Abstract
This report describes an anesthesia technique that we used to study cardiovascular anatomy and physiology with echocardiography and cardiac magnetic resonance (CMR) in 46 African clawed frogs (Xenopus laevis) (n = 24 for electrocardiography and n = 22 for CMR). For administration of anesthesia, 3 holding tanks, one each for transportation, sedation, and recovery, were filled with filtered water, with 0.05% buffered tricaine methasulfonate solution (MS-222) added into the sedation tank. Fifteen minutes after the frog was placed in the sedation tank, a paper towel was soaked in MS-222 solution, and the frog was placed in a supine position and rolled 3 to 4 times in the soaked paper with the head and legs exposed. Vital signs were monitored and recorded throughout the procedure. After imagining, frogs were unrolled from the paper towel, placed in the recovery tank, and later returned to their home tank. Monitoring was discontinued when the frogs resumed typical activity. No mortality or complications were observed in frogs that underwent this procedure. Mean duration ± 1 SD of anesthesia induction was 12 ± 5 min in the echocardiography group and 14 ± 6 min in the CMR group. The mean duration of anesthesia maintenance was 60 ± 18 min in the echocardiography group and 118 ± 37 min in the CMR group. An additional dose of anesthesia was necessary during maintenance for 9 of 24 (37%) frogs in the echocardiography group and 6 of 22 (27%) frogs in the CMR group. At the end of the procedure, the mean oxygen saturation was 66 ± 9% in the echocardiography group and 85 ± 6% in the CMR group, and heart rate was 48 ± 13 beats/min in the echocardiography group and 42 ± 7 beats/min in the CMR group. We conclude that the anesthesia technique of immersion in MS-222 is suitable for performing echocardiography and CMR imaging in this species without complications.
Abbreviations: CMR, cardiac magnetic resonance; MS-222, tricaine methasulfonate; nsd, no significant difference
MESH terms: Anesthesia, Echocardiography, Cardiac Magnetic Resonance Imaging, Single Ventricle, Tricaine, Xenopus laevis
Introduction
African clawed frogs (Xenopus laevis) have a single ventricle sustaining both the systemic and pulmonary circulations, a feature similar to that found in 15% of children who have complex congenital heart defects that result functionally in a single ventricle. The long-term outcomes of children born with functionally univentricular hearts are complicated by the combination of heart failure and cyanosis, dramatically reducing their life expectancy and severely compromising their quality of life.2,5,7,8,10,11,21 Reports of patients born with a single ventricle who survive to adulthood without surgical intervention are exceptionally rare.5,28 Children born with a functionally univentricular heart generally require at least 2 to 3 staged operations to allow the single ventricle to pump oxygenated blood through the systemic circulation, while the desaturated blood flows passively from the superior and inferior vena cava directly through the lungs.5,11,21 This artificial circulation causes high systemic venous pressure; the chronic elevation of the systemic venous pressure may result in liver failure, renal failure, protein-losing enteropathy, and plastic bronchitis.2,8,10,28
Mathematical and computational fluid dynamic models have been used to study the blood flow distribution in a single ventricle and to plan for a better type of circulation to possibly improve the efficiency of blood flows and clinical outcomes.6,29 Research on functionally univentricular hearts would greatly benefit from studies on animals born with a single ventricle. The only such animals available in nature are certain amphibians, such as Xenopus laevis, and reptiles.15 The Xenopus laevis heart has a single ventricular chamber receiving oxygenated blood from the lungs and draining into the left atrium, with the less saturated blood returning from the body and draining into the right atrium. The heart distributes the blood, mixed in the single ventricular chamber, to the pulmonary and systemic circulations through a single ventricular outlet, the conus arteriosus; the conus arteriosus divides into right and left truncus arteriosus, each of which has a lateral branch for perfusion of the lungs and skin, a medial one for perfusion of the body, and a central one for perfusion the head.3,25,27 Morphologic studies published over 50 y ago investigated the peculiar anatomy of the frog heart and tried to speculate on the cardiovascular function based on the morphology.4,14,30 However, those observations have only recently been correlated with the pathophysiology of human hearts having a single ventricle.16,23
In Xenopus laevis, atmospheric gas exchange occurs through 3 different respiratory mechanisms: cutaneous, bucco-pharyngeal, and pulmonary.27 Cutaneous respiration occurs continuously, whether the frog is in or out of water; when the frog is under water or hibernating, it is the only mode of respiration. Bucco-pharyngeal respiration is used on land, with the mouth permanently closed and the nostrils open. The oxygen supply provided by pulmonary respiration is supplemented by respiration from the skin and the buccal cavity.27 Amphibians have a substantially lower metabolic rate than mammals, including humans; this is reflected in the lower requirements for oxygen transport and cardiac output.27 Despite the compensations of oxygen supply provided by the skin and mouth, the heart of Xenopus laevis functions with a single ventricle, as in children with complex congenital heart defects, yet Xenopus laevis nevertheless thrive and live for 25 to 30 years.
A previous report investigated the electrophysiology of the Xenopus laevis heart18 and another studied heart anatomy using echocardiography.1 However, to the best of our knowledge, Xenopus laevis cardiovascular anatomy and physiology have not been studied in detail by use of echocardiography and cardiac magnetic resonance (CMR). The purpose of our study was to describe a technique of anesthesia that can be used to conduct noninvasive imaging procedures, such as echocardiography and CMR, in Xenopus laevis.
Materials and Methods
Animals.
From January to December 2021, a total of 46 female Xenopus laevis frogs (Nasco, Fort Atkinson, WI), aged one year and 10 mo, underwent anesthesia for imaging procedures, either echocardiography (n = 24) or CMR (n = 22). All procedures were approved by the IACUC of The University of Texas Health Science Center at Houston and were in accordance with the Guide for the Care and Use of Laboratory Animal Care.17 Frogs were maintained in facilities fully accredited by AAALAC International. A color photograph was taken of each frog, showing its unique identifying markings. Photographs were kept in a catalog for use in identification of individual frogs.
Housing.
The housing system consisted of 2 50-L tanks on a recirculating life-support system (Aquaneering, San Diego, CA), with each tank holding 8 to 10 frogs at any given time. In this system, water exits each tank via a surface skimming overflow, passing through a 20-µm mesh filter to remove uneaten food and feces before being collected in the dirty sump. A pump then directs the prefiltration water through a fluidized biologic bed filter and a degassing column to a filtration sump. A second pump then sends the filtered water through a 50-µm fine-particulate filter, a 100-W UV sterilizer, and a flow-through chiller back to the individual tanks.
The housing room was maintained between 19.5 and 20.5 °C, and the water temperature between 17.5 and 18.0 °C. Frogs were maintained in a 12:12-h light:dark cycle. Frogs were fed 15 mL of Nasco Frog Brittle (Nasco, Fort Atkinson, WI) per tank 3 times a week.
Transportation.
A cart with 3 tanks (Figure 1) was used to transport the frogs from the housing room to the imaging suites, either for echocardiography or CMR. The transportation, sedation, and recovery tanks were respectively filled with 4, 2, and 8 L of filtered system water. A 0.05% solution of tricaine ethyl 3-aminobenzoate methanesulfonate (MS-222, Sigma Aldrich, St. Louis, MO) was mixed thoroughly into the water of the sedation tank to achieve a dose of 1.5 g per 1 L of system water and was buffered with 4.8 g of sodium bicarbonate to achieve a pH of 7.0 to 7.4.
Figure 1.

From left to right: sedation tank, transport tank, and recovery tank.
The photo catalog was used to identify the frogs captured for imaging. Selected frog(s) were placed in the transportation tank and taken to the imaging suite with the lids closed and tanks covered with a drape. One or 2 frogs were transported for imaging each day, based on requests from of the sonographer and/or CMR investigator.
Anesthesia procedure.
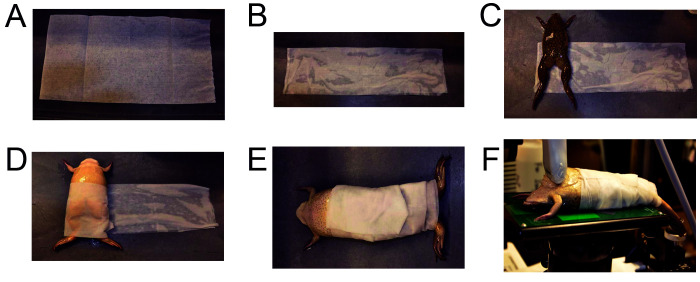
Once in the echocardiography or CMR imaging suite, the frog was removed from the transportation tank and placed in the sedation tank for 10 to 15 min with the lid open. At the end of this induction period, the frog was placed on its back with the head raised and the nostrils above the water line to assess alertness and depth of anesthesia. Absence of a pain response was verified via toe pinch to check for adequacy of sedation while the frog was still in the sedation tank. If a toe reflex was present, the frog remained in the sedation tank for an additional period, generally ranging from 2 to 5 min, to ensure adequate sedation. Once a toe reflex was absent, a paper towel (Figure 2A) was soaked in the MS-222 solution, and lightly squeezed of excess fluid. The paper towel was then stretched out on the transportation cart and folded on itself 3 times (Figure 2B).The sedated frog was then removed from the sedation tank and weighed; weight was documented in the health record and anesthesia sheet. The frog was then placed at one end of the folded damp paper towel, in supine position (Figure 2C), and rolled 3 to 4 times in the paper towel like a ‘burrito,’ with the head and front legs exposed above the rolled paper towel and the hind legs below (Figures 2D). Electrocardiography and respiratory rate leads were attached to the skin for monitoring purposes, and the frog was then handed to the echocardiography or CMR investigator (Figure 2E)and positioned for the procedure (Figure 2F). Vital signs were monitored throughout the entire procedure. This procedure was repeated as needed, with a new immersion in the sedation tank after the procedure. Oxygen saturation and heart rate were measured and recorded at the end of all imaging sessions using a neonatal oxygen saturometer with adhesive pulse oximeter sensors (Masimo Device Company, Irvine, CA) placed on the legs. The ambient temperature in the echocardiography room was 22 °C, while the CMR room was 17 °C. The frogs did not receive any supplemental heating or cooling during imaging and were left to adapt to the respective room temperatures of the different rooms used for the imaging. As our protocol did not include invasive procedures, we did not perform any evaluation of the frogs’ metabolic rates, which would have required an invasive approach.
Figure 2.
Dry paper towel (A) and folded paper towel that has been dipped into the sedation tank and soaked with tricaine solution (B). Frog placed on an MS-222-soaked paper towel (C) and wrapped in a ‘burrito’ fashion (D). Frog with ‘burrito’ wrap completed and ready for echocardiography (E) and placed supine on platform of echocardiography machine for imaging (F).
Consistent with safety regulations for the use of MS-222, personnel wore gloves throughout the entire procedure. The MS-222 was discarded as required by institutional procedures.
Recovery.
Once the imaging was completed, the frog was unrolled from the paper towel, placed in the recovery tank, and monitored for adverse signs such as the inability to swim or maintain the head above water. Once fully recovered, the frog was placed in the transportation tank and returned to its home tank. Monitoring was discontinued when the frog returned to its typical activity (that is, swimming and interacting with the other frogs). Another assessment was performed 24 h later to verify normal recovery.
For each frog, a separate anesthesia sheet was used to record all details of the induction, maintenance, and recovery of the anesthesia procedure, with sequential recording of the time of each event, including the need to administer additional anesthesia. Feeding was restarted 24 h after imaging, and frogs were reweighed after 48 h and then at monthly intervals.
Statistical analysis.
A t-test was used to compare results between the 2 groups of Xenopus laevis (echocardiography and CMR), and statistical significance was accepted for P< 0.05. Descriptive statistics are presented as mean ± SD.
Results
No mortality or complications were observed for the entire duration of this study, including during transportation, anesthesia, and recovery. All frogs recovered well and remained in good condition for the duration of the study.
No statistical difference was found between the mean body weight ± SD of Xenopus laevis that underwent echocardiography (173 ± 19 g, range: 140 to 218 g) or CMR (174 ± 24 g, range: 149 to 218 g).
The mean duration of anesthesia induction was 12 ± 5 min (range: 6 to 23 min) for the echocardiography group and 14 ± 6 min (range: 6 to 20 min) for the CMR group (nsd), while the mean duration of anesthesia maintenance was 60 ± 18 min (range: 22 to 85 min) for the echocardiography group and 118 ± 37 min (range: 60 to 183 min) for the CMR group (P< 0.001). In the echocardiography group, 9 of 24 (37.5%) frogs required an additional dose of anesthesia during maintenance, while in the CMR group 6 of 22 (27%) required an additional dose (nsd; P = 0.47).
At the end of the procedure, the mean oxygen saturation was 66 ± 9% for the echocardiography group and 85 ± 6% for the CMR group (P< 0.001), and the heart rate was 48 ± 13 beats/min for the echocardiography group and 42 ± 7 beats/min for the CMR group (nsd). During the investigations, the lowest heart rate reached by a frog in the echocardiography group was 12 beats/min, while in the CMR group the lowest rate was 4 beats/min. Despite the differences in room temperature between the 2 investigations, no difference in speed or completion of recovery was observed between the 2 groups.
Discussion
The presence in Xenopus laevis of a double-inlet single ventricle with a single outlet has stimulated our interest due toits similarities to the cardiovascular anatomy of children born with complex congenital heart defects.5,28 However, in contrast to the life span of 25 to 30 y for Xenopus laevis, patients with a single ventricle, despite repeated cardiac interventions, have a short life expectancy and a poor quality of life.2,5,7,8,10,11,21,28 Although morphologic studies published over 50 y ago speculated on the cardiovascular function based on the peculiar anatomy of the frog heart,4,14,30 heart function in Xenopus laevis has only recently been studied with regard to the pathophysiology of human hearts with a single ventricle.16,23
In our literature review, we found very little information on frog heart anatomy as studied using with echocardiography,30 and, to the best of our knowledge, detailed information on the cardiovascular anatomy and physiology investigated with echocardiography and cardiac magnetic resonance (CMR) has not yet been reported. In particular, we are not aware of studies aiming to understand the mechanism for the perfect balance between systemic and pulmonary circulation in Xenopus laevis, which allows unrestricted life activities for many years.
General anesthesia was necessary to acquire all desired information using either echocardiography or CMR. In our experience, the duration of general anesthesia was up to 1.5 h for echocardiography, and up to 3 h for CMR. Therefore, we sought a method that would provide safe anesthesia for imaging procedures in Xenopus laevis while maintaining stable cardiovascular and respiratory parameters for the entire duration of the procedures.
Various types of anesthesia have been used for Xenopus laevis, including MS-222 and benzocaine gel,3,9,12,13,17,19,22,24,26 intramuscular ketamine,9,12,24 clove oil (Eugenol),9,12,24 propofol,9,12,13,24 etomidate,31 and hypothermia.20 Selection of an anesthetic protocol for a procedure on Xenopus laevis requires consideration of the type and duration of the procedure being performed and all available options. Amphibians offer veterinarians an option for anesthesia administration that is not available in higher vertebrates: immersion. The highly vascular integument of an amphibian is generally capable of absorbing different anesthetics at a rate allowing for prolonged anesthesia. Consequently, immersion is the method most frequently used to administer anesthesia in Xenopus laevis research.
Topical anesthetics can be applied directly onto the integument of Xenopus laevis. This approach has the advantage of avoiding a dilution effect and therefore smaller volumes and doses can be used. However, a potential disadvantage is a greater risk for toxicity if drugs are rapidly absorbed through the integument. The most common anesthetics used for topical application are isoflurane24 and benzocaine,17,24 although this route is rarely reported. Inhalant anesthetics can also be used to anesthetize amphibians, delivered via oxygen flow through the respiratory tract using tracheal intubation and mechanical ventilation. However, due to the relatively small size of the cervical region and upper airway in Xenopus laevis as compared with other amphibians and potential complications such as single lung intubation and desiccation, this method is rarely used.24
Based on our literature review, and considering our needs to maintain stable cardiovascular and respiratory parameters while avoiding any type of muscle paralysis, our choice was to use the immersion technique for anesthesia with MS-222 as the selected anesthetic. We used a ‘burrito’ method of anesthesia, with the frog wrapped in a paper towel soaked in the MS-222 solution; this practice kept the skin wet and allowed continuous anesthetic absorption during the entire procedure. In our study, fewer than half of Xenopus laevis required an additional dosage of anesthesia to complete the investigational procedure, when lasting more than one hour.
This technique allowed cardiovascular and respiratory stability of the duration necessary to acquire the information required from echocardiography and CMR. The only difference observed between the 2 groups was that percutaneous oxygen saturation at the end of the procedure was lower for the group undergoing echocardiography as compared with the group undergoing CMR (respectively, 66 ± 9% compared with 85 ± 6%, P< 0.001). We attributed this difference to the higher ambient temperature in the echocardiography room as compared with the CMR room (respectively, 22 °C and 17 °C). Because of peripheral vasodilatation caused by a higher temperature, the left-to-right shunting of the single ventricle, which maintains a good balance between pulmonary and systemic blood flow at optimal ambient temperatures, fell, thereby reducing the normal QP/QS ratio between pulmonary blood flow (QP) and systemic blood flow (QS). This in turn may have resulted in the lower oxygen saturation observed in the echocardiography group. However, despite this reduction in systemic perfusion relative to the pulmonary circulation, no negative consequence was observed in any of the frogs, and all had full recoveries after the procedures regardless of the environmental temperature to which they were exposed.
The singularity of the cardiovascular circulation in Xenopus laevis attracted our interest because of the similarities with complex congenital heart defects in humans. To improve our knowledge of Xenopus laevis, which is currently limited to the morphology of the heart and great vessels, we performed noninvasive evaluations using echocardiography and CMR to learn more about this unusual and complex pathophysiology. Anesthesia provided by immersion in MS-222 allowed us to perform a large number of imaging procedures without complications. This technique of anesthesia can be easily reproduced and used for similar investigations in Xenopus laevis and would be a safe and reliable method for noninvasive procedures requiring a period of 2 to 3 h. We have no data regarding its potential use for invasive procedures
Acknowledgments
We are very thankful to Callie Lazarine, for the technical assistance received during this study.
References
- 1.Bartlett HL, Escalera RB, Patel SS, Wedemeyer EW, Volk KA, Lohr JL, Reinking BE. 2010. Echocardiographic Assessment of Cardiac Morphology and Function in Xenopus. Comp Med 60:107–113. [PMC free article] [PubMed] [Google Scholar]
- 2.Buchhorn R, Bartmus D, Buhre W, Bursch J. 2001. Pathogenetic mechanisms of venous congestion after the Fontan procedure.Cardiol Young 11:161–168. https://doi.org/10.1017S1047951101000051. [DOI] [PubMed] [Google Scholar]
- 3.Burggren WW, Warburton S. 2007. Amphibians as Animal Models for Laboratory Research in Physiology. ILAR J 48:260–269. 10.1093/ilar.48.3.260. [DOI] [PubMed] [Google Scholar]
- 4.Chambers WN, Criscittiello MG, Goodale F. 1961. Cor triloculare biatriatum. Survival to adult life. Circulation 23:91–101. 10.1161/01.CIR.23.1.91. [DOI] [PubMed] [Google Scholar]
- 5.Corno AF, Becker AE, Bulterijs AHK, Lam J, Nijveld A, Schuller JL, Marcelletti C. 1982. Univentricular heart: Can we alter the natural history? Ann Thorac Surg 34:716–726. 10.1016/S0003-4975(10)60917-4. [DOI] [PubMed] [Google Scholar]
- 6.Corno AF, Vergara C, Subramanian C, Johnson RA, Passerini T, Veneziani A, Formaggia L, Alphonso N, Quarteroni A, Jarvis JC. 2010. Assisted Fontan procedure: animal and in vitro models and computational fluid dynamics study. Interact Cardiovasc Thorac Surg 10:679–684. 10.1510/icvts.2009.223024. [DOI] [PubMed] [Google Scholar]
- 7.de Level MR, Deanfield JE. 2010. Four decades of Fontan palliation. Nat Rev Cardiol 7:520–527. 10.1038/nrcardio.2010.99. [DOI] [PubMed] [Google Scholar]
- 8.Dennis M, Zannino D, du Plessis K, Bullock A, Disney PJS, Radford DJ, Hornung T, Grigg L, Cordina R, d’Udekem Y, Celermajer DS. 2018. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol 71:1009–1017. 10.1016/j.jacc.2017.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Gentz EJ. 2007. Medicine and surgery of amphibians. ILAR J 48:255–259. 10.1093/ilar.48.3.255. [DOI] [PubMed] [Google Scholar]
- 10.Gewillig M, Brown SC. 2016. The Fontan circulation after 45 years: update in physiology. Heart 102:1081–1086. 10.1136/heartjnl-2015-307467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannico S, Corno AF, Marino B, Cicini MP, Gagliardi MG, Amodeo A, Picardo S, Marcelletti C. 1992. Total extracardiac right heart bypass. Circulation 86(5 Suppl): II110–117. [PubMed] [Google Scholar]
- 12.Green SL. 2010. Anesthesia, p 110–112. In: The laboratory Xenopus sp. London: CRC Press. [Google Scholar]
- 13.Guénette SA, Beaudry F, Vachon P. 2008. Anesthetic properties of Propofol in African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 47:35–38. [PMC free article] [PubMed] [Google Scholar]
- 14.Haberich FJ. 1965. The functional separation of venous and arterial blood in the univentricular frog heart. Ann N Y Acad Sci 127:459–476. 10.1111/j.1749-6632.1965.tb49419.x. [DOI] [PubMed] [Google Scholar]
- 15.Hicks JW. 2002. The physiological and evolutionary significance of cardiovascular shunting patterns in reptiles. News Physiol Sci 17:241–245. 10.1152/nips.01397.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hillman SS, Hedrick MS, Kohl ZF. 2014. Net cardiac shunts in anuran amphibians: physiology or physics? J Exp Biol 217:2844–2847. 10.1242/jeb.105536. [DOI] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 18.Knight VA, Richardson DR, Makoba B. 1989. Use of frog ventricle to examine mechanical and electrical activity of heart. Am J Physiol 256:S9–S13. 10.1152/advances.1989.256.6.S9. [DOI] [PubMed] [Google Scholar]
- 19.Koustubhan P, Kaplan DL, Levin M. 2013. Humane anesthesia and pain management in amphibian limb surgery of Rana pipiens. Cold Spring Harb Protoc 2013(2):149–155. 10.1101/pdb.prot071977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lillywhite HB, Shine R, Jacobson E, DeNardo DF, Gordon MS, Navas CA, Wang T, Seymour RS, Storey MB, Heatwole H, Heard D, Brattstorm B, Burghardt GM. 2017. Anesthesia and euthanasia of amphibians and reptiles used in scientific research: should hypothermia and freezing be prohibited? Bioscience 67:53–61. 10.1093/biosci/biw143. [DOI] [Google Scholar]
- 21.Marcelletti C, Corno AF, Giannico S, Marino B. 1990. Inferior vena cava to pulmonary artery extracardiac conduit: A new form of right heart bypass.J Thorac Cardiovasc Surg 100:228–232. 10.1016/S0022-5223(19)35562-X. [DOI] [PubMed] [Google Scholar]
- 22.Medler S. 2019. Anesthetic MS-222 eliminates nerve and muscle activity in frogs used for physiology teaching laboratories. Adv Physiol Educ 43:69–75. 10.1152/advan.00114.2018. [DOI] [PubMed] [Google Scholar]
- 23.Meyer SL, Jongbloed MR, Ho SY, Bartelings MM, McCarthy KP, Uemura H, Ebels T. 2017. Intracardiac anatomical relationships and potential for streaming in double inlet left ventricles. PLOS One 12:e0188048. 10.1371/journal.pone.0188048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell MA. 2009. Anesthetic considerations for amphibians. J Exot Pet Med 18:40–49. 10.1053/j.jepm.2008.11.006. [DOI] [Google Scholar]
- 25.O’Rourke DP, Rosenbaum MD. 2015. Biology and diseases of amphibians, p 931–965. In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, editors. Laboratory Animal Medicine, Third Edition. Boston (MA): Academic Press. 10.1016/B978-0-12-409527-4.00018-3. [DOI] [Google Scholar]
- 26.Paduano M, Colafrancesco KC, Wong SA, Caldwell MS, Gridi-Papp M. 2013. The response of gray Treefrogs to anesthesia by tricaine methanesulfonate (TMS or MS-222). ISRN Zool 2013:635704. 10.1155/2013/635704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pokhrel P. 2016. Respiratory system in frogs. https://microbiologynotes.com/respiratory-system-frog/.
- 28.Poterucha JT, Anavekar NS, Egbe AC, Julsrud PR, Connolly HM, Ammash NM, Warnes CA. 2016. Survival and outcomes of patients with unoperated single ventricle. Heart 102:216–222. 10.1136/heartjnl-2015-308440. [DOI] [PubMed] [Google Scholar]
- 29.Rijnberg FM, Hazekamp MG, Wentzel JJ, de Koning PJH, Westenberg JJM, Jongbloed MRM, Blom NA, Roest AAW. 2018. Energetics of blood flow in cardiovascular disease concept and clinical implications of adverse energetics in patients with a Fontan circulation. Circulation 137:2393–2407. 10.1161/CIRCULATIONAHA.117.033359. [DOI] [PubMed] [Google Scholar]
- 30.Sharma HL. 1961. The circulatory mechanism and anatomy of the heart of the frog, Rana pipiens. J Morphol 109:323–349. 10.1002/jmor.1051090307. [DOI] [PubMed] [Google Scholar]
- 31.Smith BD, Vail KJ, Carroll GL, Taylor MC, Jeffery ND, Vemulapalli TH, Elliott JJ. 2018. Comparison of Etomidate, Benzocaine, and MS222 anesthesia with and without subsequent Flunixin Meglumine analgesia in African clawed frogs (Xenopus Laevis). J Am Assoc Lab Anim Sci 57:202–209. [PMC free article] [PubMed] [Google Scholar]