Abstract
Objective
To characterise uncontrolled severe asthma and compare the disease burden with the general and asthmatic populations.
Design
Retrospective observational study using a national sample of a French healthcare database (Echantillon Généraliste des Bénéficiaires (EGB)).
Setting
The EGB, an anonymised permanent sample of health insurance databases, representing 1/97th of the French population.
Participants
Patients (≥12 years) were selected in year 2014 and followed 2 years. A cohort of patients with uncontrolled severe asthma was defined using an algorithm based on peer-reviewed literature and Global Initiative for Asthma recommendations. Index date was the occurrence of the first marker of uncontrolled asthma. This cohort was matched with two control cohorts, general population and asthmatic controls, on baseline characteristics.
Main outcomes measures
Mortality, healthcare use and associated costs were studied in the 2 years of follow-up.
Results
Among 467 716 individuals in the EGB, 16 588 patients with asthma were identified, including 739 (4.5%) with uncontrolled severe disease. The survival probability at 2 years for patients with uncontrolled severe asthma (92.0%) was lower than in the general population cohort (96.6%; relative risk of death: 2.35; 95% CI: 1.70 to 3.29; p<0.0001) and tended to be lower than in the control asthmatic cohort (94.3%; p=0.07). Emergency department visits and hospitalisations were higher in patients with uncontrolled severe asthma than in the general population (64.7% vs 34.9%; p<0.0001) and asthmatic controls (64.7% vs 55.2%; p=0.0002). Other components of healthcare use (medical and paramedical visits, medications) were increased in patients with uncontrolled severe asthma compared with control populations. These increases translated into higher costs (p<0.0001 for both comparisons).
Conclusions
This study demonstrates the huge burden of uncontrolled severe asthma in terms of mortality, morbidity and healthcare resource consumption compared with other patients with asthma and with the general population and emphasises the importance of appropriate management in this high-risk population.
Keywords: Asthma, Epidemiology, HEALTH ECONOMICS
Strengths and limitations of this study
The study was conducted using a well-recognised and robust populational medical administrative database that confers many advantages, such as the completeness of mortality data and comprehensiveness of healthcare reimbursed for all patients.
This is the first study, to our knowledge, to specifically compare patients with uncontrolled severe asthma with the overall asthmatic population, in the French population.
Comparability of studied groups was ensured by careful matching process.
The lack of clinical data was mitigated by the use of a comprehensive algorithm to identify the uncontrolled severe asthmatic population with great care in the definitions of asthma, severity and control to ensure the accuracy of the cohorts.
The criteria requiring continuous insurance coverage may have resulted in a marginally lower number of younger patients being included due to student-specific insurance offered during school years.
Introduction
Asthma is one of the most common chronic diseases and is a major cause of morbidity. This heterogeneous disease is characterised by its chronic inflammatory nature which can lead to airway remodelling and presents with varied levels of severity and control. While a majority of patients with asthma have mild to moderate disease according to Global Initiative for Asthma (GINA) criteria, between 3% and 10% present a severe asthma, which may be life-threatening, particularly due to severe and potentially fatal exacerbations.1 2 Despite implementation of an optimal management strategy, many patients with severe asthma are not able to achieve disease control, leading to poor quality of life and significant social and health burdens. Therefore, asthma represents an important public health issue given its impact on work productivity (ie, absenteeism and presenteeism) and the costs associated with disease management and healthcare resource utilisation.3–7
In the context of the rapidly evolving landscape of expensive GINA step 5 asthma treatments, it is crucial to generate up-to-date data on the real-life population burden of uncontrolled severe asthma in adolescents and adults and to understand the relative impact of the disease in patients with severe asthma compared with the general and non-severe asthmatic populations. The framework of the French Health Data Hub project provided the opportunity to access the public medico-administrative database to address this specific issue.
The RESONANCE study was undertaken to: (1) characterise uncontrolled severe asthma in patients aged 12 years and older and (2) compare the disease burden in terms of mortality, healthcare utilisation and associated costs within this population to two series of matched controls from the general population and the asthmatic population (ie, total asthmatic population excluding those with uncontrolled severe asthma).
Methods
Study design
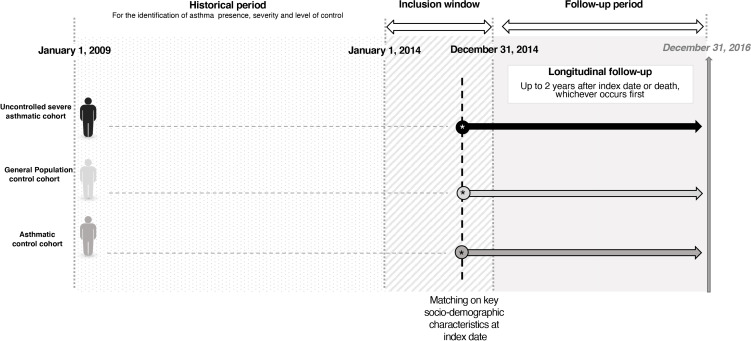
A retrospective, non-interventional cohort study was carried out using a large populational database from France, the Echantillon Généraliste des Bénéficiaires (EGB). All individuals aged 12 years and over in the EGB were included in the source population. A cohort of adolescent and adult patients who had uncontrolled severe asthma during 2014 were identified. Their index date was the date of the first event identifying non-control during 2014. Two control cohorts were defined, one from the general population and another of patients with asthma. For each selected control, the assigned index date was the calendar index date of respective matched patients from the uncontrolled severe asthma cohort.
Historical information on comorbidities, healthcare use and treatments received was collected up to 5 years preceding the index date to assess baseline characteristics. Sociodemographic characteristics were collected at index date for the three cohorts. Cohorts were followed up to 2 years after the index date or death, whichever occurs first, to assess the outcomes of interest (figure 1).
Figure 1.
RESONANCE study design.
Data sources
The EGB is an anonymised permanent sample of health insurance databases representing 1/97th of the entire French population.8 9 Given the universal healthcare system in France, the EGB is a sizeable and representative sample of all subjects covered by various social security schemes, and now includes nearly 660 000 beneficiaries.
The EGB contains information on demographics (age, sex, area of residence and so on); outpatient care reimbursement (including drug dispensing); medical care and reimbursement received in outpatient and hospital settings; specific information on the right to universal supplementary health coverage (CMU-c); whether beneficiaries are affected by a long-term condition (affection de longue durée; ALD) and the date of death. The database benefits from the interlinkage of several administrative data sources, thereby allowing an assessment of beneficiaries’ healthcare utilisation over time.
Subjects
Patients with uncontrolled severe asthma were identified using an algorithm based on events occurring in 2014 (online supplemental figure S1). This algorithm was designed following a literature review,10–12 and GINA management recommendations,13 in collaboration with an expert scientific committee composed of three respiratory specialists.
bmjopen-2021-060160supp001.pdf (794.7KB, pdf)
Patients with evidence of another chronic pulmonary disease, including chronic obstructive pulmonary disease (COPD), identified through International Classification of Diseases, 10th Revision codes related to hospital diagnoses or ALD, were excluded (online supplemental file 1).
Controls from general population were randomly selected among all individuals, besides patients with uncontrolled severe asthma, present in the EGB in 2014. Controls were matched with patients in the uncontrolled severe asthma cohort on age, sex and CMU-c status at index date. Up to three controls per case were selected. Direct matching quality was assessed by comparing standardised differences between both cohorts (online supplemental table S1).
Individuals in the asthma cohort were randomly selected within the total asthmatic population present in the EGB in 2014, excluding those with uncontrolled severe asthma. Individuals were matched with patients in the uncontrolled severe asthma cohort on a 1:1 ratio, using nearest neighbour propensity score matching. Propensity scores incorporated 45 variables recommended by the expert scientific committee, including sociodemographic characteristics, comorbidities and history of healthcare use prior to index date. Standardised differences were used to assess matching quality (online supplemental table S2).
Collected data and outcomes
The following sociodemographic and clinical characteristics were considered:
Up to 5 years prior to index date: comorbidities, treatments received, healthcare utilisation (medical visits, emergency department (ED) visits, hospitalisations).
At index date: age, sex, CMU-c status.
-
During follow-up:
Date of death, if applicable.
Drugs of interest: asthma treatments and other classes of interest (online supplemental file 1).
Healthcare utilisation: medical and paramedical visits (general medicine, respiratory specialists, otolaryngology, nursing support, physiotherapy), ED visits and hospitalisations (all-cause and asthma-related).
Direct medical costs associated with medications and healthcare use (outpatient and hospital settings).
The primary objective of this study was to assess the number and proportion of patients with uncontrolled severe asthma aged 12 and older. Sociodemographic and clinical characteristics were provided for this population.
The secondary objective was to assess all-cause mortality and survival probability, healthcare use and associated costs in the 2 years after index date in the uncontrolled severe asthma cohort compared with the two matched control cohorts (general population and asthmatic controls).
Statistical analyses
Continuous variables were described using means, SD, medians and ranges, while categorical variables were described as frequencies.
Crude cumulative all-cause mortality rates at 2 years were obtained for all cohorts using the Kaplan-Meier methodology, whereby the probability of survival from index date through follow-up was estimated. The comparative analyses of mortality, healthcare utilisation and associated costs between the primary cohort and matched controls were conducted using paired Student’s t-tests or Wilcoxon signed-rank tests for quantitative variables according to their distribution, and McNemar tests for qualitative variables. A post hoc analysis using a Cox regression model was built to compare mortality between the uncontrolled severe asthmatic cohort and general population. The final model included the following variables: population (uncontrolled severe asthmatic cohort vs general population), age, history of cardiovascular disease, diabetes, psychiatric disease and cancer. Interaction terms between the variable ‘population’ and other covariates included in the model were tested. Interaction between population and history of psychiatric disease was significant and was thus included in the final model, with other variables listed above. Costs were evaluated from the collective perspective, and all costs collected between 2014 and 2016 were reevaluated to correspond to 2018 € using the 2018 consumer price index (health data index 4011-E, published by national institute of statistics and economic studies, Institut National de la Statistique et des Études Économiques), since 2018 was the date of data access. All analyses were performed using the SAS V.9.4 software package (SAS Institute).
Patient and public involvement statement
Patients and the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Population selection
Of the 467 716 adolescents and adults present in the EGB, a total of 16 588 patients with asthma were identified and 5025 of these exhibited a marker of uncontrolled disease in 2014 (online supplemental figure S1). Among these, 739 patients with uncontrolled severe asthma were identified (57% women; mean age: 62.0 years; 10.0% with CMU-c) (online supplemental tables S1 and S2).
A total of 2217 matched individuals were selected to build the control cohort from the general population (online supplemental table S1). The control asthmatic population (all patients with asthma excluding those with uncontrolled severe asthma) included 739 patients matched to patients with uncontrolled severe asthma using propensity scores (53.0% women; mean age: 63.2 years; 7.7% with CMU-c) (online supplemental table S2). The majority of the 45 variables defined for matching were successfully balanced (online supplemental table S2 and figure S2).
Mortality
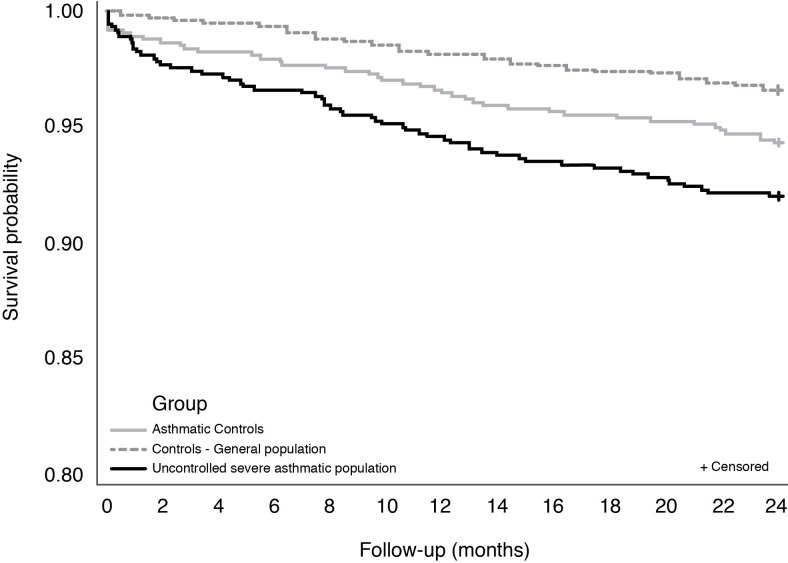
In total, 59 patients with uncontrolled severe asthma died during the 2-year follow-up period. The probability of survival at 2 years in this cohort was 92.0%, which was lower than that observed in the matched control cohort from the general population (96.6%; relative risk (RR) of death: 2.35; 95% CI: 1.70 to 3.29; p<0.0001). An increased mortality risk was notably seen among those aged 50 to <60 years old (RR: 18.0; 95% CI: 2.2 to 148.2), and those aged 60 to <70 years old (RR: 4.1; 95% CI: 1.9 to 8.7). While the asthmatic control cohort had a numerically higher survival probability at 2 years than the uncontrolled severe asthmatic cohort, the difference was not significant (94.3% vs 92.0%; p=0.0747). However, the analysis of mortality by age group showed a higher risk of mortality in patients aged 60 to <70 years (8% vs 2% deaths in uncontrolled severe asthmatic cohort vs asthmatic control cohort; RR: 4.1; 95% CI: 1.2 to 14.0; online supplemental figure S3).
Compared with both control cohorts, the increased risk of mortality in uncontrolled severe asthma cohort was observed early, and became higher during follow-up (figure 2).
Figure 2.
24-Month survival for the uncontrolled severe asthmatic cohort versus general population and asthmatic control cohorts.
Due to a significant interaction between population (uncontrolled severe asthma or general population) and history of psychiatric disease in the Cox model assessing mortality risk, impact of population was assessed by history of psychiatric disease, and impact of history of psychiatric disease was assessed by population (online supplemental table S3). Uncontrolled severe asthma increased mortality risk, only for patients without history of psychiatric disease (HR: 3.25; 95% CI: 2.14 to 4.92). The difference in mortality risk between patients with uncontrolled severe asthma and general population was not significant in patients with history of psychiatric disease (HR: 1.22; 95% CI: 0.66 to 2.26). Comorbidities (age, history of cardiovascular disease, diabetes and cancer) increased mortality risk, especially cancer (HR: 2.40; 95% CI: 1.64 to 3.50). Among subjects without history of psychiatric disease, overall survival was lower in the uncontrolled severe asthmatic cohort compared with general population, with a difference of 10% at 24 months (online supplemental figure S4).
Healthcare utilisation
Table 1 shows the level of healthcare utilisation for all three cohorts during follow-up. The proportion of patients requiring medical care in the hospital setting at least once during follow-up was higher in the uncontrolled severe asthmatic population compared with both control populations.
Table 1.
Comparison of the results for mortality and main healthcare use per patient during the 2-year follow-up
| Cohort of uncontrolled severe asthmatics (n=739) |
General population cohort (n=2217) |
P value | Asthmatic control cohort (n=739) |
P value | |
| Deaths, n (%) | 59 (8.0) | 75 (3.4) | p<0.0001 | 42 (5.7) | p=0.0747 |
| Deaths by age group, n (%) |
p=0.0393 | p=0.0366 | |||
| <20 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 20 to <30 | 0 (0.0) | 1 (1.3) | 0 (0.0) | ||
| 30 to <40 | 0 (0.0) | 1 (1.3) | 0 (0.0) | ||
| 40 to <50 | 0 (0.0) | 1 (1.3) | 0 (0.0) | ||
| 50 to <60 | 6 (10.2) | 1 (1.3) | 1 (2.4) | ||
| 60 to <70 | 15 (25.4) | 11 (14.7) | 3 (7.1) | ||
| 70 to <80 | 14 (23.7) | 16 (21.3) | 10 (23.8) | ||
| 80 to <90 | 15 (25.4) | 20 (26.7) | 16 (38.1) | ||
| ≥90 | 9 (15.3) | 24 (32.0) | 12 (28.6) | ||
| Mean age at death (σ) | 76.1 (12.5) | 79.7 (14.5) | p=0.0266 | 83.0 (9.7) | p=0.0037 |
| Min–max | 51.0–102.0 | 25.0–100.0 | 55.0–102.0 | ||
| At least one medical care act done at hospital, n (%) | 478 (64.7) | 774 (34.9) | p<0.0001 | 408 (55.2) | p=0.0002 |
| ED visit | 318 (43.0) | 465 (21.0) | p<0.0001 | 247 (33.4) | p=0.0002 |
| All-cause hospitalisation | 409 (55.3) | 556 (25.1) | p<0.0001 | 349 (47.2) | p=0.0023 |
| Asthma-related hospitalisations (asthma and severe exacerbation)* | 27 (3.7) | 0 (0.0) | NA | 12 (1.6) | p=0.0163 |
| Asthma-related hospitalisations with at least one visit in intensive care unit | 3 (0.4) | 0 (0.0) | NA | 1 (0.1) | 0.3173 |
| At least one medical visit during follow-up, n (%) | 708 (95.8) | 1995 (90.0) | p<0.0001 | 709 (95.9) | p=0.8981 |
| General practitioner | 602 (81.5) | 1628 (73.4) | p<0.0001 | 620 (83.9) | p=0.2185 |
| Respiratory specialist | 94 (12.7) | 53 (2.4) | p<0.0001 | 42 (5.7) | p<0.0001 |
| Ear–nose–throat specialist | 67 (9.1) | 122 (5.5) | p<0.0001 | 37 (5.0) | p=0.0030 |
| At least one paramedical visit during follow-up, n (%) | 600 (81.2) | 1390 (62.7) | p<0.0001 | 546 (73.9) | p=0.0011 |
| Nurse consultation | 531 (71.9) | 1184 (53.4) | p<0.0001 | 496 (66.7) | p=0.0353 |
| Physiotherapy consultation | 320 (43.3) | 673 (30.4) | p<0.0001 | 259 (35.0) | p=0.0013 |
| At least one filled prescription for an asthma medication during follow-up, n (%) | 726 (98.2) | 257 (11.6) | p<0.0001 | 638 (86.3) | p<0.0001 |
| Short-acting bronchodilator | 606 (82.0) | 100 (4.5) | p<0.0001 | 396 (53.6) | p<0.0001 |
| Long-acting bronchodilator: LAMA/LABA | 316 (42.8) | 53 (2.4) | p=0.0004 | 190 (25.7) | p<0.0001 |
| Long-acting bronchodilator: xanthines | 56 (7.6) | 2 (0.1) | p<0.0001 | 10 (1.4) | p<0.0001 |
| Anti-inflammatory medication (ICS±OCS) | 506 (68.5) | 115 (5.2) | p<0.0001 | 273 (36.9) | p<0.0001 |
| Anti-inflammatory medication (ICS±OCS) and long-acting bronchodilator | 626 (84.7) | 128 (5.8) | p<0.0001 | 413 (55.9) | p<0.0001 |
| At least one visit to a GP, respiratory specialist or allergy specialist, followed within 10 days by a filled prescription of OCS, n (%) | 481 (65.1) | 447 (20.2) | p<0.0001 | 229 (31.0) | p<0.0001 |
| At least one filled prescription of a medication of interest during follow-up, n (%) | 727 (98.4) | 1847 (83.3) | p<0.0001 | 678 (91.7) | p<0.0001 |
| Intranasal antihistamines | 22 (3.0) | 13 (0.6) | p<0.0001 | 10 (1.4) | p=0.0339 |
| Ocular antihistamines | 43 (5.8) | 80 (3.6) | p=0.0004 | 27 (3.7) | p=0.0523 |
| Systemic antihistamines | 488 (66.0) | 675 (30.4) | p<0.0001 | 273 (36.9) | p<0.0001 |
| Intranasal corticosteroids | 420 (56.8) | 567 (25.6) | p<0.0001 | 230 (31.1) | p<0.0001 |
| Nonsteroidal anti-inflammatory drugs | 407 (55.1) | 1083 (48.8) | p<0.0001 | 317 (42.9) | p<0.0001 |
| Antibiotics | 670 (90.7) | 1323 (59.7) | p<0.0001 | 501 (67.8) | p<0.0001 |
| Proton-pump inhibitors | 506 (68.5) | 980 (44.2) | p<0.0001 | 348 (47.1) | p<0.0001 |
| Psychotropic drugs | 426 (57.6) | 840 (37.9) | p<0.0001 | 320 (43.3) | p<0.0001 |
| Topical corticosteroids | 235 (31.8) | 512 (23.1) | p<0.0001 | 145 (19.6) | p<0.0001 |
| Topical immunosuppressants | 2 (0.3%) | 1 (0.0) | p=0.0588 | 0 (0.0%) | NA |
*Excluding stays of less than one night (or Z codes)
ED, emergency department; GP, general practitioner; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; NA, not applicable; OCS, oral corticosteroid.
A twofold increase in ED visits and all-cause or asthma-related hospitalisations was observed for patients with uncontrolled severe asthma compared with the general population (64.7% vs 34.9%; p<0.0001). Patients with uncontrolled severe asthma also had an increased use of other main components of care, such as medical visits (95.8% vs 90.0%; p<0.0001) and paramedical visits (81.2% vs 62.7%; p<0.0001) compared with the general population. An increased healthcare use was also highlighted in comparison with the asthmatic cohort, since patients with uncontrolled severe asthma had more ED visits and hospitalisations (64.7% vs 55.2%; p=0.0002), and paramedical visits (81.2% vs 73.9%; p=0.0011), compared with asthmatic controls.
For drug prescriptions, the proportion of patients receiving medications was higher in patients with uncontrolled severe asthma compared with matched individuals from the general population cohort, both in terms of asthma-related treatments including oral corticosteroid therapy (98.2% vs 11.6%; p<0.0001), and other treatments, such as antibiotics, nonsteroidal anti-inflammatory drugs and psychotropic drugs (98.4% vs 83.3%; p<0.0001). Patients with uncontrolled severe asthma also had higher medication use than the asthmatic controls both for asthma-related treatments (98.2% vs 86.3%; p<0.0001), and other medications (98.4% vs 91.7%; p<0.0001).
Costs
Increase of healthcare use was translated into higher costs. Healthcare use mean costs per patient during the 2-year follow-up were higher for patients with uncontrolled severe asthma (€14 020) compared with the general population (€3564; p<0.0001) or asthmatic controls (€6418; p<0.0001), regardless of the expenditure (table 2).
Table 2.
Comparison of costs (reimbursement amounts, in Euros) associated with hospital and outpatient use, per patient, over a 2-year period after the index date (patients with uncontrolled severe asthma vs general population and asthmatic control cohorts)
| Costs per patient | Cohort of patients with uncontrolled severe asthma (n=739) |
General population cohort (n=2217) |
P value | Asthmatic control cohort (n=739) |
P value | |
| Hospitalisation | Mean (σ) | 8163 (20 669) | 1800 (7991) | p<0.0001 | 3589 (13 702) | p<0.0001 |
| Median(Q1-Q3) | 426 (0–5854) | 0 (0–0) | 0 (0–1284) | |||
| Min–max | 0–246 483 | 0–176 423 | 0–190 457 | |||
| ED visit without hospitalisation | Mean (σ) | 12 (28) | 6 (18) | p<0.0001 | 9 (33) | p<0.0001 |
| Median (Q1–Q3) | 0 (0–25) | 0 (0–0) | 0 (0–0) | |||
| Min–max | 0–319 | 0–273 | 0–671 | |||
| Medical visits | Mean (σ) | 643 (1068) | 368 (437) | p<0.0001 | 496 (658) | p<0.0001 |
| Median (Q1–Q3) | 491 (278–786) | 265 (113–499) | 376 (217–612) | |||
| Min–max | 0–24 882 | 0–6861 | 0–11 547 | |||
| Laboratory tests | Mean (σ) | 241 (321) | 154 (266) | p<0.0001 | 203 (285) | p=0.0131 |
| Median (Q1–Q3) | 145 (53–295) | 85 (0–180) | 126 (54–237) | |||
| Min–max | 0–3137 | 0–5727 | 0–2461 | |||
| Paramedical visits | Mean (σ) | 1406 (4827) | 747 (2986) | p<0.0001 | 968 (3236) | p<0.0001 |
| Median (Q1–Q3) | 176 (12–689) | 18 (0–288) | 42 (0–469) | |||
| Min–max | 0–65 527 | 0–50 060 | 0–36 145 | |||
| Medications | Mean (σ) | 3076 (9131) | 199 (410) | p<0.0001 | 721 (747) | p<0.0001 |
| Median (Q1–Q3) | 1525 (1037–2185) | 58 (12–220) | 523 (246–957) | |||
| Min–max | 0–95 453 | 0–5855 | 0–9472 | |||
| Total costs | Mean (σ) | 14 020 (24 076) | 3564 (9540) | p<0.0001 | 6418 (15 109) | p<0.0001 |
| Median (Q1–Q3) | 4625 (2492–13 897) | 825 (297–2286) | 1905 (1020–4906) | |||
| Min–max | 105–253 160 | 0–190 380 | 0–193 294 |
ED, emergency department.
The average cost of a patient with uncontrolled severe asthma who died was significantly higher than that of a surviving patient (€28 009 vs €11 851; p<0.0001). Primary drivers of this increase in patients with uncontrolled severe asthma were hospitalisations (fivefold and twofold increases vs the general and asthmatic populations, respectively), drugs of interest (16-fold and fourfold increases vs the general and asthmatic populations, respectively) and paramedical fees (twofold and 1.4-fold increases vs the general and asthmatic populations, respectively).
Discussion
Given the lack of recent data assessing the impact of uncontrolled severe asthma on public health, the RESONANCE study provided important data characterising this population and comparing it to the general and asthmatic populations in terms of morbidity and mortality, as well as healthcare use and associated costs. To our knowledge, this is the first study to specifically compare patients with uncontrolled severe asthma with the overall asthmatic population, in the French population.
The clinical burden of uncontrolled severe asthma was demonstrated through the increased risk of mortality, use of additional treatments (asthma-related treatment and other associated treatments) and hospitalisations during the 2-year follow-up period in comparison with matched patients from the general and asthmatic populations. The average cost of uncontrolled severe asthma for healthcare system during the 2 years of follow-up represented nearly four times (mean cost difference of €10 456 per patient) and more than two times (mean cost difference of €7602 per patient) that of matched patients from the general population and from asthmatic control cohort, respectively, over the same period. Bourdin et al found an annually incremental cost of $5276 for healthcare system when comparing patients with severe asthma with the general population, although a 2-year extrapolation would be biased due to the non-annual rate of care seeking in the study population.12 However, no specific assessment of uncontrolled severe asthma was performed.
Hospitalisations were the main driver of healthcare use and costs, in agreement with other studies assessing the economic burden of asthma.4 7 14 Costs during follow-up could have been influenced by the cost of hospitalisation occurring at index date, since hospitalisation related to asthma was part of the selection criteria to identify asthma non-control; however, influence of this phenomenon was limited since only 5.1% of patients were selected based solely on this criterion.
This study highlighted the excess of healthcare costs during the last months of life in uncontrolled severe asthma, accounting for part of the excess cost associated with this condition overall, given their higher mortality rate during the follow-up period.
Our study also assessed the size of the uncontrolled severe asthma population. The 739 patients identified among the total population of 16 588 patients with asthma suggest that this small but high-risk population represented 4.5% of all patients with asthma in France in 2014. When extrapolating to the entire adolescent and adult French population, this represents a prevalence of 0.15% (ie, 86 342 patients; 95% CI: 80 341 to 92 789), which is slightly lower than other severe asthma populational estimates that used different definitions of asthma severity and did not account for asthma non-control.12 15
The uncontrolled severe asthmatic population was identified by means of a comprehensive algorithm, with great care in the definitions of asthma, severity and control to ensure the accuracy of the cohorts and palliate the lack of clinical data. Asthma was defined with robust data, such as asthma-related hospitalisations and complications, asthma-related full coverage for a long-term condition and specific treatments.
Two cohorts were compared with patients with uncontrolled severe asthma. The objective of the general population control cohort was to highlight the absolute burden of uncontrolled severe asthma in the population. Matching on key sociodemographic characteristics aimed to control for potential confounding bias and ensure comparability between populations, which was confirmed by acceptable standardised differences. In addition, a Cox regression model was developed in post hoc analysis to compare mortality risk between the uncontrolled severe asthmatic cohort and the general population: uncontrolled severe asthma was associated with increased mortality, only for patients without history of psychiatric disease. This increased risk was not found in patients with history of psychiatric disease, however, the statistical model was only adjusted for age, history of cardiovascular disease, diabetes and cancer but not for other potential confounders such as psychotropic drugs use since we did not aim at assessing factors associated with mortality in subjects with a history of psychiatric disease.
The asthmatic control cohort was selected and matched with patients with uncontrolled severe asthma using propensity scores to control potential confounders, using all available and relevant variables. A conservative approach was used, since some potential confounders included in propensity scores may be preponderant in patients with uncontrolled severe asthma, leading to a potential minimisation of the burden of asthma lack of control and severity. More, given that this control population includes severe but controlled or non-controlled but non-severe patients with asthma, it is not a population exclusively comprising low-risk patients.
The study was conducted using the EGB, a well-recognised and robust populational medical administrative database that confers many advantages, such as the completeness of mortality data and comprehensiveness of healthcare reimbursed for all patients. Of note, the EGB does not include clinical information such as visit reason, diagnoses made outside hospitals, biological test results or patients’ anthropometric data.
The challenges associated with the use of administrative databases have been well documented.16 In this study, the difficulties in selecting the patients with uncontrolled severe asthma were associated with potential measurement bias related to coding errors in hospital diagnoses; this source of error is likely limited and non-differential between groups. The algorithms assumed that filled prescriptions were proxies to treatment use; however, it is not possible to confirm that a filled prescription has been taken.
Potential indications of asthma treatments for other chronic pulmonary diseases such as COPD were accounted for, since an exclusion criterion based on ALD declarations or hospitalisations related to other chronic pulmonary diseases was considered. Despite this, some patients with COPD may still have been included given the relatively old age of the asthmatic cohorts. This would have occurred if ALD for COPD has not been declared, or if a patient has never been hospitalised during the last 5 years. Criterion requiring continuous insurance coverage may have resulted in a lower number of younger patients being included due to student-specific insurance offered during school years; however, the impact of this limit on the results is likely minimal.
Patients have been assigned to their group of interest (uncontrolled severe asthma, other patients with asthma, general population) at index date. Some patients may have evolved from non-asthma to asthma, or from asthma to uncontrolled severe asthma during follow-up and this was not considered in statistical analyses. However, given the duration of follow-up of 2 years, impact of such misclassification of patients during follow-up is probably limited.
The EGB offers the advantage of a limited number of patients lost to follow-up given the universal healthcare system present in France. It also has a few limits: while most patients were covered by the general scheme, patients covered by other plans may have not been selected due to the later integration of those plans in the EGB. Also, the algorithms used to define asthma and uncontrolled asthma excluded patients who died at the hospital around the time of index date, thereby potentially excluding the most severe and uncontrolled patients. Finally, additional residual confounding factors may persist despite the matching processes.
This large study allowing the comparison between patients with uncontrolled severe asthma and other patients with asthma and the general population, highlighted that severe asthma associated with non-control significantly affects mortality, healthcare use and associated costs. This study emphasises that close attention should be paid to ensure appropriate management of patients with severe asthma and monitoring of the level of control of asthma symptoms.
Conclusions
This database study demonstrates the huge burden of uncontrolled severe asthma in terms of mortality, morbidity and healthcare resource consumption compared with other patients with asthma and the general population. These findings emphasise the importance of appropriate management in this high-risk population.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the assistance of Anaïs Havet (PharmD, PhD) and Lucile Dheyriat (PharmD), of stève consultants for assistance in drafting of this manuscript, which was funded by Sanofi.
Footnotes
Contributors: All authors participated in the interpretation of the data, provided critical feedback and final approval for submission, and took responsibility for the accuracy, completeness and protocol adherence of data and analyses. SB is the guarantor of the study, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to concept and design; acquisition, analysis or interpretation of data. AdL and SB contributed to drafting of the manuscript. NR, PD, GG, CC, VP, AM and LV contributed to critical revision of the manuscript for important intellectual content. AdL, SB and CC contributed to statistical analysis. SB, VP, AM and LV contributed to administrative, technical or material support. All authors contributed to supervision.
Funding: This work was supported by Sanofi-Aventis France.
Competing interests: PD has received honoraria and/or research grants from ALK, Mylan-Viatris, Stallergenes, ThermoFisher Scientific, AstraZeneca, GSK, Novartis, Menarini and Regeneron. GG has received honoraria from ALK, Novartis, AstraZeneca, GSK, Sanofi and Chiesi for conferences or advisory board meetings. NR has received research funding from Boehringer Ingelheim, GSK, Pfizer and Novartis and honoraria from Boehringer Ingelheim, Pfizer, Novartis, Teva, GSK, AstraZeneca, Chiesi, Sanofi and Zambon. LV, AM, VP are Sanofi employee and may hold shares and/or stock options in the company. No other disclosures were reported.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Used data were anonymised to ensure confidentiality at the patient level. Data handling, processing and analysis were carried out in alignment and with authorisations from an independent committee (CEREES, Comité d’Expertise pour les Recherches, les Études et les Évaluations dans le domaine de la Santé; favourable opinion on 14 June 2018; TPS 31996) and from the French National Data Protection Agency (CNIL, Commission Nationale de l’Informatique et des Libertés; Authorisation DR-2018-097).
References
- 1.Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol 2000;106:1033–42. 10.1067/mai.2000.111307 [DOI] [PubMed] [Google Scholar]
- 2.Israel E, Reddel HK, Severe RHK. Severe and difficult-to-treat asthma in adults. N Engl J Med 2017;377:965–76. 10.1056/NEJMra1608969 [DOI] [PubMed] [Google Scholar]
- 3.Yaghoubi M, Adibi A, Safari A, et al. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med 2019;200:1102–12. 10.1164/rccm.201901-0016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Neill S, Sweeney J, Patterson CC, et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British thoracic Society difficult asthma registry. Thorax 2015;70:376–8. 10.1136/thoraxjnl-2013-204114 [DOI] [PubMed] [Google Scholar]
- 5.Godard P, Chanez P, Siraudin L, et al. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J 2002;19:61–7. 10.1183/09031936.02.00232001 [DOI] [PubMed] [Google Scholar]
- 6.Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm 2016;22:848–61. 10.18553/jmcp.2016.22.7.848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med 2009;9:24. 10.1186/1471-2466-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne 2015;36:411–7. 10.1016/j.revmed.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 9.Bezin J, Duong M, Lassalle R, et al. The National healthcare system claims databases in France, SNIIRAM and EGb: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017;26:954–62. 10.1002/pds.4233 [DOI] [PubMed] [Google Scholar]
- 10.Catherine Quantin and Caisse nationale d’Assurance maladie des travailleurs salariés (CNAMTS). Etude des algorithmes de définition de pathologies dans le système national d’information inter-régimes de l’assurance maladie (SNIIRAM). Deuxième partie: Recensement des équipes utilisant les données du SNIIRAM, ainsi que des algorithmes d’identification de pathologies et des travaux publiés 2015. https://assurance-maladie.ameli.fr/sites/default/files/2014_etude-algorithmes-definition-pathologies-partie-2_cartographie.pdf [Google Scholar]
- 11.Catherine Quantin and Caisse nationale d’Assurance maladie des travailleurs salariés (CNAMTS). Etude des algorithmes de définition de pathologies dans le système national d’information inter-régimes de l’assurance maladie (SNIIRAM). Première partie : Les algorithmes d’identification de pathologies utilisés par la cartographie des patients et des dépenses, développée par la Caisse nationale de l’Assurance maladie des travailleurs salariés 2015. https://assurance-maladie.ameli.fr/sites/default/files/2014_etude-algorithmes-definition-pathologies-partie-1_cartographie.pdf [Google Scholar]
- 12.Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract 2019;7:1477–87. 10.1016/j.jaip.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 13.Global Initiative for Asthma . GINA report: global strategy for asthma management and prevention, 2018: 35–88. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf [Google Scholar]
- 14.Jalaludin BB, Smith MA, Chey T, et al. Risk factors for asthma deaths: a population-based, case-control study. Aust N Z J Public Health 1999;23:595–600. 10.1111/j.1467-842X.1999.tb01543.x [DOI] [PubMed] [Google Scholar]
- 15.Enquête Santé et Protection Sociale (ESPS) . Corrélations entre asthme déclaré et remboursements de médicaments dans l’enquête ESPS 2006, 2012. Available: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/asthme/documents/rapport-synthese/correlations-entre-asthme-declare-et-remboursements-de-medicaments-dans-l-enquete-esps-2006.-peut-on-proposer-des-indicateurs-de-suivi-de-la-prise [Accessed Nov 2021].
- 16.Jacob C, Haas JS, Bechtel B, et al. Assessing asthma severity based on claims data: a systematic review. Eur J Health Econ 2017;18:227–41. 10.1007/s10198-016-0769-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060160supp001.pdf (794.7KB, pdf)
Data Availability Statement
No data are available. All data relevant to the study are included in the article or uploaded as supplemental information.