Abstract
Agricultural development, extensive industrialization, and rapid growth of the global population have inadvertently been accompanied by environmental pollution. Water pollution is exacerbated by the decreasing ability of traditional treatment methods to comply with tightening environmental standards. This review provides a comprehensive description of the principles and applications of electrochemical methods for water purification, ion separations, and energy conversion. Electrochemical methods have attractive features such as compact size, chemical selectivity, broad applicability, and reduced generation of secondary waste. Perhaps the greatest advantage of electrochemical methods, however, is that they remove contaminants directly from the water, while other technologies extract the water from the contaminants, which enables efficient removal of trace pollutants. The review begins with an overview of conventional electrochemical methods, which drive chemical or physical transformations via Faradaic reactions at electrodes, and proceeds to a detailed examination of the two primary mechanisms by which contaminants are separated in nondestructive electrochemical processes, namely electrokinetics and electrosorption. In these sections, special attention is given to emerging methods, such as shock electrodialysis and Faradaic electrosorption. Given the importance of generating clean, renewable energy, which may sometimes be combined with water purification, the review also discusses inverse methods of electrochemical energy conversion based on reverse electrosorption, electrowetting, and electrokinetic phenomena. The review concludes with a discussion of technology comparisons, remaining challenges, and potential innovations for the field such as process intensification and technoeconomic optimization.
1. Introduction
1.1. Conventional Methods of Water Purification
It is estimated that four billion people live in localities which are, for at least one month of the year, under conditions of severe water scarcity.1,2 One increasingly common method used to secure supplies of potable water is desalination, and so the development of desalination systems that are energy and infrastructure efficient is a critical technological challenge.3 In the most general sense, desalination is a process that is used to remove ions, colloidal particles, chemical compounds, and organic matter, referred to hereafter by the single term contaminants, from saline water. Existing methods for desalination can be broadly categorized into physical methods and chemical methods.3
Physical methods include distillation,4−6 freezing (or freeze–thaw) desalination,7,8 (liquid-phase) solvent extraction,9 membrane processes,10−12 solar desalination,13−15 and wave-powered desalination.16 Distillation, a process which appears to have been used by early experimentalists of the classical era such as Aristotle,17 involves separation of water from contaminants across the interface between a gas and a liquid by selective boiling and condensation. Modern implementations of this method include multi-stage flash distillation, multiple effect distillation, vapor compression, and humidification dehumidification, all of which are in essence a sequence of countercurrent heat exchangers.5,18−24 In a similar way to distillation, freezing desalination also uses a phase change (freezing and melting) to separate water from contaminants.25,26 Solvent extraction is used to separate contaminants based on their relative solubilities in two immiscible liquids, normally water (polar) and an organic solvent (nonpolar), where transport is driven by gradients in the chemical potential of the contaminants.27 Membrane processes are diverse in that the kind of (semipermeable) membrane used can be tuned based on the target contaminant from which the water is to be removed. These processes include microfiltration, ultrafiltration, nanofiltration, reverse osmosis (RO), and forward osmosis (FO), and they are distinct primarily in the pore sizes of the corresponding membrane.11,28−38 During operation, water is driven across a membrane by an input of mechanical work (or by a gradient in osmotic pressure in the case of FO) to retain the contaminants in a concentrated brine. Like distillation, solar desalination is said to have been employed by humans for thousands of years, originally by Greek mariners and Persian alchemists.39,40 The basis of this technology is not distinct from distillation or membrane processes; it is simply a means to generate the energy that these methods require: that is, heat for distillation or electricity for membrane processes. Wave-powered desalination is similar in principle to solar desalination methods in that it generates electricity (by the motion of submerged buoys in this case) to run a desalination process based on RO.16 Although these technologies are mature and are cost-effective for the desalination of seawater (as well as other concentrated solutions), they are inherently inefficient and energy intensive when used to treat dilute feeds or to selectively remove target contaminants from a concentrated feed, as explained in the following section. It is in these situations that selectively removing trace amounts of a desired species is preferable to indiscriminately concentrating all dissolved species in a brine.
Chemical methods, which tend to be selective in molecular separations, can be classified into two major types: the first, discussed in this section, involves no electrochemistry (i.e., the established chemical methods), and the second, introduced in sections 1.3 and 2, is based on electrokinetic and electrochemical phenomena. Established chemical methods include precipitation,41 coagulation flocculation,42 adsorption,43,44 ultraviolet, ozone, and chlorine disinfection,45 aeration,46 and ion exchange.47,48 Precipitation involves the creation of a solid (the precipitate) from a solution using a chemical referred to as the precipitant.49,50 Similarly, coagulation flocculation involves the addition of compounds (typically metallic salts) that promote the clumping of fines into larger floc, which can be readily separated from water by sedimentation, filtration, or flotation.51,52 Adsorption is a physicochemical phenomenon that is used to remove contaminants by binding them to the surfaces of an adsorbent material.44,53 Disinfection technologies in general are used to kill bacteria, viruses, and other disease-causing pathogens present in water. The most common disinfection treatments are based on ultraviolet radiation, chlorination, and ozonation, all of which inactivate the waterborne pathogen by disrupting its cellular functions.45 Aeration of water is achieved by passing air through the liquid and is typically used to remove iron or organic matter, dispel certain dissolved gases, or oxidize dissolved or suspended compounds.54,55 Ion exchange represents a broad class of processes where ions are exchanged between an electrolyte solution and a solid ion exchanger, such as polymeric resin, chelating agents, zeolites, clay, and montmorillonite.47,48,56−62 Ion exchange is in general a reversible process, where the ion exchanger is regenerated using a wash solution. Altogether, these traditional chemical methods are based on either chemical reactivity (precipitation, adsorption, and chelation), affinity for charged or functionalized surfaces (coagulation flocculation and ion exchange), or susceptibility to oxidative degradation (disinfection and aeration) of the contaminants.
In practice, a water treatment process often combines several of the methods described above to improve the quality of water and make it suitable for a specific end use. Figure 1 shows the process diagram of a representative municipal water treatment facility, in which the water treated contains high levels of hardness and iron.63 As described in ref (63), raw water is taken from wells and sent to an aerator, where contact with air removes volatile solutes (e.g., H2S, CO2, CH4) and odorous substances (e.g., CH3SH, bacterial metabolites). Contact with oxygen further promotes iron removal by oxidizing soluble Fe(II) to insoluble Fe(III). After aeration, lime is added (as CaO or Ca(OH)2) to raise the pH and cause the precipitation of Ca2+ and Mg2+. Precipitates of these hardness ions settle from the water in the primary basin, and much of the remaining solid material is suspended and requires the addition of coagulants (e.g., Fe(III), Al2(SO4)3) to settle. Activated silica or synthetic polyelectrolytes (e.g., poly(sodium styrenesulfonate), poly(acrylic acid)) may also be added to induce coagulation or flocculation. Settling of colloidal particles occurs in the secondary basin after the addition of carbon dioxide to lower the pH. Sludge from both basins is then pumped to a lagoon, and the water is finally chlorinated, filtered, and pumped to the water mains.
Figure 1.
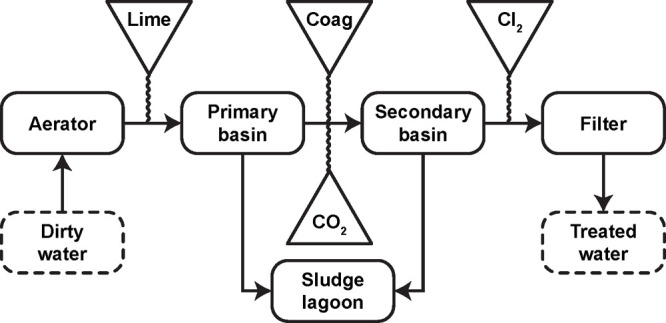
Process diagram of a representative municipal water treatment facility. This process combines several of the methods described in section 1.1 to improve the quality of water and make it suitable for domestic use. Adapted with permission from ref (63). Copyright 2001 CRC.
1.2. Limitations of Conventional Methods
Almost all of the methods introduced in section 1.1 have seen commercial success for a range of applications across numerous industries. Each of these methods, however, has applications and operating constraints outside of which the use of an alternative process would be more practical. For example, thermal distillation has historically been the dominant means of seawater desalination, but the most prevalent technology used today in large-scale desalination plants is RO because of its high energy efficiency and small footprint.33,64 This technology works by pumping the feed at pressures above the osmotic pressure of the solution through a membrane permeable only to water molecules (3–5 MPa for seawater).12 RO has been optimized over several decades of development for the desalination of concentrated feeds like seawater,38,65 and modern seawater RO (SWRO) plants currently require under 3 kW h m–3 of energy when including all pre- and post-treatment steps.66−69 While RO is the best available solution for city-scale seawater desalination, RO systems demonstrate poor scaling of energy demand with decreasing feed concentration, as demonstrated in Figure 2.70 For example, brackish water RO (BWRO) plants require nearly the same energy input as SWRO plants (1–3 kW h m–3), despite the fact that brackish water is less concentrated than seawater by about an order of magnitude.71 According to the van’t Hoff equation, osmotic pressure is linearly proportional to concentration for dilute solutions, which suggests that the energy demand for BWRO should be about an order of magnitude lower than for SWRO. Friction losses in RO systems, however, do not scale with salt concentration, but rather with the amount of water transported across the membrane. This intrinsic feature of RO explains the relatively high losses and poor energy efficiency of BWRO compared to SWRO.72 Because desalination of brackish water is a promising solution for water scarcity, and because removal of trace contaminants from dilute feeds is an important capability, technologies whose energy demand scales with feed concentration would be more desirable than RO for these applications.
Figure 2.
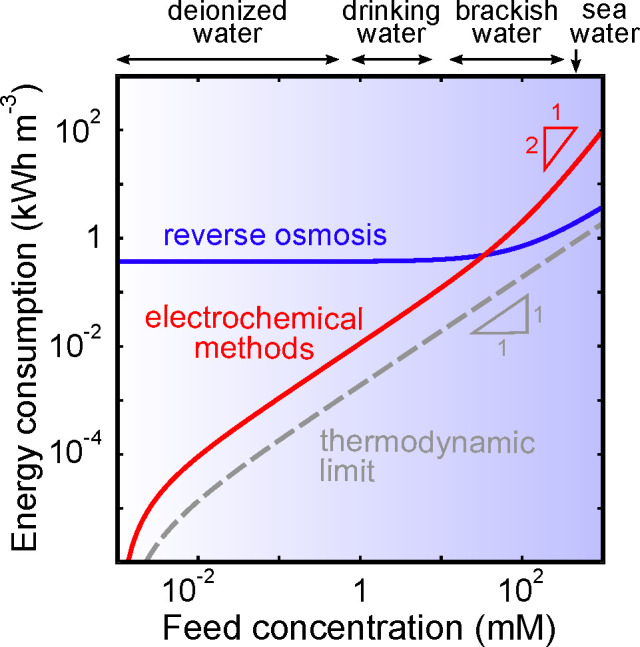
Estimates of
the volumetric energy consumed by RO and a generic
electrochemical process based on the analysis in section 6.1, specifically eqs 17 and 18. These estimates assume that the feed is desalinated to a
final concentration of 1 μM; here, γ = 0.5 (water
recovery, defined as the fraction of the feed recovered as permeate)
and 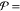 10 L h–1 m–2 (productivity). Energies are compared
to the thermodynamic limit, represented by the dashed curve, and are
reported in units of kW h m–3 of diluate.
10 L h–1 m–2 (productivity). Energies are compared
to the thermodynamic limit, represented by the dashed curve, and are
reported in units of kW h m–3 of diluate.
Another notable drawback of RO plants is that they require large capital expenditures and mature infrastructure, which limits their utility for small-scale applications or in remote locations.66 Moreover, it is difficult to downscale RO systems because high-pressure pumps and resilient plumbing are required at any scale to pressurize the feed in excess of the osmotic pressure. Facile downscaling to smaller, less-expensive plants that can be introduced into small residential areas and communities would help water treatment technologies further penetrate the market. Inexpensive, small-scale plants may in fact be the most appropriate solution for developing and off-grid locations, where water scarcity is severe and commonplace, and where infrastructure may be underdeveloped or nonexistent. Small-scale portable desalination units are also in demand by industrial facilities, by mobile military units and vessels, in recreational spaces, and in the travel industry.73,74 To meet these growing and diverse needs for purified water, the technological focus should extend beyond RO by including scalable systems with lower energy demands and more flexible infrastructure requirements for treatment of brackish water and dilute feeds.
Many of the drawbacks of RO, particularly when used to treat dilute feeds, can be overcome by using the physicochemical methods introduced in section 1.1. These methods, however, have their own limitations and often require nonreusable chemicals. Solvent extraction can be both efficient and cost-effective in separating hazardous contaminants from benign feeds, but this process requires large volumes of organic extractants and sometimes toxic solvents, and the entrainment of phases yields low-quality effluents.75,76 Precipitation is another simple and cost-effective process that is commonly used to remove toxic heavy metals from water, but it produces large amounts of sediment and sludge that is often difficult and expensive to dispose of. Precipitation is also ineffective at removing ions that are present at low concentration, and its utility may be limited when the water is contaminated with multiple metals.76,77 Coagulation flocculation is often employed after precipitation to remove solid particulates from water, and this method could also be used to capture larger particles and inactivate biological agents.78,79 The applicability of this method is limited, however, because it requires nonreusable inorganic coagulants which are usually toxic.76,80 Ion exchange, on the other hand, offers a wider range of simple and well-established commercial products, many of which can be regenerated for repeated use.76,77,81 The performance of ion exchange resins and chelating agents, however, is sensitive to variations in pH, and some of these agents react with dissolved metal ions to form soluble metal complexes that lead to secondary pollution.76,77,82
1.3. Emerging Electrochemical Methods
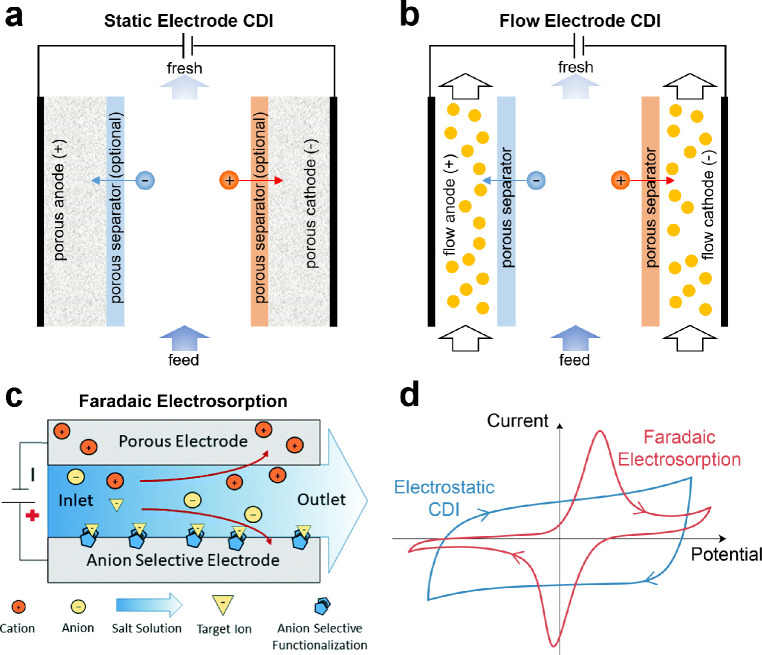
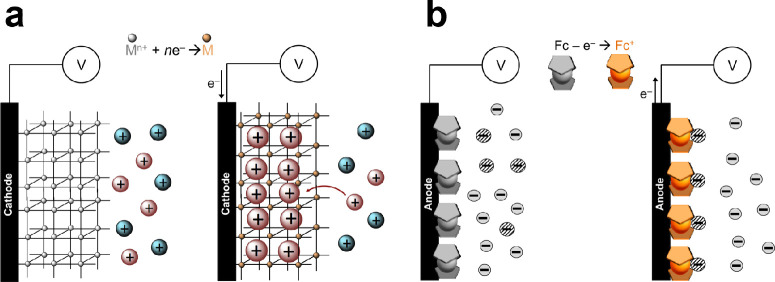
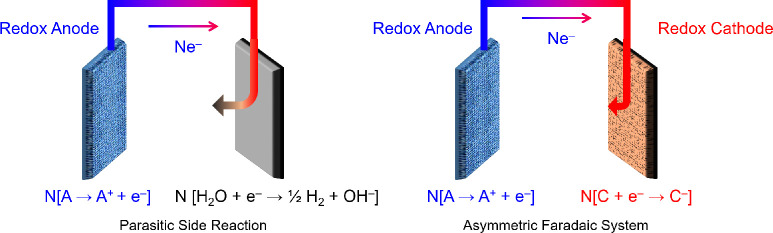
A variety of innovative techniques based either on electrokinetics or electrosorption have been proposed for water purification and ion separations, and these techniques have given rise to emerging electrochemical methods.83−91 The recent discovery of deionization shock waves in microchannels92,93 and porous media,87,94,95 for instance, inspired a new area of research in electrokinetic methods for deionization. Parallel developments in materials science have uncovered a wealth of novel electrode chemistries, where the electrosorption of ions is promoted by Faradaic reactions, to replace carbon, conventionally the material of choice in capacitive systems.86,96 These innovations have not only enhanced deionization capacity but have also imparted molecular selectivity to the electrodes. Recent examples of Faradaic platforms for water purification are based on electrochemical reduction of target contaminants,97,98 electrochemical switching of ion exchange,99−101 and molecularly selective removal of ions,86,96,102 uncharged compounds,103,104 and biomolecules (e.g., proteins).105 Many of these advances have relied on Faradaic compounds with immobilized surfaces to achieve superior electrochemical performance and chemical specificity. By modulating the binding interactions at the surfaces, the affinity of the electrodes can be tuned specifically for minority components in a feed, which in practice may be either highly valuable or seriously toxic.
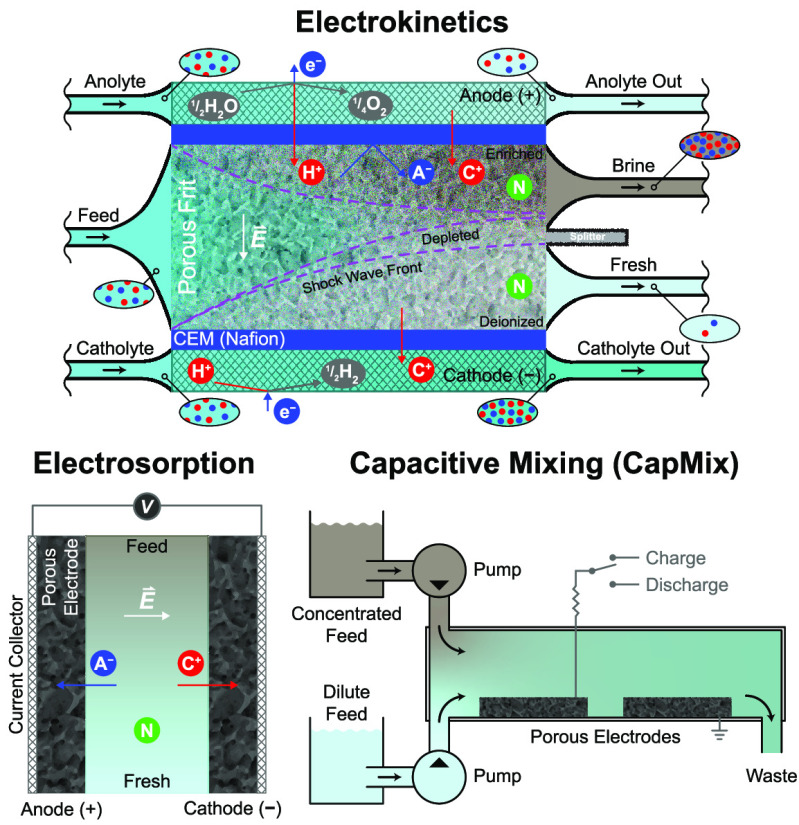
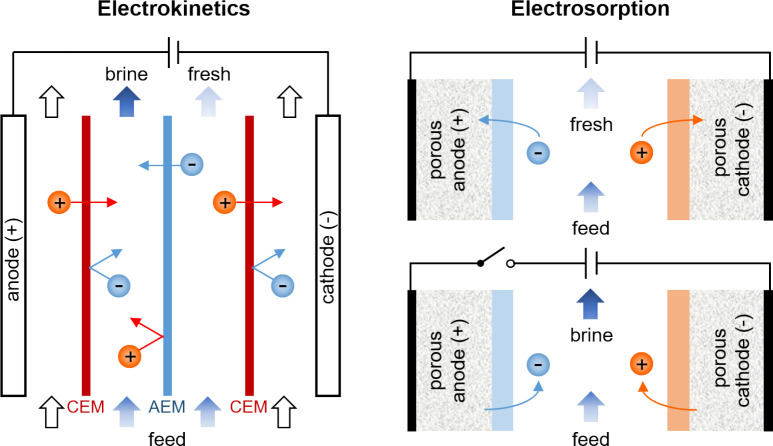
Emerging electrochemical methods include electrodeionization (EDI, sometimes called hybrid ion exchange ED),61,106−108 shock electrodialysis (shock ED),87,109 capacitive deionization (CDI),110,111 battery deionization (BDI),112,113 and Faradaic electrosorption.86,96 These technologies are unique from all of the others discussed so far in that removal of contaminants is based on their response to electric fields in solution or electrochemical reactions at electrodes. Electrochemical systems use applied electrical currents to remove contaminants from the feed by either driving separations in bulk electrolytes,61,114 electrochemically trapping them in electric double layers (EDLs),90,110,115 or intercalating them in solid electrodes (e.g., materials composed of two-dimensional, layered structures).116−118 The first of these mechanisms is governed by electrokinetics, and the second and third are forms of electrosorption, as explained in Figure 3. The primary input of energy to these systems is an applied electric potential, which makes these processes scalable without the need to be operated at extreme temperatures or pressures. Energy dissipation in electrochemical systems, however, arises from three general sources:357,1173 (i) ohmic resistance, due to hydrodynamic drag acting on moving ions in electrolytes or membranes,72 as well as electronic resistance in porous electrodes and current collectors; (ii) Faradaic reaction resistance at electrode–electrolyte interfaces, leading to activation overpotential; and (iii) concentration polarization, associated with limitations in ion diffusion. These losses all scale with the number of ions removed rather than the amount of solution process, as shown in Figure 2. These systems therefore tend to be more energy efficient compared to physical methods (and are molecularly selective) when used to treat brackish water and dilute feeds.
Figure 3.
Electrokinetics and electrosorption are the two main mechanisms by which contaminants are separated in any nondestructive electrochemical process. Electrokinetic processes, which are typically continuous, involve transport of charged or uncharged but dielectric119,120 species in an electrolyte in response to an applied electric field, and so removal of contaminants relies on effective mass transfer. Electrokinetic methods like EDI and shock ED use porous materials in the feed channels to increase ion removal and improve energy efficiency when the feed is dilute. Electrosorption processes are cyclic and encompass all phenomena in which the binding of contaminants is aided by an applied electric field. In addition to effective transport, electrosorption relies on favorable reaction kinetics and thermodynamics.
Among the existing electrochemical methods, electrodialysis (ED) has been studied and used for water desalination for decades, and several ED desalination plants for treatment of brackish water are currently operational in the U.S.114,121−125 The past decade, however, has seen the emergence of several novel electrochemical systems for water purification with unique functionalities and working principles compared to ED, and these are EDI,126 shock ED,87 CDI,115,127,128 and Faradaic electrosorption.86 This review summarizes the development of these novel technologies to form the basis for the emerging field of electrochemical systems for water purification and ion separations. For completeness, we also briefly discuss related microfluidic technologies, which have been reviewed in detail elsewhere.129,130 Microfluidic systems, which are driven by electrical energy, have throughputs at the scale of nanoliters and may be difficult to scale up to the volumes needed for deployment for human consumption, agriculture, or industry. Electrochemical systems instead are based on cells and stacks made of components like ion exchange membranes (IEMs), porous dielectric separators, and porous electrodes that can be produced in large areas as flat sheets or films, and thus these systems naturally have clearer pathways for scale up.
1.4. Outline of This Review
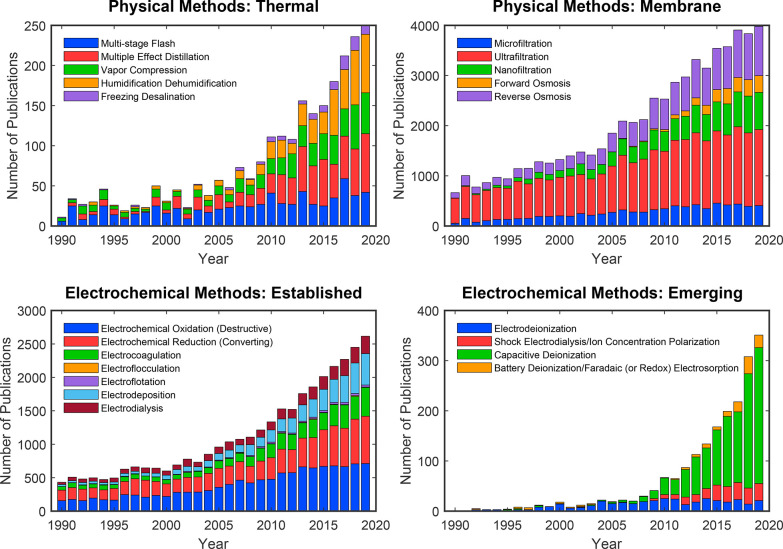
Conventional electrochemical methods, which exploit Faradaic electron transfer reactions at electrodes to drive chemical or physical transformations, have been previously reviewed, particularly in their use for removal of organic matter,131 organic compounds,132−137 inorganic contaminants,138−142 and microorganisms143−145 as well as for degradation of various contaminants and micropollutants.123,146−149 The technologies on which many of these publications focus are well established and currently used in industry. We begin the review by introducing these established electrochemical transformation methods and the broad range of applications for which they are used (section 2). We then build on the existing literature and related reviews68,105,115,118,123,126,150−155 by emphasizing the principles and applications of emerging electrochemical methods for desalination, water purification, and ion separations (sections 3 and 4). As shown in Figure 4, several of these emerging methods have developed only in the last 10 years and have been reviewed either briefly or independently of other methods. To provide a foundation for our discussion, we examine recent developments in electrokinetic phenomena and electrosorption for water purification and selective ion separations. See refs (76) and (118) for tables that summarize the advantages and disadvantages of techniques used for water purification, including many of the emerging methods discussed here.
Figure 4.
Number of publications per year for various methods used for water purification or ion separations. The technologies considered here are broadly classified as either physical (top row) or electrochemical (bottom row). This review focuses on electrochemical methods, and special attention is given to the emerging methods, including shock ED, CDI, and Faradaic electrosorption. The literature search was performed using Elsevier’s Scopus database with the search field TITLE-ABS(···).
In section 3, we explain (nondestructive) methods based on electrokinetics, which may either include or exclude membranes. Two methods in the class of membrane-based systems are ED and EDI, where the membrane plays a critical role in the removal of charged species. Ions can be separated from the bulk fluid directly, however, by virtue of the electrokinetic phenomena, without much contribution from the membrane itself. Key developments have also been made in the class of “semimembraneless” systems (i.e., systems in which the feed is partitioned into fresh and brine streams across a deionization shock wave that functions as a virtual interface), which include microfluidic (or nanofluidic) concentration polarization and shock ED. These methods are both based on the phenomenon of concentration polarization, which arises due to extreme gradients in the concentration of ions in solution. In section 4, we describe electrosorption, the phenomenon that is responsible for sorption in electrochemical systems, and how this process is used for desalination and molecular separations. (These separations are often nondestructive, but in certain cases, they may involve electrochemical conversion or degradation of the contaminants.) This explanation is followed by an overview of CDI techniques as well as recent innovations in Faradaic (or redox-active) materials and their broad use in chemical and environmental processes. With these methods explained, we introduce inverse methods of energy conversion that convert gradients in salinity to energy (section 5). We conclude by discussing the energetics, thermodynamic and technological challenges, and prospects of electrochemical methods for water purification and ion separations (sections 6 and 7).
2. Electrochemical Transformations
In the general areas of water purification and wastewater treatment, a variety of electrochemical processes have been developed to remove contaminants ranging from ions to colloidal particles. For example, chemical coagulation flocculation, flotation, precipitation, and redox (reduction and oxidation) can be improved by applying electric fields.156−158 In this section, we discuss nonelectrosorptive electrochemical methods that are well established and reviewed extensively in the literature, namely electrochemical oxidation, electrochemical reduction, electrocoagulation, electroflotation, and electrodeposition.98,146,156−160 These processes involve Faradaic reactions at electrodes to drive chemical or physical transformations of ionic or molecular solutes. For example, electrochemical redox reactions are used primarily when the objective is to degrade or convert nonbiodegradable organic contaminants and certain inorganic compounds (e.g., cyanides, thiocyanates, sulfides) and disinfect water. In section 3, we examine both traditional and emerging electrokinetic methods based on ED, which rely on coupled transport phenomena in electrolyte solutions. We then discuss in section 4 electrosorption systems (both capacitive and Faradaic) for selective ion removal based on various functional materials, including porous carbon, inorganic crystals, and polymers.90,115
2.1. Electrochemical Oxidation
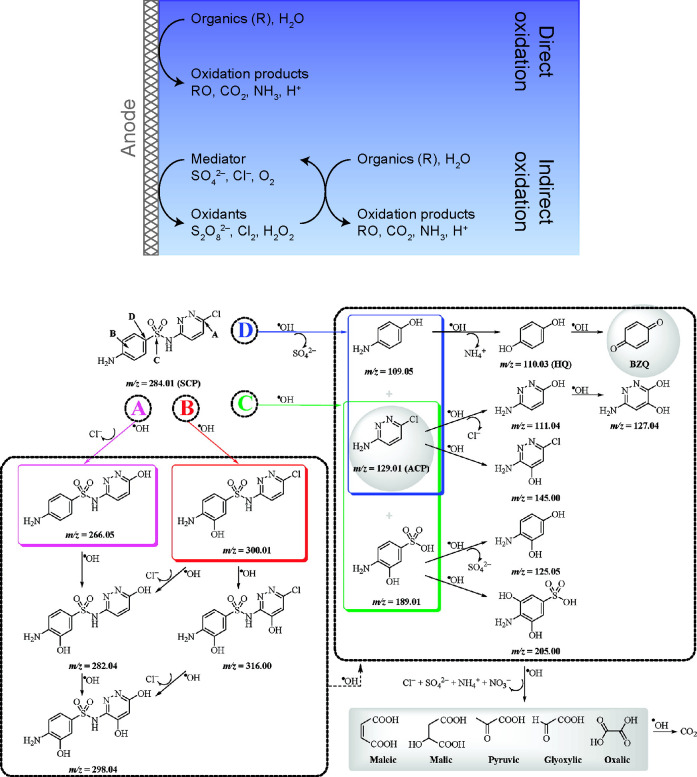
Electrochemical oxidation is a chemical reaction involving the loss of one or more electrons by an atom or a molecule at the anode when an electrical current is passed through the system.146,161,162 In the context of water treatment, electrochemical oxidation generates reactive oxidizing agents called free radicals that interact with the contaminants and degrade them, as explained in Figure 5.123 Superoxide (O2•–), hydroperoxyl (HO2•), hydroxyl (HO•), and sulfate (SO4•–) radicals are examples of reactive agents that can degrade organic and organometallic contaminants by initiating a radical oxidation chain (see refs (123), (146), and (163−167) for lists of common reaction pathways by which these radicals are formed).136,168−170 Superoxide and hydroxyl are two of the most important radicals in free-radical chemistry, and they are both believed to be key species in oxidative processes.163,164,171 While superoxide is normally a nucleophile and reducing agent,164,172 it exists in equilibrium with the hydroperoxyl radical, which can behave as an oxidizing agent in various biological and chemical reactions.173−177 (In general, superoxide is a weak reducing agent, but in the presence of solids or cosolvents that are less polar than water (e.g., H2O2), superoxide becomes reactive and can degrade halogenated aliphatic compounds,178,179 including perfluorocarboxylic acids,180,181 via nucleophilic attacks.182) The hydroxyl radical oxidizes both organic and inorganic compounds with high reaction rates, such that its action occurs only in the region where it is produced.183 The degradation process begins with formation of the reactive oxidizer, followed by initial attacks on target molecules and their breakdown into biodegradable intermediates. Subsequent attacks on these intermediates by the oxidizer can lead to their mineralization (i.e., production of water, carbon dioxide, and inorganic salts), as shown in Figure 5.123,146 Another class of oxidizers is obtained by the oxidation of chloride ions to generate active chlorine (Cl2), which may disproportionate to hypochloric acid (HClO) or hypochlorite (ClO–) depending on the pH. Although these species can effectively oxidize various contaminants in real wastewaters (e.g., landfill leachates, textile effluents, olive oil wastewater, tannery wastewater),97 this approach has the drawback of producing chlorinated organic compounds during the electrolysis, which is the main limitation of electrochemical oxidation.97,184 These chlorinated byproducts increase the toxicity of the effluent because they tend to be much more persistent than what is initially present in the feed.185−187 In the absence of chloride electrolytes, however, electrochemical oxidation can be reliably used for disinfection, wastewater treatment, groundwater treatment, soil remediation, wastewater sludge conditioning, and odor and taste removal.146,188−190
Figure 5.
Electrochemical oxidation is an established destructive, nonelectrosorptive method that is used to degrade most organic contaminants and certain inorganic compounds. (top) The method involves formation of reactive oxidizers that interact with contaminants either at the anode surface (direct oxidation) or in the bulk (indirect, or mediated, oxidation).123 Reproduced with permission from ref (191). Copyright 2017 Royal Society of Chemistry. (bottom) Complex organic compounds, such as the sulfonamide antibiotic sulfachloropyridazine, are degraded and mineralized by the attack of reactive oxidizers at key reaction sites (designated by the letters A–D). In this example, hydroxyl radicals can attack four unique sites on sulfachloropyridazine to yield different primary cyclic byproducts, all of which are eventually transformed into CO2. Reproduced with permission from ref (192). Copyright 2012 American Chemical Society.
In the 1990s, researchers became increasingly aware that the anode material is an especially important consideration in the design and optimization of electrochemical oxidation processes.193−196 (The cathodes are usually stainless steel plates, platinum meshes, or carbon felt electrodes.) The results obtained by several groups indeed demonstrated that the choice of anode influences the selectivity and efficiency of organic compound oxidation.165,197 According to a model proposed by Comninellis,194 anode materials are divided into active anodes (e.g., carbon, platinum, iridium oxides, ruthenium oxides) and nonactive anodes (e.g., antimony-doped tin oxide, lead dioxide, boron-doped diamond).97 Active anodes have low oxygen evolution overpotential and are good electrocatalysts for oxygen evolution, while nonactive anodes have high oxygen evolution overpotential and are poor electrocatalysts for oxygen evolution. Anodes based on boron-doped diamond (BDD) have received considerable attention due to their chemical stability, high electrical conductivity, resistance to corrosion even in harsh environments, and wide window of electric potential.198 As a result, BDD is generally viewed as one of the most effective and energy efficient anodes for mineralization of organic contaminants, although its use in practice is limited due to high manufacturing costs.198 Moreover, BDD (as well as other anodes) promotes the oxidation of chloride to chlorate (ClO3–) and perchlorate (ClO4), which are water-soluble disinfection byproducts that are exceedingly mobile in aqueous solutions and are highly persistent under typical water conditions.97,146 Because disinfection byproducts in drinking water are usually regulated, their concentrations should be monitored and controlled when performing in-line electrolysis.
Other prominent anodes include dimensionally stable anodes (DSAs, also called mixed metal oxide electrodes) and substoichiometric titanium oxide anodes.97,199,200 DSAs are fabricated by coating a substrate such as titanium with several kinds of metal oxides, including RuO2, IrO2, and PtO2. These anodes exhibit high conductivity and corrosion resistance, and recent studies showed that doping DSAs with metal and nonmetal elements can further improve their performance.201−204 Moreover, the use of nanotechnology has gained traction in the field of electrode fabrication to increase the porosity and active surface area of the anodes.205,206 Anodes based on substoichiometric titanium oxide (TinO2n–1) also display high conductivity and corrosion resistance, and their many advantages and long service life have led to their broad use in fuel cells, lead-acid batteries, and, most recently, wastewater treatment.207−210 Ganiyu et al. prepared a Magnéli-phase Ti4O7 electrode by plasma deposition and compared it to DSA and BDD anodes for the degradation of the beta-blocker propranolol211 and the analgesic paracetamol.212 These studies showed that the Ti4O7 electrode can achieve similar or better removal of organic carbon compared to DSAs and BDD. Several methods have been reported in the literature to improve the performance of substoichiometric titanium oxide electrodes, and these methods include plasma spraying,213 which produces doped functional coatings, and high-temperature sintering, which produces an electrode with extensively interconnected macropores.210
Most anodes used in electrochemical oxidation produce highly active hydroxyl radicals on their surfaces,214 and treatment of wastewater with these materials requires adequate flow of the contaminants toward them. When the concentration of pollutants near the anode is low, the process rate is limited by mass transfer of these species to the surface of the electrode.97 Common methods to overcome this limitation include gas sparging,215 incorporation of turbulence promoters,97 and use of nanoengineered materials.216−218 Most importantly, the efficiency of electrochemical oxidation can be improved with the indirect (or mediated) oxidation method, which avoids the production of oxygen by generating precursors that are transformed to active oxidizers. Persulfate (S2O82–), percarbonate (C2O6), and hydrogen peroxide (H2O2) are examples of precursors that can be produced using BDD anodes.219−221 These species are relatively stable at ambient conditions and generate highly active inorganic radicals that enable mediated oxidation of organic contaminants.97 Michaud et al. experimentally tested the production of precursors using BDD and observed that persulfate is produced with high current efficiency when the electrolyte is concentrated in sulfate (SO42–) and the process temperature is low.219 The persulfate precursor is activated to generate sulfate radicals, and this step requires a transition metal catalyst or sufficient energy.222 Activated persulfate can then oxidize organic compounds, and this approach has been used to treat groundwater and soils contaminated with biorefractory organic species.97
In recent years, there have been significant advances in the design, synthesis, and use of nanostructured electrodes for electrochemical oxidation.218 Nanoengineered materials exhibit new and improved properties, such as an increase in the number of active sites and an improvement in electrical conductivity, and these materials can promote heterogeneous catalysis at electrode surfaces.218 According to Du et al., a wide range of nanostructured cathodes have been reported in literature, and they can be divided into four categories: cathodes based on carbon nanomaterials such as carbon nanotubes (CNTs) and graphene,223,224 carbon cathodes doped with heteroatoms such as fluorine and nitrogen,225,226 metals or metal oxides deposited on carbon,227−229 and metal oxide cathodes.230,231 In most studies, these electrodes are used for electrosynthesis of H2O2 via oxygen reduction, and the structural morphology and composition of functional groups largely affect cathode performance.224,232,233 Du et al. also explain that there exists a large variety of nanostructured anodes, which can be divided into four categories similar to those of the nanostructured cathodes: anodes based on carbon nanomaterials such as CNTs and nanostructured BDD,234−237 anodes doped with heteroatoms such as fluorine and boron,238,239 metals or metal oxides deposited on carbon,240−242 and metal or metal oxide anodes.243−246 Most articles published on nanostructured electrodes report improvements in the kinetics of HO• production and pollutant oxidation due to the synergistic effects of greater stability, electrical conductivity, electrochemical reactivity, and active-site exposure.218 Even though nanoengineering has enabled major advances in improving electrode stability, more efforts are needed to demonstrate stability for long-term use, and standard protocols must be established to assess the lifetime and reliability of these systems.218
Over the past two decades, scale up of anode systems has gained attention, where the focus has been on increasing the throughput of laboratory-scale systems while retaining performance and reliability.247,248 At the same time, electrochemical advanced oxidation processes have been developed to improve the efficacy and applicability of conventional electrochemical oxidation.146,249−251 These specialized variants of electrochemical oxidation introduce Fenton’s reaction chemistry,192,252,253 photoelectrocatalysis,254 sonoelectrolysis,146 and aerobic or anaerobic digestion (using microbial electrochemical technologies)255−258 to the standard process.259 In conventional electrochemical oxidation, the reactive oxidizers are often (but not always) produced at the anode surface; the advanced variants facilitate additional generation of oxidizers in the bulk.146,197 Although these oxidative processes are more widely studied and used (because they usually lead to mineralization of the contaminants), treatments based on electrochemical reduction have been gaining interest because they enable partial recovery of chemicals as well as production of value-added substances.97
2.2. Electrochemical Reduction
Electrochemical reduction, the complementary process to electrochemical oxidation, is a chemical reaction involving the gain of one or more electrons by an atom or a molecule at the cathode when an electrical current is passed through the system.123 Similar to electrochemical oxidation, electrochemical reduction can occur either directly on the surface of the cathode or indirectly in the bulk by the action of a reducing agent generated at one of the electrodes.98 This process is typically used to treat water contaminated with heavy metal ions (see section 2.5 also),123,260 inorganic anions (e.g., bromate, perchlorate),261,262 or halogenated organic compounds (e.g., organic volatile halides, chlorofluorocarbons, polychlorohydrocarbons, polyhalophenols)97,98 by converting these species into more benign products. As shown in Figure 6, the mechanism of this conversion usually involves the removal of halogen atoms or the reduction of aldehydes and ketones to produces less toxic species.97,123
Figure 6.
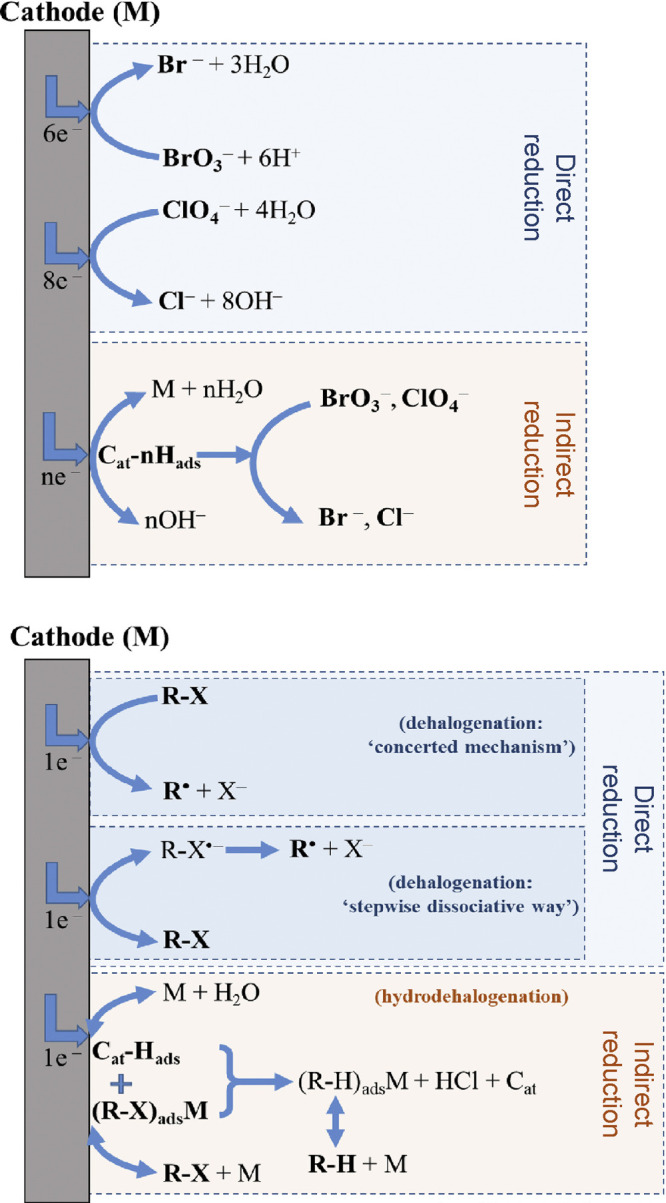
Electrochemical reduction is an established conversion method that is used to treat oxidized contaminants, such as (top) inorganic and (bottom) organic halides (R-X). The method involves formation of high energy electrons or reactive species that interact with contaminants either at the cathode surface (direct reduction) or in the bulk (indirect reduction). M refers to the cathode material (i.e., the catalyst, Cat); Cat-Hads, (R-X)adsM, and (R-H)adsM are the hydrogen atom, organic halide, and dehalogenated organic compound (R-H), respectively, adsorbed on the cathode. Reproduced with permission from ref (98). Copyright 2020 Elsevier.
Among the major parameters that determine the efficiency of electrochemical reduction are catalyst loading, cathode potential, and water quality.98 Generally, an increase in catalyst loading improves reduction activity, though only up to a limit beyond which activity either steadies or even decreases as the distance for electron transfer increases.263 In the case of nanosized catalysts such as palladium, the particles can aggregate at higher loading, which results in excessive local evolution of hydrogen bubbles that restrict access of the contaminants to the catalyst.264 As is the case for catalyst loading, a reaction will have an optimal operating cathode potential above which the abundance of hydrogen bubbles produced could inhibit adsorption of contaminants.265,266 Electrochemical reduction is also sensitive to the quality and characteristics of the feed, which influence performance and electrode lifetime.98 Performance typically improves at lower pH (due to increased formation of adsorbed hydrogen),267,268 at higher ionic strength (due to smaller EDLs),98 and in the absence of certain species (e.g., organic matter, electrocatalyst poisons, competing ions).264,269
The choice of electrode is another critical design parameter that influences the mechanism of electrochemical reduction, as it impacts the reaction pathway, selectivity, and energy consumption.97,98 An effective catalyst enables strong bonding on the surface of the substrate.98 From among the many materials investigated to date, electrodes based on silver, nickel, and carbon hold prominent positions due to their high electrocatalytic activity, robustness, and inexpensiveness with respect to conversion of halogenated contaminants.97,98 Noble metals such as palladium, platinum, and ruthenium are also effective materials for electrocatalytic hydrodehalogenation,263 especially when combined with other elements to produce bimetallic catalysts.270−272 These bimetallic catalysts can even be modified by adding nanosized or anchored materials to further improve their electrocatalytic efficiency.263,273,274 The main limitation of metallic catalysts, however, is their high cost, which makes the use of carbon-based materials an attractive alternative.98,275 Some of the most effective carbon-based catalysts involve nanostructured polymer coatings that selectively adsorb halogenated compounds,276,277 but the electrocatalytic activity of modified carbon materials in general is low.278 Activity can be improved by combining nanostructured carbons such as reduced graphene oxide (RGO) with metallic electrodes.264
In general, electrochemical reduction is an effective method not only for treatment of pollutants, such as volatile organic halides and chlorofluorocarbons, but also for their transformation into value-added products.97 This capability can be achieved by selective removal of halogens as well as by carboxylation or carbonylation of the organic compounds. The combination of electrochemical reduction and electrosynthesis is another way by which wastewater can be treated and upgraded for synthesis of value-added organic products.279,280 Moving forward, it will be important to assess and improve the stability and lifetime of electrocatalytic materials for use in practical applications.
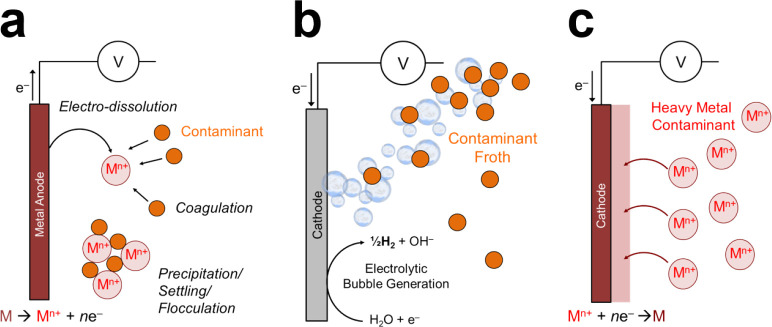
2.3. Electrocoagulation and Electroflocculation
The two-step process of electrocoagulation and electroflocculation relies on the dissolution of metal anodes to induce the formation of flocs, which trap contaminants and enable their removal by settling, sedimentation, precipitation, or flotation.156,281−285 This method was patented in 1906 by Dieterich for treatment of sewage in London and bilge water from ships using iron and aluminum as sacrificial anodes.286,287 As shown in Figure 7, the crucial electrochemically mediated step relies on in situ oxidation of the appropriate metal surfaces (often aluminum or iron)288 to produce metal ions which then form flocs that facilitate the removal of solids, organic species, and inorganic compounds.158 What follows is essentially ordinary coagulation: the (typically negative) surface charge of the contaminants is neutralized, which destabilizes them and causes them to form aggregates that can be removed by settling, sedimentation, precipitation, or flotation.289 Electrocoagulation is regularly used in industrial applications, such as removal of heavy metals, remediation of wastewater, and treatment of produced water.290,291,1269 To improve the performance of electrocoagulation for wastewater treatment, numerous studies have sought to integrate this technology with other processes, such as peroxidation or a more specialized biological process, both of which facilitate the removal of organic matter.292
Figure 7.
Established nondestructive, nonelectrosorptive methods of water purification. (a) Electrocoagulation and electroflocculation is a two-step process by which metal anodes are dissolved to induce formation of flocs that trap contaminants for removal by settling, sedimentation, precipitation, or flotation. (b) Electroflotation produces bubbles by water redox to transport lighter contaminants or flocs by flotation. (c) Electrodeposition is based on the electrochemical deposition of metal ions in solution onto an electrode.
Electrocoagulation has been extensively studied, often using iron electrodes, for specialty separations and wastewater treatment, particularly to remove light organic pollutants such as oils, dyes, and humic particles.293 In addition, electrocoagulation can be used with aluminum electrodes to remove heavy metal ions, including Ag+, Zn2+, Cu2+, Ni2+, and Cr6+,294−296 as well as halide ions.297 Compared to classical coagulation, electrocoagulation has several advantages that lower its operating cost. For example, the cationic coagulant is generated in situ by a chemical reaction on the sacrificial electrode, and this feature limits the introduction of counterions from chemical reactants that contribute to the formation of sludge.298 Electrocoagulation thus requires no separation of unreacted counterions from the chemical coagulant in solution, which are usually removed to meet discharge standards.299 The reduced formation of sludge also lowers expenses associated with handling and disposal of this waste. Another advantage of electrocoagulation is that the electrochemical reactions that drive this process produce OH–, which eliminates the need for external chemical agents to regulate pH.300 These reactions also produce gaseous H2, which could be captured and used subsequently as a fuel.301
In contrast to classical flocculation, which requires the input of large amounts of chemicals, electroflocculation relies on Faradaic (anodic) dissolution to dose the system with the coagulant, a property that enables finer control and easier handling of the process. Complexation of the released metal ions, such as Al3+ or Fe3+, with hydroxides produces monomeric (Al(OH)2+ and Al(OH)2+) or polymeric species (Al13(OH)34) that then flocculate with organic compounds (e.g., dyes) and further promotes precipitation by either sedimentation or flotation.302 Despite their advantages, however, electrocoagulation and electroflocculation exhibit operational challenges, such as electrode passivation, sludge deposition on the electrode, nonuniform dissolution of the anode, and inconsistent production of the coagulant, all of which undermine performance under long-term, continuous operation.303 The sacrificial anode is also consumed over time, which necessitates periodic replacement of the electrode, and the high concentration of residual (metal) ions requires a post-treatment step prior to discharge.304 But because of their operational simplicity, low cost, and versatility, electrocoagulation and electroflocculation remain active areas of research.305
2.4. Electroflotation
Electroflotation is often used in conjunction with electrocoagulation and electroflocculation for electrochemical–physical separations.306−309 Electroflotation relies on the electrolytic process of water redox, in which bubbles are formed to transport lighter contaminants or flocs by flotation. The key driving Faradaic reaction for electroflotation is the electrolysis of water at both electrodes (O2 evolves at the anode and H2 at the cathode). The first proposed use of electroflotation is attributed to Elmore in a patent from 1905 for mining separations,310 and this process has since been expanded to handle a range of contaminants such as oils and low-density suspended particles in mining water and groundwater, among others.306 The primary limitation of electroflotation is the difficulty of controlling the uniformity of bubble evolution.158,311 Careful optimization of current densities and voltage windows is therefore crucial for effective performance.139,282,308,312
Electroflotation has several features that are attractive for applications in water treatment. For example, this process can be used to recover valuable components from wastewater without the need for chemical reagents.313 The reason is that electroflotation generates gaseous O2 and H2, which are more active than the gases used in conventional flotation (e.g., natural gas, air, N2). Electroflotation can also produce bubbles with diameters of 1–30 μm, which leads to better dispersion, finer distribution, and longer residence times of the bubbles in solution.314−316 This feature facilitates the flotation of fine particles in a way that is difficult to achieve by classical flotation. Finally, the energy consumption of electroflotation is in the range of 0.1–0.5 kW h m–3, and it decreases as the electrical conductivity of the solution increases.314
2.5. Electrodeposition
The process of electrodeposition is synonymous with electroplating and is one of the earliest applications of electrochemistry, especially in metallurgical processing.139,317−319 While many of these plating methods are major sources of heavy metal pollution, the same electrochemical principles have been used to treat industrial wastewater and sometimes even to recover and reuse discharged materials. As an example, copper electrodeposition is commonly used to print circuit boards and manufacture electronics. Recovery of copper from spent parts, however, has also been an application of electrochemical methods that combine leaching, ED, and electrodeposition from wastewater.320,321
Electrodeposition involves the application of cathodic overpotentials to induce the electrochemical deposition (or reduction) of metal ions in solution onto an electrode. In other words, this process is direct electrochemical reduction of metal ions adsorbed on an electrode surface. Electrodeposition can effectively handle both copper and arsenic wastes, often with the production of pure elemental copper depending on the electrochemical parameters.322,323 This method has also been used for secondary recovery of residual copper from low-content tailings derived from waste electrical cable.324 These applications of electrodeposition rely on the same principle of removing metal ions from aqueous solutions that is used to charge aqueous metal flow batteries, such as zinc–air,325 zinc–bromine,326 zinc–iron,327 and lithium–air batteries.328
2.6. Challenges and Limitations
Established nonelectrosorptive processes exhibit irreversible side reactions that consume significant amounts of energy and reduce current efficiency.287 Depending on the complexity of the electrochemical matrix, a number of byproducts can be produced, some of which passivate the electrodes and further diminish performance.282,287,329 For example, even though there were attempts as early as the late 1800s to implement electrocoagulation and electroflotation at scale, these processes never evolved into mainstream technologies because of their prohibitively high operating costs.282 Electrocoagulation and electroflotation have also lacked systematic studies aimed at scaling up the processes and optimizing their operating parameters.282,305 These methods, however, remain promising for localized and small-scale applications, and they continue to offer interesting avenues for scientific research in interfacial science, electrochemical engineering, and reactor design.
3. Electrokinetic Separations
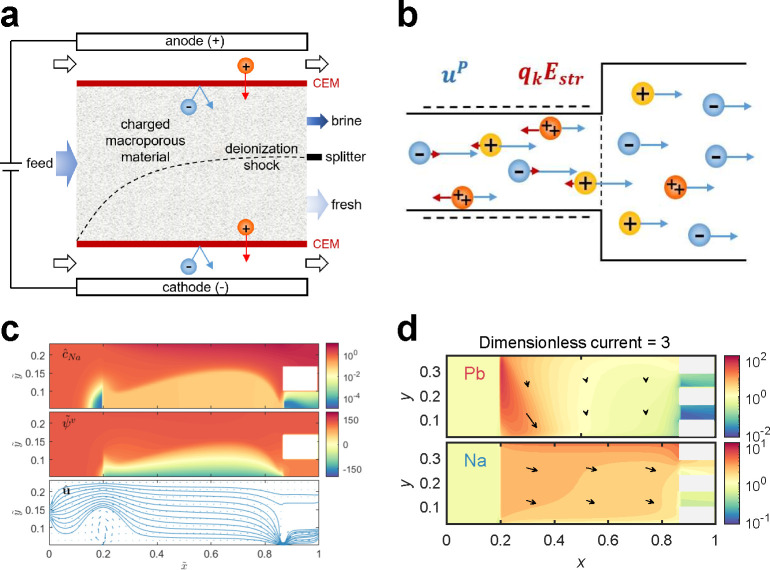
When current is applied across a pair of electrodes, ions and larger particles in solution are transported by electromigration and electrokinetic phenomena,91,330−332 such as surface conduction, electroosmosis, and electrophoresis. Water purification by electrokinetics is based on the transport of contaminants in an electrolyte, and methods of this kind can be used to remove both organic159,333−335 and inorganic68,115,153,336 ions from water. Electrokinetic methods such as ED, EDI, and shock ED are continuous and involve an electric field that is perpendicular to the direction of fluid flow. These methods also include IEMs to fractionate the feed into diluate and concentrate streams. EDI and shock ED are similar in that a porous material (e.g., ion exchange resin beads, ceramics, clays, porous glass) is used to enhance mass transfer across the liquid to the solid phase. This unique feature of EDI and shock ED allows for currents beyond the ideal diffusion-limited current and makes these methods well suited to remove trace contaminants from dilute feeds. In this section, we briefly discuss the operating principles and basic physics of ED and EDI. We then focus on the emerging method known as shock ED, which is being reviewed for the first time. Our discussion of shock ED is preceded by an examination of the key developments in microfluidics and electrokinetic modeling that inspired the invention of shock ED as a method for water purification and ion separations. We conclude this section with a discussion of fouling phenomena and methods to overcome them in electrokinetic systems.
3.1. Electrodialysis
3.1.1. Basic Principles of Electrodialysis
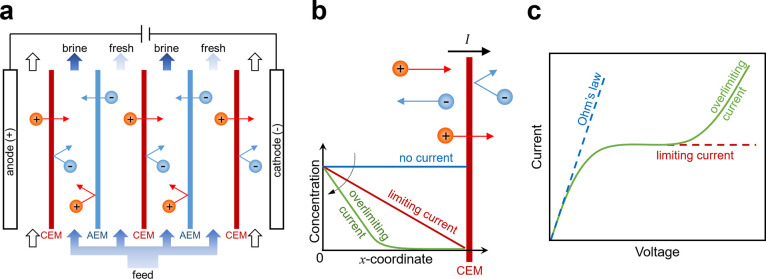
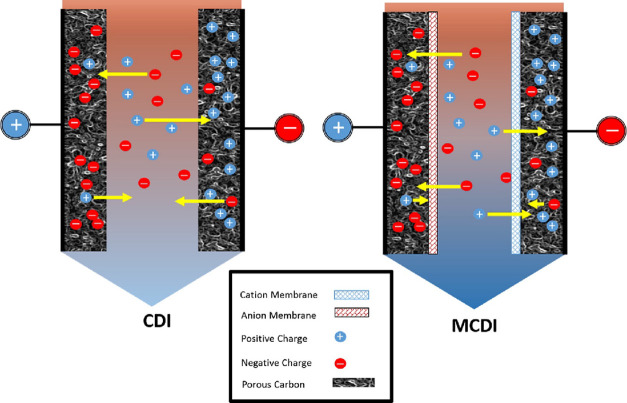
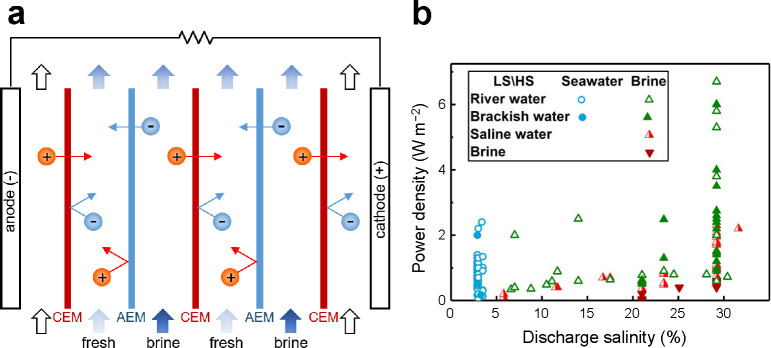
ED is a membrane-based electrochemical method that was developed in the 1950s114,337 for desalination of brackish water and for ion separations.338−343 The operating principles and underlying physics of this method are summarized in Figure 8 (see refs (152 and 153) for detailed reviews of membrane phenomena in ED). The process begins by passage of the feed through a stack of parallel, nonporous cation and anion exchange membranes (CEMs and AEMs; see refs (344 and 345) for detailed reviews of IEMs and the state of their development). At the same time, direct current (DC) is applied across the stack, perpendicular to the direction of flow to separate ions in alternating channels of fresh water (diluate) and brine (concentrate). In the sections where diluate is produced, cations pass through CEMs and anions through AEMs on the opposite side, which lowers the concentration of uncharged salt in a boundary layer that grows into the channel downstream. In the sections where concentrate is produced, anions are retained by CEMs and cations by AEMs, which results in boundary layers of increasing salt concentration.338,346,347 Once the boundary layers span the entirety of the channels, a condition termed “fully developed” forced convection,348 the dissolved salts have been effectively transported from diluate channels into neighboring concentrate channels for discharge.
Figure 8.
Operating principles and underlying physics of ED. (a) The feed flows through a stack of channels separated by alternating CEMs and AEMs, across which a current is applied. These channels are filled with spacer material that physically separates the membranes, promotes turbulent mixing, and inhibits ICP and the formation of laminar boundary layers near the membranes.349,350 The concentration profiles of the ions exhibit axial growth of boundary layers of depletion and enrichment in the diluate and concentrate channels, respectively. Adapted with permission from ref (346). Copyright 1968 Elsevier. (b) Ion fluxes near a CEM and the associated convection–diffusion boundary layers, which become more strongly depleted with increasing current until the diffusion limit is reached. (c) The current–voltage relationship reaches a plateau at the limiting current, while overlimiting current, which results in an extended zone of ion depletion, is observed at higher voltages.
Depletion and enrichment of salt in unsupported electrolytes lead to self-generated diffusional electric fields that impede the transport of active ionic species through the phenomenon of ion concentration polarization (ICP). These electric fields arise to maintain electroneutrality via redistribution of the inactive (typically oppositely charged) ionic species. The additional internal voltage drop associated with variations in salt concentration relative to that of electrical conduction in a uniform bulk electrolyte (Ohm’s law) is termed the concentration overpotential. When the salt concentration tends to zero, the concentration overpotential diverges and leads to a diffusion-limited current, as long as the transport is dominated by electrodiffusion in a neutral unsupported electrolyte without the creation of any additional ions.331,351,352 For example, the mobilities of cations and anions in an electrolyte are comparable but may differ considerably in an IEM or a nanochannel with charged surfaces.353 Salt depletion dramatically increases the electrical resistivity of the solution, which leads to significant departures from Ohm’s law as shown in Figure 8, and limits the achievable rate of ion removal by ED.338−341,354 The current–voltage relationship for ICP in a symmetric (z:z) binary electrolyte resembles that of an ideal diode:
| 1 |
where I is current, Ilim is the diffusion-limited current (or limiting current),95z is charge, V is voltage, F is Faraday’s constant, R is the universal gas constant, and T is temperature. The maximum diffusion-limited current, Ilim, is approached when a large potential difference (relative to the thermal voltage, RT/F ≈ 26 mV at room temperature), V, is applied to the system and as the salt concentration in the diluate sections approaches zero.
The theory of ED was pioneered by Peers,355 after which it was extended for arbitrary transference numbers by Rosenberg and Tirell356 as well as for fully developed convection by Sonin and Probstein.346 A useful approximation for the limiting current is given by the Peers equation,
| 2 |
where c0 is the bulk salt concentration, D is the effective salt diffusivity for coupled diffusion and electromigration (usually taken to be the ambipolar diffusivity in a dilute, binary electrolyte,357,358 although concentration dependent corrections can be significant359), τα is an effective transference number for counterions that selectively cross the solution–membrane interface (τα = 1 for an ideal membrane or electrode),339−341τβ is the transference number for counterions in the solution, and δ is the effective boundary layer thickness. This thickness scales as δ/H ∼ Pe–1/3 in the Lévêque approximation,338,346,348,360 where Pe = UH/D is the Péclet number, defined by the channel spacing, H, and characteristic fluid velocity, U. This theory is based on a boundary layer analysis of the steady convection–diffusion equation for an uncharged binary electrolyte,338,348 where the flow is assumed to be fully developed and unidirectional and axial diffusion is neglected, as is usual for forced convection in straight pipes and channels.348,361 Similar boundary layer approximations can be derived for membraneless flow batteries with forced convection over (selective) redox electrodes instead of IEMs.362 Extensions for turbulent flow,363 including effects of screen spacers to promote turbulent mixing,364 were incorporated in a general equivalent circuit model of ED by Belfort and Guter.365 Theoretical models were also used to calculate the pH profile366 and analyze ion selectivity (e.g., K+ versus Ca2+,347 NO3– versus Cl–367) in ED.
In practical systems, the ideal diffusion-limited current is always exceeded when the applied voltage is sufficiently high, and possible mechanisms of this “overlimiting current” have been extensively studied in membrane science.153,368 In bulk liquid electrolytes, there are two main kinds of mechanisms responsible for overlimiting current: electrochemical and electrokinetic.369 Electrochemical mechanisms involve charge regulation370−376 and self-ionization of water molecules,72,355,377 both of which may lead to current induced membrane discharge378 (i.e., loss of ion selectivity) and in turn passage of co-ions that would otherwise be repelled by the membrane. Electrokinetic mechanisms are based on the Rubinstein–Zaltzman electroconvective instability, where the EDL (either in379 or out of equilibrium380,381) between the depleted solution and the membrane becomes hydrodynamically unstable to electroosmosis and in turn drives bulk vortices that transport ions to the membrane faster than by diffusion.382−387 In ED, electroconvective mixing can enable overlimiting current and improve the transport of ions,388 although at the expense of greater energy consumption compared to operating below the limiting current. These observations led the scientific community to develop ways to control electroconvection, including the use of topological heterogeneity, for example by patterning the surface of an IEM,387,389−392 and pulsed electric fields.393−397 Phenomena resembling electroconvection also arise in metal electrodeposition, and they strongly influence the formation of patterns.398,399 Understanding and controlling these instabilities in bulk electrolytes is a grand challenge, the solution to which could lead to novel electrochemical systems that benefit from operating in the exotic regime of overlimiting current despite the higher energy demand.
The efficiency of ED depends on device structure (e.g., spacer thickness and geometry, number of cell pairs in the stack), membrane properties (e.g., material chemistry, concentration of the fixed ionic moiety),364,400−404 electrode design (e.g., capacitive flow or membrane electrode, electrode redox couple),405 operating conditions (e.g., electric potential, current density, hydrodynamics, temperature), and feed composition.114,406 On the basis of their structure, commercial IEMs are classified as either homogeneous or heterogeneous.407 A homogeneous IEM generally displays higher conductivity and permselectivity compared to a heterogeneous membrane because the latter comprises a larger insulating phase in its matrix as a result of the fabrication method.408,409 A heterogeneous IEM, on the other hand, often has greater mechanical strength and is less expensive to manufacture.410 The heterogeneous structure of such a membrane also promotes electroconvection and mass transfer by localizing the migration of ions through the conductive parts of the membrane.387,411−415 Because a commercial ED stack may contain 300–500 cell pairs,416 the conductivity of the membrane will largely determine the overall conductivity of the stack, which in turn influences the energy consumption of the process. Another critical feature of IEMs is their resistance to the formation of deposits (e.g., organic fouling, inorganic scaling), which degrade the performance of the membranes and negatively affect the quality of the water produced.417,418 Much research has therefore been devoted to developing IEMs that have improved antifouling properties,419−422 as discussed in section 3.6.
3.1.2. Electrodialysis With Bipolar Membranes
One class of ED systems that remains an active area of research is based on bipolar membranes, which facilitate water dissociation without the need for chemicals.114,344,423−425 Bipolar membranes are fabricated by combining a CEM and an AEM, with a hydrophilic contact region separating the two IEMs. It is at this contact region where water is split into H+ and OH–, which migrate into acid and base compartments, respectively, when an electric potential is applied. The primary benefits of using bipolar membranes with ED are that they catalyze the production of H+ and OH– at voltages lower than what is needed for standard electrolysis at an electrode, reduce the amount of concentrate generated, and increase the recyclability of the waste by recovering ions from the feed as acids and bases.58,426,427 The use of a bipolar membrane, however, also introduces an electrical resistance to the system that lowers the current efficiency. This decrease in efficiency is overcome by lowering layer resistance and incorporating weak ion exchange groups in the membrane.428 As a result, the energy required to produce H+ and OH– with commercially available bipolar membranes is nearly equal to the theoretical minimum value428 (the theory of bipolar membranes is discussed extensively in refs (425), (428−432)).
ED systems with bipolar membranes have several applications, one of which is the recovery of acids and bases from salts produced by chemical reactions or neutralization.433−436 Because ions must first be separated to produce these acids and bases (e.g., Li+ for LiOH, Na+ for NaOH, SO42– for H2SO4), this process desalinates a concentrated feed in the same way as conventional ED.434,437−439 The first industrial process to employ ED with bipolar membranes was developed by Aqualytics to recover HF and HNO3 from pickling baths in the steel industry.434,440 Although commercial use of this technology remains limited due to the high cost, low permselectivity, and short lifetime of existing bipolar membranes,114,434 novel applications are rapidly emerging, such as pH control, carbon capture, production and recovery of ammonia, and energy storage.425
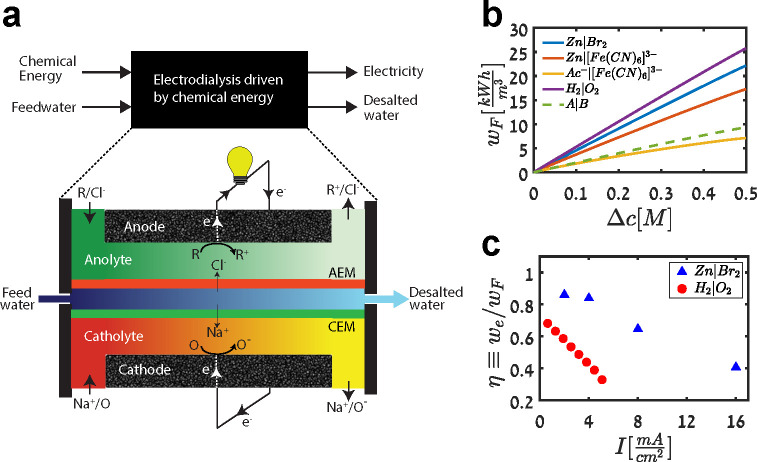
3.1.3. Electrodialysis Driven by Chemical Energy
Recently, chemical energy was used in ED to simultaneously drive separations and generate electricity (Figure 9a).441−444 Inspired by microbial desalination cells,445,446 these ED systems are driven by inorganic redox couples, but they do not actually employ microbes because the use of microorganisms can limit electricity production and salt removal rates.447 ED cells driven by chemical energy can simultaneously produce clean water and generate electricity by performing a combined reaction–separation process that is thermodynamically spontaneous. Atlas et al. calculated the maximum available energy from this combined process, as shown in Figure 9b, and quantified the thermodynamic efficiency of cells driven by chemical energy, as shown in Figure 9c.447 The authors also found that, for certain chemistries, up to 25 kW h m–3 of electricity can be produced. This technology can thus generate significant excess electricity, well above what is that needed for pre- and post-treatment of the feed, which is approximately 1 kW h m–3 for seawater.68
Figure 9.
Operating principles of chemical energy ED. (a) Schematic of an ED cell driven by chemical energy, where a reductant R and oxidant O are used to drive desalination and generate electricity. Adapted with permission from ref (441). Copyright 2019 Elsevier. (b) Calculations of the maximum available energy from an ED cell driven by chemical energy for various redox couples. (c) Measured thermodynamic efficiency of ED cells driven by hydrogen–oxygen and zinc–bromine redox couples. Reproduced with permission from ref (447). Copyright 2020 The Electrochemical Society.
The concept of chemical energy ED was tested with a variety of redox chemistries, including zinc–bromine,441 zinc–air,154 aluminum–air,443 hydrogen–oxygen,447−451 and acid–base452 couples. In particular, the hydrogen–oxygen couple is promising, as it relies on relatively inexpensive gas-phase reactants, and the product of the chemical reaction is simply water (Figure 9a); cells that use the hydrogen–oxygen chemistry are termed “desalination fuel cells.”444,447 Other chemistries that rely on liquid-phase reactants or that produce a waste product complicate disposal of the brine.441,443 The hydrogen–oxygen chemistry, however, exhibits relatively low thermodynamic efficiency relative to other chemistries, such as zinc–bromine, mainly due to losses at electrodes attributed to (platinum) catalyst poisoning by halide ions in the brine (Figure 9c).447,453 Therefore, a crucial area of research is the design and development of inexpensive catalyst materials tailored to long-term operation in desalination fuel cells. Asokan et al. demonstrated the use of chloride-tolerant, iron-based catalysts for oxygen reduction in a desalination fuel cell, which opens the field of nonplatinum group metal catalysts for these systems.454
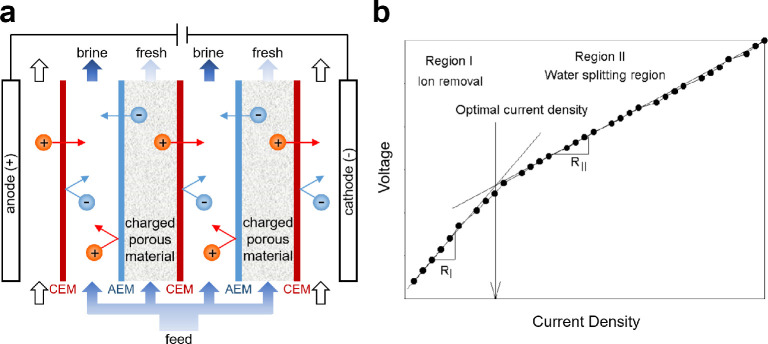
3.2. Electrodeionization
EDI, shown in Figure 10a, is typically used to generate highly pure products by processing feeds with low levels of dissolved solids (e.g., RO permeate).126,455−459 This method originated in the late 1950s with the intent of enabling extreme deionization of contaminated feeds by packing the channels of an ED stack with charged porous media or conductive ion exchange resin beads.61,106 The purpose of these conductive materials is to reduce the ICP observed in ED by enhancing transport of ions via electrokinetic phenomena (see Figure 11c and Figure 13). As in ED, ions are depleted in the diluate compartments of an EDI stack and are concentrated in the adjacent (concentrate) compartments because of the permselective properties of the IEMs. To deionize the electrolyte while maintaining reasonable conductivity across the stack, however, a conductive material such as ion exchange resin is needed to lower the resistivity of the electrolyte in the diluate compartments.61,460 As shown in Figure 10b, there is an optimal current density that is believed to coincide with the limiting current introduced in section 3.1.461 Another, more subtle reason ion exchange resin is used in the diluate compartments in EDI is to regulate the pH of the product streams by exploiting the relationship between the applied electrical current and the equilibrium concentrations of H+ and OH– in solution.462
Figure 10.
Design and operating principles of EDI. (a) As in ED, ions are depleted in the diluate compartments of an EDI stack and are concentrated in the adjacent (concentrate) compartments due to the permselective properties of the IEMs. In EDI, however, the use of a charged porous material (usually ion exchange resin) enables extreme deionization by boosting the conductivity of the electrolyte in the diluate compartments. (b) Typical current–voltage relationship in EDI, which reveals the existence of two regions with distinct resistivities (characterized by the slope of the graph). Reproduced with permission from ref (461). Copyright 2005 Springer.
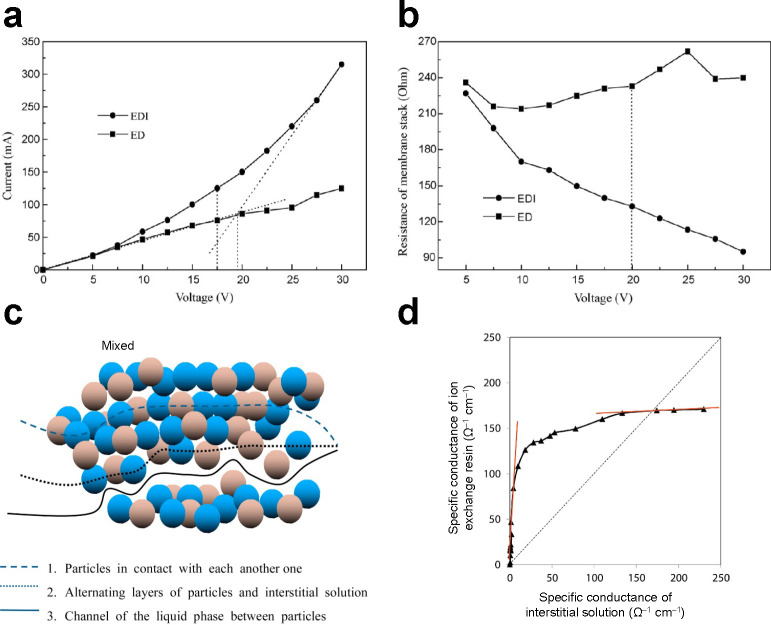
Figure 11.
Role of ion-exchange resin in EDI. Comparison of (a) current–voltage and (b) resistance–voltage relationships in ED and EDI. Reproduced with permission from ref (464). Copyright 2009 Elsevier. (c) Schematic of the three pathways for charge transport in a mixed bed. (d) Specific conductance of the ion exchange resin versus that of the interstitial solution. Reproduced with permission from ref (469). Copyright 2015 Elsevier.
Figure 13.
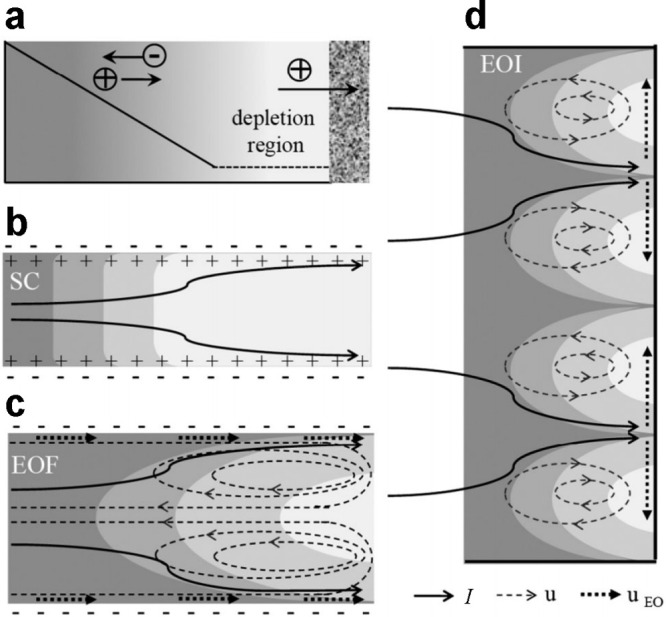
Schematic representations of common mechanisms to sustain overlimiting current in a microchannel between a reservoir on the left and a CEM on the right. The volume average conductivity in (a) exhibits classical linear diffusion (continuous line) up to a region of depletion (dashed line), where charge carriers are transported by (b) surface conduction, (c) electroosmosis, or (d) electroconvection (visualized experimentally by Gu et al.564). The relative contributions of these effects depend on the width of the channel, such that surface conduction dominates in narrow channels and electroconvection in wide ones. Reproduced with permission from ref (527). Copyright 2011 American Physical Society.
As illustrated in Figure 8c, a typical ED cell exhibits a dramatic increase in electrical resistivity when salt is depleted from the diluate compartments. Because the concentration of ions in these compartments is smaller near the membranes than it is in the bulk, water dissociation occurs and reduces current efficiency.463,464 The use of ion exchange resin (whether as loose beads or as a wafer83,465,466) in EDI mitigates this effect by promoting transport in a conductive medium, which functions as a bridge for ions between membrane pairs. The current–voltage curves in Figure 11a,b show that EDI maintains increasingly higher conductivity (or lower resistivity) than ED as a function of applied voltage.126 Pathways of charge transfer in the ion exchange bed of an EDI cell are often studied using the porous plug model467−469 introduced by Wyllie and co-workers.470 The overall conductivity of the ion exchange bed is the sum of the conductivities of the ion exchange resin and interstitial solution. These conductivities are sustained via three different pathways, namely the resin (solid), the interstitial solution (liquid), or both (solid–liquid), as shown in Figure 11c. Alvarado et al. studied these pathways using an EDI cell as a mixed bed to treat a synthetic solution containing chromium at concentrations up to 250 mg L–1.469 In this system, nearly 82.9% of the electric charge was transported by the combined solid–liquid pathway, whereas only 0.6% was transported by the interstitial solution alone. In total, the ion exchange resin, alone or in combination with the interstitial solution, contributed to approximately 94% of ion transport, which underscores the crucial role of ion exchange in driving charge transfer in EDI (Figure 11d).
In addition to facilitating the migration of ions, ion exchange resin enables operation beyond the limiting current, at which diffusion of ions becomes the rate-limiting step in electrochemical separations.471 Operating in the regime of overlimiting current leads to water dissociation and exotic electrokinetic phenomena like the electroconvective instability,368,472,473 as was microscopically visualized by Park and Kwak as well as Stockmeier et al.474,475 In EDI, water dissociates due to the presence of bipolar zones formed at points of contact between resin particles and either other resin particles or IEMs.26,465 As the ion exchange resin traps ions, H+ and OH– produced by water dissociation act as charge carriers and regenerate the resin through the process of electroregeneration.106 Water dissociation is also important when complete removal of weakly ionized species, such as silicon and boron, is needed to produce ultrapure water. The OH– generated by this dissociation reacts with silicon and boron as follows:476
| 3a |
| 3b |
| 3c |
Once the neutral species SiO2 and H3BO3 become ionized, they are readily transported into the concentrate compartment and discharged.
Another useful function of the ion exchange resin in EDI is that, as a selective medium, it preferentially removes ions based on their affinity to the resin.57 Selective separation of ions with similar charge and size can be achieved by controlling the mobility of these ions with a complexing agent.477,478 For example, Taghdirian et al. used a complexing agent made of ethylenediaminetetraacetic acid (EDTA) to separate Ni2+ from Co2+.478 This complexing agent formed a strong bond with Ni2+, which produced a negatively charged complex whose mobility was inhibited in the bed of cation exchange resin. On the other hand, Co2+ remained a free cation that could readily enter the gel phase of the resin. By removing Co2+, the molar ratio of Ni2+ to Co2+ in the solution was increased from 3 to over 150.478
Although the microscopic mechanisms of EDI have received much less attention compared to those of ED, there have been several efforts to mathematically model107,459,479−482 and numerically simulate483−486 the process of EDI. Early descriptions of EDI proposed that the removal of ions occurs in two steps.487 First, ions diffuse from the bulk to the liquid–solid (resin) interface, where counterions are exchanged with mobile ions on the resin. Second, the adsorbed counterions are transported toward and across the corresponding IEM, where they are released into the concentrate compartments. Removal of ions is controlled by the rate of diffusion from the aqueous phase to the surface of the solid.126,487 This rate is determined by the properties of the solid surface, the thickness of the liquid layer through which ions diffuse, and the concentration gradient between the two phases. When a current is applied beyond what is needed for the electromigration of ions to the surfaces of the resin, water molecules dissociate into H+ and OH–, which replace the ions that have adsorbed on the resin.126
3.3. Electrokinetics in Nanochannels and Membranes
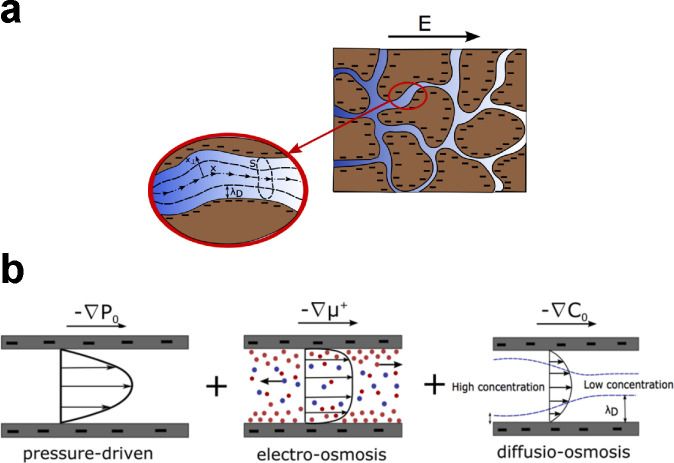
While nanofabricated devices are difficult to manufacture at scale, the scalable polymeric IEMs used in ED, EDI, and shock ED are essentially made of a network of nanoscale pores with high charge densities, as shown in Figure 12a.337,344,488,489 This nanoscale structure promotes selectivity based on the charge of an ion and on chemical interactions between the ion and the pore walls.490 IEMs are an integral component of many electrochemical methods of ion separations, and this fact underpins the development of membrane properties like conductivity and selectivity.337,344,491,492 There also exist exciting opportunities for enhanced separations as well as reduction in fluidic resistance using engineered nanoscale conduits like CNTs493−496 and graphene,497−499 but the success of these technologies in practice requires a high density of channels and a scalable and controlled fabrication process.
Figure 12.
Electrokinetic transport in charged porous media. (a) Schematic of a charged porous medium with solid (brown) and porous (blue) domains. Ion and fluid transport occur along the center axes of pores. The thickness of the EDLs and the amount of surface charge influence the electrokinetic coupling. (b) Fluid can flow due to gradients in pressure, electric field, or electrolyte concentration. Ion separations use these modes of transport to impart selectivity based on electric charge. Reproduced with permission from ref (507). Copyright 2017 American Chemical Society.
A membrane or surface in contact with an electrolyte solution either already has or will acquire a net charge, and under most circumstances, this surface charge is balanced (or screened) in the liquid by a diffuse layer of oppositely charged ions.331,332 This concept of an “electric double layer” was originally proposed by Helmholtz,500 later refined by Gouy and Chapman501,502 and revised once more by Stern.503 The local diffuse charge leads to electroosmosis and diffusio-osmosis in response to an electric field and concentration gradient, respectively.91 The equations that govern transport in charged nanoporous media are therefore coupled, such that gradients in pressure, concentration, or electric potential (Figure 12b) lead to combined fluxes in fluid flow, salt transport, and electrical current.504 Gross and Osterle described the set of coupled transport phenomena by the Poisson–Nernst–Planck–Stokes (PNPS) equations, with assumptions of local equilibrium and electroneutrality in the cross sections of pores.505 This approach presents a unified theoretical framework to describe ion and fluid transport in charged porous media down to the nanoscale, and it can be used to model ED, EDI, and shock ED. Recent work extended these results to numerically solve the PNPS equations506 and describe nanofluidic transport in nanopores.504 Systems modeled in this way include homogeneous networks with pores connected in series or in parallel as well as heterogeneous networks with pores of varying cross section.507−509 The homogenization of the PNPS equations to heterogeneous porous media revealed complex flow patterns and vortices due to parallel connectivity in these materials.510 Other models incorporated chemical interactions, mismatch in the diffusion coefficients,511 multicomponent electrolytes,512−514 electroviscous effects,515 nonionic solutes,511 salts with asymmetric valences,516,517 reactions of fixed charges,518,519 temperature gradients,520 electron conducting521 or polarizable nanopores,522 and dielectric effects512,513,523−525 to describe the distribution of ions and solvent molecules in the pores. Analytical approximations to the nonlinear transport equations are valid when the surface potentials are small526 or when the EDLs are overlapping.504
Perhaps the most fundamental principle of nanofluidic transport is electroneutrality, which is an implicit assumption embedded in the models previously discussed that dictates that the charge within a pore must balance the charge on the pore walls. Indeed, in deionization processes such as EDI and shock ED, the strong tendency toward electroneutrality in charged nanopores allows for residual conductivity, even as the concentration of ions in the bulk goes to zero, because counterions screening the surface charge are always present at a concentration determined by this charge.94,353,527,528 Levy et al., however, recently showed that one-dimensional confinement of ions to an isolated nanochannel in a dielectric matrix can lead to breakdown of cross-sectional electroneutrality, as the screening length diverges exponentially with decreasing ion concentration.529 Under these conditions, which are typical of biological ion channels,1270 the role of ion-specific chemical interactions becomes more significant than that of electroneutrality, although this order of importance is reversed in membranes where the distance between parallel channels becomes smaller than their lengths.1271 In addition to energy barriers to enter a pore, intrapore energy barriers can govern ion transport in one-dimensional pores, where ions are confined to the molecular scale.530
Other open questions exist about the structure and dynamics of ions and solvent molecules in the EDL, particularly at high ionic strength531,532 or in the case of extreme confinement.490 Discrepancies between molecular dynamics (MD) simulations and continuum electrokinetics predictions occur for channels with widths of ∼1 nm.490,533 As another example, overscreening of surface charge at high concentration can lead to reversal of electroosmotic flow,534,535 and a high surface charge density can cause electric charge to crowd an interface.532,536,537 A theoretical explanation of these phenomena requires consideration of electrostatic correlations in the EDL that go beyond the mean-field electrostatic potential.534,538,539 Transport models of dense ionic solutions can account for these correlations as well as coupled transport modes due to friction between pairs of species. For example, transport equations based on the Stefan–Maxwell model have been used to describe the coupled fluxes of ions and solvent molecules passing through nanoporous membranes.540−543 Regardless of the model, fixed surface charge influences the distribution of ions in a nanopore and in turn the observed transport properties. The regulation of surface charge via chemical reactions also affects transport dynamics.372,375,544−547 While the surface charge is usually assumed to be in equilibrium, nonequilibrium reactions can participate in electrokinetic transport.548,549
3.4. Ion Concentration Polarization in Microfluidics
The physical phenomenon that enables shock ED, the deionization shock wave, was first discovered and studied in the seemingly distinct context of ICP in microfluidic devices.92,93,353,550−556 What we now understand to be stationary deionization shock waves were observed by Wang et al. as early as 2005 in a microchannel near a nanochannel junction and used to trap and concentrate biomolecules in cross-flow, though without a theoretical explanation of the mechanism.557 In 2010, Kim et al. reported a microfluidic system that used ICP to desalinate seawater by applying current in the direction of flow and across a nanochannel junction,554 albeit at very low efficiency and in nanoliter volumes.555 A sharp, stationary depletion region was observed behind the nanojunction, and the deionized fluid below the shock was separated from the enriched brine into different microchannels. Separation of all charged solutes into the brine channel was also claimed,558 although this would appear to contradict the theory of electrodiffusiophoresis,559 which predicts the dominance of electrophoresis (motion in the direction of the Coulomb force) over diffusiophoresis (climbing salt concentration gradients, regardless of the charge sign) and suggests that fast-moving, positively charged particles in the deionized channel may not have been detected in the experiments.
The breakthrough in understanding microfluidic ICP that paved the way for shock ED occurred in 2009, when Mani, Zangle, and Santiago theoretically modeled92 and experimentally observed93 propagating ion concentration shock waves for the first time in a microchannel–nanochannel device with negatively charged surfaces. Two microchannels were filled with a stagnant electrolyte and were separated by a nanochannel with thick, overlapping EDLs, which behaved like a CEM to induce ICP and initiate a shock wave of salt depletion in one direction and salt enrichment in the other. Mathematical modeling revealed how the nonlinear drift arising from electromigration (competing with electrodiffusion) can create breaking waves of salt concentration and propagating shocks,92 analogous to the shock waves that arise in isotachophoresis560,561 and capillary electrophoresis.562,563
3.5. Shock Electrodialysis
3.5.1. Deionization Shock Waves in Microstructures
While developments in microfluidics have been critical to advancing the scientific understanding of ICP under strong confinement, microfabricated lab-on-a-chip devices are neither scalable nor efficient for macroscopic ion separations and water treatment. To produce any meaningful volume of water, a prohibitively large number of channels or devices would need to be fabricated and operated in parallel. Porous media, on the other hand, represent much more compact systems of interconnected microchannels in parallel that can be manufactured at scale, and these include ion exchange resin beads, ceramics, clays, and porous glass. Moreover, theoretical and experimental work have demonstrated that overlimiting current can in fact be sustained in charged porous media.94,95,565−568
In 2011, Mani and Bazant demonstrated the propagation of deionization shock waves in porous microstructures by theory and simulation.94 At the same time, Dydek et al. described the transport processes that enable overlimiting current in a charged microchannel, as shown in Figure 13.527 This work explained that electroosmosis dominates the transport of ions when an electrolyte is confined to small pores (∼100 μm). When the pores are even smaller (∼1 μm), surface conduction becomes the dominant mechanism of overlimiting current. Experimental visualizations by Nam et al. later confirmed the theoretical predictions of the regimes in which surface conduction and electroosmosis sustain overlimiting current in a microchannel.556,569 These observations and discoveries served as the foundation for developing a novel technology that can separate ions by deionization shock waves in porous media. Deionization shocks have also proven useful in controlling the high-rate electrodeposition of metals in charged nanopores,567 where shock electrodeposition can suppress dendritic growth570 and enable resistive switching memory571 and rechargeable metal batteries.572
In 2013, Deng et al. reported experimental evidence for overlimiting current, sustained by surface conduction and electroosmosis, and deionization shock waves in a charged porous material.95 This study was the first to observe these phenomena in porous media, and it demonstrated the feasibility of shock ED as a new platform to desalinate water and perform electrochemical separations.95,573 The basic design of the original shock ED system used a silica glass frit (weakly charged porous material) sandwiched between two electrodes (which can be thought of as strongly charged porous material; recall the microchannel–nanochannel geometry of Mani et al.92), as shown in Figure 14. (Note that the porous material and the IEM must have surface charges of the same sign. This condition ensures the propagation of depleted and enriched regions.93) Because overlimiting current is sustained in shock ED to form a deionization shock wave near the cathodic CEM, as shown in Figure 14c, it is possible to deionize the feed to concentrations well below what could be achieved by standard ED. The experimental work by Deng et al. indeed demonstrated that shock ED can produce deionized water with concentrations of ∼10 μM (see Figure 14d).95 In the same year, the nonlinear dynamics of ICP in porous media were mathematically described by Dydek et al. using a homogenized model (which assumes that the EDLs are thin relative to the nominal pore size), with emphasis on water treatment by shock ED.528 These two publications also proposed a scalable shock ED system that would be operated continuously.
Figure 14.
Prototype “button cell” used for shock ED. (a) Rectangular cross section and (b) photograph of the button cell prototype. This device is a cylindrical macroporous material submerged in an electrolyte and placed between copper electrodes. Electrical current is driven by the electrodeposition of copper onto the bottom electrode. (c) Current–voltage relationship in shock ED with 1 mM aqueous CuSO4 as the electrolyte; the effect of the sign of surface charge is also shown. Dashed lines represent experimental data, and solid lines represent the fit to a theoretical model. (d) Energy cost per unit volume of deionized water with (dashed lines) and without (solid lines) the series resistances due to the reservoir and electrodes. Reproduced with permission from ref (95). Copyright 2013 American Chemical Society.
The original shock ED system by Deng et al. was a radially symmetric button cell that had to be operated in batch mode.95 This geometry made it difficult to scale up the system, and it was clear to the authors at the time that a continuous process was more desirable. The second generation of shock ED devices, built by Schlumpberger et al. then improved by Conforti and Alkhadra, had a rectangular geometry and was shown to remove more than 99.9% of the salt present in solution.87,574 This new system used a silica frit sandwiched between two CEMs and included a splitter at the outlet between the two membranes to partition the flow into a diluate and a concentrate, as shown in Figures 15 and 16a. In the range of concentrations tested using binary electrolytes of monovalent ions, Schlumpberger et al. showed that desalination was a function of only dimensionless current:
| 4 |
where z+ and c+ correspond to the charge and concentration of the cation and Q is the volumetric flow rate of the feed. This definition of Ilim can be interpreted as the rate of advection of positive charge across the device; it is assumed that the flux of anions is zero across ideal CEMs. The authors also described the effect of electroosmosis on the fraction of liquid recovered as desalinated water from the contaminated feed, often referred to in the literature as the water recovery. Because the zeta potential of the glass frit is negative, the applied electric field induces electroosmosis in the same direction348,575 (i.e., toward the depleted region near the cathode) and as a result increases water recovery (to above 80% at high current87). This effect resembles the function of an electroosmotic pump originally used in microfluidic devices576,577 and later studied in relation to ICP in microchannels.578
Figure 15.
Shock ED as a continuous process. (left) Photographs and (right) illustration of the device that shows assembly: a working device consists of electrodes, wire, and a porous material sandwiched between identical IEMs. Inlet (outlet) streams are labeled contaminated, anolyte (anolyte out), and catholyte (catholyte out); fluid leaving the top edge of the porous material is split into fresh and brine streams. Reproduced with permission from ref (574). Copyright 2019 American Chemical Society.
Figure 16.
Operating principles and representative simulation results of shock ED. (a) Ions are transported by an electric field perpendicular to the flow, and the electrical current is driven by water redox at the electrodes. (b) Schematic to illustrate ion transport by advection (blue arrows) and streaming potential (red arrows) at the interface between a charged channel and the outlet. uP is the hydrodynamic flow velocity, and qkEstr is an electrostatic force generated by the streaming potential, where qk is electric charge and Estr is electric field. Reproduced with permission from ref (581). Copyright 2021 American Chemical Society. (c) Steady-state profiles of concentration, c, electric potential, ψ, and velocity, u, at a dimensionless current of five. The velocity profile shows distortion of the depletion zone by electroosmotic vortices. The feed to all channels is 10 mM NaCl aqueous solution at a pH of seven. Reproduced with permission from ref (580). Copyright 2021 Elsevier. (d) Contour plots of dimensionless, depth-averaged concentration and flux vectors for Na+ and Pb2+ at six sample locations in the porous material. Reproduced with permission from ref (581). Copyright 2021 American Chemical Society.
To improve the mathematical model developed by Dydek et al. following these modifications,528 Schlumpberger et al. considered the role of linear electroosmotic flow and captured some aspects of deionization and water recovery observed experimentally.579 The model, however, significantly overestimates the extent of deionization and underestimates the overlimiting conductance. Recently, Tian et al. developed a more comprehensive model of shock ED that is valid for multicomponent electrolytes (irrespective of EDL thickness), captures the phenomena of electroosmosis, diffusioosmosis, and water dissociation, and incorporates more realistic boundary conditions.580 This model also describes the role of electroosmotic vortices at the inlet and outlet of the system, and it considers the effect of hydronium transport. Figure 16c shows the profiles of concentration, electric potential, and velocity obtained from numerical simulations, which have enabled quantitative predictions of deionization and overlimiting conductance that agree with the experimental data in ref (87).
3.5.2. Selective Separations by Shock Electrodialysis
The design introduced by Schlumpberger et al. was the first example of a scalable device that could be used for shock ED.87 Recent work by Čížek et al. upgraded this design by using a multistack device with AEMs and various porous materials to remove Na+.582 In both studies, however, only binary electrolytes of monovalent ions were considered, which encouraged examination of selective removal of multivalent ions. This research began with a systematic study in which Mg2+ was selectively removed from an aqueous mixture of NaCl and MgCl2.109,583 For a feed with Na+ and Mg2+ in molar proportions of nine to one, more than 99% of the Mg2+ was removed, even though the total desalination was only 68%. This outcome implied that the divalent ion was selectively removed in the presence of a competing monovalent ion. The results of this work inspired further research into the kinds of contaminants that can be targeted by shock ED in the presence of competing ions. The first of these studies used shock ED in two passes to desalinate artificial seawater (3.5 wt %), from which 99.8% of the salt fed was rejected and more than 99.99% of the Mg2+ was removed, as shown in Figure 17a.584 These results also revealed selectivity toward Mg2+, which was preferentially removed relative to all other species by at least one (Mg2+:K+) and up to nearly two orders of magnitude (Mg2+:Ca2+). Scaled (retention) selectivity in the fresh stream was calculated as a function of dimensionless current between each pair of unique species i and j using the equation109
| 5 |
For example, if Sj:i was greater than one, then species j was selectively removed relative to species i. Despite the high rejection of salt and extreme selectivity toward Mg2+, desalination of seawater by shock ED was energy intensive. It was therefore concluded that a more suitable application of this technology would be to target trace contaminants in dilute feeds, such as toxic heavy metals in drinking water or radioactive ions in the process water of a nuclear reactor (i.e., water used in the boiler or for cooling).
Figure 17.
Representative results for selective ion separations by shock ED. (a) Concentration per pass (top and middle) and scaled selectivity (bottom) as functions of dimensionless current for the most abundant cations in seawater. Reproduced with permission from ref (584). Copyright 2020 Elsevier. (b) Deionization per pass (bottom) and cumulative deionization (top) at a dimensionless current of five for three cations normally present in the process water of light-water nuclear reactors. Reproduced with permission from ref (574). Copyright 2019 American Chemical Society. (c) Normalized concentration of Pb2+ and water recovery as functions of dimensionless current for removal of Pb2+ from water in the presence of excess competing Na+. Reproduced with permission from ref (581). Copyright 2021 American Chemical Society.
Nuclear fission is a common method to produce energy, although this process leads to contamination of the process water with several dissolved species, some of which are radioactive.585,586 It is often desirable to selectively accumulate the radionuclides to reduce the volume of nuclear waste and facilitate its containment or disposal.587−589 Shock ED was thus used to selectively and continuously remove cobalt and cesium from a feed of dissolved lithium, cobalt, cesium, and boric acid.574 This formulation modeled the contaminated water normally found in light-water nuclear reactors and in other nuclear processes.590−593Figure 17b shows deionization per pass and cumulative deionization for each species (with the exception of boron, which was present predominantly as the electrically neutral boric acid) in three passes. In each of the first two passes, all three species were removed in nearly equal proportions, but in the third pass, Co2+ was preferentially removed. Overall, the three-step process led to a high cumulative deionization for each species, ranging from 96.3% for Cs+ to 99.6% for Co2+. On the basis of these results, a clear and consistent trade-off was observed between the extent of purification (as well as water recovery) and the energy demand of the process. In general, however, the energy demand of this process (which has not yet been optimized) was low, ranging between 1.8 and 4.8 kW h m–3, because only charged species were targeted and essentially no energy was expended, removing boric acid, the most abundant species in the mixture.
Similar to radioactive species, heavy metals can damage normal functions of the human body and lead to heavy metal poisoning. Affordable and effective removal of toxic heavy metals from water, especially in the presence of excess competing ions, has been a longstanding goal in environmental science and engineering.594 Tian et al. recently demonstrated low-cost, continuous, and selective removal of Pb2+ from simulated drinking water using shock ED.581 As shown in Figure 17c, this process removed over 98% of Pb2+ (to safe levels595 below 1 ppb) with a water recovery of 70% and at an energy cost of 0.1 kW h m–3. At the same time, only 40% of Na+ was removed, which maintained electrolyte conductivity and lowered the energy demand of the process relative to nonselective methods.
Although these selective separations have been successfully demonstrated in practice, the basic physical mechanisms of selective ion removal by shock ED are still under investigation. Using the mathematical model of shock ED in ref (580), Tian et al. proposed several possible mechanisms for selective removal of multivalent cations. These mechanisms include greater separation of multivalent cations by the deionization shock wave, their high affinity to the negatively charged surfaces of the porous medium where transport is slow (due to the condition of no slip at solid surfaces), and a strong resistance to their emergence in the fresh product due to streaming potentials.596 The last two of these mechanisms are shown schematically in Figure 16b. Tian et al. also used this model to explain the selective removal of Mg2+ relative to Na+ observed in refs (109 and 584). A recent publication adapted this model to simulate and interpret selective removal of Pb2+ in the presence of excess competing Na+ (see Figure 17c).581Figure 16d shows representative simulation results of concentration profiles and ion fluxes for both Pb2+ and Na+. Indeed, the greater flux of Pb2+ relative to Na+ out of the feed and into the cathode stream (see Figure 16b) underpins the selective removal of Pb2+ by shock ED.
3.6. Fouling in Electrokinetic Systems
Fouling is the accumulation of unwanted material (known as foulant), such as mineral scale, organic compounds, colloids, and biomass, on solid surfaces. In electrokinetic systems, fouling typically occurs on the surfaces of membranes417 and porous media (e.g., ion exchange resin),597,598 which diminishes performance and increases electrical resistance. If left uncontrolled, fouling may introduce additional problems, such as increased pressure drop, flow blockages and instabilities, material fatigue, and premature system failure. In this section, we discuss how fouling occurs in electrokinetic systems as well as common methods used to prevent and control it.
Mineral scale is deposited on membrane surfaces (or within the membrane itself) when ions such as Ca2+, Mg2+, HCO3–, and SO4 precipitate (or sometimes crystallize) from solution as solid salts.417,599Figure 18 shows scanning electron microscopy (SEM) images of deposits of substrates on both CEM and AEM surfaces, particularly on the faces in contact with the concentrate.600,601 In the concentrate compartments of ED and EDI cells, the concentration of an ion can approach or even exceed its saturation point, which leads to precipitation of the ion. Turek et al. developed a model to predict the formation of mineral scale by computing the saturation level of divalent cations in the concentrate compartment of an ED cell.602 Precipitation of minerals also depends on the pH and temperature of the electrolyte.417,601,603,604 For example, a basic electrolyte is concentrated in OH–, which reacts with Ca2+ and Mg2+ as follows:417
| 6a |
| 6b |
Figure 18.
SEM images of deposits of CaCO3, Ca(OH)2, and Mg(OH)2 on the surfaces of three IEMs (MK-40 and MK-40MOD are CEMs; MA-41 is an AEM). Reproduced with permission from ref (601). Copyright 2018 Elsevier.
As shown in Figure 19, these reactions may occur via several pathways, many of which begin with the dissociation of water into H+ and OH– in the boundary layers of the diluate compartment.368,605 (see ref (606) for a more detailed explanation of the reaction pathways in Figure 19). Cifuentes-Araya et al. demonstrated that scaling of the CEM can be reduced by using a modified cell configuration and pulsed electric fields to control water dissociation.606−608
Figure 19.
Pathways of mineral scaling on CEMs and AEMs in ED, many of which are initiated by water dissociation in the boundary layers of the diluate compartment. Reproduced with permission from ref (606). Copyright 2014 Elsevier.
Colloids, which are nondissolved suspended solids (e.g., silt granules, clay minerals, colloidal silica, metal nanoparticles, organic colloids), represent another common source of membrane fouling.417 The key property of colloids that makes them potential foulants is their charged surfaces, which can lead to electrostatic interactions with oppositely charged IEMs.609−611 For example, Lee et al.609 and Mondor et al.611 reported that colloidal silica deposits irreversibly on the surfaces of AEMs and forms so-called “cake” layers that cannot be removed by chemical cleaning.612 Similar to colloids, organic compounds (e.g., humic substances, proteins, carbohydrates, organic acids) exhibit electrostatic interactions with the fixed charge of an IEM that can cause organic fouling.613−617 Unlike colloidal fouling, however, organic fouling may also occur via hydrophobic interactions between the foulant and membrane.417,618−620
Although chemical and mechanical procedures can be used to remove foulants from membrane surfaces,616 cleaning protocols increase operating costs and user intervention. Frequent cleaning may also damage the membrane and shorten its lifetime. Several strategies have thus been developed to reduce the amount of cleaning and maintenance required, the earliest of which was to periodically reverse the electrical polarity in ED (electrodialysis reversal) and EDI (electrodeionization reversal) systems.418,458,621−623 Membranes with intrinsic antifouling properties have also been explored as a means to reduce cleaning requirements.419−422,624 Engineering membranes that are resistant to fouling requires careful design of chemical composition, surface hydrophobicity and roughness, and the surface charge of functional groups.417 For instance, increasing the hydrophilicity of IEMs by modifying their surfaces with sulfonyl groups reduces the formation of mineral scale.625 Similarly, the introduction of polyelectrolyte layers inhibits organic fouling by electrostatic repulsion.626 Other methods to make membranes hydrophilic involve the use of additives such as inorganic particles,419,420,625,627 some of which could even be placed inside the membrane to modify its entire matrix. Membrane surfaces can also be made homogeneous to improve their resistance to fouling. For example, heterogeneous IEMs exhibit a dramatic reduction in scale deposition after being coated with a homogeneous layer of (hydrophobic) Nafion.601 The improved resistance to scaling was attributed to enhanced electroconvective mixing, suppressed water dissociation, and decreased crystal growth at the membrane surfaces.601,628
4. Electrosorptive Separations
Electrosorption refers to the adsorption of dissolved species from a solvent onto the surfaces or into the bulk of a charged electrode and is driven by an applied voltage. The ability to cyclically trap and release ions without the need for chemical additives or swings in temperature and pressure is a major advantage of this process. The performance of devices that employ electrosorption depends on both effective transport of species to the electrode and adequate storage capacity by the electrode. When subjected to an electric potential, the surface of an electrode becomes polarized, and the mechanism by which electrosorption occurs depends on both the identity of the dissolved species and the electrode properties. For instance, ions in an electrolyte can be electrosorbed within the EDL that forms on a polarized surface, and they can be released by either attenuating or reversing the applied electric field. This mechanism is the basis of CDI with porous carbon electrodes, as shown in Figure 20a.629 A number of emerging applications use CDI for water purification, including desalination of brackish water and selective electrosorption of target ions.115,150,630−632 While electrosorption is generally limited to removal of charged species, porous carbons have the well-known ability to adsorb organic compounds, which can enable simultaneous removal of salt and uncharged organic contaminants by CDI.633 Many novel designs and cell architectures have been invented and characterized, such as membrane CDI (MCDI, see Figure 33)70,634−637 and flow electrode CDI (FCDI, Figure 20b).638−642 MCDI can improve cell cycle life and energy efficiency, and FCDI enables continuous desalination using a single CDI cell. Moreover, the structure of CDI cells can be categorized as either flow-between (FB) or flow-through electrode (FTE), depending on the direction of the feed. In FB cells, the feed flows between the two porous electrodes, while in FTE cells, the feed flows directly through the electrodes..643−645
Figure 20.
Schematics of various electrosorption processes. (a) Static electrode CDI has electrodes that are rigid solids, such as porous carbon and intercalation materials, whereas (b) flow electrode CDI has electrodes that are made of a suspension (or slurry) of carbon beads in an electrolyte. When oriented vertically, FCDI is often referred to as fluidized bed CDI, in which the flow of suspended carbon is impeded by gravity to establish a densely packed fluidized bed. (c) Faradaic electrosorption comprises redox-active electrodes that can selectively remove target ions. Reproduced with permission from ref (646). Copyright 2020 Royal Society of Chemistry. (d) Typical cyclic voltammograms for electrostatic electrosorption, where double-layer charging is associated with relatively constant capacitance over a wide range of potentials, versus Faradaic electrosorption (or electrochemical adsorption), where redox reactions that transfer electrons between certain ions and the electrode yield peaks in the voltammogram at specific potentials.
Figure 33.
Comparison between the designs of conventional CDI and MCDI. Membranes in the latter repel co-ions and prevent them from escaping to the bulk, which in turn attracts more counterions to preserve electroneutrality. Reproduced with permission from ref (1032). Copyright 2014 American Chemical Society.
Selective ion electrosorption is an emerging and promising application of electrosorption technologies.154,632,642 Compared to other methods such as RO and ED, standard CDI is membraneless and its active elements are the electrodes. These properties enable unique functionalities and mechanisms of selectivity not accessible to membrane-based systems. For example, the selectivity of inexpensive and widely available activated porous carbon electrodes in CDI can be tuned by various parameters such as electrode voltage, chemical charge, pore size, and cell charging time.647−651 Through design and selection of functional materials, technologies for selective electrosorption are now well developed and can be used to separate both charged and neutral species from water.
Faradaic electrosorption, in particular, relies on the transfer of electrons to a distinct redox-active species bound to the electrode, which changes the oxidation state of that electrode and enables tunable binding of ions based on chemical affinity.96,652,653 Systems for Faradaic electrosorption include crystalline intercalation materials, which operate on the same principles as lithium-ion batteries for charge storage, and redox-active polymers, which selectively interact with species through single-site binding. By changing the molecular structure of the functional material, the mechanism of binding can be adapted to a specific class of ions. In this section, we describe the general principles of electrosorption in CDI and provide a review of porous carbon, intercalation, and emerging redox-active electrodes for desalination and selective separations.
4.1. Capacitive Deionization With Porous Carbon Electrodes
4.1.1. Electrostatic Electrosorption in Electric Double Layers
The origins of CDI and electrosorption can be traced back to the work of Helmholtz, who in 1853 developed the first EDL theory to describe a monolayer of counterions at the surface of an electrode in terms of a mechanism of charge storage similar to a capacitor.500 In the early 1900s, Gouy revised this theory by claiming the electrolyte in the EDL to be mostly diffuse and by allowing both co-ions and counterions to spread over a large distance.501 Chapman further improved the model by deriving the EDL differential capacitance for a symmetric binary electrolyte,502 and Stern introduced a series capacitance to account for surface solvation.503 The Gouy–Chapman–Stern (GCS) theory emerging from these developments has become the most widely used model to understand EDLs, and it is relevant for many electrosorption systems, especially in the dilute limit. The theory of electrocapillarity and EDLs was extensively discussed by Grahame in 1947,654 and there have since been several publications addressing the application of this theory to electrosorption and molecular separations.150,655,656
CDI models were first proposed by Johnson and Newman in 1971 based on an equivalent circuit for charging of EDLs in porous electrodes, and the models included an empirical factor for salt removal efficiency.657 The first self-consistent microscopic theory for EDL charging and salt removal, however, was not developed until the work of Bazant, Thornton, and Ajdari in 2004.658 This theory was motivated by the modeling of induced-charge electrokinetic phenomena,120,532 where large applied voltages at blocking porous electrodes, including voltage steps658 and alternating voltages,659 lead to nonlinear double-layer responses such as transient salt depletion and space charge formation. From this work, the first mathematical model for CDI with porous carbon electrodes, which bridged EDL theory with applications in water purification and desalination, was developed in 2010 by Biesheuvel and Bazant660 and validated by direct numerical simulations with corrections for surface conduction.661 Further extensions of the theory by Biesheuvel et al. include the addition of multicomponent asymmetric electrolytes, a generalized Frumkin–Butler–Volmer (FBV) model for parasitic Faradaic side reactions, a modified Donnan model for strongly overlapped EDLs, and a model for attractive ion image forces in metallic micropores.662−665
Storage of ions in EDLs for the purpose of water purification was first proposed and demonstrated in 1960. In the decades that followed, this area of research advanced slowly, although interest has grown quickly since 2010 with breakthroughs in theoretical modeling, experimental methods, material performance, and surface modifications.115,154,632,666 The most widely used materials for ion electrosorption are microporous carbons due to their broad commercial availability, electrochemical stability, and high electrical conductivity.115 Prominent examples of microporous carbons include activated carbon, carbon aerogels, graphene, and CNTs.629 Early theoretical frameworks of electrosorption in these materials are rooted in EDL models for planar surfaces (e.g., Helmholtz, GCS).658,660,667 One of the main theoretical advances in the past decade was to treat the EDLs in carbon micropores as EDLs in an IEM because geometric confinement in both cases leads to EDL overlapping. Current theories also assume a uniform potential in the liquid phase of a micropore as well as a Donnan potential drop between a micropore and its adjacent macropore. With these innovations, numerous modified Donnan-type models have been proposed and validated.664,668−670 One benefit of such EDL models is their simplicity, which facilitates their integration into an electrode or cell-level transport theory.671
4.1.2. Ion Selectivity
Ions constitute a major subset of contaminants found in water. When present even at low concentration, ions like F–, CrO42–, AsO4, Hg2+, and Pb2+ can pose a threat to the health of humans and animals.672−674 For this reason, researchers have studied and developed platforms for targeted removal of ionic contaminants using CDI.675−687 Selective adsorption by CDI can also be employed to recover valuable elements, such as lithium,688−696 phosphorus,697−702 and nitrogen.701,703−710 In this section, we briefly review several experimental works that focus on selective separation of ions from multicomponent solutions using porous carbon electrodes. In particular, we focus on studies that involve either two monovalent ions or one monovalent and one divalent ion, and we exclude studies that involve mixtures of more than two competing ions because of the complexity of these systems.711−720 We then discuss the quantification of ion selectivity via a separation factor. Next, we consider the use of composite and functionalized electrodes for enhancement of ion selectivity. Lastly, we present the foundations of selectivity modeling at equilibrium. Several studies focus on theories that capture selectivity based on charge, size, or affinity, which are not reviewed in detail here.649,651,664
Early work by Avraham et al. reported the possibility of selective removal of ions by tuning the pore size of microporous carbons.721 In this work, the electrodes were modified by chemical vapor deposition (CVD) using toluene as a precursor. This treatment influenced the outer surface of the micropores and reduced their size, so only small enough ions could access the pores. Results of this study demonstrated facile storage of the smaller, monovalent cation (Na+) with reduced storage of larger, divalent cations (Ca2+ and Mg2+). A follow-up study by Noked et al. also used this CVD approach for selective removal of NO3– from a mixture of NO3 and Cl–.722 NO3– and Cl– are hydrated anions of similar size, but the openings of the pores were controlled for NO3 to preferentially enter by virtue of stereoselectivity. That is, the size and structure of unhydrated NO3– was what determined the capacity for electrosorption. Cerón et al. tuned the pore size of hierarchical carbon aerogel monolith electrodes by adjusting the activation time to toggle selectivity toward either Na+ or Ca2+.723 In this study, Ca2+ was nearly completely excluded from the small pores of the electrode. In the large pores, however, MD simulations suggested that Ca2+ selectivity is limited at high applied potential due to the competition between volume exclusion and electrostatic forces as well as the more favorable desolvation of Na+. Zhang et al. studied cation selectivity via pore sieving, charge, and desolvation effects, and they also examined time-dependent ion swapping.650 Han et al. further explored the impact of ion size and pore characteristics on selectivity.724 Eliad et al. showed that the capacity of an EDL can be independent of ion size when the pores are much larger than the ions, which effectively disables ion sieving.725
In addition to ion sieving, other widely employed strategies to selectively separate contaminants leverage the influence of ion charge and size on the EDL structure in micropores. Gabelich et al., for example, studied two different electrodes of aerogel carbon with relatively large nominal pore sizes (approximately 4 and 9 nm) and showed that charge had the greatest effect on electrosorption capacity.726 They also found that smaller, monovalent ions showed improved storage relative to larger, divalent ions. These results prompted additional research, for example by Hou et al., who combined grand canonical Monte Carlo (GCMC) simulations with experiments using electrodes made of carbon aerogel.727 When two ions of the same charge but different sizes were present in solution (e.g., K+ and Li+), the smaller ions favorably screened the surface charge while occupying a smaller volume in the liquid phase. This result was also predicted by using the closed-form Boublik–Mansoori–Carnahan–Starling–Leland (BMCSL) equation of state to model hydrated ions in the micropore EDLs as hard spheres,649 building on earlier work using either local-density approximations for ion crowding in thin EDLs532 or the Carnahan–Starling equation for overlapping EDLs664 and consistent with later experimental observations.683,690,728
Ion separations in CDI are complicated, however, and cannot be fully explained by molecular or continuum models of equilibrium EDLs, especially in the presence of multivalent ions. For example, when a monovalent ion competes with a divalent ion (e.g., K+ and Ca2+), the GCMC simulations of Hou et al. predicted preferential electrosorption of the divalent ion (Ca2+), but this result was not observed experimentally. This observation was later attributed to ion-specific electrosorption dynamics by Zhao et al.,648 who developed a general theory of time-dependent ion-selectivity in CDI, based on the interplay of voltage-dependent adsorption capacities in the EDL and ion-specific transport resistances in the electrolyte. The theory was validated by experiments involving mixtures of Na+ and Ca2+, which also confirmed the prediction that selectivity toward Ca2+ would become high only after several hours of charging in their system.648 To design more selective CDI electrodes for concentrated mixtures of multivalent ions, it may be important to extend these models to account for electrostatic correlations and hydration forces.729 These effects can be incorporated using either local approximations based on the Bazant–Storey–Kornyshev (BSK) equation534,535,538,539 or nonlocal weighted density approximations730 based on fundamental measure theory731 and charged-shell electrolyte models.732
Separations of anions using CDI have also been studied. Tang et al. observed moderate selectivity of Cl– relative to F– under constant voltage charging,683 a result later observed and validated theoretically under constant current charging.733 Selective removal of NO3– is a particularly common topic. Chen et al. studied a mixture of Cl– and NO3 and showed that Cl– is preferentially electrosorbed at early times, whereas Cl– is displaced by NO3– in the EDLs at later times.734 The time dependence of selectivity observed by Chen et al. was not observed for a mixture of SO4 and Cl–, from which neither anion was preferentially removed despite the difference in charge.734 In contrast, Li et al. later observed dynamic replacement of Cl– by both NO3– and SO4 in separate experiments, and they suggested that selectivity and capacity are in fact determined by the hydration ratio, namely the ratio of the hydrated radius to the ionic radius.735 This work reported that monovalent ions with lower hydration ratios exhibit greater electrosorption relative to other monovalent ions and that divalent ions are preferentially stored over monovalent ions at equilibrium, which supports the results of Zhao et al.648 Nonetheless, there appear to be multiple mechanisms at play for the selective separation of NO3– from Cl–. In addition to the work by Chen et al. and Li et al.,734,735 Oyarzun et al. modeled the intrinsic selectivity of electrodes treated with cetyltrimethylammonium bromide (CTAB) toward NO3 using surface group–ion equilibrium constants, and they obtained an observable NO3– selectivity factor of 6.5 relative to Cl–.651 Using MD simulations, Hawks et al. examined NO3 selectivity in slit-shaped, subnanometer pores with approximately the same size as the hydrated diameters of NO3– and Cl–. This study attributed the high selectivity to the slit-like shape and low hydration energy of NO3, which allows its hydration shell to be removed more readily than those of Cl– and SO42–.716 Mubita et al. found that the high selectivity of NO3 relative to Cl– was maintained even when the pore size exceeded that of the hydrated ion.736
Selectivity between competing
ions can be quantified by a separation
factor, defined as the ratio of the molar electrosorption of ion i (Γi) to that of ion j (Γj) scaled by the respective
feed concentrations,  and
and  :649
:649
| 7 |
If βi:j > 1, ion i is
preferentially removed relative to ion j. We note
that eq 7, as it is written,
does not hold for batch mode operation because the feed concentrations
vary during charging and discharging. Considering the hydrated radii
of the ions studied by Nightingale,737Figure 21 shows that βi:j for competing monovalent ions, with
ion i being the smaller of the two, is typically
between one and five. One exception is for NO3–, which is larger than
both Cl– and Br– but is selectively
electrosorbed relative to these monatomic anions. For competing divalent
(i) and monovalent (j) ions, βi:j is time dependent and can range from below 1 to more than 10.648 Occasionally, selectivity is reported using
a relation similar to the scaled retention selectivity introduced
in section 3.5.2 for shock ED (see eq 5). The two definitions are related by rewriting the separation factor
as 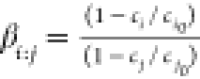 provided the system is operated in a single
pass; here, ci and cj are the concentrations in
the desalinated product (see ref (738) for details on sample collection). Unlike in
shock ED, selectivity in (membraneless) CDI arises due to the storage
of ions in electrode pores. It is therefore preferable in CDI to use eq 7 because it explicitly
accounts for ion electrosorption and also because effluent concentrations
can vary in time even if pore concentrations reach steady state.
provided the system is operated in a single
pass; here, ci and cj are the concentrations in
the desalinated product (see ref (738) for details on sample collection). Unlike in
shock ED, selectivity in (membraneless) CDI arises due to the storage
of ions in electrode pores. It is therefore preferable in CDI to use eq 7 because it explicitly
accounts for ion electrosorption and also because effluent concentrations
can vary in time even if pore concentrations reach steady state.
Figure 21.
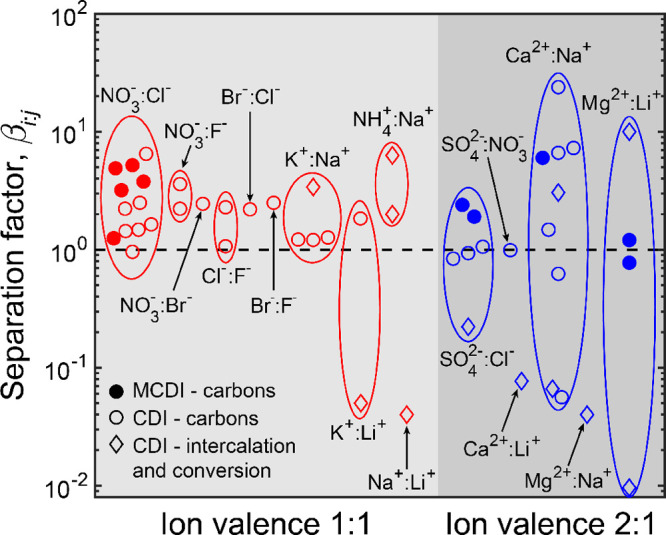
Separation factor βi:j calculated using eq 7 in MCDI, CDI with porous carbons, and CDI with intercalation or conversion electrodes for pairs of competing anions and cations. βi:j > 1 implies selectivity toward ion i, βi:j < 1 implies selectivity toward ion j, and βi:j = 1 (dashed line) corresponds to no selectivity. Competing monovalent ions (ion valence 1:1) typically display βi:j between 1 and 10. Competing divalent and monovalent ions (ion valence 2:1) display a wider range of βi:j, typically between 0.01 and 24. References for valence 1:1 ion pairs in alphabetical order: Br–:Cl–,735 Br–:F–,735 Cl–:F–,683,735 K+:Li+,688,690 K+:Na+,712,728,735,739 Na+:Li+,688 NH4+:Na+,740 NO3:Br–,735 NO3–:Cl– (MCDI,678,680 CDI651,709,734,735), and NO3:F–.709,735 References for valence 2:1 ion pairs: Ca2+:Li+,688 Ca2+:Na+ (MCDI,741 CDI,647,648,712,723 intercalation or conversion742,743) Mg2+:Li+,688,689,744 Mg2+:Na+,742 SO42–:Cl–,734,745,746 and SO4:NO3–.709
In addition to examining the mechanisms of selective electrosorption, researchers have sought to increase electrode capacity toward a specific ion by using composite carbon electrodes embedded with materials like polymers and metal oxides.678,680,747 Kim et al. demonstrated this concept by coating the surface of a carbon anode with anion exchange resin that is selective toward NO3–, which more than doubled the separation of NO3 to Cl– compared to the commercial AEM Neosepta.680 By applying constant current to the device, the same group demonstrated even greater separation using the IEMs and composite electrodes reported by Kim and Choi.678 Similarly, Tang et al. showed slight preferential removal of SO42– by using an MCDI system operated at constant current.748 It is important to distinguish composite electrodes, where materials are affixed directly to carbon, from MCDI, where free-standing membranes are layered onto the electrodes. MCDI can be used for selective removal of ions, as shown in Figure 21, but because ion selectivity in MCDI is primarily determined by the membranes rather than by the electrodes, we do not discuss this method further in this section. Besides using composites to tune selectivity, researchers have explored chemical treatments to functionalize electrode micropores for enhanced selectivity. Guyes et al. oxidized the negative electrode to add carboxylic groups to enhance ion selectivity based on size, which increased the selectivity factor of K+ relative to Li+ from approximately 1 to 1.84.690 In a later study, Guyes et al. used a cell with a sulfonated cathode to increase the selective removal of Na+ relative to Ca2+ at early charging times and high charging voltages.647 Uwayid et al. then published the first demonstration of perfect Ca2+ selectivity relative to Na+ in a CDI cell using a sulfonated cathode with long charging times and nonzero discharging voltages (i.e., only Ca2+ was removed during charging).749
The effects of ion size, chemical charge, and affinity can be mathematically modeled using continuum theories. Here, we briefly review the principles of modified Donnan (mD) EDL theory to describe some aspects of selectivity in porous carbon electrodes with micropores larger than the hydrated ions present in solution. In this framework, we spatially average over micropore volume to neglect the local pore structure and simplify the governing equations. We do not cover CDI models based on transport equations, which are needed to describe time-dependent electrosorption and selectivity.647,750 An alternative yet related framework to the mD model, termed the amphoteric Donnan (amph-D) model, considers the electrodes to comprise separate regions of positive and negative surface charge.751
The mD model employs the Donnan approximation of spatially constant micropore potential and assumes equilibrium between the macropores and micropores, which results in a Boltzmann distribution for concentration:
| 8 |
The terms ci,kmi and ci,k are the micropore and
macropore concentrations, respectively, of ion i in
electrode k = A (anode) or C (cathode), ΔϕD,k is the dimensionless
difference in potential between the micropores and macropores, Δμi,kex is the dimensionless difference
in excess chemical potential between the micropores and macropores,
and zi is valence; all
potentials are scaled by the thermal voltage (see section 3.1.1). For single-pass operation
and at cell equilibrium, it is usually assumed that the macropore
concentration equals the feed concentration: 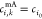 . The term
. The term 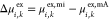 accounts for nonideal effects, such as
an affinity between the electrode surface and specific ions,752 image forces acting on ions,664 and hard-sphere interactions between ions.649,690
accounts for nonideal effects, such as
an affinity between the electrode surface and specific ions,752 image forces acting on ions,664 and hard-sphere interactions between ions.649,690
According to eq 8, higher background (macropore) concentration of a particular ion increases its micropore concentration. In the case of two competing ions with equal valence and excess potential, the ion with higher background concentration will be stored in larger quantities. In a charged cell, ΔϕD,C is negative and so cations are stored in the cathode, while ΔϕD,A is positive and so anions are stored in the anode. Because the Donnan potential is multiplied by valence, multivalent (|zi| > 1) counterions are preferentially stored relative to monovalent (|zi| = 1) counterions. As captured by the term Δμi,kex, a larger excess potential reduces ion concentration in micropores. Considering effects of volume exclusion due to finite ion size, a small ion has a lower excess potential and is thus preferentially stored relative to a larger ion with equal valence.649 The value of Δμi,k is also affected by electrode properties such as the chemical charge of surface groups, which can be tuned to improve selectivity toward target ions.690 Moreover, it was recently demonstrated that volume exclusion may enhance selective removal of larger ions with higher valence due to the complex interplay between electrostatic forces and volume-exclusion effects.749
4.1.3. Energy Consumption
Energy consumption
by CDI has been extensively studied over the past five years.90,636,753−761 Recently, Hawks et al. proposed metrics and methodologies to compare
energy consumption between different CDI systems and other desalination
technologies.738 They suggested that a
target separation should be implemented across all systems being compared
by specifying feed concentration, c0,
concentration reduction, Δc, and water recovery,
γ. The systems can then be compared in terms of volumetric energy
consumption (in units of W h m–3 of
treated water) versus productivity,  , defined as the throughput of fresh water
scaled by the projected cross-sectional area of the electrode and
multiplied by the number of cells (see eq 24).738 A related
metric to quantify energy demand was used by Lin,762 namely the specific energy consumption, SEC, scaled by
either the amount of adsorbed salt759,761
, defined as the throughput of fresh water
scaled by the projected cross-sectional area of the electrode and
multiplied by the number of cells (see eq 24).738 A related
metric to quantify energy demand was used by Lin,762 namely the specific energy consumption, SEC, scaled by
either the amount of adsorbed salt759,761
| 9 |
or the volume of produced water738,762
| 10 |
where Ech is energy consumption during charging, Msalt is the molecular weight of the salt, Q is volumetric flow rate, and tch is charging time. Another common metric in the literature is energy normalized adsorbed salt (ENAS),90,706,738 which equals SECion–1. To ensure repeatable results, energy consumption should be determined from the CDI cycle after the cell has reached a dynamic steady state.90 Dynamic steady state is usually reached after 3–5 cycles, after which the system exhibits steady behavior and the amount of salt adsorbed during charging equals the amount released during discharging.
In CDI, energy can be recovered from the discharging step to reduce the total energy consumption.756 Energy recovery is often quantified as the ratio of energy recovered during discharging to energy consumed during charging.763−767 Early studies of energy recovery used either only the consumer unit, a CDI cell,766 or only the storage unit, a buck-boost converter,763−765 and reported recovery ratios of up to 83%. Kang et al. later examined a system comprising both a CDI cell and a buck-boost converter, which achieved recovery ratios of up to 50% and that decreased with lower feed concentrations and faster desalination rates.767 Oyarzun et al. emphasized the importance of utilization efficiency, defined as the recovery efficiency over a full cycle, including charging and discharging of the DC–DC converter used for energy storage.768 The nature of the power source used to drive the CDI cell is also an important consideration. As demonstrated by Tan et al.,769−771 photovoltaics are an adequate power source due to the low applied voltage required for CDI cell charging (<1.2 V), which makes CDI a candidate for off-grid desalination.
The energy efficiency of CDI systems is normally reported as a thermodynamic efficiency, ηthermo (see eq 21),90,150,757,761,762 defined as the ratio of the theoretical minimum energy needed to achieve a target separation (see eq 19) to the energy consumed in practice. For comparisons of ηthermo to be meaningful, the systems being compared should have the same productivity and realized ion separation.761,772 Porada et al. presented an MCDI cell with an efficiency of 16.04% at a productivity of 11.8 L h–1 m–2 and current density of 18.5 mA m–2.636 Hemmatifar et al. analyzed a membraneless cell designed to achieve high efficiency with total recovery of the energy released during discharging.90 This system achieved an efficiency of 8.89% at a productivity of 3 L h–1 m–2 and current density of 4 mA m–2; increasing productivity to 6 L h–1 m–2 and current density to 8 mA m–2 lowered the efficiency to 6.81%. Ramachandran et al. reported a cell with an efficiency of 8% when operated at constant current and 5% when operated at constant voltage, assuming total energy recovery.760 A comprehensive review of CDI efficiencies was presented recently,761,762 and it reported values of up to approximately 6%.750,773
Three efficiency indicators have been proposed to study mechanisms of charge and energy loss: Coulombic efficiency, charge efficiency,90,738,774−776 and flow efficiency.636,657,758,760 Coulombic efficiency quantifies the ratio of charge released during discharging to charge delivered to the cell during charging. Values below one are due to parasitic Faradaic reactions that can occur during charging,777 such as oxygen reduction at the cathode and carbon oxidation at the anode (see eq 11). Parasitic reactions, which can degrade salt storage capacity in the electrodes, are more likely to occur when operating at higher charging voltages and for longer times.755,756,778−781 Charge efficiency is defined as the ratio of moles of salt removed from the feed to moles of electrons transferred between the electrodes during discharging,775,782,783 and typical charge efficiencies in CDI systems are in the range of 60–90%.115,784 Many strategies have been explored to improve charge efficiency, such as using high discharge voltages (with 0.3 V as a minimum value),756,785 chemically functionalizing electrodes,784 or layering IEMs over the electrodes, as in MCDI.70,786 Flow efficiency is defined as moles of salt removed from the feed divided by moles of salt adsorbed by the electrodes,760 and it captures unwanted effects like direct mixing of diluate and concentrate when switching between charging and discharging. Hawks et al. demonstrated that flow efficiency can be improved by adding a high throughput flush step between charging and discharging.758
In addition to energy losses caused by electrons participating in side reactions, there are also resistive losses caused by electronic and ionic resistances.753,754,761,787 Electronic losses arise mainly from resistances of the external circuit components, contact resistance between current collectors and electrodes, and resistances in the solid phase of the electrodes.757,761 Electronic losses can be reduced by improving contact between the current collectors and electrodes,761 which can be achieved by surface treatments of the current collectors and electrodes757 or by using conductive epoxies.753 Electronic losses are expected to be greater when the cell is operated at constant voltage, as current can be high at early times.90,755,756 Ionic losses occur mainly due to the resistance of the electrolyte in the separator and electrode. Common methods to lower these losses are to reduce spacer thickness or increase spacer porosity.757 Although operating at constant current is often considered more energy efficient than operating at constant voltage,755,788 Dykstra et al. showed that the reverse can be true when operating at certain conditions.756
Qin et al. recently analyzed brackish water desalination by RO and by CDI, and they concluded that RO is much more energy efficient, with no expected change in the future.789,790 However, several serious issues with this analysis were later identified, including unrealistic values of electrical resistance, unphysical trends in energy consumption, and inaccurate predictions of performance.791 Porada et al. also highlighted that the comparison by Qin et al. was done using inconsistent definitions of salt rejection.636 While the common definition given by Rj = 1 – cD/c0 (where cD is diluate concentration) was used for CDI, salt rejection in RO was defined as Rj = 1 – cD/cB (where cB is brine concentration).789 Porada et al. thus repeated the analysis with consistent definitions of salt rejection and demonstrated that CDI can indeed be competitive with RO for brackish water desalination, especially when water recovery is high.636
Many applications in desalination and water purification demand high water recovery to reduce the volume of waste generated.636,636,757,760,761,789 It was recently shown that water recovery in CDI could be significantly improved, with only a small increase in energy consumption, by decreasing the discharging flow rate.760,792 For example, Ramachandran et al. found that setting the discharging flow rate to 10% of the charging flow rate was most suitable in their study,760 and Tan et al. used a stopped flow discharge process.792 Water recovery can also be improved by adjusting the operating parameters, which include charging and discharging times as well as charging and discharging voltages (or currents).756 Another method to increase water recovery is by flowing the brine product through the cell as a washing solution during discharging.793
4.1.4. Flow Electrode Capacitive Deionization
Research and commercialization efforts in CDI have grown significantly over the past 10 years, and much of this growth has been catalyzed by the invention of new architectures and designs for CDI systems.115,794,795 In 2011, Duduta et al.1272 introduced semisolid lithium-ion flow batteries with flowable intercalation electrodes, a concept that has since been applied to other rechargeable batteries1273−,1275 as well as CDI. In 2013, Jeon et al. introduced flow electrodes for CDI, which were a suspension of carbon particles that are transported along with the flowing electrolyte, as shown in Figure 20a.638 FCDI offers two major improvements to CDI with static electrodes, namely the ability to operate the system continuously rather than cyclically and an increased capacity for salt storage.115 Transforming CDI into a continuous process requires discharge of the carbon particles (and formation of the brine) downstream, either in a mixing tank or in a second device.641,796−803 FCDI has also demonstrated the possibility of desalinating feeds that are more concentrated than what is processed by static electrode CDI.638,804
FCDI is sometimes used to concentrate inorganic nutrients and organic molecules for later recovery.699,700,805−808 Bian et al. introduced FCDI as an alternative to a complex and costly nutrient removal system for the extraction and up-concentration of inorganic nutrients, such as NH4+, NO3, and PO43–, from wastewater.805 Although the system achieved a high recovery of NH4 (89–99%), NO3– (83–99%), and PO4 (49–91%), it required post-treatment to further separate inorganic nutrients from desalinated salts (e.g., NaCl). Several modifications of FCDI then enabled the recovery of inorganic nutrients, especially phosphorus. For instance, PO43– was selectively recovered as H3PO4 by leveraging inevitable side reactions like the generation of H+ by water splitting.806 In this study, operating the system between 1.5 and 2.1 V resulted in the dissociation of water, while both PO4 and Cl– were adsorbed by the positively charged carbon particles. As a result, both H3PO4 and H+ were produced at the same electrode. During regeneration, the electrode released most of the adsorbed Cl– back into the original chamber, whereas neutral H3PO4 remained in the electrolyte (with a selectivity factor of > 2 relative to Cl–) to produce a solution rich in phosphorus.806 Moreover, Zhang et al. recovered PO43– with magnetic carbon particles (impregnated with Fe3O4) that showed strong affinity toward phosphorus.700 This study proposed a two-step process for the recovery of highly purified PO4 by concentrating the ion using FCDI and crystallizing it into vivianite (a hydrated iron phosphate mineral) using a fluidized bed crystallization column. The applications of FCDI have expanded to include recovery of carboxylates, such as acetate and oxalate, downstream of advanced oxidation processes.807
In contrast to solid carbon electrodes which have electrical conductivities in excess of 1000 mS cm–1, flow electrodes have electrical conductivities mostly below 10 mS cm–1 because a suspension of carbon particles can form a largely discontinuous network for electron transport.666,809 The electrical conductivity of flow electrodes is a function of the amount of suspended carbon, typically quantified by the weight fraction of carbon in the electrode (i.e., the dry weight of carbon particles divided by the total weight of the electrode). Increasing the fraction of carbon boosts electrical conductivity, although slurries used as flow electrodes become viscous and difficult to pump when the fraction of carbon exceeds about 20 wt %.666 On the other hand, low electrical conductivity adversely impacts performance, particularly in the extreme case that the slurry does not percolate electric charge. In this limit, carbon particles through much of the network cannot be charged and do not store salt, and the mechanism of desalination reverts to that of an ED system driven by a steady Faradaic current.639,810 This functionality is sometimes mistaken for capacitive storage of salt in carbon particles, which has led to spurious reports of unusually high salt adsorption capacities of flow electrodes.810,811
To overcome the limitations of weakly conductive flow electrodes, researchers have proposed new platforms that increase the loading of suspended carbon. These platforms include fluidized bed electrodes, a combination of these electrodes and slurry particles, conductive chemical additives, and redox electron mediators.641,809,812−816 Fluidized bed electrodes are distinct from slurries used as flow electrodes because the former uses large (∼100 μm), porous beads made of carbon that are pumped with the electrolyte vertically upward. The weight of the beads causes them to travel more slowly than the surrounding fluid, which leads to a densely packed electrode where the fraction of carbon can reach over 30 wt %.641 Although these fluidized beds can be dense, the electrical conductivity often remains less than 1 mS cm–1.809 One way to further increase the loading of carbon beads is to enrich the bed with a slurry of smaller particles, which feel a negligible force due to gravity. Cohen et al. showed that although this slurry itself could have a conductivity greater than 1 mS cm–1, the conductivity of the combined system would only just reach 1 mS cm–1.809 This result suggests that a more detailed understanding is needed of the relationship between the properties of carbon particles and the conductivity of the electrolyte. Ma et al. found that by adding redox-active quinone to a flow electrode with carbon particles of 100 μm loaded at 1 wt %, the rate of salt removal can be increased in potentiostatic mode.812 Similarly, other researchers have attempted to increase the conductivity of flow electrodes by introducing conductive additives such as multiwalled CNTs and carbon black.813,814 Recently, Halfon and Suss demonstrated a system that comprised metal particles under two operating modes: the first was a static mode in which conductivity exceeded 10 000 mS cm–1, and the second was a flow mode in which the particles were discharged.817 In the future, this concept may be adapted to FCDI by replacing the metal particles with carbon.
The latest advances in the field of FCDI have focused on scaling up, discovering innovative alternate applications, and better understanding the overall process. Today, FCDI is scaled up by either fabricating a stack with multiple unit cells or using three-dimensional honeycomb cells with multiple channels that are interconnected by porous carbon supports.818−821 At the same time, researchers have adapted FCDI for capacitive neutralization desalination, double displacement reactions,822 ion separations,704,707,823−828 and resource recovery.699,700 To better understand the process, theoretical models have been proposed,800,829,830 and the governing transport mechanisms have been studied.831 Overall, FCDI has the potential to be used for water purification, but for this field to reach its potential, the community must develop ways to either improve or compensate for the low electrical conductivity of flow electrodes.
4.1.5. Parasitic Faradaic Reactions
Faradaic side reactions in an electrochemical system are often (but not always) undesirable and can limit the performance and stability of the process.652,832 The two reactions that are believed to have the greatest impact on the performance of CDI are carbon oxidation at the positive electrode and oxygen reduction at the negative electrode:779,780
| 11 |
Managing the oxidative step in the corrosion of carbon plays a key role in the long term stability of a CDI device, as the electrodes are repeatedly charged and discharged. While this reaction can lower Coulombic efficiency, reduce the conductivity of the electrode, and distort its microporous structure, the most severe effect is likely the production of carboxyl surface groups (COO–).652,778,782 As the concentration of these surface groups increases in the micropores of the positive electrode, so does the concentration of co-ions to compensate for the increased negative charge.784 The result is that a larger fraction of the applied current is consumed during charging to expel co-ions from the positive electrode, which lowers charge efficiency. This phenomenon was observed in the form of “inversion peaks” in the effluent concentration profile during charging after several cycles.778,784 In addition, X-ray photoelectron spectroscopy revealed that, after cycling, the positive electrode was oxidized to the point that its surface was highly oxygenated,778 and measurements of pH indicated asymmetric electrosorption as well as irreversible consumption of charge.779 Although salt removal significantly decreases due to corrosion of the positive electrode,833 charge storage capacitance often remains largely unaffected.834 For a cell with an oxidized cathode, it was found that the anode was relatively stable during cycling, but the cathode degraded due to loss of its negative chemical charge.690,781 Uwayid et al. recently compared the lifetimes of oxidized cathodes and sulfonated cathodes, and they found that the sulfonic acid groups led to a more stable cell.835
When no measures are taken to mitigate this corrosion, a CDI cell is often limited to 50 cycles before the capacity for salt adsorption exhibits a significant loss,779,781,836 where FTE CDI cells are reported to degrade faster than FB cells.640,837 Known strategies to mitigate corrosion include periodically reversing cell polarity to slow the oxidation of carbon,778,838 optimizing the voltage window to reduce electrode degradation,839,840 reducing the concentration of dissolved oxygen (DO) by sparging with gaseous nitrogen,778 decorating the electrodes with titania to enhance electrochemical reduction and prevent DO from participating in corrosion reactions,836 treating the surfaces of the positive electrode to make it negatively charged (inverted CDI),838,841,842 and reducing the charging time to inhibit Faradaic reactions.647,781 Other proposed methods include recovering the electrodes by thermal treatment834 and adding an AEM between the main channel and the positive electrode to both reduce corrosion and extend cycle life.115,635,843 While the positive electrode may still corrode, its capacitance can be preserved, as the AEM is the active element in this desalination.
By measuring the concentration of DO in the product, researchers discovered that oxygen is reduced at the negative electrode during charging, while the concentration of DO recovers during discharging.779,780 It was proposed that DO reduction at the cathode contributes to corrosion of the anode carbon because it causes asymmetry in the electric potential778 and produces hydrogen peroxide (see eq 11),640,780,844 which accelerates oxidation of the positive electrode.836 The reduction of oxygen, which dominates when the applied voltage is less than approximately 1 V, can severely diminish Coulombic efficiency under certain conditions.779 The main approach to suppress this parasitic reaction is to dilute the DO by displacing it with gaseous nitrogen.778 As with corrosion of carbon, the rate at which oxygen is reduced is lowered in MCDI systems635 because the membranes impede transport of DO inside the device.
Besides the two reactions in eq 11, several other parasitic reactions may occur, although they usually have a minor impact on the performance of the cell.652,686 One such reaction is electrolysis of water, which produces gaseous oxygen at the positive electrode and gaseous hydrogen at the negative electrode. The fact that this reaction is sluggish on carbon electrodes, however, reduces its impact on desalination by CDI. Moreover, electrolysis of water can be prevented by operating the system at or below the standard potential of water electrolysis (1.23 V). Recent studies of Faradaic side reactions in CDI with carbon electrodes have enabled researchers to limit the destructive effects of these reactions.781,837,839 While the use of IEMs can reduce the impact of side reactions and increase cycle life, future work in this area should focus on delivering stable electrodes without the need for potentially costly membranes.
4.1.6. Fouling in Capacitive Deionization with Porous Carbon Electrodes
CDI devices with porous carbon electrodes are susceptible to fouling, which may either occur on electrode surfaces or block electrode pores. In MCDI devices, fouling can also occur on membrane surfaces or inside the membrane structure. Here, we discuss common types of fouling and their mechanisms in CDI; we limit the discussion to fouling of electrodes, as membrane fouling was explained in section 3.6. Fouling of electrodes by organic matter has received the most attention to date, and researchers generally report that salt capacity is reduced in the presence of organic matter.558,726,845−851 Zhang et al. showed that the level of dissolved organic matter can determine the extent of fouling. In particular, treatment of lake water with low levels of organic matter led to little fouling and only a 13% loss in capacity over 2 weeks, whereas treatment of lake water with high levels of organic matter led to a 75% loss in capacity in the same amount of time.846 Organic fouling also increases energy consumption due to adsorption of organic species that block active sites on the electrodes and inhibit fluid flow.846,851 For example, Chen et al. found deposits of alginate gel and humic acid in the bulk electrode structure, and these deposits were more noticeable in the presence of Na+ and Ca2+.851 The same authors observed similar deposits on the surfaces of IEMs in MCDI, although the alginate gels formed in the presence of Ca2+ were denser than those formed in the presence of Na+. Organic foulants can also be adsorbed within carbon pores, although this adsorption may not significantly impact performance. Liu et al. found that while humic acid was adsorbed in both microporous and mesoporous activated carbons, salt capacity decreased by only 5% over 30 cycles.848
Mineral scale can form on the electrodes in CDI when ions such as Ca2+, Mg2+, HCO3–, and SO4 are present in solution. Several studies found that scaling by Ca2+ and Mg2+ is minor,845,846 although Mossad et al. observed that Fe3+, even at concentrations as low as 2 mg L–1, formed deposits which reduced salt removal by 7.4% and flow rate by 13% over 30 hours.845 Moreover, Pb2+ and Cu2+ poorly desorb during cell discharging, which indicates that these species can remain bound to the electrode.852
A number of techniques have been studied to control fouling and prolong device lifetime, several of which are similar to the methods discussed in section 3.6. Pretreatment involves removing foulants and scalants upstream of CDI using chemical additives and antiscaling compounds. Often, these compounds are added directly to the feed, although in some cases they may interfere with the electrosorption process. Special electrodes (e.g., TiO2–RGO nanocomposites853) can also be designed to have intrinsic antifouling properties. Finally, cleaning solutions such as citric acid, NaOH, and HCl are used to regularly remove deposits and restore performance. A more detailed review of methods to control fouling in CDI can be found in ref (854).
4.2. Capacitive Deionization With Intercalation and Conversion Electrodes
4.2.1. Faradaic Electrosorption Involving Electron Transfer Reactions
CDI cells have historically relied on porous carbon electrodes110 because carbons are inexpensive, widely available, and capable of reversible positive and negative polarization across a wide range of potentials.855−858 Despite these advantages, however, carbon electrodes have several limitations. First, the extent to which a porous carbon can store salt (expressed as the mass of stored material per unit mass of material available for storage, often in mg g–1) is limited and rarely exceeds 20 mg g–1 for a typical salt like NaCl. Second, carbon electrodes must be charged to a relatively high voltage of 0.8–1.2 V for the amount of salt stored per unit charge to be meaningful. Third, the anode is unstable when exposed to water with a high concentration of DO, as discussed in section 4.1.5.837,859 Researchers have thus sought to develop novel materials that can address the shortcomings of porous carbons.860,861
One class of intercalation materials traps ions between the closely spaced layers of the electrode mainly by electrostatic forces, although these materials often exhibit redox reactions as well. Examples of such materials include the inorganic compounds MXene128,862−864 and MoS2 (shown in Figure 22a,b),865−868 both of which can be used as a cathode and, to a lesser extent, as an anode in CDI cells. Intercalation in these materials is largely driven by the electrostatic attraction between the ions and electrode, with only limited influence of redox reactions, as signified by wide “box-like” cyclic voltammograms such as the one shown in Figure 22b. MXenes have so far demonstrated electrosorption capacities of NaCl of up to 45 mg g–1 and charge efficiencies greater than 90%,862,863 but degradation caused by oxidation and hydrolysis is a limiting factor.869 Furthermore, these materials are susceptible to deformation (e.g., swelling) during operation because intercalation and ion exchange can alter the interlayer spacing,870 as also observed in porous carbons adsorbing larger ions from room-temperature ionic liquids.871 In MoS2, salt capacity and charge efficiency have been shown to increase with increasing salt concentration in the feed, a trend opposite to what is observed in porous carbons.866
Figure 22.
Representative intercalation and conversion electrodes used for electrochemical separations. Structural image and cyclic voltammetry of (a,b) MoS2, (c,d) NMO, and (e,f) Ag/AgCl. (a,b) Reproduced with permission from ref (865). Copyright 2015 Springer Nature. (c) Reproduced with permission from ref (872). Copyright 2015 Royal Society of Chemistry. (d) Reproduced with permission from ref (843). Copyright 2014 Royal Society of Chemistry. (e,f) Reproduced with permission from ref (873). Copyright 2020 Elsevier.
A second class of cells that do not use porous carbons involve asymmetric combinations of an electrostatic insertion cathode with a Faradaic conversion anode, such as Ag/AgCl,874,875 Bi/BiOCl,876 or MnO2.877,878 In this design, an important feature is that the metallic phase donates or accepts electrons in a redox reaction where ions are adsorbed from the liquid phase. The most familiar example of this process is the oxidation of Ag with Cl–, which produces the solid salt AgCl on the surface of the electrode until the Ag is fully converted. As shown in Figure 22e,f, the reversible electron transfer reaction is associated with rate-dependent, symmetrically shifted peaks in the cyclic voltammograms, which are the most familiar waveforms observed in voltammetry.879 Another such reaction is that of Bi with Cl– to form BiOCl according to the chemical equation,876
| 12 |
and Bi-based electrodes enable high storage of Cl– (∼80 mg g–1).876 MnO2 is also used as an anode because of its stability, high capacity for storage, and inhibition of parasitic side reactions,877 and the redox reaction in which MnO2 participates is analogous to that observed for Prussian Blue analogues (PBAs, discussed in more detail below).880 Similarly, RuO2 and TiO2 increase electrosorption capacity when deposited onto a porous carbon electrode.881,882 Overall, these inorganic materials are functional and selective, although they are limited in that only the primary atom in the host structure is reactive and that reactions of this kind can influence chemical structure.
Another class of intercalation materials is based on redox-active solid insertion electrodes, which enable ion storage throughout the (typically crystalline) solid bulk of the material.860,883,884 In this section, redox-active crystalline solids are distinguished from redox-active polymers, which are discussed as a separate class of materials for Faradaic electrosorption in section 4.3. The reactions that enable ion storage in solid insertion electrodes typically require less than 1 V.715,843,880,885 Moreover, a high diffusivity of ions within the crystal structure is desirable to facilitate ion insertion into the electrode, which increases the capacity of adsorption and reduces energy consumption.88 High rates can also be achieved using nanoparticles with small diffusion lengths, as in most intercalation batteries.357,886,887 The enhanced salt capacity in redox electrodes was described theoretically by He et al., who generalized the EDL theory used for capacitive CDI electrodes to include redox-active groups.888
So far, three major classes of redox-active materials have been explored for use in CDI, namely Na2Mn5O10 (NMO, shown in Figure 22c,d),843,872,889 metal phosphates,885,890 and PBAs.99,880,891 PBAs are analogues of Prussian Blue in which a fraction of the iron is replaced by another metal like nickel or copper, and they are widely investigated because of their potential longevity, high specific capacity, and facile intercalation of cations, particularly K+.715,739,892−896 Other materials have also been introduced in recent years, including V2O5,897 TiS2,898 and sodium superionic conductors (NASICON), the last of which includes NMO. When subjected to an applied voltage, cations are selectively and reversibly inserted into the negatively charged crystal structure of these materials, whereas anions are electrostatically rejected. As the electrodes are charged more negatively (at increasingly positive cathodic potentials), the redox-active atoms are reduced further and more cations are drawn into the pores, which leads to desalination of the water surrounding the electrodes.88 In practice, these materials are often combined with anodes such as Ag/AgCl and carbon to selectively adsorb the anions.874,875,899,900
Hybrid CDI (HCDI), first introduced by Lee et al.,843 demonstrates this idea by pairing a redox-active electrode with a porous carbon anode.694,877,881,896,901 Smith and Dmello discovered that redox-active materials can even be used as anodes as long as the two electrodes are separated by an IEM.127 In the case of electrodes that selectively capture cations (e.g., NMO, metal phosphates, PBAs), the membrane must be an AEM, which functions as the anion selective surface in the device.116,127,739 The AEM in this symmetric design partitions the feed into two channels in a way that resembles ED, except the electric field is still cyclic. This mode of operation was mathematically modeled by Singh et al.88
Recent advances in electrosorption with intercalation materials have provided opportunities for combined theoretical and experimental studies,86,96,127,631,888 which build on earlier models of ion intercalation in porous electrodes of lithium-ion batteries.357,777,886,887 Redox-active systems have an electric charge that depends on the applied potential, and this variable charge yields storage capacity and ionic selectivity.105,902 In 2017, Smith used nickel hexacyanoferrate (NiHCF) and NMO electrodes in an ED cell and developed the first model to account for two-dimensional ion transport in ED with intercalation electrodes.116 In 2018, He et al. proposed the first model to account for the thermodynamics of electrosorption based on variable chemical charge at electrodes whose surfaces were decorated with redox-active species.888 This same framework was adapted to generate an equivalent circuit model to predict and validate the electroanalytical performance of intercalation materials.903 He et al. further expanded the theory of electrosorption by redox-active materials by developing equivalent circuit models and a model based on coupled diffusion, convection, and electromigration with surface reaction kinetics.903,904 Singh et al. also presented a theory for CDI with porous electrodes comprising nanoparticles made of a redox-active intercalation material, and the authors described the dynamics of this system in terms of concentration of the product, distribution of intercalated ions, cell potential, and energy consumption.88 These models, however, neglect variations in ion concentration inside the particles and assume fast reactions with quasi-equilibrium adsorption isotherms. Inspired by recent progress in modeling intercalation batteries with multiphase layered materials,777,886,887,905,906 it would be interesting to further consider the effects of coupled ion–electron transfer reactions, mechanical deformations, and phase transformations resulting from Faradaic electrosorption in porous electrodes.
Considering the many recent advances in intercalation materials, models, and cell designs summarized here, it is naturally useful to compare their desalination performances to those of traditional porous carbons. In a comparison of nine electrode materials, Pothanamkandathil et al. found that energy demands for NiHCF were lower than those of carbon materials when desalinating a 20 mM feed of NaCl without energy recovery; they were comparable, however, when assuming complete energy recovery.907 Metzger et al. analyzed CDI with only intercalation electrodes and found that this CDI module costs approximately 27% of a typical MCDI module and is nearly four times smaller in volume due to the larger capacity of intercalation electrodes.908 Liu et al. showed, however, that intercalation electrodes are more susceptible to declines in performance from organic fouling.848
4.2.2. The Role of Electron Transfer in Ion Intercalation
It is a common misconception that ion intercalation in solid host materials is a physical electroadsorption process, whenever the ion does not itself participate in redox reactions. Indeed, lithium-ion batteries derive their name from the fact that Li+ is inserted reversibly in each electrode without being reduced to metallic lithium, effectively shuttling back and forth during battery cycling like a “rocking chair.” This picture, reminiscent of physical adsorption, might appear to be inconsistent with peaked voltammograms for lithium-ion battery materials, which clearly signify redox reactions, similar to those shown in Figure 22d for intercalation electrodes used in electrochemical separations.
In the context of lithium-ion batteries, the resolution of this paradox began with the discovery of Bai and Bazant in 2014 that ion intercalation can be limited by solid–solid electron transfer reactions that reduce and oxidize nearby transition metal ions in the host crystal.909 The authors constructed Tafel plots (logarithm of current versus overpotential) for the high-rate cathode material LixFePO4 in quantitative agreement with those predicted by the quantum mechanical Marcus theory of electron transfer,777,910,911 specifically the Marcus–Hush–Chidsey formula for electron transfer from a metallic electrode (Figure 23a),912,913 and they postulated a rate-limiting step of electron transfer from the carbon coating to the iron redox site, Fe2+⇌ Fe3+ + e– (Figure 23b). They also showed that the temperature-independent curvature of the Tafel plot (Figure 23a) is controlled by the reorganization energy, λ, of the local “solvent” (dielectric solid environment), which is well approximated by the Marcus estimate for outer-sphere electron transfer:
| 13 |
based on the optical and static dielectric constants of the solid, εop and εs, respectively, as well as the effective radius of the reactant a0 and the distance for electron transfer d, each set to the Fe–O bond distance assuming direct contact of FePO4 octahedra with a metallic (and sufficiently thick) carbon coating.
Figure 23.
CIET mechanism for ion intercalation in redox-active solid electrodes. (a) Curved Tafel plots consistent with Marcus–Hush–Chidsey theory, indicating electron transfer limitation in LiFePO4. (b) Sketch of lithium ion transfer (IT) coupled to electron transfer (ET) from the carbon coating to the nearest iron redox site. (c) Excess chemical potential landscape for CIET, combining the classical ion transfer coordinate with the solvent reorganization coordinate for quantum mechanical electron transfer. (a,b) Adapted with permission from ref (909). Copyright 2014 Nature Research. (c) Adapted with permission from ref (914). Copyright 2021 Elsevier.
The emerging understanding is that both mechanisms occur simultaneously, as ion intercalation in redox-active solids occurs by coupled ion–electron transfer (CIET).914 In this new picture, classical transfer of an ion over the intercalation energy barrier (dominated by the ion–electron Coulomb energy and the local dielectric response of the interface) facilitates the instantaneous quantum mechanical transfer of an electron tunneling between a localized state in the host crystal and a delocalized state in the electrode (Figure 23c). CIET theory is becoming widely used in modeling lithium-ion batteries,887 and it could also be used to guide the design of Faradaic electrosorption systems from microscopic first principles. In contrast to the empirical Butler–Volmer equation, CIET theory predicts a reaction limited current, which can be modified by tuning the electrostatic and dielectric properties of the electrode–electrolyte interface and the electronic structure of the electrode.914,915 For example, the theory attributes the enhanced intercalation rate in LixFePO4 achieved experimentally by anionic surface charge modification916 to lowering of the ion-transfer barrier of lithium. CIET theory also reveals the roles of dielectric properties and crystal structure of the intercalation host, including generalizations of eq 13 for the CIET barrier.914
4.2.3. Ion Selectivity in Intercalation Materials
Unlike the selectivity in capacitive materials described in section 4.1.2, selectivity in redox-active intercalation materials is achieved by insertion of specific ions into the crystalline structure, as shown in Figure 24a. Cyclic voltammetry of NaTi2(PO4)3 and Na4Mn9O18 indeed showed nearly one order of magnitude greater capacity for Na+ compared to K+, Ca2+, and Al3+. It was also concluded that the rate at which ions were removed from a dilute feed was limited by their transport from the bulk to the surfaces of the electrodes as well as by the concentration of noninserting (or electrochemically inert) ions.
Figure 24.
Overview of methods for water purification involving intercalation and conversion processes by (a) crystalline redox materials and (b) polymeric, single-site redox materials. Adapted with permission from ref (631). Copyright 2016 Wiley-VCH.
In addition to the innovations already described in this section, several research groups developed novel device architectures and designs with the intent of selectively removing specific ions. For example, Pasta et al. created an asymmetric system in which one electrode was made of (redox-active) Na2Mn5O10 and the other of (metallic) AgCl to desalinate seawater.711 In contrast to carbon-based electrodes which are slightly selective toward K+, this system could selectively remove Na+ with a separation factor of approximately 2.8 relative to K+. Separation factors of more than ten were also reported for electrodes made of (redox-active) LiMn2O4 in electrolytes comprising Li+ as well as the competing cations Na+, K+, Ca2+, and Mg2+.688 Kim et al. reported an even higher separation factor of 16 for Na+ relative to K+ in the presence of competing ions such as Ca2+ and Mg2+ by using (redox-active) Na0.44MnO2 and AgCl electrodes.927 Among the most extreme separations of Li+, however, were those achieved by Trócoli et al.,917 who reported separation factors of 240 relative to Na+ and 320 relative to K+ by using (redox-active) LiFePO4 and AgCl electrodes.918,919 Recent studies that used PBAs for water desalination showed selectivity factors between 2.2 and 3.4 for K+ relative to Na+.715,739,895 Although PBAs are usually selective toward monovalent cations, Singh et al. used the PBA vanadium hexacyanoferrate (VHCF) to preferentially remove Ca2+ in the presence of Na+ with a selectivity factor of 3.5.896
Intercalation materials that are selective toward anions have also been examined in conventional CDI systems for the selective removal of PO43– and F–. For instance, Hong et al.920 recently introduced ZnAl-layered double hydroxide (LDH, commonly used as an adsorbent for PO4) to an electrode material that is itself selective toward PO43–.921,922 LDH was composited with RGO to increase conductivity, and the LDH/RGO composite electrode (with activated carbon as the counter electrode) was used for selective separation of PO4. This composite electrode is selective toward PO43– because as RGO boosts the conductivity of the composite electrode, the electron density of the transition metal sites in LDH is effectively reduced. The reduced metal sites then allow PO4 to form inner-sphere complexations via an exchange reaction of ligands between the OH– groups of the transition metal sites and PO43–. As a result, the LDH/RGO composite electrode is selective toward PO4 (with a selectivity factor of 6.1), even in the presence of Cl– at 10 times the concentration of PO43–.920 Similarly, hydroxyapatite (HA) was composited with RGO and used to selectively remove F–:923 as RGO boosts the conductivity, the RGO/HA composite electrode forms a region concentrated with anions near the electrode, where ion exchange occurs efficiently between OH– in the HA metal sites and F–.923,924 With this composite electrode, the CDI system exhibited five times greater removal of F– compared to an equivalent system with activated carbon electrodes when both were applied to an equimolar solution of F–, Cl–, and NO3.923
4.2.4. Electrochemical Systems Design With Redox Reactions
Various cell architectures have been developed to use intercalation and conversion materials more efficiently. For instance, HCDI (shown in Figure 25a) is designed to increase the extent of desalination by using the Faradaic electrode as a working electrode. Because this design slows desalination, a capacitive carbon electrode is included to mitigate the reduction in the rate of ion removal.843,885,925 HCDI systems usually comprise intercalation or conversion materials, such as Na4Mn9O18843 and Na2FeP2O7,885 as the negative electrode to selectively capture Na+, along with porous carbon and an AEM as the positive electrode to capture the anions. Na4Mn9O18 and Na2FeP2O7 could be categorized as one-dimensional insertion materials that store and diffuse cations, especially Na+, in their tunnel structures.926,927 Compared to a system with ordinary capacitive electrodes (which had a capacity of 13.5 mg g–1 and a maximum removal rate of 0.048 mg g–1 s–1), Lee et al. demonstrated major improvements in the capacity (31.2 mg g–1) and maximum removal rate (0.066 mg g–1 s–1) by using HCDI with an NMO electrode, as shown in Figure 26a.843 The HCDI system comprising Na2FeP2O7 was also compared to an MCDI system based on capacity and rate capability using a CDI Ragone plot (see Figure 26b).885 The Ragone plot of the HCDI system showed improvement in the capacity of ion removal by up to 150% relative to the MCDI system, which was attributed to the high capacity of Na2FeP2O7. Moreover, the HCDI system achieved a rate capability comparable to that of its MCDI counterpart.885 The hybrid use of electrodes has also been shown to enhance desalination performance in FCDI systems. As illustrated in Figure 25b, Chang et al. used copper hexacyanoferrate (CuHCF, a PBA) as the flow-negative electrode and activated carbon as the flow-positive electrode.928 This system exhibited a higher rate of salt removal (up to 0.12 mg cm–2 min–1) and a greater efficiency (up to 96%) compared to a system with only activated carbon electrodes.
Figure 25.
Schematic illustrations of CDI systems that use intercalation and conversion materials as electrodes. Adapted with permission from ref (929). Copyright 2020 Multidisciplinary Digital Publishing Institute.
Figure 26.
Representative results and mechanisms of CDI with intercalation and conversion electrodes. (a) Ion removal capacity and maximum removal rate in HCDI applied to a 10 mM solution of NaCl; the system is operated at 1.2 V for 15 min during the capture step and at −1.2 V for 15 min during the release step. Reproduced with permission from ref (843). Copyright 2014 Royal Society of Chemistry. (b) Ragone plot of HCDI (with Na2FeP2O7) and MCDI for feed concentrations of 10 mM and 100 mM. Reproduced with permission from ref (885). Copyright 2016 Elsevier. (c) Mechanism and (d) performance metrics of RCDI applied to a 600 mM solution of NaCl; the system is operated at a cell voltage of 200 mV. (c) Reproduced with permission from ref (715). Copyright 2017 American Chemical Society. (d) Reproduced with permission from ref (930). Copyright 2019 Royal Society of Chemistry.
Recently, several studies introduced dispersed redox species in various multichannel architectures to further improve performance.812,931−934 By using two IEMs to provide different environmental conditions between the feed and electrolyte, the redox reaction can occur without contaminating the feed.812,929,931−934 For instance, the use of redox-active species significantly improves charge transfer in FCDI by overcoming some of the limitations of charge transfer between current collectors and particles in the flow electrodes (see Figure 25c; details are provided in section 4.1.4).812,931 Although many studies have suggested increasing either carbon content or salt concentration to reduce the internal resistance of the electrode channels, the conductivity of these channels could be compromised if the carbon particles were to aggregate.812,935 Ma et al. thus proposed the use of hydroquinone (H2Q) as an electron mediator that shuttles electrons from the current collector to the particles in the flow electrodes.812 As a result, the deionization system exhibited an increase of 131% in the average rate of salt removal compared to the same system in the absence of H2Q.812 Kim et al. used redox species as an additional means for salt removal in the multichannel redox system (see Figure 25d).932 In this system, the redox reaction between Fe(CN6)3– and Fe(CN6)4– further remove ions from the feed to maintain bulk electroneutrality in the electrolyte,929 and the redox species are continuously regenerated as the redox couple circulates the electrodes. Overall, the desalination performance of this system (with a capacity of 67.8 mg g–1) was improved by more than a factor of three compared to the same system in the absence of the redox species (with a capacity of 20.0 mg g–1).932
Another active area of research is on the development of various configurations of battery desalination,711,715,873,876,930,936−939 where both capacitive electrodes are replaced by intercalation or conversion materials. This concept, shown in Figure 25e, was first introduced by Pasta et al., who used NMO to capture Na+ and Ag to capture Cl–.711 Following this study, several intercalation and conversion materials have been used in desalination battery systems to overcome the limited capacity of capacitive electrodes. Electrodes used to capture Na+ include NMO,711 NaTi2(PO4)3,876,938 and PBAs,940 and electrodes used to capture Cl– include Ag and Bi. Because conversion electrodes have high theoretical specific capacities, they are typically used as counter electrodes.711,876,938,940−942 Conversion reactions, however, can lead to poor electrode stability due to repeated volume expansion and contraction over many cycles.943 Despite this drawback, battery desalination systems have been used to recover Li+ using selective electrodes such as LMO941 and LiFePO4942 because of the structural advantages these materials exhibit toward Li+. For instance, the tetrahedral sites of LMO and LiNi0.5Mn1.5O4 are narrowly spaced and enable selective removal of Li+ relative to larger cations.944−946 Although the use of Faradaic materials can significantly increase the capacity of salt removal, they tend to limit the rate at which salt is removed.117,947 Initial studies showed that CDI with activated carbon permits an applied current greater than 1 mA cm–2,115 whereas intercalation electrodes limit the applied current to ∼100 μA cm–2.115,715,739
Lee et al. recently proposed a multichannel desalination battery, as shown in Figure 25f, to overcome the mass transfer limitations of traditional desalination batteries.939 The multichannel system was designed to have two independent channels (one for both electrodes and one for the feed) by placing an IEM at each interface.939,948 The two electrodes (NaNiHCF and Ag) were exposed to a concentrated solution (1 M of NaCl) to reduce the resistance of the system, where the high salinity of the electrolyte significantly improved the capacity (52.9 mg g–1) and removal rate (0.0576 mg g–1 s–1).939 Lee et al. also demonstrated the continuous battery desalination system known as “rocking-chair” CDI (RCDI; Figure 25g).715 This configuration is usually made of two different PBAs, such as NaNiHCF and NaFeHCF, separated by an AEM. During the charging step, the working electrode (NaFeHCF) captures cations, while anions move to the counter electrode (NaNiHCF) to maintain bulk electroneutrality. During the discharging step, the working electrode is regenerated while the counter electrode captures cations, which enables continuous desalination with a removal capacity of 59.9 mg g–1 (Figure 26c).715,936,937 In the same manner, the cation selective electrodes can be replaced with anion selective conversion electrodes such as Ag/AgCl for even greater continuous desalination (85873 to 115930 mg g–1) at a low operating voltage (≈ 200 mV; Figure 26d).873,930 During oxidation of the Ag electrode, Ag reacts with Cl– in solution to form AgCl by breaking Ag–Ag bonds. Because both the oxidation and reduction peaks of Ag/AgCl occur at a low cell potential (220 mV versus SHE), this Faradaic process requires only a small input of energy (ranging between 2.5 kBT ion–1930 and 10 kJ mol–1 of salt873). By adapting these cell architectures to existing designs, the desalination performance of conventional systems can be greatly improved using intercalation and conversion electrodes.
4.3. Electrosorption by Redox-Active Polymers
As illustrated in Figure 24, Faradaic redox reactions occur when there is transfer of n electrons at the surface of an electrode that changes the oxidation state of a reactant.949,950 The equilibrium thermodynamics of these reversible Faradaic reactions are governed by the Nernst equation.777,951−953 In this review, the notion of a Faradaic process encompasses reactions at electrodes regardless of the phase in or across which the reactants are moving or are present. For instance, a Faradaic process could be either a redox event at an electrode surface86 or a variety of side reactions in which the electrons are transported across the electrolyte (e.g., water splitting, oxygen reduction).89,954 Faradaic processes, combined with rich redox chemistry, have been extensively studied for a variety of applications, including energy storage,955,956 bioelectrochemistry,957 sensing,958−960 and electrocatalysis.961,962 As explained in section 2, nonelectrosorptive electrochemical methods rely on Faradaic reactions, be it through dissolution of a metal electrode or changes in the oxidation state of a dissolved species for plating. In the context of water purification by capacitive electrosorption, electron transfer and redox reactions are common, even without the use of external electroactive species. If left unregulated, these reactions could be parasitic and in turn degrade the electrodes, produce undesired byproducts, and reduce the efficiency of the primary electrochemical process.150,954
In this section, we focus on electrosorption promoted by redox reactions at active polymer electrodes, which boost energy efficiency and, more importantly, enable molecular selectivity. Materials with intrinsic Faradaic properties have long been studied and used for applications in energy storage.963,964 Batteries, for example, store electric charge by Faradaic intercalation reactions and by changes in the oxidation state of a crystalline solid subjected to an electric field. These synthetic materials confine the redox reactions to within the solid electrode itself, without loss of electrons or transfer of ions across the electrolyte. Moreover, the surface reactions that occur usually promote specific binding of ions by creating sites of fixed charge that enable noncovalent interactions, such as hydrogen bonding, charge transfer processes, and hydrophobic transitions. From the perspective of electrochemical engineering, Faradaic materials also enable greater control of the window of operating voltages and suppress leakage currents and parasitic side reactions that would otherwise be detrimental to the longevity of the system.902 The properties and applications of redox-active polymers are described in the sections below.
4.3.1. Overview of Redox-Active Polymers
Electrochemically tunable redox polymers, defined by the IUPAC as polymers with groups that can be either oxidized or reduced, play a key role as stimuli-responsive materials in many chemical and biochemical applications.965 These materials have several properties that can be modulated electrochemically, such as reactivity,966 mechanical actuation,967 sensing,968 and energy storage.969 As shown in Figure 27, the redox group can exist either directly on the backbone of the polymer chain, as is the case of polyanilines, polypyrroles, and polyquinones, or in pendant groups like ferrocene.970 Redox-active polymers with electroactive units in the main chain are often conjugated971 and comprise π-bonds that enable semiconductivity. While some of these semiconducting polymers have linear backbones, such as polyacetylene, many have conjugated backbones made of aromatic groups, such as polyaniline (PANI) and polypyrrole (PPy). Both PANI and PPy have been used extensively in electrosorption, particularly for enhanced CDI.96,972−976 Redox polymers with active units in pendant groups, like pendant-group metallopolymers, are charged by a combination of bounded diffusion and electron hopping (or free diffusion).977 Although these materials are less electrically conductive, they exhibit unique electronic properties and charge transfer interactions that make them highly selective toward target ions.96,102,978
Figure 27.
Chemical structures of representative redox-active and conducting polymers used to study selective electrochemical separations.
Faradaic electrosorption relies on a change in the oxidation state of the electrode by electron transfer, which in turn influences the environment of the material. In a complementary manner, the solvent and immediate environment affect the behavior of redox polymers.979,980 The oxidation or reduction of a redox polymer, for example, creates a fixed charge that selectively binds counterions by either ion exchange or insertion and release of the ions.86,96,979−982 For redox systems that favor oxidation, the following chemical reactions are observed:
| 14a |
| 14b |
The first reaction describes a redox polymer P+ (e.g., PPy) in which the weakly bound ion X– is exchanged with the substituent ion An–. The second reaction describes a redox polymer Pn that is initially uncharged but, after undergoing oxidation (e.g., the transition from ferrocene to ferrocenium), can bind the counterion An– to a cationic group. In this process of binding, the affinity of the ions to the electrodes influences the electrochemical kinetics of adsorption. Ions that are strongly bound often facilitate electron transfer and shift the formal potentials, which makes the use of tunable redox polymers well suited for selective electrosorption and sensing.978,983,984 Redox polymers, especially pendant-group metallopolymers, can also modulate electrode potentials, which prevents excursions in potential that would otherwise lead to side reactions. The suppression of parasitic side reactions (e.g., water splitting) improves performance and enables better control of water chemistry, as explained in Figure 28.102,902,985 In the following sections, we describe recent advances in redox-active polymers for the separation of common pollutants like ions and uncharged contaminants.
Figure 28.
Suppression of side reactions using an asymmetric redox electrode configuration. Adapted with permission from ref (902). Copyright 2017 Royal Society of Chemistry.
4.3.2. Molecular Selectivity of Redox-Active Polymers
The main advantage of molecularly selective technologies is the ability to target a minority species in the presence of excess competing molecules. Redox-active polymers have facilitated the realization of this capability by enabling selective electrosorption and reversible release at fixed electric potentials. Because of their favorable electrochemical kinetics, electroactive polymers have been considered for various applications in analytical sensing.986−989 The mechanisms of selective ion sorption onto conducting polymers have been studied since the advent of ion exchange voltammetry.990−992 For example, electroactive polymer films can be used as selective sensors by preconcentrating them with a certain counterion from the contacting solution phase.993,994 Conducting polymers (e.g., PANI, PPy),995,996 redox polymers,982,997−999 and polymers entrapping redox units1000−1002 have also been used for ion doping. These materials represent the archetypal systems of modified electrodes used during the development of electroanalytical chemistry.951 The mechanisms of selectivity vary depending on the chemical structure of the redox monomer, the hydrophobicity of the polymer, and the electrostatic properties of the ions themselves. In general, the combination of controlled electric potentials and tailored molecular selectivity makes electroactive polymers a promising platform for energy efficient purification of water, recovery of valuable resources, and separation of fine chemicals.
Metallopolymers are one class of polymers that display electrochemically switchable properties, possess favorable electron transfer kinetics, and enable a diverse array of synthetic pathways for structural modification and tuning.1003−1005 Recently, these materials were used as heterogeneous coatings on porous carbon to improve the capacity for energy storage. In particular, the noncovalent conjugation of polyvinylferrocene (denoted as PVF) with CNTs was demonstrated by both organic solution deposition and electrodeposition.1006,1007 The resulting systems displayed high capacitances (1450 F g–1) and energy densities (79.5 W h kg–1).1007 Through similar electrochemical methods, the charged moieties of metallopolymers can be used to capture and release anions efficiently. Films of PVF are oxidized in water over a range of positive potentials near 0.3 V versus Ag/AgCl,1008 which is characteristic of stable electron transfer kinetics. Depending on the heterogeneity of the film, however, the applied voltage needed to fully charge all immobilized ferrocene units can be higher (in the range of 0.6–0.8 V versus Ag/AgCl), as was indicated by the large peak separation of different redox films in various electrolytes.
Redox-based electrosorption is cyclical and operates by electrochemical swings to capture target ions. During adsorption, electrodes become positively charged due to the formation of oxidized sites. Captured ions are then released by reversing the polarity due to the reduction of the redox adsorbent, as demonstrated in Figure 29a. The redox of PVF, for example, has been used to selectively extract organic anions such as carboxylates, phosphonates, and sulfonates from water in the presence of excess ions and without the use of chemical additives.96,902,978,1009 Because many organic micropollutants like pesticides (e.g., metolachlor-based pesticide), microplastics, and pharmaceuticals (e.g., ethinyloestradiol contraceptive, propranolol hydrochloride beta blocker) are negatively charged,1010−1012 electrosorption of negatively charged species is carried out by charging a redox polymer at the anode. In such a process, the functionalized redox electrode is immersed in an electrolyte comprising the minority organic anion (in concentrations ranging from 0.1 to 3 mM) as well as excess competing ions like ClO4– and Cl– (in concentrations exceeding 100 mM). The transformation of ferrocene to ferrocenium results in a positively charged surface, which then electrostatically draws anions in solution to that surface. Systems based on PVF were also found to be stable over numerous cycles, and they generally display molecular selectivity, efficient use of energy, and a high capacity for uptake and storage of charge.902 One example, shown in Figure 30a, is a system that paired a PVF anode with a polyanthraquinone (PAQ) cathode, which was effective compared to other systems at separating carboxylate, as quantified by an electrosorption capacity as high as 157 mg of anion per gram of adsorbent.1009Figure 30b reveals a relationship between the amount of oxidized species and the applied potential, and it shows that the utilization efficiency of ferrocene was lower than 100% during charging, which may be attributed to either active sites being inaccessible or redox units being kinetically trapped. In municipal wastewater, trace amounts of micropollutants usually exist in the range from micrograms to below a nanogram per liter.1013 Future work demonstrating the effectiveness of redox-based electrochemical separations toward trace micropollutants will thus provide insight into industrial feasibility.
Figure 29.
Electrochemical control of redox-active polymers for selective electrosorption. (a) Illustration of the concept of redox-based capture and release of a target anion. Reproduced with permission from ref (978). Copyright 2016 Wiley-VCH. (b) Selective separation of chromium at a PVF electrode; the schematic shows both charging and discharging steps as well as the reactions that occur at each counter electrode. (c) Results of adsorption uptake of chromium on PVF working electrodes as a function of potential. Reproduced with permission from ref (1014). Copyright 2018 Nature Research. (d) Calculated selectivity of individual anions (0.5 mM each) relative to a competing anion (20 mM of ClO4–) after 3 h of adsorption on PVF-CNT at 0.8 V versus Ag/AgCl. Reproduced with permission from ref (1015). Copyright 2020 Wiley-VCH.
Figure 30.
Operating principles of pseudocapacitive separation of ions. (a) Asymmetric PVF/PAQ system for electroregulated, selective recovery of lithiated carboxylates. (b) Variations in charge and ferrocene utilization efficiency (FUE) as functions of the applied potential. (c) Electrosorption capacity of this system compared to other supercapacitors reported in the literature (denoted by the light-colored symbols). Reproduced with permission from ref (1009). Copyright 2016 American Chemical Society.
As illustrated in Figure 29, redox-active electrodes made of PVF were recently used to selectively extract toxic heavy metal oxyanions from water, namely chromate (CrO42–), arsenate (AsO4), and vanadate (VO43–).102,1014,1015 The concentrations of chromium and arsenic were as low as 100 μg L–1, and the experiments were performed in the presence of secondary wastewater components and excess competing ions at 200 times the concentration of the target ions. This process displayed high ion uptake (>100 mg of metal per gram of adsorbent; see Figure 29c), fast kinetics (<5 min of equilibrium uptake) at moderate potentials (≈ 0.8 V versus Ag/AgCl), and nearly complete electrochemical reversibility. The redox-based mechanisms of electrosorption for PVF were inferred from its electrochemical behavior, where an increase in the uptake of anions was correlated with an increase in redox charging.1014 In situ measurements using transmission electron microscopy (TEM) supported the hypothesis that the insertion of ions into redox films was the main mechanism for selectivity, and there was no evidence of plating or phase change of the transition metal during electrosorption.1014 Further analysis revealed that Cr(VI) (hexavalent) was reduced to the less toxic Cr(III) (trivalent) during the release step due to thermodynamic speciation at the electrified surface, favored by regulation of solvent pH at the counter electrode.1014 This Faradaic reaction worked to the benefit of water purification because Cr(VI) (often produced in industrial processes) is carcinogenic, unlike Cr(III) which is an essential element in the human diet.1016−1018
When using metallopolymers for electrosorption, one of the main mechanisms of selectivity is charge transfer. Quantum calculations of electronic structure have pointed to a strong correlation between charge transfer at the redox moieties and the binding energy of the participating anion.978,1014 For example, the binding energies of Cl–, ClO4–, CrO4, and HAsO42– with ferrocenium were calculated to be 2.90, 3.47, 5.53, and 4.73 kcal/mol, respectively. These computations were performed using density functional theory (DFT) with corrections for entropic and solvation effects. Analysis of the binding of ferrocenium to anions, with supporting evidence from nuclear magnetic resonance (NMR) spectroscopy, has also underscored the role of cyclopentadienyl ligands on intermolecular binding. Once oxidized, ferrocenium (both the metal and the ligand) becomes a strong acceptor of charge that is selective toward anions. This selectivity was observed in both aqueous and organic solvents (e.g., acetonitrile), particularly during the removal of carboxylates in the presence of competing anions like hexafluorophosphate.978,1009,1019,1020 The introduction of selective chemical interactions superimposing electrostatics enables selective separations by functionalized redox-metallopolymer electrodes.
More recently, radical-based redox polymers were also found to be highly effective at molecular separations for water purification. Kim et al. showed that TEMPO-based copolymers are effective at selective adsorption of per- and polyfluoroalkyl substances (PFAS) based on their affinity toward these polymers.1021 PFAS contaminants are highly persistent and difficult to both capture and degrade due to their unique physicochemical properties. In the work by Kim et al., redox copolymers with amine functional groups and redox-active nitroxyl radical groups were used to achieve high selectivity and uptake of various PFAS compounds, with separation factors exceeding 500 relative to Cl–. Furthermore, the use of BDD as the counter electrode reduced energy consumption and enabled degradation of PFAS, which was selectively adsorbed on the redox polymer electrode, in the electrode regeneration step. By tuning the ratio of amines to radical groups, the electrochemical regeneration and selectivity toward PFAS could be further improved.1021
4.3.3. Redox Separation of Uncharged Pollutants
Redox systems have recently been used to capture and release uncharged contaminants from water via hydrophilic-to-hydrophobic transitions. Many environmental micropollutants are uncharged pharmaceutical and personal care products (PPCPs) that are hazardous to humans and animals.1022−1025 PPCPs have unique physicochemical properties and are often present in water in trace quantities, which complicates the removal of these contaminants by conventional methods such as adsorption, precipitation, biological treatment, and membrane filtration.1024,1026−1028 As shown in Figure 31, Faradaic electrodes have been used to selectively capture uncharged micropollutants based on the electrochemically tunable affinity of these redox-active electrodes to the contaminants.
Figure 31.
Faradaic systems with electrochemically tunable affinity for controlled capture of uncharged contaminants. (a) Redox of a ferrocene copolymer for separation of butanol from water. Reproduced with permission from ref (1029). Copyright 2011 American Chemical Society. (b) Comparison of the mechanisms of selectivity in CDI, RMSS, and ETAS. (c) Multistage electrochemical concentration of uncharged species using the ETAS process. Reproduced with permission from ref (103). Copyright 2018 Royal Society of Chemistry.
Prior work on chemical switching in redox polymers, shown in Figure 31a, revealed the possibility of extracting an uncharged compound such as butanol from water based on its affinity to a copolymer of hydroxybutyl methacrylate (HBMA) and vinylferrocene (VF).1029 The selectivity of the redox gel to butanol in the reduced state was reported in terms of a separation factor equal to the ratio of the equilibrium distribution coefficients (see eq 15) of butanol and water, which was shown to exceed 5. This selectivity was attributed to the hydrophilic-to-hydrophobic transition and preferential swelling of the solvent within the gel.1029 The concepts presented in this system served as the basis for electrochemically tunable affinity separations (ETAS), which leverage the redox switchable hydrophobicity of polymers to achieve targeted separations. Figure 31b compares the principles of electrostatic CDI, redox-mediated selective separations (RMSS), and ETAS.103 In ETAS, a nanostructured blend of PVF and PPy was used to remove and concentrate a collection of uncharged organic pollutants, including dyes like methyl orange, endocrine disruptors like ethinylestradiol, and toxic chemicals like dichlorophenol and bisphenol. The PVF/PPy electrodes were synthesized by simultaneous electropolymerization of pyrrole and electrodeposition of PVF, which yielded a homogeneous coating of the PVF/PPy system onto a carbon cloth, as shown in Figure 32.103,1030 These nanostructured hybrid materials also possess supercapacitive properties (>500 F g–1) superior to those of their individual components (PVF with 79 F g–1 and PPy with 27.3 F g–1), without the use of carbon nanomaterials.1030 Differential selectivity in ETAS was quantified using the distribution coefficient KD:
| 15 |
where Qe is the adsorption capacity (in mg g–1) and ce is the concentration of the target contaminant at equilibrium. The distribution coefficient is a function of the applied electric potential (see Figure 32f) and the physicochemical properties of the contaminants.103 As shown in Figure 31c, ETAS was implemented in a multistage cyclic batch process to concentrate target uncharged species by several orders of magnitude. The principle of hydrophobic transitions was taken a step further by using complementary asymmetric electrodes.104
Figure 32.
Imaging and analytical characterization of PVF/PPy coatings on a carbon cloth. (a) Schematic illustration of the ETAS adsorbent made of PVF/PPy coated on a flexible carbon substrate. Images of the PVF/PPy system using (b) SEM, (c) TEM, (d) energy dispersive X-ray spectroscopy (EDS) N mapping, and (e) EDS Fe mapping. (f) Adsorption isotherms of PVF/PPy at different potentials. Open circles represent experimental data and solid lines are fits based on the Freundlich adsorption model. Reproduced with permission from ref (103). Copyright 2018 Royal Society of Chemistry.
4.3.4. Electrode Design for Faradaic Electrosorption
As explained in section 4.1.5, parasitic reactions compromise the performance of electrochemical systems.778,1031 These unwanted side reactions lower current efficiency and negatively impact the chemistry of the solution, for example, by producing hydroxides which lower selectivity toward anions. Asymmetric configurations of the electrodes have thus been proposed to mitigate parasitic reactions and improve efficiency. Innovations in this area encompass both Faradaic and non-Faradaic systems and involve the use of asymmetric membranes in CDI configurations,1032 differential functionalization of the electrodes,1033,1034 or implementation of asymmetric redox chemistries.96,902,985 In one such example, the performance of CDI was improved by placing IEMs at the electrodes to improve selective adsorption of counterions and to prevent co-ions from escaping into the bulk, as shown in Figure 33.668,1032
Within the context of Faradaic systems, symmetric and asymmetric configurations have been proposed to improve charge storage in pseudocapacitors1035−1037 and batteries.1038−1040 Similar principles hold with regard to electroseparations: that is, the better the capacity for energy storage, the more ions will be separated and retained. Moreover, systems in which electron transfer occurs at a fixed, well-defined redox potential can help control the voltage window to limit side reactions and inhibit competing Faradaic reactions that result in current leakage.902 These desirable properties can be achieved using asymmetric electrodes made of redox-active metallopolymers, as illustrated in Figure 34. Faradaic intercalation in crystalline systems can also be used to improve the efficiency of electrosorption. For example, symmetric electrodes of CuHCF have been used to reduce the amount of energy needed for desalination (down to 0.02 kW h m–3 for a feed with an initial concentration of 25 mM and a final concentration of 17 mM).937 This system was operated at a much lower cell voltage (i.e., 0.6 V) compared to a standard CDI cell, which reduced parasitic side reactions.937
Figure 34.
Asymmetric Faradaic systems for selective electrochemical separations. (a) Schematic of a Faradaic system with redox-active polymer electrodes, namely a ferrocene metallopolymer (PVF) for the anode and a cobaltocenium metallopolymer for the cathode. (b) Characterization of the electrochemical system and corresponding redox reactions by cyclic voltammetry. Reproduced with permission from ref (902). Copyright 2017 Royal Society of Chemistry.
Asymmetric configurations have the advantage of increasing energy storage at each of the electrodes by promoting the appropriate electron transfer reactions, namely oxidation at the anode during charging and reduction at the cathode. In the case of electrosorption, asymmetric Faradaic designs have been used to capture anions at the anode and cations at the cathode.86,96,154,711 One example of redox-active systems that enabled selective electrochemical separations was made of ferrocene (PVF) and cobaltocenium metallopolymers on opposite electrodes, respectively.902 The two electrodes operated within the window of water stability and, more importantly, their redox reactions allowed for selective capture of anions from solution and subsequent control of the voltage window with 96% current efficiency. By carrying out these redox reactions at low voltages (i.e., 0.4 and −0.6 V versus Ag/AgCl for the anode and cathode, respectively), the pH of the system was stable and there was significant improvement in the efficiency with which anions were removed from dilute solutions. Furthermore, the selective properties of the PVF system were preserved upon capture of anions by preventing the formation of hydroxides.902 In subsequent studies, it was shown that the organometallic cobalt center could be tuned using a tetraphenyl ligand,1041 which enabled simultaneous recovery of cations and anions.902
More recently, hexacyanoferrate (HCF) electrodes were combined with redox-active polymers to achieve selective electrochemical separations that can be carried out with extremely small swings in voltage (<0.1 V) by redox matching at the electrodes.985 As shown in Figure 35a, both anions and cations were selectively captured by combining an electrode made of PVF with another made of HCF. The redox potential of the cathode was shown to be tunable based on the content of mixed metal (see Figure 35b), which enabled structural control of the window of operating voltages down to ∼0.1 V at a current density of 1 A m–2 for a two-cell system. This feature also improved anion selectivity and separation efficiency, as demonstrated in Figure 35c,d, which highlights the potential of hybrid redox polymers with crystal electrodes for efficient separations.985
Figure 35.
Asymmetric redox system using iron active centers for selective separations. (a) Schematic of the asymmetric Faradaic cell, in which a ferrocene metallopolymer is oxidized at the anode (blue) and an HCF crystal is reduced at the cathode (red). (b) Cyclic voltammetry shows that the HCF electrode can be structurally tuned to control the redox potential. (c) Cell potential during electrosorption and (d) recovery of molybdenum anions for different electrode chemistries. Reproduced with permission from ref (985). Copyright 2020 Wiley-VCH.
In addition to their high efficiency, asymmetric redox systems combine separations and reactions to chemically transform harmful contaminants into benign species. By combining an electrosorption working electrode with an electrocatalytic counter electrode, Kim et al. found that As(III) (trivalent) can be captured and transformed into As(V) (pentavalent), as shown in Figure 36a.102 The former is a naturally and artificially occurring contaminant in groundwater that is highly toxic, even though it is often present at low concentrations.1042−1044 Capturing this contaminant in the presence of competing species and transforming it into As(V) is a major challenge in water purification. Because of the affinity of PVF for charge transfer anions, PVF-CNT composite electrodes were used to capture AsO43– and AsO3 during oxidation of the working electrode.978,1014 The counter electrode was coated with poly-TEMPO methacrylate (PTMA), which comprises stable nitroxide radicals that undergo oxidation by one-electron transfer. This system efficiently reduced the concentration of a contaminated solution from 100 ppb to below 10 ppb, the recommended maximum total exposure.1045 Moreover, separation factors of over 49 relative to competing Cl– were achieved during the capture of these oxyanions. Upon release, the PVF electrode liberated As(III), and the counter electrode transformed the anions into As(V) in both batch and flow configurations. By taking advantage of these redox processes, contaminants were simultaneously separated and converted, as described in Figure 36. This dual process reduced energy consumption to 0.45 kW h mol–1 of converted As and increased energy efficiency by an order of magnitude compared to systems that decouple separations from reactions.102 This framework is similar to the use of PVF with a platinum counter electrode to capture and transform Cr(VI) into the less harmful Cr(III). In the latter process, however, the redox transformation occurs at a single PVF electrode which adsorbs Cr(VI) during oxidative charging and catalyzes its reduction during discharging, while water splitting occurs at the counter electrode to regulate pH.1014 A study recently revealed the role of oxygen in ferrocene-mediated conversion of arsenic, where production of peroxide aided the regeneration of active ferrocenium units.1046 The study also showed how PVF systems can be used for efficient remediation of groundwater contaminated with arsenic.
Figure 36.
Simultaneous capture and conversion of As(III) into As(V) using redox-active electrodes. (a) Schematic of the asymmetric electrochemical system with electrodes made of PVF (electroadsorbent) and PTMA (electrocatalyst). (b) Flow diagram explaining the speciation of arsenic in this system. Reproduced with permission from ref (102). Copyright 2020 Wiley-VCH.
Asymmetric systems have been designed with complementary hydrophilic-to-hydrophobic transitions to increase the capacity of adsorption of uncharged organic contaminants.104 As an example, one electrode was coated with the PVF/PPy hybrid introduced in section 4.3 and the other with PPy containing the amphiphilic surfactant dioctyl sulfosuccinate (DOSS, known commercially as AOT). When paired, these two electrodes exhibit hydrophilic-to-hydrophobic transitions that are complementary and occur at opposite electrodes. That is, the PVF/PPy electrode becomes hydrophilic when oxidized and hydrophobic when reduced, whereas the PPy/AOT electrode becomes hydrophobic when oxidized and hydrophilic when reduced. As shown in Figure 37, this design enables capture of hydrophobic molecules like Sudan Orange G (SOG), which is released from both loaded electrodes during charging (oxidation at PVF/PPy and reduction at PPy/AOT).104 The PPy/AOT electrode was developed to achieve superhydrophobic properties and strong π–π interactions with uncharged aromatic species.1047 During electrostatic charging, the surfactant dopants are reoriented to modulate hydrophobicity, an interaction that was explored in detail using DFT and MD simulations.1047 While discharging, the asymmetric system of PVF/PPy and PPy/AOT was found to be nearly 20 times more energy efficient than systems based on activated carbon (12 J g–1 of adsorbent for the asymmetric redox system and 235 J g–1 of adsorbent for activated carbon).
Figure 37.
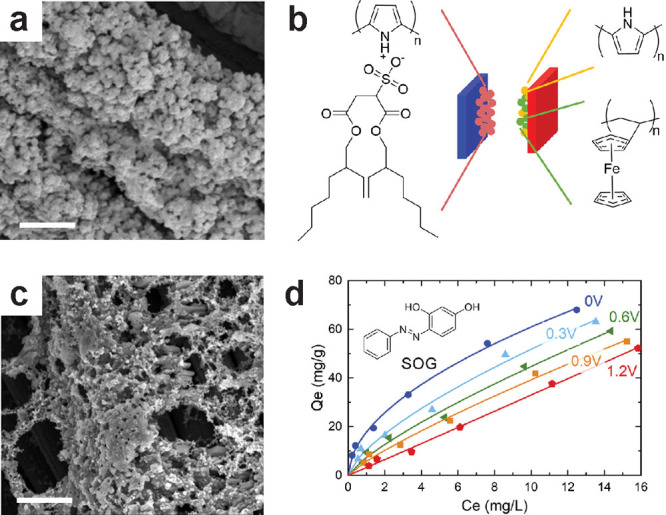
Asymmetric electrochemical system with tunable hydrophobicity. Complementary redox-active electrodes made of (a) PVF/PPy and (c) PPy/AOT nanostructures; scale bars are 10 μm. (b) Schematic of the asymmetric system with electrochemically modulated affinity toward uncharged organic molecules. (d) Adsorption isotherms of SOG for various applied potentials. Filled markers represent experimental data, and solid lines are fits based on the Freundlich adsorption model. Reproduced with permission from ref (104). Copyright 2019 American Chemical Society.
Finally, these asymmetric systems have been redesigned into flow configurations, in which CNTs coated with PVF and PPy (doped with the anionic surfactant dodecylbenzenesulfonate, DBS) were used as the electrodes to selectively remove organic contaminants.1048 This system integrated in situ ultraviolet, conductivity, and pH sensors for online monitoring, as shown in Figure 38, and it displayed a high capacity and rate of salt adsorption compared to CDI systems in the literature.1049 On the basis of dynamic measurements of adsorption and release, the flow platform was also selective toward benzoate, a compound representative of toxic carboxylates, in the presence of competing electrolytes, which were 50 times more concentrated than the target anion. This work demonstrated the feasibility of using asymmetric redox designs as on-site systems for water purification.
Figure 38.
Selective adsorption of organic anions in a flow cell with asymmetric redox-active electrodes. (a) Schematic of the continuous electrosorption system with integrated sensing. (b) Cyclic voltammetry of carbon substrates functionalized with PVF-CNT, PPy-DBS-CNT, and CNT. Reproduced with permission from ref (1048). Copyright 2020 Elsevier.
5. Inverse Methods of Energy Conversion
Generating clean, renewable energy is just as important as producing purified water for environmental sustainability. In this section, we introduce what are known as “inverse methods” of energy conversion and draw parallels between these systems and the methods of water purification discussed above. The electrochemical inverse methods that we describe here include reverse ED, capacitive mixing (CapMix), and battery mixing (BattMix). These technologies are similar in that they retrieve energy from the mixing of two streams between which there is a gradient in concentration (or more precisely in chemical potential), such as river water and seawater. They differ, however, in how they are designed and operated, but as we will show in this section, reverse ED, CapMix, and BattMix are in fact just ED, CDI, and Faradaic electrosorption, respectively, operated in reverse. For completeness, we also review electrokinetic conversion of mechanical energy to electrical energy based on harvesting the streaming current produced by pressure-driven flow in charged microchannels or porous media.
Although the scope of this review is limited to electrochemical methods, we note that there exists a physical, membrane-based inverse method known as pressure-retarded osmosis (PRO), which converts the free energy of mixing into useful electrical power.1050−1055 In this process, two streams of different concentrations are separated by a semipermeable membrane that allows only water to pass by osmosis. The imbalance in chemical potential across the membrane promotes transport of water from diluate to concentrate. This osmotic flow can be stopped if enough hydraulic pressure is applied to the concentrate. The applied pressure needed to maintain osmotic equilibrium is called osmotic pressure and is determined entirely by the composition of the solution. PRO thus occurs as long as the applied pressure is less than the osmotic pressure, and because water flows against this gradient in hydraulic pressure, the free energy of mixing can be converted into mechanical work.
5.1. Inverse Electrokinetic Methods
5.1.1. Reverse Electrodialysis
In reverse
ED, the system is fed dilute and concentrated streams that are partitioned
by alternating AEMs and CEMs. This stack is sandwiched between metal
electrodes that are connected through an external load to which power
is delivered, as shown in Figure 39.1056,1057 Transport of cations across the CEMs and anions across the AEMs
produces a flux of electric charge between the electrodes. The magnitude
of the resulting electric potential is related to the difference in
chemical potentials of the salt in adjacent streams, and the voltages
across each membrane are additive.1056−1058 Natural runoffs and
rivers in coastal areas are abundant sources of both dilute feed that
is usually left to mix with seawater and energy that is produced without
capture.1059 It was estimated that 2.3 MJ
of work could theoretically be extracted from each cubic meter of
river water that flows into the sea.1056,1059,1060 With the typical discharges of all rivers combined,
the net power produced by this unconventional source of energy was
estimated to be between 2.4 and 2.6 TW.1056,1061 Of course, not all of this energy could be harvested by reverse
ED, but the question is exactly how much can be extracted? Forgacs
conservatively predicted a yield of 0.35 MJ per cubic meter of river
water,1062 Audinos arrived at a similar
yield and calculated an energy recovery of 21% (excluding losses due
to pumping and power inversion),1063 and
Jagur-Grodzinski and Kramer experimentally determined a yield between
0.25 and 0.6 MJ per cubic meter of river water.1064 Długołȩcki et al. then
derived an equation to calculate the maximum power output  based on the properties of the system and
its components:1065
based on the properties of the system and
its components:1065
| 16 |
where N is the number of
membrane pairs, A is effective membrane area, αavg is average membrane permselectivity, a is thermodynamic activity,  is membrane resistance (in Ω m2), w is compartment width, κ is electrolyte conductivity, and subscripts “c” and
“d” represent the concentrate and diluate, respectively.
is membrane resistance (in Ω m2), w is compartment width, κ is electrolyte conductivity, and subscripts “c” and
“d” represent the concentrate and diluate, respectively.
Figure 39.
Operating principles and empirical results of reverse ED. (a) Dilute and concentrated streams are passed through a stack of alternating AEMs and CEMs. Differences in the chemical potentials of adjacent streams generate an electric potential across each membrane, and the total voltage is the sum of the potential differences across each membrane. In most reverse ED systems, reversible redox couples (e.g., Fe2+/Fe3+, [Fe(CN)6]4–/[Fe(CN)6]3–) in a supporting electrolyte (e.g., NaCl–HCl) are used as electrode streams to convert ion flux into electrical current.1066−1068 (b) Power density versus discharge salinity for various pairs of low salinity (LS) and high salinity (HS) feeds. Reproduced with permission from ref (1069). Copyright 2018 Elsevier.
Early studies of reverse ED showed that little energy is recovered relative to what is theoretically available. Post et al. later investigated how much energy can be recovered in reverse ED by distinguishing between internal and external losses of energy and by focusing exclusively on the former1059 (external losses arise due to pumping and power inversion and can only be quantified in a full-scale, optimized system). In the literature, it is explained that internal losses arise due to ionic shortcut currents,1070 internal resistances (e.g., friction losses, pumping requirements, electrochemical overpotentials),1064,1071,1072 concentration polarization,1056,1073 and osmotic transport.1074 Internal losses must therefore be reduced to improve energy efficiency, which can be achieved by developing improved electrode systems (to lower electrochemical overpotentials),1075 introducing air bubbles to the feed (to decrease ionic shortcut currents),1059,1070 improving stack hydrodynamics and spacer design (to reduce concentration polarization),1073 and designing spiral wound modules (to decrease membrane area).1076,1077 Using some of these principles, Post and Veerman et al. designed systems that, in the best case, recovered more than 80% of the work made available by mixing artificial river water and seawater.1059,1070 Several other studies1069 in the past decade also focused on optimizing the performance of reverse ED systems by modeling and developing new IEMs,1078−1081 spacers,1082 and electrodes.1083 At the same time, researchers focused on improving the overall operation of the stack,1084,1085 understanding the impact of fouling and mitigating it (see section 3.6),1086−1089 and combining reverse ED with desalination technologies.1090−1095
The fastest growing areas of research over the past five years, however, are related to applications for energy storage,1096,1097 developing micro- and nanofluidic reverse ED systems,1098,1099 and pilot studies for commercial use.1100−1103 Pilot studies are essential to bridge the gap between experimentation at the laboratory scale and implementation at the industrial scale. So far, pilot plants for reverse ED exist in only a few countries, including The Netherlands and Italy. The first of these plants was commissioned in 2014 in Afsluitdijk, a major dam in The Netherlands, and it features a dyke that is 32 km long which separates the IJssel Lake and the Wadden Sea.1069 This plant produces electrical energy (the amount of which has not been made available to the public) from controlled mixing of fresh water with seawater. The pilot plant in Italy was commissioned near the Ettore e Infersa Saltworks in Trapani, and it mixes saturated brine from the saltworks with brackish water from a nearby shoreline well to produce an average of 0.8 W m–2 of membrane in total.1069,1100,1101 Future work in this area will largely focus on improving power density, performing complete cost analyses, and building plants with greater capacity.1057,1074
5.1.2. Electrokinetic Energy Conversion
Although this review focuses on the interplay of electrical and chemical energy, we discuss here how linear electrokinetic phenomena (see section 3.3) can also be exploited to directly convert mechanical energy to electrical energy via the forced motion of liquid electrolytes in charged microchannels and pores. In 1964, Osterle introduced the basic idea of mechanical electrokinetic energy conversion by harvesting the streaming current produced by pressure-driven flow in a charged capillary.1104 Morrison and Osterle then estimated a maximum efficiency of 0.9% for ultrafine water–glass capillaries.1105 Gross and Osterle comprehensively analyzed energy conversion by linear electrokinetic phenomena (recently extended by Peters et al.504) and estimated a maximum electroosmotic conversion efficiency of 4%.505 This approach had attracted relatively little attention until its rediscovery in the context of microfluidics.1106 The possibility of measuring and controlling the streaming potential in single nanochannels renewed interest in advancing this classical method through nanoscale engineering.1107−1110 Indeed, van der Heyden et al. predicted theoretical conversion efficiencies of up to 12%1108 and experimentally demonstrated 3% efficiency in 75 nm-thick silica glass nanochannels using dilute KCl electrolytes.1109 Considering conduction through the Stern layer can further reduce the theoretically predicted efficiency.1111 Recent advances with soft, flexible microchannels have allowed for practical applications of electrokinetic energy conversion in wearable and portable self-powered devices.1112,1113
Significant developments have also followed from theoretical predictions of massive enhancement of electrokinetic phenomena for charged surfaces with hydrodynamic slip.1114−1116 Ren and Stein predicted that hydrodynamic slip could increase nanofluidic energy conversion up to 40% for hydrophobic surfaces with slip lengths on the order of tens of nanometers and up to 70% in CNTs or graphitic systems based on reported apparent slip lengths.1117 These theoretical values have proven difficult to achieve in electrokinetic energy conversion with homogeneous solid–liquid interfaces, and there are lingering signs of knowledge gaps in our understanding of electrokinetic phenomena under nanoconfinement.490 For example, the apparent slip observed for CNTs is absent in crystallographically similar boron nitride nanotubes with different electronic structures.496 As a possible alternative to homogeneous surfaces, Bagha et al.1118 predicted that micropatterned, superhydrophobic surfaces with high slip and charged liquid–gas interfaces could increase efficiency at larger length scales, and this concept remains a subject of research.1119−1121
5.1.3. Reverse Electrowetting
Electrocapillary phenomena involve interactions between liquid electrolyte interfaces and charged solid surfaces or electrodes. The classical example is electrowetting, where an applied voltage changes the surface tension of a droplet via energy stored in EDL capacitors and thus manipulates the shape of an electrolyte droplet.1122,1123 Krupenkin and Taylor first proposed the inverse effect of reverse electrowetting to convert mechanical energy to electrical energy, where immiscible electrolyte droplets were compressed by the motion of parallel-plate electrodes.1124 Kolomeisky and Kornyshev suggested an alternative approach based on the reversible penetration of a liquid electrolyte into a solvophobic porous electrode, which could be used in a “double layer shoe” to harvest electricity from walking motion.1125 Analogous to the use of moving wire electrodes in CDI for desalination,855 capacitive rotors have been proposed to generate alternating current from mechanical motion by exploiting a time-varying EDL capacitance.1126
5.2. Inverse Electrosorption Methods
5.2.1. CapMix
In 2009, Brogioli proposed a method (later called “capacitive double layer expansion,” or CDLE) to extract energy from the concentration gradient between two feeds, as shown in Figure 40.1127 Unlike reverse ED and PRO, CDLE was founded on the concept of an electrochemical capacitor (obtained by immersing two electrodes in an electrolyte),151,1128,1129 which is the basis of water purification in CDI technologies.110 CDLE can therefore be described as CDI operated in reverse: current is applied to the capacitor when filled with salt water, co-ions are repelled and counterions are attracted at each electrode, and EDLs are formed to store electric charge. After the EDLs are charged (an input of energy) the cell is flushed with fresh water to increase the electric potential, and in turn the electrostatic energy, of the system. The capacitor is then discharged (an output of energy) after which the cell is flushed with salt water to decrease the electric potential for a new cycle. This four-step cycle is demonstrated in Figure 40, and it reveals the possibility of extracting surplus energy due to a concentration gradient in the capacitor.
Figure 40.
Schematic and operating procedure of CDLE, the original CapMix system. (a) The device comprises two electrodes made of porous activated carbon, and this pair of electrodes behaves as a capacitor that can be charged and discharged. Two reservoirs contain solutions with different concentrations that are pumped to the cell where they are mixed. (b) Graphical explanation of the four-step operating procedure of CDLE. In phase A, the electrical circuit is closed and the cell is charged; in phase B, the circuit is opened and the cell is flushed with fresh water to increase the electric potential; in phase C, the circuit is closed and the cell is discharged; and in phase D, the circuit is opened and the cell is flushed with salt water to decrease the electric potential. Reproduced with permission from ref (1127). Copyright 2009 American Physical Society.
Brogioli explained the dynamics of EDLs in CDLE using the GCS theory501−503,1130 and deduced that this process can achieve an energy output of 0.44 kW h per cubic meter of fresh water. By cycling the system at a rate of 1 Hz, the power output of CDLE would be comparable to that of membrane technologies for energy production. In 2011, Brogioli et al. assessed the performance of CDLE in a larger prototype and observed an efficiency of up to 20 times what was reported originally in ref (1127).1131 To analyze the dynamics of CDLE and improve subsequent designs of these prototypes, Rica et al. mathematically modeled the electrodiffusion of ions as well as their adsorption and desorption in porous electrodes.1132,1133 These models, which accounted for adsorption of both charge and salt, were based on a macroscopic formulation of ion diffusion and Ohm’s law in porous media.
The original design by Brogioli had intrinsic technical deficiencies in that it was sensitive to impurities like DO and exhibited self-discharge.1134 Several research groups improved the efficiency of energy conversion by introducing IEMs to the supercapacitor flow cell.1135−1139 This system, which came to be known as capacitive Donnan potential (CDP), combined elements of reverse ED and Brogioli’s capacitive method to both produce an electric potential across an IEM and store charge in the EDLs of porous electrodes.1140 The method of Sales et al. was based on a fixed external resistance, and their work was followed by a study in which Liu et al. supplemented the charging cycle using an external power supply to ultimately draw more power.1141 Shortly afterward, Brogioli et al. discovered that activated carbon behaves as a polarizable electrode on short time scales but reverts to its spontaneous (self) potential on long time scales, which limits the maximum power output of CapMix systems that use activated carbon as electrodes.1142,1143 The authors attributed this effect to leakage of stored charge caused by unwanted electrochemical reactions.
To overcome the limitations of traditional carbon electrodes, researchers examined new geometries and arrangements for both the electrodes and IEMs, which enabled continuous operation666,1144 and increased power output.1145,1146 These advancements were accompanied by the use of novel materials for the electrodes, such as porous activated carbon coated with polyelectrolytes, to reduce the leakage current.1147−1151 These so-called “soft electrodes” generate electrical energy in response to changes in both the capacitance of the EDL and the Donnan potential of the polyelectrolyte coat during exchange of electrolytes.
5.2.2. BattMix
In 2011, La Mantia et al. introduced a novel electrochemical system called the “mixing entropy battery” that extracted energy from a concentration gradient but then stored the energy in the bulk crystal structure of the electrodes.889 The novelty of this BattMix system was in the use of a redox-active material, namely NMO, for the cathode (with Ag/AgCl for the anode), which improved the performance and efficiency of energy harvesting compared to supercapacitor electrodes made of activated carbon.1152 The BattMix cell is first charged by applying an electric field that electrochemically transports Na+ and Cl– from the corresponding electrode into a dilute electrolyte. The cell is then discharged by exchanging this electrolyte for a concentrated one, which drives Na+ to intercalate into the MnO2 cathode and Ag to be oxidized to Ag+ at the anode. Because the energy produced during discharging is greater than that expended during charging, net-positive energy can be harvested from this controlled mixing of two electrolytes. Following La Mantia et al., the scientific community developed and tested other redox-active materials (see section 4.2), such as PBAs,1153−1157 inorganic compounds of metals,1158−1160 and copper ammonia redox couples,1161,1162 as well as conventional battery electrodes, such as zinc and silver.1163,1164
Like capacitive electrodes in CapMix, redox-active electrodes in BattMix are electrical accumulators that can store and release charge, and these two methods are broadly classified as accumulator mixing technologies.1165 Marino et al. examined the electrochemical kinetics of these technologies and determined that supercapacitors (CapMix) can deliver a larger specific power than batteries (BattMix).1166 The authors also observed that the kinetics of capacitive electrodes are controlled by the diffusion of ions in the electrolyte,1133 whereas the kinetics of redox-active electrodes are controlled by the diffusion of intercalated sodium.1167 To limit the shortcomings of each class of materials, Lee et al. designed a hybrid accumulator mixing system that comprised a battery electrode (NMO), a capacitive electrode (activated carbon), and an AEM.1168 This system extracted an amount of energy per unit membrane area three times greater than that achieved by previous CapMix designs, including those with IEMs. Tan and Zhu also built a hybrid accumulator mixing system using CuHCF for the cathode and Bi/BiOCl for the anode.1149 Instead of including an IEM, however, Tan and Zhu coated the electrodes with a polyelectrolyte material to boost power output. Future work in the area of electrochemical energy harvesting includes creating better and more cost-effective materials, improving device configuration and assembly, and carrying out pilot studies.1169
6. Performance Comparisons and Process Intensification
Now that we have presented and discussed the basic principles of emerging electrochemical methods for water purification, we compare the performance of these technologies by quantifying energy demand, energy efficiency, and performance trade-offs. A fair and comprehensive comparison of these metrics is generally difficult, and only a few studies have been published which mainly compare one electrochemical method (typically ED or CDI) with RO. In this section, we expand the existing work on performance comparisons by quantifying similarities and differences between the electrochemical methods discussed in this review. From these comparisons, we highlight the kinds of applications where each technology could provide the greatest benefit. Finally, we conclude this section by discussing pathways and challenges associated with scale up as well as opportunities for process intensification by combining electrochemical methods to achieve unique capabilities.
6.1. Energy Demand
Electrochemical methods have the potential to lower the cost of processing brackish water (<15 g of total dissolved solids per liter) as well as feeds that are dilute (<1 g of total dissolved solids per liter) but contaminated with hazardous substances.123,1170 Unlike conventional desalination methods (e.g., distillation, RO), electrochemical processes are well suited for these tasks because their demand for electrical energy is often proportional to the amount of salt removed (for instance by electrokinetics or electrosorption) and not the volume of water treated. To demonstrate how this distinction influences energy demand, Hemmatifar1171 compared RO with a generic electrochemical process (EC) based on the analyses in refs (1170 and 1172). RO consumes energy, ÊRO (per unit volume of permeate), primarily in the form of mechanical work needed to overcome of the osmotic pressure of the feed:
| 17 |
where ΔP and Δπ are the differences in hydrodynamic and osmotic
pressures, respectively, and γ is water recovery. The hydrodynamic
pressure difference is related to productivity, 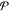 (volumetric throughput normalized by active
surface area, in units of L h–1 m–2), and membrane permeability, Lp (in units of m s–1 Pa–1), by Fick’s law:
(volumetric throughput normalized by active
surface area, in units of L h–1 m–2), and membrane permeability, Lp (in units of m s–1 Pa–1), by Fick’s law: 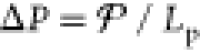 . The osmotic pressure difference is related
to the difference in salt concentration, Δc, across the membrane by the van’t Hoff equation: Δπ = iRTΔc,
where i (≈ 2) is the van’t Hoff factor.
. The osmotic pressure difference is related
to the difference in salt concentration, Δc, across the membrane by the van’t Hoff equation: Δπ = iRTΔc,
where i (≈ 2) is the van’t Hoff factor.
In contrast to RO, electrochemical processes consume electrical energy to transport ions and activate electrochemical reactions. These processes are subject to losses in the form of overpotentials, which represent energy lost as heat to the surroundings. Overpotentials in electrochemical systems are grouped into three categories, namely activation (the potential difference above the equilibrium value needed to produce a current in a redox event), concentration (the potential difference across a diffusion layer near the electrode), and resistance (the potential difference caused by resistances in conducting ions and electrons).1173 Hemmatifar showed that the energy demand, ÊEC (per unit volume of diluate), can be approximated based on ionic and Ohmic (resistive) losses:90
| 18 |
where I is current (assumed
to be constant under galvanostatic operation) and 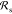 is the equivalent resistance of ionic and
Ohmic losses in series. This approximation holds unless the separation
is slow and parasitic reactions dominate. Electrical current is related
to the change in concentration as I ≈ FQΔc/Λ, where Λ is cycle
efficiency. The quantity Λ equals the ratio of salt removed
to electric charge fed (both in moles), and it is used to empirically
account for coupled inefficiencies due to charge transfer, EDLs, and
fluid flow.1171,1174Figure 2 presents estimates of the volumetric energy
consumed by RO and EC using the equations above and the parameters
in ref (1171).
is the equivalent resistance of ionic and
Ohmic losses in series. This approximation holds unless the separation
is slow and parasitic reactions dominate. Electrical current is related
to the change in concentration as I ≈ FQΔc/Λ, where Λ is cycle
efficiency. The quantity Λ equals the ratio of salt removed
to electric charge fed (both in moles), and it is used to empirically
account for coupled inefficiencies due to charge transfer, EDLs, and
fluid flow.1171,1174Figure 2 presents estimates of the volumetric energy
consumed by RO and EC using the equations above and the parameters
in ref (1171).
These results confirm that although the energy required by RO is a linear function of concentration when the feed is salty (and Δπ is large compared to ΔP), it becomes nearly independent of concentration in the dilute limit. Thus, for feeds that would be classified as either brackish water or fresh water, ÊRO is bounded by the mechanical work needed to sustain the hydrodynamic pressure, which is independent of concentration. The energy demand of EC, on the other hand, scales approximately as (Δc)2 over the entire range of concentrations, which implies that electrochemical processes should in theory be significantly less energy intensive than RO for desalination of dilute feeds. Although the estimates in this analysis are based on a specific set of parameters (e.g., water recovery, productivity, cycle efficiency), the general trend in energy consumption can be generalized to any electrochemical method that removes solute from solvent. Electrochemical processes thus offer a unique opportunity to efficiently purify brackish water and other dilute but contaminated feeds.
6.2. Energy Efficiency
Although we have shown that electrochemical methods should theoretically be efficient in the dilute limit, the energy demand in practice has so far been tens or hundreds of times the thermodynamic minimum (see Figures 2 and 42). These values correspond to thermodynamic efficiencies, ηthermo (ratio of the free energy of separation to the input of electrical work), of only a few percent, which reveals the extent of dissipation (or energy loss) in state-of-the-art electrochemical systems. This gap between the thermodynamic limit and real efficiencies, however, is expected for emerging methods and presents an opportunity for the improvement of electrochemical technologies. In the early days of RO, for example, thermal distillation used to be the state-of-the-art method to desalinate seawater. But from the 1970s to 2008, the consumption of electrical power by RO systems was reduced by almost 90%, from approximately 16 to 1.8 kW h m–3, which is near the theoretical minimum energy of 1.1 kW h m–3 needed to desalinate seawater with a water recovery of 50%.68 These improvements resulted in the widespread implementation of RO, which today is considered the leading process for seawater desalination based on installed capacity and annual growth.12
Figure 42.
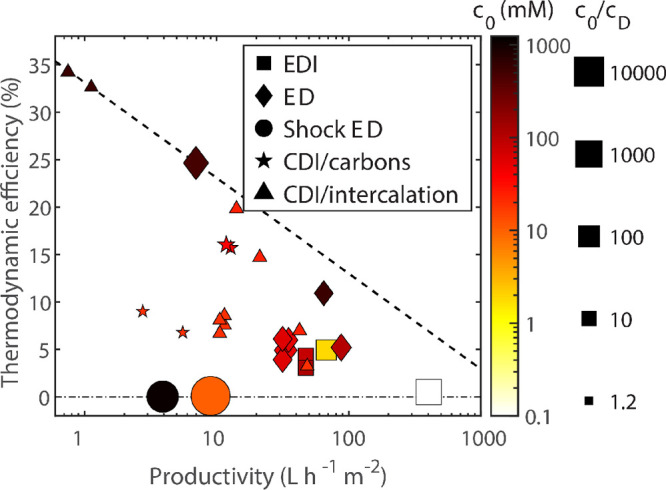
Thermodynamic energy
efficiency as a function of productivity for
various electrochemical methods performing laboratory-scale desalination
of brackish and dilute water. Representative data are presented for
EDI (squares),623,1184,1185 ED (diamonds),342,1186−1189 shock ED (circles),87,584 and CDI with either carbon electrodes
(stars)636,757 or intercalation electrodes (triangles).715,907,937 The color and size of each data
marker represent feed concentration and the ratio c0/cD (see eq 19), respectively. The dashed line
represents an (approximate) empirical efficiency limit and is given
by 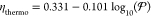 , where
, where 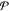 is in units of L h–1 m–2.
is in units of L h–1 m–2.
Dissipation in electrochemical systems leads to inefficiency and may be caused by the dynamics of electrokinetics and electrosorption, ionic and Ohmic losses, parasitic losses due to charge transfer, hydrodynamic dispersion and fluid mixing, or mechanical work required to pump the electrolyte. We emphasize that all electrochemical processes exhibit a complex trade-off between kinetics (measured in terms of productivity) and energetics.90,738,757,759 As predicted by the simple model in eq 18, a fast process (one that is operated at high current) consumes more energy but achieves the same level of separation as a slow process,759 although this fact holds for all desalination processes because more entropy is generated when operating farther from equilibrium.72,1175 Similarly, a slow process may be inefficient because of the dominance of parasitic side reactions,90,757 and so it is critical to understand these trade-offs and quantify the kinetics and energetics of the separation method under consideration.738
With these general guidelines established, researchers have sought to develop efficient electrochemical processes by engineering improved systems, materials, and operating conditions. Systems to improve energy efficiency were designed, such as cyclic recovery of stored energy, reduction of series resistances,753,757 and use of membranes in electrosorption methods.750,1176 Materials to suppress parasitic reactions were developed, including redox-active electrodes96 and intercalation materials,116,127,937 and operating parameters were tuned, like flow rate, current, and voltage.750,755−757,760,1177,1178 The use of IEMs directly adjacent to the electrodes in electrosorption was first reported by Andelman and Walker,1179 and this approach was since pursued by many researchers.634,750,1176,1180,1181 The selectivity introduced by these membranes increases charge efficiency by allowing for voltage reversal and increasing the capacity of adsorption.1181 One disadvantage of using IEMs, however, is that they can incur additional capital costs that exceed those of all other components in an electrochemical device.738
To suppress parasitic side reactions, which often severely compromise efficiency,90 researchers have developed and used redox-active electrodes that guide the transfer of electrons toward redox groups.96 For example, organometallic and metallopolymeric electrodes were used in an asymmetric Faradaic CDI system and led to no appreciable changes in the pH of the treated solution, which indicated that water reactions were indeed suppressed.902 The authors also demonstrated stability of pH when the system was operated in batch mode before complete oxidation of the electrodes. A second class of materials that has been developed to control parasitic side reactions are intercalation electrodes.937 For instance, Kim et al. developed a battery desalination system using identical sodium intercalation electrodes, namely CuHCF, with one or more IEMs to partition successive channels of diluate and concentrate. A triple-stack device with five IEMs showed a 10-fold reduction in energy consumption compared to an MCDI device operated under the same conditions. Again, these improvements in performance must be weighed against the capital cost incurred by using additional membranes.
The third standard approach to improve energy efficiency is to optimize the operating conditions of the process. One example is reported by Hemmatifar et al., who showed that careful selection of the operating conditions in CDI can increase the thermodynamic efficiency up to 9%.757 These high efficiencies were achieved by operating the system at constant current, recovering energy during discharging, balancing the rates of charge transfer and fluid transport (which represent the rates of adsorption and ion advection, respectively), limiting the window of voltages to between 0.4 and 1 V (higher voltages trigger side reactions and smaller ones diminish charge efficiency), and lowering series resistances by using current collectors made of titanium mesh separated by thin (30 μm) spacers. In another study, Ramachandran et al. proposed the use of alternating electrical current and successfully removed much of the salt fed without compromising energy efficiency.1178 The alternating current was sustained at the intrinsic resonant frequency of the system (equivalent to the time constant of an RC circuit), which was shown to be inversely proportional to the geometric mean of residence time and charging time. In a similar way to electrical current, the flow profile can be better controlled to improve performance, particularly in systems that are cyclic like CDI and Faradaic electrosorption. The main issue that arises in systems with uncontrolled flow profiles is hydrodynamic dispersion, which inadvertently mixes diluate (produced during adsorption) and concentrate (produced during desorption). A recent study showed that hydrodynamic dispersion (a phenomenon that increases mass diffusivity by an amount proportional to the square of the average fluid velocity) can be inhibited by reducing the flow rate during the discharging step.760 Using this approach, water recovery was increased to about 90% (compared to 50% with the flow rate fixed) without compromising desalination, productivity, or energy efficiency. In fact, the increase in water recovery enhanced the thermodynamic efficiency by up to a factor of three. Management of electrical input and fluid flow can thus improve existing electrochemical systems at minimal cost.
6.3. Performance Trade-offs
Our discussion in the previous section would be incomplete without quantifying the performance of electrochemical methods because there are underlying trade-offs between kinetics and energetics in any separation process. For this reason, the desalination community uses productivity and energy consumption (per unit volume of diluate) as the two core measures to both quantify performance126,1182,1183 and estimate the capital and operating costs of a plant.738,759 In this section, we examine the relationship between these two parameters and how they collectively influence thermodynamic efficiency.
Constraining the extent of desalination, productivity and (volumetric) energy consumption reveal complex coupling, as shown in Figure 41. This plot condenses data obtained from 30 CDI experiments reported in ref (1171) into a single graph of energy versus productivity. As noted by Hawks et al.738 and Wang et al.,759 these quantities depend on conditions like water recovery, feed concentration, and average reduction in concentration. The data in Figure 41 are interpolated to obtain a curve that corresponds to the following conditions: water recovery is 50%, feed concentration is 20 mM, and product concentration is 15.5 mM. The free energy of separation, ΔĜ (per unit volume of diluate), is known and given by
| 19 |
where the summation is taken over all ions
in solution, 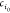 is the feed concentration of ion i, and
is the feed concentration of ion i, and 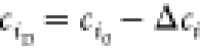 and
and 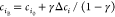 are the concentrations of ion i in the diluate and concentrate (brine), respectively. For a univalent
binary electrolyte of a single salt, eq 19 can be rewritten as757
are the concentrations of ion i in the diluate and concentrate (brine), respectively. For a univalent
binary electrolyte of a single salt, eq 19 can be rewritten as757
| 20 |
The free energy of separation can then be used to determine the thermodynamic efficiency of a given desalination process:
| 21 |
where Ê is the energy consumed per unit volume of treated water.
Figure 41.
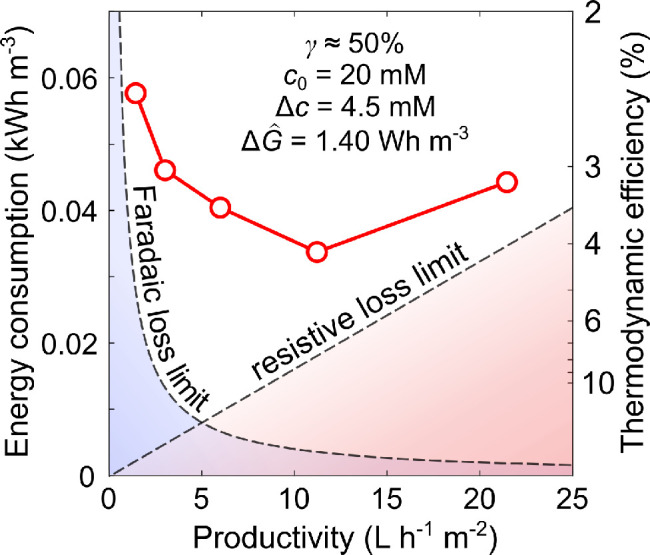
Graphical explanation of the relationship between productivity, energy consumption, and thermodynamic efficiency. Values were obtained by interpolation of data from 30 CDI experiments reported in ref (1171). In this plot, water recovery (γ), feed concentration (c0), and average reduction in concentration (Δc) are prescribed. The dashed straight line and dashed curved line show qualitative lower limits imposed by resistive and Faradaic losses, respectively.
Figure 41 shows the relationship between productivity and energy consumption in a general electrosorption process. This nonmonotonic behavior can be explained by the fact that energy consumption is governed primarily by resistive losses at high current and can be approximated as
| 22 |
where A is total active surface
area and 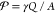 . If cycle efficiency and total resistance
are constant, energy consumption and productivity would be directly
proportional (see refs (738 and 759) for similar conclusions). The dashed straight line in Figure 41 shows a qualitative
lower limit imposed by resistive losses that dominates when productivity
is high. At low current, on the hand, energy consumption is determined
mainly by Faradaic losses90 and can be
approximated as
. If cycle efficiency and total resistance
are constant, energy consumption and productivity would be directly
proportional (see refs (738 and 759) for similar conclusions). The dashed straight line in Figure 41 shows a qualitative
lower limit imposed by resistive losses that dominates when productivity
is high. At low current, on the hand, energy consumption is determined
mainly by Faradaic losses90 and can be
approximated as
| 23 |
where the charging current was replaced by the Faradaic leakage current, IF. This simple model, represented by the dashed curved line in Figure 41, shows that energy consumption is inversely proportional to productivity. With the extent of desalination fixed, thermodynamic efficiency can be improved by carefully balancing the counteracting resistive and Faradaic losses.90
6.4. Desalination
In this section, we quantify the energy consumed by various electrochemical methods for desalination based on data reported in the literature. Figure 42 shows that the energy needed to desalinate brackish water by these methods ranges mostly between 10 and 100 times the theoretical minimum requirement. In contrast, optimized SWRO systems come within a factor of two (excluding pre- and post-treatment steps) of the thermodynamic limit.68 As a result, much research is focused on improving electrochemical systems to reliably desalinate brackish water and remove trace contaminants from dilute feeds with energy inputs closer to the thermodynamic limit. Accomplishment of this task requires innovation and optimization of advanced technologies for ion separations. Electrochemical methods are well positioned to be at the forefront of this effort because, as shown in Figures 2 and 42, they can efficiently desalinate brackish water and dilute feeds under a broad range of operating conditions.
Figure 42 plots the thermodynamic efficiency (eq 21) versus productivity for several electrochemical systems used for desalination. Here, we use the definition of productivity given by Hawks et al.:738
| 24 |
where VD is the volume of diluate, A is the projected membrane or electrode area of a single cell or a cell pair, and n is the number of cells or cell pairs (an alternative definition, which is not used here, is based on total membrane area and sets n = 1; this definition differs from eq 24 by a factor of two). The methods considered in Figure 42 include EDI (squares), ED (diamonds), shock ED (circles), and CDI with either carbon (stars) or intercalation (triangles) electrodes. The color of each marker represents feed concentration, and marker size corresponds to the ratio c0/cD, which quantifies salt rejection. The data in Figure 42 reveal a trade-off between energy consumption and productivity, where systems with higher productivity generally exhibit lower efficiency. We also observe a quantitative trend where all data points fall below a straight line, which we argue is an empirical efficiency limit at this time for desalination by the electrochemical methods considered in this review. Devices near this line are among the most efficient electrochemical systems currently used for desalination. In the future, further development and optimization will lead to devices that can surpass this empirical limit.
On the basis of Figure 42, CDI with membranes and intercalation electrodes as well
as ED are the most efficient electrochemical systems for desalination,
with maximum values of 34.2% for CDI 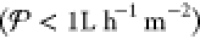 and 24.6% for ED
and 24.6% for ED 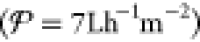 . At the same time, these systems have primarily
been used to remove small quantities of salt (c0/cD equals up to 5.6 for carbon
CDI, 1.5 for intercalation CDI, and 44 for ED). In contrast, methods
like EDI and shock ED display lower energy efficiency but have mainly
been used to deionize water (c0/cD equals up to 500 for EDI and 104 for shock ED). For nearly all the systems presented in Figure 42, water recovery
ranges between 31% and 86% (in this context, water recovery is defined
as the ratio of diluate volume to total feed volume, including electrode
streams). One ED system, however, achieved only 4% water recovery
(ηthermo = 4.91% and
. At the same time, these systems have primarily
been used to remove small quantities of salt (c0/cD equals up to 5.6 for carbon
CDI, 1.5 for intercalation CDI, and 44 for ED). In contrast, methods
like EDI and shock ED display lower energy efficiency but have mainly
been used to deionize water (c0/cD equals up to 500 for EDI and 104 for shock ED). For nearly all the systems presented in Figure 42, water recovery
ranges between 31% and 86% (in this context, water recovery is defined
as the ratio of diluate volume to total feed volume, including electrode
streams). One ED system, however, achieved only 4% water recovery
(ηthermo = 4.91% and 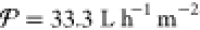 ) due to the high flow rates of the electrode
streams, which were not recirculated.1187
) due to the high flow rates of the electrode
streams, which were not recirculated.1187
6.5. Scale Up and Optimization
6.5.1. Electrodialysis
As explained in section 3.1, the cost of desalination by ED depends on the concentration of ions in the feed. In electromembrane processes, ion concentration determines current density and membrane area, both of which contribute to capital and operating costs and influence process efficiency. For example, a more conductive membrane results in faster ion transport, lower stack resistance, and less energy consumption. Faster ion transport also reduces the membrane area required by the device. Meanwhile, to separate the various components of a feed, the system needs a selective membrane. Numerous studies have focused on preparing IEMs with improved electrochemical properties, for example by employing functional nanomaterials and advanced surface modifications.343,1190−1192 These efforts have shown noticeable improvements in the electrochemical properties of IEMs and ultimately in the performance of ED systems.124 Today, ED stacks can operate at industrial throughputs and are commercially available from many companies, including PCCell, Suez Water Technologies and Solutions, Pure Water Group, and Hydro Volta. These ED systems have been deployed for a variety of applications, including brine desalination, wastewater treatment, nutrient recovery (e.g., nitrogen, phosphorus), whey and sugar demineralization, juice deacidification, and wine stabilization.125,416,434,1193−1198
In most of these applications, ED systems are subject to organic fouling, as discussed in section 3.6.1199 Strong hydrophobic interactions between the membrane and organic species can lead to irreversible adsorption and an increase in membrane resistance.1198,1199 Surface modifications with polyelectrolytes have thus attracted much interest in the design of fouling-resistant IEMs.344,626,1200,1201 These modifications significantly increase the hydrophilicity of membrane surfaces and are simple to perform. Today, IEMs are manufactured by several companies, including DuPont (Nafion), Chemours (Nafion), ASTOM (Neosepta), AGC Chemicals (Selemion), and FUMATECH BWT (Fumasep), and much research has been devoted to improving the durability and lowering the cost of these membranes.417,422,1202,1203
In practice, an ED stack may comprise hundreds of membrane pairs to achieve a sufficient membrane area when operating at high throughput.416 A large number of membrane pairs and compartments, however, increases the resistance of the stack, and so ED is usually built with thin spacers and gaskets (thicknesses are commonly in the range of 0.5–2.0 mm) to lower this resistance.1204 The narrow channels and compartments created by these thin spacers also induce a high cross-flow velocity, which elevates the pressure difference between the diluate and concentrate compartments. This pressure difference must be controlled to prevent excessive stress on the membranes.1205 Another effective way to decrease stack resistance and improve performance is to use thinner membranes.1065,1206 Reducing the thickness of the membranes, however, must be accompanied by improving their mechanical properties, as thinner membranes can become mechanically delicate. Profiled (or patterned) membranes are some of the latest developed IEMs that are used to boost the performance of electromembrane processes. With their nonflat, patterned surfaces, profiled membranes enhance fluid mixing at interfaces and in turn improve ion transport.1207 In the future, ED may even require no spacer, which would further reduce the stack resistance and fabrication cost of ED.
6.5.2. Electrodeionization
One aspect of EDI that remains an important research topic is resin configuration.482 Devices with mixed-bed configurations are commonly used to boost the removal of ions present at low concentrations by exploiting water dissociation. Random contact between cation and anion exchange resins in this configuration, however, may negatively impact ion transport due to reverse junction leakage.1208 Separated and layered beds are alternative configurations that improve current efficiency by providing more pathways for ion transport,1208 although the stack may need additional compartments or special spacer designs to maintain the resin in layers.
The loose resin beads in EDI can accumulate at the end of compartments or near the electrolyte outlets, which increases the pressure drop across the device.1209 Accumulation of resin beads can also lead to an uneven distribution of current and suboptimal ion transport. One solution to this problem is to introduce ion exchange resin wafers, which are made of resin beads bound and immobilized into a porous matrix.83,456,465,1210−1212 These wafers ensure a mechanically stable distribution of the resin and prevent accumulation of resin beads. The binder material used in resin wafers could be either insulating83 or conducting,466 and water splitting could be promoted by incorporating a catalyst in Janus bipolar resin wafers.465
Additional design features have been introduced to EDI systems to further improve their performance. One example, known as fractional EDI, involves the division of an EDI cell into a sequence of stages to optimize the distribution of electrical current.481,1213 A fractional EDI cell comprises two or more separated pairs of electrodes to divide the power supply into amounts appropriate for each pair. In this way, the energy consumption can be lowered, and selective separations based on the charge of the impurities can be achieved.1213 Moreover, this design is better at limiting the formation of mineral scale because concentrates from different fractions are separated, which prevents interaction between multivalent ions from one fraction and the OH– generated in another.
6.5.3. Shock Electrodialysis
The principles discussed in sections 6.5.1 and 6.5.2 are also relevant to the engineering of shock ED, although scaled-up commercial systems have yet to be demonstrated. As in ED or EDI, stacks of repeated layers may serve to lower the energy cost of driving electrical current at the electrodes relative to that of driving separations in all the layers,1214 although it may be possible to achieve similar efficiency at lower cost and complexity by using fewer but thicker porous layers at the centimeter scale.87,95 Performance of shock ED may also be improved by varying the charge and microstructure of the porous material.528,582,1215 Similar hierarchical porous media as in EDI, such as immobilized beds of ion exchange resin,83,465,466 could be used to boost electromigration (compared to surface conduction in a glass frit) and promote shock formation and ion separations in the depletion zone. It may also be possible to lower capital costs and improve performance by replacing the IEMs used to initiate shock waves with thin, nanoporous ceramic or polymer layers analogous to the nanochannel junctions used in microfluidic ICP.93,550,554
6.5.4. Capacitive Deionization
Today, CDI systems are available from several water technology companies. The first to develop a commercial CDI system was Voltea (founded in 2006), and it was followed by other companies including Ur-Water, Atlantis Technologies, Idropan dell’Orto Depuratori, EST Water Technologies, Siontech, and InnoDI Water Technologies.1216,1217 As discussed in section 4.1.3, energy consumption by CDI has been extensively studied, and recent research demonstrated the possibility of combining photovoltaics and batteries to power a pilot CDI plant for remote applications.792 Because pumping also contributes to the total energy demand, there has been growing interest in process design to reduce pumping costs.792 For example, Nordstrand et al. recently designed a parallel arrangement of cells with symmetrically distributed flows to maintain a low pressure drop across the system.645 Another challenge during scale up of CDI is to make the process continuous, which has been the focus of FCDI802,818,819,1218 and multichannel MCDI929,948,1219,1220 systems. The latter enables continuous production of both fresh and brine streams by periodically switching the products of the middle and side channels.1219 Technoeconomic analyses of CDI also show the importance of achieving long system lifetimes, as capital costs (e.g., electrodes, membranes, frames, current collectors) are expected to outweigh operating costs (e.g., electricity, materials, labor).772,1221,1222 In particular, it is necessary to understand and mitigate electrode degradation652,782 (see section 4.1.5) and cell fouling (see section 4.1.6). Several other publications on CDI optimization and pilot systems provide additional design rules and principles for scale up.645,771,792,818,1217,1218,1220,1223−1226
6.5.5. Faradaic Electrosorption
Redox-based separation technologies remain an emerging area of research, and there are only a few preliminary studies on scale up and technoeconomic analysis. In one example, Joo et al. demonstrated a pilot electrochemical system using LiMn2O4 to selectively recover lithium from the desalination concentrate produced by RO and membrane distillation.1227 This continuous system comprised 14 pairs of λ-MnO2 and Ag electrodes arranged in parallel, and it was sequentially submerged in solutions for capturing, washing, and cleaning to recover the lithium. The system processed the desalination concentrate at a flow rate of 250 L h–1 and produced an up-concentrated lithium solution with 88% purity and an enrichment factor of 1800. Metzger et al. conducted a comprehensive technoeconomic analysis on MCDI, HCDI, and intercalation CDI.908 This analysis showed that, compared to MCDI, intercalation CDI can achieve higher removal capacity and is more cost-effective and energy efficient.908,1228 To treat equal volumes of water with similar performance, for example, an intercalation CDI module would cost only 27% of an MCDI module and would be four times smaller in size.
It has been shown, for certain applications, that electrochemical systems with redox reactions can be more cost-effective than conventional desalination methods. Kim et al. designed a system with redox-active electrodes to valorize proteins from whey waste and performed a preliminary technoeconomic analysis.1229 To produce equal volumes of whey protein, the redox desalination system consumed up to 72% less energy than ED by driving redox reactions rather than water splitting. Moreover, the use of fewer IEMs in the redox system relative to ED lowered capital costs by 62%.1229 Further progress in redox separation technologies requires a focus on device scale up, uncomplicated and economical synthesis of electrodes, long-term stability, and innovative stacking methods.1230
6.6. Process Intensification
Many of the methods discussed in this review are still under development, and it is anticipated that innovations in materials science, device engineering, and process design will continue to advance these technologies. In addition to these advancements, process intensification can be undertaken to achieve more holistic (or global) improvements in electrochemical methods for water purification. Process intensification is any integration of unit operations, functions, and phenomena that leads to a smaller, cleaner, safer, and more efficient technology.1231,1232 Emerging electrochemical methods provide new opportunities for process intensification by combining these systems with either other emerging methods or established technologies that are complementary in function.574,1233,1234 In an electrochemical method, the electric field enables selectivity and interfacial control, promotes fast reaction rates, and reduces energy consumption.1233,1235 Advances in electrocatalysis have revealed that existing thermal and chemical methods can in fact be replaced by novel electrochemical processes.1236,1237 Because of the importance of product purification, waste processing, and materials recycling, separations based on electrokinetics and electrosorption can play a key role in process intensification. Examples of process intensification for electroseparations include integration of reaction and separation using redox interfaces (as shown in Figure 29)102,1014 as well as selective recovery of target species from waste streams using shock ED.574
The dual nature of electrochemical systems, highlighted by cells with asymmetrically configured electrodes, provides a new conceptual platform for process intensification.102,902,1014 Although some Faradaic reactions are unwanted and can compromise efficiency, others can drive separations and promote catalysis.96,652 In section 4.3, we explained that molecular design of Faradaic processes enables electrosorptive removal of heavy metal oxyanions and simultaneous redox transformation at the counter electrode. In one such example, As(III) was captured and transformed into As(V) using PVF and PTMA as electrodes,102 and in another, Cr(VI) was captured and transformed into the less harmful Cr(III) using PVF and platinum as electrodes.1014 Both of these systems involved electron transfer, which activated the redox units, and Faradaic reactions, which transformed the target metals into more benign products. Asymmetric combinations of intercalation systems and BDD electrodes have also been proposed for removal of both lithium and organic pollutants from industrial wastewater, as shown in Figure 43.1238 Li+ was recovered (in excess of 98%) using an MnO2 electrode with high selectivity toward Li+ relative to Na+, and more than 65% of the organic pollutant was decomposed at the stainless steel counter electrode. Kim et al. observed a trade-off, however, between the utilization efficiency of LiMn2O4 and the rate at which Li+ could be recovered.1238 Moving forward, we expect a growing set of concepts that combine separations and reactions for water purification.
Figure 43.
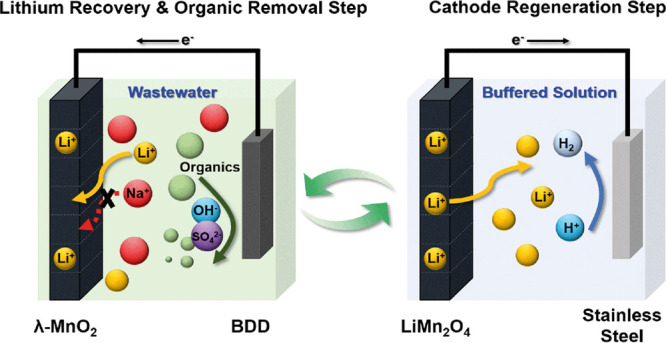
Schematic of an electrochemical system with LiMn2O4 and BDD as electrodes for removal of both lithium and organic pollutants from industrial wastewater. Reproduced with permission from ref (1238). Copyright 2018 Royal Society of Chemistry.
Electrokinetic methods can also be used for molecular separations to effectively remove harmful contaminants or recover valuable targets.478,574 For example, Li+ can be selectively captured from a multicomponent mixture and recycled (or reused elsewhere) by integrating CDI with intercalation materials88,652 as a second step following shock ED.574 As shown in Figure 44, process intensification of this kind can in principle be achieved in two steps. In the first step, shock ED is used to concentrate waste in the brine, from which Li+ is selectively captured in the CDI unit by intercalation into an appropriate electrode such as LiFePO4 or LiMn2O4.688,918,1239−1241 During this process, all cations are driven toward the intercalation electrode, but Li+ will be predominantly inserted into its crystal lattice because the vacancies in FePO4 are well fitted for this small monovalent cation. Moreover, the anions are inserted into a porous carbon electrode, so fluid leaving the device will be depleted of Li+ and its counterion(s). In the second step, Li+ and its counterion(s) are released from the electrodes by reversing the direction of the applied electric field, and the fresh stream produced by shock ED is passed through the CDI unit to collect these ions.
Figure 44.
Process intensification of shock ED (left) by integrating CDI (right) to recycle Li+ in two steps. The first step is selective capture of Li+ in the intercalation electrode of a CDI unit from the brine discharged by shock ED. The second step is release of Li+ into the fresh stream produced by shock ED by reversing the direction of the applied field. Cations other than Li+ and H+ are labeled C+, anions are labeled A–, and neutral species (unaffected by the electric fields) are labeled N; streams are colored based on the relative concentration of ions. Reproduced with permission from ref (574). Copyright 2019 American Chemical Society.
Process intensification of electrochemical methods not only enables targeted separations but also dramatically reduces energy consumption. By comparing the energetics of sequential and coupled Faradaic processes, Kim et al. lowered the energy needed to remove arsenic from 2.2 to 0.45 kW h mol–1, as shown in Figure 45.102 The basis of this energy integration was to reduce parasitic side reactions and improve current efficiency by using asymmetric redox systems. Relative to systems with nonselective electrodes (e.g., CDI with porous carbon electrodes), energy efficiency was improved by nearly an order of magnitude. Similarly, in recent work on PFAS capture by electrochemical mediation with TEMPO-based polymers, an asymmetric system of TEMPO copolymers with a BDD counter electrode achieved reactive separation of PFAS.1021 During release of the PFAS by the redox polymer, the BDD electrode simultaneously broke down PFAS with little energy consumption and a defluorination efficiency over 50%. Electroseparations can therefore enable processes that would otherwise be prohibitively expensive or complex, such as purification of dilute feeds, recovery of metals, valorization of wastes, and isomeric separations of biochemicals and pharmaceutical compounds.105,154,1234,1242 As shown in this review, electrokinetics and electrochemistry can improve energy efficiency and reduce secondary waste, which enables cleaner and more sustainable processes for water purification.
Figure 45.
Analysis of energy integration and process intensification using redox electrochemistry with coated electrodes for reactive separations. Reproduced with permission from ref (102). Copyright 2020 Wiley-VCH.
7. Conclusions and Outlook
Electrochemical methods for water purification use applied electric fields to remove contaminants by either degrading or converting them through redox reactions, driving their separations in a bulk electrolyte, electrostatically trapping them in an EDL (where they may undergo electrochemical reactions), or intercalating them in solid electrodes. The ability to remove contaminants directly from water, as opposed to removing the water from the contaminants, is the property that enables most advantages of these methods. Nondestructive electrochemical methods, which are based on electrokinetic (ED, EDI, ICP, shock ED) and electrosorption (CDI, Faradaic electrosorption) processes, tend to be more energy efficient compared to physical methods when used for molecular separations or purification of dilute feeds. These systems have other attractive features including compactness, molecular selectivity, versatility, decreased generation of secondary waste, and the ability to combine reactions and separations. The selectivity and versatility of electrochemical systems enables unique combinations of technologies for exotic separations and recovery of high-value elements. In particular, the dual nature of these systems provides a new conceptual platform for process intensification of existing methods. Significant advances, however, are still needed both at the fundamental level (e.g., to impart higher molecular selectivity to electrodes for emerging applications) and in process engineering through the development of new hybrid systems for higher energy efficiency. Comprehensive work on technoeconomic analysis is also needed for these emerging methods to provide a comparative evaluation against other treatment technologies and identify limitations, areas of opportunity, and protocols for optimal operation. Here, we highlight emerging research directions, challenges, and opportunities in electrochemical methods for water purification, ion separations, and energy conversion.
7.1. Materials Design for Multicomponent Separations
Despite advances in redox-active materials, which have enabled new selectivity and higher uptake, there remain significant challenges in both fundamental studies and new areas of application.
7.1.1. Multifunctional Redox Materials
To enhance selectivity and overcome existing limitations, one promising approach has been to combine several distinct chemical groups. While redox homopolymers are efficient for applications that demand ion selectivity,96 redox copolymers extend these capabilities by combining orthogonal properties. For example, redox copolymers have enhanced electrochemical regeneration, chemical binding of rare-earth elements,1243 and control of hydrophobicity, affinity, and electrostatics within redox adsorbents for PFAS molecules and other micropollutants.1021,1244,1245 Nanostructured combination of two distinct redox polymers has also enabled the reversible electrochemical binding and control of neutral molecules through redox-tunable hydrophobicity.103 Similar concepts of hybrid materials may be generalized to redox crystals and various multicomponent separations, in which reversibility or selectivity could be enhanced through hybridization to achieve properties beyond the base chemical structure. Moving forward, we envision these multifunctional materials will provide a versatile platform for extraction of multiple desired elements simultaneously.
7.1.2. Computational Design and Operando Electrochemical Tools for Understanding Interactions
To guide the bottom-up design of materials, computational studies play a key role in the selection of chemistry and tuning of morphology. Tools such as MD716 and electronic structure calculations1246 have been critical in understanding selectivity in ultramicroporous carbon and redox-active materials, respectively. We envision molecular simulation tools will increase in utility due to the need for a greater understanding of selective interactions and their underlying mechanisms. In addition to computational tools, we expect that the integration of operando electrochemical tools such as vibrational spectroscopy will elucidate interfacial structure and binding mechanisms. These molecular tools, along with macroscopic transport modeling, can help advance the performance of electrochemical separation methods at multiple scales.
7.1.3. Advances in Membrane Materials
Advances in membrane materials can improve the efficiency of electrokinetic processes. Membrane fouling and poor stability under certain operating conditions remain longstanding challenges in electrokinetic systems. Besides, redox materials in electrokinetic systems could pass the membranes and contaminate the treated water. The introduction of more durable, cost-effective, and selective membranes is thus needed for more efficient and reliable electrokinetic systems. Structured nanomaterials are expected to play an important role in improving membrane performance.1247,1248 Moreover, ion selectivity can be modulated through fine control of the structure, pore size, water permeability, and functional groups of the membranes.1248,1249
7.1.4. Multicomponent Mixtures for Separations
Many of the recent studies on selective removal and resource recovery have been performed using idealized simulated solutions. Real industrial and municipal effluents, however, involve complex speciation of ions and organic compounds, which affects both the selectivity and the lifetime of electrodes and membranes. Municipal effluents are often complex multicomponent mixtures that comprise organic substances, inorganic ions, and sometimes biological species,145,1250 many of which could form unwanted deposits. Groundwater often contains significant organic matter, and the solution can exhibit a range of ionic strengths and pHs. As a result, there is a need to evaluate the performance of functional materials when exposed to real samples1251 to quantify reliability and stability (e.g., electrochemical, chemical, mechanical). At a fundamental level, these studies may reveal the need for materials modification such as the use of antifouling coatings, which can provide a tunable balance between uptake, selectivity, and stability.
Nutrient recovery and metal recovery are two areas of emerging interest in the development of molecularly selective materials. There have been significant advances in the design of selective materials for nutrient recovery, especially using nanostructured porous carbons.716 Improving selectivity further requires a deeper understanding of solvation, sterics, and electronic structure interactions due to the similarity in electric charge between nutrient species (particularly NO3–) and competing ions. At the same time, many electrosorption systems are still highly dependent on pH during separations,1252,1253 and because many oxyanions have complex speciations that depend on pH, there have been approaches that leverage electrochemical swings in pH for the recovery of nutrients such as PO4.806,1253
The recovery of critical elements from mining, recycling, and industrial wastewater streams is another area of emerging importance for selective separations. Many of these transition metals are present in small amounts and are surrounded by excess competing species of similar valence and structure, which makes ion-selective electrosorption a “needle-in-a-haystack” challenge.1246,1254 Functionalized carbons and redox-active electrodes have made significant strides toward efficient metal recovery and heavy metal removal.646,1255,1256 However, there are key challenges still to be overcome, such as achieving higher selectivity between different transition metals within multicomponent mixtures. Rare-earth elements, for example, are often found in the presence of each other as well as other transition metals,1257 which makes their separation a difficult and costly task. Many competing metals are electroactive at moderate potentials (e.g., copper, lead), and they can often interfere with selective electrosorption processes. Therefore, pretreatment and tuning of electrochemical potentials will be needed to selectively purify multicomponent mixtures.
7.2. Intensifying Water Treatment Processes Through Hybrid Approaches
Electrochemical methods can assist in process intensification by decreasing waste, lowering solvent use, and reducing the number of unit operations. In particular, areas for integration include the direct combination of electrochemical methods with renewable energy sources as well as the development of single-cell reactive separations.
7.2.1. Integrating Renewable Energy Sources and Electrochemical Methods
Coupled with global environmental crises, the development of sustainable manufacturing processes has become a major goal for industries. In this regard, combining electrochemical processes with renewable energy sources (e.g., solar panels) can reduce energy consumption and carbon intensity.1258 Recently, electrochemical methods for water purification have been hybridized with dye-sensitized solar cells for direct conversion of light to electricity.1259,1260 The photoanode uses desalinated chloride to generate reactive chlorine species, which can treat wastewater, while the cathode produces molecular hydrogen.1261 The combination of photoelectrochemistry and redox-flow desalination enables continuous treatment, even in the absence of an external energy source. These systems demonstrate opportunities for the development of processes that integrate energy conversion and electrochemical separations.
7.2.2. Coupling Reaction and Separation
For persistent contaminants, degradation of these species is as important as their removal from water. The coupling of reaction and separation can be a powerful approach for providing modular water treatment, as in the case of capture and breakdown of PFAS.985,1262 The integration of membrane technologies and electrochemical advanced oxidation processes has gained much attention for treatment of organic pollutants in wastewater. In these hybrid processes, a conductive membrane serves as a flow-through anode, which filters the wastewater and drives oxidation of the organic contaminants and pharmaceutical residues.1263 Moreover, integration of redox processes and magnetic nanoparticles enables selective separation of various organic and inorganic micropollutants.1245 We envision that emerging electrosorption and electrokinetic systems coupled with advanced oxidation processes can provide efficient process intensification for wastewater treatment.
7.3. Translation to Practical Applications
While there have been extensive studies of carbon electrode stability,1264,1265 many of the emerging Faradaic materials have only been examined using idealized solutions. Demonstration of stability and reliability using real samples with relevant electrolyte concentrations is essential. For desalination, electrode stability can be improved by doping the carbon and creating hybrid materials.1265,1266 It is also important to design materials that suppress parasitic reactions and function at lower potentials, especially when the side reactions involve chloride or oxygen, which often produce unwanted byproducts.652 Proof of economic feasibility is also critical for commercialization of electrochemical systems. Recently, there has been an increase in the number of publications on life cycle assessment and technoeconomic analysis.1221,1267,1268 These technoeconomic analyses show that the costs of electrodes and membranes are key contributors to the overall capital and operating costs.908,1221 In practice, technoeconomic analysis will provide a comprehensive framework for assessing feasible electrochemical approaches for water treatment.
In summary, electrochemical systems for water purification and resource recovery are already commercialized in many industries to treat water contaminated with various kinds of waste. Whether or not the emerging purification methods meet their expectations in the areas of water desalination, remediation, and separations, it seems likely that several of these methods will eventually be employed. In the near term, we expect that a deeper knowledge of the transport phenomena and electrochemical kinetics that govern these methods will facilitate their engineering and optimization. In the long term, work directed toward understanding and improving the design of these processes at scale may guide how commercial prototypes should be built for a given application.
Acknowledgments
Part of this work was performed in the cooperation framework of Wetsus, European Centre of Excellence for Sustainable Water Technology. Wetsus is cofunded by the Dutch Ministry of Economic Affairs and Ministry of Infrastructure and Environment, the Province of Fryslân, and the Northern Netherlands Provinces (www.wetsus.eu). I Gede Wenten acknowledges financial support from the Indonesian Ministry of Research and Technology/National Agency for Research and Innovation and the Indonesian Ministry of Education and Culture under the World Class University program managed by ITB. J. Pedro de Souza acknowledges support from the NSF Graduate Research Fellowship (award no. 1,122,374). We thank Dr. Ali Hemmatifar for his significant contribution to the writing of this manuscript.
Glossary
Abbreviations
- AEM
anion exchange membrane
- H2Q
hydroquinone
- AOT
brand name of DOSS
- HA
hydroxyapatite
- BattMix
battery mixing
- HBMA
hydroxybutyl methacrylate
- BDD
boron doped diamond
- HCDI
hybrid capacitive deionization
- BDI
battery deionization
- HCF
hexacyanoferrate
- BMCSL
Boublik–Mansoori–Carnahan–Starling–Leland
- HS
high salinity
- BSK
Bazant–Storey–Kornyshev
- ICP
ion concentration polarization
- BWRO
brackish water reverse osmosis
- IEM
ion exchange membrane
- CapMix
capacitive mixing
- LDH
layered double hydroxide
- CDI
capacitive deionization
- LS
low salinity
- CDLE
capacitive double layer expansion
- MCDI
membrane capacitive deionization
- CDP
capacitive Donnan potential
- MD
molecular dynamics
- CEM
cation exchange membrane
- NASICON
sodium superionic conductors
- CIET
coupled ion–electron transfer
- NMO
sodium manganese oxide
- CNT
carbon nanotube
- NMR
nuclear magnetic resonance
- CTAB
cetyltrimethylammonium bromide
- PANI
polyaniline
- CVD
chemical vapor deposition
- PAQ
polyanthraquinone
- DBS
dodecylbenzenesulfonate
- PBA
Prussian blue analogue
- DC
direct current
- PFAS
polyfluoroalkyl substances
- DFT
density functional theory
- PNPS
Poisson–Nernst–Planck–Stokes
- DO
dissolved oxygen
- PPCP
pharmaceutical and personal care product
- DOSS
dioctyl sulfosuccinate
- PPy
polypyrrole
- DSA
dimensionally stable anode
- PRO
pressure-retarded osmosis
- EC
electrochemical process
- PTMA
poly-TEMPO methacrylate
- ED
electrodialysis
- PVF
polyvinylferrocene
- EDI
electrodeionization
- RC
resistor–capacitor
- EDL
electric double layer
- RCDI
rocking-chair capacitive deionization
- EDS
energy dispersive X-ray spectroscopy
- RGO
reduced graphene oxide
- EDTA
ethylenediaminetetraacetic acid
- RMSS
redox-mediated selective separations
- ENAS
energy normalized adsorbed salt
- RO
reverse osmosis
- ETAS
electrochemically tunable affinity separations
- SEC
specific energy consumption
- FB
flow-between
- SEM
scanning electron microscopy
- FBV
Frumkin–Butler–Volmer
- SHE
standard hydrogen electrode
- FCDI
flow electrode capacitive deionization
- SOG
Sudan Orange G
- FO
forward osmosis
- SWRO
seawater reverse osmosis
- FTE
flow-through electrode
- TEM
transmission electron microscopy
- FUE
ferrocene utilization efficiency
- TEMPO
(CH2)3(CMe2)2NO
- GCMC
grand canonical Monte Carlo
- VF
vinylferrocene
- GCS
Gouy–Chapman–Stern
Biographies
Mohammad A. Alkhadra graduated from UC San Diego in 2018 with both a B.S. and an M.S. in Chemical Engineering, after which he moved to Cambridge, MA, to begin his Ph.D. at MIT in the same major. At MIT, Mo focuses on ion separations using shock ED under the supervision of Prof. Martin Z. Bazant.
Xiao Su received his B.S. in Chemical Engineering from the University of Waterloo in 2011 and his Ph.D. in Chemical Engineering from MIT in 2017. He joined the faculty in the Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana–Champaign (UIUC) in 2019, where he focuses on electrochemical separations and process intensification. His research has been recognized by an NSF CAREER award in 2019 and the ACS Victor K. LaMer Award in 2020.
Matthew E. Suss heads the Cleantech Innovations Laboratory in the Faculty of Mechanical Engineering and Wolfson Department of Chemical Engineering at Technion—Israel Institute of Technology and is affiliated with the Grand Technion Energy Program and Grand Water Research Institute. Prof. Suss serves as a member of the Israel National Research Center for Electrochemical Propulsion (INREP) and as an Israeli delegate to the European Federation of Chemical Engineering (EFCE) working group on electrochemical engineering. Prof. Suss has won numerous national and international research awards, including the prestigious Alon Fellowship and an ARCHES award.
Huanhuan Tian joined the Department of Chemical Engineering at MIT in 2018 and is pursuing her Ph.D. under the supervision of Prof. Martin Z. Bazant. She received her B.S. and M.S. with honors in 2018 from the Department of Engineering Mechanics at Tsinghua University in China. Huanhuan is interested in issues related to energy and the environment, and her current research focuses on mathematical modeling of shock ED for selective ion separations.
Eric N. Guyes is a Ph.D. candidate at Technion under the supervision of Prof. Matthew E. Suss. He arrived at Technion in 2013 as a Fulbright Scholar and later joined Prof. Suss’s group in 2014. His doctoral research focuses on the mechanisms and applications of selective ion removal in CDI for water desalination and treatment applications.
Amit N. Shocron is a Ph.D. candidate at Technion under the supervision of Prof. Matthew E. Suss. He received his B.S. and M.S. in Mechanical Engineering also at Technion. His research focuses on CDI for ion selective water treatment and water desalination.
Kameron M. Conforti received his Ph.D. in Chemical Engineering at MIT in 2019, after which he worked as a Postdoctoral Associate in the Department of Mechanical Engineering at MIT. He is currently a Senior Research Engineer in the Competency Research Lab at Saint-Gobain Research North America, where he specializes in modeling heat and mass transfer.
J. Pedro de Souza is a Ph.D. candidate in the Department of Chemical Engineering at MIT. He received his B.S. in chemical engineering at the University of Texas at Austin in 2016. He works with Prof. Martin Z. Bazant on theoretical modeling of the equilibrium structure and transport of ions at charged interfaces.
Nayeong Kim received her B.S. in Chemical and Biological Engineering from the University of Wisconsin–Madison in 2017 and her M.S. in the same major from Seoul National University in 2020 under the supervision of Prof. Jeyong Yoon. She is pursuing her Ph.D. at UIUC under the supervision of Prof. Xiao Su, where she focuses on environmental electrochemistry and integrated electrochemical separations.
Michele Tedesco has a Ph.D. in Chemical Engineering and is currently a scientific project manager at Wetsus. His research focuses on IEMs and related processes, including ED and reverse/bipolar ED, from fundamental modeling to experimental investigation.
Khoiruddin Khoiruddin is an assistant professor in the Department of Chemical Engineering at Institut Teknologi Bandung (ITB), Indonesia. He received his Ph.D. in Chemical Engineering from ITB in 2018. Formerly, he was a process engineer at the membrane company GDP Filter Indonesia from 2009 to 2018. His research interests include design of IEMs and applications of electromembrane processes.
I Gede Wenten is a professor in chemical engineering at ITB, Indonesia. He received his Ph.D. in Chemical Engineering from the Technical University of Denmark (DTU) in 1995. He has extensive experience in membrane technology, both in the industrial and academic settings, with a career spanning over three decades. His research interests include design, preparation, and fouling control of membranes.
Juan G. Santiago received his Ph.D. in Mechanical Engineering from UIUC in 1995. His research includes the development of microsystems for on-chip chemical and biochemical analysis, methods for DNA quantification and hybridization, and CDI. He is a Fellow of the American Physical Society, a Fellow of the American Society of Mechanical Engineering, and a Fellow of the American Institute for Medical and Biological Engineering. He serves and has served as an editor of several journals and cofounded several companies in microfluidics.
T. Alan Hatton is the Ralph Landau Professor and Director of the David H. Koch School of Chemical Engineering Practice at MIT. He earned his B.S. and M.S. from the University of Natal, Durban, and his Ph.D. from the University of Wisconsin–Madison in 1981. As part of the MIT Energy Initiative, Prof. Hatton codirects the Center for Carbon Capture, Utilization, and Storage. His research focuses on the development of electrochemical processes to facilitate chemical separations and to mediate the transformation of capture waste into useful commodity chemicals.
Martin Z. Bazant is the Edwin G. Roos (1944) Chair Professor of Chemical Engineering and Mathematics at MIT. He has made seminal theoretical contributions in electrokinetics and electrochemistry with applications in energy storage, water purification, and microfluidics. Prof. Bazant’s awards include the 2015 Alexander Kuznetsov Prize in Theoretical Electrochemistry, the 2018 Andreas Acrivos Award for Professional Progress in Chemical Engineering (AIChE), and the 2019 MITx Prize for Teaching and Learning in Massive Open Online Courses. He consults extensively for industry and serves as the Chief Scientific Advisor for Saint-Gobain Research North America in Massachusetts.
The authors declare no competing financial interest.
References
- Mekonnen M. M.; Hoekstra A. Y. Four billion people facing severe water scarcity. Science Advances 2016, 2, e1500323 10.1126/sciadv.1500323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra A. Y.The Water Footprint of Modern Consumer Society; Routledge, 2019. [Google Scholar]
- Kucera J.Desalination: Water from Water; John Wiley & Sons, 2019. [Google Scholar]
- Al-Shammiri M.; Safar M. Multi-effect distillation plants: state of the art. Desalination 1999, 126, 45–59. 10.1016/S0011-9164(99)00154-X. [DOI] [Google Scholar]
- Khawaji A. D.; Kutubkhanah I. K.; Wie J.-M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. 10.1016/j.desal.2007.01.067. [DOI] [Google Scholar]
- Brogioli D.; La Mantia F.; Yip N. Y. Thermodynamic analysis and energy efficiency of thermal desalination processes. Desalination 2018, 428, 29–39. 10.1016/j.desal.2017.11.010. [DOI] [Google Scholar]
- Boysen J. E.; Stevens B. G.. Demonstration of the Natural Freeze–Thaw Process for the Desalination of Water from the Devils Lake Chain to Provide Water for the City of Devils Lake; US Department of the Interior, Bureau of Reclamation, Technical Service Center, 2004. [Google Scholar]
- Xie C.; Zhang L.; Liu Y.; Lv Q.; Ruan G.; Hosseini S. S. A direct contact type ice generator for seawater freezing desalination using LNG cold energy. Desalination 2018, 435, 293–300. 10.1016/j.desal.2017.04.002. [DOI] [Google Scholar]
- Kiezyk P.; Mackay D. Waste water treatment by solvent extraction. Canadian Journal of Chemical Engineering 1971, 49, 747–752. 10.1002/cjce.5450490607. [DOI] [Google Scholar]
- Van der Bruggen B.; Vandecasteele C. Distillation vs. membrane filtration: overview of process evolutions in seawater desalination. Desalination 2002, 143, 207–218. 10.1016/S0011-9164(02)00259-X. [DOI] [Google Scholar]
- Cath T. Y.; Childress A. E.; Elimelech M. Forward osmosis: principles, applications, and recent developments. Journal of membrane science 2006, 281, 70–87. 10.1016/j.memsci.2006.05.048. [DOI] [Google Scholar]
- Fritzmann C.; Löwenberg J.; Wintgens T.; Melin T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. 10.1016/j.desal.2006.12.009. [DOI] [Google Scholar]
- Garcia-Rodriguez L.; Palmero-Marrero A. I.; Gómez-Camacho C. Comparison of solar thermal technologies for applications in seawater desalination. Desalination 2002, 142, 135–142. 10.1016/S0011-9164(01)00432-5. [DOI] [Google Scholar]
- Lienhard J. H.; Thiel G. P.; Warsinger D. M.; Banchik L. D.. Low Carbon Desalination: Status and Research, Development, and Demonstration Needs, Report of a Workshop Conducted at the Massachusetts Institute of Technology in Association with the Global Clean Water Desalination Alliance; Massachusetts Institute of Technology, 2016.
- Godart P. Design and simulation of a heat-driven direct reverse osmosis device for seawater desalination powered by solar thermal energy. Applied Energy 2021, 284, 116039. 10.1016/j.apenergy.2020.116039. [DOI] [Google Scholar]
- Davies P. Wave-powered desalination: resource assessment and review of technology. Desalination 2005, 186, 97–109. 10.1016/j.desal.2005.03.093. [DOI] [Google Scholar]
- Liebmann A. J. History of distillation. J. Chem. Educ. 1956, 33, 166. 10.1021/ed033p166. [DOI] [Google Scholar]
- Aly S. Energy savings in distillation plants by using vapor thermo-compression. Desalination 1984, 49, 37–56. 10.1016/0011-9164(84)80011-9. [DOI] [Google Scholar]
- Al-Radif A. Review of various combinations of a multiple effect desalination plant (MED) and a thermal vapour compression unit. Desalination 1993, 93, 119–125. 10.1016/0011-9164(93)80099-9. [DOI] [Google Scholar]
- Al-Najem N. M.; Darwish M.; Youssef F. Thermovapor compression desalters: energy and availability—analysis of single-and multi-effect systems. Desalination 1997, 110, 223–238. 10.1016/S0011-9164(97)00101-X. [DOI] [Google Scholar]
- Parekh S.; Farid M.; Selman J.; Al-Hallaj S. Solar desalination with a humidification-dehumidification technique—a comprehensive technical review. Desalination 2004, 160, 167–186. 10.1016/S0011-9164(04)90007-0. [DOI] [Google Scholar]
- Narayan G. P.; Sharqawy M. H.; Summers E. K.; Lienhard J. H.; Zubair S. M.; Antar M. A. The potential of solar-driven humidification–dehumidification desalination for small-scale decentralized water production. Renewable and Sustainable Energy Reviews 2010, 14, 1187–1201. 10.1016/j.rser.2009.11.014. [DOI] [Google Scholar]
- Narayan G. P.; Sharqawy M. H.; Lienhard J. H. V; Zubair S. M. Thermodynamic analysis of humidification dehumidification desalination cycles. Desalination and Water Treatment 2010, 16, 339–353. 10.5004/dwt.2010.1078. [DOI] [Google Scholar]
- Dahmani K.; Kherroub D. E.; Belloul M.. Materials Research and Applications; Springer, 2021; pp 251–261. [Google Scholar]
- Rich A.; Mandri Y.; Bendaoud N.; Mangin D.; Abderafi S.; Bebon C.; Semlali N.; Klein J.-P.; Bounahmidi T.; Bouhaouss A.; Veesler S. Freezing desalination of sea water in a static layer crystallizer. Desalination and Water Treatment 2010, 13, 120–127. 10.5004/dwt.2010.983. [DOI] [Google Scholar]
- Lu Z.; Xu L.. Freezing desalination process. In Thermal Desalination Processes 2010, 2. [Google Scholar]
- Gupta V. K.; Ali I.; Saleh T. A.; Nayak A.; Agarwal S. Chemical treatment technologies for waste-water recycling—an overview. Rsc Advances 2012, 2, 6380–6388. 10.1039/c2ra20340e. [DOI] [Google Scholar]
- Belfort G.; Davis R. H.; Zydney A. L. The behavior of suspensions and macromolecular solutions in crossflow microfiltration. Journal of membrane science 1994, 96, 1–58. 10.1016/0376-7388(94)00119-7. [DOI] [Google Scholar]
- Rautenbach R.; Vossenkaul K.; Linn T.; Katz T. Waste water treatment by membrane processes—new development in ultrafiltration, nanofiltration and reverse osmosis. Desalination 1997, 108, 247–253. 10.1016/S0011-9164(97)00032-5. [DOI] [Google Scholar]
- Schaefer A.; Fane A. G.; Waite T. D.. Nanofiltration: Principles and Applications; Elsevier, 2005. [Google Scholar]
- Peñate B.; García-Rodríguez L. Current trends and future prospects in the design of seawater reverse osmosis desalination technology. Desalination 2012, 284, 1–8. 10.1016/j.desal.2011.09.010. [DOI] [Google Scholar]
- Kucera J.Reverse Osmosis: Industrial Processes and Applications; John Wiley & Sons, 2015. [Google Scholar]
- Wenten I.; Khoiruddin K. Reverse osmosis applications: prospect and challenges. Desalination 2016, 391, 112–125. 10.1016/j.desal.2015.12.011. [DOI] [Google Scholar]
- Imbrogno J.; Belfort G. Membrane desalination: where are we, and what can we learn from fundamentals?. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 29–64. 10.1146/annurev-chembioeng-061114-123202. [DOI] [PubMed] [Google Scholar]
- Zeman L. J.; Zydney A.. Microfiltration and Ultrafiltration: Principles and Applications; CRC Press, 2017. [Google Scholar]
- Aryanti P.; Hakim A.; Widodo S.; Widiasa I.; Wenten I. Prospect and Challenges of Tight Ultrafiltration Membrane in Drinking Water Treatment. IOP Conference Series: Materials Science and Engineering 2018, 395, 012012. 10.1088/1757-899X/395/1/012012. [DOI] [Google Scholar]
- Van der Bruggen B.Fundamental Modelling of Membrane Systems; Elsevier, 2018; pp 25–70. [Google Scholar]
- Biesheuvel P.; Porada S.; Elimelech M.; Dykstra J. Tutorial review of reverse osmosis and electrodialysis. J. Membr. Sci. 2022, 647, 120221. 10.1016/j.memsci.2021.120221. [DOI] [Google Scholar]
- Qiblawey H. M.; Banat F. Solar thermal desalination technologies. Desalination 2008, 220, 633–644. 10.1016/j.desal.2007.01.059. [DOI] [Google Scholar]
- Kalogirou S. A.Solar Energy Engineering: Processes and Systems; Academic Press, 2013. [Google Scholar]
- Wang L. K.; Vaccari D. A.; Li Y.; Shammas N. K.. Physicochemical Treatment Processes; Springer, 2005; pp 141–197. [Google Scholar]
- Jiang J.-Q. The role of coagulation in water treatment. Current Opinion in Chemical Engineering 2015, 8, 36–44. 10.1016/j.coche.2015.01.008. [DOI] [Google Scholar]
- Bose P.; Bose M. A.; Kumar S. Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc and cyanide. Adv. Environ. Res. 2002, 7, 179–195. 10.1016/S1093-0191(01)00125-3. [DOI] [Google Scholar]
- Faust S. D.; Aly O. M.. Adsorption Processes for Water Treatment; Elsevier, 2013. [Google Scholar]
- Collivignarelli M. C.; Abbà A.; Benigna I.; Sorlini S.; Torretta V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustainability 2018, 10, 86. 10.3390/su10010086. [DOI] [Google Scholar]
- Rosso D.; Larson L. E.; Stenstrom M. K. Aeration of large-scale municipal wastewater treatment plants: state of the art. Water Sci. Technol. 2008, 57, 973–978. 10.2166/wst.2008.218. [DOI] [PubMed] [Google Scholar]
- Dabrowski A.; Hubicki Z.; Podkościelny P.; Robens E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. 10.1016/j.chemosphere.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang J. Advances in cement solidification technology for waste radioactive ion exchange resins: A review. Journal of hazardous materials 2006, 135, 443–448. 10.1016/j.jhazmat.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Choppin G.; Morgenstern A. Radionuclide separations in radioactive waste disposal. Journal of Radioanalytical and Nuclear Chemistry 2000, 243, 45–51. 10.1023/A:1006754927614. [DOI] [Google Scholar]
- Ashley N. V.; Roach D. J. Review of biotechnology applications to nuclear waste treatment. J. Chem. Technol. Biotechnol. 1990, 49, 381–394. 10.1002/jctb.280490409. [DOI] [PubMed] [Google Scholar]
- Ghernaout B.; Ghernaout D.; Saiba A. Algae and cyanotoxins removal by coagulation/flocculation: A review. Desalination and Water Treatment 2010, 20, 133–143. 10.5004/dwt.2010.1202. [DOI] [Google Scholar]
- Verma A. K.; Dash R. R.; Bhunia P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of environmental management 2012, 93, 154–168. 10.1016/j.jenvman.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Carrott P.; Ribeiro Carrott M.; Suhas Low-cost adsorbents: growing approach to wastewater treatment—a review. Critical reviews in environmental science and technology 2009, 39, 783–842. 10.1080/10643380801977610. [DOI] [Google Scholar]
- Kim H. D.; Kim K. H. Water purification using aeration techniques in the lagoon. Applied Mechanics and Materials 2014, 675-677, 602–607. 10.4028/www.scientific.net/AMM.675-677.602. [DOI] [Google Scholar]
- Dardor D.; Minier-Matar J.; Janson A.; AlShamari E.; Adham S. The effect of Hydrogen sulfide oxidation with ultraviolet light and aeration on sour water treatment via membrane contactors. Sep. Purif. Technol. 2020, 236, 116262. 10.1016/j.seppur.2019.116262. [DOI] [Google Scholar]
- Elwakeel K. Z. Environmental application of chitosan resins for the treatment of water and wastewater: a review. J. Dispersion Sci. Technol. 2010, 31, 273–288. 10.1080/01932690903167178. [DOI] [Google Scholar]
- Hubicki Z.; Kołodyńska D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. Ion Exchange Technologies 2012, 7, 193–240. 10.5772/51040. [DOI] [Google Scholar]
- Iizuka A.; Yamashita Y.; Nagasawa H.; Yamasaki A.; Yanagisawa Y. Separation of lithium and cobalt from waste lithium-ion batteries via bipolar membrane electrodialysis coupled with chelation. Sep. Purif. Technol. 2013, 113, 33–41. 10.1016/j.seppur.2013.04.014. [DOI] [Google Scholar]
- Kaur M.; Zhang H.; Martin L.; Todd T.; Qiang Y. Conjugates of Magnetic Nanoparticle–Actinide Specific Chelator for Radioactive Waste Separation. Environ. Sci. Technol. 2013, 47, 11942–11959. 10.1021/es402205q. [DOI] [PubMed] [Google Scholar]
- Shkolnikov V.; Bahga S. S.; Santiago J. G. Desalination and hydrogen, chlorine, and sodium hydroxide production via electrophoretic ion exchange and precipitation. Phys. Chem. Chem. Phys. 2012, 14, 11534–11545. 10.1039/c2cp42121f. [DOI] [PubMed] [Google Scholar]
- Arar Ö.; Yüksel Ü.; Kabay N.; Yüksel M. Various applications of electrodeionization (EDI) method for water treatment—A short review. Desalination 2014, 342, 16–22. 10.1016/j.desal.2014.01.028. [DOI] [Google Scholar]
- Pham T. C. T.; Docao S.; Hwang I. C.; Song M. K.; Choi D. Y.; Moon D.; Oleynikov P.; Yoon K. B. Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energy Environ. Sci. 2016, 9, 1050–1062. 10.1039/C5EE02843D. [DOI] [Google Scholar]
- Manahan S. E.Fundamentals of Environmental Chemistry; CRC Press, 2001; pp 1–42. [Google Scholar]
- Qasim M.; Badrelzaman M.; Darwish N. N.; Darwish N. A.; Hilal N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. 10.1016/j.desal.2019.02.008. [DOI] [Google Scholar]
- Shannon M. A.; Bohn P. W.; Elimelech M.; Georgiadis J. G.; Marinas B. J.; Mayes A. M.. Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific, 2010; pp 337–346. [Google Scholar]
- Sauvet-Goichon B. Ashkelon desalination plant - A successful challenge. Desalination 2007, 203, 75–81. 10.1016/j.desal.2006.03.525. [DOI] [Google Scholar]
- Semiat R. Energy Issues in Desalination Processes. Environ. Sci. Technol. 2008, 42, 8193–8201. 10.1021/es801330u. [DOI] [PubMed] [Google Scholar]
- Elimelech M.; Phillip W. A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. 10.1126/science.1200488. [DOI] [PubMed] [Google Scholar]
- Kim J.; Park K.; Yang D. R.; Hong S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Applied Energy 2019, 254, 113652. 10.1016/j.apenergy.2019.113652. [DOI] [Google Scholar]
- Zhao R.; Porada S.; Biesheuvel P. M.; Van der Wal A. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. 10.1016/j.desal.2013.08.017. [DOI] [Google Scholar]
- Zhou J.; Chang V. W. C.; Fane A. G. Environmental life cycle assessment of brackish water reverse osmosis desalination for different electricity production models. Energy Environ. Sci. 2011, 4, 2267–2278. 10.1039/c0ee00828a. [DOI] [Google Scholar]
- Spiegler K. S.; El-Sayed Y. M. The energetics of desalination processes. Desalination 2001, 134, 109–128. 10.1016/S0011-9164(01)00121-7. [DOI] [Google Scholar]
- Ayoub J.; Alward R. Water requirements and remote arid areas: the need for small-scale desalination. Desalination 1996, 107, 131–147. 10.1016/S0011-9164(96)00158-0. [DOI] [Google Scholar]
- Song J.; Li T.; Wright-Contreras L.; Law A. W.-K. A review of the current status of small-scale seawater reverse osmosis desalination. Water international 2017, 42, 618–631. 10.1080/02508060.2017.1330841. [DOI] [Google Scholar]
- Azwanida N. N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 2167–0412. 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- Crini G.; Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters 2019, 17, 145–155. 10.1007/s10311-018-0785-9. [DOI] [Google Scholar]
- Zhang Y.; Bian T.; Zhang Y.; Zheng X.; Li Z. Chelation resin efficient removal of Cu (II), Cr (III), Ni (II) in electroplating wastewater. Fullerenes, Nanotubes and Carbon Nanostructures 2018, 26, 765–776. 10.1080/1536383X.2018.1498481. [DOI] [Google Scholar]
- Drikas M.; Chow C. W.; House J.; Burch M. D. Using coagulation, flocculation, and settling to remove toxic cyanobacteria. Journal-American Water Works Association 2001, 93, 100–111. 10.1002/j.1551-8833.2001.tb09130.x. [DOI] [Google Scholar]
- Zhang S.; Zheng H.; Tang X.; Zhao C.; Zheng C.; Gao B. Sterilization by flocculants in drinking water treatment. Chemical Engineering Journal 2020, 382, 122961. 10.1016/j.cej.2019.122961. [DOI] [Google Scholar]
- Teh C. Y.; Budiman P. M.; Shak K. P. Y.; Wu T. Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. 10.1021/acs.iecr.5b04703. [DOI] [Google Scholar]
- de Wolf C. A.; Bang E.; Bouwman A.; Braun W.; De Oliveira E.; Nasr El-Dinothers H.. Evaluation of Environmentally Friendly Chelating Agents for Applications in the Oil and Gas Industry; SPE International Symposium and Exhibition on Formation Damage Control, 2014.
- Chen J. P.Decontamination of Heavy Metals: Processes, Mechanisms, And Applications; CRC Press, 2012. [Google Scholar]
- Pan S.-Y.; Snyder S. W.; Ma H.-W.; Lin Y. J.; Chiang P.-C. Development of a resin wafer electrodeionization process for impaired water desalination with high energy efficiency and productivity. Acs Sustainable Chemistry & Engineering 2017, 5, 2942–2948. 10.1021/acssuschemeng.6b02455. [DOI] [Google Scholar]
- Tarpeh W. A.; Barazesh J. M.; Cath T. Y.; Nelson K. L. Electrochemical stripping to recover nitrogen from source-separated urine. Environ. Sci. Technol. 2018, 52, 1453–1460. 10.1021/acs.est.7b05488. [DOI] [PubMed] [Google Scholar]
- Mu L.; Wang Y.; Tarpeh W. A. Validation and Mechanism of a Low-Cost Graphite Carbon Electrode for Electrochemical Brine Valorization. ACS Sustainable Chem. Eng. 2020, 8, 8648–8654. 10.1021/acssuschemeng.0c01485. [DOI] [Google Scholar]
- Su X.; Hatton T. A. Electrosorption at functional interfaces: from molecular-level interactions to electrochemical cell design. Phys. Chem. Chem. Phys. 2017, 19, 23570–23584. 10.1039/C7CP02822A. [DOI] [PubMed] [Google Scholar]
- Schlumpberger S.; Lu N. B.; Suss M. E.; Bazant M. Z. Scalable and Continuous Water Deionization by Shock Electrodialysis. Environmental Science & Technology Letters 2015, 2, 367–372. 10.1021/acs.estlett.5b00303. [DOI] [Google Scholar]
- Singh K.; Bouwmeester H.; De Smet L.; Bazant M.; Biesheuvel P. Theory of water desalination with intercalation materials. Physical review applied 2018, 9, 064036. 10.1103/PhysRevApplied.9.064036. [DOI] [Google Scholar]
- He D.; Wong C. E.; Tang W. W.; Kovalsky P.; Waite T. D. Faradaic Reactions in Water Desalination by Batch-Mode Capacitive Deionization. Environmental Science & Technology Letters 2016, 3, 222–226. 10.1021/acs.estlett.6b00124. [DOI] [Google Scholar]
- Hemmatifar A.; Palko J. W.; Stadermann M.; Santiago J. G. Energy breakdown in capacitive deionization. Water Res. 2016, 104, 303–311. 10.1016/j.watres.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Squires T. M. Particles in electric fields. Fluids, Colloids and Soft Materials: An Introduction to Soft Matter Physics 2016, 59–79. 10.1002/9781119220510.ch5. [DOI] [Google Scholar]
- Mani A.; Zangle T. A.; Santiago J. G. On the propagation of concentration polarization from microchannel-nanochannel interfaces Part I: analytical model and characteristic analysis. Langmuir 2009, 25, 3898–3908. 10.1021/la803317p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangle T. A.; Mani A.; Santiago J. G. On the propagation of concentration polarization from microchannel-nanochannel interfaces Part II: numerical and experimental study. Langmuir 2009, 25, 3909–3916. 10.1021/la803318e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A.; Bazant M. Z. Deionization shocks in microstructures. Phys. Rev. E 2011, 84, 061504. 10.1103/PhysRevE.84.061504. [DOI] [PubMed] [Google Scholar]
- Deng D.; Dydek E. V.; Han J.-H.; Schlumpberger S.; Mani A.; Zaltzman B.; Bazant M. Z. Overlimiting current and shock electrodialysis in porous media. Langmuir 2013, 29, 16167–16177. 10.1021/la4040547. [DOI] [PubMed] [Google Scholar]
- Su X.; Hatton T. A. Redox-electrodes for selective electrochemical separations. Adv. Colloid Interface Sci. 2017, 244, 6–20. 10.1016/j.cis.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Comninellis C.; Chen G.. Electrochemistry for the Environment; Springer, 2010; Vol. 2015; Chapter 12. [Google Scholar]
- Mousset E.; Doudrick K. A review of electrochemical reduction processes to treat oxidized contaminants in water. Current Opinion in Electrochemistry 2020, 22, 221–227. 10.1016/j.coelec.2020.07.008. [DOI] [Google Scholar]
- Lilga M. A.; Orth R. J.; Sukamto J.; Haight S.; Schwartz D. Metal ion separations using electrically switched ion exchange. Sep. Purif. Technol. 1997, 11, 147–158. 10.1016/S1383-5866(97)00017-8. [DOI] [Google Scholar]
- Jeerage K. M.; Schwartz D. T. Characterization of cathodically deposited nickel hexacyanoferrate for electrochemically switched ion exchange. Sep. Sci. Technol. 2000, 35, 2375–2392. 10.1081/SS-100102344. [DOI] [Google Scholar]
- Dong H.; Wei L.; Tarpeh W. A. Electro-assisted regeneration of pH-sensitive ion exchangers for sustainable phosphate removal and recovery. Water Res. 2020, 184, 116167. 10.1016/j.watres.2020.116167. [DOI] [PubMed] [Google Scholar]
- Kim K.; Cotty S.; Elbert J.; Chen R.; Hou C.-H.; Su X. Asymmetric Redox-Polymer Interfaces for Electrochemical Reactive Separations: Synergistic Capture and Conversion of Arsenic. Adv. Mater. 2020, 32, 1906877. 10.1002/adma.201906877. [DOI] [PubMed] [Google Scholar]
- Mao X.; Tian W.; Ren Y.; Chen D.; Curtis S. E.; Buss M. T.; Rutledge G. C.; Hatton T. A. Energetically efficient electrochemically tunable affinity separation using multicomponent polymeric nanostructures for water treatment. Energy Environ. Sci. 2018, 11, 2954–2963. 10.1039/C8EE02000K. [DOI] [Google Scholar]
- Ren Y.; Mao X.; Hatton T. A. An Asymmetric Electrochemical System with Complementary Tunability in Hydrophobicity for Selective Separations of Organics. ACS Central Science 2019, 5, 1396–1406. 10.1021/acscentsci.9b00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.; Hübner J.; Kauke M. J.; Dalbosco L.; Thomas J.; Gonzalez C. C.; Zhu E.; Franzreb M.; Jamison T. F.; Hatton T. A. Redox Interfaces for Electrochemically Controlled Protein–Surface Interactions: Bioseparations and Heterogeneous Enzyme Catalysis. Chem. Mater. 2017, 29, 5702–5712. 10.1021/acs.chemmater.7b01699. [DOI] [Google Scholar]
- Wood J.; Gifford J.; Arba J.; Shaw M. Production of ultrapure water by continuous electrodeionization. Desalination 2010, 250, 973–976. 10.1016/j.desal.2009.09.084. [DOI] [Google Scholar]
- Hakim A. N; Khoiruddin; Wenten I. G Mechanism of Ion Transfer in Electrodeionization (EDI) System. Adv. Sci. Lett. 2017, 23, 5640–5642. 10.1166/asl.2017.8789. [DOI] [Google Scholar]
- Rathi B. S.; Kumar P. S.; Parthiban R. A review on recent advances in electrodeionization for various environmental applications. Chemosphere 2022, 289, 133223. 10.1016/j.chemosphere.2021.133223. [DOI] [PubMed] [Google Scholar]
- Conforti K. M.; Bazant M. Z. Continuous ion-selective separations by shock electrodialysis. AIChE J. 2020, 66, e16751 10.1002/aic.16751. [DOI] [Google Scholar]
- Oren Y. Capacitive deionization (CDI) for desalination and water treatment—past, present and future (a review). Desalination 2008, 228, 10–29. 10.1016/j.desal.2007.08.005. [DOI] [Google Scholar]
- Huyskens C.; Helsen J.; de Haan A. Capacitive deionization for water treatment: Screening of key performance parameters and comparison of performance for different ions. Desalination 2013, 328, 8–16. 10.1016/j.desal.2013.07.002. [DOI] [Google Scholar]
- Guo L.; Huang Y. X.; Ding M.; Leong Z. Y.; Vafakhah S.; Yang H. Y. A high performance electrochemical deionization method to desalinate brackish water with an FePO4/RGO nanocomposite. Journal of Materials Chemistry A 2018, 6, 8901–8908. 10.1039/C8TA01361F. [DOI] [Google Scholar]
- Guo L.; Mo R. W.; Shi W. H.; Huang Y. X.; Leong Z. Y.; Ding M.; Chen F. M.; Yang H. Y. A Prussian blue anode for high performance electrochemical deionization promoted by the Faradaic mechanism. Nanoscale 2017, 9, 13305–13312. 10.1039/C7NR03579A. [DOI] [PubMed] [Google Scholar]
- Strathmann H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. 10.1016/j.desal.2010.04.069. [DOI] [Google Scholar]
- Suss M.; Porada S.; Sun X.; Biesheuvel P.; Yoon J.; Presser V. Water desalination via capacitive deionization: what is it and what can we expect from it?. Energy Environ. Sci. 2015, 8, 2296–2319. 10.1039/C5EE00519A. [DOI] [Google Scholar]
- Smith K. C. Theoretical evaluation of electrochemical cell architectures using cation intercalation electrodes for desalination. Electrochim. Acta 2017, 230, 333–341. 10.1016/j.electacta.2017.02.006. [DOI] [Google Scholar]
- Suss M. E.; Presser V. Water desalination with energy storage electrode materials. Joule 2018, 2, 10–15. 10.1016/j.joule.2017.12.010. [DOI] [Google Scholar]
- Suresh A.; Hill G. T.; Hoenig E.; Liu C. Electrochemically mediated deionization: a review. Molecular Systems Design & Engineering 2021, 6, 25–51. 10.1039/D0ME00090F. [DOI] [Google Scholar]
- Bazant M. Z.; Squires T. M. Induced-charge electrokinetic phenomena: theory and microfluidic applications. Phys. Rev. Lett. 2004, 92, 066101. 10.1103/PhysRevLett.92.066101. [DOI] [PubMed] [Google Scholar]
- Bazant M. Z.; Squires T. M. Induced-charge electrokinetic phenomena. Curr. Opin. Colloid Interface Sci. 2010, 15, 203–213. 10.1016/j.cocis.2010.01.003. [DOI] [Google Scholar]
- Goldstein A. L. Electrodialysis on the American continent. Desalination 1979, 30, 49–58. 10.1016/S0011-9164(00)88432-5. [DOI] [Google Scholar]
- Bernardes A.; Rodrigues M.; Ferreira J.. Electrodialysis and Water Reuse; Springer, 2016. [Google Scholar]
- Sillanpää M.; Shestakova M.. Electrochemical Water Treatment Methods: Fundamentals, Methods and Full Scale Applications; Butterworth-Heinemann, 2017. [Google Scholar]
- Campione A.; Gurreri L.; Ciofalo M.; Micale G.; Tamburini A.; Cipollina A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. 10.1016/j.desal.2017.12.044. [DOI] [Google Scholar]
- Gurreri L.; Tamburini A.; Cipollina A.; Micale G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. 10.3390/membranes10070146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado L.; Chen A. Electrodeionization: Principles, Strategies and Applications. Electrochim. Acta 2014, 132, 583–597. 10.1016/j.electacta.2014.03.165. [DOI] [Google Scholar]
- Smith K. C.; Dmello R. Na-Ion Desalination (NID) Enabled by Na-Blocking Membranes and Symmetric Na-Intercalation: Porous-Electrode Modeling. J. Electrochem. Soc. 2016, 163, A530–A539. 10.1149/2.0761603jes. [DOI] [Google Scholar]
- Srimuk P.; Kaasik F.; Kruner B.; Tolosa A.; Fleischmann S.; Jackel N.; Tekeli M. C.; Aslan M.; Suss M. E.; Presser V. MXene as a novel intercalation-type pseudocapacitive cathode and anode for capacitive deionization. Journal of Materials Chemistry A 2016, 4, 18265–18271. 10.1039/C6TA07833H. [DOI] [Google Scholar]
- Balcajin O.; Noy A.; Fornasiero F.; Grigoropoulos C. P.; Holt J. K.; In J. B.; Kim S.; Park H. G.. Nanotechnology Applications for Clean Water; Elsevier, 2009; pp 77–93. [Google Scholar]
- Roelofs S. H.; van den Berg A.; Odijk M. Microfluidic desalination techniques and their potential applications. Lab Chip 2015, 15, 3428–3438. 10.1039/C5LC00481K. [DOI] [PubMed] [Google Scholar]
- Särkkä H.; Vepsäläinen M.; Sillanpää M. Natural organic matter (NOM) removal by electrochemical methods—A review. J. Electroanal. Chem. 2015, 755, 100–108. 10.1016/j.jelechem.2015.07.029. [DOI] [Google Scholar]
- Martínez-Huitle C. A.; Rodrigo M. A.; Sirés I.; Scialdone O. Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem. Rev. 2015, 115, 13362–13407. 10.1021/acs.chemrev.5b00361. [DOI] [PubMed] [Google Scholar]
- Chaplin B. P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci.: Proc. Impacts 2014, 16, 1182–1203. [DOI] [PubMed] [Google Scholar]
- Panizza M.; Bocca C.; Cerisola G. Electrochemical treatment of wastewater containing polyaromatic organic pollutants. Water Res. 2000, 34, 2601–2605. 10.1016/S0043-1354(00)00145-7. [DOI] [Google Scholar]
- Martínez-Huitle C. A.; Brillas E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Applied Catalysis B: Environmental 2009, 87, 105–145. 10.1016/j.apcatb.2008.09.017. [DOI] [Google Scholar]
- Sirés I.; Brillas E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ. Int. 2012, 40, 212–229. 10.1016/j.envint.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Brillas E.; Martínez-Huitle C. A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Applied Catalysis B: Environmental 2015, 166, 603–643. 10.1016/j.apcatb.2014.11.016. [DOI] [Google Scholar]
- Grimm J.; Bessarabov D.; Sanderson R. Review of electro-assisted methods for water purification. Desalination 1998, 115, 285–294. 10.1016/S0011-9164(98)00047-2. [DOI] [Google Scholar]
- Chen G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. 10.1016/j.seppur.2003.10.006. [DOI] [Google Scholar]
- Pulkka S.; Martikainen M.; Bhatnagar A.; Sillanpää M. Electrochemical methods for the removal of anionic contaminants from water–a review. Sep. Purif. Technol. 2014, 132, 252–271. 10.1016/j.seppur.2014.05.021. [DOI] [Google Scholar]
- Alsheyab M.; Jiang J.-Q.; Stanford C. On-line production of ferrate with an electrochemical method and its potential application for wastewater treatment–A review. Journal of Environmental Management 2009, 90, 1350–1356. 10.1016/j.jenvman.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Särkkä H.; Bhatnagar A.; Sillanpää M. Recent developments of electro-oxidation in water treatment—a review. J. Electroanal. Chem. 2015, 754, 46–56. 10.1016/j.jelechem.2015.06.016. [DOI] [Google Scholar]
- Martínez-Huitle C. A.; Brillas E. Electrochemical alternatives for drinking water disinfection. Angew. Chem., Int. Ed. 2008, 47, 1998–2005. 10.1002/anie.200703621. [DOI] [PubMed] [Google Scholar]
- Kraft A. Electrochemical water disinfection: a short review. Platinum metals review 2008, 52, 177–185. 10.1595/147106708X329273. [DOI] [Google Scholar]
- Garcia-Segura S.; Ocon J. D.; Chong M. N. Electrochemical oxidation remediation of real wastewater effluents—a review. Process Safety and Environmental Protection 2018, 113, 48–67. 10.1016/j.psep.2017.09.014. [DOI] [Google Scholar]
- Sirés I.; Brillas E.; Oturan M. A.; Rodrigo M. A.; Panizza M. Electrochemical advanced oxidation processes: today and tomorrow. A review. Environmental Science and Pollution Research 2014, 21, 8336–8367. 10.1007/s11356-014-2783-1. [DOI] [PubMed] [Google Scholar]
- Miklos D. B.; Remy C.; Jekel M.; Linden K. G.; Drewes J. E.; Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water research 2018, 139, 118–131. 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- Trojanowicz M.; Bojanowska-Czajka A.; Bartosiewicz I.; Kulisa K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)–a review of recent advances. Chemical Engineering Journal 2018, 336, 170–199. 10.1016/j.cej.2017.10.153. [DOI] [Google Scholar]
- Khan S.; Sayed M.; Sohail M.; Shah L. A.; Raja M. A. Advanced oxidation and reduction processes. Advances in Water Purification Techniques 2019, 135–164. 10.1016/B978-0-12-814790-0.00006-5. [DOI] [Google Scholar]
- Porada S.; Zhao R.; van der Wal A.; Presser V.; Biesheuvel P. M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. 10.1016/j.pmatsci.2013.03.005. [DOI] [Google Scholar]
- Yip N. Y.; Brogioli D.; Hamelers H. V.; Nijmeijer K. Salinity gradients for sustainable energy: primer, progress, and prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. 10.1021/acs.est.6b03448. [DOI] [PubMed] [Google Scholar]
- Bazinet L.; Geoffroy T. R. Electrodialytic processes: Market overview, membrane phenomena, recent developments and sustainable strategies. Membranes 2020, 10, 221. 10.3390/membranes10090221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenten I.; Khoiruddin K.; Alkhadra M. A.; Tian H.; Bazant M. Z. Novel ionic separation mechanisms in electrically driven membrane processes. Adv. Colloid Interface Sci. 2020, 284, 102269. 10.1016/j.cis.2020.102269. [DOI] [PubMed] [Google Scholar]
- Srimuk P.; Su X.; Yoon J.; Aurbach D.; Presser V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nature Reviews Materials 2020, 5, 517–538. 10.1038/s41578-020-0193-1. [DOI] [Google Scholar]
- Marek J. State-of-the-Art Water Treatment in Czech Power Sector: Industry-Proven Case Studies Showing Economic and Technical Benefits of Membrane and Other Novel Technologies for Each Particular Water Cycle. Membranes 2021, 11, 98. 10.3390/membranes11020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren J.; Syversen U. State-of-the-art electroflocculation. Filtration and Separation 1995, 32, 153–156. 10.1016/S0015-1882(97)84039-6. [DOI] [Google Scholar]
- Alinsafi A.; Khemis M.; Pons M.-N.; Leclerc J.-P.; Yaacoubi A.; Benhammou A.; Nejmeddine A. Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Proc.: Proc. Intensification 2005, 44, 461–470. 10.1016/j.cep.2004.06.010. [DOI] [Google Scholar]
- Mollah M. Y. A.; Schennach R.; Parga J. R.; Cocke D. L. Electrocoagulation (EC) - science and applications. Journal of Hazardous Materials 2001, 84, 29–41. 10.1016/S0304-3894(01)00176-5. [DOI] [PubMed] [Google Scholar]
- Rodrigo M.; Oturan N.; Oturan M. A. Electrochemically assisted remediation of pesticides in soils and water: a review. Chem. Rev. 2014, 114, 8720–8745. 10.1021/cr500077e. [DOI] [PubMed] [Google Scholar]
- Azimi A.; Azari A.; Rezakazemi M.; Ansarpour M. Removal of heavy metals from industrial wastewaters: a review. ChemBioEng. Reviews 2017, 4, 37–59. 10.1002/cben.201600010. [DOI] [Google Scholar]
- Liu C.; Wu T.; Hsu P.-C.; Xie J.; Zhao J.; Liu K.; Sun J.; Xu J.; Tang J.; Ye Z.; Lin D.; Cui Y. Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 2019, 13, 6431–6437. 10.1021/acsnano.8b09301. [DOI] [PubMed] [Google Scholar]
- Wang F.; Stahl S. S. Electrochemical oxidation of organic molecules at lower overpotential: Accessing broader functional group compatibility with electron-proton transfer mediators. Accounts of chemical research 2020, 53, 561–574. 10.1021/acs.accounts.9b00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L.; Xu L. J. Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Critical reviews in environmental science and technology 2012, 42, 251–325. 10.1080/10643389.2010.507698. [DOI] [Google Scholar]
- Hayyan M.; Hashim M. A.; AlNashef I. M. Superoxide ion: generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- Martínez-Huitle C. A.; Rodrigo M. A.; Scialdone O.. Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann, 2018. [Google Scholar]
- Xia X.; Zhu F.; Li J.; Yang H.; Wei L.; Li Q.; Jiang J.; Zhang G.; Zhao Q. A review study on sulfate-radical-based advanced oxidation processes for domestic/industrial wastewater treatment: degradation, efficiency, and mechanism. Front. Chem. 2020, 8, 592056. 10.3389/fchem.2020.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Sin A.; Nam H.; Park Y.; Lee H.; Han C. Advanced oxidation processes for microplastics degradation: A recent trend. Chemical Engineering Journal Advances 2022, 9, 100213. 10.1016/j.ceja.2021.100213. [DOI] [Google Scholar]
- Moreira F. C.; Boaventura R. A.; Brillas E.; Vilar V. J. Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Applied Catalysis B: Environmental 2017, 202, 217–261. 10.1016/j.apcatb.2016.08.037. [DOI] [Google Scholar]
- Du J.; Zhang B.; Li J.; Lai B. Decontamination of heavy metal complexes by advanced oxidation processes: A review. Chin. Chem. Lett. 2020, 31, 2575–2582. 10.1016/j.cclet.2020.07.050. [DOI] [Google Scholar]
- da Silva S. W.; Welter J. B.; Albornoz L. L.; Heberle A. N. A.; Ferreira J. Z.; Bernardes A. M. Advanced Electrochemical Oxidation Processes in the Treatment of Pharmaceutical Containing Water and Wastewater: a Review. Curr. Pollution Rep. 2021, 7, 121–124. [Google Scholar]
- Sen C.; Packer L.; Hänninen O.. Handbook of Oxidants and Antioxidants in Exercise; Elsevier, 2000. [Google Scholar]
- Halliwell B.; Gutteridge J. M.. Free Radicals in Biology and Medicine; Oxford University Press, 2015. [Google Scholar]
- Bielski B. H.; Cabelli D. E.; Arudi R. L.; Ross A. B. Reactivity of HO2/O- 2 radicals in aqueous solution. Journal of physical and chemical reference data 1985, 14, 1041–1100. 10.1063/1.555739. [DOI] [Google Scholar]
- Zhang H.; Wu J.; Wang Z.; Zhang D. Electrochemical oxidation of crystal violet in the presence of hydrogen peroxide. J. Chem. Technol. Biotechnol. 2010, 85, 1436–1444. 10.1002/jctb.2447. [DOI] [Google Scholar]
- R Buettner G. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anti-Cancer Agents Med. Chem. 2011, 11, 341–346. 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Z.; Gao Z.; Zhang L.; He W.; Yin J. J. Core–shell structure dependent reactivity of Fe@ Fe2O3 nanowires on aerobic degradation of 4-chlorophenol. Environ. Sci. Technol. 2013, 47, 5344–5352. 10.1021/es4005202. [DOI] [PubMed] [Google Scholar]
- Dimić D. S.; Milenković D. A.; Avdović E. H.; Nakarada DJ. J.; Dimitrić Marković J. M. D.; Marković Z. S. Advanced oxidation processes of coumarins by hydroperoxyl radical: An experimental and theoretical study, and ecotoxicology assessment. Chemical Engineering Journal 2021, 424, 130331. 10.1016/j.cej.2021.130331. [DOI] [Google Scholar]
- Watts R. J.; Bottenberg B. C.; Hess T. F.; Jensen M. D.; Teel A. L. Role of reductants in the enhanced desorption and transformation of chloroaliphatic compounds by modified Fenton’s reactions. Environ. Sci. Technol. 1999, 33, 3432–3437. 10.1021/es990054c. [DOI] [Google Scholar]
- Teel A. L.; Watts R. J. Degradation of carbon tetrachloride by modified Fenton’s reagent. Journal of Hazardous Materials 2002, 94, 179–189. 10.1016/S0304-3894(02)00068-7. [DOI] [PubMed] [Google Scholar]
- Mitchell S. M.; Ahmad M.; Teel A. L.; Watts R. J. Degradation of perfluorooctanoic acid by reactive species generated through catalyzed H2O2 propagation reactions. Environmental Science & Technology Letters 2014, 1, 117–121. 10.1021/ez4000862. [DOI] [Google Scholar]
- Bai L.; Jiang Y.; Xia D.; Wei Z.; Spinney R.; Dionysiou D. D.; Minakata D.; Xiao R.; Xie H.-B.; Chai L.. Mechanistic Understanding of Superoxide Radical-Mediated Degradation of Perfluorocarboxylic Acids; Environmental Science & Technology, 2021. [DOI] [PubMed] [Google Scholar]
- Watts R. J.; Teel A. L. Hydroxyl radical and non-hydroxyl radical pathways for trichloroethylene and perchloroethylene degradation in catalyzed H2O2 propagation systems. Water research 2019, 159, 46–54. 10.1016/j.watres.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Buxton G. V.; Greenstock C. L.; Helman W. P.; Ross A. B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/ ·O– in Aqueous Solution. Journal of physical and chemical reference data 1988, 17, 513–886. 10.1063/1.555805. [DOI] [Google Scholar]
- Naumczyk J.; Szpyrkowicz L.; Zilio-Grandi F. Electrochemical treatment of textile wastewater. Water Sci. Technol. 1996, 34, 17–24. 10.2166/wst.1996.0258. [DOI] [Google Scholar]
- Comninellis C.; Nerini A. Anodic oxidation of phenol in the presence of NaCl for wastewater treatment. J. Appl. Electrochem. 1995, 25, 23–28. 10.1007/BF00251260. [DOI] [Google Scholar]
- Martinez-Huitle C. A.; Ferro S. Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. 10.1039/B517632H. [DOI] [PubMed] [Google Scholar]
- Li H.; Ni J. Electrogeneration of disinfection byproducts at a boron-doped diamond anode with resorcinol as a model substance. Electrochimica acta 2012, 69, 268–274. 10.1016/j.electacta.2012.02.098. [DOI] [Google Scholar]
- Li H.; Long Y.; Zhu X.; Tian Y.; Ye J. Influencing factors and chlorinated byproducts in electrochemical oxidation of bisphenol A with boron-doped diamond anodes. Electrochim. Acta 2017, 246, 1121–1130. 10.1016/j.electacta.2017.06.163. [DOI] [Google Scholar]
- Carboneras M. B.; Cañizares P.; Rodrigo M. A.; Villaseñor J.; Fernandez-Morales F. J. Improving biodegradability of soil washing effluents using anodic oxidation. Bioresource technology 2018, 252, 1–6. 10.1016/j.biortech.2017.12.060. [DOI] [PubMed] [Google Scholar]
- Urtiaga A.; Rueda A.; Anglada Á.; Ortiz I. Integrated treatment of landfill leachates including electrooxidation at pilot plant scale. Journal of Hazardous Materials 2009, 166, 1530–1534. 10.1016/j.jhazmat.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Zöllig H.; Remmele A.; Morgenroth E.; Udert K. M. Removal rates and energy demand of the electrochemical oxidation of ammonia and organic substances in real stored urine. Environ. Sci.: Water Res. Technol. 2017, 3, 480–491. 10.1039/C7EW00014F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirany A.; Sirés I.; Oturan N.; Özcan A.; Oturan M. A. Electrochemical treatment of the antibiotic sulfachloropyridazine: kinetics, reaction pathways, and toxicity evolution. Environ. Sci. Technol. 2012, 46, 4074–4082. 10.1021/es204621q. [DOI] [PubMed] [Google Scholar]
- Comninellis C.; Pulgarin C. Electrochemical oxidation of phenol for wastewater treatment using SnO2, anodes. J. Appl. Electrochem. 1993, 23, 108–112. 10.1007/BF00246946. [DOI] [Google Scholar]
- Comninellis C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 1994, 39, 1857–1862. 10.1016/0013-4686(94)85175-1. [DOI] [Google Scholar]
- Gandini D.; Mahé E.; Michaud P. A.; Haenni W.; Perret A.; Comninellis C. Oxidation of carboxylic acids at boron-doped diamond electrodes for wastewater treatment. J. Appl. Electrochem. 2000, 30, 1345–1350. 10.1023/A:1026526729357. [DOI] [Google Scholar]
- Wala M.; Simka W. Effect of Anode Material on Electrochemical Oxidation of Low Molecular Weight Alcohols—A Review. Molecules 2021, 26, 2144. 10.3390/molecules26082144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Huitle C. A.; Panizza M. Electrochemical oxidation of organic pollutants for wastewater treatment. Current Opinion in Electrochemistry 2018, 11, 62–71. 10.1016/j.coelec.2018.07.010. [DOI] [Google Scholar]
- Panizza M.; Cerisola G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. 10.1021/cr9001319. [DOI] [PubMed] [Google Scholar]
- Oturan N.; Ganiyu S. O.; Raffy S.; Oturan M. A. Sub-stoichiometric titanium oxide as a new anode material for electro-Fenton process: application to electrocatalytic destruction of antibiotic amoxicillin. Applied Catalysis B: Environmental 2017, 217, 214–223. 10.1016/j.apcatb.2017.05.062. [DOI] [Google Scholar]
- Qiao J.; Xiong Y. Electrochemical oxidation technology: A review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants. Journal of Water Process Engineering 2021, 44, 102308. 10.1016/j.jwpe.2021.102308. [DOI] [Google Scholar]
- Palma-Goyes R.; Vazquez-Arenas J.; Torres-Palma R.; Ostos C.; Ferraro F.; González I. The abatement of indigo carmine using active chlorine electrogenerated on ternary Sb2O5-doped Ti/RuO2-ZrO2 anodes in a filter-press FM01-LC reactor. Electrochim. Acta 2015, 174, 735–744. 10.1016/j.electacta.2015.06.037. [DOI] [Google Scholar]
- Duan X.; Zhao C.; Liu W.; Zhao X.; Chang L. Fabrication of a novel PbO2 electrode with a graphene nanosheet interlayer for electrochemical oxidation of 2-chlorophenol. Electrochim. Acta 2017, 240, 424–436. 10.1016/j.electacta.2017.04.114. [DOI] [Google Scholar]
- Zuo J.; Zhu J.; Zhang M.; Hong Q.; Han J.; Liu J. Synergistic photoelectrochemical performance of La-doped RuO2-TiO2/Ti electrodes. Appl. Surf. Sci. 2020, 502, 144288. 10.1016/j.apsusc.2019.144288. [DOI] [Google Scholar]
- Wang W.; Wang K.; Hao W.; Zhang T.; Liu Y.; Yu L.; Li W. Preparation of Ti-based Yb-doped SnO2–RuO2 electrode and electrochemical oxidation treatment of coking wastewater. Journal of Rare Earths 2022, 40, 763–771. 10.1016/j.jre.2021.04.001. [DOI] [Google Scholar]
- Zhang C.; Tang J.; Peng C.; Jin M. Degradation of perfluorinated compounds in wastewater treatment plant effluents by electrochemical oxidation with Nano-ZnO coated electrodes. J. Mol. Liq. 2016, 221, 1145–1150. 10.1016/j.molliq.2016.06.093. [DOI] [Google Scholar]
- Man S.; Bao H.; Yang H.; Xu K.; Li A.; Xie Y.; Jian Y.; Yang W.; Mo Z.; Li X. Preparation and characterization of Nano-SiC doped PbO2 electrode for degradation of toluene diamine. J. Alloys Compd. 2021, 859, 157884. 10.1016/j.jallcom.2020.157884. [DOI] [Google Scholar]
- Kasian O.; Luk’Yanenko T.; Demchenko P.; Gladyshevskii R.; Amadelli R.; Velichenko A. Electrochemical properties of thermally treated platinized Ebonex® with low content of Pt. Electrochim. Acta 2013, 109, 630–637. 10.1016/j.electacta.2013.07.162. [DOI] [Google Scholar]
- Geng P.; Su J.; Miles C.; Comninellis C.; Chen G. Highly-ordered Magnéli Ti4O7 nanotube arrays as effective anodic material for electro-oxidation. Electrochim. Acta 2015, 153, 316–324. 10.1016/j.electacta.2014.11.178. [DOI] [Google Scholar]
- Ioroi T.; Yasuda K. Highly reversal-tolerant anodes using Ti4O7-supported platinum with a very small amount of water-splitting catalyst. J. Power Sources 2020, 450, 227656. 10.1016/j.jpowsour.2019.227656. [DOI] [Google Scholar]
- Lin H.; Niu J.; Liang S.; Wang C.; Wang Y.; Jin F.; Luo Q.; Huang Q. Development of macroporous Magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly-and perfluoroalkyl substances. Chemical Engineering Journal 2018, 354, 1058–1067. 10.1016/j.cej.2018.07.210. [DOI] [Google Scholar]
- Ganiyu S. O.; Oturan N.; Raffy S.; Esposito G.; van Hullebusch E. D.; Cretin M.; Oturan M. A. Use of sub-stoichiometric titanium oxide as a ceramic electrode in anodic oxidation and electro-Fenton degradation of the beta-blocker propranolol: degradation kinetics and mineralization pathway. Electrochim. Acta 2017, 242, 344–354. 10.1016/j.electacta.2017.05.047. [DOI] [Google Scholar]
- Ganiyu S. O.; Oturan N.; Raffy S.; Cretin M.; Causserand C.; Oturan M. A. Efficiency of plasma elaborated sub-stoichiometric titanium oxide (Ti4O7) ceramic electrode for advanced electrochemical degradation of paracetamol in different electrolyte media. Sep. Purif. Technol. 2019, 208, 142–152. 10.1016/j.seppur.2018.03.076. [DOI] [Google Scholar]
- Han Z.; Xu Y.; Zhou S.; Zhu P. Preparation and electrochemical properties of Al-based composite coating electrode with Ti4O7 ceramic interlayer for electrowinning of nonferrous metals. Electrochim. Acta 2019, 325, 134940. 10.1016/j.electacta.2019.134940. [DOI] [Google Scholar]
- Iniesta J.; Michaud P.; Panizza M.; Cerisola G.; Aldaz A.; Comninellis C. Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim. Acta 2001, 46, 3573–3578. 10.1016/S0013-4686(01)00630-2. [DOI] [PubMed] [Google Scholar]
- Anglada A.; Urtiaga A.; Ortiz I. Contributions of electrochemical oxidation to waste-water treatment: fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. 10.1002/jctb.2214. [DOI] [Google Scholar]
- Reddy C. V.; Reddy K. R.; Harish V. a.; Shim J.; Shankar M.; Shetti N. P.; Aminabhavi T. M. Metal-organic frameworks (MOFs)-based efficient heterogeneous photocatalysts: synthesis, properties and its applications in photocatalytic hydrogen generation, CO2 reduction and photodegradation of organic dyes. Int. J. Hydrogen Energy 2020, 45, 7656–7679. 10.1016/j.ijhydene.2019.02.144. [DOI] [Google Scholar]
- Elgarahy A. M.; Elwakeel K. Z.; Akhdhar A.; Hamza M. F. Recent advances in greenly synthesized nanoengineered materials for water/wastewater remediation: an overview. Nanotechnol. Environ. Eng. 2021, 6, 1–24. 10.1007/s41204-021-00104-5. [DOI] [Google Scholar]
- Du X.; Oturan M. A.; Zhou M.; Belkessa N.; Su P.; Cai J.; Trellu C.; Mousset E. Nanostructured electrodes for electrocatalytic advanced oxidation processes: From materials preparation to mechanisms understanding and wastewater treatment applications. Applied Catalysis B: Environmental 2021, 296, 120332. 10.1016/j.apcatb.2021.120332. [DOI] [Google Scholar]
- Michaud P.-A.; Mahé E.; Haenni W.; Perret A.; Comninellis C. Preparation of peroxodisulfuric acid using boron-doped diamond thin film electrodes. Electrochem. Solid-State Lett. 1999, 3, 77. 10.1149/1.1390963. [DOI] [Google Scholar]
- Serrano K.; Michaud P.; Comninellis C.; Savall A. Electrochemical preparation of peroxodisulfuric acid using boron doped diamond thin film electrodes. Electrochim. Acta 2002, 48, 431–436. 10.1016/S0013-4686(02)00688-6. [DOI] [Google Scholar]
- Saha M. S.; Furuta T.; Nishiki Y. Electrochemical synthesis of sodium peroxycarbonate at boron-doped diamond electrodes. Electrochem. Solid-State Lett. 2003, 6, D5. 10.1149/1.1576050. [DOI] [Google Scholar]
- Wang J.; Wang S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chemical Engineering Journal 2018, 334, 1502–1517. 10.1016/j.cej.2017.11.059. [DOI] [Google Scholar]
- Hasanzadeh A.; Khataee A.; Zarei M.; Zhang Y. Two-electron oxygen reduction on fullerene C60-carbon nanotubes covalent hybrid as a metal-free electrocatalyst. Sci. Rep. 2019, 9, 13780. 10.1038/s41598-019-50155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Meng H.; Wang Y.; Shi L.; Wang X.; Chai S. Fabrication of graphene@ graphite-based gas diffusion electrode for improving H2O2 generation in Electro-Fenton process. Electrochim. Acta 2018, 260, 112–120. 10.1016/j.electacta.2017.11.048. [DOI] [Google Scholar]
- Su P.; Zhou M.; Lu X.; Yang W.; Ren G.; Cai J. Electrochemical catalytic mechanism of N-doped graphene for enhanced H2O2 yield and in-situ degradation of organic pollutant. Applied Catalysis B: Environmental 2019, 245, 583–595. 10.1016/j.apcatb.2018.12.075. [DOI] [Google Scholar]
- Wang W.; Lu X.; Su P.; Li Y.; Cai J.; Zhang Q.; Zhou M.; Arotiba O. Enhancement of hydrogen peroxide production by electrochemical reduction of oxygen on carbon nanotubes modified with fluorine. Chemosphere 2020, 259, 127423. 10.1016/j.chemosphere.2020.127423. [DOI] [PubMed] [Google Scholar]
- Carneiro J. F.; Rocha R. S.; Hammer P.; Bertazzoli R.; Lanza M. Hydrogen peroxide electrogeneration in gas diffusion electrode nanostructured with Ta2O5. Applied Catalysis A: General 2016, 517, 161–167. 10.1016/j.apcata.2016.03.013. [DOI] [Google Scholar]
- Carneiro J. F.; Silva F. L.; Martins A. S.; Dias R. M.; Titato G. M.; Santos-Neto A. J.; Bertazzoli R.; Lanza M. R. Simultaneous degradation of hexazinone and diuron using ZrO2-nanostructured gas diffusion electrode. Chemical Engineering Journal 2018, 351, 650–659. 10.1016/j.cej.2018.06.122. [DOI] [Google Scholar]
- Paz E. C.; Aveiro L. R.; Pinheiro V. S.; Souza F. M.; Lima V. B.; Silva F. L.; Hammer P.; Lanza M. R.; Santos M. C. Evaluation of H2O2 electrogeneration and decolorization of Orange II azo dye using tungsten oxide nanoparticle-modified carbon. Applied Catalysis B: Environmental 2018, 232, 436–445. 10.1016/j.apcatb.2018.03.082. [DOI] [Google Scholar]
- Yuan S.; Gou N.; Alshawabkeh A. N.; Gu A. Z. Efficient degradation of contaminants of emerging concerns by a new electro-Fenton process with Ti/MMO cathode. Chemosphere 2013, 93, 2796–2804. 10.1016/j.chemosphere.2013.09.051. [DOI] [PubMed] [Google Scholar]
- He Y.; Ma Y.; Meng J.; Zhang X.; Xia Y. Dual electrochemical catalysis of Bi2Mo3O12/Ti cathode for hydrogen peroxide production in electro-Fenton system. Journal of catalysis 2019, 373, 297–305. 10.1016/j.jcat.2019.04.005. [DOI] [Google Scholar]
- Pitchai R.; Thavasi V.; Mhaisalkar S. G.; Ramakrishna S. Nanostructured cathode materials: a key for better performance in Li-ion batteries. J. Mater. Chem. 2011, 21, 11040–11051. 10.1039/c1jm10857c. [DOI] [Google Scholar]
- Rajkumar C.; Veerakumar P.; Chen S.-M.; Thirumalraj B.; Lin K.-C. Ultrathin sulfur-doped graphitic carbon nitride nanosheets as metal-free catalyst for electrochemical sensing and catalytic removal of 4-nitrophenol. ACS Sustainable Chem. Eng. 2018, 6, 16021–16031. 10.1021/acssuschemeng.8b02041. [DOI] [Google Scholar]
- Li Z.; Shen C.; Liu Y.; Ma C.; Li F.; Yang B.; Huang M.; Wang Z.; Dong L.; Wolfgang S. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton. Applied Catalysis B: Environmental 2020, 260, 118204. 10.1016/j.apcatb.2019.118204. [DOI] [Google Scholar]
- dos Santos Cunha G.; de Souza-Chaves B. M.; Bila D. M.; Bassin J. P.; Vecitis C. D.; Dezotti M. Insights into estrogenic activity removal using carbon nanotube electrochemical filter. Sci. Total Environ. 2019, 678, 448–456. 10.1016/j.scitotenv.2019.04.342. [DOI] [PubMed] [Google Scholar]
- Pleskov Y. V.; Krotova M.; Elkin V.; Varnin V.; Teremetskaya I.; Saveliev A.; Ralchenko V. Benzene oxidation at diamond electrodes: Comparison of microcrystalline and nanocrystalline diamonds. ChemPhysChem 2012, 13, 3047–3052. 10.1002/cphc.201101059. [DOI] [PubMed] [Google Scholar]
- Vecitis C. D.; Gao G.; Liu H. Electrochemical carbon nanotube filter for adsorption, desorption, and oxidation of aqueous dyes and anions. J. Phys. Chem. C 2011, 115, 3621–3629. 10.1021/jp111844j. [DOI] [Google Scholar]
- Cao J.; Zhao H.; Cao F.; Zhang J.; Cao C. Electrocatalytic degradation of 4-chlorophenol on F-doped PbO2 anodes. Electrochim. Acta 2009, 54, 2595–2602. 10.1016/j.electacta.2008.10.049. [DOI] [Google Scholar]
- Bessegato G. G.; Cardoso J. C.; Zanoni M. V. B. Enhanced photoelectrocatalytic degradation of an acid dye with boron-doped TiO2 nanotube anodes. Catal. Today 2015, 240, 100–106. 10.1016/j.cattod.2014.03.073. [DOI] [Google Scholar]
- Chen Y.; Zhang G.; Ma J.; Zhou Y.; Tang Y.; Lu T. Electro-oxidation of methanol at the different carbon materials supported Pt nano-particles. international journal of hydrogen energy 2010, 35, 10109–10117. 10.1016/j.ijhydene.2010.07.170. [DOI] [Google Scholar]
- Li S.-H.; Zhao Y.; Chu J.; Li W.-W.; Yu H.-Q.; Liu G. Electrochemical degradation of methyl orange on Pt–Bi/C nanostructured electrode by a square-wave potential method. Electrochim. Acta 2013, 92, 93–101. 10.1016/j.electacta.2013.01.012. [DOI] [Google Scholar]
- Li D.; Guo X.; Song H.; Sun T.; Wan J. Preparation of RuO2-TiO2/Nano-graphite composite anode for electrochemical degradation of ceftriaxone sodium. Journal of hazardous materials 2018, 351, 250–259. 10.1016/j.jhazmat.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Chang X.; Thind S. S.; Chen A. Electrocatalytic enhancement of salicylic acid oxidation at electrochemically reduced TiO2 nanotubes. ACS Catal. 2014, 4, 2616–2622. 10.1021/cs500487a. [DOI] [Google Scholar]
- Kim C.; Kim S.; Choi J.; Lee J.; Kang J. S.; Sung Y.-E.; Lee J.; Choi W.; Yoon J. Blue TiO2 nanotube array as an oxidant generating novel anode material fabricated by simple cathodic polarization. Electrochim. Acta 2014, 141, 113–119. 10.1016/j.electacta.2014.07.062. [DOI] [Google Scholar]
- Yoon J.-H.; Shim Y.-B.; Lee B.-s.; Choi S.-Y.; Won M.-S. Electrochemical degradation of phenol and 2-chlorophenol using Pt/Ti and boron-doped diamond electrodes. Bulletin of the Korean Chemical Society 2012, 33, 2274–2278. 10.5012/bkcs.2012.33.7.2274. [DOI] [Google Scholar]
- Huang L.; Li D.; Liu J.; Yang L.; Dai C.; Ren N.; Feng Y. Construction of TiO2 nanotube clusters on Ti mesh for immobilizing Sb-SnO2 to boost electrocatalytic phenol degradation. Journal of Hazardous Materials 2020, 393, 122329. 10.1016/j.jhazmat.2020.122329. [DOI] [PubMed] [Google Scholar]
- Dos Santos E. V.; Sena S. F. M.; da Silva D. R.; Ferro S.; De Battisti A.; Martínez-Huitle C. A. Scale-up of electrochemical oxidation system for treatment of produced water generated by Brazilian petrochemical industry. Environmental Science and Pollution Research 2014, 21, 8466–8475. 10.1007/s11356-014-2779-x. [DOI] [PubMed] [Google Scholar]
- Chaplin B. P. The prospect of electrochemical technologies advancing worldwide water treatment. Accounts of chemical research 2019, 52, 596–604. 10.1021/acs.accounts.8b00611. [DOI] [PubMed] [Google Scholar]
- Stirling R.; Walker W. S.; Westerhoff P.; Garcia-Segura S. Techno-economic analysis to identify key innovations required for electrochemical oxidation as point-of-use treatment systems. Electrochim. Acta 2020, 338, 135874. 10.1016/j.electacta.2020.135874. [DOI] [Google Scholar]
- Coha M.; Farinelli G.; Tiraferri A.; Minella M.; Vione D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chemical Engineering Journal 2021, 414, 128668. 10.1016/j.cej.2021.128668. [DOI] [Google Scholar]
- Ganiyu S. O.; Sable S.; El-Din M. G. Advanced oxidation processes for the degradation of dissolved organics in produced water: A review of process performance, degradation kinetics and pathway. Chemical Engineering Journal 2022, 429, 132492. 10.1016/j.cej.2021.132492. [DOI] [Google Scholar]
- Brillas E.; Sirés I.; Oturan M. A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. 10.1021/cr900136g. [DOI] [PubMed] [Google Scholar]
- Nidheesh P.; Gandhimathi R. Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 2012, 299, 1–15. 10.1016/j.desal.2012.05.011. [DOI] [Google Scholar]
- García-Espinoza J. D.; Robles I.; Durán-Moreno A.; Godínez L. A. Photo-assisted electrochemical advanced oxidation processes for the disinfection of aqueous solutions: A review. Chemosphere 2021, 274, 129957. 10.1016/j.chemosphere.2021.129957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwer A. H.; Khan M. D.; Khan M. Z.; Joshi R.. Modern Age Waste Water Problems; Springer, 2020; pp 339–360. [Google Scholar]
- Pérez-García J. A.; Bacame-Valenzuela F. J.; Hernández A. P.; Castañeda-Zaldivar F.; Espejel-Ayala F.; Reyes-Vidal Y.. The Future of Effluent Treatment Plants; Elsevier, 2021; pp 287–306. [Google Scholar]
- Cheng S.; Mao Z.; Sun Y.; Yang J.; Yu Z.; Gu R. A novel electrochemical oxidation-methanogenesis system for simultaneously degrading antibiotics and reducing CO2 to CH4 with low energy costs. Science of The Total Environment 2021, 750, 141732. 10.1016/j.scitotenv.2020.141732. [DOI] [PubMed] [Google Scholar]
- Ramírez-Vargas C. A.; Prado A.; Arias C. A.; Carvalho P. N.; Esteve-Núñez A.; Brix H. Microbial electrochemical technologies for wastewater treatment: principles and evolution from microbial fuel cells to bioelectrochemical-based constructed wetlands. Water 2018, 10, 1128. 10.3390/w10091128. [DOI] [Google Scholar]
- Oturan M. A.; Aaron J.-J. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Critical Reviews in Environmental Science and Technology 2014, 44, 2577–2641. 10.1080/10643389.2013.829765. [DOI] [Google Scholar]
- Peng H.; Guo J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environ. Chem. Lett. 2020, 18, 2055–2068. 10.1007/s10311-020-01058-x. [DOI] [Google Scholar]
- Yang Q.; Yao F.; Zhong Y.; Wang D.; Chen F.; Sun J.; Hua S.; Li S.; Li X.; Zeng G. Catalytic and electrocatalytic reduction of perchlorate in water–A review. Chemical Engineering Journal 2016, 306, 1081–1091. 10.1016/j.cej.2016.08.041. [DOI] [Google Scholar]
- Garcia-Segura S.; Lanzarini-Lopes M.; Hristovski K.; Westerhoff P. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications. Applied Catalysis B: Environmental 2018, 236, 546–568. 10.1016/j.apcatb.2018.05.041. [DOI] [Google Scholar]
- Sun C.; Baig S. A.; Lou Z.; Zhu J.; Wang Z.; Li X.; Wu J.; Zhang Y.; Xu X. Electrocatalytic dechlorination of 2, 4-dichlorophenoxyacetic acid using nanosized titanium nitride doped palladium/nickel foam electrodes in aqueous solutions. Applied Catalysis B: Environmental 2014, 158, 38–47. 10.1016/j.apcatb.2014.04.004. [DOI] [Google Scholar]
- Mao R.; Zhao X.; Lan H.; Liu H.; Qu J. Graphene-modified Pd/C cathode and Pd/GAC particles for enhanced electrocatalytic removal of bromate in a continuous three-dimensional electrochemical reactor. Water research 2015, 77, 1–12. 10.1016/j.watres.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Mousset E.; Puce M.; Pons M.-N. Advanced Electro-Oxidation with Boron-Doped Diamond for Acetaminophen Removal from Real Wastewater in a Microfluidic Reactor: Kinetics and Mass-Transfer Studies. ChemElectroChem. 2019, 6, 2908–2916. 10.1002/celc.201900182. [DOI] [Google Scholar]
- Mao R.; Zhao X.; Lan H.; Liu H.; Qu J. Efficient electrochemical reduction of bromate by a Pd/rGO/CFP electrode with low applied potentials. Applied Catalysis B: Environmental 2014, 160, 179–187. 10.1016/j.apcatb.2014.04.040. [DOI] [Google Scholar]
- Gennero De Chialvo M. R.; Chialvo A. Kinetics of hydrogen evolution reaction with Frumkin adsorption: re-examination of the Volmer–Heyrovsky and Volmer–Tafel routes. Electrochimica Acta 1998, 44, 841–851. 10.1016/S0013-4686(98)00233-3. [DOI] [Google Scholar]
- Yao F.; Yang Q.; Sun J.; Chen F.; Zhong Y.; Yin H.; He L.; Tao Z.; Pi Z.; Wang D.; Li X. Electrochemical reduction of bromate using noble metal-free nanoscale zero-valent iron immobilized activated carbon fiber electrode. Chem. Eng. J. 2020, 389, 123588. 10.1016/j.cej.2019.123588. [DOI] [Google Scholar]
- Sun C.; Lou Z.; Liu Y.; Fu R.; Zhou X.; Zhang Z.; Baig S. A.; Xu X. Influence of environmental factors on the electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on nTiN doped Pd/Ni foam electrode. Chemical Engineering Journal 2015, 281, 183–191. 10.1016/j.cej.2015.06.113. [DOI] [Google Scholar]
- Wong M. S.; Alvarez P. J.; Fang Y.-l.; Akçin N.; Nutt M. O.; Miller J. T.; Heck K. N. Cleaner water using bimetallic nanoparticle catalysts. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology 2009, 84, 158–166. 10.1002/jctb.2002. [DOI] [Google Scholar]
- Nutt M. O.; Hughes J. B.; Wong M. S. Designing Pd-on-Au bimetallic nanoparticle catalysts for trichloroethene hydrodechlorination. Environ. Sci. Technol. 2005, 39, 1346–1353. 10.1021/es048560b. [DOI] [PubMed] [Google Scholar]
- Lan H.; Mao R.; Tong Y.; Liu Y.; Liu H.; An X.; Liu R. Enhanced electroreductive removal of bromate by a supported Pd–In Bimetallic catalyst: kinetics and mechanism investigation. Environ. Sci. Technol. 2016, 50, 11872–11878. 10.1021/acs.est.6b02822. [DOI] [PubMed] [Google Scholar]
- Lou Z.; Zhou J.; Sun M.; Xu J.; Yang K.; Lv D.; Zhao Y.; Xu X. MnO2 enhances electrocatalytic hydrodechlorination by Pd/Ni foam electrodes and reduces Pd needs. Chemical Engineering Journal 2018, 352, 549–557. 10.1016/j.cej.2018.07.057. [DOI] [Google Scholar]
- Song S.; Liu Q.; Fang J.; Yu W. Enhanced electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on in situ prepared Pd-anchored Ni(OH)2 bifunctional electrodes: synergistic effect between H· formation on Ni(OH)2 and dechlorination steps on Pd. Catalysis Science & Technology 2019, 9, 5130–5141. 10.1039/C9CY01359H. [DOI] [Google Scholar]
- Gennaro A.; Isse A. A.; Bianchi C. L.; Mussini P. R.; Rossi M. Is glassy carbon a really inert electrode material for the reduction of carbon–halogen bonds?. Electrochemistry communications 2009, 11, 1932–1935. 10.1016/j.elecom.2009.08.021. [DOI] [Google Scholar]
- Zhang Y.; Mu S.; Deng B.; Zheng J. Electrochemical removal and release of perchlorate using poly (aniline-co-o-aminophenol). J. Electroanal. Chem. 2010, 641, 1–6. 10.1016/j.jelechem.2010.01.021. [DOI] [Google Scholar]
- Ding L.; Li Q.; Cui H.; Tang R.; Xu H.; Xie X.; Zhai J. Electrocatalytic reduction of bromate ion using a polyaniline-modified electrode: An efficient and green technology for the removal of BrO3–in aqueous solutions. Electrochimica acta 2010, 55, 8471–8475. 10.1016/j.electacta.2010.07.062. [DOI] [Google Scholar]
- Bellomunno C.; Bonanomi D.; Falciola L.; Longhi M.; Mussini P.; Doubova L.; Di Silvestro G. Building up an electrocatalytic activity scale of cathode materials for organic halide reductions. Electrochimica acta 2005, 50, 2331–2341. 10.1016/j.electacta.2004.10.047. [DOI] [Google Scholar]
- Gütz C.; Bänziger M.; Bucher C.; Galvão T. R.; Waldvogel S. R. Development and scale-up of the electrochemical dehalogenation for the synthesis of a key intermediate for NS5A inhibitors. Org. Process Res. Dev. 2015, 19, 1428–1433. 10.1021/acs.oprd.5b00272. [DOI] [Google Scholar]
- Möhle S.; Zirbes M.; Rodrigo E.; Gieshoff T.; Wiebe A.; Waldvogel S. R. Modern electrochemical aspects for the synthesis of value-added organic products. Angew. Chem., Int. Ed. 2018, 57, 6018–6041. 10.1002/anie.201712732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik E. A.; Carlson D. A.; Eikum A. S.; Gjessing E. T. Electrocoagulation of potable water. Water research 1984, 18, 1355–1360. 10.1016/0043-1354(84)90003-4. [DOI] [Google Scholar]
- Holt P. K.; Barton G. W.; Mitchell C. A. The future for electrocoagulation as a localised water treatment technology. Chemosphere 2005, 59, 355–367. 10.1016/j.chemosphere.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Mickova I. L. Advanced electrochemical technologies in wastewater treatment. Part I: electrocoagulation. Am. Sci. Res. J. Eng., Technol. Sci. 2015, 14, 233–257. [Google Scholar]
- Mickova I. L. Advanced electrochemical technologies in wastewater treatment. Part II: electro-flocculation and electro-flotation. Am. Sci. Res. J. Eng., Technol. Sci. 2015, 14, 273–294. [Google Scholar]
- Nidheesh P.; Kumar A.; Babu D. S.; Scaria J.; Kumar M. S. Treatment of mixed industrial wastewater by electrocoagulation and indirect electrochemical oxidation. Chemosphere 2020, 251, 126437. 10.1016/j.chemosphere.2020.126437. [DOI] [PubMed] [Google Scholar]
- Dieterich A. E.Electric water-purifier. U.S. US823671A, 1906.
- Moreno C H. A.; Cocke D. L.; Gomes J. A.; Morkovsky P.; Parga J.; Peterson E.; Garcia C. Electrochemical reactions for electrocoagulation using iron electrodes. Ind. Eng. Chem. Res. 2009, 48, 2275–2282. 10.1021/ie8013007. [DOI] [Google Scholar]
- Canizares P.; Carmona M.; Lobato J.; Martinez F.; Rodrigo M. A. Electrodissolution of aluminum electrodes in electrocoagulation processes. Ind. Eng. Chem. Res. 2005, 44, 4178–4185. 10.1021/ie048858a. [DOI] [Google Scholar]
- Ofir E.; Oren Y.; Adin A. Electroflocculation: the effect of zeta-potential on particle size. Desalination 2007, 204, 33–38. 10.1016/j.desal.2006.03.533. [DOI] [Google Scholar]
- Darban A.; Shahedi A.; Taghipour F.; Jamshidi-Zanjani A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. 10.1016/j.coelec.2020.05.009. [DOI] [Google Scholar]
- Al-Hanif E. T.; Bagastyo A. Y. Electrocoagulation for drinking water treatment: a review. IOP Conf. Ser.: Earth Environ. Scii. 2021, 623, 012016. 10.1088/1755-1315/623/1/012016. [DOI] [Google Scholar]
- Bandaru S. R. S.; van Genuchten C. M.; Kumar A.; Glade S.; Hernandez D.; Nahata M.; Gadgil A. Rapid and Efficient Arsenic Removal by Iron Electrocoagulation Enabled with in Situ Generation of Hydrogen Peroxide. Environ. Sci. Technol. 2020, 54 (10), 6094–6103. 10.1021/acs.est.0c00012. [DOI] [PubMed] [Google Scholar]
- Tahreen A.; Jami M. S.; Ali F. Role of electrocoagulation in wastewater treatment: A developmental review. Journal of Water Process Engineering 2020, 37, 101440. 10.1016/j.jwpe.2020.101440. [DOI] [Google Scholar]
- Mahesh S.; Prasad B.; Mall I. D.; Mishra I. M. Electrochemical degradation of pulp and paper mill wastewater. Part 1. COD and color removal. Ind. Eng. Chem. Res. 2006, 45, 2830–2839. 10.1021/ie0514096. [DOI] [Google Scholar]
- Heidmann I.; Calmano W. Removal of Zn(II), Cu(II), Ni(II), Ag(I) and Cr(VI) present in aqueous solutions by aluminium electrocoagulation. Journal of Hazardous Materials 2008, 152, 934–941. 10.1016/j.jhazmat.2007.07.068. [DOI] [PubMed] [Google Scholar]
- Merzouk B.; Gourich B.; Sekki A.; Madani K.; Chibane M. Removal turbidity and separation of heavy metals using electrocoagulation-electroflotation technique A case study. Journal of Hazardous Materials 2009, 164, 215–222. 10.1016/j.jhazmat.2008.07.144. [DOI] [PubMed] [Google Scholar]
- Zongo I.; Leclerc J. P.; Maiga H. A.; Wethe J.; Lapicque F. Removal of hexavalent chromium from industrial wastewater by electrocoagulation: A comprehensive comparison of aluminium and iron electrodes. Sep. Purif. Technol. 2009, 66, 159–166. 10.1016/j.seppur.2008.11.012. [DOI] [Google Scholar]
- Shen F.; Chen X. M.; Gao P.; Chen G. H. Electrochemical removal of fluoride ions from industrial wastewater. Chem. Eng. Sci. 2003, 58, 987–993. 10.1016/S0009-2509(02)00639-5. [DOI] [Google Scholar]
- Ölmez T. The optimization of Cr (VI) reduction and removal by electrocoagulation using response surface methodology. Journal of Hazardous Materials 2009, 162, 1371–1378. 10.1016/j.jhazmat.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Mamelkina M. A.; Cotillas S.; Lacasa E.; Sáez C.; Tuunila R.; Sillanpää M.; Häkkinen A.; Rodrigo M. A. Removal of sulfate from mining waters by electrocoagulation. Sep. Purif. Technol. 2017, 182, 87–93. 10.1016/j.seppur.2017.03.044. [DOI] [Google Scholar]
- Wang C.-T.; Chou W.-L.; Kuo Y.-M. Removal of COD from laundry wastewater by electrocoagulation/electroflotation. Journal of hazardous materials 2009, 164, 81–86. 10.1016/j.jhazmat.2008.07.122. [DOI] [PubMed] [Google Scholar]
- Phalakornkule C.; Sukkasem P.; Mutchimsattha C. Hydrogen recovery from the electrocoagulation treatment of dye-containing wastewater. Int. J. Hydrogen Energy 2010, 35, 10934–10943. 10.1016/j.ijhydene.2010.06.100. [DOI] [Google Scholar]
- Can O. T.; Bayramoglu M.; Kobya M. Decolorization of reactive dye solutions by electrocoagulation using aluminum electrodes. Ind. Eng. Chem. Res. 2003, 42, 3391–3396. 10.1021/ie020951g. [DOI] [Google Scholar]
- Ingelsson M.; Yasri N.; Roberts E. P. Electrode Passivation, Faradaic Efficiency, and Performance Enhancement Strategies in Electrocoagulation—A Review. Water Res. 2020, 187, 116433. 10.1016/j.watres.2020.116433. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura S.; Eiband M. M. S.; de Melo J. V.; Martínez-Huitle C. A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. 10.1016/j.jelechem.2017.07.047. [DOI] [Google Scholar]
- Hakizimana J. N.; Gourich B.; Chafi M.; Stiriba Y.; Vial C.; Drogui P.; Naja J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. 10.1016/j.desal.2016.10.011. [DOI] [Google Scholar]
- Chen X. M.; Chen G. H.; Yue P. L. Novel electrode system for electroflotation of wastewater. Environ. Sci. Technol. 2002, 36, 778–783. 10.1021/es011003u. [DOI] [PubMed] [Google Scholar]
- Ge J. T.; Qu J. H.; Lei P. J.; Liu H. J. New bipolar electrocoagulation-electroflotation process for the treatment of laundry wastewater. Sep. Purif. Technol. 2004, 36, 33–39. 10.1016/S1383-5866(03)00150-3. [DOI] [Google Scholar]
- Mollah M. Y. A.; Morkovsky P.; Gomes J. A. G.; Kesmez M.; Parga J.; Cocke D. L. Fundamentals, present and future perspectives of electrocoagulation. Journal of Hazardous Materials 2004, 114, 199–210. 10.1016/j.jhazmat.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kolesnikov V.; Il’in V.; Kolesnikov A. Electroflotation in wastewater treatment from oil products, dyes, surfactants, ligands, and biological pollutants: a review. Theoretical Foundations of Chemical Engineering 2019, 53, 251–273. 10.1134/S0040579519010093. [DOI] [Google Scholar]
- Elmore A. S.Apparatus for concentrating ores. U.S. US865334A, 1907.
- Khosla N.; Venkatachalam S.; Somasundaran P. Pulsed electrogeneration of bubbles for electroflotation. J. Appl. Electrochem. 1991, 21, 986–990. 10.1007/BF01077584. [DOI] [Google Scholar]
- Emamjomeh M. M.; Sivakumar M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. Journal of environmental management 2009, 90, 1663–1679. 10.1016/j.jenvman.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Matis K.; Peleka E. Alternative flotation techniques for wastewater treatment: focus on electroflotation. Sep. Sci. Technol. 2010, 45, 2465–2474. 10.1080/01496395.2010.508065. [DOI] [Google Scholar]
- Mohtashami R.; Shang J. Q. Electroflotation for Treatment of Industrial Wastewaters: A Focused Review. Environmental Processes 2019, 6, 325–353. 10.1007/s40710-019-00348-z. [DOI] [Google Scholar]
- Peleka E. N.; Gallios G. P.; Matis K. A. A perspective on flotation: a review. J. Chem. Technol. Biotechnol. 2018, 93, 615–623. 10.1002/jctb.5486. [DOI] [Google Scholar]
- Tadesse B.; Albijanic B.; Makuei F.; Browner R. Recovery of fine and ultrafine mineral particles by electroflotation–A review. Mineral Processing and Extractive Metallurgy Review 2019, 40, 108–122. 10.1080/08827508.2018.1497627. [DOI] [Google Scholar]
- Agrawal A.; Kumari S.; Sahu K. K. Iron and Copper Recovery/Removal from Industrial Wastes: A Review. Ind. Eng. Chem. Res. 2009, 48, 6145–6161. 10.1021/ie900135u. [DOI] [Google Scholar]
- Vasudevan S.; Oturan M. A. Electrochemistry: as cause and cure in water pollution-an overview. Environmental Chemistry Letters 2014, 12, 97–108. 10.1007/s10311-013-0434-2. [DOI] [Google Scholar]
- Rahimi M.; Schoener Z.; Zhu X.; Zhang F.; Gorski C. A.; Logan B. E. Removal of copper from water using a thermally regenerative electrodeposition battery. Journal of hazardous materials 2017, 322, 551–556. 10.1016/j.jhazmat.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Peng C.; Liu Y.; Bi J.; Xu H.; Ahmed A.-S. Recovery of copper and water from copper-electroplating wastewater by the combination process of electrolysis and electrodialysis. Journal of hazardous materials 2011, 189, 814–820. 10.1016/j.jhazmat.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Guimarães Y. F.; Santos I. D.; Dutra A. J. Direct recovery of copper from printed circuit boards (PCBs) powder concentrate by a simultaneous electroleaching–electrodeposition process. Hydrometallurgy 2014, 149, 63–70. 10.1016/j.hydromet.2014.06.005. [DOI] [Google Scholar]
- Janin A.; Zaviska F.; Drogui P.; Blais J. F.; Mercier G. Selective recovery of metals in leachate from chromated copper arsenate treated wastes using electrochemical technology and chemical precipitation. Hydrometallurgy 2009, 96, 318–326. 10.1016/j.hydromet.2008.12.002. [DOI] [Google Scholar]
- Sulonen M. L. K.; Kokko M. E.; Lakaniemi A. M.; Puhakka J. A. Simultaneous removal of tetrathionate and copper from simulated acidic mining water in bioelectrochemical and electrochemical systems. Hydrometallurgy 2018, 176, 129–138. 10.1016/j.hydromet.2018.01.023. [DOI] [Google Scholar]
- Anastassakis G. N.; Bevilacqua P.; De Lorenzi L. Recovery of residual copper from low-content tailings derived from waste electrical cable treatment. Int. J. Miner. Process. 2015, 143, 105–111. 10.1016/j.minpro.2015.09.011. [DOI] [Google Scholar]
- Müller S.; Holzer F.; Haas O. Optimized zinc electrode for the rechargeable zinc–air battery. Journal of applied electrochemistry 1998, 28, 895–898. 10.1023/A:1003464011815. [DOI] [Google Scholar]
- Lai Q.; Zhang H.; Li X.; Zhang L.; Cheng Y. A novel single flow zinc–bromine battery with improved energy density. J. Power Sources 2013, 235, 1–4. 10.1016/j.jpowsour.2013.01.193. [DOI] [Google Scholar]
- Gong K.; Ma X.; Conforti K. M.; Kuttler K. J.; Grunewald J. B.; Yeager K. L.; Bazant M. Z.; Gu S.; Yan Y. A zinc–iron redox-flow battery under $100 per kW h of system capital cost. Energy Environ. Sci. 2015, 8, 2941–2945. 10.1039/C5EE02315G. [DOI] [Google Scholar]
- Horstmann B.; Danner T.; Bessler W. G. Precipitation in aqueous lithium–oxygen batteries: a model-based analysis. Energy Environ. Sci. 2013, 6, 1299–1314. 10.1039/c3ee24299d. [DOI] [Google Scholar]
- Gilroy D.; Conway B. Kinetic theory of inhibition and passivation in electrochemical reactions. J. Phys. Chem. 1965, 69, 1259–1267. 10.1021/j100888a028. [DOI] [Google Scholar]
- Lyklema J.; Minor M. On surface conduction and its role in electrokinetics. Colloids Surf., A 1998, 140, 33–41. 10.1016/S0927-7757(97)00266-5. [DOI] [Google Scholar]
- Lyklema J.Fundamentals of Interface and Colloid Science: Soft Colloids; Elsevier, 2005; Vol. 5. [Google Scholar]
- Israelachvili J. N.Intermolecular and Surface Forces; Academic Press, 2015. [Google Scholar]
- Ricart M.; Pazos M.; Gouveia S.; Cameselle C.; Sanroman M. Removal of organic pollutants and heavy metals in soils by electrokinetic remediation. Journal of Environmental Science and Health Part A 2008, 43, 871–875. 10.1080/10934520801974376. [DOI] [PubMed] [Google Scholar]
- Ribeiro A. B.; Mateus E. P.; Rodríguez-Maroto J.-M. Removal of organic contaminants from soils by an electrokinetic process: the case of molinate and bentazone. Experimental and modeling. Sep. Purif. Technol. 2011, 79, 193–203. 10.1016/j.seppur.2011.01.045. [DOI] [PubMed] [Google Scholar]
- Handojo L.; Wardani A.; Regina D.; Bella C.; Kresnowati M.; Wenten I. Electro-membrane processes for organic acid recovery. RSC Adv. 2019, 9, 7854–7869. 10.1039/C8RA09227C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D.; Fu R.; Li Q. Removal of inorganic contaminants in soil by electrokinetic remediation technologies: A review. Journal of Hazardous Materials 2021, 401, 123345. 10.1016/j.jhazmat.2020.123345. [DOI] [PubMed] [Google Scholar]
- Strathmann H.Ion-Exchange Membrane Separation Processes; Elsevier, 2004. [Google Scholar]
- Probstein R.Physicochemical Hydrodynamics: An Introduction; John Wiley & Sons: New York, 1994. [Google Scholar]
- Hellferich F.Ion Exchange; McGraw-Hill: New York, 1962. [Google Scholar]
- Hwang S.-T.; Kammermeyer K.. Membranes in Separation; Wiley: New York, 1975. [Google Scholar]
- Nikonenko V.; Yaroslavtsev A.; Pourcelly G. In Ion Transfer in and through Charged Membranes; Ciferri A., Perico A., Eds.; John Wiley & Sons, 2012; pp 267–336. [Google Scholar]
- Chen Q.-B.; Wang J.; Liu Y.; Zhao J.; Li P. Novel energy-efficient electrodialysis system for continuous brackish water desalination: Innovative stack configurations and optimal inflow modes. Water Res. 2020, 179, 115847. 10.1016/j.watres.2020.115847. [DOI] [PubMed] [Google Scholar]
- Ladole M. R.; Patil S. S.; Paraskar P. M.; Pokale P. B.; Patil P. D.. Sustainable Materials and Systems for Water Desalination; Springer, 2021; pp 15–38. [Google Scholar]
- Xu T. Ion exchange membranes: State of their development and perspective. Journal of membrane science 2005, 263, 1–29. 10.1016/j.memsci.2005.05.002. [DOI] [Google Scholar]
- Luo T.; Abdu S.; Wessling M. Selectivity of ion exchange membranes: A review. Journal of membrane science 2018, 555, 429–454. 10.1016/j.memsci.2018.03.051. [DOI] [Google Scholar]
- Sonin A.; Probstein R. A hydrodynamic theory of desalination by electrodialysis. Desalination 1968, 5, 293–329. 10.1016/S0011-9164(00)80105-8. [DOI] [Google Scholar]
- Kim Y.; Walker W. S.; Lawler D. F. Competitive separation of di-vs. mono-valent cations in electrodialysis: Effects of the boundary layer properties. water research 2012, 46, 2042–2056. 10.1016/j.watres.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Deen W.Analysis of Transport Phenomena, 2nd ed.; Oxford University Press: Oxford, 2012. [Google Scholar]
- Geissler S.; Heits H.; Werner U. Description of fluid flow through spacers in flat-channel filtration systems. Filtration & separation 1995, 32, 538–544. 10.1016/S0015-1882(97)84109-2. [DOI] [Google Scholar]
- Balster J.; Stamatialis D.; Wessling M. Membrane with integrated spacer. J.Membr. Sci. 2010, 360, 185–189. 10.1016/j.memsci.2010.05.011. [DOI] [Google Scholar]
- Dukhin S. S.; Deriagin B. V.. Electrokinetic Phenomena, 1974. [Google Scholar]
- Dukhin S. S. Electrokinetic phenomena of the second kind and their applications. Advances in colloid and interface science 1991, 35, 173–196. 10.1016/0001-8686(91)80022-C. [DOI] [Google Scholar]
- Yaroshchuk A. What makes a nano-channel? A limiting-current criterion. Microfluid. Nanofluid. 2012, 12, 615–624. 10.1007/s10404-011-0902-6. [DOI] [Google Scholar]
- Bouhidel K.-E.; Benslimane S. Ion exchange membrane modification by weak electrolytes and glycine: reduction and elimination of the concentration polarization plateau in electrodialysis. Desalination 2006, 199, 67–69. 10.1016/j.desal.2006.03.143. [DOI] [Google Scholar]
- Peers A.Disc. Faraday Soc. 21. 1956. [Google Scholar]
- Rosenberg N.; Tirrell C. Limiting currents in membrane cells. Industrial & Engineering Chemistry 1957, 49, 780–784. 10.1021/ie50568a047. [DOI] [Google Scholar]
- Newman J.; Thomas-Alyea K. E.. Electrochemical Systems; John Wiley & Sons, 2012. [Google Scholar]
- Bird R. B.; Stewart W. E.; Lightfoot E. N.; Klingenberg D. J.. Introductory Transport Phenomena; Wiley: New York, 2015; Vol. 1. [Google Scholar]
- Mareev S.; Butylskii D.; Kovalenko A.; Petukhova A.; Pismenskaya N.; Dammak L.; Larchet C.; Nikonenko V. Accounting for the concentration dependence of electrolyte diffusion coefficient in the Sand and the Peers equations. Electrochim. Acta 2016, 195, 85–93. 10.1016/j.electacta.2016.02.098. [DOI] [Google Scholar]
- Lévêque M.Les Lois de la Transmission de Chaleur par Convection. Doctoral Dissertation, 1928. [Google Scholar]
- Kundu P. K.; Cohen I. M.. Fluid Mechanics; Elsevier, 2001. [Google Scholar]
- Braff W. A.; Buie C. R.; Bazant M. Z. Boundary layer analysis of membraneless electrochemical cells. J. Electrochem. Soc. 2013, 160, A2056–A2063. 10.1149/2.052311jes. [DOI] [Google Scholar]
- Cowan D. A.; Brown J. H. Effect of turbulence on limiting current in electrodialysis cells. Industrial & Engineering Chemistry 1959, 51, 1445–1448. 10.1021/ie50600a026. [DOI] [Google Scholar]
- Belfort G.; Guter G. A. An experimental study of electrodialysis hydrodynamics. Desalination 1972, 10, 221–262. 10.1016/S0011-9164(00)82001-9. [DOI] [Google Scholar]
- Belfort G.; Guter G. An electrical analogue for electrodialysis. Desalination 1968, 5, 267–291. 10.1016/S0011-9164(00)80104-6. [DOI] [Google Scholar]
- Atlas I.; Wu J.; Shocron A. N.; Suss M. E. Spatial variations of pH in electrodialysis stacks: Theory. Electrochim. Acta 2022, 413, 140151. 10.1016/j.electacta.2022.140151. [DOI] [Google Scholar]
- Mubita T.; Porada S.; Biesheuvel P.; van der Wal A.; Dykstra J. Strategies to increase ion selectivity in electrodialysis. Sep. Purif. Technol. 2022, 292, 120944. 10.1016/j.seppur.2022.120944. [DOI] [Google Scholar]
- Nikonenko V. V.; Kovalenko A. V.; Urtenov M. K.; Pismenskaya N. D.; Han J.; Sistat P.; Pourcelly G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. 10.1016/j.desal.2014.01.008. [DOI] [Google Scholar]
- Nikonenko V. V.; Pismenskaya N. D.; Belova E. I.; Sistat P.; Huguet P.; Pourcelly G.; Larchet C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. Colloid Interface Sci. 2010, 160, 101–123. 10.1016/j.cis.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Westall J.; Hohl H. A comparison of electrostatic models for the oxide/solution interface. Adv. Colloid Interface Sci. 1980, 12, 265–294. 10.1016/0001-8686(80)80012-1. [DOI] [Google Scholar]
- Marinova K.; Alargova R.; Denkov N.; Velev O.; Petsev D.; Ivanov I.; Borwankar R. Charging of oil- water interfaces due to spontaneous adsorption of hydroxyl ions. Langmuir 1996, 12, 2045–2051. 10.1021/la950928i. [DOI] [Google Scholar]
- Behrens S. H.; Grier D. G. The charge of glass and silica surfaces. J. Chem. Phys. 2001, 115, 6716–6721. 10.1063/1.1404988. [DOI] [Google Scholar]
- Nap R. J.; Božič A. L.; Szleifer I.; Podgornik R. The role of solution conditions in the bacteriophage PP7 capsid charge regulation. Biophysical journal 2014, 107, 1970–1979. 10.1016/j.bpj.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safinya C. R.; Radler J.. Handbook of Lipid Membranes: Molecular, Functional, and Materials Aspects; CRC Press, 2018; Chapter 9. [Google Scholar]
- Trefalt G.; Behrens S. H.; Borkovec M. Charge regulation in the electrical double layer: ion adsorption and surface interactions. Langmuir 2016, 32, 380–400. 10.1021/acs.langmuir.5b03611. [DOI] [PubMed] [Google Scholar]
- Tian H.; Wang M. Electrokinetic mechanism of wettability alternation at oil-water-rock interface. Surf. Sci. Rep. 2017, 72, 369–391. 10.1016/j.surfrep.2018.01.001. [DOI] [Google Scholar]
- Kharkats Y. I.; Sokirko A. Theory of the effect of migration current exaltation taking into account dissociation-recombination reactions. Journal of electroanalytical chemistry and interfacial electrochemistry 1991, 303, 27–44. 10.1016/0022-0728(91)85113-4. [DOI] [Google Scholar]
- Andersen M.; van Soestbergen M.; Mani A.; Bruus H.; Biesheuvel P.; Bazant M. Current-induced membrane discharge. Phys. Rev. Lett. 2012, 109, 108301. 10.1103/PhysRevLett.109.108301. [DOI] [PubMed] [Google Scholar]
- Rubinstein I.; Zaltzman B. Equilibrium Electroconvective Instability. Phys. Rev. Lett. 2015, 114, 114502. 10.1103/PhysRevLett.114.114502. [DOI] [PubMed] [Google Scholar]
- Rubinstein I.; Zaltzman B. Extended space charge in concentration polarization. Advances in colloid and interface science 2010, 159, 117–129. 10.1016/j.cis.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Zaltzman B.; Rubinstein I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173–226. 10.1017/S0022112007004880. [DOI] [Google Scholar]
- Rubinstein S.; Manukyan G.; Staicu A.; Rubinstein I.; Zaltzman B.; Lammertink R.; Mugele F.; Wessling M. Direct observation of a nonequilibrium electro-osmotic instability. Phys. Rev. Lett. 2008, 101, 236101. 10.1103/PhysRevLett.101.236101. [DOI] [PubMed] [Google Scholar]
- Yossifon G.; Chang H.-C. Selection of nonequilibrium overlimiting currents: universal depletion layer formation dynamics and vortex instability. Phys. Rev. Lett. 2008, 101, 254501. 10.1103/PhysRevLett.101.254501. [DOI] [PubMed] [Google Scholar]
- Pham S.; Li Z.; Lim K.; White J.; Han J. Direct numerical simulation of electroconvective instability and hysteretic current-voltage response of permselective membrane. Phys. Rev. E 2012, 86, 046310. 10.1103/PhysRevE.86.046310. [DOI] [PubMed] [Google Scholar]
- Kwak R.; Pham V.; Lim K.; Han J. Shear flow of an electrically charged fluid by ion concentration polarization: scaling laws for convection vortices. Phys. Rev. Lett. 2013, 110, 114501. 10.1103/PhysRevLett.110.114501. [DOI] [PubMed] [Google Scholar]
- Chang H.-C.; Yossifon G.; Demekhin E. A. Nanoscale electrokinetics and microvortices: How microhydrodynamics affects nanofluidic ion flux. Annu. Rev. Fluid Mech. 2012, 44, 401–426. 10.1146/annurev-fluid-120710-101046. [DOI] [Google Scholar]
- Davidson S. M.; Wessling M.; Mani A. On the dynamical regimes of pattern-accelerated electroconvection. Sci. Rep. 2016, 6, 22505. 10.1038/srep22505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B.; Gu Z.; Liu W.; Huo P.; Zhou Y.; Rubinstein S.; Bazant M.; Zaltzman B.; Rubinstein I.; Deng D. Electro-osmotic instability of concentration enrichment in curved geometries for an aqueous electrolyte. Physical Review Fluids 2020, 5, 091701. 10.1103/PhysRevFluids.5.091701. [DOI] [Google Scholar]
- Wessling M.; Morcillo L. G.; Abdu S. Nanometer-thick lateral polyelectrolyte micropatterns induce macrosopic electro-osmotic chaotic fluid instabilities. Sci. Rep. 2015, 4, 4294. 10.1038/srep04294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghmans F.; Evdochenko E.; Stockmeier F.; Schneider S.; Smailji A.; Tiwari R.; Mikosch A.; Karatay E.; Kuhne A.; Walther A.; Mani A.; Wessling M. 2D Patterned Ion-Exchange Membranes Induce Electroconvection. Advanced Materials Interfaces 2019, 6, 1801309. 10.1002/admi.201801309. [DOI] [Google Scholar]
- Zyryanova S.; Mareev S.; Gil V.; Korzhova E.; Pismenskaya N.; Sarapulova V.; Rybalkina O.; Boyko E.; Larchet C.; Dammak L.; Nikonenko V. How Electrical Heterogeneity Parameters of Ion-Exchange Membrane Surface Affect the Mass Transfer and Water Splitting Rate in Electrodialysis. Int. J. Mol. Sci. 2020, 21, 973. 10.3390/ijms21030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butylskii D.; Moroz I.; Tsygurina K.; Mareev S. Effect of Surface Inhomogeneity of Ion-Exchange Membranes on the Mass Transfer Efficiency in Pulsed Electric Field Modes. Membranes 2020, 10, 40. 10.3390/membranes10030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishchuk N. Perspectives of the electrodialysis intensification. Desalination 1998, 117, 283–295. 10.1016/S0011-9164(98)00120-9. [DOI] [Google Scholar]
- Mishchuk N.; Koopal L.; Gonzalez-Caballero F. Intensification of electrodialysis by applying a non-stationary electric field. Colloids Surf., A 2001, 176, 195–212. 10.1016/S0927-7757(00)00568-9. [DOI] [Google Scholar]
- Lemay N.; Mikhaylin S.; Bazinet L. Voltage spike and electroconvective vortices generation during electrodialysis under pulsed electric field: Impact on demineralization process efficiency and energy consumption. Innovative food science & emerging technologies 2019, 52, 221–231. 10.1016/j.ifset.2018.12.004. [DOI] [Google Scholar]
- Lemay N.; Mikhaylin S.; Mareev S.; Pismenskaya N.; Nikonenko V.; Bazinet L. How demineralization duration by electrodialysis under high frequency pulsed electric field can be the same as in continuous current condition and that for better performances?. J. Membr. Sci. 2020, 603, 117878. 10.1016/j.memsci.2020.117878. [DOI] [Google Scholar]
- Gonzalez-Vogel A.; Rojas O. J. Exploiting electroconvective vortices in electrodialysis with high-frequency asymmetric bipolar pulses for desalination in overlimiting current regimes. Desalination 2020, 474, 114190. 10.1016/j.desal.2019.114190. [DOI] [Google Scholar]
- Huth J.; Swinney H.; Mccormick W.; Kuhn A.; Argoul F. Phys. Rev. E 1995, 51, 3444–3458. 10.1103/PhysRevE.51.3444. [DOI] [PubMed] [Google Scholar]
- Bai P.; Li J.; Brushett F. R.; Bazant M. Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229. 10.1039/C6EE01674J. [DOI] [Google Scholar]
- He Y.; Ge L.; Ge Z.; Zhao Z.; Sheng F.; Liu X.; Ge X.; Yang Z.; Fu R.; Liu Z.; Wu L.; Xu T. Monovalent cations permselective membranes with zwitterionic side chains. J. Membr. Sci. 2018, 563, 320–325. 10.1016/j.memsci.2018.05.068. [DOI] [Google Scholar]
- Chandra A.; Bhuvanesh E.; Mandal P.; Chattopadhyay S. Surface modification of anion exchange membrane using layer-by-layer polyelectrolytes deposition facilitating monovalent organic acid transport. Colloids Surf., A 2018, 558, 579–590. 10.1016/j.colsurfa.2018.09.013. [DOI] [Google Scholar]
- Merino-Garcia I.; Kotoka F.; Portugal C. A.; Crespo J. G.; Velizarov S. Characterization of poly (Acrylic) acid-modified heterogenous anion exchange membranes with improved monovalent permselectivity for RED. Membranes 2020, 10, 134. 10.3390/membranes10060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahdab Y. D.; Rehman D.; Lienhard J. H. Brackish water desalination for greenhouses: Improving groundwater quality for irrigation using monovalent selective electrodialysis reversal. J. Membr. Sci. 2020, 610, 118072. 10.1016/j.memsci.2020.118072. [DOI] [Google Scholar]
- Ahdab Y. D.; Rehman D.; Schücking G.; Barbosa M.; Lienhard J. H. Treating irrigation water using high-performance membranes for monovalent selective electrodialysis. ACS ES&T Water 2021, 1, 117–124. 10.1021/acsestwater.0c00012. [DOI] [Google Scholar]
- Tian H.; Wang Y.; Pei Y.; Crittenden J. C. Unique applications and improvements of reverse electrodialysis: A review and outlook. Applied Energy 2020, 262, 114482. 10.1016/j.apenergy.2019.114482. [DOI] [Google Scholar]
- Liu L.; Cheng Q. Mass transfer characteristic research on electrodialysis for desalination and regeneration of solution: A comprehensive review. Renewable and Sustainable Energy Reviews 2020, 134, 110115. 10.1016/j.rser.2020.110115. [DOI] [Google Scholar]
- Wenten I.; Khoiruddin K.; Wardani A. K.; Widiasa I.. Synthetic Polymeric Membranes for Advanced Water Treatment, Gas Separation, and Energy Sustainability; Elsevier, 2020; pp 71–101. [Google Scholar]
- Ariono D; Khoiruddin; Subagjo; Wenten I G. Heterogeneous structure and its effect on properties and electrochemical behavior of ion-exchange membrane. Mater. Res. Express 2017, 4, 024006. 10.1088/2053-1591/aa5cd4. [DOI] [Google Scholar]
- Andreeva M.; Gil V.; Pismenskaya N.; Nikonenko V.; Dammak L.; Larchet C.; Grande D.; Kononenko N. Effect of homogenization and hydrophobization of a cation-exchange membrane surface on its scaling in the presence of calcium and magnesium chlorides during electrodialysis. J. Membr. Sci. 2017, 540, 183–191. 10.1016/j.memsci.2017.06.030. [DOI] [Google Scholar]
- Hosseini S.; Rafiei N.; Salabat A.; Ahmadi A. Fabrication of new type of barium ferrite/copper oxide composite nanoparticles blended polyvinylchloride based heterogeneous ion exchange membrane. Arabian Journal of Chemistry 2020, 13, 2470–2482. 10.1016/j.arabjc.2018.06.001. [DOI] [Google Scholar]
- Mareev S.; Butylskii D. Y.; Pismenskaya N.; Larchet C.; Dammak L.; Nikonenko V. Geometric heterogeneity of homogeneous ion-exchange Neosepta membranes. Journal of membrane science 2018, 563, 768–776. 10.1016/j.memsci.2018.06.018. [DOI] [Google Scholar]
- Vasil’eva V.; Goleva E.; Pismenskaya N.; Kozmai A.; Nikonenko V. Effect of surface profiling of a cation-exchange membrane on the phenylalanine and NaCl separation performances in diffusion dialysis. Sep. Purif. Technol. 2019, 210, 48–59. 10.1016/j.seppur.2018.07.065. [DOI] [Google Scholar]
- Belloň T.; Slouka Z. Overlimiting behavior of surface-modified heterogeneous anion-exchange membranes. J. Membr. Sci. 2020, 610, 118291. 10.1016/j.memsci.2020.118291. [DOI] [Google Scholar]
- Gil V.; Porozhnyy M.; Rybalkina O.; Butylskii D.; Pismenskaya N. The Development of Electroconvection at the Surface of a Heterogeneous Cation-Exchange Membrane Modified with Perfluorosulfonic Acid Polymer Film Containing Titanium Oxide. Membranes 2020, 10, 125. 10.3390/membranes10060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akberova E.; Vasil’eva V. Effect of the resin content in cation-exchange membranes on development of electroconvection. Electrochem. Commun. 2020, 111, 106659. 10.1016/j.elecom.2020.106659. [DOI] [Google Scholar]
- Al-Amshawee S.; Yunus M. Y. B. M.; Azoddein A. A. M.; Hassell D. G.; Dakhil I. H.; Hasan H. A. Electrodialysis desalination for water and wastewater: A review. Chemical Engineering Journal 2020, 380, 122231. 10.1016/j.cej.2019.122231. [DOI] [Google Scholar]
- Mikhaylin S.; Bazinet L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Advances in colloid and interface science 2016, 229, 34–56. 10.1016/j.cis.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Hansima M.; Makehelwala M.; Jinadasa K.; Wei Y.; Nanayakkara K.; Herath A. C.; Weerasooriya R. Fouling of ion exchange membranes used in the electrodialysis reversal advanced water treatment: A review. Chemosphere 2020, 263, 127951. 10.1016/j.chemosphere.2020.127951. [DOI] [PubMed] [Google Scholar]
- Tong X.; Zhang B.; Chen Y. Fouling resistant nanocomposite cation exchange membrane with enhanced power generation for reverse electrodialysis. J. Membr. Sci. 2016, 516, 162–171. 10.1016/j.memsci.2016.05.060. [DOI] [Google Scholar]
- Li Y.; Shi S.; Cao H.; Zhao Z.; Su C.; Wen H. Improvement of the antifouling performance and stability of an anion exchange membrane by surface modification with graphene oxide (GO) and polydopamine (PDA). J. Membr. Sci. 2018, 566, 44–53. 10.1016/j.memsci.2018.08.054. [DOI] [Google Scholar]
- Liu Y.; Yang S.; Chen Y.; Liao J.; Pan J.; Sotto A.; Shen J. Preparation of water-based anion-exchange membrane from PVA for anti-fouling in the electrodialysis process. J. Membr. Sci. 2019, 570, 130–138. 10.1016/j.memsci.2018.10.011. [DOI] [Google Scholar]
- Bdiri M.; Larchet C.; Dammak L. A Review on Ion-exchange Membranes Fouling and Antifouling During Electrodialysis Used in Food Industry: Cleanings and Strategies of Prevention. Chemistry Africa 2020, 3, 609–633. 10.1007/s42250-020-00178-9. [DOI] [Google Scholar]
- Huang C.; Xu T. Electrodialysis with bipolar membranes for sustainable development. Environ. Sci. Technol. 2006, 40, 5233–5243. 10.1021/es060039p. [DOI] [PubMed] [Google Scholar]
- Nikonenko V.; Urtenov M.; Mareev S.; Pourcelly G. Mathematical Modeling of the Effect of Water Splitting on Ion Transfer in the Depleted Diffusion Layer Near an Ion-Exchange Membrane. Membranes 2020, 10, 22. 10.3390/membranes10020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärnamäe R.; Mareev S.; Nikonenko V.; Melnikov S.; Sheldeshov N.; Zabolotskii V.; Hamelers H.; Tedesco M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2020, 617, 118538. 10.1016/j.memsci.2020.118538. [DOI] [Google Scholar]
- Nagasubramanian K.; Chlanda F.; Liu K.-J. Use of bipolar membranes for generation of acid and base—an engineering and economic analysis. J. Membr. Sci. 1977, 2, 109–124. 10.1016/S0376-7388(00)83237-8. [DOI] [Google Scholar]
- Bayramoğlu G.; Tuzun I.; Celik G.; Yilmaz M.; Arica M. Y. Biosorption of mercury (II), cadmium (II) and lead (II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int. J. Miner. Process. 2006, 81, 35–43. 10.1016/j.minpro.2006.06.002. [DOI] [Google Scholar]
- Wilhelm F.; Pünt I.; Van Der Vegt N.; Wessling M.; Strathmann H. Optimisation strategies for the preparation of bipolar membranes with reduced salt ion leakage in acid–base electrodialysis. J. Membr. Sci. 2001, 182, 13–28. 10.1016/S0376-7388(00)00519-6. [DOI] [Google Scholar]
- Mafé S.; Ramırez P.; Alcaraz A. Electric field-assisted proton transfer and water dissociation at the junction of a fixed-charge bipolar membrane. Chem. Phys. Lett. 1998, 294, 406–412. 10.1016/S0009-2614(98)00877-X. [DOI] [Google Scholar]
- Zabolotskii V.; Sharafan M.; Shel’deshov N. Influence of the nature of membrane ionogenic groups on water dissociation and electrolyte ion transport: A rotating membrane disk study. Russian Journal of Electrochemistry 2008, 44, 1127–1134. 10.1134/S1023193508100078. [DOI] [Google Scholar]
- Zabolotskii V.; Sheldeshov N.; Melnikov S. Heterogeneous bipolar membranes and their application in electrodialysis. Desalination 2014, 342, 183–203. 10.1016/j.desal.2013.11.043. [DOI] [Google Scholar]
- Mareev S.; Evdochenko E.; Wessling M.; Kozaderova O.; Niftaliev S.; Pismenskaya N.; Nikonenko V. A comprehensive mathematical model of water splitting in bipolar membranes: Impact of the spatial distribution of fixed charges and catalyst at bipolar junction. J. Membr. Sci. 2020, 603, 118010. 10.1016/j.memsci.2020.118010. [DOI] [Google Scholar]
- Zhang X.; Li C.; Wang Y.; Luo J.; Xu T. Recovery of acetic acid from simulated acetaldehyde wastewaters: Bipolar membrane electrodialysis processes and membrane selection. Journal of membrane science 2011, 379, 184–190. 10.1016/j.memsci.2011.05.059. [DOI] [Google Scholar]
- Ghyselbrecht K.; Huygebaert M.; Van der Bruggen B.; Ballet R.; Meesschaert B.; Pinoy L. Desalination of an industrial saline water with conventional and bipolar membrane electrodialysis. Desalination 2013, 318, 9–18. 10.1016/j.desal.2013.03.020. [DOI] [Google Scholar]
- Ilhan F.; Kabuk H. A.; Kurt U.; Avsar Y.; Gonullu M. T. Recovery of mixed acid and base from wastewater with bipolar membrane electrodialysis—a case study. Desalination and Water Treatment 2016, 57, 5165–5173. 10.1080/19443994.2014.1002012. [DOI] [Google Scholar]
- Sun X.; Lu H.; Wang J. Recovery of citric acid from fermented liquid by bipolar membrane electrodialysis. Journal of Cleaner Production 2017, 143, 250–256. 10.1016/j.jclepro.2016.12.118. [DOI] [Google Scholar]
- Bunani S.; Arda M.; Kabay N.; Yoshizuka K.; Nishihama S. Effect of process conditions on recovery of lithium and boron from water using bipolar membrane electrodialysis (BMED). Desalination 2017, 416, 10–15. 10.1016/j.desal.2017.04.017. [DOI] [Google Scholar]
- Noguchi M.; Nakamura Y.; Shoji T.; Iizuka A.; Yamasaki A. Simultaneous removal and recovery of boron from waste water by multi-step bipolar membrane electrodialysis. Journal of Water Process Engineering 2018, 23, 299–305. 10.1016/j.jwpe.2018.04.010. [DOI] [Google Scholar]
- Lin J.; Lin F.; Chen X.; Ye W.; Li X.; Zeng H.; Van der Bruggen B. Sustainable management of textile wastewater: A hybrid tight ultrafiltration/bipolar-membrane electrodialysis process for resource recovery and zero liquid discharge. Ind. Eng. Chem. Res. 2019, 58, 11003–11012. 10.1021/acs.iecr.9b01353. [DOI] [Google Scholar]
- Cherif A. T.; Molenat J.; Elmidaoui A. Nitric acid and sodium hydroxide generation by electrodialysis using bipolar membranes. Journal of Applied Electrochemistry 1997, 27, 1069–1074. 10.1023/A:1018438710451. [DOI] [Google Scholar]
- Abu Khalla S.; Suss M. Desalination via chemical energy: An electrodialysis cell driven by spontaneous electrode reactions. Desalination 2019, 467, 257–262. 10.1016/j.desal.2019.04.031. [DOI] [Google Scholar]
- Atlas I.; Suss M. Theory of simultaneous desalination and electricity generation via an electrodialysis cell driven by spontaneous redox reactions. Electrochim. Acta 2019, 319, 813–821. 10.1016/j.electacta.2019.06.014. [DOI] [Google Scholar]
- Ghahari M.; Rashid-Nadimi S.; Bemana H. Metal-air desalination battery: Concurrent energy generation and water desalination. J. Power Sources 2019, 412, 197–203. 10.1016/j.jpowsour.2018.11.042. [DOI] [Google Scholar]
- Wang L.; Zhang Y.; Moh K.; Presser V. From capacitive deionization to desalination batteries and desalination fuel cells. Current Opinion in Electrochemistry 2021, 29, 100758. 10.1016/j.coelec.2021.100758. [DOI] [Google Scholar]
- Chen X.; Xia X.; Liang P.; Cao X.; Sun H.; Huang X. Stacked microbial desalination cells to enhance water desalination efficiency. Environ. Sci. Technol. 2011, 45, 2465–2470. 10.1021/es103406m. [DOI] [PubMed] [Google Scholar]
- Qu Y.; Feng Y.; Wang X.; Liu J.; Lv J.; He W.; Logan B. E. Simultaneous water desalination and electricity generation in a microbial desalination cell with electrolyte recirculation for pH control. Bioresource technology 2012, 106, 89–94. 10.1016/j.biortech.2011.11.045. [DOI] [PubMed] [Google Scholar]
- Atlas I.; Abu Khalla S.; Suss M. Thermodynamic Energy Efficiency of Electrochemical Systems Performing Simultaneous Water Desalination and Electricity Generation. J. Electrochem. Soc. 2020, 167, 134517. 10.1149/1945-7111/abb709. [DOI] [Google Scholar]
- Zhang Y.; Wang L.; Presser V. Electrocatalytic fuel cell desalination for continuous energy and freshwater generation. Cell Reports Physical Science 2021, 2, 100416. 10.1016/j.xcrp.2021.100416. [DOI] [Google Scholar]
- Abu Khalla S.; Atlas I.; Litster S.; Suss M. E. Desalination Fuel Cells with High Thermodynamic Energy Efficiency. Environ. Sci. Technol. 2022, 56, 1413–1422. 10.1021/acs.est.1c07288. [DOI] [PubMed] [Google Scholar]
- Amikam G.; Manor-Korin N.; Nativ P.; Gendel Y. Separation of ions from water and wastewater using micro-scale capacitive-faradaic fuel cells (CFFCs), powered by H2 (g) and air. Sep. Purif. Technol. 2020, 253, 117494. 10.1016/j.seppur.2020.117494. [DOI] [Google Scholar]
- Suss M. E.; Zhang Y.; Atlas I.; Gendel Y.; Ruck E.; Presser V. Emerging, hydrogen-driven electrochemical water purification. Electrochem. Commun. 2022, 136, 107211. 10.1016/j.elecom.2022.107211. [DOI] [Google Scholar]
- Bhat Z. M.; Pandit D.; Ardo S.; Thimmappa R.; Kottaichamy A. R.; Dargily N. C.; Devendrachari M. C.; Thotiyl M. O. An electrochemical neutralization cell for spontaneous water desalination. Joule 2020, 4, 1730–1742. 10.1016/j.joule.2020.07.001. [DOI] [Google Scholar]
- Abdalla S.; Khalla S. A.; Suss M. E. Voltage loss breakdown in desalination fuel cells. Electrochem. Commun. 2021, 132, 107136. 10.1016/j.elecom.2021.107136. [DOI] [Google Scholar]
- Asokan A.; Abu-Khalla S.; Abdalla S.; Suss M. E. Chloride-Tolerant, Inexpensive Fe/N/C Catalysts for Desalination Fuel Cell Cathodes. ACS Applied Energy Materials 2022, 5, 1743–1754. 10.1021/acsaem.1c03175. [DOI] [Google Scholar]
- Bouhidel K.-E.; Lakehal A. Influence of voltage and flow rate on electrodeionization (EDI) process efficiency. Desalination 2006, 193, 411–421. 10.1016/j.desal.2005.08.027. [DOI] [Google Scholar]
- Pan S.-Y.; Snyder S. W.; Ma H.-W.; Lin Y. J.; Chiang P.-C. Energy-efficient resin wafer electrodeionization for impaired water reclamation. Journal of Cleaner Production 2018, 174, 1464–1474. 10.1016/j.jclepro.2017.11.068. [DOI] [Google Scholar]
- Zhao C.; Zhang L.; Ge R.; Zhang A.; Zhang C.; Chen X. Treatment of low-level Cu (II) wastewater and regeneration through a novel capacitive deionization-electrodeionization (CDI-EDI) technology. Chemosphere 2019, 217, 763–772. 10.1016/j.chemosphere.2018.11.071. [DOI] [PubMed] [Google Scholar]
- Lee H.-J.; Song J.-H.; Moon S.-H. Comparison of electrodialysis reversal (EDR) and electrodeionization reversal (EDIR) for water softening. Desalination 2013, 314, 43–49. 10.1016/j.desal.2012.12.028. [DOI] [Google Scholar]
- Rathi B. S.; Kumar P. S. Electrodeionization theory, mechanism and environmental applications. A review. Environmental Chemistry Letters 2020, 18, 1209–1227. 10.1007/s10311-020-01006-9. [DOI] [Google Scholar]
- Arar Ö.; Yüksel Ü.; Kabay N.; Yüksel M. Demineralization of geothermal water reverse osmosis (RO) permeate by electrodeionization (EDI) with layered bed configuration. Desalination 2013, 317, 48–54. 10.1016/j.desal.2013.02.020. [DOI] [Google Scholar]
- Song J.-H.; Song M.-C.; Yeon K.-H.; Kim J.-B.; Lee K.-J.; Moon S.-H. Purification of a primary coolant in a nuclear power plant using a magnetic filter—electrodeionization hybrid separation system. J. Radioanal. Nuclear Chem. 2005, 262, 725–732. 10.1007/s10967-005-0500-8. [DOI] [Google Scholar]
- Ganzi G.; Egozy Y.; Giuffrida A.; Jha A. High purity water by electrodeionization performance of the ionpure continuous deionization system. Ultrapure Water 1987, 4, 43–50. [Google Scholar]
- Shaposhnik V.; Kuzminykh V.; Grigorchuk O.; Vasil’eva V. Analytical model of laminar flow electrodialysis with ion-exchange membranes. Journal of membrane science 1997, 133, 27–37. 10.1016/S0376-7388(97)00063-X. [DOI] [Google Scholar]
- Fu L.; Wang J.; Su Y. Removal of low concentrations of hardness ions from aqueous solutions using electrodeionization process. Sep. Purif. Technol. 2009, 68, 390–396. 10.1016/j.seppur.2009.06.010. [DOI] [Google Scholar]
- Jordan M. L.; Valentino L.; Nazyrynbekova N.; Palakkal V. M.; Kole S.; Bhattacharya D.; Lin Y. J.; Arges C. G. Promoting water-splitting in Janus bipolar ion-exchange resin wafers for electrodeionization. Molecular Systems Design & Engineering 2020, 5, 922–935. 10.1039/C9ME00179D. [DOI] [Google Scholar]
- Palakkal V. M.; Valentino L.; Lei Q.; Kole S.; Lin Y. J.; Arges C. G. Advancing electrodeionization with conductive ionomer binders that immobilize ion-exchange resin particles into porous wafer substrates. NPJ Clean Water 2020, 3, 5. 10.1038/s41545-020-0052-z. [DOI] [Google Scholar]
- Yeon K.; Seong J.; Rengaraj S.; Moon S. Electrochemical characterization of ion-exchange resin beds and removal of cobalt by electrodeionization for high purity water production. Sep. Sci. Technol. 2003, 38, 443–462. 10.1081/SS-120016584. [DOI] [Google Scholar]
- Mahmoud A.; Muhr L.; Grévillot G.; Valentin G.; Lapicque F. Ohmic drops in the ion-exchange bed of cationic electrodeionisation cells. Journal of applied electrochemistry 2006, 36, 277–285. 10.1007/s10800-005-9081-z. [DOI] [Google Scholar]
- Alvarado L.; Rodríguez-Torres I.; Balderas P. Investigation of Current Routes in Electrodeionization System Resin Beds During Chromium Removal. Electrochim. Acta 2015, 182, 763–768. 10.1016/j.electacta.2015.09.124. [DOI] [Google Scholar]
- Sauer M.; Southwick P.; Spiegler K.; Wyllie M. Electrical conductance of porous plugs-ion exchange resin-solution systems. Industrial & Engineering Chemistry 1955, 47, 2187–2193. 10.1021/ie50550a044. [DOI] [Google Scholar]
- Strathmann H.; Krol J.; Rapp H.-J.; Eigenberger G. Limiting current density and water dissociation in bipolar membranes. J. Membr. Sci. 1997, 125, 123–142. 10.1016/S0376-7388(96)00185-8. [DOI] [Google Scholar]
- Nikonenko V.; Mareev S.; Pis’menskaya N.; Uzdenova A.; Kovalenko A.; Urtenov M. K.; Pourcelly G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis. Russian Journal of Electrochemistry 2017, 53, 1122–1144. 10.1134/S1023193517090099. [DOI] [Google Scholar]
- Mani A.; Wang K. M. Electroconvection Near Electrochemical Interfaces: Experiments, Modeling, and Computation. Annu. Rev. Fluid Mech. 2020, 52, 509–529. 10.1146/annurev-fluid-010719-060358. [DOI] [Google Scholar]
- Park S.; Kwak R. Microscale electrodeionization: In situ concentration profiling and flow visualization. Water research 2020, 170, 115310. 10.1016/j.watres.2019.115310. [DOI] [PubMed] [Google Scholar]
- Stockmeier F.; Schatz M.; Habermann M.; Linkhorst J.; Mani A.; Wessling M. Direct 3D observation and unraveling of electroconvection phenomena during concentration polarization at ion-exchange membranes. J. Membr. Sci. 2021, 640, 119846. 10.1016/j.memsci.2021.119846. [DOI] [Google Scholar]
- Wen R.; Deng S.; Zhang Y. The removal of silicon and boron from ultra-pure water by electrodeionization. Desalination 2005, 181, 153–159. 10.1016/j.desal.2005.02.018. [DOI] [Google Scholar]
- Lounis A.; Setti L.; Djennane A.; Melikchi R. Separation of Molybdenum-Uranium by a process combining ion exchange resin and membranes. J. Appl. Sci. 2007, 7, 1963–1967. 10.3923/jas.2007.1963.1967. [DOI] [Google Scholar]
- Taghdirian H. R.; Moheb A.; Mehdipourghazi M. Selective separation of Ni (II)/Co (II) ions from dilute aqueous solutions using continuous electrodeionization in the presence of EDTA. J. Membr. Sci. 2010, 362, 68–75. 10.1016/j.memsci.2010.06.023. [DOI] [Google Scholar]
- Ervan Y.; Wenten I. G. Study on the influence of applied voltage and feed concentration on the performance of electrodeionization. Songklanakarin J. Sci. Technol. 2002, 24, 955–963. [Google Scholar]
- Monzie I.; Muhr L.; Lapicque F.; Grévillot G. Mass transfer investigations in electrodeionization processes using the microcolumn technique. Chemical engineering science 2005, 60, 1389–1399. 10.1016/j.ces.2004.10.010. [DOI] [Google Scholar]
- Hakim A. N.; Khoiruddin K.; Ariono D.; Wenten I. Ionic Separation in Electrodeionization System: Mass Transfer Mechanism and Factor Affecting Separation Performance. Separ. Purif. Rev. 2019, 49, 294–316. 10.1080/15422119.2019.1608562. [DOI] [Google Scholar]
- Otero C.; Urbina A.; Rivero E. P.; Rodríguez F. A. Desalination of brackish water by electrodeionization: Experimental study and mathematical modeling. Desalination 2021, 504, 114803. 10.1016/j.desal.2020.114803. [DOI] [Google Scholar]
- Verbeek H.; Fürst L.; Neumeister H. Digital simulation of an electrodeionization process. Computers & chemical engineering 1998, 22, S913–S916. 10.1016/S0098-1354(98)00179-3. [DOI] [Google Scholar]
- Lu J.; Wang Y.-X.; Zhu J. Numerical simulation of the electrodeionization (EDI) process accounting for water dissociation. Electrochimica acta 2010, 55, 2673–2686. 10.1016/j.electacta.2009.11.107. [DOI] [Google Scholar]
- Lu J.; Ma X.-Y.; Wang Y.-X. Numerical simulation of the electrodeionization (EDI) process with layered resin bed for deeply separating salt ions. Desalination and Water Treatment 2016, 57, 10546–10559. 10.1080/19443994.2015.1040467. [DOI] [Google Scholar]
- Zahakifar F.; Keshtkar A.; Souderjani E. Z.; Moosavian M. Use of response surface methodology for optimization of thorium (IV) removal from aqueous solutions by electrodeionization (EDI). Progress in Nuclear Energy 2020, 124, 103335. 10.1016/j.pnucene.2020.103335. [DOI] [Google Scholar]
- Glueckauf E. Electro-deionization through a packed bed. Br. Chem. Eng. 1959, 4, 646–651. [Google Scholar]
- Dobrevsky I.; Zvezdov A. Investigation of pore structure of ion exchange membranes. Desalination 1979, 28, 283–289. 10.1016/S0011-9164(00)82235-3. [DOI] [Google Scholar]
- Yaroslavtsev A. B.; Nikonenko V. V.; Zabolotsky V. I. Ion transfer in ion-exchange and membrane materials. Russian chemical reviews 2003, 72, 393–421. 10.1070/RC2003v072n05ABEH000797. [DOI] [Google Scholar]
- Faucher S.; Aluru N.; Bazant M. Z.; Blankschtein D.; Brozena A. H.; Cumings J.; Pedro de Souza J.; Elimelech M.; Epsztein R.; Fourkas J. T.; Rajan A. G.; Kulik H. J.; Levy A.; Majumdar A.; Martin C.; McEldrew M.; Misra R. P.; Noy A.; Pham T. A.; Reed M.; Schwegler E.; Siwy Z.; Wang Y.; Strano M. Critical knowledge gaps in mass transport through single-digit nanopores: a review and perspective. J. Phys. Chem. C 2019, 123, 21309–21326. 10.1021/acs.jpcc.9b02178. [DOI] [Google Scholar]
- Berezina N.; Kononenko N.; Dyomina O.; Gnusin N. Characterization of ion-exchange membrane materials: properties vs structure. Advances in colloid and interface science 2008, 139, 3–28. 10.1016/j.cis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Nagarale R.; Gohil G.; Shahi V. K. Recent developments on ion-exchange membranes and electro-membrane processes. Advances in colloid and interface science 2006, 119, 97–130. 10.1016/j.cis.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Goh P.; Ismail A.; Ng B. Carbon nanotubes for desalination: performance evaluation and current hurdles. Desalination 2013, 308, 2–14. 10.1016/j.desal.2012.07.040. [DOI] [Google Scholar]
- Das R.; Ali M. E.; Hamid S. B. A.; Ramakrishna S.; Chowdhury Z. Z. Carbon nanotube membranes for water purification: a bright future in water desalination. Desalination 2014, 336, 97–109. 10.1016/j.desal.2013.12.026. [DOI] [Google Scholar]
- Holt J. K.; Park H. G.; Wang Y.; Stadermann M.; Artyukhin A. B.; Grigoropoulos C. P.; Noy A.; Bakajin O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. 10.1126/science.1126298. [DOI] [PubMed] [Google Scholar]
- Secchi E.; Marbach S.; Niguès A.; Stein D.; Siria A.; Bocquet L. Massive radius-dependent flow slippage in carbon nanotubes. Nature 2016, 537, 210–213. 10.1038/nature19315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. N.; Karnik R. Graphene cleans up water. Nature Nanotechnol. 2012, 7, 552–554. 10.1038/nnano.2012.153. [DOI] [PubMed] [Google Scholar]
- Aghigh A.; Alizadeh V.; Wong H. Y.; Islam M. S.; Amin N.; Zaman M. Recent advances in utilization of graphene for filtration and desalination of water: a review. Desalination 2015, 365, 389–397. 10.1016/j.desal.2015.03.024. [DOI] [Google Scholar]
- Wang L.; Boutilier M. S.; Kidambi P. R.; Jang D.; Hadjiconstantinou N. G.; Karnik R. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nature Nanotechnol. 2017, 12, 509–522. 10.1038/nnano.2017.72. [DOI] [PubMed] [Google Scholar]
- Helmholtz H. v. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern, mit Anwendung auf die thierisch-elektrischen Versuche (Schluss.). Annalen der Physik 1853, 165, 353–377. 10.1002/andp.18531650702. [DOI] [Google Scholar]
- Gouy M. Sur la constitution de la charge électrique à la surface d’un électrolyte. J. Phys. Theor. Appl. 1910, 9, 457–468. 10.1051/jphystap:019100090045700. [DOI] [Google Scholar]
- Chapman D. L. A contribution to the theory of electrocapillarity. London, Edinburgh, and Dublin philosophical magazine and journal of science 1913, 25, 475–481. 10.1080/14786440408634187. [DOI] [Google Scholar]
- Stern O. Zur theorie der elektrolytischen doppelschicht. Zeit. Elektrochem. Angew. Phys. Chem, 1924, 30, 508–516. 10.1002/bbpc.192400182. [DOI] [Google Scholar]
- Peters P.; Van Roij R.; Bazant M. Z.; Biesheuvel P. Analysis of electrolyte transport through charged nanopores. Phys. Rev. E 2016, 93, 053108. 10.1103/PhysRevE.93.053108. [DOI] [PubMed] [Google Scholar]
- Gross R. J.; Osterle J. Membrane transport characteristics of ultrafine capillaries. J. Chem. Phys. 1968, 49, 228–234. 10.1063/1.1669814. [DOI] [PubMed] [Google Scholar]
- Nielsen C. P.; Bruus H. Concentration polarization, surface currents, and bulk advection in a microchannel. Phys. Rev. E 2014, 90, 043020. 10.1103/PhysRevE.90.043020. [DOI] [PubMed] [Google Scholar]
- Alizadeh S.; Mani A. Multiscale model for electrokinetic transport in networks of pores, Part I: model derivation. Langmuir 2017, 33, 6205–6219. 10.1021/acs.langmuir.6b03816. [DOI] [PubMed] [Google Scholar]
- Alizadeh S.; Mani A. Multiscale model for electrokinetic transport in networks of pores, Part II: computational algorithms and applications. Langmuir 2017, 33, 6220–6231. 10.1021/acs.langmuir.7b00591. [DOI] [PubMed] [Google Scholar]
- Alizadeh S.; Bazant M. Z.; Mani A. Impact of network heterogeneity on electrokinetic transport in porous media. J. Colloid Interface Sci. 2019, 553, 451–464. 10.1016/j.jcis.2019.06.023. [DOI] [PubMed] [Google Scholar]
- Mirzadeh M.; Zhou T.; Amooie M. A.; Fraggedakis D.; Ferguson T. R.; Bazant M. Z. Vortices of electro-osmotic flow in heterogeneous porous media. Physical Review Fluids 2020, 5, 103701. 10.1103/PhysRevFluids.5.103701. [DOI] [Google Scholar]
- Sasidhar V.; Ruckenstein E. Electrolyte osmosis through capillaries. J. Colloid Interface Sci. 1981, 82, 439–457. 10.1016/0021-9797(81)90386-6. [DOI] [Google Scholar]
- Yaroshchuk A.; Bruening M. L.; Zholkovskiy E. Modelling nanofiltration of electrolyte solutions. Adv. Colloid Interface Sci. 2019, 268, 39–63. 10.1016/j.cis.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Lanteri Y.; Szymczyk A.; Fievet P. Membrane potential in multi-ionic mixtures. J. Phys. Chem. B 2009, 113, 9197–9204. 10.1021/jp901110c. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Pintauro P. N. Multicomponent space-charge transport model for ion-exchange membranes. AIChE journal 2000, 46, 1177–1190. 10.1002/aic.690460610. [DOI] [Google Scholar]
- Huisman I. H.; Prádanos P.; Calvo J. I.; Hernández A. Electroviscous effects, streaming potential, and zeta potential in polycarbonate track-etched membranes. J. Membr. Sci. 2000, 178, 79–92. 10.1016/S0376-7388(00)00485-3. [DOI] [Google Scholar]
- Sasidhar V.; Ruckenstein E. Anomalous effects during electrolyte osmosis across charged porous membranes. J. Colloid Interface Sci. 1982, 85, 332–362. 10.1016/0021-9797(82)90003-0. [DOI] [Google Scholar]
- Shang W.-J.; Wang X.-L.; Yu Y.-X. Theoretical calculation on the membrane potential of charged porous membranes in 1–1, 1–2, 2–1 and 2–2 electrolyte solutions. Journal of membrane science 2006, 285, 362–375. 10.1016/j.memsci.2006.09.005. [DOI] [Google Scholar]
- Ramirez P.; Aguilella-Arzo M.; Alcaraz A.; Cervera J.; Aguilella V. Theoretical description of the ion transport across nanopores with titratable fixed charges. Cell Biochem. Biophys. 2006, 44, 287–312. 10.1385/CBB:44:2:287. [DOI] [PubMed] [Google Scholar]
- Manghi M.; Palmeri J.; Yazda K.; Henn F.; Jourdain V. Role of charge regulation and flow slip in the ionic conductance of nanopores: An analytical approach. Phys. Rev. E 2018, 98, 012605. 10.1103/PhysRevE.98.012605. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Wang Q.; Zeng M.; Zhao C. Thermoelectric effect and temperature-gradient-driven electrokinetic flow of electrolyte solutions in charged nanocapillaries. Int. J. Heat Mass Transfer 2019, 143, 118569. 10.1016/j.ijheatmasstransfer.2019.118569. [DOI] [Google Scholar]
- Zhang L.; Biesheuvel P.; Ryzhkov I. Theory of ion and water transport in electron-conducting membrane pores with pH-dependent chemical charge. Physical Review Applied 2019, 12, 014039. 10.1103/PhysRevApplied.12.014039. [DOI] [Google Scholar]
- Ryzhkov I. I.; Lebedev D. V.; Solodovnichenko V. S.; Minakov A. V.; Simunin M. M. On the origin of membrane potential in membranes with polarizable nanopores. Journal of membrane science 2018, 549, 616–630. 10.1016/j.memsci.2017.11.073. [DOI] [Google Scholar]
- Bontha J.; Pintauro P. N. Water orientation and ion solvation effects during multicomponent salt partitioning in a Nafion cation exchange membrane. Chemical engineering science 1994, 49, 3835–3851. 10.1016/0009-2509(94)00205-3. [DOI] [Google Scholar]
- Lanteri Y.; Szymczyk A.; Fievet P. Influence of steric, electric, and dielectric effects on membrane potential. Langmuir 2008, 24, 7955–7962. 10.1021/la800677q. [DOI] [PubMed] [Google Scholar]
- Szymczyk A.; Sbaï M.; Fievet P.; Vidonne A. Transport properties and electrokinetic characterization of an amphoteric nanofilter. Langmuir 2006, 22, 3910–3919. 10.1021/la051888d. [DOI] [PubMed] [Google Scholar]
- Hawkins Cwirko E.; Carbonell R. Transport of electrolytes in charged pores: analysis using the method of spatial averaging. J. Colloid Interface Sci. 1989, 129, 513–531. 10.1016/0021-9797(89)90466-9. [DOI] [Google Scholar]
- Dydek E. V.; Zaltzman B.; Rubinstein I.; Deng D.; Mani A.; Bazant M. Z. Overlimiting current in a microchannel. Physical review letters 2011, 107, 118301. 10.1103/PhysRevLett.107.118301. [DOI] [PubMed] [Google Scholar]
- Dydek E. V.; Bazant M. Z. Nonlinear dynamics of ion concentration polarization in porous media: The leaky membrane model. AIChE J. 2013, 59, 3539–3555. 10.1002/aic.14200. [DOI] [Google Scholar]
- Levy A.; de Souza J. P.; Bazant M. Z. Breakdown of electroneutrality in nanopores. J. Colloid Interface Sci. 2020, 579, 162–176. 10.1016/j.jcis.2020.05.109. [DOI] [PubMed] [Google Scholar]
- Maffeo C.; Bhattacharya S.; Yoo J.; Wells D.; Aksimentiev A. Modeling and simulation of ion channels. Chem. Rev. 2012, 112 (12), 6250–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza J. P.; Levy A.; Bazant M. Z. Electroneutrality breakdown in nanopore arrays. Phys. Rev. E 2021, 104, 044803. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Wang Z.; Epsztein R.; Zhan C.; Li W.; Fortner J. D.; Pham T. A.; Kim J.-H.; Elimelech M. Intrapore energy barriers govern ion transport and selectivity of desalination membranes. Sci. Adv. 2020, 6, abdd9045 10.1126/sciadv.abd9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornyshev A. A. Double-Layer in Ionic Liquids: Paradigm Change?. J. Phys. Chem. B 2007, 111 (20), 5545–5557. [DOI] [PubMed] [Google Scholar]
- Bazant M. Z.; Kilic M. S.; Storey B. D.; Ajdari A. Towards an understanding of induced-charge electrokinetics at large applied voltages in concentrated solutions. Advances in colloid and interface science 2009, 152, 48–88. 10.1016/j.cis.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Qiao R.; Aluru N. R. Ion concentrations and velocity profiles in nanochannel electroosmotic flows. J. Chem. Phys. 2003, 118, 4692–4701. 10.1063/1.1543140. [DOI] [Google Scholar]
- Storey B. D.; Bazant M. Z. Effects of electrostatic correlations on electrokinetic phenomena. Phys. Rev. E 2012, 86, 056303. 10.1103/PhysRevE.86.056303. [DOI] [PubMed] [Google Scholar]
- Stout R. F.; Khair A. S. A continuum approach to predicting electrophoretic mobility reversals. J. Fluid Mech. 2014, 752, R1. 10.1017/jfm.2014.354. [DOI] [Google Scholar]
- Kilic M. S.; Bazant M. Z.; Ajdari A. Steric effects in the dynamics of electrolytes at large applied voltages. I. Double-layer charging. Phys. Rev. E 2007, 75, 021502. 10.1103/PhysRevE.75.021502. [DOI] [PubMed] [Google Scholar]
- Kilic M. S.; Bazant M. Z.; Ajdari A. Steric effects in the dynamics of electrolytes at large applied voltages. II. Modified Poisson-Nernst-Planck equations. Phys. Rev. E 2007, 75, 021503. 10.1103/PhysRevE.75.021503. [DOI] [PubMed] [Google Scholar]
- Bazant M. Z.; Storey B. D.; Kornyshev A. A. Double layer in ionic liquids: Overscreening versus crowding. Phys. Rev. Lett. 2011, 106, 046102. 10.1103/PhysRevLett.106.046102. [DOI] [PubMed] [Google Scholar]
- de Souza J. P.; Bazant M. Z. Continuum theory of electrostatic correlations at charged surfaces. J. Phys. Chem. C 2020, 124, 11414–11421. 10.1021/acs.jpcc.0c01261. [DOI] [Google Scholar]
- Wesselingh J.; Vonk P.; Kraaijeveld G. Exploring the Maxwell-Stefan description of ion exchange. Chemical Engineering Journal and The Biochemical Engineering Journal 1995, 57, 75–89. 10.1016/0923-0467(94)02932-6. [DOI] [Google Scholar]
- de Lint W. B. S.; Benes N. E. Predictive charge-regulation transport model for nanofiltration from the theory of irreversible processes. J. Membr. Sci. 2004, 243, 365–377. 10.1016/j.memsci.2004.06.041. [DOI] [Google Scholar]
- Tedesco M.; Hamelers H.; Biesheuvel P. Nernst-Planck transport theory for (reverse) electrodialysis: II. Effect of water transport through ion-exchange membranes. J. Membr. Sci. 2017, 531, 172–182. 10.1016/j.memsci.2017.02.031. [DOI] [Google Scholar]
- Oren Y.; Biesheuvel P. Theory of ion and water transport in reverse-osmosis membranes. Physical Review Applied 2018, 9, 024034. 10.1103/PhysRevApplied.9.024034. [DOI] [Google Scholar]
- Biesheuvel P.; Bazant M. Analysis of ionic conductance of carbon nanotubes. Phys. Rev. E 2016, 94, 050601. 10.1103/PhysRevE.94.050601. [DOI] [PubMed] [Google Scholar]
- Secchi E.; Niguès A.; Jubin L.; Siria A.; Bocquet L. Scaling behavior for ionic transport and its fluctuations in individual carbon nanotubes. Physical review letters 2016, 116, 154501. 10.1103/PhysRevLett.116.154501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangara R.; Brown D.; van Swol F.; Petsev D. Electrolyte solution structure and its effect on the properties of electric double layers with surface charge regulation. J. Colloid Interface Sci. 2017, 488, 180–189. 10.1016/j.jcis.2016.10.084. [DOI] [PubMed] [Google Scholar]
- Ramirez P.; Manzanares J. A.; Cervera J.; Gomez V.; Ali M.; Nasir S.; Ensinger W.; Mafe S. Surface charge regulation of functionalized conical nanopore conductance by divalent cations and anions. Electrochim. Acta 2019, 325, 134914. 10.1016/j.electacta.2019.134914. [DOI] [Google Scholar]
- Werkhoven B.; Everts J. C.; Samin S.; Van Roij R. Flow-induced surface charge heterogeneity in electrokinetics due to Stern-layer conductance coupled to reaction kinetics. Physical review letters 2018, 120, 264502. 10.1103/PhysRevLett.120.264502. [DOI] [PubMed] [Google Scholar]
- Werkhoven B.; Samin S.; van Roij R. Dynamic Stern layers in charge-regulating electrokinetic systems: three regimes from an analytical approach. European Physical Journal Special Topics 2019, 227, 2539–2557. 10.1140/epjst/e2019-800120-3. [DOI] [Google Scholar]
- Kim S. J.; Wang Y.-C.; Lee J. H.; Jang H.; Han J. Concentration polarization and nonlinear electrokinetic flow near a nanofluidic channel. Physical review letters 2007, 99, 044501. 10.1103/PhysRevLett.99.044501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J.; Li L. D.; Han J. Amplified electrokinetic response by concentration polarization near nanofluidic channel. Langmuir 2009, 25, 7759–7765. 10.1021/la900332v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J.; Song Y.-A.; Han J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: theory, fabrication, and applications. Chem. Soc. Rev. 2010, 39, 912–922. 10.1039/b822556g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangle T. A.; Mani A.; Santiago J. G. Theory and experiments of concentration polarization and ion focusing at microchannel and nanochannel interfaces. Chem. Soc. Rev. 2010, 39, 1014–1035. 10.1039/b902074h. [DOI] [PubMed] [Google Scholar]
- Kim S. J.; Ko S. H.; Kang K. H.; Han J. Direct seawater desalination by ion concentration polarization. Nature Nanotechnol. 2010, 5, 297–301. 10.1038/nnano.2010.34. [DOI] [PubMed] [Google Scholar]
- Kim S. J.; Ko S. H.; Kang K. H.; Han J. Erratum: Direct seawater desalination by ion concentration polarization. Nature Nanotechnol. 2013, 8, 609. 10.1038/nnano.2013.148. [DOI] [PubMed] [Google Scholar]
- Nam S.; Cho I.; Heo J.; Lim G.; Bazant M. Z.; Moon D. J.; Sung G. Y.; Kim S. J. Experimental verification of overlimiting current by surface conduction and electro-osmotic flow in microchannels. Physical review letters 2015, 114, 114501. 10.1103/PhysRevLett.114.114501. [DOI] [PubMed] [Google Scholar]
- Wang Y.-C.; Stevens A. L.; Han J. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Analytical chemistry 2005, 77, 4293–4299. 10.1021/ac050321z. [DOI] [PubMed] [Google Scholar]
- Kim Y.-J.; Hur J.; Bae W.; Choi J.-H. Desalination of brackish water containing oil compound by capacitive deionization process. Desalination 2010, 253, 119–123. 10.1016/j.desal.2009.11.022. [DOI] [Google Scholar]
- Rica R. A.; Bazant M. Z. Electrodiffusiophoresis: Particle motion in electrolytes under direct current. Phys. Fluids 2010, 22, 112109. 10.1063/1.3496976. [DOI] [Google Scholar]
- Křivánková L.; Pantůčková P.; Boček P. Isotachophoresis in zone electrophoresis. Journal of Chromatography A 1999, 838, 55–70. 10.1016/S0021-9673(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Jung B.; Bharadwaj R.; Santiago J. G. On-chip millionfold sample stacking using transient isotachophoresis. Analytical chemistry 2006, 78, 2319–2327. 10.1021/ac051659w. [DOI] [PubMed] [Google Scholar]
- Ghosal S.; Chen Z. Nonlinear waves in capillary electrophoresis. Bulletin of mathematical biology 2010, 72, 2047–2066. 10.1007/s11538-010-9527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahga S. S.; Santiago J. G. Coupling isotachophoresis and capillary electrophoresis: a review and comparison of methods. Analyst 2013, 138, 735–754. 10.1039/C2AN36150G. [DOI] [PubMed] [Google Scholar]
- Gu Z.; Xu B.; Huo P.; Rubinstein S. M.; Bazant M. Z.; Deng D. Deionization shock driven by electroconvection in a circular channel. Physical Review Fluids 2019, 4, 113701. 10.1103/PhysRevFluids.4.113701. [DOI] [Google Scholar]
- Yaroshchuk A.; Zholkovskiy E.; Pogodin S.; Baulin V. Coupled concentration polarization and electroosmotic circulation near micro/nanointerfaces: Taylor–Aris model of hydrodynamic dispersion and limits of its applicability. Langmuir 2011, 27, 11710–11721. 10.1021/la201354s. [DOI] [PubMed] [Google Scholar]
- Yaroshchuk A. Over-limiting currents and deionization “shocks” in current-induced polarization: Local-equilibrium analysis. Advances in colloid and interface science 2012, 183, 68–81. 10.1016/j.cis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Han J.-H.; Khoo E.; Bai P.; Bazant M. Z. Over-limiting current and control of dendritic growth by surface conduction in nanopores. Sci. Rep. 2015, 4, 7056. 10.1038/srep07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuck M.; Bazant M. Z. Homogenization of the Poisson–Nernst–Planck equations for ion transport in charged porous media. SIAM Journal on Applied Mathematics 2015, 75, 1369–1401. 10.1137/140968082. [DOI] [Google Scholar]
- Choi J.; Baek S.; Kim H. C.; Chae J.-H.; Koh Y.; Seo S. W.; Lee H.; Kim S. J. Nanoelectrokinetic Selective Preconcentration Based on Ion Concentration Polarization. BioChip Journal 2020, 14, 100–109. 10.1007/s13206-020-4109-3. [DOI] [Google Scholar]
- Han J.-H.; Wang M.; Bai P.; Brushett F. R.; Bazant M. Z. Dendrite suppression by shock electrodeposition in charged porous media. Sci. Rep. 2016, 6, 28054. 10.1038/srep28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-H.; Muralidhar R.; Waser R.; Bazant M. Z. Resistive switching in aqueous nanopores by shock electrodeposition. Electrochimica acta 2016, 222, 370–375. 10.1016/j.electacta.2016.10.188. [DOI] [Google Scholar]
- Zhi J.; Li S.; Han M.; Chen P. Biomolecule-guided cation regulation for dendrite-free metal anodes. Science Advances 2020, 6, eabb1342 10.1126/sciadv.abb1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D.; Aouad W.; Braff W. A.; Schlumpberger S.; Suss M. E.; Bazant M. Z. Water purification by shock electrodialysis: Deionization, filtration, separation, and disinfection. Desalination 2015, 357, 77–83. 10.1016/j.desal.2014.11.011. [DOI] [Google Scholar]
- Alkhadra M. A.; Conforti K. M.; Gao T.; Tian H.; Bazant M. Z. Continuous Separation of Radionuclides from Contaminated Water by Shock Electrodialysis. Environ. Sci. Technol. 2020, 54, 527–536. 10.1021/acs.est.9b05380. [DOI] [PubMed] [Google Scholar]
- Matijevic E.; Good R. J.. Surface and Colloid Science; Springer Science & Business Media, 2012; Vol. 12. [Google Scholar]
- Culbertson C. T.; Ramsey R. S.; Ramsey J. M. Electroosmotically induced hydraulic pumping on microchips: differential ion transport. Anal. Chem. 2000, 72, 2285–2291. 10.1021/ac9912202. [DOI] [PubMed] [Google Scholar]
- McKnight T. E.; Culbertson C. T.; Jacobson S. C.; Ramsey J. M. Electroosmotically induced hydraulic pumping with integrated electrodes on microfluidic devices. Anal. Chem. 2001, 73, 4045–4049. 10.1021/ac010048a. [DOI] [PubMed] [Google Scholar]
- Suss M. E.; Mani A.; Zangle T. A.; Santiago J. G. Electroosmotic pump performance is affected by concentration polarizations of both electrodes and pump. Sensors and Actuators A: Physical 2011, 165, 310–315. 10.1016/j.sna.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpberger S.; Smith R. B.; Tian H.; Mani A.; Bazant M. Z. Deionization shocks in crossflow. AIChE J. 2021, 67, e17274 10.1002/aic.17274. [DOI] [Google Scholar]
- Tian H.; Alkhadra M. A.; Bazant M. Z. Theory of shock electrodialysis I: Water dissociation and electrosmotic vortices. J. Colloid Interface Sci. 2021, 589, 605–615. 10.1016/j.jcis.2020.12.125. [DOI] [PubMed] [Google Scholar]
- Tian H.; Alkhadra M. A.; Conforti K.; Bazant M. Z.. Continuous and Selective Removal of Lead from Drinking Water by Shock Electrodialysis. In Environment Science & Technology: Water, 2021. [DOI] [PubMed] [Google Scholar]
- Čížek J.; Cvejn P.; Marek J.; Tvrzník D. Desalination Performance Assessment of Scalable, Multi-Stack Ready Shock Electrodialysis Unit Utilizing Anion-Exchange Membranes. Membranes 2020, 10, 347. 10.3390/membranes10110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti K. M.Continuous Ion-Selective Separation by Shock Electrodialysis. Ph.D. Thesis. Massachusetts Institute of Technology, 2019. [Google Scholar]
- Alkhadra M. A.; Gao T.; Conforti K. M.; Tian H.; Bazant M. Z. Small-scale desalination of seawater by shock electrodialysis. Desalination 2020, 476, 114219. 10.1016/j.desal.2019.114219. [DOI] [Google Scholar]
- Menyah K.; Wolde-Rufael Y. CO2 emissions, nuclear energy, renewable energy and economic growth in the US. Energy Policy 2010, 38, 2911–2915. 10.1016/j.enpol.2010.01.024. [DOI] [Google Scholar]
- Saidi K.; Ben Mbarek M. Nuclear energy, renewable energy, CO2 emissions, and economic growth for nine developed countries: Evidence from panel Granger causality tests. Progress in Nuclear Energy 2016, 88, 364–374. 10.1016/j.pnucene.2016.01.018. [DOI] [Google Scholar]
- Lister D. The Transport of Radioactive Corrosion Products in High-Temperature Water II. The Activation of Isothermal Steel Surfaces. Nuclear Science and Engineering 1976, 59, 406–426. 10.13182/NSE76-7. [DOI] [Google Scholar]
- Honda T.; Izumiya M.; Minato A.; Ohsumi K.; Matsubayashi H. Radiation buildup on stainless steel in a boiling water reactor environment. Nuclear Technology 1984, 64, 35–42. 10.13182/NT84-A33325. [DOI] [Google Scholar]
- Choo K.-H.; Kwon D.-J.; Lee K.-W.; Choi S.-J. Selective removal of cobalt species using nanofiltration membranes. Environ. Sci. Technol. 2002, 36, 1330–1336. 10.1021/es010724q. [DOI] [PubMed] [Google Scholar]
- Kikuchi M.; Ga E.; Funabashi K.; Yusa H.; Uchida S.; Fujita K. Removal of radioactive cobalt ion in high temperature water using titanium oxide. Nucl. Eng. Des. 1979, 53, 387–392. 10.1016/0029-5493(79)90065-7. [DOI] [Google Scholar]
- Cox B.; Wu C. Transient effects of lithium hydroxide and boric acid on Zircaloy corrosion. Journal of nuclear materials 1995, 224, 169–178. 10.1016/0022-3115(95)00043-7. [DOI] [Google Scholar]
- Sengupta S. K.; Hooper E.; Dubost E.. Processing of Nuclear Power Plant Waste Streams Containing Boric Acid (Technical Report); International Atomic Energy Agency, 2019; p 49.
- Liu X.; Wu J.; Wang J. Removal of Cs (I) from simulated radioactive wastewater by three forward osmosis membranes. Chemical Engineering Journal 2018, 344, 353–362. 10.1016/j.cej.2018.03.046. [DOI] [Google Scholar]
- Ryan P. B.; Huet N.; MacIntosh D. L. Longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environ. Health Perspect. 2000, 108, 731–735. 10.1289/ehp.00108731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lead in Drinking Water; World Health Organization, 2012.
- Tian H.; Alkhadra M. A.; Bazant M. Z. Theory of shock electrodialysis II: Mechanisms of selective ion removal. J. Colloid Interface Sci. 2021, 589, 616–621. 10.1016/j.jcis.2020.11.127. [DOI] [PubMed] [Google Scholar]
- Abrams I.Studies in Environmental Science; Elsevier, 1982; Vol. 19; pp 213–224. [Google Scholar]
- Anupkumar B.; Rao T.; Satpathy K. Microbial fouling of an anion exchange resin. Current Sci. 2001, 80, 1104–1107. [Google Scholar]
- Asraf-Snir M.; Gilron J.; Oren Y. Gypsum scaling of anion exchange membranes in electrodialysis. J. Membr. Sci. 2016, 520, 176–186. 10.1016/j.memsci.2016.07.013. [DOI] [Google Scholar]
- Zhao D.; Lee L. Y.; Ong S. L.; Chowdhury P.; Siah K. B.; Ng H. Y. Electrodialysis reversal for industrial reverse osmosis brine treatment. Sep. Purif. Technol. 2019, 213, 339–347. 10.1016/j.seppur.2018.12.056. [DOI] [Google Scholar]
- Andreeva M.; Gil V.; Pismenskaya N.; Dammak L.; Kononenko N.; Larchet C.; Grande D.; Nikonenko V. Mitigation of membrane scaling in electrodialysis by electroconvection enhancement, pH adjustment and pulsed electric field application. J. Membr. Sci. 2018, 549, 129–140. 10.1016/j.memsci.2017.12.005. [DOI] [Google Scholar]
- Turek M.; Waś J.; Mitko K. Scaling prediction in electrodialytic desalination. Desalination and Water Treatment 2012, 44, 255–260. 10.1080/19443994.2012.691747. [DOI] [Google Scholar]
- Casademont C.; Farias M. A.; Pourcelly G.; Bazinet L. Impact of electrodialytic parameters on cation migration kinetics and fouling nature of ion-exchange membranes during treatment of solutions with different magnesium/calcium ratios. Journal of membrane Science 2008, 325, 570–579. 10.1016/j.memsci.2008.08.023. [DOI] [Google Scholar]
- Ben Salah Sayadi I.; Sistat P.; Ben Amor M.; Tlili M. Brackish water desalination by electrodialysis: CaCO3 scaling monitoring during batch recirculation operation. Int. J. Chem. Reactor Eng. 2013, 11, 517–525. 10.1515/ijcre-2013-0082. [DOI] [Google Scholar]
- Tanaka Y. Water dissociation in ion-exchange membrane electrodialysis. J. Membr. Sci. 2002, 203, 227–244. 10.1016/S0376-7388(02)00011-X. [DOI] [Google Scholar]
- Cifuentes-Araya N.; Astudillo-Castro C.; Bazinet L. Mechanisms of mineral membrane fouling growth modulated by pulsed modes of current during electrodialysis: Evidences of water splitting implications in the appearance of the amorphous phases of magnesium hydroxide and calcium carbonate. J. Colloid Interface Sci. 2014, 426, 221–234. 10.1016/j.jcis.2014.03.054. [DOI] [PubMed] [Google Scholar]
- Mikhaylin S.; Nikonenko V.; Pourcelly G.; Bazinet L. Intensification of demineralization process and decrease in scaling by application of pulsed electric field with short pulse/pause conditions. Journal of membrane science 2014, 468, 389–399. 10.1016/j.memsci.2014.05.045. [DOI] [Google Scholar]
- Dufton G.; Mikhaylin S.; Gaaloul S.; Bazinet L. Positive impact of pulsed electric field on lactic acid removal, demineralization and membrane scaling during acid whey electrodialysis. International journal of molecular sciences 2019, 20, 797. 10.3390/ijms20040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-J.; Park J.-S.; Kang M.-S.; Moon S.-H. Effects of silica sol on ion exchange membranes: Electrochemical characterization of anion exchange membranes in electrodialysis of silica sol containing-solutions. Korean Journal of Chemical Engineering 2003, 20, 889–895. 10.1007/BF02697294. [DOI] [Google Scholar]
- Korngold E. Prevention of colloidal-fouling in electrodialysis by chlorination. Desalination 1971, 9, 213–216. 10.1016/S0011-9164(00)80031-4. [DOI] [Google Scholar]
- Mondor M.; Ippersiel D.; Lamarche F.; Masse L. Fouling characterization of electrodialysis membranes used for the recovery and concentration of ammonia from swine manure. Bioresource technology 2009, 100, 566–571. 10.1016/j.biortech.2008.06.072. [DOI] [PubMed] [Google Scholar]
- Yiantsios S.; Karabelas A. The effect of colloid stability on membrane fouling. Desalination 1998, 118, 143–152. 10.1016/S0011-9164(98)00110-6. [DOI] [Google Scholar]
- Tanaka N.; Nagase M.; Higa M. Organic fouling behavior of commercially available hydrocarbon-based anion-exchange membranes by various organic-fouling substances. Desalination 2012, 296, 81–86. 10.1016/j.desal.2012.04.010. [DOI] [Google Scholar]
- De Jaegher B.; Larumbe E.; De Schepper W.; Verliefde A.; Nopens I. Data on ion-exchange membrane fouling by humic acid during electrodialysis. Data in Brief 2020, 31, 105763. 10.1016/j.dib.2020.105763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jaegher B.; Larumbe E.; De Schepper W.; Verliefde A.; Nopens I. Colloidal fouling in electrodialysis: A neural differential equations model. Sep. Purif. Technol. 2020, 249, 116939. 10.1016/j.seppur.2020.116939. [DOI] [Google Scholar]
- Talebi S.; Chen G. Q.; Freeman B.; Suarez F.; Freckleton A.; Bathurst K.; Kentish S. E. Fouling and in-situ cleaning of ion-exchange membranes during the electrodialysis of fresh acid and sweet whey. Journal of Food Engineering 2019, 246, 192–199. 10.1016/j.jfoodeng.2018.11.010. [DOI] [Google Scholar]
- Persico M.; Mikhaylin S.; Doyen A.; Firdaous L.; Nikonenko V.; Pismenskaya N.; Bazinet L. Prevention of peptide fouling on ion-exchange membranes during electrodialysis in overlimiting conditions. J. Membr. Sci. 2017, 543, 212–221. 10.1016/j.memsci.2017.08.039. [DOI] [Google Scholar]
- Verliefde A. R.; Cornelissen E. R.; Heijman S. G.; Hoek E. M.; Amy G. L.; Bruggen B. V. d.; Van Dijk J. C. Influence of solute- membrane affinity on rejection of uncharged organic solutes by nanofiltration membranes. Environ. Sci. Technol. 2009, 43, 2400–2406. 10.1021/es803146r. [DOI] [PubMed] [Google Scholar]
- Lee H.-J.; Hong M.-K.; Han S.-D.; Cho S.-H.; Moon S.-H. Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 2009, 238, 60–69. 10.1016/j.desal.2008.01.036. [DOI] [Google Scholar]
- Guo H.; Xiao L.; Yu S.; Yang H.; Hu J.; Liu G.; Tang Y. Analysis of anion exchange membrane fouling mechanism caused by anion polyacrylamide in electrodialysis. Desalination 2014, 346, 46–53. 10.1016/j.desal.2014.05.010. [DOI] [Google Scholar]
- Katz W. E. The electrodialysis reversal (EDR) process. Desalination 1979, 28, 31–40. 10.1016/S0011-9164(00)88124-2. [DOI] [Google Scholar]
- Chao Y.-M.; Liang T. A feasibility study of industrial wastewater recovery using electrodialysis reversal. Desalination 2008, 221, 433–439. 10.1016/j.desal.2007.04.065. [DOI] [Google Scholar]
- Sun X.; Lu H.; Wang J. Brackish water desalination using electrodeionization reversal. Chemical Engineering and Processing: Process Intensification 2016, 104, 262–270. 10.1016/j.cep.2016.03.014. [DOI] [Google Scholar]
- Tamersit S.; Bouhidel K.-E.; Zidani Z. Investigation of electrodialysis anti-fouling configuration for desalting and treating tannery unhairing wastewater: Feasibility of by-products recovery and water recycling. Journal of environmental management 2018, 207, 334–340. 10.1016/j.jenvman.2017.11.058. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Chen Q.; Ren L.; Wang J. Fabrication of hydrophilic cation exchange membrane with improved stability for electrodialysis: An excellent anti-scaling performance. J. Membr. Sci. 2021, 617, 118618. 10.1016/j.memsci.2020.118618. [DOI] [Google Scholar]
- Mulyati S.; Takagi R.; Fujii A.; Ohmukai Y.; Maruyama T.; Matsuyama H. Improvement of the antifouling potential of an anion exchange membrane by surface modification with a polyelectrolyte for an electrodialysis process. J. Membr. Sci. 2012, 417, 137–143. 10.1016/j.memsci.2012.06.024. [DOI] [Google Scholar]
- Khoiruddin K.; Ariono D.; Subagjo S.; Wenten I. Improved anti-organic fouling of polyvinyl chloride-based heterogeneous anion-exchange membrane modified by hydrophilic additives. Journal of Water Process Engineering 2021, 41, 102007. 10.1016/j.jwpe.2021.102007. [DOI] [Google Scholar]
- Gil V.; Andreeva M.; Jansezian L.; Han J.; Pismenskaya N.; Nikonenko V.; Larchet C.; Dammak L. Impact of heterogeneous cation-exchange membrane surface modification on chronopotentiometric and current–voltage characteristics in NaCl, CaCl2 and MgCl2 solutions. Electrochim. Acta 2018, 281, 472–485. 10.1016/j.electacta.2018.05.195. [DOI] [Google Scholar]
- Cheng Y.; Hao Z.; Hao C.; Deng Y.; Li X.; Li K.; Zhao Y. A review of modification of carbon electrode material in capacitive deionization. RSC Adv. 2019, 9, 24401–24419. 10.1039/C9RA04426D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying T.-Y.; Yang K.-L.; Yiacoumi S.; Tsouris C. Electrosorption of ions from aqueous solutions by nanostructured carbon aerogel. J. Colloid Interface Sci. 2002, 250, 18–27. 10.1006/jcis.2002.8314. [DOI] [PubMed] [Google Scholar]
- Su X.; Hatton T. A. Electrosorption. Kirk-Othmer Encyclopedia of Chemical Technology 2016, 1–11. 10.1002/0471238961.koe00022. [DOI] [Google Scholar]
- Gamaethiralalage J.; Singh K.; Sahin S.; Yoon J.; Elimelech M.; Suss M.; Liang P.; Biesheuvel P.; Zornitta R. L.; de Smet L. Recent advances in ion selectivity with capacitive deionization. Energy Environ. Sci. 2021, 14, 1095–1120. 10.1039/D0EE03145C. [DOI] [Google Scholar]
- Lester Y.; Shaulsky E.; Epsztein R.; Zucker I. Capacitive deionization for simultaneous removal of salt and uncharged organic contaminants from water. Sep. Purif. Technol. 2020, 237, 116388. 10.1016/j.seppur.2019.116388. [DOI] [Google Scholar]
- Lee J. B.; Park K. K.; Eum H. M.; Lee C. W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. 10.1016/j.desal.2006.01.011. [DOI] [Google Scholar]
- Tang W.; He D.; Zhang C.; Kovalsky P.; Waite T. D. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes. Water research 2017, 120, 229–237. 10.1016/j.watres.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Porada S.; Zhang L.; Dykstra J. Energy consumption in membrane capacitive deionization and comparison with reverse osmosis. Desalination 2020, 488, 114383. 10.1016/j.desal.2020.114383. [DOI] [Google Scholar]
- Yang J.; Bu Y.; Liu F.; Zhang W.; Cai D.; Sun A.; Wu Y.; Zhou R.; Zhang C. Potential Application of Membrane Capacitive Deionization for Heavy Metal Removal from Water: A Mini-Review. Int. J. Electrochem. Sci. 2020, 15, 7848–7859. 10.20964/2020.08.98. [DOI] [Google Scholar]
- Jeon S.-i.; Park H.-r.; Yeo J.-g.; Yang S.; Cho C. H.; Han M. H.; Kim D. K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. 10.1039/c3ee24443a. [DOI] [Google Scholar]
- Ma J.; He C.; He D.; Zhang C.; Waite T. D. Analysis of capacitive and electrodialytic contributions to water desalination by flow-electrode CDI. Water research 2018, 144, 296–303. 10.1016/j.watres.2018.07.049. [DOI] [PubMed] [Google Scholar]
- Zhang C.; He D.; Ma J.; Tang W.; Waite T. D. Comparison of Faradaic reactions in flow-through and flow-by capacitive deionization (CDI) systems. Electrochim. Acta 2019, 299, 727–735. 10.1016/j.electacta.2019.01.058. [DOI] [Google Scholar]
- Doornbusch G. J.; Dykstra J. E.; Biesheuvel P. M.; Suss M. E. Fluidized bed electrodes with high carbon loading for water desalination by capacitive deionization. Journal of Materials Chemistry A 2016, 4, 3642–3647. 10.1039/C5TA10316A. [DOI] [Google Scholar]
- Zhang X.; Zuo K.; Zhang X.; Zhang C.; Liang P. Selective ion separation by capacitive deionization (CDI) based technologies: a state-of-the-art review. Environ. Sci. Water Res. Technol. 2020, 6, 243–257. 10.1039/C9EW00835G. [DOI] [Google Scholar]
- Suss M. E.; Baumann T. F.; Bourcier W. L.; Spadaccini C. M.; Rose K. A.; Santiago J. G.; Stadermann M. Capacitive desalination with flow-through electrodes. Energy Environ. Sci. 2012, 5, 9511–9519. 10.1039/c2ee21498a. [DOI] [Google Scholar]
- Remillard E. M.; Shocron A. N.; Rahill J.; Suss M. E.; Vecitis C. D. A direct comparison of flow-by and flow-through capacitive deionization. Desalination 2018, 444, 169–177. 10.1016/j.desal.2018.01.018. [DOI] [Google Scholar]
- Nordstrand J.; Dutta J. Design principles for enhanced up-scaling of flow-through capacitive deionization for water desalination. Desalination 2021, 500, 114842. 10.1016/j.desal.2020.114842. [DOI] [Google Scholar]
- Chen R.; Sheehan T.; Ng J. L.; Brucks M.; Su X. Capacitive deionization and electrosorption for heavy metal removal. Environ. Sci.: Water Res. Technol. 2020, 6, 258–282. 10.1007/978-981-15-9605-6. [DOI] [Google Scholar]
- Guyes E. N.; Shocron A. N.; Chen Y.; Diesendruck C. E.; Suss M. E. Long-lasting, monovalent-selective capacitive deionization electrodes. NPJ Clean Water 2021, 4, 22. 10.1038/s41545-021-00109-2. [DOI] [Google Scholar]
- Zhao R.; Van Soestbergen M.; Rijnaarts H.; Van der Wal A.; Bazant M.; Biesheuvel P. Time-dependent ion selectivity in capacitive charging of porous electrodes. J. Colloid Interface Sci. 2012, 384, 38–44. 10.1016/j.jcis.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Suss M. E. Size-based ion selectivity of micropore electric double layers in capacitive deionization electrodes. J. Electrochem. Soc. 2017, 164, E270 10.1149/2.1201709jes. [DOI] [Google Scholar]
- Zhang Y.; Peng J.; Feng G.; Presser V. Hydration shell energy barrier differences of sub-nanometer carbon pores enable ion sieving and selective ion removal. Chemical Engineering Journal 2021, 419, 129438. 10.1016/j.cej.2021.129438. [DOI] [Google Scholar]
- Oyarzun D. I.; Hemmatifar A.; Palko J. W.; Stadermann M.; Santiago J. G. Ion selectivity in capacitive deionization with functionalized electrode: Theory and experimental validation. Water Research X 2018, 1, 100008. 10.1016/j.wroa.2018.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; He D.; Ma J.; Tang W.; Waite T. D. Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review. Water research 2018, 128, 314–330. 10.1016/j.watres.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Yoon H.; Lee J.; Kim S.; Yoon J. Review of concepts and applications of electrochemical ion separation (EIONS) process. Sep. Purif. Technol. 2019, 215, 190–207. 10.1016/j.seppur.2018.12.071. [DOI] [Google Scholar]
- Grahame D. C. The electrical double layer and the theory of electrocapillarity. Chem. Rev. 1947, 41, 441–501. 10.1021/cr60130a002. [DOI] [PubMed] [Google Scholar]
- De Levie R. On porous electrodes in electrolyte solutions: I. Capacitance effects. Electrochimica acta 1963, 8, 751–780. 10.1016/0013-4686(63)80042-0. [DOI] [Google Scholar]
- Burt R.; Birkett G.; Zhao X. A review of molecular modelling of electric double layer capacitors. Phys. Chem. Chem. Phys. 2014, 16, 6519–6538. 10.1039/c3cp55186e. [DOI] [PubMed] [Google Scholar]
- Johnson A. M.; Newman J. Desalting by Means of Porous Carbon Electrodes. J. Electrochem. Soc. 1971, 118, 510. 10.1149/1.2408094. [DOI] [Google Scholar]
- Bazant M. Z.; Thornton K.; Ajdari A. Diffuse-charge dynamics in electrochemical systems. Phys. Rev. E 2004, 70, 021506. 10.1103/PhysRevE.70.021506. [DOI] [PubMed] [Google Scholar]
- Højgaard Olesen L. H.; Bazant M. Z.; Bruus H. Strongly nonlinear dynamics of electrolytes in large ac voltages. Phys. Rev. E 2010, 82, 011501. 10.1103/PhysRevE.82.011501. [DOI] [PubMed] [Google Scholar]
- Biesheuvel P.; Bazant M. Nonlinear dynamics of capacitive charging and desalination by porous electrodes. Phys. Rev. E 2010, 81, 031502. 10.1103/PhysRevE.81.031502. [DOI] [PubMed] [Google Scholar]
- Mirzadeh M.; Gibou F.; Squires T. M. Enhanced charging kinetics of porous electrodes: Surface conduction as a short-circuit mechanism. Physical review letters 2014, 113, 097701. 10.1103/PhysRevLett.113.097701. [DOI] [PubMed] [Google Scholar]
- Biesheuvel P.; Fu Y.; Bazant M. Z. Diffuse charge and Faradaic reactions in porous electrodes. Phys. Rev. E 2011, 83, 061507. 10.1103/PhysRevE.83.061507. [DOI] [PubMed] [Google Scholar]
- Biesheuvel P.; Fu Y.; Bazant M. Electrochemistry and capacitive charging of porous electrodes in asymmetric multicomponent electrolytes. Russian Journal of Electrochemistry 2012, 48, 580–592. 10.1134/S1023193512060031. [DOI] [Google Scholar]
- Biesheuvel P.; Porada S.; Levi M.; Bazant M. Z. Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 2014, 18, 1365–1376. 10.1007/s10008-014-2383-5. [DOI] [Google Scholar]
- Shocron A. N.; Suss M. E. The effect of surface transport on water desalination by porous electrodes undergoing capacitive charging. J. Phys.: Condens. Matter 2017, 29, 084003. 10.1088/1361-648X/29/8/084003. [DOI] [PubMed] [Google Scholar]
- Porada S.; Weingarth D.; Hamelers H. V. M.; Bryjak M.; Presser V.; Biesheuvel P. M. Carbon flow electrodes for continuous operation of capacitive deionization and capacitive mixing energy generation. Journal of Materials Chemistry A 2014, 2, 9313–9321. 10.1039/c4ta01783h. [DOI] [Google Scholar]
- Gabitto J.; Tsouris C. Volume averaging study of the capacitive deionization process in homogeneous porous media. Transport in Porous Media 2015, 109, 61–80. 10.1007/s11242-015-0502-0. [DOI] [Google Scholar]
- Biesheuvel P.; Zhao R.; Porada S.; Van der Wal A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. 10.1016/j.jcis.2011.04.049. [DOI] [PubMed] [Google Scholar]
- Porada S.; Bryjak M.; Van Der Wal A.; Biesheuvel P. Effect of electrode thickness variation on operation of capacitive deionization. Electrochim. Acta 2012, 75, 148–156. 10.1016/j.electacta.2012.04.083. [DOI] [Google Scholar]
- Wang L.; Biesheuvel P.; Lin S. Reversible thermodynamic cycle analysis for capacitive deionization with modified Donnan model. J. Colloid Interface Sci. 2018, 512, 522–528. 10.1016/j.jcis.2017.10.060. [DOI] [PubMed] [Google Scholar]
- Shocron A. N.; Suss M. E. Should we pose a closure problem for capacitive charging of porous electrodes?. EPL (Europhysics Letters) 2020, 130, 34003. 10.1209/0295-5075/130/34003. [DOI] [Google Scholar]
- Akpor O. B.; Muchie M. Remediation of heavy metals in drinking water and wastewater treatment systems: Processes and applications. Int. J. Phys. Sci. 2010, 5, 1807–1817. [Google Scholar]
- Chaudhary V.; Kumar M.; Sharma M.; Yadav B. Fluoride, boron and nitrate toxicity in ground water of northwest Rajasthan, India. Environmental monitoring and assessment 2010, 161, 343–348. 10.1007/s10661-009-0750-y. [DOI] [PubMed] [Google Scholar]
- Mohod C. V.; Dhote J. Review of heavy metals in drinking water and their effect on human health. Int. J. Innovative Res. Sci., Eng. Technol. 2013, 2, 2992–2996. [Google Scholar]
- Xu P.; Drewes J. E.; Heil D.; Wang G. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res. 2008, 42, 2605–2617. 10.1016/j.watres.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Broséus R.; Cigana J.; Barbeau B.; Daines-Martinez C.; Suty H. Removal of total dissolved solids, nitrates and ammonium ions from drinking water using charge-barrier capacitive deionisation. Desalination 2009, 249, 217–223. 10.1016/j.desal.2008.12.048. [DOI] [Google Scholar]
- Li H.; Zou L.; Pan L.; Sun Z. Using graphene nano-flakes as electrodes to remove ferric ions by capacitive deionization. Sep. Purif. Technol. 2010, 75, 8–14. 10.1016/j.seppur.2010.07.003. [DOI] [Google Scholar]
- Kim Y.-J.; Choi J.-H. Selective removal of nitrate ion using a novel composite carbon electrode in capacitive deionization. Water Res. 2012, 46, 6033–6039. 10.1016/j.watres.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Mossad M.; Zou L. A study of the capacitive deionisation performance under various operational conditions. Journal of Hazardous Materials 2012, 213-214, 491–497. 10.1016/j.jhazmat.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Kim Y.-J.; Kim J.-H.; Choi J.-H. Selective removal of nitrate ions by controlling the applied current in membrane capacitive deionization (MCDI). J. Membr. Sci. 2013, 429, 52–57. 10.1016/j.memsci.2012.11.064. [DOI] [Google Scholar]
- Macías C.; Lavela P.; Rasines G.; Zafra M. C.; Tirado J. L.; Ania C. O. Improved electro-assisted removal of phosphates and nitrates using mesoporous carbon aerogels with controlled porosity. J. Appl. Electrochem. 2014, 44, 963–976. 10.1007/s10800-014-0705-z. [DOI] [Google Scholar]
- Wang H.; Na C. Binder-Free Carbon Nanotube Electrode for Electrochemical Removal of Chromium. ACS Appl. Mater. Interfaces 2014, 6, 20309–20316. 10.1021/am505838r. [DOI] [PubMed] [Google Scholar]
- Tang W.; Kovalsky P.; Cao B.; Waite T. D. Investigation of fluoride removal from low-salinity groundwater by single-pass constant-voltage capacitive deionization. Water Res. 2016, 99, 112–121. 10.1016/j.watres.2016.04.047. [DOI] [PubMed] [Google Scholar]
- Tang W.; Kovalsky P.; He D.; Waite T. D. Fluoride and nitrate removal from brackish groundwaters by batch-mode capacitive deionization. Water Res. 2015, 84, 342–349. 10.1016/j.watres.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Lu L.; Cai Z.; Ren Z. J. Individual and competitive removal of heavy metals using capacitive deionization. Journal of Hazardous Materials 2016, 302, 323–331. 10.1016/j.jhazmat.2015.09.064. [DOI] [PubMed] [Google Scholar]
- Lado J. J.; Pérez-Roa R. E.; Wouters J. J.; Tejedor-Tejedor M. I.; Federspill C.; Ortiz J. M.; Anderson M. A. Removal of nitrate by asymmetric capacitive deionization. Sep. Purif. Technol. 2017, 183, 145–152. 10.1016/j.seppur.2017.03.071. [DOI] [Google Scholar]
- Hu C.; Dong J.; Wang T.; Liu R.; Liu H.; Qu J. Nitrate electro-sorption/reduction in capacitive deionization using a novel Pd/NiAl-layered metal oxide film electrode. Chemical Engineering Journal 2018, 335, 475–482. 10.1016/j.cej.2017.10.167. [DOI] [Google Scholar]
- Lee D.-H.; Ryu T.; Shin J.; Ryu J. C.; Chung K.-S.; Kim Y. H. Selective lithium recovery from aqueous solution using a modified membrane capacitive deionization system. Hydrometallurgy 2017, 173, 283–288. 10.1016/j.hydromet.2017.09.005. [DOI] [Google Scholar]
- Shi W.; Liu X.; Ye C.; Cao X.; Gao C.; Shen J. Efficient lithium extraction by membrane capacitive deionization incorporated with monovalent selective cation exchange membrane. Sep. Purif. Technol. 2019, 210, 885–890. 10.1016/j.seppur.2018.09.006. [DOI] [Google Scholar]
- Guyes E. N.; Malka T.; Suss M. E. Enhancing the ion-size-based selectivity of capacitive deionization electrodes. Environ. Sci. Technol. 2019, 53, 8447–8454. 10.1021/acs.est.8b06954. [DOI] [PubMed] [Google Scholar]
- Ha Y.; Jung H. B.; Lim H.; Jo P. S.; Yoon H.; Yoo C. Y.; Pham T. K.; Ahn W.; Cho Y. Continuous lithium extraction from aqueous solution using flow-electrode capacitive deionization. Energies 2019, 12, 2913. 10.3390/en12152913. [DOI] [Google Scholar]
- Siekierka A.; Tomaszewska B.; Bryjak M. Lithium capturing from geothermal water by hybrid capacitive deionization. Desalination 2018, 436, 8–14. 10.1016/j.desal.2018.02.003. [DOI] [Google Scholar]
- Siekierka A.; Bryjak M. Novel anion exchange membrane for concentration of lithium salt in hybrid capacitive deionization. Desalination 2019, 452, 279–289. 10.1016/j.desal.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka A.; Bryjak M. Selective sorbents for recovery of lithium ions by hybrid capacitive deionization. Desalination 2021, 520, 115324. 10.1016/j.desal.2021.115324. [DOI] [Google Scholar]
- Kim B.; Seo J. Y.; Chung C.-H. Electrochemical Desalination and Recovery of Lithium from Saline Water upon Operation of a Capacitive Deionization Cell Combined with a Redox Flow Battery. ACS ES&T Water 2021, 1, 1047–1054. 10.1021/acsestwater.1c00014. [DOI] [Google Scholar]
- Hu B.; Shang X.; Nie P.; Zhang B.; Yang J.; Liu J. Lithium ion sieve modified three-dimensional graphene electrode for selective extraction of lithium by capacitive deionization. J. Colloid Interface Sci. 2022, 612, 392–400. 10.1016/j.jcis.2021.12.181. [DOI] [PubMed] [Google Scholar]
- Huang X.; He D.; Tang W.; Kovalsky P.; Waite T. D. Investigation of pH-dependent phosphate removal from wastewaters by membrane capacitive deionization (MCDI). Environ. Sci.: Water Res. Technol. 2017, 3, 875–882. 10.1039/C7EW00138J. [DOI] [Google Scholar]
- Jiang J.; Kim D. I.; Dorji P.; Phuntsho S.; Hong S.; Shon H. K. Phosphorus removal mechanisms from domestic wastewater by membrane capacitive deionization and system optimization for enhanced phosphate removal. Proc. Safe. Environ. Protect. 2019, 126, 44–52. 10.1016/j.psep.2019.04.005. [DOI] [Google Scholar]
- Zhang C.; Cheng X.; Wang M.; Ma J.; Collins R.; Kinsela A.; Zhang Y.; Waite T. D. Phosphate recovery as vivianite using a flow-electrode capacitive desalination (FCDI) and fluidized bed crystallization (FBC) coupled system. Water Res. 2021, 126, 194. 10.1016/j.psep.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Wang M.; Xiao W.; Ma J.; Sun J.; Mo H.; Waite T. D. Phosphate selective recovery by magnetic iron oxide impregnated carbon flow-electrode capacitive deionization (FCDI). Water Res. 2021, 189, 116653. 10.1016/j.watres.2020.116653. [DOI] [PubMed] [Google Scholar]
- Shocron A.; Guyes E.; Biesheuvel P.; Rijnaarts H.; Suss M.; Dykstra J. Electrochemical removal of amphoteric ions. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2108240118 10.1073/pnas.2108240118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornitta R. L.; Ruotolo L. A.; de Smet L. C. High-Performance Carbon Electrodes Modified with Polyaniline for Stable and Selective Anion Separation. Sep. Purif. Technol. 2022, 290, 120807. 10.1016/j.seppur.2022.120807. [DOI] [Google Scholar]
- Kim Y. J.; Kim J. H.; Choi J. H. Selective removal of nitrate ions by controlling the applied current in membrane capacitive deionization (MCDI). J. Membr. Sci. 2013, 429, 52–57. 10.1016/j.memsci.2012.11.064. [DOI] [Google Scholar]
- Zhang C.; Ma J.; Song J.; He C.; Waite T. D. Continuous Ammonia Recovery from Wastewaters Using an Integrated Capacitive Flow Electrode Membrane Stripping System. Environ. Sci. Technol. 2018, 52, 14275–14285. 10.1021/acs.est.8b02743. [DOI] [PubMed] [Google Scholar]
- Fang K.; Gong H.; He W.; Peng F.; He C.; Wang K. Recovering ammonia from municipal wastewater by flow-electrode capacitive deionization. Chem. Eng. J. 2018, 348, 301–309. 10.1016/j.cej.2018.04.128. [DOI] [Google Scholar]
- Oyarzun D. I.; Hemmatifar A.; Palko J. W.; Stadermann M.; Santiago J. G. Adsorption and capacitive regeneration of nitrate using inverted capacitive deionization with surfactant functionalized carbon electrodes. Sep. Purif. Technol. 2018, 194, 410–415. 10.1016/j.seppur.2017.11.027. [DOI] [Google Scholar]
- Zhang C.; Ma J.; Waite T. D. Ammonia-Rich Solution Production from Wastewaters Using Chemical-Free Flow-Electrode Capacitive Deionization. ACS Sustainable Chem. Eng. 2019, 7, 6480–6485. 10.1021/acssuschemeng.9b00314. [DOI] [Google Scholar]
- Sakar H.; Celik I.; Balcik-Canbolat C.; Keskinler B.; Karagunduz A.. Ammonium removal and recovery from real digestate wastewater by a modified operational method of membrane capacitive deionization unit. J. Cleaner Prod. 2019, 215.1415–1423. 10.1016/j.jclepro.2019.01.165 [DOI] [Google Scholar]
- Gan L.; Wu Y.; Song H.; Zhang S.; Lu C.; Yang S.; Wang Z.; Jiang B.; Wang C.; Li A. Selective removal of nitrate ion using a novel activated carbon composite carbon electrode in capacitive deionization. Sep. Purif. Technol. 2019, 212, 728–736. 10.1016/j.seppur.2018.11.081. [DOI] [Google Scholar]
- Fang K.; He W.; Peng F.; Wang K. Ammonia recovery from concentrated solution by designing novel stacked FCDI cell. Sep. Purif. Technol. 2020, 250, 117066. 10.1016/j.seppur.2020.117066. [DOI] [Google Scholar]
- Pasta M.; Wessells C.; Cui Y.; La Mantia F. A desalination battery. Nano Lett. 2012, 12, 839–843. 10.1021/nl203889e. [DOI] [PubMed] [Google Scholar]
- Hou C.-H.; Huang C.-Y. A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization. Desalination 2013, 314, 124–129. 10.1016/j.desal.2012.12.029. [DOI] [Google Scholar]
- Yeo J.-H.; Choi J.-H. Enhancement of nitrate removal from a solution of mixed nitrate, chloride and sulfate ions using a nitrate-selective carbon electrode. Desalination 2013, 320, 10–16. 10.1016/j.desal.2013.04.013. [DOI] [Google Scholar]
- Kim S.; Yoon H.; Shin D.; Lee J.; Yoon J. Electrochemical selective ion separation in capacitive deionization with sodium manganese oxide. J. Colloid Interface Sci. 2017, 506, 644–648. 10.1016/j.jcis.2017.07.054. [DOI] [PubMed] [Google Scholar]
- Lee J.; Kim S.; Yoon J. Rocking chair desalination battery based on Prussian Blue electrodes. Acs Omega 2017, 2, 1653–1659. 10.1021/acsomega.6b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks S. A.; Cerón M. R.; Oyarzun D. I.; Pham T. A.; Zhan C.; Loeb C. K.; Mew D.; Deinhart A.; Wood B. C.; Santiago J. G.; Stadermann M.; Campbell P. G. Using ultramicroporous carbon for the selective removal of nitrate with capacitive deionization. Environ. Sci. Technol. 2019, 53, 10863–10870. 10.1021/acs.est.9b01374. [DOI] [PubMed] [Google Scholar]
- Dong Q.; Guo X.; Huang X.; Liu L.; Tallon R.; Taylor B.; Chen J. Selective removal of lead ions through capacitive deionization: Role of ion-exchange membrane. Chemical Engineering Journal 2019, 361, 1535–1542. 10.1016/j.cej.2018.10.208. [DOI] [Google Scholar]
- Seo S. J.; Jeon H.; Lee J. K.; Kim G. Y.; Park D.; Nojima H.; Lee J.; Moon S. H. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res. 2010, 44, 2267–2275. 10.1016/j.watres.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Hassanvand A.; Chen G. Q.; Webley P. A.; Kentish S. E. A comparison of multicomponent electrosorption in capacitive deionization and membrane capacitive deionization. Water Res. 2018, 131, 100–109. 10.1016/j.watres.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Sahin S.; Zuilhof H.; Zornitta R. L.; de Smet L. C. Enhanced monovalent over divalent cation selectivity with polyelectrolyte multilayers in membrane capacitive deionization via optimization of operational conditions. Desalination 2022, 522, 115391. 10.1016/j.desal.2021.115391. [DOI] [Google Scholar]
- Avraham E.; Yaniv B.; Soffer A.; Aurbach D. Developing Ion Electroadsorption Stereoselectivity, by Pore Size Adjustment with Chemical Vapor Deposition onto Active Carbon Fiber Electrodes. Case of Ca2+/Na+ Separation in Water Capacitive Desalination. J. Phys. Chem. C 2008, 112, 7385–7389. 10.1021/jp711706z. [DOI] [Google Scholar]
- Noked M.; Avraham E.; Bohadana Y.; Soffer A.; Aurbach D. Development of Anion Stereoselective, Activated Carbon Molecular Sieve Electrodes Prepared by Chemical Vapor Deposition. J. Phys. Chem. C 2009, 113, 7316–7321. 10.1021/jp811283b. [DOI] [Google Scholar]
- Cerón M. R.; Aydin F.; Hawks S. A.; Oyarzun D. I.; Loeb C. K.; Deinhart A.; Zhan C.; Pham T. A.; Stadermann M.; Campbell P. G. Cation Selectivity in Capacitive Deionization: Elucidating the Role of Pore Size, Electrode Potential, and Ion Dehydration. ACS Appl. Mater. Interfaces 2020, 12, 42644–42652. 10.1021/acsami.0c07903. [DOI] [PubMed] [Google Scholar]
- Han L.; Karthikeyan K. G.; Anderson M. A.; Gregory K. B. Exploring the impact of pore size distribution on the performance of carbon electrodes for capacitive deionization. J. Colloid Interface Sci. 2014, 430, 93–99. 10.1016/j.jcis.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Eliad L.; Salitra G.; Soffer A.; Aurbach D. Ion Sieving Effects in the Electrical Double Layer of Porous Carbon Electrodes: Estimating Effective Ion Size in Electrolytic Solutions. J. Phys. Chem. B 2001, 105, 6880–6887. 10.1021/jp010086y. [DOI] [Google Scholar]
- Gabelich C. J.; Tran T. D.; Suffet I. H. Electrosorption of Inorganic Salts from Aqueous Solution Using Carbon Aerogels. Environ. Sci. Technol. 2002, 36, 3010–3019. 10.1021/es0112745. [DOI] [PubMed] [Google Scholar]
- Hou C.-H.; Taboada-Serrano P.; Yiacoumi S.; Tsouris C. Electrosorption selectivity of ions from mixtures of electrolytes inside nanopores. J. Chem. Phys. 2008, 129, 224703. 10.1063/1.3033562. [DOI] [PubMed] [Google Scholar]
- Dykstra J.; Dijkstra J.; van der Wal A.; Hamelers H.; Porada S. On-line method to study dynamics of ion adsorption from mixtures of salts in capacitive deionization. Desalination 2016, 390, 47–52. 10.1016/j.desal.2016.04.001. [DOI] [Google Scholar]
- Misra R. P.; de Souza J. P.; Blankschtein D.; Bazant M. Z. Theory of surface forces in multivalent electrolytes. Langmuir 2019, 35, 11550–11565. 10.1021/acs.langmuir.9b01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin Y. Electrostatic correlations: from plasma to biology. Rep. Prog. Phys. 2002, 65, 1577. 10.1088/0034-4885/65/11/201. [DOI] [Google Scholar]
- Roth R. Fundamental measure theory for hard-sphere mixtures: a review. J. Phys.: Condens. Matter 2010, 22, 063102. 10.1088/0953-8984/22/6/063102. [DOI] [PubMed] [Google Scholar]
- de Souza J. P.; Goodwin Z. A.; McEldrew M.; Kornyshev A. A.; Bazant M. Z. Interfacial layering in the electric double layer of ionic liquids. Phys. Rev. Lett. 2020, 125, 116001. 10.1103/PhysRevLett.125.116001. [DOI] [PubMed] [Google Scholar]
- Tang W.; Kovalsky P.; Cao B.; He D.; Waite T. D. Fluoride removal from brackish groundwaters by constant current capacitive deionization (CDI). Environ. Sci. Technol. 2016, 50, 10570–10579. 10.1021/acs.est.6b03307. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Zhang H.; Wu C.; Wang Y.; Li W. A study of electrosorption selectivity of anions by activated carbon electrodes in capacitive deionization. Desalination 2015, 369, 46–50. 10.1016/j.desal.2015.04.022. [DOI] [Google Scholar]
- Li Y.; Zhang C.; Jiang Y.; Wang T.-J.; Wang H. Effects of the hydration ratio on the electrosorption selectivity of ions during capacitive deionization. Desalination 2016, 399, 171–177. 10.1016/j.desal.2016.09.011. [DOI] [Google Scholar]
- Mubita T. M.; Dykstra J. E.; Biesheuvel P. M.; van der Wal A.; Porada S. Selective adsorption of nitrate over chloride in microporous carbons. Water Res. 2019, 164, 114885. 10.1016/j.watres.2019.114885. [DOI] [PubMed] [Google Scholar]
- Nightingale E. R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. 10.1021/j150579a011. [DOI] [Google Scholar]
- Hawks S. A.; Ramachandran A.; Porada S.; Campbell P. G.; Suss M. E.; Biesheuvel P. M.; Santiago J. G.; Stadermann M. Performance Metrics for the Objective Assessment of Capacitive Deionization Systems. Water Res. 2019, 152, 126–137. 10.1016/j.watres.2018.10.074. [DOI] [PubMed] [Google Scholar]
- Porada S.; Shrivastava A.; Bukowska P.; Biesheuvel P. M.; Smith K. C. Nickel Hexacyanoferrate Electrodes for Continuous Cation Intercalation Desalination of Brackish Water. Electrochim. Acta 2017, 255, 369–378. 10.1016/j.electacta.2017.09.137. [DOI] [Google Scholar]
- Kim T.; Gorski C. A.; Logan B. E. Ammonium Removal from Domestic Wastewater Using Selective Battery Electrodes. Environmental Science & Technology Letters 2018, 5, 578–583. 10.1021/acs.estlett.8b00334. [DOI] [Google Scholar]
- Choi J.; Lee H.; Hong S. Capacitive deionization (CDI) integrated with monovalent cation selective membrane for producing divalent cation-rich solution. Desalination 2016, 400, 38–46. 10.1016/j.desal.2016.09.016. [DOI] [Google Scholar]
- Singh K.; Qian Z.; Biesheuvel P.; Zuilhof H.; Porada S.; de Smet L. C. Nickel hexacyanoferrate electrodes for high mono/divalent ion-selectivity in capacitive deionization. Desalination 2020, 481, 114346. 10.1016/j.desal.2020.114346. [DOI] [Google Scholar]
- Xu Y.; Zhou H.; Wang G.; Zhang Y.; Zhang H.; Zhao H. Selective Pseudocapacitive Deionization of Calcium Ions in Copper Hexacyanoferrate. ACS Appl. Mater. Interfaces 2020, 12, 41437–41445. 10.1021/acsami.0c11233. [DOI] [PubMed] [Google Scholar]
- Shang X.; Hu B.; Nie P.; Shi W.; Hussain T.; Liu J. LiNi0.5Mn1.5O4-based hybrid capacitive deionization for highly selective adsorption of lithium from brine. Sep. Purif. Technol. 2021, 258, 118009. 10.1016/j.seppur.2020.118009. [DOI] [Google Scholar]
- Zuo K.; Kim J.; Jain A.; Wang T.; Verduzco R.; Long M.; Li Q. Novel Composite Electrodes for Selective Removal of Sulfate by the Capacitive Deionization Process. Environ. Sci. Technol. 2018, 52, 9486–9494. 10.1021/acs.est.8b01868. [DOI] [PubMed] [Google Scholar]
- Chang J.; Li Y.; Duan F.; Su C.; Li Y.; Cao H. Selective removal of chloride ions by bismuth electrode in capacitive deionization. Sep. Purif. Technol. 2020, 240, 116600. 10.1016/j.seppur.2020.116600. [DOI] [Google Scholar]
- Kim J. S.; Jeon Y. S.; Rhim J. W. Application of poly(vinyl alcohol) and polysulfone based ionic exchange polymers to membrane capacitive deionization for the removal of mono- and divalent salts. Sep. Purif. Technol. 2016, 157, 45–52. 10.1016/j.seppur.2015.11.011. [DOI] [Google Scholar]
- Tang W.; He D.; Zhang C.; Waite T. D. Optimization of sulfate removal from brackish water by membrane capacitive deionization (MCDI). Water Res. 2017, 121, 302–310. 10.1016/j.watres.2017.05.046. [DOI] [PubMed] [Google Scholar]
- Uwayid R.; Guyes E. N.; Shocron A. N.; Gilron J.; Elimelech M.; Suss M. E. Perfect divalent cation selectivity with capacitive deionization. Water Res. 2022, 210, 117959. 10.1016/j.watres.2021.117959. [DOI] [PubMed] [Google Scholar]
- Zhao R.; Biesheuvel P. M.; van der Wal a. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 2012, 5, 9520–9527. 10.1039/c2ee21737f. [DOI] [Google Scholar]
- Gaoa X.; Porada S.; Omosebi A.; Liu K.-L.; Biesheuvel P. M.; Landon J. Complementary surface charge for enhanced capacitive deionization. Water Res. 2016, 92, 275–282. 10.1016/j.watres.2016.01.048. [DOI] [PubMed] [Google Scholar]
- Hemmatifar A.; Stadermann M.; Santiago J. G. Two-Dimensional Porous Electrode Model for Capacitive Deionization. J. Phys. Chem. C 2015, 119, 24681–24694. 10.1021/acs.jpcc.5b05847. [DOI] [Google Scholar]
- Qu Y.; Baumann T. F.; Santiago J. G.; Stadermann M. Characterization of Resistances of a Capacitive Deionization System. Environ. Sci. Technol. 2015, 49, 9699–9706. 10.1021/acs.est.5b02542. [DOI] [PubMed] [Google Scholar]
- Dykstra J.; Zhao R.; Biesheuvel P.; van der Wal A. Resistance identification and rational process design in Capacitive Deionization. Water Res. 2016, 88, 358–370. 10.1016/j.watres.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Qu Y.; Campbell P. G.; Gu L.; Knipe J. M.; Dzenitis E.; Santiago J. G.; Stadermann M. Energy consumption analysis of constant voltage and constant current operations in capacitive deionization. Desalination 2016, 400, 18–24. 10.1016/j.desal.2016.09.014. [DOI] [Google Scholar]
- Dykstra J. E.; Porada S.; van der Wal A.; Biesheuvel P. M. Energy consumption in capacitive deionization – Constant current versus constant voltage operation. Water Res. 2018, 143, 367–375. 10.1016/j.watres.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Hemmatifar A.; Ramachandran A.; Liu K.; Oyarzun D. I.; Bazant M. Z.; Santiago J. G. Thermodynamics of Ion Separation by Electrosorption. Environ. Sci. Technol. 2018, 52, 10196–10204. 10.1021/acs.est.8b02959. [DOI] [PubMed] [Google Scholar]
- Hawks S. A.; Knipe J. M.; Campbell P. G.; Loeb C. K.; Hubert M. A.; Santiago J. G.; Stadermann M. Quantifying the flow efficiency in constant-current capacitive deionization. Water Res. 2018, 129, 327–336. 10.1016/j.watres.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Wang L.; Lin S. Intrinsic tradeoff between kinetic and energetic efficiencies in membrane capacitive deionization. Water Res. 2018, 129, 394–401. 10.1016/j.watres.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Ramachandran A.; Oyarzun D. I.; Hawks S. A.; Stadermann M.; Santiago J. G. High water recovery and improved thermodynamic efficiency for capacitive deionization using variable flowrate operation. Water research 2019, 155, 76–85. 10.1016/j.watres.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Wang L.; Dykstra J. E.; Lin S. Energy Efficiency of Capacitive Deionization. Environ. Sci. Technol. 2019, 53, 3366–3378. 10.1021/acs.est.8b04858. [DOI] [PubMed] [Google Scholar]
- Lin S. Energy Efficiency of Desalination: Fundamental Insights from Intuitive Interpretation. Environ. Sci. Technol. 2020, 54, 76–84. 10.1021/acs.est.9b04788. [DOI] [PubMed] [Google Scholar]
- Alkuran M.; Orabi M.. Utilization of a buck boost converter and the method of segmented capacitors in a CDI water purification system. In 2008 12th International Middle-East Power System Conference, 2008; pp 470–474.
- Pernía A. M.; Norniella J. G.; Martín-Ramos J. A.; Díaz J.; Martínez J. A. Up–Down Converter for Energy Recovery in a CDI Desalination System. IEEE Transactions on Power Electronics 2012, 27, 3257–3265. 10.1109/TPEL.2011.2180926. [DOI] [Google Scholar]
- Pernía A. M.; Alvarez-Gonzalez F. J.; Prieto M. A. J.; Villegas P. J.; Nuno F. New Control Strategy of an Up–Down Converter for Energy Recovery in a CDI Desalination System. IEEE Transactions on Power Electronics 2014, 29, 3573–3581. 10.1109/TPEL.2013.2280814. [DOI] [Google Scholar]
- Długołȩcki P.; Van Der Wal A. Energy recovery in membrane capacitive deionization. Environ. Sci. Technol. 2013, 47, 4904–4910. 10.1021/es3053202. [DOI] [PubMed] [Google Scholar]
- Kang J.; Kim T.; Shin H.; Lee J.; Ha J.-I.; Yoon J. Direct energy recovery system for membrane capacitive deionization. Desalination 2016, 398, 144–150. 10.1016/j.desal.2016.07.025. [DOI] [Google Scholar]
- Oyarzun D. I.; Hawks S. A.; Campbell P. G.; Hemmatifar A.; Krishna A.; Santiago J. G.; Stadermann M. Energy transfer for storage or recovery in capacitive deionization using a DC-DC converter. J. Power Sources 2020, 448, 227409. 10.1016/j.jpowsour.2019.227409. [DOI] [Google Scholar]
- Zhang W.; Mossad M.; Yazdi J. S.; Zou L. A statistical experimental investigation on arsenic removal using capacitive deionization. Desalination and Water Treatment 2016, 57, 3254–3260. 10.1080/19443994.2014.981761. [DOI] [Google Scholar]
- Wu T.; Wang G.; Dong Q.; Zhan F.; Zhang X.; Li S.; Qiao H.; Qiu J. Starch Derived Porous Carbon Nanosheets for High-Performance Photovoltaic Capacitive Deionization. Environ. Sci. Technol. 2017, 51, 9244–9251. 10.1021/acs.est.7b01629. [DOI] [PubMed] [Google Scholar]
- Tan C.; He C.; Tang W.; Kovalsky P.; Fletcher J.; Waite T. D. Integration of photovoltaic energy supply with membrane capacitive deionization (MCDI) for salt removal from brackish waters. Water Res. 2018, 147, 276–286. 10.1016/j.watres.2018.09.056. [DOI] [PubMed] [Google Scholar]
- Hand S.; Guest J. S.; Cusick R. D. Technoeconomic Analysis of Brackish Water Capacitive Deionization: Navigating Tradeoffs between Performance, Lifetime, and Material Costs. Environ. Sci. Technol. 2019, 53, 13353–13363. 10.1021/acs.est.9b04347. [DOI] [PubMed] [Google Scholar]
- Kang J.; Kim T.; Jo K.; Yoon J. Comparison of salt adsorption capacity and energy consumption between constant current and constant voltage operation in capacitive deionization. Desalination 2014, 352, 52–57. 10.1016/j.desal.2014.08.009. [DOI] [Google Scholar]
- Avraham E.; Noked M.; Bouhadana Y.; Soffer A.; Aurbach D. Limitations of charge efficiency in capacitive deionization: II. On the behavior of CDI cells comprising two activated carbon electrodes. J. Electrochem. Soc. 2009, 156, P157. 10.1149/1.3193709. [DOI] [Google Scholar]
- Avraham E.; Noked M.; Bouhadana Y.; Soffer A.; Aurbach D. Limitations of charge efficiency in capacitive deionization processes III: The behavior of surface oxidized activated carbon electrodes. Electrochim. Acta 2010, 56, 441–447. 10.1016/j.electacta.2010.08.056. [DOI] [Google Scholar]
- Shanbhag S.; Whitacre J. F.; Mauter M. S. The Origins of Low Efficiency in Electrochemical De-Ionization Systems. J. Electrochem. Soc. 2016, 163, E363–E371. 10.1149/2.0181614jes. [DOI] [Google Scholar]
- Bazant M. Z. Theory of chemical kinetics and charge transfer based on nonequilibrium thermodynamics. Accounts of chemical research 2013, 46, 1144–1160. 10.1021/ar300145c. [DOI] [PubMed] [Google Scholar]
- Bouhadana Y.; Avraham E.; Noked M.; Ben-Tzion M.; Soffer A.; Aurbach D. Capacitive deionization of NaCl solutions at non-steady-state conditions: Inversion functionality of the carbon electrodes. J. Phys. Chem. C 2011, 115, 16567–16573. 10.1021/jp2047486. [DOI] [Google Scholar]
- Holubowitch N.; Omosebi A.; Gao X.; Landon J.; Liu K. Quasi-Steady-State Polarization Reveals the Interplay of Capacitive and Faradaic Processes in Capacitive Deionization. ChemElectroChem. 2017, 4, 2404–2413. 10.1002/celc.201700082. [DOI] [Google Scholar]
- He D.; Wong C. E.; Tang W.; Kovalsky P.; Waite T. D. Faradaic Reactions in Water Desalination by Batch-Mode Capacitive Deionization. Environmental Science & Technology Letters 2016, 3, 222–226. 10.1021/acs.estlett.6b00124. [DOI] [Google Scholar]
- Uwayid R.; Seraphim N. M.; Guyes E. N.; Eisenberg D.; Suss M. E. Characterizing and mitigating the degradation of oxidized cathodes during capacitive deionization cycling. Carbon 2021, 173, 1105–1114. 10.1016/j.carbon.2020.11.045. [DOI] [Google Scholar]
- Cohen I.; Avraham E.; Noked M.; Soffer A.; Aurbach D. Enhanced Charge Efficiency in Capacitive Deionization Achieved by Surface-Treated Electrodes and by Means of a Third Electrode. J. Phys. Chem. C 2011, 115, 19856–19863. 10.1021/jp206956a. [DOI] [Google Scholar]
- Gao X.; Omosebi A.; Landon J.; Liu K. Enhancement of charge efficiency for a capacitive deionization cell using carbon xerogel with modified potential of zero charge. Electrochem. Commun. 2014, 39, 22–25. 10.1016/j.elecom.2013.12.004. [DOI] [Google Scholar]
- Biesheuvel P. M.; Hamelers H. V. M.; Suss M. E. Theory of Water Desalination by Porous Electrodes with Immobile Chemical Charge. Colloids and Interface Science Communications 2015, 9, 1–5. 10.1016/j.colcom.2015.12.001. [DOI] [Google Scholar]
- Kim T.; Dykstra J.; Porada S.; van der Wal A.; Yoon J.; Biesheuvel P. Enhanced charge efficiency and reduced energy use in capacitive deionization by increasing the discharge voltage. J. Colloid Interface Sci. 2015, 446, 317–326. 10.1016/j.jcis.2014.08.041. [DOI] [PubMed] [Google Scholar]
- McNair R.; Szekely G.; Dryfe R. A. Ion-exchange materials for membrane capacitive deionization. ACS ES&T Water 2021, 1, 217–239. 10.1021/acsestwater.0c00123. [DOI] [Google Scholar]
- Liu X.; Shanbhag S.; Natesakhawat S.; Whitacre J. F.; Mauter M. S. Performance Loss of Activated Carbon Electrodes in Capacitive Deionization: Mechanisms and Material Property Predictors. Environ. Sci. Technol. 2020, 54, 15516–15526. 10.1021/acs.est.0c06549. [DOI] [PubMed] [Google Scholar]
- Han L.; Karthikeyan K. G.; Gregory K. B. Energy Consumption and Recovery in Capacitive Deionization Using Nanoporous Activated Carbon Electrodes. J. Electrochem. Soc. 2015, 162, E282–E288. 10.1149/2.0431512jes. [DOI] [Google Scholar]
- Qin M.; Deshmukh A.; Epsztein R.; Patel S. K.; Owoseni O. M.; Walker W. S.; Elimelech M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. 10.1016/j.desal.2019.01.003. [DOI] [Google Scholar]
- Qin M.; Deshmukh A.; Epsztein R.; Patel S. K.; Owoseni O. M.; Walker W. S.; Elimelech M. Response to comments on “comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 462, 48–55. 10.1016/j.desal.2019.04.004. [DOI] [Google Scholar]
- Ramachandran A.; Oyarzun D. I.; Hawks S. A.; Campbell P. G.; Stadermann M.; Santiago J. G.. Comments on “Comparison of Energy Consumption in Desalination by Capacitive Deionization and Reverse Osmosis”. Desalination, 2019, 461, 30–36. [Google Scholar]
- Tan C.; He C.; Fletcher J.; Waite T. D. Energy recovery in pilot scale membrane CDI treatment of brackish waters. Water Res. 2020, 168, 115146. 10.1016/j.watres.2019.115146. [DOI] [PubMed] [Google Scholar]
- García-Quismondo E.; Santos C.; Soria J.; Palma J.; Anderson M. A. New Operational Modes to Increase Energy Efficiency in Capacitive Deionization Systems. Environ. Sci. Technol. 2016, 50, 6053–6060. 10.1021/acs.est.5b05379. [DOI] [PubMed] [Google Scholar]
- Subramani A.; Jacangelo J. G. Emerging desalination technologies for water treatment: a critical review. Water research 2015, 75, 164–187. 10.1016/j.watres.2015.02.032. [DOI] [PubMed] [Google Scholar]
- Jia B.; Zhang W. Preparation and application of electrodes in capacitive deionization (CDI): a state-of-art review. Nanoscale Res. Lett. 2016, 11, 64. 10.1186/s11671-016-1284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duduta M.; Ho B.; Wood V. C.; Limthongkul P.; Brunini V. E.; Carter W. C.; Chiang Y. M. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 2011, 1 (4), 511–516. [Google Scholar]
- Park M.; Ryu J.; Wang W.; Cho J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2017, 2 (1), 1–18. [Google Scholar]
- Chen H.; Lu Y. C. A High-Energy-Density Multiple Redox Semi-Solid-Liquid Flow Battery. Adv. Energy Mater. 2016, 6 (8), 150–183. [Google Scholar]
- Narayanan T. M.; Zhu Y. G.; Gencer E.; McKinley G.; Shao-Horn Y. Low-cost manganese dioxide semi-solid electrode for flow batteries. Joule 2021, 5 (11), 2934–2954. [Google Scholar]
- Jeon S.-i.; Yeo J.-g.; Yang S.; Choi J.; Kim D. K. Ion storage and energy recovery of a flow-electrode capacitive deionization process. Journal of Materials Chemistry A 2014, 2, 6378–6383. 10.1039/c4ta00377b. [DOI] [Google Scholar]
- Hatzell K. B.; Iwama E.; Ferris A.; Daffos B.; Urita K.; Tzedakis T.; Chauvet F.; Taberna P.-L.; Gogotsi Y.; Simon P. Capacitive deionization concept based on suspension electrodes without ion exchange membranes. Electrochem. Commun. 2014, 43, 18–21. 10.1016/j.elecom.2014.03.003. [DOI] [Google Scholar]
- Rommerskirchen A.; Gendel Y.; Wessling M. Single module flow-electrode capacitive deionization for continuous water desalination. Electrochem. Commun. 2015, 60, 34–37. 10.1016/j.elecom.2015.07.018. [DOI] [Google Scholar]
- Zhang C.; Wu L.; Ma J.; Pham A. N.; Wang M.; Waite T. D. Integrated Flow-Electrode Capacitive Deionization and Microfiltration System for Continuous and Energy-Efficient Brackish Water Desalination. Environ. Sci. Technol. 2019, 53, 13364–13373. 10.1021/acs.est.9b04436. [DOI] [PubMed] [Google Scholar]
- Rommerskirchen A.; Linnartz C. J.; Müller D.; Willenberg L. K.; Wessling M. Energy Recovery and Process Design in Continuous Flow-Electrode Capacitive Deionization Processes. ACS Sustainable Chem. Eng. 2018, 6, 13007–13015. 10.1021/acssuschemeng.8b02466. [DOI] [Google Scholar]
- Ma J.; Liang P.; Sun X.; Zhang H.; Bian Y.; Yang F.; Bai J.; Gong Q.; Huang X. Energy recovery from the flow-electrode capacitive deionization. J. Power Sources 2019, 421, 50–55. 10.1016/j.jpowsour.2019.02.082. [DOI] [Google Scholar]
- Zhang C.; Ma J.; Wu L.; Sun J.; Wang L.; Li T.; Waite T. D. Flow Electrode Capacitive Deionization (FCDI): Recent Developments, Environmental Applications, and Future Perspectives. Environ. Sci. Technol. 2021, 55, 4243–4267. 10.1021/acs.est.0c06552. [DOI] [PubMed] [Google Scholar]
- Yang F.; He Y.; Rosentsvit L.; Suss M. E.; Zhang X.; Gao T.; Liang P. Flow-electrode capacitive deionization: A review and new perspectives. Water Res. 2021, 200, 117222. 10.1016/j.watres.2021.117222. [DOI] [PubMed] [Google Scholar]
- Rommerskirchen A.; Linnartz C. J.; Egidi F.; Kendir S.; Wessling M. Flow-electrode capacitive deionization enables continuous and energy-efficient brine concentration. Desalination 2020, 490, 114453. 10.1016/j.desal.2020.114453. [DOI] [Google Scholar]
- Bian Y.; Chen X.; Lu L.; Liang P.; Ren Z. J. Concurrent nitrogen and phosphorus recovery using flow-electrode capacitive deionization. ACS Sustainable Chem. Eng. 2019, 7, 7844–7850. 10.1021/acssuschemeng.9b00065. [DOI] [Google Scholar]
- Xu L.; Yu C.; Tian S.; Mao Y.; Zong Y.; Zhang X.; Zhang B.; Zhang C.; Wu D. Selective recovery of phosphorus from synthetic urine using flow-electrode capacitive deionization (FCDI)-based technology. ACS ES&T Water 2021, 1, 175–184. 10.1021/acsestwater.0c00065. [DOI] [Google Scholar]
- Yu C.; Xu L.; Mao Y.; Zong Y.; Wu D. Efficient recovery of carboxylates from the effluents treated by advanced oxidation processes using flow-electrode capacitive deionization in short-circuited closed-cycle operation. Sep. Purif. Technol. 2021, 275, 119151. 10.1016/j.seppur.2021.119151. [DOI] [Google Scholar]
- Fang K.; Peng F.; San E.; Wang K. The impact of concentration in electrolyte on ammonia removal in flow-electrode capacitive deionization system. Sep. Purif. Technol. 2021, 255, 117337. 10.1016/j.seppur.2020.117337. [DOI] [Google Scholar]
- Cohen H.; Eli S. E.; Jõgi M.; Suss M. E. Suspension Electrodes Combining Slurries and Upflow Fluidized Beds. ChemSusChem 2016, 9, 3045–3048. 10.1002/cssc.201601008. [DOI] [PubMed] [Google Scholar]
- Nativ P.; Badash Y.; Gendel Y. New insights into the mechanism of flow-electrode capacitive deionization. Electrochem. Commun. 2017, 76, 24–28. 10.1016/j.elecom.2017.01.008. [DOI] [Google Scholar]
- Gendel Y.; Rommerskirchen A. K. E.; David O.; Wessling M. Batch mode and continuous desalination of water using flowing carbon deionization (FCDI) technology. Electrochem. Commun. 2014, 46, 152–156. 10.1016/j.elecom.2014.06.004. [DOI] [Google Scholar]
- Ma J.; He D.; Tang W.; Kovalsky P.; He C.; Zhang C.; Waite T. Development of redox-active flow electrodes for high-performance capacitive deionization. Environ. Sci. Technol. 2016, 50, 13495–13501. 10.1021/acs.est.6b03424. [DOI] [PubMed] [Google Scholar]
- Yang S.; Park H.-r.; Yoo J.; Kim H.; Choi J.; Han M. H.; Kim D. K. Plate-Shaped Graphite for Improved Performance of Flow-Electrode Capacitive Deionization. J. Electrochem. Soc. 2017, 164, E480–E488. 10.1149/2.1551713jes. [DOI] [Google Scholar]
- Liang P.; Sun X.; Bian Y.; Zhang H.; Yang X.; Jiang Y.; Liu P.; Huang X. Optimized desalination performance of high voltage flow-electrode capacitive deionization by adding carbon black in flow-electrode. Desalination 2017, 420, 63–69. 10.1016/j.desal.2017.05.023. [DOI] [Google Scholar]
- Akuzum B.; Agartan L.; Locco J.; Kumbur E. Effects of particle dispersion and slurry preparation protocol on electrochemical performance of capacitive flowable electrodes. J. Appl. Electrochem. 2017, 47, 369–380. 10.1007/s10800-017-1046-5. [DOI] [Google Scholar]
- Akuzum B.; Singh P.; Eichfeld D. A.; Agartan L.; Uzun S.; Gogotsi Y.; Kumbur E. C. Percolation characteristics of conductive additives for capacitive flowable (semi-solid) electrodes. ACS Appl. Mater. Interfaces 2020, 12, 5866–5875. 10.1021/acsami.9b19739. [DOI] [PubMed] [Google Scholar]
- Halfon E. B.; Suss M. E. Measurements of the electric conductivity of an electrode as it transitions between static and flowable modes. Electrochem. Commun. 2019, 99, 61–64. 10.1016/j.elecom.2018.12.016. [DOI] [Google Scholar]
- Yang S.; Jeon S. I.; Kim H.; Choi J.; Yeo J. G.; Park H. R.; Kim D. K. Stack Design and Operation for Scaling Up the Capacity of Flow-Electrode Capacitive Deionization Technology. ACS Sustainable Chem. Eng. 2016, 4, 4174–4180. 10.1021/acssuschemeng.6b00689. [DOI] [Google Scholar]
- Cho Y.; Lee K. S.; Yang S.; Choi J.; Park H.-r.; Kim D. K. A novel three-dimensional desalination system utilizing honeycomb-shaped lattice structures for flow-electrode capacitive deionization. Energy Environ. Sci. 2017, 10, 1746–1750. 10.1039/C7EE00698E. [DOI] [Google Scholar]
- Ma J.; Ma J.; Zhang C.; Song J.; Dong W.; Waite T. D. Flow-electrode capacitive deionization (FCDI) scale-up using a membrane stack configuration. Water research 2020, 168, 115186. 10.1016/j.watres.2019.115186. [DOI] [PubMed] [Google Scholar]
- Linnartz C. J.; Rommerskirchen A.; Walker J.; Plankermann-Hajduk J.; Köller N.; Wessling M. Membrane-electrode assemblies for flow-electrode capacitive deionization. J. Membr. Sci. 2020, 605, 118095. 10.1016/j.memsci.2020.118095. [DOI] [Google Scholar]
- Linnartz C. J.; Rommerskirchen A.; Wessling M.; Gendel Y. Flow-Electrode Capacitive Deionization for Double Displacement Reactions. ACS Sustainable Chem. Eng. 2017, 5, 3906–3912. 10.1021/acssuschemeng.6b03086. [DOI] [Google Scholar]
- Wang M.; Hou S.; Liu Y.; Xu X.; Lu T.; Zhao R.; Pan L. Capacitive neutralization deionization with flow electrodes. Electrochim. Acta 2016, 216, 211–218. 10.1016/j.electacta.2016.09.026. [DOI] [Google Scholar]
- Nativ P.; Lahav O.; Gendel Y. Separation of divalent and monovalent ions using flow-electrode capacitive deionization with nanofiltration membranes. Desalination 2018, 425, 123–129. 10.1016/j.desal.2017.10.026. [DOI] [Google Scholar]
- He C.; Ma J.; Zhang C.; Song J.; Waite T. D. Short-Circuited Closed-Cycle Operation of Flow-Electrode CDI for Brackish Water Softening. Environ. Sci. Technol. 2018, 52, 9350–9360. 10.1021/acs.est.8b02807. [DOI] [PubMed] [Google Scholar]
- Song J.; Ma J.; Zhang C.; He C.; Waite T. D. Implication of non-electrostatic contribution to deionization in flow-electrode CDI: Case study of nitrate removal from contaminated source waters. Front. Chem. 2019, 7, 146. 10.3389/fchem.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Zhang Y.; Collins R. N.; Tsarev S.; Aoyagi N.; Kinsela A. S.; Jones A. M.; Waite T. D. Flow-Electrode CDI Removes the Uncharged Ca-UO2-CO3 Ternary Complex from Brackish Potable Groundwater: Complex Dissociation, Transport, and Sorption. Environ. Sci. Technol. 2019, 53, 2739–2747. 10.1021/acs.est.8b07157. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Zhang J.; Luo K.; Xing W.; Du J.; Dong Y.; Li X.; Tang W. Effective fluoride removal from brackish groundwaters by flow-electrode capacitive deionization (FCDI) under a continuous-flow mode. Sci. Total Environ. 2022, 804, 150166. 10.1016/j.scitotenv.2021.150166. [DOI] [PubMed] [Google Scholar]
- Rommerskirchen A.; Alders M.; Wiesner F.; Linnartz C. J.; Kalde A.; Wessling M. Process model for high salinity flow-electrode capacitive deionization processes with ion-exchange membranes. J. Membr. Sci. 2020, 616, 118614. 10.1016/j.memsci.2020.118614. [DOI] [Google Scholar]
- Wang L.; Zhang C.; He C.; Waite T. D.; Lin S. Equivalent film-electrode model for flow-electrode capacitive deionization: Experimental validation and performance analysis. Water Res. 2020, 181, 115917. 10.1016/j.watres.2020.115917. [DOI] [PubMed] [Google Scholar]
- Rommerskirchen A.; Kalde A.; Linnartz C. J.; Bongers L.; Linz G.; Wessling M. Unraveling charge transport in carbon flow-electrodes: Performance prediction for desalination applications. Carbon 2019, 145, 507–520. 10.1016/j.carbon.2019.01.053. [DOI] [Google Scholar]
- Eifert L.; Jusys Z.; Behm R.; Zeis R. Side reactions and stability of pre-treated carbon felt electrodes for vanadium redox flow batteries: A DEMS study. Carbon 2020, 158, 580–587. 10.1016/j.carbon.2019.11.029. [DOI] [Google Scholar]
- Cohen I.; Avraham E.; Bouhadana Y.; Soffer A.; Aurbach D. Long term stability of capacitive de-ionization processes for water desalination: The challenge of positive electrodes corrosion. Electrochim. Acta 2013, 106, 91–100. 10.1016/j.electacta.2013.05.029. [DOI] [Google Scholar]
- Li B.; Zheng T.; Ran S.; Sun M.; Shang J.; Hu H.; Lee P. H.; Boles S. T. Performance Recovery in Degraded Carbon-Based Electrodes for Capacitive Deionization. Environ. Sci. Technol. 2020, 54, 1848–1856. 10.1021/acs.est.9b04749. [DOI] [PubMed] [Google Scholar]
- Uwayid R.; Diesendruck C. E.; Suss M. E. Emerging investigator series: a comparison of strong and weak-acid functionalized carbon electrodes in capacitive deionization. Environ. Sci.: Water Res. Technol. 2022, 8, 949–956. 10.1039/D1EW00967B. [DOI] [Google Scholar]
- Srimuk P.; Zeiger M.; Jäckel N.; Tolosa A.; Krüner B.; Fleischmann S.; Grobelsek I.; Aslan M.; Shvartsev B.; Suss M. E.; Presser V. Enhanced performance stability of carbon/titania hybrid electrodes during capacitive deionization of oxygen saturated saline water. Electrochim. Acta 2017, 224, 314–328. 10.1016/j.electacta.2016.12.060. [DOI] [Google Scholar]
- Cohen I.; Avraham E.; Bouhadana Y.; Soffer A.; Aurbach D. The effect of the flow-regime, reversal of polarization, and oxygen on the long term stability in capacitive de-ionization processes. Electrochim. Acta 2015, 153, 106–114. 10.1016/j.electacta.2014.12.007. [DOI] [Google Scholar]
- Gao X.; Omosebi A.; Landon J.; Liu K. Voltage-Based Stabilization of Microporous Carbon Electrodes for Inverted Capacitive Deionization. J. Phys. Chem. C 2018, 122, 1158–1168. 10.1021/acs.jpcc.7b08968. [DOI] [Google Scholar]
- Lu D.; Cai W.; Wang Y. Optimization of the voltage window for long-term capacitive deionization stability. Desalination 2017, 424, 53–61. 10.1016/j.desal.2017.09.026. [DOI] [Google Scholar]
- Lee J.-H.; Bae W.-S.; Choi J.-H. Electrode reactions and adsorption/desorption performance related to the applied potential in a capacitive deionization process. Desalination 2010, 258, 159–163. 10.1016/j.desal.2010.03.020. [DOI] [Google Scholar]
- Gao X.; Omosebi A.; Landon J.; Liu K. Surface charge enhanced carbon electrodes for stable and efficient capacitive deionization using inverted adsorption–desorption behavior. Energy Environ. Sci. 2015, 8, 897–909. 10.1039/C4EE03172E. [DOI] [Google Scholar]
- Gao X.; Omosebi A.; Holubowitch N.; Landon J.; Liu K. Capacitive Deionization Using Alternating Polarization: Effect of Surface Charge on Salt Removal. Electrochim. Acta 2017, 233, 249–255. 10.1016/j.electacta.2017.03.021. [DOI] [Google Scholar]
- Lee J.; Kim S.; Kim C.; Yoon J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. 10.1039/C4EE02378A. [DOI] [Google Scholar]
- Kim T.; Yu J.; Kim C.; Yoon J. Hydrogen peroxide generation in fl ow-mode capacitive deionization. J. Electroanal. Chem. 2016, 776, 101–104. 10.1016/j.jelechem.2016.07.001. [DOI] [Google Scholar]
- Mossad M.; Zou L. Study of fouling and scaling in capacitive deionisation by using dissolved organic and inorganic salts. Journal of hazardous materials 2013, 244, 387–393. 10.1016/j.jhazmat.2012.11.062. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Mossad M.; Zou L. A study of the long-term operation of capacitive deionisation in inland brackish water desalination. Desalination 2013, 320, 80–85. 10.1016/j.desal.2013.04.010. [DOI] [Google Scholar]
- Wang C.; Song H.; Zhang Q.; Wang B.; Li A. Parameter optimization based on capacitive deionization for highly efficient desalination of domestic wastewater biotreated effluent and the fouled electrode regeneration. Desalination 2015, 365, 407–415. 10.1016/j.desal.2015.03.025. [DOI] [Google Scholar]
- Liu X.; Whitacre J. F.; Mauter M. S. Mechanisms of humic acid fouling on capacitive and insertion electrodes for electrochemical desalination. Environ. Sci. Technol. 2018, 52, 12633–12641. 10.1021/acs.est.8b03261. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zhang C.; Bai L.; Xie B.; Gan Z.; Xing J.; Li G.; Liang H. Scaling behavior of iron in capacitive deionization (CDI) system. Water research 2020, 171, 115370. 10.1016/j.watres.2019.115370. [DOI] [PubMed] [Google Scholar]
- Wang T.; Bai L.; Zhang C.; Zhu X.; Xing J.; Guo Y.; Wang J.; Lin D.; Li G.; Liang H. Formation mechanism of iron scale in membrane capacitive deionization (MCDI) system. Desalination 2020, 495, 114636. 10.1016/j.desal.2020.114636. [DOI] [Google Scholar]
- Chen L.; Wang C.; Liu S.; Hu Q.; Zhu L.; Cao C. Investigation of the long-term desalination performance of membrane capacitive deionization at the presence of organic foulants. Chemosphere 2018, 193, 989–997. 10.1016/j.chemosphere.2017.11.130. [DOI] [PubMed] [Google Scholar]
- Li Y.; Stewart T. C.; Tang H. L. A comparative study on electrosorptive rates of metal ions in capacitive deionization. Journal of water process engineering 2018, 26, 257–263. 10.1016/j.jwpe.2018.10.021. [DOI] [Google Scholar]
- Zhang W.; Jia B. Toward anti-fouling capacitive deionization by using visible-light reduced TiO2/graphene nanocomposites. MRS Commun. 2015, 5, 613–617. 10.1557/mrc.2015.65. [DOI] [Google Scholar]
- Liu E.; Lee L. Y.; Ong S. L.; Ng H. Y. Treatment of industrial brine using capacitive deionization (CDI) towards zero liquid discharge–challenges and optimization. Water Res. 2020, 183, 116059. 10.1016/j.watres.2020.116059. [DOI] [PubMed] [Google Scholar]
- Porada S.; Weinstein L.; Dash R.; Van Der Wal A.; Bryjak M.; Gogotsi Y.; Biesheuvel P. Water desalination using capacitive deionization with microporous carbon electrodes. ACS Appl. Mater. Interfaces 2012, 4, 1194–1199. 10.1021/am201683j. [DOI] [PubMed] [Google Scholar]
- Porada S.; Borchardt L.; Oschatz M.; Bryjak M.; Atchison J.; Keesman K.; Kaskel S.; Biesheuvel P.; Presser V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. 10.1039/c3ee42209g. [DOI] [Google Scholar]
- Huang Z.-H.; Yang Z.; Kang F.; Inagaki M. Carbon electrodes for capacitive deionization. Journal of Materials Chemistry A 2017, 5, 470–496. 10.1039/C6TA06733F. [DOI] [Google Scholar]
- Ratajczak P.; Suss M. E.; Kaasik F.; Béguin F. Carbon electrodes for capacitive technologies. Energy Storage Materials 2019, 16, 126–145. 10.1016/j.ensm.2018.04.031. [DOI] [Google Scholar]
- Li B.; Zheng T.; Ran S.; Lee P.-H.; Liu B.; Boles S. T. Role of metastable-adsorbed charges in the stability degradation of carbon-based electrodes for capacitive deionization. Environ. Sci.: Water Res.; Technol. 2018, 4, 1172–1180. 10.1039/C8EW00323H. [DOI] [Google Scholar]
- Singh K.; Porada S.; de Gier H.; Biesheuvel P.; de Smet L. Timeline on the application of intercalation materials in Capacitive Deionization. Desalination 2019, 455, 115–134. 10.1016/j.desal.2018.12.015. [DOI] [Google Scholar]
- Han B.; Cheng G.; Wang Y.; Wang X. Structure and functionality design of novel carbon and faradaic electrode materials for high-performance capacitive deionization. Chemical Engineering Journal 2019, 360, 364–384. 10.1016/j.cej.2018.11.236. [DOI] [Google Scholar]
- Srimuk P.; Halim J.; Lee J.; Tao Q.; Rosen J.; Presser V. Two-dimensional molybdenum carbide (MXene) with divacancy ordering for brackish and seawater desalination via cation and anion intercalation. ACS Sustainable Chem. Eng. 2018, 6, 3739–3747. 10.1021/acssuschemeng.7b04095. [DOI] [Google Scholar]
- Bao W.; Tang X.; Guo X.; Choi S.; Wang C.; Gogotsi Y.; Wang G. Porous cryo-dried MXene for efficient capacitive deionization. Joule 2018, 2, 778–787. 10.1016/j.joule.2018.02.018. [DOI] [Google Scholar]
- Shen X.; Xiong Y.; Hai R.; Yu F.; Ma J. An All-MXene-Based Integrated Membrane Electrode Constructed using Ti3C2Tx as an Intercalating Agent for High Performance Desalination. Environ. Sci. Technol. 2020, 54, 4554–4563. 10.1021/acs.est.9b05759. [DOI] [PubMed] [Google Scholar]
- Acerce M.; Voiry D.; Chhowalla M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nature Nanotechnol. 2015, 10, 313–318. 10.1038/nnano.2015.40. [DOI] [PubMed] [Google Scholar]
- Srimuk P.; Lee J.; Fleischmann S.; Choudhury S.; Jackel N.; Zeiger M.; Kim C.; Aslan M.; Presser V. Faradaic deionization of brackish and sea water via pseudocapacitive cation and anion intercalation into few-layered molybdenum disulfide. Journal of Materials Chemistry A 2017, 5, 15640–15649. 10.1039/C7TA03120C. [DOI] [Google Scholar]
- Xing F.; Li T.; Li J.; Zhu H.; Wang N.; Cao X. Chemically exfoliated MoS2 for capacitive deionization of saline water. Nano Energy 2017, 31, 590–595. 10.1016/j.nanoen.2016.12.012. [DOI] [Google Scholar]
- Cai Y.; Zhang W.; Fang R.; Zhao D.; Wang Y.; Wang J. Well-dispersed few-layered MoS2 connected with robust 3D conductive architecture for rapid capacitive deionization process and its specific ion selectivity. Desalination 2021, 520, 115325. 10.1016/j.desal.2021.115325. [DOI] [Google Scholar]
- Huang S.; Mochalin V. N. Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal Solutions. Inorg. Chem. 2019, 58, 1958–1966. 10.1021/acs.inorgchem.8b02890. [DOI] [PubMed] [Google Scholar]
- Verger L.; Natu V.; Ghidiu M.; Barsoum M. W. Effect of Cationic Exchange on the Hydration and Swelling Behavior of Ti3C2Tz MXenes. J. Phys. Chem. C 2019, 123, 20044–20050. 10.1021/acs.jpcc.9b04546. [DOI] [Google Scholar]
- Kaasik F.; Tamm T.; Hantel M. M.; Perre E.; Aabloo A.; Lust E.; Bazant M. Z.; Presser V. Anisometric charge dependent swelling of porous carbon in an ionic liquid. Electrochemistry communications 2013, 34, 196–199. 10.1016/j.elecom.2013.06.011. [DOI] [Google Scholar]
- Han M. H.; Gonzalo E.; Singh G.; Rojo T. A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries. Energy Environ. Sci. 2015, 8, 81–102. 10.1039/C4EE03192J. [DOI] [Google Scholar]
- Ahn J.; Lee J.; Kim S.; Kim C.; Lee J.; Biesheuvel P.; Yoon J. High performance electrochemical saline water desalination using silver and silver-chloride electrodes. Desalination 2020, 476, 114216. 10.1016/j.desal.2019.114216. [DOI] [Google Scholar]
- Chen F.; Huang Y.; Guo L.; Ding M.; Yang H. Y. A dual-ion electrochemistry deionization system based on AgCl-Na0.44MnO2 electrodes. Nanoscale 2017, 9, 10101–10108. 10.1039/C7NR01861D. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Guo L.; Ding M.; Huang Y.; Yang H. Y. Ultrahigh-Desalination-Capacity Dual-Ion Electrochemical Deionization Device Based on Na3V2(PO4)3@C–AgCl Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 40540–40548. 10.1021/acsami.8b14014. [DOI] [PubMed] [Google Scholar]
- Nam D.-H.; Choi K.-S. Bismuth as a new chloride-storage electrode enabling the construction of a practical high capacity desalination battery. J. Am. Chem. Soc. 2017, 139, 11055–11063. 10.1021/jacs.7b01119. [DOI] [PubMed] [Google Scholar]
- Wu T.; Wang G.; Wang S.; Zhan F.; Fu Y.; Qiao H.; Qiu J. Highly stable hybrid capacitive deionization with a MnO2 anode and a positively charged cathode. Environmental Science & Technology Letters 2018, 5, 98–102. 10.1021/acs.estlett.7b00540. [DOI] [Google Scholar]
- Yang J.; Zou L.; Song H.; Hao Z. Development of novel MnO2/nanoporous carbon composite electrodes in capacitive deionization technology. Desalination 2011, 276, 199–206. 10.1016/j.desal.2011.03.044. [DOI] [Google Scholar]
- Compton R. G.; Banks C. E.. Understanding Voltammetry; World Scientific, 2018. [Google Scholar]
- Karyakin A. A. Prussian blue and its analogues: electrochemistry and analytical applications. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis 2001, 13, 813–819. . [DOI] [Google Scholar]
- Ma X.; Chen Y.-A.; Zhou K.; Wu P.-C.; Hou C.-H. Enhanced desalination performance via mixed capacitive-Faradaic ion storage using RuO2-activated carbon composite electrodes. Electrochim. Acta 2019, 295, 769–777. 10.1016/j.electacta.2018.10.180. [DOI] [Google Scholar]
- Ding M.; Fan S.; Huang S.; Pam M. E.; Guo L.; Shi Y.; Yang H. Y. Tunable Pseudocapacitive Behavior in Metal–Organic Framework-Derived TiO 2 @Porous Carbon Enabling High-Performance Membrane Capacitive Deionization. ACS Applied Energy Materials 2019, 2, 1812–1822. 10.1021/acsaem.8b01839. [DOI] [Google Scholar]
- Yu F.; Wang L.; Wang Y.; Shen X.; Cheng Y.; Ma J. Faradaic reactions in capacitive deionization for desalination and ion separation. Journal of Materials Chemistry A 2019, 7, 15999–16027. 10.1039/C9TA01264H. [DOI] [Google Scholar]
- Sood A.; Poletayev A. D.; Cogswell D. A.; Csernica P. M.; Mefford J. T.; Fraggedakis D.; Toney M. F.; Lindenberg A. M.; Bazant M. Z.; Chueh W. C. Electrochemical Ion Insertion: From Atoms to Devices. Nat. Rev. Mater. 2021, 6, 847–867. 10.1038/s41578-021-00314-y. [DOI] [Google Scholar]
- Kim S.; Lee J.; Kim C.; Yoon J. Na2FeP2O7 as a novel material for hybrid capacitive deionization. Electrochim. Acta 2016, 203, 265–271. 10.1016/j.electacta.2016.04.056. [DOI] [Google Scholar]
- Ferguson T. R.; Bazant M. Z. Nonequilibrium thermodynamics of porous electrodes. J. Electrochem. Soc. 2012, 159, A1967. 10.1149/2.048212jes. [DOI] [Google Scholar]
- Smith R. B.; Bazant M. Z. Multiphase porous electrode theory. J. Electrochem. Soc. 2017, 164, E3291 10.1149/2.0171711jes. [DOI] [Google Scholar]
- He F.; Biesheuvel P.; Bazant M. Z.; Hatton T. A. Theory of water treatment by capacitive deionization with redox active porous electrodes. Water research 2018, 132, 282–291. 10.1016/j.watres.2017.12.073. [DOI] [PubMed] [Google Scholar]
- La Mantia F.; Pasta M.; Deshazer H. D.; Logan B. E.; Cui Y. Batteries for Efficient Energy Extraction from a Water Salinity Difference. Nano Lett. 2011, 11, 1810–1813. 10.1021/nl200500s. [DOI] [PubMed] [Google Scholar]
- Shanbhag S.; Bootwala Y.; Whitacre J. F.; Mauter M. S. Ion transport and competition effects on NaTi2(PO4)3 and Na4Mn9O18 selective insertion electrode performance. Langmuir 2017, 33, 12580–12591. 10.1021/acs.langmuir.7b02861. [DOI] [PubMed] [Google Scholar]
- Ikeshoji T. Separation of alkali metal ions by intercalation into a prussian blue electrode. J. Electrochem. Soc. 1986, 133, 2108. 10.1149/1.2108350. [DOI] [Google Scholar]
- Bocarsly A. B.; Sinha S. Effects of surface structure on electrode charge transfer properties: Induction of ion selectivity at the chemically derivatized interface. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 1982, 140, 167–172. 10.1016/0368-1874(82)85310-0. [DOI] [Google Scholar]
- Deakin M. R.; Byrd H. Prussian Blue coated quartz crystal microbalance as a detector for electroinactive cations in aqueous solution. Anal. Chem. 1989, 61, 290–295. 10.1021/ac00179a003. [DOI] [Google Scholar]
- Rassat S. D.; Sukamto J. H.; Orth R. J.; Lilga M. A.; Hallen R. T. Development of an electrically switched ion exchange process for selective ion separations. Sep. Purif. Technol. 1999, 15, 207–222. 10.1016/S1383-5866(98)00102-6. [DOI] [Google Scholar]
- Erinmwingbovo C.; Palagonia M. S.; Brogioli D.; La Mantia F. Intercalation into a Prussian blue derivative from solutions containing two species of cations. ChemPhysChem 2017, 18, 917–925. 10.1002/cphc.201700020. [DOI] [PubMed] [Google Scholar]
- Singh K.; Li G.; Lee J.; Zuilhof H.; Mehdi B. L.; Zornitta R. L.; de Smet L. C. Divalent Ion Selectivity in Capacitive Deionization with Vanadium Hexacyanoferrate: Experiments and Quantum-Chemical Computations. Adv. Funct. Mater. 2021, 31, 2105203. 10.1002/adfm.202105203. [DOI] [Google Scholar]
- Lee J.; Srimuk P.; Aristizabal K.; Kim C.; Choudhury S.; Nah Y.-C.; Mücklich F.; Presser V. Pseudocapacitive Desalination of Brackish Water and Seawater with Vanadium-Pentoxide-Decorated Multiwalled Carbon Nanotubes. ChemSusChem 2017, 10, 3611–3623. 10.1002/cssc.201701215. [DOI] [PubMed] [Google Scholar]
- Srimuk P.; Lee J.; Tolosa A.; Kim C.; Aslan M.; Presser V. Titanium Disulfide: A Promising Low-Dimensional Electrode Material for Sodium Ion Intercalation for Seawater Desalination. Chem. Mater. 2017, 29, 9964–9973. 10.1021/acs.chemmater.7b03363. [DOI] [Google Scholar]
- Tang X.; Liu H.; Su D.; Notten P. H.; Wang G. Hierarchical sodium-rich Prussian blue hollow nanospheres as high-performance cathode for sodium-ion batteries. Nano Research 2018, 11, 3979–3990. 10.1007/s12274-018-1979-y. [DOI] [Google Scholar]
- Huang Y.; Chen F.; Guo L.; Zhang J.; Chen T.; Yang H. Y. Low energy consumption dual-ion electrochemical deionization system using NaTi2 (PO4) 3-AgNPs electrodes. Desalination 2019, 451, 241–247. 10.1016/j.desal.2018.02.006. [DOI] [Google Scholar]
- Byles B. W.; Cullen D. A.; More K. L.; Pomerantseva E. Tunnel structured manganese oxide nanowires as redox active electrodes for hybrid capacitive deionization. Nano Energy 2018, 44, 476–488. 10.1016/j.nanoen.2017.12.015. [DOI] [Google Scholar]
- Su X.; Tan K. J.; Elbert J.; Ruttiger C.; Gallei M.; Jamison T. F.; Hatton T. A. Asymmetric Faradaic systems for selective electrochemical separations. Energy Environ. Sci. 2017, 10, 1272–1283. 10.1039/C7EE00066A. [DOI] [Google Scholar]
- He F.; Bazant M. Z.; Hatton T. A.. Equivalent Circuit Model for Electrosorption with Redox Active Materials. arXiv 2020, arXiv:2101.00091, 10.48550/arXiv.2101.00091. [DOI] [Google Scholar]
- He F.; Bazant M. Z.; Hatton T. A. Theory of Faradaically Modulated Redox Active Electrodes for Electrochemically Mediated Selective Adsorption Processes. J. Electrochem. Soc. 2021, 168, 053501. 10.1149/1945-7111/abfefd. [DOI] [Google Scholar]
- Ferguson T. R.; Bazant M. Z. Phase transformation dynamics in porous battery electrodes. Electrochim. Acta 2014, 146, 89–97. 10.1016/j.electacta.2014.08.083. [DOI] [Google Scholar]
- Smith R. B.; Khoo E.; Bazant M. Z. Intercalation kinetics in multiphase-layered materials. J. Phys. Chem. C 2017, 121, 12505–12523. 10.1021/acs.jpcc.7b00185. [DOI] [Google Scholar]
- Pothanamkandathil V.; Fortunato J.; Gorski C. A. Electrochemical Desalination Using Intercalating Electrode Materials: A Comparison of Energy Demands. Environ. Sci. Technol. 2020, 54, 3653–3662. 10.1021/acs.est.9b07311. [DOI] [PubMed] [Google Scholar]
- Metzger M.; Besli M. M.; Kuppan S.; Hellstrom S.; Kim S.; Sebti E.; Subban C. V.; Christensen J. Techno-economic analysis of capacitive and intercalative water deionization. Energy Environ. Sci. 2020, 13, 1544–1560. 10.1039/D0EE00725K. [DOI] [Google Scholar]
- Bai P.; Bazant M. Z. Charge transfer kinetics at the solid–solid interface in porous electrodes. Nat. Commun. 2014, 5, 3585. 10.1038/ncomms4585. [DOI] [PubMed] [Google Scholar]
- Marcus R. A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599. 10.1103/RevModPhys.65.599. [DOI] [Google Scholar]
- Kuznetsov A. M.; Ulstrup J.. Electron Transfer in Chemistry and Biology: An Introduction to the Theory; John Wiley & Sons Ltd, 1999. [Google Scholar]
- Chidsey C. E. D. Free-Energy and Temperature-Dependence of Electron-Transfer at the Metal-Electrolyte Interface. Science 1991, 251, 919–922. 10.1126/science.251.4996.919. [DOI] [PubMed] [Google Scholar]
- Zeng Y.; Smith R. B.; Bai P.; Bazant M. Z. Simple formula for Marcus–Hush–Chidsey kinetics. J. Electroanal. Chem. 2014, 735, 77–83. 10.1016/j.jelechem.2014.09.038. [DOI] [Google Scholar]
- Fraggedakis D.; McEldrew M.; Smith R. B.; Krishnan Y.; Zhang Y.; Bai P.; Chueh W. C.; Shao-Horn Y.; Bazant M. Z. Theory of coupled ion-electron transfer kinetics. Electrochim. Acta 2021, 367, 137432. 10.1016/j.electacta.2020.137432. [DOI] [Google Scholar]
- Fraggedakis D.; Bazant M. Z. Tuning the stability of electrochemical interfaces by electron transfer reactions. J. Chem. Phys. 2020, 152, 184703. 10.1063/5.0006833. [DOI] [PubMed] [Google Scholar]
- Park K.-S.; Xiao P.; Kim S.-Y.; Dylla A.; Choi Y.-M.; Henkelman G.; Stevenson K. J.; Goodenough J. B. Enhanced charge-transfer kinetics by anion surface modification of LiFePO4. Chem. Mater. 2012, 24, 3212–3218. 10.1021/cm301569m. [DOI] [Google Scholar]
- Trócoli R.; Battistel A.; Mantia F. L. Selectivity of a Lithium-Recovery Process Based on LiFePO4. Chem.-Eur. J. 2014, 20, 9888–9891. 10.1002/chem.201403535. [DOI] [PubMed] [Google Scholar]
- Liu C.; Li Y.; Lin D.; Hsu P.-C.; Liu B.; Yan G.; Wu T.; Cui Y.; Chu S. Lithium Extraction from Seawater through Pulsed Electrochemical Intercalation. Joule 2020, 4, 1459–1469. 10.1016/j.joule.2020.05.017. [DOI] [Google Scholar]
- He X.; Kaur S.; Kostecki R. Mining Lithium from Seawater. Joule 2020, 4, 1357–1358. 10.1016/j.joule.2020.06.015. [DOI] [Google Scholar]
- Hong S.; Yoon H.; Lee J.; Kim C.; Kim S.; Lee J.; Lee C.; Yoon J. Selective phosphate removal using layered double hydroxide/reduced graphene oxide (LDH/rGO) composite electrode in capacitive deionization. J. Colloid Interface Sci. 2020, 564, 1–7. 10.1016/j.jcis.2019.12.068. [DOI] [PubMed] [Google Scholar]
- Seftel E.; Ciocarlan R.; Michielsen B.; Meynen V.; Mullens S.; Cool P. Insights into phosphate adsorption behavior on structurally modified ZnAl layered double hydroxides. Appl. Clay Sci. 2018, 165, 234–246. 10.1016/j.clay.2018.08.018. [DOI] [Google Scholar]
- He H.; Kang H.; Ma S.; Bai Y.; Yang X. High adsorption selectivity of ZnAl layered double hydroxides and the calcined materials toward phosphate. J. Colloid Interface Sci. 2010, 343, 225–231. 10.1016/j.jcis.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Park G.; Hong S.; Lee C.; Lee J.; Yoon J. Selective fluoride removal in capacitive deionization by reduced graphene oxide/hydroxyapatite composite electrode. J. Colloid Interface Sci. 2021, 581, 396–402. 10.1016/j.jcis.2020.07.108. [DOI] [PubMed] [Google Scholar]
- Yu X.; Tong S.; Ge M.; Zuo J. Removal of fluoride from drinking water by cellulose@ hydroxyapatite nanocomposites. Carbohydr. Polym. 2013, 92, 269–275. 10.1016/j.carbpol.2012.09.045. [DOI] [PubMed] [Google Scholar]
- Agartan L.; Hayes-Oberst B.; Byles B. W.; Akuzum B.; Pomerantseva E.; Kumbur E. C. Influence of operating conditions and cathode parameters on desalination performance of hybrid CDI systems. Desalination 2019, 452, 1–8. 10.1016/j.desal.2018.10.025. [DOI] [Google Scholar]
- Sauvage F.; Baudrin E.; Tarascon J.-M. Study of the potentiometric response towards sodium ions of Na0.44–xMnO2 for the development of selective sodium ion sensors. Sens. Actuators, B 2007, 120, 638–644. 10.1016/j.snb.2006.03.024. [DOI] [Google Scholar]
- Kim H.; Shakoor R. A.; Park C.; Lim S. Y.; Kim J.-S.; Jo Y. N.; Cho W.; Miyasaka K.; Kahraman R.; Jung Y.; Choi J. W. Na2FeP2O7 As a Promising Iron-Based Pyrophosphate Cathode for Sodium Rechargeable Batteries: A Combined Experimental and Theoretical Study. Adv. Funct. Mater. 2013, 23, 1147–1155. 10.1002/adfm.201201589. [DOI] [Google Scholar]
- Chang J.; Duan F.; Cao H.; Tang K.; Su C.; Li Y. Superiority of a novel flow-electrode capacitive deionization (FCDI) based on a battery material at high applied voltage. Desalination 2019, 468, 114080. 10.1016/j.desal.2019.114080. [DOI] [Google Scholar]
- Kim N.; Lee J.; Kim S.; Hong S. P.; Lee C.; Yoon J.; Kim C. Short review of multichannel membrane capacitive deionization: Principle, current status, and future prospect. Applied Sciences 2020, 10, 683. 10.3390/app10020683. [DOI] [Google Scholar]
- Srimuk P.; Husmann S.; Presser V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater. RSC Adv. 2019, 9, 14849–14858. 10.1039/C9RA02570G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu Tran N. A.; Phuoc N. M.; Yoon H.; Jung E.; Lee Y.-W.; Kang B.-G.; Kang H. S.; Yoo C.-Y.; Cho Y. Improved Desalination Performance of Flow-and Fixed-Capacitive Deionization using Redox-Active Quinone. ACS Sustainable Chem. Eng. 2020, 8, 16701–16710. 10.1021/acssuschemeng.0c06651. [DOI] [Google Scholar]
- Kim N.; Hong S. P.; Lee J.; Kim C.; Yoon J. High-desalination performance via redox couple reaction in the multichannel capacitive deionization system. ACS Sustainable Chem. Eng. 2019, 7, 16182–16189. 10.1021/acssuschemeng.9b03121. [DOI] [Google Scholar]
- Dai J.; Wang J.; Hou X.; Ru Q.; He Q.; Srimuk P.; Presser V.; Chen F. Dual-Zinc Electrode Electrochemical Desalination. ChemSusChem 2020, 13, 2792. 10.1002/cssc.202000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.; Wang J.; Feng C.; Ma J.; Waite T. D. Low energy consumption and mechanism study of redox flow desalination. Chem. Eng. J. 2020, 401, 126111. 10.1016/j.cej.2020.126111. [DOI] [Google Scholar]
- Yang S.; Choi J.; Yeo J.-g.; Jeon S.-i.; Park H.-r.; Kim D. Flow-electrode capacitive deionization using an aqueous electrolyte with a high salt concentration. Environ. Sci. Technol. 2016, 50, 5892–5899. 10.1021/acs.est.5b04640. [DOI] [PubMed] [Google Scholar]
- Ahn J.; Kim S.; Jeon S.-i.; Lee C.; Lee J.; Yoon J. Nafion-coated Prussian blue electrodes to enhance the stability and efficiency of battery desalination system. Desalination 2021, 500, 114778. 10.1016/j.desal.2020.114778. [DOI] [Google Scholar]
- Kim T.; Gorski C.; Logan B. Low energy desalination using battery electrode deionization. Environmental Science & Technology Letters 2017, 4, 444–449. 10.1021/acs.estlett.7b00392. [DOI] [Google Scholar]
- Wang L.; Mu C.; Li H.; Li F. A dual-function battery for desalination and energy storage. Inorganic Chemistry Frontiers 2018, 5, 2522–2526. 10.1039/C8QI00704G. [DOI] [Google Scholar]
- Lee J.; Lee J.; Ahn J.; Jo K.; Hong S.; Kim C.; Lee C.; Yoon J. Enhancement in desalination performance of battery electrodes via improved mass transport using a multichannel flow system. ACS Appl. Mater. Interfaces 2019, 11, 36580–36588. 10.1021/acsami.9b10003. [DOI] [PubMed] [Google Scholar]
- Nam D.-H.; Lumley M.; Choi K.-S. A Desalination Battery Combining Cu3[Fe(CN)6]2as a Na-Storage Electrode and Bi as a Cl-Storage Electrode Enabling Membrane-Free Desalination. Chem. Mater. 2019, 31, 1460–1468. 10.1021/acs.chemmater.9b00084. [DOI] [Google Scholar]
- Kim S.; Joo H.; Moon T.; Kim S.-H.; Yoon J. Rapid and selective lithium recovery from desalination brine using an electrochemical system. Environ. Sci.: Proc. Impacts 2019, 21, 667–676. 10.1039/C8EM00498F. [DOI] [PubMed] [Google Scholar]
- Pasta M.; Battistel A.; La Mantia F. Batteries for lithium recovery from brines. Energy Environ. Sci. 2012, 5, 9487–9491. 10.1039/c2ee22977c. [DOI] [Google Scholar]
- Li Q.; Zheng Y.; Xiao D.; Or T.; Gao R.; Li Z.; Feng M.; Shui L.; Zhou G.; Wang X.; Chen Z. Faradaic Electrodes Open a New Era for Capacitive Deionization. Adv. Sci. 2020, 7, 2002213. 10.1002/advs.202002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiba H.; Zettsu N.; Nakayama M.; Oishi S.; Teshima K. Defect formation energy in spinel LiNi0 · 5Mn1 · 5O4 – δ using ab initio DFT calculations. J. Phys. Chem. C 2015, 119, 9117–9124. 10.1021/acs.jpcc.5b01661. [DOI] [Google Scholar]
- Lawagon C.; Nisola G.; Cuevas R.; Torrejos R.; Kim H.; Lee S.-P.; Chung W.-J. Li1–xNi0.5Mn1.5O4/Ag for electrochemical lithium recovery from brine and its optimized performance via response surface methodology. Sep. Purif. Technol. 2019, 212, 416–426. 10.1016/j.seppur.2018.11.046. [DOI] [Google Scholar]
- Kim J.-S.; Lee Y.-H.; Choi S.; Shin J.; Dinh H.-C.; Choi J. An electrochemical cell for selective lithium capture from seawater. Environ. Sci. Technol. 2015, 49, 9415–9422. 10.1021/acs.est.5b00032. [DOI] [PubMed] [Google Scholar]
- Tang W.; Liang J.; He D.; Gong J.; Tang L.; Liu Z.; Wang D.; Zeng G. Various cell architectures of capacitive deionization: Recent advances and future trends. Water research 2019, 150, 225–251. 10.1016/j.watres.2018.11.064. [DOI] [PubMed] [Google Scholar]
- Kim N.; Lee J.; Hong S.; Lee C.; Kim C.; Yoon J. Performance analysis of the multi-channel membrane capacitive deionization with porous carbon electrode stacks. Desalination 2020, 479, 114315. 10.1016/j.desal.2020.114315. [DOI] [Google Scholar]
- Stock J. T.; Orna M. V.. Electrochemistry, Past and Present; ACS Publications, 1989; Vol. 390 10.1021/bk-1989-0390. [DOI] [Google Scholar]
- Zumdahl S. S.; DeCoste D. J.. Chemical Principles; Nelson Education, 2012. [Google Scholar]
- Bard A. J.; Faulkner L. R.. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, 2001. [Google Scholar]
- Netz R. R.; Orland H. Beyond Poisson-Boltzmann: Fluctuation effects and correlation functions. Eur. Phys. J. E 2000, 1, 203–214. 10.1007/s101890050023. [DOI] [Google Scholar]
- Attard P.Thermodynamics and Statistical Mechanics: Equilibrium by Entropy Maximisation; Academic Press, 2002. [Google Scholar]
- Zhang C. Y.; He D.; Ma J. X.; Tang W. W.; Waite T. D. Faradaic reactions in capacitive deionization (CDI) - problems and possibilities: A review. Water Res. 2018, 128, 314–330. 10.1016/j.watres.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Shukla A.; Sampath S.; Vijayamohanan K. Electrochemical supercapacitors: energy storage beyond batteries. Current Sci. 2000, 79, 1656–1661. [Google Scholar]
- Khattak A. M.; Ghazi Z. A.; Liang B.; Khan N. A.; Iqbal A.; Li L.; Tang Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. Journal of Materials Chemistry A 2016, 4, 16312–16317. 10.1039/C6TA05784E. [DOI] [Google Scholar]
- Armstrong F. A.; Hill H. A. O.; Walton N. J. Direct electrochemistry of redox proteins. Acc. Chem. Res. 1988, 21, 407–413. 10.1021/ar00155a004. [DOI] [Google Scholar]
- Chen P. H.; McCreery R. L. Control of electron transfer kinetics at glassy carbon electrodes by specific surface modification. Anal. Chem. 1996, 68, 3958–3965. 10.1021/ac960492r. [DOI] [Google Scholar]
- Drummond T. G.; Hill M. G.; Barton J. K. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192–1199. 10.1038/nbt873. [DOI] [PubMed] [Google Scholar]
- Kang X. H.; Wang J.; Wu H.; Aksay I. A.; Liu J.; Lin Y. H. Glucose Oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. 10.1016/j.bios.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Costentin C.; Robert M.; Saveant J. M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. 10.1039/C2CS35360A. [DOI] [PubMed] [Google Scholar]
- Solis B. H.; Hammes-Schiffer S. Proton-Coupled Electron Transfer in Molecular Electrocatalysis: Theoretical Methods and Design Principles. Inorg. Chem. 2014, 53, 6427–6443. 10.1021/ic5002896. [DOI] [PubMed] [Google Scholar]
- Lukatskaya M. R.; Dunn B.; Gogotsi Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. 10.1038/ncomms12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousse T.; Belanger D.; Long J. W. To Be or Not To Be Pseudocapacitive?. J. Electrochem. Soc. 2015, 162, A5185–A5189. 10.1149/2.0201505jes. [DOI] [Google Scholar]
- Gracia R.; Mecerreyes D. Polymers with redox properties: materials for batteries, biosensors and more. Polym. Chem. 2013, 4, 2206–2214. 10.1039/c3py21118e. [DOI] [Google Scholar]
- Lyons M. E. Electrocatalysis using electroactive polymers, electroactive composites and microheterogeneous systems. Analyst 1994, 119, 805–826. 10.1039/an9941900805. [DOI] [Google Scholar]
- Harris R. D.; Auletta J. T.; Motlagh S. A. M.; Lawless M. J.; Perri N. M.; Saxena S.; Weiland L. M.; Waldeck D. H.; Clark W. W.; Meyer T. Y. Chemical and electrochemical manipulation of mechanical properties in stimuli-responsive copper-cross-linked hydrogels. ACS Macro Lett. 2013, 2, 1095–1099. 10.1021/mz4004997. [DOI] [PubMed] [Google Scholar]
- Kim J.; Kim J. H.; Ariga K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. 10.1016/j.joule.2017.08.018. [DOI] [Google Scholar]
- Janoschka T.; Martin N.; Martin U.; Friebe C.; Morgenstern S.; Hiller H.; Hager M. D.; Schubert U. S. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 2015, 527, 78–81. 10.1038/nature15746. [DOI] [PubMed] [Google Scholar]
- Horie K.; Baron M.; Fox R. B.; He J.; Hess M.; Kahovec J.; Kitayama T.; Kubisa P.; Marechal E.; Mormann W.; Stepto R. F. T.; Tabak D.; Vohlidal J.; Wilks E. S.; Work W. J. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. 10.1351/pac200476040889. [DOI] [Google Scholar]
- Heeger A. J. Semiconducting polymers: the third generation. Chem. Soc. Rev. 2010, 39, 2354–2371. 10.1039/b914956m. [DOI] [PubMed] [Google Scholar]
- Yan C.; Zou L.; Short R. Single-walled carbon nanotubes and polyaniline composites for capacitive deionization. Desalination 2012, 290, 125–129. 10.1016/j.desal.2012.01.017. [DOI] [Google Scholar]
- Yan C.; Kanaththage Y. W.; Short R.; Gibson C. T.; Zou L. Graphene/Polyaniline nanocomposite as electrode material for membrane capacitive deionization. Desalination 2014, 344, 274–279. 10.1016/j.desal.2014.03.041. [DOI] [Google Scholar]
- Wang Y.; Zhang L.; Wu Y.; Xu S.; Wang J. Polypyrrole/carbon nanotube composites as cathode material for performance enhancing of capacitive deionization technology. Desalination 2014, 354, 62–67. 10.1016/j.desal.2014.09.021. [DOI] [Google Scholar]
- Gu X.; Yang Y.; Hu Y.; Hu M.; Huang J.; Wang C. Facile fabrication of graphene–polypyrrole–Mn composites as high-performance electrodes for capacitive deionization. Journal of Materials Chemistry A 2015, 3, 5866–5874. 10.1039/C4TA06646D. [DOI] [Google Scholar]
- Zornitta R. L.; García-Mateos F. J.; Lado J. J.; Rodríguez-Mirasol J.; Cordero T.; Hammer P.; Ruotolo L. A. High-performance activated carbon from polyaniline for capacitive deionization. Carbon 2017, 123, 318–333. 10.1016/j.carbon.2017.07.071. [DOI] [Google Scholar]
- Akhoury A.; Bromberg L.; Hatton T. A. Interplay of electron hopping and bounded diffusion during charge transport in redox polymer electrodes. J. Phys. Chem. B 2013, 117, 333–342. 10.1021/jp302157g. [DOI] [PubMed] [Google Scholar]
- Su X.; Kulik H. J.; Jamison T. F.; Hatton T. A. Anion-Selective Redox Electrodes: Electrochemically Mediated Separation with Heterogeneous Organometallic Interfaces. Adv. Funct. Mater. 2016, 26, 3394–3404. 10.1002/adfm.201600079. [DOI] [Google Scholar]
- Bruckenstein S.; Fensore A. T.; Hillman A. R. Time-resolved mono-anion, di-anion, and solvent transfers into a poly(vinylferrocene)-modified electrode. J. Electrochem. Soc. 1998, 145, L24–L26. 10.1149/1.1838269. [DOI] [Google Scholar]
- Jureviciute I.; Bruckenstein S.; Hillman A. R. Counter-ion specific effects on charge and solvent trapping in poly(vinylferrocene) films. J. Electroanal. Chem. 2000, 488, 73–81. 10.1016/S0022-0728(00)00190-X. [DOI] [Google Scholar]
- Schiavon G.; Zotti G.; Comisso N.; Berlin A.; Pagani G. Ion exchange in the electrochemical switching of polypyrroles in acetonitrile by the electrochemical quartz crystal microbalance. Electrolyte incorporation by hydrogen bonding of anions to pyrrole. J. Phys. Chem. 1994, 98, 4861–4864. 10.1021/j100069a015. [DOI] [Google Scholar]
- Arca M.; Mirkin M. V.; Bard A. J. Polymer films on electrodes. 26. Study of ion transport and electron transfer at polypyrrole films by scanning electrochemical microscopy. J. Phys. Chem. 1995, 99, 5040–5050. 10.1021/j100014a026. [DOI] [Google Scholar]
- Beer P. D.; Gale P. A.; Chen G. Z. Mechanisms of electrochemical recognition of cations, anions and neutral guest species by redox-active receptor molecules. Coord. Chem. Rev. 1999, 185–186, 3–36. 10.1016/S0010-8545(98)00246-X. [DOI] [Google Scholar]
- Beer P. D.; Gale P. A. Anion recognition and sensing: The state of the art and future perspectives. Angew. Chem., Int. Ed. 2001, 40, 486–516. . [DOI] [PubMed] [Google Scholar]
- Tan K.-J.; Su X.; Hatton T. A. An Asymmetric Iron-Based Redox-Active System for Electrochemical Separation of Ions in Aqueous Media. Adv. Funct. Mater. 2020, 30, 1910363. 10.1002/adfm.201910363. [DOI] [Google Scholar]
- Pickup P. G. Conjugated metallopolymers. Redox polymers with interacting metal based redox sites. J. Mater. Chem. 1999, 9, 1641–1653. 10.1039/a902244i. [DOI] [Google Scholar]
- Ramanavičius A.; Ramanavičienė A.; Malinauskas A. Electrochemical sensors based on conducting polymer—polypyrrole. Electrochimica acta 2006, 51, 6025–6037. 10.1016/j.electacta.2005.11.052. [DOI] [Google Scholar]
- Lange U.; Roznyatovskaya N. V.; Mirsky V. M. Conducting polymers in chemical sensors and arrays. Analytica chimica acta 2008, 614, 1–26. 10.1016/j.aca.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Pan L.; Yu G.; Zhai D.; Lee H. R.; Zhao W.; Liu N.; Wang H.; Tee B. C.-K.; Shi Y.; Cui Y.; Bao Z. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 9287–9292. 10.1073/pnas.1202636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenscheid M. W.; Ghatakroy A. R.; Moore R. B.; Penner R. M.; Szentirmay M. N.; Martin C. R. Sensors from polymer modified electrodes. Journal of the Chemical Society-Faraday Transactions I 1986, 82, 1051–1070. 10.1039/f19868201051. [DOI] [Google Scholar]
- Toniolo R.; Comisso N.; Bontempelli G.; Schiavon G. Potential Shifts at Electrodes Coated with Ion-Exchange Polymeric Films. Talanta 1994, 41, 473–478. 10.1016/0039-9140(94)80154-1. [DOI] [PubMed] [Google Scholar]
- Ugo P.; Moretto L. M. Ion-exchange voltammetry at polymer-coated electrodes: Principles and analytical prospects. Electroanalysis 1995, 7, 1105–1113. 10.1002/elan.1140071202. [DOI] [Google Scholar]
- Espenscheid M. W.; Martin C. R. Electroactive ion-exchange polymers. J. Electroanal. Chem. Interfacial Chem. 1985, 188, 73–84. 10.1016/S0022-0728(85)80051-6. [DOI] [Google Scholar]
- Martin C. R.; Espenscheid M. W.; Ghatakroy A. R. The electrochemistry of electroactive ionomers. J. Electrochem. Soc. 1986, 133, C128. [Google Scholar]
- Kang E. T.; Neoh K. G.; Tan K. L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. 10.1016/S0079-6700(97)00030-0. [DOI] [Google Scholar]
- Lin Y. H.; Cui X. L.; Bontha J. Electrically controlled anion exchange based on polypyrrole and carbon nanotubes nanocomposite for perchlorate removal. Environ. Sci. Technol. 2006, 40, 4004–4009. 10.1021/es052148u. [DOI] [PubMed] [Google Scholar]
- Anson F. C.; Ohsaka T.; Saveant J. M. Kinetics of Electron-Transfer Cross-Reactions within Redox Polymers - Coatings of a Protonated Polylysine Co-Polymer with Incorporated Electroactive Anions. J. Am. Chem. Soc. 1983, 105, 4883–4890. 10.1021/ja00353a007. [DOI] [Google Scholar]
- Watanabe M.; Longmire M. L.; Murray R. W. A study of ferrocene diffusion dynamics in network poly (ethylene oxide) polymer electrolyte by solid-state voltammetry. J. Phys. Chem. 1990, 94, 2614–2619. 10.1021/j100369a071. [DOI] [Google Scholar]
- Hillman A. R.; Hughes N. A.; Bruckenstein S. Solvation phenomena in polyvinylferrocene films - effect of history and redox state. J. Electrochem. Soc. 1992, 139, 74–77. 10.1149/1.2069204. [DOI] [Google Scholar]
- Bruce J. A.; Wrighton M. S. Electrostatic Binding of Electroactive and Non-Electroactive Anions in a Surface-Confined, Electroactive Polymer - Selectivity of Binding Measured by Auger-Spectroscopy and Cyclic Voltammetry. J. Am. Chem. Soc. 1982, 104, 74–82. 10.1021/ja00365a017. [DOI] [Google Scholar]
- Martin C. R.; Rubinstein I.; Bard A. J. Polymer films on electrodes. 9. Electron and mass transfer in Nafion films containing tris (2, 2’-bipyridine) ruthenium (2+). J. Am. Chem. Soc. 1982, 104, 4817–4824. 10.1021/ja00382a014. [DOI] [Google Scholar]
- Shi M. L.; Anson F. C. High sensitivity of electron transfer rates within nafion coatings saturated with Os(bpy)(3)(2+) to the extent of hydration of the coating. J. Electrochem. Soc. 1995, 142, 4205–4214. 10.1149/1.2048485. [DOI] [Google Scholar]
- Hardy C. G.; Zhang J.; Yan Y.; Ren L.; Tang C. Metallopolymers with transition metals in the side-chain by living and controlled polymerization techniques. Prog. Polym. Sci. 2014, 39, 1742–1796. 10.1016/j.progpolymsci.2014.03.002. [DOI] [Google Scholar]
- Gallei M.; Elbert J.. Functional Metallosupramolecular Materials; Royal Society of Chemistry, 2015; pp 120–148. [Google Scholar]
- Gallei M.; Rüttiger C. Recent Trends in Metallopolymer Design: Redox-Controlled Surfaces, Porous Membranes, and Switchable Optical Materials Using Ferrocene-Containing Polymers. Chem.–Eur. J. 2018, 24, 10006–10021. 10.1002/chem.201800412. [DOI] [PubMed] [Google Scholar]
- Mao X.; Rutledge G. C.; Hatton T. A. Polyvinylferrocene for noncovalent dispersion and redox-controlled precipitation of carbon nanotubes in nonaqueous media. Langmuir 2013, 29, 9626–9634. 10.1021/la401440w. [DOI] [PubMed] [Google Scholar]
- Mao X.; Simeon F.; Achilleos D. S.; Rutledge G. C.; Hatton T. A. Metallocene/carbon hybrids prepared by a solution process for supercapacitor applications. Journal of Materials Chemistry A 2013, 1, 13120–13127. 10.1039/c3ta13361c. [DOI] [Google Scholar]
- Ju H.; Leech D. Electrochemistry of poly(vinylferrocene) formed by direct electrochemical reduction at a glassy carbon electrode. Journal of the Chemical Society, Faraday Transactions 1997, 93, 1371–1375. 10.1039/a606680a. [DOI] [Google Scholar]
- Achilleos D. S.; Hatton T. A. Selective molecularly mediated pseudocapacitive separation of ionic species in solution. ACS Appl. Mater. Interfaces 2016, 8, 32743–32753. 10.1021/acsami.6b07605. [DOI] [PubMed] [Google Scholar]
- Fotopoulou K. N.; Karapanagioti H. K. Surface properties of beached plastic pellets. Marine environmental research 2012, 81, 70–77. 10.1016/j.marenvres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Galloway T. S.; Cole M.; Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- Alsbaiee A.; Smith B. J.; Xiao L.; Ling Y.; Helbling D. E.; Dichtel W. R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. nature 2016, 529, 190–194. 10.1038/nature16185. [DOI] [PubMed] [Google Scholar]
- Clara M.; Strenn B.; Gans O.; Martinez E.; Kreuzinger N.; Kroiss H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water research 2005, 39, 4797–4807. 10.1016/j.watres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Su X.; Kushima A.; Halliday C.; Zhou J.; Li J.; Hatton T. A. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat. Commun. 2018, 9, 4701. 10.1038/s41467-018-07159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmatifar A.; Ozbek N.; Halliday C.; Hatton T. A. Electrochemical selective recovery of heavy metal vanadium oxyanion from continuously flowing aqueous streams. ChemSusChem 2020, 13, 3865–3874. 10.1002/cssc.202001094. [DOI] [PubMed] [Google Scholar]
- Dimos V.; Haralambous K.; Malamis S. A review on the recent studies for chromium species adsorption on raw and modified natural minerals. Critical reviews in environmental science and technology 2012, 42, 1977–2016. 10.1080/10643389.2011.574102. [DOI] [Google Scholar]
- Skowron J.; Konieczko K. Occupational exposure to chromium (VI) compounds. Medycyna Pracy 2015, 66, 407–427. 10.13075/mp.5893.00200. [DOI] [PubMed] [Google Scholar]
- Pawełczyk A.; Božek F.; Grabas K. Impact of military metallurgical plant wastes on the population’s health risk. Chemosphere 2016, 152, 513–519. 10.1016/j.chemosphere.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Whittell G. R.; Manners I. Metallopolymers: New multifunctional materials. Adv. Mater. 2007, 19, 3439–3468. 10.1002/adma.200702876. [DOI] [Google Scholar]
- Vorotyntsev M. A.; Vasilyeva S. V. Metallocene-containing conjugated polymers. Adv. Colloid Interface Sci. 2008, 139, 97–149. 10.1016/j.cis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Kim K.; Baldaguez Medina P.; Elbert J.; Kayiwa E.; Cusick R. D.; Men Y.; Su X. Molecular Tuning of Redox-Copolymers for Selective Electrochemical Remediation. Adv. Funct. Mater. 2020, 30, 2004635. 10.1002/adfm.202004635. [DOI] [Google Scholar]
- Escher B. I.; Fenner K. Recent advances in environmental risk assessment of transformation products. Environ. Sci. Technol. 2011, 45, 3835–3847. 10.1021/es1030799. [DOI] [PubMed] [Google Scholar]
- Thomaidi V. S.; Stasinakis A. S.; Borova V. L.; Thomaidis N. S. Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case-study. Journal of hazardous materials 2015, 283, 740–747. 10.1016/j.jhazmat.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Tijani J. O.; Fatoba O. O.; Babajide O. O.; Petrik L. F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: a review. Environmental chemistry letters 2016, 14, 27–49. 10.1007/s10311-015-0537-z. [DOI] [Google Scholar]
- Xu Y.; Liu T.; Zhang Y.; Ge F.; Steel R. M.; Sun L. Advances in technologies for pharmaceuticals and personal care products removal. Journal of materials chemistry A 2017, 5, 12001–12014. 10.1039/C7TA03698A. [DOI] [Google Scholar]
- Alexander J. T.; Hai F. I.; Al-aboud T. M. Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. Journal of environmental management 2012, 111, 195–207. 10.1016/j.jenvman.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Benner J.; Helbling D. E.; Kohler H.-P. E.; Wittebol J.; Kaiser E.; Prasse C.; Ternes T. A.; Albers C. N.; Aamand J.; Horemans B.; Springael D.; Walravens E.; Boon N. Is biological treatment a viable alternative for micropollutant removal in drinking water treatment processes?. Water Res. 2013, 47, 5955–5976. 10.1016/j.watres.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Besha A. T.; Gebreyohannes A. Y.; Tufa R. A.; Bekele D. N.; Curcio E.; Giorno L. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: A review. Journal of environmental chemical engineering 2017, 5, 2395–2414. 10.1016/j.jece.2017.04.027. [DOI] [Google Scholar]
- Akhoury A.; Bromberg L.; Hatton T. A. Redox-responsive gels with tunable hydrophobicity for controlled solubilization and release of organics. ACS Appl. Mater. Interfaces 2011, 3, 1167–1174. 10.1021/am200002b. [DOI] [PubMed] [Google Scholar]
- Tian W.; Mao X.; Brown P.; Rutledge G. C.; Hatton T. A. Electrochemically nanostructured polyvinylferrocene/polypyrrole hybrids with synergy for energy storage. Adv. Funct. Mater. 2015, 25, 4803–4813. 10.1002/adfm.201501041. [DOI] [Google Scholar]
- Shapira B.; Avraham E.; Aurbach D. Side reactions in capacitive deionization (CDI) processes: the role of oxygen reduction. Electrochim. Acta 2016, 220, 285–295. 10.1016/j.electacta.2016.10.127. [DOI] [Google Scholar]
- Omosebi A.; Gao X.; Landon J.; Liu K. Asymmetric electrode configuration for enhanced membrane capacitive deionization. ACS Appl. Mater. Interfaces 2014, 6, 12640–12649. 10.1021/am5026209. [DOI] [PubMed] [Google Scholar]
- Gao X.; Landon J.; Neathery J. K.; Liu K. Modification of carbon xerogel electrodes for more efficient asymmetric capacitive deionization. J. Electrochem. Soc. 2013, 160, E106–E112. 10.1149/2.111309jes. [DOI] [Google Scholar]
- Jo H.; Kim K. H.; Jung M.-J.; Park J. H.; Lee Y.-S. Fluorination effect of activated carbons on performance of asymmetric capacitive deionization. Appl. Surf. Sci. 2017, 409, 117–123. 10.1016/j.apsusc.2017.02.234. [DOI] [Google Scholar]
- Li H.; Yu M.; Wang F.; Liu P.; Liang Y.; Xiao J.; Wang C.; Tong Y.; Yang G. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. 10.1038/ncomms2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Kong D.; Zhang J.; Wang Y.; Chen T.; Cheng C.; Yang H. Y. 3D hierarchical Co3O4 @ Co3S4 nanoarrays as cathode materials for asymmetric pseudocapacitors. Journal of Materials Chemistry A 2016, 4, 3287–3296. 10.1039/C5TA09344A. [DOI] [Google Scholar]
- Boota M.; Gogotsi Y. MXene—conducting polymer asymmetric pseudocapacitors. Adv. Energy Mater. 2019, 9, 1802917. 10.1002/aenm.201802917. [DOI] [Google Scholar]
- Amatucci G. G.; Badway F.; Du Pasquier A.; Zheng T. An asymmetric hybrid nonaqueous energy storage cell. J. Electrochem. Soc. 2001, 148, A930–A939. 10.1149/1.1383553. [DOI] [Google Scholar]
- Qu Q.; Zhang P.; Wang B.; Chen Y.; Tian S.; Wu Y.; Holze R. Electrochemical performance of MnO2 nanorods in neutral aqueous electrolytes as a cathode for asymmetric supercapacitors. J. Phys. Chem. C 2009, 113, 14020–14027. 10.1021/jp8113094. [DOI] [Google Scholar]
- Wang Y.; Song Y.; Xia Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. 10.1039/C5CS00580A. [DOI] [PubMed] [Google Scholar]
- Ruttiger C.; Pfeifer V.; Rittscher V.; Stock D.; Scheid D.; Vowinkel S.; Roth F.; Didzoleit H.; Stuhn B.; Elbert J.; Ionescu E.; Gallei M. One for all: cobalt-containing polymethacrylates for magnetic ceramics, block copolymerization, unexpected electrochemistry, and stimuli-responsiveness. Polym. Chem. 2016, 7, 1129–1137. 10.1039/C5PY01845E. [DOI] [Google Scholar]
- Shaw W. D.; Walker M.; Benson M. Treating and drinking well water in the presence of health risks from arsenic contamination: results from a US hot spot. Risk Analysis: An International Journal 2005, 25, 1531–1543. 10.1111/j.1539-6924.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- Pokhrel D.; Bhandari B.; Viraraghavan T. Arsenic contamination of groundwater in the Terai region of Nepal: an overview of health concerns and treatment options. Environ. Int. 2009, 35, 157–161. 10.1016/j.envint.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Camacho L. M.; Gutiérrez M.; Alarcón-Herrera M. T.; Villalba M.d L.; Deng S. Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere 2011, 83, 211–225. 10.1016/j.chemosphere.2010.12.067. [DOI] [PubMed] [Google Scholar]
- Arsenic in Drinking Water: Background Document for Development of WHO Guidelines for Drinking Water Quality; World Health Organization, 2011.
- Song Z.; Garg S.; Ma J.; Waite T. D. Selective Arsenic Removal from Groundwaters Using Redox-Active Polyvinylferrocene-Functionalized Electrodes: Role of Oxygen. Environ. Sci. Technol. 2020, 54, 12081–12091. 10.1021/acs.est.0c03007. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Lin Z.; Mao X.; Tian W.; Van Voorhis T.; Hatton T. A. Superhydrophobic, Surfactant-doped, Conducting Polymers for Electrochemically Reversible Adsorption of Organic Contaminants. Adv. Funct. Mater. 2018, 28, 1801466. 10.1002/adfm.201801466. [DOI] [Google Scholar]
- He F.; Hemmatifar A.; Bazant M. Z.; Hatton T. A. Selective adsorption of organic anions in a flow cell with asymmetric redox active electrodes. Water Res. 2020, 182, 115963. 10.1016/j.watres.2020.115963. [DOI] [PubMed] [Google Scholar]
- Fan H.Theoretical and experimental study of electrochemically mediated adsorption processes. Ph.D. Thesis. Massachusetts Institute of Technology, 2019. [Google Scholar]
- Loeb S.; Van Hessen F.; Levi J.; Ventura M.. The osmotic power plant. In 11th Intersociety Energy Conversion Engineering Conference. 1976; pp 51–57.
- Loeb S. One hundred and thirty benign and renewable megawatts from Great Salt Lake? The possibilities of hydroelectric power by pressure-retarded osmosis. Desalination 2001, 141, 85–91. 10.1016/S0011-9164(01)00392-7. [DOI] [Google Scholar]
- Achilli A.; Childress A. E. Pressure retarded osmosis: from the vision of Sidney Loeb to the first prototype installation. Desalination 2010, 261, 205–211. 10.1016/j.desal.2010.06.017. [DOI] [Google Scholar]
- Logan B. E.; Elimelech M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. 10.1038/nature11477. [DOI] [PubMed] [Google Scholar]
- Helfer F.; Lemckert C.; Anissimov Y. G. Osmotic power with pressure retarded osmosis: theory, performance and trends–a review. J. Membr. Sci. 2014, 453, 337–358. 10.1016/j.memsci.2013.10.053. [DOI] [Google Scholar]
- Chung T.-S.; Wan C. F.. Membrane Technology for Osmotic Power Generation by Pressure Retarded Osmosis; CRC Press, 2020. [Google Scholar]
- Weinstein J. N.; Leitz F. B. Electric power from differences in salinity: the dialytic battery. Science 1976, 191, 557–559. 10.1126/science.191.4227.557. [DOI] [PubMed] [Google Scholar]
- Yasukawa M.; Suzuki T.; Higa M.. Membrane-Based Salinity Gradient Processes for Water Treatment and Power Generation; Elsevier, 2018; pp 3–56. [Google Scholar]
- Turek M.; Bandura B. Renewable energy by reverse electrodialysis. Desalination 2007, 205, 67–74. 10.1016/j.desal.2006.04.041. [DOI] [Google Scholar]
- Post J. W.; Hamelers H. V.; Buisman C. J. Energy recovery from controlled mixing salt and fresh water with a reverse electrodialysis system. Environ. Sci. Technol. 2008, 42, 5785–5790. 10.1021/es8004317. [DOI] [PubMed] [Google Scholar]
- Norman R. S. Water salination: a source of energy. Science 1974, 186, 350–352. 10.1126/science.186.4161.350. [DOI] [PubMed] [Google Scholar]
- Wick G. L.; Schmitt W. R. Prospects for renewable energy from the sea. Mar. Technol. Soc. J. 1977, 11, 16–21. [Google Scholar]
- Forgacs C. Recent developments in the utilization of salinity power. Desalination 1982, 40, 191–195. 10.1016/S0011-9164(00)88683-X. [DOI] [Google Scholar]
- Audinos R.Electric power produced from two solutions of unequal salinity by reverse electrodialysis. Indian J. Chem. 1992, 31A, 348–354. [Google Scholar]
- Jagur-Grodzinski J.; Kramer R. Novel process for direct conversion of free energy of mixing into electric power. Industrial & Engineering Chemistry Process Design and Development 1986, 25, 443–449. 10.1021/i200033a016. [DOI] [Google Scholar]
- Długołȩcki P.; Nymeijer K.; Metz S.; Wessling M. Current status of ion exchange membranes for power generation from salinity gradients. J. Membr. Sci. 2008, 319, 214–222. 10.1016/j.memsci.2008.03.037. [DOI] [Google Scholar]
- Veerman J.; Saakes M.; Metz S. J.; Harmsen G. Reverse electrodialysis: evaluation of suitable electrode systems. J. Appl. Electrochem. 2010, 40, 1461–1474. 10.1007/s10800-010-0124-8. [DOI] [Google Scholar]
- Scialdone O.; Guarisco C.; Grispo S.; D’Angelo A.; Galia A. Investigation of electrode material–redox couple systems for reverse electrodialysis processes. Part I: Iron redox couples. J. Electroanal. Chem. 2012, 681, 66–75. 10.1016/j.jelechem.2012.05.017. [DOI] [Google Scholar]
- Scialdone O.; Albanese A.; D’Angelo A.; Galia A.; Guarisco C. Investigation of electrode material–redox couple systems for reverse electrodialysis processes. Part II: Experiments in a stack with 10–50 cell pairs. Journal of electroanalytical chemistry 2013, 704, 1–9. 10.1016/j.jelechem.2013.06.001. [DOI] [Google Scholar]
- Mei Y.; Tang C. Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. 10.1016/j.desal.2017.10.021. [DOI] [Google Scholar]
- Veerman J.; Post J.; Saakes M.; Metz S.; Harmsen G. Reducing power losses caused by ionic shortcut currents in reverse electrodialysis stacks by a validated model. J. Membr. Sci. 2008, 310, 418–430. 10.1016/j.memsci.2007.11.032. [DOI] [Google Scholar]
- Lacey R. Energy by reverse electrodialysis. Ocean engineering 1980, 7, 1–47. 10.1016/0029-8018(80)90030-X. [DOI] [Google Scholar]
- Post J. W.; Veerman J.; Hamelers H. V.; Euverink G. J.; Metz S. J.; Nymeijer K.; Buisman C. J. Salinity-gradient power: Evaluation of pressure-retarded osmosis and reverse electrodialysis. Journal of membrane science 2007, 288, 218–230. 10.1016/j.memsci.2006.11.018. [DOI] [Google Scholar]
- Długołȩcki P.; Gambier A.; Nijmeijer K.; Wessling M. Practical potential of reverse electrodialysis as process for sustainable energy generation. Environ. Sci. Technol. 2009, 43, 6888–6894. 10.1021/es9009635. [DOI] [PubMed] [Google Scholar]
- Moreno J.; Grasman S.; Van Engelen R.; Nijmeijer K. Upscaling reverse electrodialysis. Environ. Sci. Technol. 2018, 52, 10856–10863. 10.1021/acs.est.8b01886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burheim O. S.; Seland F.; Pharoah J. G.; Kjelstrup S. Improved electrode systems for reverse electro-dialysis and electro-dialysis. Desalination 2012, 285, 147–152. 10.1016/j.desal.2011.09.048. [DOI] [Google Scholar]
- Wen T.; Solt G.; Sun Y. Spirally wound electrodialysis (SpED) modules. Desalination 1995, 101, 79–91. 10.1016/0011-9164(95)00011-P. [DOI] [Google Scholar]
- Wright N. C.; Winter A. G. Design of spiral-wound electrodialysis modules. Desalination 2019, 458, 54–65. 10.1016/j.desal.2019.01.010. [DOI] [Google Scholar]
- Vermaas D. A.; Saakes M.; Nijmeijer K. Power generation using profiled membranes in reverse electrodialysis. Journal of membrane science 2011, 385, 234–242. 10.1016/j.memsci.2011.09.043. [DOI] [Google Scholar]
- Hong J. G.; Chen Y. Evaluation of electrochemical properties and reverse electrodialysis performance for porous cation exchange membranes with sulfate-functionalized iron oxide. J. Membrane Sci. 2015, 473, 210–217. 10.1016/j.memsci.2014.09.012. [DOI] [Google Scholar]
- Gurreri L.; Ciofalo M.; Cipollina A.; Tamburini A.; Van Baak W.; Micale G. CFD modelling of profiled-membrane channels for reverse electrodialysis. Desalination and water treatment 2015, 55, 3404–3423. 10.1080/19443994.2014.940651. [DOI] [Google Scholar]
- Altıok E.; Kaya T. Z.; Güler E.; Kabay N.; Bryjak M. Performance of Reverse Electrodialysis System for Salinity Gradient Energy Generation by Using a Commercial Ion Exchange Membrane Pair with Homogeneous Bulk Structure. Water 2021, 13, 814. 10.3390/w13060814. [DOI] [Google Scholar]
- He Z.; Gao X.; Zhang Y.; Wang Y.; Wang J. Revised spacer design to improve hydrodynamics and anti-fouling behavior in reverse electrodialysis processes. Desalination and Water Treatment 2016, 57, 28176–28186. 10.1080/19443994.2016.1186569. [DOI] [Google Scholar]
- Lee S.-Y.; Jeong Y.-J.; Chae S.-R.; Yeon K.-H.; Lee Y.; Kim C.-S.; Jeong N.-J.; Park J.-S. Porous carbon-coated graphite electrodes for energy production from salinity gradient using reverse electrodialysis. J. Phys. Chem. Solids 2016, 91, 34–40. 10.1016/j.jpcs.2015.12.006. [DOI] [Google Scholar]
- Zhu X.; He W.; Logan B. E. Influence of solution concentration and salt types on the performance of reverse electrodialysis cells. Journal of membrane science 2015, 494, 154–160. 10.1016/j.memsci.2015.07.053. [DOI] [Google Scholar]
- Tedesco M.; Brauns E.; Cipollina A.; Micale G.; Modica P.; Russo G.; Helsen J. Reverse electrodialysis with saline waters and concentrated brines: a laboratory investigation towards technology scale-up. J. Membr. Sci. 2015, 492, 9–20. 10.1016/j.memsci.2015.05.020. [DOI] [Google Scholar]
- Winzeler H. B.; Belfort G. Enhanced performance for pressure-driven membrane processes: the argument for fluid instabilities. J. Membr. Sci. 1993, 80, 35–47. 10.1016/0376-7388(93)85130-O. [DOI] [Google Scholar]
- Vaselbehagh M.; Karkhanechi H.; Takagi R.; Matsuyama H. Biofouling phenomena on anion exchange membranes under the reverse electrodialysis process. J. Membr. Sci. 2017, 530, 232–239. 10.1016/j.memsci.2017.02.036. [DOI] [Google Scholar]
- Rijnaarts T.; Moreno J.; Saakes M.; de Vos W.; Nijmeijer K. Role of anion exchange membrane fouling in reverse electrodialysis using natural feed waters. Colloids and surfaces A: Physicochemical and engineering aspects 2019, 560, 198–204. 10.1016/j.colsurfa.2018.10.020. [DOI] [Google Scholar]
- Bodner E.; Saakes M.; Sleutels T.; Buisman C.; Hamelers H. The RED Fouling Monitor: A novel tool for fouling analysis. Journal of membrane science 2019, 570, 294–302. 10.1016/j.memsci.2018.10.059. [DOI] [Google Scholar]
- Luo X.; Cao X.; Mo Y.; Xiao K.; Zhang X.; Liang P.; Huang X. Power generation by coupling reverse electrodialysis and ammonium bicarbonate: Implication for recovery of waste heat. Electrochemistry communications 2012, 19, 25–28. 10.1016/j.elecom.2012.03.004. [DOI] [Google Scholar]
- Kim Y.; Logan B. E. Hydrogen production from inexhaustible supplies of fresh and salt water using microbial reverse-electrodialysis electrolysis cells. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16176–16181. 10.1073/pnas.1106335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Krantz W. B.; Cornelissen E. R.; Post J. W.; Verliefde A. R.; Tang C. Y. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Applied energy 2013, 104, 592–602. 10.1016/j.apenergy.2012.11.064. [DOI] [Google Scholar]
- Cusick R. D.; Kim Y.; Logan B. E. Energy capture from thermolytic solutions in microbial reverse-electrodialysis cells. Science 2012, 335, 1474–1477. 10.1126/science.1219330. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Liu Y.-Y.; Xue C.; Yang Y.-L.; Zhang W.-M. Energy self-sufficient desalination stack as a potential fresh water supply on small islands. Desalination 2015, 359, 52–58. 10.1016/j.desal.2014.12.010. [DOI] [Google Scholar]
- Wang Q.; Gao X.; Zhang Y.; He Z.; Ji Z.; Wang X.; Gao C. Hybrid RED/ED system: Simultaneous osmotic energy recovery and desalination of high-salinity wastewater. Desalination 2017, 405, 59–67. 10.1016/j.desal.2016.12.005. [DOI] [Google Scholar]
- Van Egmond W.; Saakes M.; Porada S.; Meuwissen T.; Buisman C.; Hamelers H. The concentration gradient flow battery as electricity storage system: Technology potential and energy dissipation. J. Power Sources 2016, 325, 129–139. 10.1016/j.jpowsour.2016.05.130. [DOI] [Google Scholar]
- Tufa R. A.; Pawlowski S.; Veerman J.; Bouzek K.; Fontananova E.; di Profio G.; Velizarov S.; Goulão Crespo J.; Nijmeijer K.; Curcio E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. 10.1016/j.apenergy.2018.04.111. [DOI] [Google Scholar]
- Guo W.; Cao L.; Xia J.; Nie F.-Q.; Ma W.; Xue J.; Song Y.; Zhu D.; Wang Y.; Jiang L. Energy harvesting with single-ion-selective nanopores: a concentration-gradient-driven nanofluidic power source. Adv. Funct. Mater. 2010, 20, 1339–1344. 10.1002/adfm.200902312. [DOI] [Google Scholar]
- Cao L.; Guo W.; Ma W.; Wang L.; Xia F.; Wang S.; Wang Y.; Jiang L.; Zhu D. Towards understanding the nanofluidic reverse electrodialysis system: well matched charge selectivity and ionic composition. Energy Environ. Sci. 2011, 4, 2259–2266. 10.1039/c1ee01088c. [DOI] [Google Scholar]
- Tedesco M.; Scalici C.; Vaccari D.; Cipollina A.; Tamburini A.; Micale G. Performance of the first reverse electrodialysis pilot plant for power production from saline waters and concentrated brines. J. Membr. Sci. 2016, 500, 33–45. 10.1016/j.memsci.2015.10.057. [DOI] [Google Scholar]
- Tedesco M.; Cipollina A.; Tamburini A.; Micale G. Towards 1 kW power production in a reverse electrodialysis pilot plant with saline waters and concentrated brines. J. Membr. Sci. 2017, 522, 226–236. 10.1016/j.memsci.2016.09.015. [DOI] [Google Scholar]
- Farrell E.; Hassan M. I.; Tufa R. A.; Tuomiranta A.; Avci A. H.; Politano A.; Curcio E.; Arafat H. A. Reverse electrodialysis powered greenhouse concept for water-and energy-self-sufficient agriculture. Applied Energy 2017, 187, 390–409. 10.1016/j.apenergy.2016.11.069. [DOI] [Google Scholar]
- Tamburini A.; Tedesco M.; Cipollina A.; Micale G.; Ciofalo M.; Papapetrou M.; Van Baak W.; Piacentino A. Reverse electrodialysis heat engine for sustainable power production. Applied Energy 2017, 206, 1334–1353. 10.1016/j.apenergy.2017.10.008. [DOI] [Google Scholar]
- Osterle J. F. Electrokinetic Energy Conversion. Journal of Applied Mechanics 1964, 31, 161–164. 10.1115/1.3629580. [DOI] [Google Scholar]
- Morrison F. Jr; Osterle J. Electrokinetic energy conversion in ultrafine capillaries. J. Chem. Phys. 1965, 43, 2111–2115. 10.1063/1.1697081. [DOI] [Google Scholar]
- Yang J.; Lu F.; Kostiuk L. W.; Kwok D. Y. Electrokinetic microchannel battery by means of electrokinetic and microfluidic phenomena. Journal of Micromechanics and Microengineering 2003, 13, 963. 10.1088/0960-1317/13/6/320. [DOI] [Google Scholar]
- van der Heyden F. H.; Stein D.; Dekker C. Streaming currents in a single nanofluidic channel. Physical review letters 2005, 95, 116104. 10.1103/PhysRevLett.95.116104. [DOI] [PubMed] [Google Scholar]
- Van der Heyden F. H.; Bonthuis D. J.; Stein D.; Meyer C.; Dekker C. Electrokinetic energy conversion efficiency in nanofluidic channels. Nano Lett. 2006, 6, 2232–2237. 10.1021/nl061524l. [DOI] [PubMed] [Google Scholar]
- van der Heyden F. H.; Bonthuis D. J.; Stein D.; Meyer C.; Dekker C. Power generation by pressure-driven transport of ions in nanofluidic channels. Nano Lett. 2007, 7, 1022–1025. 10.1021/nl070194h. [DOI] [PubMed] [Google Scholar]
- Bocquet L.; Charlaix E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 2010, 39, 1073–1095. 10.1039/B909366B. [DOI] [PubMed] [Google Scholar]
- Davidson C.; Xuan X. Effects of Stern layer conductance on electrokinetic energy conversion in nanofluidic channels. Electrophoresis 2008, 29, 1125–1130. 10.1002/elps.200700549. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhan K.; Wang S.; Hou X. Soft interface design for electrokinetic energy conversion. Soft Matter 2020, 16, 2915–2927. 10.1039/C9SM02506E. [DOI] [PubMed] [Google Scholar]
- Xiao K.; Jiang L.; Antonietti M. Ion transport in nanofluidic devices for energy harvesting. Joule 2019, 3, 2364–2380. 10.1016/j.joule.2019.09.005. [DOI] [Google Scholar]
- Joly L.; Ybert C.; Trizac E.; Bocquet L. Hydrodynamics within the electric double layer on slipping surfaces. Physical review letters 2004, 93, 257805. 10.1103/PhysRevLett.93.257805. [DOI] [PubMed] [Google Scholar]
- Joly L.; Ybert C.; Trizac E.; Bocquet L. Liquid friction on charged surfaces: From hydrodynamic slippage to electrokinetics. J. Chem. Phys. 2006, 125, 204716. 10.1063/1.2397677. [DOI] [PubMed] [Google Scholar]
- Silkina E. F.; Asmolov E. S.; Vinogradova O. I. Electro-osmotic flow in hydrophobic nanochannels. Phys. Chem. Chem. Phys. 2019, 21, 23036–23043. 10.1039/C9CP04259H. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Stein D. Slip-enhanced electrokinetic energy conversion in nanofluidic channels. Nanotechnology 2008, 19, 195707. 10.1088/0957-4484/19/19/195707. [DOI] [PubMed] [Google Scholar]
- Bahga S. S.; Vinogradova O. I.; Bazant M. Z. Anisotropic electro-osmotic flow over super-hydrophobic surfaces. Journal of fluid mechanics 2010, 644, 245–255. 10.1017/S0022112009992771. [DOI] [Google Scholar]
- Belyaev A. V.; Vinogradova O. I. Electro-osmosis on anisotropic superhydrophobic surfaces. Physical review letters 2011, 107, 098301. 10.1103/PhysRevLett.107.098301. [DOI] [PubMed] [Google Scholar]
- Fan B.; Bandaru P. Tensorial Modulation of Electrokinetic Streaming Potentials on Air and Liquid Filled Surfaces. Langmuir 2019, 35, 14812–14817. 10.1021/acs.langmuir.9b02841. [DOI] [PubMed] [Google Scholar]
- Vinogradova O. I.; Silkina E. F.; Bag N.; Asmolov E. S. Achieving large zeta-potentials with charged porous surfaces. Phys. Fluids 2020, 32, 102105. 10.1063/5.0024718. [DOI] [Google Scholar]
- Mugele F.; Baret J.-C. Electrowetting: from basics to applications. J. Phys.: Condens. Matter 2005, 17, R705. 10.1088/0953-8984/17/28/R01. [DOI] [Google Scholar]
- Mugele F.; Heikenfeld J.. Electrowetting: Fundamental Principles and Practical Applications; John Wiley & Sons, 2018. [Google Scholar]
- Krupenkin T.; Taylor J. A. Reverse electrowetting as a new approach to high-power energy harvesting. Nat. Commun. 2011, 2, 448. 10.1038/ncomms1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeisky A. B.; Kornyshev A. A. Current-generating ‘double layer shoe’ with a porous sole. J. Phys.: Condens. Matter 2016, 28, 464009. 10.1088/0953-8984/28/46/464009. [DOI] [PubMed] [Google Scholar]
- Haimov E.; Chapman A.; Bresme F.; Holmes A. S.; Reddyhoff T.; Urbakh M.; Kornyshev A. A. Theoretical demonstration of a capacitive rotor for generation of alternating current from mechanical motion. Nat. Commun. 2021, 12, 3678. 10.1038/s41467-021-23891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogioli D. Extracting renewable energy from a salinity difference using a capacitor. Physical review letters 2009, 103, 058501. 10.1103/PhysRevLett.103.058501. [DOI] [PubMed] [Google Scholar]
- Aricò A.; Bruce P.; Scrosati B.; Tarascon J.; van Schalkwijk W. Nanostructured materials for advanced energy conversion and storage devices. Nature materials 2005, 4, 366–377. 10.1038/nmat1368. [DOI] [PubMed] [Google Scholar]
- Simon P.; Gogotsi Y.. Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific, 2010; pp 320–329. [Google Scholar]
- Hunter R. J.Introduction to Modern Colloid Science; Oxford University Press: Oxford, UK, 1993; Vol. 7. [Google Scholar]
- Brogioli D.; Zhao R.; Biesheuvel P. A prototype cell for extracting energy from a water salinity difference by means of double layer expansion in nanoporous carbon electrodes. Energy Environ. Sci. 2011, 4, 772–777. 10.1039/c0ee00524j. [DOI] [Google Scholar]
- Rica R. A.; Brogioli D.; Ziano R.; Salerno D.; Mantegazza F. Ions transport and adsorption mechanisms in porous electrodes during capacitive-mixing double layer expansion (CDLE). J. Phys. Chem. C 2012, 116, 16934–16938. 10.1021/jp3059849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rica R. A.; Ziano R.; Salerno D.; Mantegazza F.; Bazant M. Z.; Brogioli D. Electro-diffusion of ions in porous electrodes for capacitive extraction of renewable energy from salinity differences. Electrochim. Acta 2013, 92, 304–314. 10.1016/j.electacta.2013.01.063. [DOI] [Google Scholar]
- Brogioli D.; La Mantia F.. Interface Science and Technology; Elsevier, 2018; Vol. 24; pp 87–117. [Google Scholar]
- Sales B.; Saakes M.; Post J.; Buisman C.; Biesheuvel P.; Hamelers H. Direct power production from a water salinity difference in a membrane-modified supercapacitor flow cell. Environ. Sci. Technol. 2010, 44, 5661–5665. 10.1021/es100852a. [DOI] [PubMed] [Google Scholar]
- Burheim O.; Sales B.; Schaezle O.; Liu F.; Hamelers H.. Auto generative capacitive mixing of sea and river water by the use of membranes. ASME 2011 International Mechanical Engineering Congress and Exposition (IMECE2011), Denver, CO, 2011; pp 483–492.
- Sales B. B.; Liu F.; Schaetzle O.; Buisman C. J.; Hamelers H. V. Electrochemical characterization of a supercapacitor flow cell for power production from salinity gradients. Electrochimica acta 2012, 86, 298–304. 10.1016/j.electacta.2012.05.069. [DOI] [Google Scholar]
- Burheim O.; Sales B.; Schaetzle O.; Liu F.; Hamelers H. Auto generative capacitive mixing for power conversion of sea and river water by the use of membranes. J. Energy Resour. Technol. 2013, 135, 011601. 10.1115/1.4007717. [DOI] [Google Scholar]
- Smolinska-Kempisty K.; Siekierka A.; Bryjak M. Interpolymer ion exchange membranes for CapMix process. Desalination 2020, 482, 114384. 10.1016/j.desal.2020.114384. [DOI] [Google Scholar]
- Bijmans M.; Burheim O.; Bryjak M.; Delgado A.; Hack P.; Mantegazza F.; Tenisson S.; Hamelers H. Capmix-deploying capacitors for salt gradient power extraction. Energy Procedia 2012, 20, 108–115. 10.1016/j.egypro.2012.03.013. [DOI] [Google Scholar]
- Liu F.; Schaetzle O.; Sales B. B.; Saakes M.; Buisman C. J.; Hamelers H. V. Effect of additional charging and current density on the performance of Capacitive energy extraction based on Donnan Potential. Energy Environ. Sci. 2012, 5, 8642–8650. 10.1039/c2ee21548a. [DOI] [Google Scholar]
- Brogioli D.; Ziano R.; Rica R.; Salerno D.; Kozynchenko O.; Hamelers H.; Mantegazza F. Exploiting the spontaneous potential of the electrodes used in the capacitive mixing technique for the extraction of energy from salinity difference. Energy Environ. Sci. 2012, 5, 9870–9880. 10.1039/c2ee23036d. [DOI] [Google Scholar]
- Rica R. A.; Ziano R.; Salerno D.; Mantegazza F.; Van Roij R.; Brogioli D. Capacitive mixing for harvesting the free energy of solutions at different concentrations. Entropy 2013, 15, 1388–1407. 10.3390/e15041388. [DOI] [Google Scholar]
- Hatzell M. C.; Hatzell K. B.; Logan B. E. Using flow electrodes in multiple reactors in series for continuous energy generation from capacitive mixing. Environmental Science & Technology Letters 2014, 1, 474–478. 10.1021/ez5003314. [DOI] [Google Scholar]
- Iglesias G. R.; Fernández M. M.; Ahualli S.; Jiménez M. L.; Kozynchenko O. P.; Delgado Á. V. Materials selection for optimum energy production by double layer expansion methods. J. Power Sources 2014, 261, 371–377. 10.1016/j.jpowsour.2013.12.125. [DOI] [Google Scholar]
- Delgado A.; Ahualli S.; Fernández M.; González M.; Iglesias G.; Vivo-Vilches J.; Jiménez M. Geometrical properties of materials for energy production by salinity exchange. Environmental Chemistry 2017, 14, 279–287. 10.1071/EN16210. [DOI] [Google Scholar]
- Ahualli S.; Jimenez M.; Fernández M. M.; Iglesias G.; Brogioli D.; Delgado A. V. Polyelectrolyte-coated carbons used in the generation of blue energy from salinity differences. Phys. Chem. Chem. Phys. 2014, 16, 25241–25246. 10.1039/C4CP03527E. [DOI] [PubMed] [Google Scholar]
- Fernandez M.; Wagterveld R.; Ahualli S.; Liu F.; Delgado A.; Hamelers H. Polyelectrolyte-versus membrane-coated electrodes for energy production by capmix salinity exchange methods. J. Power Sources 2016, 302, 387–393. 10.1016/j.jpowsour.2015.10.076. [DOI] [Google Scholar]
- Tan G.; Zhu X. Polyelectrolyte-Coated Copper Hexacyanoferrate and Bismuth Oxychloride Electrodes for Efficient Salinity Gradient Energy Recovery in Capacitive Mixing. Energy Technol. 2019, 8, 1900863. 10.1002/ente.201900863. [DOI] [Google Scholar]
- Ahualli S.; Orozco-Barrera S.; Fernández M. d. M.; Delgado Á. V.; Iglesias G. R. Assembly of soft electrodes and ion exchange membranes for capacitive deionization. Polymers 2019, 11, 1556. 10.3390/polym11101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias G.; Ahualli S.; Delgado A.; Arenas-Fernández P.; Fernández M. Combining soft electrode and ion exchange membranes for increasing salinity difference energy efficiency. J. Power Sources 2020, 453, 227840. 10.1016/j.jpowsour.2020.227840. [DOI] [Google Scholar]
- Ye M.; Pasta M.; Xie X.; Cui Y.; Criddle C. S. Performance of a mixing entropy battery alternately flushed with wastewater effluent and seawater for recovery of salinity-gradient energy. Energy Environ. Sci. 2014, 7, 2295–2300. 10.1039/C4EE01034E. [DOI] [Google Scholar]
- Jia Z.; Wang B.; Song S.; Fan Y. A membrane-less Na ion battery-based CAPMIX cell for energy extraction using water salinity gradients. RSC Adv. 2013, 3, 26205–26209. 10.1039/c3ra44902e. [DOI] [Google Scholar]
- Hosein Tehrani S. H. M.; Seyedsadjadi S. A.; Ghaffarinejad A. Application of electrodeposited cobalt hexacyanoferrate film to extract energy from water salinity gradients. RSC Adv. 2015, 5, 30032–30037. 10.1039/C5RA03909F. [DOI] [Google Scholar]
- Gomes W. J.; de Oliveira C.; Huguenin F. Energy harvesting by nickel prussian blue analogue electrode in neutralization and mixing entropy batteries. Langmuir 2015, 31, 8710–8717. 10.1021/acs.langmuir.5b01419. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Kim T.; Rahimi M.; Gorski C. A.; Logan B. E. Integrating Reverse-Electrodialysis Stacks with Flow Batteries for Improved Energy Recovery from Salinity Gradients and Energy Storage. ChemSusChem 2017, 10, 797–803. 10.1002/cssc.201601220. [DOI] [PubMed] [Google Scholar]
- Ye M.; Pasta M.; Xie X.; Dubrawski K. L.; Xu J.; Liu C.; Cui Y.; Criddle C. S. Charge-Free Mixing Entropy Battery Enabled by Low-Cost Electrode Materials. ACS omega 2019, 4, 11785–11790. 10.1021/acsomega.9b00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.; Rahimi M.; Logan B. E.; Gorski C. A. Evaluating battery-like reactions to harvest energy from salinity differences using ammonium bicarbonate salt solutions. ChemSusChem 2016, 9, 981–988. 10.1002/cssc.201501669. [DOI] [PubMed] [Google Scholar]
- Kim T.; Rahimi M.; Logan B. E.; Gorski C. A. Harvesting energy from salinity differences using battery electrodes in a concentration flow cell. Environ. Sci. Technol. 2016, 50, 9791–9797. 10.1021/acs.est.6b02554. [DOI] [PubMed] [Google Scholar]
- Kim T.; Logan B. E.; Gorski C. A. High power densities created from salinity differences by combining electrode and Donnan potentials in a concentration flow cell. Energy Environ. Sci. 2017, 10, 1003–1012. 10.1039/C7EE00188F. [DOI] [Google Scholar]
- Zhang F.; Liu J.; Yang W.; Logan B. E. A thermally regenerative ammonia-based battery for efficient harvesting of low-grade thermal energy as electrical power. Energy Environ. Sci. 2015, 8, 343–349. 10.1039/C4EE02824D. [DOI] [Google Scholar]
- Zhang F.; LaBarge N.; Yang W.; Liu J.; Logan B. E. Enhancing low-grade thermal energy recovery in a thermally regenerative ammonia battery using elevated temperatures. ChemSusChem 2015, 8, 1043–1048. 10.1002/cssc.201403290. [DOI] [PubMed] [Google Scholar]
- Marino M.; Misuri L.; Carati A.; Brogioli D. Proof-of-concept of a zinc-silver battery for the extraction of energy from a concentration difference. Energies 2014, 7, 3664–3683. 10.3390/en7063664. [DOI] [Google Scholar]
- Marino M.; Misuri L.; Carati A.; Brogioli D. Boosting the voltage of a salinity-gradient-power electrochemical cell by means of complex-forming solutions. Appl. Phys. Lett. 2014, 105, 033901. 10.1063/1.4890976. [DOI] [Google Scholar]
- Cipollina A.; Micale G.. Sustainable Energy from Salinity Gradients; Woodhead Publishing, 2016; pp 181–218. [Google Scholar]
- Marino M.; Misuri L.; Ruffo R.; Brogioli D. Electrode kinetics in the “capacitive mixing” and “battery mixing” techniques for energy production from salinity differences. Electrochim. Acta 2015, 176, 1065–1073. 10.1016/j.electacta.2015.07.069. [DOI] [Google Scholar]
- Kim D. J.; Ponraj R.; Kannan A. G.; Lee H.-W.; Fathi R.; Ruffo R.; Mari C. M.; Kim D. K. Diffusion behavior of sodium ions in Na0.44MnO2 in aqueous and non-aqueous electrolytes. J. Power Sources 2013, 244, 758–763. 10.1016/j.jpowsour.2013.02.090. [DOI] [Google Scholar]
- Lee J.; Yoon H.; Lee J.; Kim T.; Yoon J. Extraction of Salinity-Gradient Energy by a Hybrid Capacitive-Mixing System. ChemSusChem 2017, 10, 1600–1606. 10.1002/cssc.201601656. [DOI] [PubMed] [Google Scholar]
- Hasan K. N. M.; Khai T. X.; Kannan R.; Zakaria Z. A.. Harnessing “Blue energy”: A Review on Techniques and Preliminary Analysis; MATEC Web of Conferences. 2017; p 04013. [Google Scholar]
- Pan S. Y.; Snyder S. W.; Lin Y. J.; Chiang P. C. Electrokinetic desalination of brackish water and associated challenges in the water and energy nexus. Environ. Sci.: Water Res. Technol. 2018, 4, 613–638. 10.1039/C7EW00550D. [DOI] [Google Scholar]
- Hemmatifar A.Energy Consumption and Salt Adsorption in Capacitive Deionization. Ph.D. Thesis. Stanford University, 2018. [Google Scholar]
- Suss M.Capacitive Water Desalination with Hierarchical Porous Electrodes. Ph.D. Thesis. Stanford University, 2013. [Google Scholar]
- O’Hayre R.; Cha S.; Colella W.; Prinz F.. Fuel Cell Fundamentals; Wiley: New York, 2006; pp 59–92. [Google Scholar]
- Ramachandran A.; Hemmatifar A.; Hawks S. A.; Stadermann M.; Santiago J. G. Self similarities in desalination dynamics and performance using capacitive deionization. Water Res. 2018, 140, 323–334. 10.1016/j.watres.2018.04.042. [DOI] [PubMed] [Google Scholar]
- Mistry K. H.; McGovern R. K.; Thiel G. P.; Summers E. K.; Zubair S. M.; Lienhard J. H. Entropy generation analysis of desalination technologies. Entropy 2011, 13, 1829–1864. 10.3390/e13101829. [DOI] [Google Scholar]
- Zhao Y.; Wang Y.; Wang R.; Wu Y.; Xu S.; Wang J. Performance comparison and energy consumption analysis of capacitive deionization and membrane capacitive deionization processes. Desalination 2013, 324, 127–133. 10.1016/j.desal.2013.06.009. [DOI] [Google Scholar]
- Choi J.-H. Comparison of constant voltage (CV) and constant current (CC) operation in the membrane capacitive deionisation process. Desalination and Water Treatment 2015, 56, 921–928. 10.1080/19443994.2014.942379. [DOI] [Google Scholar]
- Ramachandran A.; Hawks S. A.; Stadermann M.; Santiago J. G. Frequency analysis and resonant operation for efficient capacitive deionization. Water Res. 2018, 144, 581–591. 10.1016/j.watres.2018.07.066. [DOI] [PubMed] [Google Scholar]
- Andelman M. D.; Walker G. S.. Charge Barrier Flow-through Capacitor. U.S. US6709560B2, 2004.
- Li H.; Gao Y.; Pan L.; Zhang Y.; Chen Y.; Sun Z. Electrosorptive desalination by carbon nanotubes and nanofibres electrodes and ion-exchange membranes. Water Res. 2008, 42, 4923–4928. 10.1016/j.watres.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Biesheuvel P.; Van der Wal A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. 10.1016/j.memsci.2009.09.043. [DOI] [Google Scholar]
- Cohen-Tanugi D.; McGovern R. K.; Dave S. H.; Lienhard J. H.; Grossman J. C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. 2014, 7, 1134–1141. 10.1039/C3EE43221A. [DOI] [Google Scholar]
- Park H. B.; Kamcev J.; Robeson L. M.; Elimelech M.; Freeman B. D. Maximizing the Right Stuff: The Trade-off between Membrane Permeability and Selectivity. Science 2017, 356, eaab0530. 10.1126/science.aab0530. [DOI] [PubMed] [Google Scholar]
- Alvarado L.; Torres I. R.; Chen A. Integration of ion exchange and electrodeionization as a new approach for the continuous treatment of hexavalent chromium wastewater. Sep. Purif. Technol. 2013, 105, 55–62. 10.1016/j.seppur.2012.12.007. [DOI] [Google Scholar]
- Liu Y.; Wang J.; Xu Y.; Wu B. A deep desalination and anti-scaling electrodeionization (EDI) process for high purity water preparation. Desalination 2019, 468, 114075. 10.1016/j.desal.2019.114075. [DOI] [Google Scholar]
- Ortiz J. M.; Expósito E.; Gallud F.; García-García V.; Montiel V.; Aldaz V. A. Desalination of underground brackish waters using an electrodialysis system powered directly by photovoltaic energy. Sol. Energy Mater. Sol. Cells 2008, 92, 1677–1688. 10.1016/j.solmat.2008.07.020. [DOI] [Google Scholar]
- Wright N. C.; Shah S. R.; Amrose S. E.; Winter A. G. A robust model of brackish water electrodialysis desalination with experimental comparison at different size scales. Desalination 2018, 443, 27–43. 10.1016/j.desal.2018.04.018. [DOI] [Google Scholar]
- Doornbusch G.; Tedesco M.; Post J.; Borneman Z.; Nijmeijer K. Experimental investigation of multistage electrodialysis for seawater desalination. Desalination 2019, 464, 105–114. 10.1016/j.desal.2019.04.025. [DOI] [Google Scholar]
- Yan H.; Wang Y.; Wu L.; Shehzad M. A.; Jiang C.; Fu R.; Liu Z.; Xu T. Multistage-batch electrodialysis to concentrate high-salinity solutions: Process optimization, water transport, and energy consumption. J. Membr. Sci. 2019, 570, 245–257. 10.1016/j.memsci.2018.10.008. [DOI] [Google Scholar]
- Tong X.; Zhang B.; Fan Y.; Chen Y. Mechanism exploration of ion transport in nanocomposite cation exchange membranes. ACS Appl. Mater. Interfaces 2017, 9, 13491–13499. 10.1021/acsami.7b01541. [DOI] [PubMed] [Google Scholar]
- Fan H.; Huang Y.; Yip N. Y. Advancing the conductivity-permselectivity tradeoff of electrodialysis ion-exchange membranes with sulfonated CNT nanocomposites. J. Membr. Sci. 2020, 610, 118259. 10.1016/j.memsci.2020.118259. [DOI] [Google Scholar]
- Alabi A.; Cseri L.; Al Hajaj A.; Szekely G.; Budd P.; Zou L. Electrostatically-coupled graphene oxide nanocomposite cation exchange membrane. J. Membr. Sci. 2020, 594, 117457. 10.1016/j.memsci.2019.117457. [DOI] [Google Scholar]
- Fidaleo M.; Moresi M. Electrodialysis applications in the food industry. Adv. Food Nutr. Res. 2006, 51, 265–360. 10.1016/S1043-4526(06)51005-8. [DOI] [PubMed] [Google Scholar]
- Vera E.; Sandeaux J.; Persin F.; Pourcelly G.; Dornier M.; Ruales J. Deacidification of passion fruit juice by electrodialysis with bipolar membrane after different pretreatments. Journal of food engineering 2009, 90, 67–73. 10.1016/j.jfoodeng.2008.06.003. [DOI] [Google Scholar]
- Šímová H.; Kysela V.; Černín A. Demineralization of natural sweet whey by electrodialysis at pilot-plant scale. Desalination and Water Treatment 2010, 14, 170–173. 10.5004/dwt.2010.1023. [DOI] [Google Scholar]
- Bazinet L.; Doyen A.; Roblet C.. Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Elsevier, 2013; pp 202–218. [Google Scholar]
- Wang Y.; Jiang C.; Bazinet L.; Xu T.. Separation of Functional Molecules in Food by Membrane Technology; Elsevier, 2019; pp 349–381. [Google Scholar]
- Kim N.; Jeon J.; Chen R.; Su X. Electrochemical separation of organic acids and proteins for food and biomanufacturing. Chem. Eng. Res. Des. 2022, 178, 267–288. 10.1016/j.cherd.2021.12.009. [DOI] [Google Scholar]
- Lindstrand V.; Sundström G.; Jönsson A.-S. Fouling of electrodialysis membranes by organic substances. Desalination 2000, 128, 91–102. 10.1016/S0011-9164(00)00026-6. [DOI] [Google Scholar]
- Han L. Current Strategies for the Design of Anti-fouling Ion-Exchange Membranes. Membrane Technology Enhancement for Environmental Protection and Sustainable Industrial Growth 2021, 13–25. 10.1007/978-3-030-41295-1_2. [DOI] [Google Scholar]
- Vaselbehagh M.; Karkhanechi H.; Mulyati S.; Takagi R.; Matsuyama H. Improved antifouling of anion-exchange membrane by polydopamine coating in electrodialysis process. Desalination 2014, 332, 126–133. 10.1016/j.desal.2013.10.031. [DOI] [Google Scholar]
- Wang W.; Fu R.; Liu Z.; Wang H. Low-resistance anti-fouling ion exchange membranes fouled by organic foulants in electrodialysis. Desalination 2017, 417, 1–8. 10.1016/j.desal.2017.05.013. [DOI] [Google Scholar]
- Ran J.; Wu L.; He Y.; Yang Z.; Wang Y.; Jiang C.; Ge L.; Bakangura E.; Xu T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. 10.1016/j.memsci.2016.09.033. [DOI] [Google Scholar]
- Tanaka Y.Progress in Filtration and Separation; Elsevier, 2015; pp 207–284. [Google Scholar]
- Mohammadi R.; Tang W.; Sillanpää M. A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis. Desalination 2021, 498, 114626. 10.1016/j.desal.2020.114626. [DOI] [Google Scholar]
- Brauns E. Salinity gradient power by reverse electrodialysis: effect of model parameters on electrical power output. Desalination 2009, 237, 378–391. 10.1016/j.desal.2008.10.003. [DOI] [Google Scholar]
- Pawlowski S.; Crespo J. G.; Velizarov S. Profiled ion exchange membranes: A comprehensible review. International journal of molecular sciences 2019, 20, 165. 10.3390/ijms20010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski A.; Zhang G.; Strathmann H.; Eigenberger G. Production of high-purity water by continuous electrodeionization with bipolar membranes: influence of concentrate and protection compartment. Sep. Purif. Technol. 2008, 60, 86–95. 10.1016/j.seppur.2007.07.052. [DOI] [Google Scholar]
- Wenten I G.; Khoiruddin; Arfianto F.; Zudiharto Bench scale electrodeionization for high pressure boiler feed water. Desalination 2013, 314, 109–114. 10.1016/j.desal.2013.01.008. [DOI] [Google Scholar]
- Zheng X.-Y.; Pan S.-Y.; Tseng P.-C.; Zheng H.-L.; Chiang P.-C. Optimization of resin wafer electrodeionization for brackish water desalination. Sep. Purif. Technol. 2018, 194, 346–354. 10.1016/j.seppur.2017.11.061. [DOI] [Google Scholar]
- Hestekin C. N.; Hestekin J. A.; Paracha S.; Morrison G.; Pakkaner E.; Moore J.; Santos de Souza L. S.; Stephens S.; Atchley C.; Kurtz I. Simulating nephron ion transport function using activated wafer electrodeionization. Commun. Mater. 2020, 1, 20. 10.1038/s43246-020-0016-3. [DOI] [Google Scholar]
- Ulusoy Erol H. B.; Hestekin C. N.; Hestekin J. A. Effects of resin chemistries on the selective removal of industrially relevant metal ions using wafer-enhanced electrodeionization. Membranes 2021, 11, 45. 10.3390/membranes11010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D.; Jagannathan V.; Bisht N. S.. Use of unique fractional electrodeionization in power plant applications. Power Plant Chemistry 2009, 11, 1. [Google Scholar]
- Chehayeb K. M.; Farhat D. M.; Nayar K. G.; Lienhard J. H. Optimal design and operation of electrodialysis for brackish-water desalination and for high-salinity brine concentration. Desalination 2017, 420, 167–182. 10.1016/j.desal.2017.07.003. [DOI] [Google Scholar]
- Marek J.; Čížek J.; Tvrzník D. Optimizing porous material in shock electrodialysis unit. Desalin. Water Treat. 2019, 170, 38–45. 10.5004/dwt.2019.24504. [DOI] [Google Scholar]
- Gupta S. S.; Islam M. R.; Pradeep T.. Advances in Water Purification Techniques: Meeting the Needs of Developed and Developing Countries; Elsevier, 2018. [Google Scholar]
- Xing W.; Liang J.; Tang W.; He D.; Yan M.; Wang X.; Luo Y.; Tang N.; Huang M. Versatile applications of capacitive deionization (CDI)-based technologies. Desalination 2020, 482, 114390. 10.1016/j.desal.2020.114390. [DOI] [Google Scholar]
- He C.; Lian B.; Ma J.; Zhang C.; Wang Y.; Mo H.; Waite T. D. Scale-up and Modelling of Flow-electrode CDI Using Tubular Electrodes. Water Res. 2021, 203, 117498. 10.1016/j.watres.2021.117498. [DOI] [PubMed] [Google Scholar]
- Kim C.; Srimuk P.; Lee J.; Aslan M.; Presser V. Semi-continuous capacitive deionization using multi-channel flow stream and ion exchange membranes. Desalination 2018, 425, 104–110. 10.1016/j.desal.2017.10.012. [DOI] [Google Scholar]
- Lee J.; Lee J.; Hong S. W.; Kim C.; Yoon J. Parametric study of multichannel desalination battery for low-energy electrochemical deionization of brackish water. Desalination 2021, 515, 115188. 10.1016/j.desal.2021.115188. [DOI] [Google Scholar]
- Hasseler T. D.; Ramachandran A.; Tarpeh W. A.; Stadermann M.; Santiago J. G. Process design tools and techno-economic analysis for capacitive deionization. Water Res. 2020, 183, 116034. 10.1016/j.watres.2020.116034. [DOI] [PubMed] [Google Scholar]
- Liu X.; Shanbhag S.; Bartholomew T. V.; Whitacre J. F.; Mauter M. S. Cost Comparison of Capacitive Deionization and Reverse Osmosis for Brackish Water Desalination. ACS ES&T Engineering 2021, 1, 261–273. 10.1021/acsestengg.0c00094. [DOI] [Google Scholar]
- Bales C.; Kovalsky P.; Fletcher J.; Waite T. D. Low cost desalination of brackish groundwaters by Capacitive Deionization (CDI)—Implications for irrigated agriculture. Desalination 2019, 453, 37–53. 10.1016/j.desal.2018.12.001. [DOI] [Google Scholar]
- Shen Y.-Y.; Sun S.-H.; Tsai S.-W.; Chen T.-H.; Hou C.-H. Development of a membrane capacitive deionization stack for domestic wastewater reclamation: A pilot-scale feasibility study. Desalination 2021, 500, 114851. 10.1016/j.desal.2020.114851. [DOI] [Google Scholar]
- Wang Q.; Fang K.; He C.; Wang K. Ammonia removal from municipal wastewater via membrane capacitive deionization (MCDI) in pilot-scale. Sep. Purif. Technol. 2022, 286, 120469. 10.1016/j.seppur.2022.120469. [DOI] [Google Scholar]
- Jeon S.-i.; Kim N.; Jo K.; Ahn J.; Joo H.; Lee C.; Kim C.; Yoon J. Improvement in the desalination performance of membrane capacitive deionization with a bipolar electrode via an energy recovery process. Chemical Engineering Journal 2022, 439, 135603. 10.1016/j.cej.2022.135603. [DOI] [Google Scholar]
- Joo H.; Kim S.; Kim S.; Choi M.; Kim S.-H.; Yoon J. Pilot-scale demonstration of an electrochemical system for lithium recovery from the desalination concentrate. Environ. Sci.: Water Res. Technol. 2020, 6, 290–295. 10.1039/C9EW00756C. [DOI] [Google Scholar]
- Reale E. R.; Regenwetter L.; Agrawal A.; Dardon B.; Dicola N.; Sanagala S.; Smith K. C. Low porosity, high areal-capacity Prussian blue analogue electrodes enhance salt removal and thermodynamic efficiency in symmetric Faradaic deionization with automated fluid control. Water Research X 2021, 13, 100116. 10.1016/j.wroa.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.; Jeon J.; Elbert J.; Kim C.; Su X. Redox-mediated electrochemical desalination for waste valorization in dairy production. Chemical Engineering Journal 2022, 428, 131082. 10.1016/j.cej.2021.131082. [DOI] [Google Scholar]
- Hand S.; Cusick R. D. Emerging investigator series: capacitive deionization for selective removal of nitrate and perchlorate: impacts of ion selectivity and operating constraints on treatment costs. Environ. Sci.: Water Res. Technol. 2020, 6, 925–934. 10.1039/C9EW01105F. [DOI] [Google Scholar]
- Baldea M. From process integration to process intensification. Comput. Chem. Eng. 2015, 81, 104–114. 10.1016/j.compchemeng.2015.03.011. [DOI] [Google Scholar]
- Keil F. J. Process intensification. Reviews in Chemical Engineering 2018, 34, 135–200. 10.1515/revce-2017-0085. [DOI] [Google Scholar]
- Kim Y.-h.; Park L. K.; Yiacoumi S.; Tsouris C. Modular chemical process intensification: a review. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 359–380. 10.1146/annurev-chembioeng-060816-101354. [DOI] [PubMed] [Google Scholar]
- Su X. Electrochemical Interfaces for Chemical and Biomolecular Separations. Curr. Opin. Colloid Interface Sci. 2020, 46, 77–93. 10.1016/j.cocis.2020.04.005. [DOI] [Google Scholar]
- Schiffer Z. J.; Manthiram K. Electrification and decarbonization of the chemical industry. Joule 2017, 1, 10–14. 10.1016/j.joule.2017.07.008. [DOI] [Google Scholar]
- Verma S.; Lu S.; Kenis P. J. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nature Energy 2019, 4, 466–474. 10.1038/s41560-019-0374-6. [DOI] [Google Scholar]
- Barton J. L. Electrification of the chemical industry. Science 2020, 368, 1181–1182. 10.1126/science.abb8061. [DOI] [PubMed] [Google Scholar]
- Kim S.; Kim J.; Kim S.; Lee J.; Yoon J. Electrochemical lithium recovery and organic pollutant removal from industrial wastewater of a battery recycling plant. Environ. Sci.: Water Res. Technol. 2018, 4, 175–182. 10.1039/C7EW00454K. [DOI] [Google Scholar]
- Zhao Z.; Si X.; Liu X.; He L.; Liang X. Li extraction from high Mg/Li ratio brine with LiFePO4/FePO4 as electrode materials. Hydrometallurgy 2013, 133, 75–83. 10.1016/j.hydromet.2012.11.013. [DOI] [Google Scholar]
- He L.; Xu W.; Song Y.; Luo Y.; Liu X.; Zhao Z. New Insights into the Application of Lithium-Ion Battery Materials: Selective Extraction of Lithium from Brines via a Rocking-Chair Lithium-Ion Battery System. Global Challenges 2018, 2, 1700079. 10.1002/gch2.201700079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Yang H.; Wang Y.; Sha Z. Review on the electrochemical extraction of lithium from seawater/brine. J. Electroanal. Chem. 2019, 850, 113389. 10.1016/j.jelechem.2019.113389. [DOI] [Google Scholar]
- Su X.; Bromberg L.; Tan K.-J.; Jamison T. F.; Padhye L. P.; Hatton T. A. Electrochemically mediated reduction of Nitrosamines by Hemin-Functionalized Redox electrodes. Environmental Science & Technology Letters 2017, 4, 161–167. 10.1021/acs.estlett.7b00059. [DOI] [Google Scholar]
- Vapnik H.; Elbert J.; Su X. Redox-copolymers for the recovery of rare earth elements by electrochemically regenerated ion-exchange. Journal of Materials Chemistry A 2021, 9, 20068–20077. 10.1039/D1TA03334D. [DOI] [Google Scholar]
- Medina P. B.; Cotty S.; Kim K.; Elbert J.; Su X. Emerging investigator series: electrochemically-mediated remediation of GenX using redox-copolymers. Environ. Sci.: Water Res. Technol. 2021, 7, 2231–2240. 10.1039/D1EW00544H. [DOI] [Google Scholar]
- Tan K.-J.; Morikawa S.; Phillips K. R.; Ozbek N.; Hatton T. A. Redox-Active Magnetic Composites for Anionic Contaminant Removal from Water. ACS Appl. Mater. Interfaces 2022, 14, 8974–8983. 10.1021/acsami.1c21466. [DOI] [PubMed] [Google Scholar]
- Chen R.; Feng J.; Jeon J.; Sheehan T.; Rüttiger C.; Gallei M.; Shukla D.; Su X. Structure and Potential-Dependent Selectivity in Redox-Metallopolymers: Electrochemically Mediated Multicomponent Metal Separations. Adv. Funct. Mater. 2021, 31, 2009307. 10.1002/adfm.202009307. [DOI] [Google Scholar]
- Alabi A.; AlHajaj A.; Cseri L.; Szekely G.; Budd P.; Zou L. Review of nanomaterials-assisted ion exchange membranes for electromembrane desalination. NPJ Clean Water 2018, 1, 10. 10.1038/s41545-018-0009-7. [DOI] [Google Scholar]
- Razmjou A.; Asadnia M.; Hosseini E.; Habibnejad Korayem A.; Chen V. Design principles of ion selective nanostructured membranes for the extraction of lithium ions. Nat. Commun. 2019, 10, 5793. 10.1038/s41467-019-13648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunuguntla R. H.; Henley R. Y.; Yao Y.-C.; Pham T. A.; Wanunu M.; Noy A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. 10.1126/science.aan2438. [DOI] [PubMed] [Google Scholar]
- Daghrir R.; Drogui P.; Tshibangu J. Efficient treatment of domestic wastewater by electrochemical oxidation process using bored doped diamond anode. Sep. Purif. Technol. 2014, 131, 79–83. 10.1016/j.seppur.2014.04.048. [DOI] [Google Scholar]
- Sholl D. S.; Lively R. P. Exemplar Mixtures for Studying Complex Mixture Effects in Practical Chemical Separations. JACS Au 2022, 2, 322. 10.1021/jacsau.1c00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook W.; Chakrabarti M.; Aroua M.; Khan G.; Ali B.; Islam M.; Abu Hassan M. A. Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: a review. Desalination 2012, 285, 1–13. 10.1016/j.desal.2011.09.029. [DOI] [Google Scholar]
- Bian Y.; Chen X.; Ren Z. J. pH dependence of phosphorus speciation and transport in flow-electrode capacitive deionization. Environ. Sci. Technol. 2020, 54, 9116–9123. 10.1021/acs.est.0c01836. [DOI] [PubMed] [Google Scholar]
- Kim K.; Candeago R.; Rim G.; Raymond D.; Park A.-H. A.; Su X. Electrochemical approaches for selective recovery of critical elements in hydrometallurgical processes of complex feedstocks. Iscience 2021, 24, 102374. 10.1016/j.isci.2021.102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W.; Zhang Y. Sustainable electrochemical extraction of metal resources from waste streams: from removal to recovery. ACS Sustainable Chem. Eng. 2020, 8, 4693–4707. 10.1021/acssuschemeng.9b07007. [DOI] [Google Scholar]
- Kim K.; Raymond D.; Candeago R.; Su X. Selective cobalt and nickel electrodeposition for lithium-ion battery recycling through integrated electrolyte and interface control. Nat. Commun. 2021, 12, 6554. 10.1038/s41467-021-26814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L. E. O. C.; Mansur M. B. Hydrometallurgical separation of rare earth elements, cobalt and nickel from spent nickel–metal–hydride batteries. J. Power Sources 2010, 195, 3735–3741. 10.1016/j.jpowsour.2009.12.071. [DOI] [Google Scholar]
- Mir N.; Bicer Y. Thermodynamic modeling of a combined photo-electrodialysis-chloralkali system for sustainable desalination. Desalination 2021, 499, 114822. 10.1016/j.desal.2020.114822. [DOI] [Google Scholar]
- Mohandass G.; Kim T.; Krishnan S. Continuous Solar Desalination of Brackish Water via a Monolithically Integrated Redox Flow Device. ACS ES&T Engineering 2021, 1, 1678–1687. 10.1021/acsestengg.1c00266. [DOI] [Google Scholar]
- Chen F.; Karthick R.; Zhang Q.; Wang J.; Liang M.; Dai J.; Jiang X.; Jiang Y. Exploration of a photo-redox desalination generator. Journal of Materials Chemistry A 2019, 7, 20169–20175. 10.1039/C9TA06307B. [DOI] [Google Scholar]
- Kim S.; Piao G.; Han D. S.; Shon H. K.; Park H. Solar desalination coupled with water remediation and molecular hydrogen production: a novel solar water-energy nexus. Energy Environ. Sci. 2018, 11, 344–353. 10.1039/C7EE02640D. [DOI] [Google Scholar]
- Santiago A. R.; Baldaguez Medina P. B.; Su X. Electrochemical remediation of perfluoroalkyl substances from water. Electrochim. Acta 2022, 403, 139635. 10.1016/j.electacta.2021.139635. [DOI] [Google Scholar]
- Pan Z.; Song C.; Li L.; Wang H.; Pan Y.; Wang C.; Li J.; Wang T.; Feng X. Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: Recent advances and future prospects. Chemical Engineering Journal 2019, 376, 120909. 10.1016/j.cej.2019.01.188. [DOI] [Google Scholar]
- Jo K.; Baek Y.; Kim S.; Hong S. P.; Yoon J. Evaluation of long-term stability in capacitive deionization using activated carbon electrodes coated with ion exchange polymers. Korean Journal of Chemical Engineering 2020, 37, 1199–1205. 10.1007/s11814-020-0530-5. [DOI] [Google Scholar]
- Duan F.; Du X.; Li Y.; Cao H.; Zhang Y. Desalination stability of capacitive deionization using ordered mesoporous carbon: effect of oxygen-containing surface groups and pore properties. Desalination 2015, 376, 17–24. 10.1016/j.desal.2015.08.009. [DOI] [Google Scholar]
- Srimuk P.; Zeiger M.; Jackel N.; Tolosa A.; Kruner B.; Fleischmann S.; Grobelsek I.; Aslan M.; Shvartsev B.; Suss M. E.; Presser V. Enhanced performance stability of carbon/titania hybrid electrodes during capacitive deionization of oxygen saturated saline water. Electrochim. Acta 2017, 224, 314–328. 10.1016/j.electacta.2016.12.060. [DOI] [Google Scholar]
- Vineyard D.; Hicks A.; Karthikeyan K.; Davidson C.; Barak P. Life cycle assessment of electrodialysis for sidestream nitrogen recovery in municipal wastewater treatment. Cleaner Environmental Systems 2021, 2, 100026. 10.1016/j.cesys.2021.100026. [DOI] [Google Scholar]
- Fernandez-Gonzalez C.; Dominguez-Ramos A.; Ibañez R.; Irabien A. Sustainability assessment of electrodialysis powered by photovoltaic solar energy for freshwater production. Renewable and Sustainable Energy Reviews 2015, 47, 604–615. 10.1016/j.rser.2015.03.018. [DOI] [Google Scholar]