrinsic excitatory input via the ON pathway (Table 3; Figure 4; Ecker et al., 2010; Estevez et al., 2012; Schmidt et al., 2014; Schroeder et al., 2018). Additionally, the extrinsic receptive field properties of M4 cells show center-surround organization and nonlinear spatial summation (Estevez et al., 2012).
Based on studies of ON-α cells, M4 cells are presumed to receive bipolar input predominately from Type 6 ON cone bipolar cells with minor input from Type 7 (Schwartz et al., 2012). M4 cells also form connections with polyaxonal amacrine cells via Cx36-mediated gap junctions, a connection which assists in global object perception (Roy, Kumar, & Bloomfield, 2017). Interestingly, the M4 type physiology is affected by the application of melatonin, which binds to MT1 receptors on M4 cells (Pack, Hill, & Wong, 2015). As such, the M4 physiology is influenced by circadian rhythm control of melatonin expression (Pack et al., 2015), making them uniquely suited to play a role in image-forming vision without isolation from the circadian system.
5.3 |. Projections and functions of the M4 type
In the mouse, the M4 type was the first to directly challenge the dogma that melanopsin ganglion cells’ only role is in mediating non-image-forming vision. M4 cells send axons to the ventromedial sector of the dLGN, and as such are positioned to influence pattern vision functions such as contrast sensitivity, spatial acuity, and stimulus tracking (Table 4; Figure 4; Ecker et al., 2010; Estevez et al., 2012; Schmidt et al., 2014; Schroeder et al., 2018; Sonoda, Lee, Birnbaumer, & Schmidt, 2018). While M4 cells are not involved in all of these functions, they are able to discriminate contrast in the absence of functional rods and cones (Ecker et al., 2010; Sonoda et al., 2018). Similarly, when non-M1 cells are selectively ablated, mice exhibit significant loss of contrast sensitivity, further supporting a primary role for M4 cells in contrast detection (Schmidt et al., 2014).
However, other image-forming visual functions, including stimulus tracking and spatial acuity, were found to be mediated by rods and cones (Schroeder et al., 2018). Because the loss of rods and cones causes severe impairment of spatial vision that results in blindness, mice with outer retinal degeneration are often used for studying the role of melanopsin ganglion cells in both image-forming and non-image-forming vision (Foster & Hankins, 2002; Lucas, Douglas, et al., 2001; Lucas, Freedman, et al., 2001; Lucas, Freedman, Munoz, Garcia-Fernandez, & Foster, 1999; Mrosovsky & Hattar, 2003; Mrosovsky et al., 2001; Panda et al., 2003; Semo et al., 2003; Yoshimura & Ebihara, 1996). As such, outer retinal photoreceptors are clearly essential for spatial vision, but the role of M4 melanopsin ganglion cells cannot be discounted as an important component of image-forming vision, particularly for contrast detection.
Moreover, it appears that detecting contrast in environmental stimuli begins at the level of the retina with M4 melanopsin ganglion cells: The weak intrinsic melanopsin light response exhibited by light-adapted M4 cells is actually critically important to the physiology of the M4 type (Sonoda et al., 2018). The small depolarization caused by the melanopsin light response in M4 cells is sufficient to close potassium (K+) leak channels, which are partly responsible for maintaining low resting membrane potential (Sonoda et al., 2018). The closing of K+ leak channels slightly raises the membrane potential of M4 cells, making them more readily excitable (Sonoda et al., 2018). This function of melanopsin is very distinct from the function of melanopsin in the M1 type, as the melanopsin light response actually decreases excitability (Sonoda et al., 2018). This slight increase in excitability may seem negligible, but it increases the contrast sensitivity of M4 cells, and therefore has a distinct impact on contrast detection (Sonoda et al., 2018). In this way, the M4 type defies convention and uses the intrinsic light response of melanopsin to shape an image-forming visual circuit (Sonoda et al., 2018).
Surprisingly, a recent study has found that M4 cells in light-adapted retinas are also color opponent: inhibited by green light and excited by blue light (Sonoda et al., 2019). The ramifications of this are discussed in the next section, as the M5 was the first color opponent melanopsin ganglion cell type to be discovered (Stabio, Sabbah, et al., 2018).
6 |. THE M5 TYPE
6.1 |. Morphology and mosaics of the M5 type
The idea that a fifth type of melanopsin ganglion cell might exist came from the development of a more sensitive Cre-based melanopsin reporter line (Opn4Cre/+ Z/EG+/−; Ecker et al., 2010), as M5 cells have very low levels of melanopsin immunoreactivity (Figure 1a). However, it was many years until this new M5 cell was fully morphologically and physiologically characterized as a true and unique type.
M5 cells are relatively compact, with small, spherical somas that are 14.2 ± 0.4 μm in diameter (mean ± SEM; n = 47; Tables 1 and 2; Figure 1b) and a dendritic field size of 224 ± 6.6 μm (mean ± SEM; n = 47; Tables 1 and 2; Figure 1c; Ecker et al., 2010; Stabio, Sabbah, et al., 2018). Similar to M4 cells, M5 dendrites stratify in the ON sublamina of the IPL, below but proximal to the ON ChAT band (Figure 2). However, M5 dendritic arbors are much more complex and bushy than the previously discovered M1–M4 types, branching an average of 52 times (Tables 1 and 2; Figures 1d and 2; Ecker et al., 2010; Stabio, Sabbah, et al., 2018). M4 cells in the temporal retina slightly resemble this morphology, but M5s consistently have more branch points, smaller somas, and are SMI-32 negative (Sonoda et al., 2019). Like the M4 type, M5 cells tile the retina, but their retinal distribution has not been directly tested. Even so, preliminary data suggests that the M5 type is asymmetrically distributed to the ventral retina, where s-opsin is dominantly expressed (Figure 3; Hughes et al., 2013; Stabio, Sabbah, et al., 2018; Stabio, Sondereker, et al., 2018).
6.2 |. Retinal connections of the M5 type
Although M5 cells can be distinguished from other types by morphology alone, their most striking property is in the functional domain: M5 cells were the first melanopsin ganglion cell type found to exhibit center-surround chromatic opponency. The M5 cell has a dominant UV-ON center that reflects the contribution of the dedicated UVcone-selective Type 9 ON bipolar cell, but also receives input from mixed-opsin bipolars (Stabio, Sabbah, et al., 2018). The longwavelength OFF-surround appears to be mediated at least partly by a GABAergic amacrine cell that presynaptically inhibits the bipolar drive to the M5 cell (Stabio, Sabbah, et al., 2018). In the presence of synaptic blockade, M5 cells exhibit weak intrinsic light responses consistent with weak melanopsin immunostaining, which is only detectible with amplification methods (Figure 1a; Ecker et al., 2010; Stabio, Sabbah, et al., 2018). Thus, M5 cells are dominated by extrinsic synaptic input (Table 3; Figure 4; Stabio, Sabbah, et al., 2018; Zhao et al., 2014), but how the extrinsic and intrinsic responses of M5 cells are integrated to influence mouse behavior is unknown. While chromatic opponency has been documented in multiple melanopsin ganglion cell types in the primate (Dacey et al., 2005), only the M4 and M5 types exhibit color opponent physiology in the mouse (Quattrochi et al., 2019; Sonoda et al., 2019; Stabio, Sabbah, et al., 2018).
6.3 |. Projections and functions of the M5 type
M5 cells project to the dLGN and are thus well-positioned to influence the cortical visual pathway (Table 2; Figure 4; Stabio, Sabbah, et al., 2018; Zhao et al., 2014). Their projections to other brain regions remain unknown, but can be inferred by indirect evidence from other studies (Table 3) (Ecker et al., 2010; Quattrochi et al., 2019). However, if and how M4 and M5 cells contribute to the mouse’s behavioral capacity for color discrimination is still an avenue for exploration. It is possible that M4 and M5 cells might provide chromatic information to non-image-forming centers, as some neurons in the mouse SCN and OPN have been reported to have s-opsin ON and m/l-opsin OFF opponency, but this remains to be directly tested (Hayter & Brown, 2018; Walmsley et al., 2015). Moreover, neurons in image-forming brain targets like the dLGN exhibit color opponent responses, which might be the result of M4 and M5 cell inputs (Denman, Siegle, Koch, Reid, & Blanche, 2017). As such, more studies on the behavioral contribution of the M4 and M5 types to color perception are needed. Nevertheless, M5 cells are yet another type that defies the non-image-forming stereotype of melanopsin ganglion cells, adding to the growing evidence that image-forming and non-image-forming visual domains are not as distinct as once imagined (Figure 4).
7 |. THE M6 TYPE
7.1 |. Morphology and mosaics of the M6 type
The M6 is the most recently discovered type of melanopsin ganglion cell, which was characterized using the Cdh3-GFP mouse line and was also found in the OPN4Cre/+ Z/EG+/− mouse line (Quattrochi et al., 2019). Similar to M4 and M5 cells, M6 cells have very low melanopsin immunoreactivity and require amplification methods for visualization (Figure 1a; Quattrochi et al., 2019). The M6 cell resembles the M3 in some respects, as it has dendrites stratifying in both the ON and OFF sublamina of the IPL (Figure 2; Quattrochi et al., 2019). M6 cells stratify in the ON sublamina below but proximal to the ON ChAT band, and these dendrites make up a more substantial arbor (84%) than those stratifying in the OFF sublamina of the IPL, which often consists of only a few dendrites (Quattrochi et al., 2019). However, dendrites of M6 cells can also be seen diving between the ON and OFF sublamina (Quattrochi et al., 2019).
In many other respects, the M6 type more closely resembles the M5 type. Like M5 cells, M6 cells have small somas and small dendritic fields (12.7 ± 0.3 μm and 216 ± 5.1 μm, respectively; mean ± SEM; n = 34; Tables 1 and 2; Figure 1; Quattrochi et al., 2019). The dendritic arbors of M6 cells are similarly compact but more complex, branching on average 100 times, which is more than any other type (Tables 1 and 2; Figure 1d; Quattrochi et al., 2019). The branches have a more tangled and spiny appearance than those of other types often crossing over other branches or turning abruptly back toward the soma. Although most M6 cells have been documented in the ventral retina (Figure 3; Quattrochi et al., 2019), it cannot be discounted that this distribution might be a product of the Cdh3-GFP mouse line that was used, and as such the distribution of M6 cells in the retina requires further examination.
7.2 |. Retinal connections of the M6 type
The bistratified nature of the M6 type suggests that these cells might be ON–OFF cells and respond to light onset as well as light offset, but this is not the case (Quattrochi et al., 2019). Responses of M6 cells are dominated by extrinsic synaptic input from the ON pathway, with only a very weak intrinsic melanopsin response (Table 3; Figure 4; Quattrochi et al., 2019). Their extrinsic response is sustained with strong center-surround receptive field organization, and M6 dendrites are positioned to receive information from Types 6, 7, 8, and 9 ON cone bipolar cells (Quattrochi et al., 2019). As M6 cells stratify in the OFF sublamina of the IPL but do not exhibit OFF responses, it is very likely that they receive light ON information via ectopic en passant synapses on their outer-stratifying dendrites, much like the M1 type, but this has not been definitively shown (Dumitrescu et al., 2009).
7.3 |. Projections and functions of the M6 type
Because the M6 cell is the newest addition to the melanopsin family, the function of this type has not yet been determined. However, M6 axons do innervate the dLGN, indicating a possible role in image-forming vision (Table 4; Figure 4; Quattrochi et al., 2019). Some evidence suggests that M6 cells also project to the core of the OPN, the posterior pretectal nucleus (PPN), the vLGN and the IGL, and thus may also influence many non-image-forming functions. However, the projections to these brain areas might also be from M5 cells, since a minority (14%) of GFP+ cells labeled in the Cdh3-GFP mouse line were M5 cells (Table 4; Quattrochi et al., 2019). However, further studies will undoubtedly continue to clarify the potential image-forming and non-image-forming functions of this bistratified melanopsin ganglion cell.
8 |. DISCUSSION
8.1 |. Melanopsin ganglion cell types are relatively consistent across mammalian species
The importance of a heterogeneous population of melanopsin ganglion cells to mammalian vision is evidenced by the fact that many of these types have also been found in rat, human, primate, and even tree shrew (Table 5). The M1–M5 types have been found and wellcharacterized in the rat retina, and their morphologies and physiologies are consistent with those described in mouse (Reifler, Chervenak, Dolikian, Benenati, Meyers, et al., 2015b). Intrinsically photosensitive ON-α RGCs have also been found in the ground squirrel (Schmidt et al., 2014), paralleling the M4 type found in mouse, rat, and human retinas.
TABLE 5.
A summary of the known melanopsin ganglion cell types across species
Note. Shaded blocks indicate the presence of a melanopsin ganglion cell type in a particular species, and white blocks indicate the absence of evidence for a particular type in the respective species. While six types of melanopsin ganglion cells have been found in the mouse, thus far only five have been identified in the rat retina, and only the equivalents of M1 and M2 types have been found in macaque retina. Although the equivalents of M1–M4 types have been documented in human retinas, it is quite possible that further research and amplification immunohistochemistry techniques might uncover remaining types in these species.
In tree shrew retinas, the M1-like cells are the displaced, S1-stratifying, TH-positive melanopsin ganglion cells, and this unique type has not been found in other species. M3-like cells have also been found in tree shrew retinas, but they are much more common than the rare bistratified M3 in mouse, and include cells with somas in both the GCL and INL. Summarized information from the following: (Baver et al., 2008; Belenky et al., 2003; Berson et al., 2010; Berson et al., 2002; Dacey et al., 2005; Ecker et al., 2010; Estevez et al., 2012; Gamlin et al., 2007; Hannibal, Christiansen, Heegaard, Fahrenkrug, & Kiilgaard, 2017; Hannibal et al., 2014; Hattar et al., 2006; Hattar et al., 2002; Jusuf, Lee, Hannibal, & Grunert, 2007; Liao et al., 2016; Muller et al., 2010; Nasir-Ahmad, Lee, Martin, & Grunert, 2019; Pu, 1999; Quattrochi et al., 2019; Reifler, Chervenak, Dolikian, Benenati, Meyers, et al., 2015b; Schmidt et al., 2014; Schmidt & Kofuji, 2009, 2010, 2011; Schmidt et al., 2008; Sonoda et al., 2018; Stabio, Sabbah, et al., 2018; Warren et al., 2003; R. S. Young & Kimura, 2008).
Melanopsin ganglion cells have recently been identified in tree shrew retinas, with M1, M2, and M3-like morphologies (E. N. Johnson et al., 2019). Similar to the M2 type in the mouse, monostratified inner melanopsin ganglion cells in the tree shrew exhibit low levels of immunoreactivity and have somas in the GCL with dendrites in the S5 sublamina of the IPL (E. N. Johnson et al., 2019). This M2-like type composes the minority of melanopsin positive cells. Interestingly, the remaining two types of melanopsin ganglion cells in tree shrew deviate in morphology from those found in both mouse and human. In tree shrew retinas, a bistratified melanopsin ganglion cell type exists with somas in the GCL and dendrites in both the S1 and S5 layers of the IPL. This type makes up the majority of melanopsin ganglion cells with somas in the GCL in this species, which is in stark contrast to the rare bistratified M3 in mouse. Additionally, a minority of bistratified melanopsin ganglion cells have somas in the INL, and as such are termed displaced bistratified cells. But perhaps the most interesting melanopsin ganglion cell in the tree shrew is similar to displaced M1 cells in mouse and human; this type has somas in the INL and dendrites that stratify in S1 of the IPL. However, these cells are unique in that they express TH, and as such are possibly the first documented dopaminergic ganglion cells (E. N. Johnson et al., 2019).
Early classification of melanopsin ganglion cell types in humans and primates resulted in two groups: outer-stratifying and inner-stratifying cells (Dacey et al., 2005; Hannibal, Christiansen, Heegaard, Fahrenkrug, & Kiilgaard, 2017; Jusuf, Lee, Hannibal, & Grunert, 2007; Liao et al., 2016; Nasir-Ahmad, Lee, Martin, & Grunert, 2019). Outer-stratifying cells have high levels of melanopsin immunoreactivity and dendrites in the OFF sublamina of the IPL, while inner-stratifying cells have less melanopsin immunoreactivity and dendrites in the ON sublamina, paralleling the M1 and M2 type, respectively (Dacey et al., 2005; Hannibal et al., 2017; Jusuf et al., 2007; Liao et al., 2016; Nasir-Ahmad et al., 2019). Interestingly, the majority of melanopsin ganglion cells are outer-stratifying cells, and those with somas displaced to the INL make up almost half of this type’s population (Dacey et al., 2005; Esquiva, Lax, Perez-Santonja, Garcia-Fernandez, & Cuenca, 2017; Jusuf et al., 2007; Liao et al., 2016; Nasir-Ahmad et al., 2019; Ortuno-Lizaran et al., 2018). A subpopulation of gigantic M1 cells, which stratify in the OFF sublamina of the IPL but have very large somas, has also been documented in the human (Hannibal et al., 2017). Similar to the mouse retina, human retinas have M4 cells and very few M3 cells, but there is as of yet no documentation of M5 or M6 cells (Hannibal et al., 2017). This is perhaps due to the extremely low level of melanopsin immunoreactivity that is a hallmark of these types, as the studies did not utilize amplification methods for melanopsin immunostaining as has been done in the mouse.
In humans, the melanopsin ganglion cell population composes between 0.2% (Dacey et al., 2005) and 0.75% (Hannibal et al., 2017) of all RGCs. But despite their small contribution to the RGC population, melanopsin ganglion cells exert control over the pupillary light reflex and circadian rhythms in both humans and primates (Adhikari, Feigl, & Zele, 2016; Barrionuevo & Cao, 2016; Barrionuevo et al., 2014; Cao, Nicandro, & Barrionuevo, 2015; Feigl & Zele, 2014; Gamlin et al., 2007; Gooley et al., 2012; Spitschan, Jain, Brainard, & Aguirre, 2014; R. S. Young & Kimura, 2008; Zele, Feigl, Smith, & Markwell, 2011). While the evidence for human circadian rhythm regulation by melanopsin ganglion cells is less direct than in the mouse, many studies have found that loss of melanopsin ganglion cells in diseases like Parkinson’s and Alzheimer’s correlates with circadian rhythm pathology (Feng, Li, Yu, Liu, & Zhao, 2016; La Morgia et al., 2016; Lax, Ortuno-Lizaran, Maneu, Vidal-Sanz, & Cuenca, 2019; Ortuno-Lizaran et al., 2018). Blind individuals also retain the ability to suppress the production of melatonin in response to light, indicating that a type of blindness exists in which light information is still relayed to the circadian system, most likely through melanopsin ganglion cells that remain intact (Czeisler et al., 1995; Hull et al., 2018). Moreover, light also activated prefrontal and thalamic brain regions in blind patients, suggesting that this melanopsin ganglion cell response to blue light might also have effects on cognition and alertness (Vandewalle et al., 2013).
Unsurprisingly, melanopsin ganglion cells in humans and primates project to non-image-forming brain targets like the SCN and the pretectal olivary nucleus (PON) (Dacey et al., 2005; Hannibal et al., 2014). The relationship between melanopsin ganglion cells and DACs also appears to be conserved in primates and humans, allowing DACs to regulate melanopsin ganglion cell sensitivity in accordance with environmental light levels (Hannibal et al., 2017; Liao et al., 2016). Consistent with mouse melanopsin ganglion cell projections, primate and human melanopsin ganglion cells send axons to image-forming areas of the brain like the SC and the LGN, suggesting a possible role in image-forming vision (Dacey et al., 2005; Ecker et al., 2010; Hannibal et al., 2014; Hatori et al., 2008; Hattar et al., 2006; Liao et al., 2016; Zhao et al., 2014).
It is important to note that this is only indirect evidence of melanopsin ganglion cell involvement in image-forming vision. As such, a gap in knowledge still exists, and the exact effects of melanopsin ganglion cell responses in generating actual visual representations of the environment are only just beginning to be elucidated. Thus, the goal of many recent studies has been to tease out the distinct influence of melanopsin ganglion cells on image-forming functions such as contrast detection, color discrimination, the enhancement of environmental scenes, and brightness perception.
8.2 |. The influence of melanopsin ganglion cells on contrast detection and color discrimination
Some of the most compelling evidence of melanopsin ganglion cell involvement in image-forming vision was uncovered in a double knockout (Gnat−/− Cnga3−/−) mouse model, in which mice were still able to discern coarse patterns in the absence of functional rods and cones (Ecker et al., 2010). As they were presumably the only photosensitive cells remaining in the retina, this image-forming ability to crudely detect contrast was attributed to melanopsin ganglion cells. Surprisingly, a paper published a few months later found a reproducible ERG response in a triple knockout (Gnat−/− Cnga3−/− Opn4−/−) mouse model that was due to a residual functional rod pathway (Allen, Cameron, Brown, Vugler, & Lucas, 2010). Still, the contrast detection behavior seen in double knockout (Gnat−/− Cnga3−/−) mice disappeared almost completely in triple knockout (Gnat−/− Cnga3−/− Opn4−/−) mice, providing further evidence that this image-forming behavior is the result of intact melanopsin ganglion cell responses and not to the residual functional rod pathway present in the triple knockout (Gnat−/− Cnga3−/− Opn4−/−) mouse model (Ecker et al., 2010). This was later confirmed with the discovery that M4 cells are capable of detecting contrast in the absence of rods and cones (Sonoda et al., 2018) and that ablation of non-M1 melanopsin ganglion cells results in deficits in contrast sensitivity. Overall, these studies indicate an essential role for M4 cells in pattern vision, most likely via projections to the dLGN (Estevez et al., 2012; Schmidt et al., 2014).
Another large breakthrough came in the discovery of color opponent melanopsin ganglion cells first in primates (Dacey et al., 2005), and later in mice (Sonoda et al., 2019; Stabio, Sabbah, et al., 2018). Interestingly, primate melanopsin ganglion cells are inhibited by s-cones and excited by l-cones and m-cones (Dacey et al., 2005), which is opposite the sign of murine M5 cells (Sonoda et al., 2019; Stabio, Sabbah, et al., 2018). In the mouse, neurons in both image-forming (dLGN) and non-image-forming (SCN and OPN) brain targets exhibit color opponent responses, which may be the result of M4 and M5 cell inputs (Denman et al., 2017; Hayter & Brown, 2018; Walmsley et al., 2015). These studies have even demonstrated that color discrimination input to these regions of the brain is essential for normal circadian photoentrainment and pupillary light reflex. Although M4 and M5 cells project to the dLGN, no distinct behavioral tests have been done to demonstrate their impact on color discrimination, and as such, this remains to be explored.
8.3 |. dLGN-projecting melanopsin ganglion cells provide a more accurate depiction of visual stimuli
Because melanopsin ganglion cells send axonal projections to the dLGN, they have the potential to influence image-forming vision by affecting the physiology of the neurons in the dLGN. While projection to the dLGN does not guarantee a role in image-forming vision, the dLGN is a precursor to the visual cortex, and inputs that affect the physiology of the dLGN are likely important for image-forming visual behaviors (Brown et al., 2010). As such, melanopsin ganglion cells have been found to influence the image-forming visual system by providing substantial input to roughly 40% of neurons in the LGN (Brown et al., 2010). This influence can be expanded to include the encoding of spatial information, as roughly 20% of the neurons in the dLGN respond to melanopsin-specific pattern stimuli (Allen, Storchi, Martial, Bedford, & Lucas, 2017).
The loss of rods and cones causes severe impairment of spatial vision that results in blindness, and as such, mice with outer retinal degeneration can be used as a tool to study the role of melanopsin ganglion cells in both image-forming and non-image-forming vision. These types of studies have found that melanopsin ganglion cells are able to relay spatial information to the dLGN in the absence of functional rods and cones (Procyk et al., 2015). While this study did not include an analysis of which type might be responsible for this ability, current knowledge about melanopsin ganglion cell types suggest that it is likely to be non-M1-driven, as these types primarily project to the dLGN (Procyk et al., 2015). However, recent evidence of Brn3b+ M1 cells projecting to the dLGN does not eliminate them as the subpopulation responsible for this phenomenon (Li & Schmidt, 2018). This suggests that melanopsin ganglion cells could provide very low-resolution spatial vision to mice with outer retinal degeneration. But their ability to do so would be limited by the duration of their sustained ON response to light; mere kinetics would prevent accurate temporal tracking of visual stimuli (Brown et al., 2012; Procyk et al., 2015).
This does not discount the potential for melanopsin ganglion cells to facilitate brightness detection in normal, healthy retinas. By encoding irradiance, melanopsin ganglion cells are able to initiate environmental light responses through manipulation of baseline firing of the dLGN, even in the absence of rods and cones (Brown et al., 2010; Davis et al., 2015; Storchi et al., 2015). Melanopsin ganglion cells can also influence dLGN processing by using environmental light levels to adjust conepathway sensitivities, creating a more reliable depiction of visual stimuli during the day (Allen et al., 2014). Similarly, activation of melanopsin ganglion cells increases the reliability of encoding spatial patterns and contrast (Allen et al., 2017), since the knockout of melanopsin in mice results in less vibrant encoding of environmental scenes (Allen et al., 2014). While the type (or types) of melanopsin ganglion cells responsible for such influences remains undetermined, these findings indicate an indispensable melanopsin-driven impact on the depiction of visual stimuli in normal, healthy retinas. Thus, melanopsin-mediated irradiance coding, which was largely thought as a non-image-forming visual function, can be repurposed by LGN-projecting melanopsin ganglion cells to make a significant impact on image-forming vision.
8.4 |. Melanopsin ganglion cell responses contribute to brightness perception in humans
Melanopsin ganglion cell responses to melanopsin-wavelengthspecific stimuli have been shown to reach the visual cortex in humans, and as such have the potential to influence image-forming vision (Spitschan et al., 2017). Recent research has also demonstrated a direct role for melanopsin ganglion cells in human image-forming vision, even insofar as to conclude that their responses are important for any facet of vision that requires the accurate measurement of environmental brightness (Brown et al., 2010; Yamakawa, Tsujimura, & Okajima, 2019). One study found that human subjects without functional rods and cones could selectively identify the presence of 480 nm light, even describing it as a feeling of “brightness” (Zaidi et al., 2007). Another found that human subjects with intact rods and cones perceived an increase in brightness as melanopsin-specific stimuli were increased (Brown et al., 2012).
Melanopsin ganglion cells may accomplish this brightness detection by working in tandem with cone photoreceptors. In healthy retinas, melanopsin ganglion cells contribute to human vision by enhancing the brightness information that is encoded by cones (Allen, Martial, & Lucas, 2019; Brown et al., 2012; Horiguchi, Winawer, Dougherty, & Wandell, 2013; Saito, Miyamoto, Uchiyama, & Murakami, 2018; Spitschan et al., 2017; Spitschan et al., 2014; Yamakawa et al., 2019; Zele, Adhikari, Cao, & Feigl, 2019; Zele, Adhikari, et al., 2018). Specifically, responses from melanopsin ganglion cells interact with cone-mediated signals to enhance contrast sensitivity and unique white perception (Cao, Chang, & Gai, 2018; Zele, Adhikari, et al., 2019). However, even on their own, melanopsin ganglion cells in humans are capable of pattern detection and can enhance the appearance of everyday images (Allen et al., 2019).
Because of the presence of the fovea, the spatial distribution of melanopsin ganglion cells is substantially different in the human retina as compared to that of the mouse retina. There are high concentrations of melanopsin ganglion cells in the parafoveal region, but melanopsin ganglion cells are absent from the fovea itself (Esquiva et al., 2017; Hannibal et al., 2017; Lax et al., 2019; Liao et al., 2016; Nasir-Ahmad et al., 2019). While there are still a large number of melanopsin ganglion cells in the peripheral retina, they are significantly less concentrated than in the parafoveal region (Hannibal et al., 2017; Liao et al., 2016; Nasir-Ahmad et al., 2019). As such, their role in image-forming vision is different in the central retina when compared to the peripheral retina, particularly for color opponency. In the peripheral retina, melanopsin ganglion cells contribute to color perception by enhancing m- and l-cone signals and opposing s-cone responses (Cao et al., 2018; Dacey et al., 2005; Zele, Feigl, et al., 2018). Subjects have reported experiencing an enhanced “yellowness” with increased melanopsin stimulation, suggesting that melanopsin ganglion cell activation can alter color perception (Cao et al., 2018; Spitschan et al., 2017). The unique ability of melanopsin ganglion cells to report ambient light levels for long periods of time may also support cone-mediated color perception through long-term estimation of colors in the environment, thus stabilizing color perception of objects (Zele, Feigl, et al., 2018). Recent studies have even called for a revision of the trichromatic model of color vision to a tetrachromatic model in the peripheral retina, as melanopsin appears to be a significant player in color perception outside of the fovea (Cao et al., 2018; Horiguchi et al., 2013).
8.5 |. Melanopsin ganglion cell impact on both branches of the visual system continues to expand
In the early years after melanopsin was first discovered in dermal melanophores of Xenopus laevis (Provencio et al., 1998), melanopsin was almost exclusively associated with non-image-forming visual functions like circadian rhythms and the pupillary light reflex. Although melanopsin-expressing ganglion cells are critical for these non-image-forming visual functions, recent studies have shown that their reach extends far beyond non-image-forming vision in humans, primates, and rodents. Moreover, the fact that a single melanopsin ganglion cell type—Brn3b+ M1, M2, and potentially M5 and M6—sends axonal projections to both image-forming and non-image-forming brain targets indicates that the two functional realms of the visual system are much more intertwined than previously thought (Tables 4 and 6; Figure 4).
TABLE 6.
A summary of melanopsin ganglion cell type characteristics in the mouse
| Type | M1 | M2 | M3 | M4 | M5 | M6 |
|---|---|---|---|---|---|---|
| Melanopsin staining with conventional immunohistochemistry | Strong | Mid to low | Mid to low | Undetectable without amplification | Undetectable without amplification | Undetectable without amplification |
| Average soma size (μm; mean ± SEM) | 15.7 ± 0.5 | 18.2 ± 0.6 | 16.3 ± 1.2 | 24.2 ± 1.5 (n) 21.3 ± 0.7 (t) |
14.2 ± 0.4 | 12.7 ± 0.3 |
| Presence of displaced somas | Yes | No | Yes | No | No | No |
| Average dendritic field size (μm; mean ± SEM) | 347.9 ± 19.7 | 383.8 ± 18.0 | 463.2 ± 27.4 | 371.9 ± 22.7 (n) 207.6 ± 6.9 (t) |
224 ± 6.6 | 216 ± 5.1 |
| Average branch points; mean ± SEM) | 10.2 ± 1.6 | 19.4 ± 1.9 | Undetermined | 34.5 ± 2.8 (n) 30.4 ± 1.5 (t) |
52.1 ± 1.9 | 100 ± 4.6 |
| Retinal distribution Bias | Dorsal | Dorsal | Undetermined | Temporal-dorsal | Potentially ventral | Potentially ventral |
| Function (NIF, IF, both) | NIF; potentially both | NIF; potentially both | Potentially IF | IF | IF; potentially both | IF; potentially both |
Note. Values for soma size, dendritic field size, and branch points were calculated from the values in Table 1 and are mean ± SEM. NIF = non-image-forming vision; IF = image-forming vision. For the M4 column, (n) = M4 cells in the nasal retina and (t) = M4 cells in the temporal retina. Summarized information from the following: (Baver et al., 2008; Belenky et al., 2003; Berson et al., 2002; Berson et al., 2010; Brown et al., 2010; Chen et al., 2011; Ecker et al., 2010; Estevez et al., 2012; Gooley et al., 2001; Gooley et al., 2003; Guler et al., 2008; Hatori et al., 2008; Hattar et al., 2002; Hattar et al., 2006; Hu et al., 2013; Jain et al., 2012; Li & Schmidt, 2018; Muller et al., 2010; Pu, 1999; Quattrochi et al., 2019; Schmidt et al., 2008; Schmidt et al., 2014; Schmidt & Kofuji, 2009, 2010, 2011; Schroeder et al., 2018; Sollars et al., 2003; Sonoda et al., 2018; Sonoda et al., 2019; Sonoda & Schmidt, 2016; Stabio, Sabbah, et al., 2018; Viney et al., 2007; Warren et al., 2003; Wassle et al., 2009; Zhao et al., 2014).
As types of melanopsin ganglion cells continue to be characterized, the role of melanopsin ganglion cells continues to diversify, revealing additional functions in image-forming and non-image-forming vision. It is therefore increasingly necessary to acknowledge the potential influence of melanopsin ganglion cells on image-forming behaviors as their impact on both image-forming and non-image-forming vision continues to expand. Although the visual system is still divided into two functional domains, it appears that lines of communication can travel across both, resulting in an overlap of information that is complementary rather than redundant. As research of these cells continues, the evidence will undoubtedly continue to illustrate ever-expanding roles for melanopsin ganglion cells in image-forming and non-image-forming vision.
ACKNOWLEDGMENT
We would like to thank Jessica Onyak for her unwavering assistance and support.
Funding information
Karl Kirchgessner Foundation; National Eye Institute, Grant/Award Number: R15EY026255
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Adhikari P, Feigl B, & Zele AJ (2016). Rhodopsin and Melanopsin contributions to the early Redilation phase of the post-illumination pupil response (PIPR). PLoS One, 11(8), e0161175. 10.1371/journal.pone.0161175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AE, Cameron MA, Brown TM, Vugler AA, & Lucas RJ (2010). Visual responses in mice lacking critical components of all known retinal phototransduction cascades. PLoS One, 5(11), e15063. 10.1371/journal.pone.0015063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AE, Martial FP, & Lucas RJ (2019). Form vision from melanopsin in humans. Nature Communications, 10(1), 2274. 10.1038/s41467-019-10113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AE, Storchi R, Martial FP, Bedford RA, & Lucas RJ (2017). Melanopsin contributions to the representation of images in the early visual system. Current Biology, 27(11), 1623–1632 e1624. 10.1016/j.cub.2017.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AE, Storchi R, Martial FP, Petersen RS, Montemurro MA, Brown TM, & Lucas RJ (2014). Melanopsin-driven light adaptation in mouse vision. Current Biology, 24(21), 2481–2490. 10.1016/j.cub.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, & Hattar S (2010). Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nature Ne roscience, 13(9), 1107–1112. 10.1038/nn.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, & Hattar S (2008). Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proceedings of the National Academy of Sciences of the United States of America, 105(50), 19998–20003. 10.1073/pnas.0808312105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, … Robbins JT (2000). The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron, 27(3), 513–523. 10.1016/s0896-6273(00)00062-3 [DOI] [PubMed] [Google Scholar]
- Barnard AR, Hattar S, Hankins MW, & Lucas RJ (2006). Melanopsin regulates visual processing in the mouse retina. Current Biology, 16(4), 389–395. 10.1016/j.cub.2005.12.045 [DOI] [PubMed] [Google Scholar]
- Barrionuevo PA, & Cao D (2016). Luminance and chromatic signals interact differently with melanopsin activation to control the pupil light response. Journal of Vision, 16(11), 29. 10.1167/16.11.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo PA, Nicandro N, McAnany JJ, Zele AJ, Gamlin P, & Cao D (2014). Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Investigative Ophthalmology & Visual Science, 55(2), 719–727. 10.1167/iovs.13-13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ, & Pickard GE (2008). Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. The European Journal of Neuroscience, 27(7), 1763–1770. 10.1111/j.1460-9568.2008.06149.x [DOI] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, & Pickard GE (2003). Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. The Journal of Comparative Neurology, 460(3), 380–393. 10.1002/cne.10652 [DOI] [PubMed] [Google Scholar]
- Berson DM, Castrucci AM, & Provencio I (2010). Morphology and mosaics of Melanopsin-expressing retinal ganglion cell types in mice. Journal of Comparative Neurology, 518(13), 2405–2422. 10.1002/cne.22381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, & Takao M (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science, 295(5557), 1070–1073. 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- Bleckert A, Schwartz GW, Turner MH, Rieke F, & Wong RO (2014). Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Current Biology, 24(3), 310–315. 10.1016/j.cub.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, & Volgyi B (2009). The diverse functional roles and regulation of neuronal gap junctions in the retina. Nature Reviews. Neuroscience, 10(7), 495–506. 10.1038/nrn2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, … Lucas RJ (2010). Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biology, 8(12), e1000558. 10.1371/journal.pbio.1000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Tsujimura S, Allen AE, Wynne J, Bedford R, Vickery G, … Lucas RJ (2012). Melanopsin-based brightness discrimination in mice and humans. Current Biology, 22(12), 1134–1141. 10.1016/j.cub.2012.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Chang A, & Gai S (2018). Evidence for an impact of melanopsin activation on unique white perception. Journal of the Optical Society of America. A, Optics, Image Science, and Vision, 35(4), B287–B291. 10.1364/JOSAA.35.00B287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Nicandro N, & Barrionuevo PA (2015). A five-primary photostimulator suitable for studying intrinsically photosensitive retinal ganglion cell functions in humans. Journal of Vision, 15(1), 15 11 27. 10.1167/15.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Steiner R, Blattner P, Oelhafen P, Gotz T, & Cajochen C (2011). Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert? PLoS One, 6(1), e16429. 10.1371/journal.pone.0016429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, & Hattar S (2011). Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature, 476(7358), 92–95. 10.1038/nature10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew KS, Renna JM, McNeill DS, Fernandez DC, Keenan WT, Thomsen MB, … Hattar S (2017). A subset of ipRGCs regulates both maturation of the circadian clock and segregation of retinogeniculate projections in mice. eLife, 6. 10.7554/eLife.22861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, … Rizzo JF 3rd. (1995). Suppression of melatonin secretion in some blind patients by exposure to bright light. The New England Journal of Medicine, 332(1), 6–11. 10.1056/NEJM199501053320102 [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, … Gamlin PD (2005). Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature, 433(7027), 749–754. 10.1038/nature03387 [DOI] [PubMed] [Google Scholar]
- Dasilva M, Storchi R, Davis KE, Grieve KL, & Lucas RJ (2016). Melanopsin supports irradiance-driven changes in maintained activity in the superior colliculus of the mouse. The European Journal of Neuroscience, 44(6), 2314–2323. 10.1111/ejn.13336 [DOI] [PubMed] [Google Scholar]
- Davis KE, Eleftheriou CG, Allen AE, Procyk CA, & Lucas RJ (2015). Melanopsin-derived visual responses under light adapted conditions in the mouse dLGN. PLoS One, 10(3), e0123424. 10.1371/journal.pone.0123424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwig A, Majumdar S, Ahern K, LaVail MM, Edwards R, Hnasko TS, & Copenhagen DR (2013). Glutamatergic neurotrans mission from melanopsin retinal ganglion cells is required for neonatal photoaversion but not adult pupillary light reflex. PLoS One, 8(12), e83974. 10.1371/journal.pone.0083974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman DJ, Siegle JH, Koch C, Reid RC, & Blanche TJ (2017). Spatial Organization of Chromatic Pathways in the mouse dorsal lateral geniculate nucleus. The Journal of Neuroscience, 37(5), 1102–1116. 10.1523/JNEUROSCI.1742-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Coutanson C, Knoblauch K, Lahouaoui H, Leviel V, Rey C, … Cooper HM (2013). The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cellular and Molecular Life Sciences, 70(18), 3435–3447. 10.1007/s00018-013-1338-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, & Yau KW (2010). Intrinsically photosensitive retinal ganglion cells. Physiological Reviews, 90(4), 1547–1581. 10.1152/physrev.00013.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, & Menaker M (2002). Circadian rhythms of dopamine in mouse retina: The role of melatonin. Visual Neuroscience, 19(5), 593–601. 10.1017/s0952523802195058 [DOI] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, & Menaker M (2002). Circadian rhythmicity in dopamine content of mammalian retina: Role of the photoreceptors. Journal of Neurochemistry, 83(1), 211–219. 10.1046/j.1471-4159.2002.01149.x [DOI] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, & Berson DM (2009). Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: Contacts with dopaminergic amacrine cells and melanopsin ganglion cells. The Journal of Comparative Neurology, 517(2), 226–244. 10.1002/cne.22158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, … Hattar S (2010). Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron, 67(1), 49–60. 10.1016/j.neuron.2010.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel AJ, Kapur K, & Do MTH (2017). Biophysical variation within the M1 type of ganglion cell photoreceptor. Cell Reports, 21(4), 1048–1062. 10.1016/j.celrep.2017.09.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquiva G, Lax P, Perez-Santonja JJ, Garcia-Fernandez JM, & Cuenca N (2017). Loss of Melanopsin-expressing ganglion cell subtypes and dendritic degeneration in the aging human retina. Frontiers in Aging Neuroscience, 9, 79. 10.3389/fnagi.2017.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, … Berson DM (2012). Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. The Journal of Neuroscience, 32(39), 13608–13620. 10.1523/JNEUROSCI.1422-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl B, & Zele AJ (2014). Melanopsin-expressing intrinsically photosensitive retinal ganglion cells in retinal disease. Optometry and Vision Science, 91(8), 894–903. 10.1097/OPX.0000000000000284 [DOI] [PubMed] [Google Scholar]
- Feng R, Li L, Yu H, Liu M, & Zhao W (2016). Melanopsin retinal ganglion cell loss and circadian dysfunction in Alzheimer’s disease (review). Molecular Medicine Reports, 13(4), 3397–3400. 10.3892/mmr.2016.4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, … Hattar S (2018). Light affects mood and learning through distinct retina-brain pathways. Cell, 175(1), 71–84 e18. 10.1016/j.cell.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk AS, Tam SKE, Brown LA, Vyazovskiy VV, Bannerman DM, & Peirson SN (2018). Light and cognition: Roles for circadian rhythms, sleep, and arousal. Frontiers in Neurology, 9, 56. 10.3389/fneur.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, & Hankins MW (2002). Non-rod, non-cone photoreception in the vertebrates. Progress in Retinal and Eye Research, 21(6), 507–527. [DOI] [PubMed] [Google Scholar]
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, & Menaker M (1991). Circadian photoreception in the retinally degenerate mouse (rd/rd). Journal of Comparative Physiology. A, 169(1), 39–50. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, & Foster R (1999). Regulation of mammalian circadian behavior by non-rod, non-cone, Ocular Photoreceptors. Science, 284 (5413), 502–504. 10.1126/science.284.5413.502 [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, & Dacey DM (2007). Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Research, 47(7), 946–954. 10.1016/j.visres.2006.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemel J, Lin X, Collins R, Veenstra RD, & Beyer EC (2008). Cx30.2 can form heteromeric gap junction channels with other cardiac connexins. Biochemical and Biophysical Research Communications, 369(2), 388–394. 10.1016/j.bbrc.2008.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Ho Mien I, St Hilaire MA, Yeo SC, Chua EC, van Reen E, … Lockley SW (2012). Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. The Journal of Neuroscience, 32(41), 14242–14253. 10.1523/JNEUROSCI.1321-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, & Saper CB (2001). Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neuroscience, 4(12), 1165. 10.1038/nn768 [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, & Saper CB (2003). A broad role for melanopsin in nonvisual photoreception. The Journal of Neuroscience, 23(18), 7093–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, & Morin LP (2008). Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One, 3(9), e3153. 10.1371/journal.pone.0003153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, … Hattar S (2008). Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature, 453(7191), 102–105. 10.1038/nature06829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Christiansen AT, Heegaard S, Fahrenkrug J, & Kiilgaard JF (2017). Melanopsin expressing human retinal ganglion cells: Subtypes, distribution, and intraretinal connectivity. The Journal of Comparative Neurology, 525(8), 1934–1961. 10.1002/cne.24181 [DOI] [PubMed] [Google Scholar]
- Hannibal J, & Fahrenkrug J (2002). Melanopsin: A novel photopigment involved in the photoentrainment of the brain’s biological clock? Annals of Medicine, 34(5), 401–407. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Ostergaard J, Georg B, Heegaard S, Larsen PJ, & Fahrenkrug J (2004). Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Investigative Ophthalmology & Visual Science, 45 (11), 4202–4209. 10.1167/iovs.04-0313 [DOI] [PubMed] [Google Scholar]
- Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D, & Gamlin PD (2014). Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. The Journal ofComparative Neurology, 522(10), 2231–2248. 10.1002/cne.23588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, & Maywood ES (2003). A clockwork web: Circadian timing in brain and periphery, in health and disease. Nature Reviews. Neuroscience, 4(8), 649–661. 10.1038/nrn1177 [DOI] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, … Panda S (2008). Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One, 3(6), e2451. 10.1371/journal.pone.0002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, & Berson DM (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. The Journal of Comparative Neurology, 497(3), 326–349. 10.1002/cne.20970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, & Yau KW (2002). Melanopsin-containing retinal ganglion cells: Architecture, projections And intrinsic photosensitivity. Science, 295(5557), 1065–1070. 10.1126/science.1069609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayter EA, & Brown TM (2018). Additive contributions of melanopsin and both cone types provide broadband sensitivity to mouse pupil control. BMC Biology, 16(1), 83. 10.1186/s12915-018-0552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi H, Winawer J, Dougherty RF, & Wandell BA (2013). Human trichromacy revisited. Proceedings of the National Academy of Sciences of the United States of America, 110(3), E260–E269. 10.1073/pnas.1214240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Hill DD, & Wong KY (2013). Intrinsic physiological properties of the five types of mouse ganglion-cell photoreceptors. Journal of Neurophysiology, 109(7), 1876–1889. 10.1152/jn.00579.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Watson TS, Foster RG, Peirson SN, & Hankins MW (2013). Nonuniform distribution and spectral tuning of photosensitive retinal ganglion cells of the mouse retina. Current Biology, 23(17), 1696–1701. 10.1016/j.cub.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JT, Czeisler CA, & Lockley SW (2018). Suppression of melatonin secretion in totally visually blind people by ocular exposure to white light: Clinical characteristics. Ophthalmology, 125(8), 1160–1171. 10.1016/j.ophtha.2018.01.036 [DOI] [PubMed] [Google Scholar]
- Jagannath A, Hughes S, Abdelgany A, Pothecary CA, Di Pretoro S, Pires SS, … Peirson SN (2015). Isoforms of Melanopsin mediate different behavioral responses to light. Current Biology, 25(18), 2430–2434. 10.1016/j.cub.2015.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V, Ravindran E, & Dhingra NK (2012). Differential expression of Brn3 transcription factors in intrinsically photosensitive retinal ganglion cells in mouse. The Journal of Comparative Neurology, 520(4), 742–755. 10.1002/cne.22765 [DOI] [PubMed] [Google Scholar]
- Jain V, Srivastava I, Palchaudhuri S, Goel M, Sinha-Mahapatra SK, & Dhingra NK (2016). Classical photoreceptors are primarily responsible for the pupillary light reflex in mouse. PLoS One, 11(6), e0157226. 10.1371/journal.pone.0157226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EN, Westbrook T, Shayesteh R, Chen EL, Schumacher JW, Fitzpatrick D, & Field GD (2019). Distribution and diversity of intrinsically photosensitive retinal ganglion cells in tree shrew. The Journal of Comparative Neurology, 527(1), 328–344. 10.1002/cne.24377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T, … Copenhagen DR (2010). Melanopsin-dependent light avoidance in neonatal mice. Proceedings of the National Academy of Sciences of the United States of America, 107(40), 17374–17378. 10.1073/pnas.1008533107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HR, Peterson BB, Dacey DM, Hattar S, & Chen SK (2013). Recurrent axon collaterals of intrinsically photosensitive retinal ganglion cells. Visual Neuroscience, 30(4), 175–182. 10.1017/S0952523813000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Lee SC, Hannibal J, & Grunert U (2007). Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. The European Journal of Neuroscience, 26(10), 2906–2921. 10.1111/j.1460-9568.2007.05924.x [DOI] [PubMed] [Google Scholar]
- Keeler C (1927). Iris movements in blind mice. American Journal of Physiology, 81(1), 107–112. [Google Scholar]
- Ko CH, & Takahashi JS (2006). Molecular components of the mammalian circadian clock. Human Molecular Genetics, 15(2), R271–R277. 10.1093/hmg/ddl207 [DOI] [PubMed] [Google Scholar]
- La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, … Carelli V (2016). Melanopsin retinal ganglion cell loss in Alzheimer disease. Annals of Neurology, 79(1), 90–109. 10.1002/ana.24548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, … Lucas RJ (2010). Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron, 66(3), 417–428. 10.1016/j.neuron.2010.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax P, Ortuno-Lizaran I, Maneu V, Vidal-Sanz M, & Cuenca N (2019). Photosensitive melanopsin-containing retinal ganglion cells in health and disease: Implications for circadian rhythms. International Journal of Molecular Sciences, 20(13), 3164. 10.3390/ijms20133164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzerini Ospri L, Prusky G, & Hattar S (2017). Mood, the circadian system, and Melanopsin retinal ganglion cells. Annual Review of Neuroscience, 40, 539–556. 10.1146/annurev-neuro-072116031324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, & Schmidt TM (2018). Morphological identification of Melanopsin-expressing retinal ganglion cell subtypes in mice. Methods in Molecular Biology, 1753, 275–287. 10.1007/978-14939-7720-8_19 [DOI] [PubMed] [Google Scholar]
- Lee SK, Sonoda T, & Schmidt TM (2019). M1 intrinsically photosensitive retinal ganglion cells integrate rod and Melanopsin inputs to signal in low light. Cell Reports, 29(11), 3349–3355 e3342. 10.1016/j.celrep.2019.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, … Hattar S (2012). Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature, 491(7425), 594–598. 10.1038/nature11673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, & Hattar S (2014). Light as a central modulator of circadian rhythms, sleep and affect. Nature Reviews. Neuroscience, 15(7), 443–454. 10.1038/nrn3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, & Schmidt TM (2018). Divergent projection patterns of M1 ipRGC subtypes. The Journal of Comparative Neurology, 526(13), 2010–2018. 10.1002/cne.24469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HW, Ren X, Peterson BB, Marshak DW, Yau KW, Gamlin PD, & Dacey DM (2016). Melanopsin-expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. The Journal of Comparative Neurology, 524(14), 2845–2872. 10.1002/cne.23995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, & Gooley JJ (2006). Circadian photoreception: Spotlight on the brain. Current Biology, 16(18), R795–R797. 10.1016/j.cub.2006.08.039 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, & Foster RG (2001). Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience, 4(6), 621–626. 10.1038/88443 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Lupi D, Munoz M, David-Gray ZK, & Foster RG (2001). Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice. Behavioural Brain Research, 125(1–2), 97–102. 10.1016/s0166-4328(01)00274-1 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, & Foster RG (1999). Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science, 284(5413), 505–507. 10.1126/science.284.5413.505 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, & Yau KW (2003). Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science, 299(5604), 245–247. 10.1126/science.1077293 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, … Brainard GC (2014). Measuring and using light in the melanopsin age. Trends in Neurosciences, 37(1), 1–9. 10.1016/j.tins.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, & Foster RG (2008). The acute lightinduction of sleep is mediated by OPN4-based photoreception. Nature Neuroscience, 11(9), 1068–1073. 10.1038/nn.2179 [DOI] [PubMed] [Google Scholar]
- Meyer A, Tetenborg S, Greb H, Segelken J, Dorgau B, Weiler R, … Dedek K (2016). Connexin30.2: in vitro interaction with Connexin36 in HeLa cells and expression in AII Amacrine cells and intrinsically photosensitive ganglion cells in the mouse retina. Frontiers in Molecular Neuroscience, 9, 36. 10.3389/fnmol.2016.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, & Lenn NJ (1972). A retinohypothalamic projection in the rat. The Journal of Comparative Neurology, 146(1), 1–14. 10.1002/cne.901460102 [DOI] [PubMed] [Google Scholar]
- Morin LP (2015). A path to sleep is through the eye. eNeuro, 2(2). 10.1523/ENEURO.0069-14.2015ENEURO.0069-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N, & Hattar S (2003). Impaired masking responses to light in melanopsin-knockout mice. Chronobiology International, 20(6), 989–999. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Lucas RJ, & Foster RG (2001). Persistence of masking responses to light in mice lacking rods and cones. Journal of Biological Rhythms, 16(6), 585–588. 10.1177/074873001129002277 [DOI] [PubMed] [Google Scholar]
- Muindi F, Zeitzer JM, Colas D, & Heller HC (2013). The acute effects of light on murine sleep during the dark phase: Importance of melanopsin for maintenance of light-induced sleep. The European Journal of Neuroscience, 37(11), 1727–1736. 10.1111/ejn.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller LP, Do MT, Yau KW, He S, & Baldridge WH (2010). Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. The Journal of Comparative Neurology, 518(23), 4813–4824. 10.1002/cne.22490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir-Ahmad S, Lee SCS, Martin PR, & Grunert U (2019). Melanopsin-expressing ganglion cells in human retina: Morphology, distribution, and synaptic connections. The Journal of Comparative Neurology, 527(1), 312–327. 10.1002/cne.24176 [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, & Iuvone PM (2000). Diurnal metabolism of dopamine in the mouse retina. Brain Research, 870(1–2), 118–125. 10.1016/s0006-8993(00)02409-4 [DOI] [PubMed] [Google Scholar]
- Noseda R, Copenhagen D, & Burstein R (2018). Current understanding of photophobia, visual networks and headaches. Cephalalgia, 39(13), 1623–1634. 10.1177/0333102418784750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, & Burstein R (2010). A neural mechanism for exacerbation of headache by light. Nature Neuroscience, 13(2), 239–245. 10.1038/nn.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuno-Lizaran I, Esquiva G, Beach TG, Serrano GE, Adler CH, Lax P, & Cuenca N (2018). Degeneration of human photosensitive retinal ganglion cells may explain sleep and circadian rhythms disorders in Parkinson’s disease. Acta Neuropathologica Communications, 6 (1), 90. 10.1186/s40478-018-0596-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack W, Hill DD, & Wong KY (2015). Melatonin modulates M4-type ganglion-cell photoreceptors. Neuroscience, 303, 178–188. 10.1016/j.neuroscience.2015.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Paul DL, Bloomfield SA, & Volgyi B (2010). Connexin36 is required for gap junctional coupling of most ganglion cell subtypes in the mouse retina. The Journal of Comparative Neurology, 518(6), 911–927. 10.1002/cne.22254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, … Hogenesch JB (2003). Melanopsin is required for non-image-forming photic responses in blind mice. Science, 301(5632), 525–527. 10.1126/science.1086179 [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, … Kay SA (2002). Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science, 298(5601), 2213–2216. 10.1126/science.1076848 [DOI] [PubMed] [Google Scholar]
- Pires SS, Hughes S, Turton M, Melyan Z, Peirson SN, Zheng L, … Halford S (2009). Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. The Journal of Neuroscience, 29(39), 12332–12342. 10.1523/JNEUROSCI.2036-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, … Zhang DQ (2016). M1 ipRGCs influence visual function through retrograde signaling in the retina. The Journal of Neuroscience, 36(27), 7184–7197. 10.1523/JNEUROSCI.3500-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyk CA, Eleftheriou CG, Storchi R, Allen AE, Milosavljevic N, Brown TM, & Lucas RJ (2015). Spatial receptive fields in the retina and dorsal lateral geniculate nucleus of mice lacking rods and cones. Journal of Neurophysiology, 114(2), 1321–1330. 10.1152/jn.00368.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, & Rollag MD (1998). Melanopsin: An opsin in melanophores, brain, and eye. Proceedings of the National Academy of Sciences of the United States of America, 95(1), 340–345. 10.1073/pnas.95.1.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, & Rollag MD (2000). A novel human opsin in the inner retina. The Journal of Neuroscience, 20(2), 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, & Castrucci AM (2002). Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature, 415(6871), 493. 10.1038/415493a [DOI] [PubMed] [Google Scholar]
- Pu M (1999). Dendritic morphology of cat retinal ganglion cells projecting to suprachiasmatic nucleus. The Journal of Comparative Neurology, 414 (2), 267–274. [PubMed] [Google Scholar]
- Quattrochi LE, Stabio ME, Kim I, Ilardi MC, Michelle Fogerson P, Leyrer ML, & Berson DM (2019). The M6 cell: A small-field bistratified photosensitive retinal ganglion cell. Journal of Comparative Neurology, 527(1), 297–311. 10.1002/cne.24556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, & Menaker M (1990). Transplanted suprachiasmatic nucleus determines circadian period. Science, 247(4945), 975–978. 10.1126/science.2305266 [DOI] [PubMed] [Google Scholar]
- Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, … Lang RA (2013). A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature, 494(7436), 243–246. 10.1038/nature11823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Li BY, Wachter RD, … Wong KY (2015a). All spiking, sustained ON displaced Amacrine cells receive gap-junction input from Melanopsin ganglion cells. Current Biology, 25(21), 2878. 10.1016/j.cub.2015.10.039 [DOI] [PubMed] [Google Scholar]
- Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Meyers BS, Demertzis ZD, … Wong KY (2015b). The rat retina has five types of ganglion-cell photoreceptors. Experimental Eye Research, 130, 17–28. 10.1016/j.exer.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna JM, Chellappa DK, Ross CL, Stabio ME, & Berson DM (2015). Melanopsin ganglion cells extend dendrites into the outer retina during early postnatal development. Developmental Neurobiology, 75(9), 935–946. 10.1002/dneu.22260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna JM, Weng S, & Berson DM (2011). Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nature Neuroscience, 14(7), 827–829. 10.1038/nn.2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, & Weaver DR (2001). Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology, 63, 647–676. 10.1146/annurev.physiol.63.1.647 [DOI] [PubMed] [Google Scholar]
- Roy K, Kumar S, & Bloomfield SA (2017). Gap junctional coupling between retinal amacrine and ganglion cells underlies coherent activity integral to global object perception. Proceedings of the National Academy of Sciences of the United States of America, 114(48), E10484–E10493. 10.1073/pnas.1708261114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, & O’Hara BF (2002). Role of melanopsin in circadian responses to light. Science, 298(5601), 2211–2213. 10.1126/science.1076701 [DOI] [PubMed] [Google Scholar]
- Rupp AC, Ren M, Altimus CM, Fernandez DC, Richardson M, Turek F, … Schmidt TM (2019). Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. eLife, 8. 10.7554/eLife.44358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Miyamoto K, Uchiyama Y, & Murakami I (2018). Invisible light inside the natural blind spot alters brightness at a remote location. Scientific Reports, 8(1), 7540. 10.1038/s41598-01825920-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, Prusky GT, & Hattar S (2014). A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron, 82(4), 781–788. 10.1016/j.neuron.2014.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, & Hattar S (2011). Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends in Neurosciences, 34(11), 572–580. 10.1016/j.tins.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, & Kofuji P (2009). Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. The Journal of Neuroscience, 29(2), 476–482. 10.1523/JNEUROSCI.4117-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, & Kofuji P (2010). Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. The Journal of Neuroscience, 30(48), 16262–16271. 10.1523/JNEUROSCI.3656-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, & Kofuji P (2011). Structure and function of Bistratified intrinsically photosensitive retinal ganglion cells in the mouse. Journal of Comparative Neurology, 519(8), 1492–1504. 10.1002/cne.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Taniguchi K, & Kofuji P (2008). Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. Journal of Neurophysiology, 100(1), 371–384. 10.1152/jn.00062.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MM, Harrison KR, Jaeckel ER, Berger HN, Zhao X, Flannery MP, … Wong KY (2018). The roles of rods, cones, and Melanopsin in Photoresponses of M4 intrinsically photosensitive retinal ganglion cells (ipRGCs) and Optokinetic visual behavior. Frontiers in Cellular Neuroscience, 12, 203. 10.3389/fncel.2018.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Maxeiner S, Kruger O, Willecke K, & Weiler R (2005). Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. The Journal of Comparative Neurology, 490(1), 29–39. 10.1002/cne.20621 [DOI] [PubMed] [Google Scholar]
- Schwartz GW, Okawa H, Dunn FA, Morgan JL, Kerschensteiner D, Wong RO, & Rieke F (2012). The spatial structure of a nonlinear receptive field. Nature Neuroscience, 15(11), 1572–1580. 10.1038/nn.3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Foster RG, Lucas RJ, & Hankins MW (2003). Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Current Biology, 13(15), 1290–1298. 10.1016/s0960-9822(03)00510-4 [DOI] [PubMed] [Google Scholar]
- Semo M, Peirson S, Lupi D, Lucas RJ, Jeffery G, & Foster RG (2003). Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. The European Journal of Neuroscience, 17(9), 1793–1801. 10.1046/j.1460-9568.2003.02616.x [DOI] [PubMed] [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, & Pickard GE (2003). Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Visual Neuroscience, 20(6), 601–610. 10.1017/s0952523803206027 [DOI] [PubMed] [Google Scholar]
- Sondereker KB, Onyak JR, Islam SW, Ross CL, & Renna JM (2017). Melanopsin ganglion cell outer retinal dendrites: Morphologically distinct and asymmetrically distributed in the mouse retina. The Journal of Comparative Neurology, 525(17), 3653–3665. 10.1002/cne.24293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda T, Lee SK, Birnbaumer L, & Schmidt TM (2018). Melanopsin Phototransduction is repurposed by ipRGC subtypes to shape the function of distinct visual circuits. Neuron, 99(4), 754–767 e754. 10.1016/j.neuron.2018.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda T, Okabe Y, & Schmidt TM (2019). Overlapping morphological and functional properties between M4 and M5 intrinsically photosensitive retinal ganglion cells. The Journal of Comparative Neurology. 10.1002/cne.24806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda T, & Schmidt TM (2016). Re-evaluating the role of intrinsically photosensitive retinal ganglion cells: New roles in image-forming functions. Integrative and Comparative Biology, 56(5), 834–841. 10.1093/icb/icw066 [DOI] [PubMed] [Google Scholar]
- Spitschan M, Bock AS, Ryan J, Frazzetta G, Brainard DH, & Aguirre GK (2017). The human visual cortex response to melanopsin-directed stimulation is accompanied by a distinct perceptual experience. Proceedings of the National Academy of Sciences of the United States of America, 114(46), 12291–12296. 10.1073/pnas.1711522114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitschan M, Jain S, Brainard DH, & Aguirre GK (2014). Opponent melanopsin and S-cone signals in the human pupillary light response. Proceedings of the National Academy of Sciences of the United States of America, 111(43), 15568–15572. 10.1073/pnas.1400942111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabio ME, Sabbah S, Quattrochi LE, Ilardi MC, Fogerson PM, Leyrer ML, … Berson DM (2018). The M5 cell: A color-opponent intrinsically photosensitive retinal ganglion cell. Neuron, 97(1), 251. 10.1016/j.neuron.2017.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabio ME, Sondereker KB, Haghgou SD, Day BL, Chidsey B, Sabbah S, & Renna JM (2018). A novel map of the mouse eye for orienting retinal topography in anatomical space. The Journal of Comparative Neurology, 526(11), 1749–1759. 10.1002/cne.24446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, & Weitz CJ (2007). Physiological importance of a circadian clock outside the suprachiasmatic nucleus. Cold Spring Harbor Symposia on Quantitative Biology, 72, 307–318. 10.1101/sqb.2007.72.053 [DOI] [PubMed] [Google Scholar]
- Storchi R, Milosavljevic N, Eleftheriou CG, Martial FP, Orlowska-Feuer P, Bedford RA, … Lucas RJ (2015). Melanopsin-driven increases in maintained activity enhance thalamic visual response reliability across a simulated dawn. Proceedings of the National Academy of Sciences of the United States of America, 112(42), E5734–E5743. 10.1073/pnas.1505274112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li N, & He S (2002). Large-scale morphological survey of mouse retinal ganglion cells. The Journal of Comparative Neurology, 451(2), 115–126. 10.1002/cne.10323 [DOI] [PubMed] [Google Scholar]
- Tam SK, Hasan S, Hughes S, Hankins MW, Foster RG, Bannerman DM, & Peirson SN (2016). Modulation of recognition memory performance by light requires both melanopsin and classical photoreceptors. Proceedings of the Biological Sciences, 283(1845), 20162275. 10.1098/rspb.2016.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, … Bourgin P (2009). Melanopsin as a sleep modulator: Circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biology, 7(6), e1000125. 10.1371/journal.pbio.1000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufford AR, Onyak JR, Sondereker KB, Lucas JA, Earley AM, Mattar P, … Cayouette M (2018). Melanopsin retinal ganglion cells regulate cone photoreceptor lamination in the mouse retina. Cell Reports, 23(8), 2416–2428. 10.1016/j.celrep.2018.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente-Soriano FJ, Garcia-Ayuso D, Ortin-Martinez A, Jimenez-Lopez M, Galindo-Romero C, Villegas-Perez MP, … Vidal-Sanz M (2014). Distribution of melanopsin positive neurons in pigmented and albino mice: Evidence for melanopsin interneurons in the mouse retina. Frontiers in Neuroanatomy, 8, 131. 10.3389/fnana.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hook MJ, Wong KY, & Berson DM (2012). Dopaminergic modulation of ganglion-cell photoreceptors in rat. The European Journal of Neuroscience, 35(4), 507–518. 10.1111/j.1460-9568.2011.07975.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle G, Collignon O, Hull JT, Daneault V, Albouy G, Lepore F, … Carrier J (2013). Blue light stimulates cognitive brain activity in visually blind individuals. Journal of Cognitive Neuroscience, 25(12), 2072–2085. 10.1162/jocn_a_00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, … Roska B (2007). Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Current Biology, 17 (11), 981–988. 10.1016/j.cub.2007.04.058 [DOI] [PubMed] [Google Scholar]
- Vugler AA, Redgrave P, Hewson-Stoate NJ, Greenwood J, & Coffey PJ (2007). Constant illumination causes spatially discrete dopamine depletion in the normal and degenerate retina. Journal of Chemical Neuroanatomy, 33(1), 9–22. 10.1016/j.jchemneu.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Walmsley L, Hanna L, Mouland J, Martial F, West A, Smedley AR, … Brown TM (2015). Colour as a signal for entraining the mammalian circadian clock. PLoS Biology, 13(4), e1002127. 10.1371/journal.pbio.1002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, & Robinson DW (2003). Intrinsic light responses of retinal ganglion cells projecting to the circadian system. The European Journal of Neuroscience, 17(9), 1727–1735. 10.1046/j.1460-9568.2003.02594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Puller C, Muller F, & Haverkamp S (2009). Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. The Journal of Neuroscience, 29(1), 106–117. 10.1523/JNEUROSCI.4442-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S, Estevez ME, & Berson DM (2013). Mouse ganglion-cell photoreceptors are driven by the most sensitive rod pathway and by both types of cones. PLoS One, 8(6), e66480. 10.1371/journal.pone.0066480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY (2012). A retinal ganglion cell that can signal irradiance continuously for 10 hours. The Journal of Neuroscience, 32(33), 11478–11485. 10.1523/JNEUROSCI.1423-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Graham DM, & Berson DM (2007). Synaptic influences on rat ganglion-cell photoreceptors. The Journal of Physiology, 582(1), 279–296. 10.1113/jphysiol.2007.133751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa M, Tsujimura SI, & Okajima K (2019). A quantitative analysis of the contribution of melanopsin to brightness perception. Scientific Reports, 9(1), 7568. 10.1038/s41598-019-44035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, & Ebihara S (1996). Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+)mice. Journal of Comparative Physiology. A, 178(6), 797–802. [DOI] [PubMed] [Google Scholar]
- Young MJ, & Lund RD (1994). The anatomical substrates subserving the pupillary light reflex in rats: Origin of the consensual pupillary response. Neuroscience, 62(2), 481–496. 10.1016/0306-4522(94)90381-6 [DOI] [PubMed] [Google Scholar]
- Young RS, & Kimura E (2008). Pupillary correlates of light-evoked melanopsin activity in humans. Vision Research, 48(7), 862–871. 10.1016/j.visres.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama J, Doi M, Borrelli E, & Sassone-Corsi P (2006). Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proceedings of the National Academy of Sciences of the United States of America, 103(16), 6386–6391. 10.1073/pnas.0510691103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, … Lockley SW (2007). Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Current Biology, 17(24), 2122–2128. 10.1016/j.cub.2007.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zele AJ, Adhikari P, Cao D, & Feigl B (2019). Melanopsin driven enhancement of cone-mediated visual processing. Vision Research, 160, 72–81. 10.1016/j.visres.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Zele AJ, Adhikari P, Feigl B, & Cao D (2018). Cone and melanopsin contributions to human brightness estimation. Journal of the Optical Society of America. A, Optics, Image Science, and Vision, 35(4), B19–B25. 10.1364/JOSAA.35.000B19 [DOI] [PubMed] [Google Scholar]
- Zele AJ, Feigl B, Adhikari P, Maynard ML, & Cao D (2018). Melanopsin photoreception contributes to human visual detection, temporal and colour processing. Scientific Reports, 8(1), 3842. 10.1038/s41598-018-22197-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zele AJ, Feigl B, Smith SS, & Markwell EL (2011). The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One, 6(3), e17860. 10.1371/journal.pone.0017860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, & McMahon DG (2012). Melanopsin mediates retrograde visual signaling in the retina. PLoS One, 7(8), e42647. 10.1371/journal.pone.0042647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, & McMahon DG (2008). Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences of the United States of America, 105(37), 14181–14186. 10.1073/pnas.0803893105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang H, Wu S, Liu Q, & Wang N (2017). Regulation of Reentrainment function is dependent on a certain minimal number of intact functional ipRGCs in rd mice. Journal of Ophthalmology, 2017, 1–8. 10.1155/2017/6804853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Stafford BK, Godin AL, King WM, & Wong KY (2014). Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. The Journal of Physiology, 592(7), 1619–1636. 10.1113/jphysiol.2013.262782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wong KY, & Zhang DQ (2017). Mapping physiological inputs from multiple photoreceptor systems to dopaminergic amacrine cells in the mouse retina. Scientific Reports, 7(1), 7920. 10.1038/s41598-017-08172-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


