Abstract
Introducing transition-metal components to ceria (CeO2) is important to tailor the surface redox properties for a broad scope of applications. The emergence of high-entropy oxides (HEOs) has brought transformative opportunities for oxygen defect engineering in ceria yet has been hindered by the difficulty in controllably introducing transition metals to the bulk lattice of ceria. Here, we report the fabrication of ceria-based nanocrystals with surface-confined atomic HEO layers for enhanced catalysis. The increased covalency of the transition-metal–oxygen bonds at the HEO–CeO2 interface promotes the formation of surface oxygen vacancies, enabling efficient oxygen activation and replenishment for enhanced CO oxidation capabilities. Understanding the structural heterogeneity involving bulk and surface oxygen defects in nanostructured HEOs provides useful insights into rational design of atomically precise metal oxides, whose increased compositional and structural complexities give rise to expanded functionalities.
Short abstract
Oxygen defect engineering of ceria nanocrystals is realized by building surface-confined high-entropy oxide layers, which exhibit enhanced CO oxidation capabilities.
Introduction
Activation of lattice oxygen in ceria (CeO2), usually associated with the formation and migration of oxygen vacancies, is fundamentally important for catalysis,1 energy conversion,2 magnetics,3 sensing, and biomedical applications.4,5 Introducing foreign metal species has been demonstrated to be an effective route to modulating the defect chemistry of ceria to access desirable properties.6 First-row transition-metal elements, including Cu, Co, Fe, Ni, and Mn, are intriguing promoters to activate lattice oxygen in CeO2 as substitutional dopant and/or interfacial domains.7 In comparison with binary and ternary rare-earth oxides that are chemically inert, transition-metal substitution induces catalytically active centers that efficiently trigger a variety of molecular transformations.8,9 For example, coordination-unsaturated copper species that are atomically dispersed on ceria yield high activity and stability for oxidation,10 hydrogenation,11 desulfurization,12 and water-gas shift reactions,13 as well as multiple electrocatalytic and photocatalytic reactions.14,15
The emergence of high-entropy oxides (HEOs) has introduced a distinct and transformative paradigm to manipulate the creation of oxygen defects in ceria-based oxides.16 Different from high-entropy alloys (HEAs) with single-site occupancies, independent cation and anion sublattices in HEOs have greater structural tunabilities.17−19 The enhanced configurational entropy gives rise to a higher endurance for lattice distortion. This allows the incorporation and stabilization of a higher concentration of aliovalent metal cations in the oxide lattice, which promotes the activation of lattice oxygen and formation of active species.20 Additionally, the sluggish kinetics of HEOs introduced improved thermal and chemical stabilities for the metal species under reaction conditions.21,22 The entropy-maximization principle has also been extended to the self-regeneration of the HEO-supported metal catalysts.23,24
Nonetheless, the applicability of this entropy-induced stabilization strategy is limited for ceria-based mixed oxides. The drastic size difference between the first-row transition metal and cerium atoms prompts the formation of oxide–oxide interfaces with inherent structural heterogeneities, which presents a fundamentally distinct scenario relative to the rare-earth-metal-based ceria solid solutions.25 The associated influence on the oxygen defects of ceria has remained elusive, mainly due to the inherent complexities in oxygen-containing structures and difficulties in controllably creating and accurately identifying different types of oxygen defects. Various oxygen-deficient CeO2−δ polymorphs within the Ce2O3–CeO2 composition range can be formed upon oxygen loss.6 Frenkel-type oxygen defects, which involve displacement of oxygen atoms from the octahedral interstitial sites in the fluorite lattice, are also common in CeO2.26 Discerning these stoichiometric and nonstoichiometric oxygen defects on the surface and in the bulk of ceria-based HEOs is thus challenging yet crucial, particularly for nanocatalysis. Accessibility to surface oxygen defects determines the reaction pathways for low-temperature molecular conversions, whereas the pathways in the bulk favor oxygen transport for electrocatalysis and high-temperature thermocatalysis.27,28 Fundamental insights are also desired to unveil and leverage the cation–oxygen-vacancy interactions in entropy-stabilized oxides for rational catalyst design.
Recent studies have observed pronounced local structural heterogeneities involving distinct surface and bulk domains of HEOs during synthesis and catalysis processes.20 Instead of the formation of entropy-stabilized single-phase solid solutions,29 building surface-confined HEO domains provides an alternative to tailor the surface reactivities of ceria via oxygen defect engineering. Here, we demonstrate the feasibility of constructing atomically thin HEO layers on ceria for enhanced catalysis. Neutron diffraction coupled with a pair-distribution function (PDF) analysis enables an atomistic investigation of the HEO–CeO2 interface, which identifies both the intrinsic interstitial oxygen defects in the bulk lattice and the extrinsic oxygen vacancies on the surface of CeO2. The strong covalency of the transition-metal–oxygen bonds facilitates the formation of surface-confined oxygen vacancies, which promotes efficient oxygen activation and replenishment for enhanced CO oxidation capabilities. Elucidating the structural heterogeneity involving surface and bulk oxygen defects in HEO nanocrystals lays the foundation for regulating oxygen defects in a broad range of complex oxides with expanded functionalities. For instance, controllably introducing different types of oxygen defects in nanoscale HEOs offers a potential entryway to modulating the electrochemical behaviors and engineering the oxide–oxide heterojunctions.
Results and Discussion
Synthesis and Characterizations of Ceria-Based Nanocrystals
Ceria-based nanocrystals with tunable compositions were prepared using colloidal synthesis. During the synthesis, cerium and transition-metal oleates decompose in the mixture of octadecene and oleylamine at 220 °C and ultimately form metal oxide nanoparticles.30 In addition to pristine CeO2 nanoparticles, 5 atom % Cu-substituted CeO2 (denoted Cu-CeO2) and 5 atom % Cu- and 5 atom % Co-co-substituted CeO2 (denoted CuCo-CeO2), corresponding to binary copper–ceria and ternary copper–cobalt–ceria systems, respectively, were also fabricated.10,31,32 Diversifying the dopant species can improve their dispersions on ceria.33,34 Further extension to entropy-stabilized multicomponent oxides brings elevated transition-metal contents stabilized in the ceria lattice due to the enhanced entropic effects, as revealed by recent studies.21,22,35 We thereby prepared CeO2 simultaneously substituted with Cu, Co, Fe, Ni, and Mn (5 atom % for each element, denoted CuCoFeNiMn-CeO2) to interrogate the entropic effects of the transition-metal promoters. Kinetic control in colloidal solutions warrants facile nucleation and growth of high-entropy nanocrystals at low temperatures,36,37 where phase segregation would induce an enthalpic penalty. The surface-capped oleylamine ligands, which are absent for high-temperature solid-state materials, can be another factor that fine-tunes the formation pathway of the HEO nanocrystals.
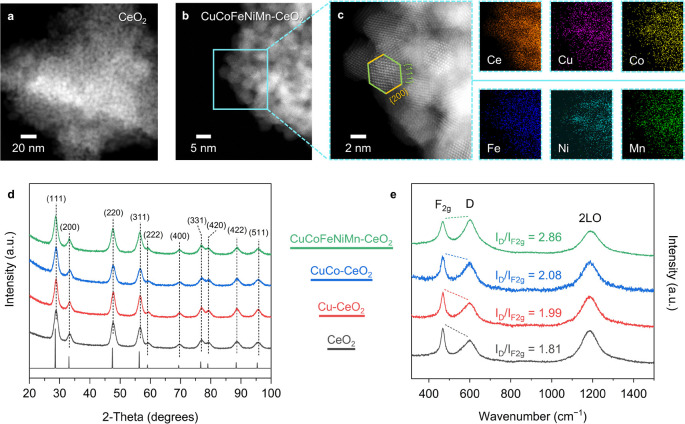
As shown by the transmission electron microscopy (TEM) images with corresponding fast Fourier transform (FFT) patterns (Figures S1 and S2), the as-synthesized colloidal CeO2 nanocrystallites are approximately 5 nm in size, with a truncated-octahedral shape exposing the {200} and {111} facets.31,38 The ceria-based colloidal nanocrystals were then annealed in air at 500 °C to remove the surface capping ligands for further characterization (Figure S3 and Tables S1 and S2). The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images with the corresponding STEM-energy dispersive X-ray spectroscopy (EDS) element maps show that the annealed samples retain the truncated-octahedral morphology with uniform elemental distributions (Figure 1a–c). The annealed powders also become darker with elevated substitution levels, transitioning from pale yellow for CeO2 to dark brown for CuCoFeNiMn-CeO2 (Figure S4). This color transition can be ascribed to the decreased band gaps along with transition-metal incorporation, which extends the absorption edge to lower energies and may bring enhanced photocatalytic activities.39
Figure 1.
HAADF-STEM images of the annealed (a) CeO2 and (b, c) CuCoFeNiMn-CeO2 nanocrystals with EDS elemental maps showing a uniform distribution of the transition-metal elements. The terminated {111} and {200} facets in the truncated-octahedral shape are highlighted in green and yellow, respectively. (d) XRD patterns of the annealed transition-metal-substituted CeO2 nanocrystals and the simulated reference pattern for comparison.40 (e) UV Raman spectra of the annealed ceria-based samples with an excitation wavelength of 325 nm. The relative peak heights of F2g and D are highlighted by dashed lines, with the intensity ratio (ID/IF2g) estimated according to Lorentzian fitting.
Figure S5 and Figure 1d display the powder X-ray diffraction (XRD) data for the colloidal and annealed CeO2, Cu-CeO2, CuCo-CeO2, and CuCoFeNiMn-CeO2 samples, with a reference pattern for bulk CeO2 for comparison.40 Diffraction peaks corresponding to the CeO2 phase can be identified for all of the samples, confirming retention of the host fluorite structure upon transition-metal incorporation. Notably, CuCoFeNiMn-CeO2 only exhibits the characteristic diffraction peaks matching well with the fluorite structure of CeO2, while additional crystalline peaks corresponding to the CuO phase emerge for the annealed copper-ceria sample with comparable transition-metal concentrations (∼25 atom %) (Figure S6). This suggests that diversifying the transition-metal components mitigates the formation of the segregated transition-metal oxides in the bulk form. According to the Gibbs free energy equation (ΔGmix = ΔHmix −TΔSmix), the enhanced configurational entropy (ΔSmix) lowers the overall Gibbs free energy (ΔGmix) through forming a homogeneous, randomly distributed phase,41 which inhibits phase segregation and prompts better dispersions of the transition-metal species.
Figure 2e displays the ultraviolet (UV) Raman spectra of the annealed CeO2 samples measured with a 325 nm excitation wavelength, which probes the dynamic interaction between the metal and oxygen atoms in ceria with a strong resonance effect.42 CeO2 exhibits the characteristic F2g and defect-induced D modes at 467 and 599 cm–1, respectively, together with the 2LO mode centered at 1188 cm–1. In comparison, the D and 2LO modes of CuCoFeNiMn-CeO2 exhibit an ∼4 cm–1 red shift relative to those of the other samples, while the F2g mode remains unchanged at ∼467 cm–1, implying substitution-induced modulation of the fluorite lattice (Table S3). Moreover, the relative intensity ratio between the D and F2g modes (ID/IF2g), which is indicative of the oxygen defect concentration in ceria,43 increases from CeO2 to CuCoFeNiMn-CeO2. Concentration of the oxygen defects in ceria is thus positively correlated with the overall concentration of the transition-metal substituents. This is further supported by the Raman spectra with 532 nm excitation wavelength, as the D mode of CuCoFeNiMn-CeO2 is observably stronger than those of the other samples (Figure S7 and Table S4).
Figure 2.
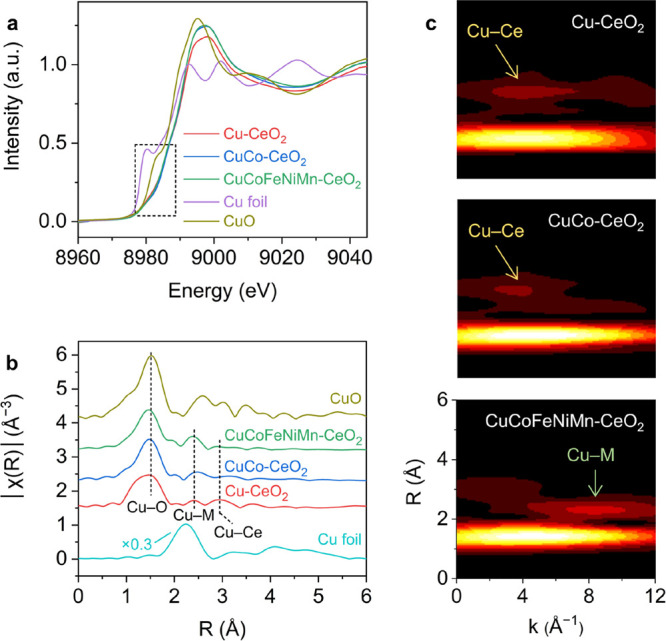
(a) Normalized Cu K-edge XANES spectra of the ceria-based samples with Cu and CuO references, with the pre-edge region (∼8980 eV) being highlighted. (b) Cu K-edge EXAFS spectra of the ceria-based samples with Cu and CuO references. The peaks corresponding to Cu–O, Cu–M, and Cu–Ce shells are highlighted for comparison. (c) Wavelet transforms of EXAFS for Cu-CeO2, CuCo-CeO2, and CuCoFeNiMn-CeO2.
Atomistic Insights into Oxygen Defects in Ceria-Based HEO Nanocrystals
The above results show the capability to create and modify oxygen defects in CeO2 nanocrystals by introducing transition-metal substituents and cosubstituents. However, the long-range, macroscopic characteristics derived from the XRD pattern and Raman spectra can hardly be utilized to precisely pinpoint the origin of the observed structure difference, especially for nanoscale materials.44 Possessing much smaller sizes in compariosn with that of cerium, the transition-metal substituents induce severe local distortions with short-range, nonperiodic features that underpin the local structures and the correlated functional properties.45 In our case, the pronounced surface-dependent properties derived from the high surface-area-to-volume ratio of nanocrystals affords the opportunity to differentiate bulk and surface characteristics. We resorted to synchrotron- and neutron-based techniques to elucidate the nature, type, and concentration of the oxygen defects, which helps identify the metal–oxygen correlations in the ceria-based nanocrystals.
X-ray absorption spectroscopy (XAS) was used to study the chemical environments of the metal cations. The Ce LIII edge remains almost identical for all the samples (Figure S8), indicating that the bulk Ce3+/Ce4+ ratio remains unaltered upon transition-metal substitution. This is consistent with the unchanged surface Ce3+/Ce4+ ratio as supported by the X-ray photoelectron spectroscopy (XPS) results of the Ce 3d region (Figure S9). We then focused on Cu, which is involved in all the three substituted samples, to acquire energetic insights. Figure 2a displays the Cu K-edge XANES spectra. The measurably higher absorption energy (E0) values for Cu-CeO2 (8991.5 eV), CuCo-CeO2 (8991.6 eV), and CuCoFeNiMn-CeO2 (8991.2 eV) relative to that for CuO (8990.3 eV) and the pre-edge features corresponding to the 1s → 3d transitions collectively indicate the existence of Cu2+.10 A previous study has attributed the oddly positive energy shift of Cu to the emergence of Cu3+ species,31 while Lancaster and colleagues recently suggested that the strong covalency of the Cu–ligand bonding could be the main trigger.46 For pristine CeO2, the empty Ce 4f and filled O 2p energy levels, which are above and below the Fermi level, respectively, are the main contributors to the covalent interactions.47 Incorporating Cu and other first-row transition-metal cosubstituents alters the hybridization and energy level of the Ce and O orbitals, which will be discussed later.
The local coordination geometries of Cu were analyzed by fitting their Fourier-transformed extended X-ray absorption fine structure (EXAFS) spectra (Figure 2b and Figure S10 and Table S5). Cu-CeO2, CuCo-CeO2, and CuCoFeNiMn-CeO2 all possessed the main Cu–O shell at ∼1.9 Å with reduced magnititude and a lower Cu coordination number (CN) of ∼2–3, reflecting the existence of transition-metal oxide motifs with small domains. Cu-CeO2 also exhibited a minor Cu–Ce contribution at 3.26 Å (CN ≈ 3.0), which is attributed to the Cu–[Ox]–Ce structure with strong copper–ceria interactions.48 Further wavelet transforms suggest that the Cu–Ce contribution weakens with an increased amount of the transition-metal substituents, while an additional coordination shell emerges at lower R and higher k values (Figure 2c). It is challenging to unambiguously identify the atoms giving rise to each individual scattering path, especially in the case of high-entropy CuCoFeNiMn-CeO2, where different transition-metal atoms share similar weight numbers. On the basis of the copper–ceria interfacial perimeter model,13 we tentatively assign this additional shell as the Cu–Cu distance (or Cu–M, where M represents Co, Fe, Ni, or Mn). XANES and EXAFS analyses together reveal that the atomically dispersed transition-metal substituents are strongly coordinated with the ligand oxygen from the CeO2 domain.
Neutron diffraction, which relies on short-range nuclear interactions and thus possesses a high detection sensitivity for light elements,49 was employed to identify the positions of the oxygen atoms in the transition-metal-substituted CeO2 nanocrystals. According to the Rietveld refinements (Figures S11–S14 and Table S6), no (crystalline) impurity phase was observed for CeO2, Cu-CeO2, and CuCo-CeO2, whereas a minor rock salt, sub-nanometer (<5 Å) HEO phase emerged for CuCoFeNiMn-CeO2.50 Moreover, no substantial changes were observed for the refined lattice parameters of CeO2 among these four samples. This affords direct and unambiguous evidence showing the atomic-level structural heterogeneity, where the transition-metal substituents reside on the CeO2 surface. Lattice shrinkage would be expected if Ce4+ (Ce3+) in the bulk lattice were replaced by the transition-metal cations with smaller sizes. In addition, the surface enrichment of Ce3+ was also observed in the STEM-electron energy loss spectroscopy (EELS) analysis, as evidenced by the appearance of low-energy features at the Ce M4,5 edge (Figure S15). Taken together, these results point to a structural picture where the substituted metal cations are embedded on the surface lattice of ceria, which constructs atomically thin HEO layers in CuCoFeNiMn-CeO2.
The unexpected local structural disorder and interfacial effects between ultrasmall domains, which can hardly be detected using conventional techniques, play decisive roles in regulating the surface properties of high-entropy materials.51,52 PDF analysis was applied to interpret this surface-confined transition-metal–ceria interface on the basis of the neutron diffraction data. Different from Rietveld refinements that focus on Bragg diffraction peaks, PDF analysis utilizes both Bragg scattering and diffuse scattering information and thereby possesses high sensitivities to short- and intermediate-range structures in nanoscale and amorphous systems.53−56 As exhibited in Figure 3a, the first negative G(r) peak at around 0.96–1.0 Å is assigned to the surface-attached O–H bonds, given the negative coherent nuclear scattering length of hydrogen. The peak at ∼1.95 Å is attributed to the nearest transition-metal–oxygen (M–O) correlation, which splits from a single peak to multiple deconvoluted peaks for CuCoFeNiMn-CeO2 that correspond to different M–O bond lengths. This manifests the structure disorder in the surface-confined HEO layers. The nearest Ce–O and O–O correlations are located at ∼2.35 and ∼2.70 Å, respectively. Frenkel-type oxygen defects, which are common in the bulk lattice of CeO2 due to the displacement of the oxygen anions along the ⟨111⟩ or ⟨110⟩ directions,26 were introduced to the structure model for a short-range PDF analysis (Figures S16–S18). The concentrations of the interstitial oxygen defects were refined to be 9.3%, 12.0%, 13.6%, and 13.1% for CeO2, Cu-CeO2, CuCo-CeO2 and CuCoFeNiMn-CeO2, respectively (Table S7). The concentration of the intrinsic interstitial oxygen defects in the bulk lattice thus remains almost unaltered, due to the comparable sizes and morphologies of the ceria-based nanocrystals. Variation of the transition-metal substituents and cosubstituents situated on the CeO2 surface thus has a negligible influence on the formation of oxygen defects deep within the bulk lattice.
Figure 3.
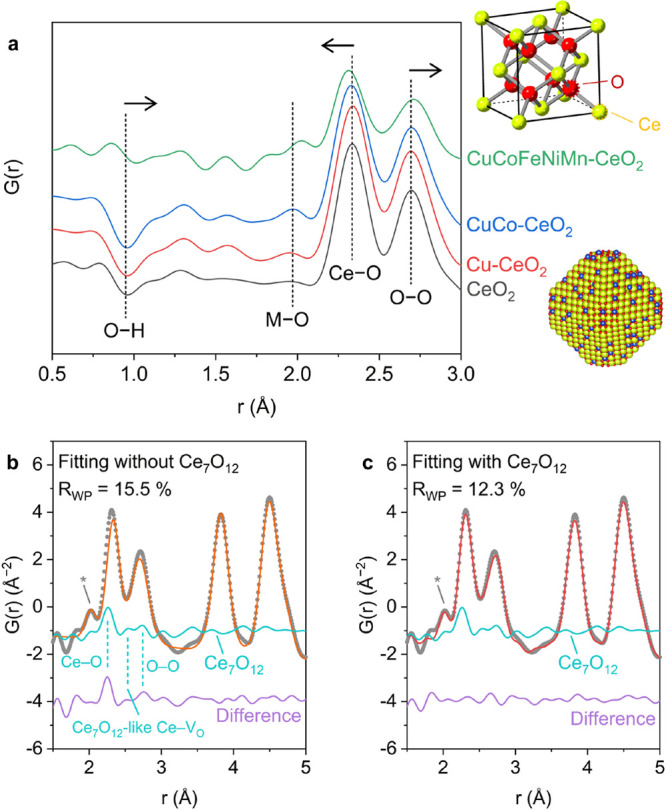
(a) Short-range neutron diffraction data for the transition-metal-substituted CeO2 samples. The peaks corresponding to the O–H, M–O, Ce–O, and O–O correlations are noted for comparison. Insets showing the structure model of the fluorite structure of CeO2 and illustrative model of the surface-substituted CeO2 nanocrystals are provided at the top and bottom, respectively. Short-range PDF analysis of CuCoFeNiMn-CeO2 (b) excluding and (c) including the Ce7O12 phase. The contribution from the rock salt HEO domains, which is less than 5 Å as shown in Figure S21, is highlighted using an asterisk.
Interestingly, the nearest Ce–O bond becomes shorter with transition-metal substitution, while the nearest O–O distance shifts toward higher r values (Figure 3a). This shift becomes non-negligible for CuCoFeNiMn-CeO2 and results in a discrepancy between the experimental and fitting results (Figure 3b). As the average fluorite structure model still well describes the intermediate-range PDF data above 5 Å (Figures S19 and S20), this opposite trend for the shift of the nearest Ce–O and O–O distances indicates an abundance of the surface oxygen defects in the HEO layers that drastically differ from the interstitial defects in the bulk fluorite lattice. Due to the small domain size, a large portion of the surface oxygen atoms from the CeO2 domain are directly bonded to the transition-metal cations, resulting in pronounced surface-dependent phenomena for the high-entropy CuCoFeNiMn-CeO2 sample.
To corroborate this hypothesis, we selected C–Ce2O3+δ, Ce7O12, and Ce11O20, three common CeO2–x polymorphs with different types of oxygen vacancies,40 as an additional surface phase to the short-range PDF analysis of CuCoFeNiMn-CeO2. The Ce7O12 phase, a rhombohedral structure with periodic arrangements of oxygen vacancies perpendicular to the {111} planes,57 was identified as the one that best describes the oxygen sublattice at the HEO–ceria interface. As displayed in Figure 3b, the difference between the simulated and experimental curves show three features in the range of 2–3 Å, which match well with the Ce–O, Ce7O12-like Ce–VO (partially vacant oxygen sites), and Ce–O correlations in the Ce7O12 structure, respectively. The PDF fitting for the CuCoFeNiMn-CeO2 data including the Ce7O12 phase yields a noticeable improvement (Figure 3c and Figure S21), demonstrating enrichment of surface-confined, oxygen-deficient phases. Accordingly, we were able to estimate the concentration of the Ce7O12-like oxygen vacancies for CeO2 (<1%), Cu-CeO2 (3.0%), CuCo-CeO2 (5.0%), and CuCoFeNiMn-CeO2 (13.4%) (Table S8 and Figure S22). In contrast to the relatively constant concentration of the interstitial oxygen defects, significantly more surface-confined oxygen vacancies are produced along with the formation of surface-confined HEO atomic layers in CuCoFeNiMn-CeO2. This also suggests two orthogonal strategies to modulate the amount of the bulk and surface oxygen defects in ceria. Tuning the geometric parameters such as size and exposed facet facilitates the modification of the intrinsic, interstitial oxygen defects in the bulk lattice, while modulating the transition-metal substituents enables engineering of the extrinsic, surface-confined oxygen vacancies.
Computational Investigation of Formation of the Surface Oxygen Vacancies of Ceria-Based Nanocrystals
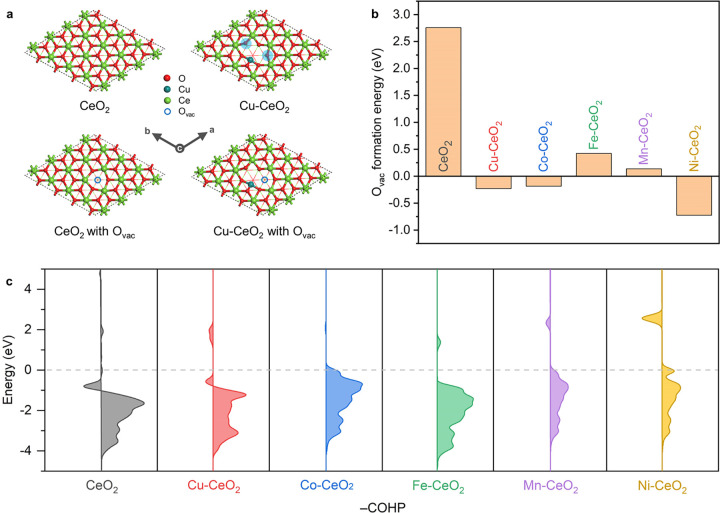
The oxygen vacancy formation energy has been shown to be an important descriptor for the reactivity of lattice oxygen on oxide surfaces. We hypothesize that, when the surface-confined HEOs are formed in CuCoFeNiMn-CeO2, oxygen vacancy formation in the CeO2 surface becomes more favorable, leading to the Ce7O12-like Ce–VO species. To test this hypothesis and elucidate the correlation between formation of the transition-metal–oxygen bond and modulation of surface oxygen vacancies, we performed density functional theory (DFT) calculations at the GGA+U level based on a simplified model for the transition-metal-functionalized CeO2 surfaces, using the oxygen vacancy formation energy (Evac) as a descriptor.
Because the truncated-octahedral shape of the ceria-based nanocrystals prominently exposes the {111} facet of CeO2 (Figure 1), we use the (111) surface structure of CeO2 as our starting model to probe how a surface substitutional doping by a 3d metal changes Evac (Figure 4a).58 One can see from Figure 4b that the Evac value of M-CeO2-(111) is much lower than that of the pristine CeO2-(111) for M = Cu, Co, Fe, Mn, Ni. The key reason is that substitution of M for Ce on the surface creates 2-fold-coordinated oxygen atoms (Figure 4a and Figure S23), which are more active than the 3-fold-coordinated oxygens on the pristine CeO2-(111). The negative Evac values, especially for Ni-CeO2-(111), indicate the favorable thermodynamic driving force for creating surface oxygen vacancies. The sluggish diffusion kinetics that are characteristic of HEOs59 may further enhance the availability of the oxygen vacancies.
Figure 4.
(a) Optimized surface structures of CeO2-(111) and Cu-CeO2-(111) with and without oxygen vacancies. (b) Calculated oxygen vacancy formation energies (Evac) of the pristine and transition-metal-substituted CeO2-(111). (c) Calculated COHP between the cerium and oxygen atoms on the surfaces of the pristine and transition-metal-substituted CeO2-(111). The colored areas on the left and right correspond to the antibonding and bonding orbitals, respectively, and the Fermi level is noted using a dashed gray line.
Bader charge analysis was performed to elucidate the oxidation state in Cu-CeO2, with Cu2O and CuO as references. We found that Cu in Cu-CeO2 is slightly more oxidized in comparison to Cu in CuO (Figure S24), consistent with the observed positive energy shift of the Cu K-edge XANES spectra for Cu-CeO2 (Figure 2a). We further investigated the change in the surface Ce–O bonding with M, using a crystal orbital Hamilton population (COHP) analysis (Figure 4c). One can see that the antibonding states of the CeO2-(111) surface are either decreasing or pushed to above the Fermi level after substitutional doping by M. In other words, the Ce–O bonds are more strengthened in M-CeO2 than in CeO2. The computational results suggest that the formation of surface oxygen vacancies is thermodynamically favored in the high-entropy ceria nanocrystals. In comparison with Ce3+/Ce4+, the transition-metal cations possess lower oxidation states and smaller ionic radii, resulting in structural heterogeneities at the transition-metal–ceria interface. The strong bond covalency redistributes the charge on lattice oxygen and facilitates generation of the oxygen vacancies on the CeO2 surface/subsurface instead of the interstitial vacancies in the bulk.
CO Oxidation Performance of Ceria-Based Nanocrystals
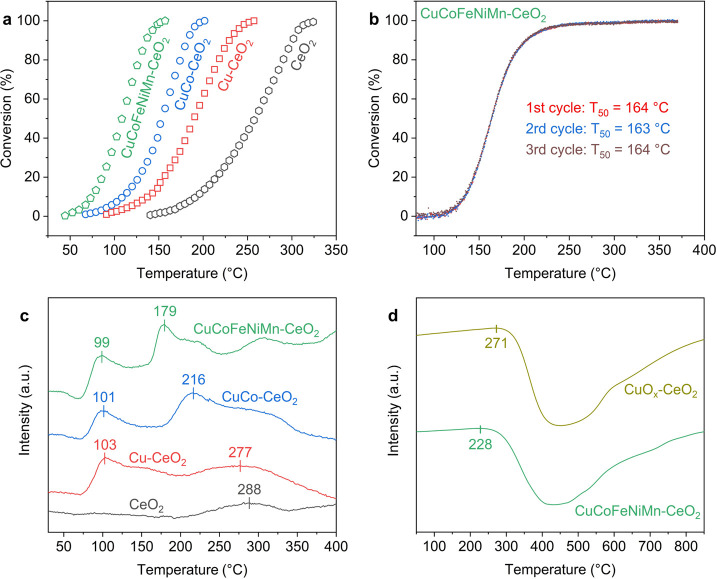
The surface oxygen vacancies induced by transition-metal substitution not only facilitate removal of oxygen atoms but also promote refilling of the vacant sites by adsorbed oxygen species. CO oxidation, a key technological process to treat automotive exhaust streams,60,61 was selected as a model reaction to study the associated redox properties. The “light-off” curves show that introducing transition-metal species lowers the onset and complete conversion temperatures for CO oxidation (Figure 5a). The T50 values, the temperatures at which 50% of CO is converted to CO2, are 257, 190, 155, and 109 °C for CeO2, Cu-CeO2, CuCo-CeO2, and CuCoFeNiMn-CeO2, respectively. The steady-state measurements at 80 °C show the same trend (Figure S25a). Following the Mars–van Krevelen mechanism,34 CO molecules are adsorbed and oxidized by the activated lattice oxygen on the CeO2 surface, where the accompanying oxygen vacancies are repeatedly filled by the dissociated oxygen molecules in the atmosphere. Notably, the CO oxidation activity is positively correlated with the concentration of the Ce7O12-like surface oxygen vacancies in the transition-metal-substituted CeO2 nanocrystals. The apparent activation energies were calculated in the range of 48.6–66.8 kJ/mol (Figure S25b), consistent with previously reported values.8 This also validates that the activity difference originates from the different amouns of active sites that share the same Mars–van Krevelen mechanism. We further applied the optimal CuCoFeNiMn-CeO2 catalyst to the treatment of exhaust streams, which showed compelling catalytic activity and stability under harsh conditions (GHSV ≈ 200000 h–1), as displayed in Figure 5b and Figure S26. Inherent compositional diversities in HEOs afford separate active metal sites for adsorption and conversion of competing reactant molecules, which include CO, CO2, hydrocarbons, NOx, and H2O in the exhaust streams. This points out a new direction for the design of CeO2-based noble-metal-free catalysts for enhanced catalysis, where relevant studies have mainly focused on improving the CO oxidation activity or the performance of preferential oxidation of CO in excess hydrogen (PROX).8,10,31,33,48 In addition to the catalytic activity, the sluggish kinetics of HEOs may reinforce the resistance to hydrocarbon inhibitions and catalyst stability during long-term operations, both of which are pivotal for industrial exhaust treatments.
Figure 5.
(a) CO oxidation light-off curves for the transition-metal-substituted CeO2 samples, measured with 20 mg of the catalysts in 1% CO balanced with dry air at a flow rate of 12 mL min–1. (b) CO oxidation light-off curves under simulated exhaust conditions (0.2% CO, 0.01% NO, 0.083% C2H4, 0.033% C3H6, 0.11% C3H8, 6.0% CO2, 12.0% O2, and 6.0% H2O balanced with Ar) using the CuCoFeNiMn-CeO2 catalyst for three repeated cycles, measured with 111 mg of the catalyst at a flow rate of 370 mL min–1 (GHSV ≈ 200000 h–1). (c) CO-TPR profiles of the transition-metal-substituted CeO2 catalysts with the CO consumption amount being highlighted. (d) Evolution of the 18O2 species for CuCoFeNiMn-CeO2 and CuOx-CeO2 during the TPOIE measurement, where the onset temperature is noted for comparison.
CO temperature-programmed reduction (CO-TPR) was conducted to evaluate the reducibility of the transition-metal-substituted CeO2 nanocrystals. As shown in Figure 5c, Cu-CeO2 exhibits two peaks at 103 and 277 °C, corresponding to the reduction of the CuOx clusters weakly bound to CeO2 and the Cu–[Ox]–Ce structure with strong covalent copper–ceria interactions, respectively.48 While the position of the first peak remains at ∼100 °C, the second peak observably shifts toward lower temperatures for CuCo-CeO2 (216 °C) and CuCoFeNiMn-CeO2 (179 °C). This suggests that the incorporation of the additional transition-metal species other than Cu facilitates reduction of the M–[Ox]–Ce structures, allowing activation and utilization of surface lattice oxygen at lower temperatures. In parallel, the CO consumption amount also increases from Cu-CeO2 (256 μmol g–1) and CuCo-CeO2 (294 μmol g–1) to CuCoFeNiMn-CeO2 (562 μmol g–1), highlighting the enlargement of the HEO–CeO2 heterointerface for CO activation. A similar trend was observed for the H2-TPR data (Figure S25c), whereas differentiation of the different reduction peaks became challenging, presumably due to the stronger reducing ability of H2 in comparison with that of CO. In light of the above experimental and computational studies, we infer that the strong covalency of the M–O bonds in the M–[Ox]–Ce structures leads to the formation and stabilization of surface oxygen vacancies. With maximized active sites, the constructed HEO layers on ceria enable efficient oxygen activation and replenishment for enhanced CO conversions.
To unveil the entropic effects in oxygen defect formation and correlated lattice oxygen activation, we applied temperature-programmed oxygen isotope exchange (TPOIE) to compare the oxygen exchange behaviors of the high-entropy oxide (CuCoFeNiMn-CeO2) and binary oxide (CuOx-CeO2) with comparable transition-metal concentrations (Table S2).62 As shown in Figure 5d, the consumption of 18O2 started at 228 °C for CuCoFeNiMn-CeO2, observably lower than that for CuOx-CeO2 (271 °C). A similar trend was observed for the evolution of the 16O2 and 16O18O signals (Figure S27), which demonstrates the enhanced oxygen exchange capability for CuCoFeNiMn-CeO2 relative to that for CuOx-CeO2. Meanwhile, the normalized oxygen capacity for CuCoFeNiMn-CeO2 is around the same (104%) as that for CuOx-CeO2. The TPOIE results indicate that the surface-confined atomic HEO layers enriched with Ce7O12-like oxygen vacancies facilitate the exchange between gas-phase oxygen and surface lattice oxygen of CeO2. The enhanced configurational entropy affords a distinct route to activating surface lattice oxygen, benefiting the formation, migration, and utilization of active oxygen species for oxidation reactions. This brings opportunities to tackle issues that are considered challenging for conventional mixed-oxide catalysts.
Conclusions
Given the broad scope of potential applications for ceria-based nanomaterials, it is pivotal to tailor the oxygen defects in CeO2 through a rational structure and composition design. As a distinct strategy to tune the oxygen defects in ceria, entropy stabilization has been mostly limited to single-phase solid solutions. Here, we demonstrate that the surface-confined HEO atomic layers, where different transition metals are randomly embedded and stabilized on the surface lattice of ceria, have profound effects on the creation of oxygen defects. The formation of the bulk interstitial oxygen defects is dependent on the size and geometry of the ceria nanocrystals, whereas tuning of the cluster surface defects relies on the transition-metal–CeO2 interface engineering. With enhanced bond covalency and maximized active sites, the CuCoFeNiMn-CeO2 sample exhibits optimal CO oxidation capabilities. These atomistic insights into the surface and bulk structures of ceria-based HEO nanocrystals have important implications for oxygen defect engineering in reducible metal oxides.63 For example, the ability to control the surface and bulk oxygen defects of nanoscale HEOs is important in the exploration of new topological materials, where the interior electronic and magnetic properties are completely different from those on the surface or at the oxide–oxide interface.64 More work is anticipated to unravel the metal–oxygen covalency in mixed and entropy-stabilized metal oxides, as the atomic-scale structural heterogeneities may benefit lattice oxygen activation for catalytic and sensing applications.
Acknowledgments
This work was supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science Program. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Neutron total scattering measurements were conducted at the NOMAD beamline at the Spallation Neutron Source, Oak Ridge National Laboratory, which was sponsored by the Scientific User Facilities Division, Office of Basic Sciences, U.S. Department of Energy. Part of the work including Raman and STEM was conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility. We thank Yujia Bian for the assistance during XAS experiments at the 10-BM beamline at the Advanced Photon Source at Argonne National Laboratory and Harry M. Meyer III at Oak Ridge National Laboratory for XPS acquisition and analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.2c00340.
Additional experimental and calculation details and additional figures and tables as described in the text (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Trovarelli A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. - Sci. Eng. 1996, 38, 439–520. 10.1080/01614949608006464. [DOI] [Google Scholar]
- Yang C.; Wong R. A.; Hong M.; Yamanaka K.; Ohta T.; Byon H. R. Unexpected Li2O2 Film Growth on Carbon Nanotube Electrodes with CeO2 Nanoparticles in Li–O2 Batteries. Nano Lett. 2016, 16, 2969–2974. 10.1021/acs.nanolett.5b05006. [DOI] [PubMed] [Google Scholar]
- Ackland K.; Coey J. M. D. Room Temperature Magnetism in CeO2—A Review. Phys. Rep. 2018, 746, 1–39. 10.1016/j.physrep.2018.04.002. [DOI] [Google Scholar]
- Jasinski P.; Suzuki T.; Anderson H. U. Nanocrystalline Undoped Ceria Oxygen Sensor. Sens. Actuators, B 2003, 95, 73–77. 10.1016/S0925-4005(03)00407-6. [DOI] [Google Scholar]
- Han S. I.; Lee S.; Cho M. G.; Yoo J. M.; Oh M. H.; Jeong B.; Kim D.; Park O. K.; Kim J.; Namkoong E.; Jo J.; Lee N.; Lim C.; Soh M.; Sung Y.-E.; Yoo J.; Park K.; Hyeon T. Epitaxially Strained CeO2/Mn3O4 Nanocrystals as an Enhanced Antioxidant for Radioprotection. Adv. Mater. 2020, 32, 2001566. 10.1002/adma.202001566. [DOI] [PubMed] [Google Scholar]
- Montini T.; Melchionna M.; Monai M.; Fornasiero P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. 10.1021/acs.chemrev.5b00603. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Polo-Garzon F.; Bao Z.; Moon J.; Huang Z.; Chen H.; Chen Z.; Yang Z.; Chi M.; Wu Z.; Liu J.; Dai S. Manipulating Copper Dispersion on Ceria for Enhanced Catalysis: A Nanocrystal-Based Atom-Trapping Strategy. Adv. Sci. 2022, 9, 2104749. 10.1002/advs.202104749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.-W.; Du P.-P.; Zou S.-H.; He H.-Y.; Wang R.-X.; Jin Z.; Shi S.; Huang Y.-Y.; Si R.; Song Q.-S.; Jia C.-J.; Yan C.-H. Highly Dispersed Copper Oxide Clusters as Active Species in Copper-Ceria Catalyst for Preferential Oxidation of Carbon Monoxide. ACS Catal. 2015, 5, 2088–2099. 10.1021/cs5014909. [DOI] [Google Scholar]
- Zhang P.; Lu H.; Zhou Y.; Zhang L.; Wu Z.; Yang S.; Shi H.; Zhu Q.; Chen Y.; Dai S. Mesoporous MnCeOx Solid Solutions for Low Temperature and Selective Oxidation of Hydrocarbons. Nat. Commun. 2015, 6, 8446. 10.1038/ncomms9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.-Z.; Wang W.-W.; Li S.-Q.; Fu X.-P.; Wang X.; Wu K.; Si R.; Ma C.; Jia C.-J.; Yan C.-H. Construction of Active Site in a Sintered Copper–Ceria Nanorod Catalyst. J. Am. Chem. Soc. 2019, 141, 17548–17557. 10.1021/jacs.9b05419. [DOI] [PubMed] [Google Scholar]
- Lin L.; Yao S.; Liu Z.; Zhang F.; Li N.; Vovchok D.; Martínez-Arias A.; Castañeda R.; Lin J.; Senanayake S. D.; Su D.; Ma D.; Rodriguez J. A. In Situ Characterization of Cu/CeO2 Nanocatalysts for CO2 Hydrogenation: Morphological Effects of Nanostructured Ceria on the Catalytic Activity. J. Phys. Chem. C 2018, 122, 12934–12943. 10.1021/acs.jpcc.8b03596. [DOI] [Google Scholar]
- Li Z.; Flytzani-Stephanopoulos M. Cu–Cr–O and Cu–Ce–O Regenerable Oxide Sorbents for Hot Gas Desulfurization. Ind. Eng. Chem. Res. 1997, 36, 187–196. 10.1021/ie960245d. [DOI] [Google Scholar]
- Chen A.; Yu X.; Zhou Y.; Miao S.; Li Y.; Kuld S.; Sehested J.; Liu J.; Aoki T.; Hong S.; Camellone M. F.; Fabris S.; Ning J.; Jin C.; Yang C.; Nefedov A.; Wöll C.; Wang Y.; Shen W. Structure of the Catalytically Active Copper–Ceria Interfacial Perimeter. Nat. Catal 2019, 2, 334–341. 10.1038/s41929-019-0226-6. [DOI] [Google Scholar]
- Yang K.; Li D.-F.; Huang W.-Q.; Xu L.; Huang G.-F.; Wen S. Origin of Enhanced Visible-Light Photocatalytic Activity of Transition-Metal (Fe, Cr and Co)-Doped CeO2: Effect of 3d Orbital Splitting. Appl. Phys. A 2017, 123, 96. 10.1007/s00339-016-0700-9. [DOI] [Google Scholar]
- Wang Y.; Chen Z.; Han P.; Du Y.; Gu Z.; Xu X.; Zheng G. Single-Atomic Cu with Multiple Oxygen Vacancies on Ceria for Electrocatalytic CO2 Reduction to CH4. ACS Catal. 2018, 8, 7113–7119. 10.1021/acscatal.8b01014. [DOI] [Google Scholar]
- Rost C. M.; Sachet E.; Borman T.; Moballegh A.; Dickey E. C.; Hou D.; Jones J. L.; Curtarolo S.; Maria J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. 10.1038/ncomms9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y.; Li S.; Qian Y.; Zhu W.; Yuan H.; Jiang P.; Guo R.; Wang L. High-Entropy Alloys as a Platform for Catalysis: Progress, Challenges, and Opportunities. ACS Catal. 2020, 10, 11280–11306. 10.1021/acscatal.0c03617. [DOI] [Google Scholar]
- Feng D.; Dong Y.; Zhang L.; Ge X.; Zhang W.; Dai S.; Qiao Z. Holey Lamellar High-Entropy Oxide as an Ultra-High-Activity Heterogeneous Catalyst for Solvent-free Aerobic Oxidation of Benzyl Alcohol. Angew. Chem. Int. Ed 2020, 59, 19503–19509. 10.1002/anie.202004892. [DOI] [PubMed] [Google Scholar]
- Gu K.; Wang D.; Xie C.; Wang T.; Huang G.; Liu Y.; Zou Y.; Tao L.; Wang S. Defect-Rich High-Entropy Oxide Nanosheets for Efficient 5-Hydroxymethylfurfural Electrooxidation. Angew. Chem. Int. Ed. 2021, 60, 20253–20258. 10.1002/anie.202107390. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Dai S. High-Entropy Materials for Catalysis: A New Frontier. Sci. Adv. 2021, 7, eabg1600 10.1126/sciadv.abg1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Yao Y.; Huang Z.; Xie P.; Liu Z.; Yang M.; Gao J.; Zeng K.; Brozena A. H.; Pastel G.; Jiao M.; Dong Q.; Dai J.; Li S.; Zong H.; Chi M.; Luo J.; Mo Y.; Wang G.; Wang C.; Shahbazian-Yassar R.; Hu L. Denary Oxide Nanoparticles as Highly Stable Catalysts for Methane Combustion. Nat. Catal. 2021, 4, 62–70. 10.1038/s41929-020-00554-1. [DOI] [Google Scholar]
- Xu H.; Zhang Z.; Liu J.; Do-Thanh C.-L.; Chen H.; Xu S.; Lin Q.; Jiao Y.; Wang J.; Wang Y.; Chen Y.; Dai S. Entropy-Stabilized Single-Atom Pd Catalysts via High-Entropy Fluorite Oxide Supports. Nat. Commun. 2020, 11, 3908. 10.1038/s41467-020-17738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S.; Ma X.; Shu Y.; Bao J.; Zhang Q.; Chen M.; Zhang P.; Dai S. Self-Regeneration of Supported Transition Metals by a High Entropy-Driven Principle. Nat. Commun. 2021, 12, 5917. 10.1038/s41467-021-26160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Sun Y.; Yang S.; Wang H.; Dmowski W.; Egami T.; Dai S. Self-Regenerative Noble Metal Catalysts Supported on High-Entropy Oxides. Chem. Commun. 2020, 56, 15056–15059. 10.1039/D0CC05860B. [DOI] [PubMed] [Google Scholar]
- Chen D.; Guan Z.; Zhang D.; Trotochaud L.; Crumlin E.; Nemsak S.; Bluhm H.; Tuller H. L.; Chueh W. C. Constructing a Pathway for Mixed Ion and Electron Transfer Reactions for O2 Incorporation in Pr0.1Ce0.9O2–x. Nat. Catal. 2020, 3, 116–124. 10.1038/s41929-019-0401-9. [DOI] [Google Scholar]
- Mamontov E.; Egami T. Structural Defects in a Nano-Scale Powder of CeO2 Studied by Pulsed Neutron Diffraction. J. Phys. Chem. Solids 2000, 61, 1345–1356. 10.1016/S0022-3697(00)00003-2. [DOI] [Google Scholar]
- Chen J.; Arandiyan H.; Gao X.; Li J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. 10.1007/s10563-015-9191-5. [DOI] [Google Scholar]
- Grimaud A.; Diaz-Morales O.; Han B.; Hong W. T.; Lee Y.-L.; Giordano L.; Stoerzinger K. A.; Koper M. T. M.; Shao-Horn Y. Activating Lattice Oxygen Redox Reactions in Metal Oxides to Catalyse Oxygen Evolution. Nat. Chem. 2017, 9, 457–465. 10.1038/nchem.2695. [DOI] [PubMed] [Google Scholar]
- Li T.; Yao Y.; Ko B. H.; Huang Z.; Dong Q.; Gao J.; Chen W.; Li J.; Li S.; Wang X.; Shahbazian-Yassar R.; Jiao F.; Hu L. Carbon-Supported High-Entropy Oxide Nanoparticles as Stable Electrocatalysts for Oxygen Reduction Reactions. Adv. Funct. Mater. 2021, 31, 2010561. 10.1002/adfm.202010561. [DOI] [Google Scholar]
- Lee S. S.; Zhu H.; Contreras E. Q.; Prakash A.; Puppala H. L.; Colvin V. L. High Temperature Decomposition of Cerium Precursors to Form Ceria Nanocrystal Libraries for Biological Applications. Chem. Mater. 2012, 24, 424–432. 10.1021/cm200863q. [DOI] [Google Scholar]
- Elias J. S.; Risch M.; Giordano L.; Mansour A. N.; Shao-Horn Y. Structure, Bonding, and Catalytic Activity of Monodisperse, Transition-Metal-Substituted CeO2 Nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. 10.1021/ja509214d. [DOI] [PubMed] [Google Scholar]
- Binder A. J.; Toops T. J.; Unocic R. R.; Parks J. E.; Dai S. Low-Temperature CO Oxidation over a Ternary Oxide Catalyst with High Resistance to Hydrocarbon Inhibition. Angew. Chem. Int. Ed. 2015, 54, 13263–13267. 10.1002/anie.201506093. [DOI] [PubMed] [Google Scholar]
- Lu J.; Wang J.; Zou Q.; He D.; Zhang L.; Xu Z.; He S.; Luo Y. Unravelling the Nature of the Active Species as Well as the Doping Effect over Cu/Ce-Based Catalyst for Carbon Monoxide Preferential Oxidation. ACS Catal. 2019, 9, 2177–2195. 10.1021/acscatal.8b04035. [DOI] [Google Scholar]
- Kim K.; Han J. W. Mechanistic Study for Enhanced CO Oxidation Activity on (Mn,Fe) Co-Doped CeO2 (111). Catal. Today 2017, 293–294, 82–88. 10.1016/j.cattod.2016.11.046. [DOI] [Google Scholar]
- Chen H.; Jie K.; Jafta C. J.; Yang Z.; Yao S.; Liu M.; Zhang Z.; Liu J.; Chi M.; Fu J.; Dai S. An Ultrastable Heterostructured Oxide Catalyst Based on High-Entropy Materials: A New Strategy toward Catalyst Stabilization via Synergistic Interfacial Interaction. Appl. Catal. B Environ. 2020, 276, 119155. 10.1016/j.apcatb.2020.119155. [DOI] [Google Scholar]
- McCormick C. R.; Schaak R. E. Simultaneous Multication Exchange Pathway to High-Entropy Metal Sulfide Nanoparticles. J. Am. Chem. Soc. 2021, 143, 1017–1023. 10.1021/jacs.0c11384. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhan X.; Bueno S. L. A.; Shafei I. H.; Ashberry H. M.; Chatterjee K.; Xu L.; Tang Y.; Skrabalak S. E. Synthesis of Monodisperse High Entropy Alloy Nanocatalysts from Core@shell Nanoparticles. Nanoscale Horiz. 2021, 6, 231–237. 10.1039/D0NH00656D. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Jin Q.; Chan S. W. Ceria Nanoparticles: Size, Size Distribution, and Shape. J. Appl. Phys. 2004, 95, 4319–4326. 10.1063/1.1667251. [DOI] [Google Scholar]
- Ma R.; Zhang S.; Wen T.; Gu P.; Li L.; Zhao G.; Niu F.; Huang Q.; Tang Z.; Wang X. A Critical Review on Visible-Light-Response CeO2-Based Photocatalysts with Enhanced Photooxidation of Organic Pollutants. Catal. Today 2019, 335, 20–30. 10.1016/j.cattod.2018.11.016. [DOI] [Google Scholar]
- Kümmerle E. A.; Heger G. The Structures of C–Ce2O3+δ, Ce7O12, and Ce11O20. J. Solid State Chem. 1999, 147, 485–500. 10.1006/jssc.1999.8403. [DOI] [Google Scholar]
- Oses C.; Toher C.; Curtarolo S. High-Entropy Ceramics. Nat. Rev. Mater. 2020, 5, 295–309. 10.1038/s41578-019-0170-8. [DOI] [Google Scholar]
- Taniguchi T.; Watanabe T.; Sugiyama N.; Subramani A. K.; Wagata H.; Matsushita N.; Yoshimura M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. 10.1021/jp9049457. [DOI] [Google Scholar]
- Wu Z.; Li M.; Howe J.; Meyer H. M.; Overbury S. H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. 10.1021/la101723w. [DOI] [PubMed] [Google Scholar]
- Holder C. F.; Schaak R. E. Tutorial on Powder X-Ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. 10.1021/acsnano.9b05157. [DOI] [PubMed] [Google Scholar]
- Schmitt R.; Nenning A.; Kraynis O.; Korobko R.; Frenkel A. I.; Lubomirsky I.; Haile S. M.; Rupp J. L. M. A Review of Defect Structure and Chemistry in Ceria and Its Solid Solutions. Chem. Soc. Rev. 2020, 49, 554–592. 10.1039/C9CS00588A. [DOI] [PubMed] [Google Scholar]
- Dimucci I. M.; Lukens J. T.; Chatterjee S.; Carsch K. M.; Titus C. J.; Lee S. J.; Nordlund D.; Betley T. A.; MacMillan S. N.; Lancaster K. M. The Myth of d8 Copper(III). J. Am. Chem. Soc. 2019, 141, 18508–18520. 10.1021/jacs.9b09016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamatsu H.; Mukoyama T.; Adachi H. Ionic and Covalent Bonds in CeO2 Crystal. Chem. Phys. Lett. 1995, 247, 168–172. 10.1016/0009-2614(95)01185-7. [DOI] [Google Scholar]
- Wang W. W.; Yu W. Z.; Du P. P.; Xu H.; Jin Z.; Si R.; Ma C.; Shi S.; Jia C. J.; Yan C. H. Crystal Plane Effect of Ceria on Supported Copper Oxide Cluster Catalyst for CO Oxidation: Importance of Metal-Support Interaction. ACS Catal. 2017, 7, 1313–1329. 10.1021/acscatal.6b03234. [DOI] [Google Scholar]
- Luo S.; Li M.; Fung V.; Sumpter B. G.; Liu J.; Wu Z.; Page K. New Insights into the Bulk and Surface Defect Structures of Ceria Nanocrystals from Neutron Scattering Study. Chem. Mater. 2021, 33, 3959–3970. 10.1021/acs.chemmater.1c00156. [DOI] [Google Scholar]
- Zhang J.; Yan J.; Calder S.; Zheng Q.; McGuire M. A.; Abernathy D. L.; Ren Y.; Lapidus S. H.; Page K.; Zheng H.; Freeland J. W.; Budai J. D.; Hermann R. P. Long-Range Antiferromagnetic Order in a Rocksalt High Entropy Oxide. Chem. Mater. 2019, 31, 3705–3711. 10.1021/acs.chemmater.9b00624. [DOI] [Google Scholar]
- Jiang B.; Bridges C. A.; Unocic R. R.; Pitike K. C.; Cooper V. R.; Zhang Y.; Lin D.-Y.; Page K. Probing the Local Site Disorder and Distortion in Pyrochlore High-Entropy Oxides. J. Am. Chem. Soc. 2021, 143, 4193–4204. 10.1021/jacs.0c10739. [DOI] [PubMed] [Google Scholar]
- Santodonato L. J.; Zhang Y.; Feygenson M.; Parish C. M.; Gao M. C.; Weber R. J. K.; Neuefeind J. C.; Tang Z.; Liaw P. K. Deviation from High-Entropy Configurations in the Atomic Distributions of a Multi-Principal-Element Alloy. Nat. Commun. 2015, 6, 5964. 10.1038/ncomms6964. [DOI] [PubMed] [Google Scholar]
- Billinge S. J. L. Nanoscale Structural Order from the Atomic Pair Distribution Function (PDF): There’s Plenty of Room in the Middle. J. Solid State Chem. 2008, 181, 1695–1700. 10.1016/j.jssc.2008.06.046. [DOI] [Google Scholar]
- Liu H.; Liu H.; Lapidus S. H.; Meng Y. S.; Chupas P. J.; Chapman K. W. Sensitivity and Limitations of Structures from X-Ray and Neutron-Based Diffraction Analyses of Transition Metal Oxide Lithium-Battery Electrodes. J. Electrochem. Soc. 2017, 164, A1802–A1811. 10.1149/2.0271709jes. [DOI] [Google Scholar]
- Marchbank H. R.; Clark A. H.; Hyde T. I.; Playford H. Y.; Tucker M. G.; Thompsett D.; Fisher J. M.; Chapman K. W.; Beyer K. A.; Monte M.; Longo A.; Sankar G. Structure of Nano-Sized CeO2 Materials: Combined Scattering and Spectroscopic Investigations. ChemPhysChem 2016, 17, 3494–3503. 10.1002/cphc.201600697. [DOI] [PubMed] [Google Scholar]
- Liu J.; Olds D.; Peng R.; Yu L.; Foo G. S.; Qian S.; Keum J.; Guiton B. S.; Wu Z.; Page K. Quantitative Analysis of the Morphology of {101} and {001} Faceted Anatase TiO2 Nanocrystals and Its Implication on Photocatalytic Activity. Chem. Mater. 2017, 29, 5591–5604. 10.1021/acs.chemmater.7b01172. [DOI] [Google Scholar]
- Wilkens H.; Schuckmann O.; Oelke R.; Gevers S.; Schaefer A.; Bäumer M.; Zoellner M. H.; Schroeder T.; Wollschläger J. Stabilization of the Ceria ι-Phase (Ce7O12) Surface on Si(111). Appl. Phys. Lett. 2013, 102, 111602. 10.1063/1.4795867. [DOI] [PubMed] [Google Scholar]
- Krcha M. D.; Janik M. J. Challenges in the Use of Density Functional Theory to Examine Catalysis by M-Doped Ceria Surfaces. Int. J. Quantum Chem. 2014, 114, 8–13. 10.1002/qua.24548. [DOI] [Google Scholar]
- Sun Y.; Dai S. High-Entropy Catalysts: Supremacy of Diversity. Chem. Catal 2021, 1, 490–492. 10.1016/j.checat.2021.06.019. [DOI] [Google Scholar]
- Fu Q.; Li W.-X.; Yao Y.; Liu H.; Su H.-Y.; Ma D.; Gu X.-K.; Chen L.; Wang Z.; Zhang H.; Wang B.; Bao X. Interface-Confined Ferrous Centers for Catalytic Oxidation. Science 2010, 328, 1141–1144. 10.1126/science.1188267. [DOI] [PubMed] [Google Scholar]
- Fu Q.; Yang F.; Bao X. Interface-Confined Oxide Nanostructures for Catalytic Oxidation Reactions. Acc. Chem. Res. 2013, 46, 1692–1701. 10.1021/ar300249b. [DOI] [PubMed] [Google Scholar]
- Bao Z.; Fung V.; Moon J.; Hood Z. D.; Rochow M.; Kammert J.; Polo-Garzon F.; Wu Z. Revealing the Interplay between “Intelligent Behavior” and Surface Reconstruction of Non-Precious Metal Doped SrTiO3 Catalysts during Methane Combustion. Catal. Today 2022, 10.1016/j.cattod.2022.03.012. [DOI] [Google Scholar]
- Li Y.; Lin L.; Gao L.; Mu R.; Fu Q.; Bao X. Predominance of Subsurface and Bulk Oxygen Vacancies in Reduced Manganese Oxide. J. Phys. Chem. C 2021, 125, 7990–7998. 10.1021/acs.jpcc.1c01203. [DOI] [Google Scholar]
- Chakhalian J.; Millis A. J.; Rondinelli J. Whither the Oxide Interface. Nat. Mater. 2012, 11, 92–94. 10.1038/nmat3225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.