Abstract
Sphingomonas (formerly Pseudomonas) paucimobilis UT26 utilizes γ-hexachlorocyclohexane (γ-HCH), a halogenated organic insecticide, as a sole source of carbon and energy. In a previous study, we showed that γ-HCH is degraded to chlorohydroquinone (CHQ) and then to hydroquinone (HQ), although the rate of reaction from CHQ to HQ was slow (K. Miyauchi, S. K. Suh, Y. Nagata, and M. Takagi, J. Bacteriol. 180:1354–1359, 1998). In this study, we cloned and characterized a gene, designated linE, which is located upstream of linD and is directly involved in the degradation of CHQ. The LinE protein consists of 321 amino acids, and all of the amino acids which are reported to be essential for the activity of meta-cleavage dioxygenases are conserved in LinE. Escherichia coli overproducing LinE could convert both CHQ and HQ, producing γ-hydroxymuconic semialdehyde and maleylacetate, respectively, with consumption of O2 but could not convert catechol, which is one of the major substrates for meta-cleavage dioxygenases. LinE seems to be resistant to the acylchloride, which is the ring cleavage product of CHQ and which seems to react with water to be converted to maleylacetate. These results indicated that LinE is a novel type of meta-cleavage dioxygenase, designated (chloro)hydroquinone 1,2-dioxygenase, which cleaves aromatic rings with two hydroxyl groups at para positions preferably. This study represents a direct demonstration of a new type of ring cleavage pathway for aromatic compounds, the hydroquinone pathway.
γ-Hexachlorocyclohexane (γ-HCH; also called γ-BHC and lindane) is a halogenated organic insecticide which has been used worldwide. Because of its toxicity and long persistence in soil, most countries have prohibited the use of γ-HCH. However, many contaminated sites still remain throughout the world. Moreover, some countries are presently using γ-HCH for economic reasons, and new sites are continually being contaminated.
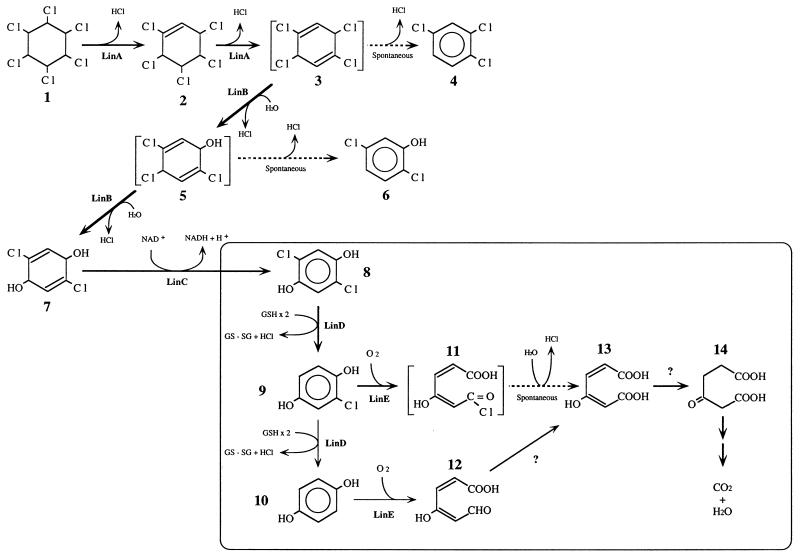
Sphingomonas (formerly Pseudomonas) paucimobilis UT26 utilizes γ-HCH as a sole source of carbon and energy (12). UT26 degrades γ-HCH through the pathway shown in Fig. 1 (24, 27, 28). γ-HCH is likely converted by two steps of dehydrochlorination via γ-pentachlorocyclohexene (γ-PCCH) to 1,3,4,6-tetrachloro-1,4-cyclohexadiene (1,4-TCDN). This is productively metabolized to 2,5-dichloro-2,5-cyclohexadiene-1,4-diol (2,5-DDOL) by two steps of hydrolytic dehalogenation. 2,5-DDOL is further degraded to 2,5-dichlorohydroquinone (2,5-DCHQ) and 2,5-DCHQ is dechlorinated to CHQ or HQ, then to be mineralized. Two dead-end products, 1,2,4-trichlorobenzene (1,2,4-TCB) and 2,5-dichlorophenol (2,5-DCP), are also produced in this pathway.
FIG. 1.
Proposed assimilation pathway of γ-HCH in S. paucimobilis UT26. Compounds: 1, γ-HCH; 2, γ-PCCH; 3, 1,4-TCDN; 4, 1,2,4-TCB; 5, 2,4,5-DNOL; 6, 2,5-DCP; 7, 2,5-DDOL; 8, 2,5-DCHQ; 9, CHQ, 10, HQ; 11, acylchloride; 12, γ-HMSA; 13, maleylacetate; 14, β-ketoadipate.
In previous studies, we cloned and sequenced four genes involved in the γ-HCH degradation in UT26 (11, 23, 27, 28). The linA gene encodes γ-HCH dehydrochlorinase (LinA), which converts γ-HCH to 1,2,4-TCB via γ-PCCH. LinA shows no homology to known proteins (11). The linB gene encodes 1,4-TCDN chlorohydrolase (LinB), which converts 1,4-TCDN to 2,5-DDOL via 2,4,5-trichloro-2,5-cyclohexadiene-1-ol (2,4,5-DNOL). LinB shows significant similarity to hydrolytic dehalogenase (DhlA) from Xanthobacter autotrophicus GJ10 (13). The linC gene encodes 2,5-DDOL dehydrogenase, which converts 2,5-DDOL to 2,5-DCHQ (28). LinC shows homology to the members of the short-chain alcohol dehydrogenase family (29). The linD gene encodes 2,5-DCHQ dechlorinase, which converts 2,5-DCHQ to HQ via CHQ, and its activity rises in the presence of glutathione. LinD shows similarity to some members of class theta glutathione S-transferase family (23). We also showed that linA, linB, and linC were constitutively expressed (11, 27, 28), whereas linD was inducibly expressed in the presence of its substrate (23).
In this study, we describe the isolation and characterization of linE gene which is directly involved in the degradation of CHQ, one of the intermediates of γ-HCH degradation pathway in S. paucimobilis UT26. We show that the protein product of linE is a novel type of meta-cleavage dioxygenase, (chloro)hydroquinone 1,2-dioxygenase, which cleaves the aromatic ring with two hydroxyl groups at para positions preferably.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Sphingomonas strains, Pseudomonas strains, and Escherichia coli were grown in Luria broth (21) or on W minimal medium (12). Cultures were incubated at 30°C for Sphingomonas and Pseudomonas strains and at 37°C for E. coli strains. Antibiotics were used at final concentrations of 50 μg/ml for ampicillin and kanamycin, 25 μg/ml for nalidixic acid, and 20 μg/ml for tetracycline.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| S. paucimobilis | ||
| UT26 | HCH+ Nalr | 12 |
| UT103 | HCH− Nalr ΔlinD genome::Tn5 | 26 |
| UT116 | HCH− Nalr ΔlinD ΔlinE genome::Tn5 | 26 |
| P. putida PpY101 | Met Nalr | 19 |
| E. coli | ||
| MV1190 | Δlac-proAB thi supE Δsrl-recA306::Tn10 F′ traD36 proAB lacIqZΔM15 | 39 |
| HB101 | F−hsdS recA ara proA lacY galK rpsL xyl mtl supE | 21 |
| Plasmids | ||
| pRK2013 | ColE1::RK2 Tra+ Kmr | 7 |
| pKTY320::Tn5 | Apr Cm::Tn5 (Kmr Bler Strr) | 25 |
| pKS13 | RK2 replicon, cos Mob+ Tcr | 19 |
| pUC18 | pMB9 replicon, Apr | 39 |
| pUC118 | pMB9 replicon, Apr | 39 |
| pHSG399 | pMB9 replicon, Cmr | 39 |
| pAQN | pMB9 replicon, lacIqaqn Apr | 38 |
| pKSM1920 | pKS13 with about 20 kb of UT26 DNA containing linD | This study |
| pKSM208 | pKS13 with about 20 kb of UT26 DNA containing linD | This study |
| pSS1 | pUC18 containing a 1.3-kb SacI-SacI fragment upstream of linD | This study |
| pSS2 | pUC18 containing a 7-kb SacI-SacI fragment upstream of linD | This study |
| pLE1 | pUC18 carrying a 1.3-kb blunted PstI-NaeI fragment including linE at its SmaI site. linE is the same direction as the lac promoter | This study |
| pLE11 | pHSG399 carrying a 5.5-kb PstI-PstI fragment from which NaeI-NaeI fragment is removed. linE is the same direction as the lac promoter | This study |
| pPP55 | pUC18 carrying a 5.5-kb PstI-PstI fragment including linE at its PstI site. linE is the same direction as the lac promoter. | This study |
| pMYLE2 | pAQN carrying the EcoRI-NaeI fragment of pLE11 | This study |
| pCY385 | pUC18 carrying xylE | 20 |
| pLEH162A | pUC18 containing linE mutant (H162A) | This study |
| pMYH162A | pAQN containing linE mutant (H162A) | This study |
| pLEH229A | pUC18 containing linE mutant (H229A) | This study |
| pMYH229A | pAQN containing linE mutant (H229A) | This study |
| pLEE278A | pUC18 containing linE mutant (E278A) | This study |
| pMYE278A | pAQN containing linE mutant (E278A) | This study |
HCH+, grown on γ-HCH. aqn, aqualysin I gene of Thermus aquaticus.
Isolation of DNA.
Plasmid DNA of E. coli was isolated by the alkaline lysis method of Maniatis et al. (21) and, if needed, purified by a cesium chloride-ethidium bromide density gradient centrifugation. Total DNAs from Sphingomonas and Pseudomonas strains were isolated as described previously (25).
Assay for CHQ degradation activity.
Cosmid clones with LinD activity were assayed for CHQ degradation activity. A small quantity of each colony was picked and suspended in 100 μl of the assay solution (20 mM phosphate buffer [pH 7.0] containing CHQ at 1 μg/ml). The solution was incubated for 12 to 18 h at 30°C for Pseudomonas putida and at 37°C for E. coli, 500 μl of ethyl acetate was added, and the mixture was vortexed for 1 min. After centrifugation, the ethyl acetate layer was recovered. Five microliters of this extract was used for gas chromatography-mass spectroscopy (GC-MS) analysis. CHQ degradation activity was detected as the disappearance of the peak for CHQ.
To measure CHQ degradation activity of Sphingomonas strains, CHQ solution (20 mM potassium-phosphate buffer [pH 7.0] containing CHQ and ascorbic acid (each at a final concentration of 100 μM) was added to each whole cell (100 mg [wet weight]/ml). The mixture was extracted with ethyl acetate at specified times and analyzed by GC-MS. CHQ degradation activity was detected as the disappearance of the peak for CHQ.
CHQ was purchased from Aldrich (Milwaukee, Wis.), and a stock solution was made by dissolving the compound in ethanol.
GC-MS analyses.
GC-MS analysis was performed as described previously (23). The column temperature was increased from 80 to 160°C at a rate of 5°C/min and then from 160 to 260°C at a rate of 10°C/min. The carrier gas flow rate was 20 ml/min.
Nucleotide sequence determination.
Nucleotide sequences were determined by the dideoxy-chain termination method with a LI-COR model 4000L DNA sequencing system (LI-COR, Lincoln, Neb.).
Southern blot analysis.
Southern blot analysis was performed with the ECL (enhanced chemiluminescence) gene detection system (Amersham, Arlington Heights, Ill.) according to the protocol provided by the manufacturer.
Northern blot analysis.
Northern blot analysis was performed as described previously (23). 2,5-DCHQ, CHQ, and HQ (10 μM each) were used as inducers.
Analysis of the linE gene product.
For overexpression of the linE gene product, plasmid pMYLE2 was constructed from pAQN (38). Plasmid pAQN was digested with EcoRI and HindIII to replace the 1.8-kb aqualysin coding fragment with the 1.3-kb EcoRI-HindIII fragment including the linE gene from pLE1. In pMYLE2, the linE gene is expressed under the control of the tac promoter. Expression is repressed tightly by the lacIq gene product which is produced from the same plasmid, without IPTG (isopropyl-β-d-thiogalactopyranoside). Overexpression of linE was achieved as described previously (11) by using E. coli MV1190 containing pMYLE2. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done as described previously (27).
Analysis of the metabolites.
E. coli overproducing LinE was suspended in 20 mM potassium-phosphate buffer (pH 7.0) containing 100 ppm of (C)HQ and was incubated at 37°C with shaking for 12 h. Cells were removed by centrifugation, and the supernatant was extracted with ethyl acetate after acidification by HCl. Following evaporation of the ethylacetate layer, the substances were trimethylsilylated by N-methyl-N-trimetylsilyl-trifluoroacetamide (Nacalai Tesque, Kyoto, Japan) at 65°C. The resultant samples were analyzed by GC-MS. E. coli MV1190 was used as a negative control.
O2 consumption assays using crude cell extract.
Cells overproducing LinE or XylE were suspended in 20 mM Tris-HCl buffer (pH 7.5) and were disrupted by sonication (Sonifier; Branson, Danbury, Conn.). After centrifugation (12,000 × g) at 4°C for 10 min, the supernatant was used as the crude cell extract. Three hundred microliters of crude extract was diluted in 2.7 ml of O2-saturated 20 mM potassium-phosphate buffer (pH 7.0). Substrates were diluted to 100 mM with ethanol. The assay was started by injecting 1 μl of substrate solution to the diluted crude extract. The consumption of O2 was measured with an O2 electrode system (MD-1000; Iijima Electronics Co., Aichi, Japan). The protein concentration of the crude extract was measured with a protein assay kit (Bio-Rad, Hercules, Calif.). One unit of activity was defined as the amount which consumes 1 μmol of O2 in 1 min. Values reported are those from which the value of the endogeneous oxygen consumption was subtracted.
Site-directed mutagenesis.
Site-directed mutagenesis was performed by using an LA PCR in vitro mutagenesis kit (Takara, Kyoto, Japan). Primers used for the mutants were 5′-GTCCAGCTGGCAAAGCCC-3′ for H162A, 5′-CGCGGCGGCATGAACTTGG-3′ for H229A, and 5′-GACCGAGGCCGCGAACAAC-3′ for E278A.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper are registered with the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB021867.
RESULTS
CHQ degradation activity of the LinD-less mutant.
We previously cloned the linD gene, whose product (LinD) is responsible for the conversion of 2,5-DCHQ to CHQ and CHQ to HQ (23). However, the conversion of CHQ to HQ by LinD seems not to be essential for the degradation pathway of γ-HCH in UT26, because the conversion rate of CHQ to HQ is much lower than that of 2,5-DCHQ to CHQ (23). Therefore, we investigated whether UT26 has another gene involved in CHQ degradation. The CHQ degradation activity of UT103, the LinD-less mutant (22, 23, 26), was tested. UT103 had the same extent of CHQ degradation activity as UT26 (100 mg [wet weight] of both strains degraded 100 nmol of CHQ within 1 h), indicating that UT26 has another gene (designated linE) for CHQ degradation. We also tested the CHQ degradation activity of UT116, carrying a deletion of the linD gene and its flanking region (23, 26). UT116 had no LinE activity, suggesting that the putative linE gene resides near the linD gene.
Screening of a cosmid clone which has CHQ degradation activity.
In a previous study, we obtained six clones of P. putida PpY101, each of which holds a 20- to 30-kbp insert containing the linD gene (23). We tested each clone for CHQ degradation activity (LinE activity) and found that two of the six clones (one carrying pKSM1920 and one carrying pKSM208) had LinE activity. This activity was not detected when we used E. coli HB101 carrying pKSM1920 and pKSM208.
Subcloning and sequence analysis of the linE gene.
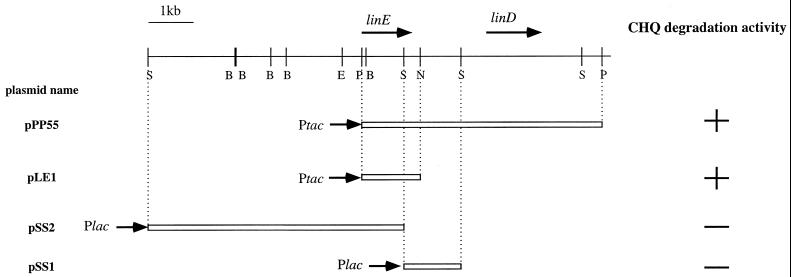
Subcloning analysis revealed that the 5.5-kb PstI-PstI fragment containing the linD gene and 1.3-kb PstI-NaeI fragment were responsible for LinE activity (Fig. 2). One open reading frame (ORF) of reasonable size (963 bp) was found in the 1.3-kb PstI-NaeI fragment (Fig. 2). As this ORF is preceded by a putative Shine-Dalgarno sequence, we designated it linE. The linE gene encodes a polypeptide of 321 amino acids, and its deduced molecular mass is 36.0 kDa. Neither a sequence which shows a high level of similarity to the promoter sequence in E. coli nor one which is expected to form a stem-loop structure to function as a terminator was found around the linE gene. The G+C content of the linE gene is 60.1%, which is close to the total G+C content of a type strain of S. paucimobilis (65%) (31); the G+C contents of linB, linC, and linD are 64.3% (27), 62.5% (28) and 60.8% (23), respectively.
FIG. 2.
Restriction map and deletion analysis of the region around the linD gene. Directions of transcription by the lac promoter are indicated by arrows. The procedure for the measurement of the LinE activity is described in Materials and Methods. The deduced location and direction of transcription of the linE gene are indicated by the arrow above the restriction map. B, BamHI, E, EcoRI; N, NaeI; P, PstI; S, SacI.
Southern blot analysis of the above-mentioned six cosmids containing the linD gene by using the 1.3-kb PstI-NaeI fragment containing linE as a probe revealed that only two cosmids which expressed LinE activity in P. putida (pKSM1920 and pKSM208) contained the whole linE gene (data not shown). Northern blot analysis revealed that linE is inducibly expressed in the presence of CHQ, HQ, and 2,5-DCHQ (data not shown).
Overexpression of the linE gene in E. coli and identification of its protein product.
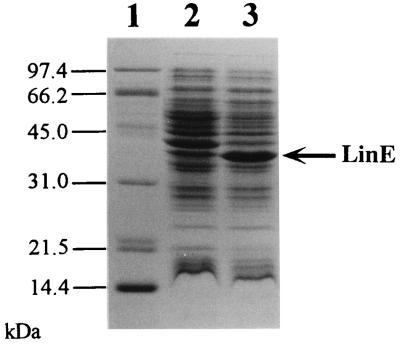
To identify the protein product of the linE gene, we constructed plasmid pMYLE2, in which the linE gene was under the control of the tac promoter. E. coli MV1190 transformed with this plasmid was incubated with or without IPTG, and total proteins were analyzed by SDS-PAGE. An overproduced protein band corresponding to about 36 kDa was observed in the IPTG-treated cells (Fig. 3, lanes 2 and 3). The molecular mass of this protein was almost equal to that deduced from the nucleotide sequence of the linE gene, thus confirming that the product of linE is a protein with a molecular mass of about 36 kDa.
FIG. 3.
Expression of linE under the tac promoter in E. coli. Lanes: 1, molecular mass markers; 2 and 3, total proteins of E. coli containing pMYLE2 not induced by IPTG and induced by IPTG, respectively. LinE protein is indicated by the arrow. The conditions for induction are described in Materials and Methods.
Homology search analysis of the LinE protein.
The computer search revealed that two genes and their protein products, pcpA of Sphingomonas chlorophenolica (41) (recently the sequence of PcpA was revised) and orf88′ of Methylobacterium extorquens AM1 (37), showed significant similarity to linE and LinE (46 and 37% amino acid identity, respectively) (Fig. 4). LinE also showed high levels of similarity to ORFs of Bacillus subtilis (YkcA, YodE, and YdfO [28, 29, and 23% amino acid identity, respectively]), but their functions are unknown. PcpA is involved in the degradation of pentachlorophenol (PCP) in S. chlorophenolica (41). It is known that PcpA is a periplasmic protein induced by PCP, although its function is unknown. Orf88′ of M. extorquens AM1 shares homology with only the N terminus of the LinE protein, because it is the product of a partial ORF which is located at the 3′ end of the published sequence, and consists of 88 amino acids. orf88′ resides near the pqqEF operon, which is responsible for the synthesis of pyrroloquinolinequinone. The putative product of orf88′ shares some identity with some members of catechol 2,3-dioxygenases (C23Os) (37). Therefore, we aligned LinE with some meta-cleavage dioxygenases (Fig. 4).
FIG. 4.
Alignment between LinE, its homologs, and some meta-cleavage dioxygenases as obtained by CLUSTAL X and modified according to the crystal structure of BphC. The residues involved in Fe(II) binding (#) and in its activity ($) in BphC of Pseudomonas sp. strain KKS102 are shown, as are the residues referred to as conserved residues in reference 6) (∗). The identities and similarities between LinE and each dioxygenase are shown following each sequence. The alignment was generated by using the CLUSTAL X sequence alignment program. Abbreviations: LinE_UT26, S. paucimobilis UT26, LinE; PcpA_SPCP, S. chlorophenolica, PcpA (41); Orf88′, M. extorquens AM1, Orf88′ (37); BphC_KKS, Pseudomonas sp. strain KKS102, 2,3-dihydroxybiphenyl 1,2-dioxygenase (19); XylE_JI, P. aeruginosa JI104, C23O (20); CatE_PSHV, Pseudomonas sp. strain HV3, C23O (42); Cdo2_MT5, P. putida MT-15, C23O (18); TodE_PSF1, P. putida F1, 3-methylcatechol 2,3-dioxygenase (43).
Three-dimensional structures of two kinds of meta-cleavage dioxygenases, 2,3-dihydroxybiphenyl 1,2-dioxygenases (BphC), from Pseudomonas sp. strain KKS102 (35) and Burkholderia cepacia LB400 (9), were determined. The important residues for the Fe(II) binding and its enzymatic activity were proposed from these data (Fig. 4). Eltis and Bolin have made an alignment of 23 meta-cleavage dioxygenases including the above two BphCs (6). Alignment of LinE with some of these dioxygenase (Fig. 4) revealed that all of the residues for Fe(II) binding (His162, His229, and Glu278 [LinE numbering]) are conserved. Although the level of similarity around the whole sequence is low, it is likely that LinE belongs to the meta-cleavage dioxygenase family. We also investigated the sequence similarity between LinE and gentisate 1,2-dioxygenases (8, 40), whose substrate (gentisate) has a hydroquinone structure, but there is not significant sequence similarity between LinE and known gentisate 1,2-dioxygenases.
HQ degradation activity of E. coli overproducing LinE.
We previously observed that when HQ was incubated with UT26 with shaking, the color of the solution turned yellow, like the meta-cleavage product of catechol, and the accumulation of the yellow compound corresponded to the increase of the absorption peak at 320 nm (26). When E. coli overproducing LinE was incubated in potassium-phosphate buffer containing HQ with shaking, similar results were obtained: disappearance of HQ, increase of the absorption peak at 320 nm, and accumulation of the yellow compound. When E. coli not expressing linE was used as a control, the color of the solution turned red, apparently as a result of autooxidants of HQ. These results suggest that LinE can degrade HQ in addition to CHQ.
LinE cleaves the aromatic ring of (C)HQ with consumption of O2.
Because amino acids at the catalytic sites of meta-cleavage dioxygenases are conserved in LinE, we investigated the possibility that LinE functions as a ring cleavage dioxygenase.
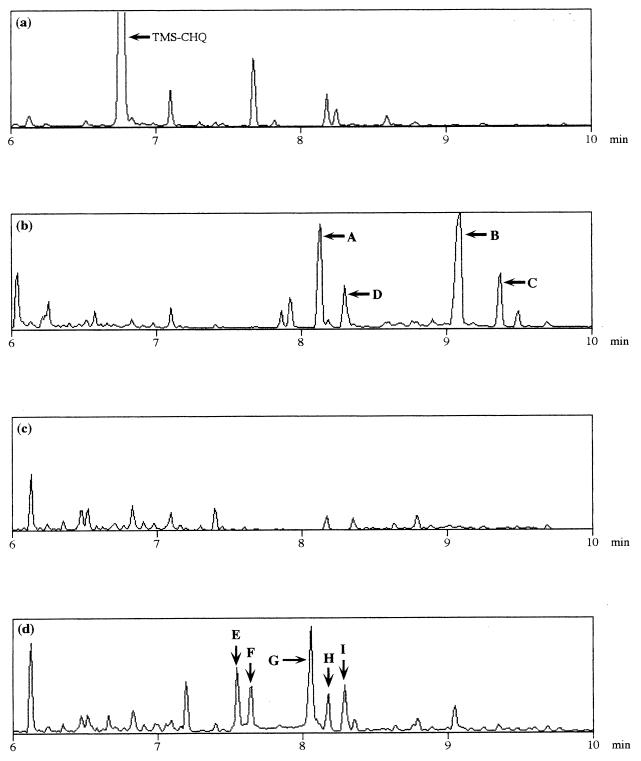
To identify the metabolites of CHQ and HQ produced by the LinE protein, each substrate was incubated, with shaking, with resting E. coli cells overproducing LinE. The metabolites were extracted by ethyl acetate after acidification, then trimethylsilylated, and analyzed by GC-MS. The mass spectra of the peaks which specifically appeared when the cells were incubated with CHQ (Fig. 5a and b, peaks A through D) were identical to trimethylsilylated maleylacetate (peak A through C) and trimethylsilylated 3-oxoadipate (β-ketoadipate) (peak D) (Table 2) (33). The appearance of three peaks for maleylacetate appeared to be due to its isomers (33). When the authentic 3-oxoadipate was also trimethylsilylated and analyzed by GC-MS, the retention time and mass spectrum were identical to those of peak D in Fig. 5b (Table 2). The other peaks (for example, the peak whose retention time is 6.1 min in Fig. 5b) were not reproducible. The mass spectra of the peaks which appeared when cells were incubated with HQ (Fig. 5c and d, peaks E through I) were characteristic of those of trimethylsilylated γ-hydroxymuconic semialdehyde (γ-HMSA). The appearance of five peaks also seemed to be due to its isomers.
FIG. 5.
GC-MS analysis of intermediates of (C)HQ by LinE reaction. (a) E. coli MV1190 incubated with CHQ; (b) E. coli MV1190(pMYLE2) incubated with CHQ; (c) E. coli MV1190 incubated with HQ; (d) E. coli MV1190(pMYLE2) incubated with HQ. After incubation, all samples were extracted by ethyl acetate followed by trimethylsilylation. The mass spectrum of each peak is indicated in Table 2.
TABLE 2.
Mass spectra of the peaks in Fig. 5
| Peaka | Mass spectrum m/z (relative intensity) |
|---|---|
| A, B, C | 374 (0.46), 359 (8.58), 315 (5.08), 257 (100), 241 (2.56), 197 (3.02), 147 (47.16), 123 (8.13), 95 (14.41), 73 (71.15) |
| D | 376 (8.65), 361 (61.96), 317 (26.81), 286 (53.72), 259 (15.51), 243 (25.93), 231 (48.03), 169 (70.72), 147 (71.03), 125 (38.33), 97 (17.79), 73 (100) |
| Authentic β-ketoadipate | 376 (7.73), 361 (59.74), 317 (27.87), 286 (53.82), 259 (16.18), 243 (27.78), 231 (46.83), 169 (63.55), 147 (64.13), 125 (47.89), 97 (19.76), 73 (100) |
| E, F, G, H, I | 286 (2.13), 271 (62.34), 257 (7.73), 243 (10.15), 169 (70.51), 153 (34.31), 147 (50.42), 143 (60.95), 73 (100) |
All peaks are those of trimethylsilylated samples.
Next, we investigated O2 consumption during the reaction by LinE. We used crude cell extracts of E. coli MV1190 overproducing LinE and of E. coli MV1190 overproducing XylE (C23O of Pseudomonas aeruginosa JI104 [20]) as a control. The results (Table 3) indicated that LinE consumed O2 when CHQ and HQ were added but not when catechol was added. On the other hand, XylE consumed O2 only when catechol was added. The activity of LinE against (C)HQ was much lower than that of XylE against catechol. Possibly because LinE formed inclusion bodies when overproduced in E. coli (data not shown) and in the resultant crude cell extract, there seemed to be very little soluble LinE.
TABLE 3.
Substrate specificities of crude cell extracts overproducing LinE or XylE against (C)HQ and catechol and activities of LinE mutants
| Protein | Sp act (U/mg of protein) fora:
|
||
|---|---|---|---|
| CHQ | HQ | Catechol | |
| LinE | 8.8 × 10−1 | 2.7 × 10−1 | ND |
| XylE | ND | ND | 99 |
| LinE H162A | ND | NT | NT |
| LinE H229A | ND | NT | NT |
| LinE E278A | ND | NT | NT |
ND, not detectable; NT, not tested.
These results indicated that LinE is a novel type of meta-cleavage dioxygenase, (C)HQ 1,2-dioxygenase, which cleaves aromatic rings with two hydroxyl groups at para rather than ortho positions.
Site-directed mutagenesis.
For further confirmation that LinE is a member of meta-cleavage dioxygenases, we constructed three kinds of mutants. His162, His229, and Glu278, which correspond to the putative Fe(II) binding residues, were changed to alanine and designated H162A, H229A, and E278A, respectively. Activities of E. coli cell extracts producing mutant or wild-type LinE were measured by using CHQ as a substrate (Table 3). We confirmed by SDS-PAGE analysis that LinE and its mutants were synthesized in the same quantity. Activities of the mutants were not detected, indicating the importance of these amino acids for the activity of LinE, like other meta-cleavage dioxygenases.
DISCUSSION
We have cloned and sequenced the linE gene and partially characterized its protein product, LinE. Our findings suggest that LinE is a novel type of meta-cleavage dioxygenase which cleaves the aromatic ring with two hydroxyl groups at para positions. “meta cleavage” describes the manner of cleavage of the ring fission dioxygenases, which cleave outside two adjacent hydroxyl groups. Although the cleavage style of LinE does not fit the definition of meta cleavage, we categorize LinE as a member of meta-cleavage dioxygenases on the basis of its amino acid sequence similarity to them. In fact, catalytic amino acids of these dioxygenases are highly conserved in LinE. Some degradation pathways of aromatic compounds in which hydroquinones are used as a direct ring cleavage substrate have been reported (4, 36, 32), but to our knowledge there is no information about a gene or a protein responsible for cleavage of hydroquinones. LinE is the first ring cleavage enzyme which cleaves (C)HQ in preference to catechol, which is one of the general substrates for meta-cleavage dioxygenases. In fact, we showed that C23O (XylE) cleaves catechol rather than (C)HQ. We believe that LinE recognizes two hydroxyl groups at para positions. This study represents the direct demonstration of a new degradation pathway for aromatic compounds, the hydroquinone pathway. Next, we plan to purify and characterize the LinE protein.
The homology search showed that LinE does not exhibit a high level of similarity to known enzymes except for some proteins. One of them, Orf88′ of M. extorquens AM1, has homology to some C23Os, although orf88′ is an incomplete ORF and its function is unknown (37). The alignment between LinE and meta-cleavage dioxygenases showed that LinE has nearly all residues for an active center and Fe(II) binding which are well conserved among the meta-cleavage dioxygenases. LinE also has a high level of similarity to PcpA of S. chlorophenolica, whose function is not known (41). Chanama and Crawford discussed the function of PcpA as 2,6-DCHQ chlorohydrolase (3). However, we suspect that PcpA is also a kind of (chloro)hydroquinone dioxygenase because of its sequence similarity to LinE. We had also isolated the pcpA gene from S. chlorophenolica independently as the gene which is responsible for 2,5-DCHQ degradation and showed that PcpA also had a ring cleavage dioxygenase activity (30).
The metabolites of (C)HQ were also identified. LinE cleaves CHQ between two carbon atoms (C-1 and C-2) which are substituted by hydroxyl group and chlorine group, respectively. The product, acylchloride, seems to react with water to form the resultant product, maleylacetate. In this study, however, β-ketoadipate was also detected. The conversion of maleylacetate to β-ketoadipate may not to be due to LinE because when we used partially purified LinE, the peak of β-ketoadipate did not appear (22). The cause of conversion of maleylacetate to β-ketoadipate in E. coli is unknown. Maleylacetate reductase, which converts maleylacetate to β-ketoadipate, was found in some degradation pathways of aromatic compounds (5, 15, 17, 34). We are now trying to identify the gene encoding maleylacetate reductase from UT26. So far, we have not found a region homologous to maleylacetate reductase in the flanking regions of linE and linD. As for acylchloride converted from CHQ by LinE, a previous study reported that when 3-chlorocatechol was cleaved by C23O, the resultant metabolite, acylchloride, bound with the C23O and inactivated it (1). C23O of P. putida GJ31, however, can avoid the suicidal inactivation by acylchloride which was formed from 3-chlorocatechol by its own activity, and the resultant acylchloride reacts with water to form 2-hydroxymuconate (16). From our results, LinE can also avoid the inactivation by the acylchloride, and the acylchloride is likely to react with water to form maleylacetate.
From this and previous studies, S. paucimobilis converts γ-HCH to (C)HQ by LinA, LinB, LinC, and LinD, and then (C)HQ is cleaved by LinE in a meta-cleavage manner. The resultant metabolite, maleylacetate, appears to be degraded by the following β-ketoadipate pathway, which is known as part of the ortho-cleavage degradation pathway of catechol or 1,2,4-trihydroxybenzene (10). The pathway from CHQ to γ-HMSA via HQ may not be a major pathway because the conversion rate of CHQ to HQ by LinD is very slow (23), and the substrate specificity of LinE against HQ is lower than that for CHQ (Table 3). We are now trying to identify the gene involved in the degradation of γ-HMSA. However, the possibility that γ-HMSA is a dead-end product in the γ-HCH degradation pathway of UT26 as 1,2,4-TCB and 2,5-DCP cannot be excluded.
ACKNOWLEDGMENTS
We thank A. Kitayama for the gift of plasmid pCY385. This work was carried out by using the facilities of the Biotechnology Research Center, The University of Tokyo.
K.M. was financially supported by research fellowships from the Japan Society for the Promotion of Science for Young Scientists. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Bartels I, Knackmuss H J, Reineke W. Suicide inactivation of catechol 2,3-dioxygenase from Pseudomonas putida mt-2 by 3-halocatechols. Appl Environ Microbiol. 1984;47:500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candidus S, van Pee K H, Lingens F. The catechol 2,3-dioxygenase gene of Rhodococcus rhodochrous CTM: nucleotide sequence, comparison with isofunctional dioxygenases and evidence for an active-site histidine. Microbiology. 1994;140:321–330. doi: 10.1099/13500872-140-2-321. [DOI] [PubMed] [Google Scholar]
- 3.Chanama S, Crawford R L. Mutational analysis of pcpA and its role in pentachlorophenol degradation by Sphingomonas (Flavobacterium) chlorophenolica ATCC 39723. Appl Environ Microbiol. 1997;63:4833–4838. doi: 10.1128/aem.63.12.4833-4838.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darby J M, Taylor D G, Hopper D J. Hydroquinone as the ring-fission substrate in the catabolism of 4-ethylphenol and 4-hydroxyacetophenone by Pseudomonas putida JD1. J Gen Microbiol. 1987;133:2137–2146. [Google Scholar]
- 5.Daubaras D L, Danganan C E, Hubner A, Ye R W, Hendrickson W, Chakrabarty A M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by Burkholderia cepacia strain AC1100: evolutionary insight. Gene. 1996;179:1–8. doi: 10.1016/s0378-1119(96)00326-5. [DOI] [PubMed] [Google Scholar]
- 6.Eltis L D, Bolin J T. Evolutionary relationships among extradiol dioxygenases. J Bacteriol. 1996;178:5930–5937. doi: 10.1128/jb.178.20.5930-5937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuenmayor S L, Wild M, Boyes A L, Williams P A. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J Bacteriol. 1998;180:2522–2530. doi: 10.1128/jb.180.9.2522-2530.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S, Eltis L D, Timmis K N, Muchmore S W, Bolin J T. Crystal structure of the biphenyl-cleaving extradiol dioxygenase from a PCB-degrading pseudomonad. Science. 1995;270:976–980. doi: 10.1126/science.270.5238.976. [DOI] [PubMed] [Google Scholar]
- 10.Harwood C S, Parales R E. The beta-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 11.Imai R, Nagata Y, Fukuda M, Takagi M, Yano K. Molecular cloning of a Pseudomonas paucimobilis gene encoding a 17-kilodalton polypeptide that eliminates HCl molecules from γ-hexachlorocyclohexane. J Bacteriol. 1991;173:6811–6819. doi: 10.1128/jb.173.21.6811-6819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai R, Nagata Y, Senoo K, Wada H, Fukuda M, Takagi M, Yano K. Dehydrochlorination of γ-hexachlorocyclohexane (γ-BHC) by γ-BHC-assimilating Pseudomonas paucimobilis. Agric Biol Chem. 1989;53:2015–2017. [Google Scholar]
- 13.Janssen D B, Pries F, Ploeg J V, Kazemier B, Terpstra P, Witholt B. Cloning of 1,2-dichloroethane degradation genes of Xanthobacter autotrophicus GJ10 and expression and sequencing of the dhlA gene. J Bacteriol. 1989;171:6791–6799. doi: 10.1128/jb.171.12.6791-6799.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasberg T, Daubaras D L, Chakrabarty A M, Kinzelt D, Reineke W. Evidence that operons tcb, tfd, and clc encode maleylacetate reductase, the fourth enzyme of the modified ortho pathway. J Bacteriol. 1995;177:3885–3889. doi: 10.1128/jb.177.13.3885-3889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasberg T, Seibert V, Schlomann M, Reineke W. Cloning, characterization, and sequence analysis of the clcE gene encoding the maleylacetate reductase of Pseudomonas sp. strain B13. J Bacteriol. 1997;179:3801–3803. doi: 10.1128/jb.179.11.3801-3803.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaschabek S R, Kasberg T, Müller D, Mars A E, Janssen D B, Reineke W. Degradation of chloroaromatics: purification and characterization of a novel type of chlorocatechol 2,3-dioxygenase of Pseudomonas putida GJ31. J Bacteriol. 1998;180:296–302. doi: 10.1128/jb.180.2.296-302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaschabek S R, Reineke W. Degradation of chloroaromatics: purification and characterization of maleylacetate reductase from Pseudomonas sp. strain B13. J Bacteriol. 1993;175:6075–6081. doi: 10.1128/jb.175.19.6075-6081.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keil H, Lebens M R, Williams P A. TOL plasmid pWW15 contains two nonhomologous, independently regulated catechol 2,3-oxygenase genes. J Bacteriol. 1985;163:248–255. doi: 10.1128/jb.163.1.248-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimbara K, Hashimoto T, Fukuda M, Koana T, Takagi M, Oishi M, Yano K. Cloning of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J Bacteriol. 1989;171:2740–2747. doi: 10.1128/jb.171.5.2740-2747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitayama A, Achioku T, Yanagawa T, Kanou K, Kikuchi M, Ueda H, Suzuki E, Nishimura H, Nagamune T, Kawakami Y. Cloning and characterization of extradiol aromatic ring-cleavage dioxygenases of Pseudomonas aeruginosa JI104. J Ferment Bioeng. 1996;82:217–223. [Google Scholar]
- 21.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 22.Miyauchi, K., Y. Nagata, and M. Takagi. Unpublished data.
- 23.Miyauchi K, Suh S K, Nagata Y, Takagi M. Cloning and sequencing of a 2,5-dichlorohydroquinone reductive dehalogenase gene which is involved in the degradation of γ-hexachlorocyclohexane in Sphingomonas paucimobilis. J Bacteriol. 1998;180:1354–1359. doi: 10.1128/jb.180.6.1354-1359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagasawa S, Kikuchi R, Nagata Y, Takagi M, Matsuo M. Aerobic mineralization of γ-HCH by Pseudomonas paucimobilis UT26. Chemosphere. 1993;26:1719–1728. [Google Scholar]
- 25.Nagata Y, Imai R, Sakai A, Fukuda M, Yano K, Takagi M. Isolation and characterization of Tn5-induced mutants of Pseudomonas paucimobilis UT26 defective in γ-hexachlorocyclohexane dehydrochlorinase (LinA) Biosci Biotechnol Biochem. 1993;57:703–709. doi: 10.1271/bbb.57.703. [DOI] [PubMed] [Google Scholar]
- 26.Nagata Y, Miyauchi K, Suh S, Futamura A, Takagi M. Isolation and characterization of Tn5-induced mutants of Sphingomonas paucimobilis defective in 2,5-dichlorohydroquinone degradation. Biosci Biotechnol Biochem. 1996;60:689–691. [Google Scholar]
- 27.Nagata Y, Nariya T, Ohtomo R, Fukuda M, Yano K, Takagi M. Cloning and sequencing of a dehalogenase gene encoding an enzyme with hydrolase activity involved in the degradation of gamma-hexachlorocyclohexane in Pseudomonas paucimobilis. J Bacteriol. 1993;175:6403–6410. doi: 10.1128/jb.175.20.6403-6410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata Y, Ohtomo R, Miyauchi K, Fukuda M, Yano K, Takagi M. Cloning and sequencing of a 2,5-dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase gene involved in the degradation of gamma-hexachlorocyclohexane in Pseudomonas paucimobilis. J Bacteriol. 1994;176:3117–3125. doi: 10.1128/jb.176.11.3117-3125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neidle E, Hartnett C, Ornston L N, Bairoch A, Rekik M, Harayama S. cis-diol dehydrogenases encoded by the TOL pWW0 plasmid xylL gene and the Acinetobacter calcoaceticus chromosomal benD gene are members of the short-chain alcohol dehydrogenase superfamily. Eur J Biochem. 1992;204:113–120. doi: 10.1111/j.1432-1033.1992.tb16612.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohtsubo, Y., K. Miyauchi, K. Kanda, T. Hatta, H. Kiyohara, T. Senda, Y. Nagata, Y. Mitsui, and M. Takagi. PcpA, which is involved in the degradation of pentachlorophenol in Sphingomonas chlorophenolica ATCC 39723, is a novel type of ring-cleavage dioxygenase. FEBS Lett., in press. [DOI] [PubMed]
- 31.Palleroni N J. Genus I, Pseudomonas. In: Kreig N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. p. 198. [Google Scholar]
- 32.Prakash D, Chauhan A, Jain R K. Plasmid-encoded degradation of p-nitrophenol by Pseudomonas cepacia. Biochem Biophys Res Commun. 1996;224:375–381. doi: 10.1006/bbrc.1996.1036. [DOI] [PubMed] [Google Scholar]
- 33.Rieble S, Joshi D K, Gold M H. Purification and characterization of a 1,2,4-trihydroxybenzene 1,2-dioxygenase from the basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1994;176:4838–4844. doi: 10.1128/jb.176.16.4838-4844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seibert V, Stadler-Fritzsche K, Schlomann M. Purification and characterization of maleylacetate reductase from Alcaligenes eutrophus JMP134(pJP4) J Bacteriol. 1993;175:6745–6754. doi: 10.1128/jb.175.21.6745-6754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senda T, Sugiyama K, Narita H, Yamamoto T, Kimbara K, Fukuda M, Sato M, Yano K, Mitsui Y. Three-dimensional structures of free form and two substrate complexes of an extradiol ring-cleavage type dioxygenase, the BphC enzyme from Pseudomonas sp. strain KKS102. J Mol Biol. 1996;255:735–752. doi: 10.1006/jmbi.1996.0060. [DOI] [PubMed] [Google Scholar]
- 36.Spain J C, Gibson D T. Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl Environ Microbiol. 1991;57:812–819. doi: 10.1128/aem.57.3.812-819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Springer A L, Ramamoorthi R, Lidstrom M E. Characterization and nucleotide sequence of pqqE and pqqF in Methylobacterium extorquens AM1. J Bacteriol. 1996;178:2154–2157. doi: 10.1128/jb.178.7.2154-2157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terada I, Kwon S-T, Miyata Y, Matsuzawa H, Ohta T. Unique precursor structure of an extracellular protease, aqualysin I, with NH2- and COOH-terminal pro sequences and processing in E. coli. J Biol Chem. 1990;265:6576–6581. [PubMed] [Google Scholar]
- 39.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 40.Werwath J, Arfmann H A, Pieper D H, Timmis K N, Wittich R M. Biochemical and genetic characterization of a gentisate 1, 2-dioxygenase, from Sphingomonas sp. strain RW5. J Bacteriol. 1998;180:4171–4176. doi: 10.1128/jb.180.16.4171-4176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xun L, Orser C S. Purification of a Flavobacterium pentachlorophenol-induced periplasmic protein (PcpA) and nucleotide sequence of the corresponding gene (pcpA) J Bacteriol. 1991;173:2920–2926. doi: 10.1128/jb.173.9.2920-2926.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yrjala K, Paulin L, Kilpi S, Romantschuk M. Cloning of cmpE, a plasmid-borne catechol 2,3-dioxygenase-encoding gene from the aromatic- and chloroaromatic-degrading Pseudomonas sp. HV3. Gene. 1994;138:119–121. doi: 10.1016/0378-1119(94)90792-7. [DOI] [PubMed] [Google Scholar]
- 43.Zylstra G J, Gibson D T. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli. J Biol Chem. 1989;264:14940–14946. [PubMed] [Google Scholar]